Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a12791
RESEARCH ARTICLE
Formulation of eucalyptus oil-zinc sulphide hybrid nanoemulsion and evaluation of its larvicidal potential against Aedes aegypti
Komalpreet Kaur SandhuI, II; Nisha VashishatI; Devinder Kaur KocherI
IDepartment of Zoology, Punjab Agricultural University, Ludhiana, India
IIDepartment of Zoology, Akal University, Talwandi Sabo, Bathinda, India
ABSTRACT
The growing threat of vector-borne diseases and their associated health risks has prompted the nanotechnology-based investigations. The present study aimed to use a nanotechnological application with larvicidal potential against Aedes aegypti by preparing aqueous hybrid nanoemulsion of zinc sulphide nanoparticles and Eucalyptus globulus oil. The mean droplet size of prepared and most stable hybrid nanoemulsion (9.5 ppm) was found to be 60 ± 10.00 nm with rectangular shape. The hybrid nanoemulsion exhibited LC50 and LC90 values of 7.63 and 9.22 ppm, respectively, against larval stages of Aedes aegypti. The findings obtained from the larvicidal assay were corroborated with SEM, histological and biochemical profiles of Aedes larvae after treating with hybrid nanoemulsion. Under simulated conditions, nanohybrid treatment demonstrated optimum larvicidal potency after 48 hours of exposure. Further, biosafety studies of hybrid nanoemulsion were carried out against Scapholebris kingi and this larvicidal concentration of nanohybrid was found to be non-toxic to this species. Thus, the following research explains the larvicidal efficacy of zinc sulphide-based hybrid nanoemulsion of eucalyptus oil formulated during the present study is a step towards safe and efficient approach against the dengue-spreading vector mosquito, Aedes aegypti.
Keywords: biosafety, essential oils, mosquito control, simulated conditions, zinc sulphide ]
INTRODUCTION
Aedes aegypti (Linnaeus, 1762) is responsible for transmitting diseases like dengue, chikungunya and zika in tropical and sub-tropical countries. This mosquito breeds on stagnant water existing in artificial containers (Vezzani 2007). In more than 100 countries, dengue is endemic (Pessoa et al. 2018) and major cause of hospitalisation (Santosh Kumar et al. 2018). The mosquito transmits diseases to more than 700 million people every year (Taubes 2000). Therefore, the control of this vector mosquito is an important public health concern (Magro et al. 2019) and until now, only few vaccines exist against arboviruses and especially for humans are still under clinical trials. Vector control measures include mainly the chemical methods and to some extent use of biocontrol agents for their immature and adult stages (George et al. 2015). In this context, insecticides are the most widely used products. But their usage in bulk is non-selective, toxic to non-target species, harmful for the environment and also results in mosquito resistance (Margulis-Goshen et al. 2013).
Various scientific studies reported the larvicidal properties of phytoconstituents and plant-based extracts with varied potential (Piplani et al. 2019). The main drawback of using essential oils as a larvicidal agent in the natural habitat of larvae, which are water bodies, is immiscibility of water and oil. This problem can be overcome by downsizing the oils using nanotechnology and by making oil nanoemulsions. Furthermore, researchers have examined the efficacy of different metal nanoparticles against insect vectors and revealed the potency of nanoparticles (NPs) in vector control. Out of these silver, gold, palladium, copper, zinc, silica and carbon nanoparticles have been best exemplified for mosquito control (Minal & Prakash 2019).
Recently, nanoformulations of heteroleptic metal complexes have been developed as potential larvicides against Ae. aegypti. Metal complexes of Cu (II), Co (II) and Fe (III) with heteroleptic ligands viz. 1,2,4-triazole-dithiocarbamate, triphenyl phosphine and isothiocyanate in different ratios prepared and converted into water dispersed nano-formulations were found to act as better larvicidal agents due to lower toxicity to non-target organisms and higher water dispersibility (Gumber et al. 2017). Oil in water nanoemulsion of carbendazim (a fungicide) showed effective anti-larval activity against Culex mosquito, as compared to organophosphates like temephos (Gumber et al. 2017). Different reports on antimicrobial and anti-larval activities of transition metal complexes derived from amino acid Schiff bases have also been well documented and as compared to other bioactive metals, copper complexes with best potential against Ae. aegypti (Santha Lakshmi & Geetha 2016). Recently, Azadirachta indica leaf extract mediated bimetallic copper-zinc NPs have been found to show larvicidal potential against Culex quinquefasciatus (Minal & Prakash 2019).
Green synthesis of copper oxide nanoparticles (CuO NPs) has also shown the larvicidal potential against Ae. aegypti (Selvan et al. 2018) and these CuO NPs were found to result in malformations of the larvae and pupae of cotton leafworm, Spodoptera littorals (Shaker et al. 2016). When Scadoxus multiflorus leaf powder aqueous extract was used as a capping and stabilising agent during the synthesis of pure zinc oxide nanoparticles (ZnO NPs), it acted as larvicidal and ovicidal agent against Ae. aegypti (Dhabi & Arasu 2018). Antilarval activity of ZnO NPs using Momordica charantia leaf extract against Anopheles stephensi, Culex quinquefasciatus was reported by Gandhi et al. (2017).
The bioefficacy and safety of metal sulphide nanoparticles are of interest for public health use. Therefore, the present study was planned to synthesise and test the larvicidal efficacy of Eucalyptus globulus oil based nanohybrid emulsions having ZnO NPs against Ae. aegypti along with testing its safety against other non-target organisms which in the present study was Scapholebris kingi.
MATERIALS AND METHODS
Preparation of hybrid nanoemulsions (nanohybrids)
Synthesis of ZnS nanoparticles
A 20 ml aqueous solution of sodium sulphide (0.001 M) was mixed dropwise into equal quantity of zinc acetate solution (0.001 M) under ultrasonic irradiation at temperature of 40.25 °C having pulse of 05 on and 01 off and amplitude of 60%, which was continued for additional 30 minutes to obtain zinc sulphide nanoparticles (0.0005 M, 48.74 ppm).
