Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904
Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.39 n.2 Stellenbosch 2018
http://dx.doi.org/10.21548/39-2-3156
RESEARCH NOTE
The Use of SO2 to Bind Acetaldehyde in Wine: Sensory Implications
C. Coetzee; A. Buica; W.J. du Toit*
Department of Viticulture and Oenology, Stellenbosch University, Private Bag X1, Matieland 7602, South Africa
ABSTRACT
It is thought that the formation of hydroxysulphonate when sulphur dioxide is added to wine containing free acetaldehyde negates the sensory impact of the latter compound, but little research has been done on this. Descriptive analyses were employed using a trained sensory panel to assess the sensory effect of sulphur dioxide and acetaldehyde as single compounds and in combination in model wine. The addition of acetaldehyde or sulphur dioxide as singular compounds led to large increase in especially the green apple or sulphur descriptors respectively. When these two compounds were added in equimolar concentrations, the green apple description decreased drastically; however, a prominent sulphur description was still noted. It thus seems that hydroxysulphonate also has a sulphur-like aroma. The hydroxysulphonate did not influence the perception of a prominent ester, isoamyl acetate, in model wine. A low pH influences the perception of sulphur when sulphur dioxide is present on its own, but this is not the case with hydroxysulphonate. The implications of these results for wine production are discussed further.
Keywords: Acetaldehyde, sulphur dioxide, oxidised aroma
INTRODUCTION
Acetaldehyde is considered the principal compound responsible for the particular aroma of wine subjected to oxidative ageing (Zea et al., 2015; Coetzee et al., 2016b). Its organoleptic influence and its ability to combine rapidly with SO2, even at low temperatures, makes this compound one of the critical oxidation markers during winemaking (Burroughs & Sparks, 1973). Acetaldehyde is produced by yeast during alcoholic fermentation (Margalit, 2012) and can also originate from the microbial activity of other microbes, such as lactic acid bacteria and acetic acid bacteria (Drysdale & Fleet, 1988; Liu & Pilone, 2000). However, some post-fermentation winemaking practices may enhance acetaldehyde formation and can lead to moderate to important increases in acetaldehyde (Jackowetz & Mira de Orduna, 2013).
The most important non-microbial production of acetaldehyde in wine is due to the oxidation of ethanol (Wildenradt & Singleton, 1974). This reaction is not direct, but rather via the coupled auto-oxidation of certain phenolic compounds. Free SO2 present in wine will prevent this oxidation by reacting with intermediate oxidation products, as well as with the formed acetaldehyde, resulting in a (supposedly) odourless sulphite combination known as hydroxysulphonate, which is stable in the acid medium (Waterhouse & Laurie, 2006). The reaction between acetaldehyde and bisulphite is rapid and, at a pH of 3.3, 98% of the acetaldehyde will be combined with the sulphite within 90 minutes. It has been estimated that only 0.04% of acetaldehyde is in the free form in the presence of 30 mg/L free SO2 (Blouin, 1966).
Odours associated with the presence of free acetaldehyde have been described as "green apple", "oxidised green apple", "grass" and "chemical" (Coetzee et al., 2016a). At low concentrations, the presence of free acetaldehyde could contribute to the pleasant, fruity aroma of a wine, but the typical oxidation-related nuances will develop at higher concentrations (Zea et al., 2010; Coetzee et al., 2016a)1.5, 2.5, 4.5, and 6 years in the Montilla-Moriles region (southern Spain. The sensory effect of acetaldehyde has also been shown to have important suppressive interactions with compounds such as 3-mercaptohexan-1-ol and 3-isobutyl-2-methoxypyrazine (Coetzee et al., 2016a). When in the bound form, the sensory effect of acetaldehyde is presumably reduced (Jackowetz et al., 2011), and it is thus recommended that a sufficient level of free SO2 is maintained to ensure the fixation of acetaldehyde. However, to our knowledge, this recommendation is not supported by scientific tests.
The aim of this study was to determine the sensory effectiveness of SO2 in reducing the oxidation odour associated with acetaldehyde and to confirm whether hydroxysulphonate is odourless, as generally believed. The effect of varying pH on the perception of "sulphur" was also investigated. In addition, a brief interaction study was conducted to investigate the interactive effects of hydroxysulphonate on the perception of a common and generally abundant aromatic ester, isoamyl acetate. This ester is present in wines made from a wide range of varieties and is described as contributing a "banana" aroma (Van Wyk et al, 1979).
