Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
African Entomology
versão On-line ISSN 2224-8854
versão impressa ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a10672
RESEARCH ARTICLE
Occurrence of the potato leaf miner, Liriomyza huidobrensis (Diptera: Agromyzidae), and parasitoids in potato fields and natural vegetation of the Western Cape province, South Africa
Thabu MugalaI; Diedrich VisserII; Antoinette P. MalanI; Pia AddisonI
IDepartment of Conservation Ecology and Entomology, Faculty of AgriSciences, Stellenbosch University, Stellenbosch, South Africa
IIAgricultural Research Council, ARC-VIMP, Pretoria, South Africa
ABSTRACT
The leaf miner, Liriomyza huidobrensis (Diptera: Agromyzidae) is a polyphagous fly, which infests a wide range of vegetables and ornamental plants. However, scant knowledge exists of the biology and ecology of the pest in the Western Cape province of South Africa, both of which are essential components in developing an effective management programme. Several aspects of its biology and ecology were studied in two potato [Solanum tuberosum L. (Solanales: Solanaceae)] fields in the Sandveld region during both winter and summer months. The population densities of adult L. huidobrensis were monitored using yellow sticky traps, which were retrieved and counted once every two weeks (June 2019 to January 2020). The number of leaf miner adults caught throughout the summer and winter monitoring periods was significantly different. In addition, 50 randomly selected plants were assessed for leaf damage (in the form of punctures and mines) using presence/ absence sampling. Leaf miner persistence within the soil was confirmed through one-time soil sampling of the two fields, one month after harvesting. Leaf miner pupae were detected in the soil samples after the potatoes were harvested. However, no adult emergence was recorded among the collected pupae under laboratory conditions. The observed trend was that the traps, in both fields, caught an increasing number of leaf miners as the crops matured. Damage occurred in both the monitored fields as early as week four, after plant emergence. Of the five parasitoid species collected, Diglyphus isaea (Hymenoptera: Eulophidae) was the most abundant.
Keywords: Diglyphus isaea, plant emergence, Sandveld, Solanum tuberosum
INTRODUCTION
The potato leaf miner, Liriomyza huidobrensis (Blanchard) (Diptera: Agromyzidae), is an important pest of potatoes [Solanum tuberosum L. (Solanales: Solanaceae)] that causes substantial damage to potato crops in different parts of the world (Reitz et al. 2013). It is known to affect several plant species worldwide due to its polyphagous behaviour (CABI 2019), affecting mostly vegetable crops and weeds (López et al. 2010; Reitz et al. 2013). The term 'leaf miner' refers to those insects who live out one or more of their life stages by feeding on leaf tissue between the epidermal cells (Ayabe 2010). The leaf-mining habit causes vulnerability to predators because, when leaf miners are inside the leaves, the ability to escape predation is limited (Visser and Schoeman 2004). Other studies have, however, suggested that the leaf-mining habit is a mechanism that is used by the insect as a defence against pathogens and predatory insects (Hering 1951; Connor and Taverner 1997). An alternative hypothesis is that the susceptibility of leaf-mining insects to pathogens is, likely, due to the humid conditions within the leaf (Cornell 1989).
Liriomyza huidobrensis is a highly invasive pest and resistant to a variety of insecticides (Reitz et al. 2013; Weintraub et al. 2017). Direct damage to potato foliage is caused by oviposition punctures of the females and feeding of the larvae, which cause the leaves to become necrotic and eventually die. Chabi-Olaye et al. (2008) reported a 62% reduction in the photosynthetic ability of mined leaves, leading to high yield losses. Indirect damage results from diseases that may enter the host through puncture holes on the leaves (Deadman et al. 2000). Scant information is available on the economic injury level and the economic threshold level because of their short life cycle which makes determining economic injury challenging (Rondon 2010). However, in a study by Alves et al. (2017) an economic injury level of 0.07 and an economic threshold of 0.05 mines per plant were determined, thus indicating the need to review current control measures.
The potato, S. tuberosum, is considered an essential food tuber around the world, being grown in over 125 countries by both subsistence and commercial farmers (Caldiz et al. 2009). In South Africa, the extent ofthe area that is annually planted with potatoes is between 50 000 and 54 000 ha, mostly in the Limpopo, Western Cape, Free State and Mpumalanga provinces (DAFF 2012). However, potato production is affected by several different insect pests that reduce productivity (Oerke and Dehne 2004).
Leaf-mining activities reduce the photosynthetic potential of an infested plant, leading to plant mortality (Chabi-Olaye et al. 2008). Therefore, for effective control options, chemical insecticides have been widely used, usually being applied after the first signs of infestation (Rondon 2010; Mujica and Kroschel 2013). Liriomyza huidobrensis population densities tend to increase rapidly, with Weintraub and Horowitz (1995) reporting an increase in population densities from 38% in week 5 to 47% in week 9 after planting, in Peru. High population densities elicit applications of synthetic insecticides to the potato plants, usually on a large scale (Reitz et al. 2013), which cause elevated production costs as well as other negative impacts including a reduction in natural enemies and increasing the risk of environmental contamination (El-Wakeil et al. 2013). Some studies have recognised that outbreaks are associated with synthetic insecticide overuse (Vincini and Carmona 2006), because the natural enemies concerned are lost, resulting in an increase in leaf miner reproduction (Johnson et al. 2002).
Previous studies have demonstrated that natural enemies play a vital role in regulating the population of Liriomyza species in their native ranges, as well as in invaded areas (Rauf et al. 2000; Chen et al. 2003). To reduce the use of synthetic pesticides, biological control options (like the mass release of parasitoids) have become popular alternative treatments (Connor and Taverner 1997). Several parasitoids have been used as biological control agents against the L. huidobrensis in various parts of the world (Civelek et al. 2002).
Various studies show the efficacy of parasitoids in regulating leaf miner populations (Salvo and Valladares 2007). Over 300 known parasitoid species are associated with agromyzids (Spencer 1990). However, of the parasitoid species concerned, only 80 are associated with Liriomyza species (Liu et al. 2009; Mujica and Kroschel 2011). The parasitoids known to parasitise Liriomyza species mostly include families in the order Hymenoptera, namely Braconidae, Eulophidae and Pteromalidae (Shepard and Braun 1998; Weintraub and Horowitz 1998; Civelek et al. 2002). However, one of the commonly used parasitoids in mass release control programmes worldwide, is Diglyphus isaea (Walker) (Hymenoptera: Eulophidae), which is a solitary larval ectoparasitoid of different leaf miners, including L. huidobrensis, Liriomyza sativae Blanchard and Liriomyza trifolii (Burgess) (Ode and Heinz 2002; Liu et al. 2009). The use of parasitoids as biocontrol agents has become popular in different parts of the world (especially in Europe, USA, Asia and, in recent years, different parts of Africa) (Ode and Heinz 2002; Liu et al. 2009; Musundire et al. 2012).
