Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Industrial Engineering
On-line version ISSN 2224-7890
Print version ISSN 1012-277X
S. Afr. J. Ind. Eng. vol.33 n.4 Pretoria Dec. 2022
http://dx.doi.org/10.7166/33-4-2621
GENERAL ARTICLES
Simulation analysis of a neonatal unit with complex patient flow patterns - an enhanced model for capacity planning
T.S. PereraI,*; B.Ç. UsluII
IDepartment of Engineering and Mathematics, Sheffield Hallam University, England
IIDepartment of Industrial Engineering, Marmara University,Turkey
ABSTRACT
Steadily increasing demand requires neonatal units and networks to improve their overall capacities. Given the operational complexities involved, simulation is a popular choice for modelling patient flow and analysing its impact on resource capacities. Clinical pathways, designed to reduce variation of care and improve the quality of care for a specific group of patients, broadly define patient flow patterns. The literature points to many simulation studies that have addressed the interactions between clinical pathways and resource planning. For efficient model building, however, these simulation studies have assumed a unidirectional flow of patient - i.e., progressively moving to lower levels of care. But patient flows are much more complex. In some instances, patients may require a higher level of care than the current level of care. In such cases, bi-directional flows are created. This paper explores the impact of bidirectional flows on capacity planning. Using a real-world neonatal unit as an example, two scenarios of patient flow - i.e., unidirectional and bidirectional - are modelled and extensively analysed. This study revealed that the bidirectional flow model, which is the more realistic model, produces significantly different capacity-planning estimates. For example, the number of admission requests rejected by the unit increased by five to seven times - i.e., the uni-directional model significantly underestimates the overall capacity. The bidirectional model also revealed that there is a need to double the number of beds required for high-level care, and that bed utilisation, in general, is higher than the estimates produced by the unidirectional model. Given that there is a need to generate accurate capacity estimates to ensure better services for patients and to minimise frequent changes because of poor capacity estimates, this paper argues that bidirectional modelling should be used to produce more accurate capacity estimates.
OPSOMMING
Stadig toenemende vraag vereis neonatale eenhede en netwerke om hul algehele vermoë te verbeter. Gegewe die operasionele kompleksiteite wat betrokke is, is simulasie 'n gewilde keuse om pasiëntvloei te modelleer en die impak daarvan op hulpbronvermoë te ontleed. Kliniese prosesse, ontwerp om variasie van sorg te verminder en die kwaliteit van sorg vir 'n spesifieke groep pasiënte te verbeter, definieer breedweg pasiëntvloeipatrone. Die literatuur dui op baie simulasiestudies wat die interaksies tussen kliniese prosesse en hulpbronbeplanning aangespreek het. Vir doeltreffende modelbou het hierdie simulasiestudies egter 'n eenrigtingvloei van pasiënt veronderstel - dit wil sê, progressief beweeg na laer vlakke van sorg. Maar pasiëntvloei is baie meer kompleks. In sommige gevalle kan pasiënte 'n hoër vlak van sorg benodig as wat tans beskikbaar is. In sulke gevalle word tweerigtingstrome geskep. Hierdie studie ondersoek die impak van tweerigtingvloeie op kapasiteits-beplanning. Deur 'n werklike neonatale eenheid as 'n voorbeeld te gebruik, word twee scenario's van pasiëntvloei - d.w.s. eenrigting en tweerigting - gemodelleer en omvattend ontleed. Hierdie studie het aan die lig gebring dat die tweerigtingvloeimodel, wat die meer realistiese model is, aansienlik verskillende kapasiteits-beplanningskattings produseer. Byvoorbeeld, die aantal toelatingsversoeke wat deur die eenheid afgekeur is, het met vyf tot sewe keer toegeneem - d.w.s. die eenrigtingmodel onderskat die algehele kapasiteit aansienlik. Die tweerigtingmodel het ook aan die lig gebring dat daar 'n behoefte is om die aantal beddens wat benodig word vir hoëvlakversorging te verdubbel, en dat bedbenutting oor die algemeen hoër is as die skattings wat deur die eenrigtingmodel geproduseer word. Gegewe dat daar 'n behoefte is om akkurate kapasiteitskattings te genereer om beter dienste vir pasiënte te verseker en om gereelde veranderinge as gevolg van swak kapasiteitskattings te minimaliseer, argumenteer hierdie artikel dat tweerigtingmodellering gebruik moet word om meer akkurate kapasiteitskattings te produseer.
1. BACKGROUND
The care of newborns with medical and surgical conditions is one of the few highly specialised medical services in the healthcare sector. A typical neonatal unit consists of several cots that are equipped to support different levels of care. Intensive care (IC) cots with incubators are the most resource-intensive. High-dependency care (HDC) cots provide the next level of care. Further cots are provided for special care (SC) which are the least resource-intensive [1].
Although this study focuses on a single neonatal unit, it is essential to note that, for operational efficiencies, several neonatal units together form neonatal networks to meet regional needs collectively [2]. Capacity-planning exercises might focus on a single unit or on a network. Although this study focuses on a single neonatal unit, its findings are equally helpful when neonatal networks are analysed.
