Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a11734
RESEARCH ARTICLE
Efficacy of Beauveria bassiana against adults of Prostephanus truncatus (Horn), Sitophiluszeamais Motschulsky and Teretriusnigrescens Lewis in stored maize
MA AcheampongI; EW CorneliusI; VY EziahI; KO FeningII; KO OforiI; C StormIII; N JessopIV; B LukeV; D MooreVI; VA ClotteyVII; O PotinVIII; P GrammareVIII
IDepartment of Crop Science, University of Ghana, Legon, Accra, Ghana
IISoil and Irrigation Research Centre, University of Ghana, Accra, Ghana
IIIExosect Ltd, Colden Common, Winchester, UK
IVMagGrow, Somerford Keynes, Cirencester, UK
VCABI Europe-UK, Egham UK
VIWoodcote Road, Reading, RG47EY, UK
VIICABI, West Africa, Cantonments, Accra, Ghana
VIIIAgrauxine, S.A. 49070 Beaucouze, France
ABSTRACT
The larger grain borer, Prostephanus truncatus (Horn) and the maize weevil, Sitophilus zeamais Motschulsky continue to cause tremendous losses to stored maize. Research in the UK has identified Beauveria bassiana, IMI 389521 as a suitable control agent for grain storage pests in the UK. The pathogenicity of B. bassiana, IMI 389521 was evaluated against adult P. truncatus, S. zeamais and Teretrius nigrescens in Ghana. Fifty adults of each insect species were treated with 0.5 g dry conidia powder of this isolate at 8.65 x 108 conidia/g for 1 minute and mortality recorded daily for 14 days. The results indicated that B. bassiana, is pathogenic against P. truncatus and S. zeamais, inducing over 90% mortality by day 7. Teretrius nigrescens was, however less susceptible to the fungus with 30% mortality. To determine the most effective concentration of B. bassiana for the control of P. truncatus, a laboratory dose response experiment using four concentrations of B. bassiana (108-1011 cfu/kg maize) was also conducted. Maize grains (250 g) in separate jars were treated with the four concentrations of the product. Fifty adults of P. truncatus were placed into the jars containing the treated maize and mortality was assessed weekly for 3 weeks. The most effective dose was 1010 cfu/kg maize, which resulted in 96% and 100% mortality of P. truncatus after 2 and 3 weeks, respectively. This study shows that B. bassiana could effectively be integrated into bio-control programme of these two key pests of maize in Ghana after further field trials.
Keywords: histerid beetle, larger grain borer, maize storage, maize weevil, microbial control
INTRODUCTION
Maize is the most important staple food in Ghana. Its production, however, is constrained by low yields and high postharvest losses (MiDA 2010). Insect pests contribute about 20-50% of postharvest losses of stored maize in Ghana (Anankware et al. 2012). Several species of insect pests infest stored maize in Ghana. Some of these include the maize weevil, Sitophilus zeamais Motschulsky (Coleoptera: Curculionidae), the red flour beetle, Tribolium castaneum Herbst (Coleoptera: Tenebrionidae), the Angoumois grain moth, Sitotroga cereallela (Olivier) (Lepidoptera: Gelechiidae) and the larger grain borer (LGB), Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) (Obeng-Ofori 2008). The two most destructive species, however, are S. zeamais and P. truncatus (Vowotor et al. 2005; Obeng-Ofori 2008).
Prior to the introduction of the larger grain borer, P. truncatus, S. zeamais was the most important pest of maize in Africa (Arbogast and Mullen 1990), causing about 7-20% weight loss of stored maize in Ghana (Hall 1970). The introduction of P. truncatus has, however, tremendously increased postharvest losses and has outcompeted the former due to its tolerance to dry conditions and ability to breed in grains with lower moisture content compared to S. zeamais (Obeng-Ofori 2008; CABI 2019).
Prostephanus truncatus is an occasional important pest of stored maize in its native regions: Central America, tropical South America and extreme South of USA (Boxall 2002; CABI 2019). This pest was accidentally introduced into Africa, likely through importation of infested maize grains (Harnisch and Krall 1984), and has spread across the continent (Borgemeister et al. 2003; Gueye et al. 2008). Currently, this pest is a collective problem in 20 African countries (CABI 2019) and may establish in all countries in Sub-Saharan Africa if preventive measures are not put in place (Bergvinson and Garcia-Lara 2011). In Tanzania, where this pest was first reported (Borgemeister et al. 2003), on-farm storage losses of up to 34 and 70% over a 3 month and 4 months storage period have been reported for maize and dried cassava chips, respectively (Hodges et al. 1983; Hodges et al. 1985). It is currently the major storage pest of the two crops in Ghana (Obeng-Ofori 2008; Asante 2013) and Sub-Saharan Africa (Holst and Meikle 2003; Phiri and Otieno 2008). Some of the improved varieties of maize widely grown in Ghana including, 'Obatanpa' and 'Okomasa' with greater yields (Abdoulaye et al. 2012) are equally susceptible to this pest (Golob et al. 1999), therefore, suggesting an urgent need to search for an alternative, sustainable and environmentally friendly control measure.
Insect pests of stored produce are currently controlled using chemical insecticides (Obeng-Ofori 2011). In Ghana, broad spectrum organophosphate-pyrethroid insecticides such as pirimiphos-methyl dust and fenvalerate are usually used as protectant for stored produce (Ogbonna et al. 2014). This practice is no longer desirable due to the high cost of control, development of insecticide resistance and environmental and food safety concerns.
