Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a13141
RESEARCH ARTICLE
Effect of visible light and ultraviolet light on the pathogenicity of entomopathogenic fungi to false codling moth, Thaumatotibia leucotreta (Lepidoptera: Tortricidae) larvae
S Rossouw; LL Mathulwe; NF Stokwe; AP Malan
Department of Conservation Ecology and Entomology, Faculty of AgriSciences, Stellenbosch University, Stellenbosch, South Africa
ABSTRACT
Entomopathogenic fungi (EPF) are effective and environment-friendly insect biological control agents. Ultraviolet (UV) light is known to have an effect on the survival of fungal conidia, and natural sunlight is potentially one of the most damaging factors undermining EPF persistence and pathogenicity. This study aimed to test the infection potential of an isolate of Beauveria bassiana and five Metarhizium species after exposure to different light treatments, on soil and leaf surfaces under laboratory and field conditions, using Thaumatotibia leucotreta (Lepidoptera: Tortricidae) as the test host. Conidia were exposed either to growth light alone, which emits the same visible light as the sun, but excluding UV light, or directly exposed to UV light for 12 h. The results indicated no negative effect on the infection potential of the conidia of most species tested. The conidia of the two Metarhizium pinghaense (5HEID and TH149) isolates showed the greatest tolerance to visible light and UV radiation exposure on both soil and leaf surfaces. Exposure of M. pinghaense isolates to visible light on soil surfaces showed pathogenicity of > 80% for both isolates, and of between 58% and 88% after exposure to UV light. On leaf surfaces, three Metarhizium isolates, M. pinghaense (5HEID and TH149) and M. majus (TH153) had > 90% pathogenicity following exposure to UV light, and M. pinghaense (TH149) and M. robertsii (6EIKEN) showed greater tolerance of > 70%, under laboratory conditions. However, the pathogenicity of the EPF isolates was very low in field trials, indicating that further trials on the use of formulations and adjuvants with the isolates are needed to improve long-term persistence and efficacy under field conditions.
Keywords: Beauveria, biological control, conidia, insect pests, Metarhizium, ultraviolet light
INTRODUCTION
False codling moth (FCM), Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae), occurs naturally on the Indian Ocean islands, as well as in sub-Saharan Africa, including South Africa (CABI 2021). The insect attacks a variety of fruit crops, including citrus, peaches, and apples (Moore et al. 2015). Thaumatotibia leucotreta causes economic losses estimated at R100 million to the citrus industry in southern Africa (Moore & Kirkman 2009). The duration of the life cycle of T. leucotreta ranges between 25 and 60 days, and up to six generations a year can occur (Stofberg 1954; Georgala 1969; Daiber 1980). The insect is most active from December to June in South African agroecosystems (Moore & Kirkman 2009). The adult females of T. leucotreta lay their eggs on the fruit, which the larvae penetrate (Daiber 1989). The last larval instar drops to the soil, where it pupates (Daiber 1979b). The adult ecloses from the cocoon after 12 to 16 days at 25 °C (Daiber 1979a).
Entomopathogenic fungi (EPF) in the genera Beauveria and Metarhizium, order Hypocreales (Ascomycota), class Hyphomycetes, have been well researched and used against several pest insects in agriculture (Inglis et al. 2012; Meyling & Eilenberg 2007). Using EPF for insect control is an important tool in terms of organic agriculture and the integrated control of pests, including Coleoptera, Hemiptera and Lepidoptera, which attack economically important crops (Rodrigues et al. 2016). The use of Beauveria and Metarhizium isolates has several advantages, as they are environment-friendly, and have low toxicity to the applicator and to the environment. Local isolates of entomopathogenic fungi (EPF) have been investigated as components of an integrated pest management (IPM) system for T. leucotreta in South Africa (Goble et al. 2011; Moore 2021).
EPF infect their insect hosts directly through the cuticle (Roy et al. 2006). A conidium attaches and germinates on the host cuticle and develops a germ tube with an appressorium which penetrates the cuticle (Chandler 2017). Invasion of the circulatory system and haemolymph follows penetration. The infection in the haemolymph spreads via blastospores, which are yeast-like cells that usually kill the insect within a period of 3 to 7 days (Inglis et al. 2012). Beauveria and Metarhizium spp. produce secondary metabolites with insecticidal properties. Metarhizium spp. produce destruxins, whereas Beauveria spp. produce oosporein (Coombes et al. 2015). Previous studies found that Metarhizium anisopliae (Metch.) Sorokin (Hypocreales: Clavicipitaceae) and Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordycipitaceae) can successfully suppress agricultural pest insects, like T. leucotreta, during their soil-dwelling life stages (Coombes et al. 2016).
Although Beauveria and Metarhizium spp. are promising biological control agents of various agricultural insect pests, limited information is available on how abiotic factors such as ultraviolet (UV) light affect their persistence and infectivity (Jaronski 2010). Natural sunlight which includes different wavelengths, including solar UV radiation (UV = 10-400 nm) consisting of UV-A and UV-B, is one of the most important factors affecting the persistence of EPF (Ignoffo 1992; Fernandes et al. 2015; Acheampong et al. 2020). UV-B consists of short wavelengths between 280 and 320 nm, which is the most destructive wavelength for EPF (Fernandes et al. 2015). In contrast, UV-A consists of relatively long wavelengths between 315 and 400 nm. Wavelengths of 375-425 nm have the potential to promote photoreaction and are capable of stimulating recovery of the damaged conidia (Fernandes et al. 2015). Both direct and indirect sunlight can cause damage and the inactivation of EPF conidia (Ignoffo 1992; Rodrigues et al. 2016). The inactivation by UV light is caused mainly by strand breakage and crosslinking, as well as by the formation of labile sites on the DNA generating highly reactive radicals, like peroxides (Ignoffo 1992), and cell death (Fang & St. Leger 2012). Radiation can also negatively affect germination of conidia and early developmental stages of germination tubes (Rodrigues et al. 2016).
