Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904
Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.41 n.1 Stellenbosch 2020
http://dx.doi.org/10.21548/41-1-3705
ARTICLES
The Effect of Hot Water Treatment of Rooted Grapevine Nursery Stock on the Survival of the Root-knot Nematode, Meloidogyne javanica (Nematoda: Heteroderidae)
R Knoetze*
Agricultural Research Council, Infruitec-Nietvoorbij, Private Bag X5026, Stellenbosch 7599, South Africa
ABSTRACT
Root-knot nematodes (Meloidogyne spp.) are endoparasites which cause severe losses in grapevine. To ensure economically viable grape production, it is important that nurseries produce rooted nursery material free of plant-parasitic nematodes. Hot water treatment (HWT) at 50°C for 45 min to eliminate root-knot nematode (RKN) from rooted nursery material was investigated as a method to ensure nematode free plant material. Rooted grapevine rootstocks known to be susceptible (US 8-7 and 110 Richter), moderately resistant (1103 Paulsen and 143 B) and resistant (Ramsey) to Meloidogyne javanica were artificially infested by inoculating them with RKN eggs and larvae. After one growing season, the vines were lifted, shoots and root systems trimmed and subjected to different HWT regimes viz. 50°C for 45 min and 55°C for 20 min, while some were left as untreated controls. To evaluate plant response, each vine was planted in a pot, together with a three-week old tomato seedling as an indicator of root-knot nematode infestation. The tomato plants were removed after 12 weeks and their roots examined for the presence of M. javanica galls and egg masses. At the end of the growing season, the effects of the treatments on plant growth were assessed by determining total shoot and root mass. The results demonstrated that HWT at 55°C for 20 min significantly reduced the nematode populations in the rooted stocks, but did not eliminate the nematodes from the roots since indicator plants from HWT vines still supported a small number of galls. HWT at 55°C for 20 min also reduced the level of infestation of RKN in grapevine planting material, but resulted in a significant reduction in growth.
Key words: Grapevine, Meloidogyne, hot water treatment, root-knot nematode, control
INTRODUCTION
Root-knot nematodes (Meloidogyne spp.) (Tylenchida: Heteroderidae) are endoparasites which cause severe losses in grapevine. They penetrate the roots to feed, inducing the formation of characteristic root galls. Heavy infestation weakens the root system, restricting the ability of roots to absorb water and nutrients, leading to reduced vigour and yield (Storey et al., 2017). Nematode control in established vineyards is costly and untreated infestations can reduce the productive lifespan of a vineyard. Providing nematode-free planting material to growers is key to ensure economically viable grape production. Although nurseries use soil fumigation and chemical control throughout the year, endoparasites like root-knot nematodes (RKN) can still be transmitted by infested rooted plant material.
Hot water treatment (HWT) is an effective and practical method for the control of a number of grapevine pests and diseases in dormant grapevine cuttings and young rooted vines (Von Broembsen & Marais, 1978; Suatmadji, 1982; Loubser & Höppner, 1986; Fourie & Halleen, 2004; Gramaje et al., 2009). Barbercheck (1986) showed that HWT eliminated root-knot nematodes from grapevine nursery stock, but concerns have been raised that this HWT regime may not be sufficient to ensure that no viable RKN adults, juveniles or eggs survive in the roots of treated plants.
The South African Plant Certification Scheme for wine grapes requires plant material to be visually free of RKN infestation for certification, whilst HWT for eradication of RKN in rooted grapevine material is not prescribed in the Standard Operating Procedure (SOP) for Grapevines pertaining to rejections of graft combinations for visual symptoms of RKN (https://www.gov.za/documents/plant-improvement-act-south-african-plant-certification-scheme-wine-grapes-amendment). The current SOP does prescribe HWT at 50°C for 45 min to eliminate crown gall and aster yellows phytoplasma, or HWT at 55°C for 5 min to remove other superficial pathogens and pests. Confirmation of the efficacy of HWT at 50°C for 45 min for the elimination of RKN from rooted vines is important for the Vine Improvement Association before revising current regulations and SOPs.
The aim of this study was to test the efficacy of HWT for eliminating RKN from rooted nursery material, as well as the impact of HWT on the initial growth of the treated plants.