This necessitated the search for a biocontrol agent against this destructive pest. The histerid beetle, Teretrius nigrescens Lewis (Coleoptera: Histeridae) obtained from its native Central America, has been identified to be the most effective predator, strongly attracted to aggregation pheromones of male P. truncatus (Boye 1988; Rees et al. 1990; Scholz et al. 1998) and reducing the population of its prey under both laboratory and field conditions (Poschko 1993; Bonu-Ire 2001; Schneider et al. 2004; Bonu-Ire 2015; Muatinte and Cugala 2015). Adults and larvae of this predator feed on eggs, larvae (Poschko 1993) and occasionally adults (Bonu-Ire 2015) of P. truncatus. The wide distribution of T. nigrescens in Central America is believed to be responsible for the low pest status of the prey in its native region (Omondi et al. 2011). Accordingly, classical biological control of P. truncatus in the field using T. nigrescens has been introduced in several African countries particularly, West and East African countries; Beginning with Togo in 1991 (Biliwa et al. 1992), Ghana in 1994 (Compton and Ofosu 1994) and Kenya in 1996 (Giles et al. 1996). Notwithstanding the introduction of this predator, P. truncatus is still causing tremendous losses to stored maize in Ghana (Birkinshaw and Hodges 2000; Birkinshaw et al. 2002). Teretrius nigrescens is believed to be undergoing difficulty in establishing in Ghana (Boateng 1996). This has been partly attributed to indiscriminate bushfires (Boateng 1996; Asante 2013). Teretrius nigrescens is also known to feed on commodities with high starch content, for example wheat, sorghum and dried cassava (Poschko 1993; Bonu-Ire 2015) hence may not be entirely beneficial (Anankware et al. 2012). Holst and Meikle (2003) in a review of the impact of T. nigrescens as biocontrol agent against P. truncatus in West Africa concluded that, control using this predator might fail, as a result of the intra-specific density dependence and low population growth rate of T. nigrescens in comparison to P. truncatus. A more efficacious alternative to T. nigrescens is urgently needed. There is, therefore, a growing interest in the use of microbial pest control agents (MPCAs) as alternatives to complement other integrated pest management measures in order to improve food security in Ghana.
One such MPCA, is the entomopathogenic fungus, Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Clavipitaceae) which is a soil-borne natural enemy, used in the control of important agricultural arthropod pests globally (de Faria and Wraight 2007; van Lenteren et al. 2018; Hatting et al. 2019). Several studies have confirmed the infectivity of strains of this fungus against many storage insect pests as well as the larger grain borer (Bourassa et al. 2001; Cherry et al. 2005; Smith et al. 2006; Dhuyo and Selman 2007; Sedehi et al. 2014; Osipitan et al. 2015; Athanassiou et al. 2017; Batta 2018; Ak 2019). Even though T. nigrescens adults are mildly susceptible to B. bassiana, they are more resistant compared to P. truncatus (Bourassa et al. 2001; Dhuyo and Selman 2007; Nboyine et al. 2015). Notwithstanding, the susceptibility of T. nigrescens to B. bassiana, Dhuyo and Selman (2007) suggested that both B. bassiana and T. nigrescens could be used together for greater control of the larger grain borer.
Research in the United Kindgom (UK) has identified B. bassiana, IMI 389521 as a suitable control agent for grain storage pests in the UK (Cox et al. 2004; Wakefield et al. 2013). Although, high efficacies of this isolate against storage beetles and others, especially P. truncatus and S. zeamais, have been reported in the European Union (EU), its pathogenicity against storage pests especially, P. truncatus and S. zeamais under tropical climatic conditions, such as Ghana, is unknown.
Evaluation of the infectivity of B. bassiana, IMI 389521 for use on both P. truncatus and T. nigrescens in Ghana, is also particularly important since T. nigrescens is being reared for mass release in fields and storage barns across several regions, where the larger grain borer is still a major problem. Although no follow up study has been done to determine the establishment of T. nigrescens in maize storage barns in Ghana, this predator could come into contact with the proposed fungal strain (B. bassiana IMI 389521) when used in storage protection of grains. Thus, the susceptibility of T. nigrescens to the proposed fungal strain could be detrimental to the biocontrol programme of P. truncatus with this predator. This paper therefore determined the pathogenicity of B. bassiana, IMI 389521 to P. truncatus, S. zeamais and T. nigrescens in the laboratory. To determine the most effective concentration of B. bassiana, IMI 389521 for the control of P. truncatus in a semi-field trial, a laboratory dose response experiment using four concentrations of B. bassiana product (1 x 108 to 1 x 1011 cfu/kg maize) was also studied.
MATERIALS AND METHODS
Study site
The two studies were conducted in the Entomology laboratory of the Department of Crop Science, University of Ghana, Legon. The temperature and relative humidity (RH) in the laboratory averaged 27 ± 2 °C and 67 ± 5% RH respectively.
Source of insects, maize and fungal isolate for the study
Adult insects for both trials (unsexed P. truncatus, S. zeamais and T. nigrescens) were obtained from a continuous rearing culture established at the Plant Protection and Regulatory Service Directorate (PPRSD) of the Ministry of Food and Agriculture (MoFA), Accra. Insects were reared at 28.6 °C, 65.6% RH and 12L:12D photoperiod (Bonu-Ire 2001) in the Entomology laboratory of the Bio-control Unit, of PPRSD, MoFA, Accra. Prostephanus truncatus and S. zeamais were cultured on whole maize grains, while T. nigrescens was cultured on a mixture of maize grains, larvae, eggs and adults of P. truncatus. Insects used were approximately one week post adult eclosion.
Untreated (insecticide and fungicide-free) healthy grains of maize variety 'Obaatanpa' with an average moisture content of 11% were purchased from farmers during the harvesting period (August-September) in Accra. The moisture content was determined using a digital grain moisture meter (Protimeter Grainmaster I, Merlin Lazer Ltd, UK).
Dry conidia of B. bassiana, IMI 389521 (8.7 x 108 conidia /g) and formulated dry conidia powder (1 x 108, 1 x 109, 1 x 1010 and 1 x 1011 cfu/kg maize) were obtained from Agrauxine, France and stored at -4 °C in a refrigerator prior to use. This isolate was originally obtained from an infected Sitophilus oryzae (Linnaeus) (Coleoptera: Curculionidae) on stored wheat in a UK grain store. Molecular identification of the species of this isolate was provided in Luke (2014). Formulated conidia contained Entostat™ (Exosect*, Winchester, U.K.) and kaolinite. Entostat is an electrostatically charged powder made from Brazilian Carnauba palm, Copernica martius (Palmae) which aids in the adhesion and dispersal onto a target surface such as grain or insect cuticle (Wakefield et al. 2010).
