Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a12814
RESEARCH ARTICLE
Formulation of Metarhizium pinghaense and Metarhizium robertsii and the infection potential of the formulations against Pseudococcus viburni (Hemiptera: Pseudococcidae) after storage
LL Mathulwe; AP Malan; NF Stokwe
Department of Conservation Ecology and Entomology, Faculty of AgriSciences, Stellenbosch, South Africa
ABSTRACT
Formulation of entomopathogens refers to the mixing of various inert ingredients, like clays and mineral oils, with the active ingredients which are the entomopathogens. Successful formulation enhances the survival of the entomopathogen and eases their transportation, storage, preparation and application. The aim of this study was to develop a formulation to maintain the longevity and pathogenicity of the mass-produced conidia of local Metarhizium pinghaense and M. robertsii, for above-ground future commercial field application against Pseudococcus viburni. The objectives were to develop a cost-effective protocol for formulation of infective propagules and to test their effectiveness under laboratory conditions. The conidia of both isolates were formulated using four different oils (liquid paraffin, coconut, canola and olive oils) as liquid carriers, and diatomaceous earth as a mineral carrier. Conidial viability and pathogenicity were assessed over a period of eight weeks. Conidia formulated in oil carriers maintained a high conidial viability and survival rate of > 95% over a period of eight weeks for both isolates, relative to when formulated in mineral carriers, or when stored as dry conidial powder. The conidia in all the oil formulations were also observed to induce high mortality, ranging between 60% and 90% for M. pinghaense, and between 70% and 90% for M. robertsii, when used against P. viburni. The ability of conidia of both isolates to maintain viability and pathogenicity, following storage in the oil formulations, increased the likelihood of the local isolates being successfully integrated as biological control agents for management of P. viburni under field conditions.
Keywords: conidial viability, entomopathogenic fungi, microbial control, mineral carriers, oil carriers
INTRODUCTION
Insects are important components of agricultural ecosystems as they play a variety of key roles within such environments. However, their role as key pests results in significant damage to agricultural crops, through direct consumption and the damage resulting from disease transmission, contamination and the spoilage of crops (Shah & Pell 2003; Oerke & Dehne 2004). The management of insect pests in production areas relies primarily on the use of chemical control measures and, to some extent, on biological control (Franco et al. 2009). However, the use of chemical insecticides to control important insect pests has detrimental effects on the environment and, thus the use and development of alternative strategies that are environmentally friendly, economical, reliable and sustainable is important (Tupe et al. 2017). Biological control using entomopathogens like entomopathogenic fungi (EPF) offers a relatively suitable alternative for pest management (Moore et al. 2000; Tupe et al. 2017). EPF are known to infect and cause epizootics in a wide range of insect orders, including Hemiptera, Lepidoptera, Coleoptera, Diptera and Hymenoptera (Shah & Pell 2003; Zimmermann 2007).
Pseudococcus viburni Signoret (Hemiptera: Pseudococcidae), obscure mealybug, is an important polyphagous, cosmopolitan insect pest of deciduous fruits (Wakgari & Giliomee 2004; Charles 2011). In South Africa, P. viburni is one of the main pests of pears (Pyrus communis L.) and apples (Malus domestica L. Borkhausen) (Nel 1983; Wakgari & Giliomee 2004; Mudavanhu 2009). When feeding, both adults and nymphs of the mealybug ingest large amounts of phloem sap and excrete the excess sugar and water as a carbohydrate-rich substance known as honeydew (Franco et al. 2009; Da Silva et al. 2017a). The honeydew causes secondary damage to both the plants and fruit, as it falls on the fruit, leaves and stems, where it promotes growth of black mould. The black mould decreases rate of photosynthesis on plant leaves, which affects the host plant's development (Wood et al. 1988; Mibey 1997; Daane et al. 2012). The presence of the black mould on fruits affects their marketability, as stained fruits and fruit consignments showing the presence of the mealybug are downgraded or rejected in pack houses due to phytosanitary concerns and quarantine restrictions (Park et al. 2010).
Management of P. viburni in orchards relies mainly on the use of both biological and chemical control. In South Africa, the use of chemical insecticides is the most common control strategy for management of the mealybug (Van der Merwe 2000). However, the use of chemical insecticides has proven to be ineffective, as levels of P. viburni infestation have increased. Pseudococcus viburni has developed some level of resistance against various chemical insecticides that were previously used for its management (Blumberg & Van Driesche 2001; Mudavanhu et al. 2011). To date, commercially reared encyrtid parasitoids and sex pheromone-baited traps are used for biological control of P. viburni in orchards (Charles 1993; Blumberg & Van Driesche 2001; Mudavanhu et al. 2011; Da Silva et al. 2017b). However, the use of current biological control strategies has proven to be ineffective in suppressing population levels of P. viburni in orchards. Mathulwe et al. (2021), showed the potential of two entomopathogenic fungi isolates, Metarhizium pinghaense Chen and Guo 5HEID and Metarhizium robertsii (Metschn.) Sorokin 6EIKEN, as possible effective biological control agents ofP. viburni, inducing > 90% average mortality under laboratory conditions.
Several EPF and other biocontrol pathogens have been formulated in different ways to create wettable powders, gels, emulsions and dusts for sprays, seed treatments and dips (Fravel et al. 1998). The proper formulation of EPF conidia is important for their success as biological control agents, and inadequacies in formulation result in failure of the formulated spores to induce high mortality of the target insect pests under both laboratory and field conditions (Wraight et al. 2001; Roberts & St. Leger 2004). A shelf life of at least one-and-a-half years is required for the commercialisation of an EPF-based mycoinsecticide and for it to compete with other pest control products (Couch & Ignoffo 1981; Burges 2012). The formulation of biocontrol organisms is known to stabilise the formulated product for storage, until it is required for application. The type of formulation also depends on the biology of the entomopathogen, the target host and the cropping system involved (Fravel et al. 1998).
