Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a13244
RESEARCH ARTICLE
Response of ants, beetles and spiders to disturbance varies among taxa in a South African savannah biome
Risuna MavasaI; Inam YekwayoII; Tarombera MwabvuIII, IV; Zivanai TsvuuraI
ISchool of Life Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
IIDepartment of Biological and Environmental Sciences, Walter Sisulu University, Mthatha, South Africa
IIISchool of Biology and Environmental Sciences, University of Mpumalanga, Mbombela, South Africa
IVSchool of Life Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
Savannahs are structurally complex ecosystems consisting of a diverse community of plants and animals such as arthropods. Arthropods are essential in many ecosystem processes that help maintain life on Earth. The anthropogenic conversion of natural landscapes into croplands, residential and industrial areas has a negative impact on surface-active arthropods that have limited dispersal abilities and narrow habitat preferences. This study investigated the effect of disturbance on assemblages of ants, beetles and spiders in the savannah vegetation in Mpumalanga province, South Africa. We compared species richness, abundance and composition of these three taxa between the pristine savannah and the savannah that is exposed to a variety of anthropogenic activities (disturbed savannah). Arthropods were collected using pitfall traps in 15 sites in pristine savannah and 15 sites in disturbed savannah. We found that disturbance affects species richness and abundance of these taxa differently. Disturbance did not affect species richness of spiders and abundance of beetles, while greater species richness of ants and beetles, as well as abundance of ants and spiders was in disturbed than in pristine savannah. Furthermore, the species compositions of all taxa were different between disturbed and pristine savannah. The disturbed savannah had twice more unique indicator species than the pristine savannah. Differences in assemblages of arthropods between pristine and disturbed habitats suggest that it may be important to consider habitats in and outside protected areas in the conservation of arthropods, particularly in areas with greater percentage of natural and semi-natural landscapes occurring outside protected areas.
Keywords: Arthropods, diversity, natural-landscapes, transformed-landscapes
INTRODUCTION
The savannah is one of the most diverse biomes on Earth (Hutley and Setterfield 2018) and is characterised primarily by a layer of grasses interrupted by sparsely distributed woody vegetation (Wakeling et al. 2012; Lehmann et al. 2014). The woody plants of the savannah biome have open canopies, which allow direct sunlight to filter through to the understorey vegetation (Mucina and Rutherford 2010). The structure of the savannah biome allows several biological activities to occur both in the canopy and understorey vegetation (Simioni et al. 2003). For example, arthropods, which play an integral part in several ecosystems, are active in the canopy and understory vegetation (Simioni et al. 2003; Marquart et al. 2020).
Surface-active arthropods, which are active mostly in the understorey vegetation, form a significantly high component of the total arthropod species that have been described to date (Stork 2018; Eisenhauer et al. 2019; Seibold et al. 2019). Some of these surface-active arthropods, which include ants, beetles, spiders, cockroaches, termites and millipedes have limited dispersal abilities (Lavelle et al. 2006) and are sensitive to changes in their environment (Yekwayo and Mwabvu 2019) because of their dependence on specific microhabitats (Hill et al. 2008). For example, species diversity of ants correlates positively with vegetation cover (Pacheco et al. 2012; Silva da Costa and Schmidt 2022), while species composition of dung beetles is influenced by soil type (Daniel et al. 2022). Furthermore, variation in habitat requirements for arthropods occurs even within a taxon. For example, Pacheco et al. (2012) reported that habitats that are different structurally (grassland, savannah with scattered trees and savannah with dense tree cover or closed woodland) support different species composition of ants, while structurally similar habitats (savannah with dense tree cover and closed woodland) support similar assemblages of ants. Therefore, surface-active arthropods are confined to specific habitats with the resources that they need. The sensitivity of surface-active arthropods to changes in their environment can be used to identify aspects of the environment that are responsible for changes in their assemblages (see Hoffmann et al. 2021; Leonard et al. 2021). Additionally, changes in the assemblages of surface-active arthropods can be linked reliably to changes in their local environment because of their dispersal limitations (e.g. H0ye and Culler 2018; Arribas et al. 2021). As such, surface-active arthropods present a potentially effective tool that can assist to monitor changes in an environment which may arise as a result of anthropogenic disturbance (Maleque et al. 2009).
In South Africa, many landscapes of natural vegetation have been transformed into croplands, industrial and residential areas to support the growing human population (Wigley et al. 2010; Jewitt et al. 2015; Hyvarinen et al. 2019). Transformation of natural landscapes due to anthropogenic activities is a widespread phenomenon in many parts of the world. For example, a review by Osborne et al. (2018) revealed that vegetation structure of the savannah biome may be influenced negatively by agricultural activities, construction of roads, increased frequency and intensity of fires and replacing wild grazing with livestock grazing. The anthropogenic transformation of natural landscapes does not only affect the vegetation structure but poses a significant risk to organisms such as surface-active arthropods which have limited dispersal abilities (Hlongwane et al. 2019; Leonard et al. 2021). For example, in Germany, Seibold et al. (2019) observed that intensification of land use decreased the gamma diversity and biomass of arthropods over a 9-year period. Furthermore, anthropogenic activities such as removal of trees, fires, harvesting of fuelwood and livestock grazing, which lead to the reduction of vegetation cover, were reported to reduce abundance of insects and alter species composition of insects in a forest in Tanzania (Leweri and Ojija 2018). Similarly, burnt and encroached forests in Ghana supported low species diversity and abundance of insects (Husseini et al. 2019). Fire reduces arthropod diversity due to direct mortality and alteration of microhabitats, which become unfavourable for specialist species (Vasconcelos et al. 2009). Additionally, in a South African grassland, Pryke et al. (2016) showed that grazing by livestock not only decreases species richness of insects but also changes their composition when compared to grazing by wildlife. However, it is important to note that the management of fire and grazing are vital in shaping diversity of arthropods in the savannah biome (Thoresen et al. 2021).
The response of arthropods to anthropogenic activities sometimes varies among taxa because of variation in the environmental requirements for each arthropod group. For example, Ste-Marie et al. (2018) reported that different arthropod taxa respond differently to the proximity to the road. Abundance of collembolans that require a specific soil type decreased with proximity to the road, while the opposite was true for dipterans (associated with high plant biomass and increased moisture) and hymenopterans (associated with increased dipterans) (Ste-Marie et al. 2018). On the other hand, abundance of predatory spiders was not influenced by the proximity to the road (Ste-Marie et al. 2018). Furthermore, the effect of habitat fragmentation by roads is likely to be pronounced on surface-active arthropods that have limited dispersal ability. In this regard, Andersson et al. (2017) recorded different species composition of bees and wasps in two habitats separated by a road. Despite the negative impact of roads on arthropods, the presence of road verges can play a vital role in the conservation of ants and spiders (Kaur et al. 2019).
In a southern African savannah, Mauda et al. (2018) observed significantly lower species diversity of ants in croplands and villages than in rangelands distant from residential areas. In parts of the Mpumalanga province in South Africa, croplands in the form of subtropical fruit plantations dominate the landscape where savannah vegetation occurred previously. In addition, savannah vegetation in some areas of the province occurs outside protected areas. As a result, the vegetation is exposed to a variety of anthropogenic activities. For example, unplanned fires, fragmentation, establishment of croplands, introduction of alien plant species, harvesting of plants and plant products, grazing by livestock and being in close proximity to residential and/or industrial areas are some of the perturbations to the vegetation and the landscape (Osborne et al. 2018; Yekwayo and Mwabvu 2019). In our study savannah vegetation that is experiencing or is in close proximity to these anthropogenic activities is considered 'disturbed' savannah.