MATERIALS AND METHODS
Chemicals and spiking
The model wine consisted of distilled water, 5 g/L tartaric acid (Sigma-Aldrich, Steinheim, Germany) and 12% v/v ethanol (Illovo, Durban, South Africa), with the pH adjusted to 3.5 using sodium hydroxide (Sigma-Aldrich, Steinheim, Germany). After preparing the model wine, the composition was confirmed using a WineScan FT 120 instrument (FOSS Analytical, Denmark).
The compounds used in the sensory study were SO2, acetaldehyde and isoamyl acetate. Sulphur dioxide solution at 18% m/v (Laffort, France) was added directly to the samples. Acetaldehyde dilutions (Sigma-Aldrich, Steinheim, Germany) were prepared fresh every week to a concentration of 100 g/L using Milli-Q-Water (Millipore Filter Corp., Bedford, MA, USA) and stored at 4°C. Isoamyl acetate solution (Riedel de Haën, Seelze, Germany) was prepared fresh daily to a concentration of 3 g/L using 99.5% v/v ethanol (Merck Chemicals, South Africa). These solutions were used to spike the model wine to the desired concentration. Acetaldehyde and SO2 were added to the model wine 18 hours prior to sensory evaluation. Isoamyl acetate was added to the samples one hour prior to sensory evaluation.
Experimental design
The concentrations used in this study are shown in Table 1. Acetaldehyde concentrations (25 and 50 mg/L) were chosen based on the concentrations found in dry white wines in general (Jackowetz & Mira de Orduna, 2013). Sulphur dioxide levels were calculated (based on bisulphite molar mass) to match acetaldehyde levels in specific molar ratios. For instance, a combination of 25 mg/L acetaldehyde with 46 mg/L SO2 resulted in a 1:1 molar ratio; a combination of 25 mg/L acetaldehyde and 92 mg/L SO2 resulted in a 1:2 molar ratio. The isoamyl acetate concentration (2.5 mg/L) was chosen based on levels found in dry white wines (King et al, 2011; Benkwitz et al, 2012). Pre-screenings by experienced wine tasters were done before finalising the concentrations.
Sensory analysis
The method used for sensory analysis is based on descriptive analysis with some deviations, which are pointed out in the following sections. The sensory panel consisted of 12 judges (all female and between the ages of 27 and 64, with a mean age of 39). For reasons of human ethics, a brief explanation of the addition of flavour to the samples was given prior to testing, although care was taken to exclude any information that could have caused bias.
Descriptors were generated during the first training sessions, after which line scaling exercises were done. During training, the panellists were not informed of the composition of each sample. A range of reference standards was available for the duration of the training and testing (Table 2). Intensity rating was done using a 100 mm unstructured line scale that rated intensity from "none" to "intense". Testing was done in booths using standard ISO wine-tasting glasses. The booths had standard artificial daylight lighting and were temperature control at 20 ± 2°C. Sample glasses were marked with a unique random three-digit code for each judge, and the glasses were covered with a plastic lid prior to sensory assessment to prevent the aroma contaminating the laboratory environment. The order of the samples was random and balanced across the assessors. Along with the set of samples containing the spiked compound, a glass containing the unspiked model wine only, the "blank", was provided for comparison. Panellists evaluated the samples orthonasally only, and the data was collected on a paper ballot. Testing was done in triplicate and regular breaks between samples were encouraged, while compulsory breaks were taken between replicated sample sets to avoid fatigue.
Determination of the effect of single compound additions on sensory perception
The various concentrations of acetaldehyde and sulphur dioxide were added singularly to the model wine solution in order to confirm the sensory effect of each compound. Training was done over three sessions (one hour each; all on separate days), after which a test was conducted. During training and testing, the panel members were presented with the eight samples together with a blank glass containing model wine only.
Determination of the effect of the addition of acetaldehyde and sulphur dioxide on sensory perception
After the assessment of single compounds, acetaldehyde and sulphur dioxide were added together in the same sample at different combinations (Table 1). At this stage the judges were already familiar with the sensory characteristics of the single compounds. Training for these samples took place over seven sessions (one hour each), after which a test was conducted. Samples were tested on a comparison basis. The panel member was presented with a pivot glass, as well as the sample to be profiled (Table 3). The pivot glass consisted of a sample containing a single compound only. On the scaling sheet provided, the profiling information of the pivot glass was already available for the panel member (information gathered during the profiling of the single samples). The panel member had to compare the two samples and provide the intensity rating of each attribute in comparison to the pivot sample.
