Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of HIV Medicine
versión On-line ISSN 2078-6751
versión impresa ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.21 no.1 Johannesburg 2020
http://dx.doi.org/10.4102/sajhivmed.v21i1.1105
ORIGINAL RESEARCH
Patient acceptance of HIV testing services in rural emergency departments in South Africa
Aditi RaoI; Caitlin KennedyI; Pamela MdaII; Thomas C. QuinnIII, IV; David SteadV, VI; Bhakti HansotiI, III
IDepartment of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, United States of America
IINelson Mandela Academic Clinical Research Unit, Mthatha, South Africa
IIIDepartment of Medicine, Johns Hopkins University School of Medicine, Baltimore, United States of America
IVDivision of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, United States of America
VDepartment of Medicine, Frere and Cecilia Makiwane Hospitals, East London, South Africa
VIDepartment of Medicine, Faculty of Health Sciences, Walter Sisulu University, East London, South Africa
ABSTRACT
BACKGROUND: South Africa faces the highest burden of HIV infection globally. The National Strategic Plan on HIV recommends provider-initiated HIV counselling and testing (HCT) in all healthcare facilities. However, HIV continues to overwhelm the healthcare system. Emergency department (ED)-based HCT could address unmet testing needs.
OBJECTIVES: This study examines the reasons for accepting or declining HCT in South African EDs to inform the development of HCT implementation strategies.
METHOD: We conducted a prospective observational study in two rural EDs, from June to September 2017. Patients presenting to the ED were systematically approached and offered a point-of-care test in accordance with national guidelines. Patients demographics, presenting compaint, medical history and reasons for accepting/declining testing, were recorded. A pooled analysis is presented.
RESULTS: Across sites, 2074 adult, non-critical patients in the ED were approached; 1880 were enrolled in the study. Of those enrolled, 19.7% had a previously known positive diagnosis, and 80.3% were unaware of their HIV status. Of those unaware, 90% patients accepted and 10% declined testing. The primary reasons for declining testing were 'does not want to know status' (37.6%), 'in too much pain' (34%) and 'does not believe they are at risk' (19.9%).
CONCLUSIONS: Despite national guidelines, a high proportion of individuals remain undiagnosed, of which a majority are young men. Our study demonstrated high patient acceptance of ED-based HCT. There is a need for investment and innovation regarding effective pain management and confidential service delivery to address patient barriers. Findings support a routine, non-targeted HCT strategy in EDs.
Keywords: HIV counselling and testing; South Africa; emergency department; patient acceptance; implementation research; linkage to care.
Introduction
South Africa (SA) faces the highest burden of HIV infection globally, with 7.7 million people living with HIV (PLWH) and prevalence ranging from 12.6% to 27% across the country.1,2 In 2018, SA had 240 000 new HIV infections and 71 000 AIDS-related deaths.1 The Joint United Nations Programme on HIV/AIDS (UNAIDS) adopted the ambitious treatment target of 90-90-90, wherein by 2020 90% of all PLWH would know their status, 90% of whom would be receiving sustained antiretroviral therapy (ART), of whom 90% would have achieved viral suppression.3 Currently, in SA, an estimated 90% of PLWH know their status, of whom 68% are accessing ART, and 87% of these have achieved viral suppression.1 Over the past two decades, numerous steps have been taken by the government to deliver evidence-based interventions focusing on HIV prevention, treatment, and retention. These efforts include the expansion of condom distribution, a national voluntary medical male circumcision program, prevention of mother-to-child transmission, as well as initiatives to increase knowledge and awareness of HIV/AIDS in communities utilising healthcare, in educational infrastructures and social media.2,4 However, despite sustained efforts and innovative measures, critical coverage gaps remain, with an estimated 10% of HIV-positive South Africans unaware of their status5 and with 15% of new global infections occurring in the country.2
The first critical step to meeting the 90-90-90 target is HIV testing. Early detection of undiagnosed HIV infection followed by effective linkage to care and treatment extends life expectancy, improves the quality of life, and reduces HIV transmission.6 Since 2015, the South African National Strategic Plan on HIV, sexually transmitted infections and tuberculosis has recommended provider-initiated HIV counselling and testing (HCT) to all persons attending healthcare facilities as a standard component of medical care, including trauma, casualty, and specialty clinics.6,7 Nonetheless, the provision of HCT in healthcare facilities is often hindered by the lack of standardised training and by competing clinical care priorities that prohibit effective service delivery.5,7 In addition, resources for HCT have largely been directed to primary healthcare centres and antenatal clinics or are focused on high-risk populations such as sex workers, men who have sex with men, injection drug users, and prisoners.8,9 As a result, individuals who do not interact with the healthcare system through these channels, such as young men, often miss being tested.
In SA, 90% of the population accesses healthcare through the public sector. For 28%, the emergency department (ED), a setting that provides high-volume care, is their only point of contact.10 In the United States of America, the ED is recognised by the Centers for Disease Control and Prevention to be a crucial venue in implementing the national HIV testing strategy.11 Seminal studies have not only quantified the burden of HIV infection in EDs but also have been critical to shaping the US national strategy for HIV; they could similarly address unmet testing needs in SA.11,12,13,14 In low- and middle-income countries (LMICs), HIV prevalence in EDs may be high, for example, 19% in Papua New Guinea and 50% in Uganda.15,16 Provision of HIV testing in the ED could thus be a critical intervention in curbing the epidemic. Its acceptance in acute care settings, however, has not been widely evaluated in sub-Saharan Africa. Studies have primarily focused on rates of acceptance, without exploring the reasons behind patients' decisions. Ascertaining the perspectives of patients, especially of those who decline testing, enables the identification of barriers to service delivery and the development of effective strategies to increase HIV diagnosis and linkage to care.
In this exploratory observational study, to determine the feasibility of expanding an ED-based HIV testing strategy in SA, we investigated patient perspectives on accepting or declining HCT and quantified the burden of HIV infection in the ED while implementing the nationally recommended HCT programme. This study will assist policymakers and healthcare providers to inform the integration of HCT in the clinical care pathway and optimise HCT service delivery in this venue, resulting in early engagement in care and treatment initiation, ultimately reducing HIV-associated morbidity and mortality.
Methods
The Walter Sisulu Infectious Diseases Screening in Emergency Departments (WISE) Study was a prospective observational study. HIV counselling and testing was implemented in the EDs of the Nelson Mandela Academic Hospital (NMAH) and the Mthatha Regional Hospital (MRH) in the Eastern Cape Province, from 27 June to 03 September 2017.
Study site
The study was conducted in Mthatha, a rural town in the South African province of the Eastern Cape, a region that supports 12.6% of the country's population.10 The area faces a disproportionate burden of acute injuries and illnesses with high rates of HIV and tuberculosis.7 It is also one of SA's poorest provinces and is a key priority area for HIV research and capacity building.7 Both hospitals are affiliated with the Walter Sisulu University. Nelson Mandela Academic Hospital is a large tertiary-care referral centre with 24-h trauma services, seeing only patients requiring specialty or surgical interventions referred from other district-level facilities. Mthatha Regional Hospital is a district-level facility that provides care to walk-in patients and referrals from adjacent maternal and childcare facilities. The EDs provide 24-h coverage and see 100-150 patients daily from the surrounding 100-km catchment area. Both sites are relatively low-resourced and not equipped with an electronic medical record (EMR) system, patient tracking system or standardised triage processes. Furthermore, there are no providers specialising in emergency medicine at these sites.
Study population
Patients presenting to the ED who were aged 18 years and older and clinically stable (defined as the South African Triage Scale designation of 'non-emergent') were included in the study. Triage scores were assigned by trained study staff, based on the South African Triage Scale (SATS).17 Patients younger than 18 years, not able to provide informed consent (i.e. patients with a depressed level of consciousness or mentally altered) or undergoing active resuscitation were excluded.
Recruitment and sampling
All patients presenting to the ED during the study period who met the inclusion criteria were approached by trained HCT staff, informed of the ongoing study and offered a point-of-care HIV test. Written informed consent was sought for testing and participation in a survey that asked about reasons for accepting or declining the test. Patients with a known HIV-positive diagnosis were asked if they had access to an antiretroviral (ARV) clinic, if they were on regular treatment and whether they were aware of having developed AIDS or being virally suppressed. Data were also collected on patient demographics, presenting complaint, presenting symptoms and past medical history.
HIV counsellors approached all eligible patients in a large waiting room after they underwent initial triage and administrative processes. Patients consenting to the study were escorted to a private room for testing if possible, whereas patients assigned a bed were tested at the bedside with curtains drawn where possible. Given the lack of an EMR or patient tracking system, HCT staff placed a small dot on the folders of all patients who were approached and offered HCT. Every 4 h, the study supervisors audited the folders of patients located within the ED to ensure that all eligible patients had been approached.
Based on recent survey data from the 2017 South African national HIV prevalence study, HIV prevalence amongst South Africans of all ages was estimated at 14%.2 Our study aimed to recruit a sample size of 700 patients at each site. This would present a large enough sample to capture the variation in testing preferences in the study setting, allowing us to detect a difference of greater than 5% from the baseline estimate of 14%, assuming a two-sided α of 0.05 and 80% power, for a period of 7 weeks at each site.
Intervention
Patients were offered point-of-care HIV testing following the South African national HIV testing guidelines.7 Patients who consented to the test provided a blood sample obtained through a lancet finger prick. Following the recommended testing algorithm, patients were first tested using the Advanced Quality Anti-HIV 1&2 rapid test (InTec Products, Inc., Fujian, China). Non-reactive samples were reported as an HIV-negative result. Reactive samples were confirmed with an HIV 1/2/O Tri-line HIV rapid test (ABON Biopharm, Hangzhou, China). Confirmed reactive samples were reported as an HIV-positive result, and patients were provided with a referral letter to a local ARV clinic. Confirmed non-reactive samples were reported as an indeterminate result, and patients were counselled to repeat the test in 4-6 weeks. Counselling preceded and followed all tests and included education on HIV transmission, prevention, and management. Results were available within 10-15 min of testing, whereas counselling required an additional 10-15 min, depending on the HIV test result.
Data collection
Ten local research assistants were hired and trained in rapid point-of-care HCT, good clinical practice and data collection, and were familiarised with the study protocol before the start of the study. Research assistants and study staff worked in shifts to ensure 24-h coverage of the ED.
In tandem with offering HIV testing, HCT staff administered a brief survey. Patient responses to questions about their gender, past medical history, mode of arrival, reason for visit, presenting complaint, and symptoms were recorded as pre-determined binary or categorical options, age was recorded as free text, and reasons for accepting or declining testing were captured via pre-determined categorical options derived from the literature or as free text. Data were recorded on case report forms. These forms were scanned and uploaded onto iDatafax (DF/Net Research, Inc., Seattle, WA, USA) by trained study staff. Following validation and cleaning, data were exported into Excel v.16.9 (Microsoft, Inc., Redmond, WA, USA), and then imported into Stata v.14 (StataCorp, TX, USA) for analysis.
The outcome of interest, declining HCT, was measured as a binary variable ('no' = 0 and 'yes' = 1). The independent variables measured were age (18-30, 31-50, 51-70, 70+), sex (male, female), presenting complaint (trauma, medical), South African triage score (death, routine visit, urgent, very urgent, emergent), access to primary care (yes, no), past medical history (hypertension, coronary artery disease, tuberculosis, diabetes, asthma, chronic obstructive pulmonary disorder, cancer), visit time (within regular operating hours, 9 am to 5 pm, or out of regular operating hours), visit reason (new complaint, return visit, referral), mode of transport (self-transport, ambulance, police), presenting symptoms (pain, fever) and disposition (death, intensive care unit admission, general admission, emergent surgery, transfer, discharge, absconded).
Data analysis and statistics
Analysis was conducted on patients unaware of their status, to examine the relationship between the outcome of interest and all other independent variables. Chi-square tests were used to explore individual variable associations with declining HCT. Logistic regression analysis was conducted to assess the contribution of each variable to declining HCT. Bivariate analysis was conducted to estimate the association between the outcome and each predictor variable, as well as multivariate analysis to estimate the independent effect of each predictor variable, adjusting for all others. All variables were included in the final model, following checks for collinearity and goodness of fit and performing a best-subsets variable selection. Sub-group analysis was completed on the top reasons for accepting and declining HCT by gender.
A reference level was selected for categorical variables with multiple responses, and other levels were accordingly compared. Associations were assessed using odds ratios (ORs), 95% confidence intervals (CIs) and p-values. A p-value of ≤ 0.05 was regarded as statistically significant. A pooled analysis of data collected from both sites is presented; no significant differences were observed between the two sites (Table 1).
Ethical consideration
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board (reference number IRB00105801), the Human Research Ethics Committee from the University of Cape Town (MREC reference number 856/2015), the Human Research Committee of Walter Sisulu University (reference number 069/2015) and the Eastern Cape Department of Health. Written consent was obtained from all participants who enrolled in the study and was required for the collection of demographic data, HCT and a follow-up call for newly diagnosed patients, separately.
Results
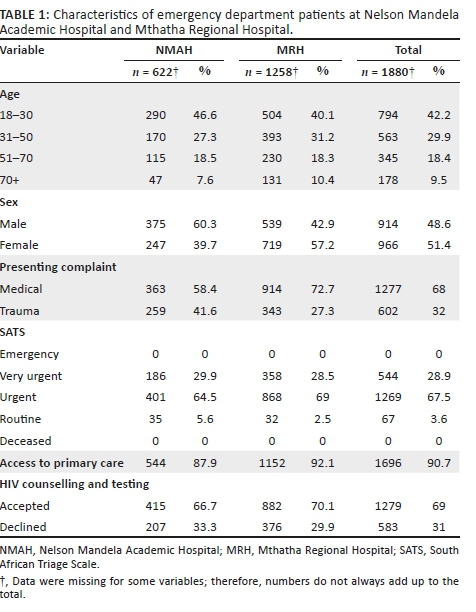
A total of 1010 patients presented to the NMAH ED between 27 June and 13 August 2017. Of these, 727 (72%) patients were approached by HCT staff, and 622 (61.6%) were enrolled in the study. A total of 3245 patients presented to the MRH ED between 24 July and 03 September 2017; of these, 1347 (41.5%) patients were approached by HCT staff, and 1258 (38.8%) were enrolled in the study (Table 1).
Across both sites, 2074 patients were approached by the HCT staff, and 1880 (90.6%) were enrolled in the study. Patients enrolled were slightly female predominant (966, 51.4%), with a median age of 33 years (interquartile range [IQR]:24-59). Most patients presented with medical complaints (1278, 67.9%), received a triage designation of 'urgent' (1269, 67.5%) and reported having access to primary care services (1696, 90.2%; Table 1).
Of the 1880 patients enrolled, 465 (24.7%) patients were aware of their HIV status (defined as a known HIV-positive diagnosis [371, 19.7%] or tested HIV negative within the last 12 months [94, 5%]), and 1415 (75.3%) patients were unaware of their HIV status. Of patients with a known HIV-positive diagnosis, 351 (94.9%) said they were regularly accessing an ARV clinic. Of patients who were regularly accessing an ARV clinic, 23 (6.5%) reported being virally suppressed, 46 (13.1%) reported not being virally suppressed and 282 (80.3%) were unsure (Figure 1). In addition, 20 (5.4%) patients who had a known HIV-positive diagnosis wanted to get retested to confirm if they were truly/still HIV positive.
Of the 1415 patients unaware of their status, 141 (10%) declined HCT, and 1274 (90%) accepted. Of the patients who accepted HCT, 159 (12.5%) were diagnosed as HIV positive, 1102 (86.5%) were diagnosed as HIV negative and 13 (1%) had an indeterminate result. The overall prevalence of HIV in the study population was 28.1%. Patients declining and those accepting HCT both largely presented with medical complaints (912, 64.5%), received a triage designation of 'urgent' (954, 67.4%), had stated access to primary care services (1255, 89.2%), had no past medical history (929, 65.7%), visited the ED outside of regular hours (799, 56.5%), had a new complaint (878, 62.4%), used self-transport (881, 62.7%), had symptoms of pain (790, 55.8%), had no symptoms of fever (1388, 98.1%), were ultimately discharged from the ED (718, 53.9%) and were aged 18-30 years (629, 44.5%; Table 2).
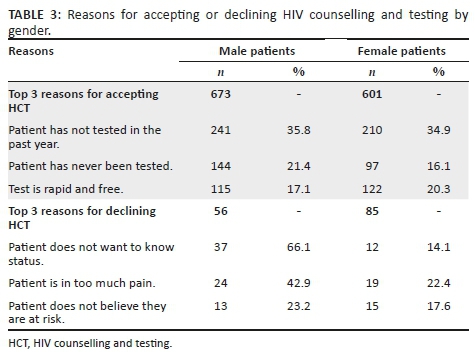
The top reasons for accepting HCT were 'has not tested in the past year' (451, 35.4%), 'has never been tested' (242, 18.9%) and 'test is rapid and free' (237, 18.6%) (Figure 1). Patients accepting testing were largely male (672, 52.7%). The top reasons for declining HCT were 'does not want to know status' (53, 37.6%), 'in too much pain' (48, 34%) and 'does not believe they are at risk' (28, 19.9%; Figure 1). Patients declining testing were largely female (85, 60.3%).
Sub-group analysis of the reasons for accepting and declining HCT by gender showed slight differences between men and women in the reported reasons (Table 3). The primary reason for accepting HCT for both men and women was 'has not tested in the past year', 35.8% and 34.9%, respectively, followed by 'has never been tested' (21.4%) for men and 'test is rapid and free' (20.3%) for women. The primary reason for declining HCT given by men was 'does not want to know status' (66.1%) and 'in too much pain' for women (22.4%), followed by 'in too much pain' for men (42.9%) and 'does not believe they are at risk' (17.6%) for women.
Univariate analysis showed that compared with male patients, female patients were more likely to decline HCT Associations were assessed using odds ratio (OR: 1.7; 95%) Confidence Intervals (CI:1.2-2.4). Patients who complained of pain compared with patients who did not (OR: 1.7; 95% CI: 1.2-2.5) and those arriving at the ED by ambulance compared to self-transport or with the police (OR: 1.4; 95% CI: 1.1-2.1) were also more likely to decline HCT. In addition, patients presenting with traumatic injuries compared with medical complaints (OR: 1.6; 95% CI: 1.1-2.2) were more likely to decline HCT. Other factors including age, triage score, access to primary care, past medical history, visit time, visit reason and final disposition did not show a statistically significant correlation with declining HCT (Table 2).
Multivariate analysis showed that patients who complained of pain compared with patients who did not (OR: 1.6; 95% CI: 1.1-2.6) were slightly more likely to decline HCT. Other variables did not show a statistically significant correlation with declining HCT (Table 2).
Discussion
We found acceptance of HCT services in the ED to be reassuringly high. Our study revealed that 90% of patients who were unaware of their HIV status accepted HIV testing. This was observed despite patients being in a clinical environment where HIV testing is not routinely offered and where patients present with acute injury or illness and are often in pain or moderate distress.11,18 These findings are consistent with results from other LMICs. In Kenya and Guyana, it was found that 97.7% and 75.5% of non-critical patients accepted HCT in the ED, respectively.19,20 Our results also highlight a substantial burden of undiagnosed HIV: 8.5% of enrolled patients were newly diagnosed as HIV positive. These patients presented to the ED for various clinical complaints, and a positive diagnosis of HIV was an incidental finding.
The two most common reasons for accepting HCT in the ED were 'has not tested in the past year', followed by 'has never been tested'. This positively implies the need for HCT in acute care settings, to cover the existing testing gap, as we are able to capture patients who are currently missed by other testing venues within the healthcare system. Recently, novel approaches for HCT - such as home-based, community-based and couples testing - have been added to traditional facility-based HCT delivery systems, with good acceptance rates.21,22 A systematic review of HCT strategies in sub-Saharan Africa reported an acceptance rate of 70% for home-based testing and 76% for community-based testing.21 However, despite the variety of testing strategies and venues, a testing gap remains, particularly amongst young adults and men.23,24 Considering that 44.1% of all patients accepting testing were young adults (18-30 years) and 52.7% were male, our study demonstrates that the ED is an opportune venue to capture this missed population.
Another factor leading to testing acceptance, reported by a fifth of patients accepting HCT, was 'the test is rapid and free'. This measure combines both cost and ease/limited time lost to testing. While it is hard to separate the two and determine which is a more significant factor, ensuring that both are addressed is a likely key to maintaining high acceptance of HCT in a fast-moving environment such as the ED. This is supported by a study in Uganda, where 25% of ED patients reported not knowing their HIV status because of the lack of access to free testing services.16 At present, HCT services are offered free of cost in all government healthcare facilities in SA; however, maintaining free services can be burdensome for the government, especially if testing services are to be further expanded. Furthermore, ensuring that testing and counselling are not time-consuming and are part of routine clinical care in the ED might address the barrier of having to seek out testing as a discrete task in itself.
Acceptance, however, was not universal. In our study population, women were significantly more likely to decline to test, whereas men were more likely to accept. Similar findings were observed in other studies examining the acceptability of testing in EDs, in both high- and low-resource settings.20,25 This might be because women are aware of having access to testing services during antenatal visits, through preventing mother-to-child transmission (PMTCT) programmes or through family planning services, and hence do not need to test in the ED.1 This is supported by the finding that more women were already aware of their HIV status; 71.6% of the patients with a known HIV-positive diagnosis were women. In addition, a significant proportion of women presenting to the ED in our context were diagnosed with pregnancy complications or injury wounds, likely justifying 'in too much pain' as the primary reason reported for women to decline HCT. On the contrary, though women were more likely to decline HCT, a majority still accepted testing when offered. While it is difficult to generalise individual motivations, factors including lack of social support and fear of stigma or rejection if tested HIV positive, especially in the presence of their partner or family accompanying them to the ED, may otherwise underlie the greater tendency of women to decline HCT.25
The top reasons reported for declining HCT in our ED, 'does not want to know status' and 'does not believe they are at risk', are interestingly established findings from high-income countries and LMICs, across healthcare settings.16,20,26,27,28,29 It could be that patients prefer uncertainty rather than facing the psychosocial consequences of an HIV-positive diagnosis, especially considering the imaginable stigma attached to such a diagnosis.30 This could be tackled through targeted pre- and post-counselling efforts. On the contrary, it is also possible that the small proportion of patients who declined to be tested are not at risk of contracting HIV and were accurately perceiving their risk. We did not include any risk measures in our survey and are thus unable to precisely indicate individuals who should have been tested.
A significant barrier to HCT in the ED and other healthcare facilities frequently described in the literature are stigma and the lack of confidentiality.20,26,31 This finding is supported by contextual knowledge, wherein anthropological studies exploring cultural perceptions and practices around HIV in Mthatha have reported pervasive stigma attached to HIV/AIDS, resulting in multiple forms of exclusion based on sexism, racism and homophobia,30 The National HIV Prevalence Survey indicated that 26% of people would not be willing to share a meal, 18% were unwilling to sleep in the same room and 6% would not speak to PLWH.2 Yet, none of the patients declining testing in our study reported reasons implying real or perceived stigma or the lack of confidentiality. The studies supporting this notion conducted in-depth interviews or had one-on-one conversations with patients, which likely allowed for deeper exploration of patient perspectives on HCT services, whereas given the patient volumes, high turnover and the lack of any coordinated processes in our study setting, it is possible that patients were less likely to report stigma as a reason for declining testing.
Pain was a notable justification for declining testing in this context and showed significant correlation through bivariate and multivariate analysis. The second most common reported reason, 'in too much pain', was not surprising, as a high proportion of cases presented with acute traumatic injuries. In addition, traumatic injuries and arriving at the ED in an ambulance, which are critical proxies to pain and the seriousness of a patient's condition, were positively correlated with declining testing. This finding is specific to declining HCT in the ED and has not been previously reported as a barrier in other testing venues. To address this barrier, the integration of pain management before HCT is recommended. If a patient's presenting complaint has been addressed by providers, and appropriate action taken, patients might be more likely to accept testing.
Linkage to care is the next critical step following testing. As part of our study, all newly diagnosed patients were counselled extensively on the importance of seeking follow-up care and were given a referral letter. Given that both NMAH and MRH see patients from a 100-km radius, it was challenging to ensure linkage to care, as it would depend on the area individuals came from and the presence and ease of access to an ART clinic. For patients who were local to Mthatha, we were able to direct them to the Gateway Clinic - an ART centre - located within the same campus as the hospital. With the consent of all patients who were newly diagnosed as HIV positive, we collected their names and contact details to conduct follow-up calls after 1 month, 6 months and a year. The follow-up calls will allow us to assess whether individuals have been able to link to care and/or what challenges they are facing in doing so. Results from the follow-up calls will be collated and analysed post-completion. Despite these challenges, 94.9% of patients with a known HIV-positive diagnosis presenting to the EDs reported having access to an ART clinic, and 85.4% of those individuals reported regularly accessing the clinic. These rates are commendable and imply a willingness of patients in this setting to seek follow-up care. However, these are self-reported statistics and could be inflated as a result of social-desirability bias.
Another interesting finding was that a small proportion of patients with a known HIV-positive diagnosis (20, 5.4%) requested a repeat test to confirm their diagnosis. Patients stated that they wanted to confirm whether they were truly HIV positive and/or if they were still HIV positive. Upon retesting, all 20 patients were HIV positive. The desire to retest when an opportunity presented could likely be a result of mistrust in the healthcare system or a result of the low health literacy in the region, which are both potential barriers to achieving high rates of testing and sustained linkage to care.30,31
There are several study limitations to consider. The protocol was to approach every patient presenting in the ED who met the inclusion criteria. However, the lack of infrastructure, systematic medical record-keeping or a patient tracking process made it challenging to retain all patients who presented to the ED. Many patients were missing from the records, whereas others were entered multiple times, making it difficult to keep count of the total number of patients. HIV counselling and testing services were provided 24 h a day, yet we were only able to approach 48% of patients who presented for care. We believe this is, in part, a result of the high volumes of patients and the quick turnaround time, as well as the time-consuming nature of counselling. Patients enrolled in the study may be a biased subset of the ED population, namely, easier to approach, spoke the same language as the HCT counsellors, had milder injuries or conditions and presented at times when the patient volume was lower. Maintaining confidentiality was challenging given the limited space - the EDs in both hospitals were in essence one big room, with beds lined up against each other. Lastly, the study was human-resource intensive. We had a team of four dedicated HCT staff at all times. Nevertheless, greater staff numbers would have allowed the capture of more study subjects. Such a situation would be difficult to sustain in a low-resource setting such as Mthatha.
To optimise our strategy and accurately capture data, given the lack of organisation and clear processes, our data were collected prospectively, whereby we relied less on recorded data and were able to capture most of it in real time. As the ED is busy and sees high patient volumes, we attempted to collect as much data as efficiently as possible, using a survey format with mostly 'yes' and 'no' questions. However, to have had a better understanding of patient perspectives, the study might have been enhanced by in-depth telephone interviews with a smaller number of patients after they had left the ED.
Conclusion
Our study demonstrated high patient acceptance of the nationally recommended HCT strategy in an ED setting. The overall adult prevalence of HIV in the ED was high at 28.1%. Patients who were male, young and not in pain or critically injured were more likely to accept HCT, critically supporting the provision of HCT in acute care settings, as it successfully captured an important demographic that has generally been missed through other testing venues. In addition, the lack of significant correlation in demographic or clinical characteristics and HCT uptake argues for a routine, non-targeted strategy in the ED. Our study further reveals the need for continued investment to ensure that HCT is widely available, with provision to effectively identify and manage pain and trauma. Finally, critical to embedding HCT in the routine clinical care offered in the ED will be the confidential conduct of HCT that permits stigma around HIV infection and testing to be appropriately addressed - something that will require further innovation and implementation research.
Acknowledgements
The authors acknowledge the staff in the Nelson Mandela Academic Hospital and Mthatha Regional Hospital emergency departments for making this research possible and the HIV Counselling and Testing team for their dedication and hard work during the study. The authors also acknowledge the contributions of Nomzamo Mvandaba for her assistance as a study coordinator for the WISE study and those of Victoria Chen and Kathryn Clark in data collection and data validation.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
B.H. conceived the original idea for the parent study and designed the protocol. A.R., P.M. and B.H. coordinated the study and data collection. A.R. carried out data analysis and prepared the manuscript. C.K., T.C.Q., D.S. and B.H. provided substantial edits and revisions.
Funding information
This research was supported by the South African Medical Research Council, the Division of Intramural Research, the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and the Johns Hopkins Center for Global Health.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, A.R. The data are not publicly available because they contain sensitive information that could compromise the privacy of research participants.
Disclaimer
All views expressed in the submitted article are the authors' own and not an official position of the institutions represented or the funders.
References
1.UNAIDS. UNAIDS data 2019. UNAIDS, Geneva, Switzerland; 2019. [ Links ]
2.Simbayi LC, Zuma K, Zungu N, et al. South African National HIV prevalence, incidence and behaviour, and communication survey, 2017. Cape Town: Human Sciences Research Council Press; 2019. [ Links ]
3.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [ Links ]
4.UNAIDS. Ending AIDS: Progress towards the 90-90-90 targets. Geneva: UNAIDS; 2017. [ Links ]
5.Johnson LF, Chiu C, Myer L, et al. Prospects for HIV control in South Africa: A model-based analysis. Glob Health Action. 2016;9(1):30314. https://doi.org/10.3402/gha.v9.30314 [ Links ]
6.DoHRoS A. National HIV counselling and testing (HCT) policy guidelines 2015. Pretoria: National Department of Health; 2015. [ Links ]
7.SANAC. Let our actions count: South Africa's National strategic plan for HIV, TB and STIs 2017-2022. Pretoria: SANAC; 2017. [ Links ]
8.Telisinghe L, Charalambous S, Topp SM, et al. HIV and tuberculosis in prisons in sub-Saharan Africa. Lancet. 2016;388(10050):1215-1227. https://doi.org/10.1016/S0140-6736(16)30578-5 [ Links ]
9.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. Lancet. 2010;376(9738):355-366. https://doi.org/10.1016/S0140-6736(10)60832-X [ Links ]
10.DoHRoS A. Mid-year population estimates. Pretoria: National Department of Health; 2015. [ Links ]
11.Rothman RE, Ketlogetswe KS, Dolan T, Wyer PC, Kelen GD. Preventive care in the emergency department: Should emergency departments conduct routine HIV screening? A systematic review. Acad Emerg Med. 2003;10(3):278-285. https://doi.org/10.1111/j.1553-2712.2003.tb02004.x [ Links ]
12.Hsieh YH, Kelen GD, Beck KJ, et al. Evaluation of hidden HIV infections in an urban ED with a rapid HIV screening program. Am J Emerg Med. 2016;34(2):180-184. https://doi.org/10.1016/j.ajem.2015.10.002 [ Links ]
13.Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: Experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33(2):147-155. https://doi.org/10.1016/s0196-0644(99)70387-2 [ Links ]
14.Haukoos JS, Rowan SE. Screening for HIV infection. BMJ. 2016;532(1):i1. https://doi.org/10.1136/bmj.i1 [ Links ]
15.Curry C, Bunungam P, Annerud C, Babona D. HIV antibody seroprevalence in the emergency department at Port Moresby General Hospital, Papua New Guinea. Emerg Med Australas. 2005;17(4):359-362. https://doi.org/10.1111/j.1742-6723.2005.00757.x [ Links ]
16.Nakanjako D, Kamya M, Daniel K, et al. Acceptance of routine testing for HIV among adult patients at the medical emergency unit at a national referral hospital in Kampala, Uganda. AIDS Behav. 2007;11(5):753-758. https://doi.org/10.1007/s10461-006-9180-9 [ Links ]
17.Twomey M, Wallis LA, Thompson ML, Myers JE. The South African Triage Scale (adult version) provides reliable acuity ratings. Int Emerg Nurs. 2012;20(3):142-150. https://doi.org/10.1016/j.ienj.2011.08.002 [ Links ]
18.Hansoti B, Kelen GD, Quinn TC, et al. A systematic review of emergency department based HIV testing and linkage to care initiatives in low resource settings. PLoS One. 2017;12(11). e0187443. https://doi.org/10.1371/journal.pone.0187443 [ Links ]
19.Waxman MJ, Muganda P, Carter EJ, Ongaro N. The role of emergency department HIV care in resource-poor settings: Lessons learned in western Kenya. Int J Emerg Med. 2008;1(4):317-320. https://doi.org/10.1007/s12245-008-0065-8 [ Links ]
20.Christensen A, Russ S, Rambaran N, Wright SW. Patient perspectives on opt-out HIV screening in a Guyanese emergency department. Int Health. 2012;4(3):185-191. https://doi.org/10.1016/j.inhe.2012.03.001 [ Links ]
21.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77-S85. https://doi.org/10.1038/nature16044 [ Links ]
22.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Group ZS. Effects of home-based voluntary counselling and testing on HIV-related stigma: Findings from a cluster-randomized trial in Zambia. Soc Sci Med. 2013;81(1):18-25. https://doi.org/10.1016/j.socscimed.2013.01.011 [ Links ]
23.Nglazi MD, Van Schaik N, Kranzer K, Lawn SD, Wood R, Bekker LG. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2012;59(3):e28-e34. https://doi.org/10.1097/QAI.0b013e31824445f0 [ Links ]
24.Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: Successes and challenges. AIDS. 2015;29(11):1401-1409. https://doi.org/10.1097/QAD.0000000000000721 [ Links ]
25.Pisculli ML, Reichmann WM, Losina E, et al. Factors associated with refusal of rapid HIV testing in an emergency department. AIDS Behav. 2011;15(4):734-742. https://doi.org/10.1007/s10461-010-9837-2 [ Links ]
26.Chimoyi L, Tshuma N, Muloongo K, Setswe G, Sarfo B, Nyasulu PS. HIV-related knowledge, perceptions, attitudes, and utilisation of HIV counselling and testing: A venue-based intercept commuter population survey in the inner city of Johannesburg, South Africa. Glob Health Action. 2015;8(1):26950. https://doi.org/10.3402/gha.v8.26950 [ Links ]
27.Schechter-Perkins EM, Koppelman E, Mitchell PM, Morgan JR, Kutzen R, Drainoni ML. Characteristics of patients who accept and decline ED rapid HIV testing. Am J Emerg Med. 2014;32(9):1109-1112. https://doi.org/10.1016/j.ajem.2014.05.034 [ Links ]
28.Christopoulos KA, Weiser SD, Koester KA, et al. Understanding patient acceptance and refusal of HIV testing in the emergency department. BMC Public Health. 2012;12(1):3. https://doi.org/10.1186/1471-2458-12-3 [ Links ]
29.Gazimbi MM. A multilevel analysis of the determinants of HIV testing in Zimbabwe: Evidence from the demographic and health surveys. HIV/AIDS Res Treat Open J. 2017;4(1):17. http://doi.org/10.17140/HARTOJ-4-124 [ Links ]
30.Leclerc-Madlala S, Simbayi LC, Cloete A. The sociocultural aspects of HIV/AIDS in South Africa. In: HIV/AIDS in South Africa 25 years on: Psychosocial perspectives. 2009; p. 13-25. [ Links ]
31.Nombembe C. Music-making of the Xhosa diasporic community: A focus on the Umguyo tradition in Zimbabwe. Cape Town: University of Witwatersrand, Faculty of Humanities, Wits School of Music in Cape Town; 2013. [ Links ]
 Correspondence:
Correspondence:
Aditi Rao
aditi.rao@jhmi.edu
Received: 15 May 2020
Accepted: 02 June 2020
Published: 22 July 2020
ORIGINAL RESEARCH
Retention in care for adolescents who were newly initiated on antiretroviral therapy in the Cape Metropole in South Africa
Brian van Wyk; Ebrahim Kriel; Ferdinand Mukumbang
School of Public Health, Faculty of Community and Health Sciences, University of the Western Cape, Cape Town, South Africa
ABSTRACT
BACKGROUND: Long-term retention of adolescents aged 10 -19 years on antiretroviral therapy (ART) is crucial to achieve viral load suppression. However, it is reported globally that adolescents have lower retention in care (RiC) on ART, compared with children and adults.
OBJECTIVES: To determine the prevalence and predictors of RiC of adolescents over 2 years following initiation onto ART in public health facilities in the Metropole District Health Services of the Western Cape province in 2013.
METHODS: Data of 220 adolescent patients who were newly initiated on ART in 2013 were extracted from the provincial electronic database, and subjected to univariate and bivariate analyses using SPSS.
RESULTS: The rate of RiC post-initiation was low throughout the study period, that is, 68.6%, 50.5% and 36.4% at 4, 12 and 24 months, respectively. The corresponding post-initiation viral load suppression levels on ART of those remaining in care and who had viral loads monitored were 84.1%, 77.4% and 68.8% at 4, 12 and 24 months, respectively. Retention in care after initiation on ART was higher amongst younger adolescents (10-14 years), compared with older adolescents (15-19 years). Male adolescents were significantly more likely to be retained, compared with females. Pregnant adolescents were significantly less likely to be retained compared with those who were not pregnant.
CONCLUSION: Key interventions are needed to motivate adolescents to remain in care, and to adhere to their treatment regimen to achieve the target of 90% viral load suppression, with specific emphasis on older and pregnant adolescents.
Keywords: HIV; AIDS; adolescents; youth; retention in care.
Introduction
Globally, it has been estimated that 190 000 (59 000-380 000) adolescents, between the ages of 10 and 19 years, were newly infected with human immunodeficiency virus (HIV) in 2018, and the total number of adolescents living with HIV (ALWH) was 1.6 million (1.1-2.3 million), which accounts for 4% of all people living with HIV (PLWH).1 Sub-Saharan Africa has the highest number of HIV-infected adolescents with about 1.5 million of them. In South Africa (SA), it is estimated that 280 000 children aged between 0 and 14 years were living with HIV in 2017, and that just under 7 million persons aged ≥ 15 years were PLWH.2 The national HIV household survey estimated HIV prevalence in 0-14-year-olds to be 3.0% and 2.4% for females and males, respectively.3 In 15-19-year-olds, it was 5.8% in females and 4.7% in males. The number of adolescents aged 15-19 years receiving antiretroviral therapy (ART) in SA increased tenfold between 2005-2008 and 2013-2016.4 This increase is attributed to perinatally infected infants surviving into adolescence and to a rising incidence of HIV in behaviourally infected 15-19-year-olds.
Despite success in ART roll-out in most countries over the last decade, acquired immune deficiency syndrome (AIDS)-related deaths amongst adolescents have increased whilst declining in other age groups.5 To prevent AIDS-related deaths, the infected must be diagnosed, receive ART and remain in care to maintain viral load (VL) suppression. This would help achieve the 90-90-90 targets of the Joint United Nations Programme on HIV and AIDS (UNAIDS).6 Retention on ART is particularly challenging for key populations, such as adolescents, amongst others, and has been noted as a global priority for action.7,8 Previous studies confirm that adherence, retention in care (RiC) and treatment outcomes for adolescents in southern Africa are worse, compared with adults.9,10,11
However, routine monitoring of HIV treatment programmes does not report RiC and treatment outcomes for adolescents (10-19 years); they report only for children (0-14 years) and adults (15 years and older).11 In this way, adolescent problems with RiC and treatment outcomes remain undetected in the monitoring of HIV programmes. It is argued that because of the general adherence problems faced by adolescents globally, specific analysis is needed to report outcomes for this age group at a health-systems level.8
Objectives
This article reports on the RiC of ALWH aged 10-19 years who were newly initiated on ART in public health facilities in the Western Cape Metropole, SA, in 2013, and the risk factors associated with remaining in care at 4, 12 and 24 months post-initiation on ART.
