Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Enology and Viticulture
On-line version ISSN 2224-7904
Print version ISSN 0253-939X
S. Afr. J. Enol. Vitic. vol.35 n.1 Stellenbosch 2014
Effect of bottle storage on colour, phenolics and volatile composition of Malvasia and Moscato white wines
A. Del CaroI,; P. PiombinoII; A. GenoveseII; L. MoioII; C. FanaraI; A. PigaI
IDipartimento di Agraria, Università degli Studi di Sassari, Viale Italia 39/A, 07100 Sassari, Italy
IIDipartimento di Agraria, Università degli Studi di Napoli Federico II, via Università 100, 80055 Portici (NA), Italy
ABSTRACT
The effect of bottle storage on the colour, phenolics and volatile composition of Malvasia and Muscat wines obtained from grapes grown in Sardinia was evaluated. Colour was evaluated by UV-VIS spectrophotometry and by tristimulus colorimetry. Polyphenols were analysed by UV-VIS spectrophotometry and HPLC-DAD. GC/MS was used to identify and quantify the content of free and bound volatile compounds. As expected, the absorbance values at 420 nm increased significantly for both wines during storage, due to oxidative browning, while difference in colour (DE*) from the beginning of storage and after 18 months was more intense in the Muscat wine than in the Malvasia wine. A significant decrease was observed in different phenolic compounds over time, especially in the Malvasia wine. In-bottle storage for 18 months at 20°C in the dark resulted in a significant decrease in all the classes of free and bound volatiles. These finding enhance knowledge regarding the effects of bottle storage on Muscat and Malvasia wines. This is of interest because, rather surprisingly, this topic has been poorly investigated in relation to these two varieties.
Key words: Bottle storage, colour, phenolics, white wines
INTRODUCTION
The continuous changes in phenolic composition, colour and volatile compounds occurring during the bottle storage of white wine have been studied extensively (González-Vinas et al., 1996; De la Presa-Owens & Noble, 1997; Cejudo-Bastante et al., 2011; Zafrilla et al., 2003; Recamales et al., 2011). It is well known that the oxidation of phenols to quinones and their polymerisation results in yellow-brown macromolecules that are responsible for wine browning reactions (Singleton, 1987; Li et al., 2008). Another reaction that potentially contributes to the browning of white wine is the conversion of flavonols into yellow xanthylium pigments that increase the absorption in the 400 to 500 nm spectra region (Es-Safi et al., 2000). In particular, oxidative browning of white wines seems to be related more to the flavanols to which it is subjected during storage than to oxidation and polymerisation reactions (Hernanz et al., 2009; Kallithraka et al., 2009). In fact, many studies report significant losses in phenolic content during bottle storage, in particular of flavan-3-ols and flavonols (Pérez-Magarino & Gonzales-San José, 2001; Zafrilla et al., 2003; Cejudo-Bastante et al., 2013). A study by Cheynier et al. (1990) showed that other compounds undergoing oxidation and browning during storage are the hydroxycinnamic acid derivatives, but controversial results were published after this study. In fact, Recamales et al. (2006) reported a decrease in the concentration of the tartaric acid esters and an increase in their respective acids, while Mayen et al. (1997) and Cejudo-Bastante et al. (2011) had opposite results.
Flavonol concentration decreases during storage due to oxidative degradation (De Beer et al., 2005), while its aglycons increase due to hydrolysis reactions (Zafrilla et al., 2003; Fang et al., 2007). White wine colour changes during storage are the result of a concomitant increase in the chroma (C*) and decrease of the hue (h*) (Recamales et al., 2006; Hernanz et al., 2009). Moreover, a shift from pale yellow to yellow-brown as a result of an increase in a* and b* values has been reported (Cejudo-Bastante et al., 2011).
With regard to colour changes, a loss of sensory attributes of young wines during storage, such as fresh, floral, citrus and fruity, and the development of new ones, like biscuit, honey, toffee, toast, nutty and others, have been reported (Skouroumounis et al., 2005; Bueno et al., 2010). This sensory evolution has been correlated with the change in several volatile compounds and it has been pointed out that chemical hydrolysis reactions result in concentration fluctuations in various fermentation-derived esters, thus resulting in the loss of the fresh fruity characters (Leino et al., 1993; González-Vinas et al., 1996; De la Presa-Owens & Noble, 1997; Recamales et al., 2011). More particularly, González-Viñas et al. (1996) provided evidence for the loss of fruity attributes after 18 months of storage under commercial conditions, while high storage temperatures result in wine aroma changes after only five days (De la Presa-Owens & Noble, 1997). Moreover, the increase in concentration of long-chain esters and the decrease of acetates, such as isoamyl acetate, isopentyl acetate and 2-phenylethyl acetate, have been demonstrated (González-Viñas et al., 1996; Cejudo-Bastante et al., 2011). In general, however, the concentration of most of the wine volatile compounds decreases after one year of bottle storage (Cejudo-Bastante et al., 2011), and the major parameters influencing the rate of volatile change in concentration during ageing are temperature (Leino et al., 1993; De la Presa-Owens & Noble, 1997; Bueno et al., 2010; Robinson et al., 2010; Recamales et al., 2011; Butzke et al., 2012; Makhotkina & Kilmartin, 2012) and wine packaging materials (Fu et al., 2009; Mentana et al., 2009; Ghidossi et al., 2012; Hopfer et al., 2012). Research done on aroma evolution has been directed mainly at non-aromatic grapes (Airen, Chardonnay, Riesling, Sauvignon blanc), while little information is present on wine produced from aromatic grapes such as Muscat and Malvasia (Pérez-Magarino et al., 2013).
