Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904
Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.44 n.1 Stellenbosch 2023
http://dx.doi.org/10.21548/44-1-makhath
ARTICLES
Susceptibility of Grapevine Sucker and Green Shoot Wounds to Trunk Disease Pathogens
G.J. MakatiniI; F. HalleenI, II; C. MutawilaI; P. MoyoI; L. MostertI, *
IDepartment of Plant Pathology, Stellenbosch University, Private Bag X1, Matieland, 7602, South Africa
IIPlant Protection Division, ARC Infruitec-Nietvoorbij, Private Bag X5026, Stellenbosch, 7599, South Africa
ABSTRACT
Grapevine trunk disease fungi infect vines through openings, primarily pruning wounds. The main objective of this study was to understand the role of sucker wounds and wounds made by the removal of green shoots from the stems of potted grapevines as potential points of infection for grapevine trunk disease pathogens. Six wine and four table grape vineyards of different ages were sampled in different production areas in the Western Cape grape region of South Africa. Isolations were made from 161 sucker wounds, and fungal pathogens were identified using morphology and DNA sequence analysis of the internal transcribed spacers (ITS1 and ITS2) and the 5.8S ribosomal RNA gene, the translation elongation factor 1alpha or the partial β-tubulin gene. The results show that 62% of the sucker wounds were infected by trunk disease pathogens, including Diaporthe ampelina, Diplodia seriata, Phaeomoniella chlamydospora, Phaeoacremonium minimum, Eutypella microtheca, Cryptovalsa ampelina and Neofusicoccum australe. Diaporthe ampelina was the most common, followed by D. seriata and P. chlamydospora, in both the wine and table grape sucker wounds. Under glasshouse conditions, wounds made by the removal of young green shoots on one-year-old potted grapevine plants were inoculated with spore suspensions of D. ampelina, E. lata, N. parvum, P. minimum and P. chlamydospora. After four months, all the inoculated pathogens could be re-isolated. This study shows that grapevine sucker and green shoot wounds are susceptible to different grapevine trunk disease pathogens and may therefore play a role in the epidemiology of trunk diseases.
Key words: Grapevine trunk diseases, pruning, sucker wounds, Vitis vinifera
INTRODUCTION
Grapevine trunk diseases are a major threat to wine and table grape (Vitis vinifera and Vitis spp.) production worldwide and their occurrence has increased significantly over the last two decades (Bertsch et al., 2013). Trunk diseases are caused by a complex group of fungi that infect and inhabit the xylem vessels of grapevines and thereafter may act individually, in synergy or in succession to produce different symptoms (Surico, 2009). The different trunk diseases include Botryosphaeria dieback (caused by species of the Botryosphaeriaceae family) (Van Niekerk et al., 2004; Úrbez-Torres et al., 2006), Eutypa dieback (caused by species of the Diatrypaceae family) (Petzoldt et al., 1981; Pitt et al., 2010; Trouillas & Gubler, 2010), Petri disease (caused by P. chlamydospora (Crous & Gams, 2000) and Phaeoacremonium spp. (Gramaje et al., 2015)), esca (caused by wood-rotting species of the Hymenochaetales, together with Petri disease fungi) (Surico, 2009, White et al., 2011; Cloete et al., 2015) and Phomopsis dieback (caused by various species of the genus Diaporthe) (Van Niekerk et al., 2005, Úrbez-Torres et al., 2013; Lesuthu et al., 2019).
Grapevine trunk diseases may affect any growth stage of the vine, although Petri disease is common in young vines (Mostert et al., 2006b). Trunk disease symptoms may include the death of spurs and cordons, internal streaking and discolouration, as well as necrosis of the wood (White et al., 2011). Vascular cankers and loss of vine vigour, which can slowly lead to the death of grapevines, are some characteristics of severely infected vines (Úrbez-Torres et al., 2006). Grapevine trunk diseases are frequently responsible for reduced lifespan and premature re-establishment of vineyards, which consequently have economic implications for growers (Wicks & Davies, 1999; Siebert, 2001; Van Niekerk et al., 2003; Bertsch et al., 2013; Gramaje et al., 2016; Kaplan et al., 2016). Most trunk disease infections occur through the exposed xylem tissue at pruning wounds, or other grapevine openings such as mechanical wounds (Halleen et al., 2010; Gramaje & Armengol, 2011; Mutawila et al., 2011).
Winter pruning wounds are regarded as the primary portals of entry for grapevine trunk disease pathogens (Rolshausen et al., 2010; Van Niekerk et al., 2011). This is due to multiple susceptible wounds being present during the dormancy period, as well as the inoculum release and dispersal events of some trunk disease pathogens, which can lead to wound infection when weather conditions are conducive (Elena & Luque, 2016). Some studies have demonstrated that spore release of some trunk disease pathogens, including Botryosphaeriaceae spp., E. lata, P. chlamydospora, P. minimum and P. inflatipes, coincided with rain events, which are predominantly associated with the winter pruning season (Elena & Luque, 2016). However, other studies have found that aerial spores of trunk disease pathogens do occur in vineyards outside the winter pruning period, particularly in summer and spring (Uddin et al. 1997; Larignon & Dubos, 2000; Eskalen & Gubler, 2001; Eskalen et al., 2004; Kuntzmann et al., 2009; Van Niekerk et al.,, 2010; Halleen et al., 2020). Spore release of D. ampelina (formerly known as Phomopsis viticola) has been associated with high rainfall during late winter and early spring (Van Niekerk et al., 2010). Uddin et al. (1997) found the highest inoculum of D. ampelina to be released during spring.
