Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.30 Pretoria 2022
http://dx.doi.org/10.17159/2254-8854/2022/a10688
SHORT COMMUNICATION
Attractiveness of white clover [Trifolium repens L.) and plum (Prunus salicina Lindl. cv. Sapphire) flower volatiles to female Frankliniella occidentalis (Pergande, 1895)
E AllsoppI, II; SY DewhirstIII; MC KnipeI; GJ PrinslooIV
IARC Infruitec-Nietvoorbij, Stellenbosch, South Africa
IIDepartment of Conservation Ecology and Entomology, Stellenbosch University, South Africa
IIIDepartment of Biological Chemistry and Crop Protection, Rothamsted Research, Harpenden, UK
IVARC Small Grain, Bethlehem, South Africa
ABSTRACT
Producers in the Western Cape province of South Africa are looking towards a push-pull strategy to reduce oviposition damage to plums by western flower thrips (WFT). White clover, Trifolium repens L., a favoured host plant of WFT, was investigated as a possible trap crop to provide the "pull" element. The attractiveness of collected volatiles of white clover flowers and of unopened (balloon stage) and open plum (Prunussalicina Lindl. cv. Sapphire) blossoms to WFT females was determined, using a Y-tube olfactometer. E-/3-Farnesene, a known attractant for WFT, was included as positive control. Clover flower and open plum blossom volatiles exhibited statistically significant attraction of 69% and 71%, respectively, to WFT females. E-/3-Farnesene and balloon stage plum blossom volatiles attracted 63% and 65% of WFT, respectively. When compared directly, 69% of WFT females chose the arm with the plum blossom volatiles over the clover flower volatiles. The clear preference shown by WFT for plum blossom volatiles indicates the use of a semiochemical to deter WFT from the plum blossoms would be required to enhance the efficacy of white clover as a trap crop in a push-pull strategy.
Keywords: air entrainment olfactometer trap crop, western flower thrip
Deciduous fruit producers in the Western Cape (South Africa) are looking for alternatives to pesticides to reduce fruit damage by western flower thrips (WFT), Frankliniella occidentalis (Pergande, 1895) (Thripidae), particularly oviposition damage (pansy spot and pitting) on plums, Prunus salicina Lindl. (Rosaceae). Western flower thrips overwinters on weeds and other plants in and around orchards, with adults moving into plum trees when the blossoms emerge, usually during August and September (Allsopp 2010). Female WFT lay eggs in the blossoms before the petals even open, which renders the application of contact insecticides during flowering ineffective (Allsopp 2010). By the time the plums are fully developed, very few WFT are present on the trees for the rest of the season. Increasing consumer demands for residue-free, sustainably produced fruit are also driving the search for alternative, eco-friendly pest management strategies.
Extensive research has been conducted on semiochemicals that attract WFT, to enhance the efficacy of monitoring traps and to attract WFT predators (Roditakis & Lykouressis 1996; Manjunatha et al. 1998; Koschier 2008; Broughton & Harrison 2012; Muvea et al. 2014; Teulon et al. 2017; Reitz et al. 2020). E-//-Farnesene is a component of many flower volatiles that has been shown to be attractive to WFT (Manjunatha et al. 1998; Pow et al. 1999; Koschier et al. 2000; Bennison et al. 2003). Methyl isonicotinate is another non-pheromone semiochemical that attracts a range of thrips species, including WFT. According to Teulon et al. (2017), it is used in conjunction with coloured sticky traps to improve trapping efficiency for indoor and outdoor crops. Further research is required to explore its potential for mass trapping, increasing the efficacy of thrips predators, lure and kill, lure and infect, trap cropping and push/pull strategies.
