Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Southern African Journal of HIV Medicine
On-line version ISSN 2078-6751
Print version ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.23 n.1 Johannesburg 2022
http://dx.doi.org/10.4102/sajhivmed.23i1.1335
REVIEW ARTICLE
Age-related differences in the vascular function and structure of South Africans living with HIV
Anisca LouwrensI; Carla M.T. FourieI, II; Shani Botha-Le RouxI, II; Yolandi BreetI, II
IHypertension in Africa Research Team (HART), School for Physiology, Nutrition and Consumer Science, Faculty of Health Sciences, North-West University, Potchefstroom, South Africa
IIMRC Research Unit for Hypertension and Cardiovascular Disease, Faculty of Health Sciences, North-West University, Potchefstroom, South Africa
ABSTRACT
BACKGROUND: As the life expectancy of people living with the HIV increases because of antiretroviral treatment (ART), their risk for vascular co-morbidities and early vascular ageing (EVA) also increases.
OBJECTIVE: We aimed to investigate whether HIV infection relates to vascular structure and function in black South African adults and whether this relationship is age dependent.
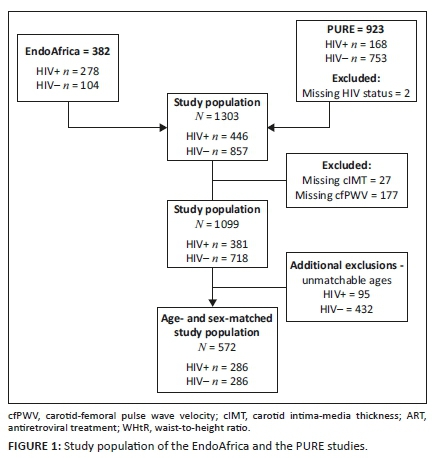
METHOD: This cross-sectional study carried out in urban and rural areas of North West province, South Africa, included 572 age- and sex-matched people living with HIV (PLWH) and without HIV. Participants from the EndoAfrica study and PURE study were stratified according to tertiles of age. Measures of vascular structure (carotid intima-media thickness) and function (carotid-femoral pulse wave velocity, central systolic blood pressure, central pulse pressure and pulse pressure amplification) were determined.
RESULTS: Blood pressure measures were lower in PLWH compared with their controls (all P ≤ 0.001), especially in the younger and middle-aged groups (all P ≤ 0.031), whilst vascular measures did not differ (all P ≥ 0.611). In multivariate linear regression analyses, vascular measures were not associated with a HIV- positive status in either the total or any of the age groups.
CONCLUSION: Black South Africans living with HIV have a less adverse blood pressure profile than their counterparts without HIV. The HIV-positive status was not associated with measures of vascular structure or function in any age group. The results suggest that HIV does not contribute to EVA in this population; however, further longitudinal investigation is warranted.
Keywords: arterial stiffness; carotid intima-media thickness; antiretroviral treatment; early vascular ageing; multi-morbidity.
Introduction
The HIV epidemic is a global health problem with South Africa contributing a large number of people living with HIV (PLWH); of the 38 million PLWH worldwide, 7.7 million reside in South Africa.1 Although communicable diseases, including HIV, were the leading cause of death in 2012,2 the aetiology of mortality in sub-Saharan Africa (SSA) has shifted to a combination of communicable and non-communicable diseases since then.3 Approximately 69.4% of the South African population older than 40 years are battling multimorbidity, which is defined as two or more simultaneously existing chronic diseases.4 Multimorbidity adversely affects vascular health, causing acceleration in the vascular ageing process.5,6,7 Physiological, vascular ageing occurs with the progression of age and is a gradual and continuous process, characterised by structural and/or mechanical changes within the vessel wall.8,9 These mechanical and structural alterations that occur with vascular ageing may result in arterial stiffness and an increased carotid intima-media thickness (cIMT),10 which can affect some individuals prematurely, a term coined early vascular ageing (EVA).11 The prevalence of EVA causes an accelerated trajectory of prematurely developing hypertension, subclinical cardiovascular damage and CVD.11 An adverse transition from physiological vascular ageing to EVA occurs with exposure to risk factors, such as HIV,12,13 and factors commonly related to the disease, namely hypertension,14 dyslipidaemia,15 chronic low-grade inflammation16 and oxidative stress. Data on EVA in PLWH, especially in SSA, are scant as the majority of studies have been carried out on HIV-1 subtype B. However, a previous study carried out by our group found an indication of accelerated vascular ageing in older, never treated HIV-positive South Africans (aged > 50 years), as well as probable early atherosclerosis and endothelial dysfunction.17 Diabetes mellitus18 and dysglycaemia,19 as well as factors related to an unhealthy lifestyle,20,21,22 also lead to acceleration of the vascular ageing process.23 Vascular comorbidities, such as arterial stiffness and atherosclerosis,5,6 as well as cardiometabolic disturbances,24,25 become increasingly evident with the prolonged life expectancy of PLWH because of the effectiveness of antiretroviral treatment (ART).26 Hanna et al. indicated that the effect of HIV on the vasculature may differ through the lifespan of PLWH.27 HIV and/or ART-related vascular alterations may contribute to EVA.8,9,10,28 However, there is a lack of literature regarding the development of EVA in PLWH from South Africa, where the vast majority are infected with HIV type-1 subtype C phenotype.29 Therefore, we investigated whether HIV infection relates to vascular structure and function in South African adults on first-line ART at different ages.
