Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Enology and Viticulture
versão On-line ISSN 2224-7904
versão impressa ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.36 no.1 Stellenbosch 2015
Phenolic compound profiles in skins of white wine and table grape cultivars grown in the national grape germplasm resource nursery of China
M.-X. ZhangI, *; C.-H. LiuII; H.-J. NanI; Z. LiIII
IHenan Institute of Science and Technology, Xinxiang, Henan Province, P.R. China 453003
IIZhengzhou Fruit Research Institute, Chinese Academy of Agriculture Science, P.R. China 450009
IIIFood Science and Human Nutrition Department, Institute of Food and Agricultural Sciences, University of Florida, USA 32611
ABSTRACT
The phenolic compound profiles in the skins of white grapes, containing ten wine grape and six table grape cultivars grown in the National Grape Germplasm Resource Nursery at the Zhengzhou Fruit Research Institute of China, were investigated using ultra-high performance liquid chromatography coupled with mass spectrometry (UHPLC-MS/MS). The objective of this study was to evaluate if phenolic compound profiles can be used as indictors to differentiate the quality of wine and table grape cultivars. Significant differences in phenolic compound profiles were observed among these grape cultivar skins. The highest content of total hydroxybenzoic acids, total hydroxycinnamic acids, total flavan-3-ols, total flavones and flavonols and total stilbenes was observed in the skin of the Canada Muscat, Rommel, Kadin parmac, Bacchus and Silvaner cultivars respectively. A great compositional difference was observed among these grape cultivars regarding the individual hydroxycinnamic acids and flavonols. Cluster analysis showed that three table grape cultivars, namely Canada Muscat, Rommel and Kadin parmac, possessed significantly different phenolic compound profiles compared to the other grape cultivars. These results suggested that phenolic compound patterns and contents played important roles in evaluating the quality of table and wine grapes and might provide useful information on grape breeding and winemaking in China.
Key words: Phenolic compounds, table grapes, wine grapes, UHPLC-MS, cluster analysis
INTRODUCTION
Phenolic compounds have been confirmed to play important roles in the quality and functional properties of grapes and wines (Haslam, 1996; Kaplan et al., 2001; Majo et al., 2005; Li et al., 2010). Phenolic compounds are structurally divided into flavonoid and non-flavonoid phenolic compounds. Flavonoids are comprised mainly of anthocyanins, flavan-3-ols, flavones and flavonols, whereas hydroxybenzoic acids, hydroxycinnamic acids and stilbenes are predominant non-flavonoids (Li et al., 2011). Phenolic compound profiles are determined primarily by grape genotypes, and their biosynthesis and accumulation are affected by development stages and growing conditions. During the winemaking process, phenolic compounds are transferred from grapes to wines and experience further evolution during wine ageing (Gil-Munoz et al., 1999; Cozzolino et al., 2004; Jin et al., 2009). Therefore, genotype determines phenolic compound profiles in different grape varieties and provides the corresponding wines with unique sensory attributes (Poudel et al., 2008; Rockenbach et al., 2011). Therefore, understanding phenolic compound profiles in grapes is critical to investigate the properties of different grape varieties. The results could provide useful information on nutritional and quality improvements of grape cultivars in China.
Compared with table grapes, wine grapes normally possess much smaller berry sizes, resulting in bigger skin surfaces (grape surface-to-volume ratio). This feature favours the accumulation of phenolic compounds in grapes and the extraction of more phenolic compounds in wines during the fermentation process (Cantos et al., 2002; Pomar et al., 2005). Moreover, we hypothesised that the phenolic compound composition and content of the grape can be considered an important factor that determines whether grapes can be consumed directly or applied for winemaking. Therefore, we selected ten wine grape and six table grape cultivars grown in the National Grape Germplasm Resource Nursery at the Zhengzhou Fruit Research Institute of China and investigated their phenolic compound profiles using ultra-high performance liquid chromatogram coupled with mass spectrometry (UHPLC-MS/MS). The National Grape Germplasm Resource Nursery at the Zhengzhou Fruit Research Institute was established in 1960, and is located at latitude 34o43' and longitude 113o39'. The average annual temperature is about 14°C. The average relative humidity and annual precipitation are 66% and 636 mm respectively. The soil type of the nursery is loam. This nursery contained more than 1 400 grape varieties and cultivars by 2010. The objectives of this study were to evaluate the effect of genotype variation on phenolic compound profiles, and further to compare the phenolic profile differences between winemaking and table grape varieties. These could provide useful information on the improvement of grape quality and nutritional values and benefit variety cultivation and breeding.
