Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.30 Pretoria 2022
http://dx.doi.org/10.17159/2254-8854/2022/a10298
RESEARCH ARTICLE
A preliminary assessment of the physiological and morphological correlates of beetle aggression in an emerging sugarcane pest, Cacosceles newmannii (Thomson, 1877) (Coleoptera: Cerambycidae)
Marion JavalI; Olivia Le MoëneII; Chanteile SmitI; Desmond Ε ConlongIII; John S TerblancheI
IDepartment of Conservation Ecology and Entomology, Faculty of AgriSciences, Stellenbosch University, South Africa
IIDivision for Neurobiology, Department of Biomedical and Clinical Sciences, Linköping University, Sweden
IIISouth African Sugarcane Research Institute, Mount Edgecombe, KwaZulu-Natal, South Africa
ABSTRACT
Understanding the morphological and physiological correlates of competitive behaviours can provide important insights into the ecology of competition, home range size and resource consumption. Here we first estimated and defined sexual dimorphism in a poorly studied African cerambycid species, Cacosceles newmannii (Thomson, 1877). We then assessed morphological and physiological attributes of male beetles in relation to their fighting behaviour. Suites of morphological and energetic measurements were carried out on adult males, the latter before and after male-male interactions. Aggressive behaviour and the outcomes of male fighting trials were assessed under controlled conditions. The species is highly sexually dimorphic in relation to mandible size. During male-male interactions, a continuum of behaviours with an increasing risk of injury and metabolic cost was observed. Grasping was prolonged in males with larger fighting apparatus, who also tended to use more energy during the encounter than males displaying other behaviours. Our results indicate that the mandible size in C. newmannii serves as an honest signal of fighting ability in this species. Additionally, energetic assessments in preparation for fighting, costs during a fight, and persistence of metabolic costs post-fighting may be useful for understanding the relative fitness costs of competition.
Keywords: dimorphism insect behaviour mandible respirometry sexual dimorphism sexual selection
INTRODUCTION
Limited access to resources may cause conflict, notably between males within a species. These conflicts have been the subject of numerous studies in various biological models. In particular, efforts have focused on understanding the decision-making process determining an individual's investment in a fight (Taylor & Elwood 2003; Chapin et al. 2019). Indeed, fighting is costly in time (i.e. lost opportunities, Hardy & Briffa 2013), potential injuries (Neat et al. 1998), predator exposure (Jakobsson et al. 1995), stress (Adamo & Parsons 2006) and has direct energetic costs (Briffa & Sneddon 2007; Boisseau et al. 2017). These energetic costs can play an important role in determining contest outcome, as they directly affect the individuals' fitness (Neat et al. 1998; Boisseau et al. 2017). Therefore, energetic expenses can influence an individual's decision-making process (Briffa & Sneddon 2007), and energetics can be considered a crucial factor in behavioural performance (Careau & Garland 2015).
Multiple factors can affect the willingness of an individual to engage in a fight. These factors include the resource holding potential (RHP), namely the capacity to obtain and retain resources (Parker 1974; Smith & Parker 1976), the importance of the resource in terms of fitness (Smith & Parker 1976; Arnott & Elwood 2008), the ownership status (Smith & Parker 1976; Leimar & Enquist 1984), an individual's previous experience (Snell-Rood & Moczek 2013; Camerlink et al. 2017) and the personality of an individual (Modlmeier et al. 2015). In some cases, opponents do not assess each other, but only rely on their RHP and their own cost/benefit balance (Arnott and Elwood 2008; Elwood & Arnott 2012; Chapin et al. 2019). In the latter instance, an individual would only win a fight if the opponent reached a limit and gave up, or was injured during the fight. On the other hand, mutual assessment models assume that contestants assess their opponent's RHP and decide whether to continue the interaction based on a cost / benefit estimation. Mutual assessment allows the protagonists to put an end to the conflict and to escape when the outcome of the fight is likely to be unfavourable to them, thus limiting the associated costs (Taylor and Elwood 2003; Hsu et al. 2006; Arnott and Elwood 2008). This assessment can be done before the start of the fight, or during the fight (sequential assessment model, e.g. Enquist et al. 1990). In consequence, repeated interactions allow the individuals to re-evaluate their opponent's RHP and the cost / benefit balance of the combat.
RHP is often positively correlated with the size of the insect, and by extension, the size of its fighting apparatus, i.e. mandibles (Huntingford & Turner 1987; Snell-Rood & Moczek 2013). The outcome of a fight may depend on this morphological trait (Snell-Rood & Moczek 2013; Vieira & Peixoto 2013; del Sol et al. 2021). In arthropods, these costly traits usually function as honest signals for potential mates (Zahavi 1980; Kotiaho 2001), with mandibles disproportionately large due to sexual selection (Andersson 1994). In Coleoptera, sexual dimorphism is common, and very well described (Kawano 2006). In many species, females have short mandibles, used to soften the substrate in which they will lay eggs, whereas males have longer mandibles, often associated with aggressive behaviour (Okada et al. 2006; Snell-Rood & Moczek 2013; Goyens et al. 2015a).
