Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.30 Pretoria 2022
http://dx.doi.org/10.17159/2254-8854/2022/a11687
RESEARCH ARTICLE
The parasitic impact of Romanomermis iyengari (Nematoda: Mermithidae) on the survival and biology of Culexpipiens (Diptera: Culicidae)
H ElbrenseI; M ShamseldeanII; WS MeshrifI; Al SeifI
IDepartment of Zoology, Faculty of Science, Tanta University, Tanta, Egypt
IIDepartment of Zoology and Agricultural Nematology, Faculty of Agriculture, Cairo University, Cairo, Egypt
ABSTRACT
The extensive use of chemical insecticides to control mosquitoes led to the development of insecticide resistance and environmental health hazards. This caused a surge in interest in eco-friendly biocontrol agents. The present study aimed to explore the susceptibility of different instar larvae of the common mosquito, Culex pipiens Linnaeus, 1758, to the mermithid nematode, Romanomermis iyengari Welch, 1964. Moreover, the effects of R. iyengari on the mosquito pupal developmental time, adult emergence, longevity, female fecundity, as well as egg-hatching rate were determined after larval treatment with an LC50 quantity of the nematode pre-parasites. Different instars of Cx. pipiens (1st-4th) were exposed separately to R. iyengari at concentrations of 1-6 pre-parasites/mosquito larva. Mortality rates of mosquito larvae were observed and the LC50 values were calculated. The estimated LC50 values for the 1st-4th larval instars were 3.18, 2.73, 3.79 and 4 pre-parasites/larva, respectively. Mean percent mortality of the 1st-4th larval instars ranged from 10-94%, 16-100%, 4-100% and 0-52%, respectively. The results indicated that exposure of 4th larval instar to the LC50 of R. iyengari pre-parasites significantly prolonged the duration of pupal development, reduced the percentage of emerged adults and reduced mosquito female fecundity compared with the control. In contrast, adult longevity and the egg-hatching rate did not differ between the control and the exposed group. In conclusion, this isolate of R. iyengari could be a promising biocontrol agent for Egyptian Cx. pipiens. Other trials are required to assess the biocontrol potential of this parasite in field conditions.
Keywords: biological control biology Mermithidae mosquito, reproductive capacity
INTRODUCTION
What would happen if every mosquito on earth was wiped out? This is not a question, but a dream of every person. Mosquitoes are vectors for a number of deadly diseases and hence a significant threat to human health (WHO 1996). They are vectors of diseases such as malaria, lymphatic filariasis, yellow fever and dengue fever (Farajollahi et al. 2011; WHO 2014). The common house mosquito, Culex pipiens Linnaeus, 1758 (Order: Diptera, Family: Culicidae) is a dominant mosquito species worldwide (Clements 1992; Elhawary et al. 2020). Therefore, it has been the target of several control programs worldwide (WHO 1996, 2014, 2020). The use of chemical insecticides has been curtailed due to hazardous residues, pollution and development of insect resistance (Chareonviriyaphap et al. 2013; Nicolopoulou-Stamati et al. 2016). Alternatives, such as biological control agents have therefore received major attention (Platzer 1981; Lacey and Orr 1994). One of the promising biological alternatives to chemical insecticides are aquatic mermithid nematodes (Platzer 2007; Paily et al. 2013; Di Battista et al. 2020). Aquatic mermithid nematodes occur in the same water bodies as the mosquito larvae. They are obligate endoparasites of at least 100 species of mosquito larvae (Platzer 2007). The ease of application, environmental safety, host specificity, lethality, practical methods of in vivo mass rearing and achievability of long-term recycling make mermithid nematodes ideal biological control agents (Abagli et al. 2019).
Until 1982, most research efforts on the mermithid nematodes infecting mosquito larvae were focused on Romanomermis culicivorax Ross & Smith, 1976 (Order: Mermithida, Family: Mermithidae). However, another species in the same genus has proven to be even more promising (Platzer 2007). This species was originally found in the haemocoele of Anopheles subpictus Grassi, 1899 larvae collected by Iyengar from Bangalore, India (Ross 1906) and subsequently from different species of Anopheles (Iyengar 1930). Later the nematode was described as Romanomermis iyengari Welch 1964. In aquatic habitats, R. iyengari, pre-parasites (second-stage juveniles) hatch from the eggs, search for their hosts, penetrate the cuticle of the mosquito larvae, develop in the haemocoele, and escape from the host larvae as post-parasites through mechanical rupture of the integument, forcing their way out of the larvae and in the process killing them. Post-parasites moult to become adults and burrow into the moist substrate at the bottom of their aquatic habitats. After copulation, eggs are laid in the soil by gravid females. The eggs embryonate and after completion of their development, hatch when submerged by water (Platzer 2007).
