Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904
Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.36 n.2 Stellenbosch 2015
Transmission of Grapevine Leafroll-associated Virus 3 (GLRaV-3): Acquisition, inoculation and retention by the mealybugs Planococcus ficus and Pseudococcus longispinus (hemiptera: Pseudococcidae)
K. KrügerI, *; D.L. SaccaggiI, III; M. van der MerweII; G.G.F. KasdorfII
IDepartment of Zoology & Entomology, University of Pretoria, Private Bag X20, Pretoria 0028, South Africa
IIARC-Plant Protection Research Institute, Private Bag X134, Pretoria 0001, South Africa
IIICurrent address: Plant Health Diagnostic Services, Department of Agriculture, Forestry and Fisheries, Private Bag X5015, Stellenbosch 7599, South Africa
ABSTRACT
The vine mealybug, Planococcus ficus (Signoret), and the longtailed mealybug, Pseudococcus longispinus (Targioni Tozzetti), are vectors of grapevine leafroll-associated virus 3 (GRLaV-3), one of the most abundant viruses associated with grapevine leafroll disease. To elucidate the transmission biology in South Africa, acquisition access periods (AAPs), inoculation access periods (IAPs) and the retention of the virus in starving and feeding first- to second instar nymphs were determined. The rootstock hybrid LN33 served as virus source and grapevines (Vitis vinifera L., cv. Cabernet franc) served as recipient plants. An AAP of 15 min or an IAP of 15 min was sufficient for Pl. ficus to acquire or transmit GLRaV-3, respectively. Nymphs of Pl. ficus retained the virus for at least eight days when feeding on a non-virus host and grapevine, and for at least two days when starving, and were then capable of transmitting it successfully to healthy grapevine plants. Nymphs of Ps. longispinus transmitted the virus after an AAP of 30 min and an IAP of 1 h. They retained the virus for at least three days when feeding on virus-free vines or starving. The GLRaV-3 infection rates of plants with Pl. ficus as vector varied with AAPs. These were lower (20 to 60%) for AAPs of 12 h or less than for AAPs of 24 h or more (80 to 100%). The findings are of importance for understanding the transmission biology of mealybug vectors and devising management strategies for grapevine leafroll.
Key words: Ampelovirus, Closteroviridae, Coccoidea, grapevine leafroll disease, Vitis vinifera
INTRODUCTION
Grapevine leafroll-associated virus 3 (GLRaV-3) is one of the most widespread of at least nine viruses associated with grapevine leafroll disease (GLD) (Martin et al., 2005; Pietersen, 2006; Akbas. et al., 2007; Almeida et al., 2013; Naidu et al., 2014). The disease, which affects the quantity and quality of yield (Goheen & Cook, 1959; Cabaleiro et al., 1999; Atallah et al., 2012), has been reported from all major grapevine-growing areas worldwide (Martelli, 1986).
The infection of leafroll-free plant material after planting in open vineyards is a major problem for the grapevine industry (Pietersen et al., 2013). Until the 1980s, GLD was thought to be transmitted by using virus-infected plant material, either as rootstock or in grafts, but then Tanne et al. (1989) and Engelbrecht and Kasdorf (1990) showed that the spread of GLRaV-3 in vineyards is also mediated by mealybugs (Pseudococcidae). Furthermore, a few soft scale (Coccidae) species have been identified as vectors of GLRaV-3 (Belli et al., 1994; Mahfoudhi et al., 2009; Krüger & Douglas-Smit, 2013). Transmission is thought to occur in a semi-persistent manner (Martelli et al., 2002; Tsai et al., 2008), although Cid et al. (2007) have suggested a circulative transmission mechanism. Importantly, first-instar nymphs, unlike other stages that are more sessile, move actively and can be dispersed by wind (Gullan & Kosztarab, 1997; Walker et al., 2004; Grasswitz & James, 2008) and are more efficient vectors than later instars and adult females (Tsai et al., 2008; Sandanayaka et al., 2013).
Both the vine mealybug, Planococcus ficus (Signoret), and the longtailed mealybug, Pseudococcus longispinus (Targioni Tozzetti) (Hemiptera: Pseudococcidae), are vectors of GLRaV-3 (e.g. Tanne et al., 1989; Engelbrecht & Kasdorf, 1990). They occur in many grapevine-growing regions throughout the world, including Africa, Europe and the USA. Planococcus ficus is the most abundant species on grapevines in South Africa, whereas Ps. longispinus usually only occurs in low numbers and in small patches in vineyards in this country (Walton & Pringle, 2004; Walton et al., 2009).