Insect removal from maize
Maize grains were stored in a freezer, at -4 °C for 48 hours. The maize was then removed, oven dried for 24 hours at 50 °C and left for a day to cool. This was done to kill residual insects in the grain.
Determination of pathogenicity of B. bassiana, IMI389521 against P. truncatus, S. zeamais andT. nigrescens
A factorial treatment combinations of dry conidia powder of B. bassiana, IMI 389521 and a control of no B. bassiana added, and three insect species. (P. truncatus, S. zeamais, T. nigrescens) were used in a completely randomised design. Dry conidia powder ofB. bassiana IMI 389521 applied at 8.65 x 108 conidia/g was used to determine its pathogenicity against the three insect species.
A total of 500 unsexed adults of P. truncatus were introduced into 10 Kilner jars (500 ml each containing 50 insects). Five 500 ml Kilner jars (5 replicates) were each treated with 0.5 g of the conidia powder of B. bassiana and 50 adult P. truncatus introduced into each jar and left to stand for 1 minute. The contents of the jars were then emptied into sterilised Petri dishes and insects removed and placed into 9 cm diameter plastic Petri dishes containing 7 g of broken maize for feeding and were incubated under room temperature. A control treatment which comprised five clean, sterilised jars containing the same number of insects without treatment with B. bassiana conidia powder were handled in a similar manner. This procedure was repeated for S. zeamais and T. nigrescens. Bioassays for all three insect species were conducted concurrently. Temperature and relative humidity were recorded daily using a data logger (EL-USB-2, Lascar Electronics Ltd UK). Mortality was recorded daily for 14 days and individual insects that remained immobile following probing with a blunt probe were considered dead. To determine whether mycosis was the cause of death, cadavers of the three insect species, including those of the control were surface sterilised in 2% sodium hypochlorite for 1 minute, followed by two rinses in sterile distilled water. Cadavers were then transferred onto Petri dishes with Whatman filter papers moistened with 1 ml sterile water, left at 27 ± 2 °C, 67 ± 5% RH and examined for external growth of fungus (Acheampong et al. 2016).
Determination of concentration of B. bassiana, IMI 389521 for the protection of stored maize against P. truncatus
A completely randomised design was used with 5 treatments (P. truncatus infested with four concentrations (1 x 108, 1 x 109, 1 x 1010 and 1 x 1011 cfu/kg maize) of formulated B. bassiana, IMI 389521 conidia powder and a negative control where no B. bassiana was added with five replicates.
Maize (1.25 kg) was treated with 4.5 g of the highest concentration (1 x 1011 cfu/kg maize) of B. bassiana and divided into five lots of 250 g each contained in a 1000 ml Kilner jar. For the other concentrations, 1.25 kg of maize each was mixed thoroughly with 3.2 g of B. bassiana at 1 x 1010 cfu/kg maize, 3 g of B. bassiana at 1 x 108 and quantity 1 x 109 cfu/ kg maize and divided into five equal parts (250 g) representing five replications. There was a negative control in which no B. bassiana product was added. The grains were left for 24 hours after which 50 unsexed adults of P. truncatus were introduced into each jar. The treatments were left at 27 ± 2 °C, 67 ± 5% RH in the laboratory. The temperature and relative humidity in the laboratory was monitored using a data logger (EL-USB-2, Lascar Electronics Ltd, UK). Mortality of P. truncatus was recorded at 7-day intervals for three weeks by emptying each jar onto laboratory trays. On each assessment day, the grains, live insects and powder were placed back into their respective jars after inspection. Dead insects, however, were removed and the cause of death was ascertained using the same procedure described above. On day 21, grains were sieved to separate grain powder from the kernel. Maize grains (500) were randomly selected from each jar. The number of damaged (grains with holes) and undamaged grains from this sample were separated, counted and weighed. Subsequently percent weight loss was determined using the count and weigh method (Boxall 1986).
Data analysis
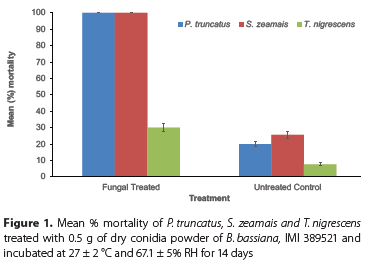
The total number of dead insects in B. bassiana and control treatments were compared using student t-test for the pathogenicity test. To compare the mortality of the three insects, percent mortality data was arcsine transformed and subjected to analysis of variance (ANOVA) to give the back transformed means presented in Figure 1. Cumulative percentage mortality data from the dose response bioassay were corrected for the corresponding control mortality using Abbott's formula (Abbott 1925), arcsine transformed, and subjected to ANOVA. The least significant difference (LSD5%) was used to compare means and this was accomplished using Genstat statistical software (12th edition).
RESULTS AND DISCUSSION
Pathogenicity of B. bassiana, IMI 389521 to P. truncatus, S. zeamais and T. nigrescens
The mortality of the treated insects was significantly higher than the untreated insects (t(28) = 6.33, p < 0.001). Analysis of a 14-day mean (%) mortality data revealed that, main effects as well as associated interaction were significant (p < 0.05) (Figure 1). There was a sharp increase in mean percentage mortality of P. truncatus and S. zeamais treated with B. bassiana conidia powder, with over 90% mortality by day 7 (data not shown), reaching 100% in day 14 (Figure 1). The mean percentage mortality of T. nigrescens, however, increased slightly to just 30% by the 14th day, when treated with the dry conidia powder of B. bassiana. The mean percentage mortality of control S. zeamais was slightly higher than the control P. truncatus. However, the mortality of treated P. truncatus and S. zeamais were the same. About 97% mycosis was observed on cadavers of the three insect species treated with B. bassiana and none on the controls, confirming fungal mycosis as cause of death. External sporulation on incubated cadavers commenced three days after incubation for all the three insect species and completely covered the cadavers after a week (Plate 1).
