Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Journal of Radiology
versión On-line ISSN 2078-6778
versión impresa ISSN 1027-202X
S. Afr. J. radiol. (Online) vol.27 no.1 Johannesburg 2023
http://dx.doi.org/10.4102/sajr.v27i1.2725
CASE SERIES
Cystic lung tuberculosis in children: A series of five cases
Chetna MishraI; Pooja AbbeyI; Rama AnandI; Varinder SinghII; Ravinder KaurIII
IDepartment of Radiology, Lady Hardinge Medical College, New Delhi, India
IIDepartment of Pediatrics, Lady Hardinge Medical College, New Delhi, India
IIIDepartment of Microbiology, Lady Hardinge Medical College, New Delhi, India
ABSTRACT
Frequent imaging manifestations of pulmonary tuberculosis are airspace or interstitial nodules with or without tree-in-bud nodules, consolidation, cavitation, ground glass opacity, miliary nodules, lymphadenopathy and pleural effusion. It is unusual to encounter cystic changes in patients with pulmonary tuberculosis, and these findings should be differentiated from other cystic lung diseases. This case series describes five cases of cystic lung disease in children with tuberculosis (TB) with illustrative chest radiography and CT findings.
CONTRIBUTION: The manuscript highlights the need to consider tuberculosis as a possible cause of acquired cystic lung disease in appropriate clinical settings, particularly in endemic regions.
Keywords: pulmonary tuberculosis in children; cystic lung disease; pneumothorax; pulmonary tuberculosis; TB.
Introduction
The diagnosis and management of pulmonary tuberculosis (PTB) in children remains challenging because the paucibacillary nature of the disease in children and immunocompromised patients makes microbiological confirmation difficult. Chest imaging plays a key role by suggesting the tuberculous aetiology for early diagnosis and treatment in children with PTB.
Pulmonary tuberculosis is classified as primary or post-primary (reactivation) TB based on age, underlying immune status and prior exposure. Infants and children are frequently affected by primary TB, which is characterised by lymphadenopathy with concomitant parenchymal abnormalities such as consolidation and pleural effusion without loculation. Post-primary (reactivation) TB is caused by re-infection or reactivation of dormant bacilli from primary infection in previously sensitised individuals.1 Post-primary (reactivation) TB primarily affects adolescents and adults and the usual imaging manifestations include airspace or interstitial nodules with or without a tree-in-bud (TIB) appearance, consolidation, cavitation and empyema.2
It is unusual to encounter cystic changes in patients with PTB. Lung cysts are well-defined, thin-walled, epithelial lined airspaces within the lung parenchyma and may arise from a variety of causes.1 Pulmonary tuberculosis with cystic changes needs to be differentiated from other cystic lung diseases and mimics, such as pneumatocoele, cavity, bronchiectasis and honeycombing. Early diagnosis enables appropriate management and reduces long-term morbidity and mortality.
This case series illustrates five cases of cystic lung changes in children with TB in the absence of human immunodeficiency virus (HIV) infection. The findings at chest radiography (CXR) and CT are presented.