For Metarhizium conidia, formulation in different types of oil products, like kerosene and mineral oils, vegetable oils and oil products that are designed for pesticide formulation, is usually preferred as the hydrophobic conidia tend to mix well in oil (Moore et al. 1995; Roberts & St. Leger 2004). Oil-based formulations of EPF are also thought to increase the efficacy of the EPF against insect pests, relative to water-based formulations, especially under conditions with low relative humidity. This is because they have an advantage of good adhesion to the hydrophobic-lipophilic cuticle surfaces of the insects (Prior et al. 1988; Bateman et al. 1993; Alves et al. 2002; Sedighi et al. 2013). However, different oil types vary in their effect on conidial survival and pathogenicity during storage (Stathers et al. 1993).
Abiotic factors in the storage environment such as temperature, relative humidity and moisture content are known also to influence conidial longevity and shelf life (Hedgecock et al. 1995; Burges 2012). However, the storage of fungal conidia in oil, whether mineral or vegetable, for extensive periods has the disadvantage of solidifying and becoming rancid at high ambient temperatures (Burges 2012).
The development of formulation and storage techniques that prevents the loss of conidial virulence and viability, while in a dormant state, is important for successful integration and commercialisation of EPF isolates as biological control agents, following conidial mass production (Daoust et al. 1983; Jaronski & Mascarin 2017). Therefore, the aim of the current study was to develop strategies to maintain the virulent nature and effectiveness of the mass-produced conidia of the local South African isolates of Metarhizium, for use in laboratory bioassays and in the field. The objective was to develop a cost-effective formulation of infective propagules, and to test the effectiveness ofthe formulated propagules against P. viburni, under laboratory conditions.
MATERIALS AND METHODS
Source of aerial conidia
The aerial conidia of both M. pinghaense 5HEID (MT895630) and M. robertsii 6EIKEN (MT380849) were obtained from Stellenbosch University. They were cultured using blastospores as inoculum and rice as a solid fermentation substrate (Mathulwe et al. 2022). The viability of each batch of conidia was assessed prior to their use in conidial formulations. Homogenous conidial suspensions containing 50 mg conidia suspended in 10 ml sterile distilled water, supplemented with 0.05% v/v Tween 20 were created, and 100 μl of the suspension was spread-plated on three Sabouraud dextrose agar (SDA) plates. The plates were incubated at a temperature of 25 °C, in complete darkness (Ekesi et al. 2002). The percentage of conidial germination was assessed by a 100 spore count from each plate, under 40x magnification, 24 h following incubation (Ekesi et al. 2002; Inglis et al. 2012). Conidia that developed a germ tube twice the length of their diameter were counted as viable, while those with shorter or no germ tubes were counted as nonviable (Inglis et al. 2012). From the three SDA plates, the average percentage of viable conidia was calculated and only the mass-produced conidia batches with a viability of >85% were used for the formulation of both the M. pinghaense and the M. robertsii isolates.
Carrier material
Liquid paraffin (B.P. 100% v/v, Pinnacle), coconut oil (Pinnacle), canola oil (Woolworths Foods), olive oil (SPAR Foods) and diatomaceous earth (MINEMA DO780) were tested for their potential as the liquid and mineral carriers of the M. pinghaense and M. robertsii conidia.
Conidial formulations
To prepare the formulations in liquid and mineral carriers, 10 mg conidia (~4.7 x 108), with > 90% viability, was added to each liquid and mineral carrier (Bukhari et al. 2011). To make consistent suspensions and mixtures, 10 ml of each liquid carrier was applied to the 10 mg dry conidia in separate 50-ml plastic Falcon centrifuge tubes for each isolate. For the mineral carrier, 10 mg conidia of each isolate were applied to 1 g of the substrate in separate 50-ml plastic Falcon centrifuge tubes. For each carrier medium, six tubes were prepared and placed at a controlled temperature of 3 °C and stored in an upright position. Six tubes containing 10 mg of unformulated dry EPF conidia were also prepared and stored at a controlled temperature of 3 °C. The viability of each formulation was tested over a period of eight weeks. After two, four and eight weeks, two tubes of each conidial formulation were tested for viability, using conidial germination tests.
Germination or conidial viability tests
EPF conidia formulated in liquid carriers were centrifuged for 10 min and the top 9.9 ml of the supernatant removed and discarded. Drops of 0.05% v/v Tween 20 in 5 ml sterile distilled water were added to each tube to make a suspension. The suspensions were then vortex mixed for 2 to 4 min to allow for proper mixing. For the dry conidia and the mineral carrier formulations, 5 ml sterile distilled water and 0.05% v/v Tween 20 were added to 10 mg conidia in 50-ml plastic Falcon centrifuge tubes. The mixture or suspension was then vortex mixed for 2 to 3 min. The conidial viability of these suspensions was checked, following the procedures outlined previously.
Efficacy of the conidial oil formulations against P. viburni
The virulence of each oil conidial formulation for both M. pinghaense and M. robertsii were tested against P. viburni and was assessed using laboratory bioassays after the 8-week storage period. Conidial suspensions of the oil formulation for use in the efficacy bioassays against P. viburni were made as described for the conidial viability tests.