MATERIALS AND METHODS
Inoculation of grapevine rootstocks
Grapevine rootstocks known to be susceptible (US 8-7 and 110 Richter), moderately resistant (1103 Paulsen and 143 B) and resistant (Ramsey) to Meloidogyne javanica (Treub, 1885) Chitwood, 1949 were grafted with Chenin blanc, callused and rooted at a commercial grapevine nursery (Vititec, Paarl). Rooted vines were planted in sandy soil in planting bags and kept in a glasshouse, set at 25°C. Twenty-one representatives of each rootstock were inoculated with M. javanica on four occasions during the 2017/18 growing season by pipetting 5 ml of a suspension containing approximately 3000 eggs and juveniles into two holes made in the soil, approximately 1.5 to 2.0 cm from the plant stem. Inoculation sites were covered by pressing the soil mixture back into place. Seven representatives of each rootstock were not inoculated (control plants) and kept separately to avoid contamination. After the first growing season, root samples were collected and pooled for each rootstock type, where-after 3 x 10 g subsamples were used to determine the level of infestation. The number of eggs per gram of roots was determined by using Riekert's adapted NaOCl method (Riekert, 1995), whereby the roots were cut into 10-mm pieces and shaken thoroughly for 4 min in a 1 % NaOCl solution. The solution was decanted through stacked sieves (75- and 25-Lim-aperture) and washed thoroughly before the eggs and juveniles were collected from the 25-Lim-aperture sieve.
Hot water treatment
After the first growing season, the vines were lifted, shoots trimmed to three buds and the root systems trimmed to approximately 150 mm. The plants were then packed into plastic bins, covered with sawdust and stored in a cold room at 5°C. Plants from each rootstock were divided into 4 groups, constituting the different treatments, which were: i) infected plants, no HWT; ii) infected plants, HWT at 50 °C for 45 min; iii) infected plants, HWT at 55 °C for 20 min and iv) non-infected plants; no HWT. There were seven replications of each treatment. Plants were submersed in thermostatically controlled hot water baths, set at the predetermined temperatures. Immediately after treatment the vines were immersed in a cold water bath at 15°C for 15 min. After treatment, the vines were planted immediately and moved to a glasshouse at 25°C.
Evaluation of the efficacy of HWT
To evaluate treatment efficacy, each vine was planted in a plastic pot containing sterilized sandy soil and placed in a glasshouse at 25°C. The pots were arranged in a randomized block design, consisting of seven blocks, with 20 pots in each block. The vines were drip-irrigated with filtered water (5 Lim filter) to avoid external contamination, as well as cross contamination between pots. Three days later, a three-week old tomato seedling (cv. Moneymaker) was planted next to each vine as indicator of RKN infestation.
Twelve weeks later, the tomato plants were removed, their roots stained with a 0.1 % food dye containing Ponceau 4R (Damasceno et al., 2016) for easier detection and then examined for the presence of root-knot nematode galls and egg masses. The number of galls and/or egg masses resulting from each grapevine-tomato combination was recorded. A value of 100 galls was assigned to heavily infested root systems, as it was not possible to accurately count the number of galls present.
Evaluation of growth response
To evaluate plant response to the HWT, budding and sprouting of the plants after planting were monitored on a weekly basis. Five months after planting (February 2019) the grapevines were removed from their pots, leaves removed and the root systems rinsed with water to be free of soil. Shoot length and fresh shoot weight were measured immediately, but the root weight was determined after overnight (12 h) drying at room temperature.
Data analyses
The experimental design was a randomised block design with seven block replications. The treatment design was a factorial with two factors, rootstocks with five levels and hot water treatment with four levels. To interpret the number of galls and/or egg masses on tomato root systems the data was subjected to an ANOVA using General Linear Models Procedure (PROC GLM) of SAS software (Version 9.4; SAS Institute Inc, Cary, USA). The kurtosis value and symmetrical histogram in the Univariate Procedure (PROC UNIVARIATE) of SAS Software indicated normality of standardized residuals. Fisher's least significant difference was calculated at the 5% level to compare treatment means (Ott & Longnecker, 2001). To analyse growth variables of infested plants, the data was subjected to an ANOVA using General Linear Models Procedure (PROC GLM) of SAS software (Version 9.4; SAS Institute Inc, Cary, USA). The Shapiro-Wilk test confirmed normality of the standardized residuals (Shapiro & Wilk, 1965). Fisher's least significant difference was calculated at the 5% level to compare treatment means (Ott & Longnecker, 2001).
RESULTS AND DISCUSSION
Infection rates
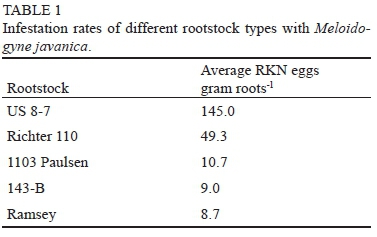
The grapevines were successfully infested with M. javanica during the first growing season. Table 1 shows the number of eggs gram roots-1 detected at the end of the growing season. The results confirmed that US 8-7 and 110 Richter were the most susceptible to M. javanica, with 1103 Paulsen and 143 B being moderately susceptible and Ramsey the least susceptible.