Metabolic rate (MR), whether at rest, during routine behaviours, or peak performance, is a well-established physiological measure in diverse taxa, known to be positively correlated with body size (Kleiber 1932; Glazier 2009). In parallel, body size is also positively correlated with some behaviours, such as patrolling, or with the home range size, and especially with the outcomes of intraspecific competition (Huntingford & Turner 1987; Snell-Rood & Moczek 2013). Recently, Videlier et al. (2019) have described sex-specific selection on resting MR in Drosophila, with males being under stronger positive directional selection than females. However, what is far less clear is whether or not these kinds of positive correlations translate into fitness costs or benefits when MR is up or down-regulated (Chown & Storey 2006; Terblanche et al. 2010), or if they translate into predictable outcomes in intra-specific sexual competition. In the handful of studies where this has been assessed, MR was correlated with dominance and the capacity to win a fight (Briffa & Sneddon 2007; Careau & Garland 2015). Furthermore, it remains unclear how costly honest signalling is, even if fights are avoided, as sustained, but lower signalling costs may accumulate quickly over time (e.g. Doubell et al. 2017). One expectation is that the ontogeny, shape, size, and weight of the fighting apparatus imply a proportional energetic investment (Kotiaho 2001; Somjee et al. 2018). Physiological state would therefore also implicitly be involved in the determination of RHP and contest success (Lailvaux & Irschick 2006). In contrast, the perceived stress of an impending fight could result in an elevated MR and hence eliminate costs saved using honest signalling. Yet another possibility is that energetic costs persist for a long period after a competitive bout, whether it escalates into full opponent contact and engagement or otherwise. Consequently, while there is much scope to integrate energy metrics into behavioural competition assessments, there is little consensus on the likely outcomes, partly due to the general paucity of such studies (Lailvaux & Irschick 2006; Careau & Garland 2015; Boisseau et al. 2017).
Here, we investigate fights between pairs of males in relation to their morphophysiological characteristics, to improve understanding of the mating system in a poorly-studied native species. Cacosceles newmannii (Thomson, 1877) is a cerambycid species native to southern Africa (Ferreira 1980). Its biology has been poorly studied until recently, with its recent emergence as a pest on sugarcane (Way et al. 2017; Javal et al. 2019a; Javal, et al. 2019b; Lehmann et al. 2021; Smit et al. 2021a, b). Its life cycle is assumed to last for two years, during which time larvae feed on organic material. Adults emerge and mate in summer, with their lifespan lasting about a month (Way et al. 2017). In this species, fighting has been observed between males. Since adult C. newmannii do not have any functional digestive system, fights are most probably not triggered by competition over feeding resources, but males could be defending a territory, or fighting over females (Snell-Rood & Moczek 2013).
We measured several morphological traits to assess sexual dimorphism and male morphs (Kawano 2006). In parallel, the correlation between morphology and MR was investigated in males. Several of the measured traits could be considered as a proxy of fighting ability (Snell-Rood & Moczek 2013). Therefore, the MR before and after male-male interaction was measured in fighting (behaviourally tested) and non-fighting (control) specimens in order to estimate the impact this interaction had on physiology. Finally, we assessed whether the measured morphophysiological traits affected the outcome of male interactions. Morphologically bigger and well-armed males were hypothesized to have a greater propensity to fight, since both self and mutual assessment would give the individual a positive signal to fight. We assumed that higher aggressive behaviour would result in greater energetic cost. This study provides a preliminary assessment of the physiological cost of intraspecific interactions in relation to several potential morphophysiological asymmetries.
MATERIALS and METHODS
Specimen collection
Cacosceles newmannii (Coleoptera: Cerambycidae) adults were collected by hand in sugarcane fields in KwaZulu-Natal (28°55'S, 31°19 'e), South Africa during summers 2017, 2018 and 2019. Live specimens were transported individually in plastic jars with perforated lids to Stellenbosch University (Western Cape, South Africa). Shredded paper was placed in the jars to provide a perching substrate and some protection from excessive movement during transport. A damp paper towel was added to each jar to provide moisture during transit. Only individuals collected in 2019 were used for the behavioural experiments, immediately after their arrival in the laboratory. The sexing of the individuals was aided by the marked morphological difference of the mandibles (Figure 1A).
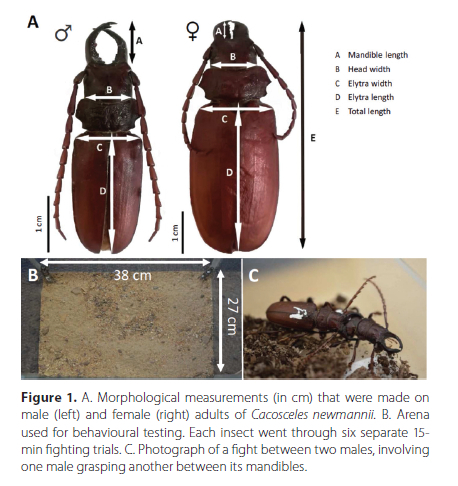
Morphometric measurements
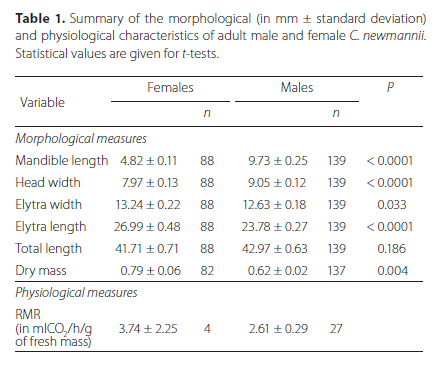
Morphological measurements were conducted on 143 dead males and 88 dead females. We measured mandible length, head width, elytra width, elytra length and total length with an electronic digital calliper (RS Component, 0.01 mm resolution) (Figure 1). Specimens were subsequently dried in an incubator at 50 °C and the body mass of selected samples checked on a regular basis until the mass stabilised (about 2 weeks). Dry mass was then determined by weighing each individual to the nearest 0.1 mg (AB104-S/Fact, Mettler Toledo International, Inc., Columbus, OH, USA).