Case series
Case 1
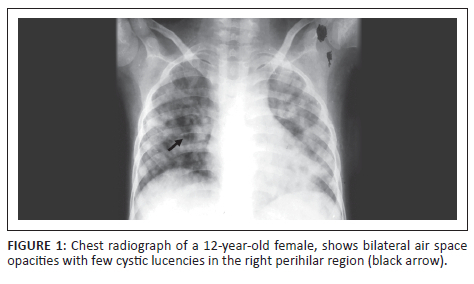
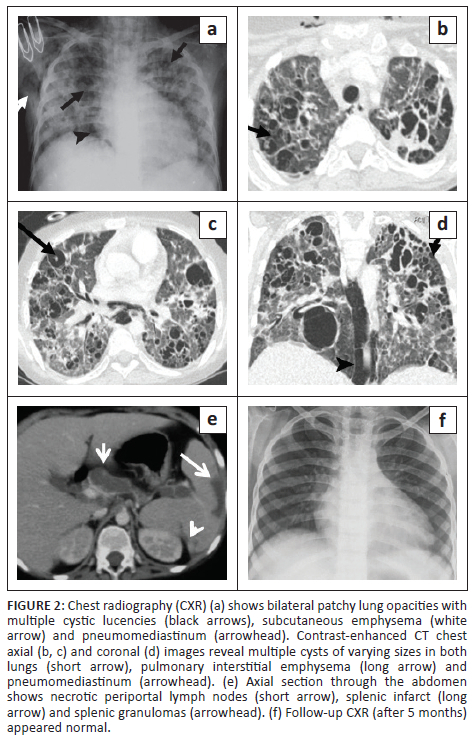
A 12-year-old girl, on treatment for autoimmune haemolytic anaemia, presented with a history of intermittent fever for 1-month, non-productive cough for 3 weeks and shortness of breath over the preceding 7 days. On examination, the patient was febrile with a heart rate (HR) of 156 beats/min and a high respiratory rate (RR) of 64/min. Chest radiography demonstrated ill-defined, bilateral air space opacities, with a few cystic lucencies in the right perihilar region (Figure 1). GeneXpert (sputum) detected mycobacterium tuberculosis (MTB) and the patient was started on antitubercular therapy (ATT). The respiratory failure necessitated ventilator support. Twenty days post-admission, the patient experienced sudden respiratory distress and a repeat CXR (Figure 2) demonstrated patchy opacities with an increased number of cystic lucencies in both lungs, along with the development of subcutaneous emphysema and pneumomediastinum. Contrast-enhanced CT (CECT) (Figure 2) revealed multiple thin-walled cysts, ground glass opacities (GGOs) and a few nodules bilaterally, along with pulmonary interstitial emphysema and pneumomediastinum. Multiple hypodense splenic granulomas and a few enlarged mediastinal and periportal lymph nodes were also seen. The patient responded well to treatment and was weaned off the ventilator within 10 days. A follow-up CXR (Figure 2) 5 months later appeared normal.
Case 2
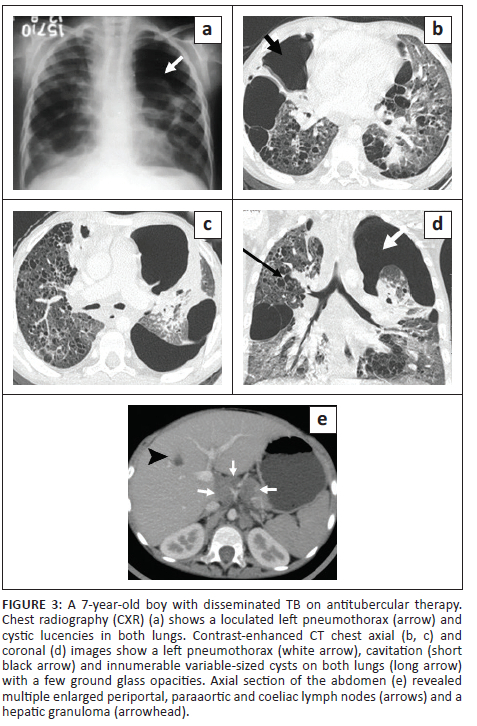
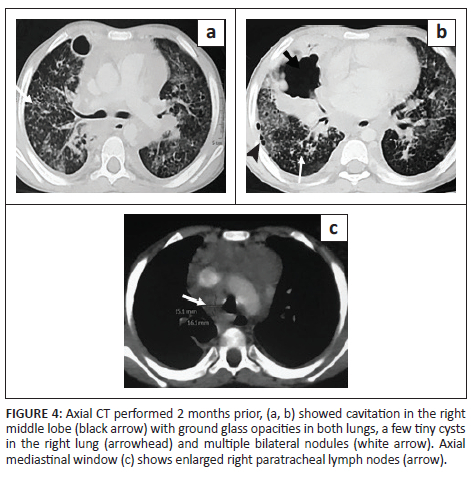
A 7-year-old boy, previously diagnosed with disseminated TB, on ATT for 3 months and on home oxygen therapy, presented to the paediatric outpatient department with complaints of intermittent increased cough from before and was found to have decreased oxygen saturation on room air. He underwent a CXR (Figure 3) that revealed a left loculated pneumothorax. The patient was admitted for assessment of multidrug resistant (MDR) TB and air leak. Contrast-enhanced CT chest (Figure 3) confirmed the left pneumothorax and revealed a thick-walled cavity in the right middle lobe and multiple cysts in both lungs. A previous CECT chest 2 months prior (Figure 4) was reviewed and revealed GGOs, a few tiny cysts, multiple bilateral