Research methods and design
Setting and study population
This study includes data of participants residing in North West province of South Africa who participated in both the EndoAfrica study (vascular endothelial dysfunction: the putative interface of emerging cardiovascular risk factors affecting populations living with and without HIV in SSA) and the Prospective Urban and Rural Epidemiology (PURE) study. Data from the EndoAfrica study were collected from 2017 to 2018, and data collection for the PURE study took place in 2015. Both the studies were designed to determine the risk of developing cardiovascular disease amongst South Africans living with and without HIV. Both studies were previously described in more detail elsewhere.30,31
The EndoAfrica study recruited participants from seven local clinics in and around Potchefstroom; the participants for the PURE study were recruited from urban and rural communities, which include Potchefstroom (urban), Ganyesa and Tlakgameng (rural). Participants in the EndoAfrica study were 18-60 years and in the PURE study were 42-88 years old. For this study, we included 286 PLWH and 286 HIV-free participants (control group). The participants in the group with HIV were matched for age and sex with those who were HIV free (Figure 1). The PLWH using treatment received first-line fixed dose combination ART.26 The exclusion criteria for both studies include PLWH using second- and third-line ART, individuals who refused HIV testing or to provide a blood sample, women who were pregnant or less than three months post-partum. Additional exclusions were made for missing data of the main variables and/or an unknown HIV status.
Questionnaire and anthropometric measurements
A standardised questionnaire provided information regarding the age, sex, lifestyle patterns (which included tobacco use and alcohol consumption) and medication use in both studies.
Anthropometric measurements were performed according to the standardised methods of the International Society for the Advancement of Kinanthropometry.32 For both studies, height (stadiometer, SECA, Hamburg, Germany [EndoAfrica]; stadiometer, Leicester height measure, SECA, Birmingham, United Kingdom [UK] [PURE]), weight (flat scale, SECA Hamburg, Germany [EndoAfrica]; Precision Health Scale, A & D Company, Japan [PURE]) and waist circumference (WC) (lufkin steel anthropometric tape, W606PM, lufkin, Apex, United States [US] [EndoAfrica]; steel tape, lufkin, cooper tools, Apex NC, US [PURE]) were measured. Body mass index (BMI) was calculated according to international standards.
Cardiovascular measurements
The brachial blood pressure, including systolic blood pressure (SBP), diastolic blood pressure (DBP), and heart rate of each seated participant were taken twice after 10 min of rest (OMRON M6 automatic digital blood pressure monitor, Omron Healthcare, Kyoto, Japan). The final measurement was used in subsequent analyses. We identified participants as hypertensive if SBP ≥ 140 mmHg and/or DBP ≥ 90 mmHg33 and/or if they used anti-hypertensive medication. Mean arterial pressure (MAP) was calculated, MAP = (SBP(2xdbp))/3.
Central SBP (cSBP), central pulse pressure (cPP) and carotid-femoral pulse wave velocity (cfPWV) were measured by arterial waveform analyses (SphygmoCor® XCEL System, AtCor Medical Pty. Ltd, Sydney, Australia). The transit-distance method was used to measure cfPWV along the descending carotid-femoral artery. The 80% rule was applied for the calculation of the distance used.34 The cfPWV measurement was performed twice and repeated a third time if the two measurements differed by more than 3 m/s. The average was used for further analyses. Pulse pressure amplification (PPA) was defined as the ratio of the amplitude of the pulse pressure between a distal and proximal location.
Carotid intima-media thickness was measured in the PURE (SonoSite Micromaxx ultrasound system, SonoSite, Inc., Bothel, WA, US) and EndoAfrica (General Electric Vivid E9, GE Vingmed Ultrasound A/S, Horten, Norway) studies. Digitalised images were analysed with carotid vessel analyser automated software (Vascular Research Tools 6, Medical imageing applications, Coralville, Iowa, US) in the EndoAfrica study and with Artery Measurements Systems software (I version 1.139, Chalmers University of Technology, Gothenburg. Sweden) in the PURE study.