A total of 30 female P. viburni adults were used to test the efficacy of the most viable formulation for both M. pinghaense and M. robertsii. Six insects were placed individually into a well of a 24-well bioassay plate, fitted with a filter paper disc, with one well left between each insect. Five plates were used per treatment. Each insect was inoculated using 50 μl of the conidial suspension, at a conidial concentration of 1 x 107 conidia/ml.
The 24-well bioassay plates were fitted with a glass cover slip on top to prevent the escape of the insects from the well, and for the maintenance of moisture. The bioassay plates were placed in 2-l white plastic ice cream containers, fitted with distilled water-moistened paper towels, to ensure relative humidity of >90%. The containers were closed and incubated at a controlled temperature of 25 ± 2 °C. The control treatment was conducted following the same procedure, except that the insects were inoculated with 50 |il sterile distilled water and 0.05 % v/v Tween 20 only. Insect mortality was recorded five days post treatment and cause of mortality was determined by surface-sterilising the insect cadavers, using 70% ethanol and sterile distilled water (Mathulwe et al. 2021). The insect cadavers were placed on water agar and incubated at a controlled temperature of 25 ± 2 °C. Four to five days following incubation, overt mycosis on cadavers was recorded. The formulation efficacy bioassay trials were repeated once more, using a fresh batch of formulated inoculum.
Data analysis
Statistical analysis of the data was done using STATISTICA version 13.5.0.17 (TIBCO Software Inc. 2018). The conidial viability data of the formulated conidia of both M. pinghaense and M. robertsii in various carrier materials was analysed using a two-way ANOVA, with time and carrier material as factors, and normal probability plots were used to check for normality of the residuals. Levene's test for homogeneity of variances was conducted, while the LSD test (least significant difference test) was done to determine a significant difference between the means. The pathogenicity data of the formulated conidia of both M. pinghaense and M. robertsii against P. viburni was analysed using non parametric Kruskal-Wallis test, and normal probability plots, the Levene test and the LSD test.
RESULTS
Germination or conidial viability tests
Over time, a steady decline in the viability of M. pinghaense conidia stored in all carrier materials was observed. The viability of the conidia stored in diatomaceous earth ranged from 93.8% ± 1.2% two weeks after formulation to 62.5% ± 5.6%, eight weeks after formulation. The viability of the unformulated dry conidia ranged between 91.8% ± 3.1% and 77.5% ± 3.3% over eight weeks, whereas it ranged between 98.8% ± 0.8% and 96.3% ± 1.2% in liquid paraffin, between 99.2% ± 0.4% and 95.3% ± 1.6% in canola oil, between 99% ± 0.8% and 93.3% ± 0.8% in olive oil, and between 98.6% ± 0.6% and 92.7% ± 2.9% in coconut oil (Figure 1).
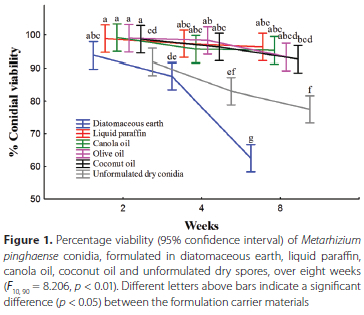
No significant difference in conidial viability was observed between the M. pinghaense conidia formulated in diatomaceous earth (93.8 ± 1.2% (mean ± SE)), liquid paraffin (98.8 ± 0.8%), canola oil (99.2 ± 0.4%,) olive oil (99 ± 0.8%), and coconut oil (98.7 ± 0.6%) carrier materials two weeks after formulation. The unformulated dry conidia (91.8 ± 3%) differed significantly in conidial viability from all carrier materials two weeks after formulation, except for the conidia formulated in diatomaceous earth (F10 90 = 8.206, p < 0.01) (Figure 1). Four weeks following formulation, a significant difference in conidial viability was observed between the fungal conidia formulated in diatomaceous earth compared to the oil carriers, but not the unformulated dry conidia. No significant difference in conidial viability between the oil carriers was found (Figure 1). Eight weeks following formulation, a significant difference in conidial viability was observed between the M. pinghaense conidia formulated in diatomaceous earth, 62.5% ± 5.6%, and the unformulated dry conidia, 77.5% ± 3.3%. The conidial viability ofthe unformulated dry conidia and the conidia in diatomaceous earth both differed significantly from the viability of the conidia formulated in liquid paraffin, 96.3% ± 1.2%, canola oil, 95.3% ± 1.6%, olive oil, 93.3% ± 0.8%, and coconut oil, 92.7% ± 2.3%. No significant difference in conidial viability among the four oil carriers was observed eight weeks following formulation (Figure 1).
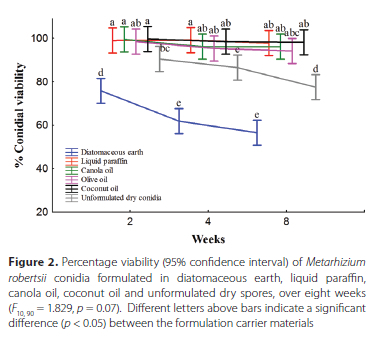
Over time, a gradual decline in the conidial viability of M. robertsii conidia formulated and stored using all six formulation methods was observed. The viability of the conidia stored in diatomaceous earth ranged between 75.8% ± 7%, two weeks after formulation, to 56.5% ± 6.4%, eight weeks after formulation. The viability of the unformulated dry conidia ranged between 90.5% ± 1.8% and 77.5% ± 3.8%, between 99% ± 0.7% and 97.8% ± 0.3% in liquid paraffin, between 99.5% ± 0.3% and 96.2% ± 1.7% in canola oil, between 98.5% ± 0.4% and 94.2% ± 3% in olive oil, and between 99.7% ± 0.3% and 98.7% ± 0.5% in coconut oil.