nodules, along with necrotic mediastinal and abdominal lymphadenopathy, and a few small hypodense lesions in the liver suggestive of non-calcified granulomas. There was an interval increase in the number and size of the lung cysts, with a decrease in the number of nodules. Sputum microscopy and culture were negative. Bronchoscopy indicated signs of chronic inflammation and destruction of the distal bronchial segments and provided abnormal early entry into numerous cavitary structures, unlike the non-diseased areas where the distal branches gradually became smaller in size and did not allow entry into the lung parenchyma. Bronchial washings sent for culture were positive for TB (rifampicin sensitive). The patient was continued on ATT and demonstrated subsequent improvement.
Case 3
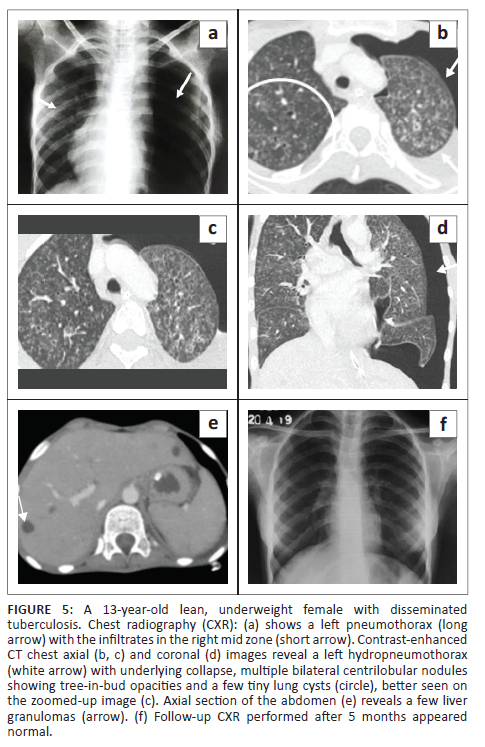
A 13-year-old emaciated, underweight girl presented with a cough for 12 days and projectile vomiting, associated with altered sensorium, blurring of vision and double vision over the preceding 4 days. There was a history of low-grade fever for 1 month associated with decreased appetite, weight loss and night sweats. The patient also had a positive contact history for pulmonary TB from her grandmother. Non-contrast CT brain at another facility (not shown) revealed communicating hydrocephalus and a hypodense fluid collection in the prevertebral space at the C2-C3 vertebral level, suggestive of a prevertebral abscess. Clinical and cerebrospinal fluid (CSF) findings suggested the diagnosis of tuberculous meningitis and the child was started on ATT. During her hospital stay, she developed sudden respiratory distress. The CXR (Figure 5) revealed a left pneumothorax with a few infiltrates in the right mid zone. An intercostal drainage (ICD) tube was subsequently placed. Sputum GeneXpert detected MTB. A CT of the chest (Figure 5) demonstrated a left hydropneumothorax with underlying collapse, multiple centrilobular nodules and a TIB pattern in both lungs, a few tiny cysts in both upper lobes, patchy consolidation in the right lower lobe, mediastinal lymph nodes with calcification, and hepatosplenomegaly with multiple liver and splenic granulomas. The patient improved on ATT and follow-up CXR (Figure 5) after 5 months revealed resolution of the radiographic findings.