This study tested the infection potential of an isolate of B. bassiana (1ARC) and isolates of five Metarhizium species after exposure of their conidia to different light treatments, namely no light, growth light and UV light, on soil and leaf surfaces under laboratory and field conditions. Larvae of T. leucotreta served as test hosts.
MATERIALS AND METHODS
Origin of EPF isolates and insects
The EPF isolates used in this study were obtained from the Stellenbosch University EPF collection (Table 1). One isolate of B. bassiana (1ARC) and five isolates of the M. anisopliae species-complex, namely Metarhizium pinghaense Chen & Guo (TH149), Metarhizium majus (Johnst.) Bisch., Rehner (TH153), Metarhizium robertsii (Metchnikoff) Sorokin (6EIKEN), Metarhizium brunneum Petch (3GREY) and M. pinghaense (5HEID) were used in the study. The conidia were cultured on Sabouraud dextrose agar (60 g) with yeast extract (1 g) (SDAY), supplemented with penicillin-streptomycin (200 μl), at a controlled temperature of ± 25 °C in a growth chamber. The last instar larvae of T. leucotreta were obtained from the insect mass-rearing facility, X-Sterile Insect Technique (XSIT) (Pty) Ltd, in Citrusdal, Western Cape province, where they were reared on an artificial diet.
PREPARATION OF CONIDIAL SUSPENSIONS
To produce homogenous conidial suspensions, conidia were harvested from two- to three-week-old surface cultures by scraping with a sterile surgical blade under sterile conditions and suspended in 20 ml sterile distilled water supplemented with 0.05% Tween 20 (Mathulwe 2019). Conidial suspensions were vortex-mixed for 3-4 min and poured through sterile organza fabric into a sterilised 100-ml glass beaker, to remove mycelium and fungal hyphae present. After being poured back into the bottles, the suspension was vortex-mixed for 60 sec. To determine the conidial concentrations for each fungal isolate, 1 ml of conidial suspension from each fungal isolate was transferred into a 10-ml McCartney bottle, containing 9 ml sterile distilled water. The bottles were sealed and vortex-mixed for 2-3 min. The conidial concentrations were determined under a compound microscope using a haemocytometer (Inglis et al. 2012).
The viability of the conidial suspensions was determined following the methodology by Inglis et al. (2012), at a concentration of 1 χ 107 conidia/ml, by spread plating 100 μl of the suspension onto three different SDA plates. The plates were sealed using Parafilm and incubated at a controlled temperature of ± 25 °C. Conidial viability was determined 24 h following incubation, by determining percentage of conidial germination from 100 spores on each plate. Only conidia that developed a germ tube were counted as being viable (i.e., alive), whereas those without a germ tube were considered non-viable (i.e., dead) (Inglis et al. 2012). Only fungal isolates with a viability of > 90% were used in trials.
Pathogenicity of entomopathogenic fungi on soil surface with no light exposure
Five soil sub-samples were collected from each of five vineyards on a table grape farm (33°26'42"S 19°38'56"E), Hex River Valley, Western Cape, South Africa. The sub-samples for each vineyard were mixed, sifted through a 5-mm mesh sieve and autoclaved. For each of the six EPF species and the control, five 250-ml plastic containers (114 mm diam.) were prepared. Each container received 50 g of soil from each of the five vineyard soil samples, for a total of 250 g soil. For the six EPF isolates and the control treatment, a total of 35 containers were used. Each container was inoculated with 8 ml of the conidial suspension of one of the six isolates, at a concentration of 1 χ 107 conidia/ml, and then thoroughly mixed. The control treatment received 8 ml of sterile distilled water only. The soil was allowed to dry for 24 h, after which it was lightly moistened using sterile distilled water. To test conidial pathogenicity, 12 last-instar larvae of T. leucotreta were added to each of the 35 containers (Coombes et al. 2015). After being sealed, the containers were incubated in a growth chamber at a controlled temperature of ± 25 °C, in complete darkness. After 7 days, all dead insects were washed with sterile distilled water, 70% alcohol and lastly rinsed with sterile distilled water, and placed on an agar-agar medium. Infection was measured against overt mycosis after 7 days (Coombes et al. 2015). The experiment was repeated on a different test date, using a fresh batch of EPF inoculum.
Pathogenicity of entomopathogenic fungi on soil surface after exposure to growth light
A similar procedure for preparation of containers with soil and soil inoculation as detailed above was followed, except that the soil samples were exposed to a growth light for a period of 12 h following inoculation with EPF, at a temperature of ± 25 °C. The growth light had a wavelength of between 400 nm and 700 nm and mimicked the visible spectrum of the sun, without UV light (Fargues et al. 1997). After exposure to the growth light, conidial pathogenicity to last-instar larvae of T. leucotreta was determined as described above. The experiment was repeated using fresh EPF inoculum for each isolate.