Several studies have examined aspects of the transmission biology of mealybug vectors of GLRaV-3 (e.g. Cabaleiro & Segura, 1997; Petersen & Charles, 1997; Douglas & Krüger, 2008; Tsai et al., 2008; Mahfoudhi et al., 2009; Tsai et al., 2010; Le Maguet et al., 2012); for reviews see Charles et al. (2006), Almeida et al. (2013) and Naidu et al. (2014). Understanding the transmission biology of GLRaV-3 by mealybug vectors is important for devising management strategies for grapevine leafroll, especially in view of increasing reports of the occurrence of GLRaV-3 and other leafroll-associated viruses (e.g. Akbas. et al., 2007; Cabaleiro et al., 2008; Golino & Almeida, 2008; Fuchs et al., 2009; Almeida et al., 2013). The aim of this study was to determine (i) acquisition and inoculation access periods and (ii) the effects of post-acquisition starving and post-acquisition feeding on the persistence of GLRaV-3 using nymphs of South African populations of Pl. ficus and Ps. longispinus.
MATERIALS AND METHODS
Insects
Individuals from a laboratory culture of Pl. ficus, established with mealybugs collected in vineyards in the Western Cape (South Africa) and maintained for several generations on butternut, a non-host of GLRaV-3, were used. A non-viruliferous culture of Ps. longispinus was established on Alocasia macrorrhizos L. (Araceae) with specimens collected from greenhouse-grown banana plants in Pretoria (Gauteng, South Africa) and maintained for several generations. Initial attempts to establish a culture on grapevine (cv. Merlot, cv. Cabernet franc) with individuals collected from grapevines in vineyards in the Western Cape failed, as Ps. longispinus seemed unable to produce successive generations on this host. The identifications of Pl. ficus and Ps. longispinus were confirmed by Ian Millar (Biosystematics Division, Agricultural Research Council - Plant Protection Research Institute (ARC-PPRI)) and by using a multiplex polymerase chain reaction (PCR) technique described by Saccaggi et al. (2008). Mealybugs were kept in an insect growth room at approximately 25°C, 65% humidity and 14 h:10 h light:dark photoperiod.
Virus source and recipient plants
Plants propagated from stem cuttings of the rootstock hybrid LN33 (1/5/2, ARC Infruitec-Nietvoorbij, South Africa) with GLRaV-3 isolate 621 (Jooste et al., 2010) served as virus source. Plants propagated from stem cuttings of GLRaV-3-free Cabernet franc vines (mixed clones) at ARC Infruitec-Nietvoorbij and Vititec (Pty) Ltd (Western Cape, South Africa) were used as recipient vines. Virus source plants and recipient plants were kept in separate insect-free greenhouse compartments at approximately 25°C, natural humidity and day-length or environment-controlled growth rooms under the same environmental conditions as the insects.
Acquisition and inoculation access periods
To determine the acquisition access period (AAP) of Pl. ficus, first- to second-instar nymphs were initially subjected to acquisition periods ranging from one to seven days on virus source plants (LN33) and then transferred to healthy recipient plants (Cabernet franc) in groups of 50 nymphs per plant for a seven-day inoculation access period (IAP). To determine the IAP, first- to second-instar nymphs were given a seven-day AAP on virus source plants and IAPs of one to seven days on virus-free plants, again in groups of 50 nymphs per plant. Originally, one and seven days were chosen for AAPs and IAPs based on a study by Cabaleiro and Segura (1997), which showed that AAPs of three and seven days, but not one day, were sufficient for Planococcus citri (Risso) to transmit GLRaV-3. However, after no apparent difference in transmission efficiency was observed within AAPs or IAPs of one to seven days, nor for the number of nymphs used per group (Douglas & Krüger, 2008), shorter AAPs and IAPs were tested with groups of 15 to 20 nymphs per plant. Virus-free first- to second-instar nymphs of Pl. ficus were given AAPs of 15 min to 12 h on the virus source plants and an IAP of four days in groups of 15 to 20 nymphs on the recipient plants. Similarly, groups of 15 to 20 virus-free first- to second-instar nymphs of Pl. ficus were given IAPs of 15 min to 12 h on recipient plants after AAPs of four days.
To test the AAPs of Ps. longispinus, first- to second-instar nymphs were allowed to feed on virus-infected vines for periods ranging from 30 min to 24 h. Mealybugs were then transferred in groups of 15 to virus-free recipient vines (cv. Cabernet franc) for at least four days. The procedure for testing IAPs was similar to that for testing acquisition access time. Virus-free nymphs were allowed to feed on virus-infected vines for two to five days and then transferred to healthy recipient plants in the manner described above, where they were given inoculation feeding periods ranging from 30 min to 24 h. Nymphs of Pl. ficus or Ps. longispinus feeding on leaves of plants or on butternut were gently disturbed with a fine paint brush until they stopped feeding. Only these nymphs were carefully transferred to and from plants. For IAPs of 48 h or more, nymphs were transferred from virus source to recipient plants using small leaf cuttings with first- to second-instar nymphs placed on leaves of each of the recipient plants. As the leaf cuttings desiccated, the mealybugs moved to leaves on the plant, usually within the first few hours. Plants exposed to virus-free Pl. ficus and Ps. longispinus nymphs served as negative controls.