In South Africa, spores of E. lata, D. ampelina and species of the Botryosphaeriaceae were trapped during spring (Van Niekerk et al., 2010). Phaeomoniella chlamydospora, P. inflatipes and P. minimum spores were trapped throughout the year in California, with the latter being trapped even in the absence of rain (Eskalen & Gubler, 2001; Eskalen et al., 2004). In French vineyards, P. chlamydospora was trapped throughout the year, whilst Pm. minimum only occurred during the vegetative period (Larignon & Dubos, 2000). Kuntzmann et al. (2009) found that spores of D. seriata and D. mutila were trapped throughout the year in French vineyards. In California, spores of the Botryosphaeriaceae were trapped from fall to spring, with the highest occurrence in winter and rarely in summer (Úrbez-Torrez et al., 2010).
In Australian vineyards, spores of Eutypa lata and Botryosphaeriaceae were detected throughout the whole year, primarily in response to rainfall (Billones-Baaijens et al., 2017). In Chile, spores of Botryosphaeriaceae were detected mostly in the fall and winter, less so in spring and not at all in summer (Valencia et al., 2015). During spring, wounds occur on grapevines from the removal of unwanted green shoots (suckers) from older wood, including the trunk, cordon and spurs of vines, as well as the removal green shoots from one-year-old canes. The availability of aerial inoculum during spring indicates that it is possible for grapevines to be infected through sucker wounds and green shoot wounds.
The infection of sucker wounds has been studied in Bordeaux, France (Lecomte et al., 2001, 2004, 2005; Lecomte & Bailey, 2011) and California, USA (Epstein et al., 2008). Lecomte and Bailey (2011) conducted surveys to assess the natural infection of E. lata on sucker wounds versus winter pruning wounds and found an overall natural infection percentage of 2.1% for sucker wounds in comparison to 13% for winter pruning wounds. Furthermore, artificial inoculation of sucker wounds made by the removal of either buds or suckers from the trunk or cordon of vines with ascospores of E. lata confirmed the susceptibility of sucker wounds to this pathogen. These results led to the conclusion that, although sucker wounds are not the primary portals of pathogen entry, they may pose a significant threat to the infection of grapevines by E. lata.
A study conducted by Epstein et al. (2008) in California further confirmed the natural infection of sucker wounds by trunk disease pathogens. In this study, wounds made by removing suckers from the trunks of 14 vines were left exposed to natural infection and the sucker wounds were analysed one year later for infection by D. seriata. The results revealed that 64% of the vines showed natural sucker wound infections by D. seriata. Sosnowski and Ayres (2022) demonstrated that spring shoot-thinning wounds, made by tearing off green shoots, can become infected when inoculated with E. lata and D. seriata spores. Although the susceptibility of grapevine sucker wounds to E. lata and D. seriata has been studied, such studies have not been conducted in South Africa, and the susceptibility of grapevine sucker wounds to a broader selection of trunk pathogens is also unknown and thus needs to be determined.
The natural susceptibility of sucker wounds to grapevine trunk disease pathogens reported in France and California, USA raises the question of the potential role of sucker wounds in trunk disease epidemiology. The objectives of this study were therefore to i) assess naturally infected sucker wounds from both wine and table grape vineyards in the Western Cape province of South Africa and ii) assess green shoot wound susceptibility towards different trunk disease pathogens.
MATERIALS AND METHODS
Sucker wound survey
Sampling and isolations
Sucker wounds were sampled from two wine grape cultivars, viz. Chenin blanc and Cabernet Sauvignon, and two table grape cultivars, Thompson Seedless and Crimson Seedless. The ages of the vineyards ranged from 10 to 15 years, with no obvious external symptoms visible. The wine grape vineyards were situated in Darling, Robertson and Stellenbosch, and the table grape vineyards were in Paarl and Piketberg, all in the Western Cape province of South Africa. Sampling was carried out from April to June in 2011 (Darling, Robertson and Paarl) and in 2012 (Stellenbosch and Piketberg), with each vineyard sampled once only. Ten vineyards were sampled and included, three each of Chenin blanc and Cabernet Sauvignon and two each of Thompson seedless and Crimson seedless. Fifteen cordons (table grapes) or spur bases (wine grapes) of one- to three-year-old wood with sucker wounds were collected from each vineyard and taken to the laboratory for fungal isolations.
Vines from which sucker wounds were collected were selected randomly throughout the vineyard. Sucker wounds were cut (leaving approximately 2 cm of cane above and below the wound) from at least 10 of the cordons (table grapes) or spur bases (wine grapes) in each vineyard. Wounds that showed wood discolouration, which is typical of trunk disease infections, as well as symptomless wounds, were analysed further. In total, fungal isolations were made from 161 sucker wounds. Wood pieces were surface-disinfected in 70% ethanol for 30 seconds, then for one minute in 3.5% sodium hypochlorite solution and again in 70% ethanol for 30 seconds. Sucker wounds were aseptically dissected longitudinally across the wound. Fungal isolations were carried out aseptically from symptomatic (browning or streaking) wood that originated from sucker wounds, with wood fragments taken from the wound scar interface. In addition, if symptoms were not present, isolations were made from tissue from the interface that seemed healthy.
