Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Journal of Radiology
versión On-line ISSN 2078-6778
versión impresa ISSN 1027-202X
S. Afr. J. radiol. (Online) vol.27 no.1 Johannesburg 2023
http://dx.doi.org/10.4102/sajr.v27i1.2677
PICTORIAL REVIEW
Imaging evaluation of the benign and malignant lesions of the floor of the mouth: Pictorial review
Ashim K. LahiriI; Charles R. DaultreyII
IDepartment of Radiology, Worcestershire Acute Hospitals NHS Trust, Worcester, United Kingdom
IIDepartment of ENT and Head and Neck Surgery, Worcestershire Acute Hospitals NHS Trust, Worcester, United Kingdom
ABSTRACT
The floor of the mouth is an important anatomical region of the oral cavity where primary benign and malignant disease processes can originate or secondary pathologies can extend into adjacent spaces. Knowledge of the anatomy is crucial for accurate localisation of pathology and understanding the spread of disease. The sublingual space is the dominant component of the floor of the mouth, bounded inferiorly by the mylohyoid muscle that separates it from the submandibular space. Imaging is immensely important to characterise and map the extent of disease, considering the fact that the bulk of the disease may be submucosal and not visible on clinical inspection.
CONTRIBUTION: The floor of the mouth is a complex anatomical region for radiological evaluation. The purpose of this pictorial review is to present an understanding of the relevant anatomy and to demonstrate the role and appropriate application of different imaging modalities. This article highlights the imaging spectrum of a wide range of various benign conditions including normal variants and a variety of malignant lesions at different tumour stages, with an aim to establish the correct diagnosis, avoid misinterpretation and help in treatment planning
Keywords: floor of mouth; sublingual space; mylohyoid; computed tomography; CT; MRI.
Introduction
Radiological evaluation of the floor of the mouth (FOM), an anatomical compartment of the oral cavity, is complex and challenging.1 The region harbours different types of tissues, including salivary glands, ducts, mucosa, submucosal soft tissues and the bony mandible and can be associated with a wide range of diseases, including congenital, inflammatory, infective, benign and malignant tumours and pseudolesions.2,3
Imaging is of immense value for evaluating submucosal disease of the FOM, which may not be completely obvious on clinical inspection.2,4 Ultrasound, CT, MRI and 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) imaging are crucial and complementary imaging techniques in diagnosis, disease mapping, treatment planning and surveillance.2,3,4,5
This manuscript reviews the pertinent anatomy, clinical contexts and distinct imaging characteristics of different pathologies and pseudolesions affecting the FOM.
Anatomy
The FOM is an important region of the oral cavity, located inferior to the oral tongue, bound anteriorly and laterally by the inner mandibular gingiva and inferiorly by the mylohyoid and geniohyoid muscles (Figure 1 and Figure 2). The posterior margin of the FOM corresponds to the attachment of the anterior tonsillar pillar to the tongue.
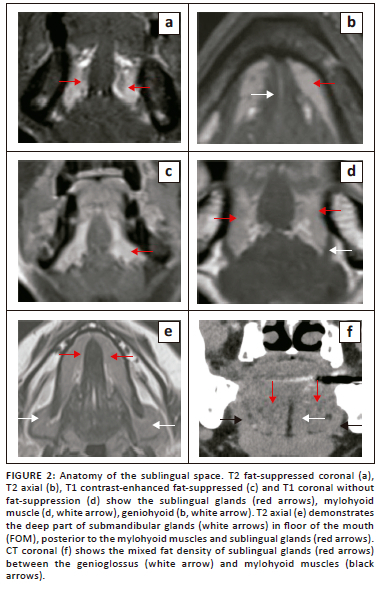
The sublingual space (SLS) is the major dominant deep neck anatomical space and is also referred to as the FOM, divided into bilateral spaces by the frenulum of the tongue.1,2,3,4 The SLS lies lateral to the genioglossus and geniohyoid muscles and superior to the mylohyoid muscle (Figure 1d and Figure 2). The contents of the SLS are fat, the sublingual glands, the lingual nerve (branch of V3 trigeminal nerve), lingual vessels, branches of the glossopharyngeal and hypoglossal nerves (IX and XII cranial nerves), the superior most part of the submandibular gland, Wharton's duct (submandibular) and part of the hyoglossus muscle.3,5,6 The SLSs communicate freely with each other anteriorly and communicate with the submandibular spaces inferiorly through the gap between the free posterior margin of the mylohyoid and hyoglossus muscles4,6,7 (Figure 1b and Figure 2e). Extension of disease can occur from the submandibular to the parpharyngeal space through the communicating channels.7,8
The sublingual glands are formed by smaller glands and are drained by short ducts in the FOM/SLSs. These glands are best visualised on T2-weighted short-tau inversion recovery (T2-STIR) and post-contrast MRI8 (Figure 2a, c). The submandibular (Wharton) ducts pierce the mylohyoid muscles and open in the FOM, on either side of the frenulum. The duct opening can be occluded by calculi or tumours and patients can present with features of the obstructive submandibular adenitis.
