Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.30 Pretoria 2022
http://dx.doi.org/10.17159/2254-8854/2022/a12055
RESEARCH ARTICLE
Distribution and assemblage structure of blackflies in the western Aures Mountains, Algeria (Diptera: Simuliidae)
Besma M DambriI, II; Farrah SamraouiI, II; Boudjéma SamraouiI, III
ILaboratoire de Conservation des Zones Humides, Université 8 Mai 1945 Guelma, Guelma, Algeria
IIDepartment of Ecology, Université 8 Mai 1945 Guelma, Guelma, Algeria
IIIDepartment of Biology, University Badji Mokhtar Annaba, Annaba, Algeria
ABSTRACT
Besides their important ecological role in flowing waters, blackflies (Diptera: Simuliidae) may pose medical and veterinary risks. For seventeen months, we surveyed the blackflies of ten localities across the Aures Mountains, in the Saharan Atlas, Algeria, and recorded eight taxa (i.e. species, species groups or species complexes). High altitude sites were dominated by the Simulium ornatum (Meigen, 1818) group, whereas sites located on the southern slope of the Aures Mountains were occupied by the eurytopic Simulium velutinum (Santos Abreu, 1922) complex and the thermophilic, pollutant-tolerant Simulium ruficorne Macquart, 1838 'A' morphotype. Co-inertia analysis was used to determine the relationship between a species' abundance and habitat types. The co-inertia analysis revealed a likely co-structure between blackfly assemblages and measured environmental descriptors (water temperature, conductivity, current velocity, bed width, etc.) in sampled habitats. This confirmed the importance of altitude as a driver of blackfly distribution. Our results also showed that there has been an increase in anthropogenic pressures on the vulnerable freshwater biota of the Aures Mountains.
Keywords: aquatic insects, co-inertia analysis, freshwater biodiversity, North Africa, river stream
INTRODUCTION
Blackflies (Diptera: Simuliidae) are an important component of lotic ecosystems (Malmqvist et al. 2004). Their larvae and pupae develop in fresh, flowing water, with high levels of dissolved oxygen, while their adults are aerial. While a few species are entirely anthophilic, most adult blackfly females feed on birds, humans and other mammals (Crosskey 1990; Currie & Adler 2008). Consequently, blackflies may be a source of nuisance (Hansford & Ladle 1979; Tabatabaei et al. 2020; Sitarz et al. 2021), or act as vectors for pathogens, such as viruses, bacteria, protozoa and nematodes. These pathogens cause human onchocerciasis, eastern equine encephalitis, and other vector-borne diseases affecting mammals and birds (Brockhouse et al. 1993; Shelley & Coscaron 2001; Reeves & Nayduch 2002; Reeves et al. 2007; Barba et al. 2019). Therefore, the medical and veterinary importance of blackflies cannot be overstated (Adler et al. 2010; Watanabe 2014).
Blackflies are also considered the most diverse fauna of stream communities with more than 2401 species reported worldwide (Adler 2021). The Palearctic is considered the most species-rich biogeographic region with 700 recorded species (Currie & Adler 2008). In North Africa, 52 nominal species have been identified, with Morocco having the highest diversity (44 species), followed by Algeria (34 species), Tunisia (18 species), Libya (5 species) and Egypt (2 species) (Belqat et al. 2018).
In Algeria, several simuliid studies have focused mainly on surveys and relied largely on alpha taxonomy and species distributions (Belazzoug & Tabet-Derraz 1980; Gagneur & Clergue-Gazeau 1988). In contrast, recent studies that surveyed different Algerian regions, have tackled both taxonomy and bionomics (Djurdjura Mountains, central Algeria: Lounaci et al. 2000a, 2000b; Tafna River Basin, north-western Algeria: Boudghane-Bendiouis et al. 2014; Ahaggar, central Sahara: Cherairia & Adler 2018; Seybouse River Basin: Cherairia et al. 2014; and El Kala Highlands, north-eastern Algeria: Samraoui et al. 2021).
The present study, focusing on the Simuliidae of the western part of the Aures Mountains, aims to survey this important family in a poorly explored region. We also used multivariate analysis to analyse the response of community composition to environmental conditions by testing for possible co-structure between measured habitat characteristics and blackfly assemblages, in line with the habitat template concept (Southwood 1977; Townsend & Hildrew 1994).
