Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Journal of Radiology
versión On-line ISSN 2078-6778
versión impresa ISSN 1027-202X
S. Afr. J. radiol. (Online) vol.27 no.1 Johannesburg 2023
http://dx.doi.org/10.4102/sajr.v27i1.2556
CASE REPORT
A rare case of small-cell neuroendocrine tumour of the lung metastasising to the urinary bladder
Humphrey MapurangaI; Siseko SiloloII; Abraham C. van WykIII; Sucari S.C. VlokI
IDepartment of Radiodiagnosis, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIDepartment of Urology, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IIIDivision of Anatomical Pathology, Faculty of Medicine and Health Sciences, National Health Laboratory Service, Stellenbosch University, Cape Town, South Africa
ABSTRACT
A 77-year-old woman with suspected lung carcinoma had multiple bladder masses and lymphadenopathy outside the normal urinary bladder drainage area. Fine needle aspiration and immunocytochemistry of the cervical lymph node complex and transurethral biopsy of the bladder masses confirmed metastatic small-cell neuroendocrine carcinoma.
CONTRIBUTION: Clinical correlation, imaging findings, tumour markers and immunohistochemistry are necessary for metastatic bladder tumour work-up
Keywords: small-cell neuroendocrine carcinoma; metastases; cystoscopy; immunohistochemistry; biopsy.
Introduction
Urinary bladder metastases are rare, accounting for 4.5% of all bladder neoplasms. Secondary bladder neoplasms can arise from direct extension from another pelvic malignancy or from distant organs as part of a more extensive disease.1 Primary malignancies with distant spread to the urinary bladder include the stomach, skin, lung and breast.2 Bladder metastases from a proven primary lung neoplasm are extremely rare with a paucity of literature on this specific entity.
Owing to the uncommon occurrence of distant bladder metastases from a non-contiguous primary source and the morphologic diversity of urothelial carcinoma of the bladder, accurate diagnosis can be challenging.
Case presentation
A 77-year-old woman known with hypertension, chronic obstructive pulmonary disease and a strong smoking history of 20 pack years, presented with a 4-month history of dysphagia, odynophagia and dysphonia. She reported chronic loss of appetite and tolerated only fluid feeds with hesitancy for solids. She had significant weight loss of approximately 20 kg over a 3-month period. At the time of presentation, she had no haematuria, flank pain, dysuria or any accompanying genitourinary symptoms. She had a previous hysterectomy and a surgical procedure for perforated peptic ulcer disease.
On physical examination, she was frail and cachectic with poor performance status: Eastern Cooperative Oncology Group grade 4 (ECOG-4). On clinical examination, a 3 cm firm, fixed and tender right cervical lymph node complex was palpated. Chest and breast examinations were normal. Review of other systems was unremarkable.
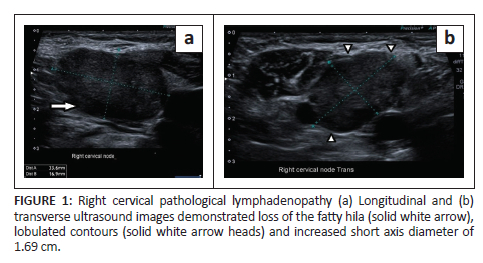
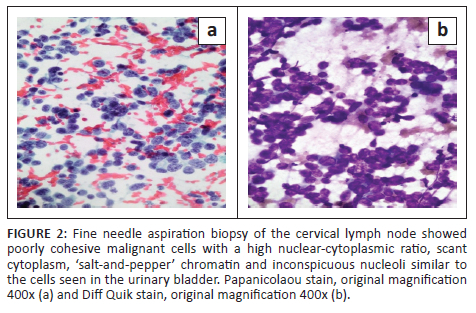
Gastroscopy demonstrated extrinsic oesophageal compression at 30 cm. Right neck ultrasound revealed a cluster of large, lobulated, hypoechoic lymph nodes with loss of fatty hila (Figure 1a & b). A lymph node fine needle aspiration biopsy proved metastatic carcinoma with morphological features suggestive of small-cell carcinoma (Figure 2a & b). Synaptophysin, chromogranin A and CD56 immunocytochemical stains were positive on the cell block, confirming neuroendocrine differentiation.