Blood sampling and biochemical analyses
Blood samples were collected, centrifuged and aliquoted according to standardised methods, and were stored in −80 C bio-freezers for future analyses. Samples collected from the rural site during the PURE study were prepared at the on-site laboratory and immediately stored on dry ice (−18 °C) for a maximum of 5 days. These samples were then transported to the laboratory and stored at −80 °C.
In both studies, the Cobas Integra® 400 Roche® Clinical System (Roche Diagnostics, Indianapolis, IN, US) was used to determine glucose, total cholesterol (TC), high-density lipoprotein (HDL), low-density lipoprotein (LDL) and triglyceride levels using an enzymatic colourimetric method. High-sensitivity C-reactive protein (CRP) was analysed with the particle-enhanced turbidimetric assay method and gamma-glutamyl transferase (GGT) with the enzymatic colourimetric assay method.
Glycated haemoglobin (HbA1c) analyses were carried out with ethylenediamine tetraacetic acid whole blood samples in the EndoAfrica (turbidimetric inhibition immunoassay; Cobus Integra® 400plus, Roche, Switzerland) and PURE (ion-exchange high-performance liquid chromatography method; D-10 Haemoglobin testing system from Bio-Rad #220-0101) studies.
In the EndoAfrica study, plasma samples were sent to the National Health Laboratory Services (NHLS) to determine the CD4+ cell count (Beckman COULTER® EPICS® XLTM Flow Cytometer, GMI Inc., Fullerton, CA, US). The finger-prick blood and a point-of-care device (PIMATM CD4, Alere, Jena, Germany) with the fixed volume cytometry analysis were used in the PURE study.
HIV testing and counselling
Pre-counselling was given to each participant in both the EndoAfrica and PURE studies before HIV testing was performed with the first-response rapid HIV test (Premier Medical Corporation Limited, Daman, India). Positive HIV results were confirmed by the SD BOLINE HIV 1 / 2 3.0 (Standard Diagnostics, INC, Korea) rapid test in the EndoAfrica study and the ABON (Biopharm Corporation Limited Hanyzhou, China) in the PURE study.
Statistical analyses
IBM® SPSS® Statistics, version 26.0 (IBM Corporation, Armonk, NY, USA) software was used for data analyses. In order to assess whether the data were normally distributed, we used graphical inspections (histograms and Q-Q plots) and numerically inspected the skewness and kurtosis of variables. Continuous data with a normal distribution are presented as arithmetic mean ± standard deviation and categorical data as proportions. For non-normally distributed data, we performed non-parametric tests and reported the median and interquartile ranges. The study population was stratified according to tertiles of age. The tertile ranges of the age groups for PLWH and without HIV, respectively, were as follows: younger ages were classified as 18-47 years versus 19-48 years, middle as 48-53 years versus 49-53 years, and older as 54-71 years versus 54-71 years. Differences between the groups were determined with independent T-tests or Mann-Whitney U-tests and Chi-square tests. Correlation analyses included Pearson or Spearman rank tests. In order to test for independent associations between the main outcome variables (cfPWV, PPA, cPP, cSBP and cIMT) and HIV infection, we performed multiple regression analyses (with the Enter method) in the total group, as well as stratified according to age tertile groups. All the models included HIV status, sex, BMI, TC, GGT, HbA1c, CRP, tobacco use, ART use and anti-hypertensive medication use. Mean arterial pressure was additionally included in the models, with cfPWV, cIMT and PPA as the dependent variables and heart rate in the model with PPA as the dependent variable. Adjustments for each dependent variable were made as follows: cfPWV (sex and MAP), cIMT (sex and MAP), cSBP (sex), cPP (sex) and PPA (sex, MAP, heart rate and height).
Ethical considerations
This study complies with the requirements of the Declaration of Helsinki, and the Health Research Ethics Committee of the North-West University approved the EndoAfrica study (NWU-00045-15-A1), the PURE study (NWU-00016-10-A1), as well as this study (NWU-00367-20-A1). All procedures were explained to the participants before any measurements were made, and all participants gave written informed consent.
Results
The characteristics of the study population are presented in Table 1. The PLWH and without HIV were matched for age (P = 0.098) and sex (P = 1.000), and their locality was comparable (P = 0.073). People living with HIV had a lower waist-to-height ratio (WtHR) and BMI (both P < 0.001) compared with the HIV-free group. With regard to cardiovascular measurements, all brachial blood pressures were lower in PLWH (all P < 0.049); however, no differences were found in either cfPWV, cIMT or PPA (all P ≥ 0.204). When comparing biochemical markers, GGT was higher in those with HIV (P < 0.001), whilst levels of glucose, glycated haemoglobin, TC, triglycerides and LDL-cholesterol (P ≤ 0.049) were lower. The CD4+ count was found to be 525.5 cells/µL for PLWH. Both tobacco use and alcohol consumption (P ≤ 0.014) were more frequent in PLWH.