Respirometry
We estimated the resting metabolic rate (RMR) in a subset of available specimens (4 females and 31 males), to look for correlations with morphological measurements. After field collection, specimens were allowed to recover for a few days in individual containers (15 χ 8 χ 8 cm, 24-26°C, 16L:8D regime). Insects were then placed individually in transparent flow-through 50 ml or 20 ml chambers to perform respirometry measurements, as described by Smit et al. (2021b). Briefly, an air compressor was used to pump ambient air through a purge gas generator to scrub carbon dioxide (CO2) and water (H2O). Downstream airflow was maintained at 200 ml min-1 (STPD) using a mass flow control valve (Sidetrak, Sierra International, USA) and directed into a calibrated Li-7000 infrared CO2/ H2O analyser. Data was logged using standard LiCor software (LiCor, Lincoln, NE, USA). The CO2 production of each beetle was recorded differentially (VCO2) in ppm. The activity was monitored using infrared activity detectors (AD-2, Sable Systems International, Las Vegas, NV, USA). Baseline recordings were taken before and after each run to correct for potential analyser drift, although this was typically non-existent. The temperature inside the respirometry chamber was controlled using a programmable circulating refrigeration bath filled with water (CC410wl, Huber, Berching, Germany). Runs lasted for 15 minutes, but only the last 10 minutes were analysed to avoid the settling-in period. Respirometry data were subsequently converted to ml CO2/h and extracted using Expedata software (version 1.9.10, Sable Systems). The highest 30 seconds (zenith) in the final 10-minute period were extracted for further analyses. Individual physiological measures were corrected for fresh mass. Given the small sample size for females, mean values of RMR are shown, but not analysed further.
Behavioural experiments
Upon arrival from the field, 14 healthy males of similar fresh mass from the 2019 sampling were randomly allocated to the behaviourally tested and the control group. Each male was weighed before the start of the experiment. Considering the relatively short life span of adults, all behavioural experiments were performed in 2 days, to avoid potential trial duration effects. Respirometry measurements were performed 24 h prior and post behavioural experiments. Control insects were handled similarly and had their RMR measured twice but did not go through fighting trials. Specimens were uniquely marked using a dab of white paint (Figure 1C) and allowed to rest for 15 minutes in a jar with a wet cotton pad to limit desiccation before behavioural experiments started.
The 21 possible pairs were formed using the seven available individuals. Consequently, each individual was involved in six separate fighting trials. They were sequentially placed in a glass container with a layer of coarse sand and peat (Figure 1B), which served as the encounter arena. The interactions between the two specimens were recorded for 15 minutes with a camera (Nikon D5100, 23.6 χ 15.6 mm CMOS sensor, AF-S DX Micro 40 mm f/2.8 lens). After 15 minutes, males were put back in their respective jars. If a specimen had to be used for several encounters in a row, it was allowed a 15-min break to rest and recover. Encounters were planned to exclude situations where a single male was involved in more than two interactions in a row, and overall we tried to avoid as much as possible using the same specimen for several interactions in a row. The video recording of the interactions allowed for behavioural scoring. We identified three different types of behavioural interactions between the males: physical contact (a specimen touches the other, but its mandibles do not move), mandible display (a specimen displays mandibular movements, with or without physical contact) and grasping (one of the specimens catches the other with its mandibles and tries to knock it over) (Figure 1C). The frequency and duration of each behaviour were scored.
Behavioural data preparation
For each individual, we accumulated the total frequency and duration ofeach behaviour emitted over the six fighting trials, and compared these data with the individual's morphophysiological parameters. In parallel, for each pair formed, we calculated the absolute difference in the behaviours observed for the two members of the pair (duration of behaviour observed for specimen 1 - duration of behaviour observed for specimen 2), and the associated difference in morphophysiological parameters. As the observed behaviour depended on the opponent encountered, we could not categorize individuals as a function of the behaviour they displayed (i.e. more or less aggressive individuals). Therefore, we could not test whether the change in RMR after the encounter differed between more or less aggressive individuals.
Statistical analyses
Statistical analyses for all data were performed in R (version 3.6.3, R Core Team 2013) and SPSS (version 26, IBM Statistics).
The significance level used for the analyses was P < 0.05.
Sex differences for morphological and physiological parameters were tested using bilateral i-tests. Linear regressions were computed for each variable as a function of the total body length, and ANCOVAs (homogeneity of slopes model) were used to compare slopes between sexes. A principal component analysis (PCA) of the morphological parameters of males and females was built using the FactoMineR (Lê et al. 2008) and factoextra (Kassambara & Mundt 2016) packages. Correlations between morphophysiological parameters within sexes were explored using Pearson correlation coefficients.
Morphophysiological differences between control and behaviourally tested insects were assessed with Mann-Whitney tests. The differences in RMR between the two groups was analysed using a two-way ANOVA for repeated measures on the factor time (before and after trial). The influence of trial order on each individual's behavioural performance over the six fighting trials was tested using Friedman's ANOVAs. Correlations between behavioural, morphological and physiological parameters for individuals and pairs were explored using Spearman correlations (Performance Analytics package; Peterson & Carl 2014). Finally, the effect of morphophysiological differences on the trial outcome within pairs of behaviourally tested insects was investigated with a Kruskal-Wallis test. In case of significance, Conover post-hoc tests compared the different outcome groups (PMCMRplus package; Pohlert, 2014).
RESULTS
Morphological and physiological measurements in males and females
The mandible size of females was significantly smaller than for males (Table 1), although the size ranges for the sexes overlapped (males: 3.71-15.4 mm; females: 2.56-7.5 mm, Figure 2). Figure 2B shows that the first axis of the PCA contrasts females characterized by longer elytra and greater dry mass, and males characterized by longer mandibles, larger heads and greater fresh mass. Total body length was not significantly different between sexes, but females were heavier (Table 1). Mandible length accounted for 11.60 ± 2.10% of the total body length for females, and 22.15 ± 3.71% for males.
