Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a10656
RESEARCH ARTICLE
The effect of pollution on the competitive dynamics of Anopheles arabiensis Patton, 1905 and Culex quinquefasciatus Say, 1823 (Diptera: Culicidae)
Alexander CSN JeanrenraudI, II; Blazenka D LetinicII; Jean MollettIII; Basil D BrookeI, II; Shüné V OliverI, II
ICentre for Emerging Zoonotic and Parasitic Diseases, National Institute for Communicable Diseases of the National Health Laboratory Service, Johannesburg, South Africa
IIWits Research Institute for Malaria, School of Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIISchool of Molecular and Cell Biology, Faculty of Science, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
Culex quinquefasciatus Say, 1823 and Anopheles arabiensis Patton, 1905 (Diptera: Culicidae) are often found breeding in the same water sources and engage in interspecific competition. Although Cx. quinquefasciatus is known to proliferate in a range of polluted environments, the ability of An. arabiensis to proliferate in polluted water has only been reported relatively recently. The effects of pollution and insecticide resistance on this competitive interaction are unknown. This study examined the effect of pollution on the dynamics of the interspecific competition. Three laboratory strains were used in this study: an insecticide susceptible and an insecticide resistant An. arabiensis, and an insecticide resistant Cx. quinquefasciatus. Larval pollutant tolerances of each strain were assessed and compared by determining the lethal concentration at 50% mortality (LC50). The larvae from each strain were exposed to either inorganic fertiliser or copper nitrate, following which eclosion success was assessed. The results showed that the insecticide resistant strains had higher emergence rates when reared in polluted conditions without competition, with the Cx. quinquefasciatus strain showing the highest rate of eclosion. This species also had a higher tolerance for metal pollution than the An. arabiensis strains. The effects of pollutants on oviposition choice were examined. Pollution altered adult oviposition choice. The effect of larval metal exposure had variable effects on the activity of metabolic detoxification enzymes. An insecticide resistant phenotype had greater tolerance to pollutants and possibly developmental advantages based on a variable detoxification response to the pollutant. Pollution can therefore alter interspecific competition dynamics between the malaria vector An. arabiensis and Cx. quinquefasciatus.
Keywords: copper nitrate, detoxification enzymes, inorganic fertiliser, maximum acceptable toxicity concentration, tolerance
INTRODUCTION
The Anopheles gambiae Patton, 1905 complex (Diptera: Culicidae) consists of nine morphologically identical species of which six are either primary or secondary vectors of malaria. Primary vector species in this complex include An. gambiae sensu stricto, An. arabiensis and An. coluzzii (Sinka et al. 2010). One of the defining characteristics of the An. gambiae complex is that member species prefer to proliferate in shallow, sunlit, temporary bodies of relatively clean water (Gillies & Meillon 1968; Sinka et al. 2010). This is in contrast to Culex mosquito species, which have been found to proliferate in heavily polluted water, such as sewage and chemically treated water of septic tanks (Calhoun et al. 2007; Charlwood 1994; Correia et al. 2012).
The level of anthropogenic pollution is increasing (Donohue & Garcia Molinos 2009; Matson et al. 2002), with Anopheles mosquitoes evidently adapting to breeding in polluted waters, most notably An. gambiae (Antonio-Nkondjio et al. 2011; Awolola et al. 2007) and An. arabiensis (Jones et al. 2012). As a result, the diversity of breeding habitats utilised by the An. gambiae complex is expanding, placing these species in increased competition with other mosquitoes, such as those of the Culex pipiens Say, 1823 complex (Diptera: Culicidae) of which Cx. quinquefasciatus is a member.
Anopheles arabiensis is a major malaria vector in southern Africa (Sinka et al. 2010). This adaptable vector poses a particular challenge to those vector control methods that are based on the indoor application of insecticides [i.e. indoor residual spraying (IRS) and insecticide-treated bed nets] (Kitau et al. 2012), enabling this species to sustain low-level outdoor transmission, known as residual malaria (Killeen 2014). Anopheles arabiensis can flourish in agricultural and urban settings, making the relationship between this species and land use important for the control of malaria transmission (Githeko et al. 1996; Ijumba & Lindsay 2001; Mwangangi et al. 2006).
Anopheles arabiensis breeds in the same habitats as the common house mosquito, Cx. quinquefasciatus. As such, these two species are subject to interspecific competition. Interspecific competition between mosquito larvae has previously been examined in laboratory and field settings (Kirby & Lindsay 2009; Reiskind & Lounibos 2009; Kweka et al. 2012). The effect of environmental pollution on this dynamic, particularly between An. arabiensis and Cx. quinquefasciatus, has not been examined. Furthermore, the role of existing insecticide resistant phenotypes in larval interspecific competition in polluted conditions has not been evaluated.
Pollutant exposure during the immature aquatic stages may have consequences for subsequent adult mosquito life history traits (Oliver & Brooke 2018a; Oliver & Brooke 2018b). Examination of the effect of agricultural and urban pollutants on both malaria vectors and non-vectors is therefore important. Due to their preferred breeding sites, members of the An. gambiae complex and the Cx. pipiens complex would therefore be subject to the same environmental stressors. This has the potential to alter disease transmission dynamics. Anopheles mosquitoes transmit malaria, while Culex mosquitoes transmit arboviruses and filarial nematodes (Bartlow et al. 2019). The effects ofcompetition on their relative abundances could translate into changes in the rates of disease transmission.
The aim of this study was to examine interspecific larval competition between An. arabiensis and Cx. quinquefasciatus in relation to common pollutants and insecticide resistance. The effects of pollution on competitive success were examined, as were the effects of levels of pollution on detoxification enzyme activity in larvae.
MATERIALS AND METHODS
Laboratory-reared Cx. quinquefasciatus and An. arabiensis strains were utilised in this study. The two strains of An. arabiensis used were SENN and SENN-DDT. The SENN-DDT strain was selected for DDT resistance specifically, but also displayed resistance to deltamethrin, permethrin, λ-cyhalothrin (pyrethroids) and malathion (organophosphate) (Oliver & Brooke 2013). An insecticide resistant strain of Cx. quinquefasciatus, QUINQS, was included in the study for comparative purposes. This strain was resistant to DDT and pyrethroids.
Original sources of founding populations for mosquito colonies
Anopheles arabiensis (SENN strain): The insecticide susceptible SENN strain was originally from Sennar, Sudan, and has been maintained as a laboratory colony at the Botha de Meillon insectary, Johannesburg, South Africa, since 1980.
Anopheles arabiensis (SENN-DDT strain): The insecticide resistant SENN-DDT strain was selected from the Sudanese SENN strain by continuous selection with 4% DDT, and has been maintained as a laboratory strain at the Botha de Meillon insectary, Johannesburg, South Africa, since 1995.