MATERIALS AND METHODS
Study area
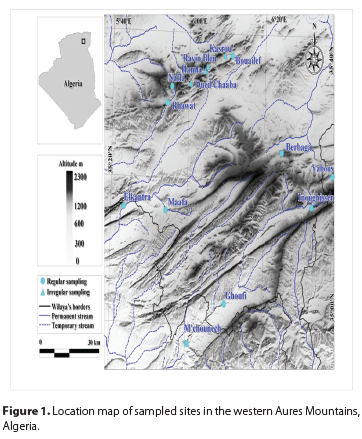
The Aures Massif, located at the eastern end of Algeria, is part of the Saharan Atlas Mountains (Figure 1). The north-east-south-west orientation of this mountain range led to the development of many valleys with in the alignment. The region covers three provinces, namely Batna, Khenchela and Biskra, an area of 2529 km2. The climate in the Aures region varies between semi-arid, with cold winters in the northern part (Batna and Khenchela), to arid, with temperate winters in the southern part (Biskra). The area of study also included a protected park, namely the Belezma National Park, which harbours the Atlas cedar Cedrus atlantica (Endl.) Manetti ex Carriere, a coniferous tree endemic to Morocco and Algeria.
The collection of blackflies was part of a comprehensive study of macroinvertebrates of the region, carried out over a period of 17 months, from April 2018 to August 2019 (Dambri et al. 2020).
Blackfly larvae and pupae were sampled monthly at ten localities (Table 1, Figure 1) using two methods. At each locality, an area of 100 m2 was kick-sampled by walking across all microhabitats and samples collected using a dip-net (25 cm diameter, 500 |im mesh size). In addition, we sampled a random set of ten cobbles with an average size of 10 cm and collected larvae and pupae using entomological forceps. Sampling covered small to medium-sized mountainous streams with different degrees of ease of access. An additional set of four localities, not easily accessible, were sampled occasionally (Table 1, Figure 1). Larvae and pupae were fixed in ethanol or Carnoy's solution, with identification performed by Prof. Peter H Adler (Clemson University).
Physicochemical sampling and environmental data
For each sampling event, we recorded the physical and chemical parameters of the water in situ using a tape (water depth and river bed width) and multi-probes: conductivity, total dissolved solids (TDS), water temperature, and pH were measured using an Adwa AD32 tester and a HANNA HI1271 pH electrode. Water samples were transported in a cooler and the remaining parameters (i.e. NO3, NO2, NH4, CO3, HCO3, Cl and O2) measured within 48 hours in the laboratory. Water velocity was estimated using a floating cork stopper timed with a stopwatch. As recurrent droughts in intermittent streams resulted in missing data, only data (temperature, conductivity, TDS, etc.) recorded from November to February were presented/analysed.
Statistical analyses
A co-inertia analysis (CIA) was performed using the ade4 package (Doledec & Chessel 1994; Dray et al. 2003) to test for co-structure between the blackfly assemblages and the measured environmental descriptors. The blackfly matrix was made up of the total abundance of each taxon at each site. Only regularly (monthly) sampled sites were included in the analysis. The vectorial correlation coefficient 'RV' of the CIA measured the overall correlation between the recorded taxa and the environmental descriptors. The RV ranged from 0 (all the taxa are independent of environmental variables) and 1 (perfect match). The significance of the RV coefficient was tested by performing a Monte-Carlo test (random permutation of the rows of both tables) (Dray et al. 2003). All statistical analyses were performed using R software version 4.0.5 (R Development Core Team 2021).
RESULTS
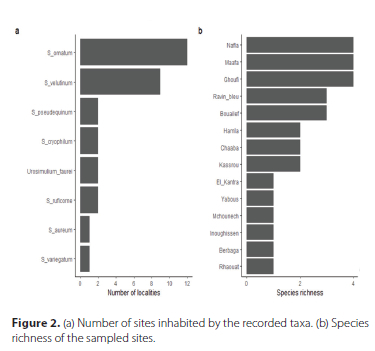
A total of 479 specimens were identified and assigned to eight taxa (i.e. species, species groups or species complexes) during this study. The Simulium ornatum (Meigen, 1818) group was the most abundant and widespread taxon in the western Aures (Figure 2a). The S. velutinum (Santos Abreu, 1922) complex was also abundant and widespread, but to a lesser extent than S. ornatum. Three sites (i.e. Nafla, Maafa and Ghoufi) had four taxa present and were the most species-rich localities (Figure 2b).