Several investigations in the last three decades have worked on the susceptibility of mosquito larvae to R. iyengari (Paily & Balaraman 2000). They identified 10 species of mosquitoes, belonging to five genera, which were susceptible to R. iyengari. Likewise, Pérez-Pacheco et al. (2015) examined the susceptibility of culicine mosquito larvae to R. iyengari. In laboratory experiments, Abagli et al. (2019) showed that all Culex quinquefasciatus Say, 1823 instar larvae were susceptible to R. iyengari infection. Moreover, Pérez-Pacheco et al. (2015) demonstrated that the 3rd instar larvae of Aedes aegypti (Linnaeus, 1762), Anopheles pseudopunctipennis Theobald and Cx. quinquefasciatus were more susceptible to parasitism than the 4th instar larvae. However, there is a lack of information on the effect of lethal concentrations of R. iyengari on the biology of the mosquito host. Therefore, the main objective of the present study was to evaluate the comparative susceptibility of different Cx. pipiens instar larvae to R. iyengari infection in the laboratory. The effects of the LC50 of this mermithid nematode on the developmental period of the mosquito pupae, the emergence of adults, adult longevity, emerged female fecundity and egg-hatching rate were also investigated.
MATERIAL AND METHODS
Mosquito source and maintenance
Culex pipiens larvae were collected from a ground-hole in the city of Tanta, Gharbia governorate, Egypt (30°48'5.339"N 30°59'36.114"E). They were transferred to the insectary in the animal facility, Faculty of Science, Tanta University. The collected larvae were identified according to Harbach (1985). The mosquitoes were reared for one generation in the laboratory at a temperature of 28 ± 2 °C, a relative humidity (RH) of 70-80% and a 12L:12D h photocycle. Mosquito larvae were reared in enamel pans (10 cm in height and 30 cm in diam.) containing dechlorinated tap water. Larvae were fed daily on a mixture of 1:3 brewer's yeast and ground wheat rusk, modified after Asahina (1964). Pupae were collected daily into glass cups (180 mL) with dechlorinated tap water and transferred to a labelled rearing cage (30 χ 30 χ 30 cm) for adult emergence. Adult mosquitoes were fed daily on cotton pads soaked in a 10% sugar solution. Female mosquitoes were fed on blood meals from restrained domestic pigeons. The care and maintenance of the pigeons were done according to the permission (IACUC-SCI-TU-0180) obtained from the Research Ethical Committee, Faculty of Science, Tanta University, Egypt. A cup containing 100 mL of dechlorinated water was provided in a mosquito cage for oviposition. Egg rafts were collected from oviposition cups, transferred into enamel pans containing water and the hatched larvae subsequently reared as above.
Romanomermis iyengari source and mass culture
The laboratory culture of R. iyengari was established from eggs obtained from the Department of Nematology, University of California, Riverside, USA. It was mass cultured in vivo using Cx. quinquefasciatus second instar larvae as a host (Platzer & Stirling 1978). As needed, an appropriate amount of egg/sand mixture was flooded with 250 mL of distilled water to induce R. iyengari egg hatching and to collect infected second-stage juveniles (pre-parasites). After a few hours, the water containing the nematodes was separated from the sand. Thereafter, the concentration of suspended pre-parasites was counted using the volumetric dilution method (Petersen & Willis 1972).
Susceptibility of mosquito larval stages to the nematode infection
The susceptibility of Cx. pipiens 1st, 2nd, 3rd and 4th instar larvae to R. iyengari were recorded using the method described by Abagli et al. (2012). Six concentrations (1, 2, 3, 4, 5, and 6 pre-parasites per mosquito larva) were used in the study. Twenty larvae from each mosquito instar were separately placed in glass cups (5 χ 10 cm) containing 99 mL of distilled water. Then, 1 mL of distilled water containing the tested concentration of R. iyengari pre-parasites was added. Parallel control groups containing 20 of the same larval instars were run using nematode-free distilled water of the same volume. This procedure was replicated five times for each larval instar and/or nematode concentration. The larvae were maintained at a temperature of 28 ± 2 °C. Three days after exposure, the larvae were washed in distilled water and transferred individually into 24-well bioassay plates containing 2 mL of distilled water. The status of the larval infection by R. iyengari was observed daily with a stereomicroscope, to ensure that larval mortality was due to the emergence of post-parasitic nematodes from mosquito larva cadavers. Bioassay plates with third and fourth instar larvae were kept in mosquito cages in anticipation of adult emergence. Mortality was recorded in each group and the LC50 value of each instar was determined.