The need for highly specialist clinical staff and the high cost of equipment mean that the overall capacity of neonatal units and networks is kept to the lowest possible level to manage the perceived demand. Given that the demand for neonatal care has been steadily increasing in recent years, neonatal units/networks are forced to adjust their resource configurations from time to time.
Capacity-planning studies, which determine how many cots/staff are needed for a given patient admission pattern and mix, have often used discrete-event simulation platforms [3-6]. However, studies so far have assumed a unidirectional flow of patients - i.e., patients gradually move to lower levels of care from the type of care required at admission. For example, a patient who needs HDC at admission will either move to SC (the next lower level of care) or leave the unit. An analysis of patient flow data gathered over a year indicated that, in 36% of instances, patients required a higher level of care after the current status of care.
Table 1 shows the type of care required by a sample of 10 patients. Patient P0004 has undergone three pairs of care steps: HdC to SC, SC to IC, and IC to HDC. Within these three pairs, the second is an example of reverse flow (SC to IC), leading to bi-directional flow. For the 212 patients, there were a total of 258 care step pairs, and 94 of them were from lower-level care to higher-level care - i.e., 36%. In neonatal units where bi-directional flow is significant, the assumption that all flows are from a higher level of care to a lower level of care may compromise the accuracy of a simulation analysis. Therefore, the primary purpose of this paper is to demonstrate how simulation models with bidirectional flows improve the accuracy and robustness of capacity estimates.
2. LITERATURE REVIEW
The main purposes of the literature review were to understand (a) how patient flows have been captured in simulation analysis, and (b) how models have been used to assist capacity-planning exercises. As the number of neonatal modelling cases reported in the literature is somewhat limited, the review was extended to include other hospital units with similar operational characteristics, such as intensive care units.
2.1. Patient flow representation
Patient flow is the movement of patients through a healthcare facility. Clinical pathways (CPWs), which are designed to reduce variation of care and improve the quality of care for a specific group of patients, broadly define patient flow patterns [7]. An extensive review by Hulshof et al. [8] has identified different flow patterns linked to CPWs in healthcare systems. From their work, it can be concluded that there are three primary patterns. Different combinations of these primary patterns create a specific flow pattern for a given CPW.
Sequential pattern - patients progress through care steps sequentially.
Cyclical pattern - patients may go through a series of pre-defined care steps several times.
Complex pattern - patients progress through a complex sequence of steps, visiting the same care step more than once randomly.
These patterns are illustrated in Figure 1 using a simple example.
While the sequential pattern creates a unidirectional flow, the other two patterns create bi-directional flows. These patterns have been used in many simulation studies. A simulation study by Demeulemeester et al. [9] and Cardoen and Demeulemeester [10] to model a generic decision scheme that represented clinical pathways for the cardiac catheterisation of children used a combination of sequential and cyclical patterns. Tanfani and Angela [11] report developing a simulation model that was based on a unidirectional flow for a surgical department. Campbell-Yeo et al. [12] discuss the use simulation model, based on a unidirectional flow, to improve the care for newborns and support families during the COVID-19 pandemic.
Asaduzzaman et al. [13] present a queuing model that aims to estimate the number of cots required in a neonatal unit for given overflow and rejection probabilities. In this study, the authors assume that the condition of infants constantly improves after the current level of care, and that they move to a lower level of care. However, if the required category of cot is unavailable at admission, a cot from a higher-level category is allocated, if one is available. For example, if an infant requires SC type care at admission and all SC beds are occupied, then an HDC bed is given, if one is available. However, the flow of infants always remains unidirectional.
Demir et al. [14] use different modelling approaches, such as stochastic modelling and system dynamic modelling, to estimate infants' length of stay (LoS) in a neonatal unit. Patient flow is depicted in Figure 2. As in the previous study, this modelling work also assumes that patient flow is unidirectional.
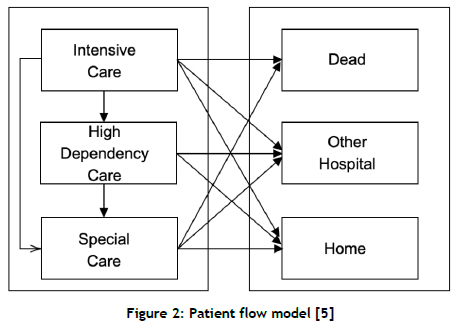
Allen et al. [15] report a comprehensive analysis of a neonatal network in the United Kingdom (UK). With a view to analysing the performance of a specific neonatal network, the authors developed a large-scale simulation model. Figure 3 shows their representation of patient flow.
This patient flow representation is effectively the same as the one used by [14]. Essentially, infants flow from high-level care to lower levels of care. Several other studies have also assumed a unidirectional flow in their modelling exercises [13, 16, 17, 18, 19].
Capacity planning in neonatal care units is challenging, as there are several highly varying parameters, such as the level of demand and the length of stay. The literature reports different approaches being deployed to estimate those parameters. Literature reviews of those parameters are presented in sections 2.2, 2.3, and 2.4 below.