Culex quinquefasciatus (QUINQS): The insecticide resistant QUINQS strain was originally from Johannesburg, South Africa, and has been maintained as a laboratory strain at the Botha de Meillon insectary, Johannesburg, South Africa, since 2010.
Rearing conditions
All larvae were fed a mixture of TetrafinTM Plus (480 mg in 15 mL dH2O) and/or a standard diet consisting of powdered dog biscuits and yeast in a 5:1 ratio (Hunt et al. 2005).
Determination of relative pollutant toxicity in Culex quinquefasciatus and Anopheles arabiensis
Dose response experiments were used to determine the lethal metal concentrations that would induce a larval mortality of 50% (LC50). The source of the metal pollutants for cadmium, copper and lead were cadmium chloride (CdCl2), copper nitrate (Cu(NO3)2) and lead nitrate (Pb(NO3)2). WondersolTM (WondersolTM All Purpose Fertiliser) was the commercial fertiliser used for testing purposes. The concentrations of nitrogen (N), phosphorus (P) and potassium (K) in WondersolTM were 103.5 g/kg (N), 23.0 g/kg (P) and 59.5 g/kg (K) respectively. In order to test the effects of N, P and K on larvae separately, rather than in combination (viz. WondersolTM), urea was used as a source of N, superphosphate as a source of P and potassium chloride as a source of K. Twenty-five fourth instar larvae were exposed to the selected concentrations for each pollutant treatment and the test replicated four times. Fourth instar larvae, the final larval stage before pupation, were selected due to their relatively large size. The relatively large size of the larvae made it easier to select equivalently sized individuals for a homogenous test population. All experiments were performed in 100 mL of distilled water (dH2O) in paper cups. All experiments were performed at 25 oC (± 2 °C) and 80% humidity (± 5%). All experiments were set up during the light period of the insectary. During the 24-hour experimental period, the larvae were exposed to a 12:12 hour light/dark cycle with a 30-minute duration for dawn/dusk. Treatment concentrations follow WHO guidance on larviciding (WHO 2005) and are detailed in Table 1a-c. Each exposure lasted 24 hours at which point mortality was recorded. This provided an indication of the ability of each strain to cope with pollution stress. Lethal concentrations (LC50s) for each strain were determined using a probit method of analysis (Finney 1952). If the control mortality exceeded 5% Abbott's formula was applied as a correction (Abbott 1925).
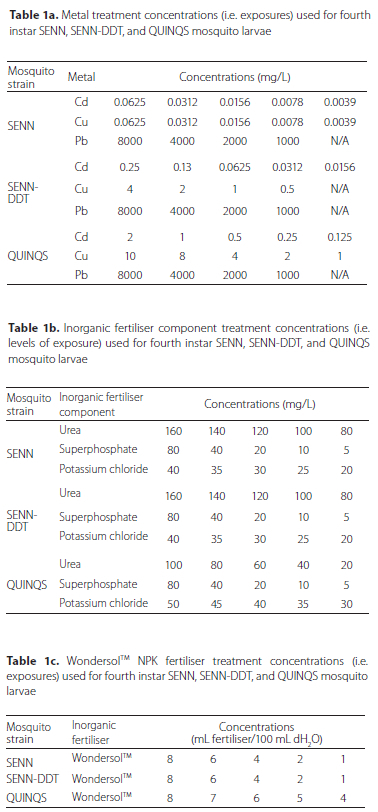
The effect of pollution on interspecific competition between Culex quinquefasciatus and Anopheles arabiensis larvae
Culex quinquefasciatus and An. arabiensis (SENN and/or SENNDDT strains) first instar larvae were placed together in containers (415 χ 295 χ 40 mm) filled with 1 L dH2O. Each tray contained two hundred larvae (one hundred from each species/strain) in combinations of either QUINQS and SENN or QUINQS and SENN-DDT. The experimental set up was replicated in triplicate with larvae emerging from different egg batches. The separation of SENN and SENN-DDT was performed to allow clear phenotypic distinction among adults, which would not be possible with the morphologically indistinguishable An. arabiensis strains and allowed enumeration of emergent adults. Controls were left untreated, while exposure trays were treated with either 0.2% WondersolTM (V:V), or 1.86 μg/L copper nitrate. The WondersolTM concentrations were based on a value equivalent to a LC25 value for all three strains. The concentration chosen for copper nitrate was the maximum acceptable toxicity concentration (MATC) for this metal. Copper nitrate was chosen as cadmium chloride proved highly toxic to the An. arabiensis strains, while lead nitrate was highly tolerated (as summarised in the LC50 data obtained from the relative pollutant toxicity study described above). Larvae were allowed to pupate and the numbers of adults emerging were counted. To control for the effect of pollution without competition, each of the strains was reared separately in polluted environments at the same concentrations used for the competition study. One hundred QUINQS, SENN and SENN-DDT first instar larvae were each placed separately into trays containing 1 L of dH2O, supplemented with either fertiliser or copper nitrate. Larvae reared in clean dH2O were used as a control. The experiment was replicated three times from three different cohorts arising from three separate egg batches.
Oviposition site choice
SENN, SENN-DDT and QUINQS adults emerging from larvae reared under standard insectary conditions were collected. Adults were collected before the age of 24 hours and segregated by sex until the experiment was performed. Samples from each strain were kept separately in a male:female ratio of 2:1 (n = 50 males, n = 25 females). They were allowed to feed to repletion on a human volunteer (co-author SVO) at three and seven days. At the age of 11 days they were allowed to oviposit, during which they were offered a choice of an egg plate containing either clean water, water polluted with 0.7% NPK fertiliser, or water polluted with 0.7 mg/L copper nitrate. Blood feeding was performed as per ethics waiver S Oliver 03-01-2018 from the University of the Witwatersrand. Both the polluted water and clean water egg plates were available in the same cage. The experiment was replicated three times, with adults arising from three independent egg batches. Each of the biological replicates consisted of three technical replicates (n = 225 females, n = 450 males).