The effectiveness of parasitism depends on the ability of the parasitoid to locate suitable hosts and to kill the larvae through consuming the host (Kruidhof et al. 2019). The highest success rate that has been reached with the use of D. isaea as a biological control agent has been through augmentative release (Ozawa et al. 2001). Previous studies suggest that the success of parasitoids varies depending on the life stage of the insect host and environmental conditions (Grabenweger et al. 2010). Environmental conditions are believed to play an important role in parasitoid efficiency, with increased insect parasitism occurring in temperate regions, compared to that of tropical regions (Hawkins et al. 1997; Klok et al. 2003; Hill et al. 2016). The rate of parasitism may also be affected by several factors including the presence of other organisms like bacteria, fungi and nematodes (Fenoglio and Salvo 2009; Fenoglio et al. 2012). Johnson and Hara (1987) argued that the effectivity of specific biological control agents may also depend on the plant species on which the leaf miner feeds.
Successful classic biological control using parasitoids on leaf miners, both in open fields and in greenhouses, have been documented (Dharmadhikari et al. 1977; García-Marí et al. 2004; Cusumano et al. 2020). However, several aspects must be considered in relation to control, such as tolerance to humidity, the ability to detect the host and host synchronisation (Wang et al. 1999; Girardoz et al. 2006). Fluctuations in temperature tend to result in the inability of parasitoids to parasitise their host successfully (Duan et al. 2014; Kalaitzaki et al. 2014).
Environmental variables such as temperature, precipitation, atmospheric pressure, turbulence and UV radiation can, potentially, influence parasitoid species distribution and the community structures of the L. huidobrensis (Hodkinson 2005). However, the direct effects of environmental factors tend to provide little explanation of their distribution patterns geographically (Lomolino 2001; Eyre et al. 2005). Recent studies indicate that factors like thermotolerance are essential when determining the seasonal abundance and the geographical distribution of most Liriomyza species (Rodríguez-Castaneda et al. 2017). However, insufficient information exists regarding the direct effects of both the abiotic and the biotic factors on L. huidobrensis abundance and survival. In this regard, local information regarding the ecology and biology of the leaf miner was, at the time of the current study, unavailable. Therefore, possessing knowledge of the mode of infestation of the L. huidobrensis, regarding, for instance, whether it utilises alternative hosts in surrounding habitats, as well as its population dynamics, overwintering mechanism and damage levels, is of great importance.
The current study was conducted to gain insight into the presence and distribution of L. huidobrensis and its parasitoids in potato fields in the Western Cape province, South Africa. The information can assist in the refinement of management programmes to improve control strategies against the pest. In the current study, various potato fields and adjacent stands of natural vegetation were monitored for the presence and prevalence of L. huidobrensis using sticky traps. In addition, the mode of infestation on leaves was investigated and whether natural adjoining hosts played a role in harboring overwintering populations. Liriomyza huidobrensis laboratory rearing and natural parasitoid occurrence were also investigated.
MATERIALS AND METHODS
Climatic conditions of the Sandveld
The Sandveld, the largest potato-growing region in the Western Cape, South Africa, is a narrow area that is situated between the Swartland and the West Coast. The region experiences a typical Mediterranean climate with hot, dry summers, and winters that range from moderate to cold and wet (Schulze et al. 2008). The average rainfall experienced within this region ranges between 300 mm and 400 mm per annum in the winter months (between April and September) (Schulze et al. 2008). The mean annual temperatures range between 16 °C and 19 °C, with temperatures reaching up to 35 °C in summer and a low of 8 °C in winter. The evaporation rates range between 5.5 mm/day and 7.35 mm/day in summer and between 1.5 mm/day and 2.3 mm/day in winter, with the values increasing towards the coast (Schulze et al. 2008). The estimated irrigation requirements for potatoes in the Sandveld is about 700 mm ofwater for the crops that are planted in September and about 610 mm for the crops that are planted in August (Schulze et al. 2008). However, the irrigation requirements tend to vary according to the rainfall, temperature, and wind conditions experienced at the time (Schulze et al. 2008; DAFF 2012).
Potato field sites
The study sites were situated at three different locations in the Sandveld region. The two sites that were used to determine the mode of infestation (damage assessments) were situated in Saamstaan (32°35'19.3"S 18°20'07.9"E and 32°34'50.8"S 18°20'05.4"E), whilst the five sites that were used as leaf collection sites were situated in Modderfontein (32°34'23.8"S 18°22'47.1"E) and Taaiboskraal (32°33'18.3"S 18°24'38.3"E, 32°33'50.6''S 18°4'25.9"E, 32°33'47.3"S 18°25'39.6"E and 32°33'32.7"S 18°25'59.0"E). The potato leaves collected were used to survey for parasitoids and as the core collection from which the colony-related material was reared (Figure 1). The alternative host survey was conducted in Philippi, Cape Town, South Africa (34°02'23.9"S 18°32'42.1"E). All fields were irrigated using centre-pivot irrigation, which is a method of crop irrigation in which the irrigation lines are installed 1 or 2 m above the canopy.
Adult population dynamics
Two potato fields on the farm Saamstaan were regularly sampled for male and female L. huidobrensis before, during and after the potato growing period as well as for related larval damage. The monitoring involved placing yellow sticky traps in the potato fields at specific distances (25 m, 50 m and 100 m) from the centre point, following transects running from the centreline to the edge, and 25 m, 50 m and 100 m distances into the surrounding natural vegetation. Each potato field consisted of four such transects, with each transect containing six sticky traps in total, being three within the potato fields and three in the surrounding natural vegetation. The traps were placed at the beginning of the growing season for the winter monitoring period, during June 2019, with the monitoring period commencing two weeks after plant emergence and lasting until October 2019 (tuber maturity). For the summer monitoring period, traps were placed in the field during October 2019, monitoring commenced two weeks after plant emergence and ended during January 2020. Traps were monitored and replaced every fortnight. Traps were taken to the laboratory where the number of L. huidobrensis were identified morphologically and counted. The identification of the leaf miners was carried out using a dissection microscope and the aid of a taxonomic key (Weintraub 2001). The surrounding vegetation consisted mainly of daisies [Arctotis breviscapa Thunb. (Asterales: Asteraceae)], Saldanha pincushion [Leucospermum tomentosum Thunb. (Proteales: Proteaceae)], coast silver oaks [Brachylaena discolor (Asterales: Asteraceae)] and Helichrysum [Helichrysum sp. (Asterales: Asteraceae)].