All morphological parameters were significantly positively correlated (Table S1). Mandible length showed a positive relationship with body length for both sexes, but was more pronounced in males than in females (scaling exponent of 0.4 and 0.1 respectively, F = 236.726, P < 0.001, Tables S1, S2, Figure 2). In males, the RMR was significantly positively correlated with all the morphological traits (Pearson correlation coefficients > 0.54, all Ps < 0.05), except for dry mass (rho = 0.27, P > 0.05) (Table S1).
Individual morphophysiological correlates of behavioural performance
The results for behavioural frequencies and durations were similar, thus we have only presented the results for duration. To account for the effect of fatigue, we compared individual behavioural performances over the six fighting trials. The duration of physical contact (χ2(5) = 2.347, P = 0.799), mandible display (;2(5) = 4.159, P = 0.527) and grasping (;2(5) = 10.387, P = 0.065) were not affected by the sequence of fighting trials. Therefore, the chronological order of fighting trials was not accounted for in further analyses.
RMRs measured before and after the trials did not differ significantly between behaviourally tested and controls specimens (F(1 = 3.464, P = 0.087). However, there was a consistent effect of time on RMR, with lower values after the trials (F(112) = 13.716, P = 0.003) (Figure 3A). We found no interaction between time and group for RMR values (F = 0.272, P = 0.611).
In the seven individuals performing behavioural tests, we found only one morphological correlate of behaviour, namely males with bigger mandibles grasped their opponent for a longer time (Spearman correlation, S = 12, P = 0.048, rho = 0.786). A contrario to the subset of males for which morphophysiological parameters were measured in Table 1, we did not find any correlations between morphological and physiological parameters in males going through fighting trials (Spearman correlation, all Ps > 0.10). Similarly, we found no correlation between behavioural and physiological traits (Spearman correlation, all Ps > 0.10).
Physiological predictors of fighting trial outcomes
During the trials, nineteen (90.48 %) of the observed pairs involved physical contact, 17 (80.95 %) mandible display and 11 (52.38 %) grasping. Eleven (57.87%) out of the 19 interactions involving physical contact and 11 (64.71%) out of the 17 interactions involving mandible display, escalated to grasping. Mandible display was directly preceded by physical contact in all cases, whereas grasping was never directly preceded by physical contact, but always by a mandible display.
In accordance with these observations, we allocated the pairs to three groups depending on the outcome of the trial: grasping, mandible display, and physical contact/no interaction. The groups did not differ in relation to the average absolute difference in morphophysiological parameters between the opponents (all P-values > 0.743). However, the absolute difference in RMR was significant between the outcome groups before the trials (χ2(2) = 5.895, P = 0.050). The members of the pairs only displaying physical contact had a lower absolute difference in RMR before the trials compared to the pairs of the two other groups (i.e. contact vs. mandible display, P = 0.046; contact vs. grasping, P = 0.014) (Figure 3B). This difference disappeared after the trials (χ2(2) = 0.464, P = 0.793; Figure 3B). Finally, the difference in behaviours exchanged by the opponents of each pair were not correlated with their difference in morphophysiological parameters (all P-values > 0.10).
DISCUSSION
This study provided a formal validation of sexual dimorphism, and a preliminary assessment of the cost of intrasexual fighting in a poorly studied cerambycid pest. More than 50% of the dyadic encounters led to a fight (i.e. grasping), which is comparable to other studies using Coleoptera [e.g. Prosopocoilus inclinatus: < 30% (Inoue & Hasegawa 2013) and Aegus chelifer: > 50% (Songvorawit et al. 2018)], and this despite the absence of females. Indeed, fighting for mates is a common behaviour in the animal kingdom, especially in insects. In Prioninae specifically, there is growing evidence that females produce pheromones to attract males, and can be recognized by males based on the composition of their cuticular hydrocarbon (Millar & Hanks 2017). However, our results show that the presence of females is not needed for fighting to occur between males.
Sexual dimorphism, function and associated strategy in C. newmannii
Females were heavier than males, despite a similar total body length. In males indeed, mandibles accounted for a large proportion of total body length. The female reproductive status could not be controlled, and samples likely included both gravid and non-gravid females. This is supported by the higher standard deviation of dry mass in our data for females.
As in many other Coleoptera species, males and females of C. newmannii showed different mandibular morphs (Kawano 2006). Females had shorter but wider mandibles, whereas males had longer, narrower mandibles. An explanation might be that the two sexes have different uses for their mandibles. Since neither males nor females have a functional digestive system (MJ, unpublished data), it is unlikely that mandibles are used for feeding purposes. The robust mandibles of females might be used to chew substrate before laying eggs, as in many Coleoptera species [e.g. Asian longhorned beetle, Anoplophora glabripennis (Haack et al. 2018); stag beetle, Dorcus rectus (Tanahashi et al. 2009)]. Males showed a strong positive correlation between mandible and body size, which is often a sign of sexual selection (Eberhard 1979). In many Coleoptera species, males can show two or more morphs, associated with different reproductive strategies (Kawano 2006). We did not find such a dimorphism in our specimens, and the continuous mandible size distribution rather suggests that the different strategies to access the resources were probably not strongly split in this species.
Behavioural correlates of morphophysiological parameters
Behaviourally tested and control insects showed similar decreases in RMR 24 h after the last trial. Therefore, the male-male interaction did not have a notable affect on an individual's energetic function. However, it could be argued that this result is of limited value, as accumulated duration does not fully represent the inclination of an individual to engage in a fight. Future studies including more individuals, or specifically manipulating the number of bouts could clarify this matter.