Simulium (Simulium) ornatum (Meigen, 1818) group
The S. ornatum group was one of the two most common taxa in this study and was found at all the sites except M'Chouneche, Bouailef and El Kantra. The water temperature was high at the last two stations. The taxon tolerated a wide range of water temperatures, but seemed to avoid localities with high water conductivity and TDS (Table 2).
Prosimulium faurei Bertrand & Grenier, 1972
First record from the Aures Mountains. Our record from the Aures region is associated with a temporary steam (Maafa) at 932 m above mean sea level (AMSL). The substrate was sandy with cobbles and the water was clean with a low flow (0.24 m s-1).
Simulium (Eusimulium) aureum (Fries, 1824) group
We recorded two larvae and one pupa from one small mountainous stream (Ravin Bleu) at 1335 m AMSL. The site was visited only once for reasons related to accessibility.
Simulium (Eusimulium) velutinum (Santos Abreu, 1922) complex
Our sampling showed that the S. velutinum complex had a wide distribution (i.e. Ghoufi, Maafa, Bouailef, Kassrou, Nafla, M'Chouneche, Hamla and Ravin Bleu) in the Aures region over a remarkable altitudinal range (i.e. 350-1700 m AMSL). Simulium velutinum s.l. has been shown to be made up of a complex of sibling species, based on the banding sequences of the polytene chromosomes from the larval salivary glands (Cherairia et al. 2014).
Simulium (Nevermannia) ruficorne Macquart, 1838 'A'
First record from the Aures Mountains. This taxon was collected from two sites (Bouailef and El Kantra) with a temperature range from 17.87-27.45 °C (Table 2). The species seemed to tolerate high values of water conductivity and TDS (Table 2). We based the identification of morphoform 'A' on gill structure (Cherairia et al. 2014).
Simulium (Nevermannia) cryophilum (Rubtsov, 1959) complex
First record from the Aures Mountains. We recorded it at three localities (i.e. Ghoufi, Maafa, and Kassrou). Characteristically, the taxon seemed to avoid localities with high water temperatures, but appeared to tolerate high water conductivity values (Table 2).
Simulium (Simulium) variegatum (Meigen, 1818) group
First record from the Aures Mountains. We collected S. variegatum larvae and pupae from two temporary streams (i.e. Maafa and Ravin Bleu) with low current velocity (0.24 m s-1 for Maafa).
Simulium (Wilhelmia) pseudequinum Seguy, 1921
We found it at two different localities (i.e. Ghoufi and Nafla) characterized by the presence of a large amount of macrophytes. In addition, the species seemed to tolerate high values of water conductivity and TDS (Table 2).
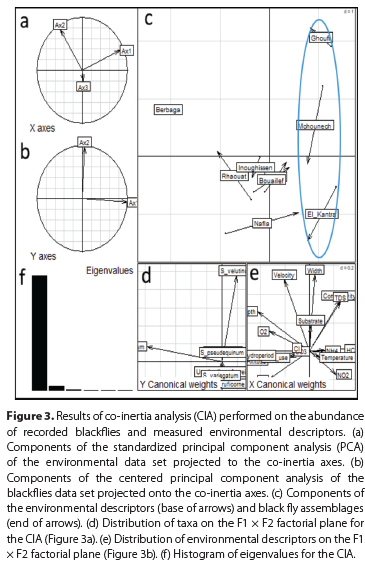
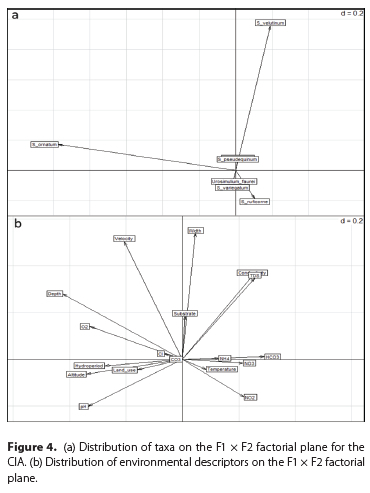
The CIA indicated the existence of a co-structure between the blackfly assemblages and the measured environmental descriptors (Figure 3a-f). The Monte-Carlo test using 10 000 replicates gave a marginally significant p-value (0.10) for the coefficient of vectorial correlation (RV = 0.42) for the CIA. This indicated a likely relationship between the distribution of black flies and the measured environmental descriptors. Axis 1, representing 96% of the total variance, separated high altitude sites from the low altitude Saharan sites, located on the southern flank of the Saharan Atlas. The high altitude sites were well oxygenated localities, with relatively deep water, dominated by taxa such as S. ornatum (Figure 4a). The low altitude sites were characterized by high water conductivity and temperature, high loads of total dissolved solids, nitrates and nitrites (Figure 4b) and were dominated by taxa such as Simulium ruficorne 'A' and the S. velutinum complex. Of lesser importance (3.5% of total inertia), Axis 2 organised the Saharan localities along increasing gradient of bed width.