For transmission experiments with Pl. ficus and Ps. longispinus, five and six plants were used per AAP and IAP respectively. The number of plants used in experiments was limited by the number of pesticide- and virus-free plants available.
To prevent Pl. ficus and Ps. longispinus nymphs moving between plants and treatments, each plant was placed in its own separate insect-proof cage, and each cage was placed on four saucers containing engine oil to avoid movement of mealybugs between cages. Experiments were undertaken in insect rearing rooms at approximately 25°C, 65% humidity and 14 h photoperiod. Experiments were replicated using different source plants and by carrying out transmissions on different days, with a negative control plant for each day and each species.
After completion of the transmission experiments, plants were treated with chlorpyrifos and imidacloprid to remove nymphs and to prevent re-infestation. Thereafter, plants were transferred to glasshouses or plant growth rooms, where they were kept at approximately 25°C and natural humidity. In addition to the negative control plants used in the experiments, virus-free grapevine plants were kept in plant growth rooms as additional negative controls throughout the study. Plants were tested for GLRaV-3 starting at the earliest six weeks after treatment, and then at various intervals until two years after treatment. Not all plants used in the transmission experiments survived the dormant period.
GLRaV-3 retention
The transmission of plant viruses by insect vectors is dependent on whether insects have been starving or feeding. The effects of post-acquisition starving and post-acquisition feeding on the retention and subsequent transmission of GLRaV-3 by Pl. ficus were determined using first- to second-instar nymphs. Nymphs were given AAPs of four to six days on LN33 cuttings and were then carefully transferred with a fine paint brush in groups of 15 to 20 to one of three treatments: (i) plastic microtubes for starving periods of 6 h to four days (nymphs did not survive for more than four days when starving), (ii) fresh Ficus benjamini (Moraceae) leaves kept in glass tubes for feeding on a virus non-host for up to eight days, or (iii) Cabernet franc plants for feeding on virus-susceptible plants for periods of two to eight days. Thereafter, nymphs were transferred to healthy Cabernet franc plants to test their ability to transmit the virus. After completion of the experiments, plants were treated with pesticides as described previously. Subsamples of mealybugs were removed from F. benjamini leaves and placed in microtubes and, together with those starved in microtubes, were frozen (-20°C) at the various time intervals for later analysis. Plants exposed to virus-free nymphs served as negative controls. Sample size ranged from three to five plants.
To examine GLRaV-3 retention by Ps. longispinus, first-to second-instar nymphs were given AAPs of two to five days on GLRaV-3 source plants. They were then transferred with a fine paint brush in groups of 10 to healthy vines or Eppendorf tubes for post-acquisition feeding or starving periods ranging from 30 min to 72 h. The nymphs were then killed by freezing at -20°C and subsequently tested for GLRaV-3 by nested RT-PCR. Ten virus-free nymphs were collected directly from the A. macrorrhizos plant, while 10 nymphs were sampled directly from the virus source plant after an AAP of at least two days to serve as negative and positive controls, respectively. Nymphs of Pl. ficus and Ps. longispinus were tested individually for the presence of GLRaV-3.
Virus detection
To determine the virus status of the GLRaV-3 source and recipient plants, plant samples were tested for GLRaV-1 to 3, grapevine virus A (GVA) and grapevine virus B (GVB) using nested reverse transcription polymerase chain reaction (nested RT-PCR), an indirect enzyme-linked immunosorb-ent assay (ELISA) for the simultaneous detection of GL-RaV-1, GLRaV-2, and GLRaV-3 in grapevines developed by D. Goszczynski (Virology Unit, ARC-PPRI), and immunosorbent electron microscopy (ISEM) (Milne & Luisoni, 1977). Plants that tested positive for GLRaV-3 only were used as virus source plants. The GLRaV-3 isolate was determined by E. Jooste (Virology Unit, ARC-PPRI).
To ensure that mealybugs were virus free, sub-samples of Pl. ficus and Ps. longispinus from the laboratory cultures were tested for GLRaV-3 using nested RT-PCR before the transmission experiments. In order to detect GLRaV-3 in individual first- to second-instar nymphs and plant samples, the method described by La Notte et al. (1997) was adopted. Nested RT-PCR was performed using the primers developed by Ling et al. (2001) and following the protocol adapted from Ling et al. (2001) by M. van der Merwe. For details of the extraction and nested RT-PCR, see Douglas and Krüger (2008). Plants were tested from six weeks to two years after GLRaV-3 transmission.
