Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of HIV Medicine
On-line version ISSN 2078-6751
Print version ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.23 n.1 Johannesburg 2022
http://dx.doi.org/10.4102/sajhivmed.v23i1.1396
ORIGINAL RESEARCH
Rifampicin resistance and mortality in patients hospitalised with HIV-associated tuberculosis
Ruan SpiesI; Charlotte SchutzII, III; Amy WardII, III; Avuyonke BalfourIII; Muki SheyII, III; Mark NicolIV; Rosie BurtonV; Bianca SossenII, III; Robert WilkinsonIII, VI, VII; David BarrIII, VIII; Graeme MeintjesII, III
IDepartment of Medicine, New Somerset Hospital, Cape Town, South Africa
IIDepartment of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
IIIWellcome Centre for Infectious Diseases Research in Africa (CIDRI-Africa) and Institute of Infectious Disease and Molecular Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
IVDivision of Infection and Immunity, School of Biomedical Sciences, University of Western Australia, Perth, Australia
VMédecins sans Frontières, Cape Town, South Africa
VIThe Francis Crick Institute, London, United Kingdom
VIIDepartment of Infectious Disease, University College London, London, United Kingdom
VIIIInstitute of Infection and Global Health, University of Liverpool, Liverpool, United Kingdom
ABSTRACT
BACKGROUND: Patients with HIV and drug-resistant tuberculosis (TB) are at high risk of death.
OBJECTIVES: We investigated the association between rifampicin-resistant TB (RR-TB) and mortality in a cohort of patients who were admitted to hospital at the time of TB diagnosis.
METHOD: Adults hospitalised at Khayelitsha Hospital and diagnosed with HIV-associated TB during admission, were enrolled between 2013 and 2016. Clinical, biochemical and microbiological data were prospectively collected and participants were followed up for 12 weeks
RESULTS: Participants with microbiologically confirmed TB (n = 482) were enrolled a median of two days (interquartile range [IQR]: 1-3 days) following admission. Fifty-three participants (11.0%) had RR-TB. Participants with rifampicin-susceptible TB (RS-TB) received appropriate treatment a median of one day (IQR: 1-2 days) following enrolment compared to three days (IQR: 1-9 days) in participants with RR-TB. Eight participants with RS-TB (1.9%) and six participants with RR-TB (11.3%) died prior to the initiation of appropriate treatment. Mortality at 12 weeks was 87/429 (20.3%) in the RS-TB group and 21/53 (39.6%) in the RR-TB group. RR-TB was a significant predictor of 12-week mortality (hazard ratio: 1.88; 95% confidence interval: 1.07-3.29; P = 0.03.
CONCLUSION: Mortality at 12 weeks in participants with RR-TB was high compared to participants with RS-TB. Delays in the initiation of appropriate treatment and poorer regimen efficacy are proposed as contributors to higher mortality in hospitalised patients with HIV and RR-TB.
Keywords: HIV-associated tuberculosis; rifampicin-resistant tuberculosis; drug-resistant tuberculosis; multi-drug resistant TB; TB; Khayelitsha Hospital.
Introduction
Drug-resistant tuberculosis (DR-TB) is a global health concern.1 In South Africa (SA), 13 005 cases of rifampicin-resistant TB (RR-TB) were identified in 2019.2,3 People living with HIV are at higher risk of acquiring DR-TB, which is associated with poorer treatment outcomes in this patient population.2,4,5,6
The treatment of DR-TB has low success rates and high mortality due to lengthy, poorly tolerated and poorly efficacious regimens.7,8 In 2018, only 58% of patients with DR-TB globally were successfully treated.1 Mortality estimates from studies conducted primarily in outpatients or in TB hospitals in high-burden settings, prior to the introduction of bedaquiline-based regimens, range from 11% to 39%.9,10,11,12,13,14 The introduction of bedaquiline-based treatment has been associated with a reduction in mortality in patients with DR-TB; however, treatment failure and mortality remain high in high-prevalence settings, with mortality still ranging from 6% to 17%.15,16,17,18,19,20,21
As most DR-TB is managed in outpatient clinics or in TB hospitals, most research on DR-TB outcomes occurs in these settings. Patients who are hospitalised with HIV-associated TB are often severely ill and inpatient case fatality rates range from 11% to 32%.22,23,24,25,26,27 In autopsy series, inpatient deaths occur with a median of 4-5 days following admission.28,29,30 There are scarce data on outcomes of patients diagnosed with HIV-associated DR-TB admitted to general hospitals.