This study thus was carried out with the aim to evaluate the effect of 18 months of bottle ageing on colour, phenolic content and free and glycosylated volatiles of two white wines obtained from two important aromatic grapes of Sardinia, namely Malvasia of Bosa, which is a synonym for Malvasia of Sardegna (number 7266 in the Vitis International Variety Catalogue), and Muscat of Sorso-Sennori, which is a synonym for Moscato Bianco (number 8193 in the Vitis International Variety Catalogue). To the best of our knowledge, no data on the bottle ageing of these wines are available in the literature.
MATERIALS AND METHODS
Wine samples
The Malvasia (MV) was produced by an important Sardinian winery, while the Muscat (MS) was obtained from a north Sardinian cooperative wine growers' association. Grape harvesting took place in the second half of October 2008 at 25.5 (MV) and 28°Brix (MS). MV grapes (around 3 600 kg) were crushed, 5 g/100 kg of SO2 were added and the must was left overnight for skin contact (12°C), after which it was drained and inoculated with selected yeast (US-01, Unistrains SrL, Sassari, Italy) at 30 g/hL. Fermentation was carried out in stainless steel tanks of 1 000 L capacity at 18 to 20°C, and racking was performed at a residual sugar level of 3°Brix. Fermentation was stopped by chilling at -3°C, followed by stabilisation.
After crushing, MS grapes (around 4 000 kg) were given 5 g/100 kg of SO2, 3 g/100 kg of pectic enzyme and 15 g/100 kg of tannins to protect the must from oxidation (Cejudo-Bastante et al., 2010), and 6 g/100 kg of ascorbic acid. After cooling at 19°C, inoculation took place at 30 g/hL, following yeast rehydration (Uva ferm Ghm, Lallemand, Montreal, Canada) in warm water for 30 min, as suggested by the manufacturer. Fermentation was carried out with skin contact in stainless steel tanks of 1 000 L capacity at 19 to 20°C, until the initial sugar content had been halved. Racking was carried out when a residual sugar level of 8°Brix was attained, while fermentation was stopped by chilling at -3°C, followed by stabilisation.
The MS and MV wines were bottled six months after winemaking. Bottles were transparent, had a capacity of 750 mL and were sealed with cork stoppers. The bottles were stored in an upright position in the dark at a controlled temperature of 20°C and sampled at the beginning of the experiment and after 18 months of bottling. We decided to store the bottles for 18 months as we think this is a reasonable period, due to the fact that the maximum storage life for the optimal quality of young white wines stored in glass bottles has been suggested to be 24 months (Gonzalez-Vinas et al., 1996).
Colour measurement
Wine colour was assessed using the CIELAB space colour system with a tristimulus colorimeter Minolta CR-300. The following parameters were evaluated: L*, an approximate measure of lightness, a* and b* coordinates, which represent the red-green colour and yellow-blue colour respectively, C* (Chroma), which is considered the quantitative attribute of colourfulness, and h*, which represents the qualitative attribute of the colour. To evaluate differences in colour between the beginning of storage and after 18 months, the DE*ai was calculated using the Euclidean distance, as reported in Gamasa et al. (2009). The absorbances at 420 and 520 nm were determined spectrophotometrically (Hewlett Packard mod. 8453, Palo Alto, CA) (Iland et al., 2004). A total of 10 readings were taken for each sample.
Analyses of polyphenols by spectrophotometry
Spectrophotometric analyses of polyphenols were carried out according to Di Stefano et al. (1989). These methods are able to eliminate interferences due to salts, sugars and proteins by isolating the phenolic compounds on a Sep-Pak C18 cartridge. A UV-VIS spectrophotometer was used (HP 8453 Palo Alto, CA). The following parameters were determined: total polyphenols (mg/L of catechin), vanillin index (mg/L of catechin) and proanthocyanidins (mg/L cyanidin). Analyses were done in triplicate.
Extraction and HPLC-DAD analyses of polyphenols
Wine samples were filtered through 0.22 μm cellulose acetate filters according to La Torre et al. (2008), and injected directly into a liquid chromatograph (Hewlett-Packard Series 1050, Palo Alto, CA) coupled with an HP 1050 diode array detector. The column used was a LiChrosphere C18, 4 mm x 250 mm, 5 μηι; 20 μL loop; 0.5 mL/min flow; mobile phase: A) 50 mM NH4H2PO4 solution brought to pH 2.6 with H3PO4, B) 80% CH3CN and 20% phase A, C) 200 mM H3PO4. The phenols were monitored at three different wavelengths: 280 nm for catechins, 316 nm for hydroxycinnamic acids and 365 nm for flavonols. The compounds were quantified by calibration with the following pure standards: gallic acid, catechin, epicatechin, protocatechuic acid, caffeic acid, p-coumaric acid, ferulic acid, tyrosol and trans-caftaric acid, all provided by Sigma Chemical Co. (St. Louis, MO). The quercetin 3-glucuronide, for which there is not a standard, was quantified as rutin equivalent and the ethyl ester of caffeic acid as caffeic acid. Every sample was analysed twice.