MATERIALS AND METHODS
Chemicals and standards
Methanol, acetic acid and acetonitrile were purchased from Fisher Scientific (Fairlawn, NJ). Ethyl acetate was obtained from the Beijing Chemical Reagent Plant (Beijing, China). The external standards of gallic acid, catechin, quercetin caffeic acid and trans-resveratrol were obtained from Sigma-Aldrich (St. Louis, MI). Millipore water was generated with the Milli-Q Element water purification system (Millipore, Bedford, MA).
Grape samples
All the fully ripened grape cultivars, including ten wine grape cultivars and six table grape cultivars, were harvested from the National Grape Germplasm Resource Nursery at the Zhengzhou Fruit Research Institute at the Chinese Academy of Agricultural Science (Zhengzhou, China) according to their physicochemical properties. The 100 berries in the grape samples were selected randomly from six to eight bunches for each cultivar, and the grape samples that were harvested were separated into skin, flesh and seed fractions immediately. Afterwards, the skin fraction was frozen using liquid nitrogen and immediately ground into a fine powder. The skin powders were stored at -80°C until analysis. The flesh portion was crushed to yield the juice and used to test the total sugar and total acid contents. The total sugar (expressed as glucose) and total acid (expressed as tartaric acid) content analyses followed National Standard method GB15038-2006. The genotype, origins and application of these grape cultivars are listed in supplementary Table 1. In particular, Bacchus, Rommel and Rose Gueen are hybrids of V. vinifera and V. labrusca, whereas the other grape cultivars originated from V. vinifera. Regarding their application, Canada Muscat, Chasselas Napoleon, Koz ousioum, Rommel, Rose Gueen and Kadin parmac are used as table grape cultivars, whereas Gamay Blanc, Sheshi I Bardhe, Bahian chirei, Bela Breza, Vloshi, Grasa de Cotnari, Silvaner, Jiubai, Bacchus and Medovec are cultivars used for wine production. The detailed physicochemical properties of these grape cultivars are shown in Table 1.
Phenolic compound extraction from grape skin
The grape skin powder (2.5 g) was mixed with 25 mL of water:ethyl acetate (1:9 v/v), and shaken at 160 rpm for 30 min at room temperature. The supernatant was collected after centrifuging. The resulting residue was mixed with 25 mL of the same solvent five more times. The resultant supernatants were pooled and the ethyl acetate fraction was collected. Subsequently, the ethyl acetate fraction was evaporated to dryness using a rotary evaporator (Shanghai Shensheng Biotech Co., Ltd, Shanghai, China) and then re-dissolved in 2.5 mL of methanol for UHPLC analysis. Each sample was conducted in triplicate.
UHPLC-MS/MS analyses
An Agilent 1200 UHPLC system consisting of an autosampler, a quaternary pump, a column compartment, a diode array detector (Agilent Technologies, Santa Clara, CA) was interfaced with MSD trap VL mass spectrometry. A reverse-phase Zorbax SB C18 column (3 χ 50 mm, 1.8 μπι, Agilent Technologies) was used to separate flavonoids with the column temperature maintained at 25°C. The binary mobile phase consisted of (A) 1% acetic acid:water (v/v) and (B) 1% acetic acid:acetonitrile (v/v). A 62 min gradient program followed a published method (Jin et al., 2009) and was used as follows: 0 to 10 min, 5 to 8% B; 10 to 18 min, 8 to 10% B; 18 to 40 min, 10 to 15% B; 40 to 50 min, 15 to 20% B; 50 to 53 min, 20 to 30% B; 53 to 58 min, 30 to 50% B; 58 to 62 min, 50 to 100% B. The injection volume was 2 μL with a flow rate of 1.0 mL/min. The wavelength on the diode array detector was 280 nm. Electrospray ionisation in negative mode was performed using nebuliser 30 psi, drying gas 10 mL/min, drying temperature 325°C, and capillary voltage 4 000 V. All scan mass spectra were recorded from m/z 100 to m/z 1 500. Catechin, quercetin, gallic acid, caffeic acid and trans-resveratrol were used as external standard to quantify flavan-3-ols, flavones and flavonols, hydroxybenzoic acid, hydroxycinnamic acid and stilbenes respectively. Data were collected and integrated using Chemstation software (Agilent Technologies, Santa Clara, CA). The identification of phenolic compounds was determined by MS data and further confirmed by comparison with a published work (Jin et al., 2009). The peak number, retention time, mass spectra and identification of the phenolic compounds are listed in supplementary Table 2.