The outcomes of a cohort of patients admitted to hospital in Khayelitsha, SA, newly diagnosed with HIV-associated TB, were previously described.31 In this cohort, RR-TB was present in 16.9% of participants who died and in 7.2% of survivors.31 In this secondary analysis, we aimed to describe the association between RR-TB and mortality in patients diagnosed with HIV-associated TB while hospitalised and to identify factors associated with mortality in patients with RR-TB.
Methods
Study design and setting
Patients admitted to Khayelitsha Hospital were enrolled to a prospective cohort study between January 2013 and October 2016. The hospital is in Khayelitsha - a large township in Cape Town, SA, with high rates of HIV, TB and DR-TB.32 Most cases of TB are managed in primary healthcare clinics; however, patients may be referred to hospital if they require inpatient care.
Participants
All patients in the emergency unit and medical wards were screened on weekdays. Adults with HIV infection, a CD4 count of < 350 cells/µL and a high clinical suspicion of TB were eligible to enrol into the study. Pregnant patients, patients who received anti-TB therapy within the past month, or patients who were recently initiated and received three or more doses of anti-TB therapy, were not eligible for enrolment. Clinical details, chest X-ray, sputum, urine and blood samples were obtained at enrolment. Additional samples, including extra-pulmonary samples such as pleural fluid, cerebrospinal fluid and lymph node aspirates, were obtained as indicated by clinical staff. Participants remained under routine clinical care and treatment decisions were made by clinical staff and not by study staff. Results of validated sputum, urine and blood TB tests that were collected by the study were communicated to the routine clinical team. Participants were assessed daily in the ward and, after discharge, were managed in primary care according to local guidelines. Participants had a telephonic follow-up at week 4 and returned for a clinical assessment at week 12.
Laboratory assays
Tuberculosis microbiology was performed by the National Health Laboratory Services (NHLS). Sputum and urine were sent for TB culture and Xpert MTB/RIF assay (Cepheid, Sunnyvale, California, United States). The study preceded the introduction of Xpert MTB/RIF Ultra, which has a higher sensitivity than Xpert MTB/RIF Ultra for pulmonary TB.33 Mycobacterial blood culture was performed by culturing 5 mL of whole blood in Myco/F Lytic (Becton Dickinson Biosciences, Franklin Lakes, New Jersey, United States) bottles for 42 days. The GenoType MTBRplus assay (Hain Lifesciences, Nehren, Germany) was used to identify Mycobacterium tuberculosis complex from the positive sputum and blood cultures and provided rifampicin and isoniazid resistance testing. RR isolates underwent susceptibility testing to second-line drugs at a referral laboratory. Between 2014 and 2016, phenotypic resistance testing was performed for amikacin and ofloxacin. From 2016 the GenoType MTBDRsl (Hain Lifesciences, Nehren, Germany) line probe assay was used to assess susceptibility to aminoglycosides and fluoroquinolones. CD4 count, HIV viral load, haemoglobin, creatinine and electrolytes, liver function and C-reactive protein (CRP) tests were performed on all participants.
Data collection and definitions
Clinical data were captured on standardised case record forms from patient interviews, hospital folders and clinical review at enrolment. The primary outcome was survival at 12 weeks. If patients could not be contacted at 12 weeks, searches of electronic records were conducted. Participants with an electronic entry indicating a clinic visit, collection of medicine or a laboratory test performed beyond 12 weeks were assumed to be alive at week 12. Participants without electronic entries at or beyond 12 weeks were classified as lost to follow-up. In this study we describe and analyse the subset of patients with microbiologically confirmed TB, defined as participants with M. tuberculosis identified by culture or Xpert MTB/RIF from any clinical sample. RR-TB was defined as rifampicin resistance on any sample using either of the genotypic tests performed at the NHLS while rifampicin-susceptible TB (RS-TB) was defined as rifampicin susceptibility on all samples.
Statistical analyses
Data were analysed using R version 4.02 (R Core Team, Vienna, Austria).
Median values with interquartile ranges were used as measures of central tendency and dispersion. Categorical variables were described using counts and proportions and were compared using the Fisher's exact test. Continuous variables were compared between study groups using the Mann-Whitney U test.
