Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Industrial Engineering
On-line version ISSN 2224-7890
Print version ISSN 1012-277X
S. Afr. J. Ind. Eng. vol.33 n.3 Pretoria Nov. 2022
http://dx.doi.org/10.7166/33-3-2812
SPECIAL EDITION
Design of cubic Ni-based alloys for use as coating in petrochemical industry - a first principles approach
M. Phasha*; M. Mulaudzi; H. Mdller
Advance Materials Division, Mintek, Randburg, South Africa
ABSTRACT
This work presents the structural stability and mechanical properties of ordered face-centred cubic (FCC) Ni-Cr binary and ternary alloys. The effects of alloying on these properties have been evaluated using the first principles density functional theory (DFT) within generalised gradient approximation (GGA). The calculated heats of formation show that the introduction of third elements (Al, Ti, Mo, Sn) improved the thermodynamic stability of the usually unstable L12 Ni3Cr phase. Unsurprisingly, ternary alloys that yielded higher mechanical properties were those alloyed with Al and Ti owing to their ability to form a very stable L12 phase with Ni. Therefore, the theoretical technique has been successfully employed to identify promising high-strength cubic Ni-based ternary intermetallic alloys that can be used as coatings.
OPSOMMING
Hierdie werk bied die strukturele stabiliteit en meganiese eienskappe van geordende gesiggesentreerde kubieke (FCC) Ni-Cr binére en ternére legerings aan. Die uitwerking van legering op hierdie eienskappe is geévalueer deur die eerste beginsels digtheid funksionele teorie (DFT) binne veralgemeende gradiéntbenadering (GGA) te gebruik. Die berekende hitte van vorming toon dat die bekendstelling van derde elemente (Al, Ti, Mo, Sn) die termodinamiese stabiliteit van die gewoonlik onstabiele L12 Ni3Cr fase verbeter het. Dit is nie verbasend dat drieledige legerings wat hoér meganiese eienskappe opgelewer het, dié wat met Al en Ti gelegeer is, dra as gevolg van hul vermoé om 'n baie stabiele L12-fase met Ni te vorm. Daarom is die teoretiese tegniek suksesvol aangewend om belowende hoésterkte kubieke Ni-gebaseerde ternére intermetaallegerings wat as bedekkings gebruik kan word, te identifiseer.
1. INTRODUCTION
Corrosion is the process in which material properties deteriorate as a result of chemical interaction with their environment. This process manifests in the form of premature failure, necessitating the maintenance of infrastructure, thus having a huge economic and environmental impact on all facets of national assets. An example of such a process is metal dusting (MD), which is more prevalent in the petrochemical and energy industries. Metal dusting is regarded as a form of carburisation that results in the degradation of alloys into a fine dust of metal particles, metal carbides, metal oxides, and graphite by localised pitting or general corrosion attack. This phenomenon occurs at high temperatures of between 300 °C and 800 °C in synthesis gas environments or, more commonly, in carburising and selectively oxidising environments [1, 2].
The application of a protective coating on metal surfaces to act as a barrier or to provide sacrificial protection is one of the available techniques for controlling corrosion. Despite intense research efforts that have led to much progress in mitigating metal dusting at high temperatures, particularly in the oil and gas industry, complete prevention remains an unresolved issue [3, 4]. The persistence of this globally challenging and unresolved issue of controlling metal dusting stems from the exposure of metallic structural materials to a syngas environment consisting mostly of carbon monoxide and hydrogen, at a temperature range of between 300 °C and 800 °C and a pressure around 20 bar. Unfortunately, the use of syngas and related gases cannot be avoided because of their typical involvement in value-add hydrocarbon conversion and greenhouse gas mitigating processes. The development of suitable materials that could help to prolong production operations in these types of conditions is thus important from both an industrial and an environmental point of view. As a result, recent research developments have identified that viable options that have the potential to offer the best resistance to aggressive MD environments via coating are: (i) the introduction of alloying elements that are able to form protective oxides, such as chromium (Cr), aluminium (Al), and silicon (Si), to the nickel based alloys; (ii) the inhibition of the catalytic effect of iron (Fe), nickel (Ni), and cobalt (Co) on the formation of solid carbon from the gas phase through the addition of copper (Cu) and tin (Sn); and (iii) the development of high-entropy alloys as coatings [5]. These options are currently being explored globally in research and development (R&D) spaces for future industrial consideration.