Statistical analyses
Data were expressed as the mean ± standard deviation. Oneway analyses of variance of mean (ANOVA) were performed using SPSS Version 16.0 Statistical Package for Windows (SPSS Corporation, Chicago, IL). A difference of p < 0.05 was considered as significant. Cluster analysis was achieved with the same software using the individual flavonoids as variables.
RESULTS AND DISCUSSION
Phenolic compounds are important compounds that contribute to good quality and favourable sensory attributes of grapes and wines (Li et al., 2009; 2011). More importantly, phenolic compounds have been demonstrated to possess health benefits for humans (Chen et al., 2009; Adams et al., 2010). Grapes contain high levels of phenolic compounds, and these functional compounds can be transferred to wine during the maceration process. During fermentation and ageing, phenolic compounds can undergo a further series of reactions that affect the sensory attributes of final wines, such as colour, mouthfeel, astringency and aroma. According to the components and properties of grape berries, grapes are divided into wine and table grapes. Wine grapes are generally used to make wine, whereas table grapes are suitable for oral consumption. The present study was conducted to investigate whether or not phenolic compounds can be used as an indicator for distinguishing wine and table grapes. To this end, ten wine grape and six table grape cultivars grown in the National Grape Germplasm Resource Nursery at the Zhengzhou Fruit Research Institute of China were selected.
Regarding the physicochemical properties (Table 1), the bunch weights of the wine and table grapes in this study were similar and ranged between 80 g and 150 g, except for Bahian chirei and Silvaner, and Chasselas Napoleon and Rose Gueen in the wine and table grape cultivars respectively. More importantly, the sugar/acid ratio is a common indicator that evaluates the ripeness of grapes and it is known that table grapes display much higher sugar/acid ratios compared to wine grapes (Table 1). The sugar/acid ratios in the table grapes varied from 30 to 40. However, the ratios in the wine grapes were between 14 and 32, except for the Bacchus grape cultivar (a ratio of 59.6).
Comparison of hydroxybenzoic and hydroxycinnamic acid
The majority of phenolic acids in grapes are hydroxybenzoic and hydroxycinnamic acids, and their derivatives such as ester forms. They are initially accumulated before veraison (Khater et al., 2011). These hydroxybenzoic and hydroxycinnamic acids and derivatives are present mainly in the skins and flesh of grape berries, and they play important roles as precursors in the synthesis of volatile phenols (Etievant, 1981; Khater et al., 2011). It also has been reported that the synthesis gene of hydroxybenzoic and hydroxycinnamic acids has the potential to regulate the biosynthesis of proanthocyanidins in berries (Romeyer et al., 1983; Kennedy et al., 2001).
Regarding the content of total hydroxybenzoic acids (Fig. 1A), the highest content was observed in Canada Muscat grape skin, whereas a similar content of total hydroxybenzoic acids was observed among the other grape cultivars. However, it should be noted that Gamay Blanc, Bahian Chirei, Vloshi, Bacchus and Medovec of the wine grape cultivars and Rommel of the table grape cultivars did not detect hydroxybenzoic acids in the skin. Compared to total hydroxycinnamic acids (Fig. 1B), the Rommel grape cultivar skin contained the highest content, followed by Medovec and Silvaner. Bela Breza and Gamay Blanc cultivars of the wine grape cultivars and Rose Gueen of the table grape cultivars did not contain hydroxycinnamic acids, whereas the other grape cultivar skins showed a comparable content of the total hydroxycinnamic acids.