From brown to dark brown vascular discoloration, 12 wood fragments (0.5 mm × 1.0 mm) were obtained from each sucker wound and plated onto 90 mm Petri dishes (four pieces per plate) containing potato dextrose agar (PDA, Biolab, Wadeville, South Africa) amended with chloromycetin (250 mg/L). The plates were incubated at ±23°C under cool white light and near-UV lights and monitored daily for four weeks. Fungal colonies resembling taxa associated with grapevine trunk diseases were hyphal-tipped and grown on PDA. Pure cultures were stored in double-sterilised distilled water (dH2O) in 14 ml McCartney bottles and kept at 4°C. Representative isolates were stored in the culture collection of the Department of Plant Pathology, Stellenbosch University, South Africa.
Fungal identification
Fungi were identified according to cultural and morphological characteristics as species of Botryosphaeriaceae (Van Niekerk et al. 2004; Úrbez-Torres et al., 2006), Diatrypaceae (Rolshausen et al., 2014; Moyo et al., 2018), Phaeoacremonium (Crous et al., 1996; Mostert et al., 2006a), Diaporthales (Mostert et al., 2001; Lesuthu et al., 2019) and Phaeomoniella chlamydospora (Crous & Gams, 2000). Genomic DNA was extracted from two-week-old fungal mycelia obtained from PDA plates. A CTAB-based DNA extraction method was used, as described by Damm et al. (2008). For the species of the Botryosphaeriaceae, Diatrypaceae and D. ampelina, the internal transcribed spacers (ITS1 and ITS2) and the 5.8S ribosomal RNA gene were amplified with the primers ITS1 and ITS4 (White et al., 1990). The PCR conditions were the same as reported by Van Niekerk et al. (2004). To confirm the species identity, the partial β-tubulin gene (TUB) was amplified for representative isolates of P. minimum and E. microtheca. Diplodia seriata was confirmed with the translation elongation factor 1 alpha. The primers EF1-728F and EF1-968R (Carbone & Kohn, 1999) were used to amplify part of the translation elongation factor 1-alpha (EF1-a) for a representative isolate of D. seriata.
The PCR was performed using the following conditions: an initial denaturing step at 94°C for 5 min, followed by 35 cycles of 45 s at 94°C, 45 s at 55°C and 1 min at 72°C, and a final step for 7 min at 72°C. The TUB was amplified for the Phaeacremonium species isolates using primers T1 (O'Donnell & Cigelnik, 1997) and Bt2b (Glass & Donaldson, 1995). The PCR conditions for the TUB gene were the same as those described by Mostert et al. (2006a). PCR products were purified using a commercial kit (MSB® Spin PCRapace 250, Invitek, Berlin, Germany) according to the manufacturer's instructions. DNA sequence analysis was carried out using the Big Dye system (version 3.1 dye terminators, Applied Biosystems, California, USA) on an ABI 3130XL Genetic Analyzer. Sequences obtained for both directions were evaluated using Geneious 3.5.6 (Biomatters Ltd, New Zealand) and edited manually using Sequence Alignment Editor v. 2.0a11. The identities of the sequences were compared by the Megablast function of the NCBI's GenBank nucleotide database. Sequences of representative isolates were deposited in GenBank (Table 1).
Susceptibility of green shoot wounds to five trunk pathogens in the glasshouse
Green shoot wounds of one-year-old canes of potted vines were used due to the difficulty of finding grapevines free from trunk disease pathogens. Two glasshouse trials were conducted to investigate the susceptibility of green shoot wounds to trunk disease pathogens. In the first trial, wine and table grape cultivars of own-rooted Chardonnay and Crimson Seedless plants were inoculated with ascospores of E. lata and conidia of P. chlamydospora. In the second trial, a broader range of trunk disease pathogens, namely, D. ampelina, E. lata, N. parvum, P. minimum and P. chlamydospora were inoculated on grafted one-year-old Chardonnay vines.
Plant cultivation
For the first glasshouse trial, own-rooted Chardonnay and Crimson Seedless plants were propagated from one-year-old dormant canes obtained from mother blocks. The procedure is described in Makatini (2014). For the second glasshouse trial, certified, grafted one-year-old Chardonnay plants were obtained from a nursery and planted in individual plastic bags. The plants were maintained at 25°C and allowed to bud in the glasshouse prior to inoculations.
Preparation of fungal inoculum for experiments
Perithecia of E. lata were obtained from a grapevine wood piece with visible stroma and identified as this species from the distinct ascus and ascospore morphology (Carter, 1991). Ascospore suspensions of 5 χ 104 ascospores/ml were made according to Kotze et al. (2011). Conidial suspensions of N. parvum (STE-U 4439) and D. ampelina (STE-U 7768) were made from pycnidia, with conidial droplets that formed on water agar plates containing sterilised pine needles after four weeks at 25°C. The pycnidia were crushed in dH2O to release the conidia, and the concentration was adjusted to 5 × 104 conidia/ml. Conidial suspensions of P. minimum (STE-U 6996) and P. chlamydospora (STE-U 6384) were made from two-week-old cultures on PDA. Mycelium blocks measuring 10 mm × 10 mm were placed in sterile dH2O and shaken vigorously to suspend the conidia, and the concentrations were adjusted as described above.
