Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904
Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.38 n.1 Stellenbosch 2017
ARTICLES
Characterisation of vernaccia Nera (Vitis vinifera L.) grapes and wine
D. FracassettiI; M. GabrielliI; O. CoronaII; A. TirelliI, *
IDepartment of Food, Environmental and Nutritional Sciences, Universitá degli Studi di Milano, Via G. Celoria 2, 20133 Milan, Italy
IIDepartment of Food Science and Technology, Universitá degli Studi di Palermo, Viale delle Scienze 11, 90128 Palermo, Italy
ABSTRACT
Vernaccia Nera (VN) is a minor Italian red grape cultivar whose oenological properties have not been investigated yet. Traditional winemaking procedures with VN can include grape drying and even triple sequential fermentations, but a rational vinification approach should be based on the grape composition. Since a comprehensive characterisation of the VN grape is still missing, the ripening of VN grapes was monitored by evaluating flavour compounds, proanthocyanidins and anthocyanins. The grapes were used to produce red wine whose chemical composition and sensory properties were assessed. Ripe VN grapes contained high amounts of extractable anthocyanins (0.88 g/kg). The most abundant anthocyanin was malvidin (56.6%), and high relative amounts of cumarate forms (11.3%) were also found. The grape skin showed a high concentration of proanthocyanidins (2 g/kg), whose degree of polymerisation was low (10.3). Epigallocatechin accounted for up to 39% of the flavan-3-ol units in the skin. Flavour compounds in the grapes included glycosylated norisoprenoids (mainly 3-oxo-(x-ionol and vomifoliolo) and benzenoids. The VN red wine showed a high concentration of anthocyanins, but the level of proanthocyanidins (0.93 g/L) was lower than expected. The spicy flavours were the notes mostly recognised in the sensory evaluation. Our data highlight the VN grape as suitable for the production of ready-to-drink or shortly aged red wine due to its high acidity and low astringency.
Key words: Vernaccia Nera, phenols, grape, ripening, winemaking
INTRODUCTION
Vernaccia Nera (VN) is an autochthonous Italian red grape cultivar growing in an overall 250 ha of vineyards, mainly in the Marche region (mid-eastern Italy). Serrapetrona (about 43°09'-12' N, 313°10'-15' E) is the area where the VN vineyards are mostly located (about 50 ha). The vineyards are located at an altitude of between 240 and 957 m.
VN grapes show morphological characters of bunch, leaf and berry very similar to Grenache grapes, and the modification of the name Grenache to Grenaccia and then to Vernaccia has been hypothesised as a possible origin of its current Italian denomination. To the best of our knowledge, there are no genetic data supporting such a hypothesis. Nonetheless, Calo et al. (2006) report the VN grape as a cultivar other than Grenache and the names are considered synonyms of each other.
Two wines with protected designation of origin (P.D.O.) are obtained from this grape cultivar, namely Serrapetrona still wine and Vernaccia di Serrapetrona sparkling red wine (Gazzetta Ufficiale n. 205, 01.09.2004a; 01.09.2004b). The latter is obtained by fermenting up to 60% of freshly harvested grapes, while the remaining 40% is dried in suitably conditioned rooms in order to reach at least 13% potential alcoholic strength by volume. The grape must obtained from the dried grapes can be either fermented or blended with the wine from the fresh grapes and then fermented. A third fermentation step can be carried out in the final bulk of the still wine, when sparkling wine is produced by the gastight-vat method. The grape drying was also adopted in the past to produce "Serrapetrona" still red wine, but it is no longer mandatory (Gazzetta Ufficiale n. 205, 01.09.2004a). The latter, uncommon traditional winemaking procedure would be consistent with grapes containing poorly astringent proanthocyanidins. However, little is known concerning the compositional properties of VN grapes, although the first ampelographic description of this cultivar was reported in 1876 (Calo et al., 2006). No data was previously presented in terms of aromatic characterisation of the grapes. Furthermore, very little information is available about the red still wine Serrapetrona. A few research papers report data concerning the flavour compounds and the sensory properties of retailed VN wine (Boselli et al, 2004; Fiorini et al, 2014). However, commercial wines can be obtained by blending up to 15% of red wine from other grape cultivars. As a consequence, the reported profile of volatiles could be affected by the blending.
This research attempted to characterise the flavonoids (anthocyanins and proanthocyanidins) in VN grapes throughout the ripening process and in Serrapetrona wine produced under controlled conditions with only VN grapes. The aromatic profile of both ripe VN grapes and wine was assessed. The sensorial evaluation was carried out on Serrapetrona still wine.
