Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a13944
RESEARCH ARTICLE
Infection of insects and persistence of Metarhizium (Hypocreales: Clavicipitaceae) species on apple bark
Letodi L. Mathulwe; Antoinette P. Malan; Nomakholwa F. Stokwe
Department of Conservation Ecology and Entomology, Faculty of AgriSciences, Stellenbosch University, Stellenbosch, South Africa
ABSTRACT
Entomopathogenic fungi (EPF) are cosmopolitan soil borne pathogens that cause epizootics in various insect orders. EPF isolates of Metarhizium brunneum and M. pinghaense have shown the potential for use as biological agents of important agricultural insect pests. The aim of the current study was to test for the persistence of M. brunneum and M. pinghaense on apple bark over a period of three weeks, under laboratory conditions. Apple bark was sprayed with conidial suspensions of both fungi, at a standard infective conidial concentration of 107 conidia/ml. The persistence, or survival, of the conidia on apple bark was measured using codling moth larvae (CM) (Cydia pomonella) and females of woolly apple aphid (WAA) (Eriosoma lanigerum) as indicator species. The results showed that conidia of M. pinghaense can induce mortality of insect pests through contact with an EPF-treated substrate, with mortality of 39% to 82% for WAA over a period of 10 days post application, and with mortality of 3% to 68% for CM over a period of 7 days, after application to apple bark. Further evaluation showed that the conidia of M. pinghaense persisted longer on apple bark, up to 63%, than did M. brunneum, up to 11%, three weeks post application of the conidial suspensions. The study provides insights into the potential persistence of fungal isolates on apple bark over time post application. Further evaluation of the persistence of the isolates on apple bark under both glasshouse and field conditions should be conducted.
Keywords: codling moth, woolly apple aphid, biological control, Metarhizium brunneum, Metarhizium pinghaense, conidia
INTRODUCTION
The soil environment is an important reservoir of a large diversity of fungal species, with various ecological functions (Shah and Pell 2003; Meyling and Eilenberg 2007). Entomopathogenic fungi (EPF) play an important role in pest insect population regulation, and are generally considered to be promising biological control agents of insect pests (Charnley and St Leger 1991; St Leger et al. 1996; Gilbert and Gill 2010). EPF are among the natural enemies that cause epizootics in arthropod species in agroecosystems, by means of inducing lethal infection in their host insects, which helps regulate pest populations (Leger et al. 1992; Roy et al. 2006; Meyling and Eilenberg 2007; Vega et al. 2009; Shahid et al. 2012).
Important EPF species with biocontrol potential are found mainly across two fungal divisions: Ascomycota and Entomophthoromycota. The fungal division, Ascomycota, contains Hypocreales fungal species, including the Metarhizium anisopliae Metch. Sorokin (Hypocreales: Clavicipitaceae) complex and the Beauveria species, of which some are commercially produced and used globally for biological control against a variety of agricultural pests (De Faria and Wraight 2007; Quesada-Moraga et al. 2007; Hatting et al. 2019). Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordycipitaceae) and M. anisopliae, which are among the most well-studied fungal species within agroecosystems, have been used to control several insect pests in some countries (Chase et al. 1986; St Leger et al. 1992; Wraight et al. 2000; Meyling and Eilenberg 2007).
The use of EPF as mycoinsecticides for insect pests is also considered as an environmentally acceptable alternative to chemical pesticides, as they tend to pose less harm to the environment (St Leger et al. 1996; Shah and Pell 2003). Their long history of use as biological control agents for controlling many pest species has been met with varying levels of success (Dedryver et al. 2010). The use of local strains of EPF against crop-damaging insects across agricultural ecosystems tends to be favoured, because the fungi are generally already adapted to the local climatic conditions (Abaajeh and Nchu 2015). EPF can cause mycosis in many different taxa of arthropods, and in almost every order of insects, as well as infect all the different life stages of the insects (Dedryver et al. 2010; Shahid et al. 2012).
Eriosoma lanigerum L. (Hausmann) (Hemiptera: Aphididae: Pemphiginae), woolly apple aphid (WAA), is an important insect pest that attacks apple trees, negatively affecting apple production on a global scale (Heunis 2001; Damavandian and Pringle 2002; Short 2003). Infestations and feeding of the root colonies of E. lanigerum on an apple root system can have a devastating effect on the plant's health, as their presence can result in damage to the root system through the formation of hypertrophic galls on the inner bark of the host plant's root system (Sandanayaka and Hale 2003; Sandanayaka et al. 2003). The formation and development of hypertrophic galls on the apple tree's root system restricts water and nutrient movement, and disrupts normal plant growth and development (Short 2003; Damavandian and Pringle 2007; Dardeau et al. 2014). Eriosoma lanigerum has also developed some level of resistance against a wide array of chemical insecticides that have been used in the past for its control (Christians 2003).