To perform the inoculation experiments, wounds were created by removing green shoots from the trunks of grapevine plantlets. These wounds are herein referred to as 'green shoot wounds'. For the first trial, green shoot wounds were created by tearing off the apical shoot (50 mm to 70 mm long) from each plant to simulate shoot removal in the vineyard. For the second trial, the green shoot wounds were created by removing the second green shoot from the apex. The reason for this was that a preliminary field trial, in which green shoot wounds were created by removing the apical shoots, failed due to dieback that occurred above the green shoot wound. Subsequently, green shoot wounds were created by removing the second green shoots from the apex. Each green shoot wound was inoculated with a 20μl spore suspension droplet (1 000 ascospores or conidia). Control plants received the same volume of sterile dH2O. The trials were laid out in a completely randomised block design, with three and two blocks for trials 1 and 2, respectively. Each block consisted of ten plants per treatment.
Fungal re-isolation and identification After three months for trial 1 and four months for trial 2, green shoot wounds were harvested and taken to the laboratory for fungal re-isolations. Wounds were surface sterilised and aseptically dissected, as described previously. Fungal isolations were performed by obtaining wood fragments from the wound scar interface (top isolation zone) and from 5 mm below or away from the first isolation point (bottom isolation zone). From each isolation position, four wood fragments (0.5 mm × 1.0 mm) were obtained from each half of the wound and plated onto PDA in 90 mm Petri dishes (eight pieces in total, four pieces per plate). The plates were incubated at approximately 25°C and monitored daily for four weeks. Inoculated fungi were processed and identified as described previously. Wound susceptibility or infection was evaluated by calculating the percentage mean pathogen incidence.
Data analysis
The incidences of the fungal re-isolations were calculated by the presence or absence of a positive (infected) wood fragment per wound and expressed as a percentage of the total number of green shoot wounds in each treatment. The data from the different trials was analysed using analysis of variance (ANOVA) and the Student's t-test to determine the least significant differences (LSD) at a 5% level of significance (P < 0.05). All data analyses were performed using SAS version 9.2 (SAS Institute Inc., SAS Campus Drive, Cary, NC, USA).
RESULTS
Sucker wound survey
Trunk disease symptoms that were observed from the surveyed sucker wounds included brown wood discolouration and streaking (Fig. 1). The trunk disease pathogens isolated from these wounds were identified as Cryptovalsa ampelina, Eutypella microtheca, Diaporthe ampelina, Diplodia seriata, Neofusicoccum australe, Phaeoacremonium minimum and Phaeomoniella chlamydospora (Table 1). Phaeomoniella chlamydospora can easily be identified based on its unique cultural and morphological characteristics and was therefore not sequenced. Of the different pathogens isolated, D. ampelina was the most common, followed by D.seriata and P. chlamydospora, in both the wine and table grape sucker wounds (Table 2). In addition, low numbers of P. minimum, E. microtheca, C. ampelina and N. australe were also isolated, although these were only from wine grapes.
Sixty-two percent of the collected wounds were found to be infected by at least one trunk pathogen species. Multiple (two and more) fungal pathogen species were obtained from 19% of the wounds (Figs 1A, 1B and 1D). There was a higher incidence of infected sucker wounds from wine grape (84%) in comparison to table grape (16%) cultivars (Table 3).
Inoculation experiments
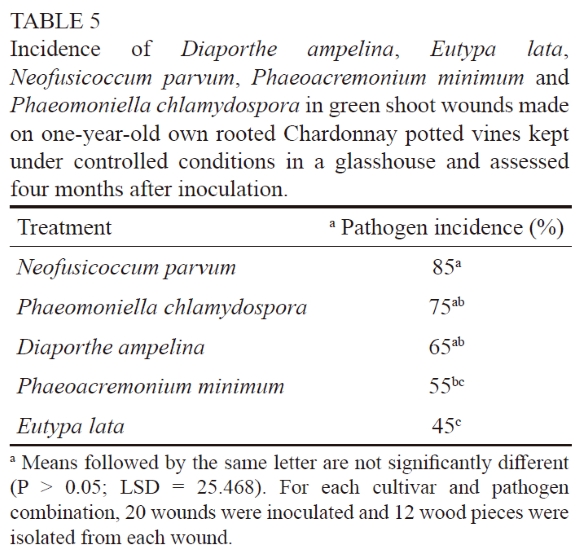
Green shoot wounds on potted Chardonnay and Crimson Seedless plants were susceptible to P. chlamydospora and E.lata. The analysis of variance did not reveal a significant cultivar × treatment interaction (P = 0.78), which indicates that both cultivars responded similarly to the two pathogens. For both Chardonnay and Crimson Seedless, significant differences were found between the pathogen treatments (P = 0.0009 for Chardonnay and P = 0.0001 for Crimson Seedless). The incidence of P. chlamydospora in inoculated green shoot wounds was significantly higher than that of E. lata in Chardonnay and Crimson Seedless (Table 4). No trunk disease pathogens were isolated from the controls. For the second trial, all of the inoculated fungi were re-isolated (Table 5). Significant differences were found between the pathogen treatments (P = 0.0018). Neofusicoccum parvum was isolated from 85% of the wounds, thus its occurrence was significantly higher than that of P. minimum (55%) and E. lata (45%). No trunk disease pathogens were re-isolated from the controls.
DISCUSSION
Sucker wounds of both wine and table grapevines were naturally infected by different trunk disease pathogens, including D. ampelina, D. seriata, P. chlamydospora, P. minimum, E. microtheca, C. ampelina and N. australe. Among these, D. ampelina, D. seriata, P. chlamydospora and P. minimum were the most commonly isolated species.
