Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
African Entomology
On-line version ISSN 2224-8854
Print version ISSN 1021-3589
AE vol.30 Pretoria 2022
http://dx.doi.org/10.17159/2254-8854/2022/a11738
ARTICLES
Identity of wasp parasitoids (Hymenoptera) attacking Pieris brassicae (Linnaeus, 1758) (Lepidoptera: Pieridae) in South Africa
Simon van NoortI, II; Jose Fernandez-TrianaIII; Hannes BaurIV, V; Mark R. ShawVI
IResearch and Exhibitions Department, South African Museum, Iziko Museums of South Africa, Cape Town, South Africa
IIDepartment of Biological Sciences, University of Cape Town, South Africa
IIICanadian National Collection of Insects, Ottawa, Canada
IVDepartment of Invertebrates, Natural History Museum Bern, Switzerland
VInstitute of Ecology and Evolution, University of Bern, Switzerland
VINational Museums of Scotland, Edinburgh, UK
ABSTRACT
The alien invasive large cabbage white, Pieris brassicae (Linnaeus, 1758) (Pieridae), although introduced into South Africa more than 27 years ago, has not dramatically expanded its distribution, possibly because it is effectively attacked by two parasitoid wasp species. Even though there is a cohort of parasitoid species associated with P. brassicae in the Northern Hemisphere, these are the only two recorded parasitoids in South Africa. We determine these parasitoid wasps as Cotesia glomerata (Linnaeus, 1758) (Braconidae: Microgastrinae) and Pteromalus puparum (Linnaeus, 1758) (Pteromalidae), and provide high resolution images and DNA barcodes to facilitate their recognition. This is the first record of C. glomerata from the Afrotropical region.
Keywords alien species biocontrol Braconidae Cotesia glomerate invasive species parasitoid wasps Pteromalidae Pteromalus puparum taxonomy
INTRODUCTION
The large cabbage white, Pieris brassicae (Linnaeus, 1758) (Pieridae), was accidentally introduced into South Africa sometime prior to its first detection in July 1994 in Sea Point, Cape Town (Claassens 1995, 1996, 1998, 2014; Gardiner 1995; Geertsema 1996; Picker and Griffiths 2011, 2017; van Noort 1996). The species is extremely common on the Cape Peninsula (Claassens 1995, 1996, 1998, 2014; Steele 1998), and the adults are present for most of the year (Prinsloo & Uys 2015), although population levels can fluctuate dramatically from year to year (Janion-Scheepers & Griffiths 2020). The species is a registered invasive species (Robinson et al. 2020), but has not spread much beyond a core south-western Cape distributional range, extending north as far as Velddrif on the Western Cape coast, and east to Keurboomstrand on the southern Cape coast, with two outlying isolated records from Walvis Bay in Namibia (iNaturalist 2021, https://www.inaturalist.org/observations?taxon_id=55401).
Although P. brassicae appears to have had limited impact on cruciferous vegetable production in South Africa, even though it has the potential to do so (Geertsema 1996), there is evidence that it is having an impact as an effective pollinator on invasive ornamental plants in South Africa, such as devil's beard (Centranthus ruber L.) and purple loosestrife (Lythrum salicaria L.) (Geerts et al. 2017; Le Roux et al. 2020). Besides cabbage, the caterpillars commonly feed on nasturtiums (Tropaeolum majus L.) and sweet alyssum [Lobularia maritima (L.) Desv.] in gardens and parks in South Africa, as well as on a common weed, Rapistrum rugosum (L.) (Wild Mustard or Turnip Weed) (Claassens 1995, 2014; Prinsloo & Uys 2017; van Noort 1996).
This butterfly, indigenous to the Palaearctic region in the northern hemisphere, is an invasive species in other areas of the southern hemisphere besides South Africa: Chile where it has been established for over 36 years (Benyamini 1996; Gardiner 1974; Neira et al. 1989), and New Zealand where it has purportedly subsequently been eradicated (Brown et al. 2019). In Europe and Chile (and formerly in New Zealand), P. brassicae larvae are commonly parasitized by the microgastrine Cotesia glomerata (Linnaeus, 1758) (Braconidae), and pupae by Pteromalus puparum (Linnaeus, 1758) (Pteromalidae) (Karnavar 1983; Peters 1991; Shaw and Fitton 1989; Shaw and Huddleston 1991; Askew and Shaw 1997; Shaw et al. 2009). Together these are the most effective parasitoids of Pieris brassicae and parasitism levels may exceptionally be as high as 100% (Peters 1991), but are usually much lower than this (Shaw et al. 2009). Both parasitoid species were originally released in New Zealand in the early 1930s as biological control agents for P. rapae (Cameron et al. 1989; Phillips et al. 2014), a misguided attempt as C. glomerata is better adapted to P. brassicae, and P. puparum has a wide host range (Shaw et al. 2009). The records of two other microgastrine braconids from P. brassicae clearly stem from host misidentifications: Cotesia rubecula (Marshall, 1885), which almost exclusively attacks Pieris rapae in nature, and Cotesia ruficrus (Haliday, 1834), which has a wide host repertoire, but does not attack P. brassicae (Shaw et al. 2009). Other Microgastrinae species mentioned in some references (e.g. Greathead & Greathead 1992; Harvey et al. 1999, see also compilation of references in