Assessment of Anopheles arabiensis SENN and SENN-DDT larval detoxification enzymes
The effect of metal pollutants on larval detoxification enzymes was assessed to determine the species-specific mechanisms for coping with elevated metal concentrations. Previous studies on the effect of metal concentrations on larval detoxification enzyme levels in SENN and SENN-DDT adults indicated that cytochrome P450, glutathione S-transferase (GST), general esterases and glutathione peroxidases were all affected (Oliver & Brooke 2018b). The effects of metal concentrations on these larval enzymes were therefore examined to assess if similar effects would be found in larvae rather than adults. Metals were selected, as there were large differences and variations in tolerance in this class of xenobiotic, based on the results obtained from the pollutant tolerance study. Furthermore, the role of enzymes in the detoxification of metals, in the adults of the strains selected for the study, have been described in earlier studies (Oliver & Brooke 2018b). Larvae were reared from hatchlings to the fourth instar, under untreated (control) and treated (metal) conditions. Metal treatments were conducted at the MATCs (0.36 μg/L CdCl2, 1.86 μg/L Cu(NO3)2, and 4.39 μg/L Pb(NO3)2) (Mireji et al. 2008; Mireji et al. 2010). Forty-eight samples were taken from each treatment, repeated for both species/strains. Larvae were rinsed in dH2O to avoid metal contamination in the homogenate. Two larvae were used per reaction. Samples were homogenised using a Qiagen' TissueLyser II. Samples were stored at -70 oC until required for further use. All biochemical assays were conducted as per Oliver & Brooke (2017). In brief, samples were assessed for metabolic detoxification enzyme activities (cytochrome P450, glutathione S-transferase and general esterase activity) and oxidative stress enzyme activity (glutathione peroxidase). Cytochrome P450 activity was measured as haeme peroxidase activity, determined by the peroxidation of 3,3',5,5'-tetramethyl benzidine, measured as an end product at 650 nm. Glutathione S-transferase activity was measured as the conjugation of chlorodinitrobenzene, measured kinetically at 340 nm. General esterase activity was measured as the conversion product of 1-Naphthol or 2-Naphthol acetate to naphthol, measured at 570 nm. All the quantified products were standardised by dividing each reaction by total amount of protein per sample. Endpoint assays were determined by calibration to a standard curve (Cytochrome C: Sigma-Aldrich Product Number C2867 at concentrations of 0, 0.25, 0.5, 1 and 2 M; 1-Naphthol: Sigma-Aldrich Product Number N1000; 2-Naphthol: Sigma-Aldrich Product Number 185507 at concentrations of 0, 25, 50, 100, 200 and 400 M). All calorimetric readings were performed using a Multiskan Ascent 96 well microplate reader (AEC Amersham: Thermo Scientific).
Statistical analysis
All statistical analyses were performed at a 95% confidence interval. Samples were tested for normality using the Shapiro-Wilks test (Shapiro & Wilk 1965). Lethal concentrations were determined using probit analysis (Finney 1952). Differences in mean enzyme activity and the ovipositional activity index (OAI) (Kramer & Mulla 1979) were determined either with a two-sample t-test, or a one-way ANOVA, with a Tukey HSD as a post hoc test (Tukey 1949). Bartlett's test for equality of variance was performed for each ANOVA (Bartlett 1937). The multiple comparisons between means in the enzyme assays were corrected by the Benjamini-Hochberg procedure. All statistical analyses were performed using either IBM SPSS version 22 (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp), or Statistix 8 (Analytical Software, Tallahassee, Fl.).
RESULTS
Relative toxicity and larval tolerance to pollutants
The capacity of fourth instar larvae to tolerate metal salts and fertiliser were used as a proxy for the capacity to cope with pollutants in nature. The LC50 values for metal salt exposures show that both SENN-DDT and QUINQS larvae coped with Cu(NO3)2 and CdCl2 stress significantly better than SENN (oneway ANOVA: cadmium: p < 0.01, F(28) = 17.5; copper: p < 0.01, F(2;8) = 18.8) (Table 2).
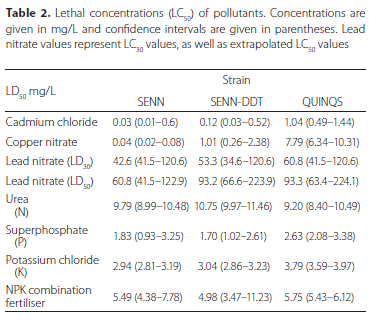
An LC50 estimate for Pb(NO3)2 was not possible for the QUINQS strain, as they were extremely tolerant to this pollutant, necessitating an LC30 estimate instead. Based on the estimates for LC30, QUINQS and SENN-DDT showed a significantly higher tolerance to Pb(NO3)2 than SENN (one-way ANOVA: p = 0.04; F = 5.21). When comparing IX^ values for QUINQS and SENN-DDT, the QUINQS strain showed a significantly higher LC30 value (two-sample t-test: p = 0.01; t = 4.85) (Table 2).
SENN-DDT showed the highest tolerance to urea (one-way ANOVA: p = 0.04, = 5.98). Tolerance to superphosphate was low across all strains, with no significant difference between them (one-way ANOVA: p = 0.13, F = 2.84,). The QUINQS strain showed the highest tolerance to potassium chloride (KCl) (one-way ANOVA: p = 0.01, F = 9.43, Tukey's critical Q = 4.34) (Figure 1C). The tolerance of the larvae to fertilisers in combination showed no significant differences between strains (one-way ANOVA: p = 0.1, F(2;8) = 3.49) (Table 2).
The effects of fertiliser and exposure to metal pollutants on adult emergence under competitive conditions
As adult emergence is a crucial measure of life history success, it can be used to assess the effect of pollutant concentrations on larvae and hence give insight into competitive success. Adult emergence was used rather than development rate for this purpose. This was because development rate is normally measured as time to pupation, and the pupae of the different species could not be accurately identified. Under control conditions, QUINQS had a greater number of adults emerging compared to both SENN and SENN DDT (one-way ANOVA: p = 0.04, F = 6.12, Benjamini-Hochberg correction: SENN vs QUINQS: p = 0.04, q = 0.04; SENN-DDT vs QUINQS: p = 0.02, q = 0.03). When all three strains were reared as single strains in water polluted with fertiliser, SENN had a significantly lower rate of emergence than QUINQS (one-way ANOVA: p = 0.03, F(2;6) = 6.43; Benjamini-Hochberg correction: SENN vs QUINQS: p = 0.01, q = 0.02; SENN-DDT vs QUINQS: p = 0.08, q = 0.08; SENN vs SENN-DDT: p = 0.2, q = 0.14). When exposed to elevated metal concentrations, both SENN and SENNDDT had a lower rate of emergence than QUINQS, although SENN-DDT had an increased rate of emergence compared to the SENN strain (one-way ANOVA: p < 0.01, F = 29.01, Benjamini-Hochberg correction: SENN vs QUINQS: p < 0.01, q < 0.01; SENNDDT vs QUINQS: p = 0.02, q = 0.02; SENN vs SENN-DDT: p < 0.01. q < 0.01) (Figure 1A).
