Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
African Entomology
versión On-line ISSN 2224-8854
versión impresa ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a14949
RESEARCH ARTICLE
Love at first bite? Pre-release surveys reveal a novel association between a native weevil and the invasive Nymphaea mexicana Zuccarini (Nymphaeaceae) in South Africa
MK ReicTCI; MP HillI; JA CoetzeeII
ICentre for Biological Control, Department of Zoology and Entomology, Rhodes University, Makhanda, South Africa
IICentre for Biological Control, Department of Botany, Rhodes University, Makhanda, South Africa
ABSTRACT
Classical biological control aims to suppress alien invasive plant populations by introducing host-specific natural enemies from the native range. This relies on the assumption that invasive plant populations in the invaded range benefit from the release of natural enemies. Pre-release surveys in the invaded range are a useful way to determine if enemy release applies to a particular invasive alien plant, and to determine what other factors may contribute to the invasion. Similarly, pre-release surveys gather information that can be used to compare invaded sites before and after the release of biological control agents and may also identify whether natural enemies have been accidentally introduced into the country. Pre-release surveys were conducted in South Africa on the invasive Nymphaea mexicana Zuccarini (Nymphaeaceae) to gather such information about this species, for which a biological control programme is being developed. There was lower diversity and abundance of herbivores in the native range compared to South Africa, suggesting that N. mexicana does experience enemy release at most sites in South Africa. This support for the enemy release hypothesis justifies the investment in biological control for its management. However, a native weevil, Bagous longulus Gyllenhal (Coleoptera: Curculionidae), was found feeding and reproducing on N. mexicana at three sites, resulting in damage to the leaves and suggesting that a novel association has formed between these species. Bagous longulus may have potential to be distributed to sites of N. mexicana where it is not present, though further investigation is necessary to confirm if its host range is suitable for this to be a safe endeavour. With the exception of sites where B. longulus was present, leaf sizes were large and damage was low, and there is no evidence that any natural enemies have been accidentally introduced from the native range. Findings such as these emphasise the importance of conducting thorough surveys during the development of biological control programmes.
Keywords: biological control, invasive alien plant, enemy release hypothesis, insect herbivores, Mexican waterlily, yellow waterlily
INTRODUCTION
To manage invasive alien plants, classical biological control makes use of host-specific insect herbivores from the native range of the alien plant to suppress populations in the invaded range (Müller-Schärer & Schaffner 2008). Biological control has been successful in controlling many weed species (McFadyen 2000; van Klinken et al. 2003; Herrick & Kok 2010; Coetzee et al. 2011, 2021) and is cost effective and environmentally friendly (de Lange & van Wilgen 2010; van Wilgen et al. 2020). Biological control for weed management relies mostly on invasion hypotheses such as the Enemy Release Hypothesis (ERH), which states that alien plants may become invasive due to reduced herbivory as a result of the lack of natural enemies in the invaded range (Keane and Crawley 2002), and the Evolution of Increased Competitive Ability (EICA) hypothesis. The EICA hypothesis states that invasive alien plants shift resource allocation from defence to growth and reproduction when they experience reduced herbivory (Blossey and Notzold 1995). While there is contrasting evidence for these hypotheses, in which there may be more complex and varied explanations for invasion success in different species (Colautti et al. 2004; Joshi and Vrieling 2005), understanding the drivers for invasions are important to determine the best means of managing problematic populations.
To initiate a biological control programme, a series of research steps should be taken to gather sufficient information about the target plant and maximise the chances that released agents will be effective at suppressing invasive populations (Jacob and Briese 2003; Sheppard et al. 2003; van Klinken and Raghu 2006). One such step is the completion of pre-release surveys, which involves identifying insect fauna associated with an invasive alien plant in its invaded range, before biological control agents are introduced. These surveys are useful to ascertain whether enemy release contributes to the plant's invasiveness (Keane and Crawley 2002; Canavan et al. 2014), and to determine whether potential biological control agents are already established in the invaded range (Dudley et al. 2006). This information is important in making decisions about measures to manage invasive alien plant populations and may save considerable time and money that would otherwise be invested in importing and testing potential biological control agents from the native range. For example, in California, U.S.A., surveys revealed that the potential biological control agent Tetramesa romana Walker (Eurytomidae) was already established on the invasive alien Arundo donax L. (Poaceae) (Dudley et al. 2008). Tetramesa romana was also found on A. donax
in South Africa, as well as another specialist herbivore that had been accidentally introduced from the native range (Canavan et al. 2014). Pre-introductory surveys can therefore provide useful information and should be prioritised especially for plants that have a long history in the invaded range and/or have been introduced multiple times.
In addition to providing useful information about the insect assemblages that occur on invasive plants in the introduced range, pre-introductory surveys are beneficial for determining the impacts of invasive plant species on native ecosystems. Acquiring such information allows biological control researchers to determine the success of biological control agents by comparing factors such as area coverage, invasive plant density, and native plant diversity, before and after the release of biological control agents (Diop and Hill 2009).
Nymphaea mexicana Zuccarini (Nymphaeaceae) is an invasive alien plant that originates in southern U.S.A. and Mexico that has become problematic in South Africa. Owing to the popularity of Nymphaea spp. in the horticultural trade, several hybrids of Nymphaea mexicana also exist in South Africa (Reid et al. 2021). The existence of hybrids can pose a challenge for biological control due to difficulties in morphological differentiation and because host-specific agents may not accept hybrids as host plants (Hoffman 2004; Urban et al. 2011; Williams et al. 2014). Research into potential biological control agents of N. mexicana is ongoing, but pre-introductory faunal surveys of N. mexicana have not previously been conducted in South Africa. Hence, in this study, pre-release surveys of sites across South Africa invaded by N. mexicana are described and discussed, with the aim of establishing baseline information for this species and determining the insect assemblages present. Although multiple landowners have indicated that the presence of N. mexicana has negative consequences, quantification of the impacts of the plant have not been carried out but are beyond the scope of this study. Surveys in the native range of this plant have already been conducted (Reid et al. 2020), so it is possible to compare the results of these surveys in the invaded range to those conducted in the native range. This study will determine the role of enemy release in N. mexicana invasions and collect valuable information to assist in the development of a biological control programme for this species, as well as make data available for comparison after the release of future biological control agents to establish their effectiveness.