Yu et al. 2016) are almost certainly incorrect and should not be considered further. On a global level P. brassicae is attacked by several other wasp species as well as fly parasitoids. Again many of the records of Ichneumonoidea cited in Yu et al. (2016), as well as in the primary literature (Greathead & Greathead 1992; Neira et al. 1989; Razmi et al. 2011; Shahram & Debjani 2013) need to be treated with great suspicion unless their identity and positive association with P. brassicae is confirmed. Apart from C. glomerata, the campoplegine ichneumonid, Hyposoter ebeninus (Gravenhorst, 1829), is another koinobiont larval parasitoid attacking P. brassicae (Shaw et al. 2016), and a Brachymeria species (Chalcididae) is also an occasional primary parasitoid of the pupa (Shaw et al. 2009). Idiobiont pupal parasitoids are usually less specialised (Shaw 1994), and in Europe Pimpla rufipes (Miller, 1759) and Apechthis compunctor (Linnaeus, 1758) are commonly reared from P. brassicae, with the gregarious Blapsidotes vicinus (Gravenhorst, 1829) being less commonly recorded from this host (Shaw 1982; Shaw et al. 2009). A number of non-host specific tachinid fly species develop as larval and larval-pupal parasitoids [Epicampocera succincta (Meigen) is, however, a Pieris specialist], some with wide host ranges e.g. species of Pales Robineau-Desvoidy, Compsilura Bouché, Phryxe Robineau-Desvoidy and Exorista Meigen regularly attack P. brassicae, and at least in the case of E. succincta outcompete C. glomerata (Shaw 1982; Shaw et al. 2009). Polyphagous Trichogramma Westwood (Trichogrammatidae) egg parasitoids commonly attack Pieris Schrank hosts in Europe (Shaw 1982). Incidence and levels of hyperparasitism in this system are currently unknown.
In South Africa, two species of Tachinidae in the genera Winthemia Robineau-Desvoidy and Exorista, and a species of Pteromalus (Pteromalidae: Chalcidoidea) (Claassens 1995, 1996), as well as a species of Cotesia (Claassens 1998) - cited as a species of Apanteles in Prinsloo & Uys (2015) and Janion-Scheepers & Griffiths (2020) - have been reared from P. brassicae. The pteromalid wasp associated with P. brassicae in South Africa was subsequently recorded as the cosmopolitan P. puparum (Claassens 1998; Picker and Griffiths 2011; Prinsloo and Uys 2015), which is a gregarious pupal endoparasitoid (Askew and Shaw 1997; Shaw 1982; Shaw 2002; Shaw et al. 2009). We here provide a species determination for the microgastrine braconid wasp as C. glomerata, which is a gregarious larval endoparasitoid (Shaw 1982; Shaw et al. 2009), and confirm the identity of Pteromalus puparum. To facilitate their future recognition we provide high resolution images of these two species reared from P. brassicae in South Africa as well as DNA barcodes for C. glomerata. All images presented here as well as supplementary images are available on www.waspweb.org.
MATERIALS and METHODS
A leg from each of three selected specimens of Cotesia reared from P. brassicae in South Africa was submitted to Barcode of Life Data System (BOLD) for barcoding. DNA extracts were obtained from single legs using a glass fibre protocol (Ivanova et al. 2006). Total genomic DNA was re-suspended in 30 μ! of dH2O, a 658 base pairs (bp) region near the 5' terminus of the CO1 gene was amplified using standard primers (LepF1-LepR1) following established protocols (http://v4.boldsystems.org/index.php), and a composite sequence was generated for all successful amplifications. All information for the sequences associated with each individual specimen barcoded can be retrieved from the BOLD system (Ratnasingham and Hebert 2007). We use the Barcode Index Number (BIN) system to discuss species limits, following the BIN concept detailed in Ratnasingham and Hebert (2013).
Images were acquired at SAMC with a Leica LAS 4.9 imaging system, comprising a Leica* Z16 microscope (using either a 2xor 5x objective) with a Leica DFC450 Camera and 0.63x video objective attached. The imaging process, using an automated Z-stepper, was managed using the Leica Application Suite V 4.9 software installed on a desktop computer. Diffused lighting was achieved using a Leica LED5000 HDI dome. All images presented in this paper, as well as supplementary images, are available at www.waspweb.org.
Depository of specimens
SAMC: Iziko South African Museum, Cape Town (curator Simon van Noort)
RESULTS
Barcoding
Of the three Cotesia samples submitted to BOLD, two rendered full DNA barcodes (658 base pairs), the third (38754_B02_ SAM-HYM-P020703; FSA1906-21), which was a dry specimen mounted in 1998 shortly after collection, returned only a 286 bp length that was determined to represent a potential contamination. The ethanol preserved specimen (38754_B03_ SAM-HYM-P020703; FSA1907-21) from this same collecting event had been stored in 96% ethanol until extraction on 27 November 2020 for submission to BOLD. The third specimen (38754_B11_SAM-HYM-P095102; FSA1915-21) was freshly collected on emergence on 1 December 2020 and killed in 96% ethanol shortly prior to submission. The Pteromalus extraction failed to produce a sufficient sequence length for barcoding (i.e. 281 base pairs).