The following trap design was created to accommodate strong wind and elevation above the canopy during the rapid growth pattern of the potato plants. Yellow sticky traps (Chempac Pty Ltd, Simondium, South Africa) were attached to trap supports using cable ties, whereupon they were tightened to allow the traps to freely rotate around the horizontal metal support (Figure 2). The trap support consisted of a metal rod approximately 4 mm in diameter and 1 m high. This original design permitted the trap to spin around the metal support, but also allowed the metal rod in the ground to spin around itself in the windy conditions that tend to prevail in the region, which prevented wind damage to the traps. To reduce the risk of the traps being blown over (as the soils of the Sandveld region are sandy and soft), white PVC pipes were inserted into the soil first, with the trap support being placed in the top opening of each pipe, which allowed its rotation in all wind directions. Each trap was continuously elevated slightly above the canopy level every two weeks, to enable line of sight from the surrounding plants.

Damage assessments and persistence after harvest
The damage assessment, which was conducted in the two potato fields surveyed, involved the random sampling of 50 plants in the field, every two weeks [adapted from Chiluwal et al. (2012), who sampled 10 plants]. The phenological stages of the potato plant were (week 1-2, establishment/sprout development, week 2-4, stolon initiation/vegetative growth, week 4-6 tuber initiation, week 6-10 tuber filling/bulking and week 10-12 tuber maturity), these were also recorded during damage assessment data collection. The selected plants were assessed for the presence of leaf mines which became visible in the lower canopy, generally from the second week after plant emergence until harvest. Any sign of L. huidobrensis damage, was noted as being present or absent.
Liriomyza huidobrensis persistence in the soil after harvest was assessed by the collection of 10 χ 1 kg soil samples from the two potato fields a month after harvest. This was done by scooping a 25 cm deep sample using a small shovel, collected at random within each of the respective potato fields. Soil samples were then taken to the laboratory, where they were inspected for the presence of pupae, with the aid of a stereomicroscope. The collected pupae were incubated at 25 °C for five weeks to allow for the eclosion of adults.
Insect-rearing and parasitoid survey
The initial L. huidobrensis colony was started from infested potato leaf samples collected from the different potato fields situated in the Sandveld to investigate the life cycle during controlled conditions. The infested leaf samples underwent incubation for six days at 25 °C and a long photoperiod (16L: 8D) in ventilated containers in the insectary located at Stellenbosch University, Department of Conservation Ecology and Entomology. All pupae that formed in the containers after six days were stored in Petri dishes until adult emergence. Perspex cages (60 cm length χ 60 cm width χ 60 cm height) were used to contain the emerging adults, the cages were ventilated through a mesh-covered entrance. Potted bean (Phaseolus vulgaris L.) and tomato (Lycopersicon esculentum L. Checha F1 Hybrid) plants, as well as two weed species (Chrysanthemum sp. and Chenopodium murale), were provided as host plants and egg laying substrates. All plants were inspected four times a week and were replaced if the leaf surfaces were punctured extensively.
Liriomyza huidobrensis-infested potato leaves were additionally collected and kept separate at a temperature of 25 °C ± 2 °C, with a long photoperiod (16L:8D). In addition, 60% ± 5% relative humidity (RH) was maintained by means of placing wet paper towels within the Perspex cages. The surrounding humidity in the insectary ranged between 70% and 75%. The temperature and photoperiod conditions were
controlled using the PlantVisor process monitor, while the humidity was measured using an HTC-1 temperature and a humidity thermometer indoor clock. Any emerging parasitoids were identified and stored in 80% ethanol. The parasitoids were identified using morphological keys (Rakhshani et al. 2011, 2012) and molecular techniques, using the CO1 region and universal primers Folmer FW (LCO1490) and Folmer Rev (HCO2198) (Folmer et al. 1994). The following PCR cycling programme was carried out: 95 °C for 1 min for 1 cycle; 95 °C for 45 sec, 51 °C for 45 sec, 72 °C for 1 min for 35 cycles; 72 °C for 3 min for 1 cycle. Sequencing was performed by Inqaba Biotec™ and edited using CLC Main Workbench software. To validate the identity of the species concerned, the NCBI Taxonomy Database was used.
Alternative host survey
Damage caused by leaf miner species from different vegetable crops and weeds in Philippi (34°02'23.9"S 18°32'42.1"E) Cape Town, South Africa were investigated. To identify leaf miners from alternative hosts, 50 infested leaves, from each of the different vegetable crops and weeds at each location were sampled. The sampling of the host plants and the weeds took place twice, on 21 March 2019 and 22 October 2019. The infested leaves were incubated in the laboratory, as described above, and eclosed flies were subsequently identified morphologically and molecularly.
Off-season sampling of L. huidobrensis in the natural vegetation
The natural vegetation near the potato field on the farm located at (32°35'19.3"S 18°20'07.9"E) was sampled for adult L. huidobrensis for 12 weeks after the potato harvest (carried out on 4 May 2020). The monitoring involved placing three yellow sticky traps in the natural vegetation at specific distances (25 m, 50 m and 100 m) from the edge along a transect, with four transects, following the same experimental design that was used to monitor adult population dynamics in potato fields. Every two weeks, the traps were replaced, with the old traps being taken to the laboratory to confirm species identity of trapped leaf miners and to count the number of adults.
Data analyses
All analyses were conducted using STATISTICA 13.3 (TIBCO Software Inc., 2017). Shapiro-Wilks test was used to assess if residuals are normally distributed. To analyse differences between L. huidobrensis numbers caught on yellow sticky traps, a mixed models ANOVA (REML) was used. Grouping variables were field (summer and winter fields monitored), location (inside potato fields, outside natural vegetation) and week (weeks monitored). To analyse the off-season sampling data, mixed models in R (REML), with transect/blocks/repetitions observed over weeks, were used. As the residuals were not normally distributed on normal probability plots, a square root transformation was used.