MATERIALS AND METHODS
Site descriptions
Initial surveys were conducted in Gauteng province in March 2020 but were then halted due to the enforcement of level 5 lockdown in South Africa due to COVID-19. The surveys resumed in the Eastern Cape and Western Cape provinces in September 2020, and were repeated in February 2021, while the Gauteng surveys were repeated in December 2020-January 2021. Thirteen sites comprising either N. mexicana (n = 10) or hybrids (n = 3) were sampled on two occasions each to account for possible seasonal differences in insect assemblages. Sites were selected to represent varying degrees of disturbance (four were in nature reserves, the remainder were either artificial ponds or recreational dams in estates or parks). At two sites, second samplings were not possible as the plants had been removed manually or with herbicides.
After the repeated sampling of these sites, one site revealed the presence of a native weevil, Bagous longulus Gyllenhal (Coleoptera: Curculionidae) feeding on N. mexicana. This weevil was identified by Riaan Stals at the South African National Collection of Insects. In light of this result, 12 additional sites across the country were included for once-off sampling to increase the likelihood of finding additional herbivores including the weevil, and to incorporate more sites in which hybrids and native Nymphaea species were growing (Figure 1).
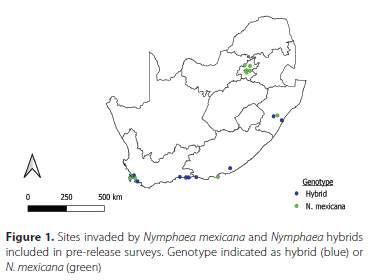
Descriptions of each site, including estimates of percent coverage by N. mexicana, notes on levels of damage (estimated from the area of leaf damage from mining, chlorosis, or chewing, in addition to petiole mining), and observations of leaf growth density (with dense leaf growth visualised when leaves were growing above water and crowding each other) were compiled to form a baseline of information with which to compare the extent of invasion at each site pre- and post-biocontrol agent release. Percent coverage and leaf size were used as metrics to compare the level of invasion between sites. The species richness of herbivorous insects found damaging N. mexicana was calculated for each site, and incidental species were excluded. Individual based rarefaction species accumulation curves, using Chao 2, ICE (incidence coverage estimator), and Jack1 estimators, were generated for the insects collected at each site using EstimateS version 8.0 (Colwell 2006). Different estimators may produce varying accuracy, so multiple indicators were selected based on their accuracy in other studies (González-Oreja et al. 2010; Martínez-Sanz et al. 2010). Species accumulation curves were generated for sampling occasions for all sites.
Water nutrient measurements
A Sanxin PC5 pH/conductivity multimeter (Shanghai San-Xin Instrumentation Inc.) was used to take readings of water parameters at each site including pH, temperature, total dissolved solids, electrical conductivity and salinity, all replicated five times. Four 50 ml plastic containers were used to collect water at the sites that were sampled twice within the year as well as two additional sites to obtain nitrate and phosphate concentration measurements. These water samples were analysed in the laboratory at the Institute for Water Research at Rhodes University, Makhanda, Eastern Cape. Water samples were analysed for orthophosphate-phosphorous (PO4-P) using a photometric phosphate Spectroquant® concentration test kit (HC399495) and a Biotek microplate reader, utilising calibration curves to calculate phosphate in μg l-1. Nitrate-nitrogen (NO3-N) was analysed according to summarised protocols from Ondrus (1996) and APHA (American Public Health Association) (1998); (Nelson Odume and Khaya Mgaba, pers. comm.).
The nitrate analysis was conducted by adding 25 ml of sample to 25 ml of distilled water in 250 ml Erlenmeyer flasks, including a blank sample. 1 ml of 1:4 HCl and 1 ml sulphanilic acid were added, followed by 1.5 g Zinc and NaCl mixed powder. The samples were then shaken thoroughly until the powder dissolved, 1 ml of buffer-colour reagent was added, before adding 1 ml sodium acetate (NaCH3COO) to each sample and mixing. The samples were left for five minutes to allow for colour development, and three replicates of 250 μ! of each sample as
well as the blank were added to a 96-well microplate and read at 540 nm in a Biotek microplate reader. The concentrations of nitrate were calculated in μg l-1 by entering the blanked readings obtained from the microplate reader into the equation obtained from a calibration curve prepared before samples were processed. If negative values were present, they were set to half of the lowest concentration value obtained to allow for statistical analyses.
Sites where nitrate and phosphate levels were tested were classified as oligotrophic (< 500 μg N l-1, < 5 μg P l-1), mesotrophic (500-2 500 μg N l-1, 5-25 μg P l-1), or eutrophic (2 500-10 000 μg N l-1, 25-250 μg P l-1) according to the South Africa Water Quality Guidelines for Aquatic Ecosystems (DWAF 1996).
Plant measurements
At each site, N. mexicana plant cover was estimated across the visible water body using a DAFOR abundance scale (Dominant: 75-100%, Abundant: 50-75%, Frequent: 25-50%, Occasional: 5-25%, Rare: 1-5%) (Kent 2012). To obtain plant coverage percentage estimates, two to three researchers (the principal investigator, and the field assistant(s)) independently estimated the coverage of N. mexicana on the visible open water body. The means of these estimates were calculated to reduce sampler bias. If other Nymphaea species were present, their percentage coverage was also estimated, and the presence of other emergent vegetation and plant species were recorded. Leaf size can be used as an indication of resource allocation to growth and is thus useful to allow comparison between sites and develop an understanding of effects of herbivory (as a source of stress resulting in declining plant size) and nutrients on populations (Poorter et al. 2012). Although crowding may affect leaf size, preliminary observations in the field determined that leaf size was still comparatively large even when the plants grew in high densities. Hence, N. mexicana leaf length (cm) was used as an indicator of leaf size and was measured using a ruler or tape measure from five random leaves at each site. These measurements were compared with similar measurements taken in U.S.A. and reported in Reid et al. (2020).