Pathogenicity of entomopathogenic fungi on soil surface after exposure to UV light
The same procedure for preparation of containers with soil and soil inoculation described above was followed, except that the soil samples were exposed to artificial UV light for a period of 12 h at an ambient temperature of ± 25 °C following inoculation with the conidial suspensions. A Sylvania Reptistar UV light, with a wavelength of between 280 nm and 400 nm was used, as it produces both UV-A and UV-B, which is as close to mimicking the UV radiation of the sun as can be attained (Rangel et al. 2006). After exposure to UV light, conidial pathogenicity to last-instar larvae of T. leucotreta was determined as described previously. The experiment was repeated using a fresh EPF inoculum for each isolate.
Pathogenicity of entomopathogenic fungi on leaves with no light exposure
Leaves were collected from the vines in vineyards where soil samples were collected and washed with 5% bleach (sodium hypochlorite) solution and sterile distilled water to remove any biological and chemical material on their surfaces. The conidial persistence and pathogenicity of EPF isolates were tested by spraying 10 ml of conidial suspensions of each EPF isolate onto five vine leaves, at a standard conidial concentration of 1 χ 107 conidia/ml. For the control treatment, five leaves were sprayed with sterile distilled water only (n = 35 leaves). The conidial suspensions on the leaves were allowed to dry for 24 h in the dark, after which the leaves were lightly sprayed with sterile distilled water and individually placed in 350-ml, 114-mm diameter containers, fitted with moist filter paper. A total of 12 T. leucotreta last instar larvae were placed on top of each leaf, and containers were sealed and incubated at ± 25 °C in a dark growth chamber. Seven days following incubation, the dead insects were surface-sterilised, following similar procedures to those outlined previously, placed on an agar-agar medium and incubated at ±25 °C. Seven days following incubation, overt mycosis on the insect cadavers was recorded. The experiment was repeated, using fresh inoculum for each EPF isolate on a different test date.
Pathogenicity of entomopathogenic fungi on leaves exposed to growth light
Similar procedures for collection of leaves and inoculation with EPF conidial suspensions as those outlined in the previous section were followed. The EPF inoculated leaves were exposed to a growth light for a period of 12 h. Thereafter conidial pathogenicity to last-instar larvae of T. leucotreta was determined as described in the preceding section. The experiment was repeated, using fresh inoculum for each EPF isolate on a different test date.
Pathogenicity of entomopathogenic fungi on leaves exposed to UV light
Similar procedures for collection of leaves and inoculation with EPF conidial suspensions as those outlined previously were followed. Following application of the conidial suspensions to the leaf surfaces, leaves were exposed to artificial UV light for a period of 12 h, at a temperature of ±25 °C. Thereafter conidial pathogenicity to last-instar larvae of T. leucotreta was determined as described for pathogenicity on leaf surfaces with no light exposure. The experiment was repeated, using fresh inoculum for each EPF isolate.
Pathogenicity of entomopathogenic fungi on leaves under field conditions
Leaves in situ were selected in a completely randomised design in one of the vineyard blocks where soil samples were collected. The two best performing isolates in the lab screening trials were selected for the field trials conducted over 24 h and over 7 days. For each trial period, 10 leaves, from various plants, were sprayed on both sides with 10 ml of 107 conidia/ml suspension of each EPF isolate and tagged, for easy visibility of treated leaves during collection. For the control treatment, 10 leaves, from various plants, were sprayed with sterile distilled water. After 24 h and 7 days, tagged leaves were removed from the vines and transferred to the laboratory. In the laboratory, each leaf was lightly sprayed with sterile distilled water and placed in a 350-ml, 114-mm diameter container, fitted with moist filter paper. Twelve T. leucotreta larvae were added to each container to determine conidial viability and pathogenicity, as described in previous sections. Larval mortality and the cause of mortality were recorded for each trial, by surface sterilisation of each dead insect using 70% ethanol and sterile distilled water. Dead insects were placed on an agar-agar medium and further incubated at ± 25 °C, and overt mycosis checked five days post incubation.
Data analysis
STATISTICA Version 13.5.0.17 (TIBCO Software Inc. 2018) was used for statistical analyses. Laboratory experiments were repeated two times and collected data was pooled prior to analysis if normal probability plots confirmed normality. The data was analysed using one-way ANOVA. For the data that did not show normality, a Kruskal-Wallis test was used. Levene's test was done to check the assumption that the variances were homogenous, while the LSD test (least significant difference test) was done to determine significant differences between the means.
RESULTS
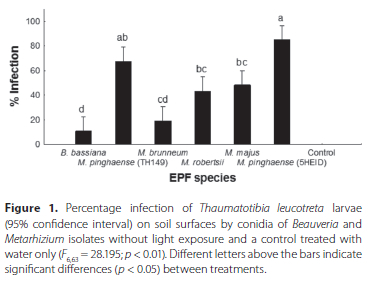
Pathogenicity of entomopathogenic fungi on soil surface with no light exposure
The data were pooled, after the t-test showed no significant differences between the main effects (dates and treatment). The ANOVA (Figure 1) showed significant differences (F663 = 28.195; p < 0.01) in percentage infection of T. leucotreta between the different treatments, with no infection observed in the control treatment. B. bassiana (1ARC) produced the lowest percentage infection of 10.83% ± 2.79% (mean ± SE), followed by M. brunneum (3GREY) with 19.17% ± 4.49%. There was no significant difference between the isolates (p > 0.05). The highest average percentage of T. leucotreta infection was obtained with the M. pinghaense (5 HEID) and M. pinghaense (TH149) isolates, namely 85.00% ± 5.09% and 67.50% ± 8.19%, respectively, with no significant difference (p > 0.05) between them. The percentage infection of T. leucotreta larvae due to M. pinghaense (TH149), 67.50% ± 8.19%, did not differ significantly from that of M. majus (TH153) with 48.33% ± 7.84%, or M. robertsii (6EIKEN) with 43.33% ± 7.43% (Figure 1).