We found that males with bigger mandibles displayed longer grasping, suggesting that males with bigger weapons are more prone to engage in a fight. This is consistent with observations for other Coleoptera species (Moczek & Emlen 2000; Okada et al. 2006; Inoue & Hasegawa 2013; Goyens et al. 2015a; Songvorawit et al. 2018). Additionally, the males exhibiting grasping behaviour and making mandible displays during the fighting trial had a higher RMR before the encounter than the males who would only display physical contact. After the encounter, this difference in RMR disappeared, as if equalized by the fighting trial, and we found no relation between the duration of antagonistic behaviours and RMR in males. This suggests, as hypothesized, that males with bigger mandibles were more aggressive and initially had more energy to invest into the fight, resulting in a greater energy loss for them than their less aggressive counterparts (Somjee et al. 2018).
On another note, it was unclear whether the three observed behaviours were antagonistic, in the sense that they predisposed the individual to fight. Physical contact might only be a recognition contact where insects exchange information. Indeed, RHP asymmetry might also be measured by the estimation of differences in cuticular hydrocarbons or pheromones, as hypothesized for other Coleoptera (Goyens et al. 2015a). In addition, pairs only exchanging physical contact had a smaller difference in RMR before the trials, a difference which disappeared afterwards. This supported the mutual assessment hypothesis, as individuals with similar energetic resources would not take the risk of investing in aggressive behaviours. Visual display of opened mandibles is a common aggressive behaviour in Coleoptera (Goyens et al. 2015b; Okamoto & Hongo 2013). Indeed, in our case only mandible display significantly directly preceded grasping. A gradation of the measurement mechanisms can therefore be envisaged, each step being riskier but making it possible to refine opponent assessment.
CONCLUSION
To conclude, the data presented here constitute an important attempt at understanding the morphological and physiological correlates of the aggressive behaviours of a poorly studied cerambycid species. These interactions might lead to territoriality, the securing of resources (e.g. food or mates), or establishment of dominance hierarchies. These behaviours could drive dispersal patrolling behaviours, and in turn, affect the size of the home range and hence, management strategies for this species.
Adult C. newmannii, and especially females, do not fly often, and tend to stay on the ground in the sugarcane and kikuyu fields from where they were collected. This made gathering a high number of healthy, live adults challenging, as specimens are difficult to see in dense vegetation, resulting in the small sample available for the behavioural part of this study. Though our results must be interpreted with some caution owing to the small sample size in the behavioural assessments, the results outlined here may form the basis for future larger studies on this emerging pest species. Behaviours are routinely assessed with small sample sizes in groups for which sampling or laboratory rearing is technically or ethically difficult, such as for large mammals, or in the context of non-manipulative studies (LaFollette 1971; De Nys et al. 2010 ; Wright et al. 2019). Reliable behavioural or physiological data for insects can in many cases be obtained with a reduced number of individuals, although further validation is necessary and useful. For example, the flight behaviour of the Asian longhorned beetle was initially measured on very few individuals (Javal et al. 2018), but the results obtained were subsequently validated in a study that used a larger sample size (Lopez et al. 2017).
While our results are preliminary, they are informative and suggest several directions to explore in future. Male intra-sexual interactions seem to follow a pattern of increasing intensity, with each additional step being costlier in terms of risk of injury and energy investment. Mutual assessment could play a role in the first steps of the interaction, when mandible size is used to assess fighting ability. However, the positive correlation between individual mandible size and duration of grasping, together with the lower difference in RMR before the trials in individuals not displaying aggressive behaviours, suggest that once a fight has been initiated, the duration would rely more on self-resources than on mutual assessment (Pinto et al. 2019). In the case of C. newmannii, the RMR of a male individual, and the length of its mandibles might set the amount of time or energy that this specific individual is willing to invest in the fight.
Finally, we found that males with bigger mandibles tended to have a bigger energetic investment in a fight than smaller males. Male-exaggerated sexual traits are usually physiologically costly (O'Brien et al. 2019) and can impact fitness in indirect ways (Basolo & Alcaraz 2003). The considerable energetic investment linked to large fighting apparatus (Kotiaho 2001) can lead to faster exhaustion, or even premature death. Longevity has not been considered here, but could be the focus of further studies, since the cost of having a large fighting apparatus, together with the cost of engaging in energy-demanding interactions could reduce the males' lifespan (Somjee et al. 2018).
ACKNOWLEDGEMENTS
We thank the SASRI Eshowe Biosecurity team who helped collect the specimens, D. Gillespie and N. Muthusamy at the South African Sugarcane Research Institute for the transfer of insects, anonymous referees, and P. Huijgens and P. Lehmann for constructive comments on an earlier version of the manuscript.
FUNDING
This research was supported by the Centre for Invasion Biology and the South African Sugarcane Research Institute (Contract number: S005221).
