Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a12593
RESEARCH ARTICLE
Dung beetles (Scarabaeidae: Scarabaeinae) attracted to carrion in Ghana, West Africa and evidence for adult food source plasticity
T Keith PhilipsI; Christie A SukhdeoII; Stewart B PeckIII
ISystematics and Evolution Laboratory, Department of Biology, Western Kentucky University, Bowling Green, KY, USA
IIDepartment of Biological Sciences, University of New Orleans, New Orleans, USA
IIIResearch and Collections Division, Entomology, Canadian Museum of Nature, Ottawa, Canada
ABSTRACT
A study on dung beetles attracted to carrion using baited pitfalls was conducted in eight Upper Guinean wet and moist forest sites as well as one savannah site in Ghana, West Africa. A total of 42 species and 1380 individuals were collected from all sites. The highest diversity was found in the Shai Hills savannah with 19 species while the lowest total of only four species was collected in the Cape Three Points forest. The forest sites combined had seven unique species while the savannah locality had 12 unique taxa. Most carrion feeders belong to the genus Onthophagus; Onthophagus liberianus made up 23% of the total catch and together with the next nine most abundant species accounted for 78% of the specimens collected. Two dung beetle tribes of African savannah species not noted as carrion feeders were strongly attracted to vertebrate carrion and included a member of the Oniticellini, Latodrepanus caelatus (Gerst.) and the Onitini, Onitis cupreus Castelnau. Additionally, a forest species of Sisyphini, Neosisyphus angulicollis Felsche, that is uncommon on carrion was attracted to carrion in large numbers. For two species, Onthophagus liberianus and O. rufopygus, studied herein and previously in the Ivory Coast, the relative attractiveness of carrion and dung in each country varied greatly, demonstrating behavioral plasticity in food choice.
Keywords: carrion beetles, diversity, food plasticity, Ivory Coast, upper Guinean forests, West African savannah
INTRODUCTION
Beetles of the subfamily Scarabaeinae are commonly known as dung beetles due to their typical specialised habit of coprophagy. However, many species appear to be opportunistic and will use alternative food sources, in particular carrion but also fungi and fruit. These resources can either be a primary food source and used as both adult and larval brood food or as a secondary or supplemental source of nutrition for the adults only (Halffter and Matthews 1966).
Reports of scarabaeines attracted to or feeding on carrion are known from all continents, but for many species this food is utilised only if dung is not readily available (Halffter & Matthews 1966). Regardless, there are some specialist species and moreover the use of carrion is a relatively common alternative food source that has evolved many times, especially within tropical forest species where large herbivores are relatively uncommon (Halffter 1991). Notably large animals can still be common in some areas of Africa where elephants, buffalo, gorillas, and okapi for example, still exist.
One aspect of the food source for various scarabaeines is the degree of exclusivity. As noted by Halffter & Mathews (1966) some species are predominantly coprophagous and occasionally necrophagous, other species have no preference, while others are mainly necrophagous, and lastly some exclusively use carrion. The preference of carrion for some dung beetles and not just dung remaining inside a carcass has also been demonstrated (Stone et al. 2021).
Most traditionally defined tribes of scarabaeines include at least one species that is either solely necrophagous or feeds upon both carrion and dung, with the notable exception of the Argentinian Eucraniini and the Old World Onitini, where no records of carrion association are known. In the Old World, carrion associated records of adults appear to be either rare or generally absent in the Gymnopleurini, Onitini, Oniticellini and the Sisyphini (see scattered records for e.g. in Balthasar 1963; Cambefort 1991a; Daniel 2020; Davis et al. 2008; Halffter & Matthews 1966; Panin 1957; Vinson 1939).
Within the New World, necrophagous specialist Scarabaeinae that use carrion as larval food are relatively abundant and include five tribes and species within nine genera; the Coprini or Ateuchini (Pedaridium and Canthidium), Dichotomiini (Uroxys), Canthonini (Canthon and Deltochilum), Onthophagini (Onthophagus) and the Phanaeini (Coprophanaeus, Megaphanaeus, and Phanaeus) as reported in various studies (e.g. Amézquita & Favila 2011; Favila 2001; Halffter 2003; Halffter & Matthews 1966; Hernández et al. 2002; Janzen 1983; Martinez 1959; Moron 1979, 1994; Morone et al. 1985; Morone et al. 1986; Silva et al. 2007).
Many other genera in this region, such as Agamopus, Ateuchus, Boreocanthon, Canthonella, Copris, Glaphyrocanthon, Malagoniella, Pedaridium, Pseudocanthon, Onthophagus, Trichillum and Uroxys, include species that are considered generalists (e.g. Novelo et al. 2006) and will use both carrion and dung or are at least attracted to carrion to various degrees (Halffter & Matthews 1966). There are also reports of eurysternines that are attracted to this food source (Luederwaldt 1910 [1911], Genier 2009) but no species appear to be carrion feeding specialists. Notably, in a longterm study in Amazonian Brazil, 34 of the 66 species attracted to dung were also collected using carrion bait and no carrion specialists were discovered (Ratcliff 2013).