Given that plantations of subtropical fruits dominate the Mpumalanga province, many studies on arthropods have focused on taxa that are economically important (for example, pollinators, pests and natural enemies) to the agricultural industry (Van den Berg et al. 1999; Bruwer et al. 2021; Smith et al. 2022). However, other surface-active arthropods are equally important to agricultural and natural landscapes because they provide several key ecological services which help to maintain ecosystem sustainability (Roy et al. 2018). We investigated the influence of disturbance on the species richness, abundance and composition of three groups of surface-active arthropods in the savannah biome of Mpumalanga province. Following the approach used in the study by Uehara-Prado et al. (2009), this study is focused on ants, beetles and spiders because these taxa are known to have adequate samples in terms of abundance and species richness. The study may be relevant for providing baseline information as little is known about surface-active arthropod assemblages in savannah landscapes that are dominated by various anthropogenic disturbances in Mpumalanga. In addition, data obtained from this study will contribute towards producing a checklist of ants, beetles and spiders occurring in the savannah biome in Mpumalanga.
MATERIALS AND METHODS
Study sites
The study was conducted at two locations, on the outskirts of the Mbombela City (OMC) and in Barberton Nature Reserve (BNR), which is approximately 20 km from the Mbombela City, in the lowveld of the Mpumalanga province, South Africa (Figure 1). Study sites on the outskirts (approximately 9 km from the city centre) of Mbombela City were categorised as 'disturbed', while those in BNR were considered 'pristine'. Sites in OMC were located on fallow land which is exposed to a variety of anthropogenic activities such as being in close proximity (±1 km) to ongoing residential, industrial and agricultural development. As such, unplanned fires, livestock grazing, collection of plant products and habitat fragmentation by roads were inevitable. In addition, there were observations of invasive alien plants such as Psidium guajava L. and Lantana camara L. in the disturbed sites. The vegetation on the outskirts of Mbombela City is dominated mainly by bushveld savannah with patches of shrubveld savannah (Mucina and Rutherford 2010). The grass layer on OMC is dominated by species in the genera Aristida, Hyparrhenia and Cymbopogon, while the woody layer is dominated by Vachellia tortilis (Forssk.) Galasso and Banfi and Sclerocarya birrea (A. Rich.) Hochst.
Unlike sites on OMC, sites in Barberton Nature Reserve were in a protected area with little human interference. Barberton Nature Reserve covers about 27 541 ha of land (Mpumalanga Tourism and Parks Agency 2019). The vegetation in BNR can be described as sour bushveld of the savannah biome (Mucina and Rutherford 2010). Some of the woody plant species observed in BNR are Grewia occidentalis L., Olea europaea subsp. africana Mill., Ruttya ovata Harv., Vachellia nilotica (L.) P. Hurter and Mabb, Diospyros sp., Euclea sp. and Ximenia sp. The grass layer was composed mainly of Heteropogon contortus (L.) P. Beauv. ex Roem. and Schult., Panicum maximum Jacq. and Hyparrhenia hirta (L.) Stapf.
At each location category (disturbed and pristine), 15 sites were selected, with an elevation ranging from 613 to 827 m above sea level. The lowveld of the Mpumalanga province experiences wet summers and dry winters with a mean annual rainfall between 600 and 700 mm (Mucina and Rutherford 2010; ARC-ISCW unpubl.). In summer, temperatures range from 13 to 40 °C in Barberton and 14 to 43 °C in Mbombela (ARC-ISCW unpubl.).
Data collection procedure
The study was conducted in austral summer (December 2018 to February 2019) when the activity of surface-active arthropods was high. Several studies have indicated that the activity and abundance of surface-active arthropods are affected significantly by changes in temperature such that they are more active in summer than in winter (Niemela et al. 1992; Botes et al. 2006; Barahona-Segovia et al. 2019; Suheriyanto et al. 2019). Therefore, sampling surface-active arthropods in summer increased the chances of making observations that are inclusive of a wide variety of species.
The pitfall trapping method was used as pitfalls are quick to install, sample continually, require periodic emptying only, and therefore, are a cost-effective method (Woodcock 2005; Samways et al. 2010). Furthermore, the pitfall trapping method allows large numbers of surface-active arthropods to be caught with minimal effort compared to other methods, such as suction sampling and foliage beating (Woodcock 2005). A pitfall trap in this study was an open-top cylindrical plastic jar of 6 cm diameter and 13.5 cm depth. Pitfall traps were quarter filled with 50% ethylene glycol and inserted into the soil with the rim of the jar level with the soil surface so that the surface-active arthropods encountered no vertical barrier. We selected 15 replicate sites for each site category of disturbed and pristine savannah, which creates potential problems of pseudo-replication because the sites are nested within a site category (Hurlbert 2004). Nonetheless, in studies where interspersion of treatments is impractical (see Rutherford et al. 2014; Mwambilwa et al. 2021), pseudo-replication is unavoidable. In this study, replicates were at least 300 m apart, which is adequate to allow for segregated sample dispersion (sensu Krebs 1999) and avoid pseudo-replication as reported in many studies of arthropods (e.g. Hlongwane et al. 2019, but see Schmidt et al. 2008 for spiders that are passively dispersed in air for up to 3 km).
Each site was a 10 x 10 m plot with six pitfall traps installed in each corner for a sum of 24 traps per site. Adjacent pitfalls in each corner were 2 m apart. Surface-active arthropods collected using pitfall traps indicate the activity patterns rather than the population density (Samways et al. 2010). Traps from the four corners of a site were pooled to form a single sample. In addition, pooling samples from all 24 traps per site reduces catch differences that may have resulted from the spatial arrangement of each pitfall trap in a site (Brennan et al. 2005; Woodcock 2005). Moreover, the pitfall trapping method may be influenced by weather conditions (Samways et al. 2010). As such, for each site we sampled over a period of 28 days, which is expected to have varying weather patterns. The sampling over 28 days was divided into four intervals, where after 7 days pitfall traps were emptied, and new sets of traps were inserted. Samples from all four intervals in the disturbed and pristine savannah were pooled to form a single sample for each site. Samples were preserved in 70% ethanol and then sorted into morpho-species and later identified to family, subfamily and genus where possible. Ants were identified using a guide by Fisher and Bolton (2016), while guides by Bouchard (2014), Picker et al. (2019), and White (1998) were used for beetles. Spiders were also identified using many guides, including Dippenaar-Schoeman (2014), Dippenaar-Schoeman and Jocqué (1997), Filmer (2011) and Holm and Dippenaar-Schoeman (2010).
Vegetation cover was measured using a modification of Botes et al. (2006). In each corner of a 10 m x 10 m plot, three 1-m2 quadrats were placed between the two rows of the 3 x 2 m grid. Percentage ground cover of each quadrat was estimated for vegetation cover and litter cover.
Data analysis
A previous study has reported that analysis that is inclusive of all arthropods showed no effect of habitat change on species richness of arthropods, while variation in arthropod response was apparent when analyses were conducted for each taxon (Yekwayo et al. 2018). As such, to reduce the impact of the most dominant arthropod taxa on the overall dataset, we compared species richness, abundance and composition between disturbed and pristine savannah vegetation for each of the three arthropod taxa (ants, beetles and spiders). Our datasets had a high percentage of singletons and doubletons, and for spiders these contributed almost half (47%) of the total morpho-species, while beetles and ants had 28% and 27%, respectively. Barlow et al. (2010) reported that inclusion of singletons and doubletons in the analyses may not provide a true reflection of how species respond to habitat change. As such, prior to analysing our data we removed singletons and doubletons. In addition, we also removed from the analyses a single morpho-species belonging to the genus Camponotus, which contributed 78% of individuals and of which 91% of those individuals were collected from one site. This high abundance in one site may have been derived from a nest in close proximity to this site. When we compared results of analyses with inclusion and exclusion of this dominant ant species, results were similar statistically for all diversity measures (species richness, abundance and composition). In this manuscript, we present results that exclude this dominant ant species.
All analyses were conducted in R. Species richness and abundance datasets were tested for normality using the Shapiro-Wilk test. We tested our data for spatial autocorrelation using the Moran's I (Gittleman and Kot 1990; Dormann et al. 2007) and the ape package (Paradis and Schliep 2019). Moran's I results showed spatial autocorrelation for species richness of ants (p < 0.001) and beetles (p < 0.05). Similarly, spatial autocorrelations were revealed for abundance of ants (p < 0.001) and spiders (p < 0.001).