MATERIALS AND METHODS
Chemicals and reagents
Methanol, ethanol, acetonitrile, ammonium acetate, trifluoroacetic acid (TFA), perchloric acid, iron (II) sulphate heptahydrate (Fe2SO4.7H2O), sulphuric acid, hydrochloric acid, malvidin, catechin, epicatechin 1-hexanol, acetovanillone, benzaldehyde, ethyl octadecanoate, ethyl hexanoate, ethyl lactate, ethyl octanoate, diethyl succinate, hexanoic acid, octanoic acid, isobutyric acid, isovaleric acid, benzoic acid, zingerone, geraniol and linalol were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium metabisulphite was purchased from J.T. Baker (Deventer, Holland). All the chemicals were at least of analytical grade. HPLC-grade water was obtained by a Milli-Q system (Millipore Filter Corp., Bedford, MA, USA).
The hydrochloric ethanol solution contained ethanol, water and hydrochloric acid 70/30/1 (v/v/v). The synthetic wine solution contained 3.0 g/L tartaric acid, 12% ethanol (v/v), and 100 mg/L SO2 at pH 3.2 adjusted with 12 M sodium hydroxide (Merck, Darmstadt, Germany).
Grapes
The 2 ha vineyard was planted in 2005 at an average altitude of440 m in the Serrapetrona area (Macerata, Italy) onto 420A rootstock with an east/south-east and west/north-north-west row direction. Grape sampling and the harvest were carried out in 2014. Weekly grape samplings were carried out, collecting 800 g of berries by cutting the pedicle to prevent any berry damage. A total of eight samplings were carried out in the time spanning from five weeks before to two weeks after the harvest. A maximum of three berries per sampled bunch were taken. The picking area of the sampled bunch (front, back, top and bottom) was varied with each sampled bunch. The samples were stored at 4°C for a maximum of 24 hours until sample preparation. Four hundred berries were used for the determination of flavonoids and aroma, in triplicate. The chemical parameters were assessed with the remaining 400 berries, in duplicate.
The climate data were provided by the official agrometeorological service, ASSAM (n.d.). The data, relating to minimum and maximum temperatures, humidity and rainfall, were acquired daily.
Winemaking
Three hundred and sixty kilograms of VN grapes were hand harvested on 2014-10-25, which was 58 days after véraison. The grapes were divided into three equal batches (120 kg each) before destemming and crushing. The musts were moved to 100 L stainless steel vats, to which 7 g/hL potassium metabisulphite was added, and the musts were inoculated with Saccharomyces cerevisiae RC212 strain (30 g/hL; DalCin, Concorezzo, Italy). The fermentation was carried out at 20 ± 2°C. In order to ensure suitable yeast nutrition, thiamine and ammonium salts were added to adjust the content of the readily assimilable nitrogen to 220 mg/L (Thiazote, Lallfort, Bordeaux Cedez, France). Manual punchdowns were performed twice a day to improve colour and phenol extraction. The alcoholic fermentations were monitored daily with density readings using a hydrometer, and the value was corrected for the temperature. At the end of the alcoholic fermentation, the wines were racked off and the pomace was gently pressed with a hydraulic press in order to recover the wine. The malolactic fermentation occurred spontaneously and completed in 30 days, after which the wines were racked and potassium metabisulphite at 100 mg/L was added. The wine was bottled in 500 mL glass bottles capped with a crown cap and stored at 15°C for two months until analyses.
Chemical analyses
Sugars, total and volatile acidity, tartaric acid, malic acid, citric acid, lactic acid, readily assimilable nitrogen (RAN), ethanol, potassium, density, total dry matter and the pH of the grapes and wine were assessed by an ISO 9000-accredited laboratory using a Grapescan™ FT 120 instrument (Foss Electric, Denmark), based on Fourier transform infrared spectroscopy. All of the analytical determinations were carried out in duplicate.
Phenol determination
Total and extractable flavonoids and anthocyanins in grapes
Total and extractable flavonoids and anthocyanins were assessed as described by Di Stefano et al. (1989) and Di Stefano and Cravero (1991). Total anthocyanins and flavonoids were extracted as follows. One hundred grape berries were homogenised by a high-speed Ultra-Turrax T25 for 3 ± 0.05 min, then 10 g of homogenate was suspended in 10 mL of hydrochloric ethanol solution and kept for 30 min at 20 ± 1°C. The sample was centrifuged at 5 000 χ g for 10 min and the supernatant was collected in a 50 mL volumetric flask, whereas the pellet was suspended in 10 mL of hydrochloric ethanol solution and then centrifuged further. The supernatant was recovered into the 50 mL volumetric flask. The hydrochloric ethanol solution was used to bring the volume to 50 mL. The extractable anthocyanins and flavonoids were obtained following the same procedure, but the synthetic wine solution was used instead of the hydrochloric ethanol solution and the extraction lasted 240 min instead of 30 min.