Methods
Design
We conducted a retrospective cohort analysis of adolescents aged 10-19 years, who were initiated on ART in 2013 in the Western Cape Metropole.
Study context
The Western Cape's ART programme has been in existence for over 10 years. The 2013 version of the ART guidelines was updated in 2016, and had consolidated adult, adolescent, paediatric and prevention of mother-to-child transmission of HIV guidelines.12 In the province of the Western Cape, most adults and children access ART services at primary healthcare facilities, such as clinics, community day centres and community health centres. At the end of June 2017, this province had 237 285 patients on ART, of whom 229 171 were aged ≥ 15 years and 8114 were aged < 15 years. The Cape Town Metropole accounts for 74.3% (167 833) of the total number of ART patients in the Western Cape, that is 162 092 aged ≥ 15 years and 5741 aged < 15 years.13 Antiretroviral therapy services are rendered at the various Western Cape facilities, as outlined in Table 1.13
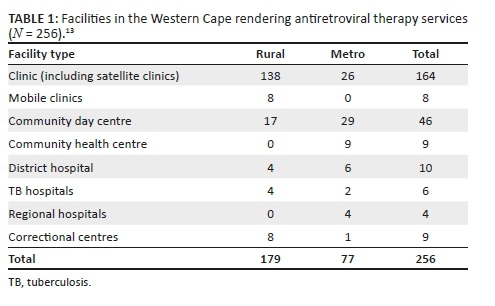
However, services rendered at these facilities vary. Some provide only adult ART services. Others offer both adult and paediatric support. Some facilities offer ART daily, whilst others provide ART only on certain days of the week. Human resource capacity also varies. Not all facilities have resident medical officers or clinical nurse practitioners. Hence, the need for outreach support arises. The capacity to care for adults in the Western Cape has improved with the implementation of the nurse-initiated management of antiretroviral treatment (NIMART) programme, whereby nurses are trained and receive structured mentorship and accreditation to initiate first-line ART. A challenge in the rural areas is the irregular access to competent and skilled clinicians to manage paediatric patients and complicated adult and adolescent patients. As previously stated, most ART services are designated as paediatric or adult. Adolescent-specific ART services are not yet part of standard care being offered at all ART facilities. Those that do are limited to services initiated and/or supported by tertiary hospitals or non-profit organisations.
Data source
Two data sources were used for this analysis: the Three Interlinked Electronic Registers.Net (TIER.Net),14 an electronic ART database developed by the University of Cape Town's Centre for Infectious Disease Epidemiology and Research, and patients' folders accessed at the treating facilities. Tier.Net is used operationally in the public health facilities of SA to monitor baseline clinical care and client outcomes over time. It is also the platform on which HIV tests are electronically captured in the public sector.15
We first visited the Tier.net platform to obtain data on all those who met the inclusion criteria. With the use of our data-capturing form, we searched for the relevant information from the Tier.net platform. Where information was missing, we accessed the patient's folder to confirm the availability of the required information.
Study participants
Data were found for 332 ALWH newly registered on ART from 29 facilities across the Western Cape Metropole and extracted from the provincial Tier.net register. Only 220 participants were included in the final analysis. Of the 112 excluded, for 68, folders could not be found in spite of making numerous attempts to trace these documents at the various facilities. Furthermore, 28 patients were incorrectly captured as new patients when they had been transferred in from other facilities; 4 patients were not adolescents, and their birth dates had been incorrectly captured; and 12 patients had been incorrectly captured as having initiated ART in 2013.
Main outcome measures and analysis
We extracted data on sociodemographic characteristics (age, sex, source of income, type of dwelling, disclosure to significant other and reported alcohol or other drug use) and clinical characteristics (CD4 count, WHO stage, pregnancy and ART regimen).
Bivariate analysis was conducted to determine the significance and strength of association between RiC at 4, 12 and 24 months and various sociodemographic and clinical characteristics. Statistical significance was tested by using the chi-square test, with significance set at p < 0.05, and where significant, the strength of association was calculated as risk ratios (RRs) with 95% confidence interval (CI), using SPSS v23. Our use of 4, 12 and 24 months rather than 6, 12 and 24 months is informed by the operational guidelines of the Western Cape's ART programme, which requires the first VL test to be conducted at 4 months and the patient to return for results in month 5.16 Patients would receive 1 month's medication if they have unsuppressed VL, and 2 months' supply if their VL is suppressed. The reason for the 4-month RiC measurement is to identify how many ALWH return for their VL tests. The subsequent RiC behaviour of the patients is measured annually.
Survival analysis was assessed with lost to follow-up (LTFU) as the outcome of interest. We did a comparative survival analysis for the age and sex of the study participants. We reported the hazard ratios and p-values. An ALWH was considered LTFU if they had not made contact with a treating healthcare facility within 90 days since their last registered contact for HIV-related treatment and care. The LTFU date was determined from the day when the patient was last seen at the clinic where they were provided with their last medication. Therefore, by using the intention-to-treat population in this study, the RiC definition was the proportion of HIV-infected adolescents alive and on ART at months 4, 12 and 24 in the entire study sample.
Ethical consideration
The protocol was approved by the University of the Western Cape Biomedical Research Ethics Committee (Reference number: BM/17/1/15) and the Government Health Impact Assessment (Reference number: WC_2017RP58_418) Committee.
Results
Of the 220 adolescents who were newly initiated on ART in 2013, the majority were 'older' adolescents, 15-19 years (n = 179, 81.4%) and female (n = 182, 82.7%) (Table 2). Most were financially supported by their families and friends (n = 129, 58.6%) and lived in a formal house (n = 116, 52.7%). As per HIV clinical treatment guidelines, the overwhelming majority (n = 182, 87%) had disclosed their HIV status to a significant other.
The median CD4 count at ART initiation was 292.5 cells/mm3 (interquartile range [IQR]: 228.8-391.3). Only two participants had no baseline CD4 count recorded. Half of the participants (n = 109) were initiated with a CD4 count between 200 cells/mm3 and 349 cells/mm3, and 19% (n = 42) had a baseline CD4 count of < 200 cells/mm3 as per the HIV treatment guidelines of 2013.
Of the 213 participants who had WHO staging done at ART initiation, 46.5% and 22.5% were WHO stages I and II, respectively, and 23.2% and 6.8% were WHO stages III and IV, respectively. As with the CD4 counts, clinical staging is taken into consideration in the universal test and treat era.
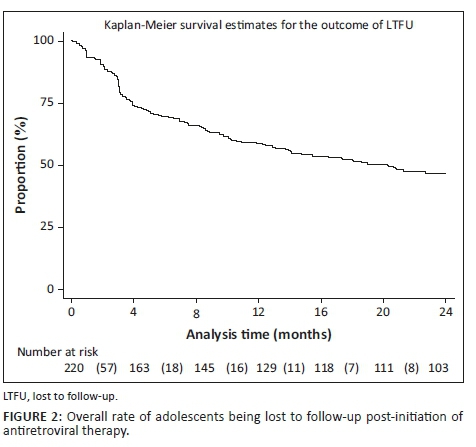
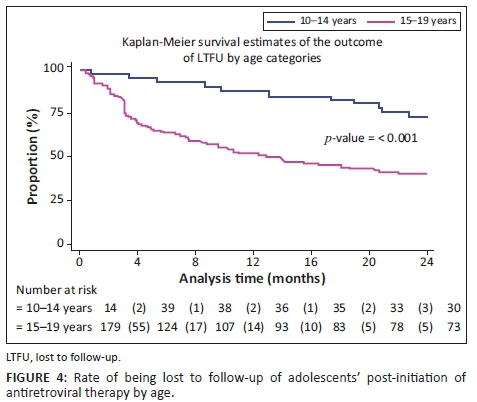
Observed RiC was low throughout the study period with 68.6%, 50.5% and 36.4% adolescents being retained in care at 4, 12 and 24 months post-initiation on ART, respectively. Figure 1 illustrates the comparison of RiC at 4, 12 and 24 months between younger (10-14 years of age) and older (15-19 years of age) adolescents (90.2% vs. 63.7%, 82.9% vs. 43.0% and 68.3% vs. 29.1%, respectively). However, RiC of the younger adolescents at month 4 was just over 90%, but the younger adolescents at months 12 and 24 fell short of 90%. The older adolescents showed poorer rates of RiC at months 4, 12 and 24, compared with the younger adolescents at the same time periods.
Table 3 shows that a significantly higher number of younger adolescents (10-14 years) were retained in care at 4, 12 and 24 months post-initiation on ART, compared with older adolescents (15-19 years). At 4 months post-initiation on ART, younger adolescents had 37% higher risk (likelihood) of RiC (RR = 1.37, 95% CI: 1.17-1.60) compared with older adolescents. At 12 months post-initiation on ART, younger adolescents had 85% higher risk of RiC (RR = 1.85, 95% CI: 1.48-2.31), compared with older adolescents, and more than two times higher risk (RR = 2.35, 95% CI: 1.73-3.20) at 24 months.
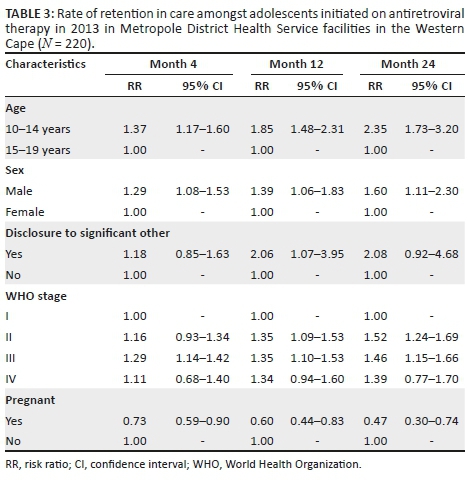
Male adolescents had higher rates of RiC post-initiation of ART at 4 months (RR = 1.29, 95% CI: 1.08-1.53), 12 months (RR = 1.39, 95% CI: 1.06-1.83) and 24 months (RR = 1.60, 95% CI: 1.11-2.30), compared with female adolescents.
Adolescents who were pregnant had significantly lower rates of RiC post-initiation of ART, compared with all other adolescents at 4 months (RR = 0.73, 95% CI: 0.59-0.90), 12 months (RR = 0.60, 95% CI: 0.44-0.83) and 24 months (RR = 0.47, 95% CI: 0.30-0.74).
Adolescents who disclosed their HIV status to a significant other were two times more likely to be retained in care at month 12 (RR = 2.06, 95% CI: 1.07-3.95) than adolescents who did not disclose to a significant other.
Adolescents classified as WHO stage I at ART initiation had significantly lower rates of RiC at 4 months post-initiation, compared with adolescents who were classified as WHO stage III (RR = 1.29, 95% CI: 1.14-1.42). Those classified as WHO stages II and IV also had better rates of RiC at month 4, compared with adolescents classified as WHO stage I at baseline, but these did not reach statistical significance. At 12 months post-initiation of ART, those who were at WHO stage II (RR = 1.35, 95% CI: 1.09-1.53) as well as WHO stage III (RR = 1.35, 95% CI: 1.10-1.53) at baseline had a 35% greater risk (likelihood) of RiC, compared with those who were WHO stage I at ART initiation. Adolescents who were at WHO stage I at baseline also showed significantly lower RiC rates at 24 months post-initiation of ART, compared with those who were at WHO stage II (RR = 1.52, 95% CI: 1.24-1.69) and WHO stage III (RR = 1.46, 95% CI: 1.15-1.66) at baseline. At 24 months post-initiation of ART, adolescents who were classified as WHO stage II (RR = 1.35, 95% CI: 1.09-1.53) and those who were WHO stage III (RR = 1.35, 95% CI: 1.10-1.53) at ART initiation had a 35% greater risk of RiC, compared with those who were WHO stage I at ART initiation. Those adolescents who were classified as WHO stage IV at ART initiation showed better RiC rates at months 4, 12 and 24 post-initiation, but none of these reached statistical significance.
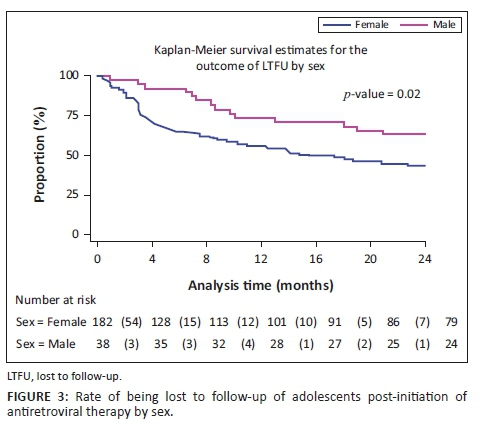
Figures 2, 3 and 4 show the survival curves of the study participants. The overall person-time at risk of being LTFU was 3303 months, with an incidence of 119/3807 (4/100) person-months. The hazard ratio for males compared with females was 0.71 (95% CI: 0.41-1.26). The hazard ratio for those aged 15-19 years compared with those aged 10-14 years was 2.53 (95% CI: 1.30-4.91).
Discussion
In 2014, the UNAIDS set a global ART target of 90-90-90. This includes the goal that 90% of persons who test HIV-positive should be initiated on ART.6 Having successfully tested and initiated ALWH onto ART, their RiC and the maintenance of VL suppression of at least 90% are the ongoing challenges. Our study reports that the overall RiC of ALWH was low throughout the 24-month observation period. Contrary to our findings, Nabukeera-Barungi et al. found that 90.4% of Ugandan adolescents demonstrated good RiC with an LTFU of only 5%.17 A meta-analysis of six South African studies also reported a relatively high ALWH retention rate of 83% (95% CI: 68% - 94%) in the first 2 years on ART.18 The results of our study are reported with the intention-to-treat population as the denominator at every time point, that is, months 4, 12 and 24, and without the exclusion of transfer-outs, LTFU patients and the numerator being those alive and on ART. The above-mentioned studies did not measure RiC in the same manner. Nabukeera-Barungi et al.17 determined RiC by dividing those still active in care by the total number that started after subtracting those who died and transferred out from both the numerator and denominator.
Younger adolescents (10-14 years) demonstrated better RiC rates, compared with the older group. This observation could be attributed to the disproportionate attention offered to younger adolescents. In spite of the unique challenges posed by adolescents and ART, there is a dearth of comprehensive health services for adolescents, including interventions to improve RiC in sub-Saharan Africa.19 Nevertheless, younger adolescents show better RiC rates because they depend, to a greater extent, on their caregivers to handle their treatment journey. In this way, the RiC of the young adolescent is an extension of the dedication and understanding of the caregiver. Another study designed to investigate the RiC rates between younger and older adolescents in Zimbabwe demonstrated no differences in attrition amongst younger versus older adolescents.20
We found that older adolescents (15-19 years) were significantly less likely to be retained in care over the first 24 months, compared with younger adolescents. This finding is congruent with the trends reported in other studies21,22 and corresponds to the transition of adolescents from paediatric to adult HIV programmes - a known high-risk period for disengagement with care.23,24,25 Several authors have argued that patient-level challenges, such as developmental delays, mental health issues, stigma and social support at home and school, must be adequately addressed for a successful transition to take place.26 A supported transition requires a skilful adult treatment team and the provision of facilitated care aimed at overcoming the disruptions of the patient-paediatric provider relationship. The loss of ancillary support is required to foster independence, the exercise of autonomy and the growth of personal responsibility.27,28
Although male adolescents constituted a smaller proportion of the study sample, on average, they had greater RiC throughout the observation period, compared with females. Just under half of the female adolescents (n = 84/182, 46%) were initiated on ART whilst pregnant. They exited care at an alarming rate, that is, 44%, 64% and 79% at 4, 12 and 24 months, respectively. These findings correspond to those of Nuwagaba-Biribonwoha et al. who found a greater rate of LTFU amongst pregnant and non-pregnant female adolescents, compared with male adolescents.29 The current study reports lower RiC rates, compared with the 76.4% RiC at 12 months noted in a recent systematic review of pregnant and post-partum women in Africa.30 This report found younger age and same-day ART initiation to be risk factors for poor retention, as was initiating during pregnancy, particularly late pregnancy.
Our findings indicate that adolescents who were classified as WHO stage IV at ART initiation showed better RiC rates at months 4, 12 and 24 post-initiation, although no statistical significance was achieved. Individuals at WHO stages III and IV are likely to remain in care because they are motivated by their health status and by the association between treatment and health outcomes. Clinicians tend to monitor individuals who are at WHO stages III and IV more closely because of other comorbidities such as tuberculosis and other opportunistic infections requiring clinical assessments. However, Matyanga et al. found that a low CD4 count and advanced HIV infection at initiation were associated with LTFU.20 We also found that adolescents classified as WHO stage I at ART initiation had significantly lower rates of RiC at 4 months post-initiation versus those with a WHO stage III. Contrary to the results in our study, another Ugandan study found that the risk of LTFU of adolescents at 12 months was significantly greater amongst those on WHO clinical stages III and IV, compared with those on WHO stages I and II.31 People living with HIV at WHO stage I hardly display signs and symptoms associated with AIDS. The literature has attributed this low RiC behaviour amongst adolescents at stage I to not feeling 'sick' or feeling 'well' as a proxy of nothing being wrong.
Although the primary focus of our study was not on pregnant, HIV-infected adolescents, many in this sub-group were captured in our sample. This could be explained by the fact that pregnant, HIV-infected adolescents are often horizontally infected and receive their positive HIV test result for the first time when booking for antenatal care. Although vertical transmission of HIV is common amongst younger ALWH, horizontal transmission is a frequent mode of transmission in older adolescents. Adolescent boys tend to not access HIV treatment because they mostly remain asymptomatic at this stage.
Interventions such as task shifting, community-based adherence support, mHealth platforms and group adherence counselling emerged as strategies in adult populations that could be adapted for adolescents.32,33 These interventions may benefit older adolescents, especially those transitioning to adult programmes that utilise them. However, the effectiveness of, for example, 'teen clubs', has had mixed results. MacKenzie et al.34 reported that Malawian ALWH who were not in a teen club were less likely to be retained than those in teen clubs. On the other hand, Munyayi and van Wyk35 found that group-based adherence interventions such as teen clubs did not improve retention rates for younger adolescents in specialised paediatric ART clinics in Namibia but did hold potential for improving rates in older adolescents. Adolescent-only clinics and monthly meetings have been shown to improve the RiC of adolescents.36 To this end, we support the calls of other authors for interventions, especially targeting older adolescents whose needs are increased during the transition period.23
Conclusion
Our study highlights low RiC for adolescents over the first 2 years after initiation on ART. Critical intervention is needed to motivate adolescents to remain in care, adhere to treatment and ultimately to achieve and maintain VL suppression (even when they are not feeling sick). Targeted interventions to address transition coordination - pre- and post-transition from paediatric to adult HIV programmes - are needed to counter older adolescents dropping out of care. Female adolescents who initiate ART whilst pregnant should receive special attention. This aligns with the increased need to provide and integrate appropriate sexual reproductive health services to ALWH. Behavioural interventions to improve adherence and RiC should ideally be embedded in community health services so that this forms part of an 'extended' HIV treatment package.
Acknowledgements
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
E.K. conducted the research under the supervision of B.v.W., and F.M. and B.v.W. drafted the first manuscript. E.K. and F.M. commented on all drafts. All authors approved the final version.
Funding information
This project was supported under a Medical Research Council of South Africa Self-Initiated Research grant.
Data availability statement
Data are available upon request to the author.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the South African Medical Research Council or University of the Western Cape.
References
1.UNICEF. Turning the tide against AIDS will require more concentrated focus on adolescents and young people [homepage on the Internet]. 2019 [cited 2019 Oct 21]. [ Links ] Available from: https://data.unicef.org/topic/hivaids/adolescents-young-people/
2.Joint United Nations Programme on HIV/AIDS (UNAIDS). Data [homepage on the Internet]. [ Links ] 2018 [cited 2019 Oct 21]. Available from: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf
3.South African National HIV prevalence, incidence, behaviour and communication survey [homepage on the Internet]. [ Links ] 2017 [cited 2019 Dec 03]. Available from: http://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_ Summary_ZA_ADS_cleared_PDFA4.pdf
4.Maskew M, Bor J, MacLeod W, Carmona S, Sherman GG, Fox MP. Adolescent HHIV treatment in South Africa's national HIV programme: A retrospective cohort study. Lancet HIV. 2019;6(11):e760-e768. https://doi.org/10.1016/S2352-3018(19)30234-6 [ Links ]
5.UNICEF. Children and AIDS [homepage on the Internet]. 2017 [cited 2018 Aug 18]. [ Links ] Available from: https://data.unicef.org/wp-content/uploads/2017/11/HIVAIDS-Statistical-Update-2017.pdf
6.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic [homepage on the Internet]. [ Links ] UNAIDS; 2014 [cited 2019 Oct 15]. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf
7.Mugglin C, Haas AD, Van Oosterhout JJ, et al. Long-term retention on antiretroviral therapy among infants, children, adolescents and adults in Malawi-term retention on antiretroviral therapy among infants, children, adolescents and adults in Malawi: A cohort study. PLoS One. 2019;14(11):e0224837. https://doi.org/10.1371/journal.pone.0224837 [ Links ]
8.Wong VJ, Murray KR, Phelps BR, Vermund SH, McCarraher DR. Adolescents, young people, and the 90-90-90 goals: A call to improve HIV testing and linkage to treatment. AIDS. 2019;31(Suppl. 3):S191-S194. https://doi.org/10.1097/QAD.0000000000001539 [ Links ]
9.Nglazi MD, Kranzer K, Holele P, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. 2012;12:21. https://doi.org/10.1186/1471-2334-12-21 [ Links ]
10.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L. Antiretroviral therapy adherence, virologic and immunological outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65-71. https://doi.org/10.1097/QAI.0b013e318199072e [ Links ]
11.Kariminia A, Law M, Davies M, et al. Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. JIAS. 2018;21:e25215. https://doi.org/10.1002/jia2.25215 [ Links ]
12.Western Cape Government Health. 2016. The Western Cape consolidated guidelines for HIV treatment: Prevention of mother-to-child transmission of HIV (PMTCT), children, adolescents and adults. Western Cape: Western Cape Government Health. [ Links ]
13.Western Cape Government Health: HAST. June 2017 ART data sign-off document. Western Cape: Western Cape Government Health; 2017. [ Links ]
14.Osler M, Hilderbrand K, Hennessey C, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. JIAS. 2014;17:18908. https://doi.org/10.7448/IAS.17.1.18908 [ Links ]
15.Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RPH. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: Analysis of routine data. PLoS One. 2020;15(1):e0227572. https://doi.org/10.1371/journal.pone.0227572 [ Links ]
16.Western Cape Government Health. Update to antiretroviral guidelines: Circular H116 of 2012 [homepage on the Internet]. [ Links ] 2012 [cited 2017 Oct 06]. Available from: http://www.doh.gov.za.
17.Nabukeera-Barungi N, Elyanu P, Asire B, et al. Adherence to antiretroviral therapy and retention in care for adolescents living with HIV from 10 districts in Uganda. BMC Infect Dis. 2015;15:520. https://doi.org/10.1186/s12879-015-1265-5 [ Links ]
18.Zanoni BC, Archary M, Buchan S, et al. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: The Cresting Wave. BMJ Global Health. 2016;1:e000004. https://doi.org/10.1136/bmjgh-2015-000004 [ Links ]
19.Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: A narrative review. J Int AIDS Soc. 2015;18(1):20049. https://doi.org/10.7448/IAS.18.1.20049 [ Links ]
20.Matyanga CMJ, Takarinda KC, Owiti P, et al. Outcomes of antiretroviral therapy among younger versus older adolescents and adults in an urban clinic, Zimbabwe. Public Health Action. 2016;6(2):97-104. https://doi.org/10.5588/pha.15.0077 [ Links ]
21.Kranzer K, Bradley J, Musaazi J, et al. Loss to follow-up among children and adolescents growing up with HIV infection: Age really matters. J Int AIDS Soc. 2017;20(1):21737. https://doi.org/10.7448/IAS.20.1.21737 [ Links ]
22.Okoboi S, Ssali L, Yansaneh A, et al. Factors associated with long-term antiretroviral therapy attrition among adolescents in rural Uganda: A retrospective study. J Int AIDS Soc. 2016;19(554):20841. https://doi.org/10.7448/IAS.19.5.20841 [ Links ]
23.Dahourou DL, Gautier-Lafaye C, Teasdale CA, et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: Challenges, youth-friendly models, and outcomes. J Int AIDS Soc. 2017;20(Suppl. 3):21528. https://doi.org/10.7448/IAS.20.4.21528 [ Links ]
24.Cervia JS. Easing the transition of HIV infected adolescents to adult care. AIDS Patient Care STDS. 2013;27(12):692-696. https://doi.org/10.1089/apc.2013.0253 [ Links ]
25.Pinzón-Iregui MC, Ibanez G, Beck-Sagué C, Halpern M, Mendoza RM. '… like because you are grownup, you do not need help': Experiences of transition from pediatric to adult care among youth with perinatal HIV infection, their caregivers, and health care providers in the Dominican Republic. J Int Assoc Provid AIDS Care. 2017;16(6):579-587. https://doi.org/10.1177/2325957417729749 [ Links ]
26.Vijayan T, Benin AL, Wagner K, et al. We never thought this would happen: Transitioning care of adolescents with perinatally acquired HIV infection from paediatrics to internal medicine. AIDS Care. 2009;21(10):1222-1229. https://doi.org/10.1080/09540120902730054 [ Links ]
27.Jones C, Ritchwood TD, Taggart T. Barriers and facilitators to the successful transition of adolescents living with HIV from pediatric to adult care in low and middle-income countries: A systematic review and policy analysis. AIDS Behav. 2019;23(9):2498-2513. https://doi.org/10.1007/s10461-019-02621-6 [ Links ]
28.Kung TH, Wallace ML, Snyder KL, et al. South African healthcare provider perspectives on transitioning adolescents into adult HIV care. SAMJ. 2016;106(8):804-808. https://doi.org/10.7196/SAMJ.2016.v106i8.10496 [ Links ]
29.Nuwagaba-Biribonwoha H, Kiragga AN, Yiannoutsos CT, et al. Adolescent pregnancy at antiretroviral therapy (ART) initiation: A critical barrier to retention on ART. J Int AIDS Soc. 2018;21:e25178. https://doi.org/10.1002/jia2.25178 [ Links ]
30.Knettel BA, Cichowitz C, Ngocho JS, et al. Retention in HIV care during pregnancy and the postpartum period in the option B+ era: A systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr. 2018;77(5):427-438. https://doi.org/10.1097/QAI.000000000001616 [ Links ]
31.Ssali L, Kalibala S, Birungi J, et al. Retention of adolescents living with HIV in care, treatment, and support programs in Uganda. 2014; Washington, DC: United States Agency for International Development. Available from: https://www.popcouncil.org/uploads/pdfs/2014HIVCore_UgandaAdolHAART.pdf. [ Links ]
32.Ridgeway K, Dulli LS, Murray KR, et al. Interventions to improve antiretroviral therapy adherence among adolescents in low- and middle-income countries: A systematic review of the literature. PLoS One. 2018;13(1):e0189770. https://doi.org/10.1371/journal.pone.0189770 [ Links ]
33.Murray KR, Dulli LS, Ridgeway K, et al. Improving retention in HIV care among adolescents and adults in low-and middle-income countries: A systematic review of the literature. PLoS One. 2017;12(9):e0184879. https://doi.org/10.1371/journal.pone.0184879 [ Links ]
34.MacKenzie RK, Van Lettow M, Gondwe C, et al. Greater retention in care amongst adolescents on antiretroviral treatment accessing 'Teen Club' an adolescent-centred differentiated care model compared with standard of care: A nested case-control study at a tertiary referral hospital in Malawi. J Int AIDS Soc. 2017;20(3):e25028. https://doi.org/10.1002/jia2.25028 [ Links ]
35.Munyayi F, Van Wyk B. The effects of teen clubs on retention in HIV care amongst adolescents in Windhoek, Namibia. S Afr J HIV Med. 2020;21(1):a1031. https://doi.org/10.4102/sajhivmed.v21i1.1031 [ Links ]
36.Izudi J, Mugenyi J, Mugabekazi M, Spector VT, Katawera A, Kekitiinwa A. Retention of HIV-positive adolescents in care: A quality improvement intervention in mid-western Uganda. BioMed Res Int. 2018;2018:1524016. https://doi.org/10.1155/2018/1524016 [ Links ]
 Correspondence:
Correspondence:
Brian van Wyk
bvanwyk@uwc.ac.za
Received: 12 Feb. 2020
Accepted: 23 May 2020
Published: 22 July 2020
ORIGINAL RESEARCH
The spectrum of electrolyte abnormalities in black African people living with human immunodeficiency virus and diabetes mellitus at Edendale Hospital, Pietermaritzburg, South Africa
Preyanka PillayI, II; Somasundram PillayIII, IV; Nobuhle MchunuV, VI
IDepartment of Internal Medicine, Greys Hospital, Pietermaritzburg, South Africa
IISchool of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIIDepartment of Internal Medicine, Edendale Hospital, Pietermaritzburg, South Africa
IVDepartment of Internal Medicine, King Edward Hospital, Durban, South Africa
VDepartment of Biostatistics, Faculty of Statistics, South African Medical Research Council, Durban, South Africa
VIDepartment of Statistics, School of Mathematics, Statistics and Computer Science, University of KwaZulu-Natal, Pietermaritzburg, South Africa
ABSTRACT
BACKGROUND: Serum electrolyte abnormalities in black African people living with human immunodeficiency virus (HIV) and diabetes mellitus (PLWH/DM) is unknown.
OBJECTIVES: The aim of this study was to analyse serum electrolytes (sodium, potassium, calcium and phosphate) and factors associated with electrolyte abnormalities in black African PLWH/DM versus HIV-uninfected patients with DM.
METHODS: We conducted a retrospective case-control study in 96 black African PLWH/DM (cases) and 192 HIV-uninfected patients with DM (controls), who were visiting the Edendale Hospital DM clinic, from 01 January 2016 to 31 December 2016. Pearson's correlation, multivariate linear and logistic regression analyses were utilised.
RESULTS: Hypocalcaemia was the most frequent electrolyte abnormality in PLWH/DM and HIV-uninfected patients with DM (31.25% vs. 22.91%), followed by hyponatraemia (18.75% vs. 13.54%). Median (IQR) corrected serum calcium levels were significantly lower in PLWH/DM compared with HIV-uninfected patients with DM (2.24 [2.18-2.30] mmol/L vs. 2.29 [2.20-2.36] mmol/L; p = 0.001). For every per cent increase in glycated haemoglobin, the odds of hyponatraemia significantly increased in both PLWH/DM (odds ratio [OR]: 1.55; 95% confidence interval [CI]: 1.19 -2.02; p = 0.003) and HIV-uninfected patients with DM (OR: 1.26; 95% CI: 1.04 -1.54; p = 0.009.
CONCLUSION: Hypocalcaemia and hyponatraemia were the most frequent electrolyte abnormalities and occurred more frequently in PLWH/DM compared with HIV-uninfected patients with DM. People living with HIV and DM have significantly lower corrected serum calcium levels compared with HIV-uninfected patients with DM. Furthermore, hyponatraemia is a marker of impaired glycaemic control.
Keywords: HIV; diabetes mellitus; electrolytes; sodium; potassium; calcium; phosphate; black African.
Introduction
Low- and middle-income countries account for 80% of the global diabetes mellitus (DM) burden.1 The International Diabetes Federation (IDF) estimates that Africa will experience the greatest global upsurge of DM by 2045.1 In 2015, DM was the second leading cause of mortality in South Africa after tuberculosis.2 Moreover, South Africa has the highest prevalence of human immunodeficiency virus (HIV) globally and recorded 7.06 million people living with HIV (PLWH) in 2017, with KwaZulu-Natal province having the majority of these cases.3,4 Following the introduction of antiretroviral therapy (ART), the prevalence of comorbid HIV and DM is on the rise because of an increase in life expectancy and the adverse metabolic effects of ART.5 A study in the United States of America (USA) determined that the prevalence of DM in PLWH was 10.3%, and the prevalence of DM was 3.8% higher in PLWH compared with the general population.6
Electrolytes play a vital role in maintaining homeostasis and are paramount in mediating enzymatic reactions, cellular function and electrical gradients.7 Patients with HIV or DM are predisposed to electrolyte abnormalities because of multifactorial pathophysiological factors.8,9 The risk of nephropathy, with subsequent electrolyte abnormalities, increases in the setting of comorbid HIV and DM. Furthermore, the black African population has distinct electrolyte physiology and a predisposition to chronic kidney disease and HIV-associated nephropathy (HIVAN).10 In addition, the use of tenofovir (TDF) increases the risk of proximal tubular dysfunction and subsequent hypokalaemia and hypophosphataemia.11
The Atherosclerosis Risk in Communities (ARIC) study concluded that African American patients had an approximately twofold greater incidence of type 2 DM compared with white patients.12 Although this racial disparity is multifactorial, lower vitamin D13 and serum potassium levels14 in the black population are being explored as possible contributory factors. Importantly, the prevalence of vitamin D deficiency in type 2 DM is disproportionately elevated in African American people compared with other ethnic groups in the USA.15
Electrolyte abnormalities are associated with increased morbidity and mortality, even if they are chronic or of mild severity and may remain clinically silent until an advanced stage.8,9 However, there is a paucity of data from Africa regarding electrolyte abnormalities in HIV or DM. Furthermore, there are no studies assessing electrolyte abnormalities in black African people living with HIV and diabetes mellitus (PLWH/DM). Determining and understanding the spectrum of electrolyte abnormalities in black African PLWH/DM are of crucial significance, particularly in South Africa, which has a large burden of HIV and DM and is undergoing an epidemiological transition in a resource-limited setting.
The objective of this retrospective case-control study was to determine, compare and identify associated factors regarding serum electrolyte abnormalities (sodium, potassium, calcium and phosphate) in black African PLWH/DM versus black African HIV-uninfected patients with DM who attended the Edendale Hospital DM clinic from 01 January to 31 December 2016.
Methods
This quantitative retrospective case-control study was conducted in 96 black African PLWH/DM (cases) and 192 black African HIV-uninfected patients with DM (controls) attending the Edendale Hospital DM clinic, Pietermaritzburg, KwaZulu-Natal, South Africa, over 1 year from 01 January to 31 December 2016. Records of patients attending the DM clinic were analysed retrospectively from datasheets. Electrolytes were measured during routine outpatient visits at the Edendale Hospital DM clinic. Black African PLWH/DM included in the study could have either type 1 or type 2 DM, be on ART or antiretroviral-naïve, have any degree of renal function that was determined by the estimated glomerular filtration rate (eGFR) and be on medication for comorbidities. Patients with incomplete records were excluded from the study. Sample sizes were determined by applying a power analysis in G*Power, which used an alpha of 0.05, a power of 0.80 and a medium effect size of 0.4. The ratio of cases and controls was 1:2, and participants were selected by random sampling, matched by eGFR. Estimated glomerular filtration rate was stratified by the Kidney Disease Outcomes Quality Initiative (KDOQI) classification. Data were anonymised with reference numbers. Variables analysed included the following:
• age (years)
• sex
• HIV status
• type of DM
• duration of DM (years)
• duration of HIV (years)
• duration of ART (years)
• type of ART
• eGFR (mL/min/1.73m2)
• levels of serum sodium, potassium, corrected calcium and phosphate (mmol/L)
• levels of glycated haemoglobin (HbA1c) (%).
The following serum electrolyte reference ranges, as per the National Health Laboratory Services (NHLS), were utilised:
• sodium: 136 mmol/L - 145 mmol/L
• potassium: 3.5 mmol/L - 5.1 mmol/L
• calcium: 2.20 mmol/L - 2.55 mmol/L
• phosphate: 0.78 mmol/L - 1.42 mmol/L
Any electrolyte values below the lower limit of normal were considered hypo-electrolyte abnormalities, whilst those above the upper limit of normal were considered hyper-electrolyte abnormalities. Corrected serum calcium levels were utilised and calculated as follows: measured total calcium (mmol/L) + 0.02 (40 [g/L] - serum albumin [g/L]). The 2017 Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) guidelines advocate for an HbA1c ≤ 7% to prevent micro- and macro-vascular complications. Therefore, in this study, adequate glycaemic control was defined as HbA1c ≤ 7%. Estimated glomerular filtration rate was calculated by using the Modification of Diet in Renal Disease (MDRD) formula. Serum electrolytes, eGFR and HbA1c were measured by using the Siemens Dimension® analyser.
Statistical analysis
Data were captured by using Microsoft Excel, version 2016 (Microsoft, USA). Statistical analyses were conducted by using Statistical Analysis Software (SAS), version 9.4 (SAS Institute Inc., Cary, NC, USA). Continuous variables were expressed as medians with interquartile ranges (IQRs). Categorical variables were expressed as frequencies and percentages. Continuous variables were compared by using the Wilcoxon rank-sum test as data were asymmetrically distributed. Categorical variables were compared by using either the Chi-square test or Fisher's exact test if there were less than five observations in any cell. Pearson's correlation coefficient assessed the correlation between HbA1c and electrolytes. Multinomial logistic regression assessed factors associated with electrolyte abnormalities. Linear regression analyses assessed the effect of measured covariates on electrolytes. Multivariable models were stratified by HIV status and were adjusted for gender, age, use of TDF, type of DM, duration of DM, duration of HIV, HbA1c and eGFR. A two-tailed value of p < 0.05 was considered to indicate statistical significance.
Ethical consideration
Ethical approval to conduct the study was obtained from the Biomedical Research and Ethics Committee (BREC) of the University of KwaZulu-Natal (reference number BE576/18). Permission was obtained from Edendale hospital to utilise the DM clinic datasheet for data collection.
Results
Descriptive data
Ninety-six black African PLWH/DM (cases) and 192 black African HIV-uninfected patients with DM (controls) were reviewed. People living with HIV and DM were significantly younger than HIV-uninfected patients with DM (median [IQR]: 46.5 [39-53.5] years vs. 56 [47-64] years; p < 0.001) (Table 1). HIV-uninfected patients had a significantly longer duration of DM compared with PLWH/DM (median [IQR]: 7 [3-13] years vs. 5 [2-9] years; p = 0.006). People living with HIV and DM had a median (IQR) duration of HIV of 7 (3-10) years and 86 (89.58%) patients were on ART, which included 65 (67.7%) patients on TDF (Table 1). Eighty-four (87.5%) PLWH/DM and 172 (89.58%) HIV-uninfected patients had type 2 DM. Seventy-three (76.0%) PLWH/DM and 153 (79.7%) HIV-uninfected patients with DM had an HbA1c > 7%, which was considered uncontrolled. The median (IQR) HbA1c amongst PLWH/DM and HIV-uninfected patients with DM was 9.45% (7.1% - 11.45%) and 9.70% (7.35% - 11.5%), respectively (p = 0.836). An eGFR less than 60 mL/min/1.73m2 was present in 12 (12.5%) PLWH/DM and 24 (12.5%) HIV-uninfected patients with DM, respectively (Table 1).