For normally distributed datasets (species richness of ants and beetles) that were spatially autocorrelated, we used the generalised least squares (GLS) regression (Dormann et al. 2007) to compare the dependent variables between disturbed and pristine savannah. To account for spatial autocorrelation on datasets that were not normally distributed (abundance of ants and spiders), we used the generalised linear mixed models (GLMM) with the penalised quasilikelihood estimation method (Dormann et al. 2007). When calculating GLMM the MASS package (Venables and Ripley 2002) and Poisson distribution (Bolker et al. 2009) were used. In our models (GLS and GLMM) we included the location (longitude and latitude) of each site as a dummy variable with an exponential correlation (Dormann et al. 2007), which was the random factor. However, the status of disturbance, percentage of leaf litter cover and percentage of vegetation cover were included as fixed factors in both GLS and GLMM.
Neither species richness of spiders (p > 0.05) nor abundance of beetles (p > 0.05) showed spatial autocorrelation. As a result, the linear model was used to determine the effect of disturbance on species richness of spiders because the data were normally distributed. However, the generalised linear model (GLM) with the negative binomial distribution in the MASS package (Venables and Ripley 2002) was used to analyse the abundance of beetles with a dataset that was not normally distributed. Boxplots were used to visualise the significant differences in species richness and abundance between disturbed and pristine savannah.
The effect of status of the savannah, percentage of leaf litter cover and vegetation cover on species composition of each taxon was determined using the manyglm function in the package mvabund (Wang et al. 2012). The negative binomial distribution was used for the GLMs for all taxa. Additionally, the Hellinger transformation from the vegan package (Oksanen et al. 2020) in R was used to create a non-metric multidimensional scaling plot for the visualisation of the species composition of ants, beetles and spiders in disturbed and pristine sites.
The indicator value analysis of the two habitats (disturbed and pristine savannah) was conducted using the function multipatt found in the indicspecies package (De Caceres and Legendre 2009). Multipatt groups species according to their association with a particular habitat, which is driven by the frequent occurrence of species and their abundance in a particular habitat type (Samways et al. 2010). Only species that are associated significantly (p < 0.05) with disturbed or pristine savannah vegetation are listed in the results of the multilevel pattern analysis.
RESULTS
In total, 308 741 specimens and 130 morpho-species of ants were identified in this study. A total of 240 790 individuals of the total abundance was from a single morpho-species (Camponotus sp.) with 219 140 individuals in this species collected from one site. Out of the total of 130 morpho-species of ants, 26 were singletons and nine were doubletons. A total of 5 013 individuals of beetles in 177 morpho-species were collected in our study. There were singletons (29) and doubletons (20) in the sample of beetles. We collected a total of 8 847 individuals in 302 morpho-species of spiders. Almost 50% (143 morpho-species) of the total morpho-species of spiders were singletons (102) and doubletons (41). The description of the results below excluded singletons and doubletons in all three taxa, as well as the dominant morpho-species in the genus Camponotus.
Out of the four subfamilies of ants collected, the Myrmicinae was the most abundant (51 900) and species rich (48) subfamily, followed by the Formicinae (9 400 individuals and 27 morpho-species). Both the Myrmicinae and Formicinae had the highest number of morpho-species and individuals in the disturbed savannah than the pristine savannah (Table S1). Although the subfamily Dorylinae had higher number of individuals (4 844) compared to the Ponerinae (1 762), the Dorylinae was the least species rich subfamily (3 morpho-species) compared to the Ponerinae (16 morpho-species). The disturbed savannah vegetation had the highest number (16) of unique morpho-species compared to the pristine savannah, which had four unique morpho-species of ants.
The most abundant families of beetles were the Scarabaeidae (3 719 individuals), Chrysomelidae (730 individuals), Carabidae (617 individuals), Elateridae (538 individuals), Curculionidae (414 individuals) and Tenebrionidae (411 individuals). Families of beetles that were represented by less than ten individuals were Anthicidae, Cantharidae, Erotylidae, Melandryidae, Meloidae, Silvanidae and Trogidae (Table S1). The most species-rich families of beetles were the Scarabaeidae (39 morpho-species), Chrysomelidae (22 morpho-species), Carabidae (13 morpho-species) and Tenebrionidae (11 morpho-species). Families of beetles that had a single morpho-species were Anobiidae, Anthicidae, Cantharidae, Erotylidae, Melandryidae, Meloidae and Trogidae (Table S1). The disturbed savannah had 16 unique morpho-species of beetles, while no unique species was recorded in the pristine savannah.
The most dominant families of spiders were the Zodariidae (2 858 individuals), Gnaphosidae (1 672 individuals), Salticidae (1 470 individuals) and Lycosidae (1 183 individuals). The least abundant families of spiders included the Microstigmatidae, Segestriidae and Sparassidae, each represented by less than ten individuals (Table S1). The Salticidae was the most species rich (38 morpho-species) family of spiders, followed by the Lycosidae (21 morpho-species), Gnaphosidae (20 morpho-species) and Zodariidae (12 morpho-species). Families of spiders with the lowest number of morpho-species (one morpho-species each) included the Caponiidae, Hahniidae, Microstigmatidae, Segestriidae and Sparassidae (Table S1). Slightly more unique morpho-species of spiders were recorded in the pristine savannah (30) compared to disturbed savannah (24).
The effect of disturbance on species richness, and abundance varied among taxa. Species richness of ants and beetles was significantly greater in the disturbed savannah compared to the pristine savannah (Table 1, Figures 2a, b). Similarly, the disturbed savannah supported greater abundance of ants and spiders (Table 1, Figures 2c, d). However, species richness of spiders and abundance of beetles were not influenced by disturbance (Table 1). Species richness of beetles and spiders, as well as abundance of ants and beetles were significantly influenced by the percentage of leaf litter cover (Table 1). However, the percentage of vegetation cover did not affect species richness nor abundance of any of the taxa (Table 1).
Species composition of all three taxa were significantly influenced by the status of the savannah (Table 2, Figure 3). Percentage of leaf litter cover significantly affected species composition of beetles and spiders only (Table 2, Figure 3). However, the percentage of vegetation cover did not affect species composition for all three taxa (Table 2).
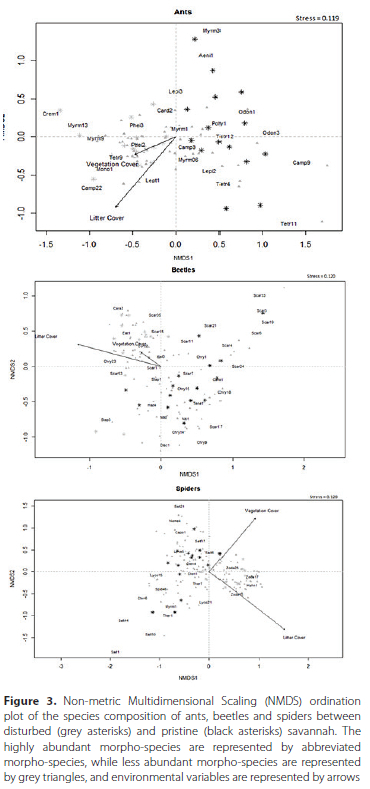
Disturbed savannah vegetation had the highest number (61) of indicator species compared to the pristine savannah, which had 25 indicator species (Table 3). Out of the three taxa, we identified a greater number (28) of ant species that were associated with the disturbed savannah, while the pristine savannah vegetation had one indicator species (Odontomachus sp. 1) (Table 3). Even though the indicator value analysis did not identify shared indicator species between pristine and disturbed savannah, a total of 74 morpho-species of ants was recorded in both habitat types.
