Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.30 Pretoria 2022
http://dx.doi.org/10.17159/2254-8854/2022/a11455
RESEARCH ARTICLE
Review of Liriomyza huidobrensis (Blanchard, 1926) (Diptera: Agromyzidae) on potatoes in South Africa, with special reference to biological control using entomopathogens and parasitoids
T MugalaI; D VisserII; AP MalanI; P AddisonI
IDepartment of Conservation Ecology and Entomology, Faculty of AgriSciences, Stellenbosch University, Stellenbosch, South Africa
IIAgricultural Research Council, ARC-VIMP, Pretoria, South Africa
ABSTRACT
Potatoes are among the four most widely consumed vegetable crops worldwide. However, a potato crop can be infested by various pests like the devastating leaf miner, Liriomyza huidobrensis (Blanchard, 1926) (Diptera: Agromyzidae). This leaf miner has, since the early 2000s, become an important pest of potatoes in South Africa. It is highly invasive, causing up to 70% damage of solanaceous crops. Direct damage results from the adult female flies feeding on the leaf mesophyll during oviposition, and the larvae mining the leaves. Indirect damage is induced through pathogens entering through perforations that act as vectors of plant diseases. Biocontrol agents, such as entomopathogenic nematodes (EPNs), entomopathogenic fungi (EPF) and parasitoids, have shown potential for control of L. huidobrensis. This review investigates the biology and morphological identification of L. huidobrensis, its host range and the potential of associated biocontrol agents, like EPNs, EPF and parasitoids, as future control options.
Keywords: biocontrol agents, entomopathogenic nematodes, entomopathogenic fungi, potato leaf miner, Diglyphus isaea
INTRODUCTION
Insect invasions from one country to another are becoming commonplace, usually due to increased movement of goods and people (Pimentel et al. 2001; Seebens et al. 2018). Many factors contribute to insect invasions worldwide, although one of the common factors is climate change (Hill et al. 2016). Most economic damage and crop losses are the result of pest invasions (Oerke & Dehne 2004). The invasions have a negative impact on food security, and thus can increase levels of poverty (Umesha et al. 2018). To reduce crop losses associated with insect pests, synthetic pesticides are commonly used. However, the overuse of pesticides often results in negative impacts, like the development of insecticide resistance, soil contamination and adverse health problems (Pretty & Pervez 2015). Integrated pest management (IPM), which is an environmentally sensitive approach, includes multiple control strategies that are effective, ecologically compatible and above all, economically feasible (Norris et al. 2002).
Potatoes are among the most consumed non-grain commodity worldwide (Lutaladio & Castaldi 2009). In South Africa, it is one of the most important vegetable crops as it accounts for 60% of the vegetables grown (Joubert et al., 2010). In 2011 South Africa's potato industry represented three percent of the total value of agricultural products in the country's GDP (DAFF 2012). Over 50% of the potatoes that are produced in South Africa are consumed locally, whilst approximately 30% are exported to nearby countries, such as Zimbabwe, Zambia and Mozambique, with the rest exported to other countries (DAFF 2012).
Liriomyza huidobrensis (Blanchard, 1926) (Diptera: Agromyzidae), commonly known as the potato leaf miner, is a devastating pest of potato (Solanum tuberosum L.; Solanales: Solanaceae) in South Africa. The potato leaf miner, which originates from Central and South America, was first detected on other continents in the 1980s. After having been detected in Europe in 1987 (Lanzoni et al. 2002; CABI 2018), it was found to have invaded South Africa by the early 2000s (Visser 2009).
Gaining an in-depth understanding of the biology and control options for a devastating pest, like the potato leaf miner, is required prior to implementation of IPM. This review provides an overview of the current information available on the biology and ecology of L. huidobrensis and management practices for the pest under South African conditions, with special emphasis on biocontrol agents and their potential implementation in IPM.
INSECTS AFFECTING POTATOES
Globally there are many insects that target potato crops. Among these, 49 important pest species were recognised by Kroschel et al. (2020). Farmers in the tropical and subtropical regions tend to have more challenges with pests when compared to farmers in temperate regions, where pest densities are generally lower (Kroschel & Schaub 2013). L. huidobrensis and Phthorimaea operculella (Zeller, 1823) (Lepidoptera: Gelechiidae) (potato tuber moth) are major insect pests of potatoes in several countries (Table 1). Liriomyza huidobrensis, which is the pest of interest in this case, can cause about 70% crop loss (Visser 2005). Some studies suggest that, in some cases, the leaf miner can cause 100% crop loss (Rondon 2010; Mujica & Kroschel 2013).
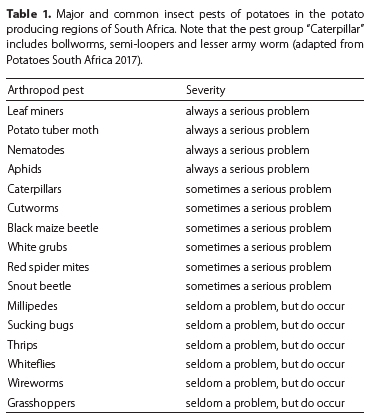
In South Africa, more than 60 arthropod species have been identified infesting potatoes (Visser 2005). However, most are considered minor or nuisance pests. In a survey conducted on more than 100 commercial potato farms across South Africa, several insect species were identified as important causes of yield loss or as commonly occurring in potato fields (Table 1) (Potatoes South Africa 2017). The four most important pest species according to their grouping (Table 1), as reported by potato farmers (in order of importance) were: leaf miners, potato tuber moth, nematodes and aphids (as virus vectors).
LEAF MINERS
More than 10 000 leaf miner species have been reported worldwide from the insect orders Coleoptera, Diptera, Hymenoptera and Lepidoptera (Hering 1951). A leaf miner is defined as an insect that feeds within the leaf tissues for at least part of its life cycle (Hering 1951). During feeding the larvae form tunnels, or mines, which are feeding channels within the parenchyma (between the epidermal tissues) of infested leaves (Figure 1) (Hering 1951; Basij et al. 2011). With the exception of oviposition sites, both the epidermis and the outer wall remain intact during larval development (Hering 1951; Weintraub et al. 2017).

The feeding pattern of the larvae may be divided into two categories, i.e., facultative and obligate (Powell 1980). Facultative leaf miners are those that feed within the leaf and externally (in the case of the last larval instars) before they pupate. Obligate leaf miners, on the other hand, feed entirely within the leaves and may even pupate within the leaves (Hering 1951; Powell 1980; Ameixa et al. 2007). Other classifications of leaf miners depend on the shape of the mines (Weintraub et al. 2017).
Some studies hypothesize that the leaf-mining habit protects the insect and, more broadly, acts as a defence against several natural enemies (Hering 1951; Connor & Taverner 1997). An alternative hypothesis suggests that leaf miners might have increased susceptibility to pathogens, due to the humidity within the leaf environment, which may be conducive to pathogens (Cornell 1989). However, Connor & Taverner (1997) found that leaf-mining insects are likely to encounter a lower pathogen incidence than those that feed externally on the foliage. The success rate attained with biological control options, like parasitoids, in relation to leaf-mining insects is greater than for externally feeding insects (Connor & Taverner 1997).
Abiotic changes affect leaf miner larval development. Therefore, the adult leaf miners' host choice is vital, due to the limited mobility of the immature stages, which means that they must feed on the plants where the eggs are laid (Zehnder & Trumble 1984; Musundire et al. 2012). Oviposition differs, depending on the insect order (Weintraub et al. 2017). Some insect orders leave puncture marks on the leaf surface when laying their eggs, while others do not (Hering 1951). In the case of Liriomyza leaf miners, a female will first make a puncture with her ovipositor, and then proceed to lay eggs in some of the punctures (Weintraub et al. 2017).
POTATO LEAF MINER, LIRIOMYZA HUIDOBRENSIS
Liriomyza, which is one of the largest genera of the order Diptera (Agromyzidae), consists of over 300 leaf miner species worldwide, of which only 23 are considered as economically important (Liu et al. 2009). Leaf mining occurs in nine different families of Diptera, with Agromyzidae having the largest number of species (Mujica & Kroschel 2011). Several leaf miner species have invaded agricultural landscapes across the world; some are key pests and cause significant damage to a variety of crops (Rauf et al. 2000).