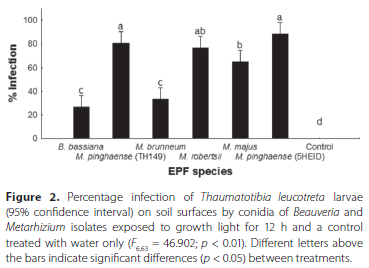
Pathogenicity of entomopathogenic fungi on soil surface after exposure to growth light
The data for the two experiments were pooled, after the t-test showed no significant differences between the main effects (dates and treatment). ANOVA showed significant differences (F663= 46.902; p < 0.01) in percentage infection of T. leucotreta between the different treatments, with no infection observed in the control treatment (Figure 2). Beauveria bassiana (1ARC) (26.67% ± 7.64%) had the lowest average percentage infection of T. leucotreta larvae followed by M. brunneum (3GREY) (33.33% ± 5.83%), with no significant difference between the isolates. The highest average percentage T. leucotreta infection was obtained using M. pinghaense (5HEID) (88.33% ± 2.22%), followed by M. pinghaense (TH149) (80.83% ± 4.31%), M. robertsii (6EIKEN) (76.67% ± 4.27%) and M. majus (TH153) (65.00% ± 5.53%) (Figure 2). No significant difference was observed between M. pinghaense (5HEID), M. robertsii (6EIKEN) and M. pinghaense (TH149) isolates, and between M. majus (TH153) and M. robertsii (6EIKEN).
Pathogenicity of entomopathogenic fungi on soil surface after exposure to UV light
The t-test showed that the two trials were not the same, and a Kruskal-Wallis ANOVA was done separately for each trial (Figure 3A and 3B). In the first trial, significant differences (F6,28 = 34.244; p < 0.01) in percentage infection were found between the treatments (Figure 3A). The highest mean percentage of T. leucotreta infection was obtained using M. pinghaense (5HEID) (88.33% ± 3.33%), followed by M. majus (TH153) (85.00% ± 4.08%), M. pinghaense (TH149) (78.33% ± 5.00%) and M. robertsii (73.33% ± 7.17%). Metarhizium pinghaense (5HEID and TH149), M. majus (TH153) and M. robertsii (6EIKEN) treatments did not differ significantly from each other (p < 0.05). The control treatment had no T. leucotreta infection. The lowest mean percentage of T. leucotreta infection was obtained with B. bassiana (1ARC) (28.33% ± 4.25%), followed by M. brunneum (3GREY) (53.33% ± 10.07%). The control, B. bassiana (1ARC) and M. brunneum (3GREY) differed significantly (p < 0.05) from all other treatments (Figure 3A).
In the second trial, significant differences in percentage infection were found between treatments (F6,28 = 17.560; p < 0.01; ANOVA) (Figure 3B), with no infection of T. leucotreta occurring in the control treatment. The lowest average percentage of T. leucotreta infection occurred with B. bassiana (1ARC) (3.33% ± 2.04%), followed by M. brunneum (3GREY) (13.33% ± 3.33%). Neither B. bassiana (1ARC) nor M. brunneum (3GREY) differed significantly from the control treatment (p > 0.05). The highest average percentage of T. leucotreta infection occurred with M. pinghaense (5HEID) (85.00% ± 3.12%), followed by M. pinghaense (TH149) (58.33% ± 12.64%), M. majus (TH153) (51.67% ± 8.50%) and M. robertsii (6EIKEN) (36.67% ± 11.96%). The average percentage of T. leucotreta infection with M. pinghaense (5HEID) differed significantly from that of the other EPF isolates and the control (Figure 3B). No significant difference in percentage infection was found between M. pinghaense (TH149), M. robertsii (6EIKEN) and M. majus (TH153).
Pathogenicity of entomopathogenic fungi on leaves with no light exposure
The t-test for overall percentage infection showed that the two trials were not the same, and Kruskal-Wallis ANOVA was undertaken separately for each trial (Figure 4A and 4B). For the first trial, significant differences in percentage infection were found between treatments (F628 = 13.205; p < 0.01) (Figure 4A). The control had zero infected T. leucotreta larvae. The lowest average percentage of T. leucotreta infections was obtained with B. bassiana (1ARC) (16.67% ± 3.73%), followed by M. majus (TH153) (58.33% ± 3.73%) and M. brunneum (3GREY) (45.00% ± 15.05%). No significant difference (p > 0.05) was found between the control and B. bassiana (1ARC). The highest average percentage of T. leucotreta infection was achieved with M. pinghaense (5HEID) (81.67% ± 8.50%), followed by M. pinghaense (TH149) (78.33% ± 8.16%) and M. robertsii (6EIKEN) (78.33% ± 12.80%). No significant difference in percentage infection was found between M. pinghaense (TH149), M. robertsii (6EIKEN), M. majus (TH153) and M. pinghaense (5HEID), and between M. brunneum (3GREY) and M. majus (TH153) (Figure 4A).
