Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
SA Journal of Radiology
On-line version ISSN 2078-6778
Print version ISSN 1027-202X
S. Afr. J. radiol. (Online) vol.27 n.1 Johannesburg 2023
http://dx.doi.org/10.4102/sajr.v27i1.2728
ORIGINAL RESEARCH
Neuroquantification enhances the radiological evaluation of term neonatal hypoxic-ischaemic cerebral injuries
Shalendra K. MisserI, II; Nobuhle MchunuIII, IV; Jan W. LotzV; Lisa KjonigsenVI; Aziz UlugVII; Moherndran ArcharyVIII
IDepartment of Radiology, Lake Smit and Partners Inc., Durban, South Africa
IIDepartment of Radiology, Faculty of Health Sciences, University of KwaZulu-Natal, Duban, South Africa
IIIBiostatistics Research Unit, South African Medical Research Council, Durban, South Africa
IVDepartment of Statistics, Faculty of Science, School of Mathematics, Statistics and Computer Sciences, University of KwaZulu-Natal, Pietermaritzburg, South Africa
VDepartment of Radiodiagnosis, Faculty of Sciences, Stellenbosch University, Cape Town, South Africa
VIMedtech, Oslo, Sweden
VIICortechs Labs, San Diego, United States of America
VIIIDepartment of Pediatrics, Faculty of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: Injury patterns in hypoxic-ischaemic brain injury (HIBI) are well recognised but there are few studies evaluating cerebral injury using neuroquantification models.
OBJECTIVES: Quantification of brain volumes in a group of patients with clinically determined cerebral palsy.
METHOD: In this retrospective study, 297 children with cerebral palsy were imaged for suspected HIBI with analysis of various cerebral substrates. Of these, 96 children over the age of 3 years with a clinical diagnosis of cerebral palsy and abnormal MRI findings underwent volumetric analyses using the NeuroQuant® software solution. The spectrum of volumetric changes and the differences between the various subtypes (and individual subgroups) of HIBI were compared.
RESULTS: Compared with the available normative NeuroQuant® database, the average intracranial volume was reduced to the 1st percentile in all patient groups (p < 0.001). Statistically significant differences were observed among the types and subgroups of HIBI. Further substrate volume reductions were identified and described involving the thalami, brainstem, hippocampi, putamina and amygdala. The combined volumes of five regions of interest (frontal pole, putamen, hippocampus, brainstem and paracentral lobule) were consistently reduced in the Rolandic basal ganglia-thalamus (RBGT) subtype.
CONCLUSION: This study determined a quantifiable reduction of intracranial volume in all subtypes of HIBI and predictable selective cerebral substrate volume reduction in subtypes and subgroups. In the RBGT subtype, a key combination of five substrate injuries was consistently noted, and thalamic, occipital lobe and brainstem volume reduction was also significant when compared to the watershed subtype.
CONTRIBUTION: This study demonstrates the value of integrating an artificial intelligence programme into the radiologists' armamentarium serving to quantify brain injuries more accurately in HIBI. Going forward this will be an inevitable evolution of daily radiology practice in many fields of medicine, and it would be beneficial for radiologists to embrace these technological innovations.
Keywords: neuroquantification; hypoxic-ischaemic; cerebral; term neonatal; magnetic resonance; artificial intelligence; brain volumetry.
Background
Hypoxic-ischaemic brain injury (HIBI) is an important cause of cerebral palsy1 worldwide, across the divide between high- and low- to-middle-income countries (LMICs).2 The patterns of injury ascribed to HIBI are well recognised,3,4,5,6,7 and the pathophysiology of these has been extensively studied through animal studies.8,9,10,11,12 Some of the injuries are related to the early phase of cell damage encountered immediately after a sentinel event or around the perinatal period. Further apoptotic injuries result from glutamate-induced delayed and secondary injuries that can be ongoing for days to weeks after the insult. These may occur with the remodelling processes innate to the developing brain.13 The result of perinatal HIBI is the structural damage of substrates that may be directly injured and others that are secondarily injured in these processes.14
Injuries sustained in the perinatal period are reflected in specific cerebral regional destruction that persists throughout the child's life. Assessment of normal and abnormal cerebral development and myelination is possible with standard MRI.15,16 The traditionally reported HIBI patterns seen on MRI pertain to the cerebral watershed territories, deep nuclear structures, periventricular white matter and multilobar cystic encephalopathy.14 In order to improve the reliability of MRI in the setting of HIBI, one must ensure the adequate preparation of patients before scanning, due attention to the use of correct imaging protocols and the recruitment of homogeneous cohorts.17 Neuroquantification software and artificial intelligence (AI) algorithms have been developed for MRI to obtain a more accurate depiction of the total burden of injury in individual patients.15
There have been few studies evaluating cerebral injury using volumetric quantification models. One such algorithm has been developed specifically for volumetry in cerebral palsy patients.18 Such AI programmes aim to improve diagnosis, stratification of brain injuries and correlation with functional outcome. This study aimed to quantify the various cerebral substrate injuries and brain volumes in a group of patients with clinically determined cerebral palsy, using accredited neuroquantification software. Specific areas of interest corresponding to those described in HIBI were targeted for volumetric measurement.