The higher occurrence of D. ampelina and D. seriata in naturally infected sucker wounds could be ascribed to the ability of these pathogens to infect green material. Since sucker wounds are made by tearing off green plant material, the wound scars expose metabolically active tissue. It is well known that D. ampelina infects green shoots and leaves of grapevines during spring (Hewitt & Pearson, 1988). Diaporthe ampelina can also infect grapevines via active or wounded buds (Hewitt & Pearson 1988; Uddin et al., 1997) and wounded green shoots (Mostert et al., 2001; Van Niekerk et al., 2005). It has been shown that species of the Botryosphaeriaceae can also infect and cause lesions on green shoots (Van Niekerk et al., 2004; Amponsah et al., 2012).
A species that has been encountered and studied less is E. microtheca. The results of a study conducted in Mexico showed that isolates of E. microtheca, obtained from cankers on grapevine cordons, were pathogenic and able to cause necrosis. However, they did not result in major symptoms in dormant canes assessed three months after inoculation (Paolinelli-Alfonso et al., 2015). In another study, done in Australia, isolates of E. microtheca obtained from other woody hosts were pathogenic and produced vascular necrosis on grapevine that was comparable in length to that caused by E. lata (Pitt et al., 2013). In South Africa, E. microtheca isolates obtained from grapevine and non-grapevine hosts were capable of causing brown vascular discolouration when inoculated artificially onto lignified and green grapevine shoots (Moyo et al., 2019). They also caused lesions that were similar in length to those of E. lata.
Higher incidences of pathogens were found on wine grape versus table grape sucker wounds. This might be due to differences in shoot thinning, trellising and pruning styles. More shoot thinning occurs on wine grapevines versus table grapevines, causing more wounds that can become infected. Wine grapes are spur pruned (usually pruned to two buds) versus cane pruning of table grapes (pruned to as many as 15 buds). Sucker wounds on wine grape spurs will be in closer proximity to older wood that could harbour fruiting structures of trunk disease pathogens. Perithecia of Phaeoacremonium have been found on old pruning wounds (Baloyi et al., 2013; Rooney-Latham et al., 2005), pycnidia of P. chlamydospora have been found on the vine cordon (Edwards & Pascoe, 2001a, 2001b; Eskalen et al., 2002; Baloyi et al., 2016) and perithecia of the Diatrypaceae on grapevine cordons and trunks (Pitt et al., 2010; Moyo et al., 2018). In addition, the infection of wine grape sucker wounds will result in more severe and faster wood degradation because of the shorter distance the pathogen has to grow to reach older wood, where wood necrosis has a bigger impact on the health or functionality of the vine.
The susceptibility of green shoot wounds to D. ampelina, E. lata, N. parvum, P. chlamydospora and P. minimum was ascertained under controlled glasshouse conditions. Although E. lata was not recovered during the survey, it was included in the inoculation experiments to compare the susceptibility of green shoots to this important trunk disease pathogen when compared to other trunk pathogens. All the pathogens could be re-isolated, showing their potential to infect green shoot wounds, with E. lata being isolated significantly less than D. ampelina, N. parvum and P. chlamydospora. This study shows that the removal of green shoots from one-year-old canes creates wounds that can become infected with trunk disease pathogens. In grapevine trunk diseases, E. lata is typically restricted to wood and is never obtained from leaves or herbaceous shoots, where symptoms are typically observed (Cardot et al., 2019). In this study, although E. lata was not recovered from naturally infected sucker wounds, it demonstrated, along with the study by Sosnowski and Ayres (2022), that E. lata is capable of infecting green shoot wounds.
Lecomte and Bailey (2011) made 'true' sucker wounds by removing suckers and buds from trunks and cordons to assess natural infection by E. lata. Although such wounds are more difficult to assess, they give a better reflection of the real risk of sucker wound infection that exists under natural conditions. However, it is more likely that such wounds are already infected prior to inoculation, depending on the age of the wounds.
CONCLUSIONS
This study shows that sucker wounds are susceptible to trunk disease pathogens and may therefore play a role in the epidemiology of these diseases. The high incidence of >D. ampelina across the surveyed locations indicates that sucker wounds are highly susceptible to this fungus. Sucker wounds are often on the cordons of the vine and infection could eventually cause death of the spurs and arms. With aerial inoculum of trunk disease pathogens available during spring, together with susceptible sucker and green shoot wounds, the protection of these wounds should be considered in the management of grapevine trunk diseases. To fully assess sucker wound susceptibility, a wider variety of rootstock and scion grapevine varieties should be evaluated for natural infections, as well as with artificial inoculations, against a range of trunk disease pathogens. Furthermore, the duration of susceptibility of these wounds could also be determined.