Extraction and gas chromatography-mass spectrometry (GC-MS) analysis of volatiles
Free and glycosylated volatile compounds were extracted from the wines according to the solid phase extraction methods proposed by Di Stefano (Di Stefano, 1991; Mateo et al., 1997) and subsequently modified (Moio et al., 2004; Piombino et al., 2010; Del Caro et al., 2012). Twenty-five mL of wine were diluted with the same amount of water and 2-octanol was added as internal standard (125 mL of a 200 mg/L methanol solution), after which it was loaded on an activated 1 g C-18 cartridge (Phenomenex, Torrence, CA) and passed through at 3 mL/min. The cartridge was then washed with 10 mL of water. The free volatile compounds were eluted with 5 mL of dichloromethane, and then 10 mL of methanol were added for the recovery of the glycoconjugated fraction (bound volatiles). The dichloromethane fraction was concentrated to dryness with Na2SO4 and then reduced to a small volume (ca. 100 μL) with nitrogen flushing.
The methanol fraction was dried with a rotary evaporator and dissolved in 5 mL of phosphate-citrate buffer containing 40 mg of Novaromtm Blanc β-glucosydase enzyme at pH 5.0 (Novozymes, Bagsvaerd, Denmark). After 16 h of incubation at 40 ± 2°C, 125 mL of an alcoholic solution of 2-octanol was added as internal standard, and the mixture containing free aglycons was loaded on a C-18 SPE cartridge. The volatiles were extracted with 5 mL of dichloromethane. The extract was dried over Na2SO4 and concentrated under N2 (1.5 L/min) for GC-MS analysis. Each extraction was carried out in triplicate.
GC-MS analysis was done with a GC/MS-QP2010 mass spectrometer (Shimadzu, Shimadzu Corp., Kyoto, Japan) in split/splitless mode and with a DB-WAX column (60m x 0.250 i.d., 0.25 μπι film thickness; J&W Scientific Inc., Folsom, CA 95360, USA). The oven temperature was set at 40°C for 5 min and then raised at 2°C/min to 220°C, and it was held at maximum temperature for 20 min. Carrier gas (He) flow was 1.02 mL/min. Injections of 1 μL were performed and the injector port and ion source were maintained at 250°C and 230°C respectively. Positive electron impact spectra were recorded at 70 eV in the range m/z 33 to 350. The identification of compounds was confirmed by injection of pure standards and comparison of their retention indices and MS data reported in the literature, and the mass spectra stored in the NIST database were compared with those obtained for each compound. Compounds for which pure reference standards were not available were identified only on the basis of their retention times and MS spectra.
Odour threshold values (OTV) reported in literature were used to calculate the odour activity value (OAV) of the most relevant volatiles detected, by dividing the concentration detected by the OTV (Guth, 1997; Ferreira et al., 2000; Vilanova & Sieiro, 2006).
Statistical analysis
Data on colour, phenolic compounds and volatile compounds were evaluated by one-way ANOVA (Statistica), with storage time being used as the group variable. Means, when significant, were separated using LSD Fisher's test (p < 0.05).
RESULTS AND DISCUSSION
Colour changes during storage
One-way ANOVA applied to the MS and MV data showed that the absorbance at 520 nm did not present significant changes during storage in both wines, while the absorbance values at 420 increased significantly (Fig. 1). This last value is widely used as a marker of white wine browning, and in fact represents the true estimation of yellow-brown pigments formed in wines during storage. Both wines showed evidence of an increase in this index due to oxidative browning (Zoecklein et al., 1995; Iland et al., 2004).
Regarding the CIELAB parameters, a not significant decrease of h* was observed for both wines, as already reported (Recamales et al., 2006). There was a significant increase of a* coordinate in the MS wine but not in the MV wine, probably due to the higher content of flavan-3-ols that could react with the glyoxylic acid that arose from tartaric acid oxidation to form more xanthylium salts (yellow-orange pigments) (Es-Safi et al., 2000). The difference in colour (DE*) from the beginning of storage to after 18 months was more intense in the MS wine than in the MV wine (6.30 and 2.98 respectively). It seems that, in general, differences in colour greater than 3 units permit the human eye to discriminate the changes in wine colour (Martinez et al., 2001; Gamasa et al., 2009).
