Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.31 Pretoria 2023
http://dx.doi.org/10.17159/2254-8854/2023/a13747
RESEARCH ARTICLE
Biology of the invasive shell lerp psyllid, Spondyliaspis cf. plicatuloides (Froggatt) (Hemiptera: Aphalaridae)
Privilege T. MakundeI, III; Bernard SlippersII, III; Samantha J. BushIII; Brett P. HurleyI, III
IDepartment of Zoology and Entomology, University of Pretoria, Pretoria, South Africa
IIDepartment of Biochemistry, Genetics and Microbiology, University of Pretoria, Pretoria, South Africa
IIIForestry and Agricultural Biotechnology Institute, University of Pretoria, Pretoria, South Africa
ABSTRACT
Spondyliaspis cf. plicatuloides (Froggatt) (Hemiptera: Aphalaridae), a shell lerp psyllid, is native to Australia but was first detected in South Africa during 2014. The insect feeds on the sap of eucalypt species and thus has the potential to become a pest in commercial Eucalyptus plantations. Information pertaining to the basic biology of this psyllid, which is important in fundamental studies of insect pests, is lacking. To investigate the biology of S. plicatuloides, the psyllid was reared under controlled glasshouse conditions on potted red-flowering gum, Corymbia ficifolia (F Muell.) KD Hill and LAS Johnson (syn. Eucalyptus ficifolia). The egg incubation period, number of nymphal instars and their developmental time, adult fecundity, adult longevity and duration of the life cycle were determined. The major diagnostic features used to differentiate nymphal instars included the number of antennal segments, wing pad development and body length. Females reached reproductive maturity 2.3 ± 0.47 days after eclosion and laid 16.2 ± 3.9 eggs on average. Reproduction was found to be sexual. The first nymphal instar took 10.7 ± 1.2 days to hatch from the egg. The nymphal instars completed their development in 22.6 ± 1.4 days under the brown scalloped shelters they secrete. The insect's total life cycle lasted 37.37 ± 1.17 days from egg to adult death. Male and female lifespans are also reported. The study provides the first information on the basic biology of S. cf. plicatuloides that will be useful for future studies on surveillance and management strategies.
Keywords: biological invasion, eucalypts, immatures, life cycle, Spondyliaspidinae
INTRODUCTION
Spondyliaspis cf. plicatuloides (Froggatt) (Hemiptera: Aphalaridae: Spondyliaspidinae), commonly known as the shell lerp psyllid, is a sap sucking insect that feeds exclusively on fully expanded leaves of eucalypts (Angophora, Corymbia and Eucalyptus) (Hollis 2004). The insect, which is native to Australia, was detected in South Africa in 2014, making it the first report of the insect outside of its native range (Bush et al. 2016). The immature stage of the insect (nymphal instars) uses anal exudates to build a brown scalloped shelter, known as a lerp, under which the individual completes its development (Hollis 2004). The size of the lerp increases with each subsequent nymphal instar (Hollis 2004). Immatures remain under the lerp until they exit as young adults. Infestation of susceptible eucalypt species is typically accompanied by direct damage from sap sucking by both adults and immature stages, which causes chlorosis and subsequently leaf necrosis and defoliation (Figure 1A-C). By covering the leaf surfaces of infested eucalypts, the lerps cause indirect damage by limiting light interception for photosynthesis (Hollis 2004). In South Africa the insect was first discovered on ornamental and street eucalypt trees (Bush et al. 2016), but it has since spread to nearly all commercial eucalypt growing regions, infesting some eucalypt species, hybrids and clones (Makunde; per. obs.). As a result, the shell lerp psyllid has the potential to be an economically important insect in South Africa.
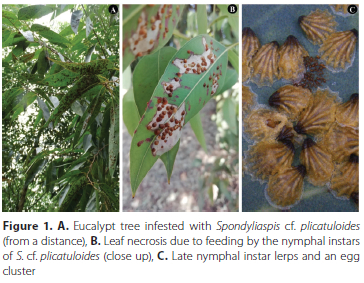
There exist substantial amounts of research published on the biology of eucalypt psyllids (Clark 1962; Azevedo and Figo 1979; Pinzón et al. 2002; Purvis et al. 2002; Santana and Zanol 2006; Angel et al. 2008; Hodkinson 2009; Soufo and Tamesse 2015; Cuello et al. 2018; Ávalos et al. 2021). However, much of the literature is focused on invasive alien eucalypt psyllids, outside of Australia. The biology of eucalypt psyllids that have not been invasive is poorly known. As a result, psyllids that have been identified for the first time as invasive alien insects, such as S. cf. plicatuloides, are typically understudied. Apart from Froggatt's original description in 1900, little is known about the insect, and almost no information about its life history or general biology has emerged since its discovery in South Africa (Bush et al. 2016). This lack of information on basic biology hinders the development of surveillance activities and pest management strategies. Furthermore, the insect's name hasn't been confirmed, as it is tentatively identified as S. cf. plicatuloides.