Analysis of electrolytes
Sodium
Hyponatraemia was the second most frequent electrolyte abnormality, which occurred in 18 (18.75%) PLWH/DM and 26 (13.54%) HIV-uninfected patients with DM (Table 1). Serum sodium was the only electrolyte significantly negatively correlated with HbA1c in both PLWH/DM (r = −0.34; p = 0.001) and HIV-uninfected patients with DM (r = −0.28; p < 0.001) (Table 2). Adjusted multinomial logistic regression analysis amongst PLWH/DM suggests that for every per cent increase in HbA1c, the odds of hyponatraemia significantly increased by 55% (odds ratio [OR]: 1.55; 95% confidence interval [CI]: 1.19-2.02; p = 0.003), whilst in HIV-uninfected patients with DM the odds of hyponatraemia significantly increased by 26% (OR: 1.26; 95% CI: 1.04-1.54; p = 0.009) (Table 3). Multivariate linear regression showed significant associations between serum sodium and HbA1c. Amongst PLWH/DM, for every per cent increase in HbA1c, serum sodium decreased by 0.51 mmol/L (β = −0.51; p = 0.004), and for every per cent increase in HbA1c amongst HIV-uninfected patients with DM, serum sodium decreased by 0.45 mmol/L (β = −0.45; p < 0.001) (Table 4).
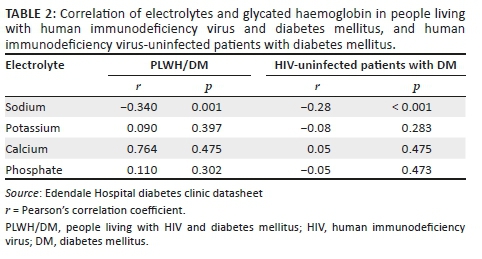
Potassium
The duration of HIV was not significantly associated with hypokalaemia on adjusted multinomial logistic regression analysis (OR: 0.85; 95% CI: 0.59-1.23; p = 0.645). Furthermore, the odds of hypokalaemia in PLWH using TDF compared with non-TDF-based ART were not significant (OR: 0.87; 95% CI: 0.06-13.12; p = 0.766). Adjusted multinomial logistic regression determined that the duration of DM was significantly associated with potassium abnormalities. In PLWH/DM, for every year increase in the duration of DM, the odds of hypokalaemia increased by 97% (OR: 1.97; 95% CI: 1.13-3.43; p = 0.025). However, in HIV-uninfected patients with DM, the odds of hyperkalaemia increased by 10% (OR: 1.10; 95% CI: 1.02-1.19; p = 0.048). Multivariate linear regression also showed significant associations between serum potassium levels and the duration of DM. For every year increase in the duration of DM, serum potassium decreased by 0.04 mmol/L amongst PLWH/DM (β = −0.04; p = 0.018) and increased by 0.01 mmol/L amongst HIV-uninfected patients with DM (β = 0.01; p = 0.042) (Table 4).
Calcium
Serum-corrected calcium was the only electrolyte with median (IQR) levels significantly lower in PLWH/DM compared with HIV-uninfected patients with DM (2.24 [2.18-2.30] mmol/L vs. 2.29 [2.20-2.36] mmol/L; p = 0.001). Furthermore, the most frequent electrolyte abnormality in PLWH/DM and HIV-uninfected patients with DM was hypocalcaemia (31.25% vs. 22.91%) (Table 1). Adjusted multinomial logistic regression in PLWH/DM and HIV-uninfected patients with DM found no factors significantly associated with hypocalcaemia or hypercalcaemia. However, multivariate linear regression analysis in HIV-uninfected patients with DM showed that for every year increase in age, serum calcium decreased by 0.01 mmol/L (β = −0.01; p = 0.031) (Table 4).
Phosphate
Adjusted multinomial logistic regression in PLWH/DM and HIV-uninfected patients with DM found no factors significantly associated with hypophosphataemia or hyperphosphataemia. Notably, the use of TDF was not significantly associated with hypophosphataemia in PLWH/DM (OR: 1.69; 95% CI: 0.20-14.12; p = 0.800). Multivariate linear regression in PLWH/DM determined that for every year increase in age, serum phosphate decreased by 0.01 mmol/L (β = −0.01; p = 0.033). Moreover, on average, serum phosphate was 0.09 mmol/L higher in women than in men amongst HIV-uninfected patients with DM, and all other variables were constant (β = 0.09; p = 0.006) (Table 4).
Discussion
Sodium
Serum sodium abnormalities in DM vary depending on the degree of water and sodium change.9 Serum glucose is an osmotically active substance; therefore, hyponatraemia in DM is mostly attributed to hyperglycaemia-induced hyper-osmolality, resulting in a dilutional effect or osmotic diuresis with hypovolemic hyponatraemia.9 Hypernatraemia may occur if water loss exceeds sodium loss.9 Our study identified serum sodium to be the only electrolyte significantly associated with HbA1c levels in both PLWH/DM and HIV-uninfected patients with DM. Furthermore, elevated HbA1c levels significantly increased the odds of hyponatraemia, with the odds being greater in PLWH/DM compared with their HIV-uninfected counterparts. Although pseudo-hyponatraemia in DM is common, hyponatraemia could be utilised as a marker of impaired DM control. Our finding regarding the association between HbA1c and serum sodium levels is comparable with that of a study conducted in India, which determined that mean (standard deviation [s.d.]) serum sodium levels were significantly lower in patients with DM compared with non-DM controls (127.92 [0.45] mmol/L vs. 135.82 [0.34] mmol/L; p = 0.0001) and that HbA1c was significantly inversely correlated with serum sodium levels (r = 0.640; p = 0.0001).7 However, no regression analysis was performed in the study conducted in India.7 Other causes of hyponatraemia in DM include side effects of drugs such as diuretics, diabetic nephropathy and the syndrome of inappropriate antidiuretic hormone secretion (SIADH).9
Our study demonstrated that PLWH/DM had a higher frequency of hyponatraemia compared with HIV-uninfected patients with DM (18.75% vs. 13.4%). The increased frequency of hyponatraemia in PLWH/DM could be attributed to the additive effect of HIV on sodium homeostasis. Hyponatraemia is a common electrolyte disorder in PLWH and a possible marker of HIV severity, as patients with hyponatraemia have significantly lower CD4 counts, higher viral loads and an increased prevalence of acquired immunodeficiency syndrome (AIDS).16 In PLWH, the main causes of hyponatraemia include opportunistic infections which predispose to SIADH, adrenal insufficiency, diarrhoea and vomiting.17 Furthermore, dysfunction of the thick ascending limb of the loop of Henle secondary to HIV and inflammation results in impaired free water clearance and dilutional hyponatraemia.8,18
People living with HIV and DM may be at a higher risk of hyponatraemia because of contributory factors from both HIV and DM. Furthermore, hyponatraemia in the black African population may indicate a greater degree of sodium imbalance compared with other ethnic groups as the black population physiologically have increased sodium retention with lower plasma renin and aldosterone levels.19,20
Potassium
Hyperkalaemia is common in DM.7,21,22 Conversely, studies conducted in Nigeria and Saudi Arabia found a predominant hypokalaemia and no significant association between serum potassium levels and glycaemic control, respectively.23,24 Similarly, our study found no significant association between HbA1c and serum potassium levels. Common causes of hyperkalaemia in DM and HIV include hyporeninaemic hypo-aldosteronism, acidosis, renal impairment and drugs such as angiotensin-converting enzyme (ACE) inhibitors, potassium-sparing diuretics and beta-blockers8,9 Hypokalaemia in DM is frequently caused by insulin administration, malabsorption, osmotic diuresis and hypomagnesaemia.9 In PLWH, hypokalaemia is commonly caused by vomiting, diarrhoea and proximal tubular dysfunction secondary to TDF.25,26,27 However, in our study, TDF was not significantly associated with hypokalaemia. This could be attributed to our study having relatively young PLWH that were on ART for a median duration of 6 years and only 12.5% of PLWH having an eGFR < 60ml/min/1.73m2, which may reduce the risk of TDF induced nephrotoxicity.
Notably, our study determined that for every unit increase in DM duration, the odds of hypokalaemia significantly increased by 97% in PLWH/DM. This could be attributed to patients with comorbid HIV and DM developing a greater degree of insulin resistance, as both conditions progress,28 and therefore require higher doses of insulin to achieve glycaemic control with a propensity for hypokalaemia. This possible contributory factor needs to be explored further as our study did not evaluate the use of insulin. In HIV-uninfected patients with DM, the likelihood of hyperkalaemia significantly increased by 10% for every unit increase in DM duration. This could be attributed to dysautonomia in long-standing DM which impairs the conversion of prorenin to renin and predisposes to hyporeninaemic hypo-aldosteronism and associated hyperkalaemia.29
Calcium
Calcium homeostasis is strongly regulated by parathyroid hormone and vitamin D. Factors contributing to hypocalcaemia in HIV and DM include vitamin D deficiency, hypoparathyroidism and hypomagnesaemia.9,30 Our study identified hypocalcaemia as the most common electrolyte abnormality in both PLWH/DM and HIV-uninfected patients with DM. Furthermore, serum calcium was the only electrolyte with median levels significantly lower in PLWH/DM compared with HIV-uninfected patients with DM. Similarly, Keuhn et al. identified mean serum calcium levels to be significantly lower in HIV-infected patients compared with controls (p < 0.0001), irrespective of serum albumin levels.30 Hypocalcaemia is also common in patients with DM, with a study in Sudan showing significantly lower mean serum calcium levels in patients with DM compared with controls (p < 0.05).31 Furthermore, vitamin D deficiency in the elderly is common despite consistent vitamin D intake and may predispose to hypocalcaemia.32 Notably, our study demonstrated a significant inverse association between serum calcium and age in HIV-uninfected patients with DM. A significant association between serum calcium and age may have not been detected in PLWH/DM as they were significantly younger. The degree of sunlight exposure was not documented in this study. Although PLWH/DM were significantly younger than HIV-uninfected patients with DM, clinically this difference in age should not usually result in a greater proportion of HIV-uninfected patients being housebound. Therefore, the significant inverse association between serum calcium levels and age in HIV-uninfected patients may be influenced by factors besides sun exposure.
The mechanism of vitamin D deficiency in HIV is multifactorial and involves the inhibitory effect of pro-inflammatory cytokines that reduces renal 1-α hydroxylation of vitamin D and the consumption of vitamin D by macrophages and lymphocytes.33 Furthermore, vitamin D has a significant immunomodulatory role, and deficiencies in PLWH are associated with lower CD4 cell counts, higher viral loads, HIV progression and an increased risk of opportunistic infections.34 Moreover, studies have suggested that vitamin D deficiency and low calcium levels result in impaired insulin synthesis and secretion with subsequent glucose intolerance and insulin resistance.15 The European AIDS Clinical Society (EACS) has vitamin D supplementation recommendations and reports that the prevalence of low vitamin D levels was up to 80% in HIV cohorts and was associated with an increased risk of osteoporosis, type 2 DM, mortality and AIDS events.35 This is in contrast to South African HIV and DM guidelines, which do not have vitamin D deficiency recommendations despite our susceptible population.36,37
Consequently, the presence of comorbid HIV and DM in the black African population potentially increases the risk of vitamin D deficiency and hypocalcaemia, which might negatively impact HIV and DM control. The role of vitamin D in the pathogenesis and control of HIV and DM and the effect of vitamin D supplementation need to be further explored, particularly in the black African population.
Phosphate
The risk of TDF-induced nephrotoxicity with isolated hypophosphataemia, proximal tubular dysfunction or Fanconi syndrome increases in the presence of renal impairment.38 This is of particular concern in patients with comorbid HIV and DM, advancing age, lower CD4 cell counts and elevated baseline creatinine levels.38 Our study did not find TDF to be significantly associated with hypophosphataemia or serum phosphate levels in PLWH/DM. This could be attributed to the fact that our study had only 12.5% of PLWH/DM with an eGFR <60 mL/min/1.73m2, patients were using ART for a median duration of only 6 years and PLWH/DM were relatively young. A study by Day et al. observed the frequency of hypophosphataemia in TDF recipients to be higher than non-TDF ART recipients (31% vs. 22%). However, no independent association was found between TDF use and the frequency or severity of hypophosphataemia.39 The recognition that hypophosphataemia in PLWH on TDF is multifactorial must be considered to avoid unnecessary TDF cessation in a resource-limited setting.
Current guidelines
This study determined that electrolyte abnormalities in black African PLWH/DM are common, with hypocalcaemia and hyponatraemia being the most frequent electrolyte abnormalities. However, the current SEMDSA guidelines only recommend that serum potassium needs to be measured at diagnosis and monitored annually.37 Furthermore, South African HIV guidelines only recommend the monitoring of serum potassium and phosphate in high-risk patients and patients with features of tubular wasting.36
Limitations
The limitations of this study included CD4 count and viral load not being documented for a majority of patients as management and monitoring of HIV occurs at designated HIV clinics. Therefore, the association between HIV control and electrolyte abnormalities could not be determined. Possible TDF-induced proximal tubular dysfunction and electrolyte loss were not assessed with urine electrolytes as they were not routinely performed in the DM clinic. Patients in this study could have been on medication or could have comorbidities which may affect electrolytes. However, by including these patients the study was more representable and reproducible as the majority of patients suffering from DM were usually part of a metabolic syndrome which requires chronic treatment to improve outcomes. In addition, the use of oral antidiabetic medication or insulin and the respective doses were not included. This could have been used to compare the DM treatment requirements in PLWH/DM and HIV-uninfected patients as both groups had similar HbA1c levels. Lastly, because of the retrospective nature of the study, causality could not be determined. However, this is a newly explored topic, and this study is useful in providing preliminary data for future prospective studies.
Conclusion
Serum electrolyte abnormalities in black African PLWH/DM are common. Hypocalcaemia and hyponatraemia were the most frequent electrolyte abnormalities and occurred more frequently in PLWH/DM compared with HIV-uninfected patients with DM. Serum calcium levels were significantly lower in black African PLWH/DM compared with HIV-uninfected patients with DM. Importantly, hyponatraemia is a potential marker of impaired glycaemic control as elevated HbA1c levels significantly increased the odds of hyponatraemia in both groups; however, the odds were greater in PLWH/DM. Ultimately, black African PLWH/DM are highly vulnerable to electrolyte abnormalities because of multifactorial pathophysiological factors. Further large prospective studies regarding electrolyte abnormalities in black African PLWH/DM will assist in identifying contributory factors and implementing tailored guidelines that could facilitate prevention, earlier detection, closer monitoring and appropriate intervention to reduce associated adverse effects in this high-risk population, particularly in the South African context.
Acknowledgements
This study contributes towards the Master of Medical Science degree of Dr Preyanka Pillay, which is supervised by Dr Somasundram Pillay.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
P.P. contributed to the conception and design of the study as well as the collection, analysis and interpretation of the data. P.P. wrote and edited the manuscript. S.P. contributed to the conception and design of the study and critically reviewed and edited the manuscript. N.M. contributed to the statistical analysis and interpretation of data; she also critically reviewed and edited the manuscript. All authors gave final approval of the version to be published.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data are available upon request from the corresponding author.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.International Diabetes Federation. IDF Diabetes Atlas, 8th ed. [ Links ] [homepage on the Internet]. Brussels: International Diabetes Federation; 2017 [cited 2019 Mar 03]. Available from: http://www.diabetesatlas.org
2.Statistics South Africa. Media release: Mortality and causes of death, 2015 [hompage on the Internet]. [ Links ] Statistics South Africa [cited 2019 Mar 03]. Available from: http://www.statssa.gov.za/?p=9604
3.UNAIDS. South Africa overview [homepage on the Internet]. [ Links ] [cited 2019 Mar 03]. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica
4.Statistics South Africa. Mid-year population estimates 2017 [homepage on the Internet]. [ Links ] [cited 2019 Mar 03]. Available from: http://www.statssa.gov.za/publications/P0302/P03022017.pdf
5.Samad F, Harris M, Puskas C, et al. Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Res Care. 2017;5(1):e000457. https://doi.org/10.1136/bmjdrc-2017-000457 [ Links ]
6.Hernandez-Romieu A, Garg S, Rosenberg E, Thompson-Paul A, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. https://doi.org/10.1136/bmjdrc-2016-000304 [ Links ]
7.Rajagopal L, Ganesan V, Abdullah S, et al. Exploring the interrelationship between electrolytes, anemia, and glycosylated hemoglobin (HbA1c) levels in type 2 diabetics. Asian J Pharm Clin Res. 2018;11(1):251. https://doi.org/10.22159/ajpcr.2017.v11i1.22533 [ Links ]
8.Musso C, Belloso W, Glassock R. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol. 2016;5(1):33-42. https://doi.org/10.5527/wjn.v5.i1.33 [ Links ]
9.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases: WJCC. 2014;2(10):488-496. https://doi.org/10.12998/wjcc.v2.i10.488 [ Links ]
10.Naicker S, Rahmania S, Kopp J. HIV and chronic kidney disease. Clin Nephrol. 2015;83(Suppl 1):32-38. https://doi.org/10.5414/cnp83s032 [ Links ]
11.Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. 2009;23(6):689-696. https://doi.org/10.1097/qad.0b013e3283262a6 [ Links ]
12.Brancati F, Kao W, Folsom A, et el. Incident type 2 diabetes mellitus in African American and white adults: The atherosclerosis risk in communities study. JAMA. 2000;283(17):2253-2259. https://doi.org/10.1001/jama.283.17.2253 [ Links ]
13.Alvarez J, Bush N, Choquette S, et al. Vitamin D intake is associated with insulin sensitivity in African American, but not European American, women. Nutr Metab. 2010;7(1):28. https://doi.org/10.1186/1743-7075-7-28 [ Links ]
14.Chatterjee R, Yeh H-C, Shafi T, et al. Serum potassium and the racial disparity in diabetes risk: The Atherosclerosis Risk in Communities (ARIC) study. Am J Clin Nutr. 2011;93(5):1087-1091. https://doi.org/10.3945/ajcn.110.007286 [ Links ]
15.Davis S. Vitamin D deficiency and type 2 diabetes in African Americans: The common denominators. Diabetes Spectrum. 2011;24(3):148. https://doi.org/10.2337/diaspect.24.3.148 [ Links ]
16.Braconnier P, Delforge M, Garjau M, Wissing K, De Wit S. Hyponatremia is a marker of disease severity in HIV-infected patients: A retrospective cohort study. BMC Infect Dis. 2017;17(1):98. https://doi.org/10.1186/s12879-017-2191-5 [ Links ]
17.Shu Z, Tian Z, Chen J, et al. HIV/AIDS-related hyponatremia: An old but still serious problem. Renal Failure. 2018;40(1):68-74. https://doi.org/10.1080/0886022x.2017.1419975 [ Links ]
18.Belloso W, de Paz Sierra M, Navarro M, Sanchez M, Perelsztein A, Musso C. Impaired urine dilution capability in HIV stable patients. Int J Nephrol. 2014;2014:1-8. https://doi.org/10.1155/2014/381985 [ Links ]
19.Sagnella G. Why is plasma renin activity lower in populations of African origin? J Hum Hypertens. 2001;15(1):17-25. https://doi.org/10.1038/sj.jhh.1001127 [ Links ]
20.Rayner B, Myers J, Opie L, Trinder Y, Davidson J. Screening for primary aldosteronism - Normal ranges for aldosterone and renin in three South African population groups. S Afr Med J. 2001;91(7):594-599. https://doi.org/10.1177/1470320312463833 [ Links ]
21.Anago E, Medehouenou T, Akpovi CD, Tchehouenou H. Electrolyte disturbances in diabetic patients in Cotonou, Benin. Int J Res Med Sci. 2016;4:5430-5435. https://doi.org/10.18203/2320-6012.ijrms20164223 [ Links ]
22.McNair P, Madsbad S, Christiansen C, Christensen M, Transbol I. Hyponatremia and hyperkalemia in relation to hyperglycemia in insulin-treated diabetic out-patients. Clin Chim Acta. 1982;120(2):243-250. https://doi.org/10.1016/0009-8981(82)90161-9 [ Links ]
23.E. Ugwuja N. A comparative study of serum electrolytes, total protein, calcium and phosphate among diabetic and HIV/AIDS patients in Abakaliki, Southeastern, Nigeria. Internet J Lab Med. 2007;2(1):1-5. https://doi.org/10.5580/15e5 [ Links ]
24.Al-Rubeaan K, Siddiqui K, Abu Risheh K, et al. Correlation between serum electrolytes and fasting glucose and Hba1c in Saudi diabetic patients. Biol Trace Element Res. 2011;144(1-3):463-468. https://doi.org/10.1007/s12011-011-9144-4 [ Links ]
25.Eshiet E, Okogun G. Plasma urea and electrolytes profile in different stages of hiv infection in Ekpoma, Nigeria. Afr J Cell Pathol. 2015;4(1):1-5. https://doi.org/10.5897/ajcpath15.001 [ Links ]
26.Narayanan Ramesh V. To study the renal and electrolyte disturbances in HIV infected patients. IOSR Jof Dent Med Sci. 2017;16(8):91-94. [ Links ]
27.Emejulu A, Onwuliri V, Ojiako O. Electrolyte abnormalities and renal impairment in asymptomatic HIV-infected patients in Owerri, South Eastern Nigeria. Aust J Basic Appl Sci. 2011;5(3):257-260. [ Links ]
28.Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;3(1):2. https://doi.org/10.1186/-1758-5996-3-2 [ Links ]
29.McFarlane S, Sowers J. Aldosterone function in diabetes mellitus: Effects on cardiovascular and renal disease. J Clin Endocrinol Metab. 2003;88(2):516-523. https://doi.org/10.1210/jc.2002-021443 [ Links ]
30.Kuehn E, Anders H, Bogner J, Obermaier J, Goebel F, Schlondorff D. Hypocalcaemia in HIV infection and AIDS. J Intern Med. 1999;245(1):69-73. https://doi.org/10.1046/j.1365-2796.1999.00407.x [ Links ]
31.Hassan S, Elsheikh W, Rahman N, ElBagir N. Serum calcium levels in correlation with glycated hemoglobin in type 2 diabetic sudanese patients. Adv Diabetes Metab. 2016;4(4):59-64. [ Links ]
32.Linnebur S, Vondracek S, Vande Griend J, Ruscin J, McDermott M. Prevalence of vitamin D insufficiency in elderly ambulatory outpatients in Denver, Colorado. Am J Geriatr Pharmacother. 2007;5(1):1-8. https://doi.org/10.1016/j.amjopharm.2007.03.005 [ Links ]
33.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64(5):226-233. https://doi.org/10.1111/j.1753-4887.2006.tb00205.x [ Links ]
34.Alvarez N, Aguilar-Jimenez W, Rugeles M. The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol. 2019;10. https://doi.org/10.3389/fimmu.2019.02291 [ Links ]
35.European AIDS Clinical Society guidelines 2018 [homepage on the Internet]; [ Links ] version 9.1:49-51. [cited 2019 Mar 03]. Available from: www.eacsociety.org/files/2018_guidelines-9.1-english.pdf
36.Meintjes G, Moorhouse M, Carmona S, et al. Adult antiretroviral therapy guidelines 2017. S Afr J HIV Med. 2017;18(1):a776. https://doi.org/10.4102/sajhivmed.v18i1.776 [ Links ]
37.SEMDSA 2017 guidelines for the management of type 2 diabetes mellitus. SEMDSA type 2 Diabetes Guidelines Expert Committee. JEMSDA [serial online] 2017 [cited 2019 Mar 03];22(1):1-196. Available from: https://www.semdsa.org.za/images/647-4385-1-pb.pdf
38.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: A double-edged sword? J Am Soc Nephrol. 2013;24(10):1519-1527. https://doi.org/10.1681/asn.2012080857 [ Links ]
39.Day S, Leake Date H, Bannister A, Hankins M, Fisher M. Serum hypophosphatemia in tenofovir disoproxil fumarate recipients is multifactorial in origin, questioning the utility of its monitoring in clinical practice. JAIDS J Acquir Immune Defic Syndr. 2005;38(3):301-304. [ Links ]
 Correspondence:
Correspondence:
Preyanka Pillay
preyankapillay@yahoo.com
Received: 26 Apr. 2020
Accepted: 07 June 2020
Published: 23 July 2020
ORIGINAL RESEARCH
Understanding adherence in virally suppressed and unsuppressed human immunodeficiency virus-positive urban patients on second-line antiretroviral treatment
Siphamandla B. GumedeI, II; Willem D.F. VenterI; Samanta T. Lalla-EdwardI
IEzintsha, a sub-division of Wits Reproductive Health and HIV Institute, University of the Witwatersrand, Johannesburg, South Africa
IIDepartment of Interdisciplinary Social Science, Public Health, Utrecht University, Utrecht, The Netherlands
ABSTRACT
BACKGROUND: Understanding antiretroviral therapy (ART) adherence may assist in designing effective support interventions
OBJECTIVES: This study elicited perspectives on how to promote treatment adherence from virologically suppressed and unsuppressed patients receiving second-line ART
METHODS: This was a cross-sectional study conducted with randomly selected patients active on second-line ART, from five public health facilities in the Johannesburg inner city. Data were collected on demographics, clinical information, participant's experiences and ART knowledge. Virological failure was defined as exceeding 1000 copies/mL
RESULTS: The study sample comprised 149 participants; of which 47.7% (n = 71) were virally unsuppressed and 69.1% (n = 103) were women; the median age of the participants was 42 years (interquartile range [IQR] 36-47 years). Experiencing medication-related difficulties in taking second-line ART (p = 0.003), finding second-line regimen more difficult to take than a first-line regimen (p = 0.001) and experiencing side effects (p < 0.001) were all subjective predictors of virological failure. Participants' recommendations for improving adherence included the introduction of a single tablet regimen (31.6%, n = 55), reducing the dosage to once daily (26.4%, n = 46) and reducing the pill size for second-line regimen (4.0%, n = 7
CONCLUSION: The results of this study highlight the importance of improving patients' knowledge about adherence and motivation to continue ART use despite the persistence of side effects and difficulties with taking medication
Keywords: adherence; viral load suppression; virological failure; antiretroviral therapy; South Africa.
Introduction
The World Health Organization (WHO) defines adherence as the degree to which a patient is able to follow a treatment plan and take medication at prescribed times.1
Factors that affect treatment adherence include changes in daily routines, forgetting to take medication, side effects, depression, being away from home, comorbidity, lack of knowledge and desire to take treatment.2,3,4 In addition, patients experiencing financial constraints, social issues such as the fear of disclosure and lack of understanding of treatment benefits are more prone to non-adherence to treatment and illnesses.5,6 Some studies have reported disclosure and relationship with the person being disclosed to as predictors of adherence.7,8
The South African government's adherence promotion strategies include routine viral load monitoring, adherence counselling, pill counting, adherence clubs and routine completion of clinical stationery.9,10,11 Despite all these antiretroviral therapy (ART) adherence strategies being implemented, treatment failure amongst patients on first- and second-line ART remains an issue.12 Lapses in ART medication adherence can lead to viral rebound with ongoing immunosuppression and viral resistance.1,13,14 However, not much is understood about the perspectives of patients regarding adherence challenges.15,16
Therefore, this study sought to describe and obtain perspectives on treatment adherence from two separate groups of patients on second-line therapy, those who were suppressed and those who were not, to understand treatment-taking behaviour.
Materials and methods
Study design and setting
This was a cross-sectional study conducted between July and August 2018 in a sub-population of patients receiving second-line ART at the end of June 2018. Five public health facilities in inner-city Johannesburg (two hospitals, one community health centre and two primary healthcare clinics) were included in the study.
Study population
The study population comprised patients aged 18 years and older who were on second-line ART for at least 1 month or longer.
Data collection
Sample selection and recruitment
We randomly sampled 10% of the population of 1500 eligible patients. The total number of active patients on second-line treatment per facility was divided by the total sample size (n = 150) to determine the interval that needed to be used to select the eligible patients. Using this formula, every nth (different for each facility) record from the register or list of active patients on second-line treatment in each facility was selected and recruited to the study until the facility sample size was reached. Once the eligible patients were identified, they were invited to participate in the study in one of the two ways: telephonically or in facility recruitment where researchers met them at the facility during their scheduled clinic visit. For the patients who refused to participate in the study, the next nth patient was recruited.
Data collection, tool and variables
A pretested semi-structured questionnaire was used, which consisted of five sections: (1) demographic data, (2) comorbidity information, (3) human immunodeficiency virus (HIV) diagnosis and care information, (4) experiences on the first-line regimen and adherence and (5) experiences on second-line regimen and adherence. Information collected included demographic information (facility name, sex, age, relationship status, employment status and education level), comorbidity information, experiences on both first- and second-line treatment, disclosure information, duration on ART, reasons for starting ART, side effects, self-reported treatment interruptions, challenges with taking second-line treatment, treatment supporter information and insights into how adherence could be improved.
Questionnaire administration
Data were collected by the principal investigator and a trained research assistant. The interviews were conducted in English as it was the most commonly spoken language in the study setting and all participants could speak it.
Data entry, cleaning and analysis
Data were captured into REDCap immediately after interviews were conducted. The research team conducted data clean-up by running data quality checks in REDCap and STATA (quantitative data). For the closed-ended questions, we assessed the association between outcome variables and selected socio-demographic and health-related characteristics. Pearson's chi-squared test was used to assess trend associations between categorical variables. Continuous data were summarised and analysed using the median and interquartile ranges (IQRs). Logistic and multiple logistic regression models (bivariate and multivariate logistic regression) were built for key outcome variables, such as viral load, difficulties in taking second-line regimen and side effects, to identify independent predictors. We reported unadjusted and adjusted odds ratios (ORs), 95% confidence interval (CI) and p-values - p-values that were less than 0.05 were considered significant. Open-ended questions were analysed using qualitative data analysis methods. Data were coded and thematic analysis was performed. Where appropriate, quotations have been included to support the reported results.
Ethical consideration
Ethical approval to conduct the study was obtained from the University of the Witwatersrand Human Research Ethics Committee (ethical clearance number: M170691). Approval was also granted by the Johannesburg Health District (DRC Ref No. 2017-08-003 and NHRD Ref No. GP_201708_030). Participants were informed that participation in the study was voluntary and that refusal would not affect their relationship with their healthcare provider or facility. All patients who agreed to participate in the study signed an informed consent form. To ensure confidentiality, there were no linkages between the data collected in the questionnaire and the patients' clinic information. Participants were reimbursed for their travel.
Results
Sample characteristics
A total of 150 out of 1500 active patients on second-line ART across the five public health facilities were interviewed (69.1%, n = 103 women). During the quality checking processes, we found that one of the participants was younger than 18 years and was subsequently omitted from the analysis. The median age of the participants was 42 years (IQR 36-47 years). Most of the participants were single (38.1%, n = 57); 30.2% (n = 45) participants were married. Nearly two-thirds of the participants were born in South Africa (61.1%, n = 91), whilst almost one-third of the participants were born in Zimbabwe (32.9%, n = 49). The majority (87.2%, n = 130) of participants had completed at least their secondary or high school-level education. A minority (8.1%, n = 12) of participants had completed tertiary qualifications; 4.7% (n = 7) participants had never attended a school. Of the total participants, 45.6% (n = 68) were unemployed. The majority of participants were identified as Christian (87.9%, n = 131). Hypertension (65.1%, n = 28/43), diabetes (9.3%, n = 4/43) and hypercholesterolaemia (9.3%, n = 4/43) were the most common concomitant conditions reported by the participants. The average distance travelled to reach a health facility was 5 km (IQR: 2 km - 15 km), with 57.7% (n = 86) participants travelling 5 km or less to reach the health facility.
Socio-demographic and virological status
Table 1 shows the socio-demographic characteristics disaggregated by virological status. Nearly half (47.7%, n = 71) of the participants interviewed had virological failure (VLF), with women accounting for 73.2% (n = 52). With regard to age, of the total unsuppressed participants, 39.4% (n = 28) were between 30 and 39 years. Of the participants with virological suppression (VLS), 29.5% (n = 23) had comorbidity compared to VLF participants (28.2%, n = 20).
Disclosure, treatment support and virological status
Almost all the participants' first disclosure of their HIV status was to a partner or relative (98.7%, n = 147 combined). More VLS participants (55.1%, n = 43) disclosed about their HIV status to a family member first, whilst most VLF participants (52.1%, n = 37) chose to disclose to their partners first. Almost no disclosure to friends was reported. Disclosure did not show any statistical significance. Participants typically (63.1%, n = 94) disclosed within 1 week after HIV diagnoses, with more VLF participants reporting early disclosure than VLS participants (64.8%, n = 46 vs. 61.5%, n = 48). Most participants (79.2%, n = 118) had treatment supporters (VLF = 80.3%, n = 57; VLS = 78.2%, n = 61). Whilst 10.1% (n = 15) of participants felt like stopping treatment completely at some point, only 3.4% (n = 5) stopped treatment for longer than 1 month, more in VLS participants, although this was not statistically significant.
Factors of virological failure and adherence
Overall, there were more participants (52.3%, n = 78) who felt that taking a second-line regimen was difficult compared to the first-line regimen, with the VLF group (66.2%, n = 47, p = 0.001) predominantly reporting this challenge. Generally, 38.3% (n = 57/149) experienced difficulties in taking the second-line regimen (p = 0.003). Of these, about two-thirds (63.2%, n = 36) were VLF participants (p = 0.003). Just under half (47.7%, n = 71/149) of the participants experienced side effects whilst taking their second-line regimen, and of these, 63.4% (n = 45) were VLF participants (p < 0.001).
Table 2 presents results from both bivariate and multivariate logistic regression analysis. No association was detected between VLF and relationship status in bivariate analysis. However, in multivariate analysis, participants who cohabit were three times more likely to have a VLF than those who are married (adjusted odds ratios [AORs] 3.1, 95% CI = 1.1-8.9; p = 0.035). Unemployed participants were two and a half times more likely to have treatment-related side effects compared to employed participants (AOR 2.5, 95% CI = 1.1-5.7; p = 0.023). Results for age did not show any statistical significance but older people were less likely to be unsuppressed.
Treatment-taking behaviour
Table 3 presents the reported treatment behaviour of the participants for the duration of receiving ART. There were more reports of treatment interruption whilst participants were on first-line treatment (n = 52). With respect to the reported treatment interruption whilst on second-line regimen, there was no distinct difference between the failing (n = 8) and suppressed (n = 5) groups. Both groups equally relied on themselves to remember to take their treatment ('naturally [I] remember taking my pills [VLF, woman, 31 years]; [I am] experienced on remembering my time' [VLS, man, 41 years]). Some of the reasons for interrupting treatment included stopping to take tuberculosis medication ('yes, interruption due to TB recurrence' [VLF, woman, 22 years]) and no medication availability whilst relocating ('I once interrupted my treatment due to the shortage of drugs as I relocated in South Africa and I did not have a proper transfer letter' [VLF, man, 38 years]). In the group with no reported second-line treatment interruption, more virologically suppressed participants (n = 21) listed using an alarm as a reminder. Several participants used the timeslots of popular local television programmes or the news (n = 14) as reminders to take their medication.
Table 4 presents the bivariate and multivariate logistic regression analyses for ART-taking behaviour. There was no association between participants' treatment-taking behaviour (i.e. medication alert, daily frequencies and pill storage) and VLF. However, participants who relied on their memory as a reminder to take medication (whilst on first-line regimen) were almost three times more likely to interrupt their first-line treatment than those who relied on an alarm (AOR: 2.6, 95% CI = 1.0-6.4, p = 0.042). There was no association found between medication alert or frequency of taking medication and second-line treatment interruption. Participants who used their handbag to store their medication were the least likely to experience second-line treatment interruption (OR: 0.2, 95% CI = 0.05-1.0, p = 0.054).
Recommendations from participants
The study participants made 175 recommendations for improving adherence (see Table 5). Coformulation in single tablets, only needing to take one dose of medication daily (preferably at night) and education about being adherent were listed as the most effective mechanisms to improve adherence on second-line treatment. Some examples of recommendations from participants include the following:
'Education should be emphasised through adherence classes. Reinforce on the benefits of ART'. (VLS, female, 38 years old)
'Availing a single-dose treatment for the second-line patients would enable them to adhere to treatment. Further ongoing education would also help'. (VLF, female, 39 years old)
Other recommendations included the development of injectable ART (n = 9, seven women and two men) and the provision of psychosocial support (particularly related to poverty and ensuring food supply):
'Should consider addressing the psychosocial needs of patients on second line as they have to adhere to treatment but sometimes they do not have enough food to eat'. (VLF, female, 45 years old)
Single recommendations to improve adherence included treatment reminders (n = 1), additional counselling (n = 1) and minimising the number of times second-line regimen is taken per day (n = 1). One patient felt that healthcare workers trusting that their patients took their medication as prescribed would promote treatment adherence. Lastly, 12 participants did not have any recommendations (mainly because of not experiencing any pill-taking challenges), as shown in the following statement:
'The current regimen is fine with me, therefore, I will suggest no change'. (VLS, male, 41 years old)
Discussion
This study sought to describe and understand treatment adherence and possible treatment support interventions from patients receiving second-line ART.
It has been reported that relationship dynamics influence ART adherence and VLS in that being married or having a committed and supportive partner tended to foster an environment for better clinical outcomes in HIV-positive people.17,18 Studies from South Africa and the United Kingdom found that HIV-positive married individuals had better clinical outcomes compared to any other relationship status.19,20 Similarly, our study found that single and unmarried people living with their partners were more likely to be virally unsuppressed.
Not statistically significant but important for consideration in adherence strengthening, our study showed that being younger was a predictor of VLF, which was congruent with previous studies.21,22,23 We noted VLS in those participants who resided further away from the health facilities. This is not in agreement with findings of studies conducted in Uganda, Ghana and Burkina Faso,24,25,26 which reported that individuals who resided closer to a health facility were more likely to seek healthcare.