In competitive conditions, both An. arabiensis strains, SENN and SENN-DDT, were at a competitive disadvantage when exposed to elevated levels of fertiliser in water, with lower rates of adult emergences than QUINQS (one-way ANOVA: p < 0.01, F(3;8) = 12.5; Benjamini-Hochberg correction: SENN vs QUINQS: p < 0.01, q < 0.01; SENN-DDT vs QUINQS: p < 0.01, q < 0.01)) (Figure 1B). No significant differences were observed in adult emergence when strains were cohabiting in water with elevated metal concentrations (Figure 1C).
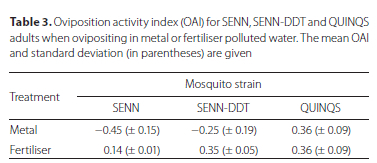
Oviposition site choice
Under control conditions, the SENN strain laid an average of 22.56 eggs per female, while the SENN DDT strain laid 14.94 eggs per female. The SENN strain laid an average of 9.95 eggs in metal-treated water and 27.19 eggs in fertiliser treated water. The SENN-DDT strain laid 13.08 eggs in metal-treated water and 19.31 eggs in fertiliser treated water. The OAI was calculated for each strain and treatment (Table 3). Metal treatment was found to be an attractant for the QUINQS strain, but a repellent for the SENN strain. Although metal concentration resulted in a negative OAI, it did not exceed the threshold for classification as ovipositioning repellent. Fertiliser, by contrast, was an attractant for SENN-DDT and QUINQS. Although the SENN strain had positive OAI values for the fertiliser treatment, the values were not large enough for it to be considered an attractant. Both treatments demonstrated that elevated concentrations of metals and fertiliser could be considered attractants, although there was no significant difference in the OAI of the treatments for the QUINQS strain (one-way ANOVA: p = 0.99, F(1;8) = 0.00). There was a significant difference between the OAI for concentrations of metal and fertiliser for the SENN (one-way ANOVA: p = 0.01, F(1;8) = 20.1) and SENN-DDT strains (one-way ANOVA: p = 0.04, F(1;8)= 9.15).
Assessment of the Anopheles arabiensis SENN and SENN-DDT enzyme detoxification systems
Cytochrome P450 activity in the An. arabiensis strains was increased by exposure to copper (two-sample t-test: SENN: p = 0.02, t = 2.36; DDT: p = 0.01, -2.66). In contrast, while lead
exposure increased activity in SENN-DDT (two-sample t-test: p < 0.01, t = -5.18), it decreased activity in SENN (two-sample t-test: p = 0.01, t = 2.64). Cytochrome P450 activity in QUINQS was always lower than for the An. arabiensis strains (one-way ANOVA: p < 0.01, F(11;288) = 36.5), with copper and lead exposure associated with significantly lower levels of activity than the control (one-way ANOVA: p = 0.03, F(2; 54) = 3.46) (Figure 2A).
Alpha esterase activity in SENN decreased after all metal treatments (one-way ANOVA: p < 0.01, F(3;95) = 5.49). Only cadmium and copper treatment significantly decreased α-esterase activity in SENN-DDT (one-way ANOVA: p < 0.01, F(3;95) = 15.4). Activity in the QUINQS strain remain unchanged regardless of metal treatment (one-way ANOVA: p = 0.51, F(3;95) = 3.46). Similarly, with β-esterases all metal treatments significantly reduced activity in the SENN strain (one-way ANOVA: p < 0.01, F(3;95) = 7.05). For SENN-DDT, only the cadmium treatment significantly reduced β-esterase activity (one-way ANOVA: p < 0.01, F(3; 95) = 10.4). Beta esterase activity in QUINQS remained unchanged regardless of treatment (oneway ANOVA: p = 0.28, F(3;95) = 1.30) (Figure 2B).
The GST activity in SENN-DDT decreased significantly after all treatments (one-way ANOVA: p < 0.01, df(3;95) = 18.4), but only for cadmium and lead treatments in SENN (two-sample t-test: cadmium: p = 0.01, t = 2.67; lead p < 0.01, t = 3.11). GST activity in the QUINQS strain was unaffected by metal treatment (oneway ANOVA: p = 0.39, F(3;95) = 1.01) (Figure 2C).
Glutathione peroxidase activity was similar to GST activity in that cadmium and lead activity decreased in SENN (one-way ANOVA: p = 0.02, F(3;95) = 3.26), while copper and cadmium activity reduced in SENN-DDT (one-way ANOVA: p = 0.02,
F(3; 95) = 5.92). As with most enzymes, the peroxidase activity in QUINQS remained unchanged (one-way ANOVA: p = 0.58, F(3; 95) = 0.65) (Figure 2D).
DISCUSSION
Interspecific competition between disease vector species has the capacity to alter the dynamics ofdisease transmission (Impoinvil et al. 2008). To date, interspecific competition studies have tended to focus primarily on competition between mosquitoes that breed in containers (e.g. Alto et al. 2013; Camara et al. 2016; Farjana et al. 2012; Lounibos et al. 2016; Noden et al. 2016; Yee et al. 2014), or inter-anopheline competition (e.g. Davies et al. 2016; Gimonneau et al. 2014; Koenraadt & Takken 2003; Schneider et al. 2000), while studies on Culex/Anopheles interspecific competition have often focussed on succession in agricultural settings (Muturi et al. 2008; Muturi et al. 2007; Mwangangi et al. 2006).
The data presented here showed a complex interaction between the aquatic stages of Culex and Anopheles mosquitoes, mediated by exposure of larvae to pollutants and the presence/absence of insecticide resistant phenotypes. In the absence of competition, insecticide resistance confers an advantage in polluted aquatic environments. The insecticide resistant SENN-DDT (An. arabiensis) and QUINQS (Cx. quinquefasciatus) strains were shown to be at an advantage, due to greater adult emergence after exposure to polluted water.
An examination of larval eclosion in polluted conditions (without competition), showed that rates of emergence for SENN-DDT in these conditions were better for both metal and fertiliser treatments than the control. For QUINQS only the fertiliser treatment resulted in an increased rate of emergence compared to the control in conditions without competition. While no effects on adult emergence (in competitive conditions) were observed for metal treatments, there was a marked difference when subjected to the fertiliser treatment. The QUINQS strain performed significantly better in terms of adult emergence following fertiliser treatment under non-competitive conditions. This carried through to the competition study. SENN, which showed no advantage under fertiliser polluted conditions, and SENN-DDT, which showed increased adult survival under polluted conditions, were subsequently disadvantaged when competing with QUINQS.