Cydia pomonella L. (Lepidoptera: Tortricidae), codling moth (CM), is a serious pest of pome fruits, with it occurring mainly in temperate areas worldwide (Vreysen et al. 2010). Females lay eggs on the fruit, leaves and twigs of their host plant. After the eggs hatch, the first-instar larvae bore into the fruit, coming to feed mainly on its inside. The last-instar larvae, having left the fruit and dropped from the host tree, tend to overwinter in a cocoon in a sheltered environment, possibly underground. Fruit infested or damaged by C. pomonella tend to drop and ripen prematurely (Pajac et al. 2011). The management of C. pomonella in orchards has relied mainly on the use of a broad range of chemical insecticides for many years (Riedl et al. 1998). However, management has shifted from the use of chemical-based pesticides only, to a more generally integrated pest management strategy, in terms of which less chemical pesticides are generally used, combined with biological control methods. The shift, which has taken place due to the development of resistance to chemical insecticides, involves the effect of chemical insecticides on the environment and on human health (Blomefield 1994; Pajac et al. 2011). The process concerned using such biological control techniques as the sterile insect technique and mating disruption, combined with employing environmentally friendly insecticides like novaluron and methoxyfenozide (Pringle et al. 2003; Vreysen et al. 2010; Odendaal et al. 2015, 2016).
Metarhizium brunneum (Petch) and Metarhizium pinghaense Chen and Guo (Ascomycota: Hypocreales: Clavicipitaceae) have both been tested and successfully used in controlling various insect pests that are present within agroecosystems. In a study conducted by Kirubakaran et al. (2018), M. pinghaense was shown to have relatively great potential for control against the rice leaf folder Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae), which is a destructive insect pest that attacks rice crops. Another study, conducted by Cossentine et al. (2010), assessed the susceptibility of apple clearwing moth, Synanthedon myopaeformis Borkhausen (Lepidoptera: Sesiidae), which is an invasive European moth species that lays its eggs on the damaged bark near graft unions and pruning cuts, to B. bassiana and M. brunneum and found that the insect pest was susceptible to infection by both EPF species.
The success of an EPF isolate in terms of managing pest insects, and in relation to its use in crop protection, partially depends on the persistence of the fungal inoculum involved, following its field application (Inyang et al. 2000). The persistence of EPF refers to the ability of the fungal isolate to remain viable in the field, on the surface where it is in contact with the host insect, for relatively long periods post application (Jaques 1983). EPF isolates that exhibit relatively long field persistence tend to have an increased probability of coming into contact with a sufficient number of target pest insects, with the isolates being capable of causing epizootics in their populations. Fungal isolates that are capable of persisting for relatively long periods are comparatively suitable for use as biological control agents (Inglis et al. 2001; Coombes et al. 2013).
The aim of the current study was to measure the persistence of M. pinghaense conidia on apple bark over time, under laboratory conditions. Results were evaluated in terms of the number of contact infections and the extent of the mortality of the insect pests involved following exposure to the apple bark treated with conidial suspensions. WAA and CM were used as indicator species for conidial persistence. The conidial persistence of M. brunneum and M. pinghaense conidia on apple bark over a period of three weeks was measured.
MATERIALS AND METHODS
Source of EPF and insects
An isolate of M. pinghaense (DO1) and of M. brunneum (3GREY) were obtained from the Stellenbosch University fungal collection. All fungal isolates were grown on Sabouraud dextrose agar (SDA), supplemented with 200 |il penicillin-streptomycin, to prevent bacterial contamination. The EPF cultures were incubated in a growth chamber, at the controlled temperature of appoximately 25 °C.
The CM last-instar larvae were obtained from stored cultures provided by Entomon Technologies (Pty) Ltd, an insect-rearing facility that is located on Welgevallen Experimental Farm in Stellenbosch, Western Cape. The larvae, which were reared from eggs, were fed on an artificial diet in a growth chamber, where they were kept under diapause conditions, with controlled temperatures of 25 °C ± 2 °C and 60% relative humidity. Once having reached the stage of last-instar larvae, the cultures were stored at temperatures of ± 6 °C, up until their use in the experimental trials. The WAA adult females, which were collected from root samples collected in an apple orchard, were kept in moist containers in the laboratory, from where they could be selected for use, as needed.