The second trial also showed significant differences (F628 = 44.310; p < 0.01; ANOVA) between treatments (Figure 4B), with no T. leucotreta infections in the control. All the treatments differed significantly (p < 0.05) from the control treatment. The highest average percentage of T. leucotreta infection was found with M. pinghaense (TH149) (100%), followed by M. pinghaense (5HEID) and M. majus (TH153), which gave the same results (95.00% ± 2.04%), and M. robertsii (6EIKEN) (85.00% ± 5.53%). No significant difference in percentage infection was found between M. pinghaense (TH149), M. robertsii (6EIKEN), M. majus (TH153) and M. pinghaense (5HEID). The lowest average percentage T. leucotreta infection was obtained with B. bassiana (1 ARC) (40.00% ± 4.08%), followed by M. brunneum (3GREY) (66.67% ± 12.64%). B. bassiana (1 ARC) and M. brunneum (3GREY) differed significantly in percentage infection from all treatments (Figure 4B).
Pathogenicity of entomopathogenic fungi on leaves exposed to growth light
The ANOVA showed significant differences between treatments CF663 = 14.946; p < 0.01). All of the EPF treatments differed significantly (p < 0.05) from the control, but not from each other (Figure 5). The lowest average percentage of T. leucotreta infection was obtained with M. majus (TH153) (57.50% ± 5.62 %), followed by M. pinghaense (5HEID) (59.17% ± 6.14%) and M. brunneum (60.83% ± 7.46%). The highest average percentage of T. leucotreta infection was attained with M. pinghaense (TH149) (72.50% ± 8.70%), followed by M. robertsii (6EIKEN) (70.00% ± 8.35%) and B. bassiana (67.50% ± 4.88%) (Figure 5).
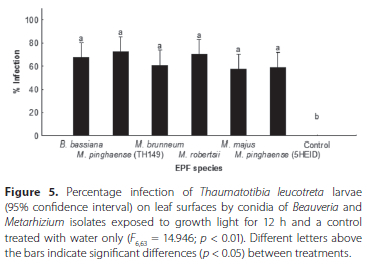
Pathogenicity of entomopathogenic fungi on leaves exposed to UV light
Significant differences were found between treatments (F6,63 = 37.950; p < 0.01) with the ANOVA (Figure 6). All treatments differed significantly from the control (p < 0.05). The highest average percentage of T. leucotreta larvae infected was obtained with M. pinghaense (5HEID) (95.00% ± 2.22%), followed by M. pinghaense (TH149) (94.17% ± 4.13%), M. majus (TH153) (91.67% ± 2.78%) and M. robertsii (6EIKEN) (91.67% ± 1.76%).
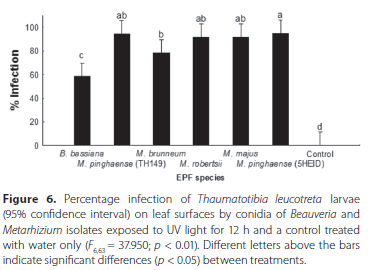
These treatments did not differ significantly from each other. The average percentage infection with M. brunneum (3GREY) (78.33% ± 7.05%) differed significantly from that of M. pinghaense (5HEID) and from B. bassiana (1ARC), which had the lowest average percentage of T. leucotreta infection (58.33% ± 11.79%) (Figure 6).
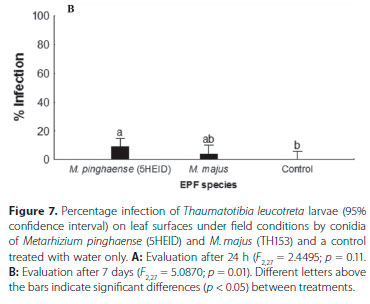
Pathogenicity of entomopathogenic fungi on leaves under field conditions
For the 24 h field trial, significant differences were found between treatments (F2,27 = 2.4495; p = 0.11; ANOVA), with zero T. leucotreta infections in the control (Figure 7A), although percentage infection of T. leucotreta was very low for all treatments. The highest average percentage of T. leucotreta infection was obtained with M. pinghaense (5HEID) (9.17% ± 4.56%), followed by M. majus (TH153) (4.17% ± 2.24%). M. pinghaense (5HEID) differed significantly (p < 0.05) from the control and from M. majus (TH153) (Figure 7A).
In the 7-day field trial, the ANOVA showed significant differences between treatments (F2,27 = 5.0870; p = 0.01). The highest average percentage of T. leucotreta infections was obtained with M. pinghaense (5HEID) (15.82% ± 5.88%), which differed significantly from the control, but not from M. majus (4.17% ± 2.24%). The control, with zero infections, did not differ significantly (p > 0.05) from M. majus (Figure 7B).
DISCUSSION
The damaging effects of sunlight, particularly UV light, on the persistence of EPF is generally seen as a major factor limiting their use in field conditions (Ignoffo 1992; Fernandes et al. 2015; Acheampong et al. 2020). However, there is evidence that not all EPF species are so sensitive to damage by UV light (Rangel et al. 2006; Fang & St. Leger 2012). This study therefore investigated the effects of growth light and UV light on the survival and pathogenicity of the conidia of six local EPF isolates.