These data indicated that Cx. quinquefasciatus should predominate when competing with Anopheles, but this has not always been observed in the field. A study of competition between An. gambiae s.s. and Cx. quinquefasciatus found little difference in life history traits between the two species, except for a decrease in adult size in An. gambiae (Kweka et al. 2012). Succession studies in a Kenyan rice field demonstrated that An. arabiensis and Cx. quinquefasciatus were the dominant species with abundances of 45% and 35.8% respectively (Muturi et al. 2007). This indicated that neither species had a marked advantage over the other in these conditions. There have been reports of different dominant species in different environments. For example, Cx. quinquefasciatus and An. arabiensis have been found to be dominant species in Kenyan rice paddies (Muturi et al. 2008; Muturi et al. 2007). This could be related to various physico-chemical parameters favouring a particular species (Jacob et al. 2005). Interspecific differences in the level of predation have also been reported for Cx. quinquefasciatus and An. Gambiae (Muturi et al. 2010), so this may also be factor in An. arabiensis.
When examining the lethal doses of the pollutants used here, QUINQS showed the highest tolerance to metal concentrations, although this did not translate into an advantage in terms of emergent numbers. Field studies have demonstrated varying tolerance to toxins, in which An. arabiensis coped better with the larvicide Agnique* (Bashir et al. 2008), while Cx. quinquefasciatus coped better with a crude extract from castor oil plant (Ricinus communis L.) (Malpighiales: Euphorbiaceae) leaves (Elimam et al. 2009a). Furthermore, leaf extracts of Calotropis procera (Aiton) Aiton fil. (Gentianales: Apocynaceae) proved more toxic to Cx. quinquefasciatus larvae than An. arabiensis and resulted in fewer eggs being laid by Cx. quinquefasciatus females, in water polluted with the extract (Elimam et al. 2009b). None of these studies took into account the insecticide resistance status of the larvae. The results of our study indicated that resistance to insecticides could provide a significant competitive advantage for resistant mosquitoes under polluted conditions.
A direct comparison of the findings in this study to other studies on interspecific competition is challenging. Examinations of An. arabiensis and Cx. quinquefasciatus interspecific competition has not been reported as often as that for intra-Aedes competition. This is notable, as An. arabiensis and Cx. quinquefasciatus have been found in the same containers more than would be expected by chance alone (Muturi et al. 2008). The effect of fertiliser and metal concentrations have also not been reported. In terms of environmental conditions, Culex larval habitats have previously been positively associated with the level of dissolved oxygen (DO) and concentration of total dissolved solids (TDS) and generally occurred in water with a higher electrical conductivity (Muturi et al. 2008). It would be useful to conduct the present study in field or semi-field conditions, as the larval habitat itself, over and above the physico-chemical parameters of the breeding site, could affect the outcome of competition (Mwangingi et al. 2008). A study of this type would be able to test the postulated competitive advantage insecticide resistant individuals have over others in polluted conditions. It is also possible that the pollutants may have a hormetic effect, as the insecticide malathion can improve life history traits in Cx. pipiens by reducing density effects (Muturi et al. 2010)
The choice of site for oviposition represents a significant ecological factor as well and may have a direct influence on how species coexist in sympatry. There was a difference in ovipositional activity between the species and two An. arabiensis strains. Both the fertiliser and metal acted as an attractant for the QUINQS strain. Metal was a repellent for the SENN strain, while fertiliser was an attractant for the SENN strain. The differences in the effect of fertiliser on oviposition site choice have been observed in the two An. arabiensis strains before (Samuel et al. 2020; Oliver 2021). This indicates that species-specific oviposition choice of site may be directly affected by the presence or absence of resistant phenotypes, and that these choices are likely to affect subsequent life histories, especially survival to adulthood. A previous study demonstrated that oviposition by An. arabiensis and Cx. quinquefasciatus in water with elevated concentrations of fertiliser was greater that in water polluted with a pesticide. Pollution also had differential effects on oviposition site choice in these species, with An. arabiensis laying more in an ammonium sulfate treatment, but Cx. quinquefasciatus laying more in a diammonium phosphate (DAP) treatment (Kibuthu et al. 2016). Although this study demonstrated interspecies differences, the resistance phenotype was not taken into account in this study. The positive effects of fertiliser application have been demonstrated in various examples, with the application of ammonium sulphate fertiliser increasing the amount of anopheline and culicine immatures in the field (Mutero et al. 2004).
Examination ofthe response oflarval detoxification enzymes to metal exposure gives some insight into how the chemoprotective system may operate. As cytochrome P450s are metal-inducible proteins (Musasia et al. 2013), it is noteworthy that the highly metal tolerant QUINQS strain showed very low basal activity for this group of enzymes. Copper and lead exposure reduces this already low level of P450 activity. At the larval stage, cytochrome P450 appears to play a crucial protective role in An. arabiensis, as most other enzyme systems are reduced in response to larval exposure to metal.
Detoxification enzyme activity in the QUINQS strain is remarkably resilient to change, with no metal treatments reducing or increasing activity, except for the reduction in cytochrome P450 activity following copper and lead exposure. Relative to the An. arabiensis strains, it appears that GST is the most important larval chemoprotective enzyme in this strain.
After all metal exposures, this was the only enzyme class that was significantly higher than that reported for the An. arabiensis strains.
SENN and SENN-DDT demonstrated markedly different tolerances to insecticide in both the adult and larval stages. It is therefore interesting to note that, with the exception of glutathione S-transferases (GSTs), the basal detoxification enzyme activity levels in both strains were the same. It is also noteworthy that the pattern of activity in the An. arabiensis glutathione peroxidases were similar to the activity patterns of GSTs, indicating that the changes observed in peroxidase activity were specifically changes in glutathione peroxidase activity, which some GSTs display (Sheehan et al. 2001). Neither of these classes (i.e. GST, peroxidases) increased in An. arabiensis larvae in response to metal exposure. Cytochrome P450 activities increased in two of three treatments in SENNDDT. This indicates that the cytochrome P450s may play a role in copper and lead tolerance in this strain.
A previous study examined the enzyme activity in SENN and SENN-DDT adults after larval exposure to the same metals at the same concentration (Oliver & Brooke 2018b). There were marked similarities in the enzyme activities of the adults of the previous study and the larvae of this study. This included a general decrease in GST activity and an increase in cytochrome P450 activity in adults. The significant reductions in activity observed in α-esterases were not observed in adults of either strain. By contrast, β-esterases in SENN-DDT adult males increased rather than decreased, as observed in the larvae. The results for the adult SENN-DDT females were all significant, but variable, with copper causing a significant increase in β-esterase activity. The generally conserved metabolic detoxification enzyme profile suggests that an increase in metal tolerance in adults is a direct consequence of increased enzyme activity induced at the larval stage in response to pollutant exposure.