Regarding the individual hydroxybenzoic acids, three hydroxybenzoic acid derivatives were identified, including the hexose ester of p-hydroxybenzoic acid, hexose ester of protecatechuic acid and hexose ester of vanillic acid (Table 2). Their distributions in these grape cultivars were significantly different. For example, the hexose ester of p-hydroxybenzoic acid was observed in the skin of two wine grape cultivars (Grasa de Cotnari and Silvaner) and one table grape cultivar (Koz ousioum). The content of this hydroxybenzoic acid derivative was not high in these three grape cultivar skins (0.10 to 0.23 mg GA/kg FW). Similarly, Canada Muscat and Koz ousioum in the table grape cultivar group contained the hexose ester of vanillic acid in the skin. It should be observed that the accumulation of this derivative was much higher in Canada Muscat skin (3.81 mg GA/kg FW) in comparison with that in Koz ousioum skin (0.10 mg GA/kg FW). Hexose ester of protocatechuic acid was present in the skin of only three wine grape cultivars (Sheshi I Bardhe, Bela Breza and Jiubai) and four table wine grape cultivars (Canada Muscat, Chasselas Napaleo, Rose Gueen and Kadi parmac) (Table 2). Within these cultivars, the contents in the wine grapes and table grapes were between 0.07 and 0.18 mg GA/kg FW, and between 0.11 and 0.35 mg GA/kg FW respectively.
Six individual hydroxycinnamic acids and their derivatives were detected, including p-coumaric acid, p-coumaric acid derivative, hexose ester of caffeic acid, ferulic acid, feruloytartaric acid, and hexose ester of ferulic acid (Table 3). Hexose ester of caffeic acid was observed only in the skin of Rommel, a table grape cultivar, with a content of 0.57 mg CA/kg FW. Bahian chirei and Vloshi wine grape cultivars contained p-coumaric acid derivative with high contents (0.71 and 1.93 mg CA/kg FW respectively), whereas this derivative was not detected in the other grape cultivar skins. Similarly, ferulic acid was observed in two wine grape cultivar skins, namely Grasa de Cotnari and Bacchus, with low contents (between 0.17 and 0.51 mg CA/ kg FW). However, the highest content of ferulic acid existed in the Canada Muscat table grape cultivar skin, at 2.92 mg CA/kg FW. Neither ferulic acid nor the hexose ester of ferulic acid was detected in several wine grape cultivar skins. These cultivars included Gamay Blanc, Sheshi I Bardhe, Bahian chirei, Bela Breza, Vloshi and Jiubai (Table 3). In the meantime, the table grape cultivars, including Koz ousioum and Rose Gueen, also did not contain ferulic acid and its hexose ester. The highest content of hexose ester of ferulic acid was observed in the skin of Medovec (5.63 mg CA/kg FW), followed by Rommel (3.37 mg CA/kg FW).
The contents of hexose ester of ferulic acid detected in the other grape skins ranged from 0.33 to 0.67 mg CA/kg FW. p-Coumaric acid was present in the skin of three wine grape cultivars, Sheshi I Bardhe, Bahian chirei and Vloshi, with the content between 0.20 and 0.39 mg CA/kg FW (Table 3). A similar content of p-coumaric acid was observed in the skin of Koz ousioum table grapes (0.31 mg CA/kg FW). It should be noted that Rommel (a table grape cultivar) grape skin contained a significantly predominant content of p-coumaric acid (4.07 mg CA/kg FW). Feruloytartaric acid was observed at high levels in the skin of Silvaner and Jiubai cultivars, but at low levels in the skin of Sheshi I Bardhe and Koz ousiooum cultivars. However, the other cultivars did not contain feruloytartaric acid in the skins.
Flavan-3-ol comparison
Flavan-3-ols exist widely in grape berries, especially in the skins and seeds (Saito et al., 1998; Jin et al., 2009)Y. Monomeric flavan-3-ols, such as (+)-catechin, (-)-epicatechin and epicatechin gallate, are important substrates for the biosynthesis of proanthocyanidins, which play important roles in the mouthfeel and astringency of grapes and wines (Jin et al., 2009; Li et al., 2011). It has been reported that flavan-3-ols initially were accumulated after fruit set and reached an accumulation peak around veraison (Kennedy et al., 2001; Khater et al., 2011). Like other flavonoids, flavan-3-ols are synthesised via phenylpropanoid metabolism and flavonoid metabolism, and VvMybPA2 was reported to regulate flavan-3-ols biosynthesis in the skins of grape berries (Bogs et al., 2005; Pfeiffer et al., 2006; Khater et al.,, 2011). Due to the limited accumulation of flavan-3-ols and their derivatives (oligomers and polymers), white grapes are always used to yield fresh wines with fruity aroma properties, whereas their favourable flavan-3-ols composition makes red grapes, such as Cabernet Sauvignon, suitable for ageing (Edwin, 1980; Li et al., 2011).