Overall, the study showed that some of the Metarhizium isolates, except M. brunneum (3GREY), exhibited better survival and pathogenicity following exposure to either growth light or UV light on soil surfaces. Rangel et al. (2006) found that some Metarhizium wild types can be relatively UV resistant,
due to nonenzymatic and enzymatic defence mechanisms. The enzymes superoxide dismutase and catalase serve to minimise the level of oxidative stress resulting from UV radiation and heat (Rangel et al. 2006). Nonenzymatic defence is attained when pigments are accumulated, which also helps against oxidative stress (Rangel et al. 2006). A major repair mechanism that serves to repair DNA damage resulting from UV radiation is known as photoreactivation, which is present in the Metarhizium genus (Fang & St. Leger 2012). Such reasons might explain the good performance in terms of survival and pathogenicity for the M. pinghaense isolates.
Further assessment of EPF conidia survival and pathogenicity on leaf surfaces following exposure to no light treatment for 24 h showed Metarhizium isolates as the most pathogenic, with high survival. The exposure of the isolates to growth light on leaf surfaces improved the survival and pathogenicity of the B. bassiana (1ARC) and M. brunneum (3GREY) isolates, which had slightly lower survival and pathogenicity when exposed to no light conditions. Similar observations were made in the trial exposing conidia of isolates on leaf surfaces to UV light for 24 h, where survival and pathogenicity of all isolates were improved.
Overall, for both the soil and leaf UV light trials, B. bassiana (1ARC) was found to yield the lowest average percentage of T. leucotreta infections, with M. pinghaense (5HEID) achieving the highest average infection. According to Brunner-Mendoza et al. (2019), M. anisopliae, M. pinghaense, M. robertsii (6EIKEN) and M. brunneum, which are closely related, have overlapping traits, including the UV protection mechanism (Fang & St. Leger 2012). However, the findings made in the current study do not conform to those made in their study, as some of the Metarhizium species, like M. brunneum (3GREY), performed poorly. According to Ortiz-Urquiza & Keyhani (2015), different strains ofM. anisopliae species complex and B. bassiana can vary in terms of the extent of their UV tolerance. Such variance might explain their poor performance in relation to those strains that are generally less UV tolerant than are others.
The above also indicates that EPF conidia require a light source to enhance their survival and infectivity potential. Onofre et al. (2001) tested three types of lights on a Metarhizium strain, Metarhizium flavoviride Gams & Roszypal (Hypocreales: Clavicipitaceae), to see what their effect would be on germination and sporulation. The study showed that, in the presence of visible light, reproduction was stimulated and the maturation of reproductive primordia occurred. Although Onofre et al. (2001) did not mention the wavelengths of the lights used, and despite M. flavoviride not being part of the six EPF tested in the current study, it is a strain of the Metarhizium genus to which five of the tested EPF belong. Oliveira et al. (2018) also tested the effect of different light exposures on the germination speed of M. robertsii. Their study described the use of a white light, with wavelengths between 390 and 700 nm, and a blue light with wavelengths between 400 and 450 nm, which were almost the same as those of the growth light used in the current study, whose wavelengths ranged between 400 and 700 nm. Both the white and blue light produced conidia that germinated much faster, which were much more pathogenic, and killed insects much faster and more frequently than when the fungi were kept in the dark with no light exposure (Oliveira et al. 2018). This might explain why the growth light and UV light trials showed higher survival and pathogenicity relative to trial with no light.
Further evaluation of the performance of the two top performing EPF isolates, M. pinghaense and M. majus (TH153), on leaf surfaces under field conditions, was based on their high survival and pathogenicity on leaf surfaces under laboratory conditions. The results showed a drastic decrease in survival and pathogenicity to < 20% on leaf surfaces under field conditions. The decline in survival and pathogenicity might be due to the two days of rainfall during the field trials, which might have washed the EPF treatments off the leaves. The decline in conidial performance of the isolates might therefore not be necessarily due to exposure sunlight over both the 24 h and seven-day periods. Kouassi et al. (2003), assessing the effect of plant type on the persistence of B. bassiana, showed that rainfall had a significant effect on conidial persistence and resulted in decline of colony forming units (CFU) on celery and lettuce leaves. Their study suggests that rainfall had washed off the EPF conidia on the plant leaves, under field conditions. Similarly, Inglis et al. (2000) assessing the influence of rain and conidial formulation on the persistence of Beauveria bassiana on potato leaves and Colorado potato beetle larvae, showed that rainfall has a substantial impact on persistence of conidia on plant leaves, with removal of high numbers of CFU, 89-95% of B. bassiana conidia, from the plant leaves within the first 15 min of rain exposure.
Although the effect of humidity was not tested in the current study, it could help to explain why the leaf field trials differed so significantly from the laboratory trials in terms of conidial survival and pathogenicity. EPF species require high air humidity to induce optimal insect mortality (Arthurs & Thomas 2001). The air humidity for the 24-h trial attained a minimum of 25% RH and a maximum of 94% RH. In contrast, the air humidity for the 7-day trial attained a minimum of 27% RH and a maximum of 100% RH. Arthurs & Thomas (2001) found that a constant humidity of 96% RH is the optimal air humidity for sporulation. Brunner-Mendoza et al. (2019) mentioned that EPF conidia require a high humidity of over 90% for germination. Such requirements could explain why the leaf field trial did so poorly compared to the soil field trials, as the humidity fluctuated far below the optimal air humidity during the leaf field trial. In contrast, under laboratory conditions at a constant temperature of 25 °C, air humidity of > 95% also remained constant throughout the tests.
