Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Enology and Viticulture
versão On-line ISSN 2224-7904
versão impressa ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.40 no.1 Stellenbosch 2019
http://dx.doi.org/10.21548/40-1-2932
ORIGINAL RESEARCH
Free radical-scavenging activity and anthocyanin profiles of Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China
B. JiangI; Z.-W. ZhangII, *
IWeinan Vocational & Technical College, Weinan 714026, China
IICollege of Oenology, Northwest A&F University, Yangling 712100, China
ABSTRACT
The present study focused on the free radical-scavenging activity and anthocyanin profiles of Cabernet Sauvignon and Merlot wines produced from four different regions in China. The anthocyanin profiles in all wine samples were analysed by HPLC-MS/MS, while the free radical-scavenging activity was estimated by the DPPH assay. The results show that the contents of phenolic subclasses and the levels of antioxidant activity in all wine samples varied greatly among cultivars and environmental factors of vine growth, and these values were the most prominent in Yuquanling regional wines. As the main components in anthocyanins, the percentages of malvidin-3-O-glucoside and its derivatives showed differences within grape cultivars in the different regional wines; these monomeric anthocyanins (not present simultaneously in the four regional wines studied within grape cultivars) had concentrations below 10 mg Mv/L. The significant correlation was obtained between DPPH-scavenging ability and the total phenolic, flavonoid and anthocyanin content. It can be concluded that this information could be used as a biochemical marker for the authenticity of the single-cultivar red wines that were produced from the four regions above.
Keywords: Wine, free radical-scavenging activity, anthocyanins profiles, HPLC-MS/MS
INTRODUCTION
As a class of phenolic compounds originating from wine grapes, anthocyanins are responsible for grape and wine colour (Tang et al., 2017). The colour of wine is one of the most important sensory properties. Therefore, it has long been recognised that the colour intensity of young red wines to some extent correlates positively with the overall wine quality. Anthocyanins accumulate in the grape skin after véraison via the phenylpropanoid biosynthetic pathway (Downey et al., 2006), and those of wine are usually extracted from the skins of grapes during crushing, pressing and fermentation.
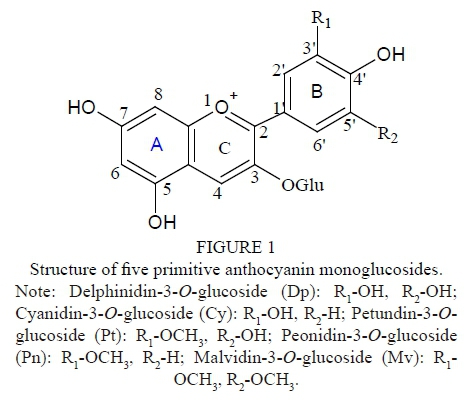
Red wines made from Vitis vinifera L. grapes normally contain five main monomeric anthocyanins, namely Dp, Cy, Pt, Pn and Mv (Fig. 1). Moreover, the main anthocyanins in the skins of red wine grape varieties are five primitive monoglucosides and their acetylated or coumaroylated derivatives, which are formed by combination with coumaric or caffeic acid (Monages et al., 2003). Most of anthocyanins are Mv and its derivatives. In addition, the anthocyanins can also be classified according to either the number of hydroxyl groups (3'-substituted anthocyanins and 3',5'-substituted anthocyanins) or methoxyl group on the B-ring, or the type of acylation (aliphatic or aromatic) (Fig. 1) (Gómez-Alonso et al., 2007). The colour characteristics of anthocyanins usually vary with these substituents. Besides genotype, management factors, winemaking and ageing conditions, and so on, it is known that climate and soil conditions are considered to be the main environmental factors determining wine characteristics and quality, resulting in different wine types. Like most other phenolic compounds, the composition and concentration of anthocyanins in red wine grapes and their corresponding wines vary with the climate and soil conditions of the vineyard. Significant differences in anthocyanin concentration between shaded clusters and sun-exposed clusters of Pinot Noir grape were found by Cortell and Kennedy (2006). Cheng et al. (2014) suggest that Cabernet Sauvignon berries from soils with less water and organic matter contain higher levels of 3',5'-substituted, O-methylated and acylated anthocyanins, which represent a positive characteristic conferring more stable pigmentation to the corresponding wine. In addition, it has been indicated that the total anthocyanins of the Malbec variety (Vitis vinifera L.) increased dramatically with higher elevations in Mendoza, Argentina (Bajda, 2007), while the skins were five times thicker at 1 500 m than at 850 m. The content of total phenolic compounds, total flavonoids and total anthocyanins increased with altitude in Cabernet Sauvignon and Merlot wines (Jin et al., 2017).