The South African Cotesia specimens match perfectly with several sequences of specimens of Cotesia glomerata from Chile, the Czech Republic and France (Figure 5). They also match fairly closely (99.5-99.6% of base pairs shared) with other sequences of C. glomerata in BOLD from Australia, Canada, the Czech Republic, Hungary, Paraguay, Spain, Switzerland and United States. Other specimens of C. glomerata in BOLD from India, Pakistan and Turkey are slightly less related (98.7% of similar base pairs), but still within the limits of what is considered as a single BIN (BOLD: AAD1110) in BOLD, which includes all specimens of C. glomerata with sequences deposited there.
Braconidae, Microgastrinae
Cotesia glomerata (Linnaeus, 1758) (Figures 1-4)
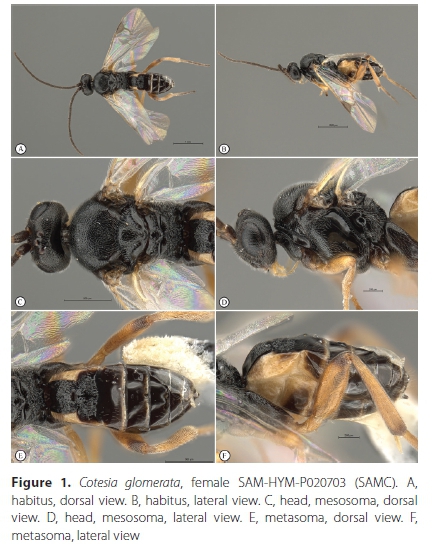
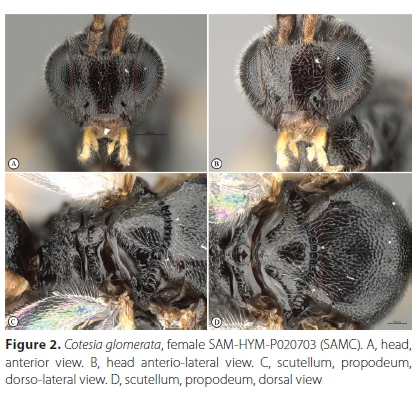


Material examined. South Africa, Western Cape Province. 6 FM: Cape Town, Sea Point, 33.917°S 18.383Έ, 8 m, 22 September 1998, A.J.M. Claassens, ex Pieris brassicae, 2 pins with caterpillar skin & cocoon cases, Cotesia sp. det. S. van Noort, 1998, SAM-HYM-P020669 (SAMC); 6 FM: Cape Town, Sea Point, 33.917°S, 18.383°E, 8 m, 28 September 1998, A.J.M. Claassens, ex Pieris brassicae, (additional specimens in ethanol), Cotesia sp. det. S. van Noort, 1998, SAM-HYM-P020671 (SAMC); 7 FM: Stellenbosch, 29 September 1998, V.B. Whitehead & V. Booth, ex Pieris brassicae, 1 pin with caterpillar case & cocoon - see material in EtOH, Cotesia sp. det. S. van Noort, 1998, SAM-HYM-P020670 (SAMC); 31 FM: Cape Town, Kirstenhof, 34.069°S 18.452Έ, 1 November 1998, S. van Noort, ex Pieris brassicae, emerged 9 November 1998, Cotesia sp. det. S. van Noort, 1998, SAM- HYM-P020703 (SAMC); 2 M: Cape Town, Devil's Peak, October 2016, N. Larsen, ex Pieris brassicae larva, SAM-HYM-P095176 (SAMC); ditto SAM-HYM-P095177 (SAMC); Muizenberg, Clifton Road, 34.101°S 18.475Έ, caterpillar collected 23 November 2020, wasp larvae emerged from host caterpillar and spun cocoon on 25 November 2020; adult wasps emerged 1 December 2020, S. van Noort, MUIZ20-COT231120, Cotesia sp. det. S. van Noort, 2020, SAM-HYM-P095102 (SAMC); ditto SAM-HYM-P095103 (SAMC); ditto SAM-HYM-P095104 (SAMC); ditto SAM-HYM-P095105 (SAMC); ditto SAM-HYM-P095106 (SAMC); ditto SAM-HYM-P095107 (SAMC); ditto SAM-HYM-P095108 (SAMC); ditto SAM-HYM-P095109 (SAMC); ditto SAM-HYM-P095110 (SAMC); ditto SAM- HYM-P095111 (SAMC); ditto SAM-HYM-P095112 (SAMC).
Diagnosis. The sequences available in BOLD support the assignment of the two South African barcodes to C. glomerata, as they cluster with sequences of that species originating from Chile, the Czech Republic and France (Figure 5).
Distribution. Australasian: Australia (ACT, NSW, QLD), New Zealand; Nearctic: Canada (BC, NB, ON, QC) and USA (CA, CO, CT, DC, FL, IL, IA, LA, MD, MA, MI, MN, NH, NJ, NY, OR, PA, SC, VT, VA, WA, WI); Neotropical: Barbados, Brazil (SP), Chile and Uruguay; Indomalayan (Oriental): China (GZ, HN, JS, SH, SN, TW, ZJ), India, Pakistan and Vietnam; Oceania: Fiji and Hawaiian Islands and Palaearctic: Armenia, Azerbaijan, Azores, Belarus, Belgium, Bulgaria, Canary Islands, China (BJ, HE, HA,
JL, LN, NM, NX, SN, XJ), Croatia, Cyprus, Czech Republic, Denmark, Egypt, Estonia, Finland, France, Georgia, Germany, Hungary, Iran, Ireland, Israel, Italy, Japan, Jordan, Kazakhstan, Korea, Latvia, Lithuania, Macedonia, Malta, Moldova, Mongolia, Morocco, Netherlands, Poland, Portugal, Romania, Russia (AD, AST, BU, KGD, KAM, KHA, KIR, KDA, KRS, MOS, PRI, ROS, SAK, SPE, SAR, TAM, VGG, VLG, YAR), Serbia, Slovakia, Spain,Sweden, Switzerland, Syria, Turkey, Ukraine, United Kingdom and Uzbekistan (Fernandez-Triana et al. 2020).