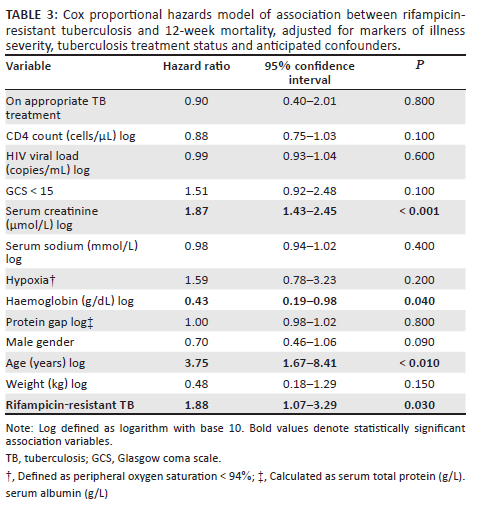
To investigate the association of RR-TB with 12-week mortality, we hypothesised that RR-TB could cause mortality through two mechanisms: (1) delayed initiation of effective therapy and (2) lower efficacy of DR-TB therapy in preventing early mortality. We therefore treated receipt of appropriate anti-TB therapy (i.e. rifampicin-based therapy for RS-TB, any DR-TB therapy for RR-TB and an individualised regimen for extremely drug-resistant [XDR] TB) as a time-dependent variable in a Cox proportional hazards model, where hazard of mortality after start of appropriate therapy was compared to hazard of mortality prior to initiation of appropriate therapy, capturing effect of delay to appropriate treatment initiation. This allowed assessment of the association between RR-TB and mortality after adjusting for an effect of RR-TB on delay to appropriate treatment initiation.
We further hypothesised that measures of disease severity at baseline (hypoxia defined as peripheral oxygen saturation < 94% on room air, serum creatinine, sodium, protein gap [PG] [defined as the difference between total protein and albumin], haemoglobin, CD4 count, HIV viral load, Glasgow coma scale [GCS] < 15 and weight) could confound TB treatment status and mortality (with patients presenting more unwell being initiated on anti-TB therapy more urgently). Finally, we considered that patient factors associated with both RR-TB and mortality, including age and sex, and the above markers of baseline disease severity could confound the relationship between RR-TB and mortality. Both these sets of variables were therefore included as covariates in the model.
Continuous predictor variables were log-transformed to resolve highly skewed distributions, and because proportional or multiplicative changes in the values of these variables were thought to be more biologically meaningful. Peripheral oxygen saturation was dichotomised to reflect a non-linear relationship between oxygen saturation and partial pressure of oxygen as parsimoniously as possible.
The hypothesised causal structure is summarised in a directed acyclic graph (Figure 1).

Observations on variables included in Cox regression were all > 95% complete except for weight which was missing in 8% of participants. All missing values were considered missing completely at random or missing at random after adjusting for other observed covariates and single-imputed for model fitting using classification and regression trees implemented with the mice package in R.
Participants lost to follow-up were censored on their last day of contact with health services. Kaplan-Meier plots were used to estimate survival over the study period, stratified by rifampicin-sensitivity status.
Ethical considerations
This study was approved by the University of Cape Town Human Research Ethics Committee (reference number 057/2013). Participants provided written informed consent where possible. The process involved in enrolling patients who did not have the capacity to provide informed consent has been described previously.
Results
Participant characteristics
A total of 482 participants with HIV-associated, microbiologically confirmed TB were included in this analysis, enrolled a median of two days (interquartile range [IQR]: 1-3 days) after admission to hospital. Fifty-three participants (11.0%) had RR-TB. Of these, 16 (30.0%) had TB resistant to rifampicin only and 32 (60.0%) had multi-drug resistant TB, three (6.0%) of whom had TB resistant to a second-line drug (one participant had XDR-TB and two participants had pre-XDR-TB). Five (9.0%) participants provided samples that were identified as RR by GeneXpert MTB/RIF but the results of further drug susceptibility testing were unavailable. In the RS-TB group, 194 (45.2%) tested positive for M. tuberculosis by Xpert MTB/RIF compared to 22 (41.5%) participants in the RR-TB group. Thirteen (2.7%) participants were lost to follow-up over the 12-week study period. Demographic and clinical characteristics, stratified by rifampicin susceptibility, are displayed in Table 1. Characteristics for the RR-TB group, stratified by vital status, are shown in Table 2.