It is well-known that the development of an optimised coating would require a material that formed stable protective phases on the surface by reactions with the process environment [6]. The protective phases could be Al2O3, Cr2O3 and SiO2, as well as some spinels (AB2O4- type metal oxides). Knowledge of the kinetics of the oxidation process is important to ensure that the protective phase grows slowly so that the coating reservoir depletion rates are low, and that interdiffusion between the coating material and the substrate occurs as slowly as possible. In addition, the thermal expansion coefficients of the coating material or protective surface scale and the substrate should be similar or close, so that the cooling and reheating stresses are minimised during temperature changes or by downtimes [6].
In terms of research approach, computational tools have lately become more accurate and reliable in predicting the properties of materials, consistent with experimental data. If applied correctly, predictive techniques are considered a useful and viable way to accelerate the development of many materials with enhanced properties. Although commercial high-temperature Ni-based superalloys (NBS) being deployed in industry so far consist of ternary and quaternary Ni-Al and Ni-Cr systems [4, 7-9], most investigations based on the density functional theory (DFT) and reported to date are about binary or ternary Ni-Cr alloys with a focus on the sigma Ni2Cr phase [10-18]. Only a few studies have been conducted to investigate the effect of a third element addition in the face-centred cubic (FCC) Ni-Cr system [19, 20]. It is thus the purpose of this study to investigate the effect of ternary element addition to the Ni-Cr system on the structure, phase stability, and elastic properties of an FCC Ni24Cr6Y2 system, where Y represents the alloying elements Al, Ti, Mo, and Sn; and to add to the scarce literature on first-principles calculations of Ni-Cr-based ternary alloys [20]. The choice of the alloying elements was based on their large atomic size, and aimed at easing the diffusion of Ni, Cr towards the surface for oxidation.
This work used the DFT-based first principles technique to carry out computational modelling calculations with the intention to identify a few key compositions for experimental consideration, instead of following tedious and expensive traditional trial and error routes. Furthermore, this study intended to serve as a foundation to use DFT methods to come up with key alloy design considerations (criteria) to guide current research work to develop better Ni-based corrosion resistance materials and coatings.
2. METHODOLOGY
All calculations were carried out using a first-principles-based code, CASTEP, which is embedded in the Materials Studio software package version 2020 [21]. Robust Vanderbilt ultrasoft pseudopotentials [22] were used to treat the ion-electron interaction within the generalised gradient approximation (GGA) [23] of Perdew-Burke-Ernzerhof (PBE) [24]. A plane wave energy cut-off of 500 eV and a k-points set of 6x6x6 were sufficient to converge the total energy of the cubic Ni24Cr6Y2 compositions. However, in order also to have a view of how the chosen alloying elements affected the thermodynamic stability of a competing and undesired sigma (a) phase, the k-point setting was altered to 17x6x12 to cater for the structural calculations of Ni4Cr2-xYx compositions with an orthorhombic crystal structure. Y represents the alloying element, which is introduced substitutionally on the Cr site.
All structures were geometry-optimised to determine their ground state using the Brayden-Fletcher-Goldfarb-Shanno (BFGS) minimisation scheme. The convergence criterion of less than 1x10-5 eV/atom, the maximum residual forces of 0.03 eV/A, the maximum residual bulk stress of 0.05 GPa, and the maximum atomic displacement of 1x10-3A were used.
With the exception of FCC Ni with space group # 225 (Fm-3m), the calculations for FCC Ni24Cr6Y2 were carried out using FCC supercells with space group #221(Pm-3m) consisting of 32 atoms; whereas those for the orthorhombic phase were done using a six-atom-based unit cell with space group # 71 (Immm) and 47 (Pmmm) for Ni2Cr and Ni4Cr2-xYx respectively. The structures described above are presented in Figure 1.