Late disclosure may hinder adherence or treatment support and subsequently yield poor clinical outcomes.27 Whilst the majority of participants (63%) disclosed their HIV status 1 week after diagnoses, about 28% took longer than 4 weeks to disclose. Early disclosure, particularly to a family member or partner, has been strongly associated with improved adherence.8,28,29 Disclosure to a family member or partner has been linked with adequate psychosocial support which in turn facilitates adherence to treatment.8,29,30,31,32 However, the findings of our study suggest that disclosure and dependence on a treatment supporter are likely not to produce desired adherence levels (and did not feature in the list of participant recommendations), indicating that disclosure and treatment support should be assessed in combination with other adherence strategies instead of as a single consideration or mechanism.33
Unsurprisingly, the more toxic the second-line multi-pill, and regimens requiring medication to be taken multiple times a day, were seen as significantly harder to take than a single tablet daily well-tolerated first-line regimen. These views were consistent with reports from other studies that attributed similar challenges to taking second-line regimen.34,35,36
Participants who did not interrupt ART mainly reported using an alarm as a reminder for taking their medication. This finding suggests the need to explore external reminder mechanisms for improving adherence in this setting, considering that about 15% of VLF participants reported not using any external reminders. Various studies have also found a trend towards better adherence amongst patients who used external reminders.37,38,39 In addition, our study showed that participants who used their handbags to store their medication were more likely to adhere to treatment. This finding is in line with other studies that have reported having a handbag to have pills all the time as the preferred ART storage by patients.40,41,42
Side effects are an important predictor of poor adherence, and cumulative toxicity associated with ART, especially in second-line regimens.43,44,45 We found that participants with VLF were more likely to have treatment-related side effects. Furthermore, those participants with side effects were more likely to be unemployed. Although this was not explored further in our study, various studies have reported that employed patients can manage their health and side effects better than their unemployed counterparts.46,47,48
Participants had ideas regarding drug formulation that may improve adherence. These included a fixed-dose combination, a dosage taken once a day and reducing the pill size. Furthermore, the participants suggested that education on the benefits of taking ART could improve adherence, whilst a few participants also suggested the implementation of injectable ART. Various studies have recommended similar strategies,49,50,51 with the effectiveness of some of these strategies being previously reported for first-line regimens.51,52
Study limitations and strengths
This study had several limitations. Firstly, the study relied on participants' self-reports, prompting a likelihood that socially desirable answers may have been provided. However, to control for this, information such as viral load, side effects and comorbidity was verified by checking participants' medical records as part of data quality checks for the study. Secondly, the clinical measure for adherence considered viral load only. Finally, the sample might be small for the results to be generalised to all patients receiving second-line ART. However, the direction and size of effect were generally consistent, suggesting that the study findings may be robust despite these limitations.
Conclusion
Participants on a second-line antiretroviral regimen had firm recommendations regarding improving adherence, largely focused on administration, reduced dosing and pill burden. The study results suggest the importance of improving patients' knowledge about treatment and adherence and motivation to continue ART use despite the persistence of side effects. Drug manufacturers and health programmers must consider such recommendations as they modify and implement new ART regimens and programmes. Lastly, treatment support interventions recommended in this study need to be tested in practice to determine their efficacy for large-scale implementation.
Acknowledgements
The authors would like to thank all the facilities and relevant health and research authorities from the City of Johannesburg for allowing the research team to engage in a partnership to strengthen health service delivery through technical assistance and research. They also thank the participants for sharing their experiences, views and recommendations, and Jean Claude Nkembi for his assistance with data collection and capture.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
S.B.G. and S.T.L.-E. designed the study and supervised the data extraction at the facilities. S.B.G. analysed the quantitative data and S.T.L.-E. analysed the qualitative data. S.B.G. interpreted the data and prepared the first draft of the manuscript, which was revised by S.T.L.-E. and W.D.F.V. All authors read and approved the final manuscript.
Funding information
This study was funded through the OPTIMIZE project. OPTIMIZE (AID-OAA-A-15-00069) is funded by the United States Agency for International Development (USAID) under the US President's Emergency Plan for AIDS Relief (PEPFAR). S.B.G. was supported by the Consortium for Advanced Research Training in Africa (CARTA). It is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No. B 8606.R02), Swedish International Development Cooperation Agency (SIDA) (Grant No. 54100113), the Developing Excellence in Leadership, Training and Science (DELTAS) Africa Initiative (Grant No. 107768/Z/15/Z) and Deutscher Akademischer Austauschdienst (DAAD). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences' (AAS) Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (the United Kingdom [UK]) and the UK government.
Data availability statement
The data sets used or analysed in this study are available from the corresponding author upon reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach [homepage on the Internet]. [ Links ] WHO Guidelines. 2013 [cited 2019 Aug 14]. Available from: https://apps.who.int/iris/bitstream/handle/10665/85321/9789241505727_eng.pdf?sequence=1
2. Fox MP, Berhanu R, Steegen K, et al. Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa. Trop Med Int Heal. 2016;21(9):1131-1137. https://doi.org/10.1111/tmi.12741 [ Links ]
3. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:1-538. https://doi.org/10.1002/14651858.CD000011.pub4 [ Links ]
4. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV [homepage on the Internet]. [ Links ] World Health Organization; 2015 [cited 2019 Aug 14]. Available from: https://apps.who.int/iris/bitstream/handle/10665/186275/9789241509565_eng.pdf?sequence=1
5. Goudge J, Ngoma B. Exploring antiretroviral treatment adherence in an urban setting in South Africa. J Public Health Policy. 2011;32:S52-S64. https://doi.org/10.1057/jphp.2011.22 [ Links ]
6. Elliott JH, Lynen L, Calmy A, et al. Rational use of antiretroviral therapy in low-income and middle-income countries: Optimizing regimen sequencing and switching. AIDS. 2008;22(16):2053-2067. https://doi.org/10.1097/QAD.0b013e328309520d [ Links ]
7. Gross R, Zheng L, Rosa A La, et al. Partner-based adherence intervention for second-line antiretroviral therapy (ACTG A5234): A multinational randomised trial. Lancet HIV. 2(1):e12-19. https://doi.org/10.1016/S2352-3018(14)00007-1 [ Links ]
8. Onoya D, Nattey C, Budgell E, et al. Predicting the need for third-line antiretroviral therapy by identifying patients at high risk for failing second-line antiretroviral therapy in South Africa. AIDS Patient Care STDS. 31(5):205-212. https://doi.org/10.1089/apc.2016.0291 [ Links ]
9. Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005;19(12):1243-1249. https://doi.org/10.1097/01.aids.0000180094.04652.3b [ Links ]
10. South African National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults [homepage on the Internet]. [ Links ] Health Policy. 2015 [cited 2019 Aug 20]. Available from: https://sahivsoc.org/Files/ARTGuidelines15052015.pdf
11. Nnambalirwa M, Govathson C, Evans D, McNamara L, Maskew M, Nyasulu P. Markers of poor adherence among adults with HIV attending Themba Lethu HIV Clinic, Helen Joseph Hospital, Johannesburg, South Africa. Trans R Soc Trop Med Hyg. 2016;110(12):696-704. https://doi.org/10.1093/trstmh/trx003 [ Links ]
12. World Health Organization. HIV drug resistance report 2017 [homepage on the Internet]. [ Links ] World Health Organization; 2017 [cited 2019 Aug 20]. Available from: https://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf?sequence=1
13. Hertogs K, Bloor S, De Vroey V, et al. A novel human immunodeficiency virus type 1 reverse transcriptase mutational pattern confers phenotypic lamivudine resistance in the absence of mutation 184V. Antimicrob Agents Chemother. 2000; 44(3):568-573. https://doi.org/10.1128/AAC.44.3.568-573.2000 [ Links ]
14. Achappa B, Madi D, Bhaskaran U, Ramapuram JT, Rao S, Mahalingam S. Adherence to antiretroviral therapy among people living with HIV. N Am J Med Sci. 2013;5(3):220-223. https://doi.org/10.4103/1947-2714.109196 [ Links ]
15. Pagès-Puigdemont N, Mangues MA, Masip M, et al. Patients' perspective of medication adherence in chronic conditions: A qualitative study. Adv Ther. 2016; 33:1740-1754. https://doi.org/10.1007/s12325-016-0394-6 [ Links ]
16. Laba TL, Lehnbom E, Brien J-A, Jan S. Understanding if, how and why non-adherent decisions are made in an Australian community sample: A key to sustaining medication adherence in chronic disease? Res Social Adm Pharm. 2015;11(2):154-162. https://doi.org/10.1016/j.sapharm.2014.06.006 [ Links ]
17. Conroy A, Leddy A, Johnson M, Ngubane T, Van Rooyen H, Darbes L. 'I told her this is your life': Relationship dynamics, partner support and adherence to antiretroviral therapy among South African couples. Cult Heal Sex. 2017;19(11):1239-1253. https://doi.org/10.1080/13691058.2017.1309460 [ Links ]
18. Johnson MO, Dilworth SE, Taylor JM, Darbes LA, Comfort ML, Neilands TB. Primary relationships, HIV treatment adherence, and virologic control. AIDS Behav. 2012;16:1511-1521. https://doi.org/10.1007/s10461-011-0021-0 [ Links ]
19. Robards J, Evandrou M, Falkingham J, Vlachantoni A. Marital status, health and mortality. Maturitas. 2012. https://doi.org/10.1016/j.maturitas.2012.08.007 [ Links ]
20. Shisana O. South African national HIV prevalence, incidence and behaviour survey, 2012 [homepage on the Internet]. [ Links ] HSRC Press; 2014 [cited 2019 Aug 21]. Available from: http://repository.hsrc.ac.za/bitstream/handle/20.500.11910/2490/8162.pdf?sequence=1&isAllowed=y
21. Hadland SE, Milloy M-J, Kerr T, et al. Young age predicts poor antiretroviral adherence and viral load suppression among injection drug users. AIDS Patient Care STDS. 2012;26(5):274-280. https://doi.org/10.1089/apc.2011.0196 [ Links ]
22. Bulage L, Ssewanyana I, Nankabirwa V, et al. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014-July 2015. BMC Infect Dis. 2017;17(1):326. https://doi.org/10.1186/s12879-017-2428-3 [ Links ]
23. Mujugira A, Celum C, Tappero JW, Ronald A, Mugo N, Baeten JM. Younger age predicts failure to achieve viral suppression and virologic rebound among HIV-1-infected persons in serodiscordant partnerships. AIDS Res Hum Retroviruses. 2016; 32(2):148-154. https://doi.org/10.1089/aid.2015.0296 [ Links ]
24. Akullian AN, Mukose A, Levine GA, Babigumira JB. People living with HIV travel farther to access healthcare: A population-based geographic analysis from rural Uganda. J Int AIDS Soc. 2016;19(1):20171. https://doi.org/10.7448/IAS.19.1.20171 [ Links ]
25. Buor D. Analysing the primacy of distance in the utilization of health services in the Ahafo-Ano South district, Ghana. Int J Health Plann Manage. 2003;18(4):293-311. https://doi.org/10.1002/hpm.729 [ Links ]
26. Schoeps A, Gabrysch S, Niamba L, Sié A, Becher H. The effect of distance to health-care facilities on childhood mortality in rural Burkina Faso. Am J Epidemiol. 2011;173(5):492-498. https://doi.org/10.1093/aje/kwq386 [ Links ]
27. Cluver LD, Hodes RJ, Toska E, et al. 'HIV is like a tsotsi. ARVs are your guns': Associations between HIV-disclosure and adherence to antiretroviral treatment among adolescents in South Africa. AIDS. 2015;29 Suppl 1:S57-65. https://doi.org/10.1097/QAD.0000000000000695 [ Links ]
28. Rochat TJ, Mkwanazi N, Bland R. Maternal HIV disclosure to HIV-uninfected children in rural South Africa: A pilot study of a family-based intervention. BMC Public Health. 2015;29 Suppl 1:S57-65. https://doi.org/10.1186/1471-2458-13-147 [ Links ]
29. Wouters E, Van Loon F, Van Rensburg D, Meulemans H. Community support and disclosure of HIV serostatus to family members by public-sector antiretroviral treatment patients in the Free State Province of South Africa. AIDS Patient Care STDS. 2009;23(5):357-364. https://doi.org/10.1089/apc.2008.0201 [ Links ]
30. Kunutsor S, Walley J, Katabira E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: A randomized controlled trial. AIDS Behav. 2011;15(8):1795-1802. https://doi.org/10.1007/s10461-011-9927-9 [ Links ]
31. Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 24(9):1273-1280. https://doi.org/10.1097/QAD.0b013e328339e20e [ Links ]
32. Duwell MM, Knowlton AR, Nachega JB, et al. Patient-nominated, community-based HIV treatment supporters: Patient perspectives, feasibility, challenges, and factors for success in HIV-infected South African adults. AIDS Patient Care STDS. 2013;27(2):96-102. https://doi.org/10.1089/apc.2012.0348 [ Links ]
33. Kanters S, Park JJH, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: A systematic review and network meta-analysis. Lancet HIV. 2017; 4(1):e31-e40. https://doi.org/10.1016/S2352-3018(16)30206-5 [ Links ]
34. Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: A systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. https://doi.org/10.1371/journal.pmed.0030438 [ Links ]
35. Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: A systematic review and meta-analysis. AIDS. 2012. https://doi.org/10.1097/QAD.0b013e328351f5b2 [ Links ]
36. Cheng Y, Nickman NA, Jamjian C, et al. Predicting poor adherence to antiretroviral therapy among treatment-naïve veterans infected with human immunodeficiency virus. Medicine. 2018;97(2):e9495. https://doi.org/10.1097/MD.0000000000009495 [ Links ]
37. Petersen ML, Wang Y, Van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: A marginal structural model analysis. Clin Infect Dis. 2007;45(7):908-915. https://doi.org/10.1086/521250 [ Links ]
38. Azia IN, Mukumbang FC, Van Wyk B. Barriers to adherence to antiretroviral treatment in a regional hospital in Vredenburg, Western Cape, South Africa. South Afr J HIV Med. 2016;17(1):476. https://doi.org/10.4102/sajhivmed.v17i1.476 [ Links ]
39. Nozaki I, Dube C, Kakimoto K, Yamada N, Simpungwe JB. Social factors affecting ART adherence in rural settings in Zambia. AIDS Care. 2011;23(7):831-838. https://doi.org/10.1080/09540121.2010.542121 [ Links ]
40. Nassir Azmach N. Adherence to antiretroviral therapy and associated factors among adult ARV users in Arba Minch Hospital, Southern Ethiopia. Cent African J Public Heal. 2017;3(2):19-26. https://doi.org/10.11648/j.cajph.20170302.12 [ Links ]
41. Stawarz K, Rodríguez MD, Cox AL, Blandford A. Understanding the use of contextual cues: Design implications for medication adherence technologies that support remembering. Digit Heal. 2016; 1;2:2055207616678707. https://doi.org/10.1177/2055207616678707 [ Links ]
42. Nkomo G, Mosalo A, Thupayagale-Tshweneagae GB. Adherence to treatment and retention to care of adult patients on antiretroviral therapy. Afr J Nurs Midwifery. 2018;20(1):1-13. https://doi.org/10.25159/2520-5293/1590 [ Links ]
43. Wasti SP, Simkhada P, Randall J, Freeman JV, Van Teijlingen E. Factors influencing adherence to antiretroviral treatment in Nepal: A mixed-methods study. PLoS One. 2012;7(5):e35547. https://doi.org/10.1371/journal.pone.0035547 [ Links ]
44. Fonsah JY, Njamnshi AK, Kouanfack C, et al. Adherence to antiretroviral therapy (ART) in Yaoundé-Cameroon: Association with opportunistic infections, depression, ART regimen and side effects. PLoS One. 2017;12(1):e0170893. https://doi.org/10.1371/journal.pone.0170893 [ Links ]
45. Johnson MO, Dilworth SE, Taylor JM, Neilands TB. Improving coping skills for self-management of treatment side effects can reduce antiretroviral medication nonadherence among people living with HIV. Ann Behav Med. 2011;41(1):83-91. https://doi.org/10.1007/s12160-010-9230-4 [ Links ]
46. Moyo F, Chasela C, Brennan AT, et al. Treatment outcomes of HIV-positive patients on first-line antiretroviral therapy in private versus public HIV clinics in Johannesburg, South Africa. Clin Epidemiol. 2016;8:37-47. https://doi.org/10.2147/CLEP.S93014 [ Links ]
47. Goodman N. The impact of employment on the health status and health care costs of working-age people with disabilities. LEAD Cent Policy Br. 2015. [ Links ]
48. Kilian R, Lauber C, Kalkan R, et al. The relationships between employment, clinical status, and psychiatric hospitalisation in patients with schizophrenia receiving either IPS or a conventional vocational rehabilitation programme. Soc Psychiatry Psychiatr Epidemiol. 2012;47(9):1381-1389. https://doi.org/10.1007/s00127-011-0451-z [ Links ]
49. Barnett W, Patten G, Kerschberger B, et al. Perceived adherence barriers among patients failing second-line antiretroviral therapy in Khayelitsha, South Africa. South Afr J HIV Med. 2013;14(4):170-176. https://doi.org/10.4102/sajhivmed.v14i4.51 [ Links ]
50. Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV/AIDS. 2013;5:1-17. https://doi.org/10.2147/HIV.S28912 [ Links ]
51. Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: A rapid systematic review. AIDS. 2014; 28:S187-S204. https://doi.org/10.1097/QAD.0000000000000252 [ Links ]
52. Ridgeway K, Dulli LS, Murray KR, et al. Interventions to improve antiretroviral therapy adherence among adolescents in low- and middle-income countries: A systematic review of the literature. PLoS One. 2018;13(1):e0189770. https://doi.org/10.1371/journal.pone.0189770 [ Links ]
 Correspondence:
Correspondence:
Siphamandla Gumede
sgumede@cartafrica.org
Received: 24 May 2020
Accepted: 13 June 2020
Published: 11 Aug. 2020
ORIGINAL RESEARCH
Feasibility of implementing same-day antiretroviral therapy initiation during routine care in Ekurhuleni District, South Africa: Retention and viral load suppression
Nolundi Mshweshwe-PakelaI; Bhakti HansotiII, III; Tonderai MabutoI, IV; Deanna KerriganV; Griffiths KubekaI; Elizabeth HahnIII; Salome CharalambousI, IV; Christopher J. HoffmannI, VI, VII
IImplementation Research Division, The Aurum Institute, Johannesburg, South Africa
IIDepartment of Emergency Medicine, Johns Hopkins School of Medicine, Baltimore, United States of America
IIIDepartment of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, United States of America
IVSchool of Public Health, University of the Witwatersrand, Johannesburg, South Africa
VDepartment of Sociology, American University, Washington, United States of America
VIDepartment of Medicine, Johns Hopkins University School of Medicine, Baltimore, United States of America
VIIDepartment of Health, Behavior, and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, United States of America
ABSTRACT
BACKGROUND: Same-day initiation (SDI) of antiretroviral therapy (ART) has been advocated as an approach to increase linkage to care and overall ART initiation. Clinical trials have demonstrated impressive benefits. However, questions regarding patient preparedness and retention in care remain for routine implementation of this approach
OBJECTIVES: In this study, we sought to describe SDI of ART during routine care delivery and compare time to ART initiation on longitudinal care outcomes
METHOD: We performed a retrospective chart review of 100 consecutive individuals, newly diagnosed with HIV, from 10 health facilities across Ekurhuleni, from January to July 2017. Records were reviewed for a period of 1 year post-diagnosis. Abstracted data included demographics, time to ART initiation, clinic visits and laboratory test results (including viral load testing
RESULTS: A total of 993 patient records were reviewed, of which 826 were included in the analysis. The majority of patients (752, 91%) had ART initiation recorded, of which 654 (79%) had ART initiated within 30 days, and 224 (27%) had SDI. Uptake of SDI of ART was higher among women (36% vs. 10.4%; p < 0.001) and in younger patients (33.7% in those < 29 years; p < 0.01). Retention in care at 6 months was achieved in 477 (58%) patients. Of those with 6-month viral loads, 350/430 (73%) had a viral load < 400 c/m. Retention in care and viral suppression were similar among those with SDI of ART and later ART initiation
CONCLUSION: Same-day initiation of ART was successfully delivered with similar retention and viral load outcomes as subsequent initiation, providing re-assurance for scale-up of this strategy in routine care
Keywords: HIV testing; primary care; SDI; ARV initiation; implementation.
Introduction
Timely linkage to antiretroviral therapy (ART) is pivotal to decreasing human immunodeficiency virus (HIV) transmission and reducing HIV-associated morbidity and mortality.1 Increasing evidence indicates that same-day initiation (SDI) of ART can increase overall linkage to care (LTC) and the proportion of patients with viral load suppression.2,3,4,5,6,7 Randomised studies from South Africa, Haiti and Lesotho have demonstrated the feasibility, acceptability and improved retention over 6 months with SDI of ART when compared with standard practices in research settings.5,8,9 In response to clinical trial evidence of the benefits of SDI of ART, the South African Department of Health announced a policy of universal testing and treatment (UTT) in public health facilities in September 2016 and endorsed SDI of ART.10 The UTT directorate specified: 'ART should be started as soon as the patient is ready and within 2 weeks', with immediate (same-day) initiation prioritised for pregnant or breastfeeding women.10 Same-day initiation of ART was further highlighted as a priority in a 2017 South African Department of Health circular.10,11 and in the 2017 World Health Organization rapid ART initiation guidelines.12
As a counterpoint to SDI of ART enthusiasm, there are concerns regarding the operational feasibility of SDI in public health facilities providing routine care. These concerns include failure to rule-out important opportunistic infections, challenges with assuring adequate renal function for some primary ART regimens and patient readiness to cope with a new HIV diagnosis and initiate life-long ART.1,13,14,15,16 Furthermore, SDI of ART may not allow time (and focussed time) to provide important HIV education and adherence counselling that may be valuable for long-term ART success.16,17,18 Moreover, a recent South African study demonstrated a reduced rate of retention among same-day initiators.19 These reports of early findings warrant further investigation to understand the impact of routine delivery of same-day ART. Lastly, SDI of ART adds a further service burden to an already overwhelmed system. The shortage of 'nurse-initiation and management of ART' (NIMART) trained staff, limited resources and long waiting times in facilities pose system-level barriers that may impede the effective implementation of SDI of ART and contribute to the negative outcomes already reported.
In spite of the challenges associated with SDI of ART, it still offers important benefits to both the patient and healthcare system. In addition to early access to a definitive management strategy, SDI obviates the need for repeat visits and is likely a more attractive option for young working adults.19 Furthermore, routinisation of SDI may in some way normalise ART initiation, by providing a similar approach to HIV management as other chronic health conditions (i.e. high blood pressure management where antihypertensive medications are often started during the clinic visit without the need for extensive repeat visits or referral to a specialised clinical setting).
This study seeks to describe our experience with SDI across 10 healthcare facilities in the Ekurhuleni District of South Africa and characterise both patients- and health facility-associated factors that impact SDI success, namely retention in care and viral load suppression.
Materials and methods
Study design
This is a retrospective clinical review of patients who presented for routine care and tested HIV positive at 10 public sector health facilities in the Ekurhuleni District of South Africa between 01 January 2017 and 31 July 2017. We abstracted clinical data from patient charts (including patient files and HIV testing registers) and electronic databases (Tier.Net20 and the South African National Health Laboratory Service [NHLS] database). All data were collected after the pathway of care was completed, there was no interaction with patients and written informed consent was not obtained. All data were de-identified prior to data capture and data analysis.
Study setting and population
The study took place in the Ekurhuleni District (Gauteng Province) of South Africa. This district has the second-highest district-level HIV prevalence (14.3%) in South Africa and is comprised of urban and peri-urban settings.21,22 The study sites consisted of six primary healthcare clinics, three community healthcare centres and one outpatient department at a district-level hospital. Facilities were selected to achieve geographic diversity within the district. All facilities had HIV counselling and testing personnel (lay counsellors) and routinely provide free HIV testing services (HTS) according to the 2015 National HIV Counselling and Testing (HCT) and the UTT policy guidelines.23,24
Sampling and data collection
We aimed to include 100 consecutive adult patients with an HIV-positive diagnosis from each of the 10 health facilities for a total sample of approximately 1000 patients. This sample size was selected to provide an estimate of LTC within 20% of the point estimate, assuming 50% LTC, an alpha of 0.05 and 80% power. Consecutive patients were identified from the HIV testing registers and were included if they were aged 18 or older and had a positive HIV test recorded in the clinic HIV testing register between January 1st and July 31st 2017. Approximately 1 year after the date of diagnosis, paper and electronic clinical records were abstracted for each selected patient, from the clinic at which HIV testing occurred. The data abstracted included sex, age, CD4 count, viral load (HIV RNA), creatinine, ART initiation, HIV care visits, tuberculosis (TB) testing, TB test results and TB preventive therapy prescribing.
Outcome measures
Linkage to care was defined as documentation of ART prescription. Same-day initiation of ART was defined as having the same date of HIV diagnosis and ART initiation.25 Rapid ART initiation that did not occur on the day of HIV testing was defined as initiation between 1 and 7 days after HIV diagnosis.12 Retention in care was defined as evidence of a care visit (in the same facility where patients had initiated ART) or HIV-related laboratory testing between 91 and 365 days after ART initiation. Six-month viral suppression was defined as a viral load of < 400 c/mL (for consistency with prior reports) between 4 and 8 months after ART initiation.
Statistical analyses
Descriptive statistics were used to explore the relationship between patient characteristics and the timing of ART initiation (same-day, 1-7, 8-30, 31-90; > 90 days). Comparisons of factors associated with the timing of ART initiation were conducted using chi-square tests with particular interest in clinic attended, sex, age group, CD4 count and TB testing. Log binomial regression modelling (used to approximate relative risk) was used to assess for associations between the timing of ART initiation and viral suppression and retention in care while adjusting for age, sex, CD4 count and facility. Data were analysed using STATA© v.14 (StataCorp, LLC, Texas).
Ethical consideration
The study was approved by the University of the Witwatersrand Human Research Ethics Committee, the Johns Hopkins University School of Medicine Institutional Review Board and the Ekurhuleni District Research Committee.
Results
A total of 993 patient records were identified and abstracted during the study period. Of these, 826 (83.2%) patients were included in the analysis and 167 (16.8%) were excluded. Reasons for exclusion included: 111 (66.5%) had ART recorded prior to testing date, 49 (29.3%) were documented to have transferred out of the healthcare facility and 7 (4.2%) were documented to have died. Of those included, 528 (63.9%) were women, and the median age was 32 years (interquartile range [IQR]; 27, 39). Of the 10 study clinics, the number of patients included in the analysis ranged from 65 to 99 per clinic.
Patient characteristics at human immunodeficiency virus testing
The majority of patients had CD4 count documentation (610, 73.8%; Table 1) with a median CD4 count of 312 cells/mm3 (IQR; 156, 490). Of those with CD4 count documentation, nearly a third had a CD4 count of ≤ 200 cells/mm3 (192, 31.5%) (Table 1). Serum creatinine was documented for less than half of the patients (414, 50.1%), of which 14 (3.4%) had an estimated glomerular filtration rate of < 50 ml/min/m2. Tuberculosis testing (GeneXpert MTB/Rif) was documented for 45 (5.4%) patients, of which 10 tested positive for TB.
Timing of antiretroviral therapy initiation
Of the 826 patients included in the analysis, ART initiation within a year of HIV testing was documented for 710 (85.9%; Table 1). Of these, the majority, 654 (92.1%), of patients initiated ART within 30 days after HIV testing (median of 8 days; IQR: 0, 15). A similar proportion of men and women initiated ART (83% and 88%, respectively; p = 0.06). Of the 10 patients testing positive for TB, seven were initiated on ART within 30 days with the remaining three initiated on ART within 90 days.
Antiretroviral therapy was initiated on the same day as HIV testing for 221 patients (31.1%). The proportion of patients initiated on ART who had SDI varied considerably by facility, ranging from 8.1% to 61% (10% - 69% for women and 0% - 45% for men). A significantly higher proportion of women, compared with men, initiated ART on the same day (36% vs. 10.4%, p < 0.001) (Table 2). Same-day initiation of ART was also more common among those aged < 29 years (102, 33.7%), compared with those aged 30-40 years (89, 26.6%), and those older than 40 years (30, 16%; p = 0.009). Lastly, a greater proportion of patients with a higher CD4 count initiated on the same-day: 42 (30%) of those with a CD4 count > 500 cells/mm3 compared with 60 (21.6%) among those with a CD4 count between 200 and 500 cells/mm3 and 23 (12%) of those with CD4 count < 200 cells/mm3 (p < 0.001; Table 3). Notably, the CD4 count results were not available to clinicians on the day of HIV testing, so decisions around SDI were not influenced by the CD4 count.
Retention in care by timing of antiretroviral therapy initiation
A total of 477 patients (67.2% of patients who initiated ART) met our definition of retention in care at 6 months. There was no association between retention in care and time of ART initiation; 69.2% (153) of those who initiated same-day ART were retained at 6 months compared with 66.1% (286) who initiated ART between 1 and 30 days post-diagnosis (p = 0.47; Table 3). In a model-adjusted for sex, age and CD4 count, time of ART initiation did not significantly impact the relative likelihood retention in care at 6 months (Table 4).
Viral load suppression by timing of antiretroviral therapy initiation
A total of 455 patients (64.1% of patients who initiated ART) had a 6-month viral load result documented, of which 359 (79%) were virally suppressed (viral load < 400 c/mL; Table 3). There was no significant association between viral suppression and time to ART initiation; 78.1% (118) of patients who initiated same-day ART were virally suppressed compared with 80.3% (216) patients who initiated ART between 1 and 30 days post-diagnosis (p = 0.5; Table 4). In a model adjusted for sex, age group and CD4 count, time of ART initiation did not significantly impact the relative likelihood of viral suppression (Table 4).
Discussion
We found that just over a quarter of patients at 10 routine care facilities in South Africa initiated same-day ART. Of those initiating ART, 58% were retained in care at the same clinic at 6 months, and 58% of those retained in care at 6 months had a viral load of < 400 c/mL (80% of those with viral load test results). There was no significant association between the timing of ART initiation and viral suppression or retention in care. Furthermore, we identified that same-day ART achieves similar retention in care and viral suppression when compared with later initiation. These findings suggest that provision of SDI does not adversely impact long-term care outcomes. It is plausible that the overall retention and viral load suppression is a superior strategy to delayed ART initiation, because of less time lost from care, by reducing the time between the testing visit and subsequent clinic visit. These are important and encouraging early findings from the routine implementation of SDI that help to expand the understanding of the use of SDI beyond clinical trial settings.
We found several patient-level characteristics that impacted the likelihood of SDI, namely sex, age and CD4 count. Healthier, young women were more likely to be initiated on ART and initiated on the same day. It is unclear if this was because of provider biases in terms of who was offered SDI of ART, or a potential pitfall when engaging older male patients into SDI of ART. It is possible that higher rates of ART initiation in women are accounted for by same-day ART among pregnant women as part of prevention of mother-to-child transmission (PMTCT); the overall lower engagement in care among men may also have contributed to our findings.26,27,28 In contrast to general ART initiation studies and an SDI study from Zimbabwe, we observed that younger patients (men and women) were more likely to have SDI compared with older patients.29 This is an intriguing finding and contrasts with findings that older individuals are more likely to initiate ART.30,31 It is plausible that SDI may have an especially important role in engaging young adult patients in care.30 Overall, the most substantial variation in SDI was by facility (10 - 69% for women and 0 - 45% for men). This variability was not associated with the type of health facility or structure, nor daily headcount, suggesting that health facility practice and provider discretion were the primary determinants of whether or not a patient received SDI. Further qualitative research within healthcare facilities to understand facility-based barriers to SDI is needed to support this initiative.
Studies of retention in care with SDI are limited to studies of PMTCT and research studies.32 Research studies on SDI have reported increased retention in care, whereas some PMTCT studies have reported a higher loss from care with same-day ART.5,6,32,33,34,35,36,37 Same-day ART may improve outcomes by reducing the cost of care and enhancing the perceived value of ART because of the immediacy of receiving the therapy.38,39 The findings of our study present a stark contrast to a recently published study led by Lilian et al., which presents data on the national impact of SDI on retention in care.19 Routinely collected data from TIER.Net (n = 32 290) were analysed for HIV-infected adults who were newly initiating ART in Johannesburg or Mopani districts between October 2017 and June 2018. Their study identified a significant increase in lost to follow-up (LTFU) at 30.1% in the SDI group compared with 22.4%, 19.8% and 21.9% among clients initiating ART 1-7 days, 8-21 days and ≥ 22 days after HIV diagnosis, respectively.19 Although LTFU was similar to our study (overall LTFU was 32.8%, 233/710), the association between the timing of ART initiation and LTFU was not found. Although our study was smaller, it benefitted from the triangulation of multiple data sources that may capture individuals missed in the Tier.Net system. This likely reveals the bias of using a single data source, which may underestimate LTC. Additionally, it is also possible that there is a heterogeneity in the success of SDI by district and even by clinic. Our study within a single district (Ekurhuleni) had more promising LTC results than those in the neighbouring districts of Johannesburg and Mopani. A deeper dive into the implementation practices across districts and their relative LTC outcomes is required to understand this phenomenon further.
This study has the strength of assessing multiple clinics that provided routine care in a real-world setting without augmentation from research or academic staff. We also used multiple sources for data collection, paper and electronic and both local and national, to the covert possibility of missing or incorrect data in one source. Some notable limitations arise from the use of chart review to assess successful LTC. Linkage to care and ART initiation required documentation in a file at the same clinic as HIV testing occurred. Patients who initiated ART at other facilities were considered as not having initiated ART. Furthermore, we also did not abstract a reason for visit at the time of HIV testing, making it impossible to compare ART for PMTCT with other initiation reasons. Finally, time to ART initiation was not randomised. As a result, more motivated individuals may have started on the same day. It is also plausible that some same-day initiators were just doing what they were told and were not motivated. Comparing early ART (1-7 days after diagnosis) with same-day ART provides a group with motivation demonstrated by a prompt return to the clinic to the potentially more heterogeneous same-day group. However, the similar outcomes between these two groups are reassuring.
Conclusion
This study demonstrates the feasibility of SDI of ART and similar outcomes with subsequent initiation. These findings are reassuring and should support continued scale-up of SDI of ART. The finding of higher SDI of ART among younger individuals may suggest that this approach is especially promising to improve care engagement among this population. Operational research should be continued to identify challenges and barriers to inform programmatic delivery of same-day ART.
Acknowledgements
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
N.M-P., T.M., C.H., D.K. and S.C. designed the research study; T.M., N.M-P, G.K. and S.C. performed the research; B.H., C.H. and E.H. analysed the data; B.H., N.M-P., T.M. and C.H. wrote the article. All authors have read and approved the final manuscript.
Funding information
The study was funded by Project SOAR (Supporting Operational AIDS Research). Project SOAR (Cooperative Agreement AID-OAA-A-14-00060) was made possible by the generous support of the American people through the United States President's Emergency Plan for AIDS Relief (PEPFAR) and United States Agency for International Development (USAID).
Data availability statement
De-identified data will be available upon written request to the corresponding author, Dr Christopher J. Hoffmann (choffmann@jhmi.edu).
Disclaimer
The contents of this article are the sole responsibility of the authors and do not necessarily reflect the views of PEPFAR, USAID or the United States Government.