The limitations to this study must be noted. The difficulty in establishing a LC50 value for lead nitrate in the Cx. quinquefasciatus strain was a notable limitation, as it was not possible to make the LC30 estimates with the same level of confidence as for an LC50 estimate. Furthermore, the analysis of all the stressors in a symmetric 1:1 manner was limited in scope. This was due to the analysis of factors as single stressors, in order to prevent confounding effects. Furthermore, the study was also limited by the lack of an insecticide susceptible Cx. quinquefasciatus strain for a comparative assessment and absence of wild counterparts for evaluation.
We concluded that pre-existing insecticide resistant phenotypes may not only have an adaptive advantage in water with elevated concentrations of fertiliser and metals, but may also respond to concentrations of pollutants at a molecular and enzymatic level. Insecticide resistant phenotypes also appear to have affected the interspecific competition dynamic, with increased insecticide resistance reducing relative competitive success in unpolluted conditions, but generally increasing success in polluted conditions. These interactions are potentially influenced by numerous ecological factors, in addition to the aforementioned molecular and enzyme-mediated responses in the larvae.
ACKNOWLEDGEMENTS
Drs Mark Paine and Rodney Hull are thanked for their comments on the manuscript.
COMPETITION STATEMENT
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
ACSN performed the experiments, collected and analysed the data and contributed to the initial draft. BDL assisted with the optimisation of the experiments and contributed to the initial drafts. JM and BDB assisted with data analysis and contributed to the initial drafts. SVO conceived the project, obtained funding and produced the final draft of the manuscript.
FUNDING SOURCES
This research was supported by the National Research Foundation of South Africa (TTK160622173523), the Research Trust of the National Health Laboratory Service (2017-DEV08-SOL01), the DST/NRF South African Research Chairs Initiative (Grant No: 64763) and the South African Medical Research Council Collaborating Centre for Multidisciplinary Research on Malaria.
ORCID IDs
Alexander CSN Jeanrenraud - https://orcid.org/0000-0002-4230-9976
Blazenka D Letinic - https://orcid.org/0000-0002-2659-4324
Basil D Brooke - https://orcid.org/0000-0002-8857-1304
Shüné V Oliver - https://orcid.org/0000-0002-2140-2725
REFERENCES
Abbott WS. 1925. A method of computing the effectiveness of an insecticide. Journal of Economic Entomology 18(2): 265-267. https://10.1093/jee/18.2.265a [ Links ]
Alto BW, Lampman RL, Kesavaraju B, Muturi EJ. 2013. Pesticide-induced release from competition among competing Aedes aegypti and Aedes albopictus (Diptera: Culicidae). Journal of Medical Entomology 50(6): 1240-1249. https://10.1603/ME12135 [ Links ]
Antonio-Nkondjio C, Fossog BT, Ndo C, Djantio BM, Togouet SZ, Awono-Ambene P, Costantini C, Wondji CS, Ranson H. 2011. Anopheles gambiae distribution and insecticide resistance in the cities of Douala and Yaounde (Cameroon): influence of urban agriculture and pollution. Malaria Journal 10(1): 154. https://10.1186/1475-2875-10-154 [ Links ]
Awolola TS, Oduola AO, Obansa JB, Chukwurar NJ, Unyimadu JP. 2007. Anopheles gambiae s.s. breeding in polluted water bodies in urban Lagos, southwestern Nigeria. Journal Vector Borne Diseases 44:2 41-244. [ Links ]
Bartlow AW, Manore C, Xu C, Kaufeld KA, Del Valle S, Ziemann A, Fairchild G, Fair JM. 2019. Forecasting zoonotic infectious disease response to climate change: Mosquito vectors and a changing environment. Veterinary Sciences 6(2): 40. https://10.3390/vetsci6020040 [ Links ]
Bartlett MS. 1937. Properties of sufficiency and statistical tests. Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences 160: 268-282. [ Links ]
Bashir A, Hassan AA, Salmah MR, Rahman WA. 2008. Efficacy of agnique (mmf) monomolecular surface film against immature stages of Anopheles arabiensis Patton and Culex spp (Diptera: Culicidae) in Khartoum, Sudan. The Southeast Asian Journal of Tropical Medicine and Public Health 39: 222-228. [ Links ]
Calhoun LM, King R, Gunarto K, Burkot TR, Jones LA, Roberts J, Avery M. 2007 Combined sewage overflows (CSO) are major urban breeding sites for Culex quinquefasciatus in Atlanta, Georgia. The American Journal of Tropical Medicine and Hygiene 77(3): 478484. https://10.4269/ajtmh.2007.77.478 [ Links ]
Camara DC, Codeco CT, Juliano SA, Lounibos LP, Riback TI, Pereira GR, Honorio NA. 2016. Seasonal differences in density but similar competitive impact of Aedes albopictus (Skuse) on Aedes aegypti (L.) in Rio de Janeiro, Brazil. PLoS One 11(6): e0157120. https://10.1371/journal.pone.0157120 [ Links ]
Charlwood JD. 1994. The control of Culex quinquefasciatus breeding in septic tanks using expanded polystyrene beads in southern Tanzania. Transactions of the Royal Society of Tropical Medicine and Hygiene 88(4): 380. https://10.1016/0035-9203(94)90390-5 [ Links ]
Correia JC, Barbosa RM, Oliveira CM, Albuquerque CM. 2012. Residential characteristics aggravating infestation by Culex quinquefasciatus in a region ofNortheastern Brazil. Revista de Saude Publica 46(6): 935-941. https://10.1590/S0034-89102013005000010 [ Links ]
Davies C, Coetzee M, Lyons CL. 2016. Effect of stable and fluctuating temperatures on the life history traits of Anopheles arabiensis and An. quadriannulatus under conditions of inter- and intra-specific competition. Parasites & Vectors 9(1): 342. https://10.1186/s13071-016-1630-2 [ Links ]
Donohue I, Garcia Molinos J. 2009. Impacts of increased sediment loads on the ecology of lakes. Biological Reviews of the Cambridge Philosophical Society 84(4): 517-531. https://10.1111/j.1469-185X.2009.00081.x [ Links ]