The quantification of anthocyanins and flavonoids was carried out spectrophotometrically by recording the UV-visible spectra in the range 230 to 700 nm using a Lambda 25 spectrophotometer (Perkin Elmer, Cetus, Norwalk, CT) and measuring the absorption values at 280 nm and 520 nm, as reported by Corona et al. (2015). The results are expressed as g catechin/kg grape and g cyanidin/kg grape for the flavonoids and anthocyanins respectively.
Total flavonoids in wine
All of the wine samples were diluted 40 times with hydrochloric ethanol solution in order to obtain an absorption value approaching 1 ± 0.5 AU at 280 nm. The absorption spectra of the diluted wine in the wavelength range 230 to 700 nm was recorded and the quantification was carried out according to Corona et al. (2015). The results are expressed as mg catechin/L, taking into account the height of the peak registered at 280 nm.
Total phenol index in wine
The absorption spectra obtained to quantify the total flavonoids content were used to measure the absorption value at 280 nm. The absorption value was corrected according to the dilution (χ 40) and used as total phenol index (Di Stefano et al, 1989).
Colour measurement in wine
Wine colour was assessed as intensity and hue, as described by Ribereau-Gayon et al. (2006). The spectrophotometric absorption values at 420 nm and 520 nm of the undiluted wine were recorded using cuvettes with a 1 mm optical path.
Quantification of proanthocyanidins in wine
Proanthocyanidins were quantified as described by Bate-Smith (1981). In two separate test tubes (reaction tube and blank tube), 2 mL of wine sample, 10.5 mL of ethanol and 12.5 mL of hydrochloric acid 37% (v/v) containing 300 mg/L of FeSO4.7 H2O were added. The reaction tube was heated in a water bath at 100°C for 50 min, while the blank tube was left to stand in the dark in ice. After the reaction, the reaction tubes were cooled down in ice for 10 min and the absorbance was recorded at 550 nm. The concentration of proanthocyanidins was calculated by multiplying the difference in absorbance between the reaction tube and the blank tube by the factor 1162.5 in order to express the result as mg cyanidin/L (Di Stefano et al, 1989). Determinations were carried out in triplicate.
Flavanolic composition of proanthocyanidins in grape tissues
The proanthocyanidin composition was assessed in fresh tissues (skin and seeds) of VN grapes by carrying out an acid-catalysed cleavage in the presence of excess phloroglucinol (phloroglucinolysis) (Kennedy & Jones, 2001). The reaction was carried out on skin and seed extracts that were prepared as follows. Twenty-five grape berries were weighed and the skins and seeds were separated from the pulp, rinsed with water and gently dried. The isolated skin and seeds were weighed and extracted separately using the following procedure. A total of 30 mL of acetone/water 60/40 (v/v) was added to the skin and seed samples, which were then homogenised and left to stand for 30 minutes at 25°C. The homogenised samples were centrifuged at 5 000 χ g for 10 min at 10°C and the supernatant was recovered. The pellet was re-dispersed in 20 mL of acetone/water 60/40 (v/v) and centrifuged at 5 000 χ g for 10 min at 10°C, and both supernatants were recovered jointly. The acetone was removed from the extract by vacuum drying and the volume was adjusted to 30 mL with the synthetic wine solution. The skin and seed extracts were diluted with hydrosulphuric acid (H2SO4) 0.01 N (1:4 v/v) and purified by a 1 g Sep-Pak C-18 cartridge (Phenomenex, Torrence, CA, USA), previously activated with methanol and H2SO4 0.01 N. The sample (5 mL) was loaded into the cartridge, followed by 5 mL of H2SO4 0.01 N and then 20 mL of ethyl ether. The proanthocyanidins were recovered by eluting with 15 mL of methanol. The organic solvent was vacuum dried and the dry material was submitted to an acid-catalysed cleavage in the presence of excess phloroglucinol (phloroglucinolysis) (Kennedy & Jones, 2001), as follows. The dried samples were suspended in 2 mL of HCl 0.1 N in MeOH containing phloroglucinol 50 g/L and ascorbic acid at 10 g/L. The solution was heated to 50°C for 25 min in a water bath and then 5 mL of sodium acetate 0.04 M were added. The proanthocyanidin cleavage products were determined by an Acquity HClass UPLC (Waters, Milford, MA, USA) system equipped with a photo diode array detector 2996 (Waters), as described by Kennedy and Taylor (2003). The separation column was a Kinetex RP18 (150 χ 2.1 mm, 2.6 μηι, 100 Á) (Phenomenex, Torrance, CA, USA) kept at 30°C. The chromatographic separation was carried out using acetic acid 2.5% (v/v) in MilliQ-treated water (solvent A) and acetonitrile (solvent B) as eluting solvents. The UPLC separation was achieved by an elution gradient (3% to 9% of solvent B in 1.5 min, 9% to 16% in 4.5 min, 16% to 50% in 13.5 min) at a flow rate of 0.85 mL/min. The sample preparation was carried out in triplicate. The results are expressed as relative abundance (%) of total flavanols. Chromatographic data acquisition and processing were performed by Empower 2 software (Waters).