Insect assemblages
Leaves and stems were investigated for feeding damage for approximately 30 minutes. During this time, estimates of leaf damage were made by visual observation: damage was rated as "high" if more than 50% of the leaf showed signs of chewing, mining, or chlorosis from sap sucking; "moderate" if between 25 and 50% of the leaves were damaged; and "low" if less than 25% of the leaf was damaged. Plants were monitored for insect feeding behaviour and mode of damage by wading through the water or accessing leaves from boats if necessary. If an insect was associated with N. mexicana damage, it was recorded. Where N. mexicana was surrounded by other vegetation, the surrounding plants were also searched for insects.
Statistical analyses
All subsequent statistical analyses were carried out in R version 4.1.0 (R Core Team 2021) with model diagnostics performed using the DHARMa package (Hartig 2021) and figures made using ggplot2 (Wickham 2016). To evaluate the impact of water chemistry variables on leaf length, a general additive model (GAM) was used from the mgcv package (Wood 2010). Leaflength (cm) was modelled as a function of pH, electrical conductivity µS), total dissolved solids (ppm), salinity (ppt) and temperature (°C). Site was included as a smoothed penalised random effect to account for non-linear variation between sites not measured by the other effects. A GAM was selected to account for non-linear trends between leaf length and the water chemistry variables. A Wald's test was used to test the significance of each factor. To assess the effect of nitrate and phosphate concentrations µg/l)
on leaf length (cm), a generalised linear mixed model was used to test leaf length as a response variable with nitrate and phosphate as predictor variables, and site as a random effect to account for variation. Significance was assessed using a Likelihood-Ratio Test (LRT).
Bagous longulus was present at seven sites, three with N. mexicana and four with hybrids. As an investigation of the effects of B. longulus presence and plant genotype on leaf length (cm), a linear mixed effects model was run using site as a random effect to account for variation, and B. longulus presence and plant genotype as factors. Models including different combinations of the fixed effects were tested against a null model to assess the best model fit using ANOVA. Assessment of statistical significance for the best model was achieved using an LRT.
To assess whether leaf length differed significantly between the native and introduced range of N. mexicana, a linear mixed effects model was used with length (cm) measurements as the response variable, range as a fixed effect, and site as a random effect, assessing significance with an LRT. Comparisons of leaf length, herbivore species richness, and observations of levels of damage were also compared with data collected from native range surveys recorded in Reid et al. (2020).
Finally, the three sites where B. longulus was reproducing on N. mexicana were compared in terms of percentage plant cover and leaf length at each of the sites. These comparisons did not involve statistical tests because of the small number of sites. Instead, general descriptive differences were noted.
RESULTS
Site descriptions
Twenty-five N. mexicana sites were surveyed, of which 15 were invaded by N. mexicana and 10 were colonised by hybrids. At most of the sites, there were low levels of damage, and if there was leaf damage, this was usually caused by generalist herbivores such as snails and aphids or was due to natural decay. The species richness at the sites was low, even after a second sampling, with a mean species richness of 1.7 ± 1.1 SD across sites compared to a mean species richness of 5.4 ± 1.5 SD in the native range (Reid et al. 2020). Florida Lake in Gauteng (26°10'36.7"S 27°54'28.8"E) had the highest species richness of five species, but nevertheless damage levels were low at this site. This contrasts with the high levels of damage at sites in the native range (Reid et al. 2020). Overall, eight natural enemy species were encountered in South Africa compared to 15 in the native range (Reid et al. 2020). Most of the species encountered in South Africa were generalists, such as Aphididae, snails, Erebidae, or crambid moths such as Parapoynx sp. Hübner (Lepidoptera: Crambidae) and most were incidental visitors. There was no evidence that any natural enemies from the native range were present in South Africa due to accidental introductions.
The mean estimated percentage coverage of N. mexicana and its hybrids in the water bodies sampled was 41.17% ± 32.36 SD. Based on the percentage coverage using the DAFOR classification, of the 25 sites surveyed, one was classified as rare, six were classified as occasional, six as frequent, five as abundant, and seven as dominant. Sites with lower coverage were either large water bodies where N. mexicana grew on the edges, sites where plants were removed through chemical or manual control, or sites where there were higher levels of damage by herbivores. A detailed description of the conditions at each site is given in Supplementary Table S1.
At three of the sites (King's Beach Skate Park: 33°58'26.4"S 25°38'39.8"E, Knysna Estate: 34°01'30.4"S 23°00'44.6"E, and Fountainhill Estate: 29°27'49.1"S 30°32'25.4"E), higher levels of leaf damage were observed compared to the other surveyed sites. Multiple feeding holes were present in the leaves, in addition to signs of mining. On closer inspection, Bagous
longulus (previously known as Pseudobagous longulus Gyllenhal (Coleoptera: Curculionidae)) was collected at these sites, and all lifecycle stages were present on N. mexicana. At King's Beach Skate Park and Knysna Estate, N. mexicana leaf growth density was less dense (by observation) and with smaller leaf sizes compared to all other surveyed sites where B. longulus was not present (Figure 2). At Fountainhill Estate in KwaZulu-Natal province, coverage by N. mexicana was only 13% compared with 56% by Nymphaea nouchali Burman (Nymphaeaceae), but leaf size did not differ considerably compared to the other sites. Bagous longulus is native to South Africa and is typically recorded on native South African Nymphaea such as N. nouchali (R. Stals pers. comm.). Bagous longulus was also found at four sites where native Nymphaea spp. were growing with N. mexicana hybrids, but lower levels of feeding damage were observed on the hybrids compared to the native Nymphaea and N. mexicana, and only early larval instars were found in the hybrid leaves.
Species accumulation curves
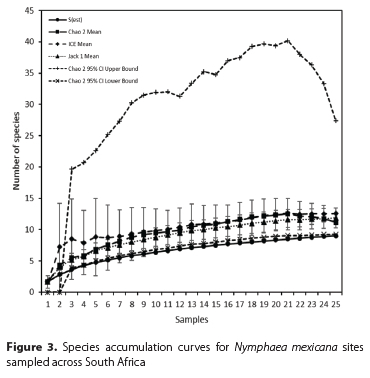
The indicators used to generate curves suggested that herbivore communities on N. mexicana were adequately sampled in South Africa. This was indicated by the S(est), ICE, Chao 2 and Jack 1 curves reaching asymptotes. The Chao 2 upper 95% confidence interval curve suggested that 18 additional species could be present (Figure 3).