Biology. Gregarious endoparasitoid of Pieris brassicae larvae.
Barcode sequences for specimen: 38754_B03_SAM-HYM-P020703 (sequence code in BOLD: FSA1907-21)
Nucleotide sequence
TTTCTATAAGATTATTAATTCGTTTAGAATTAG-GAATACCTGGAAGAT TAATTGGTAATGATCAGATT-TATAATAGAATTGTAACTTCTCATGCTTTTATTATA-ATTTTTTTTATAGTTATACCTGTAATAATTGGCG-GTTTTGGAAATTGATTAATTCCTTTGATGTTAG- GATCTCCAGATATATCTTTTCCTCGAATAAATAATATA- AGTTTTTGATTATTGATCCCTTCTTTAATATTATTAAT-TATAAGAAGATTTATTAATGTAGGAGTTGGAACTG-GTTGAACTGTTTATCCTCCTTTATCATTAATTTTAGGT-CATGGGGGAATATCAGTTGATTTAGGAATTTTTTCTT-TACATTTAGCTGGTGCATCTTCAATTATAGGTGCTGTA-AATTTTATTACTACTATTATAAATATACGTTCAAATT-TATTTAATATAGATAAAATATCTTTATTTTCTTGATCT-GTATTTATTACTGCAATTTTATTATTATTATCTTTACCT-GTTTTAGCAGGTGCTATTACTATATTATTAACTGATC-GAAATATAAATACAAGATTTTTTGATCCATCAGGTG-GAGGGGATCCAATTCTTTATCAACATTTATTT
Barcode sequences for specimen: 38754_B11_SAM-HYM-P095102 (sequence code in BOLD: FSA1915-21)
Nucleotide sequence
GGATATTAGGATTTTCTATAAGATTATTAATTCGTT- TAGAATTAGGAATACCTGGAAGATTAATTGGTAAT- GATCAGATTTATAATAGAATTGTAACTTCTCATGCTTT- TATTATAATTTTTTTTATAGTTATACCTGTAATAATTG-GCGGTTTTGGAAATTGATTAATTCCTTTGATGTTAG-GATCTCCAGATATATCTTTTCCTCGAATAAATAATATA-AGTTTTTGATTATTGATCCCTTCTTTAATATTATTAAT-TATAAGAAGATTTATTAATGTAGGAGTTGGAACTG-GTTGAACTGTTTATCCTCCTTTATCATTAATTTTAGGT-CATGGGGGAATATCAGTTGATTTAGGAATTTTTTCTT-TACATTTAGCTGGTGCATCTTCAATTATAGGTGCTGTA-AATTTTATTACTACTATTATAAATATACGTTCAAATT-TATTTAATATAGATAAAATATCTTTATTTTCTTGATCT-GTATTTATTACTGCAATTTTATTATTATTATCTTTACCT-GTTTTAGCAGGTGCTATTACTATATTATTAACTGATC-GAAATATAAATACAAGATTTTTTGATCCATCAGGTG-GAGGGGATCCAATTCTTTATCAACATTTATTT
Pteromalidae, Pteromalinae
Pteromaluspuparum (Linnaeus, 1758) (Figures 6-8)
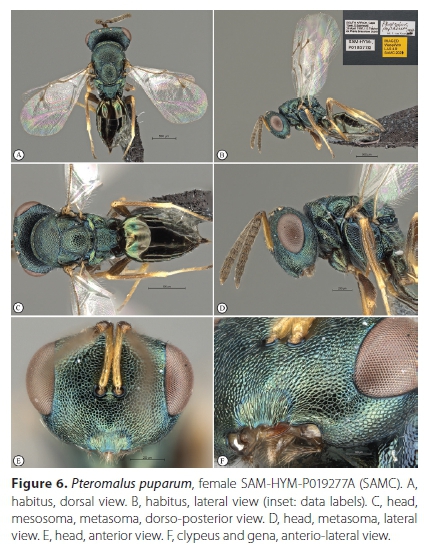
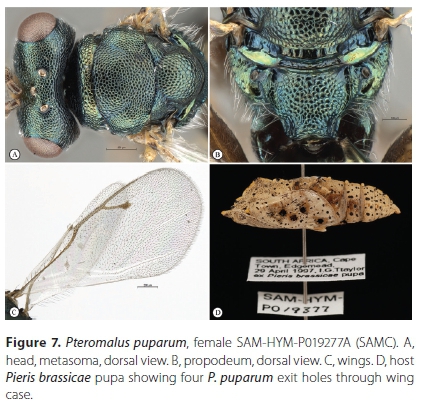
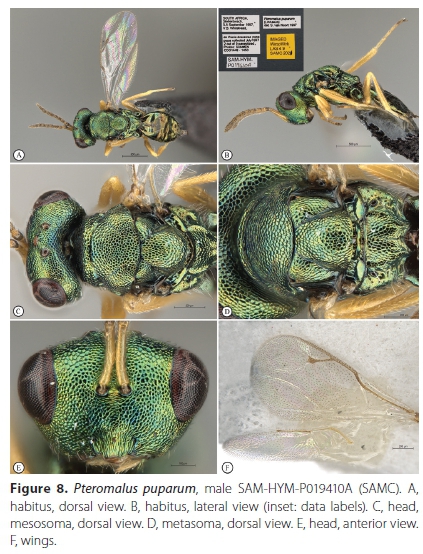
Material examined. South Africa, Western Cape Province. 9 FM: Cape Town, Pinelands, 19 October 1995, J.B. Ball, ex Pieris brassicae pupa, Pteromalus puparum det. S. van Noort, 1998, SAM-HYM-P011872 (SAMC); 10 FM: Cape Town, Edgemead, 29 April 1997, I.G.T. Taylor, ex Pieris brassicae pupa, Pteromalus puparum det. S. van Noort, 1998, SAM-HYM-P019377 (SAMC); 4 FM: Stellenbosch, pupa collected July 1997, emerged 22 August 1997, V.B. Whitehead & V. Booth, ex Pieris brassicae pupa, Pteromalus puparum det. S. van Noort, 1998, (photos: SAM EN#C001448-1450), SAM-HYM-P019409 (SAMC); 4 FM: Stellenbosch, pupa collected July 1997, emerged 23 September 1997, V.B. Whitehead & V. Booth, ex Pieris brassicae pupa, Pteromalus puparum det. S. van Noort, 1998, (photos: SAM EN#C001448-1450), SAM-HYM-P021093 (SAMC); 10 FM: Stellenbosch, pupa collected July 1997, emerged 3-6 September 1997, V.B. Whitehead & V. Booth, ex Pieris brassicae pupa (2 pupae out of 9 parasitized), Pteromalus puparum det. S. van Noort, 1998, (photos: Photo SAM EN#C001448 - 1450), SAM-HYM-P019410 (SAMC); 11 FM: Cape Town, Sea Point, 33.917°S 18.383Έ, 8 m, 1 November 1998, A.J.M. Claassens, ex Pieris brassicae pupa, Pteromalus puparum det. S. van Noort, 1998, SAM-HYM-P021092 (SAMC); 20F 1M: Kleinmond, M. Brink, MAGRIETB-160929-3, ex pupa on curtain collected 29 September 2016. Wasps emerged 7 October 2016. SAM-HYM-P088315 (SAMC).