In Figure 2 and Appendix Table 1-A1, we compared the adjusted differences in vascular measures (cfPWV, cIMT, cSBP, cPP and PPA) between the groups with and without HIV in different age tertiles (on the left), as well as across different age groups within the HIV and HIV-free groups, respectively (on the right). PLWH in the younger and middle age groups had lower cSBP (both P ≤ 0.031) and in all the age groups had lower cPP (all P ≤ 0.027) than those without HIV. Carotid-femoral pulse wave velocity, cIMT, cSBP and cPP increased, whilst PPA decreased across increased tertiles of age in both the HIV and HIV-free groups (all P ≤ 0.028).
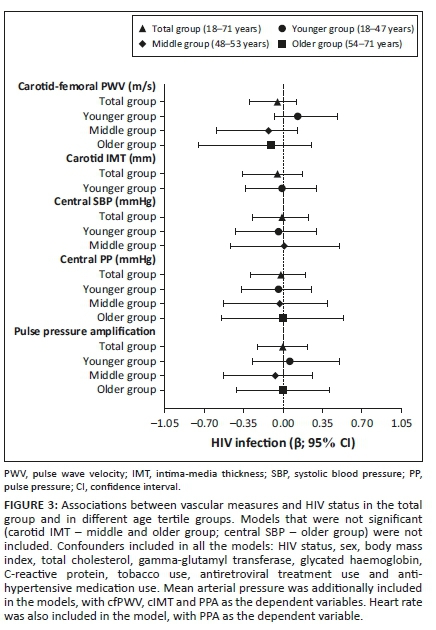
We further determined whether vascular measures associated with the HIV status in the total group and in the respective age groups (Figure 3 and Appendix Table 2-A1), whilst taking cardiovascular risk factors and ART into account. No associations were observed between any of the vascular measures and HIV infection as the main independent variable (all P ≥ 0.162).
Discussion
This study found that in a population of PLWH in South Africa, brachial blood pressure measurements (cSBP and cPP) were lower in younger and middle age groups and vascular markers (cfPWV, cIMT and PPA) were comparable with the HIV-uninfected population. In addition, we showed a lack of association between HIV infection and any of the vascular markers in any age group.
To the best of our knowledge, this was the first study to investigate age-related differences in the vascular structure and function between South Africans living with HIV and their age and sex-matched controls without HIV. We also determined whether an association between the HIV-positive status and these vascular measures exists within different age groups.
In addressing our first objective, we found that the group with HIV presented with less adverse central and brachial blood pressure profiles compared with their controls. In addition, the age-related deterioration of the vascular profile in the group with HIV was not as pronounced as expected, with cSBP being lower in the younger and middle age groups when compared with the group without HIV. Moreover, cPP was lower in PLWH in all the age groups when compared with their HIV-free counterparts. Data on the vascular profile of PLWH are controversial, and studies on this topic in a SSA context are scant. Previous studies both contradict35,36,37,38 and correspond30,39,40,41 to the study findings. Contrary to the study results, an Italian study in age-matched PLWH and individuals without HIV older than 18 years showed higher cSBP in those with HIV, which may have been attributed to renal damage.35 Msoka and colleagues concluded in a meta-analysis that cIMT, an early marker for atherosclerosis, was higher in PLWH compared with their controls in cross-sectional studies.37 The increased cIMT in the PLWH was associated with elevated levels of CRP and an adverse lipid profile.37 High CRP levels and an adverse lipid profile commonly occur in immunosuppressive states, such as HIV, where chronic low-grade systemic inflammation is known to prevail.38
Similar to our results, a narrative review of studies conducted in SSA concluded that treated PLWH had lower SBP and DBP, and hypertension compared with ART-naïve or individuals without HIV.39 We recently investigated the cardiovascular profile of PLWH and individuals without HIV in the EndoAfrica study, with a mean age of 42 years.30 The cardiovascular profile of these individuals included vascular markers similar to those in this current study, such as cfPWV, cIMT, cSBP and cPP.30 Neither the cardiovascular profile nor the CRP levels of those with HIV and participants without HIV in the EndoAfrica study differed,30 supporting the current findings that EVA was not evident in this study population with HIV. In another cross-sectional study, no increase in arterial stiffness, atherosclerosis or inflammation was found in PLWH, despite the presence of endothelial activation, when compared with ART-naïve or control groups.41