The results obtained from the pathogenicity study, indicates that Beauveria bassiana, IMI 389521 is pathogenic against adults of P. truncatus and S. zeamais. This result is consistent with the findings of Karanja et al. (unpubl.) who observed 100% and over 90% mortality of P. truncatus and S. zeamais, respectively, with over 96% kill of the former after 7 days of exposure to this same isolate in Tanzania. Smith et al. (1998) and Kassa (2002) also showed that isolates of B. bassiana that were most virulent to S. zeamais were also highly pathogenic to P. truncatus, with P. truncatus being the most susceptible. Similarly, Adane et al. (1996) demonstrated the virulence of a dry formulation of B. bassiana isolate against adults of S. zeamais in the laboratory. The most virulent isolate in their study, B. bassiana, s.l. (T190-520) at all concentrations tested (3.54 x 108, 1.77 x 109 and 3.54 x 109 conidia/25 g maize seeds) induced 100% mortality within 14 days with over 70% mortality by the 5th day. The pathogenicity study also revealed that T. nigrescens was rather less susceptible to the fungus. The resistance of adults T. nigrescens to B. bassiana relative to its prey (P. truncatus) has been previously reported (Bourassa et al. 2001; Dhuyo and Selman 2007; Nboyine et al. 2015). Although the susceptibilities of both P. truncatus and T. nigrescens are dependent on the isolate of B. bassiana used, around twofold (Bourassa et al. 2001), fourfolds and up to eightfolds (Nboyine et al. 2015) more P. truncatus are killed compared to T. nigrescens at the same conidia quantity and concentration. Following the results obtained in this study, Nboyine et al. (2015) assessed the compatibility of B. bassiana, IMI 389521 and T. nigrescens against P. truncatus in stored maize and recommended this isolate at 1 x 109 cfu/kg maize in storage systems where T. nigrescens is already well established as mortality of this predator was eightfolds less than its prey at this concentration.
Entomopathogenic fungi (the only exception being Microsporidia) infect their host by contact (Shah and Pell 2003). Infection by these fungi generally involves three steps: (a) adherence of infective propagules (for example conidia) on to host cuticle (b) germination and penetration under suitable temperature and humidity (c) proliferation within the host and sporulation on the surface of cadavers (Shah and Pell 2003; Boomsma et al. 2014). Host insect death generally resulted from massive fungal biomass due to proliferation, extensive tissue damage in addition to toxic metabolites produced by some species, for example B. bassiana (Shah and Pell 2003; Boomsma et al. 2014). The adherence and germination of conidia on the insect cuticle, which are key determinants in the initial infection process, were not determined in this study. However, we speculate this as one possible reason for the differences in susceptibilities of the three insect species (particularly P. truncatus and T. nigrescens) used in the present study. Wakefield (2006), for instance, using dry conidia powder of B. bassiana, IMI 389521 found Oryzaephilus surinamensis (Linnaeus) (strain Tram) (Coleoptera: Silvanidae) to be the most susceptible, followed by Sitophilus granarius (Linnaeus) (strain Windsor) (Coleoptera: Curculionidae) with Tribolium confusum Jacquelin du Val (strain W44) (Coleoptera: Tenebrionidae), being the most resistant to this fungus at the same conidial concentration. In the same study and using a scanning electron microscope, quantitative and qualitative differences in conidia adherence and germination on host cuticle were found between species O. surinamensis and T. confusum. At 24, 48 and 72 hours post treatments, a greater number of conidia, out of which many had germinated were found on the most susceptible species, O. surinamensis at all body areas examined, compared to T. confusum. The greater number of setae particularly on the ventral abdomen of the most susceptible species, potentially retaining high moisture on the cuticle is believed to have enhanced conidial adherence and germination, hence succumbing to infection (Wakefield 2006). Prostephanus truncatus has a heavily tuberculated body surface (prothorax and elytra) (CABI 2019), while T. nigrescens has a compact body (P5schko 1994) with an unusually hard, waxy cuticle (Boateng 1996), which may have prevented conidia adherence and subsequent penetration of its cuticle, consequently making it less susceptible to the dry conidia powder of this fungus. The results from this study suggest that B. bassiana, IMI 389521 poses no serious threat to T. nigrescens release programme in Ghana.
Response of P. truncatus to four concentrations (cfu/kg maize) of B. bassiana, IMI 389521 in the laboratory
Table 1 shows the cumulative percentage mortality of P. truncatus over a 21 day period. The two higher concentrations showed a good control of P. truncatus. Sporulation of B. bassiana on cadavers from treatments, excluding the control, confirmed mycosis as the cause of death. Mortality of P. truncatus increased with increasing days of exposure for all concentrations (Table 1). Maize grains treated with B. bassiana, IMI 389521 at 1010 and 1011 (cfu/kg maize) resulted in significantly (p < 0.05) higher mortality of adult P. truncatus compared to 108 and 109 (cfu/ kg maize). For these concentrations (1010 and 1011), mortality of P. truncatus was 65.3% and 68.9%, respectively, after 7 days of exposure reaching 95.7% and 98.7% by day 14, and finally inducing 100% mortality by day 21 (Table 1). By contrast, mortality of adult P. truncatus in B. bassiana, IMI 389521 treated grains at 108 and 109 (cfu/kg maize) were less than 13% after 7 days of exposure and remained less than 20%, for 21 days after exposure. Although, B. bassiana at 1010 and 1011 cfu/kg maize resulted in higher mortality of adult P. truncatus, there were no significant differences in mortality between these two concentrations at all days of assessments (Table 1).