Preparation of non-polar eucalyptus oil nanoemulsion
The oil was fractionated into polar and non-polar fractions. The non-polar portion of the oil was formulated in different ratios and sonicated along with water and surfactant (Kaur et al. 2019).
Preparation of nanoemulsion
The most stable oil nanoemulsion and zinc sulphide nanoparticles (ZnS NPs) were mixed in five different ratios viz. 1:1, 1:2, 1:3, 1:4 and 1:5. All the different formulations were sonicated along with a drop of Tween 20® so that metal sulphide nanoparticles were decorated on the non-polar oil nanoemulsion to make hybrid nanoemulsions.
Screening and morphological studies of hybrid nanoemulsions
Stable hybrid nanoemulsions were screened by visually observing the transparency followed by various stress tests described by Shafiq et al. (2007). The morphometric properties of zinc sulphide-based hybrid nanoemulsions (drop) on a copper grid was examined via transmission electron microscopy (TEM-Hi 7650) operating at an accelerated voltage of 80 kV in the Electron Microscopy and Nanotechnology (EMN) Laboratory, PAU, Ludhiana.
Larvicidal bioassay of Ae. aegypti larvae
Aedes aegypti larvae were collected from various peri-domestic water collections in different zones of Punjab Agricultural University, Ludhiana, Punjab (30.9010 °N 75.8573 °E) by using plastic dippers from June to August 2019. Aedes aegypti larvae identified using the standard keys given by Becker et al. (2010) and Bar & Andrew (2013). Zinc sulphide based hybrid nanoemulsions of eucalyptus oil were tested at 10.00, 9.50, 9.00, 8.50 and 8.00 ppm concentrations. Twenty early fourth instar Ae. agypti larvae per concentration were used for all the experiments. The experimental sets were kept in a Bio-Oxygen Demand incubator at 26 ± 2 °C. Control (having 100 ml de-chlorinated water only) and vehicle control (having Tween 20 in de-chlorinated water in same ratio as treatment set) sets were also run simultaneously with 20 larvae in each set. During the experimental duration, no food was provided. Dead larvae were identified from their discoloration and by probing needle in their siphon region. Mortality of the larvae in different concentrations was recorded after 3, 6, 12, 24 and 48 hours of treatment in triplicate.
Morphological studies of larvae
Morphological changes were studied by using a Scanning Electron Microscope (SEM-S3400N operated at 15 kV) at Electron Microscopy and Nanotechnology Laboratory, PAU, Ludhiana. Larvae from treated (larvae about to die) and untreated sets were primarily fixed for two hours in 2.5% glutaraldehyde solution followed by one hour fixation in 1% osmium tetroxide at 4 °C. Then, larvae were washed with 0.1 M sodium cacodylate buffer and in graded ethyl alcohol series for 15 minutes. Larvae were dried in vacuum desiccators for 1 hour and mounted on aluminium stub having a double sticky carbon tape. Larvae were covered with a thin layer of approximately 20 nm to 30 nm of gold in the iron sputter coater (E-1010).
Histological studies
For histological studies, the treated and untreated larvae were collected and washed properly in 0.9% saline solution followed by their embedding in 10% neutral buffered formalin solution. Larvae were further processed, sectioned and stained following the standard histological procedure given by Luna (1968).
Biochemical assays
The treated and control larvae were collected, washed in double distilled water, homogenised and centrifuged, the supernatant was collected and used for estimation of total proteins (Lowry et al. 1951) and activity of digestive enzymes, i.e. a-amylase, protease and lipase by the methods given by Bernfeld (1955), Elpidina et al. (2001) and Tsujita et al. (1989), respectively.
Testing the larvicidal potential of effective concentration of zinc sulfide based hybrid nanoemulsion under simulated conditions
For the conduct of experiments under simulated conditions, experiments were performed in plastic tubs (with dimensions of 45 X 34 X 26 cm) having dried leaves, soil and algae. Control, vehicle-control and treated sets were performed in triplicate by introducing 100 fourth instar Ae. aegypti larvae. Mortality was recorded after 3, 6, 12, 24 and 48 hours in all experimental sets in triplicate.
Bioefficacy of zinc sulphide-based hybrid nanoemulsions against non-target organism, Scapholebris kingi
Samples of various zooplanktons like copepods, polychaetes and cladocerans were collected from pond water using a zooplankton net and Scapholebris kingi were specifically identified on the basis of morphological characters given by Battish (1992) and Jamwal (2015). In small plastic containers, 20 S. kingi were introduced in control, vehicle-control and treated sets in triplicate. All the sets were observed for mortality and toxicity after 12, 24 and 48 hours initially and then at weekly intervals up to 21 days.
Statistical analysis
Mortality data was statistically analysed by comparing treated and control groups by using ANOVA (Duncan multiple range test; DMRT) on SPSS statistical analysis software version 16 of log probit method (Finney 1971) using the POLO (Robertson et al. 1980) software was used for calculating LC50 and LC90.
RESULTS
Screening and morphological analysis of hybrid nanoemulsions
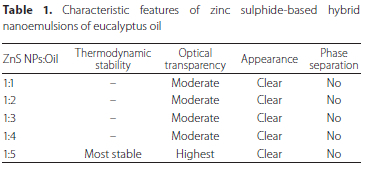
The different non-polar fractions were screened on the basis of certain parameters given in Table 1. Prepared hybrid nanoemulsions in ratio 1:5 (ZnSNPs:Oil) was found to be most stable after performing thermodynamic stability tests. TEM micrographs showed average size of droplets, i.e. 60 ± 10.00 nm with slightly irregular in shape (Figure 1). The other ratios -1:1, 1:2, 1:3 and 1:4 - did not show any coating of zinc sulphide nanoparticles (ZnS NPs) and hence were not considered for larvicidal studies.