Liriomyza huidobrensis is an invasive species, which is extremely polyphagous and is resistant to a variety of insecticides (Spencer 1973; Reitz et al. 2013; Weintraub et al. 2017). The potato leaf miner causes damage to crops both directly and indirectly. Direct damage to foliage is caused by feeding and oviposition punctures (Figure 2). In addition, infested/mined leaves become necrotic and eventually die. Chabi-Olaye et al. (2008) reported a 62% mean reduction in the photosynthetic ability of mined leaves, causing high yield losses. Indirect damage results from diseases, e.g., Alternaria spp., that enter the host through perforations made by leaf miner adults or larvae (Deadman et al. 2000). Despite L. huidobrensis itself not spreading pathogens, they do increase the probability of secondary plant pathogen infections (CABI 2018). Few studies have tried to determine economic injury level and their economic threshold, but a quick life cycle and high fertility rates make this challenging (Rondon 2010). Alves et al. (2017) obtained an economic injury level of 0.07, and an economic threshold of 0.05 mines per plant, thus indicating the high damage potential even at low population densities and consequently the need to institute control measures timeously.

Pest description and biology
Liriomyza huidobrensis can be differentiated from other Liriomyza species by its relatively dark orange-yellow head and legs (Figure 3A), which, in other species, are mostly just yellow (Visser 2015; CABI 2018). The potato leaf miner is generally small, measuring two to three millimetres in length, with a characteristic division on the second abdominal segment (Weintraub & Horowitz 1995). Adult L. huidobrensis females can lay up to 400 eggs, measuring about 0.15 to 0.30 mm, with an off-white and slightly translucent colour (Visser 2015; CABI 2018). The eggs are laid in whitish leaf punctures, usually 0.05 mm in size, made by the female, which hatch within two to five days (Wei et al. 2000; Visser 2015; IPPC 2016; CABI 2018). The larvae hatch within the leaf, where they form mines while feeding (CABI 2018). The larvae stay within their mines during their early stages (Ge et al. 2019). However, in pea plants, the larva also tends to feed on the outer surface of the pods (CABI 2018; Ge et al. 2019). There are three larval instars: the first larval instar, which is colourless at hatching, later turns pale yellow orange. The colour of the last instar is yellow-orange (Weintraub & Horowitz 1995). The larvae can reach about 3.25 mm in length before pupating (CABI 2018). The larvae of some agromyzid species have been reported as leaving one leaf for another, although such behaviour has not been reported in the case of L. huidobrensis (Parrella & Keil 1984; CABI 2018). The final instar larva makes a slit on the leaf surface to exit the mine (Visser 2015; CABI 2018). The mines are irregular in shape (which is typically serpentine) and increase in size as the larva grows (Wei et al. 2000; CABI 2018). More than one larva may feed on a single leaf, thus leading to the production of a secondary 'blotch' type mine. This usually leads to wilting of the infested leaf (Spencer 1973). Larval damage is most severe when the plant is fully-grown and is less severe during the vegetative stages of the plant (Visser 2005; CABI 2018). Actively growing leaves contain fewer leaf mines compared to that of the older leaves on the same plant (Visser 2009; Mujica 2016). Additionally, they tend to pupate on the lower surface of the leaf, but usually fall to the soil to complete their pupation period (Visser 2009, 2015). The puparium, which has an oval shape (Figure 3B), measures between 0.5 mm and 1.3 mm in length, with a brown to almost black colour (CABI 2018). The pupal stage lasts for approximately 10 days (Visser 2009). The adults are between 1.3 mm and 2.3 mm in size, with a wing length of 1.3 mm to 2.3 mm (CABI 2018). The females are generally slightly larger than the males (Weintraub et al. 2017).

Although little information exists regarding the biology of the potato leaf miner, its life cycle is typical of all agromyzid species (Weintraub & Horowitz 1995, 1996). Liriomyza males usually emerge before the females and mating usually occurs 24 h after emergence (Mujica & Cisneros 1997; Migiro et al. 2011). A single mating can fertilise all of the eggs of one female (Mujica & Cisneros 1997; Migiro et al. 2011). Most adult activity occurs in the early morning just after sunrise, and then again before sunset (Weintraub & Horowitz 1995). In South Africa, leaf miner outbreaks are usually severe during summer when temperatures are high (Adendorff 2010; Visser 2009; Weintraub et al. 2017). Additionally, in a study by Videla & Valladares (2007), it was shown that the potato plant expresses a degree of mechanical resistance against the larvae and eggs of L. huidobrensis. In young actively growing leaves, eggs and young larvae are physically "pushed out" by an increase in the multiplication rates of leaf cells, thereby exposing the immatures to predation and increasing the risk of desiccation (Videla & Valladares 2007).
Host plants
Host selection is vital for most herbivorous insects, because it determines the progeny's feeding and the female's choice of oviposition site (Maharjan & Jung 2016). So far 365 plant species, from 49 different families, have been recorded as hosts (Weintraub et al. 2017). Only 32% of the host species are cultivated food crops, with most being weeds and cultivated flowers (Weintraub et al. 2017). The leaf miner affects both field- and greenhouse-produced vegetable crops, ranging from being sporadic to being prevalent throughout a growing season (Reitz et al. 2013). Local leaf miners have a strong preference for local plant species which was observed in Argentina, where L. huidobrensis was found to prefer local vegetable crops, like bean, beet, potato, sweet pepper and celery plants, when compared to other exotic vegetables (López et al. 2010). Other studies suggest that external factors, such as temperature and humidity, play a vital role in the host preference of Liriomyza flies (Fenoglio & Salvo 2009). Another study on host preference, which was carried out in China, found that the selectivity of the leaf miner is related to nutritional and physical factors (Liu et al. 2009). Other studies have suggested that host selectivity does not depend only on the amount of chlorophyll, soluble sugars, proteins and tannic acid concentrations present, but also leaf miner adaptability to the leaf conditions (Liu et al. 2009; Weintraub et al. 2017). There is little information regarding the extent of hosts susceptible to leaf miner attack in South Africa. However, within laboratory settings, plants like common beans, onions and tomatoes have successfully hosted Liriomyza species (Musundire et al. 2012).
MANAGEMENT OF DIFFERENT LEAF MINER SPECIES WITH REFERENCE TO THE POTATO LEAF MINER
Monitoring
Liriomyza huidobrensis is included on the A2 list of quarantine pests (EPPO 2005). In South Africa, it is one of the most important potato pests (Weintraub et al. 2017). An essential part of any IPM strategy is the adoption of monitoring for key pests (CABI 2018). Monitoring can give the farmer the necessary information to make decisions pertaining to which management practices to follow (Dara 2019; Sharma et al. 2020). Effective monitoring practices help to detect the presence and abundance ofprevailing pests and may help to identify favourable conditions that facilitate increases in population abundance (Dreistadt et al. 1998; Lu et al. 2012). Monitoring of insect pests are achieved by using a variety of tools, including coloured sticky traps, light traps, pheromone traps, pitfall traps and suction traps (Epsky et al. 2008; McCravy 2018). No information is available regarding the use of sex pheromones for the potato leaf miner.
In the case ofthe potato leaf miner, the use of sticky traps coated with sticky adhesives in the monitoring of adult populations has proven to be successful. The traps have been designed in different colours, although yellow is mostly preferred (López et al. 2010). Sticky traps, however, also may trap natural enemies, such as parasitoids and lady beetles (Chavez & Raman 1987; Lu et al. 2012). In the potato plant, the adult feeding punctures present on the leaves can be used to foresee an outbreak (CABI 2018). The initial larval infestation begins in the lower third of the canopy, moving to the top canopy of the plant (Visser 2005). Hence, yellow sticky traps are usually placed at canopy level in potatoes. Thus, for fast-growing plants, the placement is usually a few inches above the canopy, while for slower growing plants just above, or at canopy level (Dreistadt et al. 1998; Atakan & Canhilal 2004). However, no study has documented how monitoring of the potato leaf miner is done in South Africa.
Chemical control
The most common method of control used against potato pests is synthetic pesticides (Rondon 2010; Mujica & Kroschel 2013). The use of contact insecticides is not effective as it only kills adult flies, thus to control the larvae growing inside the leaf, effective insecticides are systemic or translaminar (Pirtle et al. 2020). However, the use of most of these insecticides can lead to the development of insecticide resistance, increased cost of production, contamination of the environment and the loss of non-target organisms (Okoth et al. 2014). Most Liriomyza species rapidly develop resistance to certain conventional insecticides used in different countries (Weintraub et al. 2017). However, not all populations of L. huidobrensis have the same resistance profile (Weintraub et al. 2017). The larvae and adults are not susceptible to all insecticides equally, because the larval life stage is covered and protected inside the leaf, thus contact insecticides are not recommended for the control of larval populations (MacDonald 1991; Van der Staay 1992). In the early 1990s, the only effective insecticides used to control larval populations were abamectin, spinosad and cyromazine (Van der Staay 1992). Neem tree Azadirachta indica A.Juss. (Meliaceae) extract, proved to be highly effective against larvae (Weintraub & Mujica 2006). Pesticides that include pyrethroids and organophosphates are mostly ineffective against the potato leaf miner due to the development of insecticide resistance (Macdonald 1991; Weintraub & Horowitz 1995). Several insecticides containing different active ingredients have been registered against the potato leaf miner in South Africa (e.g. ciromazine, abamectin and spinosad). While the pest remains a challenge, these insecticides, if used according to the recommendations, can control, or reduce leaf miner populations (Weintraub et al. 2017).