Due to large phenolic compounds in wine, wine is considered to possess the ability to scavenge excess radicals (Liu et al., 2018). Positive correlations between total phenolics and antioxidant capacity have been reported (Gómes-Plaza et al., 2006; Orak, 2007; Jin et al., 2017). Many studies indicate that the consumption of small amounts of red wine on a regular basis reduces the risk of cardiovascular disease, atherosclerosis and certain types of cancers; this benefit is attributed to the antioxidant properties of the polyphenolic compounds (Mazza et al., 1999; Yilmaz & Toledo, 2004). Still, it is very important to determine which group of phenolic compounds is most influential in these properties of wine. Between in vitro and in vivo research trials, anthocyanins have demonstrated a noticeable ability to reduce cancer cell proliferation and to inhibit tumour formation (Hou, 2003; Lila, 2004). Anthocyanins (Kähkönen & Heinonen, 2003) and extracts containing anthocyanins (Shirahigue et al., 2010) were also reported to show radical-scavenging activity and the ability to prevent lipid peroxidation.
Unlike most other wine-producing countries, China's wine-producing areas are very scattered, with a distance of over 2 000 kilometres from west to east (Li et al., 2011). Moreover, these wine grape-growing regions of China display unique ecological conditions. All the different climate and soil characteristics give them the capability to produce various types of wines. Cabernet Sauvignon and Merlot are the world's most well-known Vitis vinifera red grape cultivars. Due to their premium quality and stronger adaptability to arid and barren lands than other grape varieties, both of them have spread across the old world and new world wine countries, from France and Italy to New Zealand, Australia and South Africa. Cabernet Sauvignon and Merlot are still the most popular varieties in each of China's wine grape-growing regions (Wang et al., 2018). However, the differences in the anthocyanin profiles of each of the monovarietal wines from four wine grape-growing regions in China remain unclear.
The aim of this work was therefore to (1) investigate and analyse the differences in the anthocyanin compounds from four regional Cabernet Sauvignon and Merlot wines, and to elucidate these wines' characteristics using their anthocyanin profiles, defined as the percentage content of each anthocyanin, and (2) evaluate the free radical-scavenging activity of four regional Cabernet Sauvignon and Merlot wines, and establish the relationship between four subclasses of phenolics and antioxidant activity in wine by correlation analysis. The present results will provide an essential basis for further fingerprinting research on these regional wines. In addition, these results also will be helpful in discovering some valuable information that could result in the production of high-quality wine in China.
MATERIALS AND METHODS
Chemicals and reagents
Folin-Ciocalteu's phenol reagent, gallic acid, catechin, 2,2'-diphenyl-1-picrylhydrazyl (DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), /»-dimethylaminocinnamaldehyde (DMACA) and malvidin-3-O-glucoside were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol (HPLC grade) was obtained from Fisher Co. (Fairlawn, NJ, USA). All other chemicals and solvents were analytical reagent grade and were purchased in China.
Sample collection and vinification
The present study was conducted using Cabernet Sauvignon and Merlot vines grafted onto SO4 rootstock from four regions that are harvested from west to east: Yuquanying of Ningxia (hereafter NXYQY) is a cool and semi-arid area in Western China; Xiangning of Shanxi (hereafter SXXN) belongs to the arid area in Northern China; Shacheng of Hebei (hereafter HBSC) is a warm and semi-arid area in Eastern China; Changli of Hebei (hereafter HBCL) is a wetter area in Eastern China. The climate data and soil conditions of the four experimental regions during the growing season of wine grapes are listed in Table 1. Vines were grown for eight years, drip irrigated, Dulong trained, with a vine spacing of 3.0 χ 1.2 m. Rows were east-west oriented. The vineyard was managed in accordance with the standard agronomic practices in the four regions.
All grape berries were harvested manually at optimum technological maturity for these vineyards in September of the year. Pre-fermentation treatments and winemaking were performed as described by Li (2002). Briefly, grapes were crushed in an experimental destemmer-crusher and then transferred to stainless steel containers. Thirty litres of each wine were produced in three replicates. Sulphur dioxide (1.5 g) and pectinase (Lallzyme Ex) (0.9 g) were added to the musts and the contents were mixed by hand. After maceration of the musts for 24 h, they were inoculated with Saccharomyces cerevisiae EC-1118 (Lallemand, Danstar Ferment AG, Switzerland) (6.0 g) according to commercial specifications. Alcoholic fermentation was carried out at 20°C to 25°C to dryness (reducing sugar < 4 g/L), which took place over a period of six to eight days, and density controls were maintained during this period. At the end of alcoholic fermentation, the wines were separated from the pomace, after which SO2 (1.5 g) was added. After fermentation, the wine samples were bottled and stored at 10°C to 15°C prior to analysis. All the samples were five months old for the analysis. Residual sugar, total acidity, pH and ethanol were analysed (Office International de la Vigne et du Vin [O.I.V.], 2018) (Table 2).