2.2. Predicting demand for neonatal care
The ability to predict future demand helps medium- and long-term capacity planning. Capan et al. [20] use two well-known statistical tools, ARIMA and linear regression, to forecast demand for neonatal care. They argue that a simple time series model that accounts for recent census levels and seasonality provides more accurate daily census predictions than the traditional approach of using a yearly average value from the previous year, and provides opportunities to reduce the underestimation of the patient census. Koestler et al. [21] report that forecasting models that use patient-specific baselines and time-varying information use most of the data that are typically available, and can substantially improve census forecasts. Villeneuve et al. [22] present the outcome of a large-scale study in which a regression model was built to forecast demand at the national level. In this study, the researchers used the index of multiple deprivation as an independent variable and as a link between social deprivation and the incidence of preterm births, which had been previously established. As can be seen from the above studies: forecasting models have been used at various levels, from a specific unit to the national level, predicting demand accurately and enabling better capacity planning. Simulation is another methodology that is used for capacity planning [23].
2.3. Predicting the length of stay
The treatment duration of patients in different neonatal care units is defined as 'length of stay' (LoS), and is one of the very important care-related outcomes for both patients and healthcare managers to evaluate costs, quality of service, and the hospital's effectiveness [24, 25]. If the length of stay can be predicted, more effective short-term capacity planning can be done. Researchers have conducted many studies predicting LoS [26, 27, 28]. Demir et al. [14] classify four basic approaches to modelling LoS durations: stochastic modelling, statistical modelling, system dynamics modelling, and discrete event simulation (DES). However, they emphasise that discrete event simulation is more widely used in solving real-life problems.
Ahalt et al. [29] study the crowding score for emergency departments, and generate a simulation model to calculate the average LoS. Elbattah and Molloy [30] propose a hybrid solution for elderly discharge planning composed of simulation and machine-learning methodologies. In their study, a discrete event simulation model is used to assess demand predictions for healthcare resources, and machine learning is added to their model to increase the model's validity. Adeyemi et al. [31] suggest that a lack of capacity is mostly associated with increased infant births. Demir et al. [5] examine the effects of different variables. Researchers in the UK have based their studies on the duration of stay of infants by analysing the duration of stay in premature infants, having been observed to be more intensive [31, 13, 32, 33].
2.4. Generating optimum resource (bed/staff) configurations
Managing an optimum resource configuration is difficult for many countries, owing to increased demand and limited hospital resources [34-41]. Researchers have used simulation and optimisation techniques to generate optimum resource profiles for a given scenario. Some of the studies in the literature, including the optimisation of hospital resources, are mentioned below.
Bachouch et al. [34] investigate the problem of hospital bed management in France. The French hospitals, limited by budget cuts, cannot effectively manage shared resources, especially the beds allocated to acute and elective patients. For this reason, nurses have to manage beds and plan their use according to demand, including acute patient cases in the emergency department. Because boarding delays the start of inpatient care, it can, in turn, result in an extended stay in hospital. Liu et al. [35] have identified a positive association between a delay in securing beds for patients and their length of stay. Patients in intensive care units are of particular interest, especially in neonatal-paediatric ICs, which are much studied by researchers [35, 36]. Inadequate capacity planning will lead not only to insufficient use of neonatal units, but also to insufficient planning for all other departments working in integration with the neonatal unit [37]. For this reason, much research has been done to increase the effectiveness of the neonatal unit. Some of these are mentioned below.
Kokangul et al. [38] focus on nurse capacity in this regard. In these units, responsibility for the care of newborns, equipment, and employee capacity is very important. If equipment and employee capacity meet the needs, the service can be provided easily. Therefore, according to Kokangul et al. [38], nurses are one of the most essential resources in the neonatal intensive care unit, and should receive specialist training. Owing to a lack of capacity (nurses, equipment, beds, etc.), the length of stay, and the random number of patients, the rejection or transfer number is determined by nurses [31, 38]. Torabipour et al. [23] study the management of hospital beds, and state that the statistic and stochastic simulation model is an efficient tool to estimate the needed number of beds, considering the LoS, admission rate, and discharge rate. According to Derienzo et al. [39], patient safety in the neonatal intensive care unit is highly dependent on critical staff work. To create a specific simulation model that can be used to estimate the number of nurses needed per shift, using specific computer techniques such as simulation is vital to overcome this problem [39, 40, 15, 39, 41, 42, 43].
As discussed above, there are numerous studies on optimising patients' clinical pathways. However, most studies have used unidirectional flow for efficient modelling, apart from a few that have included cyclical flows. Therefore, this study aims to assess whether bi-directional flow modelling is required to produce more accurate capacity estimates, thus improving service levels and minimising unnecessary changes because of poor estimates.
3. SIMULATION ANALYSIS
This patient flow simulation study uses data collected from a neonatal surgical until with 11 beds. Required operational data were extracted from a central database that includes demographic data, such as gestational age and birth weight. It also contains details of their stay, such as the date of admission, date of discharge, and level of care needed each day.