Polyphenol changes during storage
Data on the spectrophotometric analyses of polyphenols are reported in Table 1. MS had a higher content of all polyphenol classes than the MV wine, particularly of proanthocyanidins, which, due to their high oxidability (Hernanz et al., 2009; Kallithraka et al., 2009), conferred a more intense brown colour on the MS wines (Fig. 1). This content could be related both to the tannin addition after grape crushing and to the proanthocyanidin content of MS grapes. Total polyphenols did not change significantly during storage, in contrast to what has been observed in Chardonnay and Chenin blanc white wines, even if the storage temperature of these wines was different (0 °C, 15 °C and 30 °C versus 20°C) (De Beer et al., 2005). The only significant decrease was for procyanidins in the MV wine, even though their value was very low at the beginning of bottling. The decrease is generally ascribed to polymerisation, oxidation and polysaccharide interaction reactions occurring during storage (Cheynier et al., 1990; Gómez-Plaza et al., 2002; Kallithraka et al., 2009). The vanillin index remained constant in both wines, thus confirming the stability of flavanol during 12 months of storage reported by de Beer et al. (2005). HPLC analysis showed a significant decrease in different phenolic compounds over time, while a limited number of them increased (Figs 2 and 3). In particular, a significant decrease in trans-caftaric acid and a increase in caffeic and p-coumaric acid were registered for the MS wine (Fig. 2), in accordance with data in the literature (Recamales et al., 2006; Hernanz et al., 2009; Kallithraka et al., 2009; Cejudo-Bastante et al.,, 2011). The decrease in the trans-caftaric acid content could be due to degradation reactions such as hydrolysation of the esters to their corresponding acids (Ivanova et al., 2011), oxidations and complexation (Zafrilla et al., 2003). The MV wine showed a significant decrease in trans-caftaric acid and caffeic acid content and a significant increase in the caffeic ethyl ester (Fig. 3) (Hernanz et al., 2009).
MS wine underwent a significant decrease in catechin and epicatechin over time, with the latter also decreasing significantly in MV, thus confirming the data in the literature (Recamales et al., 2006; Hernanz et al., 2009; Kallithraka et al., 2009). Moreover, the decrease in flavan-3-ols during storage was due to their strong influence on the susceptibility of white wines to oxidative browning, leading to oxidation and polymerisation reactions (Simpson, 1982). During wine ageing, flavanols can react with glyoxylic acid, an oxidation product of tartaric acid, to give rise to coloured pigments such as xanthylium salts (Es-Safi et al., 2000). The results obtained here confirmed the change in colour for the two wines, in particular the MS wine, whereas the a* coordinate increased significantly during storage. The flavonol quercetin-3-glucuronide was found only in the MS wine and it decreased during storage, presumably due to oxidative degradation, precipitation or hydrolysis, as reported in other papers (Mayen et al., 1997; Zafrilla et al., 2003; Recamales, 2006; Ivanova et al., 2011). This last reaction leads to the formation of aglycons, which can precipitate more easily due to their low solubility, as reported in Zafrilla et al. (2003).
Volatile compounds
A total of 52 free volatile compounds were detected in the MV and MS wines, although two were present only in traces (Tables 2 and 3). Fifteen higher alcohols, three C-6 alcohols, thirteen terpenoids, thirteen esters, three acids, two lactones, one aldehyde, one volatile phenol and one other compound were identified. Alcohols, esters and acids were the main compounds in both wines, as reported in a previous paper (Del Caro et al., 2012).
In-bottle storage for 18 months at 20°C in the dark resulted in a significant decrease in all the classes of free volatiles (Tables 2 and 3). Only ethyl lactate and ethyl succinate increased a little, likely due to a spontaneous malolactic fermentation that occurred in the MS wine. An interesting increase in linalool and α-terpineol was detected in the MV wine. C-6 alcohols and higher alcohols underwent a significant decrease in both wines, as already observed by other authors (Recamales et al., 2011), even if an increase in C-6 alcohols has been reported by some authors and explained as the product of ester hydrolysis (Pérez-Coello et al., 2003; Makhotkina et al., 2012). The decrease in alcohols, as already reported by Oliveira et al. (2008) in relation to Alvarinho wines, may be attributed to the fact that this class of compounds is involved in different reactions, such as oxidation and esterification, resulting in a modification of the base wine aroma.
Ethyl and acetate esters decreased in both wines. The hydrolysis mechanism has been shown to explain the reduction in the ethyl esters of fatty acids and acetate esters during wine storage (Ferreira et al., 1997; Pérez-Coello et al., 2003; Roussis et al., 2005; Garde-Cerdán et al., 2008; Papadopoulou & Roussis 2008; Hernanz et al., 2009; Robinson et al., 2010; Makhotkina et al., 2012), while esterification can result in the formation of ethyl esters of branched acids during bottle storage (Ferreira et al., 1997; Pérez-Coello et al., 2003; Garde-Cerdán et al., 2008; Hernanz et al., 2009; Robinson et al., 2010; Makhotkina et al., 2012). This behaviour has been related to the pH, but also to the temperature of storage. The observed decrease in ester concentration usually corresponds to the lower perception of the fruity character, typical of young wines.
Total terpenes decreased significantly in both wines, as already reported (Rapp & Marais, 1993), but, as stated above, linalool and α-terpineol increased in the MV wine, probably due to conversion from glycosylated compounds in the case of linalool (Rapp, 1988) or from geraniol and linalool in the case of α-terpineol (Stevens et al., 1972). This evidence is interesting because of the possible positive impact of these floral aroma compounds on the sensory profile of the aged MV wine. Fatty acids decreased significantly during bottle storage, in contrast to what has been reported in the literature (Hernanz et al., 2009; Recamales et al., 2011; Pérez-Magarino et al., 2013). Minor compounds such as lactones, volatile phenols and aldehydes had also decreased by the end of the storage period.