Preparation of conidial suspensions
Fungal conidia were harvested from 2-3-week-old surface cultures by scraping, using sterile surgical blades, with the collected conidia being suspended in 20 ml of sterile distilled water, supplemented with 0.05% Tween 20, in 28-ml McCartney wide-mouth glass bottles. To produce homogenous conidial suspensions, the bottles used were closed and vortex-mixed for 3 min. The suspensions produced were poured through organza fabric into a sterilised 100-ml glass beaker, so as to remove the fungal hyphae and mycelium. Following the above-mentioned procedure, the fungal concentrate in the beaker was poured back into the 28-ml McCartney bottles and vortex-mixed for 2 min. The concentrate was then used as the conidial stock for the serial dilutions (Mathulwe et al. 2021). The methods employed by Inglis et al. (2012) were followed to quantify the conidial concentration per unit volume. Serial dilutions were conducted to obtain the desired concentration of 107 conidia/ml.
Determination of conidial viability and germination
The viability of the conidia was checked to determine the number of viable conidia per unit volume of the conidial suspension, as the number present might have influenced the efficacy of the EPF isolate. The viability of conidia was determined by means of spread-plating 100 μl of the diluted conidial suspension, at a concentration of 107 conidia/ml, on three SDA plates per isolate. Each plate, after being sealed with Parafilm, was incubated in a growth chamber at a temperature of ± 25 °C. The percentage of conidia germination was examined after 24 h from 100-conidia counts conducted on the contents of each plate, which were performed under a compound microscope at 40x magnification. The conidia that had developed a germ tube were counted as viable, or living, whereas the conidia without a germ tube were counted as dead, or as nonviable (Ekesi et al. 2002; Inglis et al. 2012). The average number of the viable conidia was calculated for the three plates used, to give the viability percentage of the conidia for the fungal isolate being tested. Fungal cultures with a viability of > 85% were used in the bioassays.
Contact infections using M. pinghaense
Pieces of apple bark, harvested from apple trees, were used in the study. After autoclaving, the bark was dried, followed by pieces of it being sprayed with a double conidial concentration of 2 x 107 conidia/ml of M. pinghaense. Then, 10 ml of conidial suspensions of the normal concentration (107 conidia/ml) were transferred to centrifuge tubes, where it was centrifuged for 20 min, following which the top 5 ml of water was removed, and the remaining 5 ml water was vortex-mixed, to mix the pellet at the bottom of the centrifuge tube. The conidial suspension was then applied to the apple bark, which was then left to dry overnight. After every 24 h, 20 pieces of the bark were lightly misted with sterile water.
The persistence and contact infection of the conidia was determined by using 10 CM larvae and 10 WAA per piece of apple bark studied. The CM larvae or the adult females of the WAA were closed in a Petri dish with moistened pieces of the bark. The Petri dishes were then placed inside 2-l plastic containers, fitted with moist paper towels, and incubated in a growth chamber at 25 °C ± 2 °C. Every day, for a period of 10 days, dead insects were removed from the dishes, surface-sterilised using 70% ethanol, and placed on a water agar medium to check for mortality and overt mycosis.
Persistence of M. pinghaense and M. brunneum conidia on apple bark
The apple bark pieces were prepared as detailed above. Thirty pieces ofthe bark were then sprayed with a conidial concentration of 107 conidia/ml of M. pinghaense and M. brunneum, respectively. Thereafter, the sprayed pieces of bark were air-dried and divided into three groups, constituting 10 pieces of bark per group, for each fungal isolate. After each piece of bark from the three groups was transferred to, and sealed within, Petri dishes (9 cm diameter) they were incubated at approx. 25 °C.
Weekly, for three weeks, 10 ofthe treated bark pieces from each fungal isolate were removed from the incubator and washed, using 5 ml sterile distilled water to collect the fungal conidia from the bark. The above was done by placing each piece of bark in a 50-ml plastic Falcon centrifuge tube containing 5 ml sterile distilled water, where it was vortex-mixed for 2 min. The apple bark was then removed from the tube, with the suspension being centrifuged for a period of 15 min. Following centrifugation, the top 1 ml of supernatant was removed, and the remaining 4 ml was mixed with the centrifuged pellet.
The persistence and pathogenicity of the conidia was determined by means of using 10 CM larvae per piece of apple bark. Each of the CM larvae was dipped in the 4 ml conidial suspension and placed in a 9-cm Petri dish fitted with filter paper, moistened using distilled water. The Petri dishes were then sealed and incubated in a growth chamber at a controlled temperature of approx. 25 °C. The same process was repeated weekly, and every day, for a period of 10 days, dead insects were removed from the Petri dishes, surface-sterilised using 70% ethanol, and placed on a water agar medium, to check for mortality and overt mycosis.
Data analysis
Analysis of the collected data was done, using the statistical software, STATISTICA, version 13.5.0.17 (TIBCO Software Inc. 2018). The data obtained was analysed, using a one-way ANOVA (analysis of variance), a comparison of means, employing LSD (least significant difference) tests, and a post-hoc test (Games-Howell post-hoc test), at 95% confidence intervals.