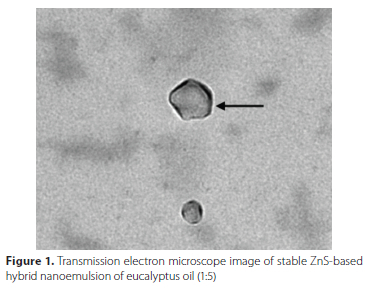
Effect of zinc sulphide-based hybrid nanoemulsions on Aedes aegypti larvae
Larvicidal effect and toxicity
Exposure of Ae. aegypti larvae to 8 ppm of stable zinc sulphide (1:5) based hybrid nanoemulsion showed 5.00 ± 0.00, 20.00 ± 5.00, 45.00 ± 5.00, 73.33 ± 15.28 and 95.00 ± 5.00 per cent mortality after 3, 6, 12, 24 and 48 hours, respectively. With the increase in concentration to 8.5 ppm, the per cent mortality was observed as 11.67 ± 2.89, 26.67 ± 2.89, 51.67 ± 2.89, 75.00 ± 5.00 and 95.00 ± 5.00 after 3, 6, 12, 24 and 48 hours respectively. The per cent larval mortality was found to increase statistically with the increase in concentration of zinc sulphide-based hybrid nanoemulsion. On exposure to 9.5 ppm of concentration of zinc sulphide based hybrid nanoemulsion, 100% mortality of Ae. aegypti larvae was observed within 24 hours. Thus, 9.5 ppm concentration was found to be statistically the most effective concentration in comparison to other concentrations, as all larvae were killed at this concentration only within 24 hours before conversion to pupae in comparison to 10 ppm concentration where 100% mortality was observed within 12 hours (Table 2).
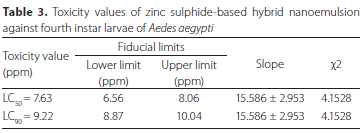
Various toxicity values, i.e. LC50 and LC90 of zinc sulphide-based hybrid nanoemulsion computed for 4th instar larvae of Ae. aegypti are given in Table 3. These values for LC50 and LC90 of zinc-based hybrid nanoemulsion were 7.63 and 9.22 ppm respectively against Ae. aegypti larvae as calculated by employing the computer programme POLO based on log-probit technique at 95% intervals (Finney 1971).
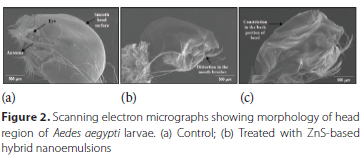
Morphological changes
Larvae exposed to effective concentration of ZnS-based hybrid nanoemulsions (9.5 ppm) were studied for morphological changes and compared with untreated larvae through the scanning electron micrographs. The modifications observed in the different body parts of the treated larvae have been described region wise as follows.
Head region - Control 4th instar larvae of Ae. aegypti were found to have smooth head surface, clearly visible eye and antennae (Figure 2a). After exposure to 9.5 ppm of ZnS-based hybrid nanoemulsion, distortion was observed in the mouth region, in particular the complete distortion of mouth brushes and constriction on the back of the head capsule was clearly visible (Figure 2b and 2c).
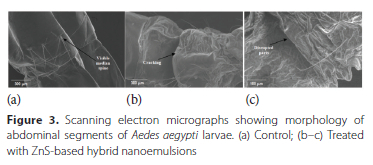
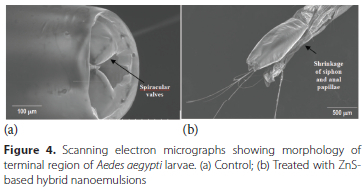
Abdominal region - Abdominal surface of control larvae was smooth as well as intact with no signs of disruption and cracking along with a clearly visible median spine (Figure 3a). Exposure of ZnS-based hybrid nanoemulsion led to reduction in thickness of exoskeleton, degradation of segmental cuticle, cracking and surface destruction of this region (Figure 3b and 3c). Terminal region - In the case of control larvae, the siphon was found to have smooth surface with very distinct spiracular valves (Figure 4a). Treatment of Ae. aegypti larvae with ZnS-based hybrid nanoemulsions resulted in shrinkage of siphon as well as anal papillae (Figure 4b).
Histological changes
ZnS-based hybrid nanoemulsion treatment to Ae. aegypti 4th instar larvae resulted in destruction of various body parts/ organs present in various regions, so these histological changes have been described region wise.
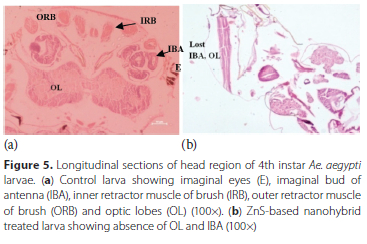
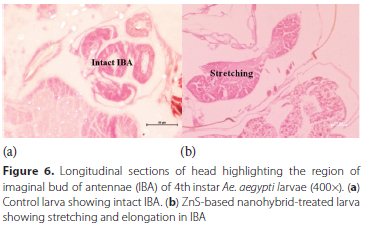
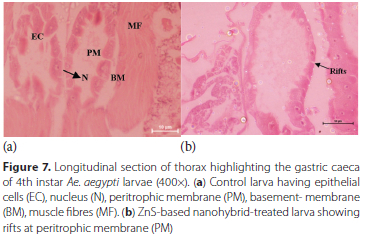
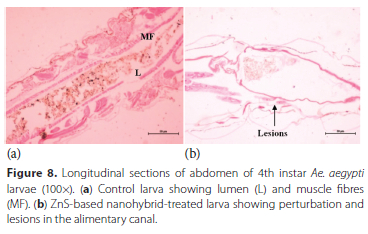
Head region - Control larvae had imaginal eyes (E), an imaginal pair of antennae buds (IBA), a pair ofbrush inner retractor muscle (IRB) and brush outer retractor muscle (ORB) and brain with a pair of optic lobes (OL), all these parts in the head region were found to have no damage (Figure 5a). Hybrid nanoemulsions-based on ZnS induced alterations in the head region structure in the form of complete absence of optic lobes, and total disintegration of the brushes of inner and outer retractor muscles (Figure 5b). Imaginal buds of antennae (IBA) were found to be intact, complete and normal in the control larva (Figure 6a) while ZnS-based hybrid nanoemulsion treatment induced stretching and elongation of the larval imaginal buds (Figure 6b). Thorax area [Stomodeum or foregut part] - In the control larvae, cylindrical epithelial cells (EC), vesicles (V), nucleus (N), peritrophic membrane (PM), basement membrane (BM) and muscle fibres (MF) were clearly visible along with well-developed microvilli (MV) present in the gastric caeca located in thorax region (Figure 7a). ZnS-based hybrid nanoemulsion treatment showed slight changes in gastric caeca, as all cells were intact, nuclei were present and the appearance of epithelial cells was the same as that observed in the control larva except the occurrence of rifts in the peritrophic membrane and breakage of epithelium cells was also observed at certain areas (Figure 7b). Abdomen - The control larva showed complete abdominal region with a clearly visible food channel showing lumen (L), muscle fibres (MF), and the gut lumen was filled with columnar cells of epithelium (Figure 8a) whereas in ZnS-based hybrid nanoemulsion-treated larvae signs of disruption in the alimentary canal were observed in the form of lesions as seen in Figure 8b.