RESULTS
Adult population dynamics
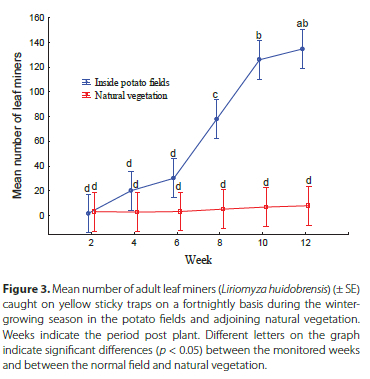
Residuals of data were not normally distributed. There was highly significant interaction effect between fieldxlocationxweek (F5,220 = 17.17, p < 0.01). Leaf miner populations were low during the winter monitoring period. The highest leaf miner abundance was detected between week 10 and 12 after plant emergence, with average trap catches of 126 and 135, respectively. Whereas the natural vegetation had very low leaf miner numbers with the highest mean catches being between week 10 and 12 of 7 and 8 flies. There were a higher number of flies caught in potato fields than in natural vegetation (Figure 3).
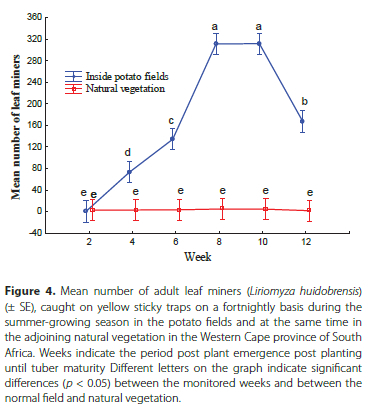
During the summer monitoring, the highest abundance of the L. huidobrensis was recorded between week 8 and 10 with mean trap catches of 311 and 312 males and females, respectively (Figure 4). However, a subsequent reduction in L. huidobrensis trapping was observed as the plants matured with mean trap catches reducing to 167. A build-up in L. huidobrensis densities was observed during both the winter and the summer monitoring periods.
Damage assessments and persistence after harvest
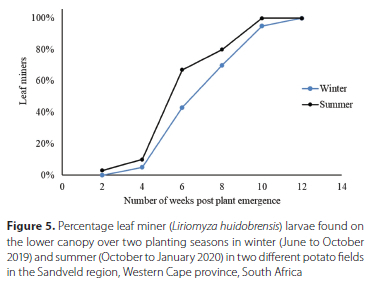
The damage assessments demonstrated that, in as early as week 4, approximately 5% of the leaves in the lower canopy contained leaf mines and punctures. The damage levels increased gradually in the monitored fields. However, leaf mines were present in the lower canopy in week 2 during the summer monitoring period and for winter as early as week 4. The infestations increased with plant maturity. Therefore, approximately seven weeks after plant emergence, 50% of the monitored plants showed evidence of leaf miner infestation (consisting of L. huidobrensis punctures and leaf mines), which was observed during both of the monitoring periods. The leaf-mining damage was caused by punctures and mines from as early as week 4, after plant emergence during winter, and during week 2, which fell in the summer monitoring period (Figure 5).
The number of pupae that were found after the winter monitoring period reached an accumulated total of 50 individuals. However, after keeping the pupae for longer than 10 days at 25 °C and for a long photoperiod (16L:8D), they were found not to eclose.
Insect-rearing and parasitoid survey
For larval rearing, bean plants proved to be relatively successful and suitable hosts, because they were the only plant species producing a second generation of L. huidobrensis, under laboratory conditions. Very few leaf punctures (egg laying sites) were noticed on the weed leaves, indicating that weeds from the potato fields that were used for rearing are likely not an ideal host for reproduction. In the laboratory, three generations were completed from the original colony on bean plants, where after the population collapsed. The recorded life cycle for three generations on the bean plants was 22, 24 and 25 days, respectively, from egg to adult. The first leaf mines appeared six days after the punctures were observed on the leaf's surface. The larval stage typically lasted between nine and 12 days, depending on the generation. The pupal stage usually lasted seven days. With regards to tomato plants and weed samples the number of generations that emerged was not quantified, due to constant collapse of the colony.
The number of parasitoids that were reared from the leaf samples were relatively low. In total, 50 parasitoids emerged, most of which were identified as D. isaea. The parasitoid species are presented in Table 1. Diglyphus isaea was the most abundant parasitoid species found in the two fields sampled in the Sandveld region.
Alternative host survey
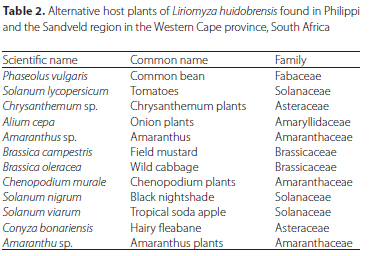
Several species of plants and weeds were identified as hosts, some of which were collected from potato fields (Table 2). Chrysanthemum sp. and C. murale were used to rear L. huidobrensis in the laboratory (Table 2). Most of the host plants found during monitoring were from the Amaranthaceae and Solanaceae. On these weeds, the L. huidobrensis were unable to produce a successive, second generation.
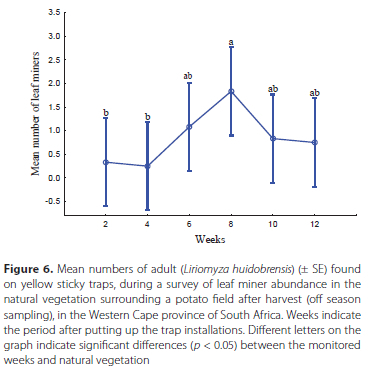
Off-season sampling of L. huidobrensis in the natural vegetation
The interaction between position*week was found to be not significant (F1442 = 0.80, p = 0.667). Significantly more flies were trapped at 25 m than 100 m distance from the potato field (F2,6 = 5.57, p = 0.043), indicating that flies most likely originate from potato fields and not surrounding vegetation. Generally, the L. huidobrensis population was low in the natural vegetation throughout the off-season monitoring period, compared to numbers caught within potato fields. The results showed that the mean trapping of the L. huidobrensis did not vary significantly between weeks (F7,21 = 0.53, p = 0.799) (Figure 6). However, there was an increase in the mean trapping of adults between weeks 4 and 8, which slowly dropped off throughout the monitoring period. The highest mean number of flies was caught in week 8.