The mylohyoid muscle is an important anatomical landmark in the FOM; it separates the SLS above from the submandibular space below, and it lies superior to the anterior belly of the digastric muscle (Figure 1 and Figure 2d). This is a paired sling-like structure, originating from the mylohyoid ridge at the medial aspect of the body of the mandible and forming the muscular floor of the oral cavity. The bulk of the muscle fibres (anterior and middle) attach to the midline raphe, which extends from the mandibular symphysis to the hyoid bone and the posterior one-fourth of the fibres attach to the anterior surface of the hyoid bone.1,6,7
Defects of the mylohyoid muscle are a common, well-recognised entity, referred to as mylohyoid boutonnieres, with a reported presence of 20% - 77% in the literature.6,7 White et al.7 confirmed mylohyoid defects in 77% of their cases; bilateral defects were observed in 67% and unilateral defects in 33%. The herniated salivary tissue is mostly from the sublingual glands and less likely from the submandibular gland, and can present as a mass in 40% of cases, with possible recurrent inflammatory changes6,7,9 (Figure 3 and Figure 4). Other lesions that can potentially dissect through the mylohyoid defect into the submandibular space include plunging ranulas, abscesses and malignancies.7,9 When evaluating the FOM on imaging, it is vital to carefully assess the integrity of the mylohyoid muscles bilaterally, which is clearly visible on the axial and coronal images.
Imaging
Imaging is absolutely essential for the characterisation of various benign and malignant lesions.1,2,3,4 Ultrasound is a common first-line imaging modality to investigate neck abnormalities, with the advantages of easy availability, non-invasiveness, high-resolution imaging and lack of radiation. Ultrasound is the first modality of choice for the assessment of the neck lumps in the paediatric population. The major drawbacks of ultrasound are that the technique is operator-dependent, and it is limited in accurately assessing the extent of deep disease. Literature reviews highlight the role of ultrasound in evaluating the FOM and oral cavity, including the depth of invasion of tongue malignancies, beyond the standard practise of assessing the neck for the thyroid gland, salivary glands, superficial lumps and ultrasound-guided procedures.1,10 Figure 4 and Figure 5 highlight the role of ultrasound in demonstrating a myelohoid boutonniere and two cases of ranula.
CT and MRI are crucial complementary imaging modalities. MRI is an excellent technique for soft tissue characterisation, disease localisation, establishing early bone marrow infiltration and perineural spread of disease. CT is distinctly superior for investigating acute infections and abscesses, demonstrating benign and malignant cortical bony changes, confirming calcification and chondroid tumour matrix, and establishing the presence of ductal calculi.3,11 However, images of the oral cavity and FOM are prone to degradation by artefacts from dental amalgam and other post-surgical metal implants, especially with CT imaging.
18F-fluorodeoxyglucose positron emission tomography/computed tomography is an immensely important problem-solving complementary imaging technique to establish primary neck disease, detect nodal disease, distant metastasis, synchronous primary tumours and recurrent or residual disease. In the presence of post-treatment changes, diagnosing recurrent or residual disease is often a diagnostic challenge.
Other helpful imaging tools are conventional sialography for salivary ductal assessment and angiographic studies for specific arterial or arterio-venous vascular abnormalities.