Elimam AM, Elmalik KH, Ali FS. 2009a. Larvicidal, adult emergence inhibition and oviposition deterrent effects of foliage extract from Ricinus communis L. against Anopheles arabiensis and Culex quinquefasciatus in Sudan. Tropical Biomedicine 26: 130-139. [ Links ]
Elimam AM, Elmalik KH, Ali FS. 2009b. Efficacy of leaves extract of Calotropis procera Ait. (Asclepiadaceae) in controlling Anopheles arabiensis and Culex quinquefasciatus mosquitoes. Saudi Journal of Biological Sciences 16(2): 95-100. https://10.1016/j.sjbs.2009.10.007 [ Links ]
Farjana T, Tuno N, Higa Y. 2012. Effects of temperature and diet on development and interspecies competition in Aedes aegypti and Aedes albopictus. Medical and Veterinary Entomology 26(2): 210217. https://10.1111/j.1365-2915.2011.00971.x [ Links ]
Finney DJ. 1952. Probit Analysis. 2nd ed. New York: Cambridge University Press. [ Links ]
Gillies, M. T. & Meillon, B. D. 1968. The Anophelinae of Africa south of the Sahara (Ethiopian Zoogeographical Region). Publications of the South African Institute for Medical Research. No. 54. Johannesburg: SAIMR. [ Links ]
Gimonneau G, Brossette L, Mamai W, Dabire RK, Simard F. 2014. Larval competition between An. coluzzii and An. gambiae in insectary and semi-field conditions in Burkina Faso. Acta Tropica 130: 155-161. https://10.1016/j.actatropica.2013.11.007 [ Links ]
Githeko AK, Service MW, Mbogo CM, Atieli FK. 1996. Resting behaviour, ecology and genetics of malaria vectors in large scale agricultural areas of Western Kenya. Parassitologia 38: 481-489. [ Links ]
Hunt RH, Brooke BD, Pillay C, Koekemoer LL, Coetzee M. 2005. Laboratory selection for and characteristics of pyrethroid resistance in the malaria vector Anopheles funestus. Medical and Veterinary Entomology 19(3): 271-275. https://10.1111/j.1365-2915.2005.00574.x [ Links ]
Ijumba JN, Lindsay SW. 2001. Impact of irrigation on malaria in Africa: paddies paradox. Medical and Veterinary Entomology 15(1): 1-11. https://10.1046/j.1365-2915.2001.00279.x [ Links ]
Impoinvil DE, Keating J, Mbogo CM, Potts MD, Chowdhury RR, Beier JC. 2008. Abundance of immature Anopheles and culicines (Diptera: Culicidae) in different water body types in the urban environment of Malindi, Kenya. Journal of Vector Ecology 33(1): 107-116. https://10.3376/1081-1710(2008)33[107:AOIAAC]2.0.CO;2 [ Links ]
Jacob BG, Arheart KL, Griffith DA, Mbogo CM, Githeko AK, Regens JL, Githure JI, Novak R, Beier JC. 2005. Evaluation of environmental data for identification of Anopheles (Diptera: Culicidae) aquatic larval habitats in Kisumu and Malindi, Kenya. Journal of Medical Entomology 42(5): 751-755. https://10.1093/jmedent/42.5.751 [ Links ]
Jones CM, Toe HK, Sanou A, Namountougou M, Hughes A, Diabate A, Dabire R, Simard F, Ranson H. 2012. Additional selection for insecticide resistance in urban malaria vectors: DDT resistance in Anopheles arabiensis from Bobo-Dioulasso, Burkina Faso. PLoS One 7(9):e45995. https://10.1371/journal.pone.0045995 [ Links ]
Kibuthu TW, Njenga SM, Mbugua AK, Muturi EJ. 2016. Agricultural chemicals: Life changer for mosquito vectors in agricultural landscapes? Parasites & Vectors 9(1): 500. https://10.1186/s13071-016-1788-7 [ Links ]
Killeen GF. 2014. Characterizing, controlling and eliminating residual malaria transmission. Malaria Journal 13(1): 330. https://10.1186/1475-2875-13-330 [ Links ]
Kirby MJ, Lindsay SW. 2009. Effect of temperature and inter-specific competition on the development and survival of Anopheles gambiae sensu stricto and An. arabiensis larvae. Acta Tropica 109(2): 118123. https://10.1016/j.actatropica.2008.09.025 [ Links ]
Kitau J, Oxborough RM, Tungu PK, Matowo J, Malima RC, Magesa SM, Bruce J, Mosha FW, Rowland MW. 2012. Species shifts in the Anopheles gambiae complex: do LLINs successfully control Anopheles arabiensis? PLoS One 7(3): e31481. https://10.1371/journal.pone.0031481 [ Links ]
Koenraadt CJ, Takken W. 2003. Cannibalism and predation among larvae of the Anopheles gambiae complex. Medical and Veterinary Entomology 17(1): 61-66. https://10.1046/j.1365-2915.2003.00409.x [ Links ]
Kweka EJ, Zhou G, Beilhe LB, Dixit A, Afrane Y, Gilbreath TM 3rd, Munga S, Nyindo M, Githeko AK, Yan G. 2012. Effects of co-habitation between Anopheles gambiae s.s. and Culex quinquefasciatus aquatic stages on life history traits. Parasites & Vectors 5(1): 33. https://10.1186/1756-3305-5-33 [ Links ]
Kramer WL, Mulla MS. 1979. Oviposition attractants and repellents of mosquitoes: oviposition responses of Culex mosquitoes to organic infusions. Environmental Entomology 8(6): 1111-1117. https://10.1093/ee/8.6.1111 [ Links ]
Lounibos LP, Bargielowski I, Carrasquilla MC, Nishimura N. 2016. Coexistence of Aedes aegypti and Aedes albopictus (Diptera: Culicidae) in Peninsular Florida two decades after competitive displacements. Journal of Medical Entomology 53(6): 1385-1390. https://10.1093/jme/tjw122 [ Links ]
Matson P, Lohse KA, Hall SJ. 2002. The globalization of nitrogen deposition: consequences for terrestrial ecosystems. Ambio 31(2): 113-119. https://10.1579/0044-7447-3L2.113 [ Links ]
Mireji PO, Keating J, Hassanali A, Mbogo CM, Muturi MN, Githure JI, Beier JC. 2010. Biological cost of tolerance to heavy metals in the mosquito Anopheles gambiae. Medical and Veterinary Entomology 24(2): 101-107. https://10.1111/j.1365-2915.2010.00863.x. [ Links ]
Mireji PO, Keating J, Hassanali A, Mbogo CM, Nyambaka H, Kahindi S, Beier JC. 2008. Heavy metals in mosquito larval habitats in urban Kisumu and Malindi, Kenya, and their impact. Ecotoxicology and Environmental Safety 70(1): 147-153. https://10.1016/j.ecoenv.2007.03.012 [ Links ]
Musasia FK, Isaac AO, Masiga DK, Omedo IA, Mwakubambanya R, Ochieng R, Mireji PO. 2013. Sex-specific induction of CYP6 cytochrome P450 genes in cadmium and lead tolerant Anopheles gambiae. Malaria Journal 12(1): 97. https://10.1186/1475-2875-12-97 [ Links ]