Cultural control
Habitat management may play an important role in increasing the activity of a leaf miner's natural enemies (Gurr et al. 2017). The use of cover crops in pest management has been advantageous for maximizing interactions between insect predators and prey (Sharma et al. 2018). However, certain weeds near crops may act as reservoirs for leaf miner pests (Schuster et al. 1991; Chen et al. 2003). The cultural control of L. huidobrensis mainly involves the use of preventive measures (Weintraub et al. 2017). These measures include weeding of the fields (clean fields), ploughing, mechanical tilling and the adoption of other phytosanitary measures like trapping and monitoring (CABI 2018). Environmentally friendly strategies that play an important role in suppressing leaf miner populations include crop rotation, the selective removal and destruction of infested plant material, both before and after harvest, and the destruction of pupae before planting (Ben Husin 2017). In terms of agricultural crops, pruning and fertilisation have played an important role in reducing the size of lepidopteran and coleopteran leaf miner populations (Ateyyat & Mustafa 2001; Johnson et al. 2011). Practices like proper fertilisation could also be used in managing potato leaf miner, since the quality of the potatoes plays a vital role in leaf miner abundance (Fenoglio & Salvo 2009). Liriomyza huidobrensis has not been widely studied in South Africa. However, various cultural control strategies should be implemented and evaluated for their efficacy in pest management.
Biological control
A major objective of biocontrol is to reduce the number of crop pests present without contaminating the environment and disturbing other organisms (Ooi 1998). Environmental contamination is reaching greater heights (Pimentel 1995). Therefore, many biocontrol agents have been developed in recent years to control pests and to eliminate the need for harmful pesticides (Bhattacharya et al. 2003; Hassan et al. 2016; Gangwar 2017). The use of natural enemies like parasitoids, predators, pathogens and viruses are key options in biological control strategies ((Hajek & Eilenberg 2018). Novel options include the use of entomopathogenic fungi (EPF) and entomopathogenic nematodes (EPNs) (Malan et al. 2018). All these options aim to control pests without the use ofharmful pesticides (Bhattacharya et al. 2003; Hassan et al. 2016).
Liriomyza species have a considerable cohort of natural enemies, with more than 80 different species reported (Liu et al. 2009). Most studies suggest that natural enemies are important in regulating Liriomyza species (Ode & Heinz 2002). The parasitoid, Diglyphus isaea (Walker, 1838) (Hymenoptera: Eulophidae), for example, is used to control agromyzid leaf miner populations in both their native and invaded areas (Rauf et al. 2000; Chen et al. 2003). In some parts of Africa, the best control of Liriomyza species achieved so far has been with augmentative release of D. isaea (Ode & Heinz 2002). In Kenya, mass production systems for parasitoids have been developed and used (Ode & Heinz 2002). In Germany, the most used parasitoid against Liriomyza in greenhouses, is Dacnusa sibirica Telenga, 1935 (Hymenoptera: Braconidae) (Leuprecht 1992). However, its effectiveness depends on the number of releases per week (between three and four releases recommended) (Leuprecht 1992). Other studies conducted in German-based greenhouses recommend the use of D. sibirica in combination with Opius pallipes Wesmael, 1835 (Hymenoptera: Braconidae) for effective control of the pests (Van der Linden 1991).
The use of biological control options helps in providing a stable and environmentally friendly pest management programme (Hajek & Delalibera 2009). The development of fungi as biocontrol agents against different pests, weeds and diseases has been an area of interest in recent years (Butt et al. 2001). Several entomopathogenic fungi (EPF) are common, and due to them being known to induce epizootics, they are very important in terms of regulating insect populations (Butt et al. 2001). EPF invade their hosts through the external cuticle, with some species being able to infect their hosts through their digestive tracts (Bonnie et al. 2004; Zimmermann 2007). The infestation process usually starts when the spores attach themselves to an insect's cuticle (Altinok et al. 2019). Spores germinate and penetrate the integument, through enzymatic degradation of the cuticle and physical pressure (Butt et al. 2001; Hajek & Delalibera 2009). After spore penetration, the fungi produce mycelia, which then ramify within the host haemocoele (Altinok et al. 2019). Due to the depletion of nutrients and the action of fungal toxins, the host dies (Butt et al. 2001; Bonnie et al. 2004). Under certain conditions hyphae, emerging from the dead cadavers, produce spores (Butt et al. 2001; Goettel et al. 2008).
The insect parasitic nematodes of the families Steinernematidae and Heterorhabditidae have been actively used since the 1990s (Poinar 1990; Navon & Ascher 2000). EPNs have been reported to show potential for use in different management strategies (Platt et al. 2020), due to their ability to locate, infect and kill several insect species actively (Campbell & Lewis 2002). The nematodes are obligate pathogens in nature, possessing a non-feeding phase that is also known as the infective juvenile (IJ) stage (Dillman et al. 2012; Shapiro-llan & Dolinski 2015). This free-living phase is the only stage that is able to survive outside the host and infect the insect host in soil substrates (Stock et al. 1999; Hazir et al. 2004). The IJs are only able to infect a host through natural openings like the mouth, the anus and the spiracles (Campbell & Lewis 2002; Hazir et al. 2004). After penetration, the IJs release a mutualistic bacterium, either through the anus or the mouth (depending on the genus) (Kaya & Gaugler 1993; Hazir et al. 2004). The released symbiotic bacteria colonise the insect and kill it within one to two days. The nematode then feeds on the bacteria and the bioconverted tissue of the dead larvae (Waterfield et al. 2009). Depending on its size, the nematode then develops through two to three more generations over a period of one to two weeks within the dead insect's body (Gõzel & Gõzel 2016).
EPF TO CONTROL LEAF MINERS
Entomopathogenic fungi (EPF) are an alternative to conventional chemical control of sap-feeding insects (Inbar & Gerling 2008). An increasing number of studies are being conducted to investigate the possibility of using biocontrol agents against leaf miners (Abd El-Salam et al. 2013). Several strains of Beauveria bassiana (Bals.-Criv.) Vuill. (Hypocreales: Cordycipitaceae) and Metarhizium anisopliae (Metschn.) Sorokin (Hypocreales: Clavicipitaceae) have been reported as virulent against dipteran pests (Hallouti et al. 2020). However, few attempts have yet been made to investigate the use of EPF against dipteran leaf miners (Quesada-Moraga et al. 2006).
Consistent results have been reported on the pathogenicity and virulence of M. anisopliae to Tuta absoluta (Meyrick, 1917) (Lepidoptera: Gelechiidae) third-instar larvae, indicating its potential for use as a biocontrol method for the control of other leaf miners, e.g., Liriomyza spp. (Inanl & Oldargc 2012; Alikhani et al. 2019). Both Metarhizium and Beauveria species have been documented as being pathogenic to eggs and larvae (Inanl & Oldargc 2012). However, not all leaf miners can be controlled by EPF and EPNs (Progar et al. 2015). Progar et al. (2015) reported an increase in the number of the invasive leaf miner, Profenusa thomsoni (Konow, 1886) (Hymenoptera: Tenthredinidae), after the application of different fungal isolates. The EPFs that have been reported to infest Liriomyza species include B. bassiana, Cordyceps fumosorosea (Wize) Kepler, B. Shrestha & Spatafora (Hypocreales: Cordycipitaceae), Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones & Samson (Hypocreales: Ophiocordycipitaceae), M. anisopliae and Akanthomyces lecanii (Zimm.) Spatafora, Kepler & B.Shrestha (Hypocreales: Cordycipitaceae) (Liu et al. 2009). Metarhizium and Beauveria species were found to be the most used EPF in previous studies (Gürlek et al. 2018).
A study by Migiro et al. (2011), investigating the pathogenicity of M. anisopliae and B. bassiana isolates against the adults of L. huidobrensis under laboratory conditions, found that all 17 isolates evaluated were pathogenic, causing mortalities of between 40% and 100% after five days of initial exposure. The LT50 of the isolates ranged from 2.6 and 5.4 days, depending on the isolate.