Time to treatment initiation and treatment status
Participants in the RS-TB group initiated appropriate treatment (rifampicin-based therapy for RS-TB, any DR-TB therapy for RR-TB and an individualised regimen for XDR-TB) a median of 1 day following enrolment (IQR: 1-2 days) compared to three days in the RR-TB group (IQR: 1-9 days), P < 0.001. Eight (1.9%) participants in the RS-TB group were not initiated on appropriate treatment during their admission, six of whom died. Six (11.3%) participants in the RR-TB group died prior to the initiation of appropriate treatment (Figure 2). Four of the six participants died within three days of enrolment. In the two remaining participants, RR-TB was confirmed on blood culture - the results of which were only available after their deaths.

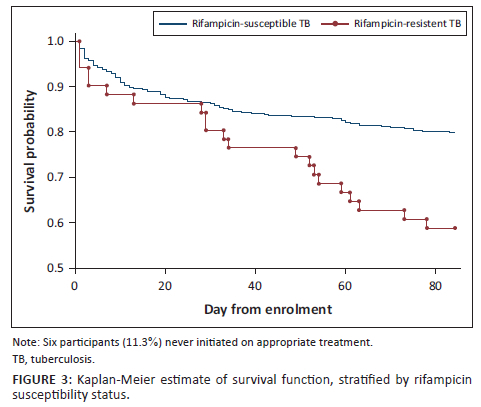
Mortality
Mortality at 12 weeks was 87/429 (20.3%) in the RS-TB group and 21/53 (39.6%) in the RR-TB group (P = 0.008). The Kaplan-Meier curve in Figure 3 demonstrates the relationship between rifampicin susceptibility and time-to-death. Table 3 depicts the results of the Cox proportional hazards model which modelled the relationship between RR-TB and 12-week mortality. RR-TB was significantly associated with 12-week mortality (hazard ratio 1.88; 95% confidence interval 1.07-3.29; P = 0.03) when adjusting for CRP, CD4 count, HIV viral load, creatinine, sodium, PG, haemoglobin, weight, GCS, hypoxia, age, sex, and TB treatment status as a time-dependent variable to adjust for treatment delay.
Discussion
In this study we describe 482 patients with HIV-associated, microbiologically confirmed TB, diagnosed while admitted to a general hospital. We found a high prevalence of RR-TB in the study cohort and a high 12-week mortality in participants with RR-TB. Rifampicin-resistant TB was significantly associated with mortality, even when controlling for markers of disease severity and delay to treatment initiation.
The mortality rate we describe is significantly higher than described in other studies of patients with HIV-associated RR-TB. Unlike our study, these studies included participants both with and without HIV, longer follow-up periods and participants in predominantly outpatient or TB hospital settings. Prospective cohorts of outpatients with RR-TB, with follow-up periods ranging 6-24 months, describe mortality rates of 9% - 31%, with HIV prevalence 38% - 85%.34,35,36,37 Retrospective cohorts of patients with RR-TB in TB hospitals describe mortality rates of 18% - 29%, with HIV prevalence 52% - 100%.38,39,40 Retrospective studies of outpatients describe mortality rates of 13% - 21%, with HIV prevalence 70% - 100%.10,12,13
In our cohort, 29% of participants with RR-TB who died did so prior to the initiation of a DR-TB regimen. This suggests that a substantial proportion of acutely ill patients with DR-TB die as inpatients, before a diagnosis of DR-TB can be confirmed, before DR-TB treatment regimens can be initiated and before they are registered on the national TB programme. These patients would contribute to the recognised gap between numbers of DR-TB diagnoses and numbers registered in the DR-TB programme.3 The authors hypothesised that the high mortality rates they observed could be driven by two, not necessarily mutually exclusive, mechanisms: delays to initiation of appropriate treatment for RR-TB and less effective therapies for RR-TB compared to RS-TB.