RESULTS
Contact infection of codling moth
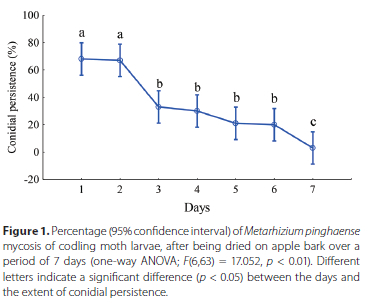
The persistence of M. pinghaense conidia on apple bark for 7 days following application is depicted in Figure 1, using CM larvae as the indicator. A significant difference in conidial persistence was observed across the 7 days of the trial (F(6,63) = 17.052, p < 0.01). No significant difference was found in the persistence of the infective conidia of M. pinghaense between the first (68% ± 13.17%) and second day (67% ± 23.59%), following application to the bark. However, a significant difference was observed between the first 2 days and days 3 to 6, as well as day 7, with a low infection rate of 3% ± 6.75%. The results showed a sharp decline of M. pinghaense conidial persistence on the bark, over the 7 days, with little infection occurring on day 7 (Figure 1).
Contact infection of the woolly apple aphid
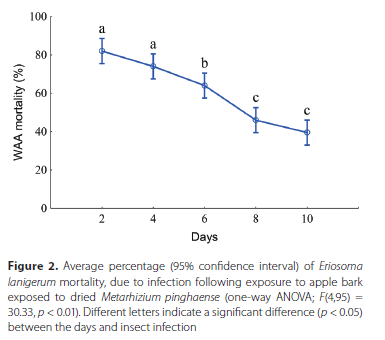
The percentage of WAA mortality, due to fungal infection, following exposure to apple bark treated with 2 x 107 conidia/ ml of M. pinghaense, for a period of 10 days, is indicated in Figure 2. The results showed a significant difference in the extent of WAA mortality over time (F(4,95) = 30.33, p < 0.01). The mortality of WAA, following exposure to apple bark treated with dried M. pinghaense, declined over time, with the highest percentage being obtained for day 2 (82% ± 15.76%), followed by the percentage obtained for day 4 (74% ± 16.67%), day 6 (64% ± 13.53%), day 8 (46% ± 15%) and day 10 (39% ± 11.91%). No significant difference was observed for mortality either between days 2 and 4, or between days 8 and 10 (p > 0.05), whereas day 6 differed significantly in mortality from all the other days (p < 0.05) forming part of the study (Figure 2).
The growth of M. pinghaense on CM larvae (Figure 3A) and on WAA adult-stage females (Figure 3B), following their exposure to apple bark treated with a concentration of 2 x 107 conidia/ml of M. pinghaense, is indicated in Figure 3.
Persistence of M. pinghaense and M. brunneum conidia on apple bark
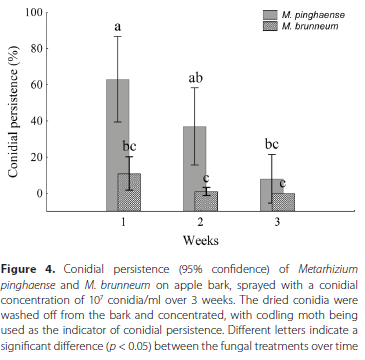
The average percentage of conidial persistence, measured by washing and concentrating the EPF conidia, and by using the infection of CM larvae as an indicator, for both M. pinghaense and M. brunneum on apple bark, showed a decline over time (Figure 4). For M. pinghaense, the results showed a significant difference in the extent of conidial persistence between the 3 weeks of the trial (F(2,27) = 9.74, p < 0.01). The first week after application showed the highest level of infection (63% ± 33.02%), which declined from the second week (37% ± 29.83%) to the third week, during which week the lowest percentage was found in conidial persistence (8% ± 18.74%). For M. brunneum, no significant difference in the average percentage of conidial persistence was observed between the 3 weeks after application (F(2,27) = 6.32, p > 0.05). A decline in persistence was observed from the first week (11% ± 12.87%) through the second week (1% ± 3.162%), with zero persistence occurring in the third week.
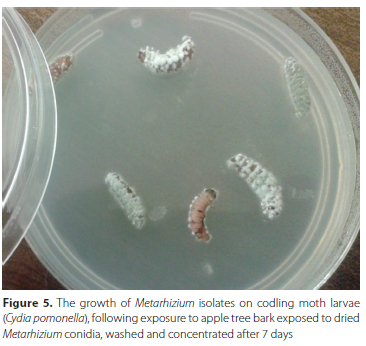
The infection of CM larvae with the Metarhizium isolates was observed by way of the hardening of the insect cadaver and by way of the growth of the hyphae and the overt mycosis taking place on the cadaver, following incubation on the water agar medium (Figure 5).