In contrast, the Old World has only three or likely four genera that are specialist necrophages. The Onthophagini have two genera with exclusive carrion feeding species, including taxa in the genus Amietina as well as many species of Onthophagus (Cambefort & Walter 1991; Hanski 1983; Hanski & Krikken 1991; Tshikae et al. 2008) and the Scarabaeus subgenus Sceliages (Bernon 1981; Forgie et al. 2002). A record from Tshikae et al. (2008) of a Catharsius species may represent a fourth tribe (Coprini) that is also a carrion specialist. Most other Old-World taxa showing an association with carrion include species of Caccobius, Neosisyphus and Sisyphus (Cambefort 1991a; Cambefort & Walter 1991). It is unclear though how many individuals were found in each case to indicate degree of attraction and whether carrion is used as a brood food or just for supplemental adult feeding.
The varieties of carrion utilised by necrophagous dung beetles are diverse in nature; species will feed upon various vertebrate land animals and fish, as well as specialist species that feed solely upon dead arthropods (Halffter & Matthews 1966). These specialists include species of Onthophagus in both Africa and southeast Asia (Hanski & Cambefort 1991; Krell et al. 1997; Brühl & Krell 2003) as well as Sceliages (Bernon 1981; Forgie et al. 2002). At least one species in the New World, Ateuchus histeroides Weber, has been reported to successfully use insect carrion for rearing offspring (Young 2006). Two taxa deserve mention as they also use animal carcasses as brood food, but unique within the dung beetles, as they are carnivores; the New World canthonines Deltochilum valgum Burmeister attacks and kills millipedes (Navajas 1950; Larsen et al. 2009) while Canthon dives Harold kills and uses reproductive leaf cutter ants (Lichti 1937; Forti et al. 2012).
When feeding upon carrion, the necrophagous Scarabaeinae work in a similar manner as their coprophagous relations; very small animal corpses can be rolled away or buried whole and utilised for food or breeding, while larger corpses can be disassembled and turned into a food or brood ball before being rolled away and buried (Hanski 1987). This consumption, dispersal and burial, of carrion serves purposes similar to that of dung use; in clearing an area of carrion, the beetles return essential nutrients to the soil and simultaneously reduce the number of potentially pathogenic species in the area by depriving them of a place to breed (Hanski 1987). Hence dung beetles feeding on both dung and carrion are extremely beneficial to the environment through their contributions, especially through the burial of their food, which has the potential to increase the return of nitrogen to soil by as much as four times, significantly improving the quality of soil for plant growth. In addition, it has been suggested that changes in dung beetle populations could have rippling effects that impact the population sizes of various dung and carrion breeding flies and parasites, thus altering the rest of the ecosystem through changes in disease and parasite prevalence (Klein 1989).
The dung beetles in Africa which are attracted to vertebrate carrion have been studied mainly in the drier regions of southern Africa (e.g. Davis 1994; Tshikae et al. 2008), North Africa (Hegazi et al. 1991) as well as savannah sites in West Africa (Cambefort 1991b). Studies in the moist or wet forests are fewer but have been done in the Tai National Park, Ivory Coast (Cambefort 1985, 1991) and at the Makokou Research Station in Gabon (Cambefort & Walter 1981, 1991). Therefore, the primary goal of this study is to further our limited knowledge on the dung beetle species attracted to vertebrate carrion in the Upper Guinean forests and also compare this fauna to a savannah habitat within Ghana, West Africa. In particular, this study enables the comparison of different protected areas of forest to indicate the level of threat from bush meat hunting. Lastly, this is a record of the diversity found in these rapidly shrinking forests and the data herein can be used to monitor future species changes; unfortunately, the dung beetle diversity in this region likely will become significantly more depauperate in the future.
MATERIALS AND METHODS
Study Sites
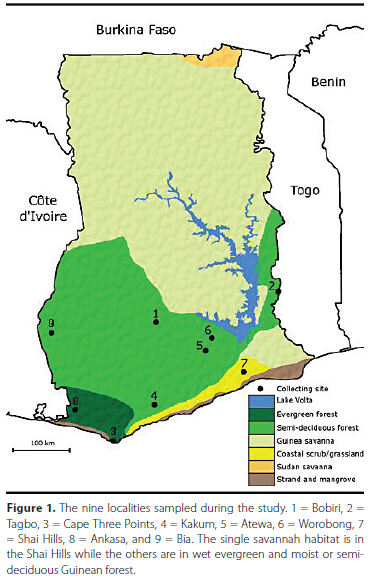
The West African Upper Guinean Forest is one of the biodiversity hotspots in the world and one of only five in Africa (Myers et al. 2000, Mittermier et al. 2011). Further, these forests can be considered the most fragmented rainforests in the world (Minnemayer 2002). The Guinean Forest historically was extensive, but the current amount of intact and disturbed forest remaining is now dramatically low (Figure S1). While field work in the southern half of Ghana was the primary survey goal, the savannah extension (Dahomey Gap) from the north to the southern coast in the southeast was an obvious and easy addition and a useful comparison. The Shai Hills Reserve is part of this extension from the north that separates the Upper Guinean Forest into a western and eastern block. Notably, there is a rainfall gradient from SW to NE from true wet evergreen forest (Ankasa and Cape Three Points), through moist forest to semi-deciduous forest zones found in all other sites.
Sampling took place in nine localities (Figure 1, Table S1) in the wet to moist and semideciduous Guinean Forest located primarily in the southcentral and southwestern part of Ghana as well as the Volta region (Tagbo) and in the Shai Hills in a location of primarily savannah near one of the more thickly forested hills. This mainly open savannah site consists of scattered scrub or low stature trees mainly of non-native Neem (Azadirachta indica A. Juss). All collections occurred once in each site during the primary rainy season in June or July over a period of three years from 2005-2007. Dung baited pitfalls using pig, cow, and human dung (10 of each with approximately 28 g of dung/trap) were also set at the same time and used in some cases for comparisons of species capture rates.
