Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Enology and Viticulture
versão On-line ISSN 2224-7904
versão impressa ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.40 no.1 Stellenbosch 2019
http://dx.doi.org/10.21548/40-1-3004
ORIGINAL RESEARCH
Effect of Gibberellic acid (GA3) inflorescence application on content of bioactive compounds and antioxidant potential of grape (Vitis L.) 'Einset Seedless' berries
M. KaplanI, * ; A. NajdaII; K. KlimekIII; A. BorowyI
IDepartment of Pomology and Nurseries, University of Life Sciences, 58 Leszczynski Street, 20-068 Lublin, Poland
IIDepartment of Vegetable Crops and Medicinal Plants, University of Life Sciences, 58 Leszczynski Street, 20-068 Lublin, Poland
IIIDepartment of Applied Mathematics and Informatics, University of Life Sciences, 28 Gleboka Street, 20-612 Lublin, Poland
ABSTRACT
Gibberellic acid (GA3) is a plant growth regulator widely used in the cultivation of seedless grape varieties to increase their yield. Hormonisation treatment has beneficial effects on yield size and quality, yet its influence on the level of biologically active compounds and grape antioxidant activity has not yet been studied extensively yet. Clusters of 11-year-old 'Einset Seedless' grapevines trained according to the single Guyot pruning style were sprayed with GA3 at 100, 200 or 300 mg/L dose once, twice or three times. Fruit harvested on 25 September were immediately examined for acidity, extract content, biologically active substances and antioxidant capacity using the DPPH test. In addition, correlations occurring between some parameters measured were calculated. Hormonisation had a negative effect on the content of extract, flavonoids and ascorbic acid, while it had no effect on the anthocyanin level. The antioxidant activity determined by the DPPH assay depended on dose and the number of treatments, and the analysed parameters were shown to decrease significantly with increasing application number. Gibberellic acid at 100 and 300 mg/L application rates had a significantly increased DPPH level compared to the control and 200 mg/L dose. The single GA3 treatment, and when applied three times, and application rates at 100 and 200 mg/L were shown to have a significant influence on phenolic acid content. The level of tannins after a single GA3 treatment and a 300 mg/L dose increased significantly.
Keywords: Seedless grape, hormonisation, DPPH, anthocyanins, correlation
INTRODUCTION
Grape consumption is strongly correlated with the reduced risk factor for developing chronic diseases, such as cardiovascular disorders and cancer (Arts & Hollman, 2005; Erdman et al., 2007; Leifert & Abeywardena, 2008; Vislocky & Fernandez, 2010; Doshi et al., 2015). This results from, among others, the presence of biologically active compounds like polyphenols, which display powerful antioxidant effects, along with anti-inflammatory, anti-carcinogenic and anti-platelet properties. Polyphenols also help dilate blood vessels, boost the immune system and play a neuroprotective role, attributed mainly to their ability to modulate and induce signalling pathways (Frankel, 1999; Stevenson & Hurst, 2007; Pezzuto, 2008; Dohadwala & Vita, 2009; Crozier et al., 2010; Vislocky & Fernandez, 2010; Xia et al., 2010; Doshi et al., 2015). Polyphenols inactivate free radicals, chelate divalent metal ions, and thus lower their oxidant potential (Scalbert et al., 2005).
The qualitative and quantitative composition, distribution and antioxidant activity of polyphenols in grapes are quite variable and depend on species, cultivar, location in berry (skin, pulp, seeds, juice), climate-soil conditions (exposure to light, temperature, soil type), agrotechnical practices (irrigation, nutrient availability, application of plant growth regulators, harvest time, berry maturity, yield and berry size) and, finally, post-harvest conditions and storage-processing techniques (Kim et al., 2003; Peña-Neira et al., 2004; Jiang et al., 2006; Montealegre et al., 2006; Orak, 2007; Xia et al., 2010; Liang et al., 2011). Polyphenols are responsible for the major sensory attributes of products and beverages of plant origin, as they are the determinants of their appearance (colour) and taste, i.e. flavour, bitterness and astringency (Tomás-Barberán & Espin, 2001; Es-Safi et al., 2007).