Determination of phenolic compounds
The total phenol (TP) content was determined by the Folin-Ciocalteu colorimetric method (Rapisarda et al., 1999). The TP concentration was expressed as milligrams of gallic acid equivalents per litre basis (mg GAE/L). The total flavonoid (TFO) and total flavanol (TFA) contents were measured separately according to a colorimetric assay (Kim et al., 2003) and the DMACA method (Li et al., 1996; Morrough et al, 1996), both of which were expressed as milligrams of catechin equivalents per litre basis (mg CTE/L). The total anthocyanin (TA) content was determined by the pH-differential method (Giusti & Worsltad, 2001) using two buffer systems - potassium chloride buffer, pH 1.0 (0.025 M) and sodium acetate buffer, pH 4.5 (0.4 M). The contents were calculated as malvidin-3-O-glucoside and expressed as milligrams of malvidin-3-O-glucoside (Mv) equivalents per litre basis (mg Mv/L).
Free radical-scavenging activity
The ability to scavenge DPPH free radicals was determined. Scavenging activity was based on the slightly modified method (Brand-Williams et al., 1995). Briefly, 0.1 mL of sample solution (with appropriate dilution as needed) was added to 3.9 mL of a 60 μM solution of DPPH in methanol. A control sample, containing the same volume of solvent in place of extract, was used to measure the maximum DPPH absorbance. After the reaction was allowed to take place in the dark for 30 min, the absorbance at 515 nm was recorded to determine the concentration of remaining DPPH. Results were expressed as Trolox equivalent antioxidant capacity ^ TE/L).
Quantitative analyses by HPLC-MS/MS
Qualitative and quantitative analyses of anthocyanin compounds were performed at the Centre for Viticulture and Oenology, College of Food Science & Engineering, China Agricultural University. The wine samples obtained above were filtered through 0.45 μπι filters (cellulose acetate and nitrocellulose, CAN) and the resulting filtrates were directly used for qualitative and quantitative analyses. For the anthocyanin compounds, an Agilent 1100 series LC-MSD trap VL, equipped with a DAD detector and reversed phase column (Kromasil C18, 250 χ 4.6, 5 μιη), was used. The solvents were: (A) aqueous 2% formic acid, and (B) acetonitrile containing 2% formic acid. The gradient was from 6% to 10% B for 4 min, from 10% to 25% B for 8 min, isocratic 25% B for 1 min, from 25% to 40% for 7 min, from 40% to 60% for 15 min, from 60% to 100% for 5 min, and from 100% to 6% for 5 min, with a flow rate of 1.0 mL/min. Injection volumes of 30 μL were used, and the detection wavelength was 525 nm. MS conditions were as follows: electrospray ionisation (ESI) interface, positive ion model, 35 psi nebuliser pressure, 10 mL/min dry gas flow rate, 350°C dry gas temperature, and scans at m/z 100 to 1 000. The anthocyanin compounds were identified by their order of elution and retention time with respect to malvidin-3-0-glucoside, and the weight of molecular ion and the fragment ion compared with standards and references (He et al., 2010).
Quantification analyses of anthocyanin
All the monomeric anthocyanins were quantified using malvidin-3-0-glucoside as standard. Gradients with eight concentrations of mixture standard (malvidin-3-0-glucoside) were set with three replications, while one group of standard curve of the concentration was made based on the average area of the malvidin-3-O-glucoside compound, and all the monomeric anthocyanins were qualified by the external standard method.
Statistical analysis
Data were expressed as the mean ± SD. Statistical analysis of the data was performed using SPSS 17.0 for Windows, with three replications of the same sample. Significant differences between wines from the four different regions were determined by Tukey's test (p < 0.05).
RESULTS AND DISCUSSION
Total content of phenolic subclasses in wine samples
The content of total phenols (TP), flavonoids (TFO), flavanols (TFA) and anthocyanins (TA) was determined for all Cabernet Sauvignon and Merlot wines from the above four regions, as listed in Table 2. The phenol concentrations vary widely in the wine samples tested.
The Cabernet Sauvignon and Merlot wines with the highest amount of TP were both from the NXYQY region, whereas both monovarietal wine samples tested from the SXXN region displayed the lowest values. For the Cabernet Sauvignon wines, the TP content in the NXYQY region was about 2.0 to 2.4 times those in the HBCL and SXXN regions, being approximately 1.2 times that in the HBSC region. For the Merlot wines, the content of TP in the NXYQY region was 1.7 to 1.9 times those in the HBCL and SXXN regions. For both monovarietal wines, the TP content in the HBCL region was not significantly (p < 0.05) higher than that in the SXXN region.
The TFO contents of Cabernet Sauvignon and Merlot wines in the NXYQY region were the highest among the four regions, followed by the HBSC region, and the HBCL and SXXN regions. Unlike the Cabernet Sauvignon wines, the Merlot wines from the HBCL and SXXN regions contained a non-significantly (p < 0.05) lower content of TFO. The content of TFA varied from 277.7 to 666.4 mg CTE/L for the Cabernet Sauvignon wines, and from 272.8 to 497.4 mg GAE/L for the Merlot wines. The TFA content of the Cabernet Sauvignon wines in the NXYQY and HBSC regions was almost double that in the SXXN region, and its content in the NXYQY regional Merlot wines was more than 70% that in the HBCL and SXXN regions. In addition, for the two monovarietal wines, there was a non-significant (p < 0.05) difference in TFA content between the HBCL and SXXN regions.