Developmental time till adult emergence and longevity
Twenty late 4th instar larvae (2 days old post-moulting) were exposed to the pre-parasites LC50 value estimated in the previous experiment. Two days later, surviving larvae were transferred to new cups with nematode-free distilled water for pupation. Control groups were run using nematode-free distilled water. This procedure was replicated five times per group. Developmental time (from late 4th instar to adult emergence) was recorded until the last pupae emerged as an adult. Mosquito adult emergence was observed until the last adult emerged from each set. The mosquito adults that survived the nematode exposure and emerged from the previous experiment were separated into new cages and used for an adult longevity assay. In this assay five pairs of adult mosquitoes were used in five replicates to follow the longevity until the death of the last adults. Another group divided into five replicates was set as a control (without nematode exposure). Adult mosquitoes were fed daily on cotton soaked in a 10% sugar solution.
Female mosquito fecundity and egg-hatching
As in the previous experiment, 10 pairs of adults that emerged from the 4th instar larvae exposed to the nematode pre-parasites LC50 were transferred to mosquito cages (30 χ 30 χ 30 cm) and allowed to mate. The females were fed a blood meal. A cup with distilled water was placed in the cage as an oviposition substrate. A control group was run with 10 pairs of adults that emerged from the original mosquito culture (not exposed to nematodes). This procedure was replicated five times. Egg rafts from each cup were collected daily and counted under a light microscope (Ray wild limited company, Germany). Thereafter, these egg-rafts were transferred to a new cup with 250 mL distilled water and left to hatch. The fecundity of the mosquito females was calculated with the following formula: (Number of laid eggs/ Number of females allowed to mate) χ 100. While the hatching rate was calculated using the following formula: (Number of hatched eggs/Number of laid eggs) χ 100.
Statistical analysis
Data obtained were expressed as mean ± standard error (SE). Response variables were checked for normality using the Shapiro-Wilk test and Bartlett's test for homogeneity of variances. The percentage mortality of larvae was analysed using a two-way analysis of variance (ANOVA). Pairwise analysis between the tested groups was carried out using multiple comparisons. The p-value was adjusted according to the Bonferroni correction to control the family-wise error rate. The effect of R. iyengari infection on pupae development time, adult emergence and longevity, female fecundity and egg-hatching rate were analysed using an unpaired t-test. The LC50 value of each instar was determined using non-linear regression analysis of normalized mortality against log concentration of pre-parasites. These analyses were carried out in GraphPad Prism version 8.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com.
RESULTS
Susceptibility of mosquito larval stages to the nematode infection
There was no mortality recorded in the control groups. Table 1 shows that all Cx. pipiens instar larvae were susceptible to R. iyengari infection. However, the susceptibility of the different instars was variable. Two-way ANOVA indicated that larval instar (p < 0.001), pre-parasite concentration (p < 0.001) and their interaction (p < 0.001) significantly affect the larval mortality. In post hoc analysis (Bonferroni), the results demonstrated that the 4th instar larva was less susceptible to R. iyengari infection (p < 0.016) than the other larval instars. For instance, at the concentration of six pre-parasites/larva, the mortality rate of 2nd and 3rd instars was 100% followed by (94.00% ± 4.00%) in the 1st instar and (52.00% ± 4.90%) in the 4th instar. Data in Table 2 confirmed that the lowest LC50 (2.73 pre-parasites/larva) was recorded for the second instar, while the highest LC50 (four pre-parasites/larva) was recorded for the 4th larval instar. Moreover, non-linear regression of Cx. pipiens mortality revealed an increase in all instars as the concentration of pre-parasites increased (Figure 1). For example, the mortality rates observed for first stage larvae at 1, 2, 3, 4, 5 and 6 pre-parasites/ larva were 10.0% ± 3.16%, 26.0% ± 4.00%, 32.00% ± 5.83%, 62.0% ± 7.35%, 84.0% ± 7.48%, and 94.0% ± 4.00%, respectively.