In conclusion, the conidia of the Metarhizium isolates were shown to be relatively tolerant to short term exposure to UV light and growth light on both leaf and soil surfaces, as all the isolates used in the study showed higher levels of pathogenicity and survival following exposure to both light sources under laboratory conditions, relative to the Beauveria isolate. However, the degree of conidial tolerance following exposure to the light sources for the different Metarhizium isolates differed, with the M. pinghaense isolates (5HEID and (TH149) showing the highest level of persistence and pathogenicity, under laboratory conditions. Therefore, further studies assessing the longterm pathogenicity and survival of the M. pinghaense isolates should be conducted under field conditions, as the isolates have shown their potential to tolerate both UV light and growth light, therefore sunlight should not be a major limiting factor. Future studies should also investigate the use of oil-formulated conidia and the use of adjuvants that will protect the conidia from adverse environmental conditions such as low humidity and extreme temperatures, to improve the efficacy of the isolates under field conditions.
ACKNOWLEDGEMENTS
The authors would like to thank Prof. D.G. Nel, from the Centre for Statistical Consultation, Stellenbosch University, for assistance with the statistical analysis.
AUTHORS' CONTRIBUTIONS
Conceptualisation: N.F. Stokwe; Data curation: S. Rossouw; Formal analysis: S. Rossouw and L.L. Mathulwe; Funding acquisition: N.F Stokwe; Investigation: S. Rossouw, L.L. Mathulwe, N.F. Stokwe and A.P. Malan; Methodology: S. Rossouw, L.L Mathulwe and A.P. Malan; Project administration: N.F. Stokwe; Resources: A.P. Malan and N.F. Stokwe; Supervision: L.L. Mathulwe, A.P. Malan and N.F. Stokwe; Writing original draft: S. Rossouw; Writing review and editing: A.P. Malan, L.L. Mathulwe and N.F. Stokwe.
ORCID IDs
Sumarie Rossouw - https://orcid.org/0000-0003-2401-6624
Letodi L. Mathulwe - https://orcid.org/0000-0002-5118-3578
Nomakholwa F. Stokwe - https://orcid.org/0000-0003-2869-5652
Antoinette P. Malan - https://orcid.org/0000-0002-9257-0312
REFERENCES
Acheampong MA, Hill MP, Moore SD, Coombes CA. 2020.UV sensitivity of Beauveria bassiana and Metarhizium anisopliae isolates under investigation as potential biological control agents in South African citrus orchards. Fungal Biology 124(5): 304-310. https://doi.org/10.1016/j.funbio.2019.08.009 [ Links ]
Arthurs S, Thomas MB. 2001. Effects of temperature and relative humidity on sporulation of Metarhizium anisopliae var. acridum in mycosed cadavers of Schistocerca gregaria. Journal of Invertebrate Pathology 78(2): 59-65. https://doi.org/10.1006/jipa.2001.5050 [ Links ]
Brunner-Mendoza C, Reyes-Montes MR, Moonjely S, Bidochka M, Toriello C. 2019. A review on the genus Metarhizium as an entomopathogenic microbial biocontrol agent with emphasis on its use and utility in Mexico. Biocontrol Science and Technology 29(1): 83-102. https://doi.org/10.1080/09583157.2018.1531111 [ Links ]
CABI. 2021. Invasive Species Compendium. Detailed coverage of invasive species threatening livelihoods and the environment worldwide. https://www.cabi.org/isc/datasheet/6904
Chandler D. 2017. Basic and applied research on entomopathogenic fungi. In: Lacey L, editor. Microbial Control of Insect and Mite Pests: From Theory to Practice. USA: Academic Press; p. 69-89. https://doi.org/10.1016/B978-0-12-803527-6.00005-6.
Coombes CA, Hill MP, Moore SD, Dames JF. 2016. Entomopathogenic fungi as control agents of Thaumatotibia leucotreta in citrus orchards: field efficacy and persistence. BioControl 61(6): 729-739. https://doi.org/10.1007/s10526-016-9756-x [ Links ]
Coombes CA, Hill MP, Moore SD, Dames JF, Fullard T. 2015. Beauveria and Metarhizium against false codling moth (Lepidoptera: Tortricidae): a step towards selecting isolates for potential development of a mycoinsecticide. African Entomology 23(1): 239242. https://doi.org/10.4001/003.023.0107 [ Links ]
Daiber CC. 1979a. A study of the biology of the false codling moth [(Cryptophlebia leucotreta (Meyr.)]: the cocoon. Phytophylactica ; 11: 151-157. [ Links ]
Daiber CC. 1979b. A study of the biology of the false codling moth [(Cryptophlebia leucotreta (Meyr.)]: the larva. Phytophylactica 11: 141-144. [ Links ]
Daiber CC. 1980. A study of the biology of the false codling moth Cryptophlebia leucotreta (Meyr.): the adult and generations during the year. Phytophylactica 12: 187-193. [ Links ]
Daiber CC. 1989. The false codling moth, Cryptophlebia leucotreta (Meyr.) (Lepidoptera, Tortricidae), in Southern Africa. Journal of Plant Diseases and Protection 96: 71-80. [ Links ]
Fang W, St. Leger RJ. 2012. Enhanced UV resistance and improved killing of malaria mosquitoes by photolyase transgenic entomopathogenic fungi. PLoS One. 7(8): e43069. https://doi.org/10.1371/journal.pone.0043069 [ Links ]