Materials and methods
A composite database was accumulated over a 6-year period, comprising 297 children with clinically recorded term neonatal hypoxic-ischaemic encephalopathy (HIE) and a clinical diagnosis of cerebral palsy, imaged by MRI in the chronic phase of evolution. Multiple sequences were performed on 1.5 T Siemens Scanners, including sagittal volumetric T1-weighted, coronal and axial T2-weighted, axial fluid attenuated inversion recovery (FLAIR), susceptibility weighted imaging (SWI), diffusion weighted imaging (DWI) and apparent diffusion coefficient (ADC), and inversion recovery sequences. All MRI studies were retrospectively and independently reviewed by the principal investigator (S.K.M.) and co-investigator (J.L.). Using the classification as proposed by Misser et al.,14 each study was evaluated to identify the HIBI subtype and record the degree of involvement of the thalami, hippocampi, basal ganglia, white matter, cortical regions, brainstem and several other critical substrates. Each investigator individually graded the selected substrates. Interobserver discordances were reviewed, discussed and resolved by the two primary readers. Categorisation of the four HIBI subtypes was undertaken, and correlation with the degree of substrate injury for each subtype and their various subgroups of injury were tabulated.
The four major HIBI subtype classifications in this study:
•The central or Rolandic basal ganglia-thalamus (RBGT) subtype, which includes patients with isolated high metabolic area injury (sensorimotor cortex, ventral posterior lateral (VPL) nucleus of the thalamus, posterior putamen and hippocampus), is subdivided into four gradations of injury, based on the degree of destruction of the parasagittal cortex19 (Figure 1). These include the mild, moderate, severe and massive paramedian injury (MPI) subgroups.
•The parasagittal or partial prolonged pattern of injury related to the destruction of the cortex and subcortical white matter in the named watershed areas with seven subgroups and/or combinations of these areas20,21 (Figure 2).
•The mixed pattern of injury incorporates a combination of high metabolic zone and watershed territory involvement.14
•Multicystic encephalomalacia follows global brain injury with spongiosis of multiple lobes and cystic change.14
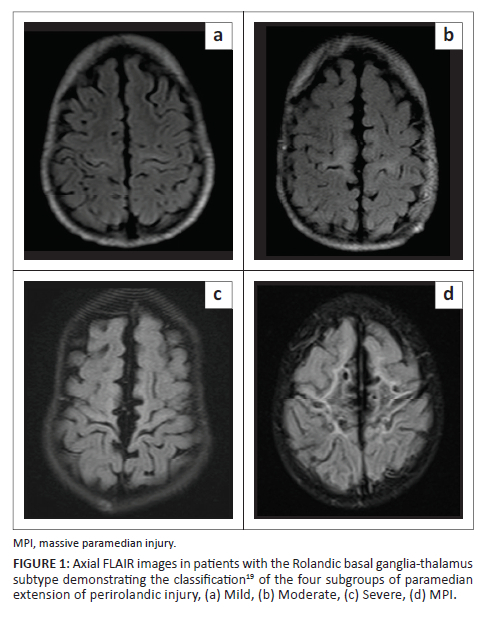
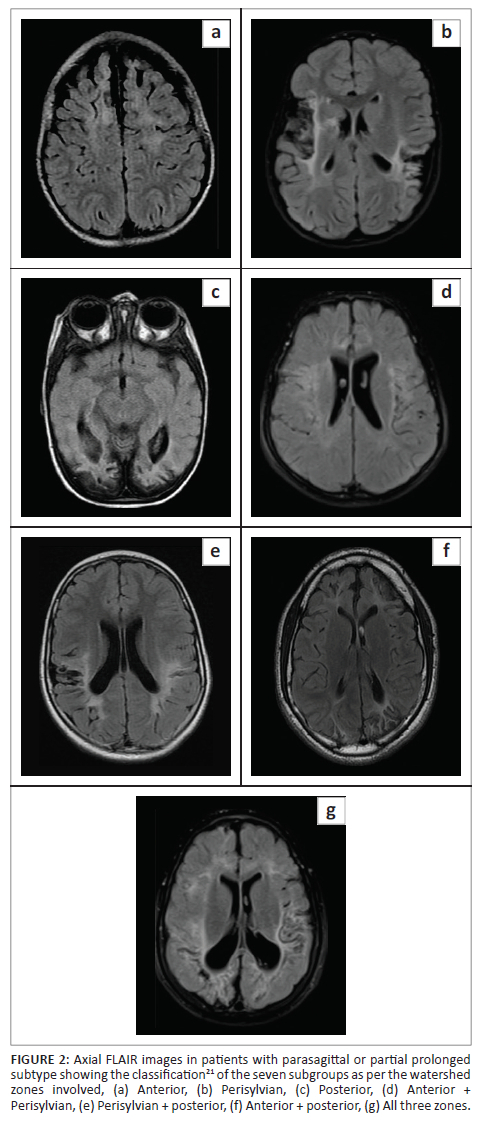
Parasagittal scoring
The parasagittal scores were calculated for each patient with substrate injuries at the parasagittal structures measured according to a previously reported grading system.19 These included the involvement of the paracentral lobule (PCL), supplementary motor areas, superior frontal gyrus, precentral gyrus, postcentral gyrus and the degree of interhemispheric fissure widening. These five specific parasagittal features were each scored from 0 to 3 using a simple scoring system: 0 = no injury, 1 = mild injury (less than a third of the structure was injured), 2 = moderate injury (more than one-third, but less than two-thirds of the structure was injured) and 3 = severe injury (more than two-thirds, with up to near-complete destruction of the substrate). The sum of these five measured values added to a potential maximum parasagittal score of 15.
Quantitative volumetric analysis