Understanding the fundamental biology and ecological needs of an insect is critical when planning management options (Taylor et al. 2018). As eucalypt psyllids are sap sucking insects that can be controlled with systemic insecticides, understanding their biology is important to identify the most vulnerable life stage. Furthermore, when using natural enemies such as parasitoids as part ofmanagement strategies, a better understanding of the target pests' biology is required to identify the most susceptible developmental stage and synchronise it with the release of the parasitoids (Angel et al. 2008).
We studied aspects ofthe basic biology of S. cf. plicatuloides in controlled conditions. Specifically, we determined the developmental time and morphometric data for the different stages of the psyllid's life cycle. We also determined the pre-oviposition period, fecundity and longevity. To our knowledge this is the first published information on the basic biology of S. cf. plicatuloides.
MATERIALS AND METHODS
Source of insects and species confirmation
Spondyliaspis cf. plicatuloides adult individuals were collected from infested leaves of Eucalyptus sideroxylon Cunn. ex Woolls in the Zoo Plot of the National Zoological Gardens, Rietondale, Pretoria, Gauteng (25°44.139' S, 28°14.435' E). Briefly, infested leaves were collected, and placed into bags, brought to the laboratory, and immediately transferred into 9.5-l labelled, unventilated plastic containers (Addis Flavour-tight seal Addis Cake Saver) lined with paper towel. The emergence of adults was monitored for 48 hours and all emerged adults were collected, sexed and used to establish a colony that was maintained in a glasshouse at the Forestry and Agricultural Biotechnology Institute (FABI), at the University of Pretoria, South Africa (25°45'19.538'' S, 28°14'7.373'' E) on two-year-old potted red-flowering gum, Corymbia ficifolia (F Muell.) KD Hill and LAS Johnson (0.5-1 m tall) under controlled conditions: 25 ± 1 °C, RH ranging from 60-70%, 13L:11D h photoperiod. Approximately 3 l of water were applied per plant every day using a watering can.
To confirm the identity of the psyllid, a section of the COI mitochondrial DNA from three adult specimens were amplified and sequenced. Total genomic DNA was extracted from individual specimens using a DNeasy* Blood and Tissue Kit (QIAGEN GmbH, Germany) according to the manufacturer's instructions. DNA concentrations were determined using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, USA) and adjusted to 2 ng/l. DNA barcoding of the COI gene was performed using PcoF1 and LepR1 primers following Park et al. (2010, 2011) protocols. The Polymerase Chain Reaction (PCR) generated a 700 bp fragment and the PCR products were sequenced using an ABI PrismTM 3100 Genetic Analyzer (Applied BioSystems, USA), separately with forward (PcoF1) and reverse (LepR1) primers. Additionally, the identity of S. cf. plicatuloides collected from various eucalypt plantations across South Africa, namely in the Eastern Cape, Gauteng, KwaZulu-Natal, Limpopo, and Mpumalanga provinces, was confirmed using the same methods described above.
For comparison, some COI sequences amplified with the PcoF 1 and LepR1 primers were obtained from the Barcode of Life Data (BOLD) System (http://www.boldsystems.org/). To edit DNA sequences, CLC Main Workbench 6.0 (CLC Bio, Denmark) and the Biological Sequence Alignment Editor (BioEdit) software (Hall 1999) version 7.0.9 were used, and an online Multiple Sequence Alignment Program (MAFFT) version 7 (http://mafftcbrc.jp/alignment/software/) was used to align the sequences (Katoh and Standley 2013).
Duration of egg incubation and nymphal instars
The duration of egg incubation (days required for eclosion to occur from the day eggs were laid) and the nymphal instars were studied on one-year-old potted Corymbia ficifolia (60-75 cm tall) in a glasshouse at 25 ± 1 °C; 60-70% relative humidity and a photoperiod of 13L:11D hours. We discovered that the psyllid could complete its life cycle on plants as young as one year old, despite the fact that it is usually found in older trees. Corymbia ficifolia plants were thoroughly examined prior to assays to rule out any prior contamination by S. cf. plicatuloides. Using a handheld entomological aspirator, newly emerged adults (yellow in colour) were isolated from the colony and sexed under a Nikon SMZ1500 stereoscopic microscope (Nikon, Tokyo, Japan) at 5-10x magnification. For this experiment, five C. ficifolia plants were used. On each plant with more than five fully expanded leaves, 20 virgin adults (10 males and 10 females) were released and confined to the plant by a zipped nylon-netting entomological sleeve (45 cm x 50 cm). Egg laying was monitored daily, and once egg laying occurred, three to four leaves per branch with egg clusters (> 60 eggs) were marked for further observation. Each leaf with an egg cluster was treated as a separate experimental unit. The entomological sleeves and adults were removed a day after egg laying. The assays were repeated six times using different plants of C. ficifolia. A temperature/ humidity logger (iButton* hygrochron, DS1923-F5#) was used to measure temperature and humidity.
The egg clusters were monitored daily until the first instars started hatching on five different plants. The incubation period for the eggs was calculated when 50% of the first nymphal instars had hatched and the adult developmental time was calculated when 50% of the immatures had become adults. Every two days after hatching until adult emergence, five lerps together with the immatures per plant were carefully lifted with a fine entomological needle and placed in 70% ethanol and stored at -20 °C for morphometric measurements. Measurements from nymphal instars included body length (measured from the head apex to the anal pore), body width (measured at the widest part), antennal length of nymphs (scape to the end of flagellomere) and width and length of lerps. All measurements were done in micrometres (|im). The number of antennal segments was also determined. Six eggs per plant were randomly selected and carefully removed with an entomological needle and used for measurements of egg length (n = 30), while three eggs per plant were used for measurements of pedicel length (n = 15).