Seedless grape varieties have been on the rise in the world grape market because of their high quality and consumer preferences, and they enjoy increasing popularity not only as table grapes, but as raisins as well (Artés-Hernández et al., 2006). One of the most promising commercial seedless cultivars that can be grown successively in cool-climate areas, such as Poland, is 'Einset Seedless', a pink grape with a unique strawberry-like flavour. This variety can be used for raisin production or for fresh consumption as table grapes. However, the natural berry size of the 'Einset Seedless' variety (± 2 g) is not large enough for table grape use and thus represents a problem for commercialisation. To overcome this problem, and to improve grape size and quality, plant growth regulators (most often gibberellic acid, GA3) are applied globally (Harrell & Williams, 1987; Dimovska et al., 2014; Nampila et al., 2010; Kaplan et al., 2017). Gibberellic acid promotes cell division, enhances earlier blooming and increases fruit size and yield. The effect of GA3 application relies on the variety, dose and application time (Khan et al., 2009; Nampila et al., 2010; Dimovska et al., 2014; Kaplan et al., 2017). The earlier studies by the present authors showed a positive response of this cultivar to gibberellic acid treatment considering fruit set, and the size of clusters and berries (Kaplan, 2011; Kaplan et al., 2017). These findings are of the utmost importance currently, because modern table grape production is expected to fully conform with the requirements of a market that is demanding improved grape quality, that is aiming for uniformly repeatable clusters, equal berry size, shape and uniform skin colour, as well as for increased resistance to transportation. An important attribute of grape berry quality has been proven to be the absence of seeds (Dimovska et al., 2014).
The influence of gibberellic acid on the content of biologically active compounds and the antioxidant potential of grapes with regard to concentration and the number of treatments on grapevines is an innovative idea. The relevant available literature does not include any experiments exploring the effect of hormonisation on the antioxidant activity level in grapes.
MATERIALS AND METHODS
Plant materials
The field experiment assessed the effect of GA3 dose and the number of treatments on the level of chosen secondary metabolites of grapes. The analysed fruit were obtained from the NOBILIS Vineyard (50o39'N; 21o34'E) located in the Sandomierska Upland in south-eastern Poland. The own-rooted vines of 'Einset Seedless' were planted in spring 2003 at a spacing of 2.0 x 1.0 m (5 000 units/ha) on lessive soil developed from loess deposits. Throughout the experiment, regular soil-protective measures against diseases, pests and weeds were carried out in compliance with the current grapevine protection programme. The grapevines were not watered. An average number of clusters per vine was 17 to 18, while grape yield averaged 3.5 kg per vine. Grapes were harvested on 2014-09-25. The grapevines were pruned in the single Guyot pruning style. The inflorescences of 11-year-old 'Einset Seedless' grapevines were treated with GA3 spray at three dose levels, viz. 100, 200 or 300 mg/L, once (seven days after full bloom, when 70% of berries in the cluster were 1 mm in diameter), twice (seven and 14 days after full bloom, when 70% berries in the cluster were 1 and 3 mm in diameter respectively) or three times (seven, 14 and 21 days after full bloom, when 70% of berries in the cluster were 1, 3 and 6 mm in diameter respectively). The solution contained 99% gibberellic acid and an adhesive and wetting SILWET Gold agent at 0.015% dose, i.e. 150 μL. The solution was prepared immediately before the treatment. The clusters were treated with a hand pump sprayer, covering the pedicels and berries thoroughly. On average, 50 mL of solution was enough to thoroughly cover all the grape clusters on a vine. The untreated grapes constituted the control.
The field experiment was set up in a randomised block design, including 10 combinations with five replications comprising plots of three grapevines each. The harvested fruit underwent laboratory tests at the Laboratory for Vegetable and Herbal Material Quality in the Department of Vegetable Crops and Medicinal Plants of the University of Life Sciences in Lublin. The research material comprised the 'Einset Seedless' grape variety ('Fredonia' χ 'Canner', Reisch et al., 1986) subjected to hormonisation with gibberellic acid (GA3).
Chemicals
All reagents and solvents were analytical grade chemicals from Merck (Darmstadt, Germany), Sigma Chemical Co. (St. Louis., MO, USA) or POCh (Gliwice, Poland). GA3 was obtained from Acros OrganicsTM (Thermo Fisher Scientific, Geel, Belgium) and SILWET Gold from Chemtura Europe Limited (Warsaw, Poland).
Physicochemical analyses
Fruit extract content was measured on harvest day using a refractometer, Abbe WAY 2W (EnviSense, Poland) while squeezing the juice from 100 representative berries collected from each combination. In order to determine biologically active compounds and antioxidant activity, grapes were transported to the laboratory on harvest day, stored in a cooler at 8°C for 16 hr, and finally underwent the chemical analyses. Titratable acidity (TA) was determined in accordance with Polish Norm PN-90/A/75101/02.