DISCUSSION
In the current study, the persistence of two EPF isolates, M. brunneum and M. pinghaense, following application to apple bark, at a standard conidial concentration of 107 conidia/ml, under laboratory conditions, was investigated. The results obtained, after washing and concentrating the conidia, showed that M. pinghaense had higher levels of conidial persistence on the apple bark over three weeks, relative to the levels obtained with M. brunneum. For both EPF isolates, the persistence of conidia on the apple bark (as indicated by CM larval infection) was also observed to decrease with an increase in the passing of time after the application. The above-mentioned results are like those that were obtained in a study conducted by Coombes (2012), assessing the persistence of fungal isolates derived from M. anisopliae and B. bassiana species, under field conditions. They found that, over time, the persistence of fungal conidia under field conditions also declined. However, the results from the current study conflict with those of Coombes (2012), who showed the extended persistence of infective EPF conidia for a minimum of 6 months in the soil, under field conditions. However, such persistence can be attributed to the high conidial concentrations used for the study involved, relative to the current study, in which only a relatively low standard concentration of 107 conidia/ml was used to test for EPF conidial persistence. Coombes (2012) also highlights that M. anisopliae-derived isolates tend to persist better under field conditions than do B. bassiana isolates. Such persistence is not exceptional, as the isolates derived from the M. anisopliae EPF species have been shown to be well adapted to surviving under fluctuating environmental conditions (Bidochka et al. 1998).
Application of M. pinghaense to apple bark has shown that the isolate might be capable of infecting the insect pests as they come into contact with the sprayed parts of the trees concerned. The fungal conidia of the species were able to persist when they were exposed on the bark at a level of > 60% for 7 days post treatment.
Although a decline in the percentage of conidial persistence was observed from 63% to 37% from week 1 to week 2, the M. pinghaense isolate offered a better chance of survival relative to M. brunneum, which did not show enhanced performance in conidial persistence, following application. Therefore, further evaluation of the conidial persistence of M. pinghaense, when applied under field conditions, should be conducted at a higher conidial concentration of > 107 conidia/ml in apple orchards. The application of M. pinghaense at a conidial concentration slightly higher than 107 conidia/ml under field conditions might serve to ensure a good probability that the insect pests will come into contact with a sufficient number of fungal conidia.
Contact infection of the WAA females, following exposure to apple tree bark treated with a concentration of 2 x 107 conidia/ml of M. pinghaense and dried over a period of 10 days, showed a decline in the persistence of the fungal species on apple bark over time, with the WAA mortality induced ranging between 82% on day 1 and 39% on day 10. The results prove that conidial persistence tends to decline over time, and that, with increased conidial concentrations, the higher are the chances of EPF persistence, and of the EPF coming into contact with a sufficient number of the target insect pest during the set period involved. Using relatively high concentrations should also help to ensure the success of the M. pinghaense isolate, in terms of causing epizootics in the pest insect's populations.
Taking into consideration that the above-mentioned results were obtained under controlled environmental conditions in the laboratory, they might be found to vary under field conditions. In the field, a variety of abiotic environmental factors, like rainfall, solar radiation, environmental temperature, wind, water availability and relative humidity, might drastically affect the efficacy of EPF against target pest insects, with them possibly affecting the persistence of the fungus over time (Inglis et al. 2001; Goble 2009). Under field conditions, the EPF have been found to be highly susceptible to the damage caused by solar radiation when they are exposed to sunlight, particularly when they are exposed to the UV-B portion (258-315 nm) of the solar spectrum, which could result in the inactivation of the fungal conidia (Inglis et al. 2001; Goble 2009). In a study conducted by Inglis et al. (1993), investigating the persistence of the EPF, B. bassiana, on the Phylloplanes of crested wheatgrass (Agropyron cristatum L. Gaertn) and alfalfa (Medicago sativa), the fungal conidia persistence was observed to decline when the conidia were exposed to solar radiation, under field conditions.
Environmental temperatures are also likely to have a significant effect on the efficacy and success of M. pinghaense against insect pests, under field conditions. Ambient temperatures are well documented as affecting the rate of infection and the time of death of insect pests that have been treated with hyphomycetous EPF, with the optimum ambient temperature requirements ranging from 20 °C to 25 °C. Temperatures of above 30 °C are likely to inhibit the vegetative growth of the EPF on infected insects (Inglis et al. 2001). Relative humidity might also affect the effectiveness of M. pinghaense conidia under field conditions, as high moisture levels are required for conidiogenesis to occur on the surfaces of the insect cuticle (Inglis et al. 2001). Reduced levels of relative humidity also tend to lower the production of conidia, thus decreasing the chances of transmission of the infective conidia from the infected insects to healthy individuals (Inglis et al. 2001; Goble 2009).