These white grape cultivars do not contain too much flavan-3-ols in the skin. The highest content of total flavan-3-ols was observed in the skin of Kadin parmac, a table grape cultivar, followed by Canada Muscat skin (Fig. 1C). It should be noted that the wine grape cultivars Gamay Blanc, Bela Breza, Vloshi, Grasa de Cotnari and Jiubai contain no flavan-3-ols in the skin, whereas the table grape cultivars, Chasselas Napoleon, Rommel and Rose Gueen, appeared to contain no flavan-3-ols in the skins.
In comparison to the individual flavan-3-ols in these grape skins (Table 4), catechin appeared to exist in many cultivars. Kadin parmac skin showed the highest catechin content (8.96 mg CE/kg FW), followed by Canada Muscat skin (6.86 mg CE/kg FW). It should be noted that these two cultivars are table grape cultivars. The wine grape cultivars that contained catechin had a content ranging from 0.62 to 1.85 mg CE/kg FW. Bahian chirei, a wine grape cultivar, was the only cultivar that contained (epi)-catechin gallate, with a content of 3.55 mg CE/kg FW (Table 4). Moreover, procyanidin dimers and trimers did not exist in almost all the grape cultivar skins. Only two table grape cultivar skins contained dimers and trimers with a relatively high content. For example, Kadin parmac skin had procyanidin dimers 1 and 2, and trimers 1 and 2 of 32.79 and 13.81, and 16.88 and 12.35 mg CE/kg FW respectively. The Canada Muscat skin only contained procyanidin dimer 1 and trimer 1, but the contents were at high levels (19.41 and 12.46 mg CE/ kg FW).
Flavone and flavonol comparison
Flavones and flavonols are accumulated as secondary metabolites in fruits, and are reported to have resistant capacity to UV and pathogens (Cuadra et al., 1997; Jin et al., 2009). They also help to contribute to grape colour via co-pigmentation with anthocyanins, and provide grapes and wines with multiple health benefits, such as antioxidant capacity, anti-cancer properties, and anti-coronary disease properties (Eiattar & Virji, 1999; Murakami et al., 2008).
Flavone and flavonol biosynthesis follows the flavonoid metabolism in grapes. Flavonol synthase appears to be the critical enzyme that regulates flavonol formation (Downey et al., 2003; Mattivi et al., 2006). Flavone synthases play primary roles in synthesising flavones (Dixon & Paiva, 1995). Furthermore, several glucosyltransferases help to conjugate the sugar moiety to aglycones (Mattivi et al., 2006).
Significant differences in the total flavone and flavonol content were observed in these grape cultivar skins (Fig. 1D). Bela Breza and Bacchus showed the highest total flavone and flavonol content in the skin among these cultivars (about 60 mg QE/kg FW), whereas Rose Gueen did not contain flavone and flavonol in the skin. The skins of the other grape cultivars had a total flavone and flavonol content ranging from about 5 to 40 mg QE/kg FW.
Regarding the individual flavones and flavonols in the skin, isoquercitrin and quercetin-3-O-glucuronide appeared to exist in almost all the grape cultivar skins, and their contents were at a high level (Table 5). For example, Bela Breza of the wine grape cultivars and Koz ousioum of the table grape cultivars showed the highest content of isoquercitrin in the skins, with contents of about 14 mg QE/ kg FW. The other cultivar skins had a content of about 1 to 7 mg QE/kg FW. It should be noted that Grasa de Cotnari and Silvaner among the wine grape cultivars and Rose Gueen of the table grape cultivars did not contain isoquercitrin in the skin. Similarly, significant differences in quercetin-3-O-glucuronide were also observed in these grape cultivar skins (Table 5). For example, the highest content of quercetin-3-O-glucuronide was observed in Bacchus skin (53.73 mg QE/ kg FW), a wine grape cultivar. However, the Gamay Blanc wine grape cultivar did not have quercetin-3-O-glucuronide in the skin. Koz ousioum turned out to be the table grape cultivar that showed the highest content of quercetin-3-O-glucuronide in the skin (24.36 mg QE/kg FW), whereas Rose Gueen skin did not contain this flavonol. The other flavones and flavonols were detected specifically in only one or two cultivars (Table 5). For example, astilbin, kaempferol-3-O-glucoside and engeletin were present in Bela Breza and Chasselas Napoleon skins. Hyperoside was observed only in the Bela Breza and Koz ousioum skins. Rommel specifically contained apigenin-O-xyloside, quercetin and quercetin-O-xyloside in the skin, whereas apigenin was observed only in the Kadin parmac cultivar skin.