Concerning bound volatiles, a total of 26 compounds, one only in traces, were detected in the MS and MV wines (Table 4 and 5). We found three C-6 alcohols, nine higher alcohols, 13 terpenes and two aldehydes. Bound compounds are flavourless precursor compounds, thus are a reservoir of flavour. After 18 months, all the compounds had undergone a drastic reduction, as shown in Tables 4 and 5. About 90% of the total bound volatiles were detected both in the MS and MV wines.
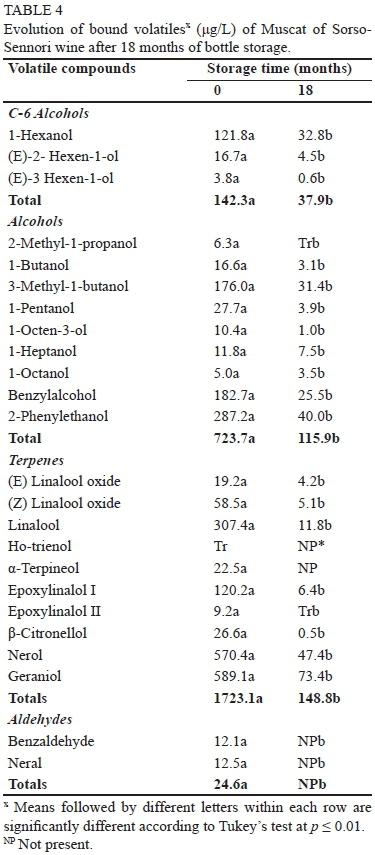
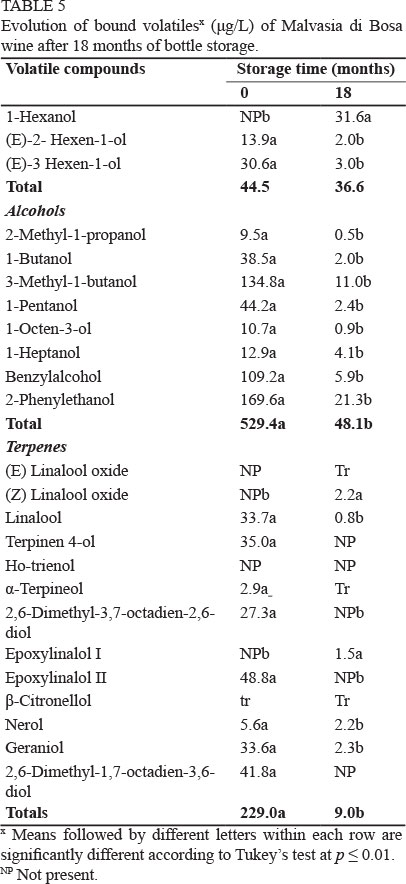
The general loss of esters and terpenes is very important in these wines, as these compounds are responsible for the fresh, floral and fruity notes of wines. Terpenes, on the other hand, are responsible for the characteristic varietal aroma of Muscat and other aromatic wines such as Malvasia (Rapp et al., 1986; Pisarnitskii, 2001; Selli et al., 2006). As can be noticed in Table 6, different aroma compounds of both wines evolved under their OTV after the storage period, thus both wines lost some of their most distinctive sensory properties. Before ageing, Muscat wine was characterised, according to the OAV (Table 6), by 3-methylbutyl acetate, ethyl octanoate, ethyl hexanoate and linalool, while Malvasia wine was particularly rich in ethyl octanoate, which may confer fruity, banana, pineapple, peach and sweet notes, and in 3-methylbutyl acetate, which may impart banana flavour (Genovese et al., 2007). After ageing, the total disappearance of geraniol can confer a floral/citric note. On the other hand, the increase of ethyl lactate in MS can enhance the overripe fruit flavour of the wine (Pérez-Coello et al., 2003).
CONCLUSIONS
This work provided new knowledge on changes during the bottle ageing of two important white wines, namely Muscat and Malvasia. Despite the reductions in compounds typical of bottle storage, we detected many changes in colour, phenolic and volatile compounds. Wine colour showed an increase of absorption in the 400 to 500 nm region and an increase of A420 due to oxidative browning. Bottle storage also influenced the phenolic content, which showed a decrease, in particular in the MV wine, of around 25%. This was probably due to the scarce antioxidant protection of the flavan-3-ols, which were present in this wine at a very low concentration.
Free and bound volatile compounds generally decreased after 18 months of storage (about 60% of free volatiles and about 90% of bound volatiles, both in the MS and MV wines), leading to a loss of the distinctive aromatic properties of these two wines, even if linalool and α-terpineol concentrations increased in the MV wine. The MV wine had the largest amount of free aromas, while the MS had a larger amount of bound volatiles. The two wines should be consumed before 18 months of storage if we want to preserve their optimal colour and, particularly, their sensory properties.