Anthocyanin profile in grapes and wine
The free anthocyanins were assessed in the grapes and wine as described by Mattivi et al. (2006). Either the total anthocyanin extract from the grapes (see paragraph on Total and extractable flavonoids and anthocyanins in grapes) or the wine samples were purified by SPE using a Sep-Pak C18 1 g cartridge (Phenomenex, Torrance, CA, USA) previously activated with methanol and water. The sample was diluted 1:1 with perchloric acid 0.3% (v/v), 5 mL were loaded into the cartridge and the anthocyanins were eluted with 10 mL of methanol after a washing step with 6 mL of perchloric acid 0.3% (v/v). The solvent was vacuum dried and the dry material was dissolved with 1 mL of methanol:water:perchloric acid (27:73:0.3 v/v/v) solution. The UPLC separation was carried out by an Acquity HClass UPLC (Waters) system coupled with a diode array detector (2996, Waters). The separation column was a Kinetex C18, 150 χ 3 mm, 2.6 μηι particle size, 100 Á pore size (Phenomenex, Torrence, CA). The flow rate was 0.5 mL/min and the column was kept at 30°C. The eluting solvents were (A) TFA 0.2% (v/v) and (B) methanol/ water/TFA 80/20/0.2 (v/v/v). Elution conditions were as follows: 30% B for 1 min, and from 30% to 85% B in 36 min. Samples were filtered through a 0.22 μm pore size PVDF membrane (Millipore, Billerica, MA) before injection. The anthocyanins were detected and quantified at 520 nm. The determination was carried out in triplicate. The quantification was carried out using the malvidin as standard. The data are expressed as relative abundance (%) for the -3-O-glucoside, -3-O-glucoside acetate and -3-O-glucoside cumarate forms of delphinidin, cyanidin, petunidin, peonidin and malvidin. Chromatographic data acquisition and processing were performed by Empower 2 software (Waters).
Determination of aromas
The berry homogenate in synthetic wine solution (see paragraph on Total and extractable flavonoids and anthocyanins in grapes) was clarified with a glycosidase-free pectolytic enzyme (0.1 g) (Rapidase X-Press, DSM, Netherlands) at room temperature for 2 h. Heptanol was added to the samples as internal standard (0.2 mL of 30 mg/L solution in 10% ethanol).
Afterwards, an aliquot was loaded onto a 5 g Sep-Pak C18 reversed-phase solid-phase extraction (SPE) cartridge (Isolute, SPE Columns, Uppsala, Sweden), previously activated with 50 mL of deionised water and then with 20 mL of methanol using a flow rate of ca. 3 mL/min. The cartridge was then rinsed with 100 mL of deionised water to remove sugars, acids and other low molecular weight polar compounds. The free fraction was then eluted with 25 mL of dichloromethane. The eluate was dried over anhydrous Na2SO4 and concentrated to about 0.2 mL under a stream of nitrogen. The extract containing free volatile compounds was immediately analysed by gas chromatography/mass spectrometry (GC/MS).