Floor of the mouth lesions
Most pathological abnormalities of the FOM are benign in nature, including developmental conditions (dermoid, epidermoid, thyroglossal cyst), ranula, lipoma, post-infective or inflammatory conditions caused by obstructive calculus disease of the submandibular duct with abscesses, sublingual adenitis and Ludwig Angina.1,3,8,11 The remaining infrequent benign lesions of the FOM are lymphatic and vascular malformations and rarely pleomorphic adenomas and benign neurogenic tumours. The mylohyoid Boutonniere lesions, seen as mylohyoid muscle defects with ectopic salivary gland tissue, are referred to as pseudolesions.6,7
The most common malignancies are squamous cell carcinoma (SCC), tumours of the salivary glands (mucoepidermoid carcinoma, adenoid cystic carcinoma) and lymphoma, which is the third most common malignant tumour.1,3,4
Cystic lesions: Ranulas
Ranulas are benign mucus retention cysts of the FOM and submandibular regions that often develop in young people but can also be seen later in life. Because of the absence of true epithelial linings, they are not true cysts and develop as a result of blockage of the sublingual gland ducts (Rivinus duct). The underlying aetiology may be idiopathic or related to previous trauma or inflammation.6,10
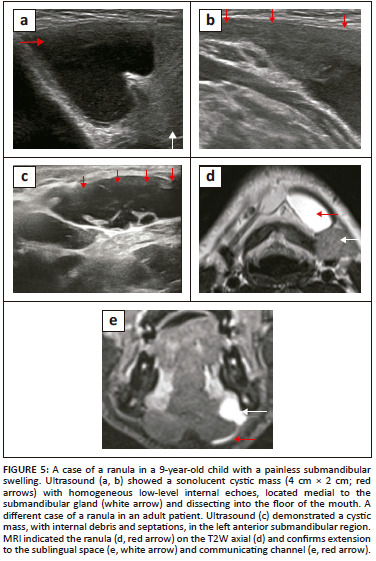
Ranulas are broadly classified into two categories: simple ranulas are confined to the SLS, bordered inferiorly by the mylohyoid muscles (Figure 6); while the plunging (diving) variety of ranula dissects into the inferior submandibular space through the communication posterior to the mylohyoid muscle or dissects through the mylohyoid muscles9,10 (Figure 7).
Ranulas can present as an intraoral mass or as a cystic, fluctuant, submandibular mass of varying size. The role of imaging is to establish the anatomical location (sublingual or plunging variety), to demonstrate the benign cystic nature of the lesion, to differentiate it from other cystic lesions of the FOM (lymphangioma, epidermoid, dermoid cyst and branchial cleft cysts), to look for integrity of the mylohyoid muscles and above all, for pre-surgical assessment with the aim of complete excision of the sublingual gland.7,10
Ultrasound is the initial imaging of choice, particularly in the paediatric age group. An uncomplicated ranula appears as a unilocular, anechoic cystic lesion, while complicated or chronic haemorrhagic ranulas demostrate varying degrees of debris and wall-thickening9,12 (Figure 5). Ultrasound may have a limited role in large plunging ranulas or suspected ranulas with indeterminate features. In evaluating plunging ranulas, CT and MRI can better demonstrate the site of communication between the sublingual and submandibular spaces posterior to the mylohyoid, where the communicating narrowed cystic tract is seen directed anteriorly. This appearance is referred to as the 'tail sign', which is diagnostic of plunging ranula10,13 (Figure 5e and Figure 7c).
Cystic lesions: Developmental
The squamous epithelium lined epidermoid and dermoid cysts and cystic teratomas of the neck are rare developmental entities, mostly seen at the FOM as slow-growing midline cysts and constitute only 1.6% to 6.9% of head and neck cystic masses.14,15,16 Ultrasound, CT and MRI can confirm the cystic nature of the lesion; however, MRI is the most sensitive modality for further characterisation and aids in differentiation from other cystic sublingual, submandibular and submental masses like ranula, cystic hygroma, lymphangioma and also, rarely, abscesses in cases of infected cysts.15
The ectoderm-lined dermoid cyst demonstrates the diagnostic features of an intracystic cluster of floating fatty corpuscles, known as the 'sack of marbles' sign, which on CT appears as a cluster of low attenuating densities and on MRI, demonstrates typical features of fat content.16
The simple, squamous epithelium lined epidermoid cyst typically appears as a midline FOM, simple, fluid-containing cyst on imaging and can attain a very large size because of slow growth14,17 (Figure 8). Restriction of diffusion is the most characteristic feature of epidermoids on MRI (Figure 8d), differentiating it from the dermoid cyst.