Each pitfall trap consisted of a square-shaped 474 ml plastic container sunk in the substrate so that the top edge was level with the soil. Water with a small amount of liquid dish soap (to act as a surfactant) was added to each container up to about 6 cm in depth to make sure all beetles attracted to the bait were captured. Bait consisted of one large-sized full chicken wing that was left at room temperature for approximately two days until there was a strong odour of decay. The wing was then wrapped in a layer of cheesecloth and then hung by string in the middle of a square piece of wire mesh (hardware cloth) with approximately 6.45 cm square holes separating the mesh wire in a grid pattern. The hardware cloth was staked down at each corner with the bait suspended within the plastic trap container. At each site, four traps were set in a grid pattern with each trap separated by a minimum of at least 50 m to avoid pseudo-replication or trap interference. Traps were set for 48 hours after which specimens were collected and stored in ethanol.
Data analysis
Data were entered into an Excel matrix with the four samples for each site and the number of each species. Diversity indices and rarefaction or species accumulation curves were produced using Estimate S (Colwell 2013 and see Colwell et al. 2004). PC-ORD version 6.0 software (McCune & Mefford 1999) was used to create non-metric multi-dimensional scaling ordination plots for site comparisons. The species matrix and dendrogram of species occurrence was created by a cluster analysis using the Bray-Curtis and group average linking method.
Voucher material is deposited in the TK Philips collection (Bowling Green, KY) and the Canadian Museum of Nature (Ottawa, ON).
RESULTS
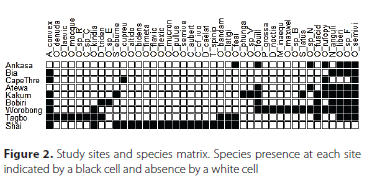
Forty-two species and 1 398 individuals of Scarabaeinae were captured in carrion baited pitfalls with an average number of species per site of 8.9 ± 4.6 during this study (Table S2). In addition to the Scarabaeinae, two species of Trogidae and one species of Hybosoridae were collected (data not included in the analyses). The majority of species (26 of 42) belong to the genus Onthophagus (Figure S2a, S2b). The next most speciose genus was Caccobius (three species) and two species each of Sisyphus, Diastellopalpus, and Milichus were also trapped. Also attracted to carrion were single species in the genera Chalconotus, Copris, Latodrepanus, Neosisyphus, Onitis, Pseudopedaria, and Tiniocellus. Rank abundance of species collected from all forest sites and the Shai Hills savannah is shown in Figure S2a while Figure S2b shows rank abundance from the forest sites only.
Overall, the highest number of species (19) were collected in the Shai Hills savannah site (Figure 2, Table 1). The most diverse forest sites were Worobong and Tagbo with 16 and 15 species each, respectively. The sites with the lowest species diversity were found in Cape Three Points and Bia with only four and five species respectively.
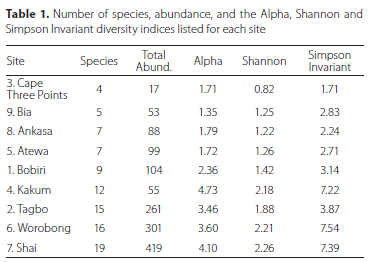
Onthophagus liberianus was the most abundant taxon with 320 individuals collected or 23% of the total catch (Figure S2a, b). Nine additional species of this genus were the next most abundant overall and including O. liberianus accounted for 1 079 or 78% of the individuals collected (Fig. 3a, 3b). Onthophagus species alone made up 1 197 of the 1 380 individuals collected or over 86% of the total.
For the diversity indices (Table 1), Kakum had the highest alpha diversity, Worobong had the highest Simpson invariant diversity, and Shai Hills had the highest Shannon diversity. A fourth site, Tagbo, was also close in these relatively high diversity values found in these three previous sites.
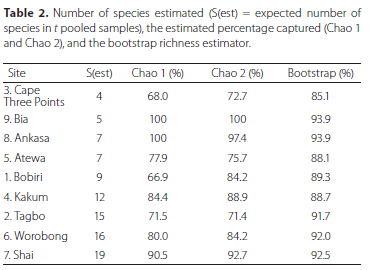
Estimation of total species captured based on the bootstrap (Table 2) ranged from a low of 85% in Cape Three Points to a maximum of 94% in Ankasa. The estimation with the broadest range is seen in the Chao 1, with the estimated percentage of species captured ranging from a low of about 67% or 68% in Bobiri and Tagbo to a high of 100% in both Ankasa and Bia. In contrast, based on species accumulation curves, it is possible that not all species attracted to carrion were sampled from any single site (Figure S3).
The species shared between sites were generally comparatively low. The lowest faunal similarities occurred between Shai Hills savannah and any one of the forest sites with no or up to two species in common excluding the notable exception of the Tagbo site which shared five species. The two most similar sites were Ankasa and Atewa which shared six of their seven total species.