Water parameters
The water parameters varied considerably between sites, indicating that N. mexicana can survive in a range of conditions. The mean (± SD) pH was 7.21 (± 0.77), with a broad range of electrical conductivity (509.4 ± 517.6 μS), total dissolved solids (361.4 ± 369.3 ppm), and salinity measurements (241.11 ± 271.15 ppt). For the sites where phosphate concentrations were tested, eight were classified as oligotrophic, six as mesotrophic, and one as eutrophic. For nitrate concentrations, seven were classified as oligotrophic, six as mesotrophic, and two as eutrophic. Intaka Island in Cape Town had the highest nitrate concentration (mean ± SD) (7080 ± 3510 μg l-1), followed by Westlakes in Cape Town (34°04'52.8"S 18°27Ί9.4'Έ) (2090 ± 2220 μg l-1) and Florida Lake in Gauteng (2000 ± 222 μg l-1), while Florida Lake also had the highest phosphate levels (79.1 ± 6.26 μg l-1), followed by Yellowwood Dam in Somerset West (21.5 ± 18.4 μg l-1), and Intaka Island (12.7 ± 9.45 μg l-1).
Leaf size
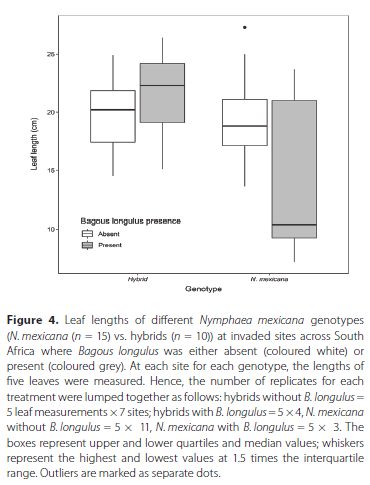
The mean leaf length of N. mexicana in the sites surveyed across the country was 18.9 cm ± 4.19 SD. Most of the sites had similar leaf sizes, but two sites, namely King's Beach Skate Park (9.78 cm ± 2.43) and Knysna Estate (9.14 cm ± 1.21), had considerably smaller leaf sizes. As a general observed trend, N. mexicana sites where B. longulus was absent had longer leaves on average (mean ± SD) (19.2 ± 3.05 cm) compared to N. mexicana sites where B. longulus was present (13.8 ± 6.58 cm) (Figure 4). This did not apply at the ten hybrid sites, where mean leaf length was similar whether B. longulus was absent (19.7 ± 2.83 cm) or present (21.4 ± 3.40 cm). For investigations of the effect of B. longulus presence and genotype on leaf length, a linear mixed effects model including an interaction between B. longulus presence and genotype was a significantly better fit than a null model and models with B. longulus presence and genotype included separately (χ2 = 4.5538, DF = 1, p < 0.05). Hence, B. longulus and genotype had a statistically significant interaction effect on N. mexicana plant length (χ2 = 4.1957, DF = 1,p < 0.05).
Analyses of the effects of water parameters on leaf length using the GAM model revealed there was a significant effect of pH on leaf length (F = 4.568, DF = 1, p < 0.05), with leaf length decreasing with increasing pH, but the remaining water parameter factors were not significant (all p-values < 0.05). This indicates that electrical conductivity, total dissolved solids, temperature, and salinity, did not significantly affect leaf length. Similarly, leaf length was not affected by differences in nitrate (χ2 = 0.0183, p = 0.8923) and phosphate (χ2= 1.4698, p = 0.2254) concentrations between sites.
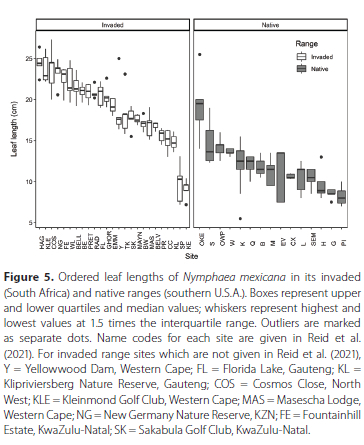
When the leaf lengths of N. mexicana in South Africa were compared with the leaf lengths in the native range (from Reid et al. 2020), leaf lengths in the invaded range (mean ± SD) (18.9 cm ± 4.19) were significantly greater (χ2 = 36.061, DF = 1, p < 0.001) than those in the native range (12.1 cm ± 3.17). This reflected a difference in length of approximately 36%. Most of the sites in the native range had smaller leaf sizes than those from the invaded range, except for Knysna Estate and King's Beach Skate Park (Figure 5). Additionally, leaves from Lake Okeechobee in the native range were bigger on average and more similar to leaf sizes from other sites in the invaded range. However, Fountainhill Estate where B. longulus was also present had similar leaf sizes to N. mexicana and hybrid sites where B. longulus was not present (Figure 5).
At sites where B. longulus was present and surviving on N. mexicana, the greatest leaf length was recorded at Fountainhill Estate (22.48 ± 1.58 cm) and the highest percentage cover by N. mexicana at Knysna Estate (60.67%), followed by King's Beach Skate Park (22.5%) and lastly Fountainhill Estate (12.5%). Furthermore, Fountainhill Estate had 56% coverage by the native N. nouchali compared to only 4% at Knysna Estate and 0% at King's Beach Skate Park.
DISCUSSION
Most of the sites invaded by N. mexicana in South Africa had dense growth, large leaves, and low levels of damage by herbivores. Twelve of the 25 surveyed sites had high (above 50%) percentage cover of the invaded water bodies. Generally, few herbivorous species were encountered, and those that were present were generalists, low in abundance, and exerted little damage. This contrasts to greater levels of herbivory, species richness, and reduced growth density and leaf sizes of N. mexicana in its native range (Reid et al. 2020). For example, five species exerted high levels of damage on N. mexicana in the native range compared to only one (B. longulus) in South Africa, which was only present at three sites (Reid et al. 2020). Species that exerted low levels of damage on N. mexicana in South Africa were generalists such as aphids or snails, in contrast to more specialist feeders in the native range. These data showing lower species richness and greater growth of N. mexicana in South Africa compared to the U.S. are similar to other studies that have compared introduced plant sizes and herbivore load in their native and invaded ranges (Memmott et al. 2000; Paynter et al. 2003; Jakobs et al. 2004). This provides evidence that enemy release, at least in part, contributes to the success of N. mexicana in South Africa. Similarly, no natural enemies from the native range were found in South Africa. It should be noted, however, that other factors likely play a significant role (Hierro et al. 2005; Vasquez & Meyer 2011; Fleming & Dibble 2015) and further studies may be necessary to determine the other factors driving N. mexicana invasions. For example, while pH seemed to have a slight effect on leaf size, no significant impacts were measured for nitrate and phosphate levels, and these measurements were not taken from all sites and have not been measured from the native range. Nevertheless, the observations obtained during this study suggest that it is likely that introducing specialist herbivores for biological control would result in a reduction in leaf size and growth density for this species in South Africa, since leaf size and density was lower in the native range and was correlated with greater levels of herbivory.