In this study, time to initiation of appropriate treatment was shorter than described in previous studies,9,41 likely reflecting access to rapid diagnostics, multiple different samples taken for TB testing, and regular monitoring available in hospital. Although the difference in time to treatment initiation between the RS-TB and RR-TB groups was statistically significant, the difference is of unknown clinical significance. Several factors may have contributed to delays in appropriate treatment initiation in the RR-TB group including delays in specimen collection (patients in the RR-TB group may have been sicker at baseline and thus sputum sample acquisition for microbiological testing may have been more difficult), in awaiting the results of TB culture and drug susceptibility testing and in initiating appropriate treatment once these became available. Previous studies have failed to demonstrate a reduction in mortality in RR-TB with a reduction in time to treatment initiation.9,42
In this study analysis, the effect of being on appropriate TB therapy on mortality during the follow-up period was unclear. This implies that the association between RR-TB and 12-week mortality observed in this cohort was not mediated by diagnostic delay. Despite the marginal difference in time to treatment initiation, the 12-week mortality in the RR-TB group was much higher. When adjusting for TB treatment status as a time-dependent variable, RR-TB remained significantly associated with 12-week mortality, suggesting that additional factors related to RR-TB, other than time to treatment initiation, were important. These additional factors could include residual confounding, but the finding is also consistent with lower efficacy of DR-TB regimens during the study period. Interestingly, the Kaplan-Meier survival curves for participants with RR-TB versus RS-TB (Figure 3) diverge at day 21 of follow-up, suggesting early factors, including delays to treatment initiation, are less important contributors to differences in mortality than later factors, such as poorer efficacy of DR-TB treatment regimens. This study preceded the introduction of bedaquiline-based regimens for patients with RR-TB and participants in our study received RR-TB regimens containing injectable second-line drugs which are poorly tolerated and which have poor efficacy.8 The introduction of bedaquiline-based regimens has resulted in promising improvements in survival in DR-TB;17 however, prospective data in hospitalised patients at high risk of early mortality are lacking. This study suggests that efficacy of evolving DR-TB regimens in seriously ill patients with HIV-associated TB is an important topic for future research.
This study has several limitations. Although our cohort was relatively large, the population of participants with RR-TB was small. The authors did not objectively measure adherence to TB treatment once participants had been discharged, potentially introducing uncertainty into their measurement of the association between treatment status and mortality. Due to the observational design of this study, the authors were unable to eliminate residual confounding when measuring the association between RR-TB and mortality and they cannot therefore make conclusions as to the causal nature of this relationship. The strengths of this study include its extensive TB testing and prospective follow-up, with vital status at 12 weeks identified for 98% of participants. This study also describes a unique, under-studied population of hospitalised patients with HIV-associated RR-TB and CD4 count < 350 cell/µL.
Conclusion
This study describes a high 12-week mortality in patients admitted to hospital at the time of TB diagnosis with HIV-associated RR-TB. We suggest that delays in treatment initiation and poorly efficacious treatment regimens, prior to the introduction of bedaquiline as standard of care, are possible contributors to mortality in this population. Hospitalised patients with DR-TB represent an under-studied group with a high risk of early mortality. Research into the contributors to mortality in this population and improved diagnostic and therapeutic strategies are required.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
G.M. and D.B. conceived the study; C.S., M.S., R.S., R.J.W. and M.N. contributed to study design. G.M. and C.S. conceived the parent study; C.S., A.W. and D.B. contributed to clinical data acquisition; G.M. and R.B. provided clinical oversight. A.B. and C.S. contributed to laboratory data acquisition. M.S. and M.N. provided laboratory oversight. R.J.W. provided laboratory facilities for storage and processing of samples. R.S. and D.B. performed data analysis with input from all co-authors and oversight from G.M. and C.S. R.S. wrote the manuscript and all co-authors reviewed and contributed to the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. D.A.B. and R.S. both verified underlying data of the study. C.S. verified all the clinical and immune data and Khayelithsa Hospital TB Study (KDHTB) data.
Funding information
C.S. was funded by the South African Medical Research Council under the National Health Scholars Programme. D.A.B. was supported by Wellcome (105165/Z/14/A) and an National Institute for Health and Care Research (UK) clinical lectureship. G.M. and M.S. were supported by Wellcome (098316, 203135, 214321/Z/18/Z and 211360/Z/18/Z), G.M. was supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa (Grant no. 64787), NRF incentive funding (UID: 85858) and the South African Medical Research Council through its TB and HIV Collaborating Centres Programme with funds received from the National Department of Health (RFA# SAMRC-RFA-CC: TB/HIV/AIDS-01-2014). R.J.W. is supported by the Francis Crick Institute, which receives funding from Wellcome (FC00110218), Cancer Research UK (FC00110218), the UK Medical Research Council (FC00110218). R.J.W. also received support from Wellcome (104803, 203135) and the National Institutes of Health (U01AI115940) and European and Developing Countries Clinical Trials (SRIA 2015-1065). For the purpose of open access, the authors have applied a CC-BY public copyright licence to any author-accepted manuscript version arising from this submission. The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of this report.