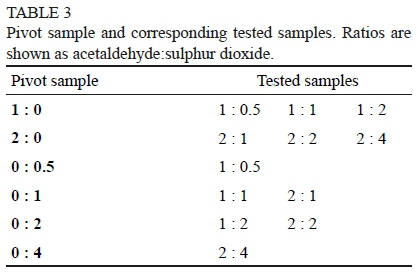
Determination of the effect of the addition of acetaldehyde and sulphur dioxide and isoamyl acetate on sensory perception
The profiling of samples containing acetaldehyde and sulphur dioxide together with isoamyl acetate was done after the profiling of samples containing acetaldehyde and sulphur dioxide only. The same range of concentrations was used; however, isoamyl acetate (2.5 mg/L) was also added to all samples.
Determination of the effect of pH on the sensory perception of the combined samples
The effect of changing pH on the sensory perception of the compounds was measured. Three different pH levels were chosen: 3.0, 3.5 and 4.0, and they were adjusted accordingly using sodium hydroxide. Samples containing 25 mg/L acetaldehyde, 46 mg/L sulphur dioxide and 2.5 mg/L isoamyl acetate singularly were profiled at the different pH levels. Samples containing a combination of compounds (25 mg/L acetaldehyde, 46 mg/L sulphur dioxide and 2.5 mg/L isoamyl acetate) were also profiled at pH levels of 3.0, 3.5 and 4.0.
SO2 analysis
Analyses of free and total SO2 were outsourced to an accredited laboratory (Vinlab Pty Ltd., Stellenbosch, South Africa). the analyses were done using the aspiration method (Amerine & Ough, 1980).
Data analysis
Assessor performance was evaluated using PanelCheck (Version V1.4.0, Nofima, Tromso, Norway) according to the workflow described by Tomic et al. (2010). For the statistical analysis of the data, one-way analysis of variance (ANOVA) was conducted to investigate differences between treatments. Post-hoc Fisher's least significant difference (LSD) tests were used to test for significance, and a p-value threshold of 0.05 (p < 0.05) was used for the determination of statistical significance.
RESULTS AND DISCUSSION
Evaluation of single compounds
The attributes and the relevant reference standards used to describe single compounds are shown in Table 2. Table 4 shows the average intensity rating for each concentration. The main attribute used to describe acetaldehyde was "green apple" (scored at the highest intensity), and it delivered intensities of 37 and 61 intensity units for the 25 mg/L and 50 mg/L of acetaldehyde respectively. The description "green apple" has also previously been used as an attribute of acetaldehyde in model wines (Coetzee et al., 2016a), and is also one of the main attributes associated with a white wine in which acetaldehyde concentrations increased due to oxidation (Coetzee et al., 2016b). The addition of SO2 resulted in the attributes "sulphur", "plastic", and "dusty", of which "sulphur" was rated at the highest intensity. For "sulphur" there were no significant differences in intensity between 23 and 46 mg/L of SO2, while "plastic" and "dusty" were rated higher at 46 mg/L compared to both 23 mg/L and 184 mg/L.
Acetaldehyde and SO2 combinations
Fig. 1 shows the intensity of the "green apple" attribute of samples containing only acetaldehyde (A25 and A50), as well as samples containing a mixture of acetaldehyde and sulphur dioxide (shown as A+S, together with the respective molar ratios). A25 delivered an average "green apple" intensity of 37, while A50 reached 61 intensity units, a statistically significant difference (Table 4). Adding sulphur dioxide at half the molar ratio of the acetaldehyde (1:0.5 and 2:1) decreased the intensity of the attribute by 38% in both cases. Adding sulphur dioxide at a molar ration of 1:1 and 2:2 led to a decrease in attribute intensity of 90% and 92% respectively. As expected, the addition of sulphur dioxide to the acetaldehyde in an equimolar ratio reduced the "green apple" aroma significantly, to less than five intensity units. Adding sulphur dioxide at double the molar ratio of the acetaldehyde (1:2 and 2:4) decreased the attribute intensity further, resulting in an decrease in intensity of 96% and 97% respectively. This confirms the effectiveness of the ability of SO2 to reduce the aroma associated with acetaldehyde.
Figure 2 shows the mean attribute intensity ratings of "sulphur" in samples containing various concentrations of sulphur dioxide alone (S23, S46, S92 and S184), and in combination with acetaldehyde (shown as A+S, together with the respective molar ratios). Adding acetaldehyde and SO2 in a ratio of 1:0.5 and 2:1 (acetaldehyde in excess) resulted in a decrease in "sulphur" by 68% (to eight intensity units) and 92% (to two intensity units) respectively. Adding the two compounds at a ratio of 1:1 or 2:2 was expected to result in very low attribute intensities. However, the results showed no significant decrease in the case of A25 + S46 (1:1) compared to S46 alone, and a smaller decrease of 27% in A50 + S92 (2:2) compared to S92 alone. Therefore, from comparing a sample containing free sulphur dioxide (S46) with a sample that should theoretically have no free SO2 available (1:1) it is evident that the aroma perception showed the two samples to have the same attribute intensity when profiling "sulphur". As demonstrated here, hydroxysulphonate has a prominent "sulphur" smell at similar intensities as a sample containing free SO2 at the same concentration (S46).