Two N. mexicana sites had comparatively sparse leaf growth, smaller leaf sizes, and higher levels of damage. These differences were attributed to the presence of B. longulus, a weevil that occurs on native Nymphaea spp. in South Africa. This weevil was found in abundance at these two sites, and all life stages were present on N. mexicana. The leaves had multiple feeding scars from adult chewing, and larval mines leading to the petiole, where late larval instars, pupae, and teneral adults were found. The abundance of B. longulus on N. mexicana at these sites and the high damage levels incurred on the plants suggests that a host expansion of a native insect onto this invasive alien plant has occurred. The leaf sizes of the sites at which B. longulus was present were comparable to the leaf sizes of sites in the native range. This could act as further evidence of the contribution of enemy release to N. mexicana invasions in South Africa and indicates promise for the possibility of using B. longulus as an augmentative biological control agent. A similar scenario, where a native insect developed a new association with an invasive plant, occurred in northern U.S.A., where Eurasian watermilfoil Myriophyllum spicatum L. (Haloragaceae) is invasive. The native weevil, Euhrychiopsis lecontei Dietz (Coleoptera: Curculionidae), was found associated with declining populations of M. spicatum at some lakes in northern U.S.A., where full lifecycles were recorded (Creed & Sheldon 1991; Newman & Biesboer 2000). Subsequent experiments showed a reduction in watermilfoil growth as a result of adult weevil feeding on the stems of leaves of the plants (Creed & Sheldon 1993). Presently in the USA, E. lecontei is successfully being used as a biocontrol agent for M. spicatum (Smith 2010; Thorstenson 2011). Before B. longulus is considered for augmentative releases it is important that its host specificity is investigated to determine the risks of non-target impacts. If B. longulus is specific to Nymphaea, there would still be risk of spill over effects on the native Nymphaea spp., but most sites invaded by N. mexicana were not colonised by native Nymphaea spp., and if they were, B. longulus was already present.
An additional site in KwaZulu-Natal also showed evidence of B. longulus damage and reproduction on N. mexicana, but the leaf sizes were more similar to sites where B. longulus populations were not sustained on exotic Nymphaea. There are five possible explanations for this: 1) Although the plants were initially identified morphologically as N. mexicana, it is possible that some hybridisation has occurred and that they are genetically different to the N. mexicana at the two other sites where B. longulus was present; 2) higher year-round temperatures at this site compared to the other sites allows greater plant growth despite herbivory by B. longulus; 3) differences in water chemistry between the sites resulted in different leaf sizes (Fountainhill Estate had slightly lower electrical conductivity, total dissolved solids, and salinity compared to the other two sites, with parameters at King's Beach Skate Park higher than the other two sites, but nitrate and pho sphate measurements were not taken, and only pH had a slight effect on leaf sizes at other sites); 4) a much greater density of N. nouchali was present at this site compared to the other two sites where the weevil was present (where there was 0% or 4% coverage of N. nouchali), resulting in a preference in B. longulus feeding on N. nouchali; 5) lack of integrated control at Fountainhill Estate (at both King's Beach Skate Park and Knysna Estate leaves were periodically removed by residents/groundskeepers in addition to the feeding by B. longulus).
The latter reason seems to be the most likely explanation in this case: mechanical and chemical removal occurred at two other sites where B. longulus was not present, but leaf sizes were still large at these sites during each sampling event, reiterating the effect of B. longulus. Hence, the combined effect of the weevils and clearing seems to have resulted in smaller leaf sizes. Nevertheless, further investigation is necessary to confirm this because although leaf size was larger at Fountainhill Estate compared to the other sites, coverage was still low. This may be the result of competition effects from N. nouchali, but this is unlikely because N. nouchali was also present at other sites in much lower abundances, usually only along the edges.
The varying genetic composition of N. mexicana in South Africa complicates the interpretation of data collected during these surveys. It is likely that multiple hybrids of N. mexicana exist in South Africa, and these differ morphologically to "pure" N. mexicana (Reid et al. 2021). Bagous longulus was prevalent at three sites that were invaded by "pure" N. mexicana but was also found at other sites where native Nymphaea spp. were growing in sympatry with N. mexicana hybrids. At these sites, minimal damage levels were recorded on the hybrid leaves: some early larval instars were recorded mining the leaves, but no later instars were present in the hybrids, despite full life cycles of B. longulus being present on the native Nymphaea. This suggests that B. longulus can survive and reproduce on N. mexicana, but not on these hybrids. Hybrid plants may display elevated, reduced, or unchanged resistance to herbivory (Fritz et al. 1994, 1999; Whitham et al. 1994) as a result of differences in chemical or morphological defences against herbivory (Fritz et al. 1999; Cheng et al. 2011). This could explain the lack of B. longulus damage on these hybrids, in contrast to the improved performance of the native weevil, E. lecontei on hybrid watermilfoil in the U.S.A. (Roley & Newman 2006). However, the watermilfoil hybrid was a cross between the invasive M. spicatum and the native M. sibiricum, whereas the hybrids in this study are crosses of exotic Nymphaea, meaning that the milfoil hybrids are more genetically similar to the native species than the Nymphaea hybrids are to the native host of B. longulus (N. nouchali). Biological control of these Nymphaea hybrids could therefore be challenging, and unlikely to succeed using augmentative releases of B. longulus. The inability of B. longulus to survive on N. mexicana hybrids with varying parentage should, however, be confirmed with further experimentation.