Case 4
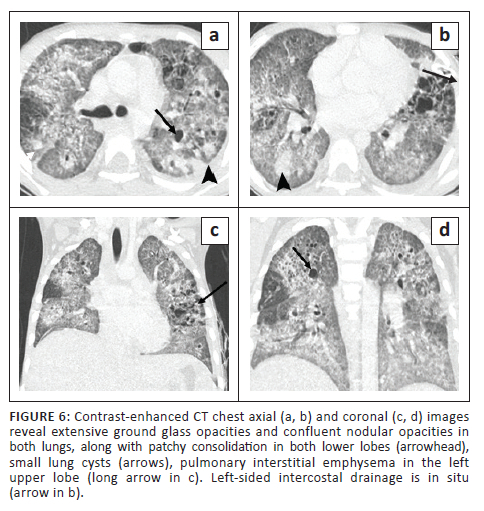
A 7-year-old girl was admitted with complaints of fever over 3 weeks, tachypnoea and cough for 4 days. In view of the respiratory distress, the child was transferred to the paediatric intensive care unit on day 5 post-admission. Mycobacterium tuberculosis was detected on sputum GeneXpert evaluation, and ATT was initiated. On day 11, the child experienced a sudden exacerbation of her respiratory distress. The CXR (not shown) revealed a left pneumothorax, for which an ICD was inserted. Subsequent CECT chest (Figure 6) revealed extensive GGOs and confluent nodular opacities in both lungs with patchy consolidation in both lower lobes, along with small cysts and bronchiolar dilatation. Other CT findings were mediastinal lymphadenopathy, pulmonary interstitial emphysema in the left upper lobe, minimal left pneumothorax with a pleural effusion, hepatosplenomegaly and hypodense non-calcified granulomas in the liver and spleen. The spleen also demonstrated a few small wedge-shaped regions of non-enhancing parenchyma suggestive of old infarcts. The patient responded well to ATT and recovered.
Case 5
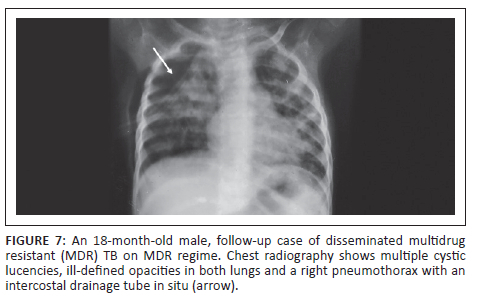
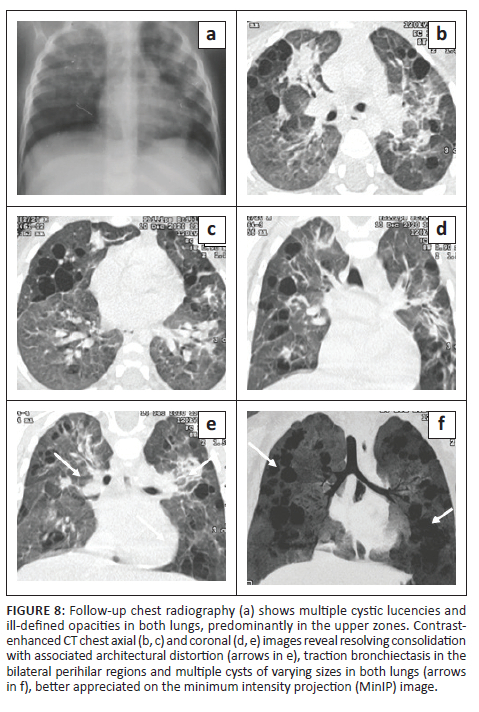
An 18-month-old male with disseminated MDR TB, on an MDR regimen for 5 months, with severe acute malnutrition and recurrent pneumothoraces was being followed up. The patient had a history of a recent admission with a right-sided pneumothorax (Figure 7) and pneumonia. He was managed with ICD tube drainage and IV antibiotics and subsequently discharged. A follow-up CXR (Figure 8) after 2 months showed multiple cystic lucencies and haziness in both lungs, predominantly in the upper zones. The CT chest (Figure 8) revealed resolving consolidation with associated architectural distortion and traction bronchiectasis in the perihilar regions along with multiple cysts of varying sizes in both lungs. The patient responded to ATT (MDR regimen) and subsequently recovered.