Statistical analysis
The ratios of the total numbers of GLRaV-3-positive plants to the total number of negative plants for the different treatments were analysed using chi-square (χ2) tests. For the analysis of AAPs and IAPs with P. ficus as vector, data on shorter (15 min to 1 h (12 h)) and longer (one to seven days) AAPs and IAPs, respectively, were pooled. The Bonferroni adjustment was used when performing multiple statistical significance tests on the same data (Sokal & Rohlf, 1995). Analyses were carried out with Statistica© (Version 11 Statsoft, Inc. 1984-2012).
RESULTS
Acquisition access and inoculation access times
Planococcus ficus
An AAP or an IAP of 15 min was sufficient to acquire GLRaV-3 or to transmit the virus respectively (Table 1). For AAPs of one to seven days, the transmission rate ranged from 80 to 100%, while transmissions for IAPs of one to seven days ranged from 25 to 80%. Comparing the proportion of infected plants with long AAPs and IAPs (one to seven days) showed that the overall transmission success of IAPs (58%) was significantly lower than that of AAPs (95%) (χ2 = 14.96, d.f. = 1, P < 0.001). For short access periods of 15 min to 1 h, transmission efficiency ranged from 20 to 60% for AAPs and from 20 to 50% for IAPs. The overall transmission success for transmission periods of 1 h or less than 24 h was 38% for AAPs and 31% for IAPs. There was no difference between the total infected plants for AAPs and IAPs for these periods (χ2 = 0.14, d.f. = 1, P = 0. 7098). In general, transmission success was higher for AAPs of 24 h or more compared to AAPs of 12 h or less (χ2 = 26.03, d.f. = 1, P < 0.001). IAP transmission success was more variable and did not differ between IAPs of 24 h or more and IAPs of 1 h or less (χ2 = 3.20, d.f. = 1, P = 0.0738).
Pseudococcus longispinus
When feeding on viruliferous vines, Ps. longispinus acquired GLRaV-3 within 30 min and was able to transmit the virus to healthy vines (Table 2). The transmission rate for AAPs ranging from 30 min to 24 h ranged from 0 to 50%. An IAP of 1 h, but not one of 30 min, was sufficient to transmit the virus to healthy plants (Table 2). The transmission efficiency for IAPs ranging from 30 min to 24 h ranged from 0 to 75%. There was no difference in the overall transmission success for AAPs (31%) and IAPs (29%) (χ2 = 0.03, d.f. = 1, P = 0.8597).
GLRaV-3 retention
Planococcus ficus
Except for nymphs starving for four and eight days, nymphs under all other experimental conditions were able to transmit GLRaV-3 (Table 3). From 24 to 48 h post-acquisition feeding, the transmission rate increased from 40 to 100% when feeding on a virus non-host, and from 60 to 100% when feeding on grapevine. However, the transmission rate declined for mealybugs feeding on a virus non-host or grapevine for four and eight days. Planococcus ficus could retain the virus for four and eight days when starving and feeding respectively on a non-virus host (Fig. 1). Forty-six percent of nymphs tested positive for GLRaV-3 after an AAP of at least four days. The percentage of GLRaV-3-positive nymphs fluctuated between 14 and 64% when starving, but declined over time from 64 to 9% when feeding on the virus non-host. Only one out of 11 individuals tested positive for GLRaV-3 after eight days on the virus non-host, and that only very weakly. Nevertheless, GLRaV-3 could be transmitted successfully to a healthy grapevine plant by Pl. ficus after feeding for eight days on a virus non-host.