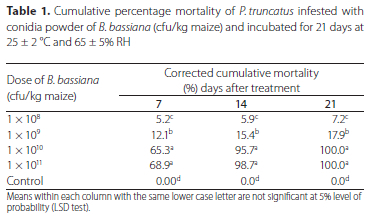
These results corroborate studies by Osipitan et al. (2014) who reported 86% mortality of P. truncatus in maize grains treated with B. bassiana (GHA) after 15 days of exposure. Kassa (2003) also observed the highest mortality of P. truncatus (98.8%) at 30 °C and 60-70% RH, 5 days after exposure to B. bassiana isolate PPRC-HH, at 3 x 108 conidia/g of maize grains. Bourassa et al. (2001) using conidial suspension of four isolates of B. bassiana at 1 x 109 conidia/ml, reported between 90-96% mortality of adult P. truncatus, 14 days post exposure at 28 °C and 55% RH, with over 83% mortality at day 6. Nboyine et al. (2015), however, reported a much lower (53%) mortality of P. truncatus on maize grains treated with B. bassiana, IMI 389521 at 1010 (cfu/kg maize), after 8 weeks of exposure at 25 °C and 35% RH. One possible reason for the observed differences in mortality may be due to the differences in laboratory conditions, especially in relative humidity observed in their study compared to those of the current study (25 ± 2 °C and 65 ± 5% RH).
Effect of B. bassiana product on grain weight loss and dust
Table 2 shows the percentage weight loss of maize grains and dust produced by P. truncatus 21 days post infection with 5. bassiana product. Grains treated with the two highest concentrations of 5. bassiana product (1010 and 1011 cfu/kg maize) recorded the lowest percentage weight loss of 0.4% and this was significantly lower than the untreated control maize (1.9%). A significantly (F = 49.7, p < 0.001) higher grain weight loss (1.7% and 1.8%) was, however, recorded on maize grains treated with the two lowest concentrations of 5. bassiana product (108 and 109 cfu/ kg maize), respectively after infestation by P. truncatus. The percentage weight loss of grains treated with 5. bassiana at 108 and 109 (cfu/kg maize) was not significantly different from the untreated control maize. Similarly, the weight of grain dust in maize treated with the two highest concentrations of 5. bassiana product (1010 and 1011 cfu/kg maize) was significantly (p < 0.05) lower than all other treatments (Table 2).
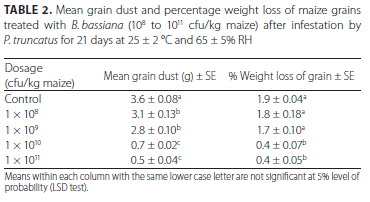
Maize grains treated with the two highest concentrations of 5. bassiana product (1010 and 1011 cfu/kg maize) resulted in lower weight loss of grains compared to 5. bassiana product at 1 x 108 and 109 (cfu/kg maize). This was not unexpected since higher mortalities were recorded in the former resulting in reduced grain damage. The higher mortalities recorded in the aforementioned were due to mycosis by 5. bassiana. The mean temperature and relative humidity of the laboratory during the trials may have supported conidia development and infection of the insects.
Although higher mortalities of LGB were recorded in maize treated with the two lowest concentrations of 5. bassiana (108 and 109 cfu/kg maize) compared to the control maize, considering that the weight loss recorded in these treatments were not significantly different from the control, the protection of maize against LGB was not adequately achieved with these two lower concentrations.
The present study showed that 5. bassiana, IMI 389521 is pathogenic against P. truncatus and S. zeamais. Teretrius nigrescens, however, is less susceptible to the fungus and it is not envisaged that 5. bassiana will have a significant negative effect on populations of T. nigrescens currently being used for management of LGB in Ghana. Due to the significant reduction in grain damage demonstrated at 1 x 1010 cfu/kg maize, there is no additional benefit of using 1011 cfu/kg maize. The minimum effective concentration, for Ghana, is therefore, between 109 and 1010 cfu/kg maize. Consequently, the two middle concentrations 109 and 1010 (cfu/kg maize) are proposed for further evaluation for the control ofLGB under both laboratory and field conditions.
CONFLICT OF INTEREST
There is no conflict of interest.
ACKNOWLEDGEMENTS
This study was funded by Bill and Melinda Gates Foundation under Grant OPP1052676 with support from Exosect-UK, Agrauxine-France, CABI/Europe-UK and CABI/WA. We are also grateful to Banga-Africa Project, University of Ghana, for funding the writing of this paper as well as Mr. Raymond Glikpo of (PPRSD/MOFA) and staff of Department of Crop Science, University of Ghana, Legon, who offered technical assistance during the trial.
ORCID ID
MA Acheampong - https://orcid.org/0000-0003-4547-6881
REFERENCES
Abbott WS. 1925. A method for comparing the effectiveness of an insecticide. Journal of Economic Entomology 18: 265-283. https://doi.org/10.1093/jee/18.2.265a [ Links ]
Abdoulaye T, Bamire AS, Wiredu AN, Baco MN, Fofana M. 2012. Characterization of maize-producing communities in Benin, Ghana, Mali and Nigeria. Drought tolerant maize for Africa (DTMA) Project Community Surveys. CIMMYT West Africa Regional Synthesis Report. https://cgspace.cgiar.org/handle/10568/88185
Acheampong MA, Cornelius EW, Eziah VY, Fening KO, Luke B, Moore D, Clottey VA, Storm C, Potin O. 2016. 5eauveria bassiana affects immature stage development of Prostephanus truncatus (Coleoptera: Bostrichidae) in stored maize. Biocontrol Science and Technology 26: 1516-1525. https://doi.org/10.1080/09583157.2016.1217394 [ Links ]
Adane K, Moore D, Archer SA. 1996. Preliminary studies on the use of 5eauveria bassiana to control Sitophilus zeamais (Coleoptera; Curculionidae), in the laboratory. Journal of Stored Product Research 32: 105-113. https://doi.org/10.1016/0022-474X(96)00009-4 [ Links ]
Ak K. 2019. Efficacy of entomopathogenic fungi against the stored-grain pests, Sitophilus granarius L. and S. oryzae L. (Coleoptera: Curculionidae). Egyptian Journal of Biological Pest Control 29: 1-7. https://doi.org/10.1186/s41938-019-0115-y [ Links ]
Anankware PJ, Fatunbi AO, Afreh-Nuamah K, Obeng-Ofori D, Ansah AF. 2012. Efficacy of the multiple-layer hermetic storage bag for biorational management of primary beetle pests of stored maize. Academic Journal of Entomology 5: 47-53. https://doi.org/10.5829/idosi.aje.2012.5.1.61332 [ Links ]
Arbogast RT, Mullen MA. 1990. Interaction of maize weevil and parasitoid (Anisopteromalus calandrae) (Hymenoptera: Pteromalidae) in small bulk of stored corn. Journal of Economic Entomology 83: 2463-2468. https://doi.org/10.1093/jee/83.6.2462 [ Links ]
Asante SK. 2013. Biological control ofthe larger grain borer, Prostephanus truncatus in northern Ghana. CSIR-Savanna Agricultural Research Institute, Nyankpala, Planning Meeting on control of LGB and other storage insect pest, Erata Hotel, Accra 24-25 April 2013.