Discussion
A variety of lung diseases can cause or mimic cysts in the lung in the paediatric population. Cystic lung disease can occur in a myriad of conditions, which can be congenital as well as acquired, such as bronchogenic cysts, intralobar pulmonary sequestrations, cystic adenomatoid malformation of the lung, tracheobronchial papillomatosis, Langerhans cell histiocytosis, lymphocytic interstitial pneumonia, post-infectious states (Pneumocystis jiroveci) and many more. Cyst-like lesions or mimics include cystic bronchiectasis, cavities, pneumatoceles and honeycombing.2,3,4 Tuberculosis as a cause of multiple thin-walled lung cysts in children is rare. To the best of our knowledge, there are only a few case reports on CT findings that describe cystic lung changes in children with tuberculosis.1,4,5
Unusual CT findings for pulmonary TB are more common in a subset of patients, especially immunocompromised patients, malnourished patients, those with autoimmune deficiency syndromes, the elderly, alcoholics and those with diabetes mellitus.6 Among the presented patients, all had varying degrees of malnutrition, but none of them were infected with HIV.
Various pathological mechanisms are postulated to be responsible for the development of cystic lesions in tuberculosis. Cyst formation may be the result of granulomatous involvement of the bronchioles with a resultant check-valve mechanism caused by mural inflammation, oedematous luminal narrowing of the bronchioles by caseous material and peribronchiolar fibrosis.1,2,3,4,5,6,7,8,9 Scarring of the larger proximal bronchi with distal dilatation and interstitial air leakage with tuberculoma rupture have also been implicated in the pathogenesis.1,3,4,5,6,7 Other potential mechanisms include healed tubercular cavities re-lined by epithelia, cystic bronchiectasis and cysts developing post-isoniazid treatment in some cases.1,4,7
Most patients with tuberculosis who develop lung cysts have extensive bilateral infiltrative and exudative disease as a result of the pneumonic process.1,4,7 All the described cases had disseminated TB (with involvement of two or more noncontiguous sites), along with diffuse, bilateral lung involvement. These cystic lung lesions are often associated with centrilobular nodules and branching opacities in surrounding areas.2,3,6 Antibiotics were administered in all five cases, and none showed superadded bacterial infection. Three cases revealed numerous nodules, GGOs and consolidations. In Case 2, two CT studies were performed 2 months apart, which demonstrated a reduction in the lung nodules on treatment, with a corresponding increase in the number of cysts. Two cases showed predominantly cystic changes, with only a few nodules. One of these (Case 5) had received 5 months of ATT for MDR TB. In the presented cases, the cystic lesions developed during isoniazid treatment, rather than before or after treatment. This is in contrast to previous literature that describes certain instances of patients developing cystic lung lesions after isoniazid treatment.7
Tuberculous cysts are prone to rupture, leading to the development of recurrent pneumothoraces or pneumomediastinum.1,2,7,9 Pneumothorax was seen in four of the cases, likely as a result of cyst rupture. However, in Case 1, both cyst rupture and additional mechanical ventilation possibly played a role in the development of pulmonary interstitial emphysema and pneumomediastinum, which is difficult to differentiate radiologically.