Trap crops or companion plants adjacent to the crop can also lure WFT away from the main crop to where it can be controlled (Cook et al. 2006). In addition, these plants can provide resources for natural enemies and interspecific competitors (Reitz et al. 2020). A cultivar of verbena (Verbena officinalis L., Verbenaceae) and another of chrysanthemum (Dendranthema sp., Asteraceae) were effective lure or trap plants for use in integrated management of WFT on ornamentals in greenhouses in the United Kingdom (Pow et al. 1998; Bennison et al. 1999; Bennison et al. 2003). A study by Pearsall (2000) concluded that none of the naturally occurring ground covers, particularly dandelion (Taraxacum officinale), were effective as trap crops under nectarine trees in British Columbia, because they were often not flowering when the nectarines started to bloom and not sufficiently attractive to lure the WFT away from the nectarine blossoms. Dandelion cover crops also did not reduce WFT density in apple blossoms or fruit injury significantly in apple orchards in central Washington, USA (Cockfield & Beers 2008). Tyler-Julian et al. (2014) evaluated common sunflower, Helianthus annuus L., (Asteraceae) as the pull component with Ultraviolet (UV)-reflective mulch and foliar applications of kaolin as the push component for managing flower thrips in field-grown bell pepper. They concluded that sunflower was not effective in reducing thrips numbers on the crop and that there was no evidence of a synergistic action between the UV-reflective mulch or kaolin and the sunflower companion plants in reducing thrips on the bell pepper crop. The companion plant Bidens alba (L.) (Asteraceae) was investigated as the pull element in a push-pull system with UV-reflective mulch and foliar applications of kaolin as the push component for managing Frankliniella spp. in field-grown tomatoes (Tyler-Julian et al. 2018). Male thrips were attracted away from the tomato crop by B. alba, where they were exposed to predation by Orius insidiosus (Say) (Hemiptera: Anthocoridae). This companion plant was, however, not successful in attracting female WFT away from the crop. Despite this, they concluded that a push-pull system with B. alba companion plants as the pull element and UV-reflective mulch and kaolin applications as push elements effectively suppressed WFT in tomato.
Logically, a trap crop for WFT in or adjacent to plum orchards should be more attractive to WFT than plum blossoms and should flower before the plums, since WFT enter blossoms even before the petals are fully opened (Allsopp 2010). Mainali & Lim (2011) found that WFT preferred complex floral shapes to simpler geometric patterns. Female WFT preferred flowers closer to the ground (up to 25 cm in height) when they began to move into nectarine orchards during spring in British Columbia and were attracted to highly scented flowers (Pearsall 2000). In the Western Cape, verbena and chrysanthemum planted outdoors do not flower early in spring when plum blossoms appear. Felland et al. (1995) reported that WFT was abundant on white clover, Trifolium repens L. (Fabaceae), while Pearsall & Myers (2000) found that white clover and alfalfa (Medicago sativa L.) supported higher numbers of WFT than a range of ground covers, including dandelion (Taraxacum officinale Weber), red clover (Trifolium pratense L.), chickweed (Stellaria media (L.)), common groundsel (Senecio vulgaris L.) and hairy vetch (Vicia villosa Roth). White clover produces flowers close to the ground and occurs in and around many orchards in the Western Cape, making it a suitable candidate for investigation as a trap crop for WFT.
To the best of our knowledge, this is the first report on the attractiveness ofwhite clover and plum blossom volatiles to WFT. A Y-tube olfactometer was used to test the attractiveness of the volatiles from white clover flowers, balloon stage (unopened) and opened plum (Prunus salicina Lindl. cv. Sapphire) blossoms to WFT females. Έ-β-Farnesene was included as a positive control to confirm that the olfactometer setup functioned properly.
A custom-made air entrainment kit, obtained from Rothamsted Research (Harpenden, United Kingdom), was used to capture the flower volatiles, according to the technique described by Blight (1990). Six month old, whole white clover plants grown in black plastic pots (15 cm diameter) and shoots of three-year old plum trees grown in 25-L ceramic pots were used for volatile capture. Plum trees were grown in pots for three years to ensure that they were well established and produced sufficient blossoms. For the first bioassay, volatiles were collected over a 48 hour period from clusters of clover flowers and shoots with either unopened (balloon stage) or fully opened plum blossoms to ensure a sufficient concentration of volatiles. A detailed description of the methods used was presented in Allsopp (2016).
Observations during this study showed that the balloon stage in plum blossoms rarely lasted more than two days, while open plum blossoms began to lose petals after two to three days. Similarly, the clover flowers began to senesce after three days. Thus, the entrainment period for flower volatiles did not exceed 48 hours. The amount of volatiles collected over 48 hours determined the number of replicates in the olfactometer bioassays.
The Έ-β-Farnesene was synthesized at Rothamsted Research, as stated in Al Abassi et al. (1998), with hexane as solvent. A concentration of 100 ng E-β-Farnesenel hexane was used, as this was demonstrated to elicit peak attraction to WFT by Manjunatha et al. (1998).
For the final bioassay comparing attractiveness of plum and clover volatiles with each other, entrainments were also done over 48 hour periods as described for the first bioassay, but using clusters of open and partially open clover flowers and plum shoots with both balloon stage and open blossoms.