Noujeim et al. (2015) carried out pathogenicity tests to determine the effect of EPF (Beauveria bassiana) and EPN (Heterorhabditis indica) Poinar, Karunakar & David, 1992 (Rhabditida: Rhabditidae) on L. huidobrensis pupae. The results showed mortalities ranging between 73% and 97% at different concentrations of B. bassiana. Migiro et al. (2011) suggested a reduction in the oviposition potential of Liriomyza flies due to the applied fungal infection, thus supporting the use of entomopathogens for the control of Liriomyza spp. The above supports the success of entomopathogenic fungi against leaf miners (T. absoluta) under local conditions which has been reported in other studies (Erasmus et al. 2021). The similarity in the bioecology between T. absoluta and L. huidobrensis indicates that there is potential for entomopathogenic fungi to be used as a biocontrol agent of L. huidobrensis.
EPNS TO CONTROL LEAF MINERS
Following the degree of success attained in the use of EPNs against soil-based insect pests, their use as pest control agents of foliage pests has dramatically increased (Lacey & Georgis 2012; Platt et al. 2020). Several characteristics make it easy for EPNs to be widely used for controlling a variety of insect pests (Kerry & Hominick 2002), including their narrow host range, easy production on a large scale and the fact that they do not contaminate the environment (Kerry & Hominick 2002; Dunn et al. 2020). EPNs can enter leaf miners through oviposition sites and feeding punctures of the adults, making foliar application a viable option (Harris et al. 1990; Steyn et al. 2019).
Williams & Walters (2000) conducted a study to determine the susceptibility of three leaf miner species, namely L. huidobrensis, Liriomyza bryoniae (Kaltenbach, 1858) (Diptera: Agromyzidae) and Chromatomyia syngenesiae (Hardy, 1849) (Diptera: Agromyzidae), to Steinernema feltiae (Filipjev, 1934) (Rhabditida: Steinernematidae). All three species were highly susceptible to the EPN and pupal production was reduced compared to the control treatment. In addition, several species belonging to the genus Steinernema and Heterorhabditis have been tested against different leaf miner species (Garcia-del-Pino et al. 2018). In a South African study, Steyn et al. (2019) investigated the susceptibility of the leaf miner, Holocacista capensis Van Nieukerken & Geertsema, 2015 (Lepidoptera: Heliozelidae) to seven locally isolated EPN species. The bioassays showed that smaller sized nematodes Heterorhabditis baujardi Phan, Subbotin, Nguyen & Moens, 2003 (Rhabditida: Rhabditidae), Heterorhabditis indica and Heterorhabditis noenieputensis Malan, Knoetze & Tiedt, 2014 were the most virulent species as compared to the larger sized nematodes from the genus Steinernema. Therefore, efficiency of smaller sized nematodes to leaf mining insects is regarded as important (Bastidas et al. 2014). Nematode species like Steinernema yirgalemense Nguyen, Tesfamariam, Gozel, Gaugler & Adams, 2004 and Steinernema feltiae could also be used against micro-insect hosts, but virulence might be reduced due to the size of the IJs (Bastidas et al. 2014).
PARASITOIDS TO CONTROL LEAF MINERS
The success of parasitoids to control leaf miner pests can be attributed to the abundance of parasitoids associated with them (Rauf et al. 2000; Chen et al. 2003). In a survey on the species composition of the host crops of leaf miners and parasitoids in Indonesia, the most common parasitoid species associated with L. huidobrensis was found to be Hemiptarsenus varicornis (Girault, 1913) (Hymenoptera: Eulophidae), with 92% infestation recorded under field conditions (Rauf et al. 2000). One of the most used parasitoids around the world is D. isaea, which is a solitary larval ectoparasitoid of a variety of leaf miner species (Ode & Heinz 2002; Liu et al. 2009).
Rates of parasitism may depend on several factors. Studies of the rates of parasitism on the horse-chestnut leaf miner in different environments, including those near forests, villages, urban parks and streets in Switzerland were conducted for both first- and second-generation parasitoids (Girardoz et al. 2006). They concluded that the rates of parasitism increased during the pupal stage of the leaf miner in the first generation and decreased in the second generation. They attributed the poor rates of parasitism to a lack of synchronization, as the parasitoids attacking the first generation were probably old or emerging from the overwintering generation.
Parasitoids are affected by several different factors, both abiotic and biotic, with thermal conditions being found to be key (Rousse et al. 2009). Sugimoto et al. (2006) compared the thermal tolerance of different native species of parasitoids in Japan, when acting as biological control agents against the leaf miner Liriomyza trifolii (Burgess, 1880) (Diptera: Agromyzidae), a leaf miner belonging to the same genus as the potato leaf miner and affecting potatoes as well. A decrease in the length of the development period was observed, as the temperature rose above 25 °C. At temperatures above 30 °C, only male parasitoids emerged. The study also showed that the effects of temperature on host feeding and parasitisation differed, depending on the experimental temperature and the parasitoid species (Sugimoto et al. 2006). In addition, in a recent study Diglyphus isaea, Eulophinae sp., Utetes africanus (Szepligeti, 1910) (Hymenoptera: Braconidae), Dacnusa sibirica and Alysiinae sp. were recorded as parasitoids of the potato leaf miner in the Sandveld region in South Africa (Mugala et al. 2021), but most of the parasitoid species identified were not native (with the exception for Utetes africanus, which was described in 1910).
ALTERNATIVE CONTROL STRATEGIES
Sterile insect technique (SIT) is an environmentally conscious control strategy that aims to reduce pest populations by releasing overwhelming numbers of sterile male insects (Dyck et al. 2005). The population is suppressed through the sterility of the F1 generation, when sterile males mate with wild females to produce non-viable offspring (Knipling 1955). The Sterile Insect Techniques (SITs) programme has been present in South Africa since the 1990s, from single pest SIT programmes to multiple insect SIT programmes in recent years, for example against tortricid and tephritid pests (Barnes et al. 2015). Sterile insect technique has been used against a few lepidopteran insects, including the tomato leaf miner T. absoluta (Tarusikirwa et al. 2020), and used against several insect pests in South Africa, including Ceratitis capitata (Wiedemann, 1824) (Diptera: Tephritidae) on deciduous fruit and table grapes, Thaumatotibia leucotreta (Meyrick, 1913) (Lepidoptera: Tortricidae) on citrus, Cydia pomonella (Linnaeus, 1758) (Lepidoptera: Tortricidae) on apples and pears, and Eldana saccharina Walker, 1865 (Lepidoptera: Pyralidae) and Chilo sacchariphagus Bojer, 1856 (Lepidoptera: Crambidae) on sugarcane (Barnes et al. 2015). The efficacy of SIT in the management of other insects, its soundness with regards to the environment and its compatibility with different control measures, warrant investigation of the potential use of SIT on potato leaf miner management in South Africa. The use of sex pheromone-based strategies, like mass trapping and mating disruption are promising techniques for use against T. absoluta (Tarusikirwa et al. 2020), but have not yet been explored for potato leaf miner control.
CONCLUSION
Outbreaks of new pests in the agricultural industry requires baseline studies to understand their ecology and distribution, prior to development and implementation ofproper management practices. A review of L. huidobrensis and its management on potatoes in South Africa is therefore vital for the enhancement of IPM. The severity of leaf miner outbreaks in South Africa is becoming a challenge and substantial losses are incurred by several farmers as a result. Potato production in South Africa faces potato leaf miner outbreaks over the summer period. The severe leaf damage, as a result of these outbreaks, highlights the need to develop potato plants mechanically resistant to the larvae and egg stages of L. huidobrensis, which could be planted during the summer period.
Insecticide overuse in potato production has led to the development of leaf miner resistance against several broad-spectrum insecticides. The latter is a reason to promote the implementation of IPM practices, including those supporting the use of EPF and EPNs. The possibility that EPNs, EPF and parasitoids can infect and colonise all the life stages of the leaf miner is a cardinal point to consider, requiring conformational research. Although previous studies on the effects of biological interventions on the potato leaf miner have concentrated on some of the larval stages, not all the life stages have been investigated in depth. For instance, in South Africa, only a few studies have been conducted regarding the potato leaf miner, especially in the Western Cape province. The current review combined the available information regarding the alternative methods of pest control of the potato leaf miner, L. huidobrensis. There is potential for most of these control strategies to be implemented locally. Furthermore, continued research will increase the current knowledge of L. huidobrensis and the use of emerging biocontrol options of Tuta absoluta in South Africa, as a reference for studies on L. huidobrensis.
ACKNOWLEDGEMENTS
The authors would like to thank The National Research Foundation and Potatoes South Africa for funding of the project.