Stilbene comparison
Stilbene has been proposed to be derived from the phenylpropanoid pathway, and it can be accumulated in response to environmental stresses in plants (Sparvoli et al., 1994, Versari et al., 2001). It has been confirmed by a number of studies that traws-resveratrol, a major stilbene accumulated in grape berries, can prevent cancer and cardiovascular diseases (Martinez & Moreno, 2000; Kuroyanagi et al., 2014).
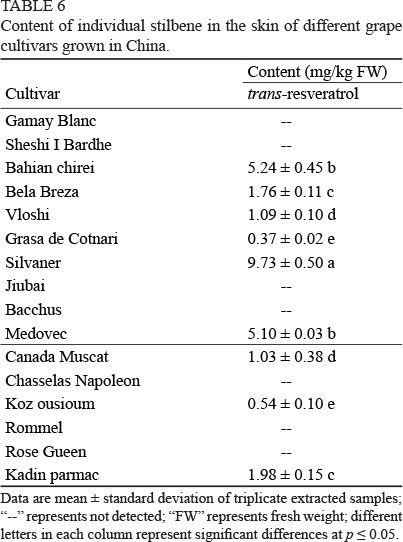
Only traws-resveratrol was detected and its content in these grape cultivar skins was different (Table 6). Gamay Blanc, Sheshi I Bardhe, Jiubai and Bacchus (wine grape cultivars) did not contain traws-resveratrol in their skins. Similarly, table grape cultivars Chasselas Napoleon, Rommel and Rose Gueen also did not contain traws-resveratrol in the skins. However, the highest traws-resveratrol content was observed in the skins of the wine grape cultivars Silvaner (9.73 mg/kg FW), followed by Bahian chirei and Medovac (about 5 mg/kg FW).
Cluster analysis
In order to better understand the phenolic compound profiles of the wine and table grape cultivars, cluster analysis was carried out using all the detected phenolic compounds as variables (Fig. 2). These grape cultivars were clearly divided into two groups in terms of phenolic compound profiles. Group A included Canada Muscat, Kadin parmac and Rommel, whereas the other cultivars were clustered in group B. It should be noted that all the grape cultivars in group A were the table grape types, although their relative distances were not close. These results suggest that their phenolic compound profiles were significantly different from the other grape cultivars, although the phenolic compound profiles in themselves were not similar. In group B, the Bela Breza cultivar was separated significantly from the other cultivars, indicating that its phenolic compound profile was distinct. Such a difference might be caused by the lack of hydroxycinnamic acids and flavan-3-ols, and by the specific flavonols, such as astilbin, kaempferol-3-O-glucoside and engeletin. The Silvaner grape cultivar also showed a long distance from the other grape cultivars in group B. This segregation might be caused by low content of hydroxycinnamic acid, flavan-3-ols and flavonol. In the meantime, much higher contents of traws-resveratrol might also contribute to such a significant difference in phenolic compound profiles. Furthermore, Bahian chirei, Vloshi and Medovec were assembled together at relatively close distances, indicating that they might have similar phenolic compound profiles. Sheshi I Bardhe and Rose Gueen, and Gammy Blanc and Grasa de Cotnari, were clustered at close distances respectively. However, these cultivars were a long distance from the Bacchus and Koz ousioum cultivars in the dendrogram. These results indicate that there was a significant variation in phenolic compound profiles in the grape cultivars.
CONCLUSIONS
In conclusion, the grape cultivar skins investigated showed significant differences in phenolic compound patterns and contents. Canada Muscat had the highest level of total hydroxybenzoic acids, whereas the highest amount of total flavan-3-ols was observed in the skin of Kadin parmac. The total flavonols and total stilbenes existed at the highest level in the skins of Bacchus and Silvaner respectively. Phenolic compound patterns and contents played important roles in differentiating the breeding and quality of these grape cultivars regarding cluster analysis. This study might provide useful references for grape breeding and winemaking in China.