References
1. Ford N, Migone C, Calmy A, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. 2018;32(1):17-23. https://doi.org/10.1097/QAD.0000000000001671 [ Links ]
2. Govindasamy D, Meghij J, Negussi EK, Baggaley RC, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low-and middle-income settings - A systematic review. J Int AIDS Soc. 2014;17(1):19032. https://doi.org/10.7448/IAS.17.1.19032 [ Links ]
3. Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: A systematic review. J Int AIDS Soc. 2012;15(2):17383. https://doi.org/10.7448/IAS.15.2.17383 [ Links ]
4. Fox MP, Rosen S. A new cascade of HIV care for the era of 'treat all'. PLoS Med. 2017;14(4):e1002268. https://doi.org/10.1371/journal.pmed.1002268 [ Links ]
5. Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: The RapIT randomised controlled trial. PLoS Med. 2016;13(5):e1002015. https://doi.org/10.1371/journal.pmed.1002015 [ Links ]
6. Koenig SP, Dorvil N, Devieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomised unblinded trial. PLoS Med. 2017;14(7):e1002357. https://doi.org/10.1371/journal.pmed.1002357 [ Links ]
7. Blanc F-X, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471-1481. [ Links ]
8. Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: A stepped-wedge cluster-randomised trial. Lancet HIV. 2016;3(11):e539-e48. https://doi.org/10.1016/S2352-3018(16)30090-X [ Links ]
9. Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a U.S. public health setting. J Acquir Immune Defic Syndr. 2017;74(1):44-51. https://doi.org/10.1097/QAI.0000000000001134 [ Links ]
10. Pillay Y, Pillay A. Implementation of the universal test and treat strategy for HIV positive patients and differentiated care for stable patients. Pretoria: BMJ Publishing Group; 2016. [ Links ]
11. SANAC. South Africa's National Strategic Plan for HIV, T.B. and STIs 2017-2022. Pretoria: South African National AIDS Council; 2017. [ Links ]
12. World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: WHO; 2017. [ Links ]
13. Schulz S, Draper H, Naidoo P. A comparative study of tuberculosis patients initiated on ART and receiving different models of TB-HIV care. Int J Tuberc Lung Dis. 2013;17(12):1558-1563. https://doi.org/10.5588/ijtld.13.0247 [ Links ]
14. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496-505. https://doi.org/10.1086/655681 [ Links ]
15. Black S, Zulliger R, Marcus R, Mark D, Myer L, Bekker L-G. Acceptability and challenges of rapid ART initiation among pregnant women in a pilot programme, Cape Town, South Africa. AIDS Care. 2014;26(6):736-741. https://doi.org/10.1080/09540121.2013.855300 [ Links ]
16. Helova A, Akama E, Bukusi EA, et al. Health facility challenges to the provision of Option B+ in western Kenya: A qualitative study. Health Policy Plan. 2016;32(2):283-291. https://doi.org/10.1093/heapol/czw122 [ Links ]
17. Chan AK, Kanike E, Bedell R, et al. Same day HIV diagnosis and antiretroviral therapy initiation affects retention in Option B+ prevention of mother-to-child transmission services at antenatal care in Zomba District, Malawi. J Int AIDS Soc. 2016;19(1):20672. https://doi.org/10.7448/IAS.19.1.20672 [ Links ]
18. Langwenya N, Phillips TK, Brittain K, Zerbe A, Abrams EJ, Myer L. Same-day antiretroviral therapy (ART) initiation in pregnancy is not associated with viral suppression or engagement in care: A cohort study. J Int AIDS Soc. 2018;21(6):e25133. https://doi.org/10.1002/jia2.25133 [ Links ]
19. Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RP. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: Analysis of routine data. PloS One. 2020;15(1):e0227572. https://doi.org/10.1371/journal.pone.0227572 [ Links ]
20. Osler M, Hilderbrand K, Hennessey C, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc. 2014;17(1):18908. https://doi.org/10.7448/IAS.17.1.18908 [ Links ]
21. Shisana O, Rehle T, Simbayi LC, et al. South African national HIV prevalence, incidence and behaviour survey. Pretoria: BMJ Publishing Group; 2012. [ Links ]
22. Zuma K, Shisana O, Rehle TM, et al. New insights into HIV epidemic in South Africa: Key findings from the National HIV prevalence, incidence and behaviour survey, 2012. Afr J AIDS Res. 2016;15(1):67-75. https://doi.org/10.2989/16085906.2016.1153491 [ Links ]
23. National Department of Health. National HIV Counselling and Testing (HCT) Policy Guidelines. Pretoria: Department of Health; 2015. [ Links ]
24. HSRC. HSRC: Research methodology and data centre, S.A. [ Links ] [homepage on the Internet]. 2018. [cited 2020 Jan 01]. Available from: http://www.hsrc.ac.za/en/departments/rmdc
25. PEPFAR. South Africa Country Operational Plan 2017(COP17): PEPFAR [homepage on the Internet]. [ Links ] 2019 [cited 2019 Jun 04]. Available from: https://www.pepfar.gov/documents/organization/272022.pdf
26. Johannessen A. Are men the losers of the antiretroviral treatment scale-up? AIDS. 2011;25(9):1225-1226. https://doi.org/10.1097/QAD.0b013e32834403b8 [ Links ]
27. Mills EJ, Beyrer C, Birungi J, Dybul MR. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med. 2012;9(2):e1001167. https://doi.org/10.1371/journal.pmed.1001167 [ Links ]
28. Taylor-Smith K, Tweya H, Harries A, Schoutene E, Jahn A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J. 2010; 22(2);49-56. https://doi.org/10.4314/mmj.v22i2.58794 [ Links ]
29. Rufu A, Chitimbire V, Nzou C, et al. Implementation of the 'Test and Treat' policy for newly diagnosed people living with HIV in Zimbabwe in 2017. Public Health Action. 2018;8(3):145-150. https://doi.org/10.5588/pha.18.0030 [ Links ]
30. Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65. https://doi.org/10.1097/QAI.0b013e318199072e [ Links ]
31. Eduardo E, Lamb MR, Kandula S, et al. Characteristics and outcomes among older HIV-positive adults enrolled in HIV programs in four sub-Saharan African countries. PloS One. 2014;9(7):e103864. https://doi.org/10.1371/journal.pone.0103864 [ Links ]
32. McNaghten A, Mneimneh AS, Farirai T, et al. Strengthening HIV test access and treatment uptake study (Project STATUS): A randomised trial of HIV testing and counseling interventions. J Acquir Immune Defic Syndr. 2015;70(4):e140. https://doi.org/10.1097/QAI.0000000000000785 [ Links ]
33. Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77. https://doi.org/10.1038/nature16044 [ Links ]
34. Genberg BL, Naanyu V, Wachira J, et al. Linkage to and engagement in HIV care in western Kenya: An observational study using population-based estimates from home-based counselling and testing. Lancet HIV. 2015;2(1):e20-e6. https://doi.org/10.1016/S2352-3018(14)00034-4 [ Links ]
35. Rosen S, Fox MP, Larson BA, et al. Simplified clinical algorithm for identifying patients eligible for immediate initiation of antiretroviral therapy for HIV (SLATE): Protocol for a randomised evaluation. BMJ Open. 2017;7(5):e016340. https://doi.org/10.1136/bmjopen-2017-016340 [ Links ]
36. Labhardt ND, Ringera I, Lejone TI, Masethothi P, Kamele M, Gupta RS, et al. Same day ART initiation versus clinic-based pre-ART assessment and counselling for individuals newly tested HIV-positive during community-based HIV testing in rural Lesotho-a randomised controlled trial (CASCADE trial). BMC Public Health. 2016;16(1):329. https://doi.org/10.1186/s12889-016-2972-6 [ Links ]
37. Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: The CASCADE randomised clinical trial. JAMA. 2018;319(11):1103-1112. https://doi.org/10.1001/jama.2018.1818 [ Links ]
38. Mabuto T, Latka MH, Kuwane B, Churchyard GJ, Charalambous S, Hoffmann CJ. Four models of HIV counseling and testing: Utilisation and test results in South Africa. PLoS One. 2014;9(7):e102267. https://doi.org/10.1371/journal.pone.0102267 [ Links ]
39. Biadgilign S, Deribew A, Amberbir A, Deribe K. Adherence to highly active antiretroviral therapy and its correlates among HIV infected pediatric patients in Ethiopia. BMC Pediatr. 2008;8(1):53. https://doi.org/10.1186/1471-2431-8-53 [ Links ]
 Correspondence:
Correspondence:
Christopher Hoffmann
choffmann@jhmi.edu
Received: 10 Mar. 2020
Accepted: 07 May 2020
Published: 20 Aug. 2020
REVIEW ARTICLE
Future approaches to clearing the latent human immunodeficiency virus reservoir: Beyond latency reversal
Alexander M.L. Hayes
Medical Sciences Division, Faculty of Clinical Medicine, University of Oxford, Oxford, United Kingdom
ABSTRACT
BACKGROUND: While combined antiretroviral therapy (cART) allows near-normal life expectancy for people living with human immunodeficiency virus (HIV), it is unable to cure the infection and so life long treatment is required
OBJECTIVES: The main barrier to curing HIV is the latent reservoir of cells, which is stable and resistant to cART
METHOD: Current approaches under investigation for clearing this reservoir propose a 'Shock and Kill' mechanism, in which active replication is induced in latent cells by latency reversal agents, theoretically allowing killing of the newly active cells
RESULTS: However, previous studies have failed to achieve depletion of the T central memory cell reservoir, are unable to target other latent reservoirs and may be causing neurological damage to participants
CONCLUSION: Future approaches to clearing the latent reservoir may bypass latency reversal through the use of drugs that selectively induce apoptosis in infected cells. Several classes of these pro-apoptotic drugs have shown promise in in vitro and ex vivo studies, and may represent the basis of a future functional cure for HIV
Keywords: HIV; latency reversal; viral reservoir; pro-apoptotic drugs; cART.
Introduction
The global HIV/AIDS pandemic has caused over 25 million deaths since its origins in the 20th century and remains without a cure. Combined antiretroviral therapy (cART) is the current treatment of choice for human immunodeficiency virus (HIV) infection1 and suppresses viral load below detectable levels with correct administration.1,2 However, lifelong treatment is required, at considerable expense to healthcare systems,1 as the cessation of therapy causes a rapid rebound of viraemia within days3 and eventual progression of the disease to AIDS. Furthermore, escape mutations of the virus are common and reduce efficacy,4,5 necessitating expensive combination therapy with multiple drugs. Viral rebound occurs due to the presence of latent viral reservoirs, rather than continuing low-level replication during cART, as shown by clonal evolutionary studies.6 On therapy cessation these latently infected cells clonally expand, seeding a population of infected cells containing intact provirus.7 Whilst cART has been successful in extending life expectancy of people living with HIV in high-income countries, this remains impaired compared to healthy adults.8 As cART is limited by mutations, is an expensive lifelong treatment and may not be available to those in countries with poorly developed healthcare systems, development of a cure for HIV is desirable. Cure research would be of considerable impact in South Africa, which has the highest number of people living with HIV in the world, at 7.7 m as of 2018, a prevalence of 20.4% in adults (UNAIDS 2019)9. In that year 77 000 deaths were recorded due to AIDS-related illnesses (UNAIDS 2019), although this number is coming down and life expectancy is rising as the cART programme continues to grow.
Reservoirs of latently infected cells are the main barrier to cART curing HIV. The first latently infected cells to be identified were CD4+ central memory T cells (TCM) containing replication-competent, integrated provirus.10 These were found to be present in patients on cART, with no change in reservoir size with continuous therapy11 as the quiescent cells do not contain actively replicating virus, and so are unaffected by cART. The TCM- reservoir is present even if cART is initiated within the first week post exposure,12 so is established early in infection. It is also extremely stable, as the latent cells have a half-life of around 44 months,13 so is unlikely to be cleared in a patient's lifetime. The TCM- reservoir contains the majority of latent cells, but other reservoirs are also present and form barriers to cure research. Several myeloid cell lines have been shown to harbour latent HIV in chronic infection and contribute to viral rebound on cART cessation.14,15 There is evidence for the presence of latent reservoirs in tissue macrophages16 and microglial cells and astrocytes in the central nervous system (CNS).17,18 There is no in vivo evidence of latently infected tissue dendritic cells but infection has been shown in vitro,19 and it has been proposed that this may be transferred to CD4+ cells via a virological synapse.20 Follicular dendritic cells have been shown to maintain a stable pool of virus on their surface without being infected, providing a latent reservoir within secondary lymphoid tissue.21 Further reservoirs have been suggested, such as within fibrocytes,20 but it is now apparent that the TCM reservoir is not the only source of latently infected cells. Potential cures must, therefore, target all identified reservoirs to be successful.
Several approaches to clearing the latent reservoirs have been investigated, with the 'Shock and Kill' combination receiving particular interest. However, recent findings seem to suggest this is unlikely to ever provide a cure for HIV22,23,24,25,26,27,28,29,30 and may have unresearched deleterious side effects.31,32,33 An emerging class of drugs targeting the apoptosis pathways in latently infected cells may allow reservoir depletion without latency reversal, and therefore merit further research as a potential cure for HIV.
Shock and Kill
A potential approach to clearing HIV latent reservoirs is 'Shock and Kill', in which the reservoir would be 'shocked' with latency-reversing agents (LRAs) to induce replication of the provirus. 'Killing' of the activated cells would then be achieved through viral cytopathic effects, CD8+ T cells, cART or other agent, resulting in clearance of the latent cells and curing the infection. This mechanism is outlined in Figure 1. Several LRAs have been developed, many using Yang's in vitro model of latently infected CD4+ TCM34 to identify compounds that selectively induce viral replication in latent cells. These include disulfiram22 and histone deacetylase inhibitors (HDACIs) such as vorinostat and panobionstat. Early in vivo studies were promising as latency reversal was demonstrated with an increase in viral gene expression in the resting cells observed with disulfiram,23,24 vorinostat25 and panbionstat26 administration. However, the key caveat is that none of these studies decreased reservoir size in vivo as the number of latent cells did not decrease following latency reversal. Whilst replication was induced in a proportion of the latent cells, this did not lead to immune or viral-mediated killing of reactivated cells.
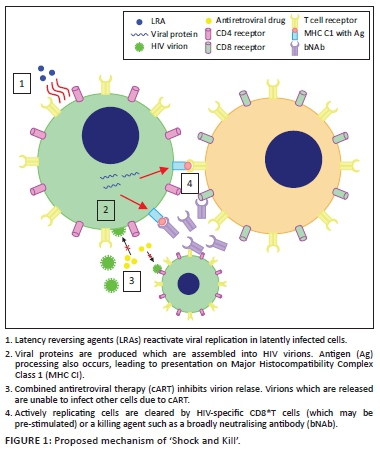
Several reasons have been proposed for the failure of LRAs alone to reduce reservoir size. The initial principle of 'Shock and Kill' was that viral cytopathic effects and lysis, due to HIV replication, aided by cytotoxic T lymphocyte (CTL)-mediated killing of actively infected cells, would be sufficient to kill reactivated, formerly latent, cells. Shan et al.27 showed that the viral cytopathic effect alone is insufficient to kill cells, and the vigorous CTL response is lost in chronic HIV infection. However, CTL stimulation increased latent cell clearance, leading to the suggestion that some form of immune stimulation may be required alongside latency reversal to achieve a reduction in reservoir size. The CTL response to reactivated cells may be insufficient to reduce reservoir size on latency reversal, due to 98% of latent T cells carrying escape mutations to CTL killing28 and T cell exhaustion in chronic infection29 reducing the efficacy of the CTL response. Furthermore, LRAs may not affect non-TCM- reservoirs30 as macrophages are resistant to the cytopathic effects of HIV, and dendritic cells do not integrate viral DNA, meaning that LRAs would be unable to cause viral replication and subsequent targeting by the immune system. It has also been proposed that continued cART may not completely inhibit infection of further cells by virions produced from reactivated, formerly latent cells.30 Thus, LRA administration may lead to infection of previously uninfected cells. It is therefore clear that LRA administration alone does not reduce reservoir size, due to a lack of viral cytopathic effects and lysis, insufficient CTL response and the potential failure of cART to suppress replication. As such, stages 3 and 4 from Figure 1 are unlikely to be successful and an additional killing agent is required to achieve the second goal of 'Shock and Kill'. This may involve immune stimulation, with natural killer (NK) cell stimulation showing some promise ex vivo35 or other agents, such as broadly neutralising antibodies (bNAbs), which appear to mediate antibody-dependent cellular cytotoxicity of activated latently infected cells in mice36 but human studies are required to assess their efficacy. Killing agents that selectively induce apoptosis of HIV-infected cells have also shown promise in vitro (see Pro-apoptotic drugs [PADs]).
Limitations of latency-reversing agents
Whilst it has been repeatedly shown that LRAs alone are unable to reduce the size of the latent HIV reservoir, administration of a killing agent alongside an LRA has been proposed as a method of clearing the reactivated cells.37 However, it seems likely that even with killing agents LRAs cannot clear enough latent cells to achieve a functional cure and may cause complications due to non-T cell HIV reservoirs. Future studies in humans to test the efficacy of killing agents administrated alongside LRAs could be carried out by administering a previously tested LRA, such as disulfiram, alongside a killing agent such as a bNAb to participants living with HIV and measuring the change in reservoir size using a technique such as TILDA.38 However, the combination of an LRA with, for example, a bNAb, is unlikely to clear the myeloid reservoir as dendritic cells are likely to be unaffected by LRAs.30 Therefore, even if functional elimination of the TCM reservoir is achieved, viral rebound on cART cessation may still occur due to the presence of the myeloid reservoir, which would likely not be cleared by current 'Shock and Kill' approaches under investigation.
Human immunodeficiency virus-associated neurological disorders (HANDs) are well-observed in patients on cART and may lead to widespread neurological impairment,39 suggesting significant damage to the CNS even when viraemia elsewhere is suppressed below detectable levels. The cause of this appears to be relatively poor blood-brain barrier (BBB) penetrance by antiretrovirals,31 allowing continued HIV replication alongside the presence of latently infected microglial cells, perivascular macrophages and astrocytes.20 Recent research suggests that impaired neurogenesis may underpin HAND persistence even with cART.32 A study on the CNS penetrance of LRAs33 showed that disulfiram and vorinostat are able to cross the BBB relatively easily. Whilst this could enable targeting of the CNS latent reservoir, it may mean that current in vivo studies of LRAs are damaging to the patients involved, as there is no reduction in latent reservoir size, whilst LRAs are able to penetrate the CNS and increase viral replication with no inhibition from antiretroviral drugs. This may potentially lead to an increase in viral protein, inflammatory cytokine (CK) and neurotoxin release from infected macrophages and microglia, which could increase the probability of HAND development (personal communication, Grant Campbell) as shown in Figure 2. Therefore, in addition to their inability to affect all HIV reservoirs, even with the accompanying administration of killing agents, LRAs may cause increased neurological impairment of patients. Furthermore, if the killing agent used in a hypothetical cure is unable to penetrate the BBB, there would be no reduction in the size of the CNS reservoir. This makes the development of a cure strategy distinct from 'Shock and Kill', involving the direct killing of latent cells without the need for latency reversal, desirable. The PAD class mentioned earlier has shown promise in achieving this.
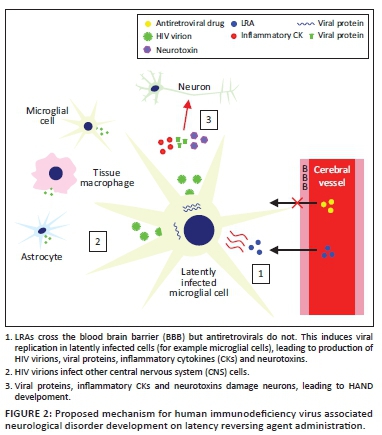
Interactions of human immunodeficiency virus with apoptosis
The human immunodeficiency virus has complex interactions with apoptosis pathways in infected cells.40 In latently infected cells the increase in longevity observed is partially the result of avoidance of apoptosis.37 A number of specific effects on apoptosis pathways have been suggested as the cause of this. The intrinsic apoptosis pathway is a response to cellular damage and involves pro-apoptotic factors causing mitochondrial outer membrane permeabilisation (MOMP), triggering the release of soluble proteins from the mitochondrial intermembrane space. These include the cytochrome c and second mitochondria-derived activator of caspase (SMAC, also known as DIABLO41). Cytochrome c binds cytosolic proteins to form the apoptosome, which activates the 'initiator' enzyme caspase-9. This, in turn, cleaves the 'executioner' enzymes caspase-3 and caspase-7 which trigger the cleavage cascade of other caspase enzymes resulting in cell death. The second mitochondria-derived activator of caspase activates this process through disinhibition of caspases 3, 7 and 9 via binding and inactivating the X-linked inhibitor of apoptosis (XIAP). The X-linked inhibitor of apoptosis binds these caspases in the absence of SMAC, inhibiting their activity and directing their degradation by the proteasome. The second mitochondria-derived activator of caspase antagonises this activity, allowing the progression of the apoptotic process.41 Several other IAPs are also inhibited by SMAC, including the baculoviral IAP repeat-containing protein 2 (BIRC, also known as cellular IAP1).41
Viral proteins, in particular Tat and Nef, cause inhibition of this intrinsic apoptosis pathway in latently infected cells. Tat upregulates host production of anti-apoptotic proteins including XIAP and Bcl2.42 The X-linked inhibitor of apoptosis inhibits caspase activity, as detailed earlier, whilst Bcl2 inhibits MOMP,43 thus preventing the release of cytochrome c and SMAC. Tat also inhibits the pro-apoptotic proteins p53 and the Bcl2-associated antagonist of cell death (BAD).40 Nef causes p21-activated kinase activation, leading to phosphorylation of BAD, thus inhibiting its pro-apoptotic activity (binding to and inhibition of Bcl2),44 and also inhibits caspase-8 and caspase-10.40 Conversely, in the CNS, it appears that Tat and Nef may induce autophagy and apoptosis in HAND pathogenesis.45,46 Expression and activation of anti-apoptotic proteins are therefore increased in latently infected cells, making this an attractive therapeutic target.
Pro-apoptotic drugs
Several classes of pro-apoptotic compounds have been developed recently for cancer treatment,47,48 as tumour cells often over-express or activate IAPs allowing the evasion of apoptosis, a key hallmark of cancer. Some of these have been shown to induce apoptosis of latent HIV infected cells in vitro, leading to speculation that PADs may contribute to a future cure.
Second mitochondria-derived activator of caspase mimetics (SMs) are a class of drugs which bind IAPs in a similar fashion to the endogenous molecule.48 They cause caspase activation via IAP binding49 and appear to reduce IAP activity through both degradation and inhibition of the proteins,50 and therefore induce apoptosis. Second mitochondrias have recently been developed for cancer treatment, inducing apoptosis48 of tumour cells which over-express or activate IAPs. The SMs birinapant, embelin and GDC-0152 were investigated for their efficacy to induce apoptosis in latently infected TCM by Campbell et al.51 All were found to cause rapid degradation of XIAP and BIRC2, which were upregulated in the infected cells, and importantly showed selective killing of HIV-infected TCM compared to uninfected TCM, with the doses required for 90% clearance of HIV-infected cells causing a small increase of TCM cell death, of 3.5% - 4.6% above basal levels. This occurred in the absence of increased virus production, despite findings in models of latency by Pache et al.52 suggesting that SMs may act as LRAs due to the reduction in BIRC2 leading to increased NFκβ signalling, resulting in an increased viral transcription. However, in latent HIV-TCM from patients undergoing cART, the Pache study showed that SMs only caused latency reversal when used in combination with an LRA such as vorinostat. Therefore, it appears that the major effect of SMs on latently infected TCM- -is to induce apoptosis rather than reactivate transcription.
Furthermore, Campbell et al. demonstrated the mechanism of apoptosis on SM administration is dependent on the induction (but not completion) of autophagy. Wortmannin (an inhibitor of early stages of autophagy) led to a reduction in apoptosis despite the degradation of IAPs by SMs. Further findings suggest that, following IAP degradation, autophagy proteins form a scaffold for the assembly of a ripoptosome-like death-inducing signaling complex (DISC), which can then initiate apoptosis via caspase 8 induction. This is an additional mechanism to the simple removal of IAP inhibition due to their degradation and may explain the selectivity of SMs for inducing death of HIV-TCM. Inhibitors of later stages of autophagy did not reduce apoptosis, suggesting that the process of autophagy itself is not required for this mechanism of cell death. Induction of autophagy is sufficient for apoptosis, as autophagy proteins allow DISC formation. IAPs are degraded by SMs in uninfected TCM but this does not lead to the same degree of cell death due to reduced autophagy induction and therefore decreased probability of DISC formation. HIV-TCM are more prone to autophagy induction than uninfected cells as a result of the effects of viral proteins. Gag and Nef interact with autophagy factors to increase autophagy induction, which in turn augments HIV yields in cells containing the actively replicating virus.53 Nef and Vif inhibit later stages of autophagy to prevent HIV degradation.53,54 Therefore, HIV-TCM are more prone to autophagy induction than uninfected cells, providing a potential explanation for the selective apoptosis observed in latently infected cells on SM administration. Over-expression or activation of IAPs in infected cells may also contribute to the selectivity of SMs.
These findings present ex vivo evidence of the selective killing of latently infected TCM by SMs, along with a convincing mechanism of how uninfected cells are spared. Clinical trials in humans may now be justified, but should be performed with caution as the side effects of these drugs in people living with HIV are unknown. A number of SMs have been used in phase I trials for various cancers, with a generally tolerable safety profile, although the occasional occurrence of cytokine release syndrome in patients is of some concern.55In vitro and ex vivo findings suggest SMs may be able to deplete the TCM reservoir in vivo, and, whilst if 90% of latently infected cells are killed (as in the Campbell study) the patient will not be cured of HIV, this eclipses reservoir clearance demonstrated by current 'Shock and Kill' approaches. However, it is unclear if these drugs would induce apoptosis in non-T cell reservoirs to the same degree, which may prevent the development of a functional cure. Future in vitro and ex vivo studies of PADs using latently infected myeloid cells from people living with HIV would be advisable to further test their suitability.
In vitro and ex vivo studies of other PADs have also shown promise. Bcl-2 antagonists such as venetoclax caused disinhibition of MOMP and led to the depletion of latent HIV-TCM from patients on cART in an ex vivo study.56 P13K/Akt inhibitors increase the expression of pro-apoptotic genes downregulated by HIV and have shown promise in predisposing latently infected macrophages and microglia to cell death.57,58 RIG-1 inducers activate an innate immune response to viral RNA, triggering apoptosis. An ex vivo study demonstrated selective depletion of latent HIV-TCM using the RIG-1 inducer acitretin.59 These classes of PADs also merit further investigation and could be used in combination with SMs in a future approach to clearing the latent reservoir to induce apoptosis in a greater portion of latently infected cells.
Conclusions
Recent 'Shock and Kill' research has failed to demonstrate significant depletion of the viral reservoir, and it remains unclear if the use of LRAs may cause HANDs. PADs may represent a promising future approach to curing HIV given their in vitro and ex vivo success in selectively causing apoptosis in latently infected cells, with SMs appearing effective against the TCM reservoir and P13K/Akt inhibitors against myeloid reservoirs. Several of these drugs have been used in clinical trials for other conditions, and further investigation into their potential for people living with HIV is merited.
Acknowledgements
Grant Campbell provided theoretical assistance via email correspondence for the limitations of the LRAs section, specifically about the potential neurological side effects LRAs may have.
Professor William James, Dunn School of Pathology, University of Oxford, was my supervisor for the review, and so offered me advice and guidance on selecting a subject area and how to go about writing the review. He has not read or edited the review since I began writing it due to university examination regulations.
Competing interests
I declare that no competing interests exists.
Author's contributions
I am the sole author for this work.
Ethical consideration
This article followed all ethical standards for carrying out research without direct contact with human or animal subjects.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of any affiliated agency of the author.
References
1. Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376(9734):49-62. https://doi.org/10.1016/s0140-6736(10)60676-9 [ Links ]
2. Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients onsuppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879-3884. https://doi.org/10.1073/pnas.0800050105 [ Links ]
3. Fischer M, Hafner R, Schneider C, et al. HIV RNA in plasma rebounds within days during structuredtreatment interruptions. AIDS. 2003;17(2):195-199. https://doi.org/10.1097/01.aids.0000042945.95433.4b [ Links ]
4. Poggensee G, Kücherer C, Werning J, et al. Impact of transmission of drug-resistant HIV on the course of infection and the treatment success. Data from the German HIV-1 seroconverter study. HIV Med. 2007;8(8):511-519. https://doi.org/10.1111/j.1468-1293.2007.00504.x [ Links ]
5. Wittkop L, Günthard H, de Wolf F, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect Dis. 2011;11(5):363-371. https://doi.org/10.1016/S1473-3099(11)70032-9 [ Links ]
6. Joos B, Fischer M, Kuster H, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci U S A. 2008;105(43):16725-16730. https://doi.org/10.1073/pnas.0804192105 [ Links ]
7. Simonetti FR, Sobolewski M, Fyne E, et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 invivo. Proc Natl Acad Sci U S A. 2016;113(7):1883-1888. https://doi.org/10.1073/pnas.1522675113 [ Links ]
8. Collaboration A TC. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293-299. https://doi.org/10.1016/S0140-6736(08)61113-7 [ Links ]
9. UNAIDS website: https://www.unaids.org/en/regionscountries/countries/southafrica [ Links ]
10. Chun TW, Stuyver L, Mizell S, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94(24):13193-13197. https://doi.org/10.1073/pnas.94.24.13193 [ Links ]
11. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295-1300. https://doi.org/10.1126/science.278.5341.1295 [ Links ]
12. Chun TW, Engel D, Berrey M, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95(15):8869-8873. https://doi.org/10.1073/pnas.95.15.8869 [ Links ]
13. Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(1):727-728. https://doi.org/10.1038/nm880 [ Links ]
14. Abbas W, Tariq M, Iqbal M, Kumar A, Herbein G. Eradication of HIV-1 from the macrophage reservoir: An uncertain goal? Viruses. 2015;7(4):1578-1598. https://doi.org/10.3390/v7041578 [ Links ]
15. Gama L, Abreu C, Shirk E, et al. SIV Latency in macrophages in the CNS. Curr Top Microbiol Immunol. 2018;417:111-130. https://doi.org/10.1007/82_2018_89 [ Links ]
16. Honeycutt JB, Thayer W, Baker C, et al. HIV persistence in tissue macrophages of humanized myeloidonly mice during antiretroviral therapy. Nat Med. 2017;23:638-643. https://doi.org/10.1038/nm.4319 [ Links ]
17. Churchill MJ, Gorry P, Cowley D, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146-152. https://doi.org/10.1080/13550280600748946 [ Links ]
18. Kramer-Hämmerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194-213. https://doi.org/10.1016/j.virusres.2005.04.009 [ Links ]
19. Kawamura T, Gulden F, Sugaya M, et al. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc Natl Acad Sci U S A. 2003;100(14):8401-8406. https://doi.org/10.1073/pnas.1432450100 [ Links ]
20. Kandathil AJ, Sugawara S, Balagopal A. Are T cells the only HIV-1 reservoir? Retrovirology. 2016;13:86. https://doi.org/10.1186/s12977-016-0323-4 [ Links ]
21. Spiegel H, Herbst H, Niedobitek G, Foss HD, Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992;140:15-22. [ Links ]
22. Xing S, Bullen C, Shroff N, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85(12):6060-6064. https://doi.org/10.1128/JVI.02033-10 [ Links ]
23. Spivak AM, Andrade A, Eisele E, et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014;58(6):883-890. https://doi.org/10.1093/cid/cit813 [ Links ]
24. Elliott JH, McMahon J, Chang C, et al. Short-term administration of disulfiram for reversal of latent HIV infection: A phase 2 dose-escalation study. Lancet HIV. 2015;2(12):e520-529. https://doi.org/10.1016/S2352-3018(15)00226-X [ Links ]
25. Archin NM, Liberty A, Kashuba A et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482-485. https://doi.org/10.1038/nature11286 [ Links ]
26. Rasmussen TA, Tolstrup M, Brinkmann C, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: A phase 1/2, single group, clinical trial. Lancet HIV. 2014;1(1):e13-e21. https://doi.org/10.1016/S2352-3018(14)70014-1 [ Links ]
27. Shan L, Deng K, Shroff N, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491-501. https://doi.org/10.1016/j.immuni.2012.01.014 [ Links ]
28. Deng K, Pertea M, Rongvaux A, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381-385. https://doi.org/10.1038/nature14053 [ Links ]
29. Chew GM, Fujita T, Webb G, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12:e1005349. https://doi.org/10.1371/journal.ppat.1005349 [ Links ]
30. Thorlund K, Horwitz MS, Fife BT, Lester R, Cameron DW. Landscape review of current HIV 'kick and kill' cure research - Some kicking, not enough killing. BMC Infect Dis. 2017;17:595. https://doi.org/10.1186/s12879-017-2683-3 [ Links ]
31. Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65-70. https://doi.org/10.1001/archneurol.2007.31 [ Links ]
32. Putatunda L, Ho WZ, Hu W. HIV-1 and compromised adult neurogenesis: Emerging evidence for a new paradigm of HAND persistence. AIDS Rev. 2019;21:11-22. https://doi.org/10.24875/AIDSRev.19000003 [ Links ]
33. Churchill MJ, Cowley DJ, Wesselingh SL, Gorry PR, Gray LR. HIV-1 transcriptional regulation in the central nervous system and implications for HIV cure research. J Neurovirol. 2015;21:290-300. https://doi.org/10.1007/s13365-014-0271-5 [ Links ]
34. Yang HC, Xing S, Shan L, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473-3486. https://doi.org/10.1172/JCI39199 [ Links ]
35. Garrido C, Abad-Fernandez M, Tuyishime M, et al. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol. 2018;92(12):e00235-18. https://doi.org/10.1128/JVI.00235-18 [ Links ]
36. Halper-Stromberg A, Lu C, Klein F, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989-999. https://doi.org/10.1016/j.cell.2014.07.043 [ Links ]
37. Kim Y, Anderson JL, Lewin SR. Getting the 'Kill' into 'Shock and Kill': Strategies to eliminate latent HIV. Cell Host Microbe. 2018;23(1):14-26. https://doi.org/10.1016/j.chom.2017.12.004 [ Links ]
38. Procopio FA, Fromentin R, Kulpa D, et al. A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine. 2015;2(8):874-883. https://doi.org/10.1016/j.ebiom.2015.06.019 [ Links ]
39. Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087-2096. https://doi.org/10.1212/WNL.0b013e318200d727 [ Links ]
40. Timilsina U, Gaur R. Modulation of apoptosis and viral latency - An axis to be well understood for successful cure of human immunodeficiency virus. J Gen Virol. 2016;97(4):813-824. https://doi.org/10.1099/jgv.0.000402 [ Links ]
41. Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7. https://doi.org/10.1101/cshperspect.a006080 [ Links ]
42. López-Huertas MR, Mateos E, Sánchez del Cojo M et al. The presence of HIV-1 Tat protein second exon delays fas protein-mediated apoptosis in CD4+ T lymphocytes: A potential mechanism for persistent viral production. J Biol Chem. 2013;288:7626-7644. https://doi.org/10.1074/jbc.M112.408294 [ Links ]
43. Llambi F, Moldoveanu T, Tait S, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44(4):517-531. https://doi.org/10.1016/j.molcel.2011.10.001 [ Links ]
44. Wolf D, Witte V, Laffert B, et al. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med. 2001;7:1217-1224. https://doi.org/10.1038/nm1101-1217 [ Links ]
45. Wu X, Dong H, Ye X, et al. HIV-1 Tat increases BAG3 via NF-κB signaling to induce autophagy during HIV-associated neurocognitive disorder. Cell Cycle. 2018;13:1614-1623. https://doi.org/10.1080/15384101.2018.1480219 [ Links ]
46. Dong H, Ye X, Zhong L, et al. Role of FOXO3 activated by HIV-1 tat in HIV-associated neurocognitive disorder neuronal apoptosis. Front Neurosci. 2019;4:44. https://doi.org/10.3389/fnins.2019.00044 [ Links ]
47. Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;150845:23. https://doi.org/10.1155/2014/150845 [ Links ]
48. Fulda S. Smac mimetics to therapeutically target IAP proteins in cancer. Int Rev Cell Mol Biol. 2017;330:157-169. https://doi.org/10.1016/bs.ircmb.2016.09.004 [ Links ]
49. Gao Z, Tian Y, Wang J, et al. A dimeric Smac/diablo peptide directly relieves caspase-3 inhibition by XIAP. Dynamic and cooperative regulation of XIAP by Smac/Diablo. J Biol Chem. 2007;282:30718-30727. https://doi.org/10.1074/jbc.M705258200 [ Links ]
50. Bertrand MJ, Milutinovic S, Dickson K, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30(6):689-700. https://doi.org/10.1016/j.molcel.2008.05.014 [ Links ]
51. Campbell GR, Bruckman RS, Chu YL, Trout RN, Spector SA. SMAC mimetics induce autophagy-dependent apoptosis of HIV-1-infected resting memory CD4+ T cells. Cell Host Microbe. 2018;24(5):689-702. https://doi.org/10.1016/j.chom.2018.09.007 [ Links ]
52. Pache L, Dutra MS, Spivak AM, et al. BIRC2/cIAP1 is a negative regulator of HIV-1 transcription and can be targeted by smac mimetics to promote reversal of viral latency. Cell Host Microbe. 2015;18(3):345-353. https://doi.org/10.1016/j.chom.2015.08.009 [ Links ]
53. Kyei GB, Dinkins C, Davis AS, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol. 2009;186(2):255-268. https://doi.org/10.1083/jcb.200903070 [ Links ]
54. Borel S, Robert-Hebmann V, Alfaisal J, et al. HIV-1 viral infectivity factor interacts with microtubule-associated protein light chain 3 and inhibits autophagy. AIDS. 2015;29(3):275-286. https://doi.org/10.1097/QAD.0000000000000554 [ Links ]
55. Boddu P, Carter BZ, Verstovek S, Pemmaraju N. SMAC mimetics as potential cancer therapeutics in myeloid malignancies. Br J Haematol. 2019;185(2):219-231. https://doi.org/10.1111/bjh.15829 [ Links ]
56. Cummins NW, Sainski AM, Dai H, et al. Prime, shock, and kill: Priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol. 2016;90:4032-4048. https://doi.org/10.1128/JVI.03179-15 [ Links ]
57. Lucas A, Kim Y, Rivera-Pabon O, et al. Targeting the PI3K/Akt cell survival pathway to induce cell death of HIV-1 infected macrophages with alkylphospholipid compounds. PLoS One. 2010;5(9):e13121. https://doi.org/10.1371/journal.pone.0013121 [ Links ]
58. Kim Y, Hollenbaugh JA, Kim DH, Kim B. Novel PI3K/Akt inhibitors screened by the cytoprotective function of human immunodeficiency virus type 1 Tat. PLoS One. 2011;6(7):e21781. https://doi.org/10.1371/journal.pone.0021781 [ Links ]
59. Li P, Kaiser P, Lampiris HW, et al. Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat Med. 2016;22:807-811. https://doi.org/10.1038/nm.4124 [ Links ]
 Correspondence:
Correspondence:
Alexander Hayes
Alexander-hayes@keble.ox.ac.uk
Received: 25 Mar. 2020
Accepted: 12 May 2020
Published: 12 Aug. 2020
ORIGINAL RESEARCH
Perspectives on oral pre-exposure prophylaxis use amongst female sex workers in Harare, Zimbabwe
Tinashe MudzvitiI, II; Anesu DhliwayoI; Byrone ChingombeIII; Bernard NgaraIV; Tsitsi G. Monera-PendukaI; Charles C. MapongaI, III, V; Gene D. MorseV, VI
ISchool of Pharmacy, University of Zimbabwe, Harare, Zimbabwe
IINewlands Clinic, Harare, Zimbabwe
IIIPopulation Services International, Harare, Zimbabwe
IVDepartment of Community Medicine, College of Health Sciences, University of Zimbabwe, Harare, Zimbabwe
VCenter for Integrated Global Biomedical Sciences, University at Buffalo, New York, United States
VITranslational Pharmacology Research Core, University at Buffalo, New York, United States
ABSTRACT
BACKGROUND: Pre-exposure prophylaxis (PrEP) could provide protection from human immunodeficiency virus (HIV) infection in sexually active persons at risk. Limited data are available in Zimbabwe with regard to the perceptions about PrEP amongst female sex workers (FSWs
OBJECTIVES: The aim of this study was to evaluate the knowledge levels of oral PrEP and the likelihood of its use amongst FSWs.
METHOD: This was a cross-sectional study in the peri-urban areas of Harare, Zimbabwe. Human immunodeficiency virus-negative FSWs were interviewed to assess their awareness of and likelihood to use PrEP. The relative importance index was used to evaluate the levels of knowledge and the likelihood of, and barriers to, PrEP use. A set of 10 questions was designed and validated that evaluated participants' understanding of PrEP. A bivariate logistic regression model was utilised to identify predictors of PrEP use.
RESULTS: A total of 131 FSWs with a median age of 25 years (interquartile range: 21-31) participated in this study. Of the 71 (54%) FSWs who had heard about PrEP, 46 (35%) participants had adequate knowledge of its use. A total of 102 (78%) participants revealed that they would be willing to continuously use PrEP if it was provided free of cost. Increasing age of the participants was associated with an increase in the likelihood of using PrEP (r = 0.0033, p = 0.038). More knowledge about PrEP increased the likelihood of its use (r = 0.21, p = 0.0153). This likelihood increased amongst participants with an unprotected sexual intercourse encounter in the preceding 3 months (r = 0.0448, p = 0.026).
CONCLUSION: Knowledge of PrEP amongst FSWs was low. To increase the uptake of PrEP, there is a need to further sensitise FSWs about this intervention. Programmes should also promote awareness training in FSW subgroups that are less likely to use PrEP.
Keywords: female sex workers; HIV; pre-exposure prophylaxis; barriers; Truvada.
Background
Whilst the adult prevalence of human immunodeficiency virus (HIV) in the general population of Zimbabwe is 15%, the prevalence in key populations is higher.1 In populations of female sex workers (FSWs), the HIV prevalence in 2013 was 50% - 70% in different parts of Zimbabwe.2 In many settings, key populations are hidden and stigmatised, and their representation in national surveillance data is limited. It has been cited that key populations and their sex partners not only make up the largest proportion of people living with HIV (PLWH) but also represent a significant proportion of new infections in sub-Saharan Africa.3
Pre-exposure prophylaxis (PrEP) can become a female-controlled HIV prevention method for FSWs and others who are unable to negotiate condom use. The Ministry of Health and Child Care (MoHCC) has developed a PrEP framework policy that prioritises access for 'at-risk' populations. Groups that need to be offered PrEP include female and male sex workers; serodiscordant couples, that is, the HIV seronegative partner; adolescent girls and young women; pregnant women in relationships with men of unknown status and high-risk men, for example, men who have sex with men (MSM); prisoners; long-distance truck drivers; and transgender people.4 A fixed-dose regimen of either Tenofovir 300 mg and Emtricitabine 200 mg (TDF/FTC) or Tenofovir 300 mg and Lamivudine 300 mg (TDF/3TC) has been recommended for once-daily oral administration during the period an individual is at risk of contracting HIV infection.4 Zimbabwe has adopted The Joint United Nations Programme on HIV and AIDS 90-90-90 global goals to help reduce new infections and end the HIV pandemic.5 This target will be difficult to reach without reducing transmission amongst high-incidence populations.