3.1. Input data
The neonatal unit used for this analysis consists of four IC beds, four HDC beds, and three SC beds. Data extracted from BadgerNet [44], a secure national system for managing neonatal data, included 212 patients who were admitted to the unit over a period of one year. Using the input data analyser in the ARENA simulation system, the distributions given in Table 2 were established.
3.2. Modelling patient flows
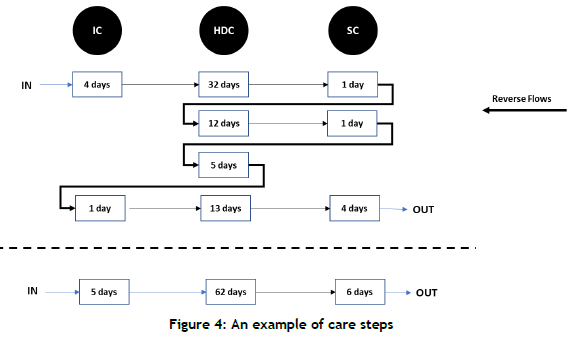
As the main aim of this work is to highlight the benefits of simulation models with bidirectional flows, two sets of simulation models were built: unidirectional and bidirectional. Figure 3 shows an example of a patient's care steps. There are three reverse flows within nine care steps, highlighted by thick arrows.
Unidirectional flow: In these models, it was assumed that patients always moved from higher levels of care to lower levels of care - i.e., from IC (if applicable) to HDC and then to SC. Taking the example shown in Figure 3, the patient flow has been transformed into three steps in Figure 4.
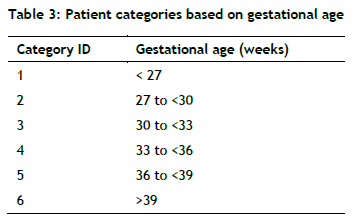
Bidirectional flow: Given the strong correlation between gestational age and the level of care needed, previous simulation studies [15] have categorised patients based on gestational age, as shown in Table 3.
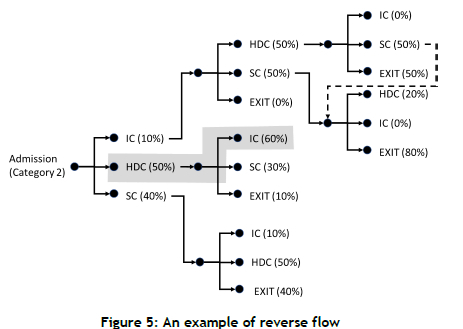
To model converse flows, a three-stage patient flow probability matrix was developed for each category of patients. Figure 4 shows patient flow paths for Category 2 patients. An example of converse flow (from HDC to IC) is highlighted. As shown in the diagram, patients who receive SC at the third stage are redirected to options for SC from the second stage. This process continues until patients exit the system. For six categories of patients, there is a total of 162 different flow patterns.
Figure 5 also highlights an example of reverse flow (the shaded area). For patients with HD as the first care, there is a 60% chance that the patient will need IC care in the second step.
When modelling bidirectional flow, it was assumed that patients requiring a lower-category bed might be allocated a bed from a higher category when all beds in the necessary category are occupied. For example, when all SC beds are occupied, a patient requiring SC care is allocated an HDC bed or an IC bed. However, the converse is not possible - e.g., an SC bed is unsuitable for a patient requiring IC care.
3.3. Model execution and validation
The models were executed for one year with a 30-day warm-up period, and each experiment with eight replications. For validation, the average length of stay (ALoS) was used [45]. The following population means were estimated to generate a null hypothesis (h0) and alternative hypothesis (H1 ) by analysing the base data set.
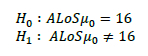
The t-parameters were estimated using the following formula.
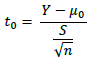
Y - the average value of replications
μ0 - population mean, estimated from the base data set
S - the standard deviation of n replications
n - the number of replications
The decision criteria of validation are that, if |t0| <,ta/2, then there is no strong evidence to reject H0. For this validation test, a is assumed to be 0.01. Parameter t0 was -0.06 and 2.74 for the unidirectional and bidirectional models respectively. The null hypothesis was accepted for both models, as ta/2 was 3.36.
3.4. Comparison of performance: unidirectional vs bidirectional flows
The primary purpose of this study is to highlight how bidirectional flow enhances the accuracy of simulation outputs used to inform capacity-planning decisions.
The following equations were used to estimate the confidence interval for each output parameter:
• Confidence interval = x± tx SEM
• SEM (standard error of the mean) = σ/IMAGEMAQUI
• n= number of replications
• x= mean of the parameter from n replications
• σ= standard deviation of the parameter from n replications
• t= t value for (n-1 ) degrees of freedom at 95% confidence level (the chosen level of confidence) Three output parameters that point to capacity constraints are analysed below.
4. RESULTS
Based on the satisfied validation (Table 3), the analysis results are as follows.
4.1. Parameter 1 - Admission requests rejected
Ideally, neonatal units should have the capacity to receive all requested admissions. But, under certain circumstances, the neonatal unit may reject some requests for admissions. These circumstances typically include instances such as:
• All beds are occupied.