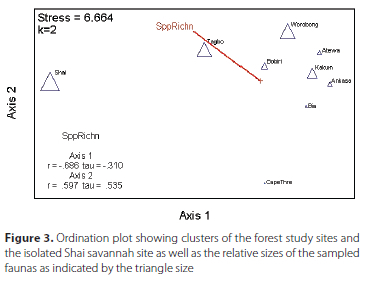
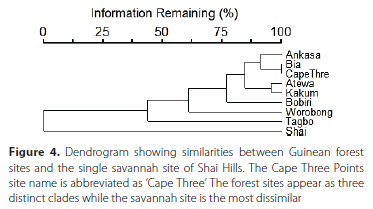
For total abundance (Table S2), the Shai Hills sampling resulted in 419 individuals collected and Worobong and Tagbo had the next highest number with 301 and 261 individuals respectively. The ordination plot (Figure 3) distinctly shows the forest study sites clustered and isolated from the Shai savannah site. Similarly, a dendrogram (Figure 4) shows the similarity among some of the forest sites (three primary clades) and the isolation of the single savannah site of Shai Hills. The clade of Ankasa, Bia, and Cape Three Points may show the influence of geography as all are located in southwestern Ghana while the sister clade of Atewa and Kakum and the two more distant (Bobiri and Worobong) are all located in south-central Ghana. The Tagbo forest, located in extreme east-central Ghana in the Volta Region, together with the Worobong site further west, is the sister clade to all other forest sites.
The Shai Hills savannah site sampling resulted in the capture of 15 individuals of Latodrepanus caelatus, a member of the Oniticellini, a tribe that generally has not had any members reported as vertebrate carrion feeders. This site also resulted in the collection of 27 individuals of Onitis cupreus, an Onitini whose members are considered to be strict dung feeders. Similarly, a notable record of a forest species of sisyphine, Neosisyphus anguilicollis was captured in five of eight forest sites and three sites had eight to 11 individuals indicating considerable attraction to carrion.
DISCUSSION
Dung beetles for the most part are coprophagous, but there are many species that are either strictly necrophagous or use both as food sources to various degrees. At the traditional tribal level classification (Philips 2008), there are records of necrophagy in all groups except for the eucraniines and the onitines. Regarding species richness, dung beetle diversity in African forests compared to savannahs is often lower. Further, the necrophagous species that specialise on vertebrate carrion are thought by some to be restricted to forest habitats and largely absent in savannahs (e.g. Cambefort & Walter 1981). Hence, when comparing the savannah Shai Hills site with those sampled in the Guinean forests, it was expected that the former location would have a less diverse fauna. However, species attracted to carrion in the Shai Hills savannah were the most diverse (19 species) and abundant (419 individuals) compared to all forest sites. The presence of mammals in the Shai savannah is easily noted, with abundant Kob antelope (Kobus kob (Erxleben)) and Chacma Baboons (Papio ursinus (Kerr)) in particular.
The rainfall gradient in Ghana reflects the forest types with the highest moisture levels in the SW (supporting wet evergreen forest) to gradually decreasing levels towards the NE (supporting moist evergreen transitioning into fully deciduous forest and then savannah). This parallels the geographical trend of similarities in species composition between reserves shown especially by the dendrogram (Figure 6) with, for example, the four forests in the SW clustered together. The ordination plot (Figure 5) also shows this species similarity between reserves as well as the general gradient in species richness with Cape Three Points, Ankasa, and Bia the least diverse in the southwest followed by Kakum, Atewa, and Bobiri successively richer to the highest diversity in the Worobong and Tagbo forest sites in the northeast.
The high variability in species richness among the reserves probably reflects various levels of disturbance to some degree. Cape Three Points and Bia appeared to be pristine habitats but the close vicinity of villages and the observations of numerous small snares as well as a bush meat hunter observed in the former suggest a high degree of pressure on the mammal fauna. In contrast, Worobong with some of the highest species diversity did have nearby villages but the people there considered monkeys sacred and do not hunt them and they were observed during our visit. Sadly, Bobiri when first visited by the senior author in 2003 was thought to have three species of monkey. This site was down to two species during a visit in 2006 when the collection for this study was done. Further, active logging was taking place during this time and some locations were heavily disturbed. During a final visit in 2009 the total primate fauna was suggested to be limited to a single species (A Boakye, E Adusei, S Boateng, pers. comm.) but it is possible there were none left by that time. Regardless, few mammals or their signs were observed in most of the forested sites during the trapping periods.
The dominance of species in the genus Onthophagus is notable with a single taxon alone, O. liberianus, accounting for 23% of the total catch. While most taxa sampled belong to tunneling clades as is typical in African forests, a few belong to the rolling guilds including three Sisyphini in the genera Neosisyphus and Sisyphus and one large Canthonini, Chalconotus convexus (Fab.).
The record of Latodrepanus caelatus attracted to vertebrate carrion in relatively large numbers may be the first substantial record of an oniticelline associated with this food source. Sampling indicated a relatively strong attraction to carrion with 15 individuals from four traps versus 29 individuals in 30 dung baited pitfalls trapped during the same visit (Philips pers. obs.). In comparison and notably, a study by Cambefort (1985) in an Ivory Coast savannah also collected this same species as well as three other closely related taxa of Oniticellini (Ixodina Roth, Latodrepanus Krikken (note Drepanellus Barbero, Palestrini, and Roggero is a recent junior synonym) and Tibiodrepanus Krikken) in large numbers (3 804) and no individuals were collected with carrion. Additionally, members of the Onitini are thought to be strict dung feeders (Davis et al. 2008), but 27 individuals of Onitis cupreus were collected with carrion bait and four traps in the Shai Hills. In comparison, 47 were collected during the same visit with 30 dung-baited pitfalls (10 each of human, cow, and pig) at the same time (Philips pers. obs.). Regardless, although there was a strong attraction of these species to carrion, it is not clear if any of these records indicate a true association defined as when carrion is used as a brood food.