For the bioassays of the attractiveness of flower volatiles compared to a control, clover flowers were collected in a field with mixed vegetation south of Stellenbosch, South Africa (33.92330°S, 18.87331°Έ). The flowers were collected every morning before olfactometer trials began and taken to the laboratory in a cooler box. A small, manual aspirator was used to collect individual WFT females in separate glass vials. For the final bioassay to compare the attractiveness of plum versus clover flower volatiles, WFT were collected in the same way from chrysanthemum flowers obtained from a commercial flower farm near Elgin, in the Western Cape (34.13601°S, 19.06642Έ), since clover flowers were not available at the time. In both cases, WFT could move freely between different host plants and were therefore not restricted to one particular host species. Field populations of WFT were used rather than laboratory reared and adapted insects, as the reaction of wild females of variable ages was expected to be a better reflection of real orchard situations. As described in Allsopp (2016), vials with WFT females were placed in a fridge at 5 °C for 10-15 minutes to immobilize the thrips. Each of the females was examined under a binocular microscope to confirm species identity (according to Mound & Kibby 1998). Thrips were kept in the glass vials at room temperature for approximately 1 hour after removal from the fridge, before the bioassay was conducted.
The attractiveness of flower volatiles from white clover, balloon stage plum blossoms, fully opened plum blossoms and of Έ-β-Farnesene to WFT females was tested separately and compared to a control (solvent). Detailed procedures for the tests were described in Allsopp (2016). For each test run, a sterile square of filter paper with 10 μl of the test odour was placed in the test arm of the olfactometer and a sterile square of filter paper with 10 μl of solvent (hexane or diethyl ether) in the control arm. To avoid positional bias, the position of the odour and control arm (right or left) was alternated every time a Y-tube was replaced. After an adjustment period of 2 min the behaviour of the WFT female was observed for a maximum of 5 min and the final choice of arm (odour or control) recorded. Females that did not move or make a choice within 5 min were discarded. The bioassays were done over a period of three weeks and 34 to 40 replicates were completed, with a single female constituting a replicate.
To compare the attractiveness of volatiles from plum blossoms (i.e. balloon stage and open combined) directly with that of clover flower volatiles, the same procedure was followed, but each arm of the olfactometer contained a square of filter paper with 10 μl of either the plum, or clover flower volatile blend. After each replicate, the Y-tube was replaced with a clean one. The odours were alternated between the right and left arms of the Y-tube after each replicate to avoid positional bias. The bioassays were conducted over a period of three weeks and 35 replicates were completed.
The data for each odour was compared separately to a ratio of 1:1 using Chi-square (χ2) tests (PROC FREQ) with SAS software (2015). The data for the final bioassay (plum vs clover flower volatiles) was also compared to a ratio of 1:1 using the same method.
The numbers of WFT females choosing the odour arm and control arm for each ofthe four treatment categories are presented in Table 1. The arm with volatile compounds of clover flowers was chosen by 68% of WFT females, which was statistically significant (χ2 = 4.81, p = 0.02). The volatile compounds of plum blossoms in full bloom also produced statistically significant results (χ2 = 6.43, p = 0.01), with 71% of WFT choosing the odour arm. Even though not statistically significant according to the Chi-square analysis, 65% of WFT chose the arm with volatiles of the balloon stage plum blossoms (χ2 = 3.60, p = 0.06), while 63% chose the arm containing Έ-β-Farnesene (χ2 = 2.31, p = 0.13).
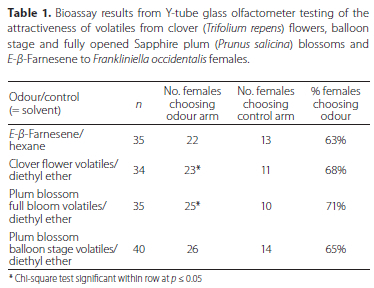
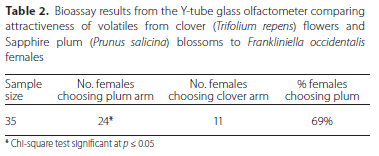
In the bioassay comparing the attractiveness of plum and clover flower volatiles to each other (Table 2), 69% of WFT females chose the arm with the balloon stage and fully opened plum blossom volatiles, which was statistically significant (χ2 = 4.83, p = 0.028).