DISCUSSION
Liriomyza huidobrensis poses a risk to all phenological stages of the potato plant (López et al. 2010). The current study took the form of a preliminary investigation into establishing the mode of infestation of the L. huidobrensis in the Sandveld region, in the Western Cape province, South Africa. During the study, the population densities of the L. huidobrensis slowly increased during early potato growth but increased more rapidly from flowering onwards, which was observed for both the winter and summer sampling period. Several studies found that the in-migrant adult populations initiated infestations in the small potato plants at the beginning of the growing season (Mujica et al. 2000). However, as the plants developed, the number of adults caught by the traps increased a week before flowering. Thus, the results from the present study are like those that were obtained by Vincini and Carmona (2006), regarding the dramatic increase in population density before flowering, and a decrease in population density during plant senescence. Results obtained for both seasons, therefore, showed a significant association between L. huidobrensis populations and potato development, as was also found by López et al. (2010). These results are like those obtained by Vincini and Carmona (2006), who concluded that L. huidobrensis is present throughout all potato development stages, with a population decrease during senescence.
The mean numbers of the leaf miners within the surrounding natural vegetation remained low throughout the two seasons (winter and summer), indicating that it is unlikely that the potential hosts present within this habitat type contribute to the spread of the L. huidobrensis to adjacent potato fields. The offseason monitoring showed the presence of very low numbers of adult leafminers in the natural vegetation after the potato harvest. As such, the study indicates the likelihood of large numbers of L. huidobrensis migrating from natural vegetation to new potato fields is relatively low. This lacks sufficient evidence to suggest that natural vegetation is used as a refuge for L. huidobrensis when potatoes are not available. Further studies should consider factors like the point of transect interception and the length of transects to broaden the existing understanding of the dynamics of the L. huidobrensis.
Generally, the survey indicated that the level of damage caused by the pest was slightly lower during winter and higher during summer. This will, however, need to be repeated on a larger scale, including more field replications to verify this trend. On average, the total amount of leaf damage exceeded 50% in as early as week 7, which coincided with the onset of the flowering period. The most severe damage was recorded on the older, lower-growing leaves of a potato plant. However, as the crop produced new leaves, additional opportunities were made available for adult fly feeding, oviposition points, and larvae development areas, resulting in an increasing population. Similar results were obtained by Mujica and Cisneros (1997), who recorded a relatively high damage index on the lower crop layer and relatively less damage on the intermediate and upper crop layers, on potatoes. The damage pattern is related to the egg extrusion phenomenon, which is related to leaf age (López et al. 2010). Egg extrusion is referred to as a hypersensitive reaction that causes hypertrophic cell growth, which eventually leads to egg ejection (Facknath 2005). Egg extrusion is very high in the young leaves and relatively low in the older leaves, because of the hypertrophic cell growth of the new leaves, which leads to egg ejection, and which exposes the eggs to the environment and to the pest's natural enemies (Facknath 2005; Videla and Valladares 2007).
The pupal persistence within the soil showed no adult emergence after subsequent incubation in the laboratory. Noujeim et al. (2013) suggest that the pupae can survive for two weeks at 4 °C but will start to die off thereafter. The failure of the pupae to emerge might have been associated with their overwintering strategy and the method of incubation and conditions provided being unsuitable, resulting in mortality of the pupae. It is possible that some temperature pre-acclimation may have been required to improve survival, as was found for this species and their adaptation to cold (Chen and Kang 2004).-
The tomato, Solanum lycopersicum and common bean, Phaseolus vulgaris proved to be useful for larval development (rearing). However, the beans were better hosts in this regard than the tomato plants, likely due to their large, leaf surface area which permitted additional punctures to be created on a single leaf. Also, bean plants are relatively fast-growing, requiring only 15 days before they became suitable for L. huidobrensis reproduction. As the host plant plays a vital role in the fitness of the larvae (Maharjan and Jung 2016), using the right host for insect rearing is cardinal. Salvo and Valladares (2009) suggest that faba bean, Vicia faba L (Fabaceae; Fabales) are suitable hosts, because they are relatively easy to grow, and they have a large surface area for the larvae to mine. Overall, the range of alternative hosts in the vegetable fields in Philippi was also considerable. Due to the above-mentioned factors, it is important to understand the dynamics of alternative hosts, as well as their ecology. The weeds in the Sandveld were not monitored, because the potato fields had little to no weeds.
In the current study D. isaea was found to be the most abundant parasitoid species. Other studies in Africa also reported the establishment of this parasitoid in southern Africa (Chabi-Olaye et al. 2008; Musundire et al. 2012). However, comprehensive studies on its diversity and potential as a biological control agent are needed. The parasitoid Utetes africanus (Wharton) (Hymenoptera: Braconidae) has no record in association with potato pests in the Western Cape, so this study is the first to report this species being reared from L. huidobrensis in South Africa. However, its association with fruit-feeding insects has been recorded, especially in terms of the olive fruit fly, Bactrocera oleae (Rossi) (Tephritidae: Diptera) (Powell et al. 2019). Dacnusa sibirica (Telenga) (Hymenoptera: Braconidae) is a parasitic wasp that is used commercially against leaf-mining larvae (CABI 2019). Records of its use exist in South Africa in relation to other leaf-mining insects, regarding a product called Minusa, which can be obtained from Koppert Biological Systems SA (Pty) Ltd. Records of Alysiinae sp. exist in South Africa, even though there are no reports, as yet, on its commercial use (Peris-Felipo and Belokobylskij 2016). Furthermore, the use of parasitoids from the Eulophidae family has been recorded against pupae of the groundnut leaf miner, Aproaerema modicella (Deventer) (Lepidoptera: Gelechiidae) (Van der Walt et al. 2009).
In conclusion, the data provided in the current study provide a preliminary indication of how severely potato fields are infested by L. huidobrensis. The low L. huidobrensis densities that were found in the immediate surrounding natural vegetation indicate that such vegetation is, most likely, not a major habitat for L. huidobrensis in the Sandveld. More likely, the build-up within the fields that has been observed to occur later in the season due to the abundant food supply found there. Additional studies need to be conducted to validate the mode of infestation that can be found under different environmental conditions and various vegetation types. The present study also confirmed that L. huidobrensis may be present at all the developmental stages of the potato plant, with a steady increase in adult abundance as the plants mature, with a peak occurrence during flowering. Lastly, as several plant hosts exist for this pest, there is a need for control measures to be adopted in terms of various weed species, especially in relation to those that do not harbour beneficial insects.
ACKNOWLEDGEMENTS
The authors would like to thank Potatoes South Africa for partial funding of this project, and National Research Foundation for supporting the student financially, and D.G. Nel, at the Centre for Statistical Consultation, Stellenbosch University.