Data availability
The data sets generated and analysed during the current study are available from the corresponding author, R.S., on reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and not an official position of their institutions.
References
1.World Health Organization. Global tuberculosis report 2021 [document on the Internet]. World Health Organization; 2020 [cited 2022 Apr 20]. Available from: https://www.who.int/teams/global-tuberculosis-programme/tb-reports/global-tuberculosis-report-2021 [ Links ]
2.Ismail NA, Mvusi L, Nanoo A, et al. Prevalence of drug-resistant tuberculosis and imputed burden in South Africa: A national and sub-national cross-sectional survey. Lancet Infect Dis. 2018;18(7):779-787. https://doi.org/10.1016/S1473-3099(18)30222-6 [ Links ]
3.World Health Organization. Tuberculosis profile: South Africa [document on the Internet]. Geneva: World Health Organisation; 2018 [cited 2021 Oct 5]. Available from: https://worldhealthorg.shinyapps.io/tb_profiles/?_inputs_&entity_type=%22country% 22&lan=%22EN%22&iso2=%22ZA%22 [ Links ]
4.Mesfin YM, Hailemariam D, Biadglign S, Kibret KT. Association between HIV/AIDS and multi-drug resistance tuberculosis: A systematic review and meta-analysis. PLoS One. 2014;9(1):e82235. https://doi.org/10.1371/journal.pone.0082235 [ Links ]
5.Samuels JP, Sood A, Campbell JR, Ahmad Khan F, Johnston JC. Comorbidities and treatment outcomes in multidrug resistant tuberculosis: A systematic review and meta-analysis. Sci Rep. 2018;8(1):1-13. https://doi.org/10.1038/s41598-018-23344-z [ Links ]
6.Bastard M, Sanchez-Padilla E, du Cros P, et al. Outcomes of HIV-infected versus HIV-non-infected patients treated for drug-resistance tuberculosis: Multicenter cohort study. PLoS One. 2018;13(3):e0193491. https://doi.org/10.1371/journal.pone.0193491 [ Links ]
7.Gandhi NR, Shah NS, Andrews JR, et al. HIV coinfection in multidrug- and extensively drug-resistant tuberculosis results in high early mortality. Am J Respir Crit Care Med. 2010;181(1):80-86. https://doi.org/10.1164/rccm.200907-0989OC [ Links ]
8.Schnippel K, Firnhaber C, Ndjeka N, et al. Persistently high early mortality despite rapid diagnostics for drug-resistant tuberculosis cases in South Africa. Int J Tuberc Lung Dis. 2017;21(10):1106-1111. https://doi.org/10.5588/ijtld.17.0202 [ Links ]
9.Evans D, Sineke T, Schnippel K, et al. Impact of Xpert MTB/RIF and decentralized care on linkage to care and drug-resistant tuberculosis treatment outcomes in Johannesburg, South Africa. BMC Health Serv Res. 2018;18(1):1-12. https://doi.org/10.1186/s12913-018-3762-x [ Links ]
10.Cox H, Hughes J, Daniels J, et al. Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis. 2014;18(4):441-448. https://doi.org/10.5588/ijtld.13.0742 [ Links ]
11.Chingonzoh R, Manesen MR, Madlavu MJ, et al. Risk factors for mortality among adults registered on the routine drug resistant tuberculosis reporting database in the Eastern Cape province, South Africa, 2011 to 2013. PLoS One. 2018;13(8):e0202469. https://doi.org/10.1371/journal.pone.0202469 [ Links ]
12.Mohr E, Cox V, Wilkinson L, et al. Programmatic treatment outcomes in HIV-infected and uninfected drug-resistant TB patients in Khayelitsha, South Africa. Trans R Soc Trop Med Hyg. 2015;109(7):425-432. https://doi.org/10.1093/trstmh/trv037 [ Links ]
13.Daniels JF, Khogali M, Mohr E, et al. Time to ART initiation among patients treated for rifampicin-resistant tuberculosis in Khayelitsha, South Africa: Impact on mortality and treatment success. PLoS One. 2015;10(11):e0142873. https://doi.org/10.1371/journal.pone.0142873 [ Links ]