Adult S. cf. plicatuloides were collected, sexed, and preserved in 70% ethanol and stored at -20 °C for morphometric measurements. Each specimen's right forewing (dorsal view) and right antennae were mounted on microscope slides. The measured parameters of the female and male adults included total body length (measured from the head apex to the tip of the folded wings), right forewing length, body width (measured at the maximum width), distance between eyes (vertex), and right antennal length (scape to the end of the flagellomere), (male n = 30; female n = 30). This method is similar to that used to investigate the life cycle of Ctenarytaina thysanura Ferris and Klyver infesting Boronia megastigma (Mensah and Madden 1993).
Images of immature stages, lerps and adults were captured using an Olympus DP21 camera system attached to a Nikon SMZ1500 stereoscopic microscope (Nikon, Tokyo, Japan). All morphometric measurements were then processed using Olympus Stream Basic image analysis software.
Pre-oviposition period, fecundity, adult longevity and egg viability assays
The newly emerged adults were collected with a handheld entomological aspirator and sexed under a Nikon SMZ1500 stereoscopic microscope (Nikon, Tokyo, Japan). The presence of a distinct external genitalia with a slender apical lobe distinguished males from females.
Additionally, males are smaller than females . Two branches of 15 Corymbia plants with five fully expanded mature leaves were enclosed by an entomological sleeve (45 cm x 50 cm) and in each sleeved branch, a pair of male and female S. cf. plicatuloides was introduced. Thereafter, egg laying was monitored on a daily basis, and the preoviposition period (the number of days from adult emergence to egg laying) was calculated based on the appearance of the first egg. The adults were monitored daily until the last female and male died to assess adult longevity (adult emergence to death). The eggs were counted and the hatching of the first nymphal instars was determined using a 40x magnifying glass (Koppert Biological Systems). The sex ratio of the offspring and egg viability (total number of eggs hatched/ total number of eggs laid) were then calculated.
Data analysis
Averages and standard deviations of pedicel length, egg length and width, pre-oviposition period, fecundity, incubation period, nymphal instar duration, longevity, adult forewing length, antennal length and life cycle duration were calculated using Excel®. Furthermore, using R 4.1.3 (R Core Team 2022), Kruskal-Wallis test with post hoc analysis were used to compare the five nymphal instars by the following parameters: body length, vertex, widest body part and antennal length. Student's t-test (Paired Two Sample for Means) in Excel® were used to determine statistical differences between female and male adults on longevity, antennae length, right forewing length, body length, widest body part and vertex.
RESULTS
Insects species confirmation
DNA barcoding using sequence data from the mt COI locus from adult specimens from the Zoo Plot of the National Zoological Gardens and each of the sites in the five provinces yielded identical sequences to those identified as Spondyliaspis plicatuloides in the BOLD System (accession numbers: BIOUG01078-G01, BIOUG01078-G02, BIOUG01078-G03, BIOUG01078-G04 and BIOUG01078-G05).
Fecundity, duration of egg incubation, egg viability and nymphal instars
Spondyliaspis cf.plicatuloides females oviposited 16.20 ± 3.90 eggs per female (n = 30) on average (± SD) with a range of 11-26 eggs. The eggs are reddish brown in colour, smooth and ovoid in shape (Figure 2A), measuring 128.01 ± 3.22 μm in width and 238.97 ± 8.89 μm in length (n = 30). A pedicel protrudes from one end of the egg which connects it to the eucalypt leaf (Figure 2B). Pedicels are responsible for water absorption and nutrient uptake in addition to supporting the eggs on the leaf surfaces (White 1968; Taylor 1992). Eggs are usually laid in clusters (Figure 2A); however, they can occasionally be found scattered singly on the leaf. The average length of the pedicel after removing the egg from the leaf was 101.61 ± 11.74 μm. The eggs are laid on the abaxial and adaxial surfaces of fully expanded leaves and the egg divides along its longitudinal axis when the first-instar nymph hatches (Figure 2C). The average egg viability of S. cf. plicatuloides eggs was 95.20 ± 3.87%.

The average (± SD) incubation period for S. cf. plicatuloides eggs at 25 °C was 10.73 ± 1.18 days. Following hatching, the first-instar nymphs move slowly for a short distance from the egg cluster on the same leaf to the adaxial or abaxial surface of the leaf until they find a feeding site. Once settled, the first-instar nymphs become sessile as each forms an individual lerp (size increases with subsequent instar). The nymphal instars, like adults, ingest eucalypt sap by inserting stylets through the stomata.