The glycoconjugates were eluted from the cartridge with 20 mL of methanol, which was under-vacuum, and evaporated to dryness at 30°C. The dried glycosidic extract was dissolved in 5 mL of citrate-phosphate buffer (0.2 M, pH 5). The enzymatic hydrolysis was carried out using 50 mg of an AR-2000 commercial preparation with glycosidase side activities (DSM Oenology, Netherlands) and incubated at 40°C for 24 h. After adding 0.2 mL of 1-heptanol (30 mg/L solution in 10% ethanol), the glycosylated precursors were extracted, following the SPE method described previously. The dichloromethane extract obtained was dried over anhydrous sodium sulphate, concentrated to 0.2 mL and stored at -20°C until analysed. All analyses were performed in duplicate. GC/MS analysis was performed with an Agilent 6890 Series GC system and an Agilent 5973 Net Work Mass Selective Detector (Milan, Italy), both equipped with a DB-WAX column (Agilent Technologies, 30 m, 0.250 mm i.d., film thickness 0.25 μm).
The GC-MS system and chromatographic conditions have been reported previously by Corona (2010). The detection was carried out by electron impact mass spectrometry in total ion current (TIC) mode using an ionisation energy of 70 eV. The mass acquisition range was m/z 30 to 330. The identification of volatiles was done by injection of commercial standards or on the basis of the data reported in the literature. Semi-quantitative data ^g/kg berries) were obtained by measuring the relative peak area of each identified compound in relation to that of the added internal standard.
Sensory analysis
A panel of 11 expert judges (three females, eight males) was enrolled for the sensory analysis of the bottled VN wine. The attributes related to the qualitative description of the VN wine were identified by the consensus method (ISO 11035, 1994).
Statistical analysis
Statistical analysis was carried out by means of STATISTICA software (Statsoft Inc., Tulsa, OK, US). The equations of the calibration curves were calculated by linear regression analysis. Differences were evaluated by the T-test (p < 0.05).
RESULTS AND DISCUSSION
The analytical data reported in this paper concern the 2014 vintage only. Therefore, they might not fully describe the properties of VN grapes. Nevertheless, the year 2014 showed weather conditions in the Serrapetrona area closely approaching the mean conditions recorded in the previous fifteen years by the official agrometeorological service (ASSAM, n.d.), and neither the vines nor the grapes showed any visible defects at harvest. The temperature and rain deviations from the mean values were between +1°C and -1°C and between +20% (i.e. + 20 mm) and -40% (i.e. -30 mm) respectively during the last 45 days before the harvest. There also were minor deviations (± 20 mm) in the water balance. The sugar content increased and the total acidity decreased regularly in the VN grapes as they ripened, and up to 260 g/L of sugars and 6.4 g/L of total acidity could be achieved in the juice (Fig. 1). However, the harvest was carried out in advance, 58 days after véraison, when the sugar content and total acidity were 198 g/L and 7.35 g/L respectively (Table 1), in order to prevent an unsuitable alcohol/tannin sensory balance.
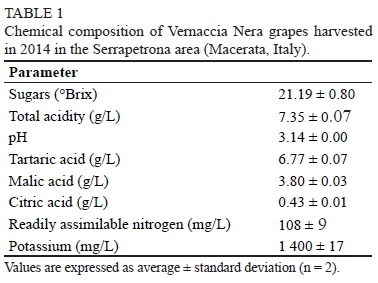
Tasting of the available VN wines on the market showed they were poor in tannins, and that a wine with a lower alcohol content and higher acidity could balance the main sensorial and tactile factors. Moreover, rain was forecasted the week after the harvest. The pH of the grape juice was consistently lower than 3.2 throughout ripening (Fig. 1) due to the poor consumption of malic acid, of which the concentration decreased along with that of tartaric acid. The high acidity was also promoted by the poor accumulation of potassium (< 1.4 g/L). Such results substantiate the suitability of VN grapes for producing red sparkling wine, as has traditionally occurred in the Serrapetrona area, and the P.D.O. conferred on this wine (Gazzetta Ufficiale n. 205, 01.09.2004b). Vintage 2014 was characterised by 184.2 mm of rain in the period June to August, with a maximum temperature of 35°C. However, the low level of rain (34 mm) in September and the mild temperature (15 to 25°C) (ASSAM, n.d.) allowed the regular ripening of the VN grapes inhibiting the development of pathogens.