LITERATURE CITED
Amponsah, N.T., Jones, E.E., Ridgeway, H.J. & Jaspers, M.V., 2012. Susceptibility of grapevine tissues to Neofusicoccum luteum conidial infection. Plant Pathol. 61, 719-729. [ Links ]
Baloyi, M.A., Eskalen, A., Mostert, L. & Halleen, F., 2016. First report of Phaeomoniella chlamydospora pycnidia as Petri disease inoculum sources in South African vineyards. Plant Dis. 100(12), 2528. [ Links ]
Baloyi, M.A., Halleen, F., Mostert, L. & Eskalen, A., 2013. First report of Togninia minima perithecia on esca- and Petri-diseased grapevines in South Africa. Plant Dis. 97(9), 1247. [ Links ]
Bertsch, C., Ramírez-Suero, M., Magnin-Robert, M., Larignon, P., Chong, J., Abou-Mansour, E., Spagnolo, A., Clément, C. & Fontaine, F., 2013. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 62(2), 243-265. [ Links ]
Billones-Baaijens, R., Ayres, M.R., Savocchia, S. & Sosnowski, M.R., 2017. Monitoring inoculum dispersal by grapevine trunk disease pathogens using spore traps. Wine Vit. J. 32(4), 46-50. [ Links ]
Carbone, I. & Kohn, L.M., 1999. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia 91(3), 553-556. [ Links ]
Cardot, C., Mappa, G., La Camera, S., Gaillard, C., Vriet, C., Lecomte, P., Ferrari, G. & Coutos-Thévenot, P., 2019. Comparison of the molecular responses of tolerant, susceptible and highly susceptible grapevine cultivars during interaction with the pathogenic fungus Eutypa lata. Front. Plant Sci. 10, 991. [ Links ]
Carter, M.V., 1991. Eutypa dieback. In: Pearson, R.C. & Goheen, C. (eds). Compendium of grape diseases. The American Phytopathological Society Press, St Paul, MN. pp. 32-34. [ Links ]
Cloete, M., Fischer, M., Mostert, L. & Halleen, F., 2015. Hymenochaetales associated with esca-related wood rots on grapevine with a special emphasis on the status of esca in South African vineyards. Phytopathol. Mediterr. 54(2), 299-312. [ Links ]
Crous, P.W. & Gams, W., 2000. Phaeomoniella chlamydospora gen. et comb. nov., a causal organism of Petri grapevine decline and esca. Phytopathol. Mediterr. 39(1), 112-118. [ Links ]
Crous, P.W., Gams, W., Wingfield, M.J. & Van Wyk, P.S., 1996. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia 88(5), 786-796. [ Links ]
Damm, U., Crous, P.W. & Fourie, P.H., 2008. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia 20(1), 87-102. [ Links ]
Edwards, J. & Pascoe, I.G., 2001a. In situ sporulation of Phaeomoniella chlamydospora in the vineyard. Phytopathol. Mediterr. 40(1), 61-66. [ Links ]
Edwards, J. & Pascoe, I.G., 2001b. Pycnidial state of Phaeomoniella chlamydospora found on Pinot Noir grapevines in the field. Australas. Plant Pathol. 30, 67. [ Links ]
Elena, G. & Luque, J., 2016. Seasonal susceptibility of grapevine pruning wounds and cane colonization in Catalonia, Spain following artificial infection with Diplodia seriata and Phaeomoniella chlamydospora. Plant Dis. 100(8), 1651-1659. [ Links ]
Epstein, L., Kaur, S. & Van der Gheynst, J.S., 2008. Botryosphaeria-related dieback and control investigated in noncoastal California grapevines. Calif. Agr. 62(4), 161-166. [ Links ]
Eskalen, A. & Gubler, W.D., 2001. Association of spores of Phaeomoniella chlamydospora, Phaeoacremonium inflatipes, and Pm. aleophilum. Phytopathol. Mediterr. 40(Suppl.), S429-S432. [ Links ]
Eskalen, A., Latham, S. & Gubler, W., 2004. Spore release of Phaeomoniella chlamydospora associated with grapevine cordons in California. Phytopathology 94(6), S28-S28. [ Links ]
Eskalen, A., Rooney, S.N. & Gubler, W.D., 2002. First report of the pycnidial state of Phaeomoniella chlamydospora, a causal agent of black measles (esca) and Petri disease in California vineyards. Phytopathology 92, S4. [ Links ]
Glass, N.L. & Donaldson, G.C., 1995. Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Appl. Environ. Microb. 61(4), 1323-1330. [ Links ]
Gramaje, D. & Armengol, J., 2011. Fungal trunk pathogens in the grapevine propagation process, potential inoculum sources, detection, identification and management strategies. Plant Dis. 95(9), 1040-1055. [ Links ]
Gramaje, D., Baumgartner, K., Halleen, F., Mostert, L., Sosnowski, M.R., Úrbez-Torres, J.R., & Armengol, J., 2016. Fungal trunk diseases: A problem beyond grapevines? Plant Pathol. 65(3), 355-356. [ Links ]
Gramaje, D., Mostert, L., Groenewald, J.Z. & Crous, P.W., 2015. Phaeoacremonium: From esca disease to phaeohyphomycosis. Fungal Biol. 119(9), 759-783. [ Links ]
Halleen, F., Baloyi, M.A., Bester, M. & Mostert, L., 2020. Aerial inoculum patterns of Petri disease pathogens in South African vineyards and rootstock mother blocks. Phytopathol. Mediterr. 59(3), 515-536. [ Links ]