The results of the olfactometer bioassays revealed that clover flowers and open plum blossoms are highly attractive to WFT females, while balloon stage plum blossoms were also attractive, but less so than the open plum blossoms. Although the degree of attraction of E-//-Farnesene was not statistically significant in this study, the 63% of WFT females that chose this odour was comparable to the results obtained by Koschier et al. (2000), where 64% of WFT chose E-//-Farnesene at a 10% concentration, while 65% chose the 1% concentration.
In the field WFT are exposed to plum blossoms and clover flowers at all stages of development at a given time. For this reason, shoots with balloon stage and fully opened blossoms and clusters of open and partially open clover flowers were used to capture the volatiles for the final bioassay to compare the attractiveness of plum and clover volatiles directly. Significantly more WFT females chose the plum volatiles over the clover volatiles (Table 2). Host selection by WFT is not solely determined by olfactory cues (Reitz et al. 2020), with WFT known to prefer more complex flowers (Mainali & Lim 2011). While the effect of flower shape in conjunction with flower volatiles was not evaluated in these bioassays, the clear preference shown by WFT for plum blossom volatiles indicates that the use of a 'push' element will probably be crucial to enhance the efficacy of white clover as a trap crop. This is in accordance with the conclusion by Pearsall (2000) that for a ground cover to be effective as a trap crop, it would have to be used in conjunction with the application of a powerful deterrent on the highly attractive nectarine blossoms.
UV-reflective mulches which disrupt the ability of thrips, including WFT, to find the host plant have been evaluated in push-pull management systems in field crops such as bell pepper (Reitz et al. 2003) and tomato (Tyler-Julian et al. 2018). Reitz et al. (2003) found that a UV-reflective mulch reduced the abundance of adult Frankliniella thrips (including WFT) in bell pepper early in the season. Tyler-Julan et al. (2018) showed that a UV-reflective mulch, combined with foliar applications of kaolin and stands of B. alba adjacent to the tomato field as a lure or companion plant, was effective in suppressing flower thrips, including WFT, on tomato. The use of such mulches during flowering to deter WFT in plum orchards in conjunction with a clover trap crop planted next to the orchard warrants investigation. The potential effects of the mulch on honeybees brought in for pollination should also be assessed.
Volatiles with repellent activities can be utilised for disruption of host finding and a number of such plant-derived compounds that repel or deter WFT have been identified (Mouden et al. 2017 and references therein). The plant essential oils thymol, methyl salicylate and carvacrol, are known to affect WFT behaviour (Koschier 2008). They were shown to be a significant deterrent to WFT oviposition in plum flowers (Allsopp et al. 2014). A major challenge to the use of such essential oils as deterrents in pest management is the development of stable suspensions to provide sustained release of effective concentrations without phytotoxic effects, particularly in open cultivation like orchards (Isman 2006; Reitz et al. 2008; Allsopp et al. 2014).
This study confirmed that white clover flower volatiles are attractive to WFT females, but those of plum blossoms were more attractive. Further bioassays are required to confirm the attractiveness of actual flowers. However, it seems likely that white clover would not be suitable for use as a trap crop until an effective deterrent is available that can be applied to the plums to provide the 'push' in a push-pull system. The potential of other flowering plants, including indigenous plants, as trap crops for WFT should also be explored.
ACKNOWLEDGEMENTS
The authors wish to thank M. Booyse of ARC (Agricultural Research Council) Biometry for statistical analysis. This research was funded by the South African Stone Fruit Producers' Association (SASPA), the South African Table Grape Industry (SATI), the Agricultural Research Council of South Africa and the National Research Foundation of South Africa (NRF-THRIP TP2009073100036). Any opinions, findings and conclusions, or recommendations expressed in any publication generated through THRIP-supported research, are those of the authors and therefore the NRF/THRIP will not accept any liability in that regard. Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (BBSRC) of the United Kingdom.