The qualitative and quantitative phenol composition of the grapes strongly affects the sensorial properties of wine. Sparkling red wines usually show a proanthocyanidin content lower than that of still red wine. This leads to minor astringency, largely due to the low pH, high acidity and the presence of carbon dioxide, which are peculiar characteristics of sparkling wine. Also, the content of the flavan-3-ols esterified with gallic acid in the proanthocyanidins should be low due to their astringent behaviour (Gibbins & Carpenter, 2013). The flavonoid content in the grapes showed minor changes during ripening, and it was highest at harvest (about 5.6 g/kg), but less than 52% of the amount was extractable, corresponding to 2.95 g/kg (Fig. 2). The flavonoid content decreased 30% just two weeks after the harvest, likely due to the lignification process taking place during grape seed maturation. The flavonoid concentrations were more constant during the ripening, with major variations after the harvest when the rain occurred in October (ASSAM, n.d.). Anthocyanins in the grapes accounted for 26.7% of the flavonoid content at harvest (Fig. 2), which was carried out under the best extractability conditions (58%), whereas the highest anthocyanin concentrations were observed about three weeks earlier. The anthocyanin pattern of VN grapes (Table 2) revealed a relative content of cumarate anthocyanins (11.3%) that was higher than the acetate forms (4.1%) that occur in Grenache grapes (referred to as Cannonau grape in Italian) (Fernandes de Oliveira & Nieddu, 2016). Nonetheless, the relative contents of both delphinidin and petunidin exceeded 8.4% and 12.5% respectively in VN grapes, whereas they accounted for 1.4% and 2.1% respectively in Grenache grapes (Fernandes de Oliveira & Nieddu, 2016). As a consequence, VN grapes could hardly be considered the same cultivar as Grenache, as suggested by Calo et al. (2006). The high amount of anthocyanins and their cumarate form can make the anthocyanins of VN quite stable to oxidation during the winemaking, leading to high colour intensity in the wine.
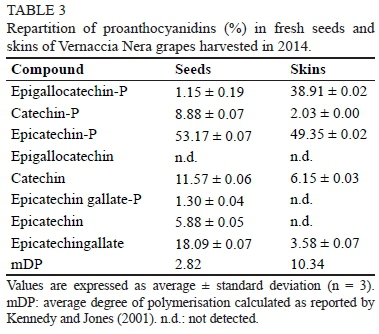
The sensorial properties of proanthocyanidins are mainly due to both their number of units and their flavan-3-ol structures (Gibbins & Carpenter, 2013). Therefore, the proanthocyanidins of the skin and seeds were characterised by phloroglucinolysis. The relative abundance of the flavan-3-ol units comprising the proanthocyanidins of VN was consistent with the composition reported on other grape cultivars (Souquet et al., 2000; Bordiga et al, 2011), where catechin and the gallic ester of epicatechin are mainly contained in the seeds, while epigallocatechin is almost missing (Table 3). Skin proanthocyanidins contained mainly epicatechin (49%) and epigallocatechin (39%), as well as minor amounts of catechin, mainly as terminal units (6.2%). The mean degrees of polymerisation were quite low compared to other grape cultivars (Souquet et al., 2000; Bordiga et al., 2011), as was the amount of epicatechin gallate. These findings could partially explain the low astringent character of the wine, either still or sparkling, obtained from VN grapes (Boselli et al., 2004).
The pattern of volatile compounds occurring in VN grapes (Tables 4 and 5) showed low levels of free compounds, mainly aldehydes, and low concentrations of polyisoprenic compounds. These latter occur in potentially useful amounts in the glycosylated form, mainly vomifoliol and 3-oxo-(x-ionone. Vernaccia Nera is far from being an aromatic or semi-aromatic cultivar, but rather shows a spicy flavour potential due to the occurrence of glycosylated norisoprenoids. Fiorini et al. (2014) reported the presence of many esters in Vernaccia di Serrapetrona sparkling red wine, but the norisoprenoid precursors were not detected in this study. Most of the esters in wine arise from yeast activity and do not belong to the varietal flavour compounds. Moreover, different analytical conditions were applied.
The oenological properties of VN grapes were also assessed by performing vinifications in triplicate. All of the alcoholic fermentations were regularly completed within eight to nine days. The malolactic fermentation occurred spontaneously and was completed within a month after the alcoholic fermentation. The main analytical parameters of the experimental wines (Table 6) did not show any evidence of undesired secondary fermentations (i.e. low volatile acidity). The total acidity value (6.1 g/L as tartaric acid), the tartaric acid content (2.5 g/L) and the pH (3.58) could indicate that VN wine is suitable for a short period of ageing.