The most common congenital lesions of the neck are thyroglossal duct cysts, which are rarely located in the FOM.18 The majority of these cysts are infrahyoid in location, with the suprahyoid site being less common, seen in only 20% of cases. A characteristic thyroglossal cyst appears as a painless, midline cystic mass in the paediatric population, the paramedian location is more common with the infrahyoid cysts. Ultrasound is the initial imaging of choice, which will show a simple, uncomplicated cyst as an anechoic, thin-walled cyst without internal solid components or vascularity. However, a more complex appearance on ultrasound would be seen in the presence of infection, haemorrhagic contents, or the rare coexistence of thyroid carcinoma within the cyst. Further imaging with CT or MR imaging would be essential to characterise more complex lesions, to demonstrate the extent of the deeper, larger lesions and for pre-surgical planning.
Floor of the mouth infection/inflammation
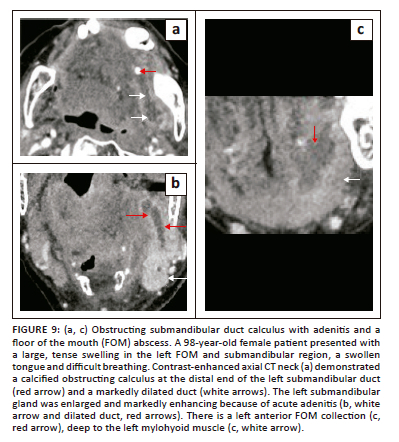
Acute infection of various neck spaces, including the FOM, is often associated with significant morbidity and mortality, particularly in elderly, diabetic and immunocompromised patients. The major underlying factors for acute FOM infections (cellulitis and abscesses) are related to salivary ductal calculus disease or odontogenic causes.8 The submandibular (Wharton) duct is a common site for intraductal calculi with the potential of causing post-obstructive suppurative adenitis and abscesses (Figure 9). Through the free anatomical communication posteriorly, cellulitis from an infected submandibular gland carries the risk of extension into the SLS and vice versa.8,11
The FOM is also involved in other acute infections of the neck spaces, like Ludwig's angina, which is a serious progressive acute bacterial infection, originating commonly from mandibular molar tooth infection. This disease entity is more common in diabetics and immunocompromised patients, and the infection can progress rapidly, causing airway compromise. Imaging demonstrates extensive cellulitis, predominantly involving the submandibular, submental and SLSs with a lesser likelihood of underlying abscess formation,11,19 as demonstrated in Figure 10.
Ultrasound is helpful for the assessment of superficial abscesses in the submandibular region, nodal disease and duct calculi. In acute settings, contrast-enhanced CT neck (from the base of the skull to the superior mediastinum) is the imaging modality of choice for confirmation and localisation of the abscesses, which appear as rim-enhancing fluid collections (Figure 11), pre-surgical planning, assessment of the patency of the major neck vessels and evaluation of the bony mandible for osteomyelitis.11,19,20 Recent studies have, however, also established the role of MRI in deep neck infection as a more accurate imaging modality because of its high soft tissue resolution.20 The major limitation is the availability of MR imaging in emergency settings.