A significant difference (p < 0.05) in the viability of M. robertsii conidia was observed between the conidia formulated in diatomaceous earth, 75.8% ± 7%, and the conidia formulated in all five oil carriers: liquid paraffin, 99% ± 0.7%; canola oil, 99.5% ± 0.3%; olive oil 98.5% ± 0.4%; coconut oil 99.7% ± 0.3%; and the unformulated dry conidia, 90.5% ± 1.8%, two weeks after formulation. The unformulated dry conidia differed significantly (p < 0.05) in viability from the conidia formulated in liquid paraffin, canola oil and coconut oil. However, no significant difference (p > 0.05) in conidial viability was observed between the conidia in olive oil and the unformulated dry conidia two weeks after formulation (two-way ANOVA, F10,90 =1.829, p = 0.07) (Figure 2). Similar observations were made four weeks following formulation, where the conidia formulated in diatomaceous earth, 61.8% ± 4%, and the unformulated dry conidia, 86.5% ± 3.2%, differed significantly from the conidia formulated in liquid paraffin, 99.2% ± 0.3%, canola oil, 96.2% ± 0.7%, olive oil, 95.3% ± 1.9%, and coconut oil, 98.5% ± 0.8%. Eight weeks after formulation, no significant difference in viability was found among the conidia in all four oil carriers: liquid paraffin, 97.8% ± 0.3%; canola oil, 96.2% ± 1.7%; olive oil, 94.2% ± 3%; and coconut oil, 98.2% ± 0.5%. The conidial viability of M. robertsii in the oil carriers differed significantly from that formulated in diatomaceous earth, 56.5% ± 6.4%, and unformulated dry conidia, 77.5% ± 3.8%, eight weeks after formulation (Figure 2).
Efficacy of oil formulations against mealybugs
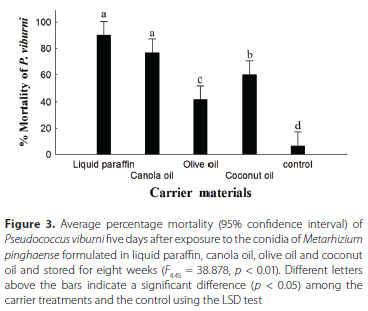
The conidial formulation of both the M. pinghaense and the M. robertsii were found to be pathogenic to P. viburni eight weeks following their storage in the four oil carriers (Figure 3). No significant difference, p > 0.05, in the average percentage mortality of P. viburni was observed between conidia of M. pinghaense formulated in liquid paraffin, 90% ± 5.1%, and in canola oil, 76.7% ± 5.1%, five days following exposure to the eight-week-old formulations. Metarhizium pinghaense conidia in liquid paraffin and canola oil differed significantly in percentage mortality from the olive oil, 41.7% ± 6.7%, and coconut oil formulations, 60% ± 5.7%. The control treatment, 6.7% ± 2.7%, differed significantly from all four oil formulations (Figure 3) (Kruskal-Wallis test, F445 = 38.8 78, p < 0.01).
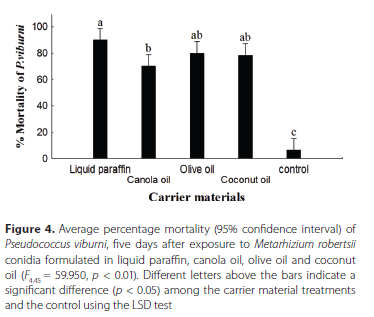
No significant difference, p > 0.05, in the average percentage mortality of P. viburni was observed among conidia of M. robertsii formulated in liquid paraffin, 90% ± 2.7%, olive oil, 80% ± 5.4%, and coconut oil, 78.3% ± 4.3%, five days following exposure to the eight-week-old formulations. No significant difference in the percentage mortality induced by the conidial formulations in olive oil, coconut oil and canola oil, 70% ± 5.4%, was observed. The M. robertsii conidia in the liquid paraffin formulation differed significantly from the canola oil formulations. The control, 6.7% ± 2.7%, differed significantly from all four oil formulations (Figure 4) (Kruskal-Wallis test, F445 = 59.9 5 0, p < 0.01).
DISCUSSION
The current study examined the potential of various oil and mineral products as carriers of mass-produced conidia of two local fungal isolates, M. pinghaense and M. robertsii, to prolong conidial survival during storage with the aim of developing a product for future use against important insect pests under field conditions. Conidia of both M. pinghaense and M. robertsii formulated in the oil carriers maintained high viability and a survival rate of > 95% over a period of eight weeks. However, the survival and viability of conidia formulated using diatomaceous earth as a mineral carrier decreased drastically over time. Conidia formulated using diatomaceous earth were also observed to have a relatively low viability and survival rate over time compared to conidia stored as unformulated dry powder. Moore & Higgins (1997) observed that conidia of M. flavoviride survived better when they were stored as dry powder, with the addition of silica gel, than in a mixture of either vegetable or mineral oils. However, in the current study, a gradual decline in the conidial viability of both M. pinghaense and M. robertsii, when stored as unformulated conidial powder, stored in <1% moisture conditions, was observed over a period of eight weeks. Decline in viability might have resulted because silica gel was not added to the conidial powder during storage.