Considering the effect of ART on metabolic markers, it was reported that the lipid profile of Chinese PLWH who used Efavirenz-based ART was less adverse than those receiving Lopinavir or Ritonavir-based ART.42 The more favourable lipid profile of the group with HIV was also found in this South African study population using Efavirenz-based ART. Although there was no obvious effect on LDL-cholesterol in the Chinese study,42 this study found lower levels of LDL-cholesterol, TC and triglycerides in the HIV group (Table 1). This study's less adverse glycaemic profile supports the rising notion that the cardiovascular profile of PLWH is less detrimental compared with their uninfected counterparts.43 These results support the findings of Shet et al., which showed better treatment outcomes for the HIV subtype C phenotype in South Africa, despite a higher baseline viral load, compared with other subtypes of HIV.44
Upon investigating whether an association exists between HIV infection and measures of vascular structure and function in different age groups, no significant results were found. In contrast with the study findings, a systematic review of studies conducted in SSA populations showed a positive association between HIV infection and atherosclerosis.45 It is known that atherosclerosis develops as a result of inflammation,46 and it is further suggested that the progression thereof is a plausible link to vasculopathy47 and EVA.28,48 However, Dillon et al. associated HIV infection with a lower SBP, DBP and BMI in a systematic review and meta-analysis on studies conducted in SSA.40 In our study population living with HIV, we found a lower cSBP and BMI compared with their counterparts without HIV in all the three age groups. However, we found no associations between cSBP and HIV infection in any of the age groups. The findings from previous studies, which found no association between HIV and arterial stiffness,49 hypertension36 or atherosclerosis,50 are in line with the study findings. Monteiro et al.49 reported a higher frequency of hypertension in the uninfected controls, which was also evident in this study.
As the carotid-femoral segment of the vasculature is highly sensitive to increases in blood pressure,51 the lower prevalence of hypertension in PLWH in this study could explain, at least in part, the absence of association of arterial stiffness with HIV status. Furthermore, PLWH using ART tend to develop cardiometabolic disturbances, resembling those found in the metabolic syndrome, such as lipodystrophy,52 dyslipidaemia53 and hyperglycaemia,54 none of which was encountered in the group with HIV of whom 76% received ART in this study.
The results on the association of HIV with vascular markers should be interpreted in light of the fact that we did not have ART adherence or viral load data. This cross-sectional study design limited us to infer cause and effect, and the study results need to be confirmed in a larger cohort. To the best of our knowledge, this was the first study to investigate the effect of age on the association between vascular structure and function and HIV infection in a South African population.
Conclusion
The results of this study revealed that HIV-positive status did not associate with measures of vascular structure and function in any of the age groups. People living with HIV did not have a worse cardiovascular profile, and EVA was not evident in these people compared with those without HIV. The study findings warrant further longitudinal investigation that may observe individual vascular changes over time to determine whether South Africans living with HIV have an increased risk of developing CVD.
Acknowledgements
The authors acknowledge all participants of both the EndoAfrica and PURE studies, as well as the students, support staff and researchers at the Hypertension Research and Training Clinic in the North-West University, South Africa.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
A.L. contributed to data acquisition, drafted the manuscript, performed statistical analyses and interpreted the results. C.M.T.F., S.B.-L.R. and Y.B. contributed to conceptualisation and the acquisition, analyses, and interpretation of the data. All authors critically revised the manuscript, gave input and final approval, and agreed to be accountable for all aspects of the work in order to ensure integrity and accuracy.
Funding information
Financial support for the EndoAfrica study conducted in North West province was received from the Department of Science and Technology in South Africa (contract number DST/CON 0133/2016), and for the PURE study from SANPAD (South Africa - Netherlands Research Programme on Alternatives in Development, GUN number 08/15), PHRI (Population Health Research Institute), the Medical Research Council of South Africa, the North-West University, Roche Diagnostics and the South African National Research Foundation (NRF, GUN numbers FA2006040700010 and 2069139).