Muturi EJ, Costanzo K, Kesavaruju B, Lampman R, Alto BW. 2010. Interaction of a pesticide and larval competition on life history traits of Culexpipiens. Acta Tropica 116: 141-146. [ Links ]
Muturi EJ, Kim CH, Jacob B, Murphy S, Novak RJ. 2010, Interspecies predation between Anopheles gambiae s.s. and Culex quinquefasciatus larvae. Journal of Medical Entomology 47(2): 287290. https://10.1093/jmedent/47.2.287 [ Links ]
Muturi EJ, Mwangangi J, Shililu J, Jacob BG, Mbogo C, Githure J, Novak RJ. 2008. Environmental factors associated with the distribution of Anopheles arabiensis and Culex quinquefasciatus in a rice agro-ecosystem in Mwea, Kenya. Journal of Vector Ecology 33(1): 56-63. https://10.3376/1081-1710(2008)33[56:EFAWTD]2.0.CO;2 [ Links ]
Muturi EJ, Mwangangi J, Shililu J, Muriu S, Jacob B, Kabiru E, Gu W, Mbogo C, Githure J, Novak R. 2007. Mosquito species succession and physicochemical factors affecting their abundance in rice fields in Mwea, Kenya. Journal of Medical Entomology 44(2): 336-344. https://10.1093/jmedent/44.2.336 [ Links ]
Mutero CM, Ng'ang'a PN, Wekoyela P, Githure J, Konradsen F. 2004. Ammonium sulphate fertiliser increases larval populations of Anopheles arabiensis and culicine mosquitoes in rice fields. Acta Tropica 89(2): 187-192. https://10.1016/j.actatropica.2003.08.006 [ Links ]
Mwangangi JM, Muturi EJ, Shililu J, Muriu SM, Jacob B, Kabiru EW, Mbogo CM, Githure J, Novak R. 2008. Contribution of different aquatic habitats to adult Anopheles arabiensis and Culex quinquefasciatus (Diptera: Culicidae) production in a rice agroecosystem in Mwea, Kenya. Journal of Vector Ecology 33(1): 129-138. https://10.3376/1081-1710(2008)33[129:CODAHT]2.0.CO;2 [ Links ]
Mwangangi J, Shililu J, Muturi E, Gu W, Mbogo C, Kabiru E, Jacob B, Githure J, Novak R. 2006. Dynamics of immature stages of Anopheles arabiensis and other mosquito species (Diptera: Culicidae) in relation to rice cropping in a rice agro-ecosystem in Kenya. Journal of Vector Ecology 31(2): 245-251. https://10.3376/1081-1710(2006)31[245:DOISOA]2.0.CO;2 [ Links ]
Noden BH, O'Neal PA, Fader JE, Juliano SA. 2016. Impact of inter- and intra-specific competition among larvae on larval, adult, and life-table traits of Aedes aegypti and Aedes albopictus females. Ecological Entomology 41(2): 192-200. https://10.1111/een.12290 [ Links ]
Oliver SV. 2021. The effect of larval cigarette exposure on the life history of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Transactions of the Royal Society of South Africa 76(2): 117-125. https://10.1080/0035919X.2021.1887004 [ Links ]
Oliver SV, Brooke BD. 2013. The effect of larval nutritional deprivation on the life history and DDT resistance phenotype in laboratory strains of the malaria vector Anopheles arabiensis. Malaria Journal 12(1): 44. https://10.1186/1475-2875-12-44 [ Links ]
Oliver SV, Brooke BD. 2017,The effect of elevated temperatures on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Malaria Journal 16(1): 73. https://10.1186/s12936-017-1720-4 [ Links ]
Oliver SV, Brooke BD. 2018a. The effect of commercial herbicide exposure on the life history and insecticide resistance phenotypes of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). Acta Tropica 188: 152-160. https://10.1016/j.actatropica.2018.08.030 [ Links ]
Oliver SV, Brooke BD. 2018b. The effect of metal pollution on the life history and insecticide resistance phenotype of the major malaria vector Anopheles arabiensis (Diptera: Culicidae). PLoS One 13(2): e0192551. https://10.1371/journal.pone.0192551 [ Links ]
Reiskind MH, Lounibos LP. 2009. Effects of intraspecific larval competition on adult longevity in the mosquitoes Aedes aegypti and Aedes albopictus. Medical and Veterinary Entomology 23(1): 62-68. https://10.HH/j.1365-2915.2008.00782.x [ Links ]
Samuel M, Brooke BD, Oliver SV. 2020. Effects of inorganic fertilizer on larval development, adult longevity and insecticide susceptibility in the malaria vector Anopheles arabiensis (Diptera: Culicidae). Pest Management Science 76(4): 1560-1568. https://10.1002/ps.5676 [ Links ]
Schneider P, Takken W, Mccall PJ. 2000. Interspecific competition between sibling species larvae of Anopheles arabiensis and An. gambiae. Medical and Veterinary Entomology 14(2): 165-170. https://10.1046/j.1365-2915.2000.00204.x [ Links ]
Shapiro SS, Wilk MB. 1965. An analysis of variance test for normality (complete samples). Biometrika 52(3-4): 591-611. https://10.1093/biomet/52.3-4.591 [ Links ]
Sheehan D, Meade G, Foley VM, Dowd CA. 2001. Structure, function and evolution of glutathione transferases: implications for classification of non-mammalian members of an ancient enzyme superfamily. The Biochemical Journal 360(1): 1-16. https://10.1042/bj3600001 [ Links ]
Sinka ME, Bangs MJ, Manguin S, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, Gething PW, Kabaria CW, et al. 2010. The dominant Anopheles vectors of human malaria in Africa, Europe and the Middle East: occurrence data, distribution maps and bionomic precis. Parasites & Vectors 3(1): 117. https://10.1186/1756-3305-3-117 [ Links ]
Tukey JW. 1949. Comparing individual means in the analysis of variance. Biometrics 5(2): 99-114. https://10.2307/3001913 [ Links ]
WHO [World Health Organisation]. 2005. Guidelines for laboratory and field testing of mosquito larvicides. Geneva: WHO [ Links ]
Yee DA, Himel E, Reiskind MH, Vamosi SM. 2014;. Implications of saline concentrations for the performance and competitive interactions of the mosquitoes Aedes aegypti (Stegomyia aegypti) and Aedes albopictus (Stegomyia albopictus). Medical and Veterinary Entomology 28(1): 60-69. https://10.1111/mve.12007 [ Links ]
 Correspondence:
Correspondence:
Shiiné Oliver
Email: shuneo@nicd.ac.za
Received: 01 April 2021
Accepted: 23 September 2021