SUPPLEMENTARY MATERIAL
Supplementary information for this article is available at https://doi.org//10.17159/2254-8854/2022/a10298
ORCID IDs
Marion Javal - https://orcid.org/0000-0001-7878-2936
Olivia Le Moëne - https://orcid.org/0000-0003-3599-0211
Chantelle Smit - https://orcid.org/0000-0001-7568-7992
Desmond E Conlong - https://orcid.org/0000-0002-1241-3430
John S Terblanche - https://orcid.org/0000-0001-9665-9405
REFERENCES
Adamo SA, Parsons NM. 2006. The emergency life-history stage and immunity in the cricket, Gryllus texensis. Animal Behaviour 72(1): 235-244. https://10.1016/j.anbehav.2006.01.011 [ Links ]
Andersson M. 1994. Sexual Selection. Princeton, NJ: Princeton University Press. https://10.1515/9780691207278 [ Links ]
Arnott G, Elwood RW. 2008. Information gathering and decision making about resource value in animal contests. Animal Behaviour 76(3): 529-542 https://10.1016/j.anbehav.2008.04.019 [ Links ]
Basolo AL, Alcaraz G. 2003.The turn of the sword: length increases male swimming costs in swordtails. Proceedings of the Royal Society B: Biological Sciences 270(1524): 1631-1636. https://10.1098/rspb.2003.2388 [ Links ]
Boisseau RP, Arthur Woods H, Goubault M. 2017. The metabolic costs of fighting and host exploitation in a seed-drilling parasitic wasp. Journal of Experimental Biology 220(21): 3955-3966. https://10.1242/jeb.160887 [ Links ]
Briffa M, Sneddon LU. 2007. Physiological constraints on contest behaviour. Functional Ecology 21(4): 627-637. https://10.1111/j.1365-2435.2006.01188.x [ Links ]
Camerlink I, Turner SP, Farish M, Arnott G. The influence of experience on contest assessment strategies. Scientific Reports 7(1): 14492. https://10.1038/s41598-017-15144-8
Careau V, Garland T. 2015. Energetics and behavior: Many paths to understanding. Trends in Ecology and Evolution 30(7): 365-366. https://10.1016/j.tree.2015.04.007 [ Links ]
Chapin KJ, Peixoto PEC, Briffa M. 2019. Further mismeasures of animal contests: a new framework for assessment strategies. Behavioural Ecology 30(5): 1177-1185. https://10.1093/beheco/arz081 [ Links ]
Chown SL, Storey KB. 2006. Linking molecular physiology to ecological realities. Physiological and Biochemical Zoology 79(2): 314-323. https://10.1086/499989 [ Links ]
De Nys HM, Bertschinger HJ, Turkstra JA, Colenbrander B, Palme R, Human AM. 2010, Vaccination against GnRH may suppress aggressive behaviour and musth in African elephant (Loxodonta africana) bulls - A pilot study. Journal of the South African Veterinary Association. 81(1): 8-15. https://10.4102/jsava.v81i1.88. [ Links ]
del Sol JF, Hongo Y, Boisseau RP, Berman GH, Allen CE, Emlen DJ. 2021. Population differences in the strength of sexual selection match relative weapon size in the Japanese rhinoceros beetle, Trypoxylus dichotomus (Coleoptera: Scarabaeidae). Evolution 75(2): 394-413. https://10.1111/evo.14101 [ Links ]
Doubell M, Grant PBC, Esterhuizen N, Bazelet CS, Addison P, Terblanche JS. 2017. The metabolic costs of sexual signalling in the chirping katydid Plangia graminea (Serville) (Orthoptera: Tettigoniidae) are context dependent: Cumulative costs add up fast. Journal of Experimental Biology 220(23): 4440-4449. https://10.1242/jeb.160036 [ Links ]
Eberhard WG. 1979. The function of horns in Podischnus agenor (Dynastiae) and other beetles. In: Blum M, Blum N, editors. Sexual selection and reproductive competition in insects. Cambridge MA: Academic Press. pp 231-258 [ Links ]
Elwood RW, Arnott G. 2012. Understanding how animals fight with Lloyd Morgan's canon. Animal Behaviour. 84(5): 1095-1102. https://10.1016/j.anbehav.2012.08.035. [ Links ]
Enquist M, Leimar O, Ljungberg T, Mallner Y, Segerdahl N. 1990. A test of the sequential assessment game: fighting in the cichlid fish Nannacara anomala. Animal Behaviour 40(1): 1-14. https://10.1016/S0003-3472(05)80660-8 [ Links ]
Ferreira GWS. 1980. The Parandrinae and the Prioninae of southern Africa (Cerambycidae, Coleoptera). Memoirs van die Nasionale Museum Bloemfontein 13: 1-335 [ Links ]
Glazier DS. 2009. Activity affects intraspecific body-size scaling of metabolic rate in ectothermic animals. Journal of Comparative Physiology B 179(7): 821-828. https://10.1007/s00360-009-0363-3 [ Links ]
Goyens J, Dirckx J, Aerts P. 2015a. Stag beetle battle behavior and its associated anatomical adaptations. Journal of Insect Behaviour 28(3): 227-244. https://10.1007/s10905-015-9495-3 [ Links ]
Goyens J, Van Wassenbergh S, Dirckx J, Aerts P. 2015b. Cost of flight and the evolution of stag beetle weaponry. Journal of the Royal Society Interface 12(106): 20150222. https://10.1098/rsif.2015.0222 [ Links ]
Haack R, Bauer L, Gao R-T, McCarthy J, Miller D, Petrice T, Poland T. 2018. Anoplophorag glabripennis within-tree distribution, seasonal development, and host suitability in China and Chicago. The Great Lakes Entomologist 39: 169-183 [ Links ]
Hardy I, Briffa M. 2013. Animal contests. Cambridge: Cambridge University Press. https://10.1017/CBO9781139051248 [ Links ]