Floor of the mouth malignancy
Squamous cell carcinoma is the most common malignancy of the oral cavity (more than 90%), most frequently involving the oral tongue and mucosal lip, followed by the floor of mouth (17% of oral cavity SCC). Other malignant conditions affecting the FOM are the minor salivary gland tumours (mucoepidermoid carcinoma, low-grade adenocarcinoma and adenoid cystic carcinoma), lymphoma and rarely, sarcomas.1,2,21,22
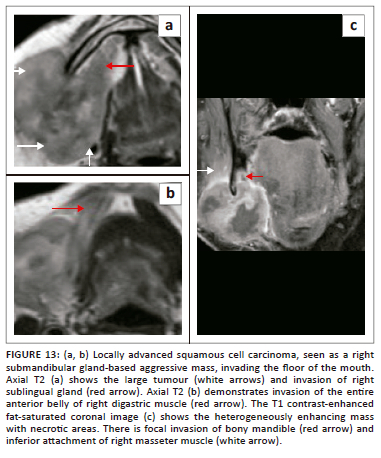
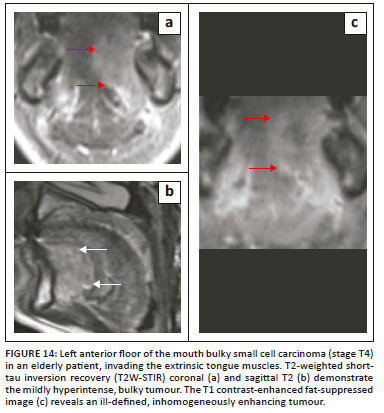
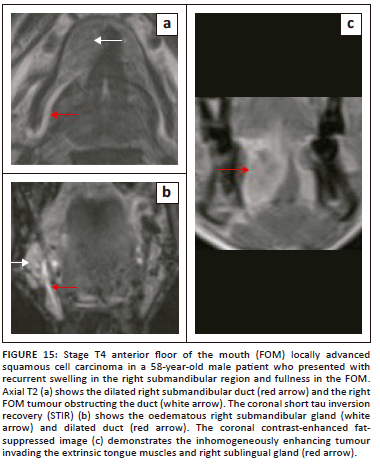
Malignancies of the mandible and submandibular gland can also invade the FOM,1,21 as demonstrated in Figure 12 (T4 SCC mandible invading the FOM) and Figure 13 (SCC of submandibular gland infiltrating the FOM). Malignancies of the FOM spread further by invading the muscles of the FOM, the mandible, the musculature of tongue, the submucosal spaces and extending along the neurovascular structures in the SLS.
CT and MRI are complementary imaging techniques to characterise and evaluate the extent of disease. MRI is an excellent technique and imaging of choice, with particular reference to the assessment of the depth of tumour invasion, which is a crucial prognostic marker for T-staging of oral cavity cancers.23,24 MRI is also very sensitive for detecting early bone marrow infiltration and establishing perineural spread of disease, which is particularly a concern for adenoid cystic cancers.22,23,25
According to the revised T-staging guidelines of the American Joint Committee on Cancer (AJCC) 2018,26 depth of invasion and tumour size are the criteria for T1 to T3 staging, full-thickness osseous destruction of the mandible and maxilla for T4a staging and invasion of the masticator spaces and skull base, and encasement of the internal carotid arteries for T4b staging (Box 1).26 Imaging of the superficial, smaller, mucosal T1 tumours poses diagnostic challenges where clinical inspection and biopsy are superior in assessment. However, MRI remains excellent in establishing spread to the tongue and FOM musculature, ductal obstruction and bone marrow invasion.
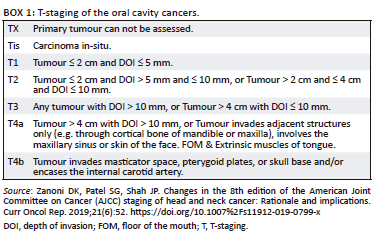
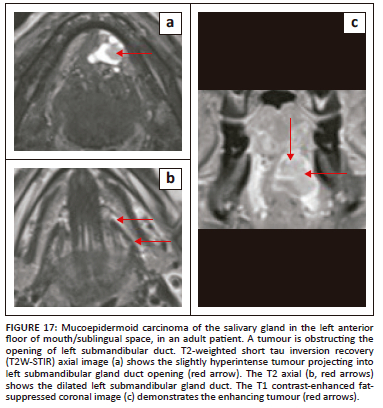
On imaging, SCC tumours typically appear as poorly defined, heterogeneously enhancing masses, most likely with cystic and necrotic components (Figure 14, Figure 15 and Figure 16). Figure 13 demonstrates a rare case of primary SCC of the submandibular gland with invasion of the muscles of the FOM and bony invasion of the mandible. Patients can present with features of recurrent sialadenitis secondary to submandibular duct obstruction at the FOM (Figure 16 and Figure 17). The most common condition mimicking malignancies of the FOM is infection of the oral cavity with associated cellulitis or abscess.21,22,25
Lymphomas contribute 14% of head and neck malignancies and the vast majority are non-Hodgkin lymphomas (96%). Lymphoma of the oral cavity is generally uncommon and constitutes only 5% of oral malignancies. Primary non-Hodgkin lymphoma of the mandible is extremely rare, accounting for only 0.6% of extranodal lymphomas and 8% of mandibular malignancies.27,28
At imaging, these rare FOM lymphomas present as characteristically bulky, homogeneous soft tissue masses with a varying degree of significant contrast enhancement and pronounced restriction of diffusion. The osseous abnormalities can appear as ill-defined lytic destruction or an infiltrative pattern with invasion and widening of the inferior alveolar nerve canal. Typical radiological findings are presented in Figure 18.