Although it is well established that effective PrEP could provide an additional safety net to sexually active persons at risk,6 limited data are available in Zimbabwe regarding the knowledge and the likelihood of PrEP use amongst FSWs. There is a need to understand both the acceptability of PrEP amongst FSWs and the factors likely to determine uptake. Most research efforts to date have focussed on clinical aspects of PrEP. Little attention has been focussed on the factors that influence FSWs' willingness to take it. It is estimated that there are 40 000 (plausibility bounds [PBs] 28 000-59 000) active FSWs in Zimbabwe, that is, 1.23% (PB: 0.86% - 1.79%) of the adult female population. A total of 20 000 (50%) are in Harare and Bulawayo.7 This study was conducted to assess the levels of knowledge, barriers to and likelihood of oral PrEP use amongst FSWs as a preventative method in reducing the risk of acquiring HIV in Harare, Zimbabwe.
Methods
Study design and setting
This was a cross-sectional study of FSWs in seven peri-urban areas of Harare province, Zimbabwe. These sites were specifically chosen because they are high-density areas, and apart from the central business district, they are areas from which FSWs frequently operate.8
Study population and recruitment
This cross-sectional study was conducted between December 2016 and February 2017 in partnership with a local private voluntary organisation (PVO) that offers PrEP services. The PVO had been offering PrEP using TDF/FTC (TruvadaTM) tablets as an HIV prevention method in six Zimbabwean districts since August 2016. This was a demonstration project introduced to inform people of the new HIV prevention strategy of the MoHCC. The primary target populations included adolescent girls and young women aged 15-24 years, FSWs, MSM and serodiscordant couples.9
Human immunodeficiency virus-negative FSWs were defined as those who had been tested for HIV in the previous 3 months and had tested negative or those who perceived themselves to be HIV-negative. The PrEP intervention that is defined for the purpose of this study refers to oral PrEP with Truvada, which is indicated for 'at-risk' individuals with a laboratory-confirmed HIV-negative result. None of the participants was already receiving PrEP from the PVO.
Snowball sampling was used to locate and enrol FSWs from the peri-urban Harare sites. A peer referral system whereby 'seed' subjects previously identified by the PVO and 'queens' (the leaders of a significant group of sex workers based on their location in a certain area) provided referrals to enable further recruitment and mobilisation of other FSWs. This technique was utilised because of laws and policies that criminalise sex work in Zimbabwe. Female sex workers were also identified during community outreach HIV testing and counselling activities by the PVO. Once identified, FSWs were asked to provide written consent and to complete a 48-item questionnaire. The questionnaire was administered by the interviewer. The questionnaire had sections enquiring about socio-demographic factors, sexual behavioural characteristics, HIV testing, PrEP knowledge, perceptions, barriers and the likelihood of its use. Sexual behavioural characteristics were investigated to determine the HIV acquisition risk profile of the FSWs. The interviews were conducted in the language that the participant was most comfortable with. 'Sufficient knowledge' was judged by means of an initial self-report and an additional nine technical questions designed to test the level of knowledge. These technical questions included the participant's knowledge that PrEP is used by HIV-negative individuals, the dosing frequency, potential drug interactions and whether there are other reproductive health benefits. Once the level of knowledge was ascertained, the participants were then educated about PrEP use. The perceived barriers that were evaluated included stigma, cost of PrEP and the side-effects of Truvada.
Data management and statistical analysis
Research Electronic Data Capture (REDCap) was used to manage data. This was hosted by the College of Health Sciences of the University of Zimbabwe. The contribution of each factor, namely, age, cost of PrEP and drug side-effects, with regard to improving PrEP uptake amongst FSWs was examined. The importance of each factor as perceived by the respondents was assessed by computing the relative importance index (RII). The RII is a statistical measure recorded on a scale of 0 < RII ≤ 1, where '0' or any value close to '0' is defined as a poor knowledge of PrEP use, the participant is less likely to use PrEP or barriers associated with PrEP are likely to affect uptake. If the RII score was '1' or close to '1', it means that participants were knowledgeable about PrEP, were more likely to use PrEP and that barriers associated with PrEP use were unlikely to affect its uptake.
The RII was computed using the equation  , where Scorei was the score for each question given by the participants and ranged from 0 to 4 (where '0' was 'very much disagree, strongly disagree or never' and '4' was 'strongly agree, very much agree or almost always'). K was the maximum possible score and n was the total number of questions. According to Johnson and LeBreton,10 RII is the proportionate contribution each predictor makes to R2 (where R2 is the extent to which the dependent variable can be predicted by the predictor variables), considering both its direct effect (correlation with the dependent variable) and its effect when combined with other variables.
, where Scorei was the score for each question given by the participants and ranged from 0 to 4 (where '0' was 'very much disagree, strongly disagree or never' and '4' was 'strongly agree, very much agree or almost always'). K was the maximum possible score and n was the total number of questions. According to Johnson and LeBreton,10 RII is the proportionate contribution each predictor makes to R2 (where R2 is the extent to which the dependent variable can be predicted by the predictor variables), considering both its direct effect (correlation with the dependent variable) and its effect when combined with other variables.
All data analysis was performed using Stata version 13 (StataCorp LP, TX, USA) software package. Bivariate linear regression was used to determine if there was a relationship between the RII scores of knowledge, likelihood and barriers (KLBs) and exploratory factor variables that were collected in the study. To identify the relationship between KLB RII scores, a matrix of Spearman correlation coefficients was used.
Ethical consideration
Prior to conducting the survey, the study protocol including the data collecting tool was reviewed and approved by the Joint Research Ethics Committee of the University of Zimbabwe and the Parirenyatwa Group of Hospitals (JREC/328/16). All participants provided written informed consent before participating in the study.
Results
A total of 131 presumed HIV-negative, adult FSWs were recruited to participate in this study, and their demographic characteristics are shown in Table 1. All study participants were self-identified as residents of the Harare province.
Participant's characteristics - Sexual risk factors and human immunodeficiency virus testing
The median number of sexual encounters by the participants was five [interquartile range (IQR): 3-6] partners per day. Half of the participants (50%) did not know their partner's HIV serostatus and only 42% of the FSWs would talk about HIV with their clients or partners. All participants perceived the use of condoms as a necessary tool when engaging in sexual activity with their partners or clients, 86% used condoms with the last three partners they encountered and 44% reported having ever had a condom burst at least once during sexual intercourse. The variables affecting HIV acquisition risk are depicted in Table 2.
Pre-exposure prophylaxis knowledge
Of the 131 participants, 71 (54%) had heard about PrEP and of those only 46 (35%) had sufficient knowledge about PrEP (RII > 0.5). Participants mostly heard about PrEP from non-governmental organisations (59%), from friends (35%) and only 6% of the participants had heard about PrEP through clinics.
Likelihood of pre-exposure prophylaxis use
The participants' responses when asked about the likelihood of PrEP use are shown in Table 3. Regardless of a likelihood to use PrEP, potential barriers were cited, such as stigma, costs, side-effects associated with the PrEP tablet and poor knowledge, as shown in Table 3.
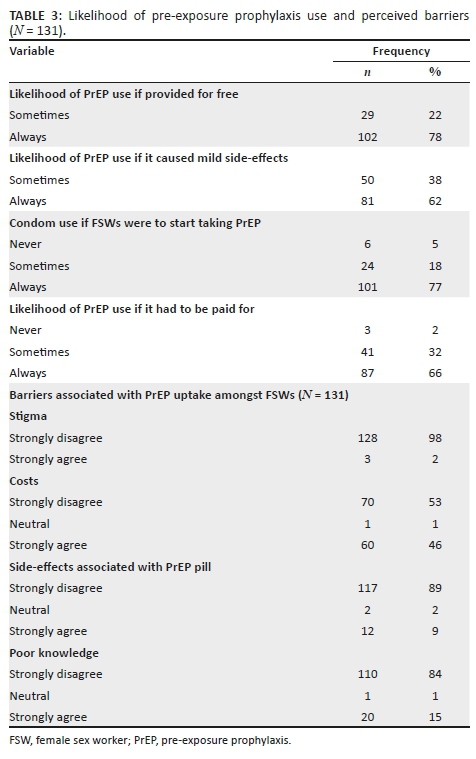
Relative importance index
On a scale of RII, the median score for PrEP knowledge was '0', indicating that participants had limited knowledge about PrEP. Participants perceived the use of PrEP as an important component in the ideal HIV prevention strategy once educated about it. In relation to the likelihood of PrEP use, the median score for the likelihood of PrEP use was 0.89 ranging from 0.48 to 1. Therefore, the likelihood of PrEP use amongst the participants was high. The RII median score for barriers associated with PrEP use was 0.29 (IQR: 0-0.63). This indicated that the barriers associated with PrEP uptake were less likely than knowledge to affect participants' use of PrEP. Results that were statistically significant in the bivariate analysis are shown in Table 4.
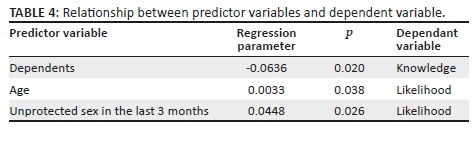
An increase in the number of dependents was associated with a reduced knowledge about PrEP, as shown in Table 4. There was a statistically significant association between age and likelihood of PrEP use. As participants became older, there was an increase in the likelihood of PrEP use. This increased amongst participants who had unprotected sex in the last 3 months.
There was, however, no statistically significant association between knowledge RII with age, marital status, education, change in place of residence, income and years of practice as a sex worker (p > 0.05).
Considering the likelihood of PrEP use amongst the participants, there was no association noted between the likelihood RII score and the number of dependents, marital status, education, change in place of residence, income and years of practice as a sex worker (p > 0.05). Spearman's rank correlation coefficient was computed to identify and test the strength of the relationship between knowledge and barriers with the likelihood of PrEP use. Table 5 shows the Spearman correlation coefficients for the relationship between RII scores of KLBs associated with PrEP use.
Considering likelihood and knowledge RII scores, there was a positive correlation whereby more knowledge about PrEP moderately increased the likelihood of PrEP use (r = 0.21, p = 0.0153). There was a negative correlation between barriers and the likelihood RII score, that is, as barriers associated with PrEP use decreased, the likelihood of PrEP use moderately increased (r = 0.23, p = 0.0074).
Discussion
In a real-world setting, the effectiveness of PrEP will depend on its acceptability, adoption and sustained use by high-risk populations. Pre-exposure prophylaxis medication will have little impact in reducing HIV infections if these components are not addressed.11 Findings from this study indicate that FSWs continue to engage in risky sexual behaviours. In the last 3 months, 53 (40%) of the participants reported having unprotected sexual intercourse with a casual partner.
In our study, 54% of FSWs had heard of PrEP before participating. In spite of having little knowledge of PrEP, the majority of FSWs were willing to use PrEP (median RII = 0.89; range 0.48-1) to reduce their risk of contracting HIV infection. In a similar study conducted in China, only 16.5% had heard of PrEP before participation, and only 1.4% had used PrEP before.12 Nevertheless, 69% and 95% of the respective FSW populations of China and India reported a willingness to use PrEP.12,13 These estimates were consistent with our findings that 89% of the participants were willing to use PrEP. Educating HIV-uninfected FSWs about PrEP is likely to support the uptake of PrEP and assist in decreasing the incidence of HIV in Zimbabwe. Pre-exposure prophylaxis programmes have been incorporated into sexually transmitted disease clinics, reproductive health programmes and genitourinary medicine clinics in high-income countries.14,15,16,17
Findings from this study indicated that there was a significant association between the likelihood of PrEP use, age and unprotected sexual intercourse in the preceding 3 months. Older participants were more likely to adopt PrEP as an HIV prevention strategy. This suggests that those who perceive themselves to be at a higher risk of HIV infection are more likely to adopt PrEP as an HIV intervention. Elmes et al. evaluated condom use by FSWs in Eastern Zimbabwe and reported that older participants were less likely to request condom use from partners.18 Older FSWs may therefore be at a higher risk of acquiring HIV and should be prioritised for PrEP access.
In spite of the high levels of interest in PrEP, potential barriers were cited including cost, side-effects and poor knowledge of PrEP use. Whilst PrEP had to be bought, 46% of the participants strongly agreed that lack of money would pose a challenge to PrEP uptake. Many participants felt that PrEP ought to be provided free in view of the severity of the HIV epidemic in Zimbabwe. The high cost of PrEP is undoubtedly a major barrier to its uptake worldwide, particularly in high-income countries where only branded TDF/FTC is available.19 In low-income countries that have established PrEP programmes, cost is not identified as a barrier to access because the medicines are free. In the Kenyan setting, FSWs were more concerned about the stigma of being tested for HIV and the barrier posed by the test with regard to the success of the intervention.20 In our study, stigma was not identified as a possible barrier to taking PrEP. This contrasted with the Kenyan setting and is a distinct finding for the Zimbabwean FSWs. With a reduced fear of stigmatisation, there is scope for improved acceptability of PrEP in FSWs.
Our results indicate that participants were generally willing to accept PrEP and adopt it as soon as it was made available. Information emerging from trial projects and open-label extensions where PrEP has been offered free of charge by knowledgeable providers suggests that uptake may be high in settings where cost and provider-related barriers had been removed.15,16,21
Our results showed that participants were willing to take PrEP even when reminded of potential side-effects. Participants cited that the side-effects of PrEP could have an impact on one's quality of life but that would not hinder taking PrEP. Only 9% of the FSWs strongly expressed that side-effects would affect their lifestyle and possibly prevent PrEP use. Sex workers in Mombasa, Kenya, voiced concerns about the potential negative side-effects of PrEP. In this qualitative study, a minority of participants were deterred from using PrEP because of the side-effects.22 Women from six US cities where female HIV infection is highly prevalent viewed PrEP as an important prevention option, provided that side-effects and cost to the consumer were minimal.23
The feasibility of oral PrEP implementation in Zimbabwe has been proven in ongoing and completed demonstration projects and clinical trials. By the end of 2017, a total of 3073 clients were initiated on PrEP in Zimbabwe. Ninety per cent of the clients initiated on PrEP were women, with the majority of them in the 25-49 years age group. The majority (52%) of the clients initiated on PrEP were FSWs. The accessibility of PrEP outside of demonstration projects has been limited.9
Whilst the sample size recruited for participation in this study was small, the information generated provides a foundation in the development of further programming for PrEP implementation in FSWs. The information was also generated from peri-urban, high-density areas in Harare (the capital city of Zimbabwe). Perceptions and knowledge levels might differ across other diverse geographical locations in Zimbabwe, and more studies need to be conducted. Because of the legal status of sex work in Zimbabwe, we used a snowball sampling technique in order to reach FSWs who might otherwise have been unwilling to participate in the study for fear of litigation. This sampling technique has the potential to introduce selection bias.
The Zimbabwean MoHCC has considered addressing the knowledge gap about PrEP in the general population through different channels whilst at the same time raising awareness to increase risk perception, especially amongst adolescent girls and young women. These considerations have been made as part of an implementation plan for PrEP in Zimbabwe between 2018 and 2020 (inclusive). Our study provides guidance on the progress made on addressing the knowledge gap about PrEP.
Conclusion
We set out to evaluate the knowledge levels of PrEP and the likelihood of its use amongst FSWs. Whilst the knowledge level was low, the majority of FSWs would be willing to use PrEP for the purpose of HIV/AIDS prevention. Non-governmental organisations are playing a major role in sensitising FSWs about PrEP. The local clinics need to increase their visibility as information dissemination institutions for PrEP. The clinics are strategically positioned to enlighten key populations about PrEP and to prescribe medication to those who might need it. Successful dissemination of information can be achieved if PrEP programmes are incorporated into other programmes within the clinics.
Acknowledgements
The authors thank all the team members, field officers and FSWs who participated in the study.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
T.M., A.D., C.C.M. and B.C. provided leadership for the project. T.M., A.D. and B.C. were responsible for conceptualisation of the study. A.D., B.N. and B.C. were responsible for data collection. T.M., C.C.M., B.N., T.G.M.-P. and A.D. were responsible for data analysis. G.D.M., T.G.M.-P. and C.C.M. provided technical expertise. T.M. and B.C. provided clinical expertise for the project. All authors contributed to the writing of the manuscript and approved the final version for publication.
Funding information
This research was made possible through core services and support from the University of Rochester Center for AIDS Research, a programme (P30 AI078498) funded by the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data availability statement
Data sets are available from the corresponding author upon request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Takarinda KC, Madyira LK, Mhangara M, et al. Factors associated with ever being HIV-tested in Zimbabwe: An extended analysis of the Zimbabwe Demographic and Health Survey (2010-2011). PLoS One. 2016;11(1):e0147828. https://doi.org/10.1371/journal.pone.0147828 [ Links ]
2.Cowan FM, Mtetwa S, Davey C, et al. Engagement with HIV prevention treatment and care among female sex workers in Zimbabwe: A respondent driven sampling survey. PLoS One. 2013;8(10):e77080. https://doi.org/10.1371/journal.pone.0077080 [ Links ]
3.Beyrer C, Baral SD, Collins C, et al. The global response to HIV in men who have sex with men. Lancet. 2016;388(10040):198-206. https://doi.org/10.1016/S0140-6736(16)30781-4 [ Links ]
4.National Medicines and Therapeutics Policy Advisory Committee (NMTPAC). Guidelines for antiretroviral therapy for the prevention and treatment of HIV in Zimbabwe, Harare: Ministry of Health and Child Care; 2016. [ Links ]
5.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90 an ambitious treatment target to help end the AIDS epidemic, Geneva: UNAIDS; 2014. [ Links ]
6.Desai M, Field N, Grant R, McCormack S. State of the art review: Recent advances in PrEP for HIV. BMJ. 2017;359:j5011. https://doi.org/10.1136/bmj.j5011 [ Links ]
7.Cowan FM, Chabata ST, Musemburi S, et al. Strengthening the scale- p and uptake of effective interventions for sex workers for population impact in Zimbabwe. J Int AIDS Soc. 2019;22 (Suppl 4):e25320. https://doi.org/10.1002/jia2.25320 [ Links ]
8.Chiyaka T, Mushati P, Hensen B, et al. Reaching young women who sell sex: Methods and results of social mapping to describe and identify young women for DREAMS impact evaluation in Zimbabwe. PLoS One. 2018;13(3):e0194301. https://doi.org/10.1371/journal.pone.0194301 [ Links ]
9.Gwavava E. Understanding the uptake and retention patterns of PrEP users in Zimbabwe by subpopulation. AIDS 2018. Amsterdam: International AIDS Society; 2018. [ Links ]
10.Johnson JW, LeBreton JM. History and use of relative importance indices in organizational research. Organ Res Methods. 2004;7(3):238-257. https://doi.org/10.1177/1094428104266510 [ Links ]
11.Brooks RA, Kaplan RL, Lieber E, Landovitz RJ, Lee SJ, Leibowitz AA. Motivators, concerns, and barriers to adoption of preexposure prophylaxis for HIV prevention among gay and bisexual men in HIV-serodiscordant male relationships. AIDS Care. 2011;23(9):1136-1145. https://doi.org/10.1080/09540121.2011.554528 [ Links ]
12.Peng B, Yang X, Zhang Y, et al. Willingness to use pre-exposure prophylaxis for HIV prevention among female sex workers: A cross-sectional study in China. HIV AIDS. 2012;4:149-158. https://doi.org/10.2147/HIV.S33445 [ Links ]
13.Reza-Paul S, Lazarus L, Doshi M, et al. Prioritizing risk in preparation for a demonstration project: A mixed methods feasibility study of oral pre-exposure prophylaxis (PREP) among female sex workers in South India. PLoS One. 2016;11(11):e0166889. https://doi.org/10.1371/journal.pone.0166889 [ Links ]
14.Cohen SE, Vittinghoff E, Bacon O, et al. High interest in pre-exposure prophylaxis among men who have sex with men at risk for HIV-infection: Baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68(4):439-448. https://doi.org/10.1097/QAI.0000000000000479 [ Links ]
15.Whetham J, Taylor S, Charlwood L, et al. Pre-exposure prophylaxis for conception (PrEP-C) as a risk reduction strategy in HIV-positive men and HIV-negative women in the UK. AIDS Care. 2014;26(3):332-336. https://doi.org/10.1080/09540121.2013.819406 [ Links ]
16.Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS. 2011;25(16):2005-2008. https://doi.org/10.1097/QAD.0b013e32834a36d0 [ Links ]
17.Dolling DI, Desai M, McOwan A, et al. An analysis of baseline data from the PROUD study: An open-label randomised trial of pre-exposure prophylaxis. Trials. 2016;17:163. https://doi.org/10.1186/s13063-016-1286-4 [ Links ]
18.Elmes J, Nhongo K, Ward H, et al. The price of sex: Condom use and the determinants of the price of sex among female sex workers in eastern Zimbabwe. J Infect Dis. 2014;210 (Suppl 2):S569-S578. https://doi.org/10.1093/infdis/jiu493 [ Links ]
19.Wilton J, Senn H, Sharma M, Tan DH. Pre-exposure prophylaxis for sexually-acquired HIV risk management: A review. HIV AIDS. 2015;7:125-136. https://doi.org/10.2147/HIV.S50025 [ Links ]
20.Mack N, Odhiambo J, Wong CM, Agot K. Barriers and facilitators to pre-exposure prophylaxis (PrEP) eligibility screening and ongoing HIV testing among target populations in Bondo and Rarieda, Kenya: Results of a consultation with community stakeholders. BMC Health Serv Res. 2014;14:231. https://doi.org/10.1186/1472-6963-14-231 [ Links ]
21.Heffron R, Celum C, Mugo N, et al., editors. High initiation of PrEP and ART in a demonstration project among African HIV-discordant couples. 21st Conference on Retroviruses and Opportunistic Infections; 2014 March 03-06; Boston, MA: International Aids Society; 2014. [ Links ]
22.Restar AJ, Tocco JU, Mantell JE, et al. Perspectives on HIV pre- and post-exposure prophylaxes (PrEP and PEP) among female and male sex workers in Mombasa, Kenya: Implications for integrating biomedical prevention into sexual health services. AIDS Educ Prev. 2017;29(2):141-153. https://doi.org/10.1521/aeap.2017.29.2.141 [ Links ]
23.Auerbach JD, Kinsky S, Brown G, Charles V. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS. 2015;29(2):102-110. https://doi.org/10.1089/apc.2014.0142 [ Links ]
 Correspondence:
Correspondence:
Tinashe Mudzviti
tinashem@newlandsclinic.org.zw
Received: 22 Oct. 2019
Accepted: 11 Jan. 2020
Published: 19 Feb. 2020
ORIGINAL RESEARCH
Feasibility of implementing a novel behavioural smoking cessation intervention amongst human immunodeficiency virus-infected smokers in a resource-limited setting: A single-arm pilot trial
Billy M. TsimaI; Precious MoediII; Joyce MaungeIII; Kitso MachanganeIII; Martha KgogwaneIII; Tebogo MudojwaIII; Joseph BastianIV; Warren BilkerV; Rebecca AshareIV; Robert SchnollIV; Robert GrossVI
IDepartment of Family Medicine and Public Health, Faculty of Medicine, University of Botswana, Gaborone, Botswana
IIPrincess Marina Hospital, Dental Department, Gaborone, Botswana
IIIBotswana UPenn Partnership, Gaborone, Botswana
IVDepartment of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, United States of America
VDepartment of Biostatistics, Epidemiology and Informatics, Perelman School of Medicine, University of Pennsylvania, Philadelphia, United States of America
VIDepartment of Medicine (ID), Perelman School of Medicine, University of Pennsylvania, Philadelphia, United States of America
ABSTRACT
BACKGROUND: Tobacco use is prevalent amongst individuals infected with human immunodeficiency virus (HIV). In resource-constrained settings, pharmacological smoking cessation interventions are unfeasible because of their high cost. There is a need to develop and evaluate behavioural interventions to address the unique challenges of tobacco use in the HIV-infected populations in these settings.
OBJECTIVES: The authors aimed to assess the feasibility and acceptability of the Behavioural Activation/Problem Solving for Smoking Cessation (BAPS-SC) intervention programme to determine whether it should be tested in an adequately powered randomised controlled trial
METHOD: The authors merged behavioural activation therapy (BAT) with the principles of problem-solving therapy to create a novel five-session counselling model to address the unique challenges of tobacco cessation amongst those infected with HIV. Feasibility measures included the rate of enrolment amongst those eligible and the retention rate and descriptive analysis of intervention acceptability. The authors' secondary outcome was 7-day point smoking prevalence abstinence, confirmed with breath carbon monoxide.
RESULTS: A total of 128 individuals were screened over 8 weeks with 50 deemed eligible and 40 enrolled (80%). Retention at week 12 was 53% (21/40). The 7-day point prevalence abstinence, co-confirmed, at week 12 was 37.5% (15/40). All respondents indicated that they would recommend BAPS-SC to other smokers who want to quit, and would be willing to participate in the programme again up to the point of exit if they did not stop smoking.
CONCLUSION: A full-scale randomised control trial comparing BAPS-SC with usual practice is warranted to evaluate the efficacy of this novel intervention in these settings.
Keywords: smoking cessation; tobacco; behaviour activation; problem solving; HIV.
Introduction
The human immunodeficiency virus (HIV) epidemic in sub-Saharan Africa has resulted in a large-scale transformation of healthcare delivery in heavily affected countries such as Botswana.1 Unfortunately, other health threats such as cardiovascular disease and cancer have emerged amongst people living with HIV/AIDS (PLWHA) partly because of the chronic inflammation of HIV that is compounded by high rates of smoking in this population.2 As such, addressing modifiable cardiovascular risk factors amongst those with HIV infection, including tobacco use, has become a critical priority.3,4
Indeed, continued smoking amongst those with HIV infection can result in serious adverse effects, including reduced effectiveness of antiretroviral (ARV) therapy.5 Controlling for medication adherence and comorbid illicit drug use, HIV-infected smokers on ARV have a significantly lower likelihood of achieving a viral response and a greater chance of viral or immunologic failure compared with their non-smoking counterparts.6 Persistent smoking amongst HIV-infected individuals may also increase progression to acquired immunodeficiency syndrome (AIDS).7 Pulmonary tuberculosis, a major public health crisis in sub-Saharan Africa, occurs at higher rates in smokers compared with non-smokers.8 Overall, the mortality gains of ARV treatment and the associated improved quality of life amongst those with HIV infection are being jeopardised by the cardiovascular and neoplastic diseases attributable to tobacco use in this population.9
Although interest in quitting smoking is high amongst PLWHA,10,11,12 particularly when HIV treatment is initiated13 and when tobacco use treatment is integrated with HIV care,14 remarkably little research has focussed on developing and testing smoking cessation interventions for PLWHA.11 Unfortunately, in low-income and developing countries, the costs of pharmacotherapy for nicotine addiction make pharmacological treatments currently inaccessible for PLWHA and further emphasise the importance of novel behavioural strategies.
Depressive symptoms are common in HIV-infected populations, often comorbid with smoking, and associated with poor smoking cessation rates.15 Behavioural activation therapy (BAT), rooted in a behavioural economic framework, has been effective at treating depression, and preliminary data in the United States of America (USA) suggests that it may also effectively address smoking.16 Behavioural activation therapy aims to increase engagement in healthy rewarding activities (i.e., alternative reinforcers) by reducing patterns of avoidance, withdrawal and inactivity, and to decrease activities that enhance the rewarding aspects of smoking (i.e., complementary reinforcers). Additionally, problem-solving approaches have been used with PLWHA to improve medication adherence and decrease depressive symptoms.17 Behavioural activation therapy and problem-solving approach may help smokers select and implement activities that will replace smoking, thereby reducing smoking rates.
The authors developed a novel counselling model incorporating elements of behavioural activation and problem solving to address the unique challenges of tobacco cessation amongst those with HIV infection. They aimed to assess the feasibility and appeal of Behavioural Activation/Problem Solving for Smoking Cessation (BAPS-SC) intervention to determine whether it should be tested in an adequately powered clinical trial.
Methods
Participant enrolment
The authors conducted a single-arm pilot trial of the BAPS-SC intervention in Botswana amongst HIV-infected individuals aged 18-65 years who smoked ≥ 5 cigarettes/day, on average, at four outpatient HIV clinics. The target accrual goal was 40 participants. This was based on the assumption that at least five participants would be enrolled per week, thus ensuring that the recruitment of human subjects into the trial is timely as this is vital to the success of the trial.18 The authors excluded participants if they reported current untreated and unstable alcohol dependence; current use or discontinuation within last 14 days of smoking cessation medications; current diagnosis of unstable and untreated major depression or current or past diagnosis of psychotic disorder; use of chewing tobacco, snuff or snus; current participation in a smoking cessation programme; or plans to use nicotine substitutes or smoking cessation treatments in the next 7 months. The restriction to 7 months was based on the anticipated period to conduct the trial so as to avoid contamination of the intervention with other smoking cessation modalities not under investigation.
A trained recruiter approached each patient in clinic to ascertain smoking status. Those acknowledging smoking were asked to participate in a questionnaire related to their smoking and offered participation in the pilot trial. Those who agreed were referred to the research assistant who arranged to meet with them to determine eligibility and administer informed consent.
Design of intervention
The authors merged BAT with the principles of problem-solving therapy to create a novel five-session counselling model to address the unique challenges of tobacco cessation amongst PLWHA (BAPS-SC). Members of the team created a formal treatment manual, which was evaluated for cultural appropriateness by Botswana co-investigators, including assessment of translation and back translation and pre-pilot testing using videoconferencing to role play. Key components of BAPS-SC include activity monitoring and rewarding activity scheduling, assessment of personal goals and values, assessment and altering of avoidance behaviour and other maladaptive coping strategies, and contingency management. Behavioural Activation/Problem Solving for Smoking Cessation focuses on reducing stress pile-up and loss of pleasure that accompanies the cessation process and on identifying and establishing environmental/social changes to promote abstinence. Behavioural Activation/Problem Solving for Smoking Cessation addresses smoking as a behaviour that prevents and restricts opportunities for contact with healthy rewarding behaviours. These changes are achieved through altering daily routines previously associated with smoking in ways that increase pleasure and mastery across life domains, reducing rumination and increasing behavioural skills to prevent return to smoking as a means of avoiding stressors.
A pre-quit session (session 1) introduces participants to: (1) self-monitoring of mood and behaviour; (2) assessment of personal values to refine the treatment plan; and (3) scheduling of substitute rewarding activities that align with their abstinence goal. At the target quit date (TQD) session (session 2), participants' experiences with abstinence are reviewed and functional analysis of behaviour is introduced, especially as it relates to smoking and avoidance patterns. Information obtained is used to help generate a tailored behavioural activation plan by using the problem-solving framework to increase rewarding activities and relationships, reduce avoidant responses to distressing experiences and facilitate successful implementation of smoking trigger management strategies. Sessions 3-5 incorporate strategies to address avoidance patterns, especially those involving smoking, and replace them with adaptive coping strategies, again by using problem solving. Sessions were conducted by telephone over a 12-week period (with the first session lasting 1 h and subsequent sessions lasting 30-45 min on average) and involved weekly homework assignments.
Data collection and management
Collected data included information related to smoking behaviour (including nicotine dependence measured by the Fagerström Test for Nicotine Dependence19), anhedonia [using the Snaith-Hamilton Pleasure Scale (SHAPS)20] and feasibility (e.g., rate of accrual and retention and appeal of the intervention). Participant accrual rate was defined as the number of participants enrolled over 8 weeks. Retention was calculated as a proportion of participants enrolled who were available at week 12 contact session. Study staff not acting as interventionists interviewed participants at baseline, week 6 and week 12 to determine if they were still smoking and whether they implemented the suggested intervention strategies. The timeline follow-back procedure assessed daily smoking between measurement time points.21 Additionally, the amount of carbon monoxide (CO) in participants' breath was tested to confirm self-reported abstinence from tobacco. Data were collected on paper and transferred to an electronic REDCap database for analysis.
Data analysis
The authors used descriptive statistics to characterise the sample. Feasibility measures included the rate of enrolment and retention and descriptive analysis of intervention acceptability (e.g., whether the participants would have enrolled in the intervention had they known what the experience would be like and whether they would refer a friend who wanted to quit smoking). The primary efficacy outcome was 7-day point prevalence abstinence at week 12 (12 weeks post-TQD), defined as self-reported abstinence for 7 days prior to the assessment and breath CO <8 ppm.22 This approach is in line with the recommendations of the working group of the Society for Research on Nicotine and Tobacco based on literature review of abstinence measures used in trials of smoking cessation intervention.23
Ethical consideration
Ethical approval was obtained from the Botswana Ministry of Health (Health Research Unit, reference number: HPDME 13/18/1).
Results
Characteristics of the sample are described in Table 1. A total of 128 individuals were screened over 8 weeks with 50 deemed eligible and 40 enrolled (80%), as shown in Figure 1. Retention at week 12 was 52.5% (21/40).
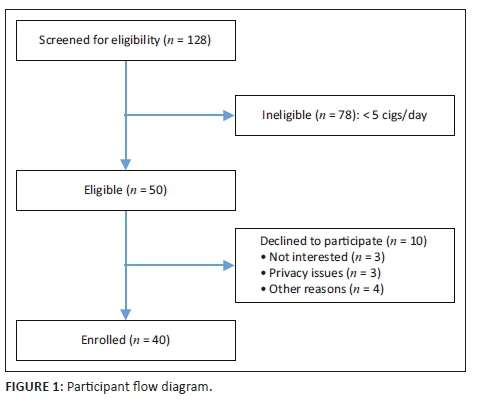
The 7-day point prevalence abstinence at week 12 was 37.5% (15/40). Notably, all respondents indicated that they would recommend BAPS-SC to other smokers who want to quit and would be willing to participate in the programme again up to the point of exit if they did not stop smoking. When a more stringent threshold of exhaled CO ≤ 4 ppm was used as the criterion for success, the quit rate in this pilot was 9/40 (22.5%).
Discussion
The authors conducted a single-arm pilot trial of the BAPS-SC intervention programme, a novel behavioural smoking cessation intervention for PLWHA in a high-HIV-burden sub-Saharan country wherein all 40 participants received the intervention. This design is ideal for studies aiming to determine if a novel intervention is appealing and feasible in the setting and warrants a full-scale clinical trial.24 The results of the CO monitoring and exit interviews suggest that BAPS-SC is likely to be efficacious.
This study's results of a high degree of smoking cessation success with BAPS-SC suggest a potentially highly impactful smoking cessation programme given that behavioural smoking cessation programmes rarely yield quit rates higher than 10% - 15%.25 There is no comparable trial that tested the intervention developed by the team of the authors with an HIV-negative cohort of smokers. Two comparisons are worth noting, and both indicate a substantial benefit from authors' intervention. Firstly, meta-analyses of behavioural interventions (without medications) for smoking cessation rooted in classic cognitive-behavioural theory show that they yield quit rates of generally less than 15% at the end of treatment.26 Secondly, MacPherson et al. (2010), in their pilot test of BAT for smoking cessation, reported an end-of-treatment quit rate of 17%.27 The authors used a higher threshold of CO to determine smoking cessation as opposed to a more stringent cut-off in the range of 3 ppm - 4 ppm recently proposed by other researchers.28 However, 8 ppm is the cut-off used in most smoking cessation clinical trials for PLWHA, so the authors chose this cut-off to compare their results to the current literature. With regard to past behavioural smoking cessation treatments for PLWHA, a cell phone-based intervention was found to be associated with significantly higher initial quit rates compared with usual care.29 Other studies including a group-based tailored intervention, motivational interventions and a web-based intervention (vs in-person or self-help) have not yielded significant increases in quit rates.30,31,32,33,34,35,36 Two pilot studies of behavioural treatments that address negative affect (depression and anxiety) show promise for PLWHA.37,38
The BAPS-SC trial proved feasible. Firstly, the target sample size of 40 participants was reached within 8 weeks of recruitment, a rate of enrolment that would make large trials feasible. The authors' findings indicated that a large-scale clinical trial would be feasible to determine the efficacy of the BAPS-SC programme in this setting where HIV prevalence is relatively high. Furthermore, smoking prevalence in the setting of their pilot study was estimated to be as high as 51% for male candidates and 6% for female candidates with HIV infection.39 There were proportionately fewer female candidates enrolled in the present study consistent with data from demographic and health surveys in sub-Saharan Africa.40 Although these smoking rates appear to be comparatively lower than reports from North America and Europe, these rates are projected to increase in the African continent whilst they are falling in other parts of the world.40,41 Given the limited behavioural health infrastructure in low middle income countries (LMICs) such as Botswana, the authors leveraged HIV clinical care sites and telephone-delivered counselling to extend the reach of skilled practitioners. The authors found this strategy to be effective and acceptable by the participants in the pilot trial.
Smoking is a major cause of morbidity and mortality, yet smokers find it very difficult to quit. If individuals quit because of this intervention, they will reduce their risk of cardiovascular disease, chronic obstructive pulmonary disease and cancers, particularly lung cancer. This will be of direct benefit to the individual participant. Further, if the authors are able to mount a full-scale trial based on the results of this pilot trial, high HIV burden LMICs such as Botswana may benefit because the intervention may become standard of care in the country, reducing disease burden caused by tobacco use. Being a behavioural intervention without pharmacological intervention, the cost is expected to be substantially less with the BAPS-SC compared with a pharmacological intervention. A formal cost-effective analysis will be needed to be undertaken following the roll out of the programme.
Results of the exit questionnaires to evaluate the acceptability of the BAPS-SC trial indicate that HIV-infected smokers in Botswana find the programme to be appealing and acceptable. The results indicate a lower-than-expected retention rate with slightly over half of the participants remaining in the study after a 12-week follow-up period. The retention rates across most smoking cessation trials generally exceed 75%.42 This may be improved by asking participants to provide contact numbers of associates such as friends and family members who can be contacted if the participant is unreachable after a number of attempts and increasing incentives to complete follow-ups. From an evidence-based medicine perspective, if efficacy of the intervention is determined to be high, implementation science methods would need to be employed in the clinical care setting to incorporate the intervention into HIV programmes. The intervention leverages the existing HIV care infrastructure and will likely facilitate scale-up in sub-Saharan African settings where HIV is common and smoking continues to emerge as a threat to HIV-positive individuals' health and survival.
Limitations
This pilot study evaluating the feasibility of BAPS-SC amongst HIV-infected smokers in Botswana has notable limitations. This was a single-arm trial, and therefore the results indicate only preliminary evidence of efficiency of the intervention and do not confirm efficacy. However, the authors' main aim was to evaluate feasibility of implementing the intervention. The planned full clinical trial informed by these results will be powered to address efficacy. Additionally, the follow-up period was of only 12 weeks as the aim was to evaluate feasibility of the novel intervention. Thus, the follow-up period was not long enough to assess long-term abstinence. However, this was a pilot study to inform a future clinical trial.
Conclusion
The results of this single-arm pilot trial demonstrate the feasibility of leveraging HIV clinical infrastructure for implementing BAPS-SC as a smoking cessation intervention programme amongst HIV-infected smokers in a resource-limited setting with high HIV burden. A full-scale clinical trial comparing BAPS-SC with standard counselling is thus warranted to evaluate the efficacy of this novel intervention in these settings.
Acknowledgements
The authors would like to thank all the participants who volunteered to participate in the study.
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
B.M.T., R.S. and R.G. designed the study and provided scientific oversight. K.M., J.M., M.K. and T.M. conducted interviews and worked on informed consent and data management. P.M., J.B., W.B. and R.A. contributed to the design of the measures and interpretation of findings. B.T. conducted the primary analysis. B.M.T., R.S. and R.G. drafted the manuscript and incorporated authors' comments. All authors critically reviewed and edited the manuscript. The final manuscript for submission was approved by all authors.