In the development of S. cf. plicatuloides, five nymphal instars were observed (Figure 3). On average, the developmental period for each of the instars; I, II, III, IV and V at 25 °C was 7.80 ± 1.56, 4.10 ± 0.31, 2.07 ± 0.37, 2.03 ± 0.32 and 6.57 ± 1.28 days, respectively and was significantly different among the nymphal instars (p < 0.001) (Table 1). The developmental duration of the first and fifth instars was greater than that of the second, third, and fourth nymphal instars (Table 1). The nymphal instars had a total mean developmental period of 22.6 ± 1.4 days between the eclosion of the first nymphal instar and emergence of the adult.
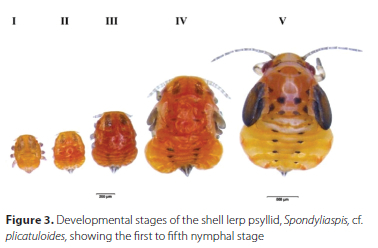
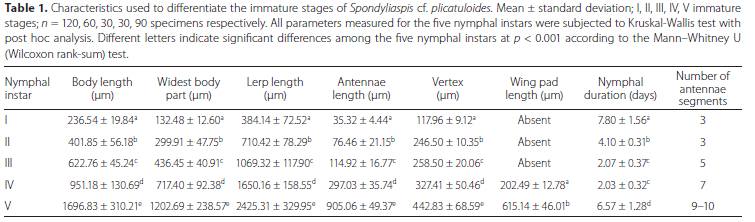
The body length, head capsule width, colouration, lerp size (length and width), forewing pad development, and number of antennal segments easily distinguished the nymphal instars (Table 1). The first-instar nymphs were completely yellow soon after hatching, with the exception of small red eyes, but turn brown after building the lerp. Turning brown may be a function of feeding, assuming they feed after choosing a site for their lerp. The second, third and fourth nymphal instars were all brown in colouration (Figure 3). The body of the fifth-instar nymph was yellowish brown with apparent black markings on the abdomen and thorax (Figure 3). Wing buds are visible or apparent in the fourth instar nymph stage, whereas those in the fifth nymphal instar are well developed and obvious (Figure 3). All stages had a flattened dorsoventral appearance. When the nymphal instars finish developing, the fifth-instar nymph leaves its lerp and settles on the leaf surface, where it moults into the adult stage. Excluding for the first and second nymphal instars, which have the same number of antennal segments, each nymphal instar has a different number of antennal segments (Table 1).
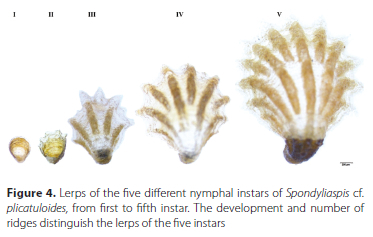
The lerps of all five instars were brown (Figure 4). The lerps of the first-instar nymphs were conical and not ridged, while the lerps of the second-instar nymphs were also conical, but with points of ridges that started to develop on the outer margins. The third, fourth and fifth instars constructed lerps in concentric layers from the hinge (point to which the lerp attach to the leaf surface) outwards. These lerps were roughly hemispheric, with constriction at the sides near the hinge. Third-instar nymphs had five-six ridges, fourth-instar nymphs had seven-eight ridges, and fifth-instar nymphs had nine-ten ridges (Figure 4). The fifth instar lerp has four raised ridges arising from the hinge. Furthermore, there are subsidiary ribs that arise between all the main ridges near the outer margin, giving the lerp a ridged appearance at the margin.
When the fifth instar emerges from the lerp, it transforms into an adult with a light-yellow body and transparent, whitish wings (Figure 5A). Shortly after, the young adults darken and turn orange, with small brown spots on the thorax and abdomen that are usually banded and give them a striped appearance and they develop a short external genitalia (Figure 5B, C and D). They either remain motionless for a few minutes near the exuvia or move slowly over the leaves as soon as they emerge. They also gulp air to expand their wings and body. Their antennae and wings are not fully opened when they start moving. The wings then vibrate until fully stretched, at which point they move quickly and bounce back when touched. The wings form a roof over the body when they are at rest.
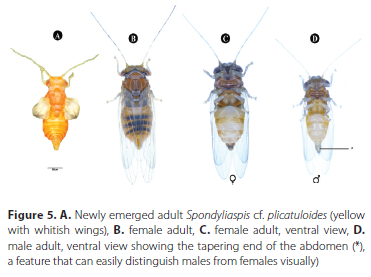
Adults morphometric measurements, longevity and sex ratio
Males and females exhibit significant differences in all morphometric measurements assessed (Student's t-test; p < 0.001). Sexual dimorphism was observed for various morphometrics and the females were 1.3 times larger than the males (Table 2). Male and female adult length (head to apex of folded wings) was 2970.82 ± 289.84 μm and 3880.30 ± 225.89 μm, respectively. Furthermore, the widest body part (abdomen) of male and female adults was 928.26 ± 98.38 |m and 1302.19 ± 127.68 μm, respectively. The vertex distances of male and female adults were 535.57 ± 32.32 |m and 652.64 ± 33.09 μm, respectively. The average antennal length of males was shorter (2076.82 ± 131.08 μm) than that of females (2309.47 ± 185.27 μm). The forewings in females (2905.04 ± 171.80 μm) of S. cf. plicatuloides are relatively longer than those of males (2325.60 ± 202.79 μm). The male has distinct external genitalia, including a slender apical lobe on the proctiger (Figure 5D).