The disturbed and pristine savannah had 14 and 13 indicator species of beetles, respectively (Table 3). There were differences in families of beetles that dominated as indicators of each type of the savannah. For example, most indicator species in the disturbed savannah came from the Carabidae, Curculionidae, Elateriade, Staphylinidae, Scarabaeidae (which was represented by a single morpho-species) and Tenebrionidae (Table 3). However, different families were identified as indicator species in pristine savannah, with the Scarabaeidae dominating (with nine morpho-species). Other families (Anobiidae, Chrysomelidae and Tenebrionidae) had a single morpho-species each (Table 3). Indicator value analysis may not have identified shared indicator species of beetles between the two habitat types because of insignificant numbers of individuals per species in each habitat type. However, 112 morpho-species of beetles were represented in both habitat types.
A total of 19 morpho-species of spiders were identified as indicators of the disturbed savannah while 11 species were associated with the pristine savannah only (Table 3). Even though some families of spiders were identified as indicators of both disturbed and pristine savannah, there were families which were unique to each type of the savannah (Table 3). There were no shared indicator species of spiders between pristine and disturbed savannah, however, 105 morpho-species were present in both habitat types.
DISCUSSION
Our results support previous studies on the importance of using a multi-taxon approach to understand the response of arthropods to changes in their environments (Gerlach et al. 2013; Yekwayo et al. 2018). Although disturbance affected species composition of all three taxa, we showed that the effect on species richness and abundance varies among taxa. Variation in species composition of ants between disturbed and pristine savannah is an indication that in our samples we may have collected different functional groups of ants, and each type of the savannah probably offered different microhabitats, thus leading to arthropod heterogeneity. For example, variation in leaf litter can affect ants differently, as Silva et al. (2011) reported that an increase in the number of leaves lead to an increase in the density of ants. However, in our study in the two environmental variables (percentage of leaf litter cover and vegetation cover) that we measured, percentage of leaf litter cover was the only variable that affected abundance of ants, while species richness and composition were not affected. Despite the fact that we did not record significant effects of the measured environmental variables, we recorded different assemblages of ants in the two types of the savannah. Previously, changes in habitat type have been reported to affect functional groups of ants differently, highlighting the different levels of specialisation (Hill et al. 2008; Santos et al. 2021). For example, more specialised ant species were negatively affected by the conversion of natural habitats to anthropogenic environments, while the opposite was true when it came to generalist and opportunistic ant species (Santos et al. 2021).
Although the level of disturbance was not categorized across sites in our study, we are of the view that the level of exposure to disturbance might not have been uniform among the sites, as such; there may have been patches of semi-natural to natural savannah within disturbed sites. Therefore, sites of the savannah that were less exposed to disturbance may have supported specialist ant species, while sites that had great exposure to disturbance could have supported generalist and opportunistic ant species. The occurrence of generalist, opportunistic and specialist ant species in the disturbed savannah could have resulted in greater species richness and abundance compared to the pristine savannah, which was probably suitable to more specialist species. Furthermore, our assumption of having sampled a greater number of generalist species and individuals than specialists is in line with Hill et al. (2008), who reported generalist and opportunistic ants as more abundant and species rich compared with specialist ants.
Our results are supported by observations of Savitha et al. (2008), who found greater species richness and abundance of ants in disturbed areas (city parks and suburbs with dry forests and a few orchards) than less disturbed sites (natural dry thorny and deciduous forests on the outskirts of the city) in Bangalore, India. Furthermore, high abundance of ants in disturbed compared to pristine savannahs in our study may indicate that human-induced activities drive species invasions and ecological change (King and Tschinkel 2008). In a large-scale experiment of ecological invasion by ants in the state of Florida, United States, King and Tschinkel (2008) found that the fire ant (Solenopsis invicta), a disturbance specialist, reduced the abundance and diversity of native ants in forest habitats after human-induced disturbance had occurred. However, in the absence of human-induced disturbance, the fire ants could not invade the forests and reduce the abundance of native ants (King and Tschinkel 2008). In addition, Berman et al. (2013) found that the invasion of yellow crazy ants (Anoplolepis gracilipes) and electric ants (Wasmannia auropunctata) in the main island of the New Caledonian archipelago was facilitated by disturbance.
Out of the 29 indicator species of ants that we identified, a morpho-species of Odontomachus was the only indicator species associated with pristine savannah, while the rest were sampled frequently in the disturbed savannah. We assume that the ground surface in the pristine savannah was less disturbed compared to the disturbed savannah. As such, the pristine savannah may have provided conducive foraging and nesting sites for Odontomachus. Odontomachus species forage and nest in leaf litter, rotten wood, as well as at the base of the trees (Fisher and Bolton 2016; Macgown et al. 2014).
The percentage of leaf litter cover significantly affected species richness, abundance and composition of beetles, even though abundance did not differ between pristine and disturbed savannah. The two types of the savannah may have provided different microhabitats, which would lead to differences in species richness and composition. Variation in species composition was evident in the results of the indicator value analysis, as 50% of the indicator species of the disturbed savannah are predators (ground and rove beetles), while 69% of the indicator species in the pristine savannah were scarabs. Species richness of predatory ground beetles may have increased in the disturbed savannah due to the association of these species with prey availability rather than specific microhabitats. That the Tenebrionidae had the highest (29%) contribution towards indicator species in the disturbed savannah could have been due to the fact that darkling beetles are scavengers that feed on a variety of food sources (Picker et al. 2019). On the other hand, frequent occurrence of scarabs in pristine savannah may be linked to greater abundance of wild grazers, which would increase dung availability. Wagner et al. (2021) reported that grazing increases species richness and abundance of dung beetles, while it alters species composition of dung beetles. Even though cattle passed through our disturbed sites infrequently, their abundance was lower than that of wild mammal grazers, such as, wildebeests in the nature reserve (pristine savannah). Pryke et al. (2016) reported that the type of grazing affects species composition and richness of dung beetles, with areas that had had wild grazers supporting greater species richness than areas that had domestic grazers. As such, the pristine savannah in our study probably had greater availability of dung, thus we recorded greater species richness of dung beetles.
Unlike the current study which investigated the overall taxa of beetles in disturbed and pristine savannah, much of the research on beetle assemblages has focused on ground beetles (Carabidae) and dung beetles (Scarabaeidae) (Hanski and Cambefort 2014; Gallé et al. 2018; Correa et al. 2019; Rahman et al. 2021). Not much data are available on overall species richness and abundance of beetles. As such, it is challenging to make comparisons among studies because of the differences in the levels of resolution, which differ from that of the present study. We identified 20 families of beetles in our study, which was much more taxa recorded than in many other studies on the composition of beetles. For example, Rahman et al. (2021) investigated the effect of disturbance on the trophic niche of six species of ground beetles in 20 tallgrass prairies and found that the response of ground beetles to disturbance varied. In particular, Rahman et al. (2021) argued that morphological traits and microhabitat preferences are important and affect how ground beetles respond to disturbance. The results from the present study suggest that overall species richness and abundance of beetles may not be an appropriate indicator of disturbance. However, reducing the number of taxa, for example, focusing on one or two families (Carabidae or Scarabaeidae), could provide more information on the possible changes in species richness and abundance resulting from habitat disturbance.
Spiders have been reported to have greater aerial dispersal because some can balloon over long distances (> 500 m; see Bishop and Riechert 1990; Schmidt et al. 2008). However, it is not possible that the similarities in species richness of spiders that we observed between pristine and disturbed savannah could be linked to their dispersal ability, as the distance between pristine and disturbed savannah was approximately > 30 km. Our study supports Moorhead and Philpott (2013), who found no significant differences in the species richness of spiders among vacant plots, community gardens and fragments of natural forests in the United States. The similarity in species richness of spiders in our study is not unusual because other studies also reported similar observations between disturbed and undisturbed habitat types (Copley and Winchester 2010; Stenchly et al. 2012; Melliger et al. 2018). The similarity in species richness of spiders in pristine and disturbed savannah may have resulted from variation in types of species (see Bell et al. 2013; Martinez et al. 2015; Swart et al. 2019; Uhey et al. 2021), which may have been due to new species colonising the disturbed habitat, thus the observed differences in species composition in our study. However, it is possible that some species of spiders in the disturbed savannah may have been represented by a greater number of individuals, thus we recorded higher abundance in disturbed than pristine savannah.