LITERATURE CITED
Bueno, M., Culleré, L., Cacho, J. & Ferreira, V., 2010. Chemical and sensory characterization of oxidative behavior in different wines. Food Res. Int. 43, 1423-1428. [ Links ]
Butzke, C.E., Vogt, E.E. & Chacon-Rodriguez, L., 2012. Effect of heat exposure on wine quality during transport and storage. J. Wine Res. 23, 15-25. [ Links ]
Cejudo-Bastante, M.J., Hermosín-Gutierrez, I., Castro-Vazquez, L.I. & Pérez-Coello, M.S., 2011. Hyperoxygenation and bottle storage of Chardonnay white wines: Effects on color-related phenolics, volatile composition, and sensory characteristics. J. Agric. Food Chem. 59, 4171-4182. [ Links ]
Cejudo-Bastante, M.J., Hermosín-Gutierrez, I. & Pérez-Coello, M.S., 2013. Monitoring of chemical parameters of oxygen-treated musts during alcoholic fermentation and subsequent bottle storage of the resulting wines. Eur. Food Res. Technol. 236, 77-88. [ Links ]
Cejudo-Bastante, M.J., Sonni, F., Chinnici, F., Versari, A., Pérez-Coello, M.S. & Riponi, C., 2010. Fermentation of sulphite-free white musts with added lysozyme and oenological tannins: Nitrogen consumption and biogenic amines composition of final wines. LWT, Food Sci. Technol., 43, 1501-1507. [ Links ]
Cheynier, V., Rigaud, J., Souquet, J., Duprat, F. & Moutounet, M., 1990. Must browning in relation to the behaviour of phenolic compounds during oxidation. Am. J. Enol. Vitic. 41, 346-349. [ Links ]
De Beer, D., Joubert, E., Gelderblom, W.C.A. & Manley, M., 2005. Changes in the phenolic composition and antioxidant activity of Pinotage, Cabernet Sauvignon, Chardonnay and Chenin blanc wines during bottle ageing. S. Afr. J. Enol. Vitic. 26, 6-15. [ Links ]
De la Presa-Owens, C. & Noble, A.C., 1997. Effect of storage at elevated temperatures on aroma of Chardonnay wines. Am. J. Enol. Vitic. 48, 310-316. [ Links ]
Del Caro, A., Fanara, C., Genovese, A., Moio, L., Piga, A. & Piombino, P., 2012. Free and enzymatically hydrolysed volatile compounds of sweet wines from Malvasia and Muscat grapes (Vitis vinifera L.) grown in Sardinia. S. Afr. J. Enol. Vitic. 1, 115-121. [ Links ]
Di Stefano, R., 1991. Proposal for a method of sample preparation for the determination of free and glycoside terpenes of grapes and wines. B. O. I. V. 721-722, 219-223. [ Links ]
Di Stefano, R., Cravero, M.C. & Gentilini, N., 1989. Metodi per lo studio dei polifenoli dei vini. L'Enotecnico 5, 83-89. [ Links ]
Es-Safi, N., Guerneve, C.L., Fulcrand, H., Cheynier, V. & Moutounet, M., 2000. Xanthylium salts formation involved in wine colour change. Int. J. Food Sci. Technol. 35, 63-74. [ Links ]
Fang, F., Li, J.M., Pan, P.H. & Huang, W.D., 2007. Determination of red wine flavonols by HPLC and effect of aging. Food Chem. 101, 428-433. [ Links ]
Ferreira, V., Escudero, A., Fernández, P. & Cacho, J.F., 1997. Changes in the profile of volatile compounds in wines stored under oxygen and their relationship with the browning process. Z. Lebensm. Unters. F. A. 205, 392-396. [ Links ]
Ferreira, V., Lopez, R. & Cacho, J.F., 2000. Quantitative determination of the odorants of young red wines from different grape varieties. J. Sci. Food Agr. 80, 1659-1667. [ Links ]
Fu, Y., Lim, L.T. & McNicholas, P.D., 2009. Changes on enological parameters of white wine packaged in bag-in-box during secondary shelf life. J. Food Sci. 74, C608-C618. [ Links ]
Gamasa, C.S., Hernandez, B., De Santiago Juan, V., Coro, A., Alfonso, S. & Dineiro, J.M., 2009. Measurement of the colour of white and rosé wines in visual tasting conditions. Eur. Food Res. Technol. 229, 263-276. [ Links ]
Garde-Cerdán, T., Marsellés-Fontanet, A.R., Arias-Gil, M., Ancín- Azpilicueta, C. & Martín-Belloso, O., 2008. Effect of storage conditions on the volatile composition of wines obtained from must stabilized by PEF during ageing without SO2. Innov. Food Sci. Emerg. 9, 469-476. [ Links ]
Genovese, A., Gambuti, A., Piombino, P. & Moio, L., 2007. Sensory properties and aroma compounds of sweet Fiano wine. Food Chem. 103, 1228-1236. [ Links ]
Ghidossi, R., Poupot, C., Thibon, C., Pons, A., Darriet, P., Riquier, L., De Revel, G. & Mietton Peuchot, M., 2012. The influence of packaging on wine conservation. Food Control 23, 302-311. [ Links ]
Gómez-Plaza, E., Gil-Munõz, R., López-Roca, J.M., Martínez-Cutillas, A. & Fernández-Fernández, J.I., 2002. Maintenance of colour composition of a red wine during storage: Influence of prefermentative practices, maceration time and storage. Lebensm. Wiss. Technol. 35, 46-53. [ Links ]