Determination of L-ascorbic acid
The fresh and comminuted grape fruits (5 g) were extracted twice for 30 min with 2.5 ml 4.0% (m/V) L-cysteine and 10.0 ml water by sonification. All aqueous extracts were combined and diluted with water to 25 mL. The samples were analysed using high performance liquid chromatography. Analyses were done with a LaChrom-Merck HPLC system with a photodiode array detector (DAD L-7450), and all separations were done in a Lichrospher 100 RP18 column (250.0 χ 4.0 mm, 5.0 μηι; Merck). The mobile phase consisted of 0.0272 g/L KH2PO4 adjusted to pH 2.40 with H3PO4, applied in isocratic elution for 30 min. The flow rate was adjusted to 1.0 mL/min. The detection wavelength was set to DAD at λ = 254.0 nm. Samples of 20.0 μL were injected. All separations were performed at 24.0°C. The peaks were assigned by spiking the samples with standard compounds and comparing the UV spectra and retention times (ascorbic acid, 5.66 min) (Najda, 2017). Calibration curves were obtained from five doses of each external standard (0.01 to 1.40 mg/mL). The regression coefficient (R2) of the calibration curve for ascorbic acid was equal to Y = 85.231, X = 18.787. The RSD value for the repeatability (n = 4) of standard solution was 0.40% (0.01 mg/mL ascorbic acid). The limits of quantitation (LOQ) and detection (LOD) of ascorbic acid were 0.16 and 0.04 mg/L respectively. All solvents used were HPLC grade (Merck). Reference standards were obtained from Sigma-Aldrich.
Total phenolic acid estimation was carried out according to the Arnov method (Polish Pharmacopoeia, 2002). One millilitre of sample was mixed with 5 ml of distilled water, 1 mL 0.5 M HCl, 1 mL of Arnov reagent and 1 mL 1M NaOH, and subsequently adjusted to 10 ml with distilled water. The absorbance was measured at 490 nm. The total phenolic acid content was expressed as caffeic acid equivalent (CAE).
Estimation of anthocyanins by means of colorimetry
Samples of raw material (1.0 g) were extracted with 50 ml HCl (1 mol/dm) and heated in a water bath for 1 hr. The obtained extract was hydrolysed with 20 ml n-butanol, and then two 10 ml n-butanol portions were added as a solution. Anthocyanin extracts were rinsed with n-butanol in a 50 mL flask. The absorbance was measured immediately at 533 nm (Milkowska & Strzelecka, 1995).
The percentage of anthocyanins, expressed as delphinidin chloride, was calculated from the expression:
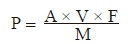
where: P = total anthocyanins (mg/100 g), A = absorbance at 533 nm, V = value of butanol phase (50 mL), F = coefficient expressed as delphinidin chloride (2.6), and m = mass of sample to be examined (mg).
Determination of antiradical activity (AA) A 0.1 mL aliquot of the methanol extract prepared above was mixed with 3.9 mL of an 80% ethanolic 0.6 mM DPPH solution. The tubes were vortexed for 15 s and allowed to stand for 180 min, as described by Cai et al. (2003). After this, the absorbance of the mixture was measured at λ = 517 nm wavelength using the HITACHI UV-Vis spectrophotometer (UV-Vis model U-2900. Shimadzu, Kyoto, Japan). Most tested compounds reacted completely within 180 min under these conditions. The reaction time for vitamin C was less than 1 min due to its fast oxidation. Ethanol (80%) was used as a blank solution, and DPPH solution without test samples (3.9 ml of DPPH + 0.1 ml of 80% ethanol) served as the control. All tests were performed in triplicate. The antiradical activity of the test samples was expressed as the median effective dose for radical scavenging activity (EC50): TP (mg) of antioxidant (test sample) required for a 50% decrease in absorbance of DPPH radicals and inhibition (%) of DPPH absorbance = (A control-Atest) x 100/A control. A plot of absorbance of DPPH vs dose of antioxidant was made to establish the standard curves (dose-response curves) and to calculate that EC50. Acontrolis the absorbance of the control (DPPH solution without the test sample), and Atestis the absorbance of the test sample (DPPH solution plus 0.1 mL of 5 μM test compound). Ascorbic acid served as a standard.
The results of the assay were expressed relative to an ascorbic acid equivalent.
Estimation of total flavonoids
The studied material was investigated for total content of flavonoids using a modified Christ and Müller method, calculated for quercetin, QE (Polish Pharmacopoeia, 2014). Absorbance was measured at 425 nm on a HITACHI U-2900 spectrophotometer.
The content of flavonoids was calculated from the equation:
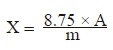
where m (g) was the amount of fresh mass
Tannin estimation
The tannins were determined by the Folin-Ciocalteu method. About 0.1 mL of the sample extract was added to a volumetric flask (10 mL) containing 7.5 mL of distilled water, 0.5 mL of Folin-Ciocalteu phenol reagent and 1 mL of 35% Na2CO3 solution, and diluted to 10 mL with distilled water. The mixture was shaken well and kept at room temperature for 30 min. A set of reference standard solutions of gallic acid (20, 40, 60, 80 and 100 μg/mL) was prepared in the same manner as described earlier. The absorbance for the test and standard solutions was measured against the blank at 725 nm with a UV/Visible spectrophotometer. The tannin content was expressed in terms of % of GAE of extract (Polish Pharmacopoeia, 2011) according to the European Pharmacopoeia (2008).