• All beds of the required category and higher categories are occupied. For example, a request may refer to a patient who requires an HD bed, but all HD and IC (higher category) beds are occupied. SC beds may be available, but they are deemed unsuitable for HD care.
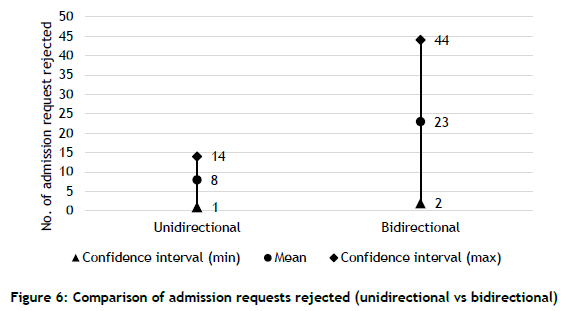
Figure 6 shows the results from two simulation scenarios: unidirectional and bidirectional. For example, the mean of the total rejections jumps from 8 to 23, which is nearly a three-fold increase. Underestimates that are generated by the unidirectional model hide the problem's true scale, and may lead to wrong decisions.
4.2. Parameter 2 - Preventable locking of higher-level care beds
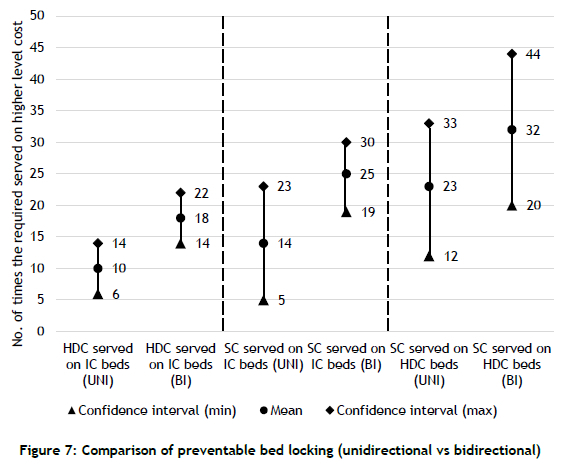
Neonatal units always attempt to allocate the required type of bed to patients. However, when this is not possible, a patient may be given a bed from a higher category. This unnecessarily locks beds that are dedicated to patients who need higher levels of care. For example, a patient requiring SC care might be allocated an HDC or IC bed when all SC beds are occupied.
As shown in Figure 7, predictions from the bidirectional model are significantly different from those from the unidirectional model. The unnecessary blocking of higher-level care beds should be avoided to ensure that patients who need higher-level care can be cared for promptly. Again, using the unidirectional model would not reveal the true scale of the problem.
4.3. Parameter 3 - Bed utilisation
Hospital managers often use bed-utilisation estimates to invest in new beds and/or to make necessary capacity adjustments in low-usage areas. The simulation model measured the utilisation of individual beds. Individual estimates were used to calculate the mean bed utilisation of each category; the mean values are shown in Figure 8.
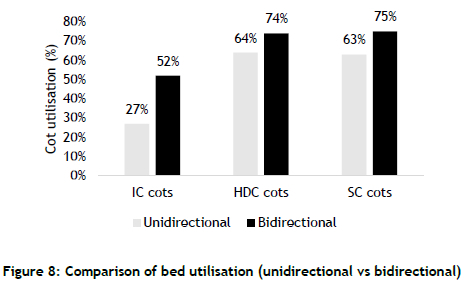
Similar to the trend set by the previous scenarios, the bidirectional model pointed to a higher level of bed utilisation for each category. However, the percentage increases were different for each bed category: 111%, 15%, and 19% respectively for IC, HDC, and SC. The unidirectional model significantly underestimated the true utilisation of IC beds. Such underestimates can lead to wrong decisions being made, such as reducing the number of IC beds when there is actually a demand for them.
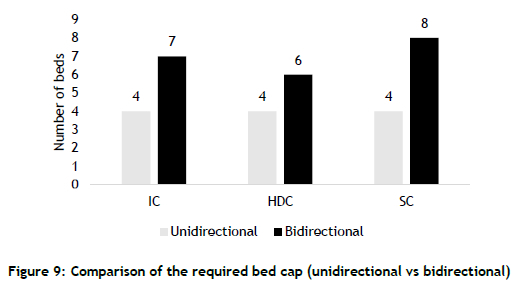
4.4. Parameter 4 - Bed capacity
The number of beds in neonatal units forms the basis of the capacity plan. The current configuration of the neonatal units consists of 11 beds (four IC beds, four HDC beds, and three SC beds). The unidirectional model estimated that 12 beds (i.e., one extra SC bed) were sufficient to serve the demand. In contrast, the bidirectional model estimated that 21 beds (seven IC beds, six HDC beds, and eight SC beds) were required to serve the demand - i.e., a 75% increase (Figure 9).