2.1. Phase stability
In order to determine the thermodynamic stability, heats of formation were calculated from Equation 1 below:
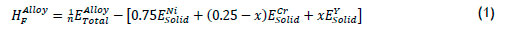
where EAlloyTotalis the total energy of the alloy composition, and ENi Solid,ECrSolid and EYSolid are the total energies of the ground-state structures of elemental Ni, Cr and that of the corresponding alloying elements, whereas x and 0.25-x refer to the fractional concentrations of the constituent elements; the total number of atoms in the structure is represented by n.
2.2. Elasticity properties
The stress-strain relation may be used to distinguish the elastic and plastic regimes of solid materials. The elastic moduli are the fundamental physical parameters that establish the stress-strain relation in the elastic regime. For an isotropic polycrystalline solid, the two independent elastic parameters are the bulk modulus (6) and the shear modulus (G). The ratio of B/G predicts the brittleness of metals, depending on the threshold value of 1.75 (ductile if above 1.75) [25]. For the cubic structures, only three elastic constants, corresponding to C11, C12, and C44, are independent [26]. The mechanical stability criteria of cubic crystals are given by the expressions in equation 2.
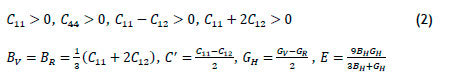
3. RESULTS AND DISCUSSION
The optimised lattice parameters and the calculated heats of formation of FCC Ni alloy compositions are listed in Table 1.
It should be noted that a small addition of Cr starts with a slight decrease in the lattice parameter at 6.25 atomic per cent (at.%), but increases afterwards. The trend of increase in the lattice parameter with the Cr addition is in agreement with available theoretical [19] and experimental data [27]. Of the studied cubic binary Ni-Cr compositions, the Ni30Cr2 composition is the most thermodynamically stable. The introduction of a third element such as Al, Ti, and Sn, which would form a stable intermetallic L12 phase with Ni on the Cr site, would improve the stability of the Ni24Cr8 composition. However, the addition of refractory element Mo would lead to a thermodynamically unstable phase, as indicated by the positive formation energy.
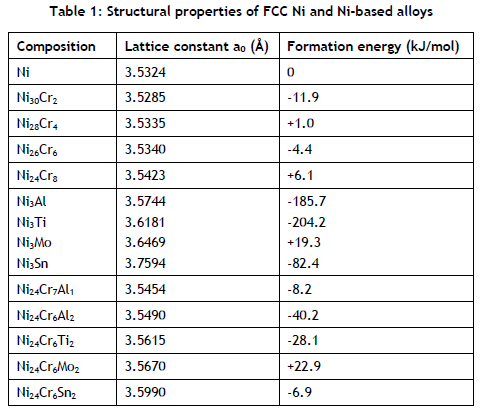
In Figure 2, the results of calculations that were conducted to investigate whether the considered alloying elements did not promote the formation of an undesired Ni2Cr σ phase are presented. The reason why this σ phase is undesirable arises from its brittleness and its much lower coefficient of thermal expansion (CTE) than the FCC phase. Despite the usefulness of low CTE in providing better high-temperature creep performance, it mismatches with that of FCC matrix, leading to strong crack-initiation points during the shutdown cycles involving cooling to room temperature. As shown in Figure 2(i), lattice parameters a and b followed a similar increasing pattern when the third element was introduced. However, the pattern for lattice parameter c was different, with the lowest value when alloyed with Al. (See Figure 2(ii).)
As shown in Figure 2(iii), all alloying elements promote the formation of a o phase with the exception of Sn. Based on their strong thermodynamic stability, as shown in Table 1, it is not surprising that Ti and Al are strong stabilisers for the o and FCC ternary phases respectively. This high thermodynamic stability of the o phase makes it difficult to avoid forming at a lower temperature, and could be the reason it has received so much attention in research.