ORCID IDs
Elleunorah Allsopp - https://orcid.org/0000-0003-1975-4999
Sarah Y Dewhirst - https://orcid.org/0000-0002-2370-5578
Goddy J Prinsloo - https://orcid.org/0000-0003-0704-8026
REFERENCES
Al Abassi S, Birkett MA, Pettersson J, Pickett JA, Woodcock CM. 1998. Ladybird beetle odour identified and found to be responsible for attraction between adults. Cellular and Molecular Life Sciences 54: 876-879. https://doi.org/10.1007/s000180050215 [ Links ]
Allsopp E. 2010. Investigation into the apparent failure of chemical control for management of western flower thrips, Frankliniella occidentalis (Pergande), on plums in the Western Cape Province of South Africa. Crop Protection 29: 824-31. https://doi.org/10.1016/j.cropro.2010.03.009 [ Links ]
Allsopp E. 2016. Developing an integrated management system for western flower thrips, Frankliniella occidentalis (Pergande), on deciduous fruit, using semiochemicals in a push-pull strategy. PhD thesis, Stellenbosch University, Stellenbosch, South Africa. [ Links ]
Allsopp E, Prinsloo GJ, Smart LE, Dewhirst SY. 2014. Methyl salicylate, thymol and carvacrol as oviposition deterrents for Frankliniella occidentalis (Pergande) on plum blossoms. Arthropod-Plant Interactions 8: 421-427. https://doi.org/10.1007/s11829-014-9323-2 [ Links ]
Bennison JA, Maulden K, Dewhirst S, Pow E, Slatter P, Wadhams LJ. 2003. Towards the development of a push-pull strategy for improving biological control of western flower thrips on chrysanthemum. In: Marullo, R. & Mound, L. (Eds) 7th International Symposium on Thysanoptera: Thrips, Plants, Tospoviruses: the Millenial Review, 2-7 July 2001, Reggio Calabria, Italy. CSIRO Entomology, Canberra, Australia. 199-206. [ Links ]
Bennison JA, Pow EM, Wadhams LJ, Maulden KA, Wardlow LR, Buxton JH. 1999. Improving biological control of western flower thrips, Frankliniella occidentalis, on greenhouse ornamentals. Proceedings sixth International Symposium on Thysanoptera, Antalya, Turkey, April 27 to May 1. Akdeniz University, Antalya, Turkey. 19-24.
Blight MM. 1990. Techniques for isolation and characterization of volatile semiochemicals of phytophagous insects. In: McCaffrey, ID & Wilson, AR. (Eds) Chromatography and isolation of insect hormones and pheromones. Plenum Press, pp 281-288.
Broughton S, Harrison J. 2012. Evaluation of monitoring methods for thrips and the effect of trap colour and semiochemicals on sticky trap capture of thrips (Thysanoptera) and beneficial insects (Syrphidae, Hemerobiidae) in deciduous fruit trees in Western Australia. Crop Protection 42:156-163. https://doi.org/10.1016/j.cropro.2012.05.004 [ Links ]
Cockfield SD, Beers EH. 2008. Management of dandelion to supplement control of western flower thrips (Thysanoptera: Thripidae) in apple orchards. Journal of the Entomological Society of British Columbia 105: 89-96. [ Links ]
Cook SM, Khan ZR, Pickett JA. 2006. The use of push-pull strategies in integrated pest management. Annual Review of Entomology 52: 375-400. https://doi.org/10.1146/annurev.ento.52.110405.091407 [ Links ]
Felland CM, Teulon DAJ, Hull LA, Polk DK. 1995. Distribution and management of thrips (Thysanoptera: Thripidae) on nectarine in the mid-Atlantic region. Journal of Economic Entomology 88: 10041011. https://doi.org/10.1093/jee/88.4.1004 [ Links ]
Isman MB. 2006. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annual Review of Entomology 51: 45-66. https://doi.org/10.1146/annurev.ento.51.110104.151146 [ Links ]
Koschier EH.2008. Essential oil compounds for thrips control - A review. Natural Product Communications 3: 1171-1182. [ Links ]
Koschier EH, De Kogel WJ, Visser JH. 2000. Assessing the attractiveness of volatile plant compounds to western flower thrips Frankliniella occidentalis. Journal of Chemical Ecology 26: 2643-2655. https://doi.org/0098-0331/00/1200-2643$18.00/0 [ Links ]
Mainali BP, Lim UT. 2011. Behavioral response of western flower thrips to visual and olfactory cues. Journal of Insect Behavior 24: 436-446. https://doi.org/10.1007/s10905-011-9267-7 [ Links ]
Manjunatha M, Pickett JA, Wadhams LJ, Nazzi F. 1998. Response of western flower thrips, Frankliniella occidentalis and its predator Amblyseius cucumeris to chrysanthemum volatiles in olfactometer and greenhouse trials. Insect Science and Its Application 18: 139144. https://doi.org/10.1017/S1742758400007773 [ Links ]
Mouden S, Sarmiento KF, Klinkhamer PGL, Leiss KA. 2017. Integrated pest management in western flower thrips: past, present and future. Pest Management Science 73: 813-822. https://doi.org/10.1002/ps.4531 [ Links ]
Mound LA, Kibby G. 1998. Thysanoptera: an identification guide. 2nd Ed. CAB International, Oxon, New York.