Data availability
The data sets used and/or analysed during the current study are available from the corresponding author, Y.B., on reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.UNAIDS. South Africa 2019: HIV and AIDS estimates [homepage on the Internet]. [cited 2021 Jun 7]. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica [ Links ]
2.Pillay-van Wyk V, Msemburi W, Dorrington R, et al. HIV/AIDS mortality trends pre and post ART for 1997-2012 in South Africa-have we turned the tide? S Afr Med J. 2019;109(11b):41-44. https://doi.org/10.7196/SAMJ.2019.v109i11b.14283 [ Links ]
3.Gouda HN, Charlson F, Sorsdahl K, et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990-2017: Results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375-e1387. https://doi.org/10.1016/S2214-109X(19)30374-2 [ Links ]
4.Academy of Medical Sciences (UK), Academy of Science of South (ASSAF). Improving the prevention and management of multimorbidity in sub-Saharan Africa [homepage on the Internet]. Proceedings report. 2020 [cited 2021 Jun 07]. Available from: https://research.assaf.org.za/handle/20.500.11911/139 [ Links ]
5.Mulè G, Mulè G, Tranchida V, et al. Aortic stiffness in HIV infection with and without antiretroviral therapy. A meta-analysis of observational studies. Artery Res. 2020;26(1):13-20. https://doi.org/10.2991/artres.k.200314.002 [ Links ]
6.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS (London, England). 2010;24(2):243-253. https://doi.org/10.1097/QAD.0b013e328333ea9e [ Links ]
7.Kwarisiima D, Atukunda M, Owaraganise A, et al. Hypertension control in integrated HIV and chronic disease clinics in Uganda in the SEARCH study. BMC Public Health. 2019;19(1):511. https://doi.org/10.1186/s12889-019-6838-6 [ Links ]
8.Lakatta EG. Age-associated cardiovascular changes in health: Impact on cardiovascular disease in older persons. Heart Fail Rev. 2002;7(1):29-49. [ Links ]
9.Ross R. The pathogenesis of atherosclerosis: A perspective for the 1990s. Nature. 1993;362(6423):801-809. https://doi.org/10.1038/362801a0 [ Links ]
10.Bots ML, Grobbee DE. Intima media thickness as a surrogate marker for generalised atherosclerosis. Cardiovasc Drugs Ther. 2002;16(4):341-351. https://doi.org/10.1023/A:1021738111273 [ Links ]
11.Laurent S, Boutouyrie P, Cunha PG, Lacolley P, Nilsson PM. Concept of extremes in vascular aging: From early vascular aging to supernormal vascular aging. Hypertension. 2019;74(2):218-228. https://doi.org/10.1161/HYPERTENSIONAHA.119.12655 [ Links ]
12.Muller L, Sewchuran T, Durand M. Prevalence of incidental premature cardiac calcifications in an HIV-infected South African population using conventional computed tomography chest radiography. SAJHIVM. 2021;22(1):1241-1248. https://doi.org/10.4102/sajhivmed.v22i1.1241 [ Links ]
13.Dubé MP, Lipshultz SE, Fichtenbaum CJ, et al. Effects of HIV infection and antiretroviral therapy on the heart and vasculature. Circulation. 2008;118(2):e36-e40. https://doi.org/10.1161/CIRCULATIONAHA.107.189625 [ Links ]
14.Xu C, Zarins CK, Pannaraj PS, et al. Hypercholesterolemia superimposed by experimental hypertension induces differential distribution of collagen and elastin. Arterioscler Thromb Vasc Biol. 2000;20(12):2566-2572. https://doi.org/10.1161/01.ATV.20.12.2566 [ Links ]
15.Fisher SD, Miller TL, Lipshultz SE. Impact of HIV and highly active antiretroviral therapy on leukocyte adhesion molecules, arterial inflammation, dyslipidemia, and atherosclerosis. Atherosclerosis. 2006;185(1):1-11. https://doi.org/10.1016/j.atherosclerosis.2005.09.025 [ Links ]
16.Touboul P-J, Hennerici M, Meairs S, et al. Mannheim carotid intima-media thickness and plaque consensus (2004-2006-2011). Cerebrovasc Dis. 2012;34(4):290-296. https://doi.org/10.1159/000343145 [ Links ]
17.Fourie C, Van Rooyen J, Pieters M, Conradie K, Hoekstra T, Schutte A. Is HIV-1 infection associated with endothelial dysfunction in a population of African ancestry in South Africa?: Cardiovascular topics. Cardiovasc J Afr. 2011;22(3):134-140. https://doi.org/10.5830/CVJA-2010-056 [ Links ]
18.Rizzoni D, Porteri E, Guelfi D, et al. Structural alterations in subcutaneous small arteries of normotensive and hypertensive patients with non-insulin-dependent diabetes mellitus. Circulation. 2001;103(9):1238-1244. https://doi.org/10.1161/01.CIR.103.9.1238 [ Links ]