ORCID IDS
T Mugala - https://orcid.org/0000-0001-6482-5974
D Visser - https://orcid.org/0000-0002-9034-0214
AP Malan - https://orcid.org/0000-0002-9257-0312
P Addison - https://orcid.org/0000-0002-8227-339X
REFERENCES
Abd El-Salam AME, Salem HA, Salem SA. 2013. Biocontrol agents against the leaf miner, Liriomyza trifolii in faba bean fields. Archives of Phytopathology and Plant Protection. 46(9): 1054-1060. https://doi.org/10.1080/03235408.2012.757857. [ Links ]
Adendorff J. 2010. The bio-ecology of the grass leaf miner, Agromyza ocularis (Diptera: Agromyzidae), on wheat and barley in the Northern Cape Province, South Africa [PhD thesis]. Bloemfonetin: University of the Free State. [ Links ]
Alikhani M, Safavi SA, Iranipour S. 2019. Effect of the entomopathogenic fungus, Metarhizium anisopliae (Metschnikoff) Sorokin, on demographic fitness of the tomato leaf miner, Tuta absoluta (Meyrick) (Lepidoptera: gelechiidae). Egyptian Journal of Biological Pest Control. 29(1): 23. https://doi.org/10.1186/s41938-019-0121-0. [ Links ]
Altinok HH, Altinok MA, Koca AS. 2019. Modes of action of entomopathogenic fungi. Current Trends in Natural Sciences. 8: 117-124. [ Links ]
Alves FM, Rocha Gonring AH, Zanuncio JC, De Sena Fernandes ME, Plata-Rueda A, Fernandes FL. 2017. Economic damage levels and treatment thresholds for leafminer insects in Solanum tuberosum crops. Crop Protection. 100: 81-86. https://doi.org/10.1016/j.cropro.2017.06.008. [ Links ]
Ameixa O, Almeida L, Gonçalves A, Neto L. 2007. Feeding behaviour of Liriomyza huidobrensis (Blanchard) and L. trifolii (Burgess) adults on bean leaves. Journal of Insect Behavior. 20(1): 137-155. https://doi.org/10.1007/s10905-006-9070-z. [ Links ]
Atakan E, Canhilal R. 2004. Evaluation of yellow sticky traps at various heights for monitoring cotton insect pests. Journal of Agricultural and Urban Entomology. 21: 15-24. [ Links ]
Ateyyat MA, Mustafa TM. 2001. Cultural control of citrus leaf miner, Phyllocnistis citrella Stainton (Lepidoptera: Gracillariidae) on lemon in Jordan. International Journal of Pest Management. 47(4): 285-288. https://doi.org/10.1080/09670870110047145. [ Links ]
Barnes BN, Hofmeyr JH, Groenewald S, Conlong DE, Wohlfarter M. 2015. The sterile insect technique in agricultural crops in South Africa: a metamorphosis.... but will it fly? African Entomology. 23(1): 1-18. https://doi.org/10.4001/003.023.0103. [ Links ]
Basij M, Askarianzaeh A, Asgari S, Moharramipou S, Rafezi R. 2011. Evaluation of resistance of cucumber cultivars to the vegetable leaf miner (Liriomyza sativae Blanchard) (Diptera: Agromyzidae) in greenhouse. Chilean Journal of Agricultural Research. 71(3): 395400. https://doi.org/10.4067/S0718-58392011000300008. [ Links ]
Bastidas B, Portillo E, San-Blas E. 2014. Size does matter: the life cycle of Steinernema spp. in micro-insect hosts. Journal of Invertebrate Pathology. 121:4 6-55. https://doi.org/10.1016/j.jip.2014.06.010. [ Links ]
Ben Husin TOA. 2017. Biological control of tomato leaf miner Tuta absoluta using entomopathogenic nematodes, [PhD thesis]. Newcastle upon Tyne: Newcastle University. http://hdl.handle.net/10443/3845. [ Links ]
Bhattacharya AK, Mondal P, Ramamurthy VV, Srivastava RP. 2003. Beauveria bassiana: a potential bioagent for innovative integrated pest management programme. In: Srivastava RP, editor. Biopesticides and bioagents in integrated pest management of agricultural Crops. Lucknow: International Book Distributing Co. [ Links ]
Bonnie HO, Pereira RM, Klingeman WE, Quigley NB, Leckie BM. 2004. Emerging concepts in plant health management: Beauveria bassiana, a dual-purpose biological control with activity against insect pests and plant pathogens. Kerala: Research Signpost. [ Links ]
Butt TM, Jackson C, Magan N. 2001. Introduction: fungal biological bio-control agents: progress, problems and potential. In: Butt TM, Jackson C, Magan N, editors. Fungi as biocontrol agents: Progress, problems and potential. Wallingford: CAB International. p. 1-8. https://doi.org/10.1079/9780851993560.0001. [ Links ]
CABI (Centre for Agriculture and Bioscience International). 2018. Invasive species compendium: Liriomyza huidobrensis (Serpentine Leafminer) [Database]. https://www.cabi.org/isc/datasheet/30956
Campbell JF, Lewis EE. 2002. Entomopathogenic nematode host-search strategies. In: Lewis EE, Campbell JF, Sukhdeo MVK, editors. The behavioural ecology of parasites. Wallingford: CAB International. p. 13-38. https://doi.org/10.1079/9780851996158.0013. [ Links ]
Chabi-Olaye A, Mujica N, Lóhr B, Kroschel J. 2008. Role of agroecosystems in the abundance and diversity of Liriomyza leaf mining flies and their natural enemies. Abstracts of the XXIII International Congress of Entomology, 6-12 July 2008, Durban, South Africa.
Chavez GL, Raman KV. 1987. Evaluation of trapping and trap types to reduce damage to potatoes by the leaf miner, Liriomyza huidobrensis (Diptera, Agromyzidae). Insect Science and Its Application;. 8(3): 369-372. https://doi.org/10.1017/S1742758400005403. [ Links ]
Chen XX, Lang FY, Xu ZH, He JH, Ma Y. 2003. The occurrence of leaf miners and their parasitoids on vegetables and weeds in Hangzhou area, Southeast China. BioControl. 48(5): 515-527. https://doi.org/10.1023/A:1025726813462. [ Links ]
Connor EF, Taverner MP. 1997. The evolution and adaptive significance of the leaf-mining habit. Oikos. 79(1): 6-25. https://doi.org/10.2307/3546085. [ Links ]
Cornell HV. 1989. Endophage-ectophage ratios and plant defense. Evolutionary Ecology. 3(1): 64-76. https://doi.org/10.1007/BF02147932. [ Links ]
DAFF. (Department of Agriculture, Forestry & Fisheries, South Africa). 2012. A profile of the South African potato market value chain. https://www.nda.agric.za/docs/amcp/potato2012.pdf
Dara SK. 2019. The new integrated pest management paradigm for the modern age. Journal of Integrated Pest Management. 10(1): 1-9. https://doi.org/10.1093/jipm/pmz010. [ Links ]
Deadman M, Khan I, Thacker J, Al-Habsi K. 2002. Interaction between leaf miner damage and leaf necrosis caused by Alternaria alternate, on potato in the Sultanate of Oman. Plant Pathology Journal. 18(4): 210-215. https://doi.org/10.5423/PPJ.2002.18.4.210. [ Links ]
Dillman AR, Chaston JM, Adams BJ, Ciche TA, Goodrich-Blair H, Stock SP, Sternberg PW. 2012. An entomopathogenic nematode by any other name. PLoS Pathogens. 8: e1002527. https://doi.org/10.1371/journal.ppat.1002527. [ Links ]
Dreistadt SH, Newman JP, Robb KL. 1998. Sticky trap monitoring of insect pests. University of California Agriculture and Natural Resources Publication 21572, Oakland.
