Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
SA Journal of Radiology
versión On-line ISSN 2078-6778
versión impresa ISSN 1027-202X
S. Afr. J. radiol. (Online) vol.18 no.1 Johannesburg oct. 2014
ORIGINAL RESEARCH
The radiological appearance of intracranial aneurysms in adults infected with the human immunodeficiency virus (HIV)
Gerrit Blignaut; Eugene Loggenberg; Coert de Vries
Department of Clinical Imaging Sciences, University of the Free State, South Africa
ABSTRACT
BACKGROUND: The global prevalence of intracranial aneurysms is estimated at 2.3%. Limited literature is available on intracranial aneurysms in HIV-infected patients.
OBJECTIVES: To describe the radiological appearance of intracranial aneurysms in HIV-positive adults.
METHOD: In this retrospective analysis of data, 23 HIV-positive patients, of which 15 (65.2%) were female, with a total of 41 aneurysms were included. The mean age was 38 years, and their median CD4 count was 305 x 106/L. Inclusion criteria comprised subarachnoid haemorrhage and confirmed intracranial aneurysms on four-vessel angiography.
RESULTS: Fifteen (65.2%) patients had a single aneurysm, of which 12 (80.0%) had a saccular appearance. Seven (46.7%) of the single aneurysms had a neck width larger than 50% of the transverse aneurysm sac size. The mean longitudinal diameter of the aneurysm sac was 4.9 mm and the transverse diameter 4.4 mm. More than half of these aneurysms occurred at the anterior communicating artery. The median CD4 count of single-aneurysm patients was 319 x 106/L. Eight patients (34.8%) had multiple aneurysms, with a total of 26 aneurysms (range 2-6 aneurysms per patient), of which 13 (50.0%) had a complex appearance. Twenty-four (92.3%) of the multiple aneurysms had a neck width larger than 50% of the transverse aneurysm sac size. The mean longitudinal diameter of the aneurysm sac was 4.0 mm and the transverse diameter 3.9 mm. The multiple aneurysms occurred more commonly in the internal carotid artery. These patients had a median CD4 count of 294 x 106/L.
CONCLUSION: HIV-associated intracranial aneurysms occur at a younger age, appear to be saccular and complex in shape, with a wide neck, and might rupture at small sizes.
Introduction
It is estimated that the global prevalence of intracranial aneurysms is approximately 2.3% in the general population.1 Human immunodeficiency virus (HIV)-associated aneurysmal vasculopathy has been described in young adults and affects predominantly the extracranial blood vessels.2 Intracranial aneurysms in HIV-positive adults are described infrequently. About 22 case reports of HIV-infected adult patients who presented with intracranial aneurysms could be located in the literature. Isolated saccular as well as fusiform aneurysms have been described in these cases.3,4,5
South Africa has a high prevalence of HIV and AIDS, with the prevalence in the Free State province being above the national average.6
The aim of our study was to describe the radiological appearance of intracranial aneurysms in HIV-positive adults who presented with subarachnoid haemorrhage (SAH) and were referred for further evaluation to the Departments of Clinical Imaging Sciences and Neurosurgery at the Universitas Academic Hospital Complex in Bloemfontein.
Method
A retrospective analysis of data was done on all HIV-positive adult patients with SAH who had confirmed intracranial aneurysms on four-vessel angiography between 01 January 2008 and 31 December 2012. Twenty-three HIV-positive patients with intracranial aneurysms, of which 15 (65.2%) were female, were included in the study. The mean age was 38 years (range 24-51 years). The median CD4 count was 305 x 106/L (range 62-1120 x 106/L) and 16 (69.6%) of the patients had a CD4 count of less than 350 x 106/L.
Digital subtraction angiography was performed on a fluoroscopic unit (Philips Allura Xper FD 20/20, Philips Electronics, Netherlands, or Siemens AXIOM Artis, Siemens AG, Germany).
Patient data and angiography reports were obtained from the interventional radiology register and the hospital information system. The HIV status and CD4 count of each patient were obtained from the hospital information system. The CD4 counts of these patients were used to determine any possible relationship between aneurysm morphology and immune status.
Only the initial examination was analysed in patients who had more than one examination. Aneurysms were counted separately in patients who had more than one aneurysm. Each aneurysm was evaluated for (1) its position, (2) number, (3) type, (4) neck size - whether larger than 50% of the transverse aneurysm diameter - and (5) longitudinal and transverse lumen diameter of the aneurysm sac. The largest diameter of each aneurysm, whether longitudinal or transverse, was used to determine the mean maximum aneurysm sac size. These assessments were done by an interventional radiology consultant. Measurements were obtained on imaging views where the aneurysm was seen best in profile. The longitudinal measurement was taken from the midpoint of the aneurysm neck to the midpoint of the aneurysm dome. The transverse diameter was the maximum transverse diameter of the aneurysm sac. The neck size was measured as close as possible to the lumen of the artery from which the aneurysm originated. These measurements are important in the decision regarding further aneurysm management.