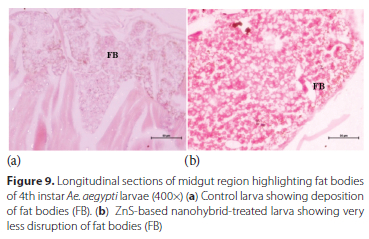
Mesenteron or midgut - The lumen content of the anterior midgut in the control larva showed well-developed fat body (FB) tissues (Figure 9a). However, much less distortion of fat bodies was observed in Ae. aegypti larvae treated with ZnS-based hybrid nanoemulsion (Figure 9b).
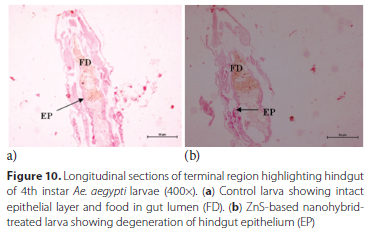
Proctodeum or hindgut - Intact epithelium layer in the hindgut region of the control larva with presence of food in the gut lumen was reported (Figure 10a). Disorganisation in the epithelial layer was observed in the larvae treated with ZnS-based hybrid nanoemulsion (Figure 10b).
Changes in biochemical parameters
Exposure to effective ZnS-based hybrid nanoemulsion (9.5 ppm) showed variable effects on the amount of proteins and activity of different digestive enzymes of Ae. aegypti larvae which are described as below.
Protein content - The mean protein content was 24.38 ± 1.24 mg/g in control larvae, whereas exposure of ZnS-based hybrid nanoemulsion resulted in significant reduction in protein contents (F = 64.030, df = 2, p < 0.05) as it was observed to be 10.56 ± 2.48 mg/g (Table 4).
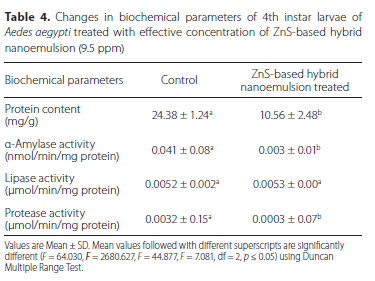
a-Amylase activity - The specific activity of a-amylase (F = 2680.627, df = 2, p < 0.05) was found to be 0.041 ± 0.08 nmol/ min/mg protein in control larval homogenate, where as in treated group it was significantly decreased to 0.003±0.01 nmol/ min/mg protein (Table 4).
Lipase activity - In the control larvae, the activity (F = 44.877, df = 2, p < 0.05) was found to be 0.0052 ± 0.002 (µmol/min/mg of protein. On the other hand, ZnS-based hybrid nanoemulsion has no effect on this enzyme, as its activity was neither decreased nor increased as compared to control larvae (Table 4). Protease activity - The activity of protease was found to decline statistically (F = 7.081, df = 2, p < 0.05) after the exposure of ZnS-based hybrid nanoemulsion as it was 0.0003 ± 0.07 (µmol/min/mg protein, in comparison to that of control set (0.0032 ± 0.15 (µmol/ min/mg protein) as shown in Table 4.
Larvicidal potential of effective concentration of zinc sulphide-based hybrid nanoemulsion under simulated conditions
Under simulated conditions, treatment of effective concentration of ZnS-based hybrid nanoemulsion (9.5 ppm) resulted in more than 90% mortality after 48 hours (Table 5). The mortality rate was found to be 19.33 ± 4.04, 32.00 ± 1.00, 51.67 ± 1.53, 79.00 ± 3.61 and 92.67 ± 2.52 per cent after 3, 6, 12, 24 and 48 hours respectively. No larval mortality was observed in control and vehicle-control sets (Table 5).
Non toxicity tests of ZnS-based hybrid nanoemulsions against Scapholebris kingi
During the present study, the exposure of selected non-target organism, i.e. S. kingi to 9.5 ppm of ZnS- based hybrid nanoemulsion resulted in its 0.00 ± 0.00 per cent mortality (Table 6). Microscopic examination of these S. kingi (after exposure) showed no morphological changes as their eyes, mouth and antennae were normal and clearly visible (Figure 11). Therefore, this nanohybrid was found to have no toxic effect on the non-target organism studied during the present research.