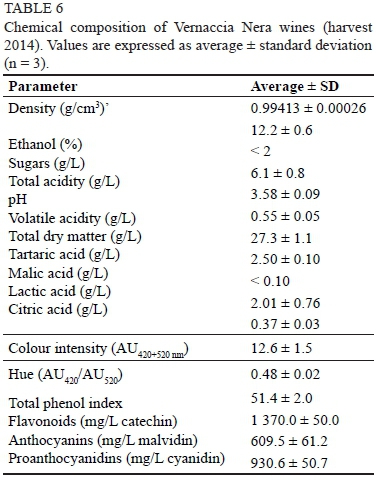
The anthocyanin content of the wine reflected the extractable anthocyanins found in the grapes (Table 6 and Fig. 2), whereas the flavonoid content (1 370 mg/L) was half of that of the grapes. Such a discrepancy was likely due to the low proanthocyanidin level in the wine (lower than 1 g/L), even if seed maceration occurred during winemaking. These data can further confirm that VN grapes are suitable to produce ready-to-drink red wine or wine aged for a short period. Our experimental wines showed a deep purple colour (A420+520 nm = 12.6 AU, A420/A520 = 0.48) (Table 6) due to both the high concentration of malvidin and the high relative content of p-cumarate anthocyanin esters (Table 2) (He et al., 2012).
Twelve experienced tasters evaluated the sensorial properties of the VN wine. Slight astringency and a bitter taste were evident, as well as spicy and red fruit notes, especially in the aftertaste. The sensory analysis was in accordance with the data found for the aromatic profile (Tables 4 and 5). In fact, the high concentration of 2-phenyl ethanol found in the wine highlighted its floral character, as well the fruity (ethyl lactate) and spicy (3-oxo-(x-ionol) notes. Our data were in accordance with Fiorini et al. (2014), who investigated the aromatic profile of sparkling VN wine. In contrast, Boselli et al. (2004) reported that a perceivable bitter taste occurred in commercial VN red wine. Moreover, the judges indicated an intense purple colour of the wine, confirming the spectrochemical data, as well as a low astringency and a good viscosity (body), which was not expected since the wine was poor in proanthocyanidin.
CONCLUSIONS
The composition of VN grapes and wine was investigated in terms of both phenolic and aromatic compounds. Our results provide evidence that VN grapes could be suitable for the production of young, deeply coloured red wine, as was evident in the high levels of free anthocyanins detected in the grapes. However, the wine lacked astringency, making it poorly suitable for barrel ageing, unless the proanthocyanidin concentrations have been increased. The study of this autochthonous cultivar could improve the production of good quality wine with a balanced aromatic profile and enhanced grape characteristics. The traditional grape drying performed in the Serrapetrona area in the past could likely be used with the aim of increasing the wine's astringency. Nonetheless, investigations of grape drying are needed in order to evaluate the characteristics of the grape itself, and of the VN wine produced after a second alcoholic fermentation.
LITERATURE CITED
ASSAM, n.d. Available at: http://www.assam.marche.it/. Last accessed: 4 August 2016. [ Links ]
Bate-Smith, E.C., 1981. Astringent tannins of the leaves of geranium species. Phytochem. 20, 211-216. [ Links ]
Bordiga, M., Travaglia, F., Locatelli, M., Coïsson, J.D. & Arlorio, M., 2011. Characterisation of polymeric skin and seed proanthocyanidins during ripening in six Vitis vinifera L. cv. Food Chem. 127, 180-187. [ Links ]
Boselli, E., Boulton, R.B., Thorngate, J.H. & Fraga, N.G., 2004. Chemical and sensory characterization of DOC red wines from Marche (Italy) related to vintage and grape cultivar. J. Agric. Food Chem. 52, 3843-3854. [ Links ]
Calo, A., Scienza, A. & Costacurta, A., 2006. Vitigni d'Italia. Calderini Edagricole, Bologna. [ Links ]
Corona, O., 2010. Wine-making with protection of must against oxidation in a warm, semi-arid terroir. S. Afr. J. Enol. Vitic. 31(1), 58-63. [ Links ]
Corona, O., Squadrito, M., Vento, G., Tirelli, A. & Di Stefano, R., 2015. Over-evaluation of total flavonoids in grape skin extracts containing sulphur dioxide. Food Chem. 172, 537-542. [ Links ]
Di Stefano, R. & Cravero, M.C., 1991. Metodo per lo studio dei polifenoli dell'uva. Riv. Vitic. Enol. 2, 37-45. [ Links ]