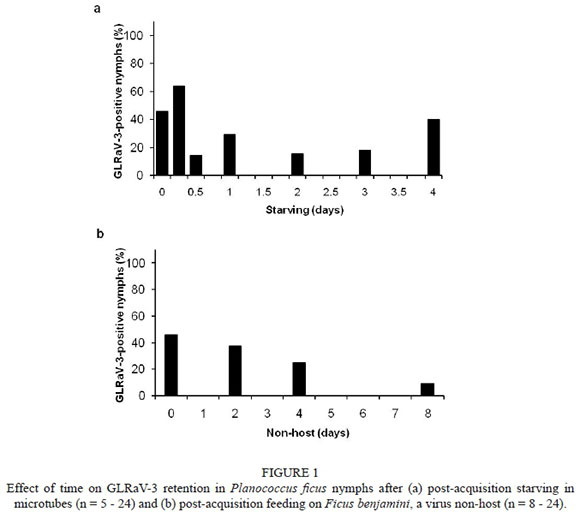
Pseudococcus longispinus
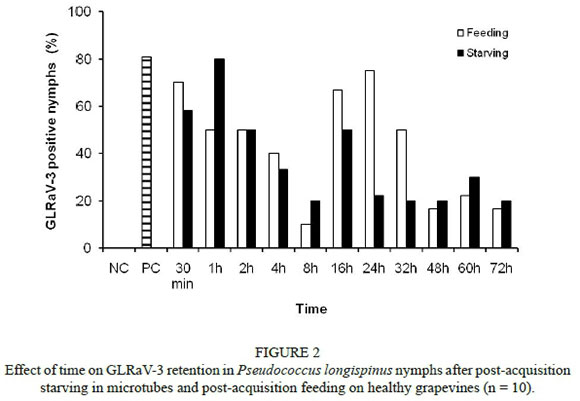
Eighty-one percent of Ps. longispinus nymphs tested positive for GLRaV-3 after an AAP of at least two days (Fig. 2). At feeding or starving times ranging from 30 min to 2 h, the virus was retained in at least 50% of the mealybugs. There was no significant difference in retention between feeding and starving mealybugs (χ2 = 6.10; d.f. = 10; P > 0.05). The percentage of infected mealybugs declined over time from 81% to 18% at 72 h, the longest retention time tested. However, the difference between infection rates at different retention times was not significant (feeding: χ2 = 18.30; d.f. = 10; P > 0.05; starving: χ2 = 17.72; d.f. = 10; P > 0.05).
Negative controls
All nymphs collected from butternut (Pl. ficus) and A. macrorrhizos (Ps. longispinus) tested negative for GLRaV-3. For all experiments, plants that served as negative controls, i.e. plants exposed to non-viruliferous nymphs and additional virus-free plants maintained together with the plants used in the experiments, tested negative for GLRaV-3 throughout.
DISCUSSION
It was shown previously that Pl. ficus nymphs can acquire or transmit GLRaV-3 after AAPs and IAPs of 1 h (Tsai et al., 2008). The results of this study show that Pl. ficus nymphs can acquire GLRaV-3 in 15 min and transmit the virus to healthy plants, and that viruliferous nymphs can inoculate healthy plants within 15 min. This is in accordance with other Hemiptera-transmitted Closteroviridae (Mar-telli & Candresse, 2014). Previous work on the transmission of GLRaV-3 by Ps. longispinus was done with AAPs or IAPs of one day or more (Petersen & Charles, 1997; Golino et al., 2002; Kuniyuki et al., 2005; Douglas & Krüger, 2008). In the current study, Ps. longispinus was able to transmit the virus to a healthy plant after an AAP of 30 min and an IAP of 1 h or more. The short AAPs and IAPs for Pl. ficus and Ps. longispinus nymphs are in contrast to the 24 h minimum feeding time observed for adult Ps. longispinus in a study on stylet penetration behaviour (Sandanayaka et al., 2013), and the average of 6 h required by Pl. citri to reach the phloem sap (Cid & Fereres, 2010).
The results of this study further show that the transmission success of GLRaV-3 by Pl. ficus nymphs is lower with AAPs of 12 h or less compared with AAPs of at least 24 h, when transmission success reached 100%. Similarly, Tsai et al. (2008) observed that transmission success peaked at 24 hours. The results for Ps. longispinus and IAPs of Pl. ficus in the current study were more variable and no clear trends were discernible. However, nymphs lose the ability to transmit GLRaV-3 after moulting. It is possible that first-instar nymphs moulted during the long AAPs of four to six days, which may have affected transmission success.
After an acquisition period of at least two days, 81% of single nymphs of Ps. longispinus exposed to viruliferous grapevines tested positive for the presence of GLRaV-3. This is a high infection rate when compared to the 46% of Pl. ficus observed in this study, and infection rates of 23% for Pl. ficus (Mahfoudhi et al., 2009) and 13 to 19% for Pl. citri (Caba-leiro & Segura, 1997). However, a previous study has shown that single first-instar nymphs of Pl. ficus and Ps. longispinus can transmit GLRaV-3 with equal efficiency (Douglas & Krüger, 2008).
GLRaV-3 has been categorised as a semi-persistently transmitted virus (Cabaleiro & Segura, 1997; Tsai et al., 2008). The results of the current study show that acquisition and inoculation periods lie in the range of 15 min to 1 h. There is no latent period, the virus is lost during moulting and is not transmitted transovarially (Tsai et al., 2008). These finding agree with the classification of GLRaV-3 as a semi-persistently transmitted virus. However, the current study has shown that, after feeding on a non-host for eight days, Pl. ficus was able to transmit GLRaV-3 to healthy vines. This is longer than the retention periods of three days previously reported for this species (Tsai et al., 2008), or the 24 h for Pl. citri (Cabaleiro & Segura, 1997), a species closely related to Pl. ficus (Downie & Gullan, 2004), when feeding on a non-host. It is possible that the nymphs did not moult - no exuviae were observed - due to slow growth on a host less suitable than grapevine. Generally, however, the reason for the long retention times is not understood (Martelli & Candresse, 2014).