The substructure volumes were measured using the software solution NeuroQuant® (Cortechs Labs, Inc., San Diego, CA, United States [US]). This AI tool is the first FDA- approved software for volumetric MRI processing. NeuroQuant® calculates the volume of different brain substructures and compares those to a large normative age- and gender-matched database to determine whether the degree of brain volume loss is statistically significant for patient age. Each patient is then allocated a percentile ranking for every volumetric measurement, compared to age-matched controls. A sample of the volumetric data acquisition process is displayed in Figure 3. These measurements were independently overseen by co-investigators (L.K. and A.U.), who were blinded to any clinical details or radiological diagnoses. Because comparable age-matched atlases are not available for children under the age of 3 years, they were therefore excluded from the volumetric analyses.
Statistical analysis
The Kolmogorov-Smirnov test was used to assess normality assumptions of the continuous variables. All variables violated the normality assumptions and were thus expressed as medians with interquartile ranges (IQR). The Kruskal-Wallis test, a non-parametric version of the ANOVA (analysis of variance) test, was used to analyse differences between the medians of two or more groups. The Mann-Whitney U test was used to analyse differences between the two groups of HIBI subtype 2 (watershed pattern). A two-tailed p < 0.05 indicated statistical significance. All statistical analyses were conducted using SAS, version 9.4 (SAS Institute). Box plot graphs were generated using GraphPad Prism Software 8.0.2 (GraphPad Software, La Jolla, CA, US).
Ethical considerations
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This retrospective study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (UKZN) (BREC/00001036/2020).
Results
A total of 96 cases were recruited for the study. Five cases were excluded as the imaging sequences did not process without errors on the neuroquantification software programme. The youngest child imaged was 3 years (owing to the threshold for the volumetric evaluation), and the oldest was 9 years of age (median age = 5 years). Categorisation of the four HIBI subtypes was undertaken, and correlation with the degree of substrate injury for each subtype and their various subgroups of injury were tabulated. There was a high degree of interobserver agreement between the two readers, with a weighted kappa of > 0.8.
There were three predominant patterns of HIBI, as displayed in Table 1. The fourth subtype was multicystic encephalomalacia, only seen in one patient. Thirty-four demonstrated the RBGT/central pattern of injury, and these were further subdivided into mild (n = 11), moderate (n = 9), severe (n = 4) and MPI (n = 10). Watershed-only injuries were identified in 35 individuals, and they were classified into seven subgroups, with anterior (n = 2), perisylvian (n = 1), posterior (n = 1), anterior + perisylvian (n = 3), perisylvian + posterior (n = 3), anterior + posterior (n = 3) and all three zones involved (n = 22). Twenty-three patients demonstrated a mixed watershed/RBGT pattern.
We found that the average intracranial volume was reduced to the 1st percentile in all patient groups (p < 0.001), across all four subtypes of HIBI (Table 1, Figure 4). Most marked was the reduction in the volume of the cortical grey matter (364.2 cm3 - 3rd percentile, p < 0.001), PCL (6.5 cm3 - 1st percentile, p = 0.001) and occipital lobe (32.2 cm3 - 1st percentile, p = 0.001) volumes.