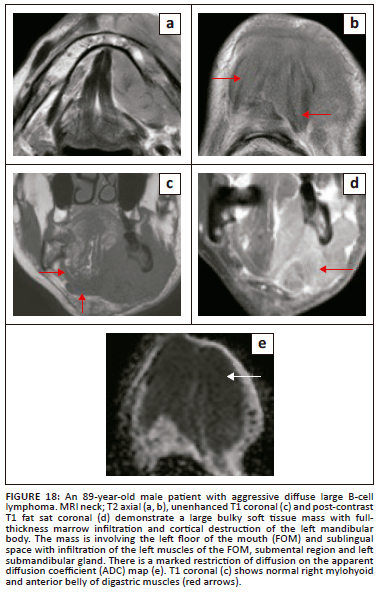
Post-treatment imaging
Interpretation of the imaging findings in the presence of post-treatment changes is often a diagnostic challenge. It is crucial for the radiologist to be aware of the time gap between the completion of treatment and imaging, as follow-up imaging is performed at least 3 months after the completion of the treatment and significant imaging variations may be present because of radiotherapy-induced oedematous changes. Furthermore, it is crucial to understand the various types of surgical procedures (type of graft repair, implants and neck dissection) that impact the imaging findings.29
MR imaging can pose a significant challenge in the differentiation of recurrent or residual disease from post-treatment changes. Although 18F-FDG PET/CT has a very high negative predictive value for excluding recurrent disease in comparison to CT and MRI (Figure 19 and Figure 20), PET imaging also has its own limitations, including the possibility of false-positive results from post-treatment changes and physiological causes,5,29 as illustrated in Figure 21.
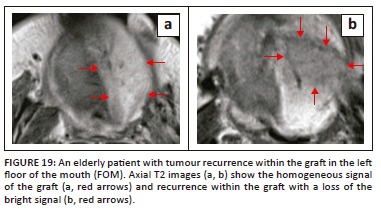
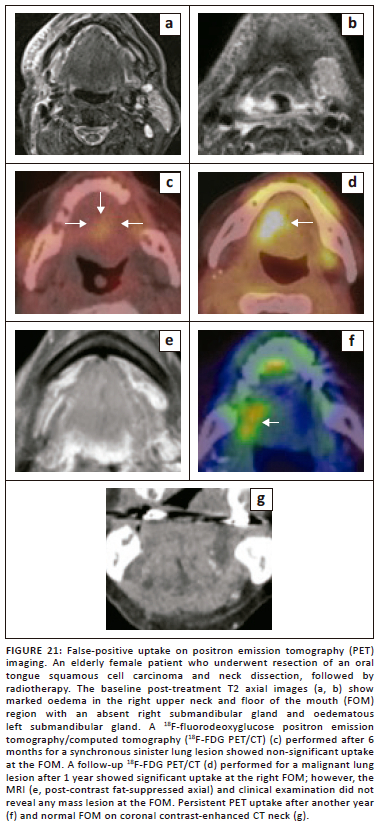
Conclusion
Precise understanding of the anatomical landmarks of the FOM and imaging findings of various benign and malignant conditions is crucial for diagnosis and assessment of the pathways of disease spread, T-staging of cancers, treatment planning and disease surveillance.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
A.K.L. is the main contributor who selected and formalised the title, finalised the topic, reviewed the literature, selected cases and prepared the manuscript, figures and legends. C.R.D. helped in manuscript preparation, literature review and reviewing the clinical and surgical parts of the cases illustrated.