The shorter AAPs and IAPs, higher transmission efficiencies and longer retention time observed in this study in comparison to Cabaleiro and Segura (1997) and Tsai et al. (2008) could be a reflection of experimental conditions, e.g. virus titre in source plants, sensitivity of detection method or GLRaV-3 isolate used. Further experiments are needed to explain the long retention time of eight days and to determine whether the GLRaV-3 isolate 621, one of the most common of the isolates recorded on grapevine in South Africa (Jooste et al., 2010), is more efficiently transmitted by Pl. ficus than less common isolates.
CONCLUSIONS
Planococcus ficus nymphs can acquire GLRaV-3 after an AAP of as little as 15 min and transmit the virus to healthy plants, and they can inoculate healthy plants after an IAP of 15 min. The nymphs retained the virus for at least eight days when feeding on a non-virus host or on virus-free grapevines, and for at least two days when starving, after which they still were able to transmit GLRaV-3 to virus-free grapevines. Pseudococcus longispinus nymphs can transmit the virus to healthy plants after an AAP of 30 min and an IAP of 1 h or more. They can retain GLRaV-3 for at least three days when starving or feeding on virus-free grapevines. The short virus acquisition times, together with the long retention times, merit further investigation. First- and second-instar nymphs are the most mobile and easily dispersible life stages of mealybugs, and a single nymph can successfully transmit GLRaV-3 to a healthy grapevine plant. In view of these findings, this study has serious implications for grapevine leafroll management strategies that rely on effective vector control.
LITERATURE CITED
Akbas , B., Kunter, B. & Ilhan, D., 2007. Occurrence and distribution of grapevine leafroll-associated viruses 1, 2, 3 and 7 in Turkey. J. Phytopathol. 155, 122-124. [ Links ]
Almeida, R.P.P., Daane, K.M., Bell, V.A., Blaisdell, G.K., Cooper, M.L., Herrbach, E. & Pietersen, G., 2013. Ecology and management of grapevine leafroll disease. Front. Microbiol. 4 (Article 94), 1-13. [ Links ]
Atallah, S.S., Gómez, M.I., Fuchs, M.F. & Martinson, T.E., 2012. Economic impact of grapevine leafroll disease on Vitis vinifera cv. Cabernet franc in Finger Lakes vineyards of New York. Am. J. Enol. Vitic. 63, 73-79. [ Links ]
Belli, G., Fortusini, A., Casati, P., Belli, L., Bianco, P.A. & Prati, S., 1994. Transmission of a grapevine leafroll associated closterovirus by the scale insect Pulvinaria vitis L. Riv. Pat. Veg. S. V. 4, 105-108. [ Links ]
Cabaleiro, C. & Segura, A., 1997. Some characteristics of the transmission of grapevine leafroll associated virus 3 by Planococcus citri Risso. Eur. J. Plant Pathol. 103, 373-378. [ Links ]
Cabaleiro, C., Couceiro, C., Pereira, S., Cid, M., Barrasa, M. & Segura, A., 2008. Spatial analysis of epidemics of grapevine leafroll associated virus-3. Eur. J. Plant Pathol. 121, 121-130. [ Links ]
Cabaleiro, C., Segura, A. & Garcia-Berrios, J.J., 1999. Effects of grapevine leafroll-associated virus 3 on the physiology and must of Vitis vinifera L. cv. Albarino following contamination in the field. Am. J. Enol. Vitic. 50, 40-44. [ Links ]
Charles, J.G., Cohen, D., Walker, J.T.S., Forgie, S.A., Bell, V.A. & Breen, K.C., 2006. A review of the ecology of grapevine leafroll associated virus type 3 (GLRaV-3). N. Z. Plant Protect. 59, 330-337. [ Links ]
Cid, M. & A. Fereres, A., 2010. Characterization of the probing and feeding behavior of Planococcus citri (Hemiptera: Pseudococcidae) on grapevine. Ann. Entomol. Soc. Am. 103, 404-417. [ Links ]
Cid, M., Pereira, S., Cabaleiro, C., Faoro, F. & Segura, A., 2007. Presence of grapevine leafroll-associated virus 3 in primary salivary glands of the mealybug vector Planococcus citri suggests a circulative transmission mechanism. Eur. J. Plant Pathol. 118, 23-30. [ Links ]