Anthocyanins are an important quality parameter for red grapes because of their significance in determining the colour of the resulting wines. In the present study, the content of TA varied from 261.5 to 400.3 mg Mv/L, with the average value being 330.3 mg Mv/L for the Cabernet Sauvignon wines, and from 157.5 to 350.3 mg Mv/L, with an average value of 246.0 mg Mv/L for the Merlot wines. The TA contents of the two monovarietal wines in the NXYQY region were the highest, and the contents in the HBCL region were the lowest. Furthermore, for the Cabernet Sauvignon wines there were non-significant (p < 0.05) differences in TA content between the NXYQY and HBSC regions, as well as between the HBCL and SXXN regions. A high concentration of anthocyanins in wine is essential for good colour, resulting in high-quality wine.
Monomeric anthocyanins are the most labile phenolic compounds in wine, typically decreasing at a rate of about 50% per year (Munoz-Espada et al., 2004). Anthocyanin extraction and stability are affected by winery production practices. Monomeric anthocyanins usually decline during maceration and fermentation, but the process may continue throughout the life of a wine. The characteristics of wine, such as SO2, pH and acetaldehyde, can influence these processes and anthocyanin interactions with other phenolic compounds. The stability of anthocyanins can be enhanced through so-called co-pigmentation. Co-pigmentation therefore plays a crucial role in wine ageing and maturation. Acylated anthocyanins containing two or more aromatic acyl groups may affect the colour through intramolecular co-pigmentation (Vivar-Quintana et al., 2002).
In order to identify the influence of ecological condition on the phenolic contents of wine samples from the four wine grape-growing regions, all the raw materials (grape berries) and wines were kept under the same conditions, including the same cultivation management and vintage, and the same winemaking techniques and ageing conditions. The results further confirm a variation in phenolic content among the wine samples tested. As is well known, the amounts of phenolic materials vary considerably in different wine grape-growing regions, depending on the grape variety and environmental factors affecting vine growth (Villano et al., 2006; Shi et al., 2016; Martínez-Gil et al., 2018). The range of data obtained is in agreement with the available literature (Li et al., 2009, 2011).
HPLC analysis of anthocyanin compounds in wine samples
A total of 37 anthocyanins were identified in both the Cabernet Sauvignon and Merlot wines, including five primitive anthocyanins and 32 derivatives (Table 3). The anthocyanin profile of grapes and wine, determined by the relative proportions of the different anthocyanins, are characteristic for each grape variety and corresponding wine. Moreover, the concentrations of different compounds varied significantly within grape cultivars according to environmental conditions (Williams & Graver, 2004; Villano et al., 2006).
The details of these anthocyanins, listed in Table 3, exhibited discrepancies in the monomeric anthocyanin composition and concentration of the four regional Cabernet Sauvignon and Merlot wines. In the Vitisvinifera L. red cultivars there are only Dp, Cy, Pt, Pn and Mv, along with the corresponding acetyl, p-coumaryl, caffeoyl, and ferulyl derivatives. Cyanidin is the precursor pigment of the other anthocyanidins, and it can be transformed into peonidin by the action of a 3'-0-methyltransferase, or into delphinidin by the action of a 3'-hydroxylase. A 3'-5'-0-methyltransferase transforms delphinidin into petunidin, and petunidin into malvidin (Vivar-Quintana et al., 2002). For the five primitive anthocyanins, the four different regional wines had the same composition within grape cultivars; these discrepancies of composition were only found in 14 anthocyanin derivatives, whose concentrations were basically below 10 mg Mv/L (except for Dp-3-0-(cis-6-0-coumaryl)-glucoside and Mv-3-0-(6-0-acetyl)-glucoside-pyruvic acid). Furthermore, the total content of monomeric anthocyanins was predominant, ranging from 197.1 to 339.6 mg Mv/L and averaging 251.8 mg Mv/L for the Cabernet Sauvignon wines, and from 129.2 to 213.0 mg Mv/L, averaging 182.4 mg Mv/L, for the Merlot wines. Next were the total content of acetylated anthocyanins, which ranged from 72.7 to 170.7 mg Mv/L and averaged 110.8 mg Mv/L for the Cabernet Sauvignon wines, and from 63.1 to 80.8 mg Mv/L, averaging 71.7 mg Mv/L, for the Merlot wines. The third class was the total content of coumarylated anthocyanins, which ranged from 29.9 to 75.1 mg Mv/L and averaged 49.2 mg Mv/L for the Cabernet Sauvignon wines, and from 32.6 to 76.7 mg Mv/L, averaging 48.5 mg Mv/L, for the Merlot wines. Blaga and Aleksandra (2010) detected 13 anthocyanins in Cabernet Sauvignon wines, with the malvidin derivatives that dominated, followed by peonidin-type anthocyanins. In the present study, we also found that malvidin derivatives dominated in all the wine samples, contributing beyond 57.65% (including Mv) to the total anthocyanins in the wines. Heredia et al. (1998) showed that the number of methoxyl and hydroxyl groups can affect the intensity and type of colour of anthocyanins: the more methoxyl groups there are, the less the redness; the more hydroxyl groups there are, the more bluish the shade. The contribution of each anthocyanin to the final anthocyanin profile was calculated based on the monoglucoside forms and expressed as a percentage (in parenthesis) in Table 3.