Statistical analysis
The results obtained in this study were analysed statistically using one-way analysis of variance and Tukey's confidence intervals. The inference was based on a significance level at p < 0.05. The estimation of correlations occurring between the qualitative parameters of the grapes was done by counting Pearson's correlation coefficients. Multidimensional analysis techniques were employed to show the similarities in the groups in such a way that the homogeneous objects could be found in the same cluster. The results of the cluster analysis are graphically shown in a dendrogram. All the statistical analyses were done using SAS Enterprise Guide 5.1. software.
RESULTS AND DISCUSSION
Table 1 presents the average monthly air temperatures and monthly precipitation totals in the 2014 growing season as compared to the multi-year average of 1998 to 2008. It can be observed that the weather conditions in the study year favoured grapevine cultivation. The average air temperature during the vegetation period was higher than the average multi-year one. Notably, an average air temperature lower than the average multi-year one was recorded only in August. The average precipitation total in the 2014 growing season was found to be higher compared to the average multi-year total. The rainfall sum in May, July and August was higher that that determined in the multi-year observations.
Sugars are one of the major components that determine fruit quality and are responsible for their sweetness. The sugar/organic acid ratio in fruit plays the most important role in the final flavour of grapes (Topalovic & Mikulic-Petkovsek, 2010). The statistical analysis indicated a significant influence of GA3 dose and application number on total extract content, vitamin C level and total acidity of the 'Einset Seedless' grape variety (Table 2). It was found that, irrespective of GA3 dose and number of treatments, the content of extract and vitamin C in the hormonised fruit was significantly lower compared to the control, except for the grapevines subjected to this treatment once. The significantly lowest level of both estimated parameters was recorded in the fruit treated with GA3 three times and with 200 mg/L GA3. The study of Al-Atrushy (2016) showed that an increase in the number of applications and dose of gibberellic acid significantly increased the total sugar level. Dimovska et al. (2014) applied GA3 twice and three times at a dose of 5, 10 and 20 mg/L and did not observe any significant effect on extract content in 'Flame Seedless' grapes.
The vitamin C content in 'Umran' grapes after 10 mg/L GA3 application was lower than in the control, whereas at higher doses, viz. 30 and 50 mg/L GA3, the hormonisation had the most beneficial impact on the parameter under study (Rachna & Singh, 2013). The vitamin C content after two applications of GA3 in seven seedless grape varieties was found to increase by 10% to 27% depending on variety, yet this was not confirmed in the present study (Gougoulias & Masheva, 2010). Awad and Al-Qurashi (2012) used gibberellic acid in the cultivation of date palms of the 'Barhee' variety and showed a positive influence of hormonisation on the vitamin C level in the fruit. The application of 100 and 150 mg/L GA3 significantly increased the vitamin C content compared to the control and the application of 50 mg/L GA3. It was found that the vitamin C level depended on the sugar content of the fruit of the grapevine variety under study. Moreover, the effect of gibberellic acid dose on vitamin C concentration was exactly the same as on sugar level.
Laszlo and Saayman (1990) and Topalovic and Mikulic-Petkovsek (2010) found that the acidity of grapes correlated with their taste, which results from the presence of tartaric and malic acid, whose contents reach as much as 90% in grapes. Total acidity in the fruits varied between 0.2 and 0.4%, and it differed significantly between the combinations under study. It was shown that grapes treated with 100 and 300 mg/L GA3 and with gibberellic acid solution applied once and twice displayed significantly higher total acidity than the control. Wholly different relationships were reported by Al-Atrushy (2016), who studied grapes subjected to hormonisation (irrespective of dose and number of treatments) and found significantly lower acidity than in the control. Similarly, Dimovska et al. (2014) applied GA3 at a dose of 20 mg/L and found that, irrespective of number of treatments, the application significantly decreased the acidity level. Rachna and Singh (2013) assessed the influence of gibberellic acid on the content of chosen biologically active compounds in fruits of Zizyphus mauritiana Lamk. cv. 'Umran' and observed an unfavourable effect of hormonisation at 50 mg/L GA3 application rate on total acidity at harvest. On the other hand, the study of Kok (2017) did not show any significant influence of GA3 applied in combination with a biostimulant on the acidity of 'Cardinal' grapes.
The antioxidant capacity of grape material is associated with the presence of secondary metabolites, i.e. phenolic acids, anthocyanins, flavonoids and tannins. The level of phenolic acids in the fruits studied depended significantly on the GA3 dose and the number of applications (Table 3). It was found that GA3 applied at 100 and 200 mg/L doses, as well as in single and three sprays, significantly increased the phenolic acid content in 'Einset Seedless' grapes.