In conclusion, the results obtained through the current study provide insights into the potential persistence of fungal isolates on the bark of apple trees. Therefore, further evaluation of the persistence of the local M. pinghaense isolate on apple bark, following application under both glasshouse and field conditions, should be conducted, and the effect of various biotic factors on the persistence of the fungal conidia should be assessed. Depending on the success of M. pinghaense under field conditions, the above provides an opportunity to use the fungal isolate in the integrated pest management of insect pests, particularly those that have developed some level of resistance against a variety of chemical insecticides.
ETHICS STATEMENT
Ethical approval not required for this type of study.
ACKNOWLEDGEMENTS
The authors would like to thank DG Nel of the Centre for Statistical Consultation, Stellenbosch University for assistance with the statistical analysis.
FUNDING
This research was funded by Hort Pome, by Hort Stone (V-18-USE-PM04) and by the Technology and Human Resources for Industry Programme (THRIP grant number: TP14062571871).
COMPETING INTERESTS
The funders had no role in the design of the study, data collection, interpretation, or decision to submit the manuscript for publication.
AUTHORS' CONTRIBUTIONS
Conceptualisation: NFS, APM, LLM. Data curation: LLM. Formal analysis: NFS, APM, LLM. Funding acquisition: NFS. Investigation: LLM. Methodology: LLM. Project administration: NFS. Supervision: NFS, AP Malan. Writing - original draft: LLM. Writing - review and editing: NFS, APM.
ORCID IDs
LL Mathulwe - https://orcid.org/0000-0002-5118-3578
AP Malan - https://orcid.org/0000-0002-9257-0312
NF Stokwe - https://orcid.org/0000-0003-2869-5652
REFERENCES
Abaajeh AR, Nchu F. 2015. Isolation and pathogenicity of some South African entomopathogenic fungi (Ascomycota) against eggs and larvae of Cydia pomonella (Lepidoptera: Tortricidae). Biocontrol Science and Technology 25(7): 828-842. https://doi.org/10.1080/09583157.2015.1019831 [ Links ]
Bidochka MJ, Kasperski JE, Wild GA. 1998. Occurrence of the entomopathogenic fungi Metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Canadian Journal of Botany 76(7): 1198-1204. https://doi.org/10.1139/b98-115 [ Links ]
Blomefield T. 1994. Codling moth resistance: is it here, and how do we manage it? Deciduous Fruit Grower 44(4): 130-132. [ Links ]
Charnley AK, St Leger R. 1991. The role of cuticle-degrading enzymes in fungal pathogenesis in insects. In: Cole GT, Hoch HC, editors. The Fungal Spore and Disease Initiation in Plants and Animals. New York: Springer US; p. 267-286. [ Links ]
Chase AR, Osborne LS, Ferguson VM. 1986. Selective isolation of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae from an artificial potting medium. Florida Entomologist 69(2): 285-292. https://doi.org/10.2307/3494930 [ Links ]
Christians GE. 2003. Identification of molecular markers linked to woolly apple aphid (Eriosoma lanigerum) (Hausmann) resistance in apple. PhD dissertation, Stellenbosch University, Stellenbosch. [ Links ]
Coombes CA. 2012. Entomopathogenic fungi for control of soil-borne life stages of false codling moth, Thaumatotibia leucotreta (Meyrick) (1912) (Lepidoptera: Tortricidae). MSc dissertation, Rhodes University, Grahamstown. [ Links ]
Coombes CA, Hill MP, Moore SD, Dames JF, Fullard T. 2013. Persistence and virulence of promising entomopathogenic fungal isolates for use in citrus orchards in South Africa. Biocontrol Science and Technology 23(9): 1053-1066. https://doi.org/10.1080/09583157.2013.819489 [ Links ]
Cossentine JE, Judd GJR, Bissett JD, Lacey LA. 2010. Susceptibility of apple clearwing moth larvae, Synanthedon myopaeformis (Lepidoptera: Sesiidae) to Beauveria bassiana and Metarhizium brunneum. Biocontrol Science and Technology 20(7): 703-707. https://doi.org/10.1080/09583151003690390 [ Links ]
Damavandian MR, Pringle KL. 2002. Development of a system for sampling population levels of subterranean Eriosoma lanigerum (Homoptera: Aphididae) in apple orchards. African Entomology. 10(2): 341-344. [ Links ]
Damavandian MR, Pringle KL. 2007. The field biology of subterranean populations of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Hemiptera: Aphididae), in South African apple orchards. African Entomology. 15(2): 287-294. https://doi.org/10.4001/1021-3589-15.2.287 [ Links ]