DISCUSSION
Among the various prevailing approaches to mosquito control, the larvicidal approach has been shown to be one of the useful and target-specific tool (Killeen et al. 2002) and helpful in reducing adult mosquito population. Many workers have evaluated synthetic formulations of herbal based larvicidal agents. Wangrawa et al. (2018) tested essential oil from Lantana camara leaves against VK7 and Kisumu strains of Anopheles gambiae and found a higher sensitivity (LC50: 7.73 µg/ml) of Kisumu strain to the tested oil compared with VK7 strain (LC50 : 25.63 µg/ml). Muturi et al. (2018) isolated essential oils from Allium sativum, Ferula asafetida to evaluate their larvicidal activity against Culex pipiens and Cu. restuans and calculated EC50 as 2.7 and 7.5 ng/ml, respectively, indicating high larvicidal potential of A. sativum oil. Andrade et al. (2018) investigated the larvicidal activity of essential oils from seven plants (Cuminum cyminum, Citrus aurantifolia, Cinnamomum verum, Syzygium aromaticum, Laurus nobilis, Lippia berlandieri and Pimpinella anisum) against Cu. quinquefasciatus larvae. In 2015, Brazilian researchers developed a nanoemulsion against 4th instar larvae of Ae. aegypti with Rosmarinus officinalis EO as an active component and mortality recorded after treatment with its 250 ppm was 80 and 90% after 24 and 48 hours respectively (Duarte et al. 2015). Previously, Prajapati et al. (2005) showed that non-emulsified R. officinalis EO had LD95 of 408 ppm after 24 hours of exposure which, in contrast, suggests the greater larvicidal efficacy of the nanoemulsion. Recently, Iranian researchers have published a work on inhibiting Anopheles stephensi, a major malaria vector in their country, through the action of Artemisia dracunculus (Asteraceae) derived nanoemulsion in EO. A nanoemulsion bioassay with LC50 or LC90 of 11.36 and 17.54 ppm was performed on 3rd and 4th larvae. P-allylanisole was reported as the main constituent of EO (Osanloo et al. 2017). Major nanoparticles synthesised by plant extracts are gold, silver, copper, copper oxide, palladium, platinum, titanium dioxide, zinc oxide, indium oxide, iron oxide, lead and selenium nanoparticles (Shah et al. 2015). Silver nanoparticles synthesis has been achieved using Kiwifruit juice, Rumex hymenosepalus extract, Annona squamosa leaf extract, Podophyllum hexandrum leaf extract, extracts of Acalypha indica Linn., Hibisicus cannabinus leaf extract, Macrotyloma uniflorum seed extract (Vidhu et al. 2011). Zinc sulphide (ZnS) nanoparticles, synthesised by sono-chemical route employing zinc chloride and sodium sulphide, displayed significant anti-fungal property against the pathogenic yeast Candida albicans (MTCC 227) at a minimum fungicidal concentration of 300 µg/ ml (Suyana et al. 2014). Supraja et al. (2016) have also reported antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated ZnO NPs. Magro et al. (2019) synthesised a novel photosensitizing hybrid nanomaterial based on chlorine-e6 and surface-active maghemite nanoparticles (SAMNs) with water self-assembly (SAMN@chlorin). A well-defined crystalline structure characterises the magnetic core, consisting of stoichiometric maghemite (n-Fe2O3). The most prominent feature of this nanomaterial is the ability to form stable colloidal suspensions in water without organic or inorganic coating to avoid aggregation and to reversibly and precisely bind organic molecules that form hybrid nanomaterials to biotechnology applications and has a photocidal potential against Ae. aegypti. Chaithong et al. (2006) studied the morphological alterations by SEM in treated and control larvae of Ae. aegypti after exposure of ethanolic extracts of Piper longum. They observed that larvae become shrunken and anal papillae were damaged, probably led to their dysfunction and intrinsically related to death of larvae; similar observations have also been recorded in our present study. Kala et al. (2019) evaluated effect of cashew nut shell liquid (CSNL) nanoemulsion on morphological characters by scanning electron microscopy (SEM) in larvae of Anopheles culicifacies which revealed the adherence of nanodroplets to larval body resulting in complete larval deformation.
Our observations coincided with other authors who considered the impact of plant extracts, their nanomaterials and other such agents on mosquitoes and even on gut section. For example, Mahmoud et al. (2019) studied larvicidal potential and histopathological changes induced after treatment of ethanolic extracts of Piper nigrum. The most characteristic disordered signs visualised in the midgut region of treated larvae were elongation and vacuolisation of epithelial cells, rupturing of the peritrophic membrane, detachment of basement membrane, disorganisation of microvilli, and furthermore, degeneration of muscle layer. Silva et al. (2008) reported histological changes in midgut of Bacillus thuringiensis var.israelensis (Bti) infected Ae. albopictus larvae, where disorganisation in intestinal cells, dilation and disintegration in rough endoplasmic reticulum with intense cytoplasmic vacuolisation was observed. Mishra et al. (2018) treated Cx. tritaeniorhynchus larvae with nanopermethrin colloidal dispersion and histological studies revealed normal midgut content (MC), EC and PM in control larvae, but these structures got damaged in treated larvae. Kala et al. (2019) revealed partial damage in epithelial cells (EC) and peritrophic membrane (PM) in cashew nut shell liquid nanoemulsion-treated Anopheles culicifacies larvae but in control larvae undamaged EC and unbroken PM were found.
Mishra et al. (2018) prepared permethrin nano-formulation against Cx. tritaeniorhynchus larvae and studied its effect on biochemical profiles. Glutathione S-transferase (GST), acetylcholine esterase (AChE) and acid and alkaline phosphatase assays were performed and decrease in cellular biomolecules and biomarker enzyme activity in the nanopermethrin-treated larvae as compared to bulk and control larvae was reported. Their results were substantiated with our results as activity of digestive enzymes decreased which led to death of the larvae. In another study by Mishra et al. (2018) prepared neem-laced urea nanoemulsion (NUNE) and larvicidal potency was studied against Ae. aegypti and Cx. tritaeniorhynchus along with few biochemical components such as total protein, lipid, carbohydrate and biomarker enzymes like GST, acid and alkaline phosphatase activity. Treated larval samples were found to have decreased activity and larvicidal toxicity tests were corroborative with biochemical profiling obtained through biochemical analysis of treated samples.