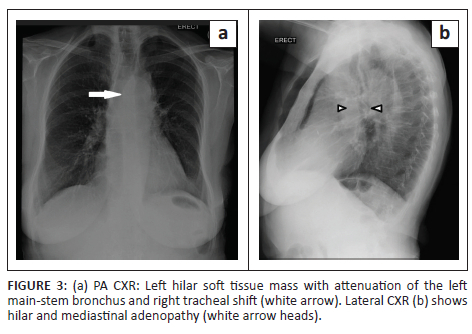
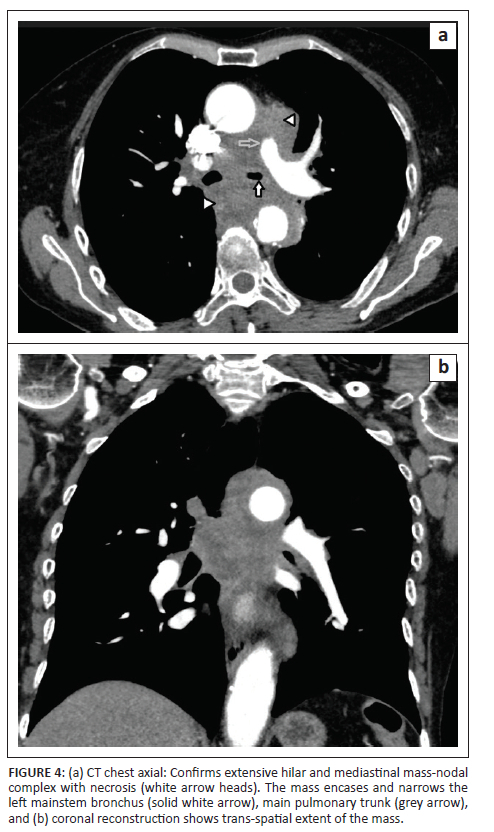
A posteroanterior chest radiograph performed at presentation revealed a left hilar mass with attenuation of the left main-stem bronchus and accompanying contralateral mediastinal shift (Figure 3). Post-contrast staging chest CT (Figure 4) showed an infiltrative, central, trans-spatial mediastinal mass encasing the left main-stem bronchus, left and right main pulmonary arteries and descending aorta. Abdomino-pelvic CT revealed multiple enhancing soft tissue mural lesions in the urinary bladder - consistent with metastases - the largest was located at the bladder base, with a similar lesion located anteriorly - in the space of Retzius - (Figure 5a), and complicated by right-sided hydro-uretero-nephrosis because of infiltration of the right vesico-ureteric junction (VUJ) by the largest deposit (Figure 5b & c). There was no abdominopelvic nodal or solid organ metastatic disease. No enhancing nodules were present in the right renal pelvis. The axial and appendicular skeletal elements were clear of metastases.
At this point, the patient was discussed at a multidisciplinary meeting and the diagnosis of central bronchus carcinoma (small-cell) with possible metastases to the urinary bladder was made. The cancer was staged radiologically as T4N3M1c. The patient was referred to the urology team for further workup of the bladder lesions and management of the right-sided hydro-uretero-nephrosis. Cystoscopy revealed several solid broad-based masses, of which the mass along the right posterior bladder wall was biopsied. Histology of the biopsy specimen revealed a small round blue cell tumour in the lamina propria with normal overlying urothelium and accompanying lymphoid aggregates. Immunohistochemical stains for broad-spectrum cytokeratin (MNF116), synaptophysin, chromogranin A and TTF-1 were all positive, confirming the diagnosis of metastatic small-cell neuroendocrine carcinoma (Figure 6a, b, c & d).