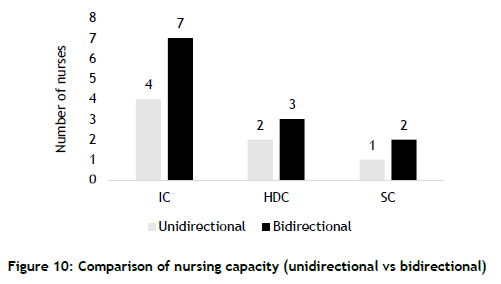
4.5. Parameter 5 - Nursing capacity
The number of nurses required to operate neonatal units is estimated from the guidelines provided by the UK's National Health Service [14]. The guideline stipulates the following allocations.
• IC beds - one nurse for each bed
• HD beds - a nurse to serve two beds
• SC beds - a nurse to serve four beds
Figure 10 shows the estimated number of nurses required to serve the bed configuration for the two scenarios. While modest increases are required in HDC and SC areas, a significant surge in nursing capacity - 75% - is required for IC.
4.6. Parameter 6 - Clinician capacity
The number and category of clinicians allocated to a neonatal unit are primarily determined by the number of beds and the patient mix. In contrast to deciding the nursing capacity, there are no strict guidelines for determining the required clinician capacity, as the requirements for each specific expertise can vary with the patient mix. As the bidirectional model forecast the need for more beds, the neonatal unit clearly required more clinicians than the number predicted by the unidirectional model.
4.7. Parameter 7 - Length-of-stay (LoS)
As the bidirectional model considered the reverse flow, the LoS estimate from the bidirectional model was about three days longer than the estimate from the unidirectional model.
As shown by the above analysis, unidirectional models can seriously underestimate the capacity requirements, leading to poor service levels, exhausted staff, and disappointed parents who cannot secure care for their newborns without inconvenient travel. Even though bidirectional models demand higher modelling efforts, they produce more accurate capacity estimates. Therefore, this study concludes that bidirectional modelling should be considered when there is a greater need for precise capacity estimates.
5. CONCLUSIONS
Simulation is often used in healthcare to justify new investments and to assess the impact of the actions taken improve matters. Therefore, it is critical that simulation models accurately represent reality. In the case of neonatal units, simulation studies so far have assumed a unidirectional and sequential flow of patients - i.e., from a higher level of care to a lower level of care - for efficient model building.
This research explores the impact of bidirectional patient flow on capacity planning for a real neonatal unit. Although simulation studies of neonatal units are sparse in the literature, there are numerous studies on optimising clinical pathways. These studies, however, have predominantly used the unidirectional flow of patients, apart from a few cases that considered a cyclical flow. The use of bidirectional flows is not evident in the literature.
Using data from a real neonatal unit, this study has demonstrated that modelling the bidirectional flow of patients significantly enhances the accuracy of capacity-planning estimates. If the results from the unidirectional model had been used to make decisions, the neonatal unit would not have attracted the required levels of investment to cater for the demand, leading to:
(a) Poor service levels as a result of insufficient resource capacities;
(b) Exhausted staff owing to inadequate numbers of nurses and clinicians;
(c) Disappointed parents who need to seek care in other localities.
This study shows that a simulation model with complex bidirectional patient flow produces the more accurate estimates that are required for capacity planning. These improved estimates not only optimise investment and operational decisions, but also bring other social benefits through the potential elimination of unnecessary processes created by wrong capacity estimates. For example, at the strategic level, a neonatal unit might underestimate its capacity, leading to unnecessary and risky travel for newborns. Underestimates could also lead to wrong decisions and deter new investments when there is a rising demand for neonatal facilities.
REFERENCES
[1] British Association of Perinatal Medicine (BAPM). 2011. Categories of care (2011). London: British Association of Perinatal Medicine. [ Links ]
[2] British Association of Perinatal Medicine (BAPM). 2010. Service standards (2010). London: British Association of Perinatal Medicine . [ Links ]
[3] Ferraro, N.M., Reamer, C.B., Reynolds, T.A., Howell, L.J., Moldenhauer, J.S. & Day, T.E. 2015. Capacity planning for maternal-fetal medicine using discrete event simulation. American Journal of Perinatology, 32(08), 761 -770. [ Links ]
[4] Fournier, D.L. & Zaric, G.S. 2013. Simulating neonatal intensive care capacity in British Columbia. Socio-Economic Planning Sciences, 47(2), 131-141. [ Links ]