The following standard views were used: anteroposterior, left anterior oblique 20°, right anterior oblique 20° and lateral for the carotid arteries, as well as Townes and lateral projections for the vertebral arteries. Additional views were obtained in some cases to get the aneurysms in profile.
The statistical analysis of data was done by the Department of Biostatistics, Faculty of Health Sciences, University of the Free State (UFS) in Bloemfontein, South Africa.
Results
The 23 patients had a total of 41 aneurysms. Fifteen (65.2%) of the patients had a single aneurysm. The remaining eight patients had multiple aneurysms (n = 26), with a range of two to six aneurysms per patient.
Nine female patients and six male patients had a single aneurysm. The mean age of patients with a single aneurysm was 39 years. The median CD4 count was 319 x 106/L. Twelve (80.0%) of the aneurysms had a saccular appearance. One patient had a fusiform aneurysm. Seven aneurysms had a neck width larger than 50% of the transverse aneurysm sac size. The aneurysm sac size had a mean longitudinal diameter of 4.9 mm (range 1.8 mm - 8.6 mm) and transverse diameter of 4.4 mm (range 1.6 mm - 13.0 mm). The mean maximum aneurysm sac size was 5.0 mm (range 1.8 mm - 13.0 mm) (see Table 1). More than half of the single aneurysms involved the anterior communicating artery (ACoA) (see Figure 1).
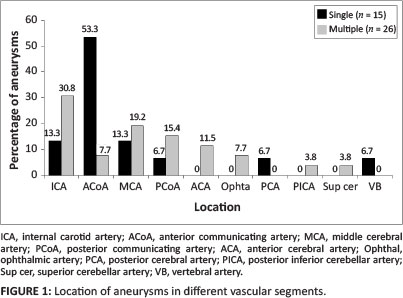
Six female patients and two male patients had multiple aneurysms. Their mean age was 37 years. The median CD4 count was 294 x 106/L. Twenty-four (92.3%) aneurysms had a neck width larger than 50% of the transverse aneurysm sac size. The mean longitudinal diameter of the aneurysm sac size was 4.0 mm (range 1.4 mm - 11.8 mm) and the transverse diameter 3.9 mm (range 1.1 mm - 9.0 mm). The mean maximum aneurysm sac size was 4.1 mm (range 1.8 mm - 11.8 mm) (see Table 1). A slight predominance for internal carotid artery (ICA) aneurysms was observed in patients who had multiple aneurysms (see Figure 1).
Twenty-two (53.7%) of all the aneurysms were saccular and only one (2.4%) was fusiform in shape. As shown in Table 1, saccular aneurysms were more common in patients with a single aneurysm (n = 12; 80.0%), whilst both complex (n = 13; 50.0%) and saccular (n = 10; 38.5%) aneurysms occurred more frequently in patients with multiple aneurysms.
With regard to specific location, as shown in Figure 1, 53.3% of the single aneurysms occurred in the anterior communicating artery (ACoA) distribution, whilst 30.8% of the multiple aneurysms occurred in the internal carotid artery (ICA).
Ethical considerations
Approval to conduct the investigation was obtained from the Ethics Committee of the Faculty of Health Sciences, University of the Free State, South Africa (Number: ETOVS 162/2011).
Discussion
Previous studies have shown that ruptured intracranial aneurysms occur more between the ages of 40 and 60 years.7,8 The peak age group for ruptured aneurysms in this study was between 35 and 45 years. Therefore, in our study, we observed that aneurysm rupture occurred at a younger age in patients with HIV infection than in the general public.
Previous studies in non-HIV-infected populations showed that single aneurysms had a higher incidence in the anterior circulation as well as ACoA,8 which was also evident in our study amongst patients with single aneurysms. The location of multiple aneurysms in the ICA and its branches, as well as the middle cerebral artery (MCA), was similar to previously reported cases. It appears that there is no difference between the location of multiple aneurysms in HIV-positive patients and the general population.
Multiple aneurysms have been proposed as a typical feature of HIV-associated aneurysms.9 Multiple aneurysms were seen in more than a third (34.8%) of our patients, of which 75% were female. These findings were also observed in other studies, which showed that multiple aneurysms occur more frequently in patients older than 40 years. However, no comment was made on the HIV-status of these patients.10,11 The mean age of our patients with multiple aneurysms was 37 years, which is considerably younger than those in the general population with multiple aneurysms (see Figure 2).