Efficacy of HWT
Data analysed with a factorial ANOVA showed significant differences (p < 0.0001) when comparing the number of galls and/or egg masses on roots of tomato indicator plants, planted next to M. javanica infected grapevines subjected to different hot water regimes (Table 2). The presence of nematodes in a tomato plant was considered proof that the HWT did not successfully eradicate all the RKN present in the roots of the grapevine plant. The number of RKN that survived in all of the HWT plants was significantly lower than in the untreated control, but it was not zero, except in the vines grafted onto Ramsey rootstocks. This means that a small number of the nematodes was not killed by the treatments and were able to produce eggs which could hatch and infest the tomato plants. Suatmadji (1982) also found that control by HWT (51.7°C for 5 min) was incomplete, as indicator plants planted next to HWT vines supported a small number of galls. This was attributed to the fact that immature nematodes were imbedded in young galls consisting of tightly packed cells, which may contribute to the nematodes surviving the treatment. Barbercheck (1986) found that HWT eliminated M. javanica from grapevine nursery stock, but it must be considered that, in that case, tomato plants were not used as indicators of RKN viability.
Eradication of the nematodes in the Ramsey material is attributed to the fact that the material was the least infested before the commencement of HWT. Thus, the combination of plant resistance and HWT seems to have contributed to effective eradication of the nematodes on this occasion. Conversely, the combination of the most susceptible rootstock (US 8-7) with the treatment of 50°C for 45 min, was the least effective of the treatment combinations (Table 2).
Considering the combined treatment averages of all the rootstocks, it is evident that the treatment of 55°C for 20 min was significantly more effective in reducing the nematode population in infested rootstocks than the 50°C for 45 min treatment (Table 2).
Growth response of vines to HWT
Cumulative sprouting percentages of the vines over a 7-week period indicated that untreated plants started sprouting in the first week after planting, whilst treated plants started sprouting in the second week. Overall, budburst of plants treated at 50°C for 45 min was delayed by one week, whilst budburst of plants treated at 55°C for 20 min was effectively delayed for two weeks. The lowest sprouting percentage was recorded for vines grafted onto Ramsey rootstocks treated at 55°C for 20 min (57%), while all the vines grafted onto Ramsey rootstocks treated at 50°C for 45 min sprouted within 3 weeks.
Data analysed with a factorial ANOVA showed no significant differences between the growth responses of different rootstocks, but the differences between treatments were highly significant (p < 0.0001). Table 3 shows the plant responses with regard to shoot length, shoot weight and root weight, regardless of rootstock. The best growth was observed in plants treated at 50°C for 45 min, but it did not differ significantly from infected plants that were not subjected to HWT. Studies carried out by Graham (2007) showed that cuttings grown in cool climates in New Zealand were susceptible to damage at 50°C for 30 min, but evidence suggests that tolerance of plants to HWT is affected by the climate in which the cuttings are grown (Waite & Morton, 2007). Von Broembsen & Marais (1978) found that treatment for 15 min to 60 min at 50°C resulted in no phytotoxic effects to dormant vines, but Loubser & Höppner (1986) reported that plant mass increase as well as root mass of HWT (50°C for 15 min) vines were significantly lower than those of untreated plants.
Shoot lengths and shoot weights of infested plants treated at 50°C for 45 minutes were significantly more than those of uninfested, untreated plants. This anomalous result can be attributed to the fact that closer inspection of the vines revealed dark-brown to black discoloration in the xylem of the root crown and basal rootstock of the vines. Fungal isolation (performed at ARC Infruitec-Nietvoorbij) detected the presence of the pathogens Phaeoacremonium aleophilum Gams, Crous, Wingfield & Mugnai (Diaporthales: Togniniaceae) and Pleurostomophora richardsiae (Nannf.) Mostert, Gams & Crous (Calosphaeriales: Pleurostomataceae), both of which are associated with Petri disease of grapevines. Fourie & Halleen (2004) found that HWT (30 min at 50°C) of dormant nursery material were effective in reducing fungal infection levels in nursery plants. It is therefore possible that HWT reduced the severity of the fungal infection in these vines, leading to the masking of any growth retardation that may have resulted from HWT at 50°C for 45 min.
The combined growth of plants treated at 55°C for 20 min was significantly less than with all of the other treatments. Gramaje et al. (2009), when investigating HWT against Petri disease pathogens, found that there was little variability in the percentages of sprouting and shoot weight after HWT, with the exception of the HWT at 54°C in which the highest reduction was obtained. In this study, it was evident from sprouting percentages, shoot length, shoot weight and root weight that vines were damaged by HWT at 55°C for 20 min.