Mdoe et al. (2014) tested the larvicidal efficacy of oil extracted from leaves of Cryptomeria japonica (Japanese cedar) against An. gambiae and observed that the rate of mortality increased with increasing dosages from 12.5 to 200 |ig/ml. They also observed 31.75 to 100% mortality of larvae under laboratory, whereas in semi field conditions the per cent mortality was 17.75 to 99.5. The LC50 and LC90 values of J. procera against larvae of An. arabiensis were 14.42 and 24.65 mg/l under laboratory conditions and 24.51 and 34.21 mg/l under semi-field or simulated conditions, respectively (Karunamoorthi et al. 2014).
Govindarajan & Benelli (2016) demonstrated lvandulyl acetate cytotoxicity on three non-target mosquito predators, Anisops bouvieri, Diplonychus indicus and Gambusia affinis and its cytotoxicity was found to be 206, 336 and 534 µg/ml, respectively. Benelli et al.(2018) has investigated the effect of essential oil extracted from Syzygium lanceolatum leaves on non-target species such as A.bouvieri, D.indicus, G.affinis and Poecilia reticulata, the LC50 ranging from 4148 to 15762 µg/ml. Nanohybrid material based on chlorine-e6 and surface-active maghemite nanoparticles (SAMNs) with water self-assembly (SAMN@ chlorin) showed a great photocidal activity against Ae. aegypti and no adverse effects was observed in Daphnia magna. Hence this nanohybrid reduced environmental concerns and may be used as a safer alternative to conventional insecticides (Magro et al. 2019). Therefore, biosafety assessment of the formulated ZnS-based hybrid nanoemulsion against non-target species depicted minimal or no toxicity proves nontoxic behaviour of applied nanohybrid which portrays its environmental benevolence as an efficient and biosafe larvicidal agent.
ACKNOWLEDGEMENTS
The authors are grateful to the Head, Department of Zoology, for providing all the necessary facilities, and Department of Science and Technology, Government of India, New Delhi, for providing infrastructural facilities under FIST grant.
ORCID IDs
Komalpreet Kaur Sandhu - https://orcid.org/0000-0003-3129-3670
Nisha Vashishat - https://orcid.org/0000-0003-0071-8955
Devinder Kaur Kocher - https://orcid.org/0000-0002-1897-8897
REFERENCES
Andrade S, Sanchez-Aldana D, Chacón-Vargas KF, Rivera-Chavira BE, Sánchez-Torres LE, Camacho AD, Nogueda-Torres B, Nevárez-Moorillon GV. 2018. Oviposition deterrent and larvicidal and pupaecidal activity of seven essential oils and their major components against Culex quinquefasciatus Say (Diptera: Culicidae): Synergism-antagonism Effects. Insects 9(1):25. https://doi.org/10.3390/insects9010025 [ Links ]
Bar A, Andrew J. 2013. Morphology and norphometry of Aedes aegypti adult mosquito. Annual Research & Review in Biology 3(1):52-69. [ Links ]
Battish SK. Freshwater Zooplankton of India. New Delhi: Oxford and IBH Publishing Ltd. p. 1-233. [ Links ]
Becker N, Petric D, Zgomba M, Boase C, Madon M, Dahl C, Kaiser A. 2010. Mosquitoes and their Control. Springer Publication, 2nd Edn. pp. 9-40. https://doi.org/10.1007/978-3-540-92874-4.
Benelli G, Rajeswary M, Govindarajan M. 2018. Towards green oviposition deterrents? Effectiveness of Syzygium lanceolatum (Myrtaceae) essential oil against six mosquito vectors and impact on four aquatic biological control agents. Environmental Science and Pollution Research 25(11): 10218-10227. https://doi.org/10.1007/s11356-016-8146-3 [ Links ]
Bernfeld P. 1955. Amylases, alpha and beta. Methods in Enzymology 1:149-158. https://doi.org/10.1016/0076-6879(55)01021-5 [ Links ]
Chaithong U, Choochote W, Kamsuk K, Jitpakdi A, Tippawangkosol P, Chaiyasit D, Champakaew D, Tuetun B, Pitasawat B. 2006. Larvicidal effect of pepper plants on Aedes aegypti (L.) (Diptera: culicidae). Journal of Vector Ecology 31(1):138-144. https://doi.org/10.3376/1081-1710(2006)31[138:LEOPPO]2.0.CO;2 [ Links ]
Dhabi NA, Arasu MV. 2018. Environmentally-friendly green approach for the production of zinc oxide nanoparticles and their antifungal, ovicidal and larvicidal properties. Nanomaterials (Basel). 8:1-13. [ Links ]
Duarte JL, Amado JRR, Oliveira AEMFM, Cruz RAS, Ferreira AM, Souto RNP, Falcão DQ, Carvalho JCT, Fernandes CP. 2015. Evaluation of larvicidal activity of a nanoemulsion of Rosmarinus officinalis essential oil. Revista Brasileira de Farmacognosia 25(2):189-192. https://doi.org/10.1016/j.bjp.2015.02.010 [ Links ]
Elpidina EN, Vinokurov KS, Gromenko VA, Rudenskaya YA, Dunaevsky YE, Zhuzhikov DP. 2001. Compartmentalization of proteinases and amylases in Nauphoeta cinerea midgut. Archives of Insect Biochemistry and Physiology 48(4):206-216. https://doi.org/10.1002/arch.10000 [ Links ]
Finney DJ. 1971. Probit analysis, 3rdEdn. Cambridge: Cambridge University Press. p. 68-72. [ Links ]
Gandhi PR, Jayaseelan C, Mary RR, Mathivanan D, Suseem SR. 2017. Acaricidal, pediculicidal and larvicidal activity of synthesized ZnO nanoparticles using Momordicacharantialeaf extract against blood feeding parasites. Experimental Parasitology 181:47-56. https://doi.org/10.1016/j.exppara.2017.07.007 [ Links ]