[5] Zouri, M., Cumpat, C., Zouri, N., Leon, M.-M., Mastaleru, A. & Ferworn, A. 2019. Decision support for resource optimization using discrete event simulation in rehabilitation hospitals. Revista de Cercetare si Interventie Sociala, 65, 82-96. [ Links ]
[6] Luo, L., Zhang, Y., Qing, F., Ding. H., Shi. Y. & Guo, H. 2018. A discrete event simulation approach for reserving capacity for emergency patients in the radiology department. BMC Health Services Research, 18(1), 1-11. [ Links ]
[7] Lawal, A.K., Rotter, T., Kinsman, L., Machotta, A., Ronellenfitsch, U., Scott, S.D., Goodridge, D., Plishka, C. & Groot, G. 2016. What is a clinical pathway? Refinement of an operational definition to identify clinical pathway studies for a Cochrane systematic review. BMC Medicine, 14(1 ), 1 -5. [ Links ]
[8] Hulshof, P.J., Kortbeek, N., Boucherie, R.J., Hans, E.W. & Bakker, P.J. 2012. Taxonomic classification of planning decisions in health care: A structured review of state of the art in OR/MS. Health Systems, 1(2), 129-175. [ Links ]
[9] Demeulemeester, E., Sermeus, W., Belien, J. & Cardoen, B. 2007. Clinical pathways and operations management: It takes two to tango. Tijdschrift voor Economie en Management, 52(3), 451-469. [ Links ]
[10] Cardoen, B. & Demeulemeester, E. 2008. Capacity of clinical pathways-a strategic multi-level evaluation tool. Journal of Medical Systems, 32(6), 443-452. [ Links ]
[11] Tánfani, E. & Testi, A. 2012. A simulation-based decision support tool to analyse clinical pathways in hospitals. In: Tànfani, E. & Testi, A. (eds), Advanced decision making methods applied to health care. International Series in Operations Research & Management Science, 173, 191-211. Milan: Springer. https://doi.org/10.1007/978-88-470-2321-5_12 [ Links ]
[12] Campbell-Yeo, M., Dol, J., Richardson, B., McCulloch, H., Hundert, A., Foye, S., ... & Whitehead, L. 2021. A co-design of clinical virtual care pathways to engage and support families requiring neonatal intensive care in response to the COVID-19 pandemic (COVES study). Journal of Neonatal Nursing, 27(6), 463-470. [ Links ]
[13] Asaduzzaman, M., Chaussalet, T.J. & Robertson, N.J. 2010. A loss network model with overflow for capacity planning of a neonatal unit. Annals of Operations Research, 178(1 ), 67-76. [ Links ]
[14] Demir, E., Lebcir, R. & Adeyemi, S. 2014. Modelling length of stay and patient flows: Methodological case studies from the UK neonatal care services. Journal of the Operational Research Society, 65(4), 532-545. [ Links ]
[15] Allen, M., Spencer, A., Gibson, A., Matthews, J., Allwood, A., Prosser, S. & Pitt, M. 2015. Right cot, right place, right time: Improving the design and organisation of neonatal care networks - a computer simulation study. Health Services and Delivery Research, 3(20). [ Links ]
[16] Khazaei, H., Mench-Bressan, N., McGregor, C. & Pugh, J.E. 2015. Health informatics for neonatal intensive care units: An analytical modeling perspective. IEEE Journal of Translational Engineering in Health and Medicine, 3, 1-9. [ Links ]
[17] Masterson, B.J., Mihara, T.G., Miller, G., Randolph, S.C., Forkner, M.E. & Crouter, A.L. 2004. Using models and data to support optimization of the military health system: A case study in an intensive care unit. Health Care Management Science, 7(3), 217-224. [ Links ]
[18] Shahani, A.K., Ridley, S. and Nielsen, M. 2008. Modelling patient flows as an aid to decision making for critical care capacities and organisation. Anaesthesia, 63(10), 1074-1080. [ Links ]
[19] Costa, A., Ridley, S., Shahani, A., Harper, P.R., De Senna, V. & Nielsen, M. 2003. Mathematical modelling and simulation for planning critical care capacity. Anaesthesia, 58(4), 320-327. [ Links ]
[20] Capan, M., Hoover, S., Jackson, E.V., Paul, D. & Locke, R. 2016. Time series analysis for forecasting hospital census: Application to the neonatal intensive care unit. Applied Clinical Informatics, 7(2), 275-289. [ Links ]
[21] Koestler, D.C., Ombao, H. & Bender, J. 2013. Ensemble-based methods for forecasting census in hospital units. BMC Medical Research Methodology, 13(1), 67. [ Links ]
[22] Villeneuve, E., Landa, P., Allen, M., Spencer, A., Prosser, S., Gibson, A., ... & Pitt, M. 2018. A framework to address key issues of neonatal service configuration in England: The NeoNet multimethods study. Health Services and Delivery Research, 6(35). [ Links ]
[23] Torabipour, A., Zeraati, H., Mohammad, A., Rashidian, A., Sari, A.A. & Sarzaiem, M.R. 2016. Bed capacity planning using stochastic simulation approach in cardiac-surgery department of teaching hospitals. Iranian Journal of Public Health, 45(9), 1208-1216. [ Links ]
[24] Appelros, P. 2007. Prediction of length of stay for stroke patients. Acta Neurologica Scandinavica, 116(1), 15-19. [ Links ]