DISCUSSION
In this study eight species, species complexes or groups were recorded in the western Aures, representing 23.5% of the known blackfly fauna of Algeria (Belqat et al. 2018). Four of the recorded taxa are new to the Aures [viz. Prosimulium faurei, Simulium (Nevermannia) ruficorne 'A', Simulium (Nevermannia) cryophilum complex and the Simulium (Simulium) variegatum group].
Previous records
The presence of the S. ornatum group has been recorded in several studies for localities across Algeria, namely the Batna Department and Bouïra Province (Edwards 1923), Biskra Province (Belazzoug & Tabet-Derraz 1980), the Oued El Haï Basin (Arigue et al. 2016), the Djurdjura Mountains (Lounaci et al. 2000b), the Tlemcen Mountains (Gagneur & Clergue-Gazeau 1988; Boudghane-Bendiouis et al. 2014), the Seybouse Basin (Cherairia et al. 2014), and El Kala, north-eastern Algeria (Samraoui et al. 2021).
Prosimulium faurei was previously found in the Tafna watershed in north-western Algeria (Gagneur & Clergue-Gazeau 1988). In north-eastern Algeria, it has been recorded in the Seybouse Basin (Cherairia et al. 2014) and the El Kala region (Samraoui et al. 2021).
The Simulium (Eusimulium) velutinum complex has been recorded in Algeria (Gagneur & Clergue-Gazeau 1988) from the Tlemcen Mountains in north-western Algeria and the Djurdjura Mountains (Lounaci et al. 2000a, 2000b). It was also recorded in the Seybouse Basin (Cherairia et al. 2014), the El Kala region (Samraoui et al. 2021) and the Aures region (Adler et al. 2015; Arigue et al. 2016).
Simulium (Nevermannia) ruficorne 'A' was previously recorded from several Algerian localities, especially those in the Sahara. It was found in habitats characterized by relatively high water temperatures (Cherairia & Adler 2018). It was also recorded in the Biskra area, Hoggar and Tassili N'Ajjer Mountains, Tlemcen Mountains, Seybouse Basin and El Kala region (Edwards 1923; Parrot 1949; Grenier & Clastrier 1960; Belazzoug & Tabet-Derraz 1980; Cherairia et al. 2014; Cherairia & Adler 2018; Samraoui et al. 2021).
The Simulium (Nevermannia) cryophilum complex is known to occur in eastern and western Algeria (Gagneur & Clergue-Gazeau 1988), the Djurdjura Mountains (Lounaci et al. 2000b; Haouchine & Lounaci 2012), the El Kala region (Samraoui et al. 2021) and the Tlemcen Mountains (Gagneur & Clergue-Gazeau 1988). Similarly, the S. variegatum group is well documented in Algeria, with records for the Djurdjura Mountains (Lounaci et al. 2000b; Haouchine & Lounaci 2012) and Tlemcen Mountains (Clergue-Gazeau et al. 1991).
Simulium pseudequinum was first recorded from Batna, Biskra, Bouira and Constantine (Edwards 1923). Thereafter, the species was collected in western Algeria from the Tlemcen Mountains (Gagneur & Clergue-Gazeau 1988; Boudghane-Bendiouis et al. 2014) and Mascara Province (Parrot 1949). It was also recorded in the Djurdjura Mountains (Lounaci et al. 2000a, 2000b; Haouchine & Lounaci 2012), the Seybouse Basin (Cherairia et al. 2014), the El Kala region (Samraoui et al. 2021) and the Aures region (Belazzoug & Tabet-Derraz 1980; Oued El Hal Basin: Arigue et al. 2016).