Dunn MD, Belur PD, Malan AP. 2020. In vitro liquid culture and optimization of Steinernema jeffreyense using shake flasks. BioControl. 65(2): 223-233. https://doi.org/10.1007/s10526-019-09977-7. [ Links ]
Dyck VA, Hendrichs J, Robinson AS. 2005. Sterile insect technique: Principles and practice in area-wide integrated pest management. Dordrecht: Springer. [ Links ]
EPPO (European and Mediterranean Plant Protection Organization). 2005. Liriomyza spp. PM 7/53(1). EPPO Bulletin. 35: 335-344. https://doi.org/10.1111/j.1365-2338.2005.00827.x. [ Links ]
Epsky ND, Morrill WL, Mankin RW. 2008. Traps for capturing insects. In: Capinera JL, editor. Encyclopedia of entomology. Dordrecht: Springer. p. 2318-2329. https://doi.org/10.1007/978-1-4020-6359-6_2523. [ Links ]
Erasmus R, Van Den Berg J, Du Plessis H. 2021. Susceptibility of Tuta absoluta (Lepidoptera: Gelechiidae) pupae to soil applied entomopathogenic fungal biopesticides. Insects. 12(6): 515. https://doi.org/10.3390/insects12060515. [ Links ]
Fenoglio MS, Salvo A. 2009. Liriomyza commelinae (Diptera: Agromyzidae): aAn alternative host for parasitoids of the leaf miner pest Liriomyza huidobrensis. International Journal of Pest Management. 55(4): 299-305. https://doi.org/10.1080/09670870902890561. [ Links ]
Gangwar RK. 2017. Role of biological control agents in integrated pest management approaches. Acta Scientific Agriculture. 1(2): 1-3. [ Links ]
Garcia-Del-Pino F, Morton A, Shapiro-Ilan D. 2018. Entomopathogenic nematodes as biological control agents of tomato pests. In: Wakil W, Brust GE, Perring TM, editors. Sustainable management of arthropod pests of tomato pests. London, UK: Academic Press. p. 269-282. https://doi.org/10.1016/B978-0-12-802441-6.00012-7 [ Links ]
Ge J, Wei J-N, Zhang D-J, Hu C, Zheng D-Z, Kang L. 2019. Pea leafminer Liriomyza huidobrensis (Diptera: Agromyzidae) uses vibrational duets for efficient sexual communication. Insect Science. 26(3): 510-522. https://doi.org/10.1111/1744-7917.12598. [ Links ]
Girardoz S, Kenis M, Quicke DLJ. 2006. Recruitment of native parasitoids by an exotic leaf miner, Cameraria ohridella: host-parasitoid synchronization and influence of the environment. Agricultural and Forest Entomology. 8(1): 49-56. https://doi.org/10.1111/j.1461-9555.2006.00281.x. [ Links ]
Goettel MS, Koike M, Kim JJ, Aiuchi D, Shinya R, Brodeur J. 2008. Potential of Lecanicillium spp. for management of insects, nematodes and plant diseases. Journal of Invertebrate Pathology. 98(3): 256-261. https://doi.org/10.1016/j.jip.2008.01.009. [ Links ]
Gözel U, Gözel C. 2016. Entomopathogenic nematodes in pest management. In: Gill H, editor. Integrated pest management (IPM): Environmentally sound pest management. London: IntechOpen. p. 55-69. https://doi.org/10.5772/63894. [ Links ]
Gürlek S, Sevim A, Sezgin F, Sevim E. 2018. Isolation and characterization of Beauveria and Metarhizium spp. from walnut fields and their pathogenicity against the codling moth, Cydia pomonella (L.) (Lepidoptera: tortricidae). Egyptian Journal of Biological Pest Control. 28(1): 50. https://doi.org/10.1186/s41938-018-0055-y. [ Links ]
Gurr GM, Wratten SD, Landis DA, You M. 2017. Habitat management to suppress pest populations: progress and prospects. Annual Review of Entomology. 62: 91-109. https://doi.org/10.1146/annurev-ento-031616-035050. [ Links ]
Hajek AE, Delalibera I. 2009. Fungal pathogens as classical biological control agents against arthropods. In: Roy HE, Vega FE, Chandler D, Goettel MS, Pell J. Wajnberg E, editors. The ecology of fungal entomopathogens. Dordrecht: Springer, p. 147-158. https://doi.org/10.1146/annurev-ento-031616-035050. [ Links ]
Hajek A E, Eilenberg J. 2012. Natural enemies: an introduction to biological control. Cambridge: Cambridge University Press. https://doi.org/10.1017/CBO9780511811838. [ Links ]
Hallouti A, Hamza MA, Zahidi A, Hammou RA, Bouharroud R, Aoumar AAB, Boubaker H. 2020. Diversity of entomopathogenic fungi associated with Mediterranean fruit fly (C. capitata) in Moroccan Argan forests and nearby area: impact of soil factors on their distribution. BMC Ecology. 20: 64. https://doi.org/10.1186/s12898-020-00334-2. [ Links ]
Harris MA, Begley JW, Warkentin DL. 1990. Liriomyza trifolii (Diptera: Agromyzidae) suppression with foliar applications of Steinernema carpocapsae (Rhabditida: Steinernematidae) and abamectin. Journal of Economic Entomology. 83(6): 2380-2384. https://doi.org/10.1093/jee/83.6.2380. [ Links ]
Hassan K, Pervin M, Mondai F, Mala M. Habitat management: a key option to enhance natural enemies of crop pest. Universal Journal of Plant Science. 2016;4(4):50-57. https://doi.org/10.13189/ujps.2016.040402.
Hazir S, Kaya HK, Stock PS, Keskin N. 2004. Entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) for biological control of soil pests. Turkish Journal of Biology. 27(4): 181-202. [ Links ]
Hering EM. 1951. The Biology of the Leaf Miners. Gravenhage: Junk. https://doi.org/10.1007/978-94-015-7196-8. [ Links ]
Hill MP, Bertelsmeier C, Clusella-Trullas S, Garnas JR, Robertson MP, Terblanche JS. Predicted decrease in global climate suitability masks regional complexity of invasive fruit fly species response to climate change. 2016;18(4):1105-1119. https://doi.org/10.1007/s10530-016-1078-5.
ínanli C, Yoldas Z, Birgücüc AK. 2012. Entomopatojen Funguslar Beauveria bassiana (Bals.) ve Metarhizium anisopliae (Metsch.)'nin Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae)'nin Yumurta ve Larva Dónemlerine Etkisi. [Effects of entomopathogenic fungi, Beauveria bassiana (Bals.) and Metarhizium anisopliae (Metsch.) on larvae and egg stages of Tuta absoluta (Meyrick) Lepidoptera: Gelechiidae]. Ege Üniversitesi Ziraat Fakültesi Dergisi. 49 (3):239-242. [ Links ]
Inbar M, Gerling D. 2008. Plant-mediated interactions between whiteflies, herbivores, and natural enemies. Annual Review of Entomology. 53(1): 431-448. https://doi.org/10.1146/annurev.ento.53.032107.122456. [ Links ]
IPPC (International Plant Protection Convention). 2016. DP 16: Genus Liriomyza. Rome: IPPC. p. DP16-DP33. [ Links ]
Johnson RAB, Owusu EO, Yawson GK. 2011. Review of the economic importance and sustainable management of the oil palm leaf miner Coelaenomenodera lameensis Berti and Mariau (Coleoptera: Chrysomelidae). Trends in Entomology. 7: 89-99. [ Links ]
Kaya HK, Gaugler R. 1993. Entomopathogenic nematodes. Annual Review of Entomology. 38(1): 181-206. https://doi.org/10.1146/annurev.en.38.010193.001145. [ Links ]
Kerry BR, Hominick W. 2002. Biological control. In: Lee DL, editor. The biology of nematodes. London: Taylor & Francis. p. 483-510. https://doi.org/10.1201/b12614-20.