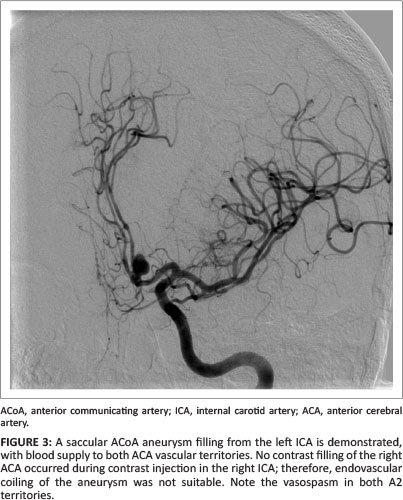
Half of the multiple aneurysms in our study had a complex configuration that included multilobar, daughter and multi-vessel take-off configurations. The majority (92.3%) of the multiple aneurysms had a neck width larger than 50% of the transverse aneurysm diameter. With morphological findings such as these, endovascular treatment becomes a challenge (see Figure 3).
Similar to our findings, saccular aneurysms are the most common type of intracranial aneurysm described in the non-HIV-infected population. Modi, Ranchod, Modi and Mochan2 reported three HIV-positive patients with fusiform type aneurysms and suggested that the characteristics of these aneurysms are distinctive of HIV-related aneurysms.
The International Study for Unruptured Intracranial Aneurysms (ISUIA trial)12 reported that the risk for SAH is very low in aneurysms with a maximum aneurysm sac diameter of less than 7 mm. Delgado Almandoz, Feasse, Crandall, et al.13 studied 1681 intracranial aneurysms and reported that the mean maximum aneurysm sac size for ruptured aneurysms was 6.5 mm. In our study, the mean maximum aneurysm sac size was smaller, with a mean diameter of 5.0 mm for single aneurysms and 4.1 mm for multiple aneurysms. From our findings, it appears that ruptured HIV-associated intracranial aneurysms are substantially smaller than those in the general population. However, this observation should be confirmed with larger comparative studies.
The mechanism is not clear, but a possible explanation could be that the aneurysm wall is prone to rupture because of a weaker structure resulting of a defective immune system. The imaging morphology of intracranial aneurysms in our study may be explained by the proposed role of HIV in aneurysm formation. Previous studies have shown that the vessel walls have intimal hyperplasia with destruction of the internal elastic lamina, medial fibrosis and loss of the muscularis layer.14 HIV viral proteins have been associated with vascular pathology. A possible link between vascular pathology and the protease inhibitor ritonavir was also identified.15 There is also the possibility that bacterial infection of the vessel wall, secondary to immune suppression, could lead to the development of aneurysms, as previously suggested with extracranial aneurysms in HIV-positive patients.16
HIV binds on the surface of CD4 cells and deactivates these cells. We could not establish a relationship between aneurysm morphology and immune status. The median CD4 count for single aneurysms was 319 x 106/L and for multiple aneurysms 294 x 106/L, although this difference was not statistical significant. Sixteen (69.6%) of our patients had a CD4 count below 350 x 106/L.
When interpreting these results, it must be taken into account that our study population was relatively small, and that we were not able to establish if these patients were on anti-retroviral therapy, what their viral load was, or if any coexistent infections were present, as this information was not available in the patient files.
Conclusion
HIV-associated intracranial aneurysms in adult patients appear to be mostly saccular and complex in shape with a wide neck. They apparently rupture at a smaller size than those in the general population, although larger comparative studies are necessary to confirm this. These findings indicate that the strength of the vessel wall is, at least in part, a function of the immune status of a person.
Acknowledgements
The authors wish to thank Dr Daleen Struwig, medical writer, Faculty of Health Sciences, UFS, for assistance with the technical and editorial preparation of the manuscript, and Mr Cornel van Rooyen, Department of Biostatistics, Faculty of Health Sciences, UFS, for statistical analysis of the data.
Competing interests
The authors declare that they have no financial or personal relationship(s) that may have inappropriately influenced them in writing this article.
Authors' contributions
G.B. (University of the Free State) was responsible for the literature search, conception and design of the study, data acquisition, analysis and interpretation of data, and drafting of the manuscript. E.L. (University of the Free State) was the study leader and revised the manuscript critically for important intellectual content. C.d.V. (University of the Free State) also revised the manuscript critically for important intellectual content. All three authors approved of the final version of the manuscript to be published.