Athanassiou GC, Rumbos CI, Sakka M, Potin O, Storm C, Dillon A. 2017. Delivering 5eauveria bassiana with electrostatic powder for the control of stored-product beetles. Pest Management Science 73: 1725-1736. https://doi.org/10.1002/ps.4522 [ Links ]
Batta YA. 2018. Efficacy of two species of entomopathogenic fungi against the stored-grain pest, Sitophilus granarius L. (Curculionidae: Coleoptera), via oral ingestion. Egyptian Journal of Biological Pest Control 28: 3-8. https://doi.org/10.1186/s41938-018-0048-x [ Links ]
Bergvinson DJ, Garcia-Lara, S. 2011. Synergistic effects of insect-resistant maize and Teretrius nigrescens on the reduction of grain losses caused by Prostephanus truncatus (Horn). Journal of Stored Products Research 47: 95-100. https://doi.org/10.1016/j.jspr.2011.01.003 [ Links ]
Biliwa A, Boye, J, Fischer HU, Helbig J. 1992. Release strategy and follow-up studies of Teretriosoma nigrescens in Togo. In: Boye J, Wright M, Laborius GA, editors. Implementation of and further research on biological control of the larger grain borer. Proceedings of an FAO/GTZ Co-ordination Meeting. 138-142. FAO, Rome, Italy, and GTZ, Eschborn, Germany.
Birkinshaw LA, Hodges RJ. 2000. Improving IPM approaches for LGB control in Africa. PhAction News 3. http://www.iita.org [ Links ]
Birkinshaw LA, Hodges RJ, Addo S, Riwa W. 2002. Can 'bad' years of damage by Prostephanus truncatus be predicted? Crop Protection 21: 783-791. https://doi.org/10.1016/S0261-2194(02)00038-8 [ Links ]
Boateng BA. 1996. Effects of maize variety and season on population dynamics of the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and the maize weevil, Sitophilus zeamais (Mots.) (Coleoptera: Curculionidae) and grain losses in the traditional 'Ewe' storage barn in Ghana. Department of Crop Science, M.Phil. thesis, University of Ghana, 90pp. [ Links ]
Boomsma JJ, Jensen AB, Meyling NV, Eilenberg, J. 2014. Evolutionary interaction networks of insect pathogenic fungi. Annual Review of Entomology 59: 467-85. https://doi.org/10.1146/annurev-ento-011613-162054 [ Links ]
Bonu-Ire MS. 2001. Evaluation of some biological control agents against the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) [M.Phil. thesis].Accra: University of Ghana. [ Links ]
Bonu-Ire MST, Captain-Esoah M, AngyiereyirI ED. 2015. Predation and parasitisation of Prostephanus truncatus by Teretrius nigrescens and Anisopteromalus calandrae respectively under controlled environmental conditions. Journal of Biology, Agriculture and Healthcare 5: 67-74. [ Links ]
Borgemeister C, Schneider H, Affognon H, Schulthess F, Bell A, Zweigert ME, Poehling H-M, Setamou M. 2003. Impact assessment of Teretrius nigrescens Lewis (Col.: Histeridae) in West Africa, a predator of the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae). 1st International Symposium on Biological Control of Arthropods, 14-18 January 2002, Honolulu, Hawaii, USA. [accessed 18 June 2021]. https://www.bugwood.org/arthropod/day5/Borgemeister.pdf
Bourassa C, Vincent C, Lomer CJ, Borgemeister C, Mauffette Y. 2001. Effects of entomopathogenic hyphomycetes against the larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and its predator, Teretriosoma nigrescens Lewis (Coleoptera: Histeridae). Journal of Invertebrate Pathology 77: 75-77. https://doiorg/10.1006/jipa.2000.4986 [ Links ]
Boxall RA. 1986. A critical review of the methodology for assessing farm-level grain losses after harvest. Tropical Development and Research Institute, London.
Boxall RA. 2002. Damage and loss caused by the larger grain borer Prostephanus truncatus. Integrated Pest Management Review 7: 105-121. https://doi.org/10.1023/A:1026397115946 [ Links ]
Boye J. 1988. Autoecological investigations on the behavior of the larger grain borer Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) in Costa Rica. [PhD thesis]. Kiel: University Kiel, G er many. [ Links ]
CABI. 2019. Invasive Species Compendium. [accessed 08 July 2021]. https://www.cabi.org/isc/datasheet/44524
Cherry AJ, Abalo P, Hell K. 2005. A laboratory assessment of the potential of different strains of the entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin and Metarhizium anisopliae (Metschnikoff) to control Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) in stored cowpea. Journal of Stored Product Research 41: 295-309. https://doi.org/10.1016/j.jspr.2004.04.002 [ Links ]
Compton JAF, Ofosu A. 1994. Biological control of the larger grain borer with Teretriosoma nigrescens, In: Compton, J.A.F. (Ed.) Quarterly Report 4/94, Ghana Larger Grain Borer Project (Research Programme Volta Region). 1-8. Ghana Ministry of Food and Agriculture, Accra, Ghana and United Kingdom Overseas Development Administration, London
Cox PD, Wakefield ME, Price N, Wildey, KB, Chambers J, Moore D, Aquino de Muro M, Bell BA. 2004. The potential use of insect-specific fungi to control grain storage pests in empty grain stores. HGCA Project Report. https://projectblue.blob.core.windows.net/media/Default/Research%20Papers/Cereals%20and%20Oilseed/pr341-final-project-report.pdf (accessed 08 July 2021).