According to previous literature, the evolution of cysts in tuberculosis may show varying severity, extent and an unpredictable outcome during the course of the disease. In some cases, cysts are reversible, while in others, the cysts remain static without progression and may persist on follow-up imaging.1,2,3,7 In this study, the cystic lesions were not evident on follow-up CXR in two cases, while one case (Case 5) showed persistent linear and fibrotic opacities on follow-up.
Conclusion
Pulmonary tuberculosis with multiple cysts should be differentiated from other cystic lung diseases. Cystic changes are among the atypical presentations of TB and should be considered as a possible cause of acquired cystic lung disease in appropriate clinical settings, especially in endemic regions. These cases require prompt management to avoid lifelong complications such as those associated with recurrent episodes of pneumothorax necessitating multiple chest tube insertions, hospitalisations and the development of irreversible fibrotic lung changes.
Acknowledgements
The authors would like to acknowledge the Department of Paediatrics, Lady Hardinge Medical College for providing clinical inputs and the Department of Microbiology, Lady Hardinge Medical College for microbiological investigations.
Competing interests
The authors declare that there are no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
C.M., P.A., R.A., V.S. and R.K. contributed significantly and equally to this work.
Ethical considerations
Informed consent was obtained from all individual participants involved in the study.
Funding information
The case series received no specific grant from any funding agency in the public, commercial and not for profit sectors.
Data availability
The authors confirm that the data supporting the findings of this research are available within the article.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Johari B, Khor BH, Musa AN, Abdul Kadir RF. Cystic lung disease as a complication of post-tuberculosis infection in young patients: A rare manifestation of a common disease. Respirol Case Rep. 2022;10(3):e0911. https://doi.org/10.1002/rcr2.911 [ Links ]
2.Perim J, Pimenta ES, Marchiori E. Cystic tuberculosis: A very unusual aspect of a common disease. Pulmonology. 2019;26(6):400-403. https://doi.org/10.1016/j.pulmoe.2019.12.001 [ Links ]
3.Ko KS, Lee KS, Kim Y, Kim SJ, Kwon OJ, Kim JS. Reversible cystic disease associated with pulmonary tuberculosis: Radiologic findings. Radiology. 1997;204(1):165-169. https://doi.org/10.1148/radiology.204.1.9205240 [ Links ]
4.Ray A, Suri JC, Sen MK, Khanna A. Cystic lung disease in tuberculosis: An unusual presentation. Lung India. 2013;30(4):351. https://doi.org/10.4103/0970-2113.120620 [ Links ]
5.Periwal P, Khanna A, Gothi R, Talwar D. Bronchocentric granulomatosis with extensive cystic lung disease in tuberculosis: An unusual presentation. Lung India. 2016;33(3):320. https://doi.org/10.4103/0970-2113.180888 [ Links ]
6.Lim KE, Wu YK, Huang SF, et al. Cystic lung changes in a patient with pulmonary tuberculosis. Tzu Chi Med J. 2010;22(2):111-114. https://doi.org/10.1016/S1016-3190(10)60051-1 [ Links ]
7.Tan LS, Tan S. Acquired lung cysts in post primary tuberculosis: An uncommon radiological finding in a common disease. Med J Malaysia. 2018;73(1):54-56. [ Links ]
8.Acharya VK, Anand R. Lung cysts in tuberculosis. Indian Acad Clin Med. 2004;5(2):202-204. [ Links ]
9.Lee HJ, Goo JM, Im JG. Rapid and irreversible cystic change of pulmonary tuberculosis in an immunocompetent adult. J Thor Imaging. 2003;18(4):254-246. https://doi.org/10.1097/00005382-200310000-00009 [ Links ]
 Correspondence:
Correspondence:
Rama Anand
rama_home@yahoo.com
Received: 28 May 2023
Accepted: 28 July 2023
Published: 28 Sept. 2023