References
1. Rinkel G, Djibuti M, Algra A, Van Gijn J. Prevalence and risk of intracranial aneurysms: A systematic review. Stroke. 1998;29:251-256. http://dx.doi.org/10.1161/01.STR.29.1.251 [ Links ]
2. Modi G, Ranchod K, Modi M, Mochan A. Human immunodeficiency virus associated intracranial aneurysms: Report of three adult patients with an overview of the literature. J Neurol Neurosurg Psychiatry. 2008;79:44-46. http://dx.doi.org/10.1136/jnnp.2006.108878 [ Links ]
3. Kossorotoff M, Touze E, Godon-Hardy S, et al. Cerebral vasculopathy with aneurysm formation in HIV-infected young adults. Neurology. 2006;66:1121-1122. http://dx.doi.org/10.1212/01.wnl.0000204188.85402.0c [ Links ]
4. Tipping B, De Villiers L, Candy S, Wainwright H. Stroke caused by human immunodeficiency virus-associated intracranial large-vessel aneurysmal vasculopathy Arch Neurol. 2006;63:1640-1642. http://dx.doi.org/10.1001/archneur.63.11.1640 [ Links ]
5. Taylor A, Lefeuvre D, Levy A, Candy S. Arterial dissection and subarachnoid haemorrhage in human immunodeficiency virus-infected patients. A report of three cases. Interv Neuroradiol. 2004;10:137-143. PMCID PMC3464403 [ Links ]
6. Human Sciences Research Council (HSRC). South African National HIV Prevalence, Incidence, Behaviour and Communication Survey [document on the Internet]. c2009 [cited 2013 March 7]. Available from http://www.mrc.ac.za/pressrelease/2009/sanat.pdf [ Links ]
7. Wilson FM, Jaspan T, Holland IM. Multiple cerebral aneurysms - A reappraisal. Neuroradiology. 1989;31:232-236. http://dx.doi.org/10.1007/BF00344349 [ Links ]
8. Keedy A. An overview of intracranial aneurysms. McGill J Med. 2006;9:141-146. PMID 18523626 [ Links ]
9. Nair R, Robbs JV, Naidoo NG, Woolgar J. Clinical profile of HIV-related aneurysms. Eur J Vasc Endovasc Surg. 2000;20:235-240. http://dx.doi.org/10.1053/ejvs.2000.1169 [ Links ]
10. Louw DJ, De Vries CS, Joubert G. Cerebral aneurysms - An audit. S Afr J Radiol. 2004;8:28-30. [ Links ]
11. Ogeng'o JA, Obimbo MM, Olabu BO, Sinkeet SR. Pattern of aneurysms among young black Kenyans. Indian J Thorac Cardiovasc Surg. 2001;27(2):70-75. http://dx.doi.org/10.1007/s12055-011-0088-2 [ Links ]
12. Wiebers DO, Whisnant JP, Huston J III, et al. Unruptured intracranial aneurysms: Natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362:103-110. http://dx.doi.org/10.1016/S0140-6736(03)13860-3 [ Links ]
13. Delgado Almandoz J, Feasse J, Crandall B, et al. Size and location of ruptured intracranial aneurysms in a consecutive series of 588 patients with first-time acute subarachnoid hemorrhage treated endovascularly at a tertiary referral medical center over a 16-year time period. Oral abstract O-008. Abstracts of the SNIS (Society of NeuroInterventional Surgery) 9th Annual Meeting. July 23-26 2012. San Diego, USA. J Neurointerv Surg. 2012;4(suppl 1):A4-A5. http://dx.doi.org/10.1136/neurointsurg-2012-010455a.8 [ Links ]
14. Shah SS, Zimmerman RA, Rorke LB, et al. Cerebrovascular complications of HIV in children. Am J Neuroradiol. 1996;17:1913-1917. PMID: 8933877 [ Links ]
15. Zhong DS, Lu XH, Conklin BS, et al. HIV protease inhibitor ritonavir induces cytotoxicity of human endothelial cells. Arterioscler Thromb Vasc Biol. 2002;22:1560-1566. http://dx.doi.org/10.1161/01.ATV.0000034707.40046.02 [ Links ]
16. Bulsara K, Ali R, Owen J. HIV and cerebral aneurysms. Neurosurg Rev. 2005;28:92-95. http://dx.doi.org/10.1007/s10143-004-0371-4 [ Links ]
 Correspondence:
Correspondence:
Gerrit Blignaut
Department of Clinical Imaging Sciences (G61)
Faculty of Health Sciences
University of the Free State
205 Nelson Mandela Drive
Bloemfontein 9300
South Africa
Email: gerritblign@yahoo.co.uk
Received: 27 Aug. 2013
Accepted: 29 Nov. 2013
Published: 04 Apr. 2014