Ecology
Two taxa, S. ornatum and S. velutinum were most widespread and abundant, dominating all other taxa recorded in the Aures. This result is similar to that obtained for the El Kala region (Samraoui et al. 2021) and the Tafna River Basin (Boudghane-Bendiouis et al. 2014), but differs from that obtained for the Seybouse River, where S. pseudequineum was the dominant taxon (Cherairia et al. 2014). Simulium pseudequineum, confined to only two sites in the Aures, is often a widespread lowland species, inhabiting streams with large bed width and high water conductivity, both in the Maghreb (Boudghane-Bendiouis et al. 2014; Cherairia et al. 2014; Samraoui et al. 2021) and the Iberian Peninsula (Gallardo-Mayenco & Toja 2002). The Seybouse River Basin may offer many opportunities for S. pseudequineum, which favours low-elevation sites, with a large river bed width and high water conductivity.
Likewise, larvae of S. velutinum were often dominant in the Aures, occupying lowland sites. A result consistent with records found elsewhere in Algeria (Boudghane-Bendiouis et al. 2014; Samraoui et al. 2021). This taxon is also known to tolerate waters with a high load of organic matter (Gallardo-Mayenco & Toja 2002). In fact, the large ecological amplitude of S. velutinum indicates the possible presence of cryptic species (Adler et al. 2015).
In contrast, S. ornatum presents a different case and it is worth noting that this taxon (group) may represent multiple cryptic species (Belqat et al. 2018), widely distributed in the western Mediterranean. The species S. ornatum is the most common and most frequently recorded species in Austrian streams and rivers (Ofenböck et al. 2002). Moreover, the ecology of this taxa seems to vary geographically. In the El Kala region, it is dominant and mainly recorded at downstream sites (Samraoui et al. 2021), while the taxon is clearly crenophilic or rhithrophilic in central and western Algeria and Morocco (Gagneur & Clergue-Gazeau 1988; Giudicelli et al. 2000; Boudghane-Bendiouis et al. 2014; this study). Elsewhere, S. ornatum was found in Pyrenean streams with slow currents, dominated by aquatic vegetation (Vincon & Clergue-Gazeau 1993). Once again, these contradictory results would be consistent with the presence of a cryptic species complex.
Despite the low statistical power of the performed CIA, due to a small sample size, the results clearly indicate the importance of altitude in driving blackfly distribution in the Aures. This result is congruent with numerous other studies in Algeria (Boudghane-Bendiouis et al. 2014; Samraoui et al. 2021) and elsewhere (Giudicelli & Dakki 1984; Vincon & Clergue-Gazeau 1993; Ya'cob et al. 2016). Undoubtedly, various environmental factors, such as temperature, dissolved oxygen, water flow, water depth, and stream width are correlated with altitude.
A good knowledge of the status and ecology of the freshwater biodiversity in the Maghreb is urgently needed, as climate change, interacting with other anthropogenic stressors, is fast disturbing freshwater communities. These disturbances are leading to a precipitous decline in both the diversity and abundance of freshwater biota (Benslimane et al. 2019), with the conditions favouring widely distributed, thermophilic species (Morghad et al. 2019). High loads of stressors, such as nitrate and nitrites, common chemical contaminants that may negatively affect human health, are now routinely found in Maghrebian streams and rivers (Abdesselam et al. 2013; Aghzar et al. 2002). These groundwater contaminants are often associated with land use, with high levels of nitrates possibly a result of the use of fertilizers in apple orchards in the Aures, and geological factors. There is thus a need to monitor freshwater biodiversity, including taxa such as blackflies that may pose medical and veterinary risks.
ACKNOWLEDGEMENTS
We are most grateful to two anonymous reviewers for their helpful comments and suggestions. We thank the Algerian Ministère de L'Enseignement Supérieur et de la Recherche Scientifique (M.E.S.R.S./D.G.R.S.T.D.) for material support.
