Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a13583
SHORT COMMUNICATION
Distribution and impact of the native South African wasp, Megastigmus transvaalensis (Hussey, 1956) (Hymenoptera: Torymidae) on the invasive Schinus terebinthifolia Raddi (Anacardiaceae) in South Africa
NL MagengeleleI; GD MartinI, II
ICentre for Biological Control, Department of Zoology and Entomology, Rhodes University, South Africa
IIAfromontane Research Unit and Department of Zoology and Entomology, University of the Free State, South Africa
ABSTRACT
Schinus terebinthifolia Raddi (Anacardiaceae) (Brazilian pepper tree) is a tree native to subtropical South America that was introduced into South Africa as an ornamental plant. Globally, it is regarded as one of the world's worst invasive trees. In South Africa the tree has acquired a native seed-feeding wasp, Megastigmus transvaalensis (Hussey, 1956) (Hymenoptera: Torymidae). The wasp's native hosts are from the Searsia F.A. Barkley genus (Anacardiaceae), but it has expanded its host range to form a new association with both S. terebinthifolia and its close relative Schinus molle L. (Anacardiaceae). In order to quantify the seed predation by M. transvaalensis on S. terebinthifolia seeds, tree populations were surveyed across the Eastern Cape and KwaZulu-Natal provinces. The wasp was present at 99% of the S. terebinthifolia populations with an average of 22% of the seeds being destroyed. In the Eastern Cape province, the highest seed damage occurred at the start of the winter months, when about 35% of seeds were damaged. This fell to less than 12% in spring and summer when the plants were flowering. Megastigmus transvaalensis was found at nearly all the S. terebinthifolia populations in South Africa, but due to the limited number of predated seeds it is unlikely to reduce population sizes or curb the spread of the invasive alien tree in South Africa.
Keywords: biological control, Brazilian pepper tree, invasive alien plant, seed parasitoid, seed predator
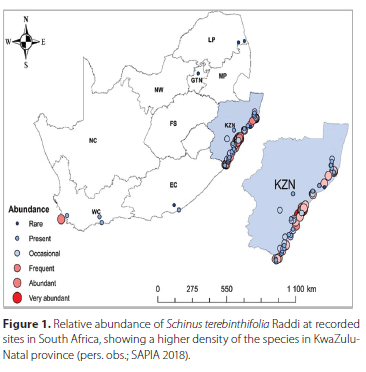
Schinus terebinthifolia Raddi (Sapindales: Anacardiaceae) (Brazilian pepper tree) is a tree native to subtropical South America that has naturalised and became invasive in more than 20 countries around the world (CABI 2020; Martin et al. 2020). Outside its native range, S. terebinthifolia is an aggressive, fast-growing invader of both natural and disturbed systems. The tree shades out and displaces native vegetation, often forming dense monocultures that reduce biological diversity (Ewel et al. 1982; Cuda et al. 2006). In South Africa, under the National Environmental Management: Biodiversity Act 10 of 2004 (DEA 2014), the tree is declared a Category 1b invader in the KwaZulu-Natal, Eastern Cape, Limpopo and Mpumalanga provinces and a Category 3 species in the remaining provinces. The 1b categorisation means that it legally requires active management interventions in the most invaded provinces (DEA 2014; Martin et al. 2020; Figure 1). South Africa has considered biological control options for the species, but no programme has yet been implemented (Martin et al. 2020).
Uncharacteristically for an invasive plant species, in South Africa the tree has acquired a new association with a native seed-feeding wasp, Megastigmus transvaalensis (Hussey 1956) (Hymenoptera: Torymidae), which is primarily associated with the indigenous Searsia F.A. Barkley genus (Anacardiaceae) (Habeck et al. 1989; Grissell & Hobbs 2000). Megastigmus transvaalensis has also been accidentally introduced into North America where the plant is invasive (Scheffer & Grissell 2003), as well as into its native range in South America (Ferreira-Filho et al. 2015). In North America, the wasp attacks the seeds of S. terebinthifolia with up to 76% of drupes damaged in Florida (Wheeler et al. 2001) and 80% in Hawaii, USA (Hight et al. 2003), while its occurrence in the native range is seen as a threat to the native S. terebinthifolia populations (Ferreira-Filho et al. 2015). The effect of the wasp on S. terebinthifolia populations in South Africa was the focus of this study, as significant seed damage could be regulating the weed, and thus influence management strategies, such as biological control.