14.Schnippel K, Shearer K, Evans D, Berhanu R, Dlamini S, Ndjeka N. Predictors of mortality and treatment success during treatment for rifampicin-resistant tuberculosis within the South African National TB Programme, 2009 to 2011: A cohort analysis of the national case register. Int J Infect Dis. 2015;39:89-94. https://doi.org/10.1016/j.ijid.2015.09.002 [ Links ]
15.Wang M-G, Wu S-Q, He J-Q. Efficacy of bedaquiline in the treatment of drug-resistant tuberculosis: A systematic review and meta-analysis. BMC Infect Dis. 2021;21(1):1-10. https://doi.org/10.1186/s12879-021-06666-8 [ Links ]
16.Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382:893-902. https://doi.org/10.1056/NEJMoa1901814 [ Links ]
17.Schnippel K, Ndjeka N, Maartens G, et al. Effect of bedaquiline on mortality in South African patients with drug-resistant tuberculosis: A retrospective cohort study. Lancet Respir Med. 2018;6(9):699-706. https://doi.org/10.1016/S2213-2600(18)30235-2 [ Links ]
18.Mbuagbaw L, Guglielmetti L, Hewison C, et al. Outcomes of bedaquiline treatment in patients with multidrug-resistant tuberculosis. Emerg Infect Dis. 2019;25(5):936. https://doi.org/10.3201/eid2505.181823 [ Links ]
19.Pym AS, Diacon AH, Tang S-J, et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2016;47(2):564-574. https://doi.org/10.1183/13993003.00724-2015 [ Links ]
20.Olayanju O, Limberis J, Esmail A, et al. Long-term bedaquiline-related treatment outcomes in patients with extensively drug-resistant tuberculosis from South Africa. Eur Respir J. 2018;51(5):1800544. [ Links ]
21.Padayatchi N, Bionghi N, Osman F, et al. Treatment outcomes in patients with drug-resistant TB-HIV co-infection treated with bedaquiline and linezolid. Int J Tuberc Lung Dis. 2020;24(10):1024. https://doi.org/10.5588/ijtld.20.0048 [ Links ]
22.Kyeyune R, Den Boon S, Cattamanchi A, et al. Causes of early mortality in HIV-infected TB suspects in an East African referral hospital. J Acquir Immune Defic Syndr. 2010;55(4):446-450. https://doi.org/10.1097/QAI.0b013e3181eb611a [ Links ]
23.Bigna JJR, Noubiap JJN, Agbor AA, et al. Early mortality during initial treatment of tuberculosis in patients co-infected with HIV at the Yaoundé Central Hospital, Cameroon: An 8-year retrospective cohort study (2006-2013). PLoS One. 2015;10(7):e0132394. https://doi.org/10.1371/journal.pone.0132394 [ Links ]
24.Subbarao S, Wilkinson KA, Van Halsema CL, et al. Raised venous lactate and markers of intestinal translocation are associated with mortality among in-patients with HIV-associated TB in rural South Africa. J Acquir Immune Defic Syndr. 2015;70(4):406-413. https://doi.org/10.1097/QAI.0000000000000763 [ Links ]
25.Meintjes G, Kerkhoff AD, Burton R, et al. HIV-related medical admissions to a South African district hospital remain frequent despite effective antiretroviral therapy scale-up. Med. 2015;94(50):e2269. [ Links ]
26.Griesel R, Stewart A, Van der Plas H, Sikhondze W, Mendelson M, Maartens G. Prognostic indicators in the World Health Organization's algorithm for seriously ill HIV-infected inpatients with suspected tuberculosis. AIDS Res Ther. 2018;15(1):1-9. https://doi.org/10.1186/s12981-018-0192-0 [ Links ]
27.Saavedra A, Campinha-Bacote N, Hajjar M, et al. Causes of death and factors associated with early mortality of HIV-infected adults admitted to Korle-Bu Teaching Hospital. Pan Afr Med J. 2017;27:48. https://doi.org/10.11604/pamj.2017.27.48.8917 [ Links ]
28.Ansari NA, Kombe AH, Kenyon TA, et al. Pathology and causes of death in a group of 128 predominantly HIV-positive patients in Botswana, 1997-1998. Int J Tuberc Lung Dis. 2002;6(1):55-63. [ Links ]