Neuroquantification of the thalamus demonstrated significant volume reduction, particularly in the RBGT subtype (1st percentile ranking). There was less significant volume reduction of the thalamic volume in the watershed (77th percentile) subtype and the mixed subtype (66th percentile). Further quantification within the RBGT subtype revealed a reduction of the thalamus volume in all RBGT or central subgroups (Figure 4).
Similarly, incremental substrate destruction could be clearly defined using measured volumes and percentile rankings for each region of interest, notably in the basal ganglia, particularly the putamina. The basal ganglia demonstrated markedly reduced overall volume (27th percentile) for patients in the RBGT subtype (Figure 4). This was more significantly reduced compared to the watershed subtype (88th percentile), where the putamen is usually spared. In the mixed injury patterns, the basal ganglia are moderately reduced (39th percentile) as there is usually some but generally less severe basal ganglia and thalamic involvement.
On average, total brainstem volume was 5.30 cm3 greater in the mild RBGT subgroup than in the MPI subgroup (β = 5.30; p = 0.002, Figure 5). The brainstem volume was also reduced in the mixed pattern group (31st percentile), largely due to the RBGT component of combined injury. Notably, the watershed pattern group demonstrated almost no reduction in brainstem volume (99th percentile). This is a critical differentiating factor between the two principal patterns (Table 2 and Table 3).
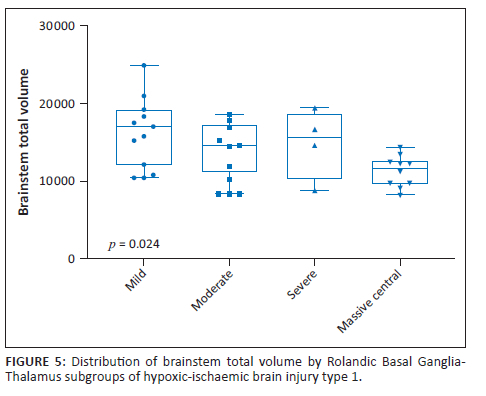
There were significant differences in total brainstem volume between the four groups, with the mild subgroup having a higher volume (Figure 5). In addition, we demonstrated a comparative decrease in brainstem volume with increase in parasagittal score. For every unit increase in the parasagittal score, the brainstem total volume significantly decreased by 0.27 units (β = −0.27; p = 0.001), (Figure 6).
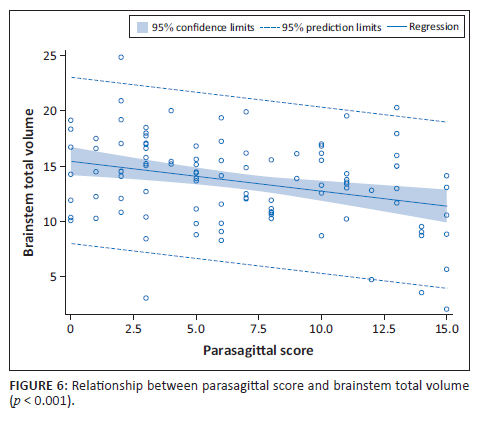
On further analysis of the RBGT subtype, we found that the parasagittal score significantly increased as the degree of severity of the RBGT pattern increased (p < 0.001) (Table 2). A marked difference in the volumes of the most severe central subgroup, called the MPI pattern, was noted, showing a reduction in volume in most of the substrates relative to the other RBGT subgroups (Table 2). As the parasagittal score increased, we noted a correlative decrease in the brainstem volume (Figure 6). This was largely isolated to the RBGT subtype.
A note is made of the inverse relationship between the PCL volumes (12 cm3, 11 cm3, 7 cm3 and 5 cm3, p < 0.001) and the progressive increase in the degree of RBGT severity (73rd, 67th, 1.5th and 1st percentiles) (Table 2). For the MPI subgroup, note the correlative most significant decrease in intracranial volume at 835 cm3 (p < 0.001) and proportional enlargement of the ventricles measured at 52 cm3 (Table 2).
Cerebral cortical volumes showed a progressive decrease in average cortical volumes (p < 0.001) and respective percentile ranking for mild = 561 cm3 (92nd percentile), moderate = 473 cm3 (79th percentile), severe = 456 cm3 (48.5th percentile) and MPI = 223 cm3 (1st percentile) (Table 2). This indicates the relative sparing of the cortex in the mild injury subgroup versus globally catastrophic cortical injury in the MPI subgroup (Figure 4).