Here we have collected useful information about sites invaded by N. mexicana in South Africa that can be used in post-release monitoring programmes to determine the success of biological control, thus fulfilling the first aim of this study. We have also determined that it is likely that enemy release contributes to the invasion of N. mexicana in South Africa, and that mean N. mexicana plant size in South Africa is greater than in its native range in USA. Furthermore, a decline in invasive N. mexicana populations has been associated with the presence of a native weevil, similar to the new association of E. lecontei in the U.S.A. (Newman & Biesboer 2000). Indeed, host range expansion of native insects to exotic plants may be more common than expected (Jahner et al. 2011; Branco et al. 2015; Sunny et al. 2015; Castagneyrol et al. 2016; Okamoto et al. 2020), but it is important to further investigate novel associations and the mechanisms underlying them, in order to understand and safely make use of these interactions for management of invasive alien plants. This study highlights the importance of conducting pre-introductory surveys in the invaded range of an alien plant. It is necessary to continue monitoring of the sites that have shown decline of N. mexicana where B. longulus is present, and to conduct further research to understand the potential of B. longulus for use in augmentative biological control.
ACKNOWLEDGEMENTS
The authors would like to thank the Natural Resources Management Programme of the Department of Environmental Affairs, South Africa; Tiso Foundation; and the Rhodes University Research Council for funding this research. Further funding for this work was provided by the South African Research Chairs Initiative of the Department of Science and Technology and the National Research Foundation of South Africa. Any opinion, finding, conclusion or recommendation expressed in this material is that of the authors and the NRF does not accept any liability in this regard. Dr Guy Sutton is thanked for his assistance and review of statistical methods in this paper. Matthew Paper, Emiel van Son, Tapiwa Mushore, and Rosali Smith are thanked for assistance with fieldwork.
ORCID IDs
MK Reid - https://orcid.org/0000-0001-7995-5878
MP Hill - https://orcid.org/0000-0003-0579-5298
IA Coetzee - https://orcid.org/0000-0002-0364-3349
CONTRIBUTOR ROLES
MK Reid: Writing - original draft; writing - review and editing; data curation; formal analysis; methodology; investigation; conceptualisation; visualisation.
MP Hill: conceptualisation, funding acquisition, resources, supervision, writing - review and editing; methodology.
JA Coetzee: conceptualisation, funding acquisition, resources, supervision, writing - review and editing; methodology.
REFERENCES
American Public Health Association (APHA). 1998. Standard methods for the examination of water and wastewater. New York: APHA. [ Links ]
Blossey B, Notzold R. 1995. Evolution of increased competitive ability in invasive nonindigenous plants: A hypothesis. Journal of Ecology. 83:887-889. https://doi.org/10.2307/2261425 [ Links ]
Branco M, Brockerhoff EG, Castagneyrol B, Orazio C, Jactel HE. 2015. Host range expansion of native insects to exotic trees increases with area of introduction and the presence of congeneric native trees. Journal of Applied Ecology. 52:69-77. https://doi.org/10.1111/1365-2664.12362 [ Links ]
Canavan K, Paterson I, Hill MP. 2014. The herbivorous arthropods associated with the invasive alien plant, Arundo donax, and the native analogous plant, Phragmites australis, in the Free State Province, South Africa. African Entomology. 22:454-459. https://doi.org/10.4001/003.022.0204 [ Links ]
Castagneyrol B, Jactel H, Brockerhoff EG, Perrette N, Larter M, Delzon S, Piou D. 2016. Host range expansion is density dependent. Oecologia. 182:779-788. https://doi.org/10.1007/s00442-016-3711-5 [ Links ]
Cheng D, Vrieling K, Klinkhamer PGL. 2011. The effect of hybridization on secondary metabolites and herbivore resistance: Implications for the evolution of chemical diversity in plants. Phytochemistry Reviews. 10:107-117. https://doi.org/10.1007/s11101-010-9194-9 [ Links ]
Coetzee JA, Hill MP. 2012. The role of eutrophication in the biological control of water hyacinth, Eichhornia crassipes, in South Africa. BioControl. 57:247-261. https://0-doi.org/10.1007/s10526-011-9426-y [ Links ]
Coetzee JA, Bownes A, Martin GD, Miller BE, Smith R, Weyl PSR, Hill MP. 2021. A review of the biocontrol programmes against aquatic weeds in South Africa. African Entomology. 29:935-964. https://doi.org/10.4001/003.029.0935 [ Links ]
Coetzee J, Hill M, Byrne M, Bownes A. 2011. A review of the biological control programmes on Eichhornia crassipes (C. Mart.) Solms (Pontederiaceae), Salvinia molesta DS Mitch. (Salviniaceae), Pistia stratiotes L. (Araceae), Myriophyllum aquaticum (Vell.) Verdc. (Haloragac.). African Entomology. 19:451-468. https://doi.org/10.4001/003.019.0202 [ Links ]
Colautti RI, Ricciardi A, Grigorovich IA, Macisaac HJ. 2004. Is invasion success explained by the enemy release hypothesis? Ecology Letters. 7:721-733. https://doi.org/10.1111/j.1461-0248.2004.00616.x [ Links ]
Colwell RK. 2006. EstimateS: statistical estimation of species richness and shared species from samples, version 8.0.
Creed RP, Sheldon SP. 1991. The potential for biological control of Eurasian watermilfoil (Myriophyllum spicatum): results of Brownington Pond, Vermont, study and multistate lake survey. Proceedings of the 25th Annual Meeting of the Aquatic Plant Control Research Program: 183-193. US Army Corps of Engineers.
Creed RP, Sheldon SP. 1993. The effect of feeding by a North American weevil, Euhrychiopsis lecontei, on Eurasian watermilfoil (Myriophyllum spicatum). Aquatic Botany. 45:245-256. https://doi.org/10.1016/0304-3770(93)90024-Q [ Links ]
De Lange WJ, van Wilgen BW. 2010. An economic assessment of the contribution of biological control to the management of invasive alien plants and to the protection of ecosystem services in South Africa. Biological Invasions. 12:4113-4124. https://doi.org/10.1007/s10530-010-9811-y [ Links ]
Diop O, Hill MP. 2009. Quantitative post-release evaluation of biological control of floating fern, Salvinia molesta D.S. Mitchell (Salviniaceae), with Cyrtobagous salviniae Calder and Sands (Coleoptera: Curculionidae) on the Senegal River and Senegal River Delta. African Entomology. 17:64-70. [ Links ]
Dudley TL, Lambert A, Kirk A. 2006. Augmentation biological control of Arundo donax. In: Hoddle MS, Johnson M, editors. Proceedings of the California Conference on Biological Control. Riverside, CA, USA; p. 141-145.