The distribution of the most common anthocyanins in the investigated Cabernet Sauvignon and Merlot wines also depends on the climatic factors of the region and on soil types: Dp was determined from 1.16 to 9.89% (mean 6.27%) in the Cabernet Sauvignon wines and from 3.52 to 9.01% (mean 5.82%) in the Merlot wines; Cy varied from 0.48 to 1.21% (mean 0.82%) in the Cabernet Sauvignon wines and from 0.33 to 0.92% (mean 0.61% ) in the Merlot wines; Pt was determined from 4.52 to 8.49% (mean 6.61%) in the Cabernet Sauvignon wines and from 4.82 to 9.39% (mean 6.97% ) for the Merlot wines; Pn varied from 2.6 to 5.97% (mean 3.81%) in the Cabernet Sauvignon wines and from 2.25 to 4.65% (mean 3.28% ) in the Merlot wines. The most prominent anthocyanin was Mv, which accounted for 40.73% (from 37.12 to 44.34%) and 37.94% (from 31.75 to 44.82%) of the total content in the Cabernet Sauvignon and Merlot wines respectively. This is in agreement with the findings of previous research (Bouzas-Cid et al., 2016; González-Neve et al., 2016; Jin et al., 2017).
The findings show that Mv was the most abundant anthocyanin in all the investigated wines. By contrast, Cy was the least abundant anthocyanin pigment, as has been demonstrated for a number of other wines (Munoz-Espada et al., 2004; Kallithraka et al., 2007). A similar profile has been reported for Syrah (Gómez-Míguez et al., 2007), but the different patterns were also observed in Cabernet Franc and Pinot Noir wines from British Columbia (Mazza et al., 1999). Another point worth mentioning is that the order of abundance based on average value of distribution if each anthocyanin was as follows: Mv > Mv-3-0-(6-0-acetyl)-glucoside > Pt > Dp > Mv-3-0-(trans-6-0-coumaryl)-glucoside or Pn.
With regard to the acylation of the glycosyl group, the distribution of non-acylated anthocyanins, with a mean of 58.24% (from 50.75 to 65.81%) for the Cabernet Sauvignon wines and 54.61% (from 51.08 to 58.06%) for the Merlot wines made it the most abundant fraction in all wine samples. The mean amount of acetylated anthocyanins was 25.50% (from 25.31 to 30.17%) for the Cabernet Sauvignon wines and 25.15% (from 23.48 to 27.04%) for the Merlot wines, followed by coumarylated anthocyanins at 11.17% (from 9.19 to 12.31%) for the Cabernet Sauvignon wines and 14.25% (from 9.94 to 19.57%) for the Merlot wines. According to the research results, it is obvious that the content of anthocyanin constituents of the monovarietal wines from four different regions differed, which may be related to the thickness of the grape skin and the climate in which the grapes grow (Crippen & Morrison, 1986; Villano et al., 2006). Environmental factors therefore could affect the biosynthesis and accumulation of each anthocyanin in the skin (Liang et al., 2014). In our study, four regional Cabernet Sauvignon and Merlot wines were made from grapes harvested at different altitudes, with a higher altitude generally corresponding to more intense sunlight, lower temperature, greater temperature difference between day and night, and more extreme environmental conditions (Mateus et al., 2001), all of which are important factors affecting the production of anthocyanins.
Correlation between radical-scavenging activity and phenolic subclasses in wine samples
The antioxidant activity of the wine samples was estimated by the ability of the sample to scavenge the stable DPPH free radicals (Roussis et al., 2005). In the DPPH scavenging assay, antioxidants reacting with DPPH produce yellow α,α-diphenyl-ß-picrylhydrazine. The degree of discoloration indicates the radical-scavenging activity of the antioxidant (Prasad et al., 2010).
The free radical-scavenging activity of all the wine samples was determined by the DPPH methods, as shown in Table 2. All wines showed a higher DPPH radical-scavenging activity. For DPPH, the values of wines varied from 4 670.4 to 6 179.4 μM TE/L for the Cabernet Sauvignon wines, and 3 857.0 to 5 304.1 μM TE/L for the Merlot wines. The range of the data obtained is in agreement with a previous study (Jin et al., 2017). The values of DPPH free radicals decreased in the order XNYQY > HBSC > SXXN > HBCL in the Cabernet Sauvignon wines and XNYQY > HBSC > HBCL > SXXN in the Merlot wines, but the antioxidant activities of the two monovarietal wines in the SXXN and HBCL regions were non-significant (p < 0.05). The results of this investigation show that the higher the concentration of antioxidant, the lower the amount of remaining DPPH free radicals and the higher the free radical-scavenging activity. The percentage of DPPH radical-scavenging activity against the content of total anthocyanins, phenolics, flavonoids and flavanols of the wine samples is plotted in Fig. 2.