Douglas, N. & Krüger, K., 2008. Transmission efficiency of grapevine leafroll-associated virus 3 (GLRaV-3) by the mealybugs Planococcus ficus and Pseudococcus longispinus (Hemiptera: Pseudococcidae). Eur. J. Plant Pathol. 122, 207-212. [ Links ]
Downie, D.A. & Gullan, P.J., 2004. Phylogenetic analysis of mealybugs (Hemiptera: Coccoidea: Pseudococcidae) based on DNA sequences from three nuclear genes, and a review of the higher classification. Syst. Entomol. 29, 238-259. [ Links ]
Engelbrecht, D.J. & Kasdorf, G.G.F., 1990. Transmission of grapevine leafroll disease and associated closteroviruses by the vine mealybug, Planococcus ficus. Phytophylactica 22, 341-346. [ Links ]
Fuchs, M., Martinson, T.E., Loeb, G.M. & Hoch, H.C., 2009. Survey for the three major leafroll disease-associated viruses in Finger Lakes vineyards in New York. Plant Dis. 93, 395-401. [ Links ]
Goheen, A.C. & Cook, J.A., 1959. Leafroll (red-leaf or rogeau) and its effects on vine growth, fruit quality, and yields. Am. J. Enol. Vitic. 10, 173-181. [ Links ]
Golino, D.A. & Almeida, R., 2008. Studies needed of vectors spreading leafroll disease in California vineyards. Calif. Agric. 62, 174. [ Links ]
Golino, D.A., Sim, S.T., Gill, R. & Rowhani, A., 2002. California mealybugs can spread grapevine leafroll disease. Calif. Agric. 56, 196-201. [ Links ]
Grasswitz, T.R. & James, D.G., 2008. Movement of grape mealybug, Pseudococcus maritimus, on and between host plants. Entomol. Exp. Appl. 129, 268-275. [ Links ]
Gullan, P.J. & Kosztarab, M., 1997. Adaptations in scale insects. Annu. Rev. Entomol. 42, 23-50. [ Links ]
Jooste, A.E.C., Maree, H.J., Bellstedt, D.U., Goszczynski, D.E., Pietersen, G. & Burger, J.T., 2010. Three genetic grapevine leafroll-associated virus 3 variants identified from South African vineyards show high variability in their 5'UTR. Arch. Virol. 155, 1997-2006. [ Links ]
Krüger, K. & Douglas-Smit, N., 2013. Grapevine leafroll-associated virus 3 (GLRaV-3) transmission by three soft scale insect species (Hemiptera: Coccidae) with notes on their biology. Afr. Entomol. 21, 1-8. [ Links ]
Kuniyuki, H., Rezende, J.A.M., De Willink, C.G., Novo, J.P.S. & Yuki, V.A., 2005. Transmissäo do grapevine leafroll-associated virus 3 pela cochonilha Pseudococcus longispinus Targioni-Tozetti (Hemiptera: Pseudococcidae). Summa Phytopathol. 31, 65-68. [ Links ]
La Notte, P., Minafra, A. & Saldarelli, P., 1997. A spot-PCR technique for the detection of phloem-limited grapevine viruses. J. Virol. Methods 66, 103-108. [ Links ]
Le Maguet, J., Beuve, M., Herrbach, E. & Lemaire, O., 2012. Transmission of six ampeloviruses and two vitiviruses to grapevine by Phenacoccus aceris. Phytopathol. 102, 717-723. [ Links ]
Ling, K.-S., Zhu, H.-Y., Petrovic, N. & Gonsalves, D., 2001. Comparative effectiveness of ELISA and RT-PCR for detecting grapevine leafroll-associated closterovirus-3 in field samples. Am. J. Enol. Vitic. 52, 21-27. [ Links ]
Mahfoudhi, N., Digiaro, M. & Dhouibi, M.H., 2009. Transmission of grapevine leafroll viruses by Planococcus ficus (Hemiptera: Pseudococcidae) and Ceroplastes rusci (Hemiptera: Coccidae). Plant Dis. 93, 999-1002. [ Links ]
Martelli, G.P., 1986. Virus and virus-like diseases of the grapevine in the Mediterranean area. FAO Plant Prot. Bull. 34, 25-42. [ Links ]
Martelli, G.P. & Candresse, T., (2014) Closteroviridae. In: Encyclopedia of Life Sciences (eLS). John Wiley & Sons Ltd, Chichester. http://www.els.net [doi: 10.1002/9780470015902.a0000747.pub3]
Martelli, G.P., Agranovsky, A.A., Bar-Joseph, M., Boscia, D., Candresse, T., Coutts, R.H.A., Dolja, V.V., Falk, B.W., Gonsalves, D., Jelkmann, W., Karasev, A.V., Minafra, A., Namba, S., Vetten, H.J., Wisler, G.C. & Yoshikawa, N., 2002. The family Closteroviridae revised. Arch. Virol. 147, 2039-2044. [ Links ]