The collecting of Latodrepanus caelatus and Onitis cupreus in a savannah habitat is significant as vertebrate carrion feeders in the Scarabaeinae are generally known only as forest denizens, especially in Africa (Cambefort & Walter 1991). The reason for the shift to a broader array of food sources in this savannah is unclear. Typically there a negative selective force for use of carrion by dung beetles in savannahs due to strong competition with vertebrate scavengers. Two species of vultures are known in the Shai Hills (Dowsett-Lemaire & Dowsett 2013) while large mammal scavengers such as hyenas in this reserve are no longer present. Hence this record may represent a shift from dung specialisation to more of a generalist feeder as reported in some of the helictopleurines in Madagascar for example (Miraldo et al. 2011).
Sisyphines in general are not thought to be strongly attracted to carrion (Davis et al. 2008). However, a notable record in this study is of a forest sisyphine, Neosisyphus angulicollis, that was captured in five of eight forest sites and in relatively large numbers in three sites (eight, nine, and 11 individuals). The number collected with all dung baits at the same time was higher (11, 45, and 232 individuals in the same sites), but those numbers notably were from 30 traps. In the Cambefort (1985) study in Tai, Ivory Coast, only two individuals of this species were collected on carrion versus 76 on dung of various types. In one study in an Ivory Coast savannah (Cambefort & Walter 1991), 744 individuals of Sisyphus seminulum Gerst. were collected on carrion but that is, in contrast, to 8 650 collected with dung bait or more than 91% of the total. Regardless, the relatively high numbers of sisyphines collected with carrion in some cases does indicate that this food source as a source of adult supplemental feeding at a minimum is being used as an alternative to dung.
Based on the species accumulation curves (Figure S3), the sampling effort may not have discovered all species attracted to carrion in any area sampled, perhaps due to only using four traps at each site. Ratcliffe (2013) in his Amazonian study noted that it took 12 weeks to collect all the dung beetles attracted to carrion. Nevertheless, it is apparent that one of the largest blocks of remaining Guinean forest in Ghana represented by Worobong may be a reasonable but slightly low approximation of the number of dung beetle species attracted to carrion in this forest ecosystem. The reason for the low numbers of only four to five species collected in Bia and Cape Three Points is unclear but one can conclude it is from low numbers of mammals due to high levels of bush-meat hunting. This hunting effect similarly has been documented on Mount Cameroon where the middle elevation had both the highest dung beetle species diversity and abundance instead of the lowest elevation as expected (Mongyeh et al. 2019).
Overall, if carrion specialists are generally more diverse and abundant in forests, one can speculate that their reduction or even possible absence in savannahs is due to the presence of many large vertebrate scavengers that eliminate this potential food source for dung beetles. It may also be due to the overall lower numbers and biomass in particular of large mammals in forests compared to savannahs and concomittant less abundant sources of dung. One should note that large herbivores in Old World forests such as forest elephants and buffalo can make up a large proportion of mammal biomass compared to that found in the Neotropics which tend to have few large bodied mammals. The only large mammals left in Ghana at the time of sampling were elephants and chimpanzees in Ankasa and elephants in Bia and Kakum. Most of the species collected in this study certainly are not specialists on carrion and being a generalist would seem to be a strategy with higher level of fitness. In contrast, many species of Onthophagus sampled, such as O. bartosi, O. fasciculiger, and O. latigibber are probable or definite carrion specialists and all have also been collected in large numbers using millipede bait in Ivory Coast (Krell et al. 1997). Notably, millipedes can be quite abundant during the rainy season and can be a reliable food source and are likely the primary food source for all of these species; none of them have ever been collected on dung in any of the sampling done in West Africa in several studies (Cambefort 1991; Davis & Philips 2005; Davis & Philips 2009). Also O. intricatus and O. semiverescens may also be part of this feeding niche in part as neither have been collected with dung (Philips pers. obs.) and 17 and five specimens respectively were collected with vertebrate carrion in this study. Notably, two additional but unidentified species of Onthophagus carrion specialists were recently found in the woodland habitat of northeast Bostwana in Chobe National Park (Tshikae et al. 2008).
Other taxa, such as O. semiviridis and O. faciculiger, have been collected with dung, carrion, and millipede (carrion) bait (Cambefort 1985; Philips pers. obs.) and appear to be generalists. Others may be primarily dung feeders and can be collected with carrion, and have not been collected using millipede bait. These include O. pullus, O. bidens, O. flaviclava, O. mucronatus as well as the onitine, Onitis cupreus. Many of the species collected in small numbers in this study are likely primarily dung feeders. This includes a species such as O. laeviceps that can be abundant in dung-baited pitfall traps (Cambefort 1985) while only a single individual was collected with carrion in this study.
Curiously, some species sampled by Cambefort (1985) in Tai National Park were found to be very abundant on carrion but much less so on dung; this was the opposite to what was found in this study in some sampling locations in Ghana (Table 3). Onthophagus liberianus and O. rufopygus were often much more numerous in carrion traps or equally common in both carrion and dung (this study and Philips pers. obs.), but in sharp contrast in Tai both species were rare in carrion (2 individuals captured) and abundant in dung (190 individuals captured). In comparing this carrion study with samples collected with dung (Philips pers. obs.), the most extreme example of differences between populations was seen in O. liberianus that was very common in carrion and absent in dung in one location (Bobiri) with the oppposite attraction pattern in another location (Cape Three Points). Hence it appears that various populations of a given species of dung beetle may be more of a generalist or specialised feeder than others and can even switch to an alternative food source depending upon available food resources in a given locality. Perhaps similarly, some New Zealand dung beetle species may have evolved a generalist diet of dung and carrion in some areas and even from marine sources via sea birds that is possibly a result of low mammal numbers (Stavert et al. 2014).