Knipling EF. 1955. Possibilities of insect control or eradication through the use of sexually sterile males. Journal of Economic Entomology.; 48(4): 459-462. https://doi.org/10.1093/jee/48.4.459. [ Links ]
Kroschel J, Schaub B. 2013. Biology and ecology of potato tuber moths as major pests of potato. In: Giordanengo P, Vincent C, Alyokhin A, editors. Insect pests of potato: Global perspectives on biology and management. Oxford: Academic Press. p. 165-192. https://doi.org/10.1016/B978-0-12-386895-4.00006-5. [ Links ]
Kroschel J, Mujica N, Okonya J, Alyokhin A. 2020. Insect pests affecting potatoes in tropical, subtropical, and temperate regions. In: Campos H, Ortiz O, editors. The potato crop. Its agricultural, nutritional and social contribution to humankind. Cham: Springer. p. 251-306 nger;. https://doi.org/10.1007/978-3-030-28683-5_8. [ Links ]
Lacey LA, Georgis R. 2012. Entomopathogenic nematodes for control of insect pests above and below ground with comments on commercial production. Journal of Nematology. 44(2): 218-225. [ Links ]
Lanzoni A, Bazzocchi G, Burgio G, Fiacconi M. 2002. Comparative life history of Liriomyza trifolii and Liriomyza huidobrensis (Diptera: Agromyzidae) on beans: effect of temperature on development. Environmental Entomology. 31(5): 797-803. https://doi.org/10.1603/0046-225X-31.5.797. [ Links ]
Leuprecht B. 1992. Liriomyza huidobrensis, a new, dangerous leaf miner. Healthy Plants. 44: 51-58. [ Links ]
Liu T, Kang L, Heinz K, Trumble J. 2009. Biological control of Liriomyza leafminers: progress and perspective. Perspectives in Agriculture, Veterinary Science, Nutrition and Natural Resources. 4(4): 1-16. https://doi.org/10.1079/PAVSNNR20094004. [ Links ]
López R, Carmona D, Vincini AM, Monterubbianesi G, Caldiz D. 2010. Population dynamics and damage caused by the leaf miner Liriomyza huidobrensis Blanchard: (Diptera:_Agromyzidae), on seven potato processing varieties grown in temperate environment. Neotropical Entomology. 39(1): 108-114. https://doi.org/10.1590/S1519-566X2010000100015. [ Links ]
Lu Y, Bei Y, Zhang J. 2012. Are yellow sticky traps an effective method for control of sweetpotato whitefly, Bemisia tabaci, in the greenhouse or field? Journal of Insect Science. 12(1): 1-13. https://doi.org/10.1673/031.012.11301. [ Links ]
Lutaladio N, Castaldi L. 2009. Potato: the hidden treasure. Journal of Food Composition and Analysis. 22(6): 491-493. https://doi.org/10.1016/j.jfca.2009.05.002. [ Links ]
Macdonald OC. 1991. Responses of the alien leaf miners Liriomyza trifolii and Liriomyza huidobrensis (Diptera: Agromyzidae) to some pesticides scheduled for their control in the UK. Crop Protection. 10(6): 509-513. https://doi.org/10.1016/S0261-2194(91)80142-3. [ Links ]
Maharjan R, Jung C. 2016. Olfactory response and feeding preference of Liriomyza huidobrensis (Diptera: Agromyzidae) to potato cultivars. Environmental Entomology. 45(5): 1205-1211. https://doi.org/10.1093/ee/nvw078. [ Links ]
Malan AP, von Diest JI, Moore SD, Addison P. 2018. Control options for false codling moth, Thaumatotibia leucotreta (Lepidoptera: Tortricidae), in South Africa, with emphasis on the potential use of entomopathogenic nematodes and fungi. African Entomology. 26(1): 14-29. https://doi.org/10.4001/003.026.0014. [ Links ]
McCravy KW. 2018. A review of sampling and monitoring methods for beneficial arthropods in agroecosystems. Insects. 9(4): 170. https://doi.org/10.3390/insects9040170. [ Links ]
Migiro L, Maniania N, Chabi-Olaye A, Wanjoya A, Vandenberg J. 2011. Effect of infection by Metarhizium anisopliae (Hypocreales: Clavicipitaceae) on the feeding and oviposition of the pea leaf miner Liriomyza huidobrensis (Diptera: Agromyzidae) on different host plants. Biological Control. 56(2): 179-183. https://doi.org/10.1016/j.biocontrol.2010.09.013. [ Links ]
Mujica N, Cisneros F. 1997. The leaf miner fly in potato: plant reaction and natural enemies as natural mortality factors. In: International Potato Centre Program Report 1997-1998. Lima: Lima, Perú: CIP (International Potato Center). p. 129-140.
Mujica N, Kroschel J. 2011. Leafminer fly (Diptera: Agromyzidae) occurrence, distribution, and parasitoid associations in field and vegetable crops along the Peruvian coast. Environmental Entomology. 40(2): 217-230. https://doi.org/10.1603/EN10170. [ Links ]
Mujica N, Kroschel J. 2013. Pest intensity-crop loss relationships for the leaf miner fly Liriomyza huidobrensis (Blanchard) in different potato (Solanum tuberosum L.) varieties. Crop Protection. 47: 6-16. https://doi.org/10.1016/j.cropro.2012.12.019. [ Links ]
Mujica N. 2016. Population dynamics of the leafminer fly Liriomyza huidobrensis (Blanchard) and its associated parasitoids in two contrasting Peruvian potato agroecologies. In: Kroschel J, editor, Ecological approaches to manage the leaf miner fly Liriomyza huidobrensis (Blanchard) in potato-based agroecosystems of Peru. Weikersheim: Margraf. p. 13-25. [ Links ]
Musundire R, Chabi-Olaye A, Kruger K. 2012. Host plant effects on morphometric characteristics of Liriomyza huidobrensis, L. sativae and L. trifolii (Diptera: agromyzidae). Journal ofApplied Entomology. 136(1-2): 97-108. https://doi.org/10.1111/j.1439-0418.2010.01597.x. [ Links ]
Navon A, Ascher KRS, editors. 2000. Bioassays of entomopathogenic microbes and nematodes. Wallingford: CABI Publishing. https://doi.org/10.1079/9780851994222.0000. [ Links ]
Norris RF, Caswell-Chen EP, Kogan M. 2002. Concepts in integrated pest management. Upper Saddle River (New Jersey): Prentice Hall. [ Links ]
Noujeim E, Sakr J, El Sayegh D, Nemer N. 2015. In vitro susceptibility of the pea leaf miner Liriomyza. Lebanese Science Journal. 16(2): 19-26. [ Links ]
Ode PJ, Heinz KM. 2002. Host-size-dependent sex ratio theory and improving mass-reared parasitoid sex ratios. Biological Control. 24(1): 31-41. https://doi.org/10.1016/S1049-9644(02)00003-8. [ Links ]
Oerke EC, Dehne HW. 2004. Safeguarding production losses in major crops and the role of crop protection. Crop Protection. 23(4): 275285. https://doi.org/10.1016/jxropro.2003.10.001. [ Links ]
Okoth CA, Deng AL, Tabu IM, Akutse KS, Fiaboe KKM. 2014. Effect of host plant on feeding, biological and morphological parameters of Liriomyza huidobrensis Blanchard (Diptera: Agromyzidae). African Entomology. 22(3): 577-588. https://doi.org/10.4001/003.022.0315. [ Links ]
Ooi PAC. 1998. Challenges to implementing vegetable IPM in Southeast Asia. The FAO Programme for Community IPM in Asia. Acta Horticulturae. 5(13): 109-118. https://doi.org/10.17660/ActaHortic.1998.513.12. [ Links ]
Parrella MP, Keil CB. 1984. Insect pest management: the lesson of Liriomyza. Bulletin of the Entomological Society of America. 30(2): 22-25. https://doi.org/10.1093/besa/30.2.22. [ Links ]
Pimentel D. 1995. Amounts of pesticides reaching target pests: environmental impacts and ethics. Ournal of Agricultural and Environmental Ethics. 8(1): 17-29. https://doi.org/10.1007/BF02286399. [ Links ]
Pimentel D, McNair S, Janecka J, Wightman J, Simmonds C, O'Connell C, Wong E, Russel L, Zern J, Aquino T, et al. 2001. Economic and environmental threats of alien plant, animal, and microbe invasions. Agriculture, Ecosystems and Environment. 84(1): 1-20. https://doi.org/10.1016/S0167-8809(00)00178-X. [ Links ]
Pirtle E, Quirk M, Umina P, Ridland P. 2020. Manangement of leafmining flies in vegetable and nursery crops in Austrialia. Hort Innovation. Available at: https://ausveg.com.au/app/uploads/2020/12/Management-Plan-Exotic-leafminers.pdf
Platt T, Stokwe NF, Malan AP. 2020. A review of the potential use of entomopathogenic nematodes to control above-ground insect pests in South Africa. South African Journal of Enology and Viticulture. 41(1): 1-16. https://doi.org/10.21548/41-1-2424. [ Links ]
Poinar GO Jr. 1990. Taxonomy and biology of Steinernema and Heterorhabditis. In: Gaugler R, Kaya HK, editors. Entomopathogenic nematodes for biological control. Boca Raton (FL): CRC Press; p. 23-61. [ Links ]
Potatoes South Africa. 2017. Descriptions of 16 potato pests in South Africa. (Fact sheets - English fact sheets). Available at: https://www.potatoes.co.za/research/research-introduction/.
Powell JA. 1980. Evolution oflarval food preferences in microlepidoptera. Annual Review of Entomology. 25(1): 133-159. https://doi.org/10.1146/annurev.en.25.010180.001025. [ Links ]
Pretty J, Bharucha Z. 2015. Integrated pest management for sustainable intensification of agriculture in Asia and Africa. Insects. 6(1):152-182. https://doi.org/10.3390/insects6010152. [ Links ]
Progar RA, Kruse JJ, Lundquist JE, Zogas KP, Rinella MJ. 2015. An entomopathogenic fungus and nematode prove ineffective for biocontrol of an invasive leaf miner Profenusa thomsoni in Alaska. Biocontrol Science and Technology. 25(4): 373-382. https://doi.org/10.1080/09583157.2014.977224. [ Links ]
Quesada-Moraga EA, Ruiz-Garcia C, Santiago-Alvarez C. 2006. Laboratory evaluation of entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae against puparia and adults of Ceratitis capitata (Diptera: tephritidae). Journal of Economic Entomology. 99(6): 1955-1966. https://doi.org/10.1093/jee/99.6.1955. [ Links ]
Rauf A, Shepard BM, Johnson MW. 2000. Leafminers in vegetables, ornamental plants and weeds in Indonesia: Surveys of host plants, species composition and parasitoids. International Journal of Pest Management. 46(4): 257-266. https://doi.org/10.1080/09670870050206028. [ Links ]
Reitz SR, Gao Y, Lei Z. 2013. Insecticide use and the ecology of invasive Liriomyza leafminer management. In: Insecticides - Development of safer and more effective technologies. London: IntechOpen. p. 235-255. https://doi.org/10.5772/53874.