REFERENCES
Abdesselam S, Halitim A, Jan A, Trolard F, Bourrié G. 2013. Anthropogenic contamination of groundwater with nitrate in arid region: case study of southern Hodna (Algeria). Environmental Earth Sciences. 70(5): 2129-2141. https://doi.org/10.1007/s12665-012-1834-5. [ Links ]
Adler PH. 2021. World blackflies (Diptera: Simuliidae): a comprehensive revision of the taxonomic and geographic inventory [2021]. [accessed 7 July 2021]. https://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf.
Adler PH, Cheke RA, Post RJ. 2010. Evolution, epidemiology, and population genetics of black flies (Diptera: simuliidae). Infection, Genetics, and Evolution. 10(7): 846-865. https://doi.org/10.1016/j.meegid.2010.07.003. [ Links ]
Adler PH, Cherairia M, Arigue SF, Samraoui B, Belqat B. 2015. Cryptic biodiversity in the cytogenome of bird-biting blackflies in North Africa. Infection, Genetics and Evolution. 29(3): 276-289. https://doi.org/10.1111/mve.12115. [ Links ]
Aghzar N, Berdai H, Bellouti A, Soudi B. 2002. Pollution nitrique des eaux souterraines au Tadla (Maroc). [Ground water nitrate pollution in Tadla (Morocco)]. Revue des Sciences de l'Eau. 15: 459-492. https://doi.org/10.7202/705465ar. [ Links ]
Arigue SF, Adler PH, Belqat B, Bebba N, Arab A. 2016. Biodiversité des mouches noires (Diptera: Simuliidae) et qualité physicochimique des eaux du bassin versant de l'oued El Haï (Aurès-Algérie). Journal of Materials and Environmental Science. 7: 4839-4849. [ Links ]
Barba M, Fairbanks EL, Daly JM. 2019. Equine viral encephalitis: prevalence, impact, and management strategies. Veterinary Medicine: Research and Reports. 10: 99-110. https://doi.org/10.2147/VMRR.S168227. [ Links ]
Belazzoug S, Tabet-Derraz O. 1980. Note sur les simulies du Tassili n'Ajjer. [Note on Simulium of Tassili n'Ajjer]. Archives de l'Institut Pasteur d'Algérie. 54: 107-108. PMID:6927586. [ Links ]
Belqat B, Adler PH, Cherairia M, Boudghane-Bendiouis CC. 2018. Inventory of the Black Flies (Diptera: Simuliidae) of North Africa. Zootaxa. 4442(2): 201-220. https://doi.org/10.11646/zootaxa.4442.2.1. [ Links ]
Benslimane N, Chakri K, Haiahem D, Guelmami A, Samraoui F, Samraoui B. 2019. Anthropogenic stressors are driving a steep decline of hemipteran diversity in dune ponds in north-eastern Algeria. Journal of Insect Conservation. 23(3): 475-488. https://doi.org/10.1007/s10841-019-00133-1. [ Links ]
Boudghane-Bendiouis CC, Abdellaoui-Hassaïne K, Belqat B, Franquet E, Hacene SB, Yadi B. 2014. Habitat characterization of black flies (Diptera: Simuliidae) in the Tafna catchment of western Algeria. Open Journal of Ecology. 4(16): 1014-1024. https://doi.org/10.4236/oje.2014.416084. [ Links ]
Brockhouse CL, Vajime CG, Marin R, Tanguay RM. 1993. Molecular identification of onchocerciasis vector sibling species in black flies (Diptera: Simuliidae). Biochemical and Biophysical Research Communications. 194(2): 628-634. https://doi.org/10.1006/bbrc.1993.1867. [ Links ]
Cherairia M, Adler PH. 2018. Genetic variation in a colonization specialist, Simulium ruficorne (Diptera: Simuliidae), the world's most widely distributed black fly. PLoS One. 13(10): e0205137. https://doi.org/10.1371/journal.pone.0205137. [ Links ]
Cherairia M, Adler PH, Samraoui B. 2014. Biodiversity and bionomics of the black flies (Diptera: simuliidae) of northeastern Algeria. Zootaxa. 3796(3796): 166-174. https://doi.org/10.11646/zootaxa.3796.L8. [ Links ]
Crosskey RW. 1990. The natural history of blackflies. Chichester: John Wiley. [ Links ]
Currie DC, Adler PH. 2008. Global diversity of black flies (Diptera: Simuliidae) in freshwater. Hydrobiologia. 595(1): 469-475. https://doi.org/10.1007/s10750-007-9114-1. [ Links ]
Dambri BM, Karaouzas I, Samraoui B, Samraoui F. 2020. Contribution to the knowledge of the caddisfly fauna of Algeria: an updated checklist of Algerian Trichoptera with new records from the Aures region. Zootaxa. 4786(2): 221-232. https://doi.org/10.11646/zootaxa.4786.2.4. [ Links ]