Dardeau F, Deprost E, Laurans F, Lainé V, Lieutier F, Sallé A. 2014. Resistant poplar genotypes inhibit pseudogall formation by the woolly poplar aphid, Phloeomyzus passerinii Sign. Trees. 28(4): 1007-1019. https://doi.org/10.1007/s00468-014-1014-1 [ Links ]
De Faria MR, Wraight SP. 2007. Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biological Control. 43(3): 237256. https://doi.org/10.1016/j.biocontrol.2007.08001 [ Links ]
Dedryver CA, Le Ralec A, Fabre, F. 2010. The conflicting relationships between aphids and men: a review of aphid damage and control strategies. Comptes Rendus Biologies. 333(6): 539-553. https://doi.org/10.1016/j.crvi.2010.03.009 [ Links ]
Ekesi S, Maniania NK, Lux SA. 2002. Mortality in three African tephritid fruit fly puparia and adults caused by the entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Science and Technology. 12(1): 7-17. https://doi.org/10.1080/09583150120093077 [ Links ]
Gilbert LI, Gill SS. 2010. Insect control: biological and synthetic agents. London: Academic Press. [ Links ]
Goble TA. 2009. Investigation of entomopathogenic fungi for control of false codling moth, Thaumatotibia leucotreta, Mediterranean fruit fly, Ceratitis capitata and Natal fruit fly, C. rosa in South African citrus. PhD dissertation, Rhodes University, Grahamstown. [ Links ]
Hatting JL, Moore SD, Malan AP. 2019. Microbial control of phytophagous invertebrate pests in South Africa: current status and future prospects. Journal of Invertebrate Pathology. 165: 54-66. https://doi.org/10.1016/j.jip.2018.02.004 [ Links ]
Heunis JM. 2001. The biology and management of aerial populations of woolly apple aphid, Eriosoma lanigerum (Hausmann) (Homoptera: Aphididae). PhD dissertation, Stellenbosch University, Stellenbosch. [ Links ]
Inglis GD, Enkerli J, Goettel MS 2012. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In: Lacey LA, editor. Manual of Techniques in Invertebrate Pathology, 2nd Edition. Academic Press. London: Academic Press; p. 189-253. [ Links ]
Inglis GD, Goettel MS, Johnson DL. 1993. Persistence of the entomopathogenic fungus, Beauveria bassiana, on phylloplanes of crested wheatgrass and alfalfa. Biological Control. 3(4): 258-270. https://doi.org/10.1006/bcon.1993.1035 [ Links ]
Inglis GD, Goettel MS, Butt TM, Strasser H. 2001. Use of hyphomycetous fungi for managing insect pests. In: Butt TM, Jackson C, Magan N, editors. Fungi as Biocontrol Agents: Progress, Problems and Potential. Wallingford: CAB International; p. 23-69. [ Links ]
Inyang EN, McCartney HA, Oyejola B, Ibrahim L, Archer SA. 2000. Effect of formulation, application and rain on the persistence of the entomogenous fungus Metarhizium anisopliae on oilseed rape. Mycological Research. 104(6): 653-661. [ Links ]
Jaques RP. 1983. The potential of pathogens for pest control. Agriculture, Ecosystems & Environment. 10(2): 101-126. [ Links ]
Kirubakaran SA, Abdel-Megeed A, Senthil-Nathan S. 2018. Virulence of selected indigenous Metarhizium pingshaense (Ascomycota: Hypocreales) isolates against the rice leaf folder, Cnaphalocrocis medinalis (Guenée) (Lepidoptera: Pyralidae). Physiological and Molecular Plant Pathology. 101: 105-115. https://doi.org/10.1016/j.pmpp.2017.06.004 [ Links ]
Mathulwe LL, Malan AP, Stokwe NF. 2021. Laboratory screening of entomopathogenic fungi and nematodes for pathogenicity against the obscure mealybug, Pseudococcus viburni (Hemiptera: Pseudococcidae). Biocontrol Science and Technology. 32(4): 397-417. https://doi.org/10.1080/09583157.2021.2010653 [ Links ]
Meyling NV, Eilenberg J. 2007. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: potential for conservation biological control. Biological Control. 43: 145-155. https://doi.org/10.1016/j.biocontrol.2007.07.007 [ Links ]
Odendaal D, Addison MF, Malan AP. 2015. Control of codling moth (Cydia pomonella) (Lepidoptera: Tortricidae) in South Africa with special emphasis on using entomopathogenic nematodes. African Entomology. 23(2): 259-274. [ Links ]
Odendaal D, Addison MF, Malan AP. 2016. Control of diapausing codling moth, Cydia pomonella (Lepidoptera: Tortricidae) in wooden fruit bins, using entomopathogenic nematodes (Heterorhabditidae and Steinernematidae). Biocontrol Science and Technology. 26(11): 1504-1515. https://doi.org/10.1080/09583157.2016.1217393 [ Links ]