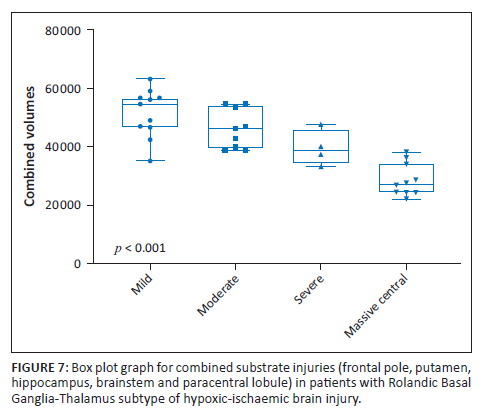
The amygdala and hippocampal formations, which are critical components of the limbic system, were systematically reduced in average volume (p < 0.001) in the RBGT subtype, measuring 2 cm3 (1st percentile) and 6 cm3 (3rd percentile), respectively. This differs quite significantly from the measured average volume of the watershed subtype patients who demonstrated 2.8 cm3 (33rd percentile) and 6 cm3 (67th percentile), respectively, for the amygdala and hippocampi. Further interrogation of the amygdala injuries in the RBGT subtype showed a severe reduction in volume in all four subgroups. The percentile ranking decreased progressively from the 3rd to 2nd to 1.5th and the 1st percentile ranks for mild through to MPI subgroups (Figure 4). We subsequently combined five regions that were commonly found to be injured (the PCL, putamen, hippocampus, frontal pole and brainstem volumes). There were statistically significant differences between the four groups of the RBGT subtype in terms of the combined total volumes of the five regions described earlier; particularly, the mild subgroup had a higher volume and the MPI subgroup showed markedly reduced combined volume (Figure 7).
In the analysis of the HIBI subtype 2 (watershed pattern), the seven subgroups were individually evaluated. Subgroup 7 (where all three watershed zones were involved) was the commonest (n = 22). The rest of the watershed subgroups (n = 13) were grouped into one comparative cohort (Table 3), to more meaningfully analyse the dataset for all these patients. The parasagittal score was much higher (12 vs 6) for subgroup 7, consistent with the more extensive destruction of the parasagittal cortex and greater substrate injury in the superior convexity of the cerebrum (p < 0.001, Table 3). Similarly, multiple substrates showed a remarkable average reduction in volume in subgroup 7 versus all other subgroups for the following regions: forebrain volume (534 cm3 vs 669 cm3), cortical volume (208 cm3 vs 339 cm3), parietal lobe volume (55.5 cm3 vs 92 cm3), primary motor cortex volume (15 cm3 vs 22.5 cm3) and primary sensory cortex volume (13 cm3 vs 22 cm3) (Table 3).
Notably, the brainstem was not reduced in volume in this subtype of HIBI (across all seven subgroups). Even in the most severe subtype of watershed injury (subgroup 7), where all three watershed zones were involved, the brainstem volumes averaged at the 99th percentile ranking (p = 0.095). In this study, the watershed subtype of HIBI was, therefore, not associated with any significant brainstem volume reduction (Table 3).
Discussion
There is increasing medicolegal litigation internationally, and the burden of HIBI is becoming even more pronounced in LMIC.2 The structural injuries of HIBI assessed visually by a radiologist are associated with poor neurodevelopmental outcomes.22 This study demonstrated a severe reduction in overall brain volume across the spectrum of HIBI subtypes when using an accredited neuroquantification software program. The average intracranial volume was reduced to the first percentile in all patient groups (p < 0.001). This is a key finding, quantitatively proving the significant morbidity of HIBI, irrespective of the subtype of injury sustained.
The complexity of brain injuries in HIBI demands the specification of the interrogating algorithms for this category of patients. Standardisation of imaging protocols is critical for obtaining usable datasets for volumetric analyses and accurately quantifying substrate injuries. A review of the available segmentation methods applicable to cerebral palsy concluded that atlas-based comparison in areas of substantial morphological injury may not suffice in accurately quantifying substrate destruction.23 In this study, the available NeuroQuant® (Cortechs Labs, Inc., San Diego, CA, US) age-matched atlases allow for the categorisation of the percentile rankings of study participants. Percentile rankings are useful in describing the degree of difference between affected children and their peers. These are useful if correlated with clinical parameters.
The typical patterns of HIBI pertain to the involvement of high metabolic substrates and watershed cerebral injuries or a combination thereof. The described patterns of HIBI, though repeated in individuals and following fairly expected substrate destruction, will produce a unique phenotype dependant on individual genetic factors, inflammatory pathways, neurotransmitter cascades and neuroplasticity.14 Serial imaging with substrate size measurement enables quantification of the end result of perinatal HIBI.4 This study, quantified brain injuries, and when measured against age-matched atlases, showed how severely the HIBI patients were affected.
For the RBGT subtype, there was a progressive increment in the volumetric measured substrate destruction. A stepwise gradation of injury was shown from the mild through the moderate, severe and MPI subgroups for the following substrates: basal ganglia (putamen), lobar volumes (parietal, occipital), cerebral cortex, PCLs and thalami. In the previously described most severe subgroup of RBGT injury, referred to as MPI, the substrate damage was most marked.