De Faria MR, Wraight SP. 2007. Mycoinsecticides and mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biological Control 43: 237-256. [ Links ]
Giles PH, Hill MG, Nang'ayo, FLO, Farrell G, Kibata GN. 1996. Release and establishment of the predator Teretriosoma nigrescens Lewis for the biological control of Prostephanus truncatus (Horn) in Kenya. African Crop Science Journal 4: 325-337. [ Links ]
Golob P, Marsland N, Nyambo B, Mutambuki K, Moshy A, Kasalile EC, Birkinshaw L, Day R. 1999. Coping strategies employed by farmers against the larger grain borer in east Africa: preliminary observation. In: Jin Z, Liang Q., Tan X, Guan, L. (Eds.) Proceedings of the 7th International Working Conference on Stored-Product Protection, 14-19 October 1998. 1772-1781. Sichuan Publishing House of Science and Technology, Chengdu, China.
Dhuyo AR, Selman BJ. 2007. Efficacy of biocontrol agents combined with insecticide against the larger grain borer Prostephanus truncatus (Horn.) (Bostrichidae: Coleoptera). Pakistan Entomologist 29: 57-62. [ Links ]
Gueye MT, Goergen G, Badiane D, Hell K, Lamboni L. 2008. First report on occurrence of the larger grain borer Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) in Senegal. African Entomology 16: 309-311. https://doi.org/10.4001/1021-3589-16.2.309 [ Links ]
Hall DW. 1970. Handling and storage of food grains in tropical and subtropical areas. FAO Agricultural Development Paper No. 90. Rome. 350 pp.
Harnisch R, Krall S. 1984. Further distribution of the larger grain borer in Africa. FAO Plant Protection Bulletin 32: 113-114. [ Links ]
Hatting JL, Moore SD, Malan AP. 2019. Microbial control of phytophagous invertebrate pests in South Africa: Current status and future prospects. Journal of Invertebrate Pathology 165: 54-66. https://doi.org/10.1016/j.jip.2018.02.004 [ Links ]
Hodges RJ, Dunstan WR, Magazini I, Golob P. 1983. An outbreak of Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) in East Africa. Protection Ecology 5: 183-194. [ Links ]
Hodges RJ, Meik J, Denton H. 1985. Infestation of dried cassava (Manihot esculenta Crantz) by Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae). Journal of Stored Products Research 21: 73-77. https://doi.org/10.1016/0022-474X(85)90024-4 [ Links ]
Holst N, Meikle W. 2003. Teretrius nigrescens against larger grain borer Prostephanus truncatus in African maize stores: biological control at work. Journal of Applied Ecology 40: 307-319. https://doi.org/10.1046/j.1365-2664.2003.00805.x [ Links ]
Kassa A. 2003. Development and testing of mycoinsecticides based on submerged spores and aerial conidia of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for control of locusts, grasshoppers and storage pests [Ph.D thesis]. Gottingen: University of Gottingen, Germany. [ Links ]
Kassa A, Zimmermann G, Stephan D, Vidal S. 2002. Susceptibility of Sitophilus zeamais (Mots.) (Coleoptera: Curculionidae) and Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) to entomopathogenic fungi from Ethiopia. Biocontrol Science and Technology 12: 727-736. https://doi.org/10.1080/0958315021000039905 [ Links ]
Luke B. 2014.Technical Study Report TSB project 154-203, Molecular identification of IMI 389521, issue date 18/06/2014, Report number X.2A-1.
Millennium Development Authority (MIDA). 2010. Investment opportunities in Ghana on maize, soya and rice. [accessed 08 July 2021]. http://gis4agricgh.net/POLICIES/Investment%20Opportunity%20in%20Ghana.pdf
Muatinte BL, Cugala DR. 2015. Monitoring the establishment and dispersal of Teretrius nigrescens Lewis (Coleoptera: Histeridae), a predator of Prostephanus truncatus Horn (Coleoptera: Bostrichidae) in Manica Province, Mozambique. African Entomology 23: 250254. https://doi.org/10.4001/003.023.0123 [ Links ]
Nboyine J, Asante SK, Nutsugah SK, Abudulai M, Ansaah-Agyapong F, Luke B, Clottey V. 2015. Biological control of the larger grain borer, Prostephanus truncatus (Horn) in stored maize using the fungal pathogen, Beauveria bassiana and the predator Teretrius nigrescens Lewis. Journal of Stored Product and Postharvest Research 6: 30-37. [accessed 08 July 2021]. https://academicjournals.org/journal/JSPPR/article-full-text-pdf/5CFAB7252678 [ Links ]
Obeng-Ofori D. 2008. Major Stored Product Arthropod Pests. In: Obeng-Ofori D, Cornelius EW, editors. Post-harvest Science and Technology. 67-91. Accrs: Smartline Publishers, Ghana. [ Links ]
Obeng-Ofori D. 2011. Protecting grains from insect infestations in Africa: Producer perceptions and practices. Stewart Postharvest Review 3: 1-8. [accessed 08 July 2021], http://ugspace.ug.edu.gh/handle/123456789/29921 [ Links ]
Ogbonna UC, Eziah VY, Owusu EO. 2014. Bioefficacy of Zingiber officinale against Prostephanus truncatus Horn (Coleoptera: Bostrichidae) infesting maize. Journal of Biopesticide 7: 177-185. http://ugspace.ug.edu.gh/handle/123456789/25161 (accessed 08 July 2021). [ Links ]
Omondi BA, Jiang N, Van den Berg J, Schulthess F. 2011. Phylogeographic structure of Teretrius nigrescens (Coleoptera: Histeridae) predator of the invasive postharvest pest Prostephanus truncatus (Coleoptera: Bostrichidae). Bulletin of Entomological Research 101: 521-532. https://doi.org/10.1017/S0007485311000113 [ Links ]
Phiri NA, Otieno G. 2008. Managing pests of stored maize in Kenya, Malawi and Tanzania, MDG Centre ESA, Nairobi, Kenya Report.