Halleen, F., Fourie, P.H. & Lombard, P.J., 2010. Protection of grapevine pruning wounds against Eutypa lata by biological and chemical methods. S. Afr. J. Enol. Vitic. 31(2), 125-132. [ Links ]
Hewitt, W.B. & Pearson, R.C., 1988. Phomopsis cane and leaf spot. In: Pearson, R.C. & Goheen, C. (eds). Compendium of grape diseases. The American Phytopathological Society Press, St Paul, MN. pp. 17-18. [ Links ]
Kaplan, J., Travadon, R., Cooper, M., Hillis, V., Lubell, M. & Baumgartner, K., 2016. Identifying economic hurdles to early adoption of preventative practices: The case of trunk diseases in California winegrape vineyards. Wine Econ. Pol. 5(2), 127-141. [ Links ]
Kotze, C., Van Niekerk, J.M., Halleen, F. & Fourie, P.H., 2011. Evaluation of biocontrol agents for grapevine pruning wound protection against trunk pathogen infection. Phytopathol. Mediterr. 50(Suppl.), S247-S263. [ Links ]
Kuntzmann, P., Villaume, S. & Bertsch, C., 2009. Conidia dispersal of Diplodia species in a French vineyard. Phytopathol. Mediterr. 48, 150-154. [ Links ]
Larignon, P. & Dubos, B., 2000. Preliminary studies on the biology of Phaeoacremonium. Phytopathol. Mediterr. 39(1), 184-189. [ Links ]
Lecomte, P. & Bailey, D.J., 2011. Studies on the infestation by Eutypa lata of grapevine spring wounds. Vitis 50(1), 35-41. [ Links ]
Lecomte, P., Cardon, S., Bastien, N. & Giry-Laterrière, S., 2004. Risques d'infection par l'eutypiose au printemps. Phytoma - Défense Végét. 576, 22-27. [ Links ]
Lecomte, P., Cardon, S., Bastien, N., Giry-Laterriere, S. & Bailey, D.J., 2005. Susceptibility of grapevine spring wounds to Eutypa lata: Further results and present epidemiological status. Phytopathol. Mediterr. 44, 105-106. [ Links ]
Lecomte, P., Clerjeau, M., Larignon, P., Bastien, N., Cardon, S., Lafitte, J. & Dubos, B., 2001. Can Eutypa lata infect grapevine through spring wounds? (Preliminary data under sheltered conditions). Phytopathol. Mediterr. 40, 483. [ Links ]
Lesuthu, P., Mostert, L., Spies, C.F.J., Moyo, P., Regnier, T. & Halleen, F., 2019. Diaporthe nebulae sp. nov. and first report of D. cynaroidis, D. novem, and D. serafiniae on grapevines in South Africa. Plant Dis. 103(5), 808-817. [ Links ]
Makatini, G.J., 2014. Role of sucker wounds as portals for grapevine trunk pathogen infections. Thesis, Stellenbosch University, Private Bag X1, 7602 Matieland (Stellenbosch), South Africa. [ Links ]
Mostert, L., Crous, P.W., Kang, J.C. & Phillips, A.J.L., 2001. Species of Phomopsis and a Libertella sp. occurring on grapevines with specific reference to South Africa: Morphological, cultural, molecular and pathological characterization. Mycologia 93(1), 146-167. [ Links ]
Mostert, L., Groenewald, J.Z., Gams, W., Summerbell, R.C. & Crous, P.W., 2006a. Taxonomy and pathology of Togninia (Diaporthales) and its Phaeoacremonium anamorphs. Stud. Mycol. 54, 1-113. [ Links ]
Mostert, L., Halleen, F., Fourie, P.H. & Crous, P.W., 2006b. A review of Phaeoacremonium species involved in Petri disease and esca of grapevines. Phytopathol. Mediterr. 45, S12-S29. [ Links ]
Moyo, P., Mostert, L. & Halleen, F., 2019. Diatrypaceae species overlap between vineyards and natural ecosystems in South Africa. Fungal Ecol. 39, 142-151. [ Links ]
Moyo, P., Mostert, L., Spies, C.F.J., Damm, U. & Halleen, F., 2018. Diversity of Diatrypaceae species associated with dieback of grapevines in South Africa, with the description of Eutypa cremea sp. nov. Plant. Dis. 102(1), 220-230. [ Links ]
Mutawila, C., Fourie, P.H., Halleen, F. & Mostert, L., 2011. Histo-pathology study of the growth of Trichoderma harzianum, Phaeomoniella chlamydospora and Eutypa lata on grapevine pruning wounds. Phytopathol. Mediterr. 50(Suppl.), S46-S60. [ Links ]
O'Donnell, K. & Cigelnik, E., 1997. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol. Phylogenet. Evol. 7(1), 103-116. [ Links ]
Paolinelli-Alfonso, M., Serrano-Gomez, C. & Hernandez-Martinez, R., 2015. Occurrence of Eutypella microtheca in grapevine cankers in Mexico. Phytopathol. Mediterr. 54(1), 86-93. [ Links ]
Petzoldt, C.H., Moller, W.J. & Sall, M.A., 1981. Eutypa dieback of grapevine, seasonal differences in infection and duration of susceptibility of pruning wounds. Phytopathology 71, 540-543. [ Links ]
Pitt, W.M., Huang, R., Trouillas, F.P., Steel, C.C. & Savocchia, S., 2010. Evidence that Eutypa lata and other diatrypaceous species occur in New South Wales vineyards. Austral. Plant Pathol. 39, 97-106. [ Links ]
Pitt, W.M., Trouillas, F.P., Gubler, W.D., Savocchia, S. & Sosnowski, M.R., 2013. Pathogenicity of diatrypaceous fungi on grapevines in Australia. Plant Dis. 97(6), 749-756. [ Links ]