ORCID IDs
T Mugala - https://orcid.org/0000-0001-6482-5974
AP Malan - https://orcid.org/0000-0002-9257-0312
P Addison - https://orcid.org/0000-0002-8227-339X
D Visser - https://orcid.org/0000-0002-9034-0214
REFERENCES
Ayabe Y. 2010. Specific mining pattern as a result of selective feeding within a leaf by the dipteran leafminer Ophiomyia maura (Diptera: Agromyzidae). Annals of the Entomological Society of America 103: 806-812. https://doi.org/10.1603/AN10049 [ Links ]
CABI (Centre for Agriculture and Bioscience International). 2019. Database: Invasive species compendium: Liriomyza huidobrensis (serpentine leafminer). https://www.cabi.org/isc/datasheet/30956
Caldiz D, Lutaladio N, Ortiz O, Haverkort A. 2009. Sustainable potato production: Guidelines for developing countries. Food and Agriculture Organization of the United Nations. http://www.fao.org/3/a-i1127e.pdf
Chabi-Olaye A., Mujica N, Löhr B, Kroschel J. 2008. Role of agroecosystems in the abundance and diversity of Liriomyza leaf mining flies and their natural enemies. In: Abstracts of the XXIII International Congress of Entomology, 6-12 July 2008, Durban, South Africa, pp. 1 -15. https://repository.up.ac.za/bitstream/handle/2263/58461/Musundire_Host_2012.pdf?sequence=3 (accessed 26 February 2021).
Chen B, Kang L. 2004. Variation in cold hardiness of Liriomyza huidobrensis (Diptera: Agromyzidae) along latitudinal gradients. Environmental Entomology 33: 155-164. https://doi.org/10.1603/0046-225X-33.2.155 [ Links ]
Chen XX, Lang FY, Xu ZH, He JH, Ma Y. 2003. The occurrence of leaf miners and their parasitoids on vegetables and weeds in Hangzhou area, Southeast China. BioControl 48: 515-527. https://doi.org/10.1023/A:1025726813462 [ Links ]
Chiluwal KN, Thapa RB, Shrestha SM, Sporleder M. 2012. Field loss assessment of potato leaf miner fly (PLMF), Liriomyza huidobrensis (Blanchard) (Diptera: Agromyzidae). Proceedings of the 4th Sustainable Agricultural Systems-Nepal Convention 4: 6. [ Links ]
Civelek HS, Yoldas Z, Weintraub P. 2002. The parasitoid complex of Liriomyza huidobrensis in cucumber greenhouses in Izmir province, Western Turkey. Phytoparasitica 30: 285-287. https://doi.org/10.1007/BF03039996 [ Links ]
Connor EF, Taverner MP. 1997. The evolution and adaptive significance of the leaf-mining habit. Oikos 79: 6-25. http://doi.org/10.2307/3546085 [ Links ]
Cornell HV. 1989. Endophage-ectophage ratios and plant defence. Evolutionary Ecology 3: 64-76. https://doi.org/10.1007/BF02147932 [ Links ]
Cusumano A, Harvey JA, Bourne ME, Poelman EH, De Boer JG. 2020. Exploiting chemical ecology to manage hyperparasitoids in biological control of arthropod pests. Pest Management Science 76: 432-443. https://doi.org/10.1002/ps.5679 [ Links ]
DAFF (Department of Agriculture, Forestry and Fisheries). 2012. A Profile of the South African Potato Market Value Chain. https://www.nda.agric.za/docs/amcp/potato2012.pdf
Deadman M, Khan I, Thacker J, Habsi KA. 2000. Interaction between leaf miner damage and leaf necrosis caused by Alternaria alternata, on potato in the Sultanate of Oman. Plant Pathology Journal 18: 210-215. http://dx.doi.org/10.5423/PPJ.2002.18.4.210 [ Links ]
Dharmadhikari PR, Perera, PACR, Hassen TMF. 1977. A short account of the biological control of Promecotheca cumingi [Coleoptera: Hispidae] the coconut leaf miner, in Sri Lanka. Entomophaga 22: 3-18. https://doi.org/10.1007/BF02372985 [ Links ]
Duan JJ, Jennings DE, Williams DC, Larson KM. 2014. Patterns of parasitoid host utilization and development across a range of temperatures: implications for biological control of an invasive forest pest. BioControl 59: 659-669. https://doi.org/10.1007/s10526-014-9604-9 [ Links ]
El-Wakeil N, Gaafar N, Sallam A, Volkmar C. 2013. Side effects of insecticides on natural enemies and possibility of integration in plant protection strategies. In: Trdan, S. (Ed.) Insecticides: Development of Safer and More Effective Technologies. 2-56. Janeza Trdine, Rijeka. https://doi.org/10.5772/3356
Eyre MD, Rushton SP, Luff, ML, Telfer MG. 2005. Investigating the relationships between the distribution of British ground beetle species (Coleoptera, Carabidae) and temperature, precipitation, and altitude. Journal of Biogeography 32: 973-983. https://doi.org/10.1111/j.1365-2699.2005.01258.x [ Links ]
Facknath S. 2005. Leaf age and life history variables of a leafminer: The case of Liriomyza trifolii on potato leaves. Entomologia Experimentalis et Applicata 115: 79-87. https://doi.org/10.1111/j.1570-7458.2005.00286.x [ Links ]
Fenoglio MS, Salvo A. 2009. Liriomyza commelinae (Diptera: Agromyzidae): An alternative host for parasitoids ofthe leafminer pest Liriomyza huidobrensis. International Journal of Pest Management 55(4): 299-305. https://doi.org/10.1080/09670870902890561 [ Links ]
Fenoglio MS, Srivastava DS, Valladares G, Cagnolo L, Salvo A. 2012. Forest fragmentation reduces parasitism via species loss at multiple trophic levels. Ecology 93: 2407-2420. https://doi.org//10.2307/41739312 [ Links ]
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R. 1994. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology 3: 294-299. [ Links ]
García-Marí F, Vercher R, Costa-Comelles J, Marzal C, Villalba M. 2004. Establishment of Citrostichus phyllocnistoides (Hymenoptera: Eulophidae) as a biological control agent for the citrus leaf miner Phyllocnistis citrella (Lepidoptera: Gracillariidae) in Spain. Biological Control 29: 215-226. https://doi.org/10.1016/S1049-9644(03)00155-5 [ Links ]
Girardoz S, Kenis M, Quicke DLJ. 2006. Recruitment of native parasitoids by an exotic leaf miner, Cameraria ohridella: host-parasitoid synchronization and influence of the environment. Agricultural and Forest Entomology 8: 49-56. https://doi.org/10.1111/j.1461-9555.2006.00281.x [ Links ]
Grabenweger G, Kehrli P, Zweimuller I, Augustin S, Avtzis N, et al. 2010. Temporal and spatial variations in the parasitoid complex of the horse chestnut leaf miner during its invasion of Europe. Biological Invasions 12(8): 1-17. https://doi.org/10.1007/s10530-009-9685-z [ Links ]
Hawkins BA, Cornell HV, Hochberg ME, 1997. Predators, parasitoids, and pathogens as mortality agents in phytophagous insect populations. Ecology 78: 2145-2152. https://doi.org/10.2307/2265951 [ Links ]
Hering, E.M. 1951. The Biology of the Leaf Miners. Junk, Gravenhage, Netherlands. 420 pp.