Anthocyanins are a group of the most important phenolic components of dark grape cultivars, as they directly affect the intensity of berry skin coloration, which is the key quality attribute determining the market value and consumer acceptance of the grapes (Kok, 2017). In addition, anthocyanins, with their strong antioxidant properties, are involved in protection against fungal and bacterial infections. Notably, anthocyanin synthesis mostly occurs only in the grape berry skin (Doshi et al., 2015). The hormonisation treatment did not significantly affect the anthocyanin level in the fruit of the grapevine variety analysed here (Table 3). This research finding is confirmed by Dimovska et al. (2014), whose study also did not show any significant effect of hormonisation on the content of these compounds in 'Flame Seedless' grape berries. Different results were reported by Gougoulias and Masheva (2010) after GA3 was applied twice, giving rise to a 30% increase in anthocyanin content in 'Kishmish Tjurkmenski' fruit. A vast body of studies indicates that the level of anthocyanins and tannins relies largely on cultivar, species, degree of maturity of the fruit, climate and site of fruit production (Mazza, 1995; Mattivi et al., 2002; Muñoz-Espada et al., 2004; Yang et al., 2009).
The antioxidant potential of extracts of the fruit analysed was determined by the DPPH method and ranged from 56.272 to 83.652 uM TE/g. This depended significantly on the number of treatments and the dose (Table 3). The treatments applied had a significantly positive effect on the parameter under study in most combinations. It was found that the control grapes and those to which 100 and 300 mg/L GA3 had been applied displayed significantly higher antioxidant activity compared to grapes treated with 200 mg/L GA3. The number of hormonisation treatments also had a significant effect on the parameter analysed compared to the control combination, since increasing the number of applications significantly decreased the antioxidant potential. Tian et al. (2011) demonstrated that 100 mg/L GA3 applied twice had an unfavourably effect on the level of antioxidant capacity measured by the DPPH assay in 'Muscat Hamburg' fruit. The authors highlighted the opposite relationship when studying the anatomical parts of the plant, that is leaf, stem and tendril. Gougoulias and Masheva (2010) noted a beneficial influence of hormonisation that increased the antioxidant potential in seedless grape cultivars by 16% to 42%.
The flavonoid content in the grapes under investigation varied between 0.083 and 0.103 mg of cyanidin 3-glucoside equivalents per 100 grapes, and differed significantly between the combinations assessed. The grapes subjected to hormonisation had significantly less flavonoids than the control (Table 3). It was observed that an increasing dose of gibberellic acid promoted a significant increase in flavonoid level. After three hormonisation treatments, the fruit displayed a significantly lower flavonoid content that the other hormonisation treatments and the control grapes. These results are consistent with those reported by Tian et al. (2011), who noted that GA3 application decreased total flavonoid content substantially in grape pulp and skin. However, contrary results were obtained by Gougoulias and Masheva (2010), who assessed 'Trakijskaperla' fruit with amber yellow grape berries and violet-red 'Kishimish Tjurkmenski' fruit after GA3 treatment applied twice. The authors observed an increase in flavonoid content by 10% and 12% respectively against the control.
Tannins occur in grape skin, seeds and pedicels. Their amount in fruit juice (must) and wine is related to grapevine viticultural practices, vine load and environmental conditions, maceration procedures and fermentation conditions (Matthew & Nuzzo, 2007). Tannins possess several vital properties that affect the coloir and colour stability of the fruit, the depth of mouthfeel and astringency (Weston, 2005). The analysis showed that hormonisation with 300 mg/L GA3 and a single application had a significant effect on tannin content. Similar relationships were reported by Awad and Al-Qurashi (2012), who treated the date palm 'Barhee' cv. with 100 and 150 mg/L of GA3. However, the available literature does not provide any data on the impact of dose and the number of GA3 sprays on the tannin level of grape berries. It was shown that the application of 300 mg/L GA3 and a single GA3 application significantly increase the level of the parameter studied, and similar relationships were found when assessing the antioxidant potential using the DPPH assay. Notably, a high tannin level was observed to be modified by a high DPPH level.
An interaction between gibberellic acid dose and number of treatments was found to significantly affect the chosen secondary metabolites in grapes of the 'Einset Seedless' variety, except for anthocyanins.
The Pearson's coefficient indicates a strong correlation between total extract content and a dose of 100 mg/L and a single application of GA3, vitamin C level and treatment applied once (Table 4). A strong negative correlation was noted between total extract content and two applications of 200 mg/L GA3; vitamin C and 300 mg/L GA3 dose; and two and three times applied sprays as well as between total acidity and dose 200 mg/L GA3. A negative correlation was observed between vitamin C content and application at 200 mg/L GA3, and between total acidity and treatment at 300 mg/L GA3 dose.