LITERATURE CITED
Adams, L.S., Phung, S., Yee, N., Seeram, N.P., Li, L. & Chen, S., 2010. Blueberry phytochemicals inhibit growth and metastatic potential of MDA- MB-231 breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 70, 3594-3605. [ Links ]
Bogs, J., Downey, M.O., Harvey, J.S., Ashton, A.R., Tanner, G.J. & Robinson, S.P., 2005. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves. Plant Physiol. 139, 652-663. [ Links ]
Cantos, E., Espin, J.C. & Tomas-Barberan, F.A., 2002. Varietal differences among the polyphenol profiles of seven table grape cultivars studied by LC- DAD-MS-MS. J. Agric. Food Chem. 50, 5691-5696. [ Links ]
Chen, L.-G., Huang, W.-T., Lee, L.-T. & Wang, C.-C., 2009. Ellagitannins from Terminalia calamansanai induced apoptosis in HL-60 cells. Toxicol. in Vitro 23, 603-609. [ Links ]
Cozzolino, D., Kwiatkowski, M.J., Parker, M., Cynkar, W.U., Dambergs, R.G., Gishen, M. & Herderich, M.J., 2004. Prediction ofphenolic compounds in red wine fermentations by visible and near infrared spectroscopy. Anal. Chim. Acta 513, 73-80. [ Links ]
Cuadra, P., Harborne, J.B. & Waterman, P.G., 1997. Increases in surface flavonols and photosynthetic pigments in Gnaphalium luteo-album in response to UV-B radiation. Phytochem. 45, 1377-1383. [ Links ]
Dixon, R.A. & Paiva, N.L. Stress-induced phenolpropanoid metabolism. Plant Cell 7, 1085-1097. [ Links ]
Downey, M.O., Harvey, J.S. & Robinson, S.P., 2003. Synthesis of flavonols and expression of flavonol synthase genes in the developing grape berries of Shiraz and Chardonnay (Vitis vinifera L.). Aust. J. Grape Wine Res. 9, 110-121. [ Links ]
Edwin, H., 1980. In vino veritas: Oligomeric procyanidins and the ageing of red wines. Phytochem. 19, 2577-2582. [ Links ]
Eiattar, T.M. & Virji, A.S., 1999. Modulating effect of resveratrol and quercetin on oral cancer cell growth and proliferation. Anti-Cancer Drugs 10, 187-194. [ Links ]
Etievant, P. ., 1981. Volatile phenol determination in wine. J. Agric. Food Chem. 29, 65-67. [ Links ]
Gil-Munoz, R., Gomez-Plaza, E., Martinez, A. & Lopez-Roca, J.M., 1999. Evolution of phenolic compounds during wine fermentation and post-fermentation: Influence of grape temperature. J. Food Compos. Anal. 12, 259-272. [ Links ]
Haslam, E., 1996. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 59, 205-215. [ Links ]
Jin, Z., He, J., Bi, H., Cui, X. & Duan, C., 2009. Phenolic compound profiles in berry skins from nine red wine grape cultivars in northwest China. Molecules 14, 4922-4935. [ Links ]
Kaplan, M., Hayek, T., Raz, A., Coleman, R., Dornfeld, L., Vaya, J. & Aviram, M., 2001. Pomegranate juice supplementation to atherosclerotic mice reduces macrophage lipid peroxidation, cellular cholesterol accumulation and development of atherosclerosis. J. Nutr. 131, 2082-2089. [ Links ]
Kennedy, J.A., Hayasaka, Y., Vidal, S., Waters, E.J. & Jones, G.P., 2001. Composition of grape skin proanthocyanidins at different stages of berry development. J. Agric Food Chem. 49, 5348-5355. [ Links ]
Khater, F., Fournand, D., Vialet, S., Meudec, E., Cheynier, V. & Terrier, N., 2011. Identification and functional characterization of cDNAs coding for hydroxybenzoate/hydroxycinnamate glucosyltransferases co-expressed with genes related to proanthocyanidin biosynthesis. J. Exp. Bot. 63, 1201-1214. [ Links ]
Kuroyanagi, G., Tokuda, H., Matsushima-Nishiwaki, R., Kondo, A., Mizutani, J., Kozawa, O. & Otsuka, T., 2014. Resveratrol suppresses prostaglandin F2a-induced osteoprotegerin synthesis in osteoblasts: Inhibition of the MAP kinase signaling. Arch. Biochem. Biophys. 542, 39-45. [ Links ]