Correlating the clinical, radiological and pathological findings, a final diagnosis of small-cell neuroendocrine carcinoma of the lung with metastases to the urinary bladder was made.
Management and outcome
Given the patient's extensive disease burden, metastatic spread and poor performance status (ECOG-4), the decision was taken by the family in conjunction with the oncology team to place the patient on palliative care. The patient was discharged and hospice care was arranged. The patient unfortunately succumbed 6 months after the initial presentation.
Discussion
Neuroendocrine tumours consist of a large heterogeneous group of malignancies derived from embryonic neural crest tissue found in various organ systems. Four major types of lung neuroendocrine tumours are described in the 2004 World Health Organization classification: typical carcinoid, atypical carcinoid, large cell neuroendocrine carcinoma and small-cell lung cancer.
Neuroendocrine tumours make up 25% of primary lung carcinomas with the most common of these being small-cell carcinoma. More than 95% of small-cell carcinomas arise from the lung, implying that a primary lung lesion should be sought for if a lesion, which is histologically confirmed as small-cell carcinoma is found within any other organ system.3
Small-cell neuroendocrine carcinomas (SNECs) of the lung are high-grade, poorly differentiated malignancies, known to be highly aggressive. They tend to arise near the bronchial region in the majority of cases. These tumours carry a poor prognosis and once diagnosed, local or distant metastases from the primary site are usually present. The mean age at presentation is 65 years, and a strong association with heavy cigarette smoking is well known.4 Although neuroendocrine tumours of the urinary bladder are much less common compared with lung neuroendocrine tumours, it is essential to consider this differential diagnosis in uropathology as this is central in guiding drug development and patient management. Bladder neuroendocrine tumours are less common than the other conventional histological genitourinary variants, namely urothelial carcinoma, adenocarcinoma and squamous cell carcinoma, constituting only 0.7% - 1% of malignant bladder cancers.5,6 They can present with haematuria and pelvic pain.
Secondary neoplasms to the urinary bladder have been known to be extremely rare. In one series of 11 cases with bladder metastases, it was noticed that the most common primary tumour was the breast. Interestingly, in the same series, none of the patients had lung carcinoma as a primary site. Of the patients in this series, 51% demised within 1 year after the diagnosis of bladder metastases.7
The radiological features of secondary bladder tumours have not been extensively described in the relevant literature. The imaging features of secondary bladder tumours closely resemble primary bladder cancers. Differentiation on imaging alone is nearly impossible with tissue sampling, immunohistochemistry and clinical history often being necessary.8 The diagnosis of SNEC is confirmed when chromogranin, synaptophysin or CD56 are positive. In the presented case, the lymph node biopsy was also positive for all these markers.
It is interesting to note that small-cell carcinoma of the urinary bladder is histologically indistinguishable from small-cell carcinoma of the lung. This has led to the proposal that a shared pathogenesis in these entities exists. A research study, however, concluded that small-cell cancers of the bladder and lung share a convergent but distinct pathogenesis and in fact the former actually arises from a cell of origin shared with urothelial bladder cancer.9
Patients with primary small-cell carcinoma of the urinary bladder require aggressive combination therapy, such as combined chemotherapy and radical cystectomy or chemotherapy and radiation therapy, to achieve cure. Those with secondary small-cell bladder cancer are usually treated with palliative chemotherapy. A cisplatin-based chemotherapy regimen is the preferred initial therapy for patients with metastatic urothelial cancer of the bladder and urinary tract, but cisplatin-related toxicity is a major drawback to this regimen, especially in patients with diminished renal function and poor performance status.10
Conclusion
While metastatic bladder cancers are rare and typically arise from contiguous sites, non-contiguous primary cancers can metastasise to the urinary bladder, although very few have been described in the literature. In particular, small-cell lung neuroendocrine cancer is extremely rare. In the workup of patients with bladder masses in the presence of a known primary, immunohistochemistry, histology and cross-sectional imaging are important in differentiating metastases to the bladder from a synchronous carcinoma and for assessing tumour genotype.