Muvea AM, Waigani MM, Kutima HL, Osiemo Z, Nyasani JO, Subramanian S. 2014. Attraction of pest thrips (Thysanoptera: Thripidae) infesting French beans to coloured sticky traps with Lurem-TR and its utility for monitoring thrips populations. International Journal of Tropical Insect Science 34: 197-206. https://doi.org/10.1017/S174275841400040X [ Links ]
Pearsall IA. 2000. Flower preference behaviour of western flower thrips in the Similkameen Valley, British Columbia, Canada. Entomologia Experimentalis et Applicata 95: 303-313. https://doi.org/0.1046/j.1570-7458.2000.00669.x [ Links ]
Pearsall IA, Myers JH. 2000. Population dynamics of western flower thrips (Thysanoptera: Thripidae) in nectarine orchards in British Columbia. Journal of Economic Entomology 93: 264-275. https://doi.org/0022-0493/00/0264-0275$02.00/0 [ Links ]
Pow EM, Bennison JA, Birkett MA, Luzniak MJ, Manjunatha M, Pickett JA, Segers IS, Wadhams LJ, Wardlow LR, Woodcock CM. 1999. Behavioural responses of western flower thrips (Frankliniella occidentalis) to host plant volatiles. Proceedings sixth International Symposium on Thysanoptera, Antalya, Turkey, April 27 to May 1. Akdeniz University, Antalaya, Turkey. 121-128.
Pow EM, Hooper AM, Luzniak MC, Pickett JA, Wadhams LJ, Bennison JA. 1998. Novel strategies for improving biological control of western flower thrips on protected ornamentals - attraction of western flower thrips to verbena plants. Proceedings Brighton Crop Protection Conference Pests and Diseases. Vol. 2. British Crop Protection Council, Farnham, UK. 417-422.
Reitz SR, Gao,Y, Kirk WDJ, Hoddle MS, Leiss KA, Funderburk JE. 2020. Invasion biology, ecology, and management of western flower thrips. Annual Review of Entomology 65: 1.1-1.21. https://doi.org/10.1146/ annurev-ento-011019-024947 [ Links ]
Reitz SR, Maiorino G, Olson S, Sprenkel R, Crescenzi A, Momol MT. 2008. Integrating plant essential oils and kaolin for the sustainable management of thrips and tomato spotted wilt on tomato. Plant Disease 92: 878-886. https://doi.org/10.1094/PDIS-92-6-0878 [ Links ]
Reitz SR, Yearby EL, Funderburk JE, Stavisky J, Momol MT, Olson SM. 2003. Integrated management tactics for Frankliniella thrips (Thysanoptera: Thripidae) in field-grown pepper. Journal of Economic Entomology 96: 1201-1214. https://doi.org/10.1603/0022-0493-96.4.1201 [ Links ]
Roditakis NE, Lykouressis DP. 1996. Prospects for the use of volatile chemicals and a new pyrrole in integrated pest management of western flower thrips. Acta Horticulturae 431: 513-520. https://doi.org/10.17660/ActaHort.1996.431.48 [ Links ]
SAS Institute. 2015. The Statistical procedure manual. North Carolina 27513: SAS Campus Drive, Cary.
Teulon DAJ, Davidson MM, Perry NB, Nielsen M-C, Castane C, Bosch D, Riudavets J, Van Tol RWHM, De Kogel WJ. 2017. Methyl isonicotinate-a non-pheromone thrips semiochemical-and its potential for pest management. International Journal of Tropical Insect Science 37: 50-56. https://doi.org/10.1017/S1742758417000030 [ Links ]
Tyler-Julian K, Funderburk J, Frantz G, Mellinger C. 2014. Evaluation of a push-pull strategy for the management of Frankliniella bispinosa (Thysanoptera: Thripidae) in bell pepper. Environmental Entomology 43: 1364-1378. https://doi.org/10.1603/en14048 [ Links ]
Tyler-Julian K, Funderburk J, Srivastava M, Olson S, Adkins S. 2018. Evaluation of a push-pull system for the management of Frankliniella species (Thysanoptera: Thripidae) in Tomato. Insects 9: 187. https://doi.org/10.3390/insects9040187 [ Links ]
 Correspondence:
Correspondence:
E Allsopp
Email: allsoppe@arc.agric.za
Received: 08 April 2021
Accepted:26 October 2021