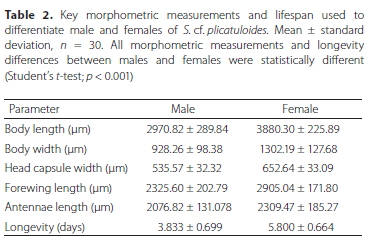
The total duration of the life cycle of S. cf. plicatuloides was 37.37 ± 1.17 days, determined by observing the egg laying to the death of the adult at 25 °C; 60-70% RH. The duration from egg laying to adult emergence from the lerp was 33.30 ± 1.44 days. Adult males and females lived an average of 3.80 ± 0.70 and 5.80 ± 0.70 days, respectively (Table 2) and the sex ratio of males to females per cluster was 1:1 (n = 30).
DISCUSSION
The current study is the first to describe the life cycle and developmental biology in the genus Spondyliaspis, and specifically Spondyliaspis cf. plicatuloides. Studying the basic and developmental biology of insects in the field is difficult due to the uncontrollable nature of the interaction of abiotic and biotic factors, which makes glasshouse studies essential.
Taxonomic revision for the genus Spondyliaspis is required as it is little studied and many uncertainties remain. The studied psyllid species was tentatively identified as Spondyliaspis cf. plicatuloides, but it could also be Spondyliaspis bancrofti (Bush et al. 2016), a type species for the genus Spondyliaspis Signoret (1879). The challenge is that Signoret did not have an adult specimen and only provided a brief description of the lerp (Taylor 1960).
The average fecundity of S. cf. plicatuloides was 16.20 ± 3.90 eggs per female, with a range of 11 to 26 eggs. This was lower compared to the fecundity of other Australian lerp forming Spondyliaspidinae such as Cardiaspina albitextura Taylor (124 eggs/female; range of 14-289) (Clark 1962), Creiis lituratus Froggatt (95 eggs/female; range of 130-148) (Angel et al. 2008), grey box Cardiaspina sp. (43.33 ± 6.40) (Hall et al. 2015), and G. brimblecombei Moore (range 50-75 eggs) (CABI 2012). Furthermore, the fecundity of S. cf. plicatuloides was even lower than that of Blastopsylla occidentalis Taylor, a free-living eucalypt psyllid (Dzokou et al. 2020). The fecundity of psyllid species is related to their life cycle type (Hodkinson 2009), with species having nymphal instars living in protective galls or lerps producing fewer eggs than those with nymphs living on exposed growing tips. The reported average fecundity per female psyllid ranges from 40 to above 1000 (Hodkinson 2009), still higher than that found in this study. Species with the lowest reported fecundity, 40-50 are found within the Spondyliaspididae and Carsidaridae, whereas those with the highest fecundity are frequently found in the Psyllidae and Triozidae (Hodkinson 2009). Temperature, day length, season and nutritional status of the host are just a few of the factors that influence fecundity (Mehrnejad and Copland 2005).
The eggs of Spondyliaspis cf. plicatuloides were 238.97 ± 8.89 urn (0.239 ± 0.09 mm) long and 128.01 ± 3.22 μm (0.128 ± 0.003 mm) wide, making them smaller than those of other Australian Spondyliaspidinae. This includes Cardiaspina albitextura (0.34 ± 0.003 mm long, 0.18 ± 0.003 mm wide), C. densitexta Taylor (0.34 ± 0.001 mm long, 0.17 ± 0.002 mm wide), C. retator Taylor (0.31 ± 0.003 mm long, 0.20 ± 0.002 mm wide), Cardiaspina sp. (nr. brunnea Taylor) (0.30 ± 0.008 mm long, 0.24 ± 0.007 mm wide), Creiis sp.1 (0.39 ± 0.003 mm long, 0.16 ± 0.003 mm wide), Glycaspis brimblecombei Moore (0.34 ± 0.002 mm long, 0.14 ± 0.002 wide), and Platyobria lewisi Taylor (0.29 mm long, 0.10 wide) (Taylor 1992). The incubation period of S. cf. plicatuloides (10.73 ± 1.18 days) fell within the general range ofpsyllids (10-20 days; Collet 2001) and with examples including Ctenarytaina thysanura (Ferris and Klyver) (10-12 days, Mensah and Madden 1993) and Blastopsylla occidentalis Taylor (10.11 ± 1.05 days, Dzokou et al. 2020). The pedicel length of S. cf. plicatuloides eggs was 101.61 ± 11.74 μm (0.102 ± 0.001 mm) on average, longer than that of C. albitextura (0.08 ± 0.0002 mm) (Spondyliaspidinae) and other Psyllidae and Triozidae species (Taylor 1992), and the same length as that of C. densitexta (0.10 ± 0.006 mm), C. retator (0.10 ± 0.002 mm), Cardiaspina sp. (nr. brunnea) (0.11 ± 0.005 mm) and G. brimblecombei (0.10 ± 0.002 mm). The egg pedicels of S. cf. plicatuloides were slightly smaller than those of Creiis sp. 1 (0.19 ± 0.002) (Taylor 1992).