The selective pressures that may account for these behavioural differences in food choice selection between populations are unclear but it most likely may be due to the relative availability of dung and carrion. Miraldo et al. (2011) discuss a general feature of dung beetle communities in tropical forests in Madagascar where a large proportion of the species have shifted from dung and either specialise on carrion or use both resources. The reason for this shift has been hypothesised due to extreme competition over dung and rapid decomposition rates in some tropical forests (Halffter 1991; Hanski 1991). Genetic drift may be an additional factor in a more rapid switch in food preference in any given population due to the small size of most of the remaining forest habitats in Ghana (Figure 1) and reduced population sizes.
To conclude, these results supply baseline data that can be used to monitor the populations and diversity of the dung beetles attracted to vertebrate carrion in the Upper Guinean Forests of Ghana. The climate crisis, deforestation, population growth and the less visible effects of bush-meat hunting that deplete the source of excrement as well as carrion for the specialist feeders does not bode well for maintaining species diversity of the dung beetles in this critical biodiversity hotspot in Ghana and elsewhere.
ACKNOWLEDGEMENTS
We first and foremost thank Henry Davis (Department of Animal Biology and Conservation Science, University of Ghana) for his assistance with logistics and field work while this study was conducted. We thank M. Adu-Asiah, Senior Wildlife Officer with the Ghana Wildlife Department, for permits to sample dung beetles. The local knowledge and technical assistance of our guides and helpers provided valuable assistance including SO Kwarteng ("Lobito"), Ezekio, Tony, and Mike in Ankasa; Thomas Quaicoe, Bernard Oudjoe and Emanuel Agquah and Justice Aban in Cape Three Points; Nicholas Boamah, Princess Ophilia Mensah, Charles Boatang, Antwi Coleman, Felix Kwakye and Philip Mensah at Bia; Cletus Nateg, John Nyame, Joseph Appiah, Baffo Peprah at Kakum; Fueini Damma and Kingsley Osei at Shai Hills; Kwasi Baffo at Atewa; Alfred Boakye, Evans Adusei, Samuel Boateng at Bobiri; Kofi Opoku and Paul Wuga at Tagbo; and Kwabena Adu-Benuah at Worobong. We also thank reviews from Adrian Davis, one anonymous reviewer, and editor James Pryke whose comments greatly improved the manuscript. The project was funded by a National Science Foundation Biological Surveys and Inventories grant (DEB-0430132).
AUTHOR CONTRIBUTIONS
T. Keith Philips - Funding acquisition, Investigation (33%), Project administration, Writing - original draft, Resources. Christie A. Sukhdeo - Formal Analysis, Writing - review and editing (10%).
Stewart B. Peck - Conceptualisation, Investigation (66%), Methodology, Writing - review and editing (5%).
ORCID IDS
T. Keith Philips - https://orcid.org/0000-0002-1190-4019
Christie A Sukhdeo - https://orcid.org/0000-0002-2899-3679
Stewart B Peck - https://orcid.org/0000-0002-3407-4978
REFERENCES
Amézquita S, Favila ME. 2011. Carrion removal rates and diel activity of necrophagous beetles (Coleoptera: Scarabaeinae) in a fragmented tropical rain forest. Environmental Entomology 40(2): 239-246. https://doi.org/10.1603/EN10203. [ Links ]
Balthasar V. 1963. Monographie der Scarabaeidae and Aphodiidae der Palaearktischen und Orientalischen Region, Coleoptera: Lamellicornia, Coprinae (Onitini, Oniticellini, Onthophagini). Tschechoslowakische Akademie der Wissenschaften, Prague. 1963:137-226.
Brashares JS, Arcese P, Sam MK. 2001. Human demography and reserve size predict wildlife extinction in West Africa. Proceedings of the Royal Society of London B 268(1484): 2473-2478. https://doi.org/10.1098/rspb.2001.1815. [ Links ]
Colwell RK. 2009. EstimateS: Statistical estimation of species richness and shared species from samples. Version 8.2. [computer program]. https://purl.oclc.org/estimates.
Colwell RK, Mao CX, Chang J. 2004. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85(10): 2717-2727. https://doi.org/10.1890/03-0557. [ Links ]
Cambefort Y. 1985. Les Coléoptères Scarabaeidae du Parc national de Taï (Cöte d'Ivoire). Revue Francaise d'Entomologie 7: 337-342. [ Links ]
Cambefort Y. 1991a. From saprophagy to coprophagy. In: Hanski I, Cambefort C, editors. Dung Beetle Ecology. Princeton (New Jersey): Princeton University Press. p. 22-35. https://doi.org/10.1515/9781400862092.22.
Cambefort Y. 1991b. Dung beetles in tropical savannahs. In: Hanski I, Cambefort C, editors. Dung Beetle Ecology. Princeton (New Jersey): Princeton University Press. p. 156-178. https://doi.org/10.1515/9781400862092.156.