3.2. Elastic properties
The calculated elastic properties of the binary and ternary FCC Ni alloy compositions are presented in Figures 3 and 4 respectively. Generally, the increase in Cr content to Ni gradually increased the elastic constants C11, C12 and C44 - more so for C11, and C44, as shown in Figure 3(i). Because of this trend, it is not surprising that the resulting computed elastic moduli (C', G, B, and E) increased with the increase in Cr concentration, reaching their maximum point at 18.75 at.% for C', G, and E, as shown in Figure 3(ii) and (iii). As expected, the corresponding B/G ratio results showed a minimum value at this composition. The strong correlation between shear moduli (C', G) and B/G seem to suggest that this B/G trend might also be linked to CTE, as Cr is known to have the lowest CTE among all refractory metals, owing to its most brittle nature.
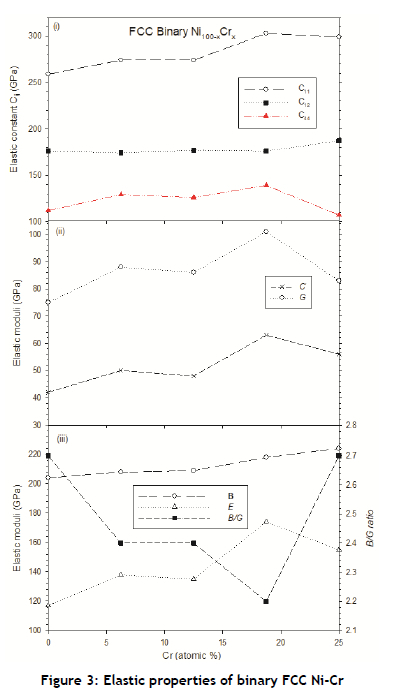
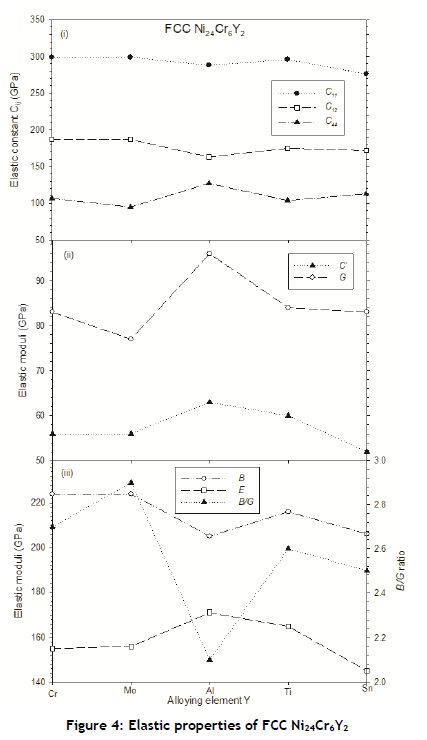
As shown in Figure 4(i), the trends for C11 and C12 were similar but opposite that of C44 when a third alloying element was introduced into the FCC phase. When comparing the elastic properties of the FCC binary Ni-Cr and ternary Ni24Cr6Y2 compositions considered in this study, we found the highest shear moduli (G, C') and Young's modulus (E) at Ni24Cr8 and Ni24Cr6Al2, signalling superior mechanical properties as well as higher mechanical stability.
4. CONCLUSION
A first-principles approach was successfully used to identify promising ordered, stable, and high-strength ordered cubic Ni-based ternary alloys that could be used as coatings in petrochemical environments. The suitable alloying elements to stabilise the Ni-Cr-based ordered cubic phase were found to be Al, Ti, and Sn, with superior mechanical properties achieved at the Ni24Cr8 and Ni24Cr6Al2 compositions. However, Al and Ti were also found to promote the formation of a deleterious σ phase, whereas Sn did not. Therefore, this study identified Sn as the viable alloying option, as it stabilised only the cubic phase, thus promoting structural coherency in the austenitic construction material used in petrochemical plants.