Dudley TL, Lambert AM, Kirk A, Tamagawa Y. 2008. Herbivores associated with Arundo donax in California. In: Julien MH, Sforza R, Bon MC, Evans HC, Hatcher PE, Hinz HL, Rector BG, editors. Proceedings of the XII International Symposium on Biological Control of Weeds, 22-27 April 2007. La Grande Motte, France: CAB International; p. 138-145.
DWAF. 1996. South African Water Quality Guidelines: Aquatic Ecosystems. 2nd Edition. Pretoria: Department of Water Affairs and Forestry Scientific Research Publishing. [ Links ]
Fleming JP, Dibble ED. 2015. Ecological mechanisms of invasion success in aquatic macrophytes. Hydrobiologia. 746:23-37. https://doi.org/10.1007/s10750-014-2026-y [ Links ]
Fritz RS, Moulia C, Newcombe G. 1999. Resistance of hybrid plants and animals to herbivores, pathogens, and parasites. Annual Review of Ecology and Systematics. 30:565-591. http://www.jstor.org/stable/221696 [ Links ]
Fritz RS, Nichols-Orians C, Brunsfeld SJ. 1994. Interspecific hybridization of plants and resistance to herbivores: hypotheses, genetics, and variable responses in a diverse herbivore community. Oecologia. 97:106-117. https://doi.org/10.1007/BF00317914 [ Links ]
González-Oreja JA, Garbisu C, Mendarte S, Ibarra A, Albizu I. 2010. Assessing the performance of nonparametric estimators of species richness in meadows. Biodiversity and Conservation. 19:1417-1436. https://doi.org/10.1007/s10531-009-9770-8 [ Links ]
Gooden B, French K, Turner PJ, Downey PO. 2009. Impact threshold for an alien plant invader, Lantana camara L., on native plant communities. Biological Conservation. 142:2631-2641. [ Links ]
Hartig F. 2021. DHARMa: Residual diagnostics for hierarchical (multilevel/mixed) regression models. R package version 0.4.4. https://CRAN.R-project.org/package=DHARMa
Herrick NJ, Kok LT. 2010. Classical biological control of weeds with Curculionidae. CAB Reviews: Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources 5:1-11. https://doi.org/10.1079/PAVSNNR20105028 [ Links ]
Hierro JL, Maron JL, Callaway RM. 2005. A biogeographical approach to plant invasions: The importance of studying exotics in their introduced and native range. Journal of Ecology. 93:5-15. https://doi.org/10.1111/j.0022-0477.2004.00953.x [ Links ]
Hill MP, Coetzee J. 2017. The biological control of aquatic weeds in South Africa: Current status and future challenges. Bothalia. 47:112. https://doi.org/10.4102/abc.v47i2.2152 [ Links ]
Hoffman JH. 2004. Biotypes, hybrids and biological control: lessons from cochineal insects on Opuntia weeds. XI International Symposium on Biological Control of Weeds. p. 283.
Jacob H, Briese DT. 2003. Improving the selection, testing and evaluation of weed biological control agents. In: Jacon HS, Briese DT, editors. Proceedings of the CRC for Australian Weed Management Biological Control of Weeds Symposium and Workshop. p. 87-98.
Jahner JP, Bonilla MM, Badik KJ, Shapiro AM, Forister ML. 2011. Use of exotic hosts by Lepidoptera: Widespread species colonize more novel hosts. Evolution. 65:2719-2724. https://doi.org/10.1111/j.1558-5646.2011.01310.x [ Links ]
Jakobs G, Weber E, Edwards PJ. 2004. Introduced plants of the invasive Solidago gigantea (Asteraceae) are larger and grow denser than conspecifics in the native range. Diversity and Distributions. 10:1119. https://doi.org/10.1111/j.1472-4642.2004.00052.x [ Links ]
Joshi J, Vrieling K. 2005. The enemy release and EICA hypothesis revisited: Incorporating the fundamental difference between specialist and generalist herbivores. Ecology Letters. 8:704-714. https://doi.org//10.1111/j.1461-0248.2005.00769.x [ Links ]
Keane RM, Crawley MJ. 2002. Exotic plant invasions and the enemy release hypothesis. Trends in Ecology and Evolution. 17:164-170. https://doi.org/10.1016/S0169-5347(02)02499-0 [ Links ]
Kent M. 2012. Vegetation Description and Analysis: A Practical Approach. 2nd Edition. University of Plymouth: John Wiley & Sons.
Martínez-Sanz C, Garciá-Criado F, Alaéz CF, Alaéz, MF. 2010. Assessment of richness estimation methods on macroinvertebrate communities of mountain ponds in Castilla y León (Spain). International Journal of Limnology. 46:101-110. https://doi.org/10.1051/limn/2010008 [ Links ]
McFadyen REC. 2000. Successes in biological control of weeds. In: Spencer NR, editor. Proceedings of the X International Symposium on Biological Control of Weeds. Montana State University, Bozeman, Montana, USA. p. 3-14.