Cambefort Y, Walter P. 1991.Dung beetles in tropical forests in Africa. In: Hanski I, Cambefort C, editors. Dung Beetle Ecology. Princeton (New Jersey): Princeton University Press. p. 198-210. https://doi.org/10.1515/9781400862092.198.
Daniel G. 2020. Overview of the biology of the dung beetle tribe of Sisyphini (Coleoptera: Scarabaeidae: Scarabaeinae). Culna - Magazine of the National Museum, Bloemfontein, South Africa. https://nationalmuseumpublications.co.za/overview-of-the-biology-of-the-dung-beetle-tribe-of-sisyphini-coleoptera-scarabaeidae-scarabaeinae/
Davis ALV, Philips TK. 2005. Effect of deforestation on a Southwest Ghana dung Bbeetle assemblage (Coleoptera: Scarabaeidae) at the periphery of Ankasa Conservation Area. Environmental Entomology 34(5): 1081-1088. https://doi.org/10.1093/ee/34.5.1081. [ Links ]
Davis ALV, Philips TK. 2009. Regional fragmentation of rain forest in West Africa and its effect on local dung beetle assemblage structure. Biotropica. 41(2): 215-220. https://doi.org/10.1111/j.1744-7429.2008.00472.x. [ Links ]
FAO and UNEP. 2020. The State of the World's Forests 2020. Forests, Biodiversity and People. Rome https://doi.org/10.4060/ca8642en.
Forti LC, Rinaldi IMP, Camargo RS, Fujihara RT, Camargo R da S, Fujihara RT. 2012. Predatory behavior of Canthon virens (Coleoptera: Scarabaeidae): A predator of leafcutter ants. Psyche: A Journal of Entomology 2012: 921465 https://doi.org/10.1155/2012/921465.
Dowsett-Lemaire F, Dowsett RJ. 2013. The birds of Shai Hills Resource Reserve, Ghana. Dowsett-Lemaire Miscellaneous Report 83: 1-12. [ Links ]
Favila M. 2001. Historia de vida y comportamiento de un escarabajo necrófago: Canthon cyanellus cyanellus LeConte (Coleoptera: Scarabaeinae). Folia Entomologica Mexicana 40: 245-278. [ Links ]
Forgie SA, Grebennikov VV, Scholtz CH. 2002. Revision of Sceliages Westwood, a millipede-eating genus of southern African dung beetles (Coleoptera: Scarabaeidae). Invertebrate Systematics 16(6): 931-955. https://doi.org/10.1071/IT01025. [ Links ]
Gámez G, Mora E, Acconcia R. 2006. Informaciones ecológicas sobre Coprophanaeus (Coprophanaeus) telamon nevinsoni Arnaud & Gamez (Coleoptera: Scarabaeinae: Phanaeini) en un sector de selva húmeda submontana en mérida, Venezuela. Acta Zoológica Mexicana (n.s.) 22(3): 95-105. [ Links ]
Génier. F. 2009. Le genre Eurysternus Dalman, 1824 (Scarabaeidae: Scarabaeinae: Oniticellini), revision taxonomique et clés de détermination illustrées. [ResearchGate - 10.13140/RG.2.1.1627.9129]
Halffter G. 1991. Historical and ecological factors determining the geographical distribution of beetles (Coleoptera: Scarabaeidae: Scarabaeinae). Folia Entomologica Mexicana 115: 195-238. https://doi.org/10.21426/B615110376. [ Links ]
Halffter G. 2003. Tribu Scarabaeini. In: Morón MA, editor. Atlas de los escarabajos de México. Vol. 2. Barcelona, Spain: Scarabaeidae, Trogidae, Passalidae y Lucanidae. Argania. p. 21-43.
Halffter G, Favila ME. 1993. The Scarabaeinae: an animal group for analyzing, inventorying and monitoring biodiversity in tropical rainforest and modified landscapes. Biology International. 27: 15-21. [ Links ]
Hanski I. 1983. Distributional ecology and abundance of dung and carrion-feeding beetles (Scarabaeidae) in tropical rain forests in Sarawak, Borneo. Acta Zoologica Fennica 167:1-45. [ Links ]
Hanski I. 1987. Nutritional ecology of dung- and carrion-feeding insects. In: Slansky F Jr, Rodrigues JG, editors. Nutritional Ecology of Insects, Mites and Spiders. Hoboken, NJ, USA: John Wiley and Sons. p. 837-884. [ Links ]
Hanski I, Cambefort Y, editors. 1991. Dung Beetle Ecology. Princeton, NJ, USA: Princeton University Press. p. 520. https://doi.org/10.1515/9781400862092. [ Links ]
Hanski I, Krikken J. 1991. South-East Asian Tropical Forests. In: Hanski I, Cambefort C, editors. Dung Beetle Ecology. Princeton (New Jersey): Princeton University Press. p. 179-210. https://doi.org/10.1515/9781400862092.179.