Hill MP, Bertelsmeier C, Clusella-Trullas S, Garnas JR, Robertson MP, Terblanche, JS. 2016. Predicted decrease in global climate suitability masks regional complexity of invasive fruit fly species response to climate change. Biological Invasions 18: 1105-1119. https://doi.org/10.1007/s10530-016-1078-5 [ Links ]
Hodkinson, I.D. 2005. Terrestrial insects along elevation gradients: species and community responses to altitude. Biological Reviews of the Cambridge Philosophical Society 80: 489-513. https://doi.org/10.1017/s1464793105006767 [ Links ]
Johnson MW, Hara AH. 1987. Influence of host crop on parasitoids (Hymenoptera) of Liriomyza spp. (Diptera: Agromyzidae). Environmental Entomology 16: 339-344. https://doi.org/10.1093/ee/16.2.339 [ Links ]
Johnson SN, Mayhew PJ, Douglas AE, Hartley SE. 2002. Insects as leaf engineers: can leaf miners alter leaf structure for birch aphids? Functional Ecology 16: 575-584. https://doi.org/10.1046/j.1365-2435.2002.00654.x [ Links ]
Kalaitzaki AP, Lykouressis DP, Perdikis DC, Alexandrakis VZ. 2014. Effect of temperature on development and survival of the parasitoid Pnigalio pectinicornis (Hymenoptera: Eulophidae) reared on Phyllocnistis citrella (Lepidoptera: Gracillariidae), Environmental Entomology 36: 497-505. https://doi.org/10.1603/0046-225X(2007)36[497:EOTODA]2.0.CO;2 [ Links ]
Klok CJ, Chown SL, Gaston KJ. 2003. The geographical range structure of the holly leaf miner. III. Cold hardiness physiology. Functional Ecology 17: 858-868. https://doi.org/10.1111/j.1365-2435.2003.00794.x [ Links ]
Kruidhof HM, Kostenko O, Smid HM, Vet LE. 2019. Integrating parasitoid olfactory conditioning in augmentative biological control: potential impact, possibilities, and challenges. Frontiers in Ecology and Evolution 7: 84. https://doi.org/10.3389/fevo.2019.00084 [ Links ]
Liu T, Kang L, Heinz K, Trumble J. 2009. Biological control of Liriomyza leafminers: progress and perspective. Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 4: 1-16. http://doi.org/10.1079/PAVSNNR20094004 [ Links ]
Lomolino MV. 2001. Elevation gradients of species-density: historical and prospective views. Global Ecology and Biogeography 10: 3-13. https://doi.org/10.1046/j.1466-822x.2001.00229.x [ Links ]
López R, Carmona D, Vincini AM, Monterubbianesi G, Caldiz D. 2010. Population dynamic and damage caused by the leaf miner Liriomyza huidobrensis (Blanchard: Agromyzidae), on seven potato processing varieties grown in temperate environment. Neotropical Entomology 39: 108-114. https://doi.org/10.1590/S1519-566X2010000100015 [ Links ]
Maharjan R, Jung C. 2016. Thermal requirements and development of the Korean population of the potato leaf miner, Liriomyza huidobrensis (Diptera: Agromyzidae). Journal of Asia-Pacific Entomology 19: 595-601. [ Links ]
Mujica N, Cisneros F. 1997. The leaf miner fly in potato: plant reaction and natural enemies as natural mortality factors. In: International Potato Centre Program Report 1997-98. 129-140. CIP, Lima.
Mujica N, Fonseca C, Suárez F, Fabian F, Marchena M, Cisneros M. 2000. Reducción del uso de insecticidas en el control de la mosca minadora Liriomyza huidobrensis Blanchard, por medio del uso de técnicas etológicas. In: Taller de Planificación, Implementación, Monitoreo y Evaluación de Programas de MIP en el Cultivo de Papa Lima. 153-162.