Ethical considerations
This article followed all ethical standards for research.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability
Data supporting the findings of this study are available from the corresponding author, A.K.L., on request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1. Law CP, Chandra RV, Hoang JK, Phal PM. Imaging the oral cavity: Key concepts for the radiologist. Br J Radiol. 2011;84(1006):944-957. https://doi.org/10.1259/bjr/70520972 [ Links ]
2. Patel S, Bhatt AA. Imaging of the sublingual and submandibular spaces. Insights Imaging. 2018;9(3):391-401. https://doi.org/10.1007/s13244-018-0615-4 [ Links ]
3. Agarwal AK, Kanekar SG. Submandibular and sublingual spaces: diagnostic imaging and evaluation. Otolaryngol Clin North Am. 2012;45(6):1311-1323. https://doi.org/10.1016/j.otc.2012.08.005 [ Links ]
4. Varghese J, Kirsch C. Magnetic resonance imaging of the oral cavity and oropharynx. Top Magn Reson Imaging. 2021;30(2):79-83. https://doi.org/10.1097/RMR.0000000000000282 [ Links ]
5. Tantiwongkosi B, Yu F, Kanard A, et al. Role of 18F-FDG PET/CT in pre and post treatment evaluation in head and neck carcinoma. World J Radiol. 2014;6(5): 177-191. https://doi.org/10.4329/wjr.v6.i5.177 [ Links ]
6. Yamamoto MO, Nakajima K, Tsuji Y, et al. Imaging of the mylohyoid muscle: Separation of submandibular and sublingual spaces. Am J Roentgenol. 2010:194(5):W431-W438. https://doi.org/10.2214/AJR.09.3516 [ Links ]
7. White DK, Davidson HC, Harnsberger HR, Haller J, Kamya A. Accessory salivary tissue in the mylohyoid boutonnière: A clinical and radiologic pseudolesion of the oral cavity. Am J Neuroradiol. 2001;22(2):406-412. [ Links ]
8. Lyle NJ, Rutherford EE, Batty VB. A pain in the neck - Imaging in neck sepsis. Clin Radiol. 2011;66(9):876-885. https://doi.org/10.1016/j.crad.2011.03.016 [ Links ]
9. Lee JY, Lee HY, Kiim HJ, et al. Plunging ranulas revisited: A CT study with emphasis on a defect of the mylohyoid muscle as the primary route of lesion propagation. Korean J Radiol. 2016;17(2):264-270. https://doi.org/10.3348/kjr.2016.17.2.264 [ Links ]
10. Koch M, Mantsopoulus K, Leibl V, et al. Ultrasound in the diagnosis and differential diagnosis of enoral and plunging ranula: a detailed and comparative analysis. J Ultrasound. 2022;26(2):487-495. https://doi.org/10.1007/s40477-022-00743-7 [ Links ]
11. Beicos AG, Nunez D. Imaging of acute head and neck infections. Radiol Clin North Am, 2012;50(1):73-83. https://doi.org/10.1016/j.rcl.2011.08.004 [ Links ]
12. Jain P, Jain R, Morton RP, et al. Plunging ranulas: high-resolution ultrasound for diagnosis and surgical management. Eur Radiol. 2010;20(6):1442-1449. https://doi.org/10.1007/s00330-009-1666-1 [ Links ]
13. Kurabayashi T, Ida M, Yasumoto M, et al. MRI of ranulas. Neuroradiology. 2000;42:917-922. https://doi.org/10.1007/s002340000341 [ Links ]
14. Mirza S, Fadi S, Napaki S, Abualruz AR. Case report of complicated epidermoid cyst of the floor of the mouth: Radiology-histopathology correlation. Qatar Med J. 2014;2014(1):12-16. https://doi.org/10.5339/qmj.2014.2 [ Links ]