Developmental time till adult emergence and longevity
The developmental time in the control Cx. pipiens pupae (time from the late 4th instar to adult emergence) was 3 ± 0.31 days. Exposure of Cx. pipiens 4th instar larvae to LC50 of R. iyengari (four pre-parasites/larva) induced a significant increase (p = 0.04, unpaired i-test) in the pupae developmental time to 4.2 ± 0.37 days (Table 3). In contrast, the percentage of emerged adults significantly decreased (p = 0.02, unpaired i-test) from 82.00% ± 4.06% in the control to 62.00% ± 5.61% in the exposed group (Table 3). Meanwhile, the longevity of emerged adults did not differ (p > 0.05, unpaired i-test) between the control and the exposed group (Table 3).

Female mosquito fecundity and egg-hatching
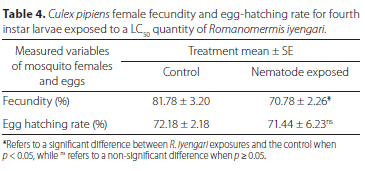
Table 4 shows that the fecundity of Cx. pipiens adult females in the control group was 81.78% ± 3.2%. Exposure of 4th instar larvae to LC50 of R. iyengari significantly (p = 0.024, unpaired i-test) decreased fecundity of emerged females to 70.78% ± 2.26%. However, no significant difference (p > 0.05, unpaired t-test) in the hatching rate of the laid eggs between the exposed and control group was detected (Table 4).
DISCUSSION
In the current study, all Cx. pipiens instar larvae were susceptible to infection by R. iyengari. The recorded mortality rates for the early instars (1st, 2nd, and 3rd) were significantly higher than the older 4th instar. Similar data was obtained by Abagli et al. (2019), who reported that Cx. quinquefasciaius 1st and 2nd instar larvae were more susceptible to infection by R. iyengari compared to the older larvae. Similarly, Pérez-Pacheco et al. (2004) showed that the 3rd instar larvae of A. pseudopunciipennis and Cx. quinquefasciaius were more susceptible to R. iyengari infection than the 4th instar larvae. Diaz et al. (2018) reported that 1st and 2nd instar larvae of Aedes albopicius (Skuse, 1894) were more susceptible to R. iyengari infection than 3rd instar larvae. The current finding that infective juveniles of R. iyengari prefer to infect early mosquito instars than older ones agreed with field parasitism data. For example, the older larvae of An. pseudopunciipennis in 16 sites in Oaxaca State, Mexico proved to be less susceptible to R. iyengari infection than younger larvae (Santamarina et al. 1999).
Successful parasitism by any biocontrol agent is a complicated process and depends not only on the aggressiveness and concentrations of the parasitic invader, but also on the physical condition of the host, behavioural defence against the parasites, body size of different hosts, developmental stages and host immunity (Petersen & Willis 1970; Dadd 1971). Our results demonstrated that the mortalities of Cx. pipiens larvae in different instars were concentration-dependent, as the greater the pre-parasitic concentrations applied, the higher the mortality observed. Our data also agree with Abagli et al. (2019), who reported a similar observation on the parasitism of another mosquito host, namely Anopheles gambiae by R. iyengari.
The insect host has a robust behaviour to avoid infection by parasites. The behaviour of the mosquito larvae as hosts of the mermithid nematodes is an important factor in successful parasitism by these biocontrol agents. Romanomermis iyengari pre-parasitic juveniles prefer to insert their needlelike odontostylet and penetrate the host larvae through the integument of the abdomen (Sanad et al. 2013). Using light and scanning electron microscopes (SEM), as well as video microscopy, Shamseldean & Platzer (1989) described in detail the penetration process of R. culicivorax, a relative species to R. iyengari, into the mosquito larvae. They have recorded the injection of a putative venom that causes temporary paralysis and cardiac arrest in the mosquito larvae during penetration of the host, which facilitates nematode entry via a wound in the host integument. That wound will be immediately sealed with an adhesive material secreted by the nematode pre-parasites while penetrating the mosquito larvae. As a behavioural response from the mosquito host, long and strong wriggle bursts may inhibit the search-piercing phase of the nematode pre-parasites (Petersen 1975). The lower susceptibility of Cx. pipiens 4th instar larvae may be explained by the violent wriggling behavioural defence by the mosquito larvae against nematode attack, making it difficult for the pre-parasitic juveniles to search and insert their stylets into the abdominal cuticle and enter the host. In addition, the thicker cuticle of 4th instar larvae make it more difficult for the pre-parasitic nematodes to insert their stylets and penetrate the host (Achinelly et al. 2004; Pérez-Pacheco et al. 2004).