[25] Baniasadi, T., Kahnouji, K., Davaridolatabadi, N. & Teshnizi, S.H. 2019. Factors affecting length of stay in children hospital in southern Iran. BMC Health Services Research, 19(1), 949. [ Links ]
[26] Liu, V., Kipnis, P., Gould, M.K. & Escobar, G.J. 2010. Length of stay predictions:Improvements through the use of automated laboratory and comorbidity variables. Medical Care, 48(8), 739-744. [ Links ]
[27] Naved, S.A., Siddiqui, S. & Khan. F.H. 2011. Apache-II score correlation with mortality and length of stay in an intensive care unit. Journal of the College of Physicians and Surgeons Pakistan, 21 (1 ), 4-8. [ Links ]
[28] Barnes, S., Hamrock, E., Toerper, M., Siddiqui, S. & Levin, S. 2016. Real-time prediction of inpatient length of stay for discharge prioritization. Journal of the American Medical Informatics Association, 23(e1), 2-10. [ Links ]
[29] Ahalt, V., Argon, N.T., Ziya, S., Strickler, J. & Mehrotra, A. 2018. Comparison of emergency department crowding scores: A discrete-event simulation approach. Health Care Management Science, 21(1), 144-155. [ Links ]
[30] Elbattah, M. & Molloy, O. 2016. Coupling simulation with machine learning: A hybrid approach for elderly discharge planning. In Proceedings of the 2016 ACM SIGSIM Conference on Principles of Advanced Discrete Simulation, pp. 47-56. [ Links ]
[31] Adeyemi, S., Demir, E., Chahed, S. & Chaussalet, T. 2010. Analysis of variability in neonatal care units: A retrospective analysis. In 2010 IEEE Workshop on Health Care Management (WHCM), pp. 16. [ Links ]
[32] Zhecheng, Z. 2014. An online short-term bed occupancy rate prediction procedure based on discrete event simulation. Journal of Hospital Administration, 3(4), 37-42. [ Links ]
[33] Abuhay, T.M., Krikunov, A.V., Bolgova, E.V., Ratova, L.G. & Kovalchuk, S.V. 2016. Simulation of patient flow and load of departments in a specialized medical center. Procedia Computer Science, 101, 143-151. [ Links ]
[34] Bachouch, R.B., Guinet, A. & Hajri-Gabouj, S. 2012. An integer linear model for hospital bed planning. International Journal of Production Economics, 140(2), 833-843. [ Links ]
[35] Liu, S.W., Chang, Y., Weissman, J.S., Griffey, R.T., Thomas, J. Nergui, S., Hamedani, A.G., Camargo, C.A. Jr & Singer, S. 2011. An empirical assessment of boarding and quality of care: Delays in care among chest pain, pneumonia, and cellulitis patients. Academic Emergency Medicine, 18(12), 1339-1348. [ Links ]
[36] Kapadia, A.S., Chan, W., Sachdeva, R., Moye, L.A. & Jefferson, L.S. 2000. Predicting duration of stay in a pediatric intensive care unit: A Markovian approach. European Journal of Operational Research, 124(2), 353-359. [ Links ]
[37] Bai, J., Fügener, A., Schoenfelder, J. & Brunner, J.O. 2018. Operations research in intensive care unit management: A literature review. Health Care Management Science, 21(1), 1-24. [ Links ]
[38] Kokangul, A., Akcan, S. & Narli, M. 2017. Optimizing nurse capacity in a teaching hospital neonatal intensive care unit. Health Care Management Science, 20(2), 276-285. [ Links ]
[39] Derienzo, C., Lenfestey, R., Horvath, M., Goldberg, R. & Ferranti, J. 2014. Neonatal intensive care unit handoffs: A pilot study on core elements and epidemiology of errors. Journal of Perinatology, 34(2), 149-152. [ Links ]
[40] Bruno, C., Angert, R., Rosen, O., Lee, C., Vega, M., Kim, M., Yu, Y., Bernstein, P.S. & Goffman, D. 2016. Simulation as a tool for improving obstetric residents' acquisition of neonatal resuscitation skills. The Journal of Maternal-Fetal & Neonatal Medicine, 29(16), 2625-2629. [ Links ]
[41] Günal, M.M. & Pidd, M. 2010. Discrete event simulation for performance modelling in health care: A review of the literature. Journal of Simulation, 4(1 ), 42-51. [ Links ]
[42] Landa, P., Sonnessa, M., Tànfani, E. & Testi, A. 2014. A discrete event simulation model to support bed management. In 2014 4th International Conference on Simulation and Modeling Methodologies, Technologies and Applications (Simultech 2014), pp. 901-912. [ Links ]
[43] Pujowidianto, N.A., Lee, L.H., Pedrielli, G., Chen, C.-H. & Li, H. 2016. Constrained optimization for hospital bed allocation via discrete event simulation with nested partitions. In 2016 Winter Simulation Conference (WSC), pp. 1916-1925. [ Links ]
[44] Gale, C. & Morris, I. 2016. The UK National Neonatal Research Database: Using neonatal data for research, quality improvement and more. Archives of Disease in Childhood - Education and Practice, 101(4), 216-218. [ Links ]
[45] Banks, J., Carson, J.S. II, Barry, L. & Nicol, D.M. 2005. Discrete-event system simulation. India: Pearson. [ Links ]
Submitted by authors 28 Sep 2021
Accepted for publication 17 Oct 2022
Available online 14 Dec 2022
* Corresponding author: t.d.perera@shu.ac.uk
ORCID® identifiers
T.S. Perera: 0000-0003-3719-7284
B.Ç. Uslu: 0000-0001-8214-825X