Martin, R.R., Eastwell, K.C., Wagner, A., Lamprecht, S. & Tzanetakis, I.E., 2005. Survey for viruses of grapevine in Oregon and Washington. Plant Dis. 89, 763-766. [ Links ]
Milne, R.G. & Luisoni, E., 1977. Rapid immune electron microscopy of virus preparation. In: Maramorosch, K. & Koprowski, H., eds: Methods in Virology, vol 6. Academic Press, New York. pp. 265 - 281. [ Links ]
Naidu, R., Rowhani, A., Fuchs, M., Golino, D. & Martelli, G.P., 2014. Grapevine leafroll: A complex viral disease affecting a high-value fruit crop. Plant Dis. 98, 1172-1185. [ Links ]
Petersen, C.L. & Charles, J.G., 1997. Transmission of grapevine leafroll-associated closteroviruses by Pseudococcus longispinus and P. calceolariae. Plant Pathol. 46, 509-515. [ Links ]
Pietersen, G., 2006. Spatio-temporal distribution dynamics of grapevine leafroll disease in Western Cape vineyards. In: Extended abstracts of the 15th Meeting of the International Council for the Study of Virus and Viruslike Diseases of the Grapevine, April 2006, Stellenbosch, South Africa. pp. 126 - 127. [ Links ]
Pietersen, G., Spreeth, N., Oosthuizen, T., Van Rensburg, A., Van Rensburg, M., Lottering, D., Rossouw, N. & Tooth, D., 2013. Control of grapevine leafroll disease spread at a commercial wine estate in South Africa: A case study. Am. J. Enol. Vitic. 64, 296-305. [ Links ]
Saccaggi, D.L., Krüger, K. & Pietersen, G., 2008. A multiplex PCR assay for the simultaneous identification of three mealybug species (Hemiptera: Pseudococcidae). Bull. Entomol. Res. 98, 27-33. [ Links ]
Sandanayaka, W.R.M., Blouin, A.G., Prado, E. & Cohen, D., 2013. Stylet penetration behaviour of Pseudococcus longispinus in relation to acquisition of grapevine leafroll virus 3. Arthropod-Plant Interact. 7, 137-146. [ Links ]
Sokal, R.R. & Rohlf, F.J., 1995 (3rd ed). Biometry. W.H. Freeman and Company, New York. [ Links ]
Tanne, E., Ben-Dov, Y. & Raccah, B., 1989. Transmission of closterolike particles associated with grapevine leafroll by mealybugs (Pseudococcidae) in Israel. In: Proc. 9th meeting of the International Council for the Study of Viruses and Virus Diseases of the Grapevine (ICVG), September 1987, Kiryat Anavin, Israel. pp. 71 - 73. [ Links ]
Tsai, C.-W., Chau, J., Fernandez, L., Bosco, D., Daane, K.M. & Almeida, R.P.P., 2008. Transmission of grapevine leafroll-associated virus 3 by the vine mealybug (Planococcus ficus). Phytopathol. 98, 1093-1098. [ Links ]
Tsai, C.-W., Rowhani, A., Golino, D.A., Daane, K.M. & Almeida, R.P.P., 2010. Mealybug transmission of grapevine leafroll viruses: An analysis of virus-vector specificity. Phytopathology 100, 830-834. [ Links ]
Walker, J.T.S., Charles, J.G., Froud, K.J. & Connolly, P., 2004. Leafroll virus in vineyards: Modelling the spread and economic impact. Hort Research Client Report No. 12795, 1-19. [ Links ]
Walton, V.M. & Pringle, K.L., 2004. A survey of mealybugs and associated natural enemies in vineyards in the Western Cape Province, South Africa. S. Afr. J. Enol. Vitic. 25, 23-25. [ Links ]
Walton, V.M., Krüger, K., Saccaggi, D.L. & Millar, I.M., 2009. A survey of scale insects (Sternorrhyncha: Coccoidea) occurring on table grapes in South Africa. J. Insect Sci. 9 (Article 47), 1-6. [ Links ]
Submitted for publication: December 2014
Accepted for publication: March 2015
* Corresponding author: E-mail address: kkruger@zoology.up.ac.za
Acknowledgements: We thank E. Jooste (ARC-Plant Protection Research Institute (ARC-PPRI)) for determining the GLRaV-3 isolate, I. Millar (ARC-PPRI) for the identification of the mealybugs, G. Malherbe (University of Pretoria) for assistance with GLRaV-3 diagnostics, R. Carstens (ARC-Infruitec-Nietvoorbij) and Vititec for providing plants, G. Pietersen (ARC-PPRI) for advice, and two anonymous reviewers for useful comments. This project was supported by Winetech, THRIP (Technology and Human Resources for Industry Programme), ARC-PPRI and the University of Pretoria