The difference in susceptibility between early and late-stage larvae to infection is probably also related to physiological factors that affect successful infection and development of the mermithid nematodes. The immune system of mosquito larvae is a potential physiological mechanism to combat pathogens that invade their haemocoele (Hillyer 2010). After invasion of their haemocoele, mosquito larvae have a diverse array of cellular and humoral immune responses (Shamseldean et al. 2006; Liu et al. 2020). It is reasonable to suggest that the fourth instar of Cx. pipiens larvae have evolved a more robust immune system than early instars to combat R. iyengari infections.
In the current study, the fourth-instar larvae of Cx. pipiens required more time to emerge as adults than the controls when exposed to the LC50 values of R. iyengari pre-parasitic juveniles. Delayed host development is a common response in insects infected by entomopathogenic nematodes. Welch & Bronskill (1962) observed that the mosquito Aedes aegypti larvae infected with Steinernema carpocapsae (Weiser, 1955) pupated one or two weeks behind the normal ones. Anopheles sp. larvae infected by Octomyomermis muspratti were also retarded in their development (Obiamiwe & MacDonald 1973). Pupal moult of mosquito larvae infected with mermithids may be delayed by an imbalance in host endocrine secretions or the presence of parasite neurosecretory compounds (Welch 1965; Petersen & Willis 1970). The observed extended period of development in the mermithid parasitised larvae is probably also related to the shortage of nutritional reserves, which are essential for development and tissue building during the pupal stage. Mermithids absorb nutrients from the host's body through their body surface (Schmidt & Platzer 1980). The parasites are therefore heavily dependent upon their host for nutritional requirements (Gordon 1981). Romanomermis culicivorax was found to deplete haemolymph proteins of Cx. pipiens to one-sixth of the control levels (Schmidt & Platzer 1978). In addition, degeneration of the mid-gut epithelium in Ae. aegypti larvae by R. culicivorax led to the starvation of infected larvae (Bailey & Gordon 1973).
Results of the current study indicated a significant reduction in the fecundity of the emerged females that survived the infection by R. iyengari. It is well documented that mermithid parasites can induce physiological changes in their hosts, leading to a reduction in host reproductive output. Gordon (1981) summarized factors that may affect female fecundity, such as the potency ofthe ovary to produce oocytes, the nutritional reserves to develop the eggs and the capacity of male sperm to fertilize the ova. The same author suggested that when adult females and/or males of insect hosts are infected, they suffer from being sterilised or biologically castrated. Parasitic castration has been defined as the destruction of gonad tissues, alteration of reproductive behaviour and disruption of hormonal balance. Other modifications of host reproductive effort may result from non-selective use ofhost energy reserves by the parasite (Baudoin 1975). The mermithid parasite can reduce the amount of energy invested by the host in reproductive activities. Hosts infected with a parasite will show a reduction in individual fitness ranging from complete loss of reproductive success to a very small decrease in reproductive success. Wülker (1975) reported several cytological changes in the gonads of chironomids and intersexuality due to mermithid parasitism. Sharp and Hunter (2008) reported that simuliid gonad development was completely inhibited by mermithid infection. One advantage of parasitic castration is that the parasite can obtain energy from host tissues, while not increasing host mortality (Obrebski 1975). The present study demonstrated that the longevity of emerged adults exposed to R. iyengari did not significantly differ from those of the control. Similarly, Di Battista et al. (2015) reported that the survival rate of adult Aedes albifasciatus (Macquart, 1838) females parasitised by Strelkovimermis spiculatus Poinar & Camino, 1986 did not differ from non-parasitised females.
The results generated through this study lead to the conclusion that all larval instars of the Egyptian strain of Cx. pipiens were susceptible to parasitism by the mermithid nematode R. iyengari. Exposure of 4th instar larvae to LC50 pre-parasitic concentrations of R. iyengari prolonged the pupal developmental time and reduced both the percentage of emerged adults and female fecundity (which reduced Cx. pipiens population size). Future field studies are needed to demonstrate the efficacy of R. iyengari under a wide range of natural environmental conditions.
ACKNOWLEDGEMENTS
The authors thank Prof. Edward G Platzer (University of California Riverside, USA) for the kind supply of the original stock of Romanomermis iyengari.