The Pearson's coefficient for the parameters determining the antioxidant activity of fruit showed a strong correlation between total phenolic acids and a dose of 100 mg/L GA3 and a treatment rate of 300 mg/L GA3, flavonoid level and number of applications, as well as between tannin content and a single GA3 treatment (Table 5). A strong negative correlation existed between total phenolic acids and a dose of 200 mg/L GA3 and the treatment applied three times, and between the DPPH parameter and 200 mg/L GA3 application, and between flavonoid content and single GA3 treatment and applied two times. The correlations were established between the anthocyanin level and a single GA3 application, the DPPH parameter and 300 mg/L GA3 application rate, the flavonoid content and 300 mg/L GA3 dose, and the tannin level and a single GA3 treatment. A negative correlation was found between total phenolic acids and a single application, anthocyanin content and a 300 mg/L GA3 application rate, and between tannin content and treatment with 200 and 300 mg/L GA3 dose.
The dendrogram shows two separate clusters, in that one object is a clear outlier (Fig. 1). This is the control, which differs distinctively from the other combinations. The latter display high similarity at the group level. These are: 100 and 300 mg/L GA3 dose (group 1) and 200 mg/L GA3 dose (group 2). Both clusters are quite similar to each other.
The dendrogram (Fig. 2) made it possible to define the similarity in the effect of the number of gibberellic acid applications on a level of antioxidant activity in the fruit. The results serve to define two separate clusters exhibiting some similarities. It was shown that a single gibberellic acid treatment and GA3 applied twice affect antioxidant activity very similarly, whereas the similarity with the control combination is noted at three times the treatment.
The PC sum (PC1 and PC2 components) of the total variation in traits for the GA3 doses reached 81.1% - 2.47% for PC1 and 28.63% for PC2 (Fig. 3a). PC1 contained the level of flavonoids, vitamin C, anthocyanins and phenolic acids, whereas PC2 contained the potential of sugars, tannins, acidity and DPPH. The control displayed a high content of extract and flavonoids, along with a low level of anthocyanins and phenolic acids. The PCA analysis showed differences between the application rates, i.e. fruit subjected to 100 mg/L GA3 treatment had a high vitamin C content. The application rates of 200 and 300 mg/L GA3 contributed to high tannin levels and, what was particularly noteworthy, 300 mg also increased acidity.
The PC sum of total variation for the analysed number of GA3 applications was 84% (60.73% and 23.7% respectively). PC1 indicates the phenolic acid level, while PC2 shows the other secondary metabolites, as well as the level of vitamin C and extract. The control fruit were characterised by a high content of flavonoids, vitamin C and extract, while the remaining components were present at low levels. The hormonisation spray, irrespective of the number of applications, had a beneficial effect on the level of phenolic acids, anthocyanins, DPPH and fruit acidity.
CONCLUSIONS
This study assessed the chosen biologically active compounds of 'Einset Seedless' grapes subjected to a gibberellic acid dose and a varied number of applications. The hormonisation treatment had an adverse effect on the content of extract, vitamin C and flavonoids in grapes. However, this treatment did not have significant influence on the anthocyanin level in the fruit of the grapevine variety under study. Antioxidant activity, as determined by the DPPH assay, depended on the dose and the number of treatments, and the analysed parameter was shown to decrease significantly with an increasing number of applications. Gibberellic acid at 100 and 300 mg/L application rates significantly increased the DPPH level compared to the control and the 200 mg/L dose. The single GA3 treatment and the treatment applied three times, and application rates of 100 and 200 mg/L ,were shown to have a significant influence on phenolic acid content. The level of tannins after a single GA3 treatment and a 300 mg/L dose increased significantly.