Okamoto K. Hongo Y,. 2013. Interspecific contests between males of two Japanese stag beetle species, Lucanus maculifemoratus and Prosopocoilus inclinatus: what overcomes a body size disadvantage? Behaviour 150(1): 39-59. https://10.1163/1568539X-00003036 [ Links ]
Hsu Y, Earley RL, Wolf LL. 2006. Modulation of aggressive behavior by fighting experience: mechanisms and contest outcomes. Biological Review of the Cambridge Philosophical Society 81(1): 33-74. https://10.1017/S146479310500686X [ Links ]
Huntingford FA, Turner A. 1987. Animal conflict. London: Hall & Chapman. [ Links ]
Inoue A, Hasegawa E. 2013. Effect of morph types, body size and prior residence on food-site holding by males of the male-dimorphic stag beetle Prosopocoilus inclinatus (Coleoptera: Lucanidae). Journal of Ethology 31(1):55-60. https://10.1007/s10164-012-0350-0 [ Links ]
Jakobsson S, Brick O, Kullberg C. 1995. Escalated fighting behaviour incurs increased predation risk. Animal Behaviour. 49(1): 235-239. https://10.1016/0003-3472(95)80172-3. [ Links ]
Javal M, Roux G, Roques A, Sauvard D. 2018. Asian Long-horned Beetle dispersal potential estimated in computer-linked flight mills. Journal of Applied Entomology 142(1-2): 282-286. https://10.1111/jen.12408 [ Links ]
Javal M, Terblanche JS, Conlong DE, Malan AP. 2019a. First screening of entomopathogenic nematodes and fungus as biocontrol agents against an emerging pest of sugarcane, Cacosceles newmannii (Coleoptera: Cerambycidae). Insects 10(4): 117. https://10.3390/insects10040117 [ Links ]
Javal M, Thomas S, Lehmann P, Barton MG, Conlong DE, Du Plessis A, Terblanche JS. 2019b. The effect ofoxygen limitation on a xylophagous insect's heat tolerance is influenced by life-stage through variation in aerobic scope and respiratory anatomy. Frontiers in Physiology 10: 1426. https://10.3389/fphys.2019.01426 [ Links ]
Kassambara A, Mundt F. 2016. Package 'factoextra'. Extract and Visualize the Results of Multivariate Data Analyses. R Foundation for Statistical Computing, Vienna. Austria. https://www.R-project.org/
Kawano K. 2006. Sexual dimorphism and the making of oversized male characters in beetles (Coleoptera). Annals of the Entomological Society of America 99(2): 327-341. https://10.1603/0013-8746(2006)099[0327:SDATMO]2.0.CO;2. [ Links ]
Kleiber M. 1932. Body size and metabolism. Hilgardia 6(11): 315-353. https://10.3733/hilg.v06n11p315 [ Links ]
Kotiaho JS. 2001. Costs of sexual traits: a mismatch between theoretical considerations and empirical evidence. Biological Review of the Cambridge Philosophical Society 76(3): S1464793101005711.https://10.1017/S1464793101005711 [ Links ]
LaFollette RM. 1971. Agonistic behaviour and dominance in confined wallabies, Wallabia rufogrisea frutica. Animal Behaviour 19(1): 93-101. https://10.1016/S0003-3472(71)80140-9 [ Links ]
Lailvaux SP, Irschick DJ. 2006. A functional perspective on sexual selection: insights and future prospects. Animal Behaviour 72(2): 263-273. https://10.1016/j.anbehav.2006.02.003 [ Links ]
Lê S, Josse J, Rennes A, Husson F. 2008. FactoMineR: An R package for multivariate analysis. JSS Journal of Statistical Software. 25(1): 1-18. [ Links ]
Lehmann P, Javal M, Du Plessis A, Terblanche JS. 2021, Using μCT in live larvae of a large wood-boring beetle to study tracheal oxygen supply during development. Journal of Insect Physiology 130: 104199. https://10.1016/j.jinsphys.2021.104199 [ Links ]
Leimar O, Enquist M. 1984. Effects of asymmetries in owner-intruder conflicts. Journal of Theoretical Biology 111(3): 475-491. https://10.1016/S0022-5193(84)80235-0 [ Links ]
Lopez VM, Hoddle MS, Francese JA, Lance DR, Ray AM. 2017. Assessing flight potential of the invasive Asian Longhorned Beetle (Coleoptera: Cerambycidae) with computerized flight mills. Journal of Economic Entomology 110(3):1070-1077. https://10.1093/jee/tox046 [ Links ]
Millar JG, Hanks LM. 2017. Chemical ecology of cerambycids. In: Qiao W, editor. Cerambycidae of the World: Biology and Pest Management. Boco Raton: CRC Press. p 291-303. https://10.1201/b21851 [ Links ]
Moczek AP, Emlen DJ. 2000. Male horn dimorphism in the scarab beetle, Onthophagus taurus: do alternative reproductive tactics favour alternative phenotypes? Animal Behaviour 59(2): 459-466. https://10.1006/anbe.1999.1342 [ Links ]
Modlmeier AP, Keiser CN, Wright CM, Lichtenstein JLL, Pruitt JN. 2015. Integrating animal personality into insect population and community ecology. Current Opinion in Insect Science 9: 77-85. https://10.1016/j.cois.2015.03.008 [ Links ]
Neat FC, Taylor AC, Huntingford FA. 1998. Proximate costs of fighting in male cichlid fish: the role of injuries and energy metabolism. Animal Behaviour 55(4): 875 -882. https://10.1006/anbe.1997.0668 [ Links ]
O'Brien DM, Boisseau RP, Duell M, McCullough E, Powell EC, Somjee U, Solie S, Hickey AJ, Holwell GI, Painting CJ, et al. (2019). Muscle mass drives cost in sexually selected arthropod weapons. Proceedings of the Royal Society B: Biological Sciences 286: 20191063. https://10.1098/rspb.2019.1063 [ Links ]