Rondon SI. 2010. The potato tuberworm: a literature review of its biology, ecology, and control. American Journal of Potato Research. 87(2): 149-166. https://doi.org/10.1007/s12230-009-9123-x. [ Links ]
Rousse P, Gourdon F, Roubaud M, Chiroleu F, Quilici S. 2009. Biotic and abiotic factors affecting the flight activity of Fopius arisanus, an egg-pupal parasitoid of fruit fly pests. Environmental Entomology. 38(3): 896-903. https://doi.org/10.1603/022.038.0344. [ Links ]
Schuster DJ, Gilreath JP, Wharton RA, Seymour PR. 1991. Agromyzidae (Diptera) leaf miners and their parasitoids in weeds associated with tomato in Florida. Environmental Entomology. 20(2): 720-723. https://doi.org/10.1093/ee/20.2.720. [ Links ]
Seebens H, Blackburn TM, Dyer EE, Genovesi P, Hulme PE, Jeschke JM, Pagad S, Pysek P, van Kleunen M, Winter M, et al. 2018. Global rise in emerging alien species results from increased accessibility of new source pools. Proceedings of the National Academy of Sciences. 115(10): E2264-E2273. https://doi.org/10.1073/pnas.1719429115. [ Links ]
Shapiro-Ilan D, Dolinski C. 2015. Entomopathogenic nematode application technology. In: Campos-Herrera R, editor. Nematode pathogenesis of insects and other pests. Sustainability in Plant and Crop Protection. Cham: Springer. p. 231-254. https://doi.org/10.1007/978-3-319-18266-7_9. [ Links ]
Sharma P, Singh A, Kahlon CS, Brar AS, Grover KK, Dia M, Steiner RL. 2018. The role of cover crops towards sustainable soil health and agriculture-A review paper. American Journal of Plant Sciences. 9(9): 1935-1951. https://doi.org/10.4236/ajps.2018.99140. [ Links ]
Sharma RA, Singh P, Setia A, Sharma AK. 2020. Insecticides and ovarian functions. Environmental and Molecular Mutagenesis. 61: 369-392. https://doi.org/10.1002/em.22355. [ Links ]
Spencer KA. 1973. Agromyzidae (Diptera) of economic importance. Series Entomologica, 9. Hague: Junk.
Steyn LAI, Addison A, Malan AP. 2019. Potential of South African entomopathogenic nematodes to control the leaf miner, Holocacista capensis (Lepidoptera: heliozelidae). South African Journal of Enology and Viticulture. 40(2): 301-309. https://doi.org/10.21548/40-2-3420. [ Links ]
Stock SP, Pryor BM, Kaya HK. 1999. Distribution of entomopathogenic nematodes (Steinernematidae and Heterorhabditidae) in natural habitats in California, USA. Biodiversity and Conservation. 8(4): 535-549. https://doi.org/10.1023/A:1008827422372. [ Links ]
Hondo T, Koike A, Sugimoto T. 2006. Comparison of thermal tolerance of seven native species of parasitoids (Hymenoptera: Eulophidae) as biological control agents against Liriomyza trifolii (Diptera: Agromyzidae) in Japan. Applied Entomology and Zoology. 41(1): 73-82. https://doi.org/10.1303/aez.2006.73. [ Links ]
Tarusikirwa VL, Machekano H, Mutamiswa R, Chidawanyika F, Nyamukondiwa C. 2020.Tuta absoluta (Meyrick) (Lepidoptera: Gelechiidae) on the "offensive" in Africa: Prospects for integrated management initiatives. Insects. 11(11): 764. https://doi.org/10.3390/insects11110764. [ Links ]
Umesha S, K. Singh P, P. Singh R. 2018. Microbial biotechnology and sustainable agriculture. Singh RK,.Mondal S, editors. Biotechnology for sustainable agriculture: Sustainable agriculture and food security. Cambridge: Woodhead. p. 185-205. https://doi.org/10.1016/B978-0-12-812160-3.00006-4.
Van Der Linden A. 1991. Biological control of the leaf miner Liriomyza huidobrensis (Blanchard) in Dutch glasshouse tomatoes. Mededelingen Faculteit Landbouwwetenschappen Rijksuniversiteit Gent. 56: 265-271. [ Links ]
Van Der Staay M. 1992. Chemical control of the larvae of the leaf miner Liriomyza huidobrensis (Blanchard) in lettuce. Mededelingen Faculteit Landbouwwetenschappen Rijksuniversiteit Gent. 57:473-478. [ Links ]
Videla M, Valladares G. 2007. Induced resistance against leaf miner eggs by extrusion in young potato plants. International Journal of Pest Management. 53(3): 259-262. https://doi.org/10.1080/09670870701439594. [ Links ]
Visser D. 2005. Guide to potato pests and their natural enemies in South Africa. ARC-Roodeplaat Vegetable and Ornamental Plants, Agricultural Research Council, Pretoria.
Visser D. 2009. A Complete Guide to Vegetable Pests in South Africa.
ARC-Roodeplaat, Vegetable and Ornamental Plant Institute, Pretoria.
Visser D. 2015, Potato. In: Uys MV, Prinsloo GL, editors. Insects of cultivated plants and natural pastures in Southern Africa. Hatfield: Entomological Society of Southern Africa. p. 48-64. [ Links ]
Waterfield NR, Ciche T, Clarke D. 2009. Photorhabdus and a host of hosts. Annual Review of Microbiology. 63(1): 557-574. https://doi.org/10.1146/annurev.micro.091208.073507. [ Links ]
Wei J, Zou L, Kuang R, He L. 2000. Influence of leaf tissue structure on host feeding selection by pea leaf miner Liriomyza huidobrensis (Diptera: agromyzidae). Zoological Studies. 39: 295-300. [ Links ]
Weintraub PG, Horowitz AR. 1995. The newest leaf miner pest in Israel, Liriomyza huidobrensis. Phytoparasitica. 23(2): 177-184. https://doi.org/10.1007/BF02980977. [ Links ]
Weintraub PG, Horowitz AR. 1996. Spatial and diel activity of the pea leaf miner (Diptera: Agromyzidae) in potatoes, Solanum tuberosum. Environmental Entomology. 25(4): 722-726. https://doi.org/10.1093/ee/25.4.722. [ Links ]
Weintraub PG, Mujica N. 2006. Note: systemic effects of a spinosad insecticide on Liriomyza huidobrensis larvae. Phytoparasitica. 34(1): 21-24. https://doi.org/10.1007/BF02981335. [ Links ]
Weintraub PG, Scheffer SJ, Visser D, Valladares G, Soares Correa A, Shepard BM, Rauf A, Murphy ST, Mujica N, MacVean C, et al. 2017. The invasive Liriomyza huidobrensis (Diptera: Agromyzidae): understanding its pest status and management globally. Journal of Insect Science. 17(1): 1-27. https://doi.org/10.1093/jisesa/iew121. [ Links ]
Williams EC, Walters KFA. 2000. Foliar application of the entomopathogenic nematode Steinernema feltiae against leaf miners on vegetables. Biocontrol Science and Technology. 10(1): 61-70. https://doi.org/10.1080/09583150029396. [ Links ]
Zehnder G, Trumble J. 1984. Host selection of Liriomyza species (Diptera: Agromyzidae) and associated parasites in adjacent plantings of tomato and celery. Environmental Entomology. 13(2): 492-496. https://doi.org/10.1093/ee/13.2.492. [ Links ]
Zimmermann G. 2007. Review on safety of the entomopathogenic fungi Beauveria bassiana and Beauveria brongniartii. Biocontrol Science and Technology.17(6): 553-596. https://doi.org/10.1080/09583150701309006. [ Links ]
 Correspondence:
Correspondence:
T Mugala
Email: tmugala@earth.ac.cr
Received: 14 June 2021
Accepted: 10 December 2021