LITERATURE CITED
Al-Atrushy, S.M.M., 2016. Effect of GA3 dose and frequency on yield and quality of 'Zark' grape. Jordan J. Agric. Sci. 12(4), 1183-1191. [ Links ]
Artés-Hernández, F., Tomás-Barberán F.A. & Artés, F., 2006. Modified atmosphere packaging preserves quality of SO2-free 'Superior seedless' table grapes. Postharvest Biol. Technol. 39, 146-154. [ Links ]
Arts, I. & Hollman, P., 2005. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 81, 317S-325S. [ Links ]
Awad, M.A. & Al-Qurashi, A.D., 2012. Gibberellic acid spray and bunch bagging increase bunch weight and improve fruit quality of 'Barhee' date palm cultivar under hot arid conditions. Sci. Hort. 138, 96-100. [ Links ]
Cai, Y.Z., Sun, M. & Corke, H., 2003. Antioxidant activity of betalains from plants of the amaranthaceae. J. Agric. Food Chem. 51(8), 2288-2294. [ Links ]
Crozier, A., Del Rio, D. & Clifford, M.N., 2010. Bioavailability of dietary flavonoids and phenolic compounds. Mol. Aspects Med. 31, 446-467. [ Links ]
Dimovska, V., Petropulos, V.I., Salamovska, A. & Ilieva, F., 2014. Flame Seedless grape variety (Vitis vinifera L.) and different concentration of gibberellic acid (GA3). Bulg. J. Agric. Sci. 20, 137-142. [ Links ]
Dohadwala, M. & Vita, J.A., 2009. Grapes and cardiovascular disease. J. Nutr. 139, 1788S-1793S. [ Links ]
Doshi, P., Adsule, P., Banerjee, K. & Oulkar, D., 2015. Phenolic compounds, antioxidant activity and insulin tropic effect of extracts prepared from grape (Vitis vinifera L.) by products. J. Food Sci. Technol. 52, 181-190. [ Links ]
Erdman, J., Balentine, D., Arab, L., Beecher, G., Dwyer, J. T., Folts, J., Harnly, J., Hollman, P., Keen, CL., Mazza, G., Messina, M., Scalbert, A., Vita, J., Williamson, G. & Burrowes J., 2007. Flavonoids and heart health. Proceedings of the ILSI North America Flavonoids Workshop. J. Nutr. 137, 718S-737S. [ Links ]
Es-Safi, N., Ghidouche, S. & Ducrot, P.H., 2007. Flavonoids: Hemisynthesis, reactivity, characterization and free radical scavenging activity. Molecules 12, 2228-2258. [ Links ]
European Pharmacopoeia, 2008 (6th ed). Council of Europe, Strasbourg. [ Links ]
Frankel, E.N., 1999. Natural phenolic antioxidants and their impact on health. In: Packer, L. (ed). Antioxidant food supplements in human health. Academic Press, London. pp. 385 - 392. [ Links ]
Gougoulias, N. & Masheva, L., 2010. Effect of gibberellic acid (GA3) on polyphenols content and antioxidative activity of some table grape varieties of Vitis vinifera L. Oxid. Commun. 33(3), 652-660. [ Links ]
Harrell, D.C. & Williams, L.E., 1987. Net CO2 assimilation rate of grapevine leaves in response to trunk girdling and gibberellic acid application. Plant Physiol. 83, 457-459. [ Links ]
Jiang, H., Ji, B.P, Liang, J.F., Zhou, F., Yang, Z.W. & Zhang, G.Z., 2006. Changes of contents and antioxidant activities of polyphenols during fruit development of four apple cultivars. Eur. Food Res. Technol. 223, 743748. [ Links ]
Kaplan, M., 2011. Effect of growth regulator application technique on quality of grapevine 'Einset Seedless' variety (In Polish). Acta Agrobot. 64(4), 189-196. [ Links ]
Kaplan, M., Najda, A., Baryla, P. & Klimek, K., 2017. Effect of gibberellic acid dose and number of treatments on yield components of "Einset Seedless" grapevine cultivar. Hort. Sci. 44(4), 195-200. [ Links ]
Khan, M., Hafeez-ur-Rahman, A., Ahmed, M., Abbas, G. & Ahmed, N., 2009. Effect of gibberellic acid on growth and fruit yield of grape cultivar 'flame seedless'. Int. J. Biol. Biotech. 6(4), 265-268. [ Links ]
Kim, D.O, Jeong, S.W. & Lee, C.Y., 2003. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 81, 321-326. [ Links ]
Kok, D., 2017. Grape growth, anthocyanin and phenolic compounds content of early ripening Cv. Cardinal table grape (V. vinifera L.) as affected by various doses of foliar biostimulant applications with gibberellic acid. ErwerbObstbau 58, 1-7. [ Links ]
Laszlo, J.C. & Saayman, D., 1990. Optimum harvesting stage for Sultanina as table grape. Decid. Fruit Grow. 40(3), 101-105. [ Links ]
Leifert, W.R. & Abeywardena, M.Y., 2008. Cardio protective actions of grape polyphenols. Nutr. Res. 28(11), 729-737. [ Links ]
Liang, Z., Sang, M., Fan, P., Wu, B., Wang, L., Duan, W. & Li, S., 2011. Changes of polyphenols, sugars, and organic acid in 5 Vitis genotypes during berry ripening. J. Food Sci. 76(9), 1231-1238. [ Links ]