FUND
This work did not receive any funds.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ORCID IDs
H Elbrense - https://orcid.org/0000-0001-9897-3772
M Shamseldean- https://orcid.org/0000-0002-9158-4229
WS Meshrif - https://orcid.org/0000-0002-1354-8046
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
AUTHOR CONTRIBUTIONS
HE, MS and AIS suggested the study idea and designed the experiments. MS provided Culex quinquefasciatus for the nematode culture. HE collected the results. WSM performed the statistical analysis. All authors wrote and approved the manuscript.
REFERENCES
Abagli AZ, Alavo TB, Perez-Pacheco R, Platzer EG. 2019. Efficacy of the mermithid nematode, Romanomermis iyengari, for the biocontrol of Anopheles gambiae, the major malaria vector in sub-Saharan Africa. Parasites & Vectors. 12(1): 253. https://doi.org/10.1186/s13071-019-3508-6. [ Links ]
Abagli AZ, Alavo TB, Platzer EG. 2012. Efficacy of the insect parasitic nematode, Romanomermis iyengari, for malaria vector control in Benin West Africa. Malaria Journal. 11(S1): P5. https://doi.org/10.1186/1475-2875-11-S1-P5. [ Links ]
Achinelly M, Micieli M, Marti G, García J. 2004. Susceptibility of neotropical mosquito larvae (Diptera: Culicidae) and nontarget aquatic organisms to the entomoparasitic nematode Strelkovimermis spiculatus Poinar & Camino, 1986 (Nematoda: Mermithidae). Nematology. 6(2): 299-302. https://doi.org/10.1163/1568541041217951. [ Links ]
Asahina S. 1964. Food material and feeding procedures for mosquito larvae. Bulletin of the World Health Organization. 31(4): 465-466. [ Links ]
Bailey CH, Gordon R. 1973. Histopathology of Aedes aegypti (Diptera: Culicidae) larvae parasitized by Reesimermis nielseni (Nematoda: Mermithidae). Journal of Invertebrate Pathology. 22(3): 435-441. https://doi.org/10.1016/0022-2011(73)90174-2. [ Links ]
Baudoin M. 1975. Host castration as a parasitic strategy. Evolution. 29(2): 335-352. https://doi.org/10.1111/j.1558-5646.1975.tb00213.x. [ Links ]
Chareonviriyaphap T, Bangs MJ, Suwonkerd W, Kongmee M, Corbel V, Ngoen-Klan R. 2013. Review of insecticide resistance and behavioral avoidance of vectors of human diseases in Thailand. Parasites & Vectors. 6(1): 280. https://doi.org/10.1186/1756-3305-6-280. [ Links ]
Clements AN. 1992. The biology of mosquitoes. Volume 1: development, nutrition and reproduction. London: Chapman & Hall. [ Links ]
Dadd RH. 1971. Size limitations on the infectibility of mosquito larvae by nematodes during filter-feeding. Journal of Invertebrate Pathology. 18(2): 246-251. https://doi.org/10.1016/0022-2011(71)90152-2. [ Links ]
Di Battista CM, Fischer S, Campos RE. 2015. Prevalence of parasitism and adult survival time of Aedes albifasciatus (Diptera: Culicidae) parasitized by Strelkovimermis spiculatus (Nematoda: Mermithidae). Journal of Vector Ecology. 40(2): 393-397. https://doi.org/10.1111/jvec.12179. [ Links ]
Di Battista CM, Fischer S, Campos RE. 2020. Susceptibility of the floodwater mosquito Aedes albifasciatus from eggs of different dormancy times to the nematode parasite Strelkovimermis spiculatus. Medical and Veterinary Entomology. 34(4): 432-439. https://doi.org/10.1111/mve.12460. [ Links ]
Díaz ZM, Garcia IG, Contreras NH, Rizo AG, Ibañez AC, Alvarez VB. 2018. Sensitivity of different larval stages of Aedes albopictus (S.) (Diptera: Culicidae) to infection by two species of mermithids nematodes in laboratory conditions. Revista Cubana de Medicina Tropical. 70(3): 83-91. [ Links ]
Elhawary N, Soliman M, Seif A, Meshrif W. 2020. Culicine mosquitoes (Diptera: Culicidae) communities and their relation to physicochemical characteristics in three breeding sites in Egypt. Egyptian Journal of Zoology. 74(74): 30-42. https://doi.org/10.21608/ejz.2020.40783.1039. [ Links ]
Farajollahi A, Fonseca DM, Kramer LD, Kilpatrick AM. 2011. "Bird biting" mosquitoes and human disease: a review of the role of Culex pipiens complex mosquitoes in epidemiology. Infection, Genetics & Evolution. 11(7): 1577-1585. https://doi.org/10.1016/j.meegid.2011.08.013. [ Links ]
Gordon, R. 1981. Mermithid nematodes: physiological relationships with their insect hosts. Journal of Nematology. 13(3): 266-274. [ Links ]
Harbach RE. 1985. Pictorial keys to the genera of mosquitoes, subgenera of Culex and the species of Culex (Culex) occurring in southwestern Asia and Egypt, with a note on the subgeneric placement of Culex deserticola (Diptera: Culicidae). Mosquito Systematics. 17: 83-107. [ Links ]
Hillyer JF. 2010. Mosquito immunity. In: Söderhäl K, editor. Invertebrate immunity. Advances in experimental medicine and biology. Boston MA: Springer. 218-238. https://doi.org/10.1007/978-1-4419-8059-5_12. [ Links ]
Iyengar MOT. 1930. Parasitic nematodes of Anopheles in Bengal. In Transactions of the 7th Congress of the Far Eastern Association of Tropical. Medicine 1927. 3: 128-135. Calcutta, India.