Diagnosis. The diagnosis of Pteromalus puparum is here cited from Baur (2015): female femora infuscate; reticulation between clypeus and malar sulcus without enlarged meshes; POL slightly greater than OOL; tentorial pit indistinct; antenna high on face, lower edge of torulus at about the middle between anterior margin of clypeus and anterior edge of anterior ocellus; mesoscutum with areoles small and only moderately enlarged in posterior part of sclerite; scutellum in lateral view moderately convex; metatibia gradually widening towards apex; female gaster obtusely pointed, rarely more than 1.6 times as long as broad (for abbreviations and terminology, see Baur 2015).
Graham's (1969) key to Pteromalus as well as the key published in Klimmek and Baur (2018) allow recognition of the species.
Distribution. The species is currently considered to be cosmopolitan (Noyes 2019). However, there are many (older) records that need reassessment and confirmation of their validity.
Biology. Gregarious pupal endoparasitoid of Pieris brassicae. The host caterpillar, in its final instar (sometimes even before it has finished feeding), or as a prepupa, is usually mounted by the female parasitoid, which often goes unnoticed. Oviposition only takes place when the soft, fresh pupal cuticle is exposed as the prepupa moults (Shaw 2002). Also commonly recorded as a parasitoid of other butterflies, in particular many species of Nymphalidae, Papilionidae and Pieridae (Graham 1969). Further supposed hosts cited in Noyes (2019), especially those involving Coleoptera, Hemiptera or Diptera, must be considered with suspicion. Pteromalus puparum has always been one of the best known species of the genus (e.g. Graham 1969). For this reason, many rearings were often attributed (often in error) to this species.
Barcode sequences. Failed.
DISCUSSION
Unfortunately the barcode extraction for P. puparum failed, but the identity of this species was confirmed by one of the authors (viz. Hannes Baur). Two of the three C. glomerata specimens submitted to BOLD produced full DNA barcodes. The specimen that failed was a dry specimen mounted in 1998 shortly after collection, but a specimen from the same collecting event that had been stored in 96% ethanol was successfully sequenced, supporting the value of different modes of specimen preservation. The haplotype variation present within the global population of C. glomerata (based on the available sequences in BOLD from specimens worldwide, depicted in Fig. 5), might indicate a variable species, or perhaps a complex of related species, but solving that will require additional studies of the world fauna and more specimens to be sequenced, which is beyond the scope of the present work. Our results confidently place the South African specimens within the present concept of C. glomerata.