Hernandez B, Maes JM, Harvey CA, Vílchez S, Medina A, Sanchez D. 2003. Abundancia y diversidad de escarabajos coprófagos y mariposas diurnas en un paisaje ganadero en el departamento de Rivas, Nicaragua. Agroforestry Americana 10: 93-102. [ Links ]
Janzen D. 1983. Seasonal change in abundance of large nocturnal dung beetles (Scarabaeidae) in a Costa Rican deciduous forest and adjacent horse pasture. Oikos 41(2): 274-283. https://doi.org/10.2307/3544274. [ Links ]
Klein BC. 1989. Effects of forest fragmentation on dung and carrion beetle communities in central Amazonia. Ecology 70(6): 1715-1725. https://doi.org/10.2307/1938106. [ Links ]
Krell F-T, Schmitt T, Linsenmair KE. 1997. Diplopod defensive secretions as attractants for necrophagous scarab beetles (Diplopoda-Insecta: Coleoptera: Scarabaeidae). Entomologica Scandinavica Supplementum 51: 281-285. [ Links ]
Minnemeyer S. 2002. An Analysis of Access into Central Africa's rainforests. Washington (DC): World Resources Institute. [ Links ]
Miraldo A, Wirta H, Hanski I. 2011. Origin and diversification of dung beetles in Madagascar. Insects 2(2): 112-127. https://doi.org/10.3390/insects2020112. [ Links ]
Mittermeier CG, Turner WR, Larsen FW, Brooks TM, Gascon C. 2011. Global biodiversity conservation: the critical role of hotspots. In: Zachos FE, Habel JC, editors. Biodiversity Hotspots: Distribution and Protection of Priority Conservation Areas. Berlin: SpringerVerlag. p. 3-22. https://doi.org/10.1007/978-3-642-20992-5_1. [ Links ]
Mongyeh ET, Philips TK, Kimbi HK, Fokam EB. 2018. Elevational and possible bushmeat exploitation effects on dung beetle (Scarabaeidae: Scarabaeinae) communities on Mount Cameroon, West Central Africa. Environmental Entomology 47(5): 1072-1082. https://doi.org/10.1093/ee/nvy112. [ Links ]
Morón MA. 1979. Fauna de coleópteros lamelicornios de la estación de Biología Tropical "Los Tuxtlas", Veracruz, UNAM, México. Anales del Instituto de Biología, UNAM. Serie Zoología 50: 375-454. [ Links ]
Morón, M.A. 1994. Fauna de Coleoptera: Lamellicornia en las montafias del noreste de Hidalgo, México. Acta Zoológica Mexicana (n. s.) 63: 7-59. [ Links ]
Morón MA, Caamal J, Canul O. 1986. Análisis de la entomofauna necrófila del área Norte de la Reserva de Biosfera "Sian Ka'an" Quintana Roo, México. Folia Entomologica Mexicana 69: 83-98. [ Links ]
Morón MA, Villalobos FJ, Deloya C. 1985. Fauna de coleópteros lamelicornios de Boca del Chajul, Chiapas, México. Folia Entomologica Mexicana 66: 57-118. [ Links ]
Miraldo A, Wirta H, Hanski I. 2011. Origin and diversification of dung beetles in Madagascar. Insects 2(2): 112-127. https://doi.org/10.3390/insects2020112. [ Links ]
Myers N, Mittermeier RA, Mittermeier CG, Da Fonseca GA, Kent J. 2000. Biodiversity hotspots for conservation priorities. Nature.403(6772): 853-858. https://doi.org/10.1038/35002501. [ Links ]
Panin S. 1957. Coleoptera Familia Scarabaeidae. Volume 10 Fauna Republicii Populare Romine Insecta. Bucarest [ROM]: Academia Republicii Populare Romine. [ Links ]
Ratcliffe BC. 2013. The dung- and carrion-feeding scarabs (Coleoptera: Scarabaeoidea) of an Amazonian blackwater rainforest: Results of a continuous, 56-week, baited-pitfall trap study. Coleopterists Bulletin 67(4): 481-520. https://doi.org/10.1649/0010-065X-67.4.481. [ Links ]
Silva FAB, Hernández MIM, Ide S, Moura RC. 2007. Comunidade de escarabeíneos (Coleoptera, Scarabaeidae) copro-necrófagos da Regiäo de Brejo Novo, Caruaru, Pernambuco, Brasil. Revista Brasileira de Entomologia 51(2): 228-233. https://doi.org/10.1590/S0085-56262007000200014. [ Links ]
Stavert JR, Gaskett AC, Scott DJ, Beggs JR. 2014. Dung beetles in an avian-dominated island ecosystem: feeding and trophic ecology. Oecologia 176(1): 259-271. https://doi.org/10.1007/s00442-014-3001-z. [ Links ]
Young OP. 2006. Laboratory studies on the feeding behavior of the putative dung beetle, Ateuchus histeroides (Coleoptera: Scarabaeidae). Journal of the New York Entomological Society 114(3): 157-169. https://doi.org/10.1664/0028-7199(2007)114[157:LSOTFB]2.0.CO;2. [ Links ]
Vinson J. 1939. On the occurrence of two species of Sisyphis in Mauritius with description of a new species and the description of a new Adoretus from Reunion. Proceedings of the Royal Entomological Society of London B. 8: 33-38. [ Links ]
Vinson J. 1951. Le Cas de Sisyphes Mauriciens (Insectes Coleopteres). Royal Society of Arts and Sciences of Mauritius 1: 106-123. [ Links ]
Wirta H, Orsini L, Hanski I. 2008. An old adaptive radiation of forest dung beetles in Madagascar. Molecular Phylogenetics and Evolution 47(3):1076-1089. https://doi.org/10.1016/j.ympev.2008.03.010. [ Links ]
 Correspondence:
Correspondence:
T Keith Philips
Email: Keith.Philips@wku.edu
Received: 20 October 2021
Accepted: 02 August 2022