15. Jain H, Singh S, Singh A. Giant Sublingual dermoid cyst in floor of the mouth. Maxillofac Oral Surg. 2012;11(2):235-237. https://doi.org/10.1007/s12663-010-0093-9 [ Links ]
16. Giarraputo L, Savastano S, D'Amore E, Baciliero U. Dermoid cyst of the floor of the mouth: Diagnostic imaging findings. Cureus. 2018;10(4):2403. https://doi.org/10.7759/cureus.2403 [ Links ]
17. Kandogan T, Koç M, Vardar E, Selek E, Sezgin O. Sublingual epidermoid cyst: A case report. J Med Case Rep. 2007;1:87. https://doi.org/10.1186/1752-1947-1-87 [ Links ]
18. Nakayama S, Kimachi K, Nakayama K, Ikebe T, Ozeki S. Thyroglossal duct cyst occurring in the floor of the mouth: Report of 2 cases. J Oral Maxillofac Surg. 2009;67:2690-2693. https://doi.org/10.1016/j.joms.2009.04.114 [ Links ]
19. Gunawan F, Ferriastuti W. Ludwig's angina: An alarming radiology challenge. Radiol Case Rep. 2022;17(9):3103-3106. https://doi.org/10.1016/j.radcr.2022.05.085 [ Links ]
20. Hiroven J, Heikkinen J, Mikko Nyman M, et al. MRI of acute neck infections: evidence summary and pictorial review. Insight Imaging. 2023;14(1):5. https://doi.org/10.1186/s13244-022-01347-9 [ Links ]
21. Montero PH, Patel SG. Cancer of the oral cavity. Surg Oncol Clin N Am. 2015;24(3):491-508. https://doi.org/10.1016/j.soc.2015.03.006 [ Links ]
22. Trotta BM, Pease CS, Rasamny JJ, et al. Oral cavity and oropharyngeal squamous cell cancer: Key imaging findings for staging and treatment planning. Radiographics. 2011;31:339-354. https://doi.org/10.1148/rg.312105107 [ Links ]
23. Baba A, Hashimoto K, Hirofumi Kuno H, et al. Assessment of squamous cell carcinoma of the floor of the mouth with magnetic resonance imaging. Jpn J Radiol. 2021;39(12):1141-1148. https://doi.org/10.1007%2Fs11604-021-01161-1 [ Links ]
24. Waech T, Pazahr S, Guarda V, et al. Measurement variations of MRI and CT in the assessment of tumor depth of invasion in oral cancer: A retrospective study. Eur J Radiol. 2021;135:109480. https://doi.org/10.1016/j.ejrad.2020.109480 [ Links ]
25. Arya S, Rane P, Deshmukh A. Oral cavity squamous cell carcinoma: role of pretreatment imaging and its influence on management. Clin Radiol. 2014;69: 916-930. https://doi.org/10.1016/j.crad.2014.04.013 [ Links ]
26. Zanoni DK, Patel SG, Shah JP. Changes in the 8th edition of the American Joint Committee on Cancer (AJCC) staging of head and neck cancer: Rationale and implications. Curr Oncol Rep. 2019;21(6):52. https://doi.org/10.1007%2Fs11912-019-0799-x [ Links ]
27. Weber AL, Rahemtullah A, Ferry JA. Hodgkin and nonHodgkin lymphoma of the head and neck: clinical, pathologic and imaging evaluation. Neuroimaging Clin N Am. 2003;13(3):371-392. https://doi.org/10.1016/s1052-5149(03)00039-x [ Links ]
28. Kwok H, Ng FH, Chau CM, Ma JKF. Multimodality imaging of extra-nodal lymphoma in the head and neck. Clin Radiol. 2022;77(8):549-559. https://doi.org/10.1016/j.crad.2022.04.017 [ Links ]
29. Tshering Vogel DW, Thoeny HC. Cross-sectional imaging in cancers of the head and neck: How we review and report. Cancer Imaging. 2016;16:20. https://doi.org/10.1186/s40644-016-0075-3 [ Links ]
 Correspondence:
Correspondence:
Ashim Lahiri
ashim_lahiri@hotmail.com
Received: 20 Mar. 2023
Accepted: 14 June 2023
Published: 31 Aug. 2023