Funding information
This study was supported by National Institutes of Health (NIH) grants that include HIV Clinical Epidemiology Training for Botswana (D43 TW00978), Penn Center for AIDS Research (P30 AI045008) and Penn Mental Health AIDS Research Center (P30 MH097488).
Data availability statement
The data used for this analysis are available from the authors upon reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Eyawo O, Franco-Villalobos C, Hull MW, et al. Changes in mortality rates and causes of death in a population-based cohort of persons living with and without HIV from 1996 to 2012. BMC Infect Dis. 2017 Feb 27;17(1):174. https://doi.org/10.1186/s12879-017-2254-7 [ Links ]
2.Magodoro IM, Esterhuizen TM, Chivese T. A cross-sectional, facility based study of comorbid non-communicable diseases among adults living with HIV infection in Zimbabwe. BMC Res Notes. 2016 Aug 02;9:379. https://doi.org/10.1186/s13104-016-2187-z [ Links ]
3.Reddy KP, Parker RA, Losina E, et al. Impact of cigarette smoking and smoking cessation on life expectancy among people with HIV: A US-based modeling study. J Infect Dis. 2016 Dec 01;214(11):1672-1681. https://doi.org/10.1093/infdis/jiw430 [ Links ]
4.Benard A, Tessier JF, Rambeloarisoa J, et al. HIV infection and tobacco smoking behaviour: Prospects for prevention? ANRS CO3 aquitaine cohort, 2002. Int J Tuberc Lung Dis. 2006 Apr 01;10(4):378-383. [ Links ]
5.Pollack TM, Duong HT, Pham TT, Do CD, Colby D. Cigarette smoking is associated with high HIV viral load among adults presenting for antiretroviral therapy in vietnam. PLoS One. 2017 Mar 07;12(3):e0173534. https://doi.org/10.1371/journal.pone.0173534 [ Links ]
6.Feldman JG, Minkoff H, Schneider MF, et al. Association of cigarette smoking with HIV prognosis among women in the HAART era: A report from the women's interagency HIV study. Am J Public Health. 2006 Jun 01;96(6):1060-1065. https://doi.org/10.2105/AJPH.2005.062745 [ Links ]
7.Nieman RB, Fleming J, Coker RJ, Harris JR, Mitchell DM. The effect of cigarette smoking on the development of AIDS in HIV-1-seropositive individuals. Aids. 1993 May 01;7(5):705-710. https://doi.org/10.1097/00002030-199305000-00015 [ Links ]
8.Bronner Murrison L, Martinson N, Moloney RM, et al. Tobacco smoking and tuberculosis among men living with HIV in johannesburg, south africa: A case-control study. PLoS One. 2016 Nov 28;11(11):e0167133. https://doi.org/10.1371/journal.pone.0167133 [ Links ]
9.Nahvi S, Cooperman NA. Review: The need for smoking cessation among HIV-positive smokers. AIDS Educ Prev. 2009 Jun 01;21(3 Suppl):14-27. https://doi.org/10.1521/aeap.2009.21.3_supp.14 [ Links ]
10.Pacek LR, Cioe PA. Tobacco use, use disorders, and smoking cessation interventions in persons living with HIV. Curr HIV AIDS Rep. 2015 Dec 01;12(4):413-420. https://doi.org/10.1007/s11904-015-0281-9 [ Links ]
11.Pacek LR, Crum RM. A review of the literature concerning HIV and cigarette smoking: Morbidity and mortality, associations with individual- and social-level characteristics, and smoking cessation efforts. Addict Res Theory. 2015 Feb 01;23(1):10-23. https://doi.org/10.3109/16066359.2014.920013 [ Links ]
12.Frazier EL, Sutton MY, Brooks JT, Shouse RL, Weiser J. Trends in cigarette smoking among adults with HIV compared with the general adult population, united states - 2009-2014. Prev Med. 2018 Jun 01;111:231-234. https://doi.org/10.1016/j.ypmed.2018.03.007 [ Links ]
13.Vidrine DJ, Frank SG, Savin MJ, et al. HIV care initiation: A teachable moment for smoking cessation? Nicotine Tob Res. 2018 Aug 14;20(9):1109-1116. https://doi.org/10.1093/ntr/ntx218 [ Links ]
14.Pacek LR, Rass O, Johnson MW. Positive smoking cessation-related interactions with HIV care providers increase the likelihood of interest in cessation among HIV-positive cigarette smokers. AIDS Care. 2017 Oct 01;29(10):1309-1314. https://doi.org/10.1080/09540121.2017.1330532 [ Links ]
15.Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. Am J Psychiatry. 2001 May 01;158(5):725-730. https://doi.org/10.1176/appi.ajp.158.5.725 [ Links ]
16.Dobson KS, Hollon SD, Dimidjian S, et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the prevention of relapse and recurrence in major depression. J Consult Clin Psychol. 2008 Jun 01;76(3):468-477. https://doi.org/10.1037/0022-006X.76.3.468 [ Links ]
17.Gross R, Bellamy SL, Chapman J, et al. Managed problem solving for antiretroviral therapy adherence: A randomized trial. JAMA Intern Med. 2013 Feb 25;173(4):300-306. https://doi.org/10.1001/jamainternmed.2013.2152 [ Links ]
18.Carter RE, Sonne SC, Brady KT. Practical considerations for estimating clinical trial accrual periods: Application to a multi-center effectiveness study. BMC Med Res Methodol. 2005 Mar 30;5:11. https://doi.org/10.1186/1471-2288-5-11 [ Links ]
19.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: A revision of the Fagerstrom tolerance questionnaire. Br J Addict. 1991 Sept 01;86(9):1119-1127. https://doi.org/10.1111/j.1360-0443.1991.tb01879.x [ Links ]
20.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton pleasure scale. Br J Psychiatry. 1995 Jul 01;167(1):99-103. https://doi.org/10.1192/bjp.167.1.99 [ Links ]
21.Brown RA, Ramsey SE, Strong DR, et al. Effects of motivational interviewing on smoking cessation in adolescents with psychiatric disorders. Tob Control. 2003 Dec 01;12(Suppl. 4):IV3-IV10. https://doi.org/10.1136/tc.12.suppl_4.iv3 [ Links ]
22.Javors MA, Hatch JP, Lamb RJ. Cut-off levels for breath carbon monoxide as a marker for cigarette smoking. Addiction. 2005 Feb 01;100(2):159-167. https://doi.org/10.1111/j.1360-0443.2004.00957.x [ Links ]
23.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: Issues and recommendations. Nicotine Tob Res. 2003 Feb 01;5(1):13-25. https://doi.org/10.1080/1462220031000070552 [ Links ]
24.Sambucini V. Comparison of single-arm vs. randomized phase II clinical trials: A Bayesian approach. J Biopharm Stat. 2015;25(3):474-489. https://doi.org/10.1080/10543406.2014.920856 [ Links ]
25.Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2017 Mar 31;3:CD001292. https://doi.org/10.1002/14651858.CD001292.pub3 [ Links ]
26.2008 PHS Guideline Update Panel, Liaisons. Treating tobacco use and dependence: 2008 update U.S. public health service clinical practice guideline executive summary. Respir Care. 2008 Sept 01;53(9):1217-1222. [ Links ]
27.MacPherson L, Tull MT, Matusiewicz AK, et al. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. J Consult Clin Psychol. 2010 Feb 01;78(1):55-61. https://doi.org/10.1037/a0017939 [ Links ]
28.Cropsey KL, Trent LR, Clark CB, Stevens EN, Lahti AC, Hendricks PS. How low should you go? Determining the optimal cutoff for exhaled carbon monoxide to confirm smoking abstinence when using cotinine as reference. Nicotine Tob Res. 2014 Oct 01;16(10):1348-1355. https://doi.org/10.1093/ntr/ntu085 [ Links ]
29.Gritz ER, Danysh HE, Fletcher FE, et al. Long-term outcomes of a cell phone-delivered intervention for smokers living with HIV/AIDS. Clin Infect Dis. 2013 Aug 01;57(4):608-615. https://doi.org/10.1093/cid/cit349 [ Links ]
30.Balfour L, Wiebe SA, Cameron WD, et al. An HIV-tailored quit-smoking counselling pilot intervention targeting depressive symptoms plus nicotine replacement therapy. AIDS Care. 2017 Jan 01;29(1):24-31. https://doi.org/10.1080/09540121.2016.1201195 [ Links ]
31.Humfleet GL, Hall SM, Delucchi KL, Dilley JW. A randomized clinical trial of smoking cessation treatments provided in HIV clinical care settings. Nicotine Tob Res. 2013 Aug 01;15(8):1436-1445. https://doi.org/10.1093/ntr/ntt005 [ Links ]
32.Ingersoll KS, Cropsey KL, Heckman CJ. A test of motivational plus nicotine replacement interventions for HIV positive smokers. AIDS Behav. 2009 Jun 01;13(3):545-554. https://doi.org/10.1007/s10461-007-9334-4 [ Links ]
33.Lloyd-Richardson EE, Stanton CA, Papandonatos GD, et al. Motivation and patch treatment for HIV+ smokers: A randomized controlled trial. Addiction. 2009 Nov 01;104(11):1891-1900. https://doi.org/10.1111/j.1360-0443.2009.02623.x [ Links ]
34.Manuel JK, Lum PJ, Hengl NS, Sorensen JL. Smoking cessation interventions with female smokers living with HIV/AIDS: A randomized pilot study of motivational interviewing. AIDS Care. 2013;25(7):820-827. https://doi.org/10.1080/09540121.2012.733331 [ Links ]
35.Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. J Acquir Immune Defic Syndr. 2012 Oct 01;61(2):208-215. https://doi.org/10.1097/QAI.0b013e3182645679 [ Links ]
36.Stanton CA, Papandonatos GD, Shuter J, et al. Outcomes of a tailored intervention for cigarette smoking cessation among latinos living with HIV/AIDS. Nicotine Tob Res. 2015 Aug 01;17(8):975-982. https://doi.org/10.1093/ntr/ntv014 [ Links ]
37.Matthews AK, Conrad M, Kuhns L, Vargas M, King AC. Project exhale: Preliminary evaluation of a tailored smoking cessation treatment for HIV-positive African American smokers. AIDS Patient Care STDS. 2013 Jan 01;27(1):22-32. https://doi.org/10.1089/apc.2012.0253 [ Links ]
38.O'Cleirigh C, Zvolensky MJ, Smits JAJ, et al. Integrated treatment for smoking cessation, anxiety, and depressed mood in people living with HIV: A randomized controlled trial. J Acquir Immune Defic Syndr. 2018 Oct 01;79(2):261-268. https://doi.org/10.1097/QAI.0000000000001787 [ Links ]
39.Mosepele M, Letsatsi V, Mokgatlhe L, Hudson FP, Gross R. Cholesterol screening and statin prescription is low among HIV-infected patients on protease-inhibitor regimens in Botswana. Open AIDS J. 2017 Jun 30;11:45-51. https://doi.org/10.2174/1874613601711010045 [ Links ]
40.Murphy JD, Liu B, Parascandola M. Smoking and HIV in sub-Saharan Africa: A 25 country analysis of the demographic health surveys. Nicotine Tob Res. 2019 Jul 17;21(8):1093-1102. https://doi.org/10.1093/ntr/nty176 [ Links ]
41.WHO Report on the Global Tobacco Epidemic, 2015. Raising taxes on tabacco [homepage on the Internet]. [ Links ] c2015 [cited 2019 Apr 10]. Available from: https://www.who.int/tobacco/global_report/2015/report/en/
42.Schnoll RA, Goelz PM, Veluz-Wilkins A, et al. Long-term nicotine replacement therapy: A randomized clinical trial. JAMA Intern Med. 2015 Apr 01;175(4):504-511. https://doi.org/10.1001/jamainternmed.2014.8313 [ Links ]
 Correspondence:
Correspondence:
Billy Tsima
btsima@hotmail.com
Received: 05 Feb. 2020
Accepted: 08 Mar. 2020
Published: 24 June 2020
Project Number: HPDME 13/18/1
^rND^sEyawo^nO^rND^sFranco-Villalobos^nC^rND^sHull^nMW^rND^sMagodoro^nIM^rND^sEsterhuizen^nTM^rND^sChivese^nT^rND^sReddy^nKP^rND^sParker^nRA^rND^sLosina^nE^rND^sBenard^nA^rND^sTessier^nJF^rND^sRambeloarisoa^nJ^rND^sPollack^nTM^rND^sDuong^nHT^rND^sPham^nTT^rND^sDo^nCD^rND^sColby^nD^rND^sFeldman^nJG^rND^sMinkoff^nH^rND^sSchneider^nMF^rND^sNieman^nRB^rND^sFleming^nJ^rND^sCoker^nRJ^rND^sHarris^nJR^rND^sMitchell^nDM^rND^sBronner Murrison^nL^rND^sMartinson^nN^rND^sMoloney^nRM^rND^sNahvi^nS^rND^sCooperman^nNA^rND^sPacek^nLR^rND^sCioe^nPA^rND^sPacek^nLR^rND^sCrum^nRM^rND^sFrazier^nEL^rND^sSutton^nMY^rND^sBrooks^nJT^rND^sShouse^nRL^rND^sWeiser^nJ^rND^sVidrine^nDJ^rND^sFrank^nSG^rND^sSavin^nMJ^rND^sPacek^nLR^rND^sRass^nO^rND^sJohnson^nMW^rND^sCiesla^nJA^rND^sRoberts^nJE^rND^sDobson^nKS^rND^sHollon^nSD^rND^sDimidjian^nS^rND^sGross^nR^rND^sBellamy^nSL^rND^sChapman^nJ^rND^sCarter^nRE^rND^sSonne^nSC^rND^sBrady^nKT^rND^sHeatherton^nTF^rND^sKozlowski^nLT^rND^sFrecker^nRC^rND^sFagerstrom^nKO^rND^sSnaith^nRP^rND^sHamilton^nM^rND^sMorley^nS^rND^sHumayan^nA^rND^sHargreaves^nD^rND^sTrigwell^nP^rND^sBrown^nRA^rND^sRamsey^nSE^rND^sStrong^nDR^rND^sJavors^nMA^rND^sHatch^nJP^rND^sLamb^nRJ^rND^sHughes^nJR^rND^sKeely^nJP^rND^sNiaura^nRS^rND^sOssip-Klein^nDJ^rND^sRichmond^nRL^rND^sSwan^nGE^rND^sSambucini^nV^rND^sLancaster^nT^rND^sStead^nLF^rND^sMacPherson^nL^rND^sTull^nMT^rND^sMatusiewicz^nAK^rND^sCropsey^nKL^rND^sTrent^nLR^rND^sClark^nCB^rND^sStevens^nEN^rND^sLahti^nAC^rND^sHendricks^nPS^rND^sGritz^nER^rND^sDanysh^nHE^rND^sFletcher^nFE^rND^sBalfour^nL^rND^sWiebe^nSA^rND^sCameron^nWD^rND^sHumfleet^nGL^rND^sHall^nSM^rND^sDelucchi^nKL^rND^sDilley^nJW^rND^sIngersoll^nKS^rND^sCropsey^nKL^rND^sHeckman^nCJ^rND^sLloyd-Richardson^nEE^rND^sStanton^nCA^rND^sPapandonatos^nGD^rND^sManuel^nJK^rND^sLum^nPJ^rND^sHengl^nNS^rND^sSorensen^nJL^rND^sMoadel^nAB^rND^sBernstein^nSL^rND^sMermelstein^nRJ^rND^sArnsten^nJH^rND^sDolce^nEH^rND^sShuter^nJ^rND^sStanton^nCA^rND^sPapandonatos^nGD^rND^sShuter^nJ^rND^sMatthews^nAK^rND^sConrad^nM^rND^sKuhns^nL^rND^sVargas^nM^rND^sKing^nAC^rND^sO'Cleirigh^nC^rND^sZvolensky^nMJ^rND^sSmits^nJAJ^rND^sMosepele^nM^rND^sLetsatsi^nV^rND^sMokgatlhe^nL^rND^sHudson^nFP^rND^sGross^nR^rND^sMurphy^nJD^rND^sLiu^nB^rND^sParascandola^nM^rND^sSchnoll^nRA^rND^sGoelz^nPM^rND^sVeluz-Wilkins^nA^rND^1A01 A02^nAnthony K.^sEnimil^rND^1A01 A02^nBrian^sEley^rND^1A01 A02^nJames^sNuttall^rND^1A01 A02^nAnthony K.^sEnimil^rND^1A01 A02^nBrian^sEley^rND^1A01 A02^nJames^sNuttall^rND^1A01 A02^nAnthony K^sEnimil^rND^1A01 A02^nBrian^sEley^rND^1A01 A02^nJames^sNuttallCASE REPORTS
The initial intravenous treatment of a human immunodeficiency virus-infected child with complicated abdominal tuberculosis
Anthony K. EnimilI, II; Brian EleyI, II; James NuttallI, II
IDepartment of Paediatrics and Child Health, College of Health Sciences, University of Cape Town, Cape Town, South Africa
IIDepartment of Paediatrics and Child Health, College of Health Sciences, Red Cross War Memorial Children's Hospital, Cape Town, South Africa
ABSTRACT
INTRODUCTION: There is very limited published experience with intravenous (IV) antituberculosis (anti-TB) and antiretroviral therapy (ART) especially in children. We have described a human immunodeficiency virus (HIV)-infected child with complicated abdominal tuberculosis who was initially treated with IV anti-TB and a partially IV ART regimen before transitioning to oral therapy
PATIENT PRESENTATION: A 3-year-old boy presented with hypovolaemic shock with a 3-day history of inability to pass stools, abdominal distension and bile-stained vomiting. Abdominal ultrasound and X-ray showed small-bowel obstruction. Human immunodeficiency virus antibody testing was positive, and Cluster of Differentiation (CD)4+ lymphocyte count was 56 cells/mL (15%). Xpert Mycobacterium tuberculosis (MTB)/Rifampicin (RIF) Ultra and TB culture on induced sputum detected MTB complex sensitive to rifampicin and isoniazid
MANAGEMENT AND OUTCOME: Following laparotomy and closure of bowel perforations, the child was commenced on IV rifampicin, moxifloxacin and amikacin. Amikacin was stopped after 3 days because of nephrotoxicity, and meropenem and IV linezolid were added. After 20 days, ART comprising IV zidovudine, oral lamivudine solution, oral lopinavir/ritonavir solution and additional oral ritonavir solution for super boosting was commenced. By day 40, the patient was well established on oral feeds and was switched to standard oral anti-TB medications. Sputum examined 1 month after starting the treatment was found culture-negative for MTB. After 4 months of treatment, the HIV viral load was < 100 copies/mL. He completed a total of 12 months of anti-TB treatment.
CONCLUSION: Despite limited experience and few available IV formulations of standard anti-TB and ARV medications, initial IV therapy may be beneficial for patients in whom oral medication is not an option
Keywords: intravenous; antituberculosis; tuberculosis; child none; ARV medications.
Introduction
There is limited published experience with the use of intravenous (IV) antituberculosis (anti-TB) drugs in patients with severe tuberculosis (TB). We describe the challenges of managing a human immunodeficiency virus (HIV) and TB co-infected child with complicated abdominal TB in whom oral treatment was not initially feasible. The aim of this report was to highlight the role of IV therapy in this context.
Patient presentation
A 3-year-old boy presented with a 3-day history of inability to pass stools, abdominal swelling and bile-stained vomiting. His mother had a history of substance abuse. The pregnancy and delivery had been un-booked. She was diagnosed HIV-positive post-partum and started on antiretroviral therapy (ART). Infant antiretroviral prophylaxis was prescribed for the child. It is not known whether HIV testing of the infant at the time of birth was performed. The mother defaulted her ART and her infant's ARV prophylaxis. The child had no history of contact with a TB source case.
On examination, the child had abdomen distension. He was in shock, pale and severely underweight. The HIV antibody test was positive on two separate samples. The CD4+ lymphocyte count on admission was 56 cells/mL (15%), haemoglobin (Hb) was 5 g/dL and the platelet count was 114 × 109/L. The chest X-ray did not suggest pulmonary TB. Sputum gene Xpert Mycobacterium tuberculosis (MTB)/RIF Ultra was positive for rifampicin-sensitive MTB. The sputum culture grew rifampicin- and isoniazid-sensitive TB. Abdominal radiography and ultrasonography showed distended small-bowel loops with no gas in the rectum, consistent with small-bowel obstruction (Figure 1).
Management and outcome
The child was given a blood transfusion and was resuscitated. At laparotomy 3 days after admission, three small-bowel perforations with faecal soiling of the abdominal cavity and 'caseous areas' of the ileum, ascending colon, mesenteric and retroperitoneal lymph nodes were identified. The abdomen was washed with saline and the perforations closed. Histology of the intra-abdominal nodes showed necrotising granulomatous inflammation.
The child was commenced on IV rifampicin, IV moxifloxacin and IV amikacin. On day 3, his creatinine concentration increased from 61 µmol/L to 133 µmol/L. Amikacin was stopped and moxifloxacin dose was adjusted according to the estimated glomerular filtration rate (eGFR) of 26.6 mL/min/1.73 m2. Intravenous meropenem and IV linezolid (LZD) were started. The patient's renal function normalised in the following 12 days. The platelet count and the Hb levels declined, and LZD was discontinued after 10 days. On day 20, ART, namely, IV zidovudine (ZDV or AZT), oral lamivudine (3TC) solution, oral lopinavir or ritonavir (LPV/r) solution and oral ritonavir solution to 'super-boost' the LPV, was introduced. The plasma LPV 'trough' concentration 1 week after starting the ART was therapeutic, namely, Cmin 9.98 mg/L. By day 30, he was tolerating small oral feeds. Intravenous meropenem was stopped and oral isoniazid was started. On day 40, he was settled on oral feeds, and oral rifampicin/isoniazid, pyrazinamide and ethambutol tablets replaced the IV rifampicin and IV moxifloxacin. Intravenous AZT was replaced with abacavir. Liver transaminases had not been significantly altered by the TB therapy or the ART. Four weeks after starting TB treatment, sputum culture was found negative for MTB. Four months after starting ART, the HIV viral load was < 100 copies/mL, and at 12 months the CD4 count was 1111 cells/mL (25%). In total, the patient received approximately 11 months of oral anti-TB therapy. His weight-for-age Z-score 3 months after the completion of the TB treatment was within the normal range, that is, above -2. This had been less than -3 before starting the treatment.
Discussion
This severely immunocompromised HIV/TB co-infected young child diagnosed with pulmonary and abdominal TB complicated by intestinal obstruction and perforation was treated with a modified, initially IV anti-TB treatment regimen before transitioning to standard oral drug-sensitive anti-TB treatment and ART. Notwithstanding the drug-associated adverse events, this initial strategy allowed the immediate provision of TB therapy while awaiting the return of normal gastrointestinal function, and this has been likely to have contributed to the positive clinical outcome.
The literature on the role of IV anti-TB treatment in patients who are unable to take oral medication is, as far as we are aware, limited to a few adult case reports.1,2 Intravenous formulations of anti-TB medications currently available in South Africa include rifampicin - this requires approval from the South African Health Products Regulatory Authority (SAPHRA) - moxifloxacin, levofloxacin, LZD, meropenem, imipenem/cilastatin and amikacin. Oral fluoroquinolones, oral LZD and IV carbapenems in combination with IV amoxicillin-clavulanic acid are also used in some rifampicin-resistant TB treatment regimens. Amikacin is no longer recommended except in extremely drug-resistant cases with few alternatives.3,4 Our patient was treated with amikacin prior to the release of these recommendations. Intravenous meropenem was used without recourse to amoxicillin-clavulanic acid. Levofloxacin was unavailable but because of less effect on Corrected QT interval (QTc) interval prolongation, it is preferred to moxifloxacin.5 Nephrotoxicity because of amikacin and haematological toxicity because of LZD resulted in the discontinuation of these drugs in our patient.6,7 The duration of IV anti-TB therapy was 40 days in this patient. Other case reports have described 40-60 days' duration of injectable anti-TB treatment with or without concomitant oral anti-TB medication.1,2
Conclusion
Intravenous anti-TB treatment must be considered in patients with complicated abdominal TB in whom oral intake is not feasible. The optimal combination of medication is uncertain and close monitoring of treatment efficacy and toxicity is essential.
Acknowledgements
The authors thank the staff of the Paediatric Infectious Diseases Unit at Red Cross War Memorial Children's Hospital for guiding the clinical management of this patient and for providing ongoing follow-up of this family.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
All authors were involved in the drafting, reviewing, editing and finalising of this article.
Ethical consideration
Written informed consent was obtained from the parents of the child described in this case report.
Funding information
The authors received no financial support for the research, authorship and publication of this article.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and not an official position of their institutions.
References
1. Boff DF, Goldani LZ. Initial combination of injectable and oral anti-tuberculosis agents for the treatment of severe disseminated tuberculosis. Trop Doct. 2013;43(4):148-150. https://doi.org/10.1177/0049475513502961 [ Links ]
2. Goldani LZ, Spessatto CO, Nunes DL, et al. Management of severe gastrointestinal tuberculosis with injectable antituberculous drugs. Trop Med Health. 2015;43(3):191-194. https://doi.org/10.2149/tmh.2015-09 [ Links ]
3. WHO consolidated guidelines on drug-resistant tuberculosis treatment [homepage on the Internet]. [ Links ] Geneva: World Health Organization; 2019 [cited 2020 Jun 1]. Available from: https://apps.who.int/iris/bitstream/handle/10665/311389/9789241550529-eng.pdf?ua=1
4. Department of Health, Republic of South Africa. Management of rifampicin-resistant tuberculosis: A clinical reference guide [homepage on the Internet]. [ Links ] 2019 [cited 2020 Jun 3]. Available from: http://www.health.gov.za/index.php/2014-03-17-09-09-38/policies-and-guidelines/category/535-2019-policies-and-guidelines
5. Tsikouris JP, Peeters MJ, Cox CD, Meyerrose GE, Seifert CF. Effects of three fluoroquinolones on QT analysis after standard treatment courses. Ann Noninvasive Electrocardiol. 2006;11(1):52-56. https://doi.org/10.1111/j.1542-474X.2006.00082.x [ Links ]
6. Garcia-Prats A, Schaaf H, Hesseling A. The safety and tolerability of the second-line injectable antituberculosis drugs in children. Expert Opin Drug Saf. 2016;15(11):1491-1500. https://doi.org/10.1080/14740338.2016.1223623 [ Links ]
7. Garcia-Prats A, Schaaf H, Draper H, et al. Pharmacokinetics, optimal dosing, and safety of linezolid in children with multidrug-resistant tuberculosis: Combined data from two prospective observational studies. PLoS Med. 2019;16(4):e1002789. https://doi.org/10.1371/journal.pmed.1002789.eCollection [ Links ]
 Correspondence:
Correspondence:
Anthony Enimil
tenimil@live.com
Received: 29 June 2020
Accepted: 11 July 2020
Published: 24 Aug. 2020
EDITORIAL
The Editor's review of articles published from August to December 2019 in the Southern African Journal of HIV Medicine
David C. Spencer
Division of Infectious Diseases, Department of Medicine, Helen Joseph Hospital, University of the Witwatersrand, Johannesburg, South Africa
August 2019
1. Woods J, Moorhouse M, Knight L. A descriptive analysis of the role of a WhatsApp clinical discussion group as a forum for continuing medical education in the Eastern Cape, South Africa. S Afr J HIV Med. 2019;20(1):a982.
Editor's comment: In the year following my internship (1976), I worked at Mseleni Hospital, then a small 120-bed hospital in a remote corner of KwaZulu-Natal (KZN) province, South Africa. For much of the time, I was the only doctor. One of the highlights was the Thursday night radio call-in to discuss cases with colleagues at Manguzi and Bethesda, similar rural hospitals in northern KZN. I was a rookie. Darryl Hackland and Pat Garde (Bethesda) and Cliff Allward (Manguzi) were my lifeline. Another was the periodic weekend fly-in of Durban-based University of KZN academics who would assist with surgery and walk through the wards with me.
This observational study of the role of a WhatsApp group gives that story of a 21st-century twist. The goal of the authors was to assess the educational value of a WhatsApp group of 166 experienced and inexperienced doctors in rural public hospitals and clinics in the Eastern Cape province of South Africa and to ask whether the WhatsApp discussion was helpful and whether informed consent and privacy rules were breached. All the patients had complicated human immunodeficiency virus/tuberculosis (HIV/TB) co-infection. The study was undertaken between January 2016 and July 2017. The WhatsApp groups were given a short questionnaire and asked to submit answers anonymously. Although 86% of respondents replied that the group had given them 'improved confidence and ability in managing sick patients', and 52% said they used the guidance they had received 'all the time', many in the WhatsApp group (n = 74/166; 45%) never actually posted a response. Moreover, whilst answers such as 'I use the guidance to manage patients', 'I refer to previous WhatsApp cases', 'I gained new clinical insights' and so on reached statistical significance, that is, suggesting that clinical confidence had been increased, high odds ratios and wide confidence intervals suggest important limitations (Table 3 in the article). Does posting patient data risk a breach of doctor-patient ethics? Eighty-nine per cent of respondents agreed with the fact that informed consent would be required before posting patient-related data; however, in reality only 52% did this. And what about those registered on the programme who never appeared to participate? Was this a valuable learning experience for them? Perhaps, an analysis of the differences between the 50% who did not post cases and the 3% who did so frequently might answer this question.
2. Mugusi SF, Mopei N, Minzi O. Adherence to combination antiretroviral therapy among orphaned children in Dar es Salaam, Tanzania. S Afr J HIV Med. 2019;20(1):a954. https://doi.org/10.4102/sajhivmed.v20i1.954
Editor's comment: Adherence to antiretroviral therapy (ART) is examined in this cross-sectional study of 216 Tanzanian orphans aged 2-14 years. All the children were HIV-positive and had been on nevirapine (NVP)-based ART for a minimum of 6 months. The study was conducted from June to September 2015. Adherence was measured in three ways: a 3-day recall of pill-taking behaviour (caregiver questioned), the historical regularity/reliability of clinic attendance and the monitoring of NVP-blood levels on study entry. Viral load levels are not supplied. Likely not available. On recall, 79.6% of children had not missed any doses, and 82.9% gave a history of regular clinic attendance. Yet, therapeutic levels of NVP, namely ≥ 3 µg/mL, were detected in only 72.2% of patients. On multivariate analysis, higher NVP levels were protective of unreliable clinic attendance (unadjusted odds ratios [uOR] 0.45, 95% confidence interval [CI] 0.21-0.95, p = 0.04) and linked positively to higher CD4 levels, namely > 25% and counts >500 cells/mm3, p = 0.001. Orphans missing both parents were at greater risk of low NVP blood levels, namely, uOR 1.37, 95% CI 0.69-2.68. Sub-therapeutic NVP levels were less likely amongst those orphans who were aware of their HIV status, that is, had experienced full disclosure (uOR 0.65, 95% CI 0.34-1.24). The limitations of this study are important: the cross-sectional design, the absence of an age-matched 'non-orphan' comparator-arm, reliance on caregiver 'self-reporting' and the wider lack of applicability of blood NVP levels to adherence management in Africa. The fact that viral loads are still not routinely available everywhere in sub-Saharan Africa is an inescapable subtext to this study. Is therapeutic NVP monitoring needed in Africa? It added value to this study. However, a wider role will be limited by costs and accessibility.
. 3. Vujanovic M, Brkic-Jovanovic N, Ilic D, et al. Associations of visceral fat thickness and anthropometric measurements with non-alcoholic fatty liver development in male patients mono-infected with human immunodeficiency virus. S Afr J HIV Med. 2019;20(1):a968. https://doi.org/10.4102/sajhivmed.v20i1.986
Editor's comment: In this article from Serbia, 88 HIV-positive men on antiretroviral therapy (ART) were enrolled in a study evaluating a link between visceral fat thickness (VFT) as measured with abdominal ultrasound and the routine anthropometric measurements of obesity, cardiovascular risk and non-alcoholic steatohepatitis (NASH). The study took place over 18 months between September 2016 and April 2018. The average age of the men was 39.9 ± 9.9 years and the following anthropometric measurements were taken: waist and hip circumference (WC, HC), waist-hip and waist-height ratios (W/HipR, W/HtR) and the body mass index (BMI). Hepatic steatosis was diagnosed on sonography. Those with steatosis were more likely to have elevated random blood glucose levels, raised BMI and raised WC, HC, W/HipR and W/HtR in addition to elevated VFT (p < 0.001). Age ≥ 38.5 years was associated with an increased risk of the condition; 90.6% of those aged > 38.5 years with a VFT > 31.98 mm had hepatic steatosis. The authors discuss these results in the context of low- and middle-income countries where access to reliable non-invasive tests for hepatic steatosis is limited. The study limitations include its cross-sectional design, the absence of women and children and the lack of detailed information on antiretrovirals used by the men and the duration of their treatment.
4. Diana NE, Feldman C. Measles in adults: A comparison of hospitalised HIV-infected and HIV-uninfected patients. S Afr J HIV Med. 2019;20(1):a877. https://doi.org/10/4102/sajhivmed.v20i1.877
Editor's comment: South Africa experienced an unusually large outbreak of measles between 2009 and 2011. In this descriptive study from the wards of the Charlotte Maxeke Johannesburg Academic Hospital, the authors present data on HIV-positive adults with laboratory-confirmed measles. Thirty-three adults with measles were identified, of whom 24 underwent HIV testing. Of the 24 tested for HIV, 18 (75%) were HIV-positive and six were HIV-negative. The remainder of the adult measles group (n = 9) were not tested. Most of the HIV-positive were women (13/18; 72%). Although the authors remarked that demographics, clinical findings and laboratory data between the HIV-positive and HIV-negative patients were similar, serious disease, for example, pneumonia and respiratory failure, was more frequent in the HIV-positive (OR 5.0, 95% CI 0.48-51.8, p = 0.34). The duration of hospital stay for the HIV-positive patients was significantly longer (p = 0.03), and of the three adult measles deaths, all were in the HIV-positive (OR 2.9, 95% CI 0.13-65.3, p = 0.56). The median CD4 count of the HIV-positive patients was 109 cells/mm3. Unfortunately, the authors do not provide further analysis, for example, individual CD4s, viral loads, antiretroviral therapy used and microbiology of the secondary infections. Do HIV-positive adults exposed to measles require re-vaccination or vaccination if this was missed in childhood? This is not addressed in this article, which is an important question. According to Loevinsohn (2019:836-844, in suggested reading below), 'the measles vaccine should be given to potentially susceptible but asymptomatic HIV-positive adults and be considered for those with symptomatic HIV infection even if NOT severely immunosuppressed'.
Suggested additional reading
-
Loevinsohn G, et al. Measles seroprevalence and vaccine responses in human immunodeficiency virus-infected adolescents and adults: A systematic review. Clin Infect Dis. 2019 Aug 16;69(5):836-844. https://doi.org/10.1093/cid/ciy980.
-
Moss WJ. Measles. Seminar. Lancet. 2017;390:2490-2502. https://doi.org/10.1016/S0140-6736(17)31463-0
-
Measles vaccination: A WHO position paper. April 2017. Recommendations. Vaccine. 2019 Jan 7;37(2):219-222. https://doi.org/10.1016/j.vaccine20178.07.066).
5. Van Elsland SL, Peters RPH, Grobbelaar C, et al. Disclosure of human immunodeficiency virus status to children in South Africa: A comprehensive analysis. S Afr J HIV Med. 2019;20(1):a884. https://doi.org/10.4102/sajhivmed.v20i1.884
Editor's comment: Recommended reading. In this cross-sectional study from the Western Cape, the authors ask the following questions: how many children know their HIV status and what factors assist our understanding of non-disclosure? It is a well-written report with data that deserve a wide audience. The total cohort was 185. All were on antiretroviral therapy and their ages ranged from 3 to 14 years. Most (145; 76.3%) had not experienced full disclosure, whilst 17 (8.9%) had experienced. A further 28 (14.7%) received 'partial' disclosure. The cross-sectional nature of the study, the small number of 'disclosed' children and the dependence on questionnaires, clinic records and caregiver's reports would have introduced limitations but the take-home messages are worth noting: disclosure was more likely to have occurred amongst older children and those whose caregivers were more highly educated. The latter were more likely to be men, although less than 10% of the study's caregivers were men. Indeed, disclosure was less likely if the caregiver was a woman, if children had detectable viral loads and if the child was still taking 'syrup' formulations of the antiretrovirals , namely, a younger group, if the child was noted by the caregiver to be non-adherent and if the child was on protease inhibitors, stavudine (d4T) and/or didanosine (ddI). This article provides the readers with credible information. Figure 1 in this article will also give HIV educators a useful outline to the important associations that promote disclosure/non-disclosure.
6. Schutz C, Ward A, Burton R, et al. False rifampicin results using Xpert MTB/RIF on urine samples in hospitalised HIV-infected patients. S Afr J HIV Med. 2019;20(1):a978. https://doi.org/10.4102/sajhivmed.v20i1.978
Editor's comment: Recommended reading. This study is from colleagues in Cape Town. Urine samples were collected prospectively from HIV-positive patients with microbiologically proven active TB in two independent cohorts between 2012 and 2016. Multiple samples from each patient - including sputum, blood, tissue and urine - were subjected to culture, gene Xpert (including Xpert Ultra) and line probe analysis (LPA). A total of 1704 urine Xpert results were available from 1171 patients. Four hundred and sixteen (24.4%; 95% CI 22.4-26.5) of the urine Xpert results were positive for Mycobacterium tuberculosis (MTB) and 43/413 (10.4%) were rifampicin resistant on Xpert analysis. Of the latter group, 30/41 were confirmed to be truly rifampicin resistant, yielding a positive predictive value of 73.2% (95% CI 57.1-85/8). Urine tests from patients NOT on TB therapy at the time of assessment gave more true-positive rifampicin-resistant Xpert results (85.7%, 95% CI 67.3-96.0) than the urine of those on TB treatment (53.8%, 95 CI 25.1-80.8). About 11/43 urine results were falsely positive for rifampicin resistance (25.6%, 95% CI 13.5-41.2). Three patients in the urine Xpert rifampicin-resistant group were found to have concurrent rifampicin-sensitive TB on alternative specimens tested simultaneously, that is, 'hetero-resistant TB'. This article is a stimulating read and feeds the reader's mind - a compulsory reading for clinicians, particularly Infectious Diseases colleagues, fellows and registrars!!
7. Ekermans P, De Gama R, Kock C, et al. An unusual case of abdominal mycobacterial infection: Case report and literature review. S Afr J HIV Med. 2019;20(1):a993. https://doi.org/10.4102/sajhivmed.v20i1.993
Editor's comment: Highly recommended. In this case report, Dr Ekermans and colleagues describe an HIV-positive 8-year-old's experience of acquired immunodeficiency syndrome in South Africa. The author does a great job of describing the difficulties in confirming the diagnosis and isolating the organism. The histological and radiographic plates are superb: clear and compelling. The author tells this story with compassion and respect for his subject. The art and science of medicine shine on these pages. This is how we learn medicine, and how we become better doctors. I loved this read and recommend it to all who read this journal.