Our findings show that both male and female S. cf. plicatuloides have five nymphal instar stages before becoming adults, with a sex ratio of 1:1, as previously reported in other psyllids (Clark 1962; Hodkinson 1974; Stechman et al. 1987; Mensah and Madden 1993; Collet 2001; Hollis 2004). The first and fifth instars' developmental duration lasted significantly longer than the second, third and fourth nymphal instars. Patil et al. (1994) reported a similar pattern for the development of Heteropsylla cubana (Crawford) and Pereira et al. (2020) on the development of G. brimblecombei. The first and second instar nymphs of S. cf. plicatuloides had three antennal segments while the third, fourth and fifth instars had five, seven and nine-ten antennal segments, respectively, as previously reported on respective instars of G. brimblecombei (Ávalos et al. 2021).
We found sexual dimorphism in S. cf. plicatuloides adult size, where females were larger and wider than males. Furthermore, males had shorter antennae than females. In addition, female forewings were noticeably longer than male forewings. The differences in morphometrics observed in adult male and female insects is due to their distinct reproductive roles (McLechlan 1986). The smaller body size and the smaller forewings of male adults may indicate reduced friction with air, thus assisting flight and location of females (Gushki et al. 2018).
Spondyliaspis cf. plicatuloides completed its life cycle at 25 °C; 60-70 % RH in 37.37 ± 1.17 days. This development time was longer than the 30 days for G. brimblecombei (Firmino-Winckler et al. 2009; Ávalos et al. 2021) at 26 °C and 27 °C, and Cardiaspina (Morgan and Taylor 1988) at 30-35 °C. Temperature influences the life cycle duration of psyllids, and these variations could be explained by the differences in rearing temperatures (Morgan and Taylor 1988). When compared to other invasive psyllids in the Ctenarytainini tribe, S. cf. plicatuloides had a shorter life cycle than Ctenarytaina spatulata Taylor (44.89 ± 1.187 days at 20 °C) (Santana and Zanol 2006), Ctenarytaina thysanura (60 days at 18-20 °C) (Mensah and Madden 1993) and Ctenarytaina eucalypti (Maskell, 1890) (149 days at 15 °C) (Pinzón et al. 2002). As a result, low temperatures may prolong the life cycle of psyllids.
Females reached reproductive maturity 2.3 ± 0.47 days after eclosion, which is consistent with other psyllids where egg maturation and oviposition occur quickly (Burts and Fischer 1967). The average longevity of S. cf. plicatuloides (3.80 ± 0.60 and 5.80 ± 0.70 days for males and females, respectively) was lower than observed in other Spondyliaspidinae, namely Cardiaspina albitextura (14 days) (Morgan and Taylor 1988) and G. brimblecombei (10.70 ± 3.74 days for males and 11.41 ± 4.20 for females) (Ávalos et al. 2021). As well as some psyllids in Ctenarytainini, for example C. spatulata (5.7 ± 1.481 days) (Santana and Zanol 2006) and C. eucalypti (70 days) (Pinzón et al. 2002). If S. cf. plicatuloides co-occurs with other destructive psyllids, reproductive data collected for other psyllids is useful because it will guide insect control specialists in optimising the best period for management.
The findings of this study are critical for developing future surveillance and Integrated Pest Management (IPM) programmes in Eucalyptus forest plantations in South Africa, and other countries where this psyllid may become invasive in the future. The key characteristics used to distinguish different nymphal instars in this study will be useful in identifying the different developmental stages in the field. In addition, knowledge on the identification and duration of the life stages can be used to inform different pest management strategies, such as the release of natural enemies that target specific life stages. Given the high diversity of psyllid species associated with eucalypts in Australia, and that only a small number of these psyllids have thus far been introduced into other continents (Makunde et al. 2020), it is likely that there will be further introductions of eucalypt-feeding psyllids in the future where the basic biology is unknown. Therefore, this study is useful in presenting established methods that could be used to investigate the biological characteristics of similar invasive eucalypt psyllids in the future.
ACKNOWLEDGEMENTS
We thank A. Hammerbacher for providing potted Corymbia ficifolia plants used in the study. We thank members of Tree Protection Co-operative Program (TPCP), DSI - NRF Centre of Excellence in Plant Health Biotechnology, University of Pretoria, South Africa for financial support.
AUTHORS' CONTRIBUTIONS
Privilege T. Makunde - Conceptualization, Methodology, Investigation, Data curation, Formal Analysis, Writing -original draft & editing.
Bernard Slippers - Funding acquisition, Conceptualization, Methodology, Supervision Writing - review & editing. Samantha J. Bush - Methodology, Writing - review & editing. Brett P. Hurley - Funding acquisition, Conceptualization, Methodology, Supervision, Writing - review & editing.