The species richness and composition of the ants, beetles and spiders can be explained in terms of the intermediate disturbance hypothesis, which suggests that species richness will increase if the disturbance is not significant enough to remove all the species in a particular site, but significant enough to allow r and K-selected species to coexist (Grime 1973; Horn 1975; Connell 1978). If disturbance results in the loss of species, perhaps through extirpation, disturbance specialists, or those with high colonising ability, may colonise the newly disturbed site rapidly (King and Tschinkel 2008). Greater colonisation by disturbance specialists results in changes in species composition but there will be no difference in the species richness of the area before and after the disturbance (Swart et al. 2019).
According to the intermediate disturbance hypothesis, moderate levels of disturbance create an environment where competitively superior species can coexist with those that are competitively inferior, resulting in high species richness (Grime 1973; Horn 1975; Connell 1978; Osman 2015). As such, the high percentage (71%) of indicator species that we recorded in the disturbed savannah compared to pristine savannah (29%) may have been due to moderate levels of disturbance. However, we did not quantify or characterise the levels of disturbance besides inferring it from observations. Previous studies have documented that species composition of surface-active arthropods is influenced by vegetation characteristics or type of vegetation (Whitmore et al. 2002; Copley and Winchester 2010; Moorhead and Philpott 2013; Hlongwane et al. 2019; Rahman et al. 2021). For example, Hlongwane et al. (2019) found that the diversity of ants in the sandstone sourveld of KwaZulu-Natal is influenced by the type of vegetation. Moreover, other studies reported also that different vegetation types support different compositions of surface-active arthropods (Mauda et al. 2018; Leonard 2021; Hoffmann et al. 2021). However, in our study none of the three taxa were significantly affected by the percentage of vegetation cover, indicating that there may be other habitat characteristics that determine assemblages of surface-active arthropods.
CONCLUSIONS
Similarities in species richness of spiders between the two types of the savannah, and greater richness of ants and beetles in disturbed than in pristine savannah was unexpected considering that pristine savannahs are structurally more complex and are assumed to provide a more diverse community of plants and microhabitats for arthropods than disturbed savannahs (Alves Da Mata and Tidon 2013; de Visser et al. 2015). However, we show that anthropogenic disturbance results in significant changes in the species composition of surface-active arthropods, and to some extent that can be related to the percentage of leaf litter cover. We show that some species of surface-active arthropods become highly abundant in habitats where there is human-induced disturbance. However, in the absence of human-induced disturbance, the disturbance specialists occur in low abundances.
The present study shows that disturbed and pristine savannahs have unique species composition of surface-active arthropods. As such, it may be essential to consider all habitat types when making efforts to conserve the biodiversity of surface-active arthropods, especially in South Africa, where a greater percentage of semi-natural habitats occur outside protected areas. This assertion is supported by the greater number of indicator species that we recorded in the disturbed than in the pristine savannah. Our results suggest strongly that disturbed habitats, where tilling no longer occurs, may be key local arthropod biodiversity hotspots in the savannah. In our study we did not quantify the levels of disturbance because of the many different types of the disturbance that we observed. Among the disturbances were the fetching of firewood and grass for thatching, hunting of birds, collecting plants for traditional medicine, fires and grazing cattle. Furthermore, there was variability in the intensity of each human activity at a particular time and area in the disturbed savannah. In future studies, characterising and examining the different disturbances individually may provide clarity on their effect on arthropods, particularly where disturbance is relatively more homogeneous than was the case at our study site.
ACKNOWLEDGEMENTS
We would like to acknowledge the Mpumalanga Tourism and Parks Agency (MTPA) for allowing us to conduct the study on their property. We thank Bongani Sibuyi, Colile Mkhatshwa, Lwazi Lubelo, Mahlatse Ngomane, Mukundi Maphiza, Nonhlanhla Sibande, Simphiwe Sibeko and Surprise Mathonsi for assisting with fieldwork. We are grateful to the National Research Foundation (Grant Number: 134117) for funding this work.
COMPETING INTERESTS
The authors declare that they have no conflict of interest
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon request.
SUPPLEMENTARY MATERIAL
Supplementary information for this article is available at https://doi.org//10.17159/2254-8854/2022/a13244
ORCID IDs
Risuna Mavasa - https://orcid.org/0000-0001-8046-120X
Inam Yekwayo - https://orcid.org/0000-0002-9722-6545
Tarombera Mwabvu - https://orcid.org/0000-0002-8947-7811
Zivanai Tsvuura - https://orcid.org/0000-0003-1852-4737
REFERENCES
Agricultural Research Council (ARC) Institute for Soil Climate and Water (ISCW). 2021. Temperature and rainfall data (unpublished). Stellenbosch: ARC-ISCW Agro-climatology division. [ Links ]
Alves da Mata R, Tidon R. 2013. The relative roles of habitat heterogeneity and disturbance in drosophilid assemblages (Diptera, Drosophilidae) in the Cerrado. Insect Conservation and Diversity. 6: 663-670. https://doi.org/10.1111/icad.12020 [ Links ]
Andersson P, Koffman A, Sjódin NE, Johansson V. 2017. Roads may act as barriers to flying insects: species composition of bees and wasps differs on two sides of a large highway. Nature Conservation 18: 47-59. https://doi.org/10.3897/natureconservation.18.12314 [ Links ]
Arribas P, Andújar C, Salces-Castellano A, Emerson BC, Vogler AP. 2021. The limited spatial scale of dispersal in soil arthropods revealed with whole-community haplotype-level metabarcoding. Molecular Ecology. 30: 48-61. https://doi.org/10.1111/mec.15591 [ Links ]
Barahona-Segovia RM, Crespin SJ, Grez AA, Veloso C. 2019. Anthropogenic thermal gradient in managed landscapes determines physiological performance and explains the edge-biased distribution of ectothermic arthropods. Forest Ecology and Management. 440: 147-157. https://doi.org/10.1016/j.foreco.2019.03.018 [ Links ]
Barlow J, Gardner TA, Louzada J, Peres CA. 2010. Measuring the conservation value of tropical primary forests: the effect of occasional species on estimates of biodiversity uniqueness. PLoS One. 5: e9609. https://doi.org/10.1371/journal.pone.0009609. [ Links ]
Bell KL, Heard TA, Manion G, Ferrier S, Van Klinken RD. 2013. The role of geography and environment in species turnover: phytophagous arthropods on a Neotropical legume. Journal of Biogeography. 40: 1755-1766. https://doi.org/10.1111/jbi.12102 [ Links ]