Rolshausen, P.E., Baumgartner, K., Travadon, R., Fujiyoshi, P., Pouzoulet, J. & Wilcox, W.F., 2014. Identification of Eutypa spp. causing Eutypa dieback of grapevine in eastern North America. Plant Dis. 98(4), 483-491. [ Links ]
Rolshausen, P.E., Úrbez-Torres, J.R., Rooney-Latham, S., Eskalen, A., Smith, R.J. & Gubler, W.D., 2010. Evaluation of pruning wound susceptibility and protection against fungi associated with grapevine trunk diseases. Am. J. Enol. Vitic. 61(1), 113-119. [ Links ]
Rooney-Latham, S., Eskalen, A. & Gubler, W.D., 2005. Occurrence of Togninia minima perithecia in esca-affected vineyards in California. Plant Dis. 89(8), 867-871. [ Links ]
Siebert, J.B., 2001. Eutypa: The economic toll on vineyards. Wines and Vines 4, 50-56. [ Links ]
Sosnowski M. & Ayres M., 2022. Spring pruning wounds are susceptible to grapevine trunk disease pathogens. Wine Vit. J. 37(2), 32-33. [ Links ]
Surico, G., 2009. Towards a redefinition of the diseases within the esca complex of grapevine. Phytopathol. Mediterr. 48(1), 5-10. [ Links ]
Trouillas, F.P. & Gubler, W.D., 2010. Pathogenicity of Diatrypaceae species in grapevines in California. Plant Dis. 94(&), 867-872. [ Links ]
Uddin, W., Stevenson, K.L. & Pardo-Schultheiss, R.A., 1997. Pathogenicity of a species of Phomopsis causing shoot blight on peach in Georgia and evaluation of possible infection courts. Plant Dis. 81(9), 983-989. [ Links ]
Úrbez-Torres, J.R., Leavitt, G.M., Voegel, T.M. & Gubler, W.D., 2006. Identification and distribution of Botryosphaeria spp. associated with grapevine cankers in California. Plant Dis. 90(12), 1490-1503. [ Links ]
Úrbez-Torres, J.R., Battany, M., Bettiga, L.J., Gispert, C., McGourty, G., Roncoroni, J., Smith, R.J., Verdegaal, P. & Gubler, W.D., 2010. Botryosphaeriaceae species spore-trapping studies in California vineyards. Plant Dis. 94(6), 717-724. [ Links ]
Úrbez-Torres, J.R., Peduto, F., Smith, R.J. & Gubler, W.D., 2013. Phomopsis dieback: A grapevine trunk disease caused by Phomopsis viticola in California. Plant Dis. 97(12), 1571-1579. [ Links ]
Valencia, D., Torres, C., Camps, R., López, E., Celis-Diez, J. & Besoain, X., 2015. Dissemination of Botryosphaeriaceae conidia in vineyards in the semiarid Mediterranean climate of the Valparaíso Region of Chile. Phytopathol. Mediterr. 54(2), 394-402. [ Links ]
Van Niekerk, J.M., Calitz, F.J., Halleen, F. & Fourie, P.H., 2010. Temporal spore dispersal patterns of grapevine trunk pathogens in South Africa. Europ. J. Plant Pathol. 127, 375-390. [ Links ]
Van Niekerk, J.M., Crous, P.W., Groenewald, J.Z., Fourie, P.H. & Halleen, F., 2004. DNA phylogeny, morphology and pathogenicity of Botryosphaeria species on grapevines. Mycologia 96(4), 781-798. [ Links ]
Van Niekerk, J.M., Fourie, P. & Halleen, F., 2003. Economic impact of Eutypa dieback on grapevines. Technical Wynboer 173, 78-80. [ Links ]
Van Niekerk, J.M., Groenewald, J.Z., Farr, D.F., Fourie, P.H., Halleen, F. & Crous, P.W., 2005. Reassessment of Phomopsis species on grapevines. Austral. Plant Pathol. 34, 27-39. [ Links ]
Van Niekerk, J.M., Halleen, F. & Fourie, P.H., 2011. Temporal susceptibility of grapevine pruning wounds to trunk pathogen infection in South African grapevines. Phytopathol. Mediterr. 50(Suppl.), S139-S150. [ Links ]
White, T.J., Bruns, T., Lee, S. & Taylor, J., 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis, M.A., Gelfand, J.J. & White, T.J. (eds). PCR protocols: A guide to methods and applications. Academic Press Inc., New York, NY. pp. 315-322. [ Links ]
White, C.-L., Halleen, F. & Mostert, L., 2011. Symptoms and fungi associated with esca in South African vineyards. Phytopathol. Mediterr. 50, S236-S246. [ Links ]
Wicks, T. & Davies, K., 1999. The effect of Eutypa on grapevine yield. Aust. Grapegrow. & Winemak. 426a, 15-16. [ Links ]
Submitted for publication: October 2022
Accepted for publication: November 2022
Acknowledgements: The authors are grateful for the financial support provided by Winetech, the National Research Foundation (NRF), Stellenbosch University (SU), the Technology and Human Resources for Industry Programme (THRIP) and the ARC Infruitec-Nietvoorbij; as well as for technical assistance from the Plant Protection Division (ARC Infruitec-Nietvoorbij). The grant holders acknowledge that the opinions, findings and conclusions or recommendations expressed in any publication generated by NRF-supported research are those of the authors, and that the NRF accepts no liability whatsoever in this regard.
* Corresponding author: E-mail addresses: lmost@sun.ac.za [Tel.: +27 (0)21 808 3397]