Doledec S, Chessel D. 1994. Co-inertia analysis: an alternative method for studying species-environment relationships. Freshwater Biology. 31(3): 277-294. https://doi.org/10.1111/j.1365-2427.1994.tb01741.x. [ Links ]
Dray S, Chessel D, Thioulouse J. 2003;Co-inertia analysis and the linking of ecological data tables. Ecology. 84(11): 3078-3089. https://doi.org/10.1890/03-0178. [ Links ]
Edwards FW. 1923On some Algerian species of Simulium. Archives de l'Institut Pasteur d'Algérie. 1: 647-653. [ Links ]
Gagneur J, Clergue-Gazeau M. Les Simulies d'Algérie (Diptera: Simuliidae). 1988. I. Premières données biogéographiques et écologiques sur les espèces de l'Ouest-Algérien. Annales de Limnologie. 24(3): 275-284. https://doi.org/10.1051/limn/1988024. [ Links ]
Gallardo-Mayenco A, Toja J. 2002. Spatio-temporal distribution of simuliids (Diptera) and associated environmental factors in two Mediterranean basins of southern Spain. Limnetica. 21(1): 47-57. https://doi.org/10.23818/limn.21.05. [ Links ]
Giudicelli J, Dakki M. 1984. Les sources du Moyen Atlas et du Rif (Maroc): Faunistique (description de deux espèces nouvelles de Trichoptères), écologie, intérêt biogéographique. Bijdragen tot de Dierkunde. 54(1): 83-100. https://doi.org/10.1163/26660644-05401007. [ Links ]
Giudicelli J, Bouzidi A, Ait Abdelaali N. 2000. Contribution à l'étude faunistique et écologique des simulies (Diptera: Simuliidae) du Maroc. IV. Les simulies du Haut Atlas. Description d'une nouvelle espèce. Annales de Limnologie. 36(1): 57-80. https://doi.org/10.1051/limn/2000005. [ Links ]
Grenier P, Clastrier J. 1960. Une simulie saharienne: Simulium ruficorne Macquart. [A Saharan simulid: Simulium ruficorne Macquart]. Archives de l'Institut Pasteur d'Algérie. 38: 329-330. PMID:13829202. [ Links ]
Hansford RG, Ladle M. 1979. The medical importance and behaviour of Simulium austeni Edwards (Diptera: Simuliidae) in England. Bulletin of Entomological Research. 69(1): 33-41. https://doi.org/10.1017/S0007485300017867. [ Links ]
Haouchine S, Lounaci A. 2012. Les macroinvertébrés benthiques des cours d'eau de Kabylie (Algérie): faunistique, écologie et répartition géographique. Bulletin de la Société Zoologique de France. 137: 133-156. [ Links ]
Lounaci A, Brosse S, Ait Mouloud S, Lounaci-Daoudi D, Mebarki N, Thomas A. 2000a. Current knowledge of benthic invertebrate diversity in an Algerian stream: a species checklist of the Sébaou River basin (Tizi-Ouzou). Bulletin de la Société d'Histoire Naturelle de Toulouse. 136: 43-55. [ Links ]
Lounaci A, Brosse S, Thomas A, Lek S. 2000b. Abundance, diversity and community structure of macroinvertebrates in an Algerian stream: the Sébaou wadi. Annales de Limnologie. 36(2): 123-133. https://doi.org/10.1051/limn/2000008. [ Links ]
Malmqvist B, Adler PH, Kuusela K, Merritt RW, Wotton RS. 2004. Black flies in the boreal biome, key organisms in both terrestrial and aquatic environments: a review. Ecoscience. 11(2): 187-200. https://doi.org/10.1080/11956860.2004.11682824. [ Links ]
Morghad F, Samraoui F, Touati L, Samraoui B. 2019. The times they are a changin': impact of land-use shift and climate warming on the odonate community of a Mediterranean stream over a 25-year period. Vie & Milieu. 69: 25-33. [ Links ]
Ofenbôck T, Moog O, Car M. 2002. Do Austrian Blackfly fauna (Diptera: Simuliidae) support the typological approach of the EU Water Framework directive? Limnologica. 32(3): 255-272. https://doi.org/10.1016/S0075-9511(02)80032-9. [ Links ]
Parrot L. 1949. Quelques notes sur les simulidés d'Algérie. [A few notes on the Algerian simulids.] Archives de l'Institut Pasteur d'Algérie. 27(3): 273-276. [ Links ]
R Development Core Team. 2021. R: A language and environment for statistical computing. Vienna, Austria.