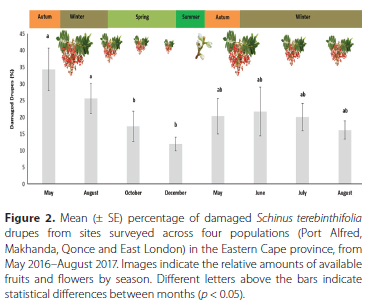
Between May 2016 and September 2017, seventy-four trees of S. terebinthifolia were sampled in the Eastern Cape (n = 27) and KwaZulu-Natal (n = 47) provinces of South Africa (Figure 1). One hundred, randomly collected drupes per tree were collected and kept in perforated jars for 28 days. After 28 days, the drupes were inspected for wasp exit holes, and those without exit holes were dissected to confirm the presence or absence of a seed predator. In addition, in the Eastern Cape province, four separate populations (Port Alfred, Makhanda, Qonce and East London) were repeatedly sampled, in the same manner, over a period of 13 months from May 2016-August 2017. In total, 26 300 seeds were examined from the 74 sites. The only seed-attacking herbivore encountered during surveys was M. transvaalensis. Across all sites, a mean 20.8% (± 2.2 SE) of sampled drupes were damaged with a maximum of 80% and a minimum of 0% recorded. More specifically, in KwaZulu-Natal province an overall of 23.2% (± 2.2 SE) of sampled drupes were damaged, with a maximum of 50% and a minimum of 0%, while, in the Eastern Cape province an overall mean of 20.1% (± 4.5 SE), with a maximum of 80% and a minimum of 0% were recorded. These percentages are lower than the mean of 44.7% (± 7.4 SE) reported by Boardman (2010), who sampled 16 sites in KwaZulu-Natal province in 2010, where a maximum of 80% and a minimum of 9% of drupes damaged per tree was recorded. However, the damage caused by M. transvaalensis on S. terebinthifolia drupes in both South African studies were lower than those in North America (Wheeler et al. 2001; Hight et al. 2003). This could possibly be as a result of escape from parasitism, as some hyperparasitoids, such Aprostocetus Westwood, 1833 species, were also collected within the perforated jars. These species were submitted to the Iziko museum (to view and obtain accession numbers see: http://www.waspweb.org/Chalcidoidea/Eulophidae/Tetrastichinae/Aprostocetus/Aprostocetus_species.htm). The abundance of these hyperparasitoids and impact on M. transvaalensis was not determined. Megastigmus transvaalensis is an excellent disperser and able to survive under a wide range of climatic conditions as it was recorded at nearly all populations sampled across the provinces during the study.
Repeated monthly sampling from four separate populations (Port Alfred, Makhanda, Qonce and East London) in the Eastern Cape province showed that when drupes were available, the highest percentage was recorded in May (35%) and the lowest recorded in December (15%) (Figure 2). No fruits could be sampled during January-April, as the trees were flowering and no suitable mature red fruits were available. There was a general decrease in seed damage from the start of winter, leading towards spring and summer, which coincided with the amount of mature red fruits present on the trees. There were no statistical differences in seed damage between the rest of the months sampled (F = 13.7; p > 0.05; ANOVA) (Figure 2). In addition, the viability of seeds without exit holes, collected in December 2016, from the four Eastern Cape province populations were tested using tetrazolium (Verma & Majee 2013) to determine how many viable seeds were available to germinate. The overall seed viability was relatively low with a mean of 26% (± 7.6 SE). More specifically the highest tested viability was recorded for seed collected in Port Alfred (48%), followed by East London (24%), Makhanda (20%) and Qonce (13%). Apart from Port Alfred these percentages were low compared to a study carried out by Dlamini et al. (2018), who reported germination percentages from 45-58% for S. terebinthifolia seeds. It is worth noting that both Panetta & McKee (1997) and Dlamini et al. (2018) found that the fruit of S. terebinthifolia have a near-obligate requirement to be ingested by frugivorous birds before the seeds can germinate. Nonetheless, the study showed that sufficient viable seeds are being produced and entering the environment to suggest that S. terebinthifolia is currently not seed limited and that there is an abundance of viable seeds in South Africa.
In the North America, M. transvaalensis has been regarded as a beneficial natural enemy of S. terebinthifolia, due to its substantial impact on the seeds (Wheeler et al. 2001; Hight et al. 2003). In contrast, in Brazil, M. transvaalensis was regarded as a problematic invasive species and was found to damage between 1% and 55% of S. terebinthifolia seeds (Ferreira-Filho et al. 2015). These high levels of seed predation were not recorded in South Africa, presumably because of the presence of alternative, possibly preferred, host plants, which are not present in North America and Brazil. The effect oftrophic interactions in the form of hyperparasitoids should also not be ignored. The low levels of seed damage, seed viability and climate suitability indicate that the invasive alien plant is likely to keep increasing in density and distribution, in the absence of further management. Therefore, biological control, if considered, should also include the use of additional seed-feeding agents.
ACKNOWLEDGEMENTS
The National Research Foundation and the Natural Resource Management Programme of the South African Department of Forestry, Fisheries and the Environment funded this research. Any opinion, finding, conclusion or recommendation expressed in this contribution is that of the authors and the NRF and DFFE do not accept any liability in this regard.