Acknowledgements
The authors would like to acknowledge Prof Richard Pitcher, Stellenbosch University Radiology Head of Department for overall guidance and support in article submission to the Health Research Ethics Committee (HREC). They would like to express their special thanks to Elvira Rohland who was very instrumental in ensuring the article meets the required minimum standard for ethical approval with the Health Research Ethics Committee (HREC).
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
H.M. conceptualised and drafted the original article. S.S.C.V. created the legends, completed final editing, article supervision and oversight. Radiological images were selected by H.M. and approved by S.S.C.V. S.S. contributed patient management details while A.C.v.W. supplied histological findings and the respective images.
Ethical considerations
Approval to conduct the study was received from the HREC, Stellenbosch University, Faculty of Medicine and Health Sciences (reference HREC: C22/07/017).
Funding information
The article received no specific grant from any funding agency in the public, commercial or not-or-profit sectors.
Data availability
No new data were created and hence no data sharing is required for this article.
Disclaimer
The professional views and opinions expressed in this article reflect those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.El-Taji O, Al-Mitwalli A, Malik F, et al. Secondary neoplasms of the urinary bladder-clinical management and oncological outcomes. Transl Androl Urol. 2021;10(6):2427-2434. https://doi.org/10.21037/tau-20-955 [ Links ]
2.Sheehan EE, Greenberg SD, Scott R Jr. Metastatic neoplasms of the bladder. J Urol. 1963;90:281-284. https://doi.org/10.1016/s0022-5347(17)64406-9 [ Links ]
3.Rekhtman N. Neuroendocrine tumors of the lung: An update. Arch Pathol Lab Med. 2010;134(11):1628-1638. https://doi.org/10.5858/2009-0583-RAR.1 [ Links ]
4.DeMarinis A, Malik F, Matin T, Rahmany Z, Putnam T, Nfonoyim J. A rare case of metastatic small cell neuroendocrine carcinoma of the lung presenting as isolated thrombocytopenia. J Community Hosp Intern Med Perspect. 2019;9(4):327-329. https://doi.org/10.1080/20009666.2019.1644916 [ Links ]
5.Bayrak BY. The clinical and uropathological aspects of neuroendocrine tumours of the bladder. J Urol Surg 2021;8(1):1-7. https://doi.org/10.4274/jus.galenos.2020.3986 [ Links ]
6.Qayoom S, Chakrabarti D, Khan F, Goel MM. Primary small cell carcinoma of the urinary bladder. BMJ Case Rep. 2019;12(9):e230185. https://doi.org/10.1136/bcr-2019-230185 [ Links ]
7.Xiao G-Q, Chow J, Unger PD. Metastatic tumors to the urinary bladder: Clinicopathologic study of 11 cases. Int J Surg Pathol. 2012;20(4):342-348. https://doi.org/10.1177/1066896911428736 [ Links ]
8.Karaosmanoglu AD, Onur MR, Karcaaltincaba M, Akata D, Ozmen MN. Secondary tumors of the urinary system: An imaging conundrum. Korean J Radiol. 2018;19(4):742-751. https://doi.org/10.3348/kjr.2018.19.4.742 [ Links ]
9.Chang MT, Penson A, Desai NB, et al. Small-Cell carcinomas of the bladder and lung are characterized by a convergent but distinct pathogenesis. Clin Cancer Res. 2018;24(8):1965-1973. https://doi.org/10.1158/1078-0432.CCR-17-2655 [ Links ]
10.Griffiths TR. Action on bladder cancer. Current perspectives in bladder cancer management. Int J Clin Pract. 2013;67(5):435-448. https://doi.org/10.1111/ijcp.12075 [ Links ]
 Correspondence:
Correspondence:
Humphrey Mapuranga
dr.hmapuranga@gmail.com
Received: 15 Sept. 2022
Accepted: 03 Mar. 2023
Published: 26 Apr. 2023