George L, Lenhart A, Toledo J, Lazaro A, Han WW, Velayudhan R, Runge Ranzinger S, Horstick O. 2015. Community-effectiveness of temephos for dengue vector control: a systematic literature review. PLoS Neglected Tropical Diseases 8: e0004006. https://doi.org/10.1371/journal.pntd.0004006 [ Links ]
Govindarajan M, Benelli G. 2016. Ecofriendly larvicides from Indian plants: effectiveness of lavandulyl acetate and bicyclogermacrene on malaria, dengue and Japanese encephalitis mosquito vectors. Ecotoxicology and Environmental Safety 133:395-402. https://doi.org/10.1016/j.ecoenv.2016.07.035 [ Links ]
Gumber K, Sidhu A, Kocher DK. Synthesis and preliminary evaluation of carbendazimnanoemulsions as larvicidal agent against Culex mosquitoes. Advances in Applied Research. 2017;9(1):7-11. https://doi.org/10.5958/2349-2104.2017.00003.1
Gumber K, Sidhu A, Kocher DK. 2017. Synthesis of Novel 1, 2, 4-triazole-DTC based metallo-phosphorus nanoformulations as larvicide against Aedes aegypti. International Research Journal of Pure Applied Chemistry 14(1):1-12. https://doi.org/10.9734/IRJPAC/2017/32958 [ Links ]
Jamwal S. 2015. Predatory potential of copepods against mosquito larvae. Ph.D. Dissertation, Punjab Agricultural University, Ludhiana, India. [ Links ]
Kala S, Sogan N, Verma P, Naik SN, Agarwal A, Patanjali PK, Kumar J. 2019. Nanoemulsion of cashew nut shell liquid bio-waste: mosquito larvicidal activity and insights on possible mode of action. South African Journal of Botany 127:293-300. https://doi.org/10.1016/j.sajb.2019.10.006 [ Links ]
Karunamoorthi K, Girmay A, Fekadu S. 2014. Larvicidal efficacy of Ethiopian ethnomedicinal plant Juniperus procera essential oil against Afrotropical malaria vector Anopheles arabiensis (Diptera: culicidae). Asian Pacific Journal of Tropical Biomedicine 4:S99-S106. https://doi.org/10.12980/APJTB.4.2014C687 [ Links ]
Kaur N, Kocher DK, Sidhu A. 2019. Synthesis and testing of Eucalyptus globulosa oil-based nanoemulsion for its larvicidal potential against Aedes aegypti. African Entomology 27(2):433. https://doi.org/10.4001/003.027.0433 [ Links ]
Killeen GF, Fillinger U, Knols BG. 2002. Advantages of larval control for African malaria vectors: low mobility and behavioral responsiveness of immature mosquito stages allow high effective coverage. Malaria Journal 1(1):8. https://doi.org/10.1186/1475-2875-1-8 [ Links ]
Luna LG. 1968. Manual of Histological Staining Methods of the Armed Forces Institute of Pathology. New York: McGraw-Hill. [ Links ]
Magro M, Bramuzzo S, Baratella D, Ugolotti J, Zoppellaro G, Chemello G, Olivotto I, Ballarin C, Radaelli G, Arcaro B, et al. 2019. Self-assembly of chlorin-e6 on y-Fe2O3 nanoparticles: application for larvicidal activity against Aedes aegypti. Journal of Photochemistry & Photobiology 194:21-31. https://doi.org/10.1016/j.jphotobiol.2019.03.004 [ Links ]
Mahmoud MD, Abd El-Bar MM, Salem AMD, Magda H, Rady MH. 2019. Larvicidal potential and ultra-structural changes induced after treatment of Culex pipiens L. (Diptera: Culicidae) larvae with some botanical extracted oils. International Journal of Mosquito Research 6(4):01-09. [ Links ]
Margulis-Goshen K, Magdassi S, Ishaaya I, Palli S, Horowitz A. 2013. Advanced Technologies for Managing Insect Pests. Dordrecht: Springer; p. 295-314. https://doi.org/10.1007/978-94-007-4497-4_15 [ Links ]
Mdoe FP, Cheng S-S, Lyaruu L, Nkwengulila G, Cheng S-T, Kweka EJ. 2014. Larvicidal efficacy of Cryptomeria japonica leaf essential oils against Anophelesgambiae. Parasites & Vectors 7(1):426. https://doi.org/10.1186/1756-3305-7-426 [ Links ]
Minal PS, Prakash S. 2019. Efficacy of bimetallic copper-zinc nanoparticles against larvae of microfilariae vector in laboratory. International Journal of Mosquito Research 8:72-75. [ Links ]
Mishra P, Balaji APB, Dhal PK, Suresh Kumar RS, Magdassi S, Margulis K, Tyagi BK, Mukherjee A, Chandrasekaran N. 2017. Stability of nano sized permethrin in its colloidal state and its effect on the physiological and biochemical profile of Culex tritaeniorhynchus larvae. Bulletin of Entomological Research 107(5):676-688. https://doi.org/10.1017/S0007485317000165 [ Links ]
Mishra P, Samuel MK, Reddy R, Tyagi BK, Mukherjee A, Chandrasekaran N. 2018. Environmentally benign nanometric neem-laced urea emulsion for controlling mosquito population in environment. Environmental Science and Pollution Research 25(3):2211-2230. https://doi.org/10.1007/s11356-017-0591-0 [ Links ]
Muturi EJ, Ramirez JL, Zilkowski B, Flor-Weiler LB, Rooney AP. 2018. Ovicidal and larvicidal effects of garlic and asafoetida essential oils Against West Nile Virus Vectors. Journal of Insect Science 18(2). https://doi.org/10.1093/jisesa/iey036 [ Links ]