The possibility of excess free SO2 still being present in the medium was considered. Samples were subjected to analysis and the results showed a free SO2 concentration of 5 mg/L in sample A25 + S46 (1:1). This confirms hydroxysulphonate as the origin of the "sulphur" smell in this specific sample, especially considering the comparison of this sample (1:1), which contains as little as 5 mg/L of free SO2, to a sample containing only SO2 at a concentration of 46 mg/L free SO2. Similar results were obtained when comparing sample 2:2 (free SO2 concentration of 10 mg/L) with sample S92. The same tendency was seen for "plastic" (results not shown).
Effect of hydroxysulphonate on the perception of isoamyl acetate
The effect of hydroxysulphonate on the perception of attributes brought by isoamyl acetate was also evaluated. The reason for choosing isoamyl acetate is that it is an ester commonly occurring in wine and is not known to react chemically with SO2 and acetaldehyde. The addition of isoamyl acetate (2.5 mg/L) to the model wine medium contributed an attribute described as "banana candy", at an intensity of 24 units. Samples containing the isoamyl acetate together with acetaldehyde and sulphur dioxide in a ration of 1:1 or 2:2 showed no significant difference in the "banana candy" intensity (results not shown). It would thus seem that there was little to no sensory interaction between hydroxysulphonate and isoamyl acetate (Coetzee et al., 2016a).
Effect of pH on the intensity of "sulphur"
The effect of varying pH on the perception of aroma attributes was also tested. The results showed a significant difference in "sulphur" in samples containing sulphur dioxide at 46 mg/L. Mean intensity ratings for "sulphur" can be seen in Fig. 3. As expected, the lowest pH (3.0) resulted in the highest rating of the attribute, at 57 intensity units, while pH 3.5 and pH 4.0 delivered significantly lower intensities, of 35 and 37 units respectively. The higher concentration of molecular SO2 (2.9, 0.9, 0.3 mg/L molecular SO2 at pH 3.0, 3.5 and 4.0 respectively) present at the lower pH (Margalit, 2012) could explain this observation. The same test was repeated with samples containing acetaldehyde and sulphur dioxide at a ratio of 1:1; however, the results did not show any significant difference in the "sulphur" intensity between the three different levels of pH (results not shown). It would seem that the pH did not have an effect on the sensory perception of hydroxysulphonate.
CONCLUSIONS
These findings can have important implications for wine producers as well as sensory scientists. Sulphur dioxide is a very efficient antioxidant additive in wines and is normally used judiciously in most wine cellars as an antioxidant. The addition of SO2, however, should not be considered as the only preventative measure. Too much oxygen contact and subsequent oxidation could result in acetaldehyde formation and, even though the reaction between acetaldehyde and sulphur dioxide effectively lowers the "green apple" odour associated with oxidation, the hydroxysulphonate formed has now been shown not to be odourless, as was believed in the past. The apparent "sulphur" smell that results due to the combination of acetaldehyde and sulphur dioxide could influence the aromatic composition of a wine. Thus, ongoing measures should be in place to prevent excessive oxygen contact with wine.
This study elucidates the role of sulphur dioxide in eliminating the "green apple" aroma and shows the contribution ("sulphur") of hydroxysulphonate to the aroma. The hydroxysulphonate did not have any sensory interactive effect on the perception of isoamyl acetate; however, wine is a complex medium with many other aroma-contributing compounds that could possibly be affected by elevated concentrations ofthe compound. Other than that, the reactivity of both sulphur dioxide and acetaldehyde with other wine constituents will have an effect. Sulphur dioxide binds with various compounds, especially aldehydes, ketones, phenolic compounds and sugars (Margalit, 2012). Depending on the binding strength, this can limit the availability of SO2 to bind with excess acetaldehyde. Acetaldehyde can also bind to other wine constituents and especially participate in acetaldehyde-mediated reactions involving phenolic material (Margalit, 2012). These reactions will influence the production of hydroxysulphonate, and subsequently the perception of the "sulphur" attribute. Future studies should thus also test the sensory implications of hydroxysulphonate in a real wine medium.