Lacey LA, Orr BK. 1994. The role of biological control of mosquitoes in integrated vector control. The American Journal of Tropical Medicine and Hygiene. 50(6) Suppl.: 97-115. https://doi.org/10.4269/ajtmh.1994.50.97. [ Links ]
Liu WT, Chen TL, Hou RF, Chen CC, Tu WC. 2020. The invasion and encapsulation of the entomopathogenic nematode, Steinernema abbasi, in Aedes albopictus (Diptera: Culicidae) larvae. Insects. 11(12): 832. https://doi.org/10.3390/insects11120832. [ Links ]
Nicolopoulou-Stamati P, Maipas S, Kotampasi C, Stamatis P, Hens L. 2016. Chemical pesticides and human health: the urgent need for a new concept in agriculture. Frontiers in Public Health. 4: 148. https://doi.org/10.3389/fpubh.2016.00148. [ Links ]
Obiamiwe BA, Macdonald WW. 1973. A new parasite of mosquitoes, Reesimermis muspratti sp. nov.(Nematoda: Mermithidae), with notes on Its life-cycle. Annals of Tropical Medicine & Parasitology.67(4): 439-444. https://doi.org/10.1080/00034983.1973.11686912. [ Links ]
Obrebski S. 1975. Parasite reproductive strategy and evolution of castration of hosts by parasites. Science. 188(4195): 1314-1316. https://doi.org/10.1126/science.1145198. [ Links ]
Paily KP, Balaraman K. 2000. Susceptibility of ten species of mosquito larvae to the parasitic nematode Romanomermis iyengari and its development. Medical and Veterinary Entomology. 14(4): 426-429. https://doi.org/10.1046/j.1365-2915.2000.00263.x. [ Links ]
Paily KP, Chandhiran K, Vanamail P, Kumar NP, Jambulingam P. 2013. Efficacy of a mermithid nematode Romanomermis iyengari (Welch) (Nematoda: Mermithidae) in controlling tree hole-breeding mosquito Aedes albopictus (Skuse) (Diptera: Culicidae) in a rubber plantation area of Kerala, India. Parasitology Research. 112(3): 1299-1304. https://doi.org/10.1007/s00436-012-3265-3. [ Links ]
Pérez-Pacheco R, Platzer EG, Woodward D, Hyman BC. 2015. Bioassays for comparative infectivity ofmermithid nematodes (Romanomermis iyengari, Romanomermis culicivorax and Strelkovimermis spiculatus) for culicine mosquito larvae. Biological Control. 80: 113-118. https://doi.org/10.1016/j.biocontrol.2014.09.012. [ Links ]
Pérez-Pacheco R, Rodriguez-Hernandez C, Lara-Reyna J, Montes-Elmont R, Ramírez-Valverde G, Martínez-Martínez L. 2004. Parasitismo de Romanomermis iyengari en larvas de tres especies de mosquitos en laboratorio y de Anopheles pseudopunctipennis en campo. Agrociencia. 38(4): 413-421. [ Links ]
Petersen JJ, Willis OR. 1970. Some factors affecting parasitism by mermithid nematodes in southern house mosquito larvae. Journal of Economic Entomology. 63(1): 175-178. https://doi.org/10.1093/jee/63.1.175. [ Links ]
Petersen JJ, Willis OR. 1972. Procedures for the mass rearing of a mermithid parasite of mosquitoes. Mosquito News. 32(2): 226-230. [ Links ]
Petersen JJ. 1975. Penetration and development of the mermithid nematode Reesimermis nielseni in eighteen species of mosquitoes. Journal of Nematology. 7(3): 207-210. [ Links ]
Platzer EG. 1981. Biological control of mosquitoes with mermithids. Journal of Nematology. 13(3): 257-262. [ Links ]
Platzer EG, Stirling AM. 1978. Improved rearing procedures for Romanomermis culicivorax [Nematode parasite of Culex pipiens, biological control agents]. In: Proceedings and Papers of the Annual Conference California Mosquito and Vector Control Association. p. 87-88.