ORCID IDs
Privilege T. Makunde - https://doi.org/0000-0001-6464-9472
Bernard Slippers - https://doi.org/0000-0003-1491-3858
Samantha J. Bush - https://doi.org/0000-0002-2420-4601
Brett P. Hurley - https://doi.org/0000-0002-8702-5547
REFERENCES
Ávalos MR, Betancur CA, Guevara G, Bacca T, Rivera AG. 2021. Life cycle and natural enemies of Glycaspis brimblecombei Moore (Hemiptera: Psyllidae) in a Eucalyptus camaldulensis Dehnhardt forest plantation (Jericó, Colombia). Boletín Científico Centro de Museos Museo de Historia Natural. 25(1):40-52. https://doi.org/10.17151/bccm.2021.25.1.3 [ Links ]
Angel P, Nichols JD, Stone C. 2008. Biology of Creiis lituratus Froggatt: (Hemiptera: Psyllidae), pest on Eucalyptus dunnii Maiden in plantations: morphology, life-cycle and parasitism. Australian Forestry. 71(4):311-316. https://doi.org/10.1080/00049158.2008.10675050 [ Links ]
Azevedo F & Figo ML. 1979. Ctenarytaina eucalyptii Mask (Homoptera, Psyllidae). Boletín del Servicio de Defensa contra Plagas 5:41-46. [ Links ]
Burts EC, Fischer WR. 1967. Mating behaviour, egg production and egg fertility in the pear psylla. Journal of Economic Entomology. 60(5):1297-1300. https://doi.org/10.1093/jee/60.5.1297 [ Links ]
Bush SJ, Slippers B, Nesser S, Harney M, Dittrich-Schróder G, Hurley BP. 2016. Six recently recorded Australian insects associated with Eucalyptus in South Africa. African Entomology. 24(2):539-544. https://doi.org/10.4001/003.024.0539 [ Links ]
CABI. 2012. Invasive Species Compendium report - Glycaspis brimblecombei (red gum lerp psyllid). Available at: http://www.cabi.org/isc/datasheet/25242. (Accessed 1 December 2021).
Clark LR. 1962. The general biology of Cardiaspina albitextura (Psyllidae) and its abundance in relation to weather and parasitism. Australian Journal of Zoology. 10(4):537-586. [ Links ]
Collet N. 2001. Biology and control of psyllids, and the possible causes for defoliation of Eucalyptus camaldulensis Dehnh (river red gum) in south-eastern Australia-a review. Australian Forestry. 64(2):88-95. https://doi.org/10.1080/00049158.2001.10676170 [ Links ]
Cuello EM, López SN, Andorno AV, Hernández CM, Botto EN. 2018. Development of Glycaspis brimblecombei Moore (Hemiptera: Aphalaridae) on Eucalyptus camaldulensis Dehnh. and Eucalyptus dunnii Maiden. Agricultural and Forest Entomology. 20(1):13-80. https://doi.org/10.1111/afe.12230 [ Links ]
Dzokou VJ, Soufo L, Tamesse JL. 2020. Biology of Blastopsylla occidentalis (Hemiptera: Psylloidea: Aphalaridae), a pest of Eucalyptus globulus (Myrtaceae) in Yaounde, Cameroon. Journal of Applied and Natural Science. 12(1):30-35. https://doi.org/10.31018/jans.v12i1.2220 [ Links ]
Firmino-Winckler DC, Wilcken CF, de Oliveira NC, de Matos CAO. 2009. Red gum lerp psyllid Glycaspis brimblecombei Moore (Hemiptera, Psyllidae) biology in Eucalyptus spp. Revista Brasileira de Entomologia. 53(1):144-146. https://doi.org/10.1590/S0085-56262009000100030 [ Links ]
Froggatt WW. 1900. Australian Psyllidae. Proceedings of the Linnean Society of New South Wales 25:250-302. [ Links ]
Gushki RS, Lashkari M, Mirzaei S. 2018. Identification, sexual dimorphism, and allometric effects of three psyllid species of the genus Psyllopsis by geometric morphometric analysis (Hemiptera, Liviidae). ZooKeys. 737:57-73. https://doi.org/10.3897/zookeys.737.11560 [ Links ]
Hall A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 41:95-98. [ Links ]
Hall AA, Gherlenda AN, Hasegawa S, Johnson SN, Cook JM, Riegler M. 2015. Anatomy of an outbreak: the biology and population dynamics of a Cardiaspina psyllid species in an endangered woodland ecosystem. Agricultural and Forest Entomology. 17(3):292-301. https://doi.org/10.1111/afe.12106 [ Links ]
Hodkinson ID. 1974. The biology of the Psylloidea (Homoptera): a review. Bulletin of Entomological Research. 64(2):325-338. https://doi.org/10.1017/S0007485300031217 [ Links ]
Hodkinson ID. 2009. Life cycle variation and adaptation in jumping plant lice (Insecta: Hemiptera: Psylloidea): a global synthesis. Journal of Natural History 43(1-2):65-179. https://doi.org/10.1080/00222930802354167 [ Links ]
Hollis D. 2004. Australian Psylloidea: Jumping Plant Lice and Lerp Insects. Australian Biological Resources Study: Canberra, Australia.