Mujica N, Kroschel J. 2011. Leafminer fly (Diptera: Agromyzidae) occurrence, distribution, and parasitoid associations in field and vegetable crops along the Peruvian coast. Environmental Entomology 40: 217-230. https://doi.org/10.1603/EN10170 [ Links ]
Mujica N, Kroschel J. 2013. Pest intensity-crop loss relationships for the leaf miner fly Liriomyza huidobrensis (Blanchard) in different potato (Solanum tuberosum L.) varieties. Crop Protection 47: 6-16. https://doi.org/10.1016/j.cropro.2012.12.019 [ Links ]
Musundire R, Chabi-Olaye A, Kruger K. 2012. Host plant effects on morphometric characteristics of Liriomyza huidobrensis, L. sativae and L. trifolii (Diptera: Agromyzidae). Journal of Applied Entomology 136: 97-108. https://doi.org/10.1111/j.1439-0418.2010.01597.x [ Links ]
Noujeim E, Sakr J, Nemer N. 2013. Potential of entomopathogenic nematodes application against Liriomyza huidobrensis Blanchard in Lebanon. IV International Symposium Agrosym. 692-698. https://doi.org/10.7251/agsy1303692n
Ode PJ, Heinz KM. 2002. Host-size-dependent sex ratio theory and improving mass-reared parasitoid sex ratios. Biological Control 24: 31-41. [ Links ]
Oerke EC, Dehne HW. 2004. Safeguarding production losses in major crops and the role of crop protection. Crop Protection 23: 275-285. [ Links ]
Ozawa A, Saito T, Ota M. 2001. Biological control of the American serpentine leafminer, Liriomyza trifolii (Burgess), on tomato in greenhouses by parasitoids. II. Evaluation of biological control by Diglyphus isaea (Walker) and Dacnusa sibirica Telenga in commercial greenhouses. Japanese Journal of Applied Entomology and Zoology 45 : 61-74. https://doi.org/10.1303/jjaez.2001.61 [ Links ]
Peris-Felipo FJ, Belokobylskij SA. 2016. New South African species of the genus Idiasta Foerster, 1863 (Hymenoptera: Braconidae: Alysiinae), with a key to the Afrotropical and Malagasy taxa. Zootaxa 4150: 566-570. https://doi.org/10.11646/zootaxa.4150.5.3 [ Links ]
Powell C, Caleca V, Sinno M, Van Staden M, Van Noort S, Rhode C, Van Asch B. 2019. Barcoding of parasitoid wasps (Braconidae and Chalcidoidea) associated with wild and cultivated olives in the Western Cape of South Africa. Genome 62: 183-199. [ Links ]
Rakhshani E, Kazemzadeh S, Starý P, Barahoei H, Kavallieratos NG, Cetkovic A, Popovic, A, Bodlah I, Tomanovic 2. 2012. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) of Northeastern Iran: Aphidiine-aphid-plant associations, key and description of a new species. Journal of Insect Science 12: 1-26. https://doi.org/10.1673/031.012.14301 [ Links ]
Rakhshani E. Tomanovic 2, Starý P, Kavallieratos NG, Ilic, M, Stankovic SS, Rajabi MN. 2011. Aphidiinae parasitoids (Hymenoptera: Braconidae) of Macrosiphoniella aphids (Hemiptera: Aphididae) in the western Palaearctic region. Journal of Natural History 45: 2559-2575. https://doi.org/10.1080/00222933.2011.597004 [ Links ]
Rauf A, Shepard BM, Johnson MW. 2010. Leaf miners in vegetables, ornamental plants and weeds in Indonesia: surveys of host plants, species composition and parasitoids. International Journal of Pest Management 46: 257-266. https://doi.org/10.1080/09670870050206028 [ Links ]
Reitz SR, Gao Y, Lei Z. 2013. Insecticide use and the ecology of invasive Liriomyza leaf miner management. In: Trdan, S. (Ed.) Insecticides -Development of Safer and More Effective Technologies 235-255 pp. https://doi.org/10.5772/53874
Rodríguez-Castaneda G, Macvean C, Cardona C, Hof AR. 2017. What limits the distribution of Liriomyza huidobrensis and its congener Liriomyza sativae in their native niche: when temperature and competition affect species' distribution range in Guatemala. Journal of Insect Science 17: 88-101. https://doi.org/10.1093/jisesa/iex059 [ Links ]
Rondon SI. 2010. The potato tuberworm: a literature review of its biology, ecology, and control. American Journal of Potato Research 87: 149-166. https://doi.org/10.1007/s12230-009-9123-x [ Links ]
Salvo A, Valladares GR. 2007. Leafminer parasitoids and pest management. Ciencia e Investigación Agraria 34: 125-142. [ Links ]
Salvo A, Valladares G. 2009. Plant-related intraspecific size variation in parasitoids (Hymenoptera: Parasitica) of a polyphagous leaf miner (Diptera: Agromyzidae). Environmental Entomology 31: 874-879. https://doi.org/10.1603/0046-225X-3L5.874 [ Links ]
Schulze RE, Maharaj M, Warburton ML, Gers CJ, Horan MJC, Kunz RP, Clark DJ. 2008. South African Atlas of Climatology and Agrohydrology. WRC Report No. 1489/1/08. Water Research Commission, Pretoria, 113 pp.
Spencer K. 1990. Host specialization in the world Agromyzidae (Diptera). Series Entomologica 45. Kluwer Academic Publisher, London, United Kingdom.
Shepard BM, Braun SAR. 1998. Seasonal incidence of Liriomyza huidobrensis (Diptera: Agromyzidae) and its parasitoids on vegetables in Indonesia. International Journal of Pest Management 44: 43-47. DOI: https://doi.org/10.1080/096708798228518 [ Links ]
Tibco Software Inc. 2017. Statistica (data analysis software system), version 13.3.
Van der Walt A, Du Plessis H, Van den Berg J. 2009. Infestation of groundnut by the groundnut leafminer, Aproaerema modicella (Deventer) (Lepidoptera: Gelechiidae) and rates of parasitization of this pest in South Africa. Crop Protection 28: 53-56. [ Links ]
Videla M, Valladares G. 2007. Induced resistance against leaf miner eggs by extrusion in young potato plants. International Journal of Pest Management 53: 259-262. https://doi.org/10.1080/09670870701439594 [ Links ]
Vincini AM, Carmona DM. 2006. Insectos. In: Caldiz, D.O. (Ed.) Producción, Cosecha y Almacenamiento de Papa en la Argentina. 165-169. McCain Argentina SA - BASF Argentina SA, Buenos Aires.
Visser D, Schoeman AS. 2004. Flight activity patterns of the potato tuber moth, Phthorimaea operculella (Zeller) (Lepidoptera: Gelechiidae). African Entomology 12: 135-139. [ Links ]
Wang X, Liu S, Guo S, Lin W. 1999. Effects of host stages and temperature on population parameters of Oomyzus sokolowskii, a larval-pupal parasitoid of Plutella xylostella. BioControl 44: 391-403. https://doi.org/10.1023/A:1009912420598 [ Links ]
Weintraub PG. 2001. Changes in the dynamics of the leaf miner, Liriomyza huidobrensis, in Israeli potato fields. International Journal of Pest Management 47(2): 95-102. https://doi.org/10.1080/09670870151130516 [ Links ]
Weintraub PG, Horowitz AR. 1995. The newest leaf miner pest in Israel, Liriomyza huidobrensis. Phytoparasitica 23: 177-184. [ Links ]
Weintraub G, Horowitz AR. 1998. Effects of translaminar versus conventional insecticides on Liriomyza huidobrensis (Diptera: Agromyzidae) and Diglyphus isaea (Hymenoptera: Eulophidae) populations in celery. Journal of Economic Entomology 91: 11801185. https://doi.org/10.1093/jee/91.5.118 [ Links ]
 Correspondence:
Correspondence:
Pia Addison
Email: Pia Addison pia@sun.ac.za
Received: 07 April 2021
Accepted: 03 July 2022