Platzer EG. 2007. Mermithid nematodes. Journal of the American Mosquito Control Association. 23(sp2): 58-64. https://doi.org/10.2987/8756-971X(2007)23[58:MN]2.0.CO;2. [ Links ]
Ross R. 1906. Notes on the parasites of mosquitoes found in India between 1895 and 1899. Epidemiology & Infection. 6(2): 101-108.https://doi.org/10.1017/S0022172400002722. [ Links ]
Sanad MM, Shamseldean MS, Elgindi AEY, Gaugler R. 2013. Host penetration and emergence patterns of the mosquito-parasitic mermithids Romanomermis iyengari and Strelkovimermis spiculatus (Nematoda: mermithidae). Journal of Nematology. 45(1): 30-38. [ Links ]
Santamarina MA, Pérez Pacheco R, Tomás Martinez SH, Enrique Cantón L, Flores Ambrosio G. 1999. The Romanomermis iyengari parasite for Anopheles pseudopunctipennis suppression in natural habitats in Oaxaca State, Mexico. Revista Panamericana de Salud Pública. 5(1): 23-28. https://doi.org/10.1590/S1020-49891999000100004. [ Links ]
Schmidt SP, Platzer EG. 1978. Hemolymph composition of mosquito larvae infected with a mermithid nematode. [Abstract]. Journal of Nematology. 10: 299. [ Links ]
Schmidt SP, Platzer EG. 1980. Changes in body tissues and hemolymph composition of Culex pipiens in response to infection by Romanomermis culicivorax. Journal of Invertebrate Pathology.; 36(2): 240-254. https://doi.org/10.1016/0022-2011(80)90030-0. [ Links ]
Shamseldean MM, Platzer EG. 1989. Romanomermis culicivorax: penetration of larval mosquitoes. Journal of Invertebrate Pathology. 54(2): 191-199. https://doi.org/10.1016/0022-2011(89)90028-1. [ Links ]
Shamseldean MM, Platzer EG, Gaugler R. 2006. Ultrastructure of the immune responses ofAnopheles quadrimaculatus to Romanomermis culicivorax (Nematoda: Mermithidae) infection. Nematropica. 36:243-250. [ Links ]
Sharp AL, Hunter FF. 2008. Chiasmate meiosis in male black fly (Diptera: Simuliidae) larvae associated with mermithid infections (Nematoda: Mermithidae). Canadian Journal of Zoology. 86(10): 1198-1202. https://doi.org/10.1139/Z08-105. [ Links ]
Welch HE. 1965. Entomophilic nematodes. Annual Revue of Entomology. 10(1): 275-302. https://doi.org/10.1146/annurev.en.10.010165.001423. [ Links ]
Welch HE, Bronskill JF. 1962. Parasitism of mosquito larvae by the nematode, DD136 (Nematoda: Neoaplectanidae). Canadian Journal of Zoology. 40(7): 1263-1268. https://doi.org/10.1139/z62-102. [ Links ]
WHO [World Health Organization]. 1996. Report of the WHO Informal Consultation on the evaluation and testing of insecticides. Geneva: World Health Organization. [ Links ]
WHO [World Health Organization]. 2014. Global status report on noncommunicable diseases. Geneva: World Health Organization. [ Links ]
WHO [World Health Organization]. 2020. Vector-borne diseases. Geneva: World Health Organization. [ Links ]
Wülker W. 1975. Parasite-induced castration and intersexualityin insects. In: Reinboth R, editor. Intersexuality in the animal kingdom. Berlin: Springer. 121-134. https://doi.org/10.1007/978-3-642-66069-6_13. [ Links ]
 Correspondence:
Correspondence:
WS Meshrif
Email: wmeshrif@science.tanta.edu.eg
Received: 7 July 2021
Accepted: 15 October 2021