CONCLUSIONS
This research showed that HWT at 50°C for 45 minutes greatly reduced the level of infestation of RKN in grapevine planting material, particularly when RKN infestation levels were low, but it did not eradicate RKN in all instances. HWT at 55°C for 20 min also reduced the level of infestation of RKN in grapevine planting material, but resulted in a significant reduction in growth and therefore cannot be recommend for the treatment of rooted grapevine nursery material.
Since HWT at 50°C for 45 min did not completely eliminate RKN from rooted material, the unqualified revision of current regulations and operating procedures cannot be recommended to the Vine Improvement Association. However, HWT can be successfully implemented in nurseries as an added measure to reduce nematode infestation, but only if due consideration is given to the prevention of infestation of rooted material with RKN. An integrated strategy for the proactive management of RKN in grapevine nurseries, which includes practices such as the filtering of irrigation water, sterilization of growing medium, general sanitation practices and the use of HWT, is advocated to provide RKN-free planting material, which will ultimately save costs for nematode control in established vineyards and prolong the productive lifespan of vineyards.
LITERATURE CITED
Barbercheck, M., 1986. Control of Meloidogyne javanica in dormant grapevine nursery stock. Phytophylactica 18, 39-40. [ Links ]
Damasceno, J.C.A., Soares A.C.F., de Jesus F.N. & Castro J.M.C., 2016. Root-knot nematode staining with artificial food dyes. Nematoda 3, 1-5. http://dx.doi.org/10.4322/nematoda.01816 [ Links ]
Fourie, P.H. & Halleen, F., 2004. Proactive control of Petri disease of grapevine through treatment of propagation material. Plant Dis. 88, 1241-5. https://doi.org/10.1094/PDIS.2004.88.11.1241 [ Links ]
Graham, A., 2007. Hot water treatment of grapevine rootstock cuttings grown in a cool climate. Phytopathol. Mediterr. 46, 124. [ Links ]
Gramaje, D., Armengol, J., Salazar, D., López-Córtes I. & Garciá-Jiménez J., 2009. Effect of hot-water treatments above 50°C on grapevine viability and survival of Petri disease pathogens. Crop Prot. 28, 280-285. https://doi.org/10.1016/j.cropro.2008.11.002 [ Links ]
Loubser, J.T. & Höppner, G.F., 1986. Control of lesion nematodes, Pratylenchus spp., in grapevine nursery material by immersion in fenamiphos solutions and hot water. South Afr. J. Enol. Vitic. 7, 3-5. [ Links ]
Ott, R.L. & Longnecker, M., 2001. An introduction to statistical methods and data analysis 5th Edition. Belmont, California, Duxbury Press. [ Links ]
Riekert, H.F., 1995. An adapted method for extraction of root-knot nematode eggs from maize root samples. Afr. Plant Prot. 1, 41-43. [ Links ]
Shapiro, S.S., Wilk, M.B. & Chen, H.J., 1968. A comparative study of various tests for normality. J. Am. Stat. Assoc. 63(324), 1343-1372. [ Links ]
Storey, S.G., Malan, A.P. & Hugo, H.J., 2017. Nematode Pests of Grapevine. In: Fourie, H., Spaull, V.W., Jones, R.K., Daneel, M.S. & de Waele, D. (Eds.) Nematology in South Africa: A view from the 21st century. Springer International, Switzerland, pp. 345 - 357. https://doi.org/10.1007/978-3-319-44210-5 [ Links ]
Suatmadji, R.W., 1982. Control of root knot nematodes, Meloidogyne javanica, in rootstocks of grapevine, Vitis vinifera, by immersion in nematicide solutions at different temperatures and in hot water. Nematol. Mediterr. 10, 119-125. [ Links ]
Von Broembsen, S. & Marais, P.G., 1978. Eradication of Phytophthora cinnamomi from grapevine by hot water treatment. Phytophylactica 10, 25-27. [ Links ]
Waite, H. & Morton, L., 2007. Hot water treatment, trunk diseases and other critical factors in the production of high-quality grapevine planting material. Phytopathol. Mediterr. 46, 5-17. [ Links ]
Submitted for publication: August 2019
Accepted for publication: November 2019
* Corresponding author: E-mail address: knoetzer@arc.agric.za
Acknowledgements: The authors would like to thank the Agricultural Research Council, Winetech and the South African Table Grape Industry for funding the research, and B. Sokomani, D. Hinds and C. Paulse for technical assistance