The different linear correlations between groups of phenolic compounds and DPPH free radical-scavenging ability of the tested wines were verified. The significant correlation was obtained between DPPH free radical-scavenging ability and TP content (Fig. 2 (A), r2 = 0.8360, p < 0.01), TFO (Fig. 2 (B), r2 = 0.8457, p < 0.01) and TA (Fig. 2 (D), r2 = 0.7254, p < 0.01). There also was a linear correlation, between DPPH scavenging ability and TFA (Fig. 2 (C), r2 = 0.5937, p < 0.01).
It is important to determine which group of phenolic compounds is more significant in the antioxidant activities of wines. Our results suggest that the amounts of TP, TFO and TA are very important for the antioxidant potency of the tested wines. However, TFA showed a weaker correlation, and these results are partially in agreement with previous reports in the literature (Minussi et al., 2003; Fernández-Pachón et al., 2006; Cimino et al., 2007). Furthermore, the difference may be dependent on the grape varieties of the tested wine samples and the complexity of the antioxidant reaction.
CONCLUSIONS
According to the results, it can be concluded that the amounts of phenolic materials and radical-scavenging activity varied considerably in the four regional Cabernet Sauvignon and Merlot wines, depending on the grape variety and the environmental factors affecting vine growth. The content of phenolic subclasses and radical-scavenging activities from the NXYQY regional wines were significantly higher than those of the other three regions tested, followed by the HBSC region. Meanwhile, a significant correlation was observed between radical-scavenging activity and phenolic subclasses (the TP, TFO and TA) for all the wine samples. The amount of phenolic subclasses is important for understanding the antioxidant potency of red wines. The mechanism by which phenolic subclasses are absorbed and metabolised in the body is currently unclear. On the other hand, their composition and concentration of anthocyanins detected in these regional Cabernet Sauvignon and Merlot wines showed discrepancies. The main components of anthocyanins, the percentage of malvidin-3-O-glucoside and its derivatives to the total content showed differences within grape cultivars from the different regional wines. Those monomeric anthocyanins that did not occur simultaneously in the four regional single-cultivar wines had concentrations below 10 mg Mv/L, except for Dp-3-O-(cis-6-O-coumaryl)-glucoside and Mv-3-O-(6-O-acetyl)-glucoside-pyruvic acid. It can be concluded that this information could be used as a biochemical marker for the authenticity of single-cultivar red wines that are produced in the above four regions (or terroirs).
LITERATURE CITED
Bajda, E., 2007. Lake County seeks to elevation high altitude wines [Online]: http://www.theelevationofwine.org [Accessed 20th December, 2009]. [ Links ]
Blaga, R. & Aleksandra, R., 2010. Free radical scavenging activity and anthocyanin profile of Cabernet Sauvignon wines from the Balkan region. Molecules 15, 4213-4226. [ Links ]
Bouzas-Cid, Y., Portu, J., Pérez-Álvarez, E.P., Gonzalo-Diago, A. & Garde-Cerdán, T., 2016. Effect of vegetal ground cover crops on wine anthocyanin content. Sci. Hortic. Amsterdam 57, 74-83. [ Links ]
Brand-Williams, W., Cuvelier, M.E. & Berset, C., 1995. Use of a free-radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 28, 25-30. [ Links ]
Cheng, G., He, Y.N., Yue, T.X., Wang, J. & Zhang, Z.W., 2014. Effects of climatic conditions and soil properties on Cabernet Sauvignon berry growth and anthocyanin profiles. Molecules 19, 13683-13703. [ Links ]
Cimino, F., Sulfaro, V., Trombetta, D., Saija, A. & Tomaino, A., 2007. Radical-scavenging capacity of several Italian red wines. Food Chem. 103, 75-81. [ Links ]
Cortell, J.M. & Kennedy, J.A., 2006. Effect of shading on accumulation of flavonoid compounds in (Vitis vinifera L.) Pinot Noir fruit and extraction in amodel system. J. Agric. Food Chem. 54, 8510-8520. [ Links ]
Crippen, D.D. & Morrison, J.C., 1986. The effects of sun exposveure on the compositional development of Cabernet Sauvignon berries. Amc J. Enol. Vitic. 37, 235-247. [ Links ]