Berman M, Andersen AN, Ibanez T. 2013. Invasive ants as back-seat drivers of native ant diversity decline in New Caledonia. Biological Invasions. 15: 2311-2331. https://doi.org/10.1007/s10530-013-0455-6 [ Links ]
Bishop L, Riechert SE. 1990. Spider colonization of agroecosystems: mode and source. Environmental Entomology. 19: 1738-1745. https://doi.org/10.1093/ee/19.6.1738 [ Links ]
Blaum N, Seymour C, Rossmanith E, Schwager M, Jeltsch F. 2009. Changes in arthropod diversity along a land use driven gradient of shrub cover in savanna rangelands: identification of suitable indicators. Biodiversity and Conservation. 18: 1187-1199. https://doi.org/10.1007/s10531-008-9498-x [ Links ]
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White JSS. 2009. Generalized linear mixed models: a practical guide for ecology and evolution. Trends in Ecology and Evolution. 24: 127-135. https://doi.org/10.1016/j.tree.2008.10.008 [ Links ]
Botes A, Mcgeoch M, Robertson H, Van Niekerk A, Davids H, Chown SL. 2006. Ants, altitude and change in the northern Cape Floristic Region. Journal of Biogeography. 33: 71-90. https://doi.org/10.1111/j.1365-2699.2005.01336.x [ Links ]
Bouchard P. 2014. The Book of Beetles: A Life-Size Guide to Six Hundred of Nature's Gems. Chicago, USA. University of Chicago Press. [ Links ]
Brennan KE, Majer JD, Moir ML. 2005. Refining sampling protocols for inventorying invertebrate biodiversity: influence of drift-fence length and pitfall trap diameter on spiders. Journal of Arachnology. 33: 681-702. https://doi.org/10.1636/M01-105.1 [ Links ]
Bruwer IJ, Giliomee JH, Pringle KL. 2021. The relationship between proboscis length and the ability of certain Heteroptera to damage macadamia kernels. African Entomology. 29: 112-124. https://doi.org/10.4001/003.029.0112 [ Links ]
Connell JH. 1978. Diversity in tropical rain forests and coral reefs. Science. 199: 1302-1310. https://doi.org/10.1126/science.199.4335.1302 [ Links ]
Copley CR, Winchester NN. 2010. Effect of disturbance and distance from a riparian corridor on spiders in a temperate rainforest. Canadian Journal of Forest Research. 40: 904-916. https://doi.org/10.1139/X10-043 [ Links ]
Correa CM, Braga RF, Louzada J, Menéndez R. 2019. Dung beetle diversity and functions suggest no major impacts of cattle grazing in the Brazilian Pantanal wetlands. Ecological Entomology. 44: 52433. https://doi.org/10.1111/een.12729 [ Links ]
Daniel GM, Noriega JA, Da Silva PG, Deschodt CM, Sole CL, Scholtz CH. Davis AL. 2022. Soil type, vegetation cover and temperature determinants of the diversity and structure of dung beetle assemblages in a South African open woodland and closed canopy mosaic. Austral Ecology. 47: 79-91. https://doi.org/10.1111/aec.13138 [ Links ]
De Caceres M, Legendre P. 2009. Associations between species and groups of sites: indices and statistical inference. Ecology. 90: 35663574. https://doi.org/10.1890/08-1823.1 [ Links ]
De Visser SN, Freymann BP, Foster RF, Nkwabi AK, Metzger KL, Harvey AW, Sinclair ARE. 2015. Invertebrates of the Serengeti: Disturbance Effects on Arthropod Diversity and Abundance. In: Anthony, RES, Kristine, LM, Simon, ARM., John, MF, editors. Serengeti IV: Sustaining Biodiversity in a Coupled Human-natural System. Chicago, U.S.A.: University of Chicago Press; p. 265-300. [ Links ]
Dippenaar-Schoeman AS. 2014. Field guide to the spiders of South Africa. Cape Town, South Africa: Lapa. [ Links ]
Dippenaar-Schoeman AS, Jocqué R. 1997. African spiders: an identification manual. Volume 9. Pretoria, South Africa: ARC-Plant Protection Research Institute. [ Links ]
Dormann CF, Mcpherson JM, Araújo MB, Bivand R, Bolliger J, Carl G, Davies RG, Hirzel A, Jetz W, Kissling WD, Kühn I, Ohlemüller R, Peres-Neto PD, Reineking B, Schröder B, Schurr FM, Wilson R. 2007. Methods to account for spatial autocorrelation in the analysis of species distributional data: a review. Ecography. 30: 609-628. https://doi.org/10.1111/j.2007.0906-7590.05171.x [ Links ]
Eisenhauer N, Bonn A, Guerra CA. 2019. Recognising the quiet extinction of invertebrates. Nature Communications. 10. https://doi.org/10.1038/s41467-018-07916-1. [ Links ]
Filmer MR. 2011. Filmer's Spiders: an identification guide for Southern Africa. Cape Town, South Africa: Penguin Random House South Africa. [ Links ]
Fisher BL, Bolton B. 2016. Ants of Africa and Madagascar: a guide to the genera. Berkeley, U.S.A: University of California Press. [ Links ]
Gallé R, Császár P, Makra T, Gallé-Szpisjak N, Ladányi Z, Torma A, Ingle K, Szilassi P. 2018. Small-scale agricultural landscapes promote spider and ground beetle densities by offering suitable overwintering sites. Landscape Ecology. 33: 1435-1446. https://doi.org/10.1007/s10980-018-0677-1 [ Links ]
Gerlach J, Samways M, Pryke J. 2013. Terrestrial invertebrates as bioindicators: an overview of available taxonomic groups. Journal of Insect Conservation. 17: 831-850. https://doi.org/org/10.1007/s10841-013-9565-9 [ Links ]
Gittleman JL, Kot M. 1990. Adaptation: statistics and a null model for estimating phylogenetic effects. Systematic Zoology. 39: 227-241. https://doi.org/10.2307/2992183 [ Links ]
Grime JP. 1973. Competitive exclusion in herbaceous vegetation. Nature. 242: 344-347. https://doi.org/10.1038/242344a0 [ Links ]
Hanski I, Cambefort Y. 2014. Dung beetle ecology. Princeton, U.S.A: Princeton University Press. [ Links ]
Hill JG, Summerville KS, Brown RL. 2008. Habitat associations of ant species (Hymenoptera: Formicidae) in a heterogenous Mississippi landscape. Environmental Enologogy. 37: 453 -463. https://doi.org/10.1603/0046-225x(2008)37[453:haoash]2.0.co;2 [ Links ]
Hlongwane ZT, Mwabvu T, Munyai TC, Tsvuura Z. 2019. Epigaeic ant diversity and distribution in the Sandstone Sourveld in KwaZulu-Natal, South Africa. African Journal of Ecology. 57: 382-393. https://doi.org/10.1111/aje.12615 [ Links ]
Hoffmann H, Peter F, Herrmann JD, Donath TW, Diekötter T. 2021. Benefits of wildflower areas as overwintering habitats for ground-dwelling arthropods depend on landscape structural complexity. Agriculture, Ecosystems and Environment. 314:107421. https://doi.org/10.1016/j.agee.2021.107421 [ Links ]
Holm E, Dippenaar-Schoeman AS. 2010. Goggo guide: the arthropods of southern Africa. Johannesburg, South Africa: Lapa. [ Links ]
Horn HS. 1975. Markovian properties of forest succession. In: Cody, ML, Diamond, JM, editors. Ecology and Evolution of Communities. Cambridge, Massachusetts, U.S.A.: Belknap Press; p.196-211. [ Links ]
Hoye TT, Culler LE. 2018. Tundra arthropods provide key insights into ecological responses to environmental change. Polar Biology. 41: 1523-1529. https://doi.org/10.1007/s00300-018-2370-x [ Links ]
Hui FKC. 2016. BORAL-Bayesian ordination and regression analysis of multivariate abundance data in R. Methods in Ecology and Evolution. 7: 744-750. https://doi.org/10.1111/2041-210X.12514 [ Links ]
Hurlbert SF. 2004. On misinterpretations of pseudoreplication and related matters: a reply to Oksanen. Oikos 104: 591-597. https://doi.org/10.1111/j.0030-1299.2004.12752.x [ Links ]