Pajac I, Pejic I, Baric B. 2011. Codling moth, Cydia pomonella (Lepidoptera: Tortricidae) -major pest in apple production: an overview of its biology, resistance, genetic structure and control strategies. Agriculturae Conspectus Scientificus. 76(2): 87-92. [ Links ]
Pringle KL, Eyles DK, Brown L. 2003. Trends in codling moth activity in apple orchards under mating disruption using pheromones in the Elgin area, Western Cape Province, South Africa. African Entomology. 11(1): 65-75. [ Links ]
Quesada-Moraga E, Navas-Cortés JA, Maranhao EA, Ortiz-Urquiza A, Santiago-Álvarez C. 2007. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycological Research. 111(8): 947-966. https://doi.org/10.1016/j.mycres.2007.06.006 [ Links ]
Riedl H, Blomefield TL, Giliomee JH. 1998. A century of codling moth control in South Africa: II. Current and future status of codling moth management. Journal of the Southern African Society for Horticultural Sciences. 8: 32-54. [ Links ]
Roy HE, Steinkraus DC, Eilenberg J, Hajek AE, Pell JK. 2006. Bizarre interactions and endgames: entomopathogenic fungi and their arthropod hosts. Annual Review of Entomology. 51: 331-357. https://doi.org/10.1146/annurev.ento.51.110104.150941 [ Links ]
Sandanayaka WRM, Hale CN. 2003. Electronically monitored stylet penetration pathway of woolly apple aphid, Eriosoma lanigerum (Homoptera: Aphididae), on apple (Malus domestica). New Zealand Journal of Crop and Horticultural Science. 31(2): 107-113. [ Links ]
Sandanayaka WRM, Bus VGM, Connolly P, Newcomb R. 2003. Characteristics associated with woolly apple aphid Eriosoma lanigerum, resistance of three apple rootstocks. Entomologia Experimentalis et Applicata. 109(1): 63-72. [ Links ]
Shah PA, Pell JK. 2003. Entomopathogenic fungi as biological control agents. Applied Microbiology and Biotechnology. 61(5): 413-423. https://doi.org/10.1007/s00253-03-1240-8 [ Links ]
Shahid AA, Rao QA, Bakhsh A, Husnain T. 2012. Entomopathogenic fungi as biological controllers: new insights into their virulence and pathogenicity. Archives of Biological Sciences. 64(1): 21-42. https://doi.org/10.2298/ABS1201021S [ Links ]
Short BD. 2003. Inaugural studies of the life history and predator/prey associations of Heringia calcarata (Loew) (Diptera: Syrphidae), a specialist predator of the woolly apple aphid, Eriosoma lanigerum (Hausmann) (Homoptera: Eriosomatidae). PhD dissertation, Virginia Polytechnic Institute and State University, Blacksburg, VA, USA. [ Links ]
St Leger RJ, Frank DC, Roberts DW, Staples RC. 1992. Molecular cloning and regulatory analysis of the cuticle-degrading-protease structural gene from the entomopathogenic fungus Metarhizium anisopliae. European Journal of Biochemistry. 204(3): 991-1001. https://doi.org/10.1111/j.1432-1033.1992.tb16721.x [ Links ]
St Leger RJ, Joshi L, Bidochka MJ, Roberts DW. 1996. Construction of an improved mycoinsecticide overexpressing a toxic protease. Proceedings of the National Academy of Sciences. 93(13): 63496354. https://doi.org/10.1073/pnas.93.13.6349 [ Links ]
TIBCO Software Inc. 2018. STATISTICA (data analysis software system), version 13.5.0.17. TIBCO Software Inc., Palo Alto, CA, USA.
Vega FE, Goettel MS, Blackwell M, Chandler D, Jackson MA, Keller S, Pell JK. 2009. Fungal entomopathogens: new insights on their ecology. Fungal Ecology. 2(4): 149-159. https://doi.org/10.1016/j.funeco.2009.05.001 [ Links ]
Vreysen MJB, Carpenter JE, Marec F. 2010. Improvement of the sterile insect technique for codling moth Cydia pomonella (Linnaeus) (Lepidoptera Tortricidae) to facilitate expansion of field application. Journal of Applied Entomology. 134(3): 165-181. https://doi.org/10.1111/j.1439-0418.2009.01430.x [ Links ]
Wraight SP, Carruthers RI, Jaronski ST, Bradley CA, Garza CJ, Galaini-Wraight S. 2000. Evaluation of the entomopathogenic fungi Beauveria bassiana and Paecilomyces fumosoroseus for microbial control of the silverleaf whitefly, Bemisia argentifolii. Biological Control. 17(3): 203-217. https://doi.org/10.1006/bcon.1999.0799 [ Links ]
 Correspondence:
Correspondence:
LL Mathulwe
Email:lukimathulwe@gmail.com
Received:15 May 2022
Accepted: 9 July 2022