Di Stefano, R., Cravero, M.C. & Gentilini, N., 1989. Metodi per lo studio dei polifenoli dei vini. L'Enotecnico 5, 83-89. [ Links ]
Dragone, G., Mussatto, S.I., Oliveira, J.M. & Teixeira, J.A., 2009. Characterisation of volatile compounds in an alcoholic beverage produced by whey fermentation. Food Chem. 112, 929-935. [ Links ]
Escudero, A., Campo, E., Farina, L., Cacho, J. & Ferreira, V., 2007. Analytical characterization of the aroma of five premium red wines. Insights into the role of odor families and the concept of fruitiness of wines. J. Agric. Food Chem. 55, 4501-4510. [ Links ]
Fernandes de Oliveira, A. & Nieddu, G., 2016. Accumulation and partitioning of anthocyanins in two red grape cultivars under natural and reduced UV solar radiation. Aust. J. Grape Wine Res. 22, 96-104. [ Links ]
Fiorini, D., Caprioli, G., Sagratini, G., Maggi, F., Vittori, S., Marcantoni, E. & Ballini, R., 2014. Quantitative profiling of volatile and phenolic substances in the wine Vernaccia di Serrapretona by development of an HS-SPME-GC-FID/MS method and an HPLC-MS. Food Anal. Method 7, 1651-1660. [ Links ]
Francis, I.L. & Newton, J.L., 2005. Determining wine aroma from compositional data. Aust. J. Grape Wine Res. 11, 114-126. [ Links ]
Gazzetta Ufficiale n. 205, 01.09.2004a. Disciplinare di produzione dei vini a denominazione di origine controllata "Serrapetrona". [ Links ]
Gazzetta Ufficiale n. 205, 01.09.2004b. Disciplinare di produzione dei vini a denominazione di origine controllata e garantita "Vernaccia di Serrapetrona". [ Links ]
Gibbins, H.L. & Carpenter, G.H., 2013. Alternative mechanisms of astringency - What is the role of saliva? J. Texture Stud. 44, 364-375. [ Links ]
Gómez-Míguez, M.J., Cacho, J.F., Ferreira, V., Vicario, I.M. & Heredia, F.J., 2007. Volatile components of Zalema white wines. Food Chem. 100, 1464-1473. [ Links ]
He, F., Liang, N.N., Mu, L., Pan, Q.H., Wang, J., Reeves, M.J. & Duan, C.Q., 2012. Anthocyanins and their variation in red wines I. Monomeric anthocyanins and their color expression. Molecules 17, 1571-1601. [ Links ]
ISO 11035, 1994. Sensory analysis - Identification and selection of descriptors for establishing a sensory profile by a multidimensional approach. [ Links ]
Kennedy, J.A. & Jones, G.P., 2001. Analysis of proanthocyanidin cleavage products following acid-catalyses in the presence of excess phloroglucinol. J. Agric. Food. Chem. 49, 1740-1746. [ Links ]
Kennedy, J.A. & Taylor, A.W., 2003. Analysis of proanthocyanidins by high performance gel permeation chromatography, J. Chromatgr. A 995, 99-107. [ Links ]
Lopez, R., Aznar, M., Cacho, J. & Ferreira, V., 2002. Quantitative determination of minor and trace volatile compounds in wine by solid-phase extraction and gas chromatography with mass spectrometric detection. J. Chromatogr. A 966, 166-177. [ Links ]
Mattivi, F., Guzzon, R., Vrhovsek, U., Stefanini, M. & Velasco, R., 2006. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 54, 7692-7702. [ Links ]
Moreno, J.A., Zea, L., Moyano, L. & Medina, M., 2005. Aroma compounds as markers of the changes in sherry wines subjected to biological ageing. Food Control 16, 333-338. [ Links ]
Peinado, R.A., Mauricio, J.C. & Moreno, J., 2006. Aromatic series in sherry wines with gluconic acid subjected to different biological aging conditions by Saccharomyces cerevisiae var. capensis. Food Chem. 94, 232-239. [ Links ]
Ribéreau-Gayon, P., Glories, Y., Maujean, A. & Dubourdieu, D., 2006 (2nd ed). Handbook of enology (Vol 2, Chapter 6). John Wiley & Sons Ltd, Chichester. [ Links ]
Souquet, J.M., Cheynier, V. & Moutounet, M., 2000. Les proanthocyanidines du raisin. Bull. OIV, 73, 601-609, 835-836. [ Links ]
Submitted for publication: August 2016
Accepted for publication: December 2016
Acknowledgements: We are grateful to Mr Matteo Cesari de Maria, for providing the grape samples and following the winemaking, and for his technical support.
* Corresponding author: E-mail address: antonio.tirelli@unimi.it