19.Gaziano TA, Abrahams-Gessel S, Gomez-Olive FX, et al. Cardiometabolic risk in a population of older adults with multiple co-morbidities in rural South Africa: The HAALSI (Health and Aging in Africa: Longitudinal studies of INDEPTH communities) study. BMC Public Health. 2017;17(1):206. https://doi.org/10.1186/s12889-017-4117-y [ Links ]
20.Pithey A, Parry C. Descriptive systematic review of sub-Saharan African studies on the association between alcohol use and HIV infection. SAHARA-J. 2009;6(4):155-169. https://doi.org/10.1080/17290376.2009.9724944 [ Links ]
21.Miguez-Burbano MJ, Burbano X, Ashkin D, et al. Impact of tobacco use on the development of opportunistic respiratory infections in HIV seropositive patients on antiretroviral therapy. Addict Biol. 2003;8(1):39-43. https://doi.org/10.1080/1355621031000069864 [ Links ]
22.Crum-Cianflone N, Roediger MP, Eberly L, et al. Increasing rates of obesity among HIV-infected persons during the HIV epidemic. PLoS One. 2010;5(4):e10106. https://doi.org/10.1371/journal.pone.0010106 [ Links ]
23.Nilsson PM. Early vascular aging (EVA): Consequences and prevention. Vasc Health Risk Manag. 2008;4(3):547-552. https://doi.org/10.2147/VHRM.S1094 [ Links ]
24.Ntsekhe M, Mayosi BM. Cardiac manifestations of HIV infection: An African perspective. Nat Clin Prac Cardiovasc Med. 2009;6(2):120-127. https://doi.org/10.1038/ncpcardio1437 [ Links ]
25.Mayosi BM, Flisher AJ, Lalloo UG, et al. The burden of non-communicable diseases in South Africa. Lancet. 2009;374(9693):934-947. https://doi.org/10.1016/S0140-6736(09)61087-4 [ Links ]
26.Solages A, Vita JA, Thornton DJ, et al. Endothelial function in HIV-infected persons. Clin Infect Dis 2006;42(9):1325-1332. https://doi.org/10.1086/503261 [ Links ]
27.Hanna DB, Guo M, Bůžková P, et al. HIV infection and carotid artery intima-media thickness: Pooled analyses across 5 cohorts of the NHLBI HIV-CVD collaborative. Clin Infect Dis. 2016;63(2):249-256. https://doi.org/10.1093/cid/ciw261 [ Links ]
28.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352(16):1685-1695. https://doi.org/10.1056/NEJMra043430 [ Links ]
29.UNAIDS. South Africa 2019: HIV and AIDS estimates [homepage on the Interet]. 2020 [2021 Nov 23]. Available from: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf [ Links ]
30.Fourie CMT, Botha-Le Roux S, Smith W, et al. Vascular function and cardiovascular risk in a HIV infected and HIV free cohort of African ancestry: Baseline profile, rationale and methods of the longitudinal EndoAfrica-NWU study. BMC Infect Dis 2020;20:473. https://doi.org/10.1186/s12879-020-05173-6 [ Links ]
31.Schutte AE, Schutte R, Huisman HW, et al. Are behavioural risk factors to be blamed for the conversion from optimal blood pressure to hypertensive status in Black South Africans? A 5-year prospective study. Int J Epidemiol. 2012;41(4):1114-1123. https://doi.org/10.1093/ije/dys106 [ Links ]
32.Silva VSd, Vieira MFS. International Society for the Advancement of Kinanthropometry (ISAK) Global: International accreditation scheme of the competent anthropometrist. Revista Brasileira de Cineantropometria & Desempenho Humano. 2020;22. https://doi.org/10.1590/1980-0037.2020v22e70517 [ Links ]
33.Unger T, Borghi C, Charchar F, et al. 2020 International Society of Hypertension global hypertension practice guidelines. Hypertension. 2020;75(6):1334-1357. https://doi.org/10.1161/HYPERTENSIONAHA.120.15026 [ Links ]
34.Van Bortel LM, Laurent S, Boutouyrie P, et al. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens. 2012;30(3):445-448. https://doi.org/10.1097/HJH.0b013e32834fa8b0 [ Links ]
35.Maloberti A, Dozio D, Betelli M, et al. Brachial and central blood pressure in HIV-infected subjects. Hypertens Res. 2015;38(6):405-412. https://doi.org/10.1038/hr.2015.25 [ Links ]
36.Lazar JM, Wu X, Shi Q, et al. Arterial wave reflection in HIV-infected and HIV-uninfected Rwandan women. AIDS Res Hum Retroviruses 2009;25(9):877-882. https://doi.org/10.1089/aid.2008.0269 [ Links ]
37.Msoka TF, Van Guilder GP, Van Furth M, et al. The effect of HIV infection, antiretroviral therapy on carotid intima-media thickness: A systematic review and meta-analysis. Life Sci 2019;235:116851. https://doi.org/10.1016/j.lfs.2019.116851 [ Links ]