Batta (2003) made similar observations to those in the current study when formulating the conidia of M. anisopliae using an invert emulsion, water-in-oil formulation, with either coconut or soybean oil. The study showed that conidial viability and survival of M. anisopliae remained high when stored in the water-in-oil formulation, compared to unformulated dry conidia. The study also showed that the conidial viability of the unformulated dry conidia of M. anisopliae, when kept at temperatures of 20 ± 1 °C and 30% ambient relative humidity, drastically declined over a period of eight weeks, from 94.5% on day zero to 1.0% by week eight. This observation differs slightly from observations made in the current study, as both the M. pinghaense and M. robertsii unformulated conidia, when stored at 3 °C, showed only a gradual decline in viability and maintained conidial viability of > 75% over a period of eight weeks.
The survival of conidia in the different types of oils used in this study varied for both M. pinghaense and M. robertsii, as different oil carriers tend to vary in their chemical properties, resulting in some carriers being more inhibitory to long-term survival of the conidia than others over time. Similarly, Stathers et al. (1993) showed that different oils, including mineral, animal and vegetable oils, tend to vary extensively in their effect on the conidial viability of M. flavoviride, when they are used as long-term storage carriers of the fungal isolate. However, oil formulations are known to prevent desiccation of conidia, as the hydrophobic conidia tend to mix well in oils, thereby prolonging their survival. The oils also reduce the sensitivity of the fungal conidia to ultraviolet radiation and improve adhesion of fungal conidia to the hydrophobic surfaces of insects and plant surfaces, when applied under field conditions (Moore et al. 1995; Humber 1997; Roberts & St. Leger 2004). Xavier-Santos et al. (2011) showed that emulsifiable oils protect the conidia of both M. robertsii and M. pinghaense from the imbibitional damage that occurs when the dehydrated conidial cells are immersed directly in water, resulting in damage to the plasma membrane. Inyang et al. (2000), in assessing the effect of formulation, application and rain on the persistence of M. anisopliae in oilseed rape, showed that the use of oil carriers appears to improve both conidial germination and insect infection.
The current study demonstrated the effectiveness of the conidia stored in various oil formulations against the obscure mealybug, as the formulated conidia of both M. pinghaense and M. robertsii were able to induce mortality of the mealybug after eight weeks of storage. The conidia of M. pinghaense induced higher mortality of P. viburni when stored in liquid paraffin and canola oil, >75%, relative to storage in either olive or coconut oil. In contrast, M. robertsii conidia induced relatively high levels of insect mortality of >70% when stored in all four of the oil types, with conidia kept in liquid paraffin inducing the highest mortality. The difference in viscosity and spreading quality the different oil might explain the difference in efficacy of oil formulations against P. viburni, as some oils might have resulted in fewer conidia per unit area on the insect's cuticle due to runoff. The interaction between the wetting agent, 0.05% v/v Tween 20, and the different oils might have affected the germination of both the oil formulated M. pinghaense and M. robertsii conidia on the P. viburni cuticle, as wetting agents in formulations are thought to also neutralise the hydrophobic interactions between the EPF conidia and the insect host cuticle and therefore affecting conidial adhesion (Boucias & Pendland 1991; Milner et al. 1991).
Polar et al. (2005), demonstrated that the effectiveness of M. anisopliae conidia for control of Boophilus microplus Canestrini (Ixodida: Ixodidae) can be improved by formulation in oils and emulsifiable adjuvant oils. Their study also showed enhanced pathogenicity of M. anisopliae stored in 10% liquid paraffin and coconut oil to B. microplus. Similarly, Sedighi et al. (2013) showed the effectiveness of three different oil-based conidial formulations of Metarhizium anisopliae var. major, when suspended in Citoweet", ADDIT* and EC", against the migratory summer generation of the sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae), under laboratory conditions. Their study showed 75%, 88% and 90% insect mortality, 10 days following inoculation with M. anisopliae var. major conidia at a conidial concentration of 2 x 108 conidia/ml, suspended in ADDIT", EC* and Citoweet", respectively. Batta (2003) showed the effectiveness of the coconut or soybean oil formulation of M. anisopliae conidia against tobacco whitefly, Bemisia tobaci Genn. (Hemiptera: Aleyrodidae), and red spider mite, Tetranychus cinnabarinus Boisd. (Trombidiformes: Tetranychidae), both of which are serious pests of field-grown eggplants, under both laboratory and field conditions. The study showed that a two-week-old oil formulation induced mortality of 100% of the nymphs of the tobacco whitefly at a conidial concentration of 5 x 106 conidia/ml, within three days of treatment under laboratory conditions, and 92.2% mortality, when tested under field conditions. Using the same conidial concentration, the oil formulation induced mortality of 82.2% and 93.3% of nymphs and adults of the red spider mite within five days of treatment under laboratory conditions, and mortality of 90.6%, when tested under field conditions.
Several entomopathogenic fungal isolates have been successfully formulated and commercialised for use against several pest insects in various production areas. Mugonza et al. (2020) showed the effectiveness of a commercial oil-formulated biopesticide of M. anisopliae (Real M. anisopliae 69 OD* - ICIPE 69, Real IPM company, Kenya Limited) against the leaf beetle, Ootheca mutabilis Sahlberg (Coleoptera: Chrysomelidae), which is an important pest of the common bean, Phaseolus vulgaris Linnaeus (Fabales: Fabaceae). They showed that the isolate induced 100% mortality of O. mutabilis at a conidial concentration of 1 x 109 conidia/ml, within seven days under laboratory conditions. The fungal isolate M. anisopliae ICIPE 69 has also been commercialised for use against other important pest insects, including thrips, mealybugs and fruit flies, supplied as oil-, powder- or water-based formulations (Mkiga et al. 2021). The Green Muscle* product (Biological Control Products SA (Pty) Ltd. (BCP)), with M. flavoviride as active ingredient, was found to induce 95% mortality in adult desert locusts at a conidial concentration of 5 x 104 conidia per insect in oil formulation, five days following application. Green Muscle*, provided in either oil formulation or as spores in silica gel as a desiccant, was also found to be effective against all nymphal instars and adults of grasshoppers, including the variegated grasshopper Zonocerus variegatus Linnaeus (Orthoptera: Pyrgomorphidae), the Africa rice grasshopper Hieroglyphus daganensis Krauss (Orthoptera: Acrididae) and locusts, Phaulacridium species, under field conditions (Langewald 1999).