Brainstem injuries in hypoxic-ischaemic encephalopathy are often underestimated as radiologists focus on the more commonly described patterns of cerebral injuries.24 Despite the brainstem rarely demonstrating signal changes on conventional sequences, this study demonstrated that the brainstem was severely reduced in volume in the RBGT group where, probably consequent on the long tract (especially corticospinal) involvement, there was a secondary reduction in the fibre tract volumes with a smaller calibre brainstem. In those children with the watershed subtype pattern of cerebral injury, there was relative sparing of the brainstem with almost no observable volume reduction across the spectrum of all seven subgroups. This is significantly different to what was found in the RBGT subtype, where the brainstem was reduced in volume to an average 15th percentile. This is an important differentiating feature not previously reported.
The thalamus has been defined as a very important neural hub, linking several diverse neural circuits in the human brain. Thalamic injuries in different HIBI subtypes are well described. The average thalamic volume for all RBGT patients was measured at the 1st percentile ranking (p < 0.001), confirming that this substrate is severely destroyed in patients sustaining this specific subtype of HIBI. Across all the RBGT subgroups, there was marked thalamic volume reduction in all patients. This confirms that injury of this substrate is a key feature of the RBGT pattern where the VPL nucleus is principally injured, and progressive necrosis of the other thalamic nuclei is seen in more severe subgroups of RBGT injury.
This study also validates, by direct substrate volume measurement, the previously purported view that the thalamus is significantly involved in the basal ganglia-thalamus pattern of injury.25 There is also relatively less destruction of the thalamus in patients who suffered the watershed pattern of injury. The previous description of the thalamus L-sign21 signifies injury of the posterolateral thalamic margin (including the thalamic reticular nucleus, lateral geniculate body and pulvinar) adjacent to the corticospinal long tract. This sign is seen in the watershed subtype or combined hypoglycaemic or partial prolonged HIBI injuries. This study predicts a less marked thalamic volume reduction in a watershed subtype of HIBI than in the RBGT subtype.
The occipital lobe, in particular, the visual cortex, has also been identified as a high metabolic zone14 of the brain, often associated with central subtype injuries. This is corroborated in the current study where there was a stepwise reduction in average occipital lobe volume as the central injury severity increased. There was a less significant but similar trend for the parietal lobe volumes in RBGT patients. The average volumetric measurement of the parietal and occipital lobes for patients in the MPI subgroup was at the 1st percentile ranking.
This study has shown that by combining five regions of interest (frontal pole, putamen, hippocampus, brainstem and PCL volumes) with particular reference to the RBGT subtype of HIBI, there was a strong correlation between the RBGT subtype and combined reduction in volume in these substrates. This was a unique finding and may provide insight into the timing and mechanism for HIBI. The possibility that each of these substrates may be injured in other pathological processes remains a reality; however, the combined injury of high metabolic substrates favours the RBGT subtype of HIBI. Further larger multicentre interrogation of this finding is warranted.
The corpus callosum (CC), although an important white matter structure, often found to be injured in children with HIBI, was excluded as a region of interest in this study. Prior studies evaluating CC volumetry confirmed injury of this commissural substrate in cases of profound HIBI.26 Measurement of corpus callosal fibre tracts is problematic due to the lack of clarity as to where one terminates the measurement at the lateral edge of the tract, that is, where does the CC actually end in its lateral aspect as it blends with the adjacent white matter. Accurate measurements of the entire CC can, therefore, not be correctly calculated. Most studies27,28 have shown the secondary effect of thinning parts of the CC as measured in midline sagittal slices. For the purposes of this study, and due to the lack of age-matched percentile callosal volumetric measurements, it was resolved to exclude CC volumetry.
An important limitation of the study is the number of patients recruited for the broad categories and subtypes of HIBI. In addition, the age threshold of 3 years limited the number of patients for whom substrate volumes could be quantified. Larger sample groups and lowering the age for neuroquantification by expanding comparative atlases would add to the power of substrate evaluations. This could be further enhanced by larger multicentre studies to compare HIBI subtypes. Multicystic encephalomalacia and severe mixed subtype patterns following global brain injury with spongiosis of multiple substrates render volumetric analysis technically difficult due to the lack of measurable substrates.