Fargues J, Rougier M, Goujet R, Smits N, Coustere C, Itier B. 1997. Inactivation of conidia of Paecilomyces fumosoroseus by near-ultraviolet (UVB and UVA) and visible radiation. Journal of Invertebrate Pathology 69(1): 70-78. https://doi.org/10.1006/jipa.1996.4637 [ Links ]
Fernandes EKK, Rangel DEN, Braga GUL, Roberts DW. 2015. Tolerance of entomopathogenic fungi to ultraviolet radiation: A review on screening of strains and their formulation. Current Genetics 61(3): 427-440. https://doi.org/10.1007/s00294-015-0492-z. [ Links ]
Georgala MB. 1969. Control of false codling moth and fruit flies in citrus orchards. South African Citrus Journal. 421: 3-7. [ Links ]
Goble TA, Dames JF, Hill MP, Moore SD. 2011. Investigation of native isolates of entomopathogenic fungi for the biological control of three citrus pests. Biocontrol Science and Technology 21(10): 1193-1211. https://doi.org/10.1080/09583157.2011.608907 [ Links ]
Ignoffo CM. 1992. Environmental factors affecting persistence of entomopathogens. The Florida Entomologist 75(4): 516-525. https://doi.org/10.2307/3496133 [ Links ]
Inglis GD, Enkerli J, Goettel MS. 2012. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In: Lacey LA, editor. Manual of techniques in invertebrate pathology. 2nd ed. London: Academic Press. p. 189-253. https://doi.org/10.1016/B978-0-12-386899-2.00007-5 [ Links ]
Inglis GD, Ivie TJ, Duke GM, Goettel MS. 2000. Influence of rain and conidial formulation on persistence of Beauveria bassiana on potato leaves and Colorado potato beetle larvae. Biological Control 18(1): 55-64. https://doi.org/10.1006/bcon.1999.0806 [ Links ]
Jaronski ST. 2010. Ecological factors in the inundative use of fungal entomopathogens. BioControl 55(1): 159-185. https://doi.org/10.1007/s10526-009-9248-3 [ Links ]
Kouassi M, Coderre D, Todorova SI. 2003. Effect of plant type on the persistence of Beauveria bassiana. Biocontrol Science & Technology 13(4): 415-427. https://doi.org/10.1080/0598315031000104532 [ Links ]
Mathulwe LL. 2019. Control of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae), using entomopathogenic fungi. MSc dissertation. Stellenbosch University, Stellenbosch. [ Links ]
Meyling NV, Eilenberg J. 2007. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biological Control 43(2): 145-155. https://doi.org/10.1016/j.BioControl2007.07.007 [ Links ]
Moore SD. 2021. Biological control of a phytosanitary pest (Thaumatotibia leucotreta): A case study. International Journal of Environmental Research and Public Health 18(3): 1198. https://doi.org/10.3390/ijerph18031198 [ Links ]
Moore S, Kirkman W. 2009. Citrus orchard sanitation with emphasis on false codling moth control. SA Fruit Journal. 7(6): 57-60. [ Links ]
Moore S, Kirkman W, Hattingh V. 2015. The host status of lemons for the false codling moth, Thaumatotibia leucotreta (Meyrick) (Lepidoptera: Tortricidae) with particular reference to export protocols. African Entomology 23(2): 519-525. https://doi.org/10.4001/003.023.0223 [ Links ]
Oliveira AS, Braga GUL, Rangel DEN. 2018. Metarhizium robertsii illuminated during mycelial growth produces conidia with increased germination speed and virulence. Fungal Biology 122(6): 555-562. https://doi.org/10.1016Ai.funbio.2017.12.009 [ Links ]
Onofre AB, Miniuk CM, de Barros NM, Azevedo JL. 2001. Growth and sporulation of Metarhizium flavoviride var. Flavoviride on culture media and lighting regimes. Scientia Agricola 58(3): 613-616. https://doi.org/10.1590/S0103-90162001000300026 [ Links ]
Ortiz-Urquiza A, Keyhani NO. 2015. Stress response signalling and virulence: insight from entomopathogenic fungi. Current Genetics 61(3): 239-249. https://doi.org/10.1007/s00294-014-0439-9 [ Links ]
Rangel DEN, Butler MJ, Torabinejad J, Anderson AJ, Braga GUL, Day AW, Roberts DW. 2006. Mutants and isolates of Metarhizium anisopliae are diverse in their relationships between conidial pigmentation and stress tolerance. Journal of Invertebrate Pathology 93(3): 170-182. https://doi.org/10.1016/j.jip.2006.06.008 [ Links ]
Rodrigues IMW, Forim MR, da Silva MFGF, Fernandes JB, Filho AB. 2016. Effects of ultraviolet radiation on fungi Beauveria bassiana and Metarhizium anisopliae, pure and encapsulated, and bio-insecticide action on Diatraea saccharalis. Advances in Entomology 4(3): 151-162. https://doi.org/10.4236/ae.2016.43016 [ Links ]
Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK. 2006. Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annual Reviews of Entomology 51(1): 331-357. https://doi.org/10.1146/annurev.ento.51.110104.150941 [ Links ]
Stofberg FJ. 1954. False codling moth of citrus. Farming in South Africa. 29: 273-276. [ Links ]
TIBCO Software Inc. 2018. STATISTICA (data analysis software system), version 13.5.0.17. Palo Alto (CA): TIBCO Software Inc. [ Links ]
 Correspondence:
Correspondence:
LL Mathulwe
Email: Mathulwell@sun.ac.za
Received:25 January 2022
Accepted: 07 September 2022