Hussein EA, El-Ghani MM, Hamdy RS, Shalabi LF. 2021. Do anthropogenic activities affect floristic diversity and vegetation structure more than natural soil properties in Hyper-Arid desert environments? Diversity. 13. https://doi.org/10.3390/d13040157 [ Links ]
Husseini R, Abubakar A, Nasare LI. 2019. Effect of anthropogenic disturbances on insect diversity and abundance in the Sinsablegbini forest reserve, Ghana. The International Journal of Developmental Biology. 6: 2026-5336. https://doi.org/10.47740/388.UDSIJD6i [ Links ]
Hutley LB, Setterfield SA. 2018. Savanna. In: Fath, B, editor. Encyclopedia of Ecology. 2nd edition. Oxford, U.K.: Elsevier BV; p. 3143-3153. [ Links ]
Hyvárinen O, Hoffman MT, Reynolds C. 2019. Vegetation dynamics in the face of a major land-use change: a 30-year case study from semiarid South Africa. African Journal of Range and Forage Science. 36: 141-150. https://doi.org/10.2989/10220119.2019.1627582 [ Links ]
Jewitt D, Goodman PS, Erasmus BF, O'connor TG, Witkowski ET. 2015. Systematic land-cover change in KwaZulu-Natal, South Africa: implications for biodiversity. South African Journal of Science. 111: 01-09. https://doi.org/10.17159/sajs.2015/20150019 [ Links ]
Kaur H, Torma A, Gallé-Szpisjak N, Seat J, Lórinczi G, Módra G, Gallé R. 2019. Road verges are important secondary habitats for grassland. Journal of Insect Conservation 2: 899-907. https://doi.org/10.1007/s10841-019-00171-9 [ Links ]
King JR, Tschinkel WR. 2008. Experimental evidence that human impacts drive fire ant invasions and ecological change. Proceedings of the National Academy of Sciences of the United States of America. 105: 20339-20343. https://doi.org/10.1073/pnas.0809423105 [ Links ]
Krebs CJ. 1999. Ecological methodology. 2nd edition. California, U.S.A: Benjamin Cummings. [ Links ]
Lavelle P, Decaens T, Aubert M, Barot SB, Blouin M, Bureau F, Margerie P, Mora P, Rossi JP. 2006. Soil invertebrates and ecosystem services. European Journal of Soil Biology. 42: S3-S15. https://doi.org/10.1016/j.ejsobi.2006.10.002 [ Links ]
Lehmann CE, Anderson TM, Sankaran M, Higgins SI, Archibald S, Hoffmann WA, Hanan NP, Williams RJ, Fensham RJ, Felfili J. 2014. Savanna vegetation-fire-climate relationships differ among continents. Science. 343: 548-552. https://doi.org/10.1126/science.1247355 [ Links ]
Leonard EE, Mast AM, Hawkins CP, Kettenring KM. 2021. Arthropod assemblages in invasive and native vegetation of Great Salt Lake wetlands. Wetlands. 41. https://doi.org/10.1007/s13157-021-01446-1 [ Links ]
Leweri C, Ojija F. 2018. Impact of anthropogenic habitat changes on insects: a case study of mount Loleza forest reserve. International Journal of Entomology Research. 3: 36-43. [ Links ]
Macgown JOE, Boudinot B, Deyrup M, Sorger DM. 2014. A review of the Nearctic Odontomachus (Hymenoptera: Formicidae: Ponerinae) with a treatment of the males. Zootaxa. 3802: 515-552. https://doi.org/10.11646/zootaxa.3802.4.6 [ Links ]
Maleque MA, Maeto K, Ishii HT. 2009. Arthropods as bioindicators of sustainable forest management, with a focus on plantation forests. Applied Entomology and Zoology. 44: 1-11. https://doi.org/10.1303/aez.2009.1 [ Links ]
Marquart A, Geissler K, Heblach J, Lobas C, Münch E, Blaum N. 2020. Individual shrubs, large scale grass cover and seasonal rainfall explain invertebrate-derived macropore density in a semi-arid Namibian savanna. Journal of Arid Environments. 176. https://doi.org/10.1016/j.jaridenv.2020.104101 [ Links ]
Martínez E, Ros M, Bonilla MA, Dirzo R. 2015. Habitat heterogeneity affects plant and arthropod species diversity and turnover in traditional cornfields. PLoS One. 10: e0128950. https://doi.org/10.1371/journal.pone.0128950 [ Links ]
Mauda EV, Joseph GS, Seymour CL, Munyai TC, Foord SH. 2018. Changes in land use alter ant diversity, assemblage composition and dominant functional groups in African savannas. Biodiversity and Conservation. 27: 947-965. https://doi.org/10.1007/s10531-017-1474-x [ Links ]
Melliger RL, Braschler B, Rusterholz HP, Baur B. 2018. Diverse effects of degree of urbanisation and forest size on species richness and functional diversity of plants, and ground surface-active ants and spiders. PLoS One. 13. https://doi.org/10.1371/journal.pone.0199245 [ Links ]
Moorhead LC, Philpott SM. 2013. Richness and composition of spiders in urban green spaces in Toledo, Ohio. Journal of Arachnology. 41: 356-363. https://doi.org/10.1636/P12-44 [ Links ]
Mpumalanga Tourism and Parks Agency. 2019. Barberton Nature Reserve. Pii Digital. Available from: http://www.mpumalanga.com/our-provincial-parks/barberton-nature-reserve. (accessed 18 September 2021)
Mucina L, Rutherford MC. 2010. The vegetation of South Africa, Lesotho and Swaziland. Pretoria, South Africa: South African National Biodiversity Institute. [ Links ]
Mwambilwa K, Kirkman KP, Tsvuura Z. 2021. Influence of burning and defoliation on Festuca costata (Nees) in the Drakensberg. African Journal of Range and Forage Science. https://doi.org/10.2989/10220119.2021.1900394
Niemelâ J, Spence JR, Spence DH. 1992. Habitat associations and seasonal activity of ground beetles (Coleoptera, Carabidae) in central Alberta. Canadian Entomologist. 124: 521-540. https://doi.org/10.4039/Ent124521-3 [ Links ]
Oksanen J, Blanchet G, Friendly M, Kindt R, Legendre P, Mcglinn D, Minchin P, O'hara B, Simpson G, Solymos P, Stevens H, Szoecs E, Wagner H. 2020. Vegan: Community Ecology Package.
Osborne CP, Charles-Dominique T, Stevens N, Bond WJ, Midgley G, Lehmann CER. 2018. Human impacts in African savannas are mediated by plant functional traits. New Phytologist. 220: 10-24. https://doi.org/10.1111/nph.15236 [ Links ]
Osman R. 2015. The intermediate disturbance hypothesis. In: Fath, B, editor. Encyclopedia of Ecology. 2nd edition. Oxford, U.K. Elsevier BV; p. 441-450. [ Links ]
Pacheco R, Vasconcelos HL. 2012. Habitat diversity enhances ant diversity in a naturally heterogeneous Brazilian landscape. Biodiversity and Conservation. 21: 797-809. https://doi.org/10.1007/s10531-011-0221-y [ Links ]
Paradis E, Schliep K. 2019. Ape 5.0: an environment for modern phylogenics and evolutionary analyses in R. Bioinformatics. 35: 526-528. https://doi.org/10.1093/bioinformatics/bty633 [ Links ]
Picker M, Griffiths C, Weaving A. 2019. Field guide to insects of South Africa. 2nd edition. Cape Town, South Africa: Penguin Random House South Africa. [ Links ]
Pryke JS, Roets F, Samways MJ. 2016. Wild herbivore grazing enhances insect diversity over livestock grazing in an African grassland system. PloS One. 11. https://doi.org/10.1371/journal.pone.0164198 [ Links ]
Rahman AU, Jones HP, Hosler SC, Geddes S, Nelson M, Barber NA. 2021. Disturbance-induced trophic niche shifts in ground beetles (Coleoptera: Carabidae) in restored grasslands. Environmental Entomology. 50: 1075-1087. https://doi.org/10.1093/ee/nvab065 [ Links ]
Roy S, Roy MM, Jaiswal AK, Baitha A. 2018. Soil arthropods in maintaining soil health: thrust areas for sugarcane production systems. Sugar Tech. 20: 376-391. https://doi.org/10.1007/s12355-018-0591-5 [ Links ]
Rutherford MC, Powrie LW, Husted LB. 2014. Herbivore-driven land degradation: consequences for plant diversity and soil in arid subtropical thicket in south-eastern Africa. Land Degradation and Development. 25: 541-553. https://doi.org/10.1002/ldr.2181 [ Links ]