Although the hot and dry Western Cape summer was hypothesised to curtail population expansion of the large cabbage white, P. brassicae, evidence that the species can enter diapause during unfavourable summer conditions in southern Europe (Held & Spieth 1999) and Chile (Benyamini 1996) has probably enabled adaptation of the South African alien population to persist in the Western Cape. The impact of this alien butterfly on local ecosystems and agriculture in South Africa is little understood, and is begging for assessment, as is the efficiency of parasitoids present in South Africa in controlling this pest butterfly species. The mode and date of introduction of the two exotic parasitoid species, C. glomerata and P. puparum is unknown, but given the latter's global distribution and multiple host species association, its presence in South Africa possibly preceded the introduction of P. brassicae circa 1994. Given that Cotesia glomerata is host-specific on only a few genera of Pieridae (Aporia, Pieris, Pontia, and more rarely Anthocharis and Euchloe) in its (presumed) Palaearctic area of origin, it is likely to have colonised South Africa subsequent to the introduction of P. brassicae, although both parasitoids could feasibly have come into South Africa together with P. brassicae, being present and developing at the time of introduction inside immature life stages of the host butterfly. This mode of parasitoid introduction has occurred elsewhere, such as in the case of the 1991 accidental introduction of the Glanville fritillary butterfly (Melitaea cinxia Linnaeus, Nymphalidae), which included the parasitoid wasp, Hyposoter hortícola (Gravenhorst) (Ichneumonidae: Campopleginae) and its associated hyperparasitoid Mesochorus cf. stigmaticus (Ichneumonidae: Mesochorinae), from the main Aland Islands in Finland to an isolated island in the archipelago (Duplouy et al. 2021). To date no indigenous African wasp parasitoids have been reared from P. brassicae, and given the length of presence of the butterfly in South Africa it appears that they may not be able to adapt to this alien species, or that the conditions for host recruitment have not yet arisen. It is perhaps surprising that no indigenous ichneumonid pimpline pupal parasitoids of butterflies, which often have wide host ranges (Shaw 1994), have been reared from P. brassicae. Conversely, it is not a surprise that local tachinid fly parasitoids with wide host ranges have recruited this exotic butterfly. Given the current globalisation phenomenon it is possible that further exotic parasitoids of P. brassicae may also establish in South Africa, although the only real contender that is a regular parasitoid of P. brassicae larvae is Hyposoter ebeninus (Shaw et al. 2016). There may actually be a need for introduction of this species as a further potential biocontrol agent of the large cabbage white, but this is contingent on future studies assessing its potential host repertoire as well as the parasitism rate of the two currently present species.
The results presented in this paper, confirming the identity of the two prevalent parasitoids attacking the large cabbage white in South Africa, provide a first step towards enabling further investigation of the impact, biology and potential expansion of this alien invasive butterfly species in the region. Further research, especially regarding parasitism of the egg and pupal stages, is expected to reveal more parasitoid species in South Africa.
ACKNOWLEDGEMENTS
Simon van Noort was funded by South African NRF (National Research Foundation) grants: GUN 2068865, GUN 61497, GUN 79004, GUN 79211, GUN 81139, and GUN 98115. Cape Nature, the Eastern Cape Department of Environmental Affairs and the Northern Cape Department of Nature and Environmental Conservation provided collecting permits for South Africa.
ORCID IDs
Simon van Noort - https://orcid.org/0000-0001-6930-9741
lose Fernandez-Triana - https://orcid.org/0000-0003-0425-0309
Hannes Baur - https://orcid.org/0000-0003-1360-3487
Mark R Shaw - https://orcid.org/0000-0002-6651-8801
REFERENCES
Askew RR, Shaw MR. 1997. Pteromalus apum (Retzius) and other pteromalid (Hym.) primary parasitoids of butterfly pupae in western Europe, with a key. Entomologist's Monthly Magazine 133: 67-72. [ Links ]
Baur H. 2015. Pushing the limits - two new species of Pteromalus (Hymenoptera, Chalcidoidea, Pteromalidae) from Central Europe with remarkable morphology. ZooKeys 514: 43-72. https://doi.org/10.3897/zookeys.514.9910 [ Links ]
Benyamini D. 1996. Pupal summer diapause in Chilean Pieris brassicae (Linnaeus, 1758) (Lepidoptera, Pieridae). Nota lepidopterologica 18 (3): 184-192. [ Links ]
Brown K, Phillips CB, Broome K, Green C, Toft R, Walker G. 2019. Feasibility of eradicating the large white butterfly (Pieris brassicae) from New Zealand: data gathering to inform decisions about the feasibility of eradication. In: C.R. Veitch, M.N. Clout, A.R. Martin, J.C. Russell and C.J. West (eds.) Island invasives: scaling up to meet the challenge. 364-369. International Union for the Conservation of Nature, Gland, Switzerland.
Cameron PJ, Hill RL, Bain J, Thomas WP. 1989. A review of biological control of invertebrate pests and weeds in New Zealand 1874 to 1987. Technical Communication 10. CAB International, Wallingford, Oxon. 424 p.