Matthew, M.A. & Nuzzo, V., 2007. Berry size and yield paradigms on grapes and wines quality. Acta Hortic. 754, 423-436. [ Links ]
Mattivi, F., Zulian, C., Nicolini, G. & Valenti L., 2002. Wine, biodiversity, technology, and antioxidants. Ann. N.Y. Acad. Sci. 957, 37-56. [ Links ]
Mazza, G., 1995. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 35(4), 341-71. [ Links ]
Milkowska, K. & Strzelecka, H., 1995. Flos Hibisci - metody identyfikacji i ocena surowca. Herba Polonica 41(1), 11-16. [ Links ]
Montealegre, R.R., Peces, R.R., Vozmediano, J.L.C., Gascueña, J.M. & Romero, E.G., 2006. Phenolic compounds in skins and seeds of ten grape Vitis vinifera varieties grown in a warm climate. J. Food Compos. Anal. 19, 687-693. [ Links ]
Muñoz-Espada, A.C., Wood, K.V., Bordelon, B. & Watkins, B.A., 2004. Anthocyanin quantification and radical scavenging capacity of Concord, Norton, and Marechal Foch grapes and wines. J, Agric. Food Chem. 52(22), 6779-6786. [ Links ]
Najda, A., 2017. Zmiennosc ontogenetyczna miety (Mentha species) czynnikiem warunkuj^cym zawartosc skladników bioaktywnych w surowcu. In Polish. University of Life Sciences, Lublin, 1, 178. [ Links ]
Nampila, R., Bing-Shiun, Ch., Ching-Cheng, Ch. & YauShiang, Y., 2010. Effect of GA3 and CPPU on berry size of seedless grapes. Hort. NCHU 35(3), 53-64. [ Links ]
Orak, H.H., 2007. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape varieties and their correlation. Sci. Hort. 111, 235-241. [ Links ]
Peña-Neira, A., Dueñas, M., Duarte, A., Hernández, T., Estrella, I. & Loyola, E., 2004. Effects of ripening stages and of plant vegetative rigor on the phenolic composition of grapes (Vitis vinifera L.) cv. Cabernet Sauvignon in the Maipo Valley (Chile). Vitis 43(2), 51-57. [ Links ]
Pezzuto, J., 2008. Grapes and human health: A perspective. J. Agric. Food Chem. 56(16): 6777-6784. [ Links ]
Polish Norm PS, PN-90 A-75101/04 - Fruit and vegetable preserves. Sample preparation and physicochemical methods of examination. Determination of total acidity (in Polish). [ Links ]
Polish Pharmacopoeia, 2002. VI, Wyd. PTFarm, Warszawa. [ Links ]
Polish Pharmacopoeia, 2011. IX, Wyd. PTFarm, Warszawa. [ Links ]
Polish Pharmacopoeia, 2014. X, Wyd. PTFarm, Warszawa. [ Links ]
Rachna & Singh, S., 2013. Effect of gibberellic acid on periodical changes in bio-chemical composition of ber cv. Umran. HortFlora Res. Spectrum 2(1): 25-29. [ Links ]
Reisch, B.I., Remaily, G.W., Pool, R.M. & Watson, J.P., 1986. 'Einset Seedless' grape. HortScience 21, 155-156. [ Links ]
Scalbert, A., Manach, C., Morand, C., Remesy, C. & Jimenez, L., 2005. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 45, 287- 306. [ Links ]
Stevenson, D.E. & Hurst, R.D., 2007. Polyphenolic phytochemicals: Just antioxidants or much more? Cell. Mol. Life Sci. 64, 2900-2916. [ Links ]
Tian, S., Wang, Y., Du, G. & Li, Y., 2011. Changes in contents and antioxidant activity of phenolic compounds during gibberellin-induced development in Vitis vinifera L. 'Muscat'. Acta Physiol. Plant. 33, 24672475. [ Links ]
Tomás-Barberán, F.A. & Espin, J., 2001. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J. Sci. Food Agric. 81, 853-876. [ Links ]
Topalovic, A. & Mikulic-Petkovsek, M., 2010. Changes in sugars, organic acids and phenolics of grape berries of cultivar Cardinal during ripening. J. Food Agric. Environ. 8(3), 223-227. [ Links ]
Vislocky, L.M. & Fernandez, M.L., 2010. Biomedical effects of grape products. Nutr. Rev. 68(11), 656-670. [ Links ]
Weston, L.A., 2005. Grape and wine tannins and phenolics, their roles in flavor, quality and human health. Proc. 29th Annual New York Wine Industry Workshop, Month? 2005, New York, USA. pp. 6 - 15. [ Links ]
Yang, J., Martinson, T.E. & Liu, R.H., 2009. Phytochemical profiles and antioxidant activities of wine grape. Food Chem. 116, 332-339. [ Links ]
Xia, E., Deng, G.F., Guo, Y.J. & Li, H.B., 2010. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 11, 622-646. [ Links ]
Submitted for publication: June 2018
Accepted for publication: August 2018
Acknowledgments: The objective of the present study was to determine the parameters affecting the content of biologically active compounds and antioxidant activity in the 'Einset Seedless' grape variety subject to the dose and number of gibberellic acid applications.
* Corresponding author: E-mail address: magdalena.kaplan@up.lublin.pl [Tel.: 48 81 5247158]