Samways MJ, Mcgeoch MA, New TR. 2010. Insect conservation: a handbook of approaches and methods. New York, U.S.A: Oxford University Press. [ Links ]
Savitha S, Barve N, Davidar P. 2008. Response of ants to disturbance gradients in and around Bangalore, India. Journal of Tropical Ecology. 49: 235-243. [ Links ]
Santos R, Dodonov P, Delabie JH. 2021. Effects of habitat conservation on ant functional groups: a global review. Sociobiology. 68: e6071. https://doi.org/10.13102/sociobiology.v68i2.6071 [ Links ]
Schmidt MH, Thies C, Nentwig W, Tscharntke T. 2008. Contrasting responses of arable spiders to the landscape matrix at different spatial scales. Journal of Biogeography. 35: 157-166. https://doi.org/10.1111/j.1365-2699.2007.01774.x [ Links ]
Seibold S, Gossner MM, Simons NK, Blüthgen N, Müller J, Ambarl D, Ammer C, Bauhus J, Fischer M, Habel JC, Linsenmair KE, Nauss T, Penone C, Prati D, Schall P, Schulze ED, Vogt J, Wóllauer S, Weisser WW. 2019. Arthropod decline in grasslands and forests is associated with landscape-level drivers. Nature. 574: 671-674. Https://doi.org/10.1038/s41586-019-1684-3 [ Links ]
Silva Da Costa MM, Schmidt FA. 2022. Gamma, alpha, and beta diversity of ant assemblages response to a gradient of forest cover in human-modified landscape in Brazilian Amazon. Biotropica. 54: 515-524. https://doi.org/10.1111/btp.13073 [ Links ]
Silva PSD, Bieber AGD, Corrêa MM, Leal IR. 2011. Do leaf-litter attributes affect the richness of leaf litter ants? Neotropical Entomology. 40: 542-457. doi.org/10.1590/S1519-566X2011000500004 [ Links ]
Simioni G, Gignoux J, Le Roux X. 2003. Tree layer spatial structure can affect savanna production and water budget: results of a 3-D model. Ecology. 84: 1879-1894. https://doi.org/10.1890/0012-9658(2003)084[1879:TLSSCA]2.0.CO;2 [ Links ]
Smith AK, Slippers B, Hurley BP, Fourie G. 2022. Diversity of Lepidoptera associated with macadamia nut damage in South Africa and development of molecular tools to monitor pest populations. Agricultural and Forest Entomology. 1-12. https://doi.org/10.1111/afe.12497
Ste-Marie E, Turney S, Buddle CM. 2018. The effect of road proximity on arthropod communities in Yukon, Canada. Arctic. 71: 89-98. https://doi.org/10.14430/arctic4702 [ Links ]
Stenchly K, Clough Y, Tscharntke T. 2012. Spider species richness in cocoa agroforestry systems, comparing vertical strata, local management and distance to forest. Agriculture, Ecosystems and Environment. 149: 189-194. https://doi.org/10.1016/j.agee.2011.03.021 [ Links ]
Stork NE. 2018. How many species of insects and other terrestrial arthropods are there on Earth? Annual Review of Entomology. 63: 31-45. https://doi.org/10.1146/annurev-ento-020117-043348 [ Links ]
Suheriyanto D, Soemarno S, Yanuwiadi B, Leksono AS, Prasetiyo DH, Permana R. 2019. Effects of season on abundance and diversity of soil arthropods in Mangli coffee plantation Kediri Regency, East Java, Indonesia. International Journal of Engineering and Technology. 8: 131-135. https://doi.org/10.14419/ijet.v8iL9.26385 [ Links ]
Swart RC, Pryke JS, Roets F. 2019. The intermediate disturbance hypothesis explains arthropod beta-diversity responses to roads that cut through natural forests. Biological Conservation. 236: 243251. https://doi.org/10.1016/j.biocon.2019.03.045 [ Links ]
Thoresen J, Vermeire ML, Venter Z, Wolfaard G, Krumins JA, Cramer M, Hawkins HJ. 2021. Fire and herbivory shape soil arthropod communities through habitat heterogeneity and nutrient cycling in savannas. Global Ecology and Conservation. 25. https://doi.org/10.1016/j.gecco.2020.e01413 [ Links ]
Uehara-Prado M, Fernandes JO, Bello AM, Machado G, Santos AJ, Vaz-De-Mello FZ, Freitas AVL. 2009. Selecting terrestrial arthropods as indicators of small-scale disturbance: a first approach in the Brazilian Atlantic Forest. Biological Conservation. 142: 1220-1228. https://doi.org/10.1016/j.biocon.2009.01.008 [ Links ]
Uhey D, Haubensak K, Hofstetter R. 2021. Mid-elevational peaks in diversity of ground-dwelling arthropods with high species turnover on the Colorado Plateau. Environmental Entomology. 50: 337-347. https://doi.org/10.1093/ee/nvaa166 [ Links ]
Van Den Berg MA, Steyn WP, Greenland J. 1999. Hemiptera occurring on macadamia in the Mpumalanga Lowveld of South Africa. African Plant Protection. 5: 89-92. [ Links ]
Vasconcelos HL, Pacheco R, Silva RC, Vasconcelos PB, Lopes CT, Costa AN, Bruna EM. 2009. Dynamics of the leaf-litter arthropod fauna following fire in a Neotropical woodland savanna. PLoS One 4. https://doi.org/10.1371/journal.pone.0007762 [ Links ]
Venables WN, Ripley BD. 2002. Statistics and computing: modern applied statistics with S. New York, U.S.A: Springer. [ Links ]
Wagner PM, Abagandura GO, Mamo M, Weissling T, Wingeyer A, BradshaW JD. 2021. Abundance and diversity of dung beetles (Coleoptera: Scarabaeoidea) as affected by grazing management in the Nebraska sandhills ecosystem. Environmental Entomology. 50: 222-231. https://doi.org/10.1093/ee/nvaa130 [ Links ]
Wakeling JL, Cramer MD, Bond WJ. 2012. The savanna-grassland 'treeline': why don't savanna trees occur in upland grasslands? Journal of Ecology. 100: 381-391. https://doi.org/10.1111/J.1365-2745.2011.01921.X. [ Links ]
Wang Y, Naumann U, Wright S, Warton D. 2012. Mvabund: an R package for model-based analysis of multivariate data. Methods in Ecology and Evolution. 3: 471-474. https://doi.org/10.1111/j.2041-210X.2012.00190.x [ Links ]
White RE. 1998. The Beetles of North America. Boston, U.S.A: Houghton Mifflin Harcourt. [ Links ]
Whitmore C, Slotow R, Crouch TE, Dippenaar-Schoeman AS. 2002. Diversity of spiders (Araneae) in a savanna reserve, Northern Province, South Africa. Journal of Arachnology. 30: 344-356. https://doi.org/10.1636/0161-8202(2002)030[0344:DOSAIA]2.0.CO;2 [ Links ]
Wigley BJ, Bond WJ, Hoffman MT. 2010. Thicket expansion in a South African savanna under divergent land use: local vs. global drivers? Global Change Biology 16: 964-976. https://doi.org/10.1111/j.1365-2486.2009.02030.x [ Links ]
Wimp GM, Martinsen GD, Floate KD, Bangert RK, Whitham TG. 2005. Plant genetic determinants of arthropod community structure and diversity. Evolution. 59: 61-69. https://doi.org/10.1111/j.0014-3820.2005.tb00894.x [ Links ]
Woodcock BA. 2005. Pitfall trapping in ecological studies. In: Leather, SR, editor. Insect Sampling in Forest Ecosystems. Oxford, U.K.: Blackwell Publishing; p. 37-57. [ Links ]
Yekwayo I, Mwabvu T. 2019. Diversity and composition of flightless arthropods on rock outcrops and adjacent vegetation in the savannah, Mpumalanga Province, South Africa. African Journal of Ecology. 57: 443-447. https://doi.org/https://doi.org/10.1111/aje.12617 [ Links ]
Yekwayo I, Pryke JS, Gaigher R, Samways MJ. 2018. Only multi-taxon studies show the full range of arthropod responses to fire. PLoS One. 13. https://doi.org/10.1371/journal.pone.0195414 [ Links ]
 Correspondence:
Correspondence:
Risuna Mavasa
Email: risunamavasa@outlook.com
Received: 10 February 2022
Accepted: 7 July 2022


