Claassens AJM. 1995. Observations on the large white, Pieris brassicae (L.) (Lepidoptera, Pieridae), a butterfly which recently established itself in the Western Cape. Metamorphosis 6(2): 86-93. [ Links ]
Claassens AJM. 1996. Further observations on Pieris brassicae (L) (Lepidoptera: Pieridae) in the Western Cape Province. Metamorphosis 7(2): 88-90. [ Links ]
Claassens AJM. 1998. A new parasitoid and host-plant of the larvae of Pieris brassicae (L) (Lepidoptera: Pieridae) in the Western Cape, South Africa. Metamorphosis 9(4): 184-185. [ Links ]
Claassens AJM. 2014. The Cabbage White: exploring our biodiversity. Veld & Flora 100: 36. [ Links ]
Duplouy A, Nair A, Nyman T, Van Nouhuys S. 2021. Long-term spatiotemporal genetic structure of an accidental parasitoid introduction, and local changes in prevalence of its associated Wolbachia symbiont. Molecular Ecology, 30: 4368-4380. https://doi.org/10.1111/mec.16065 [ Links ]
Fernandez-Triana J, Shaw MR, Boudreault C, Beaudin M, Broad GR.2020. Annotated and illustrated world checklist of Microgastrinae parasitoid wasps (Hymenoptera, Braconidae). ZooKeys 920: 1-1089.https://doi.org/10.3897/zookeys.920.39128 [ Links ]
Gardiner BOC. 1974. Pieris brassicae L. established in Chile; another palaearctic pest crosses the Atlantic (Pieridae). Journal of the Lepidopterist's Society 28: 269-277. [ Links ]
Gardiner BOC. 1995. The large cabbage white, Pieris brassicae, extends its range to South Africa. Entomologist's Record and Journal of Variation 107: 174. [ Links ]
Geerts S, Rossenrode T, Irlich U, Visser V. 2017 Emerging ornamental plant invaders in urban areas-Centranthus ruber in Cape Town, South Africa as a case study. Invasive Plant Science and Management 10: 322-331. https://doi.org/10.1017/inp.2017.35 [ Links ]
Geertsema H. 1996. The large cabbage white, Pieris brassicae, an exotic butterfly of potential threat to cabbage growers in the Western Cape, South Africa. Journal of the Southern African Society for Horticultural Sciences 6 (1): 31-34. [ Links ]
Graham MWR. De V. 1969. The Pteromalidae of North-Western Europe (Hymenoptera: Chalcidoidea). Bulletin of the British Museum (Natural History), Entomology, Supplement 16: 1-909. [ Links ]
Gravenhorst JLC. 1829. Ichneumonologia Europaea. Pars II. Vratislaviae. 989 pp.
Greathead DJ, Greathead AH. 1992. Biological control of insect pests by insect parasitoids and predators: the BIOCAT database. Biocontrol News and Information 13(4): 61-68. [ Links ]
Haliday AH.1834. Essay on parasitic Hymenoptera. Entomological Magazine 2(iii): 225-259. [ Links ]
Harvey JA, Jervis MA, Gols R, Jiang N, Vet LEM. 1999. Development of the parasitoid, Cotesia rubecula (Hymenoptera: Braconidae) in Pieris rapae and Pieris brassicae (Lepidoptera: Pieridae): evidence for host regulation. Journal of Insect Physiology 45(2): 173-182. https://doi.org/10.1016/s0022-1910(98)00113-9 [ Links ]
Held C, Spieth HR. 1999. First evidence of pupal summer diapause in Pieris brassicae L.: the evolution of local adaptedness. Journal of Insect Physiology 45(6): 587-598. [ Links ]
iNaturalist 2021. https://www.inaturalist.org/observations?taxon_id=55401 (accessed 12 July 2021).
Ivanova NV, Dewaard JR, Hebert PD. 2006. An inexpensive, automation-friendly protocol for recovering high-quality DNA. Molecular Ecology Notes 6(4): 998-1002. [ Links ]
Janion-Scheepers C, Griffiths CL. 2020. Alien terrestrial invertebrates in South Africa. In: van Wilgen B, Measey J, Richardson DM, Wilson JR, Zengeya TA, editors. Biological invasions in South Africa. 185205. Springer, Cham. https://doi.org/10.1007/978-3-030-32394-3
Karnavar G. 1983. Studies on the population control of Pieris brassicae L. by Apanteles glomeratus L. Insect Science and Its Application 4(4): 397-399. https://doi.org/10.1017/S1742758400002460 [ Links ]
Klimmek F, Baur H. 2018 An interactive key to Central European species of the Pteromalus albipennis species group and other species of the genus (Hymenoptera: Chalcidoidea: Pteromalidae), with the description of a new species. Biodiversity Data Journal 6: e27722. https://doi.org/10.3897/BDJ.6.e27722 [ Links ]
Le Roux JJ, Clusella-Trullas S, Mokotjomela TM, Mairal M, Richardson DM, Skein L, Wilson JR, Weyl OL, Geerts S. 2020. Biotic interactions as mediators of biological invasions: insights from South Africa. In: van Wilgen B, Measey J, Richardson DM, Wilson JR, Zengeya TA, editors. Biological Invasions in South Africa. 387-427. Springer, Cham. https://doi.org/10.1007/978-3-030-32394-3
Linnaeus, C. Von. 1758. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species cum characteribus, differentiis, synonymis locis. Tomus I. Editio decima, reformata. Laurnetii Salvii, Holmiae. 824 pp.
Marshall TA. 1885. Monograph of British Braconidae. Part I. Transactions of the Entomological Society of London 1885: 1-280.