Downey, M.O., Dokoozlian, N.K. & Krstic, M.P., 2006. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 57, 257-268. [ Links ]
Fernández-Pachón, M.S., Villano, D., Troncoso, A.M. & Garcia-Parrilla, M.C., 2006. Determination of the phenolic composition of sherry and table white wines by liquid chromatography and their relation with antioxidant activity. Anal. Chim. Acta 563, 101-108. [ Links ]
Gómez-Míguez, M., González-Miret, M.L. & Heredia, F.J., 2007. Evolution of colour and anthocyanin composition of Syrah wines elaborated with pre-fermentative cold maceration. J. Food Eng. 79, 271-278. [ Links ]
Giusti, M.M. & Wrolstad, R.E., 2001. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Current protocols in food analytical chemistry. Wiley, New York. pp. 577 - 590. [ Links ]
Gómes-Plaza, E., Minano, A. & Lopez-Roca, J.M., 2006. Comparison of chromatic properties, stability and antioxidant capacity of anthocyanin-based aqueous extracts from grape pomace obtained from different vinification methods. Food Chem. 97, 87-94. [ Links ]
Gómez-Alonso, S., Fernández-González, M., Mena, A., García Romero, E. & Martínez, J., 2007. Anthocyanin profile of Spanish Vitis vinifera L. red grape varieties in danger of extinction. Aust. J. Grape Wine Res. 13, 150156. [ Links ]
González-Neve, G., Favre, G., Piccardo, D. & Gil, G., 2016. Anthocyanin profile of young red wines of Tannat, Syrah and Merlot made using maceration enzymes and cold soak. Int. J. Food Sci. Technol. 51, 260-267. [ Links ]
He, J.J., Liu, Y.X., Pan, Q.H., Cui, X.Y. & Duan, C.Q., 2010. Different anthocyanin profiles of the skin and the pulp of Yan73 (Muscat Hamburg χ Alicante Bouschet) grape berries. Molecules 15, 1141-1153. [ Links ]
Heredia, F.J., Francia-Aricha, E., Rivas-Gonzalo, J.C., Vicario, I.M. & Santos-Buelga, C., 1998. Chromatic characterization of anthocyanins from red grapes. I. pH effect. Food Chem. 63, 491-498. [ Links ]
Hou, D.X., 2003. Potential mechanisms of cancer chemoprevention by anthocyanins. Curr. Mol. Med. 3, 149-159. [ Links ]
Jin, X.D., Wu, X. & Liu, X., 2017. Phenolic characteristics and antioxidant activity of Merlot and Cabernet Sauvignon wines increase with vineyard altitude in a high-altitude region. S. Afr. J. Enol. Vitic. 38, 132-143. [ Links ]
Kähkönen, M.P. & Heinonen, M., 2003. Antioxidant activity of anthocyanins and their aglycons. J. Agric. Food Chem. 51, 628-633. [ Links ]
Kallithraka, S., Mohdaly, A.A., Makris, D.P. & Kefalas, P., 2007. Determination of major anthocyanin pigment in Hellenic native grape varieties (Vitis vinifera sp.): Association with antiradical activity. J. Food Compos. Anal. 18, 375-386. [ Links ]
Kim, D.O., Chun, O.K., Kim, Y.J., Moon, H.Y. & Lee, C.Y., 2003. Quantification of polyphenolics and their antioxidant capacity in fresh plums. J. Agric. Food Chem. 51, 6509-6515. [ Links ]
Li, H., 2002. Viticulture and practices. In: College of Enology (ed.). Research progress of vine and wine. Shaanxi Agricultural Press, Xi'an. pp. 57 - 72. [ Links ]
Li, H., Wang, X.Y., Li, Y., Li, P.H. & Wang H., 2009. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 112, 454-460. [ Links ]
Li, Y.G., Tanner, G. & Larkin, P., 1996. The DMACA-HCl protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agr. 70, 89-101. [ Links ]
Li, Z., Pan, Q.H., Jin, Z.M., Mu, L. & Duan, C.Q., 2011. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 125, 77-83. [ Links ]
Liang, N.N., Zhu, B.Q., Han, S., Wang, J.H., Pan, Q.H., Reeves, M.J. & Duan, C.Q., 2014. Regional characteristics of anthocyanin and flavonol compounds from grapes of four Vitis vinifera varieties in five wine regions of China. Food Res. Int. 64, 264-274. [ Links ]
Lila, M.A., 2004. Anthocyanins and human health: An in vitro investigative approach. J Biomed. Biotechnol. 5, 306-313. [ Links ]
Liu, Y., Yan, J., Li, Q., Wang, J. & Shi, Y., 2018. Effect of training systems on accumulation of flavan-3-ols in Cabernet Sauvignon grape seeds at the North Foot of Mt. Tianshan. S. Afr. J. Enol. Vitic. 39, 35-46. [ Links ]
Martínez-Gil, A.M., Gutiérrez-Gamboa, G., Garde-Cerdán, T., Pérez-Álvarez, E.P. & Moreno-Simunovic, Y., 2018. Characterization of phenolic composition in Carignan noir grapes (Vitis vinifera L.) from six winegrowing sites in Maule Valley, Chile. J. Sci. Food Agric. 98, 274-282. [ Links ]