Conclusion
In recent years, the use of AI applications has grown exponentially worldwide and particularly in radiology. Using neuroquantification software, we have more accurately measured brain injuries in children presumed to have sustained term neonatal HIBI. All patients who suffered HIBI demonstrated reduced brain volumes, with the average intracranial volume reduced to the first percentile in all HIBI subtypes (p < 0.001). Definitive substrate injury patterns are more pronounced in certain subtypes of HIBI and in the respective subgroups. A stepwise gradation with increasing substrate volume reduction was shown in the RBGT subgroups from the mild to most severely injured. Brainstem volume reduction was isolated to the RBGT subtype and unaffected in the watershed subtype patterns of HIBI. Similarly, thalamic, amygdala and occipital lobe volume reduction was a feature of the RBGT subtype with relatively normal volumes in watershed subtypes. Combined injury with reduced volumes of five regions of interest (frontal pole, putamen, hippocampus, brainstem and PCL) was repeatedly noted in patients with RBGT subtype of HIBI.
The AI algorithms used in this study may, in the correct clinical setting, be extrapolated to support stratification of HIBI.
Acknowledgements
The authors would like to acknowledge Prof. A.J. Barkovich as the promoter and co-supervisor of this research study.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
S.K.M. was the guarantor of integrity of the entire study. M.A. was the principal supervisor and reviewer of the manuscript. S.K.M., M.A. and J.W.L. were responsible for key concepts, design and literature research. N.M. was the chief statistician and guarantor of analyses. L.K. and A.U. were involved in neuroquantification and software analysis of datasets. All authors reviewed the literature and made critical contributions at each stage of the process. S.K.M. compiled the draft, collated the data following analysis and finalised the article for publication. All the authors read and agreed on the final article.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability
Data utilised for this study are available, on request, from the picture archiving and communication system (PACS) of Lake Smit and Partners Incorporated (https://ulite.lakesmit.co.za/; contact email stewart.james@lakesmit.co.za).
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Volpe JJ. Neurology of the newborn. 6th ed. Philadelphia, PA: Saunders; 2018, Chapter 18 and 19. [ Links ]
2.Nanyunja C, Sadoo S, Mambule I, et al. Protocol for the Birth Asphyxia in African Newborns (Baby BRAiN) Study: A Neonatal Encephalopathy Feasibility Cohort Study [version 1; peer review: 2 approved]. Gates Open Res. 2022;6:10. https://doi.org/10.12688/gatesopenres.13557.1 [ Links ]
3.Barkovich AJ, Truwit CL. Brain damage from perinatal asphyxia: Correlation of MR findings with gestational age. AJNR Am J Neuroradiol. 1990;11:1087-1096. [ Links ]
4.Rutherford M, Malamateniou C, McGuinness A, Allsop J, Biarge MM, Counsell S. Magnetic resonance imaging in hypoxic-ischaemic encephalopathy. Early Hum Dev. 2010;86(6):351-360. https://doi.org/10.1016/j.earlhumdev.2010.05.014 [ Links ]
5.De Vries LS, Groenendaal F. Patterns of neonatal hypoxic-ischaemic brain injury. Neuroradiology. 2010;52(6):555-566. https://doi.org/10.1007/s00234-010-0674-9 [ Links ]
6.Miller SP, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr. 2005;146(4):453-460. https://doi.org/10.1016/j.jpeds.2004.12.026 [ Links ]
7.Sie L, Van der Knaap M, Oosting J, De Vries L, Lafeber H, Valk J. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics. 2000;31(03):128-136. https://doi.org/10.1055/s-2000-7496 [ Links ]
8.Ranck JB, Windle WF. Brain damage in the monkey, macaca mulatta, by asphyxia neonatorum. Exp Neurol 1959;1(2):131-150. https://doi.org/10.1016/0014-4886(59)90032-9 [ Links ]
9.Mota-Rojas D, Villanueva-García D, Solimano A, Muns R, Ibarra-Ríos D, Mota-Reyes A. Pathophysiology of perinatal asphyxia in humans and animal models. Biomedicines. 2022;10(2):347. https://doi.org/10.3390/biomedicines10020347 [ Links ]
10.Windle WF. Brain damage at birth. JAMA. 1968;206;1967. https://doi.org/10.1001/jama.206.9.1967 [ Links ]
11.Myers RE. Two patterns of perinatal brain damage and their conditions of occurrence. Am J Obstet Gynecol. 1972;112(2):246-276. https://doi.org/10.1016/0002-9378(72)90124-X [ Links ]