Reeves WK, Adler PH, Râtti O, Malmqvist B, Strasevicius D. 2007. Molecular detection of Trypanosoma (Kinetoplastida: Trypanosomatidae) in black flies (Diptera: Simuliidae). Comparative Parasitology. 74(1): 171-175. https://doi.org/10.1654/4243.1. [ Links ]
Reeves WK, Nayduch D. 2002. Pathogenic Bacillus from a larva of the Simulium tuberosum species complex (Diptera: Simuliidae). Journal of Invertebrate Pathology. 79: 126-128. https://doi.org/10.1016/S0022-2011(02)00016-2. [ Links ]
Samraoui B, Samraoui F, Al-Misned FA, El-Serehy HA, Adler PH. 2021. Ecological determinants of black fly assemblages of relict mountain streams in northeastern Algeria, plus new records (Diptera: simuliidae). Global Ecology and Conservation. 27: e01609. https://doi.org/10.1016/j.gecco.2021.e01609. [ Links ]
Shelley AJ, Coscaron S. 2001. Simuliid blackflies (Diptera: Simuliidae) and ceratopogonid midges (Diptera: Ceratopogonidae) as vectors of Mansonella ozzardi (Nematoda: Onchocercidae) in northern Argentina. Memorias do Instituto Oswaldo Cruz. 96(4): 451-458. https://doi.org/10.1590/S0074-02762001000400003. [ Links ]
Sitarz M, Buczek A, Buczek W. 2021. Skin lesions and systemic reactions in humans infested by blackflies (Diptera: Simuliidae) in recreational areas in southeastern Poland. Journal of Clinical Medicine. (4): 788. https://doi.org/10.3390/jcm10040788. PMID:33669296. [ Links ]
Southwood TRE. 1977. Habitat, the templet for ecological strategies. Journal of Animal Ecology. 46(2): 336-365. https://doi.org/10.2307/3817. [ Links ]
Tabatabaei F, Azarmi S, Abbaszadeh Afshar MJ, Yarizadeh H, Mohtasebi S. 2020. Blackfly fever and dermatitis caused by Simulium kiritshenkoi: a human case report in Iran. BMC Infectious Diseases. 20(1): 348. https://doi.org/10.1186/s12879-020-05070-y. [ Links ]
Townsend CR, Hildrew AG. 1994. Species traits in relation to a habitat templet for river systems. Freshwater Biology. 31(3): 265-275. https://doi.org/10.1111/j.1365-2427.1994.tb01740.x. [ Links ]
Vinçon G, Clergue-Gazeau M. 1993. Les Simulies (Diptera Simuliidae) du Sud-Ouest de l'Europe: le crénal et l'épirhithral. Annales de Limnologie. 29(2): 157-169. https://doi.org/10.1051/limn/1993014. [ Links ]
Watanabe T. 2014. Kawasaki disease with retropharyngeal edema following a blackfly bite. Case Reports in Pediatrics. 2014: 296456. https://doi.org/10.1155/2014/296456. PMID:25349761. [ Links ]
Ya'cob Z, Takaoka H, Pramual P, Low VL, Sofian-Azirun M. 2016. Distribution pattern of black fly (Diptera: Simuliidae) assemblages along an altitudinal gradient in Peninsular Malaysia. Parasites & Vectors. 9(1): 219. https://doi.org/10.1186/s13071-016-1492-7. [ Links ]
 Correspondence:
Correspondence:
B Samraoui
Email: bsamraoui@gmail.com
Received:8 August 2020
Accepted: 24 November 2021