Memmott J, Fowler SV, Paynter Q, Sheppard AW, Syrett P. 2000. The invertebrate fauna on broom, Cytisus scoparius, in two native and two exotic habitats. Acta Oecologica. 21:213-222. https://doi.org/10.1016/S1146-609X(00)00124-7 [ Links ]
Müller-Schärer H, Schaffner U. 2008. Classical biological control: Exploiting enemy escape to manage plant invasions. Biological Invasions. 10:859-874. https://doi.org/10.1007/s10530-008-9238-x [ Links ]
Newman RM, Biesboer DD. 2000. A decline of Eurasian Watermilfoil in Minnesota associated with the Milfoil Weevil, Euhrychiopsis lecontei. Journal of Aquatic Plant Management. 38:105-111. [ Links ]
Okamoto U, Shirahama S, Nasu S, Miyauchi H, Tokuda M. 2020. Host range expansion of a Polygonaceae-associated leaf beetle to an invasive aquatic plant Myriophyllum aquaticum (Haloragaceae). Arthropod-Plant Interactions. 14:491-497. https://doi.org/10.1007/s11829-020-09764-7 [ Links ]
Ondrus MG. 1996. Laboratory Experiments in Environmental Chemistry. 2nd Edition. Winniperg, Canada: Wuerz Publishing Ltd. [ Links ]
Panetta FD, Gooden B. 2017. Managing for biodiversity: Impact and action thresholds for invasive plants in natural ecosystems. NeoBiota. 34:53-66. http://dx.doi.org/10.3897/neobiota.34.11821 [ Links ]
Paynter Q, Downey PO, Sheppard AW. 2003. Age structure and growth of the woody legume weed Cytisus scoparius in native and exotic habitats: Implications for control. Journal of Applied Ecology. 40:470-480. https://doi.org/10.1046/j.1365-2664.2003.00817.x [ Links ]
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L. 2012. Biomass allocation to leaves, stems and roots: meta-analyses of interspecific variation and environmental control. New Phytologist. 193:30-50. https://doi.org/10.1111/j.1469-8137.2011.03952.x [ Links ]
Prior KM, Adams DC, Klepzig KD, Hulcr J. 2018. When does invasive species removal lead to ecological recovery? Implications for management success. Biological Invasions. 20:267-283. https://doi.org/10.1007/s10530-017-1542-x [ Links ]
R Core Team. 2021. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [ Links ]
Reid MK, Coetzee JA, Hill MP, Diaz R, Gettys LA, Cuda JP, Reid CS. 2020. Insect herbivores associated with Nymphaea mexicana (Nymphaeaceae) in southern United States: potential biological control agents for South Africa. Florida Entomologist. 103:54-63. https://doi.org/10.1653/024.103.0409 [ Links ]
Reid MK, Naidu P, Paterson ID, Mangan R, Coetzee JA. 2021. Population genetics of invasive and native Nymphaea mexicana Zuccarini: Taking the first steps to initiate a biological control programme in South Africa. Aquatic Botany. 171:103372. https://doi.org/10.1016/j.aquabot.2021.103372 [ Links ]
Roley SS, Newman RM. 2006. Developmental performance of the Milfoil Weevil, Euhrychiopsis lecontei (Coleoptera: Curculionidae), on Northern Watermilfoil, Eurasian Watermilfoil, and Hybrid (Northern X Eurasian) Watermilfoil. Environmental Entomology. 35:121-126. https://doi.org/10.1603/0046-225X-35.L121 [ Links ]
Sheppard AW, Heard TA, Briese DT. 2003. What is needed to improve the selection, testing and evaluation of weed biological control agents: workshop synthesis and recommendations. In: Jacob HS, Briese D, editors. Improving the Selection, Testing and Evaluation of Weed Biological Control Agents. p. 87-98.
Smith RA. 2010. Use of an aquatic weevil, Euhrychiopsis lecontei, as a biological control agent against Eurasian watermilfoil (Myriophyllum spicatum) in Michigan's Les Cheneaux Islands. Les Cheneaux Watershed Council. http://lescheneauxwatershed.org/images/invasive-species/wvlproj_final_jan_2010.pdf
Sunny A, Diwakar S, Sharma GP. 2015. Native insects and invasive plants encounters. Arthropod-Plant Interactions. 9:323-331. https://doi.org/10.1007/s11829-015-9384-x [ Links ]
Thorstenson AL. 2011. Biological control of Eurasian watermilfoil (Myriophyllum spicatum) using the native milfoil weevil (Euhrychiopsis lecontei). MSc. Thesis. University of Wisconsin, Stevens Point, Wisconsin.
Urban AJ, Simelane DO, Retief E, Heystek F, Williams HE, Madire LG. 2011. The invasive 'Lantana camara L.' hybrid complex (Verbenaceae): a review of research into its identity and biological control in South Africa. African Entomology. 19:315-348. https://doi.org/10.1007/s11829-015-9384-x [ Links ]
Van Klinken RD, Fichera G, Cordo H. 2003. Targeting biological control across diverse landscapes: The release, establishment, and early success of two insects on mesquite (Prosopis spp.) insects in Australian rangelands. Biological Control. 26:8-20. https://doi.org/10.1016/S1049-9644(02)00107-X [ Links ]
Van Klinken RD, Raghu S. 2006. A scientific approach to agent selection. Australian Journal of Entomology. 45:253-258. https://doi.org/10.1111/j.1440-6055.2006.00547.x [ Links ]
Van Wilgen BW, Raghu S, Sheppard AW, Schaffner U. 2020. Quantifying the social and economic benefits of the biological control of invasive alien plants in natural ecosystems. Current Opinion in Insect Science. 38:1-5. https://doi.org/10.1016/j.cois.2019.12.004 [ Links ]
Vasquez EC, Meyer GA. 2011. Relationships among leaf damage, natural enemy release, and abundance in exotic and native prairie plants. Biological Invasions. 13:621-633. https://doi.org/10.1007/s10530-010-9853-1 [ Links ]
Whitham TG, Morrow PA, Potts BM. 1994. Plant hybrid zones as centers of biodiversity: the herbivore community of two endemic Tasmanian eucalypts. Oecologia. 97:481-490. https://doi.org/10.1007/BF00325886 [ Links ]
Wickham H. 2016. Ggplot2: Elegant Graphics For Data Analysis. ISBN: 978-3-319-24277-4. New York: Springer-Verlag. https://ggplot2.tidyverse.org [ Links ]
Williams WI, Friedman JM, Gaskin JF, Norton AP. 2014. Hybridization of an invasive shrub affects tolerance and resistance to defoliation by a biological control agent. Evolutionary Applications. 7:381-393. https://doi.org/10.1111/eva.12134 [ Links ]
Wood SN. 2010. Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. Journal of the Royal Statistical Society. 73:3-36. https://doi.org/10.1111/j.1467-9868.2010.00749.x [ Links ]
 Correspondence:
Correspondence:
MK Reid
Email: megankim.reid@gmail.com
Received: 10 October 2022
Accepted:06 January 2023