12.Gunn AJ, Bennet L. Fetal hypoxia insults and patterns of brain injury: insights from animal models. Clin Perinatol. 2009;36(3):579-593. https://doi.org/10.1016/j.clp.2009.06.007 [ Links ]
13.Lai MC, Yang SN. Perinatal hypoxic-ischemic encephalopathy. J Biomed Biotechnol 2011;2011:609813. https://doi.org/10.1155/2011/609813 [ Links ]
14.Misser SK, Barkovich AJ, Lotz JW, Archary M. A pictorial review of the pathophysiology and classification of the magnetic resonance imaging patterns of perinatal term hypoxic ischemic brain injury - What the radiologist needs to know… S Afr J Radiol. 2020;24(1):1915. https://doi.org/10.4102/sajr.v24i1.1915 [ Links ]
15.Pringle C, Kilday JP, Kamaly-Asl I, Stivaros SM. The role of artificial intelligence in paediatric neuroradiology. Pediatr Radiol. 2022;52(11):2159-2172. https://doi.org/10.1007/s00247-022-05322-w [ Links ]
16.Iwasaki N, Hamano K, Okada Y, et al. Volumetric quantification of brain development using MRI. Neuroradiology. 1997;39(12):841-846. https://doi.org/10.1007/s002340050517 [ Links ]
17.Reid LB, Pagnozzi AM, Fiori S, Boyd RN, Dowson N, Rose SE. Measuring neuroplasticity associated with cerebral palsy rehabilitation: An MRI based power analysis. Int J Dev Neurosci. 2017;58:17-25. https://doi.org/10.1016/j.ijdevneu.2017.01.010 [ Links ]
18.Faria AV, Hoon A, Stashinko E, et al. Quantitative analysis of brain pathology based on MRI and brain atlases-Applications for cerebral palsy. NeuroImage. 2011;54(3):1854-1861. https://doi.org/10.1016/j.neuroimage.2010.09.061 [ Links ]
19.Misser SK, Lotz JW, Zaharie SD, et al. A proposed magnetic resonance imaging grading system for the spectrum of central neonatal parasagittal hypoxic-ischaemic brain injury. Insights Imaging. 2022;13:11. https://doi.org/10.1186/s13244-021-01139-7 [ Links ]
20.Chacko A, Andronikou S, Mian A, et al. Cortical ischaemic patterns in term partial-prolonged hypoxic-ischaemic injury - The inter-arterial watershed demonstrated through atrophy, ulegyria and signal change on delayed MRI scans in children with cerebral palsy. Insights Imaging. 2020;11(1):53. https://doi.org/10.1186/s13244-020-00857-8 [ Links ]
21.Misser SK, Lotz JW, Van Toorn R, Mchunu N, Archary M, Barkovich AJ. Thalamus L-sign: A potential biomarker of neonatal partial, prolonged hypoxic-ischemic brain injury or hypoglycemic encephalopathy? AJNR Am J Neuroradiol. 2022;43(6):919-925. https://doi.org/10.3174/ajnr.A7511 [ Links ]
22.De Vries LS, Jongmans MJ. Long-term outcome after neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal Ed. 2010;95(3):F220-F224. https://doi.org/10.1136/adc.2008.148205 [ Links ]
23.Pagnozzi AM, Gal Y, Boyd RN, et al. The need for improved brain lesion segmentation techniques for children with cerebral palsy: A review. Int J Dev Neurosci. 2015;47(Part B):229-246. https://doi.org/10.1016/j.ijdevneu.2015.08.004 [ Links ]
24.Quattrocchi CC, Errante Y, Rossi Espagnet MC, et al. Magnetic resonance imaging differential diagnosis of brainstem lesions in children. World J Radiol. 2016;8(1):1-20. https://doi.org/10.4329/wjr.v8.i1.1 [ Links ]
25.Elsingergy MM, Worede F, Venkatakrishna S, Andronikou S. Deep nuclei injury distribution in isolated "basal ganglia-thalamus" (BGT) versus combined "BGT and watershed" patterns of hypoxic-ischaemic injury (HII) in children with cerebral palsy. Clin Radiol. 2022;77(11):825-832. https://doi.org/10.1016/j.crad.2022.04.019 [ Links ]
26.Stivaros SM, Radon MR, Mileva R, et al. Quantification of structural changes in the corpus callosum in children with profound hypoxic-ischaemic brain injury. Pediatr Radiol. 2016;46(1):73-81. https://doi.org/10.1007/s00247-015-3444-3 [ Links ]
27.Andronikou S, Pillay T, Gabuza L, et al. Corpus callosum thickness in children: An MR pattern-recognition approach on the midsagittal image. Pediatr Radiol. 2015;45(2):258-272. https://doi.org/10.1007/s00247-014-2998-9 [ Links ]
28.Garhwal A, Patil A. Role of magnetic resonance imaging in biometric evaluation of corpus callosum in hypoxic ischemic encephalopathy patients. J Sci Soc. 2017;44(2):67-75. https://doi.org/10.4103/jss.JSS_30_16 [ Links ]
 Correspondence:
Correspondence:
Shalendra Misser
shalendra.misser@lakesmit.co.za
Received: 02 June 2023
Accepted: 28 Oct. 2023
Published: 26 Dec. 2023