5. ACKNOWLEDGEMENTS
This work was mostly conducted at MINTEK's computer laboratory, and so this paper is published with the permission of MINTEK. The authors would like to thank the Advanced Metals Initiative (AMI) of the Department of Science and Innovation (DSI) for financial support. Gratitude is also extended to the Centre for High-Performance Computing (CHPC) in Cape Town for allowing us to carry out the calculations using their remote computing resources.
REFERENCES
[1] P. Szakálos, "Mechanisms of metal dusting," PhD thesis, Royal Institute of Technology and Swedish Institute for Metals Research, Stockholm, Sweden, 2004. [ Links ]
[2] J. Zhang, A. Schneider, & G. Inden, Corr. Sci., vol. 45, 1329, 2003. [ Links ]
[3] M.L. Holland & H.J. de Bruyn, Int. J. Press. Vessel. Pip., vol. 66, 125, 1996. [ Links ]
[4] M.L. Holland, Corr. Paper No. 01385, Houston, TX: NACE International, 2001. [ Links ]
[5] S. Hayashi, D. Kudo, R. Nagashima, & H. Utsumi, Corr. Sci., vol. 163, 1, 2020. [ Links ]
[6] M. Schütze, M. Malessa, V. Rohr, & T. Weber, Surf. & Coat. Tech., vol. 201, 872, 2006. [ Links ]
[7] A. Mishra, D. Richesin, & R.B. Rebak, Corr. Paper No. 5802, Houston, TX: NACE International, 2001. [ Links ]
[8] H.M. Tawancy, R.B. Herchenroeder, & A.I. Asphahani, J. Metals, vol. 35, 37, 1983. [ Links ]
[9] L. Yuan, J. Liu, S. Wang, Z. Li, & Y. Xue, Mater. Sci. Eng. A, vol. 801, 140435, 2021. [ Links ]
[10] A.J. Samin & C.D. Taylor, Corros. Sci., vol. 134, 103, 2018. [ Links ]
[11] K.S. Chan, Y-M. Pan, & Y-D. Lee, Metall. Mater. Trans. A, vol. 37A, 2039, 2006. [ Links ]
[12] J.K. Chen, D. Farks, & W.T. Reynolds Jr., Acta Mater., vol. 45, 4415, 1997. [ Links ]
[13] T. Wang, L-Q. Chen, & Z-K. Liu, Metall. Mater. Trans. A, vol. 38A, 562, 2007. [ Links ]
[14] K.S. Chan, Y-D. Lee, & Y-M. Pan, Metall. Mater. Trans. A, vol. 37A, 523, 2006. [ Links ]
[15] C. Miller, R. Field, & M. Kaufman, Acta Mater., vol. 157, 1, 2018. [ Links ]
[16] R. Hu, G.M. Cheng, J.Q. Zhang, J.S. Li, T.B. Zhang, & H.Z. Fu, Intermetallics, vol. 33, 60, 2013. [ Links ]
[17] A. Verma, J.B Singh, & J.K. Chakravartty, J. Alloys Comp., vol. 639, 341, 2015. [ Links ]
[18] S. Yang, Y. Wang, Z-K. Liu, & Y. Zhong, Calphad: Comput. Coupling Ph. Diagr. Thermochem., vol. 75, 102359, 2021. [ Links ]
[19] Z-B. Yang, J. Sun, S. Lu, & L. Vitos, J. Mater. Res., vol. 33, 2763, 2018. [ Links ]
[20] A. Pathak, & A.K Singh. J. Appl. Res. Technol., vol. 15, 449, 2017. [ Links ]
[21] S. Clark, et al., Zeitschrift fuer Kristallographie vol. 220, 567, 2005. [ Links ]
* Corresponding author: MajeP@mintek.co.za
ORCID® identifiers
M. Phasha: 0000-0003-4295-9247
M.Mulaudzi: 0000-0001-8222-9420
H. Moller: 0000-0001-6075-9965