kada K, Miyanoshita A, Miyatake T. 2006. Intra-sexual dimorphism in male mandibles and male aggressive behavior in the broad-horned flour beetle Gnatocerus cornutus (Coleoptera: tenebrionidae). Journal of Insect Behaviour 19(4): 457-467. https://10.1007/s10905-006-9038-z [ Links ]
Okada K, Miyatake T. 2004. Sexual dimorphism in mandibles and male aggressive behavior in the presence and absence of females in the beetle Librodor japonicus (Coleoptera: Nitidulidae). Annals of the Entomological Society of America 97(6): 1342-1346. https://10.1603/0013-8746(2004)097[1342:SDIMAM]2.0.CO;2 [ Links ]
Parker GA. 1974. Assessment strategy and the evolution of fighting behaviour. Journal of Theoretical Biology 47(1): 223-243. https://10.1016/0022-5193(74)90111-8 [ Links ]
Peterson B, Carl P. 2014. Package 'PerformanceAnalytics'. Econometric Tools for Performance and Risk Analysis. R Foundation for Statistical Computing, Vienna. Austria. https://www.R-project.org/
Pinto NS, Palaoro AV, Peixoto PEC. 2019. All by myself? Meta-analysis of animal contests shows stronger support for self than for mutual assessment models. Biological Reviews 94(4): 12509. https://10.1111/brv.12509 [ Links ]
Pohlert T. 2014. The pairwise multiple comparison of mean ranks package. R Foundation for Statistical Computing, Vienna. Austria. https://www.R-project.org/
R Core Team. 2013. R: A language and environment for statistical computing (3.4.3). R Foundation for Statistical Computing. https://www.R-project.org/
Smit C, Javal M, Conlong DE, Hall G, Terblanche JS. 2021a. Host range determination in a novel outbreak pest of sugarcane, Cacosceles newmannii (Coleoptera: Cerambycidae, Prioninae), inferred from stable isotopes. Agricultural and Forest Entomology 23(3):378-387. https://10.1111/afe.12439 [ Links ]
Smit C, Javal M, Lehmann P, Terblanche JS. 2021b. Metabolic responses to starvation and feeding contribute to the invasiveness of an emerging pest insect. Journal of Insect Physiology 128:104162. https://10.1016/j.jinsphys.2020.104162 [ Links ]
Smith JM, Parker GA. 1976. The logic of asymmetric contests. Animal Behaviour 24(1): 159-175. https://10.1016/S0003-3472(76)80110-8 [ Links ]
Snell-Rood E, Moczek A. 2013. Horns and the role of development in the evolution of beetle contests. In: Hardy I, Briffa M, editors. Animal Contests. Cambridge: Cambridge University Press. https://10.1017/CBO9781139051248.011 [ Links ]
Somjee U, Woods HA, Duell M, Miller CW. 2018. The hidden cost of sexually selected traits: The metabolic expense of maintaining a sexually selected weapon. Proceedings of the Royal Society B: Biological Sciences 285(1891): 1685. https://10.1098/rspb.2018.1685 [ Links ]
Songvorawit N, Butcher BA, Chaisuekul C. 2018. Resource Holding Potential and the outcome of aggressive interactions between paired male Aegus chelifer chelifer (Coleoptera: Lucanidae) Stag Beetles. Journal of Insect Behaviour 31(4):347-360. https://10.1007/s10905-018-9683-z [ Links ]
Tanahashi M, Matsushita N, Togashi K. 2009. Are stag beetles fungivorous? Journal of Insect Physiology 55(11): 983-988. https://10.1016/j.jinsphys.2009.07.002 [ Links ]
Taylor PW, Elwood RW. 2003. The mismeasure of animal contests. Animal Behaviour 65(6): 1195-1202. https://10.1006/anbe.2003.2169 [ Links ]
Terblanche JS, Clusella-Trullas S, Chown SL. 2010. Phenotypic plasticity of gas exchange pattern and water loss in Scarabaeus spretus (Coleoptera: Scarabaeidae): Deconstructing the basis for metabolic rate variation. Journal of Experimental Biology 213(17): 2940-2949. https://10.1242/jeb.041889 [ Links ]
Videlier M, Rundle HD, Careau V. 2019. Sex-specific among-individual covariation in locomotor activity and resting metabolic rate in Drosophila melanogaster. American Naturalist 194(6): E164-E176. https://10.1086/705678 [ Links ]
Vieira MC, Peixoto PEC. 2013. Winners and losers: a meta-analysis of functional determinants of fighting ability in arthropod contests. Functional Ecology 27(2): 305-313. https://10.1111/1365-2435.12051 [ Links ]
Way M, Conlong D, Rutherford R, Sweby D, Gillespie D, Stramack R, Lagerwall G, Grobbelaar E, Perissinotto R. 2017. Cacosceles (Zelogenes) newmannii (Thomson) (Cerambycidae: Prioninae), a new pest in the South African sugarcane industry. Proceedings of the SASTA 90: 62-65 [ Links ]
Wright E, Galbany J, McFarlin SC, Ndayishimiye E, Stoinski TS, Robbins MM. Male body size, dominance rank and strategic use of aggression in a group-living mammal. Animal Behaviour 2019;151: 87-102. https://10.1016/j.anbehav.2019.03.011
Zahavi A. 1980. Ritualization and the evolution of movement signals. Behaviour 72(1-2): 77-80. https://10.1163/156853980X00050 [ Links ]
 Correspondence:
Correspondence:
Marion Javal
Email:marion.javal@gmail.com
Received: 27 April 2021
Accepted: 30 July 2021
Supplementary Data
The supplementary data is available in pdf: [Supplementary data]