38.Kaplan RC, Sinclair E, Landay AL, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217(1):207-213. https://doi.org/10.1016/j.atherosclerosis.2011.03.011 [ Links ]
39.Phalane E, Fourie CM, Mels CM, et al. A comparative analysis of blood pressure in HIV-infected patients versus uninfected controls residing in Sub-Saharan Africa: A narrative review. J Hypertens. 2020;34:692-708. https://doi.org/10.1038/s41371-020-0385-6 [ Links ]
40.Dillon DG, Gurdasani D, Riha J, et al. Association of HIV and ART with cardiometabolic traits in sub-Saharan Africa: A systematic review and meta-analysis. Int J Epidemiol. 2013;42(6):1754-1771. [ Links ]
41.Fourie C, Schutte A, Smith W, et al. Endothelial activation and cardiometabolic profiles of treated and never-treated HIV infected Africans. Atherosclerosis. 2015;240(1):154-160. https://doi.org/10.1016/j.atherosclerosis.2015.03.015 [ Links ]
42.Dai L, Liu A, Zhang H, et al. Impact of lopinavir/ritonavir and efavirenz-based antiretroviral therapy on the lipid profile of Chinese HIV/AIDS treatment-naïve patients in Beijing: A retrospective study. Curr HIV Res. 2019;17(5):324-334. https://doi.org/10.2174/1570162X17666191025115508 [ Links ]
43.Vos AG, Barth RE, Klipstein-Grobusch K, et al. Cardiovascular disease burden in rural Africa: Does HIV and antiretroviral treatment play a role? Baseline analysis of the Ndlovu Cohort Study. J Am Heart Assoc. 2020;9(7):e013466. https://doi.org/10.1161/JAHA.119.013466 [ Links ]
44.Shet A, Nagaraja P, Dixit NM. Viral decay dynamics and mathematical modeling of treatment response: Evidence of lower in vivo fitness of HIV-1 subtype C. J Acquir Immune Defic Syndr. 2016;73(3):245-251. https://doi.org/10.1097/QAI.0000000000001101 [ Links ]
45.Hyle EP, Mayosi BM, Middelkoop K, et al. The association between HIV and atherosclerotic cardiovascular disease in sub-Saharan Africa: A systematic review. BMC Public Health. 2017;17(1):954. https://doi.org/10.1186/s12889-017-4940-1 [ Links ]
46.Libby P. Vascular biology of atherosclerosis: Overview and state of the art. Am J Cardiol. 2003;91(3):3-6. https://doi.org/10.1016/S0002-9149(02)03143-0 [ Links ]
47.Subramanian S, Tawakol A, Burdo TH, et al. Arterial inflammation in patients with HIV. JAMA. 2012;308(4):379-386. https://doi.org/10.1001/jama.2012.6698 [ Links ]
48.Bots ML, Dijk JM, Oren A, Grobbee DE. Carotid intima-media thickness, arterial stiffness and risk of cardiovascular disease: Current evidence. J Hypertens. 2002;20(12):2317-2325. https://doi.org/10.1097/00004872-200212000-00002 [ Links ]
49.Monteiro P, Miranda-Filho D, Bandeira F, et al. Is arterial stiffness in HIV-infected individuals associated with HIV-related factors? Braz J Med Biol Resh. 2012;45(9):818-826. https://doi.org/10.1590/S0100-879X2012007500116 [ Links ]
50.Kaplan RC, Kingsley LA, Gange SJ, et al. Low CD4+ T cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS (London, England). 2008;22(13):1615. https://doi.org/10.1097/QAD.0b013e328300581d [ Links ]
51.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular diseases. Circulation. 2003;107(22):2864-2869. https://doi.org/10.1161/01.CIR.0000069826.36125.B4 [ Links ]
52.Alikhani A, Morin H, Matte S, et al. Association between lipodystrophy and length of exposure to ARTs in adult HIV-1 infected patients in Montreal. BMC Infect Dis. 2019;19(1):820. https://doi.org/10.1186/s12879-019-4446-9 [ Links ]
53.Bastard J-P, Couffignal C, Fellahi S, et al. Diabetes and dyslipidaemia are associated with oxidative stress independently of inflammation in long-term antiretroviral-treated HIV-infected patients. Diabetes Metab. 2019;45(6):573-581. https://doi.org/10.1016/j.diabet.2019.02.008 [ Links ]
54.De Wit S, Sabin C, Weber R, et al. Data collection on adverse events of anti-HIV drugs (D: A: D) study. Incidence and risk factors for new-onset diabetes in HIV-infected patients: The Data Collection on Adverse Events of Anti-HIV Drugs (D: A: D) study. Diabetes Care. 2008;31(6):1224-1229. https://doi.org/10.2337/dc07-2013 [ Links ]
 Correspondence:
Correspondence:
Yolandi Breet
21195706@nwu.ac.za
Received: 18 Oct. 2021
Accepted: 26 Nov. 2021
Published: 24 Feb. 2022
Appendix 1
