Li, Z., Pan, Q., Cui, X. & Duan, C., 2010. Optimization on anthocyanins extraction from wine grape skins using orthogonal test design. Food Sci. Biotechnol. 19, 1047-1053. [ Links ]
Li, Z., Pan, Q., Jin, Z., He, J., Liang, N. & Duan, C., 2009. Evolution of 49 phenolic compounds in shortly-aged red wines made from Cabernet Gernischt (Vitis vinifera L. cv.). Food Sci. Biotechnol. 18, 1001-1012. [ Links ]
Li, Z., Pan, Q., Jin, Z., Mu, L. & Duan, C., 2011. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 125, 77-83. [ Links ]
Majo, D.D., Giammanco, M., Guardia, M.L., Tripoli, E., Giammanco, S. & Finotti, E., 2005. Flavanones in citrus fruit: Structure-antioxidant activity relationships. Food Res. Int. 38, 1161-1166. [ Links ]
Martinez, J. & Moreno, J.J., 2000. Effect of resveratrol, a natural polyphenolic compound, on reactive oxygen species and prostaglandin production. Biochem. Pharmacol. 59, 865-870. [ Links ]
Mattivi, F., Guzzon, R., Vrhovsek, U., Stefanini, M. & Velasco, R., 2006. Metabolite profiling of grape: Flavonols and anthocyanins. J. Agric. Food Chem. 54, 7692-7702. [ Links ]
Murakami, A., Ashida, H. & Terao, J., 2008. Multitargeted cancer prevention by quercetin. Cancer Lett. 269, 315-325. [ Links ]
Pfeiffer, J., Kühnel, C., Brandt, J., Duy, D., Punyasiri, P.A.N., Forkmann, G. & Fischer, T.C., 2006. Biosynthesis of flavan 3-ols by leucoanthocyanidin 4-reductases and anthocyanidin reductases in leaves of grape (Vitis vinifera L.), apple (Malus x domestica Borkh.) and other crops. Plant Physiol. Bioch. 44, 323-334. [ Links ]
Pomar, F., Novo, M. & Masa, A., 2005. Varietal differences among the anthocyanin profiles of 50 red table grape cultivars studied by high performance liquid chromatography. J. Chromatogr. A 1094, 34-41. [ Links ]
Poudel, P.R., Tamura, H., Kataoka, I. & Mochioka, R., 2008. Phenolic compounds and antioxidant activities of skins and seeds of five wild grapes and two hybrids native to Japan. J. Food Compos. Anal. 21, 622-625. [ Links ]
Rockenbach, Gonzaga, L.V., Rizelio, V.M., Gonçalves, A.E.d.S.S., Genovese, M.I. & Fett, R., 2011. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 44, 897-901. [ Links ]
Romeyer, F.M., Macheix, J.J., Goiffon, J.J., Reminiac, C.C. & Sapis, J.C., 1983. Browning capacity of grapes. 3. Changes and importance of hydroxycinnamic acid-tartaric acid esters during development and maturation of the fruit. J. Agric. Food Chem. 31, 346-349. [ Links ]
Saito, M., Hosoyama, H., Ariga, T., Kataoka, S. & Yamaji, N., 1998. Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 46, 1460-1464. [ Links ]
Sparvoli, F., Martin, C., Scienza, A., Gavazzi, G. & Tonelli, C., 1994. Cloning and molecular analysis of structural genes involved in flavonoid and stilbene biosynthesis in grape (Vitis vinifera L.). Plant Mol. Biol. 24, 743-755. [ Links ]
Versari, A., Parpinello, G.P., Tornielli, G.B., Ferrarini, R. & Giulivo, C., 2001. Stilbene compounds and stilbene synthase expression during ripening, wilting, and UV treatment in grape cv. Corvina. J. Agric. Food Chem. 49, 5531-5536. [ Links ]
Submitted for publication: September 2014
Accepted for publication: December 2014
* Corresponding author: Mingxia Zhang, E-mail address: zhangmingx@163.com [Tel.: +86 373 3040337]; Zheng Li, E-mail address: jameslee0221@ufl.edu [Tel.: (352) 214 5315]
Acknowledgements: This research was supported by the National Natural Science Foundation of China (grant No. 31201410 to M.-X. Z.), and the Special Funds of Modern Industrial Technology System for Agriculture (nycytx-30)