Osanloo M, Amani A, Sereshti H, Abai MR, Esmaeili F, Sedaghat MM. 2017. Preparation and optimization nanoemulsion of tarragon (Artemisia dracunculus) essential oil as effective herbal larvicide against Anopheles stephensi. Industrial Crops and Products 109:214219. https://doi.org/10.1016/j.indcrop.2017.08.037 [ Links ]
Pessoa ZL, Duarte JL, Ferreira RM, Oliveira AEMFM, Cruz RAS, Faustino SMM, Carvalho JCT, Fernandes CP, Souto RNP, Araujo RS. 2018. Nanosuspension of quercetin: preparation, characterization and effects against Aedes aegypti larvae. Revista Brasileira de Farmacognosia 28(5):618-625. https://doi.org/10.1016/j.bjp.2018.07.003 [ Links ]
Piplani M, Bhagwat DP, Singhvi G, Sankaranarayanan M, Balana-Fouce R, Vats T, Chander S. 2019. Plant-based larvicidal agents: an overview from 2000 to 2018. Experimental Parasitology 199:92-103. https://doi.org/10.1016/j.exppara.2019.02.014 [ Links ]
Prajapati V, Tripathi AK, Aggarwal KK, Khanuja SPS. 2005. Insecticidal, repellent and oviposition-deterrent activity of selected essential oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresource Technology 96(16):1749-1757. https://doi.org/10.1016/j.biortech.2005.01.00 [ Links ]
Robertson JL, Russell RM, Savin NE. 1980. POLO: A User's Guide to Probit or Logit Analysis. General Technical Report PSW-038. Berkeley, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station. https://doi.org/10.2737/PSW-GTR-38
Santha Lakshmi S, Geetha K. Synthesis, crystal structure and characterization of ternary copper (II) complex derived from N-(salicylidene)-L- valine. Crystallography. 2016;2016:1-4. https://doi.org/10.1155/2016/6078543
Santosh Kumar PS, M.C A, Gupta SK, Nongkynrih B. 2018. Malaria, dengue and chikungunya in India - an update. Indian Journal of Medical Specialities. 9:25-29. https://doi.org/10.1016/j.injms.2017.12.001 [ Links ]
Selvan MS, Anand VK, Govindaraju K, Tamilselvan S, Kumar G, Subramanian K, Kannan M, Raja K. 2018. Green synthesis of copper oxide nanoparticles and mosquito larvicidal activity against dengue, zika and chikungunya causing vector Aedes aegypti. IET Nanobiotechnology 12:1-8. [ Links ]
Shafiq S, Shakeel F, Talegaonkar S, Ahmad FJ, Khar RK, Ali M. 2007. Development and bioavailabilty assessment of ramiprilnanoemulsion formulation. European Journal of Pharmaceutics and Biopharmaceutics 66(2):227-243. https://doi.org/10.1016/j.ejpb.2006.10.014 [ Links ]
Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GEJ. 2015. Green synthesis of Metallic nanoparticles via Biological entities. Materials (Basel). 8(11):7278-7308. https://doi.org/10.3390/ma8115377 [ Links ]
Shaker AM, Zaki AH, Rahim EFA, Khader MH. 2016. Novel CuO nanoparticles for pest management and pesticides degradation. Advances in Environmental Biology 10:274-283. [ Links ]
Silva VC, Pinheiro NL, Scherer PO, Falcão SS, Ribeiro VR, Mendes RMM, Chagas R, Cardozo-De-Almeida M, Dos Santos-Mallet JR. 2008. Histology and ultrastructure of Aedes albopictus larval midgut infected with Bacillus thuringiensis var. israelensis. Microscopy Research and Technique 71(9):663-668. https://doi.org/10.1002/jemt.20605 [ Links ]
Supraja N, Prasad TNVKV, Krishna GT, David E. 2016. Synthesis, characterization, and evaluation of the antimicrobial efficacy of Boswellia ovalifoliolata stem bark-extract-mediated zinc oxide nanoparticles. Applied Nanoscience 6(4):581-590. https://doi.org/10.1007/s13204-015-0472-0 [ Links ]
Suyana P, Kumar NS, Kumar D, Nair BN, Pillai SC, Mohamed A, Warrier KGK, Hareesh US. 2014. Anti fungal property of nanosized ZnS particles synthesised by sonochemical precipitation. RSC Advances. 4(17):8439-8445. https://doi.org/10.1039/c3ra46642f [ Links ]
Taubes G. 2000. Searching for a parasite's weak spot. Science. 290(5491):434-437. https://doi.org/10.1126/science.290.5491.434 [ Links ]
Tsujita T, Ninomiya H, Okuda H. 1989. p-Nitrophenyl butyrate hydrolyzing activity of hormone-sensitive lipase from bovine adipose tissue. Journal of Lipid Research 30(7):997-1004. https://doi.org/10.1016/S0022-2275(20)38302-4 [ Links ]
Vidhu VK, Aromal SA, Philip D. 2011. Green synthesis of silver nanoparticles using Macrotyloma uniflorum. Spectrochimica Acta, Part A: Molecular and Biomolecular Spectroscopy 83(1):392-397. https://doi.org/10.1016/j.saa.2011.08.051 [ Links ]
Vezzani D. 2007. Review: Artificial container-breeding mosquitoes and cemeteries: a perfect match. Tropical Medicine and International Health 12(2):299-313. https://doi.org/10.1111/j.1365-3156.2006.01781.x [ Links ]
Wangrawa DW, Badolo A, Ilboudo Z, Guelbéogo WM, Kiendrébeogo M, Nébié RCH, Sagnon N, Sanon A. 2018. Insecticidal activity of local plants essential oils against laboratory and field strains of Anopheles gambiae sl (Diptera: Culicidae) from Burkina Faso. Journal of Economic Entomology 111:2844-2853. [ Links ]
 Correspondence:
Correspondence:
Komalpreet Kaur Sandhu
Email: komalpreetkaur903@gmail.com
Received: 23 November 2021
Accepted: 07 September 2022