Katoh K & Standley DM. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. 2013. Molecular Biology and Evolution. 30(4):772-780. https://doi.org/10.1093/molbev/mst010
Makunde PT, Slippers B, Burkhardt D, de Queiroz DL, Lawson SA, Hurley BP. 2020. Current and potential threat of psyllids (Hemiptera: Psylloidea) on eucalypts. Southern Forests: a Journal of Forest Science. 82(3):233-242. https://doi.org/10.2989/20702620.2020.1813650 [ Links ]
McLechlan AJ. 1986. Sexual dimorphism in midges: Strategies for flight in the rain-pool dweller Chironomus imicola (Diptera: Chironomidae). Journal of Animal Ecology. 55(1):261-267. [ Links ]
Mehrnejad MR & Copland MJW. 2005. The seasonal forms and reproductive potential of the common pistachio psylla, Agonoscena pistaciae (Hem., Psylloidea). Journal of Applied Entomology. 129(6): 342-346. https://doi.org/10.1111/j.1439-0418.2005.00974.x [ Links ]
Mensah RK & Madden JL. 1993. Life history and biology of Ctenarytaina thysanura Ferris and Klyver (Hemiptera : Psyllidae) on Boronia megastigima Nees ex Bartl. (Rutaceae) in Tasmania. Australian Journal of Entomological Society. 32(4):327-337. [ Links ]
Morgan FD & Taylor GS. 1988. The white lace lerp in southeastern Australia. In: Berryman AA editor. Dynamics of Forest Insect Populations. Patterns, Causes, Implications. London: Plenum Press; p 130-140. https://doi.org/10.1007/978-1-4899-0789-9_7 [ Links ]
Park DS, Suh SJ, Hebert PDN, Oh HW, Hong KJ. 2011. DNA barcodes for two scale insect families, mealybugs (Hemiptera: Pseudococcidae) and armored scales (Hemiptera: Diaspididae). Bulletin of Entomological Research. 101(4): 429-434. https://doi.org/10.1017/S0007485310000714 [ Links ]
Park DS, Suh SJ, Oh HW, Hebert PDN 2010. Recovery of the mitochondrial COI barcode region in diverse Hexapoda through tRNA-based primers. BMC Genomics. 11:423. https://doi.org/10.1186/1471-2164-11-423 [ Links ]
Patil NG, Baker PS, Pollard GV. 1994. Life history parameters of the leucaena psyllid Heteropsylla cubana (Crawford) (Homoptera: Psyllidae) under various temperature and relative humidity regimes. Insect Science and Its Application. 15:293-299. https://doi.org/10.1017/S1742758400017604. [ Links ]
Pereira JM, Baldin ELL, Soliman EP, Wilcken CF. 2020. Development of the red gum lerp psyllid, Glycaspis brimblecombei (Hemiptera: Aphalaridae) in Eucalyptus spp. Scientia Forestalis. 48(127):e3283. https://doi.org/10.18671/scifor.v48n127.18 [ Links ]
Pinzón FOP, Guzmán CM, Navas NF. 2002. Contribution to the knowledge of biology, natural enemies and damage of Ctenarytaina eucalypti (Homoptera: Psyllidae). Revista Colombiana de Entomologiá. 28(2):123-128. [ Links ]
Purvis G, Chauzat MP, Segonds-Pichon A, Dunne R. 2002. Life history and phenology of the eucalyptus psyllid, Ctenarytaina eucalypti in Ireland. Annals of Applied Biology. 141(3):283-292. https://doi.org/10.1111/j.1744-7348.2002.tb00220.x [ Links ]
R Core Team. 2022. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Santana DLQ & Zanol KMR. 2006. Biologia de Ctenarytaina spatulata Taylor, 1997 (Hemiptera, Psyllidae) em Eucalyptus grandis Hill ex Maiden. Acta Biologica Paranaense. 35:47-62. [ Links ]
Soufo L & Tamesse JL. 2015. Population dynamic of Blastopsylla occidentalis Taylor (Hemiptera: Psyllidae), a psyllid pest of eucalypts. Neotropical Entomology. 44: 504-512. https://doi.org/10.1007/s13744-015-0304-7 [ Links ]
Stechman DH, Lataimaumi H, Foliaki S. 1987. Studies of Heteropsylla cubana (Crawford) in Tonga I. Leucaena Research Reports. 7(2):50-58. [ Links ]
Taylor GS. 1992. The structure of the eggs of some Australian Psylloidea (Hemiptera). Journal of the Australian Entomological Society. 31:109-117. [ Links ]
Taylor GS, Braby MF, Moir ML, Harvey MS, Sands DPA, New TR, Kitching RL, McQuillan PB, Hogendoorn K, Glatz RV, Andren M, Cook JM, Henry SC, Valenzuela I, Weinstein P. 2018. Strategic national approach for improving the conservation management of insects and allied invertebrates in Australia. Austral Entomology. 57(2):124-149. https://doi.org/10.1111/aen.12343 [ Links ]
Taylor KL. 1960. Additional information on the Australian genera of the family Psyllidae (Hemiptera: Homoptera). Australian Journal of Zoology 8(3):383-391. [ Links ]
White TCR. 1968. Uptake of water by eggs of Cardiaspina densitexta (Homoptera: Psyllidae) from leaf of host plant. Journal of Insect Physiology. 14(11):1669-1683. [ Links ]
 Correspondence:
Correspondence:
Privilege T. Makunde
Email: privilege.makunde@fabi.up.ac.za
Received: 8 April 2022
Accepted: 16 July 2022