In conclusion, the current study showed that the conidia of both M. pinghaense and M. robertsii have high survival rates and maintain their viability in the oil-based formulations, and are able to induce mortality of between 47% and 90% of P. viburni, under laboratory conditions. These formulations of M. pinghaense and M. robertsii show promise for biological control of P. viburni. If oil-based conidial formulations are to be used as mycoinsecticides against pests, further evaluation of the efficacy of all four oil-based formulations of both isolates against the obscure mealybug should be conducted under field conditions, to identify the most effective formulation, because several abiotic factors, like sunlight and fluctuation in environmental temperatures, might influence the efficacy of the conidia against the insect pest.
ACKNOWLEDGEMENTS
This research was funded by Hort Pome, Hort Stone and the Technology and Human Resources for Industry Programme (THRIP: TP14062571871) for funding of the project. The authors would like to thank D.G. Nel for assistance with the statistical analysis.
AUTHORS' CONTRIBUTIONS
Conceptualisation: N.F. Stokwe, A.P. Malan, L.L. Mathulwe; Data curation: N.F. Stokwe, A.P. Malan, L.L. Mathulwe; Formal analysis: N.F. Stokwe, A.P. Malan, L.L. Mathulwe; Funding acquisition: N.F. Stokwe; Investigation: L.L. Mathulwe; Methodology: L.L. Mathulwe; Project administration: N.F. Stokwe; Supervision: N.F. Stokwe, A.P. Malan; Writing original draft: L.L. Mathulwe; Writing review and editing: N.F. Stokwe, A.P. Malan.
ORCID IDs
Letodi L. Mathulwe - https://orcid.org/0000-0002-5118-3578
Antoinette P. Malan - https://orcid.org/0000-0002-9257-0312
Nomakholwa F. Stokwe - https://orcid.org/0000-0003-2869-5652
REFERENCES
Alves RT, Bateman RP, Gunn 1, Prior C, Leather SR. 2002. Effects of different formulations on viability and medium-term storage of Metarhizium anisopliae conidia. Neotropical Entomology 31(1): 91-99. https://doi.org/10.1590/S1519-566X2002000100013 [ Links ]
Bateman RP, Carey M, Moore DE, Prior C. 1993. The enhanced infectivity of Metarhizium flavoviride in oil formulations to desert locusts at low humidities. Annals of Applied Biology 122(1): 145-152. https://doi.Org/10.1111/j.1744-7348.1993.tb04022.x [ Links ]
Batta YA. 2003. Production and testing of novel formulations of the entomopathogenic fungus Metarhizium anisopliae (Metschinkoff) Sorokin (Deuteromycotina: hyphomycetes). Crop Protection 22(2): 415-422. https://doi.org/10.1016/S0261-2194(02)00200-4 [ Links ]
Blumberg D, Van Driesche RG. 2001. Encapsulation rates of three encyrtid parasitoids by three mealybug species (Homoptera: Pseudococcidae) found commonly as pests in commercial greenhouses. Biological Control 22(2): 191-199. https://doi.org/10.1006/bcon.2001.0966 [ Links ]
Bukhari T, Takken W, Koenraadt CJ. 2011. Development of Metarhizium anisopliae and Beauveria bassiana formulations for control of malaria mosquito larvae. Parasites & Vectors 22;4(1): 23. https://doi.org/10.1186/1756-3305-4-23 [ Links ]
Burges HD. 2012. Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments. Berlin, Germany: Springer Science & Business Media. [ Links ]
Charles JG. 1993. A survey of mealybugs and their natural enemies in horticultural crops in North Island, New Zealand, with implications for biological control. Biocontrol Science and Technology 3(4): 405418. https://doi.org/10.1080/09583159309355295 [ Links ]
Charles JG. 2011. Using parasitoids to infer a native range for the obscure mealybug, Pseudococcus viburni, in South America. BioControl 56(2): 155-161. https://doi.org/10.1007/s10526-010-9322-x [ Links ]
Couch TL, Ignoffo CM. 1981. Formulation of insect pathogens. In: Burges HD, editor. Microbial Control of Pests and Plant Diseases 1970-1980. London, U.K.: Academic Press. [ Links ]
Da Silva VCP, Nondillo A, Galzer ECW, Garcia MS, Botton M. 2017a. Effect of host plants on the development, survivorship, and reproduction of Pseudococcus viburni (Hemiptera: pseudococcidae). Florida Entomologist 100(4): 718-724. https://doi.org/10.1653/024.100.0418 [ Links ]
Da Silva VP, Garcia M, Botton M. 2017b. Biology of Blepyrus clavicornis (Compere) (Hymenoptera: Encyrtidae), a parasitoid of Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae). Revista Brasileira de Entomologia 61(3): 257-261. https://doi.org/10.1016/j.rbe.2017.05.003 [ Links ]
Daane KM, Almeida RPP, Bell VA, Walker JTS, Botton M, Fallahzadeh M, Mani M, Miano JL, Sforza R, Walton VM, et al. 2012. Biology and management of mealybugs in vineyards. In: Bostanian NJ, Vincent C, Isaacs, R, editors. Arthropod Management in Vineyards. Dordrecht, Netherlands: Springer. p. 271-307. https://doi.org/10.1007/978-94-007-4032-7_12
Daoust RA, Ward MG, Roberts DW. 1983. Effect of formulation on the viability of Metarhizium anisopliae conidia. Journal of Invertebrate Pathology 41(2): 151-160. https://doi.org/10.1016/0022-2011(83)90214-8. [ Links ]
Ekesi S, Maniania NK, Lux SA. 2002. Mortality in three African tephritid fruit fly puparia and adults caused by the entomopathogenic fungi, Metarhizium anisopliae and Beauveria bassiana. Biocontrol Science and Technology 12(1): 7-17. https://doi.org/10.1080/09583150120093077 [ Links ]