González-Viñas, M.A., Pérez-Coello, M.S., Salvador, M.D., Cabezudo, M.D. & Martín-Alvarez, P.J., 1996. Changes in the gas-chromatographic volatiles of young Airen wines during bottle storage. Food Chem. 56, 399-403. [ Links ]
Guth, H., 1997. Quantitation and sensory studies of character impact odorants of different white varieties. J. Agric. Food Chem. 45, 3027-3032. [ Links ]
Hernanz, D., Gallo, V., Recamales, A.F., Meléndez-Martínez, A.J., González-Miret, M.L. & Heredia, F.J., 2009. Effect of storage on the phenolic content, volatile composition and colour of white wines from the varieties Zalema and Colombard. Food Chem. 113, 530-537. [ Links ]
Hopfer, E., Eleber, S. & Heymann, H., 2012. The combined effects of storage temperature and packaging type on the sensory and chemical properties of Chardonnay. J. Agric. Food Chem. 60, 10743-10754. [ Links ]
Iland, P., Bruer, N., Edwards, G., Weeks, S. & Wilkes, E., 2004 (1st ed). Techniques and concepts. Chemical analysis of grapes and wine. Patrick Iland Wine, Campbelltown. [ Links ]
Ivanova, V., Vojnoski, B. & Stefova, M., 2011. Effect of the winemaking practices and aging on phenolic content of Smederevka and Chardonnay wines. Food Bioprocess Technol. 4, 1512-1518. [ Links ]
Kallithraka, S., Salacha, M.I. & Tzourou, I., 2009. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 113, 500-505. [ Links ]
La Torre, G., La Pera, L., Rando, R., Lo Turco, V., Di Bella, G., Saitta, M. & Dugo, G., 2008. Classification of Marsala wines according to their polyphenol, carbohydrate and heavy metal levels using canonical discriminant analysis. Food Chem. 110, 729-734. [ Links ]
Leino, M., Francis, I.L., Kallio, H. & Williams, P.J. 1993. Gas chromatographic headspace analysis of Chardonnay and Semillon wines after thermal processing. Z. Lebensm. F. A. 197, 29-33. [ Links ]
Li, H., Guo, A. & Wang, H., 2008 Mechanisms of oxidative browning of wine. Food Chem. 108, 1-13. [ Links ]
Makhotkina, A. & Kilmartin, P.A., 2012. Hydrolysis and formation of volatile esters in New Zealand Sauvignon blanc wine. Food Chem. 135, 486-493. [ Links ]
Makhotkina, A., Pineau, B. & Kilmartin P.A., 2012. Effect of storage temperature on the chemical composition and sensory profile of Sauvignon Blanc wines. Aus. J. Grape Wine Res. 18, 91-99. [ Links ]
Martinez, J.A., Melgosa, M., Perez, M.M., Hita, H. & Neguerela, A.I., 2001. Note. Visual and instrumental color evaluation in red wines. Food Sci. Technol. Int. 7, 439-444. [ Links ]
Mateo, J.J., Gentilini, N., Huerta, T., Jimenez, M. & Di Stefano, R., 1997. Fractionation of glycoside precursors of aroma in grape and wine. J. Chromat. A 778, 219-224. [ Links ]
Mayen, M., Baron, R., Merida, J. & Medina, M., 1997. Change in phenolic composition during accelerated browning in white wine from cv. Pedro Ximenez and cv. Baladi grapes. Food Chem. 58, 89-95. [ Links ]
Mentana, A., Pati, S., La Notte, E. & Del Nobile, M.A., 2009. Chemical changes in Apulia table wines as affected by plastic packages. Lebensm.- Wiss. Technol. 42, 1360-1366. [ Links ]
Moio, L., Ugliano, M., Gambuti, A., Genovese, A. & Piombino, P., 2004. Influence of clarification treatments on the concentrations of selected free varietal aroma compounds and glycoconjugates in Falanghina (Vitis vinifera L.) must and wine. Am. J. Enol. Vitic. 55, 7-12. [ Links ]
Oliveira, M.J, Oliveira, P., Baumes, R. & Maia, O., 2008. Changes in aromatic characteristics of Loureiro and Alvariho. J. Food Compos. Anal. 21, 695-707. [ Links ]
Papadopoulou, D. & Roussis, I.G., 2008. Inhibition of the decrease of volatile esters and terpenes during storage of a white wine and a model wine medium by glutathione and N-acetylcysteine. Int. J. Food Sci. Technol. 43, 1053-1057. [ Links ]
Pérez-Coello, M.S., González-Vinas, M.A., Garcia-Romero, E., Díaz-Maroto, M.C., & Cabezudo, M.D., 2003. Influence of storage temperature on the volatile compounds of young white wines. Food Control 14, 301-306. [ Links ]
Pérez-Magariño, S. & González-San José, M.L., 2001. Influence of commercial pectolytic preparations on the composition and storage evolution of Albillo white wine. Int. J. Food Sci. Technol. 36, 789-796. [ Links ]
Pérez-Magarino, S., Ortega-Heras, M., Martínez-Lapuente, L., Guadalupe, Z. & Ayestarán, B., 2013. Multivariate analysis for the differentiation of sparkling wines elaborated from autochthonous Spanish grape varieties: Volatile compounds, amino acids and biogenic amines. Eur. Food Res. Technol. 236, 827-841. [ Links ]