ORCID IDs
Nwabisa Magengelele - https://orcid.org/0000-0003-4747-154X
Grant Martin - https://orcid.org/0000-0001-9302-1369
REFERENCES
Boardman JN. 2010. Impact and distribution of the native South African wasp, Megastigmus transvaalensis Hussey (Hymenoptera: Torymidae) on Schinus terebinthifolius Raddi and Schinus mole L. (Anacardiaceae) in KwaZulu-Natal, South Africa. [Honours thesis]. Pietermaritzburg: School of Biological and Conservation Sciences, University of KwaZulu-Natal. [ Links ]
CA BI. 2020. Invasive species compendium Schinus terebinthifolius (Brazilian pepper tree). [accessed 14 May 2020]. https://www.cabi.org/isc/datasheet/49031.
Cuda JP, Ferriter AP, Manrique V, Medal JC, editors. 2006. Florida's Brazilian Peppertree Management Plan: Recommendations from the Brazilian Peppertree Task Force. West Palm Beach, FL, U.S.A.: Florida Exotic Pest Plant Council.
DEA (Department of Environmental Affairs). 2014. Government Gazette. https://www.sanbi.org/wp-content/uploads/2018/04/nembainvasivealienspeciesregulations-12feb2014.pdf. [accessed 23 August 2022].
Dlamini P, Zachariades C, Downs CT. 2018. The effect of frugivorous birds on seed dispersal and germination of the invasive Brazilian pepper tree (Schinus terebinthifolius) and Indian laurel (Litsea glutinosa). South African Journal of Botany. 114: 61-68. https://doi.org/10.1016/j.sajb.2017.10.009. [ Links ]
Ewel JJ, Ojima DS, Karl DA, De Busk WF. 1982. Schinus in successional ecosystems of Everglades National Park. Everglades National Park, South Florida Research Center. Homestead, FL. Report T-676.
Ferreira-Filho PJ, Pina-Rodrigues F, Silva J, Guerreiro JC, Ghiotto TC, Piotrowski I, Dias LP, Wilcken CF, Zanuncio JC. 2015. The exotic wasp Megastigmus transvaalensis (Hymenoptera, Torymidae), first record and damage on the Brazilian peppertree, Schinus terebinthifolius drupes, in São Paulo, Brazil. Anais da Academia Brasileira de Ciências. 87(4): 2091-2095. https://doi.org/10.1590/0001-3765201520140478. [ Links ]
Grissell EE, Hobbs KR. 2000. Megastigmus transvaalensis (Hussey) (Hymenoptera, Torymidae) in California, methods of introduction and evidence of host shifting. In: Hymenoptera, evolution, biodiversity and biological control. Fourth International Hymenoptera Conference, held in Canberra, Australia, January 1999. p. 267-280. Canberra: CSIRO Publishing.
Habeck DH, Bennett FD, Grissell EE. 1989. First record of a phytophagous seed chalcid from Brazilian peppertree in Florida. Florida Entomologist. 72(2): 378-379. https://doi.org/10.2307/3494922. [ Links ]
Hight SD, Horiuchi I, Vitorino MD, Wikler C, Pedrosa-Macedo JH. 2003. Biology, host specificity tests, and risk assessment of the sawfly Heteroperreyia hubrichi, a potential biological control agent of Schinus terebinthifolius in Hawaii. Biological Control. 48(4): 461-476. [ Links ]
Martin GD, Magengelele NL, Paterson ID, Sutton GF. 2020. Climate modelling suggests a review of the legal status of Brazilian pepper Schinus terebinthifolia in South Africa is required. South African Journal of Botany. 132: 95-102. https://doi.org/10.1016/j.sajb.2020.04.019. [ Links ]
Panetta FD, Mckee J. 1997. Recruitment of the invasive ornamental, Schinus terebinthifolius is dependent upon frugivores. Australian Journal of Ecology. 22(4): 432-438. https://doi.org/10.1111/j.1442-9993.1997.tb00694.x. [ Links ]
SAPIA. South African Plant Invader Atlas. 2018. Available at: https://www.arc.agric.za/arc-ppri/weeds/Pages/Geographical-Distribution-of-IAPs-in-Southern-Africa-(SAPIA).aspx. [accessed 23 August 2022].
Scheffer SJ, Grissell EE. 2003. Tracing the geographical origin of Megastigmus transvaalensis (Hymenoptera, Torymidae), an African wasp feeding on a South American plant in North America. Molecular Ecology. 12(2): 415-421. https://doi.org/10.1046/j.1365-294X.2003.01725.x. [ Links ]
Verma P, Majee M. 2013. Seed germination and viability test in tetrazolium (TZ) assay. Bio-Protocol. 3(17):e884. https://doi.org/10.21769/BioProtoc.884. [ Links ]
Wheeler GS, Massey LM, Endries M. 2001. The Brazilian peppertree drupe feeder Megastigmus transvaalensis (Hymenoptera, Torymidae): Florida distribution and impact. Biological Control. 22(2):139-148. https://doi.org/10.1006/bcon.2001.0968. [ Links ]
 Correspondence:
Correspondence:
GD Martin
Email: g.d.martin84@gmail.com
Received: 29 March 2022
Accepted: 20 April 2022