29.Cohen T, Murray M, Wallengren K, Alvarez GG, Samuel EY, Wilson D. The prevalence and drug sensitivity of tuberculosis among patients dying in hospital in KwaZulu-Natal, South Africa: A postmortem study. PLoS Med. 2010;7(6):e1000296. https://doi.org/10.1371/journal.pmed.1000296 [ Links ]
30.Wong EB, Omar T, Setlhako GJ, et al. Causes of death on antiretroviral therapy: A post-mortem study from South Africa. PLoS One. 2012;7(10):e47542. https://doi.org/10.1371/journal.pone.0047542 [ Links ]
31.Schutz C, Barr D, Andrade BB, et al. Clinical, microbiologic, and immunologic determinants of mortality in hospitalized patients with HIV-associated tuberculosis: A prospective cohort study. PLoS Med. 2019;16(7):e1002840. https://doi.org/10.1371/journal.pmed.1002840 [ Links ]
32.Medecins Sans Frontieres. Khayelitsha 2001-2011: Activity report. 10 years of HIV/TB care at primary health care level [homepage on the Internet]. 2011 [cited 2021 Oct 6]; p. 1-36. Available from: https://www.msf.org/khayelitsha-activity-report-2001-2011-10-years-hivtb-care-primary-health-care-level [ Links ]
33.Zifodya J, Kreniske J, Schiller I, et al. Xpert ultra versus Xpert MTB/RIF for pulmonary tuberculosis and rifampicin resistance in adults with presumptive pulmonary tuberculosis. Cochrane Database Syst Rev. 2021;2:CD009593. https://doi.org/10.1002/14651858.CD009593.pub5 [ Links ]
34.Berhanu R, Schnippel K, Mohr E, et al. Early outcomes of decentralized care for rifampicin-resistant tuberculosis in Johannesburg, South Africa: An observational cohort study. PLoS One. 2016;11(11):e0164974. https://doi.org/10.1371/journal.pone.0164974 [ Links ]
35.Loveday M, Wallengren K, Brust J, et al. Community-based care vs. centralised hospitalisation for MDRTB patients, KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2015;19(2):163-171. https://doi.org/10.5588/ijtld.14.0369 [ Links ]
36.Farley JE, Ram M, Pan W, et al. Outcomes of multi-drug resistant tuberculosis (MDR-TB) among a Cohort of South African patients with high HIV prevalence. PLoS One. 2011;6(7):e20436. https://doi.org/10.1371/journal.pone.0020436 [ Links ]
37.Hirasen K, Berhanu R, Evans D, Rosen S, Sanne I, Long L. High rates of death and loss to follow-up by 12 months of rifampicin resistant TB treatment in South Africa. PLoS One. 2018;13(10):e0205463. https://doi.org/10.1371/journal.pone.0205463 [ Links ]
38.Umanah TA, Ncayiyana JR, Nyasulu PS. Predictors of cure among HIV co-infected multidrug-resistant TB patients at Sizwe Tropical Disease Hospital Johannesburg, South Africa. Trans R Soc Trop Med Hyg. 2015;109(5):340-348. https://doi.org/10.1093/trstmh/trv025 [ Links ]
39.Marais E, Mlambo CK, Lewis JJ, et al. Treatment outcomes of multidrug-resistant tuberculosis patients in Gauteng, South Africa. Infect. 2013;42(2):405-413. https://doi.org/10.1007/s15010-013-0572-2 [ Links ]
40.Brust JCM, Gandhi NR, Carrara H, Osburn G, Padayatchi N. High treatment failure and default rates for patients with multidrug-resistant tuberculosis in KwaZulu-Natal, South Africa. Int J Tuberc Lung Dis. 2010;14(4):413-419. [ Links ]
41.Boyd R, Ford N, Padgen P, Cox H. Time to treatment for rifampicin-resistant tuberculosis: Systematic review and meta-analysis. Int J Tuberc Lung Dis. 2017;21(11):1173-1180. https://doi.org/10.5588/ijtld.17.0230 [ Links ]
42.Mahwire TC, Zunza M, Marukutira TC, Naidoo P. Impact of Xpert MTB/RIF assay on multidrug-resistant tuberculosis treatment outcomes in a health district in South Africa. S Afr Med J. 2019;109(4):259-263. https://doi.org/10.7196/SAMJ.2019.v109i4.13180 [ Links ]
 Correspondence:
Correspondence:
Ruan Spies
ruanspies21@gmail.com
Received: 25 Apr. 2022
Accepted: 30 June 2022
Published: 27 Sept. 2022