LITERATURE CITED
Amerine, M.A. & Ough, C.S., 1980. Methods for analysis of musts and wine. Wiley-Interscience Publication, New York. [ Links ]
Benkwitz, F., Tominaga, T., Kilmartin, P. A., Lund, C., Wohlers, M. & Nicolau, L., 2012. Identifying the Chemical Composition Related to the Distinct Aroma Characteristics of New Zealand Sauvignon blanc Wines Am. J. Enol. Vitic. 63, 1, 62-72. [ Links ]
Blouin, J., 1965. Contribution à l'étude des combinaisons de l'anhydride sulfureux dans les moüts et les vins. PhD dissertation, Université de Bordeaux. [ Links ]
Burroughs, L.F. & Sparks, A.H., 1973. Sulphite-binding power of wines and ciders. I. Equilibrium constants for the dissociation of carbonyl bisulphite compounds J. Sci. Food Agric. 24, 187-198. [ Links ]
Coetzee, C., Brand, J., Jacobson, D. & du Toit, W., 2016. Sensory effect of acetaldehyde on the perception of 3-mercaptohexan-1-ol and 3-isobutyl-2-methoxypyrazine Aust. J. Grape Wine Res. [ Links ]
Coetzee, C., Van Wyngaard, E., Suklje, K., Silva Ferreira, A.C. & Du Toit, W.J., 2016. Chemical and Sensory Study on the Evolution of Aromatic and Nonaromatic Compounds during the Progressive Oxidative Storage of a Sauvignon blanc Wine J. Agric. Food Chem. 64, 42. [ Links ]
Drysdale, G.S. & Fleet, G.H., 1988. Acetic Acid Bacteria in Winemaking: A Review Am. J. Enol. Vitic. 39, 2, 143-154. [ Links ]
Jackowetz, J.N. & Mira de Orduna, R., 2013. Survey of SO2 binding carbonyls in 237 red and white table wines Food Control 32, 2, 687-692. [ Links ]
Jackowetz, J.N., Dierschke, S. & Mira de Orduna, R., 2011. Multifactorial analysis of acetaldehyde kinetics during alcoholic fermentation by Saccharomyces cerevisiae Food Res. Int. 44, 1, 310-316. [ Links ]
King, E.S., Osidacz, P., Curtin, C., Bastian, S.E.P. & Francis, I.L., 2011. Assessing desirable levels of sensory properties in Sauvignon Blanc wines - consumer preferences and contribution of key aroma compounds Aust. J. Grape Wine Res. 17, 2, 169-180. [ Links ]
Liu, S.-Q. & Pilone, G.J., 2000. An overview of formation and roles of acetaldehyde in winemaking with emphasis on microbial implications Int. J. Food Sci. Technol. 35, 49-61. [ Links ]
Margalit, Y., 2012. Concepts in Wine Chemistry. (2nd ed.). The wine appreciation guild, San Francisco. [ Links ]
Tomic, O., Luciano, G., Nilsen, A., Hyldig, G., Lorensen, K. & Nas, T., 2010. Analysing sensory panel performance in a proficiency tests using the PanelCheck software Eur. Food Res. Technol. 230, 497-511. [ Links ]
Waterhouse, A.L. & Laurie, V.F., 2006. Oxidation of wine phenolics: A critical evaluation and hypotheses Am. J. Enol. Vitic. 57, 3, 306-313. [ Links ]
Wildenradt, H.L. & Singleton, V.L., 1974. The Production of Aldehydes as a Result of Oxidation of Polyphenolic Compounds and its Relation to Wine Aging Am. J. Enol. Vitic. 25, 2, 119-126. [ Links ]
Van Wyk, C.J., Augustyn, O.P.H., De Wet, P. & Joubert, W.A., 1979. Isoamyl Acetate -- a Key Fermentation Volatile of Wines of Vitis Vinifera CV Pinotage Am. J. Enol. Vitic. 30, 3, 167-173. [ Links ]
Zea, L., Moyano, L., Ruiz, M.J. & Medina, M., 2010. Chromatography-Olfactometry Study of the Aroma of Fino Sherry Wines Int. J. Anal. Chem. 2010, 1-5. [ Links ]
Zea, L., Serratosa, M.P., Mérida, J. & Moyano, L., 2015. Acetaldehyde as Key Compound for the Authenticity of Sherry Wines: A Study Covering 5 Decades Compr. Rev. Food Sci. Food Saf. 14, 6, 681-693. [ Links ]
Submitted for publication: August 2017
Accepted for publication: March 2018
Acknowledgments: The authors would like to thank the NRF, Thrip, DST and Winetech, for funding; Jeanne Brand, for assistance with the sensory analysis; and Prof Martin Kidd, for the statistical analysis
* Corresponding author: E-mail address: wdutoit@sun.ac.za