Miller, J. 1759. Engravings of insects, with descriptions. London. 10 plates.
Neira CM, Ruff FJ, Mundaca BN. 1989. Natural enemies of Pieris brassicae L. (Lepidoptera: Pieridae) from cultivated crucifers in Valdivia. Agro-Ciencia 5(2): 5-10. [ Links ]
Noyes JS. 2019. Universal Chalcidoidea Database. World Wide Web electronic publication. http://www.nhm.ac.uk/chalcidoids.
Peters G. 1991. Occurrence of indigenous parasitoids of the important cabbage pests in the Cologne-Bonn production region. Zeitschrift für Angewandte Zoologie 78(1): 91-99. [ Links ]
Phillips CB, Brown K, Green C, Walker G, Broome K, Toft R, Vander Lee B, Shepherd M, Bayly S, Rees J. 2014. Pieris brassicae (great white butterfly) eradication annual report 2013/14. Unpublished report, Department of Conservation, Wellington.
Picker M, Griffiths C. 2011. Alien and invasive animals: A South African perspective. Struik Nature, Cape Town.
Picker M, Griffiths C. 2017. Alien animals in South Africa - composition, introduction history, origins and distribution patterns. Bothalia 47:a2147. https://doi.org/10.4102/abc.v47i2.2147 [ Links ]
Prinsloo GL, Uys VM. 2015. Insects of cultivated plants and natural pastures in Southern Africa. Entomological Society of Southern Africa, Hatfield.
Ratnasingham S, Hebert PDN. 2007. BOLD: The Barcode of Life Data System (http://www.barcodinglife.org). Molecular ecology notes 7(3): 355-364. [ Links ]
Ratnasingham S, Hebert PDN. 2013. A DNA-based registry for all animal species: The Barcode Index Number (BIN) System. PLoS ONE 8(7): e66213. https://doi.org/10.1371/journal.pone.0066213 [ Links ]
Razmi M, Karimpour Y, Safaralizadeh MH, Safavi SA. 2011. Parasitoid complex of cabbage large white butterfly Pieris brassicae (L.) (Lepidoptera, Pieridae) in Urmia with new records from Iran. Journal of Plant Protection Research 51 (3): 248-251. [ Links ]
Robinson, T., Ivey, P., Powrie, L., Winter, P., Wong, L.J. & Pagad, S. 2020. Global register of introduced and invasive species - South Africa. Version 2.5. Invasive Species Specialist Group ISSG. Checklist dataset https://doi.org/10.15468/l6smob accessed via GBIF.org on 2020-09-13.
Shahram M, Debjani D. 2013. Taxonomic notes on common natural enemies of Pieris brassicae Linnaeus (Lepidoptera: Pieridae) belonging to hymenoptera. Annals of Entomology 31(1): 35-45. [ Links ]
Shaw MR. 1982. Parasitic Control, Section A: General information: Biology of some effective parasites. In: Feltwell, J. (ed) Large white butterfly; the biology, biochemistry and physiology of Pieris brassicae (Linnaeus) (Series Entomologica 18). 401-407. W. Junk, The Hague.
Shaw MR. 1994. Parasitoid host ranges. In: Hawkins, B.A. & Sheehan, W. (eds) Parasitoid community ecology. 111-144. Oxford University Press, Oxford.
Shaw MR. 2002. Experimental confirmation that Pteromalus apum (Retzius) (Hym., Pteromalidae) parasitizes both leaf-cutter bees (Hym., Megachilidae) and Fritillary butterflies (Lep., Nymphalidae). Entomologist's Monthly Magazine 138: 37-41. [ Links ]
Shaw MR, Fitton MG. 1989. Survey of parasitoids of British butterflies. Entomologist's Record and Journal of Variation 101: 69-71. [ Links ]
Shaw MR, Huddleston T. 1991. Classification and biology of Braconid wasps (Hymenoptera: Braconidae). Handbooks for the Identification of British Insects 7(11): 1-126. [ Links ]
Shaw MR, Stefanescu C, Van Nouhuys S. 2009. Parasitoids of European butterflies. In: Settele J.; Shreeve, T.; Konvicka, M.; van Dyck H. (eds.) Ecology of Butterflies in Europe. 130-156. Cambridge University Press.
Shaw MR, Horstmann K, Whiffin AL. 2016. Two hundred and twenty-five species of reared western Palaearctic Campopleginae (Hymenoptera: Ichneumonidae) in the National Museums of Scotland, with descript ions of new species of Campoplex and Diadegma, and records of fifty-five species new to Britain. Entomologist's Gazette 67(3): 177-222. [ Links ]
Steele B. 1998. More observations and thoughts on Pieris brassicae and its invasion of South Africa. Metamorphosis 9(3): 128-130. [ Links ]
Van Noort S. 1996. Cabbage White invades southwestern Cape. Africa - Environment & Wildlife, 4(1): 13. [ Links ]
Yu DS, Van Achterberg C, Horstmann K. 2016. Taxapad 2016. Ichneumonoidea 2015 (Biological and taxonomical information), Taxapad Interactive Catalogue Database on flash-drive. Nepean, Ottawa, Canada.
 Correspondence:
Correspondence:
Simon van Noort
Email svannoort@iziko.org.za
Received:13 July 2021
Accepted: 27 September 2021