Franco JC, Zada A, Mendel Z. 2009. Novel approaches for the management of mealybug pests: application and resistance management. In: Ishaaya I, Horowitz AR, editors. Biorational Control of Arthropod Pests. Dordrecht, Netherlands: Springer. p. 233-278. https://doi.org/10.1007/978-90-481-2316-2_10 [ Links ]
Franco JC, Zada A, Mendel Z. 2009. Novel approaches for the management of mealybug pests. In: Ishaaya I, Horowitz A, editors. Biorational Control of Arthropod Pests. Dordrecht, Netherlands: Springer. p. 233-278. https://doi.org/10.1007/978-90-481-2316-2_10 [ Links ]
Fravel DR, Connick WJ, Lewis JA. 1998. Formulation of microorganisms to control plant diseases. In: Burges HD, editor. Formulation of Microbial Biopesticides: Beneficial Microorganisms, Nematodes and Seed Treatments. Dordrecht, Netherlands: Springer. p. 187-202. https://doi.org/10.1007/978-94-011-4926-6_5 [ Links ]
Hedgecock S, Moore D, Higgins PM, Prior C. 1995. Influence of moisture content on temperature tolerance and storage of Metarhizium flavoviride conidia in an oil formulation. Biocontrol Science and Technology 5(3): 371-378. https://doi.org/10.1080/09583159550039828 [ Links ]
Humber RA. 1997. Fungi: preservation of cultures. In: Lacey LA, editor. Manual of Techniques in Insect Pathology. Cambridge, Massachusetts: Academic Press. p. 269-279. https://doi.org/10.1016/B978-012432555-5/50015-4 [ Links ]
Inglis GD, Enkerli JUERG, Goettel MS. 2012. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In: Lacey LA, editor. Manual of Techniques in Invertebrate Pathology. London, U.K.: Academic Press. p. 189-253. https://doi.org/10.1016/B978-0-12-386899-2.00007-5 [ Links ]
Inyang EN, Mccartney HA, Oyejola B, Ibrahim L, Pye BJ, Archer SA, Butt TM. 2000. Effect of formulation, application and rain on the persistence of the entomogenous fungus Metarhizium anisopliae on oilseed rape. Mycological Research 104(6): 653-661. https://doi.org/10.1017/S0953756200002641 [ Links ]
Jaronski ST, Mascarin GM. Chapter 9, Mass production of fungal entomopathogens. Microbial Control of Insect and Mite Pests: From Theory to Practice. In: Lacey LA, editor. Microbial Control of Insect and Mite Pests: From Theory to Practice. Amsterdam, Netherlands: Elsevier; 2017. p. 141-155. https://doi.org/10.1016/B978-0-12-803527-6.00009-3
Langewald J. 1999. LUBILOSA: Green Muscle User Handbook. Version 4. Biological Locust and Grasshopper Control Project. CABI. https://www.yumpu.com/en/document/read/48076284/lubilosa-green-muscle-biological-control-of-locusts
Mathulwe LL, Malan AP, Stokwe NF. Laboratory screening of entomopathogenic fungi and nematodes for pathogenicity against the obscure mealybug, Pseudococcus viburni (Hemiptera: pseudococcidae). Biocontrol Science and Technology 2022;32(4): 397-417. https://doi.org/10.1080/09583157.2021.2010653
Mathulwe LL, Malan AP, Stokwe NF. Mass Production of entomopathogenic fungi, Metarhizium robertsii and Metarhizium pinghaense, for commercial application against Insect Pests. Journal of Visual Experiment 2022 Mar 31;181(181): e63246. https://doi.org/10.3791/63246. PMID:35435892
Mibey RK. 1.2. 2.2 Scooty moulds. World Crop Pests. 1997;7:275-290. https://doi.org/10.1016/S1572-4379(97)80058-9
Mkiga AM, Mohamed SA, du Plessis H, Khamis FM, Akutse KS, Nderitu PW, Niassy S, Muriithi BW, Ekesi S. Compatibility and efficacy of Metarhizium anisopliae and sex pheromone for controlling Thaumatotibia leucotreta. Journal of Pest Science 2021;94(2): 393407. https://doi.org/10.1007/s10340-020-01281-z
Moore D, Higgins PM. Viability of stored conidia of Metarhizium flavoviride Gams and Rozsypal, produced under differing culture regimes and stored with clays. Biocontrol Science and Technology 1997;7(3): 335-344. https://doi.org/10.1080/09583159730749
Moore D, Bateman RP, Carey M, Prior C. 1995. Long term storage of Metarhizium flavoviride conidia in oil formulations for the control of locusts and grasshoppers. Biocontrol Science and Technology 5(2): 193-200. https://doi.org/10.1080/09583159550039918 [ Links ]
Moore D, Lord JC, Smith SM. 2000 Pathogens. In: Subramanyam B, Hagstrum DW, editors. Alternatives to Pesticides in Stored-Product IPM. Dordrecht, Netherlands: Kluwer Academic Publishers. p. 193227. https://doi.org/10.1007/978-1-4615-4353-4_8 [ Links ]
Mudavanhu P. 2009. An investigation into the integrated pest management of the obscure mealybug, Pseudococcus viburni (Signoret) (Hemiptera: Pseudococcidae), in pome fruit orchards in the Western Cape Province, South Africa. Doctoral dissertation, Stellenbosch University, Stellenbosch, South Africa. [ Links ]
Mugonza J, Otim MH, Egonyu JP. 2020. The comparative virulence of an atoxigenic strain of Aspergillus flavus (Eurotiales: Trichocomaceae) and the commercial ICIPE 69 Metarhizium anisopliae (Hypocreales: Clavicipitaceae) to the bean leafbeetle Ootheca mutabilis (Coleoptera: Chrysomelidae). International Journal of Tropical Insect Science 40(2): 403-411. https://doi.org/10.1007/s42690-019-00091-w [ Links ]
Nel PJ. 1983. Decidous Fruits and Vines: Pests and Diseases and their Control. David Philip, Cape Town, South Africa.