Osipitan AA, Popoola AO, Afolabi CG, Oke OA. 2015. Biological control of larger grain borer, Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) with entomopathogenic fungi Beauveria bassiana (Balsamo) Vuillemin (Hypocreales: Cordycipitaceae). International Journal of Entomology and Nematology 2: 2-8. [ Links ]
Póschko M. 1994. Research into the biology and host preference of the predator Teretriosoma nigrescens, a potential natural antagonist of the larger grain borer, Prostephanus truncatus. Eschborn: Deutsche Gesellschaft fur Technische Zusammenarbeit (GTZ) GmbH. http://www.nzdl.org/cgi-bin/library?e=d-00000-00---off-0hdl--00-0----0-10-0-0-0direct-10-4----------0-0l--11-en-50-20-about-00-0-1-00-0--4--0-0-11-10-0utfZz-8-00&cl=CL1.15&d=HASH794fac35ecff8470bcc310>=2 (accessed 08 July 2021).
Póschko, M. 1993. Biology and host specificity of Teretriosoma nigrescens Lewis (Coleoptera: Histeriadae). Ph.D. dissertation. Technical University Berlin, Germany. [ Links ]
Rees DP, Rivera RR, Rodriguez F.S.H. 1990. Observations on the ecology of Teretriosoma nigrescens (Lewis) (Col., Histeridae) and its prey Prostephanus truncatus (Horn) (Col., Bostrichidae) in the Yucatan peninsula, Mexico. Tropical Science 30: 153-165. [ Links ]
Schneider H, Borgemeister C, Setamou M, Affognon H, Bell A, Zweigert ME, Poehling H, Schulthess F. 2004. Biological control of the larger grain borer Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) by its predator Teretrius nigrescens (Lewis) (Coleoptera: Histeridae) in Togo and Benin. Biological Control 30: 241-255. https://doi.org/10.1016/j.biocontrol.2004.01.010 [ Links ]
Scholz D, Borgemeister C, Poehling M. 1998. EAG responses of Prostephanustruncatusand its predator Teretriosoma nigrescensto the borer-produced aggregation pheromone. Physiological Entomology 23: 265-273. https://doi.org/10.1046/j.1365-3032.1998.233089.x [ Links ]
Sedehi A, Sedaghatfar E, Modarres-Najafabadi SS. 2014. Studies on effect of the Beauveria bassiana on eggs and larvae of Plodia interpunctella. Canadian Journal of Basic Applied Sciences 2: 40-45. [ Links ]
Shah PA, Pell JK. 2003. Entomopathogenic fungi as biological control agents. Applied Microbiology and Biotechnology 61: 413-423. https://doi.org/10.1007/s00253-003-1240-8 [ Links ]
Smith SM, Oduor GI, Moore. D. 1998. Preliminary investigations into the potential of entomopathogenic fungi for the control of pests of stored maize. Insect Pathogens and Insect Parasitic Nematodes Bulletin 21: 53-60. [ Links ]
Smith SM, Moore D, Oduor GI, Wright DJ, Chandi EA, Agano JO. 2006. Effect of wood ash and conidia of Beauveria bassiana (Balsamo) Vuillemin on mortality of Prostephanus truncatus (Horn). Journal of Stored Products Research 42: 357-366. https://doi.org/10.1016/j.jspr.2005.06.003 [ Links ]
Van Lenteren JC, Bolckmans K, Kóhl J, Ravensberg WJ, Urbaneja A. 2018. Biological control using invertebrates and microorganisms: plenty of new opportunities. BioControl 63: 39-59. https://doi.org/10.1007/s10526-017-9801-4 [ Links ]
Vowotor KA, Meikle WG, Ayertey JN, Markham RH. 2005. Distribution of and association between the larger grain borer Prostephanus truncatus (Horn) (Coleoptera: Bostrichidae) and the maize weevil Sitophilus zeamais: Motschulsky (Coleoptera: Curculionidae) in maize stores. Journal of Stored Products Research 41: 498-512. https://doi.org/10.1016/j.jspr.2004.08.002 [ Links ]
Wakefield ME. 2006. Factors affecting storage insect susceptibility to the entomopathogenic fungus Beauveria bassiana. In: Proceedings of the 9th International Working Conference on Stored Product Protection. 15-18 October 2006. 855-862. Campinas, São Paulo, Brazil. Brazilian Post-harvest Association. [accessed 07 July 2021]. https://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.604.3876&rep=rep1&type=pdf
Wakefield ME, Moore D, Luke B, Taylor B, Storm C, Grammare P, Potin O. 2010. Progress in the development of a biopesticide for the structural treatment of grain stores. Julius-Kuhn-Archive 42: 760-765. [ Links ]
Wakefield ME, Moore D, Luke B, Taylor B, Collins DA, Storm C, Grammare P, Young R. 2013. Biopesticides for the control of storage insect pests. HGCA Project Report No. 507. [accessed 08 July 2021]. https://projectblue.blob.core.windows.net/media/Default/Research%20Papers/Cereals%20and%20Oilseed/pr507.pdf
 Correspondence:
Correspondence:
MA Acheampong
Email: maacheampong@ug.edu.gh
Received: 13 July 2021
Accepted: 3 July 2022