Mateus, N., Marques, S., Goncalves, A.C., Machado, J.M. & De Freitas, V., 2001. Proanthocyanidin composition of red Vitis vinifera varieties from the Douro valley during ripening: Influence of cultivation elevation. Am. J. Enol. Vitic. 52, 115-121. [ Links ]
Mazza, G., Fukumoto, L., Delaquis, P., Girard, B. & Ewert, B., 1999. Anthocyanins, phenolics, and color of Cabernet Franc, Merlot, and Pinot Noir wines from British Columbia. J. Agr. Food Chem. 47, 4009-4017. [ Links ]
Minussi, R.C., Rossi, M., Bologna, L., Cordi, L., Rotilio, D., Pastore, G.M. & Duran, N., 2003. Phenolic compounds and total antioxidant potential of commercial wines. Food Chem. 82, 409-416. [ Links ]
Monages, M., Nunez, V., Bartolome, B. & Gomez-Cordoves, C., 2003. Anthocyanin-derived pigments in Graciano, Tempranillo, and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 54, 163-169. [ Links ]
Morrough, M.I., Madigan, D. & Smyth, M.R., 1996. Semipreparative chromatographic procedure for the isolation of dimeric and trimeric proanthocyanidins from barley. J. Agric. Food Chem. 44, 1731-1735. [ Links ]
Munoz-Espada, A.C., Wood, K.V., Bordelon, B. & Watkins, B.A., 2004. Anthocyanin quantification and radical scavenging capacity of Concord, Norton and Marechal Foch grapes and wines. J. Agric. Food Chem. 52, 6779-6786. [ Links ]
Orak, H.H., 2007. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Sci. Hortic. 111, 235-241. [ Links ]
Office International de la Vigne et du Vin (O.I.V.), 2018. Recueil des Methods Internationales D'analyse des vins et des moüts (Vol. 2). Paris. O.I.V. [ Links ]
Prasad, K.N., Yang, B., Shi, J., Yu, C.Y., Zhao, M.M., Xue, S. & Jiang, Y.M., 2010. Enhanced antioxidant and antityrosinase activities of longan fruit pericarp by ultra-high-pressure-assisted extraction. J. Pharmaceut. Biomed. 51, 471-477. [ Links ]
Rapisarda, P., Tomaino, A., Cascio, L.R., Bonina, F., Pasquale, D.A. & Saija, A., 1999. Antioxidant effectiveness as influenced by phenolic content of fresh orange juices. J. Agric. Food Chem. 47, 4718-4723. [ Links ]
Roussis, I.G., Lambropoulos, I. & Soulti, K., 2005. Scavenging capacities of some wines and wine phenolic extracts. Food Technol. Biotech. 43, 351358. [ Links ]
Shi, P.B., Yue, T.X., Ai, L.L., Cheng, Y.F., Meng, J.F., Li, M.H. & Zhang, Z.W., 2016. Phenolic compound profiles in grape skins of Cabernet Sauvignon, Merlot, Syrah and Marselan cultivated in the Shacheng area (China). S. Afr. J. Enol. Vitic. 37, 132-138. [ Links ]
Shirahigue, L.D., Plata-Oviedo, M., Alencar, D.S.M., Darce, M., Souza, T.M.F., Oldoni, T.L.C. & Contreras-Castillo, C.J., 2010. Wine industry residue as antioxidant in cooked chicken meat. Int. J. Food Sci. Tech. 45, 863-870. [ Links ]
Tang, K., Liu, T., Han, Y., Xu, Y. & Li, J.M., 2017. The importance of monomeric anthocyanins in the definition of wine colour properties. S. Afr. J. Enol. Vitic. 38, 1-10. [ Links ]
Villano, D., Fernandez-Pachon, M.S., Troncoso, A.M. & Garcia-Parrilla, M.C., 2006. Influence of enological practices on the antioxidant activity of wines. Food Chem. 95, 394-404. [ Links ]
Vivar-Quintana, A.M., Santos-Buelga, C. & Rivas-Gonzalo, J.C., 2002. Anthocyanin-derived pigments and colour of red wines. Anal. Chim. Acta 458, 147-155. [ Links ]
Wang, X.Q., Xie, X.L., Chen, N., Wang, H. & Li, H., 2018. Study on current status and climatic characteristics of wine regions in China. Vitis 57, 9-16. [ Links ]
Williams, C.A. & Graver, R.J., 2004. Anthocyanins and other flavonoids. Nat. Prod. Rep. 21, 539-573. [ Links ]
Yilmaz, Y. & Toledo, R.T., 2004. Health aspects of functional grape seed constituents. Trends Food Sci. Tech. 15, 422-433. [ Links ]
Submitted for publication: May 2018
Accepted for publication: June 2018
Acknowledgments: The authors thank the earmarked fund of the Sci-Tech Research and Development Project of Shaanxi Province (Project 2015KJXX-98), the Sci-Tech Research and Development Project of Weinan City (Project 2015KYJ-4-3), and the Modern Agro-Industry Technology Research System of China (CARS-30-zp-09). We are also grateful to the providers of the grape samples used in the study.
* Corresponding author: E-mail address: treebaojiang@163.com