Piombino, P., Genovese, A., Gambuti, A., Lamorte, S.A., Lisanti, M.T. & Moio, L., 2010. Effects of off-vine bunches shading and cryomaceration on free and glycosylated flavours of Malvasia delle Lipari wine. Int. J. Food Sci. Technol. 45, 234-244. [ Links ]
Pisarnitskii, A.F., 2001. Formation of wine aroma and imperfections caused by minor components (review). Appl. Biochem. Microb. 37, 552-560. [ Links ]
Rapp, A., 1988. Studies on terpene compounds in wines. In: Charalambous, G. (ed). Frontiers of flavour. Elsevier Science Publisher B.V., Amsterdam. pp. 799 - 813. [ Links ]
Rapp, A. & Marais, J., 1993. The shelf life of wine: Change in aroma substances during storage and ageing of white wines. In: Charalambous, G. (ed.). Shelf life studies of foods and beverages chemical, biological, physical and nutritional aspects. Elsevier Science Publisher B.V., Amsterdam. pp. 891 - 921. [ Links ]
Rapp, A., Mandery, H. & Niebergall, H., 1986. New monoterpendiols in grape must and wine and in cultures of Botrytis cinerea. Vitis 25, 79-84. [ Links ]
Recamales, A.F., Gallo, V., Hernanz, D., Gonzalez-Miret, M.L. & Heredia, F.J., 2011. Effect oftime and storage conditions on major volatile compounds of Zalema white wine. J. Food Qual. 34, 100-110. [ Links ]
Recamales, A.F., Sayago, A., González-Miret, M.L. & Hernanz, D., 2006. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 39, 220-229. [ Links ]
Robinson, A.L., Mueller, M., Heymann, H., Ebeler, S.E., Boss, P.K., Solomon, P.S. & Trengove, R.D., 2010. Effect of simulated shipping conditions on sensory attributes and volatile composition of commercial white and red wines. Am. J. Enol. Vitic. 61, 337-347. [ Links ]
Roussis, I.G., Lambropoulos, I. & Papadopoulou, D., 2005. Inhibition of the decline of volatile esters and terpenols during oxidative storage of Muscat- white and Xinomavro-red wine by caffeic acid and N-acetyl-cysteine. Food Chem. 93, 485-492. [ Links ]
Simpson, R.F., 1982. Factors affecting oxidative browning of white wine. Vitis 21, 233-239. [ Links ]
Singleton, V.L., 1987. Oxygen with phenols and related reactions in musts, wines and model systems: Observations and practical implications. Am. J. Enol. Vitic. 38, 69-76. [ Links ]
Selli, S., Canbas, A., Cabaroglu, T., Erten, H. & Gunata, Z., 2006. Aroma components of cv. Muscat of Bornova wines and influence of skin contact treatment. Food Chem. 94, 319-326. [ Links ]
Shinohara, T., 1985. Gas chromatographic analysis of volatile fatty acids in wines. Agr. Biol. Chem. 49, 2211-2212. [ Links ]
Skouroumounis, G.K., Kwiatkowski, M.J., Francis, I.L., Oakey, H., Capone, D.L., Duncan, B., Sefton, M.A. & Waters, E.J., 2005. The impact of closure type and storage conditions on the composition, colour and flavour properties of a Riesling and a wooded Chardonnay wine during five years' storage. Aus. J. Grape Wine Res. 11, 369-377. [ Links ]
Stevens, K.L., Jurd, L. & Manners, G., 1972. Transformations of geraniol in aqueous acid solutions. Tetrahedron 28, 1939-1944. [ Links ]
Takeoka, G., Buttery, R.G., Flath, A., Terahishi, R., Wheeler, EL., Wieczorek, L. & Guetert, M., 1989. Volatile constituents of pineapple. In: Teranishi, R., Buttery, R.G. & Shahidi, F. (eds). Flavor chemistry: trends and development. ACS Symposium Series, Washington DC. pp. 221 -237. [ Links ]
Vilanova, M. & Sieiro, C., 2006. Determination of free and bound terpene compounds in Albariío wine. J. Food Compos. Anal. 19, 694-697. [ Links ]
Zafrilla, P., Morillas, J., Mulero, J., Cayuelas, J.M., Martínez-Cachá, A., Pardo, F. & Nicolás, J.M.L., 2003. Changes during storage in conventional and ecological wine: Phenolic content and antioxidant activity. J. Agric. Food Chem. 51, 4694-4700. [ Links ]
Zoecklein, B.W., Fugelsang, K.C. & Gump, B.H., 1995. Phenolic compounds and wine colour. In: Zoecklein, B.W., Fugelsang, K.C., Gump, B.H. & Nury, F.S. (eds). Wine analysis and production. Chapman & Hall, New York. pp. 115 - 151. [ Links ]
Submitted for publication: October 2013
Accepted for publication: January 2014
The authors contributed equally to this study
Aknowledgements: We kindly acknowledge the Società Cooperativa Romangia and Zarelli Vini S.r.L., for providing us with the Muscat and Malvasia wine samples, respectively
* Corresponding author: E-mail address: delcaro@uniss.it [Tel.: +39 079 229346; Fax: +39 079 229320]