Oerke EC, Dehne HW. 2004. Safeguarding production losses in major crops and the role of crop protection. Crop Protection. 23(4): 275285. https://doi.org/10.1016/j.cropro.2003.10.001 [ Links ]
Park DS, Leem YJ, Hahn KW, Suh SJ, Hong KJ, Oh HW. 2010. Molecular identification of mealybugs (Hemiptera: Pseudococcidae) found on Korean pears. J Econ Entomol. 103(1): 25-33. https://doi.org/10.1603/EC09144 [ Links ]
Polar P, Kairo MT, Moore D, Pegram R, John SA. 2005. Comparison of water, oils and emulsifiable adjuvant oils as formulating agents for Metarhizium anisopliae for use in control of Boophilus microplus. Mycopathologia. 160(2): 151-157. https://doi.org/10.1007/S11046-005-0120-4 [ Links ]
Prior C, Jollands P, Le Patourel G. 1988 Infectivity of oil and water formulations of Beauveria bassiana (Deuteromycotina: Hyphomycetes) to the cocoa weevil pest Pantorhytes plutus (Coleoptera: Curculionidae). Journal of Invertebrate Pathology 52(1): 66-72. https://doi.org/10.1016/0022-2011(88)90103-6 [ Links ]
Roberts DW, St Leger RJ. 2004. Metarhizium spp., cosmopolitan insect-pathogenic fungi: mycological aspects. Adv Appl Microbiol. 54:170. https://doi.org/10.1016/S0065-2164(04)54001-7 [ Links ]
Sedighi N, Abbasipour H, Askary H, Sheikhi Gorjan A. 2013. Effect of oil suspended conidia of Metarhizium anisopliae var. major on mortality of the sunn pest, Eurygaster integriceps Puton (Hemiptera: Scutelleridae). Arch Phytopathol Pflanzenschutz. 46(2): 128-140. https://doi.org/10.1080/03235408.2012.735083 [ Links ]
Shah PA, Pell JK. 2003. Entomopathogenic fungi as biological control agents. Appl Microbiol Biotechnol. 61(5-6): 413-423. https://doi.org/10.1007/s00253-003-1240-8 [ Links ]
Stathers TE, Moore D, Prior C. 1993. The effect of different temperatures on the viability of Metarhizium flavoviride conidia stored in vegetable and mineral oils. Journal of Invertebrate Pathology 62(2): 111-115. https://doi.org/10.1006/jipa.1993.1085 [ Links ]
TIBCO Software Inc. 2018. STATISTICA (data analysis software system), version 13.5.0.17. Palo Alto, CA, USA: TIBCO Software Inc. [ Links ]
Tupe SG, Pathan EK, Deshpande MV. 2017. Development of Metarhizium anisopliae as a mycoinsecticide: from isolation to field performance. Journal of Visual Experiment 125(125): e55272. https://doi.org/10.3791/55272 [ Links ]
Wakgari WM, Giliomee JH. 2004. Description of adult and immature female instars of Pseudococcus viburni (Hemiptera: Pseudococcidae) found on apple in South Africa. African Entomology 12:29-38. [ Links ]
Wood BW, Tedders WL, Reilly CC. 1988. Sooty mold fungus on pecan foliage suppresses light penetration and net photosynthesis. HortScience. 24:231-256. https://doi.org/10.21273/HORTSCI.23.5.851 [ Links ]
Wraight SP, Jackson MA, De Kock SL. 2001. Production, stabilization and formulation of fungal biocontrol agents. In: Butt TM, Jackson C, Magan N, editors. Fungi as Biocontrol Agents: Progress, Problems and Potential. Wallingford, U.K.: CABI. p. 253-287. https://doi.org/10.1079/9780851993560.0253 [ Links ]
Xavier-Santos S, Lopes RB, Faria M. 2011. Emulsifiable oils protect Metarhizium robertsii and Metarhizium pingshaense conidia from imbibitional damage. Biological Control 59(2): 261-267. https://doi.org/10.1016/j.biocontrol.2011.08.003 [ Links ]
Zimmermann G. 2007. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Science and Technology 17(6): 553-596. https://doi.org/10.1080/09583150701309006 [ Links ]
 Correspondence:
Correspondence:
LL Mathulwe
Email: Mathulwell@sun.ac.za
Received: 18 November 2021
Accepted: 19 September 2022