8. Atuhaire C, Taseera K, Spoor C, Cumber RY, Cumber SN. Knowledge and perceptions of male immigrants in Leeds (UK) towards male circumcision as an HIV-prevention strategy. S Afr J HIV Med. 2019;20(1):a823. https://doi.org/10.4102/sajhivmed.v20i1.823
Editor's comment: Whilst the estimated prevalence of HIV infection in the United Kingdom (UK) is low, namely, ≤ 1.5 per 1000 persons, that of UK immigrants from Eastern and Southern Africa is far higher, namely, 25-50 per 1000 persons. Would medical male circumcision (MMC) be considered by these immigrants as a means of preventing HIV transmission? Only 10 persons were interviewed in a snowball recruitment study of contacts from a local church in Leeds, UK. The participants expressed little or no knowledge of circumcision as an HIV-preventive tool. Instead and despite the group's roots from the epicentre of the epidemic, circumcision is still merely a 'rite of passage'. Whilst the study limitations are obvious, it begs the question of the universality of HIV dogma. In the West, 'Treatment as Prevention' has replaced MMC. With current dogma promoting universal 'test and treat (UTT)' and 'immediate ART for all', should we be talking about MCC in high-income countries? And what of its future in middle- and low-income regions in the face of more effective preventive measures? And how effectively has MMC changed attitudes to HIV prevention in eastern and southern Africa?
September 2019
9. Manickchund N, Du Plessis C, John M-A, et al. Case report. Emtricitabine-induced pure red cell aplasia. S Afr J HIV Med. 2019;20(1):a983. https://doi.4102/sajhivmed.v20i1.983
Editor's comment: In this article, the authors report a female patient with pure red-cell aplasia. She was 35 years of age in 2014, pregnant, anaemic at baseline (haemoglobin [Hb] = 8.2 g/dL) and had a low CD4 count (83 cells/mm3). Two months after starting first-line ART, namely, tenofovir + emtricitabine (FTC) + efavirenz, she was found to be severely anaemic: Hb = 2.2 g/dL, normocytic normochromic. Her HIV infection was under control. Workup included a bone marrow examination, which revealed a pure red cell aplasia and a positive parvovirus B-19 polymerase chain reaction (PCR). The patient received intravenous immune globulin (IVIG) for 5 days and packed red cells. Over the next 11 months, she required multiple transfusions and six more courses of IVIG. Her ART was changed and the FTC stopped: tenofovir + abacavir + efavirenz, after which the anaemia resolved. However, the patient's parvovirus B19-PCR remained positive (2017). A role for lamivudine (3TC) and emtricitabine in pure red cell aplasia has been suggested by multiple reports over the past two decades. This report is a reminder of this rare drug-related toxicity.
Suggested additional reading
-
Tsukamoto T. Hematopoietic stem/progenitor cells and the pathogenesis of HIV/AIDS. Front Cell Infect Microbiol. 2020 Feb 21;10:60. https://doi.org/10.3389/fcimb.2020.00060
-
Durandt C, Potgieter JC, Mellet J, et al. HIV and haematopoiesis. S Afr Med J. 2019 Sep 10;109(8b):40-45. https://doi.org/10.7196/SAMJ.2019.vi09:8b.13829
-
Knuesel SJ, Sawalla Guseh J II, Karp Leaf R, Ciaranello AL, Eng GM. Case 6-2018: A 35-year-old woman with headache, subjective fever, and anemia. N Engl J Med. 2018;378:753-760. https://doi.org/10.1056/NEJMcpc1712223
10. Cloete CM, Hampton J, Chetty T, et al. Evaluation of a health system intervention to improve virological management in an antiretroviral programme at a municipal clinic in central Durban. S Afr J HIV Med. 2019;20(1):a985. https://doi.org/10.4102/sajhivmed.v20i1.985
Editor's comment: 'What are the gaps in service delivery that allow for clinical failure/poor viral control?' This is a detailed, prospective clinic-based study undertaken between 2011 and 2015. The investigators divided the study into three periods: pre-intervention, intervention and post-intervention. The intervention required checking every 10th patient file (n = 1538) with (1) an in-depth file review, (2) recording of viral loads (VL) and the 'retention-in-care' status of the client and (3) an assessment of the 'viral load-management process'. Gaps were identified and interventions were implemented. Outcome measurements improved over the 4 years of the study, namely, the number of appropriate VL tests and the filing of results increased from 78% to 92% (p = 0.0009), the number of patients who accessed their VL result increased from 59% to 86% (p < 0.0001) and fewer patients, from 81% to 27%, required changes to antiretroviral therapy (ART) following the intervention. The detailed description in this report suggests a huge commitment from the clinic staff and the research team. Sustainable? The study required outside funding and the salaries of additional staff. Sustainable? Gaps are noted: continuing high patient volumes, the ongoing and urgent priority of ART-initiation, the need for and absence of dedicated pharmacists in HIV clinics and so on. The authors point out that the third UNAIDS 90 or (95)% goal is achievable, that is, reliable long-term VL suppression by 2030. This sounds optimistic. 'Is this effort reproducible on a large scale?'
11. Kummerow M, Shaddock EJ, Klipstein-Grobusch K, et al. Unexpected low frequency of respiratory symptoms in an HIV-positive urban sub-Saharan population compared to an HIV-negative control group. S Afr J HIV Med. 2019;20(1):a1010. https://doi.org/10.4102/sajhivmed.v20i1.1010
Editor's comment: This is a cross-sectional study of 547 adults living in Johannesburg between July 2016 and November 2017. Two-thirds of the patients were people living with HIV (PLWH) (n = 347, 63%). The remainder were HIV-negative matched controls. The median age of the patients was 37 years. Two-thirds (62%) were women. Recruits were asked about cough, cough accompanied by mucus or phlegm, breathlessness, wheeze and so on. The participants were also examined, had their blood tested (HIV status confirmed, HIV viral load [VL] and CD4 cell count) and completed a 'quality of life' questionnaire. The PLWH were subdivided into three groups: 'the antiretroviral therapy (ART)-naïve' (26%), 'on first-line ART' (24%) and 'on second-line ART' (50%). Those on ART were recruits from randomised controlled trials (RCTs) and demonstrated a high level (> 90%) of viral suppression. Their CD4 levels (median) were largely normal: namely, first-line ART, CD4 = 413.5 (range: 278.5-574.3) and second-line ART, CD4 = 619 (range: 429-798) cells/mm3, respectively. Those 'initiating' therapy, that is, naïve to ART when tested, also demonstrated relatively preserved median CD4 levels, namely, CD4 = 281 (range: 191-400.8) cells/mm3. Moreover, indeed, the authors note that 'the frequency of respiratory symptoms did not differ by HIV status after adjustment for age and sex'. 'Breathlessness (however) was associated with older age, female sex, obesity, a previous history of respiratory infection and airway hyper-reactivity (asthma)'. Chronic lung disease has been described in Africans living with HIV. The participants in this study were young and most had accessed reliable ART for several years, exhibited immune (peripheral CD4 cell) reconstitution and reliable viral suppression. How should HIV clinicians be monitoring the health of our patients' respiratory tract? Will the Coronavirus disease 2019 (COVID-19) pandemic require a rethink of our patients' respiratory safety?
Suggested additional reading
-
Desai SR, Nair A, Rylance J, et al. Human immunodeficiency virus-associated chronic lung disease in children and adolescents in chest radiographic and high-resolution computed tomographic findings. Clin Infect Dis. 2018;66(2):274-281. https://doi.org/10.1093/vid/cix778
-
Shaddock EJ, Richards GA, Murray J. Lung fibrosis in deceased HIV-infected patients with pneumocystis pneumonia. S Afr J HIV Med. 2012;13(2):64-67.
12. Bharuthram N, Feldman C. The diagnostic utility of bone marrow examination in an infectious diseases ward. S Afr J HIV Med. 2019;20(1):a974. https://doi/org/10.4102/sajhivmed.v20i1.974
Editor's comment: This is a retrospective review of consecutive bone marrow aspirate and trephine (BMAT) examinations of 327 patients admitted to the infectious diseases ward of a large academic hospital in Johannesburg from 2012 to 2014. Most of the patients (314;96%) were people living with HIV (PLWH). 'What is the utility of BMAT in the context of HIV infection?' A peripheral white blood cell (WBC) cytopenia of ≤ 4 × 109/L predicted a 'unique' diagnosis (odds ratio [OR] 2.38, 95% CI 1.37-4.14, p = 0.002) and the likelihood of a mycobacterial infection (OR 2.11, 95% CI 1.28-4.41, p = 0.005). 'Unique' diagnoses mean diagnoses found only on BMAT despite extensive alternate investigation or achieved before the results of other tests were available or known. Unique diagnoses occurred in 77 (23.5%) patients and were Mycobacterium tuberculosis (MTB) in 17/77 (22%) and Mycobacterium avium complex (MAC) in another three patients. Three BMATs each provided ≥ 1X 'unique' diagnosis, for example, TB and cancer. Proven or suspected mycobacterial disease accounted for 57 BMATs with granulomas, culture-proven MTB without supportive histology in 50 and MTB confirmed with granulomas in 32 patients. The limitations of this study include its retrospective format, inherent case selection bias and, sadly, the absence of newer diagnostic tools such as sputum and urine MTB-rif-resistance gene-Xpert, gene-XPert Ultra and urine lipoarabinomannan (LAM). The latter is particularly disappointing as these molecular diagnostics are currently changing the face of clinical medicine.
13. Mehta UC, Van Schalkwyk C, Naidoo P, et al. Birth outcomes following antiretroviral exposure during pregnancy: Initial results from a pregnancy exposure registry in South Africa. S Afr J HIV Med. 2019;20(1):a971. https://doi/org/10.4102/sajhivmed.v20i1.971
Editor's comment: Recommended reading. Although international first-line ART guidelines have replaced nevirapine (NVP) and efavirenz (EFV) with dolutegravir (DTG), concerns remain regarding the safety of ART in pregnancy. Dolutegravir is teratogenic in the first trimester of pregnancy. Women living with HIV and planning a family and those diagnosed with HIV in the first trimester should not use DTG. This article addresses the safety of NVP and EFV in pregnancy in a cohort of pregnant South African (SA) women.
In 2013, the SA National Department of Health promoted the introduction of a birth-outcomes registry amongst pregnant women and their infants exposed to ARVs. The authors report on the first 12 months of this programme (2013-2014). Two outcomes were assessed:
1. Major congenital malformations (CMs) following ARV exposure in the first trimester of pregnancy.
2. Adverse birth outcomes (ABOs), namely, foetal death, preterm delivery, low birth weight, small for gestational age and neonatal death, following ARV exposure at any time during pregnancy.
Data were collected at the Prince Mshiyeni Memorial Hospital in Umlazi, Durban, South Africa. A total of 10 417 pregnancies and 10 517 birth outcomes were captured. The overall prevalence of HIV infection was 4013/10 417 (38.5%). A higher prevalence was noted in women > 35 years (640/1100; 58%) and in multigravida versus primigravid women (49.2% vs. 21.9%), respectively. The numbers of major CMs were small. About one-third of cases were in infants of mothers who were on ART (11/27; 29.7%). Compared to HIV-negative pregnant women unexposed to ARVs, first-trimester exposure to efavirenz in HIV-positive women did not increase the risk of CM (risk ratio [RR] 0.87, 95% CI 0.12-6.4, p = 0.895). However, first-trimester NVP exposure may increase the risk: RR 9.2, 95% CI 2.27-37.94, p = 0.002. This finding may have been influenced by confounders (e.g. small numbers) and thus requires more data or confirmation. The risk of ABOs was greater in infants of mothers with exposure to ART at any time throughout pregnancy versus HIV-uninfected mothers (RR 1.23, 95% CI 1.14-1.31, p < 0.001) but particularly where EFV or NVP use had started before the pregnancy. This report is published at a time when guidelines are changing. The non-nucleoside reverse transcriptase inhibitors (NNRTIs) are being phased out of first-line regimens. But women unable to take DTG are likely to be given EFV or perhaps NVP. This is a high-end paper that is informative and supports the long-term role of EFV in women for whom DTG is contraindicated.
Suggested additional reading
-
Zash R, Holmes L, Diseko M, et al. Neural tube defects and antiretroviral treatment regimens in Botswana. N Eng J Med. 2019 Aug;381(9):827-840. https://doi.org/10.1056/NEJMoa1905230
-
The National Department of Health, The Republic of South Africa. 2019 antiretroviral treatment guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates. October 2019. Dolutegravir Overview, ART Initiation, p. 8. Available from: https://www.health.gov.za
October 2019
14. Cele MA, Archary M. Acceptability of short text messages to support treatment adherence among adolescents living with HIV in a rural and urban clinic in KwaZulu-Natal. S Afr J HIV Med. 2019;20(1):a976. https://doi.org/10.4102/sajhivmed.v20i1.976
Editor's comment: This article reports the results of a small, questionnaire-based, cross-sectional, pilot study of 100 adolescents (aged 12-19 years) from two clinic sites - one urban (n = 50) and the other rural (n = 50) in KwaZulu-Natal, South Africa. Poor retention in care and unreliable treatment adherence challenge the success of antiretroviral therapy (ART) in this group of patients. Will text messaging remedy the problem? The authors confirm the widespread use (88%) of mobile devices amongst rural and urban respondents. Although two-thirds of participants were willing to receive their health information messages through mobile devices, others were unwilling or undecided. Higher education was found to be linked with greater mobile device usage. But who are those - people living with HIV - who are unwilling or undecided? Did the potential breach of privacy and the risk of unsanctioned disclosure via their smartphone inform the negative response? Forty-eight per cent of the cohort had never sent health-related short message services (SMSs) to or received (fewer) such messages from their clinic or health professionals. Who are these 12-19-year-olds who are unwilling or undecided? Are these the ones who will be lost to care and fail adherence? How do we ensure these also become confident, understand their rights and are assisted to adhere to ART?
15. Van Wyk B, Davids L-A. Challenges to HIV treatment adherence among adolescents in a low socio-economic setting in Cape Town. S Afr J HIV Med. 2019;20(1):a1002. https://doi.org/10.4102/sajhivmed.v20i1.1002
Editor's comment: This is a descriptive record of challenges faced by 15 adolescents (aged 10-19 years) living with HIV since birth, and receiving support from a primary care clinic in the greater Cape Town district. The participants were interviewed in 2016 and had been on antiretroviral therapy for a minimum of 6 months. Group and individual discussion focused on barriers to and facilitators of adherence. The themes identified by the authors are not new: the conflict between school and clinic, the need for greater 'HIV-competency' of households and the provision of adolescent-friendly health services. Limitations are acknowledged: small numbers, incomplete data saturation and the absence of a wide spectrum of views including that of defaulters. However for me, the strength of this study includes the verbatim comments of the participants. For a brief moment, the reader hears what it is like to be young and stigmatised and shamed by HIV and AIDS. This is why adherence is so difficult. It is not simply a matter of healing our youth; it is rather about society and the ongoing wider response to people living with HIV.
16. Moodley K, Bill PLA, Patel VB. Motor lumbosacral radiculopathy in HIV-infected patients. S Afr J HIV Med. 2019;10(1):a992. https://doi.org/10.4102/sajhivmed.v20i1.992
Editor's comment: This is a short report of 11 young (median age = 29 years) people living with HIV naïve to antiretroviral therapy (ART), who experienced a slowly progressive, bilateral and symmetrical, isolated, lower motor neurone weakness of the lower limbs. The latter were areflexic and flaccid. The remainder of the neurological examination, including higher function, sensation and sphincter control, were normal. A diagnosis of subacute motor lumbosacral radiculopathy was made. The mean duration of symptoms was 6.5 months (interquartile range [IQR] 3-7.5 months). Six were female patients. Cerebrospinal fluid (CSF) was notable for an elevated protein and the presence of mononuclear cells. Tests for malignancy and various infecting organisms were negative. The group's median CD4 cell count was 327 cells/mm3 (IQR 146 cells/mm3 - 457 cells/mm3). Unfortunately, serum and CSF HIV viral load levels were not drawn. On magnetic resonance imaging (MRI), gadolinium enhancement was visible in the lumbar ventral roots. Electromyography (EMG) confirmed abnormal activity of the lumbar and lower limb muscles. All the patients were treated for up to 4-6 weeks, with large amounts of oral prednisone (1.5 mg/kg/day). Steroids were sometimes given for longer periods. No steroid toxicity and no intercurrent (opportunistic) infection (e.g. TB and fungal) were reported. Antiretroviral therapy was not started immediately and no patient was on ARVs at the time of diagnosis. Ninety-one per cent of patients recovered within 3.4 months. All except one with mild residual weakness were 'normal' at the final 18-month follow-up visit. The authors - from the Neurology Department of the University of KwaZulu-Natal (UKZN), Durban, South Africa - discuss the differential diagnosis and point out that since the rollout of 'Universal Test and Treat' in 2017, few additional cases have been reported.
17. Laughton B, Naidoo S, Dobbels EFMT, et al. Neurodevelopment at 11 months after starting antiretroviral therapy within 3 weeks of life. S Afr J HIV Med. 2019;20(1):a1008. https://doi.org/10.4102/sajhivmed.v20i1.1008
Editor's comment. Recommended reading. This is an important, prospective, observational study of 29 infants born to mothers living with HIV (MLWH). All infants were started on antiretroviral therapy (ART) within 21 days of their birth. Twenty-four of the mothers (83%) were on ART at the time of delivery. Twenty-three (79%) infants were females. Infant viral load (VL) level at birth was 3904 (The median infant VL level at birth was 3904 (range, 259-16,022) copies/mL. Viral suppression (VL < 400 copies/mL) on ART occurred at 19.1 weeks (median, range 15, 36) of age. The Global Griffiths Mental Development Scales (GMDS), an early neurodevelopmental assessment tool, found the infant's developmental scores to be normal at 11.5 ± 0.8 months. This was despite the fact that 9/29 (31%) infants had a detectable bloodstream VL at the time. Of the five central nervous system (CNS) domains assessed, locomotor skills scored the lowest and hearing and language the highest. The authors acknowledge that this study is small and the results are preliminary. Nonetheless, these data suggest that ART started at this extremely young age is safe and beneficial. The authors inform us that a larger study is already underway. This article is another milestone along the way to beating the virus and to the well-being of future generations,
18. Archary M, Fairlee L, Slogrove A. Opinion piece. Current perspectives on paediatric HIV management from the Mexico International AIDS Society Conference, 2019. S Afr J HIV Med. 2019;20(1):a1027. https://doi.org/10.4102/sajhivmed.v20i1.1027
Editor's comment: Recommended reading. This is a summary of presentations and discussions held at the following meetings: the 11th International Workshop on Pediatric HIV and the 5th Workshop on Children and Adolescents HIV-Exposed and Uninfected, and the International AIDS Society (IAS) Conference in Mexico, July 2019. The authors remark that despite general success in controlling vertical transmission, there were 160 000 new global paediatric HIV infections in 2018. They further add that 'sub-Saharan Africa is struggling with meeting UNAIDS 90-90-90 goals for children and adolescents living with HIV'. This is nevertheless an optimistic report that focuses on antiretroviral treatment and the prevention of vertical transmission of HIV.
New antiretrovirals and a new delivery system: GS-6207 is the first of the capsid-inhibitor ARV class but currently no paediatric studies have been reported. Adult trials are promising. Treatment = long-acting, s/c administration every 3 months. MK-8591, the first nucleoside reverse transcriptase TRANSLOCATION-inhibitor. No paediatric data but adult studies = prolonged intracellular half-life and low once a week dosing. No cross-resistance to other nucleoside-reverse transcritpase inhibitors (NRTIs). An adult fixed-dose combination (FDC) in trials: MK-8591+doravirine. A paediatric formulation is possible. Tenofovir alafenamide (TAF) in children, a new NRTI with lower renal and bone demineralisation risk and an FDC formulation for children aged > 6 years and weighing ≥ 25 Kg: TAF/FTC/elvitegravir+cobicistat. Of a variety of novel drug delivery systems, the adult transdermal adhesive micro-needle skin patches that deliver monthly cabotegravir offer new therapeutic or prevention options with potential for paediatric application.
Antiretroviral drug efficacy and safety: Birth defects. The risk of neural tube defects in infants exposed to dolutegravir (DTG) during periconception (first-trimester of pregnancy) appears to be confirmed with updated data from Botswana's Tsepamo Birth Surveillance Study. Nevertheless, the World Health Organization recommends DTG-based antiretroviral therapy (ART) for all women of child-bearing potential who are living with HIV. Women who are not pregnant must be counselled regarding contraception. And counselling of all women MUST ensure that the decision for either a DTG or an efavirenz (EFV)-based ART is a fully INFORMED DECISION and decided ahead of the start of ART. Children who are HIV-exposed but uninfected (CHEU) enrolled in the International Antiretroviral Pregnancy Registry (APR) had lower birth weight- and length-for-age compared to unexposed, uninfected children. Zimbabwe's SHINE study also reported stunting and mortality risk to CHEU (see comment summary 13 above).
Metabolic effects of ART. Exposure of adults to ART (the South African ADVANCE study) uncovered significant weight gain in all study arms but especially those given both DTG+TAF/FTC. What drug-related toxicity is emerging and what does this mean for children on these drugs?
Public health, ART and children. Paediatric retention in care and viral load (VL) suppression rates are suboptimal in southern Africa. Adolescent mortality and morbidity risk is too high. According to the International Epidemiologic Databases to Evaluate AIDS - Southern Africa (IeDEA-SA) collaboration data 2004-2017, 'children lag behind'.
Readers are encouraged to check out this 'Opinion Piece' for themselves. Its background is the success of the prevention of mother-to-child transmission of HIV. Its reality is the day-to-day management of young people living with HIV.
19. Dunlop JL, Slemming W, Schnippel K, et al. Breast abnormalities in adolescents receiving antiretroviral therapy. S Afr J HIV Med. 2019;20(1):a1017. https://doi.org/10.4102/sajhivmed.v20i1.1017
Editor's comment: This is a retrospective report of abnormal breast development in adolescents aged 10-19 years. All were on antiretroviral therapy (ART). The article reflects January-December 2014 clinical records of 631 Johannesburg-based adolescents living with HIV (ALWH). Of the patients, 37 (5.9%) had 'abnormal' breasts. Of those with abnormal breasts, most (24; 65%) were men (p = 0.043). Median duration of ART was 4.9 years. The older adolescents (p < 0.0005) and those on efavirenz (EFB)-based ART (p = 0.016) were more likely to have breast problems. Ninety-two per cent (34/37) were on EFV, whilst the remaining 8% were on lamivudine (3TC) monotherapy, that is, they were likely to have been on EFV previously. (The prevalence of EFV use amongst those with normal breasts - the comparator group - was lower, namely n = 384/594;74.3%). The use of boosted-protease inhibitors, such as lopinavir or ritonavir, atazanavir and darunavir, was not associated with breast changes. Although lipodystrophy was recorded in 46.3% and gynecomastia in 29% of those with breast changes, body mass index-confirmed overweight or obesity was found in only eight (19.5%) participants. More than 70% had viral suppression (VL < 50 copies/mL) and immune reconstitution (CD4 count ≥ 500 cells/mm3). Most of the 37 adolescents took their pills. The file review indicated that clinic physicians 'corrected' the problem by replacing EFV with nevirapine (NVP). This hardly solved the problem. The authors remark that few were referred for additional breast care. And of the three who were referred, none received any definitive intervention. This article has also limitations. Retrospective studies have a tendency to accumulate data gaps with the passage of time. Did the breast abnormalities ever resolve? The use of EFV is widespread in sub-Saharan Africa. Are these toxicities still being seen? Is anyone culpable? After all, these are iatrogenic adverse events.
20. Govender NP, Meintjes G, Mangena P, et al. Southern African HIV Clinicians Society guideline for the prevention, diagnosis and management of cryptococcal disease among HIV-infected persons: 2019 update. S Afr J HIV Med. 2019;20(1):a1030. https://doi.org/10.4102/sajhivmed.v20i1.1030
Editor's comments: I recommend this guideline paper to all. It is a must-read and is up there with the best.
21. Spencer DC, Krause R, Rossouw T, et al. Palliative care guidelines for the management of HIV-infected people in South Africa. S Afr J HIV Med. 2019;20(1):a1013. https://doi.org/10.4102/sajhivmed.v20i1.1013
Editor's comment: This is a guideline paper and I thank the colleagues who helped me with this one. If you are interested in this field of HIV medicine, please contact the HIV Clinicians Society. We are eager to start an interest group to develop the field further.
22. Kaplan S, Nteso KS, Ford N, Boulle A, Meintjes G. Loss to follow-up from antiretroviral therapy clinics: A systematic review and meta-analysis of published studies in South Africa from 2011 to 2015. S Afr J HIV Med. 2019;20(1):a984. https://doi.org/10.4102/sajhivmed.v20i1.984
Editor's comment: Recommended reading. This review article provides a meta-analysis of public sector South African antiretroviral therapy (ART) studies that address loss to follow-up (LTFU) and mortality data. This is an important article and will inform the epidemiology and practice of ART in South Africa in the years ahead.
The data are derived from 48X adult, 15X paediatric and 4X pregnancy studies completed between January 2011 and October 2015. The study limitations are acknowledged: non-homogeneous data sources, namely, clinics, hospitals, rural and urban sites; and non-standardised definitions of LTFU across sources. When is a client lost to follow-up? After 3, 6 or 12 months since their last visit? The initiation of ART itself was in flux at the time: baseline CD4 counts varied and treatment varied between private and state providers. Were 'silent transfers' excluded from the LTFU data, that is, clients who move between clinics without informing staff and who are not truly LTFU?
The median cohort study size was 3737 persons. Median adult age and baseline CD4 count at ART initiation were 35.8 years and 121 cells/mm3, respectively. The median age of ART initiation in children was 4.2 years. The 'defined' follow-up time varied from 9 weeks to 5 years and the meta-analysis indicated no difference in LTFU estimates at 3-, 6- or 12-month census. The overall median mortality at 1 year was 7.9% (IQR 4.1-11.4; range 0% - 26%) and the median LTFU at 1 year was 12.8% (IQR 7.9% - 22.0%, range 0.2% - 43.1%). Aggregate meta-analysis LTFU estimates at 1 year were 11.6% (95% CI 11.4% - 11.7%) in the adult studies, 30.0% (95% CI 28.7% - 37.4%) for the pregnant cohorts and accounted for 7.5% (95% CI 6.7% - 8.2%) of the paediatric data. The 5-year LTFU estimate was 25% (95% CI 24.8% - 25.4%) based on three adult studies. In their concluding remarks, the authors indicate their support for the standardisation of the LTFU definition to 180 days (6 months).
Thank you for reading through this summary of articles published in the Southern African Journal of HIV Medicine August-December 2019. Similar summaries are available for the first half of 2019. If you have suggestions and improvements, please contact me at editor@sajhivmed.org.za.
Dr David Spencer
Editor-in-Chief
Southern African Journal of HIV Medicine
 Correspondence:
Correspondence:
David Spencer
editor@sajhivmed.org.za
^rND^1A01^nJeremy^sNel^rND^1A02^nSipho^sDlamini^rND^1A03^nGraeme^sMeintjes^rND^1A04^nRosie^sBurton^rND^1A05^nJohn M.^sBlack^rND^1A06^nNatasha E.C.G.^sDavies^rND^nEric^sHefer^rND^1A07^nGary^sMaartens^rND^1A08 A09^nPhetho M.^sMangena^rND^nMoeketsi T.^sMathe^rND^1A10^nMahomed-Yunus^sMoosa^rND^nMuhangwi B.^sMulaudzi^rND^1A11^nMichelle^sMoorhouse^rND^1A12^nJennifer^sNash^rND^1A13 A14^nThandeka C.^sNkonyane^rND^1A15 A16^nWolfgang^sPreiser^rND^1A17^nMohammed S.^sRassool^rND^1A18 A19^nDavid^sStead^rND^1A02^nHelen^svan der Plas^rND^1A20 A21^nCloete^svan Vuuren^rND^1A21^nWillem D.F.^sVenter^rND^1A22^nJoana F.^sWoods^rND^1A01^nJeremy^sNel^rND^1A02^nSipho^sDlamini^rND^1A03^nGraeme^sMeintjes^rND^1A04^nRosie^sBurton^rND^1A05^nJohn M.^sBlack^rND^1A06^nNatasha E.C.G.^sDavies^rND^nEric^sHefer^rND^1A07^nGary^sMaartens^rND^1A08 A09^nPhetho M.^sMangena^rND^nMoeketsi T.^sMathe^rND^1A10^nMahomed-Yunus^sMoosa^rND^nMuhangwi B.^sMulaudzi^rND^1A11^nMichelle^sMoorhouse^rND^1A12^nJennifer^sNash^rND^1A13 A14^nThandeka C.^sNkonyane^rND^1A15 A16^nWolfgang^sPreiser^rND^1A17^nMohammed S.^sRassool^rND^1A18 A19^nDavid^sStead^rND^1A02^nHelen^svan der Plas^rND^1A20 A21^nCloete^svan Vuuren^rND^1A21^nWillem D.F.^sVenter^rND^1A22^nJoana F.^sWoods^rND^1A01^nJeremy^sNel^rND^1A02^nSipho^sDlamini^rND^1A03^nGraeme^sMeintjes^rND^1A04^nRosie^sBurton^rND^1A05^nJohn M^sBlack^rND^1A06^nNatasha E. C. G^sDavies^rND^nEric^sHefer^rND^1A07^nGary^sMaartens^rND^1A08 A09^nPhetho M^sMangena^rND^nMoeketsi T^sMathe^rND^1A10^nMahomed-Yunus^sMoosa^rND^nMuhangwi B^sMulaudzi^rND^1A11^nMichelle^sMoorhouse^rND^1A12^nJennifer^sNash^rND^1A13 A14^nThandeka C^sNkonyane^rND^1A15 A16^nWolfgang^sPreiser^rND^1A17^nMohammed S^sRassool^rND^1A18 A19^nDavid^sStead^rND^1A02^nHelen^svan der Plas^rND^1A20 A21^nCloete^svan Vuuren^rND^1A21^nWillem D. F^sVenter^rND^1A22^nJoana F^sWoods
GUIDELINE
Southern African HIV Clinicians Society guidelines for antiretroviral therapy in adults: 2020 update
Jeremy NelI; Sipho DlaminiII; Graeme MeintjesIII; Rosie BurtonIV; John M. BlackV; Natasha E.C.G. DaviesVI; Eric HeferVII; Gary MaartensVIII; Phetho M. MangenaIX, X; Moeketsi T. MatheXI; Mahomed-Yunus MoosaXII; Muhangwi B. MulaudziXIII; Michelle MoorhouseXIV; Jennifer NashXV; Thandeka C. NkonyaneXVI, XVII; Wolfgang PreiserXVIII, XIX; Mohammed S. RassoolXX; David SteadXXI, XXII; Helen van der PlasII; Cloete van VuurenXXIII, XXIV; Willem D.F. VenterXXV; Joana F. WoodsXXV
IHelen Joseph Hospital, Department of Medicine, University of the Witwatersrand, Johannesburg, South Africa
IIDepartment of Infectious Diseases, Faculty of Medicine, University of Cape Town, Cape Town, South Africa
IIIDepartment of Medicine, Division of Infectious Diseases and HIV Medicine, Groote Schuur Hospital, University of Cape Town, Cape Town, South Africa
IVSouthern African Medical Unit, Médecins Sans Frontières (MSF), Cape Town, South Africa
VDepartment of Medicine, Division of Infectious Diseases, Livingstone Tertiary Hospital, Port Elizabeth, South Africa
VIAnova Health Institute, Johannesburg ,South Africa
VIIPrivate Practice Medical Adviser, Johannesburg, South Africa
VIIIDepartment of Medicine, Division of Clinical Pharmacology, University of Cape Town, Cape Town, South Africa
IXDepartment of Internal Medicine, School of Medicine, Pietersburg Hospital, Polokwane, South Africa
XDepartment of Medicine, School of Medicine, University of Limpopo, Turfloop, South Africa
XIPrivate Practice, Vereeniging, South Africa
XIIDepartment of Infectious Diseases, Division of Internal Medicine, University of KwaZulu-Natal, Durban, South Africa
XIIIPrivate Practice (Phomolong Medical Centre), Rustenburg, South Africa
XIVReproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
XVSpecialist Family Physician, Amathole District Clinical Specialist Team, East London, South Africa
XVIDepartment of Infectious Diseases, Faculty of Medicine, Sefako Makgatho Health Sciences University, Pretoria, South Africa
XVIIDepartment of Medicine, Dr George Mokhari Hospital, Pretoria, South Africa
XVIIIDepartment of Medical Virology, National Health Laboratory Service, Tygerberg, South Africa
XIXDepartment of Pathology, Faculty of Medicine and Health, Stellenbosch University, Cape Town, South Africa
XXClinical HIV Research Unit, Wits Health Consortium, Johannesburg, South Africa
XXIDepartment of Medicine, Faculty of Infectious Diseases, Frere and Cecilia Makiwane Hospitals, East London, South Africa
XXIIDepartment of Medicine, Faculty of Health Sciences, Walter Sisulu University, Mthatha, South Africa
XXIIIDepartment of Internal Medicine, Military Hospital, Bloemfontein, South Africa
XXIVDepartment of Internal Medicine, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
XXVEzintsha, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
What is new in the 2020 guidelines update?
Key updates
➢ A recommendation for dolutegravir (DTG)-based therapies as the preferred first-line antiretroviral therapy (ART) option (section 11).
➢ Updated guidelines for second- and third-line ART regimens (section 13).
➢ New recommendations on the management of patients on DTG-based therapies who have an elevated viral load (section 12).
➢ A lowering of the threshold for virological failure from 1000 copies/mL to 50 copies/mL (section 8).
➢ A recommendation against routine cluster of differentiation 4 (CD4+) monitoring in patients who are clinically well once the CD4+ count is > 200 cells/µL (section 9).
➢ Updated recommendations for isoniazid preventive therapy (IPT) in human immunodeficiency virus (HIV)-positive patients (section 27).
➢ A recommendation for the use of low-dose prednisone as prophylaxis for paradoxical tuberculosis (TB) immune reconstitution inflammatory syndrome (IRIS) in TB/HIV co-infected patients commencing ART within 1 month of TB therapy (section 26).
1. Preamble
Key principles
Although many antiretroviral therapy (ART) guidelines are available internationally, the current guidelines have been written to address issues relevant to southern Africa. A major spur for the current guidelines is the introduction of dolutegravir (DTG) into first- and second-line ART regimens. Dolutegravir-based ART regimens hold much promise, although the transition inevitably challenges existing paradigms and generates additional complexities. These guidelines aim to address many of these and to update the text in general to reflect the latest evidence.
As with previous iterations, these guidelines take affordability into account, as countries in the region vary according to their low- and middle-income status. Hence, only the treatment and diagnostic options that are available in southern Africa are included. In addition, these guidelines recognise the need to bridge the gap in treatment recommendations between public and private sector programmes, considering that many patients transition between the two sectors for treatment. The format of this iteration of the guidelines has been modified to highlight each section's key points and common pitfalls. It will also be released over time as multiple stand-alone modules, designed with an emphasis on readability, ease of access and user-friendliness.
Goals of antiretroviral therapy
The goals of ART are to:
•provide maximal and durable suppression of viral load (VL)
•restore and/or preserve immune function
•reduce human immunodeficiency virus (HIV)-related infectious and non-infectious morbidity
•prolong life expectancy and improve quality of life
•prevent onward transmission of HIV
•minimise adverse effects of the treatment
These goals are achieved by suppressing viral replication completely for as long as possible, using well-tolerated and sustainable treatment undertaken with good adherence. With prolonged viral suppression, the cluster of differentiation 4 (CD4+) lymphocyte count usually increases, which is accompanied by a restoration of pathogen-specific immune function. For most patients, this results in a dramatic reduction in the risk of HIV-associated morbidity and mortality. In patients who start to receive ART with preserved CD4+ counts, ART is able to prevent the decline in CD4+ count observed in untreated patients and prevent clinical complications of HIV infection. It is still unclear whether immune function ever returns to full normality, although long-term cohorts have shown that patients who adhere well to ART have a near-normal life expectancy.1 Patient adherence to the ART regimen remains a key focus and challenge.
Stopping antiretroviral therapy
Antiretroviral therapy should not be stopped unless there is an extremely compelling reason to do so. In most cases where drug toxicities develop, switching the culprit drug(s) should be attempted instead. If non-nucleoside reverse transcriptase inhibitor (NNRTI)-based therapy is stopped, then we generally do not recommend 'covering the tail' with an additional 5-7 days of nucleoside reverse transcriptase inhibitors (NRTIs). There is little evidence for this in patients on long-term ART, and the intracellular half-life of drugs, such as tenofovir disoproxil fumarate (TDF), in any case approximates that of the NNRTIs.2 It is important to ensure that the VL is suppressed before substituting a single drug for toxicity; otherwise, resistance may develop to the new drug, consequently compromising future regimens. However, single-drug substitutions can be done in the first few months of ART without measuring the VL, as the VL may take up to 6 months to become suppressed.
We strongly advise against lamivudine (3TC) monotherapy 'holding regimens' in patients who have virological failure. Such regimens can be associated with a rapid fall in CD4+ count. When prescribing ART, the objective should always be to provide a regimen that could achieve virological suppression.
2. Nucleoside or nucleotide reverse transcriptase inhibitor class of antiretroviral drugs
Key points
➢ The recommended nucleoside or nucleotide reverse transcriptase inhibitor (NRTI) drugs for first-line therapy are tenofovir disoproxil fumarate (TDF) and either 3TC or emtricitabine (FTC).
➢ Patients with a creatinine clearance rate (CrCl) < 50 mL/min should generally be started on abacavir (ABC) instead of TDF for first-line therapy.
➢ Zidovudine (AZT) should only be used in special circumstances as a first-line drug.
➢ Tenofovir disoproxil fumarate can cause renal failure or a renal-tubular wasting syndrome. Creatinine monitoring at regular intervals is recommended.
➢ Abacavir can cause a fatal hypersensitivity reaction in patients with HLA-B*5701. If feasible, this allele should be excluded prior to starting ABC, although it is very rare in people of African descent.
➢ Zidovudine can cause anaemia and neutropenia, and regular monitoring of haemoglobin (Hb) and neutrophil counts is recommended for the first 6 months.
Available nucleoside or nucleotide reverse transcriptase inhibitors
Nucleoside reverse transcriptase inhibitors and nucleotide reverse transcriptase inhibitors (NtRTIs) work by acting as nucleotide base analogues. Following incorporation into the deoxyribonucleic acid (DNA) chain by HIV's reverse transcriptase enzyme, they block further chain elongation. A summary of NRTIs is provided in Table 1, and the appropriate baseline investigations and required monitoring are presented in Table 2. Nucleoside reverse transcriptase inhibitors may be available as single tablets or in fixed-dose combination (FDC). The latter is recommended where possible to decrease the overall pill burden. Many NRTIs require dose adjustment in patients with renal failure (see section 21).

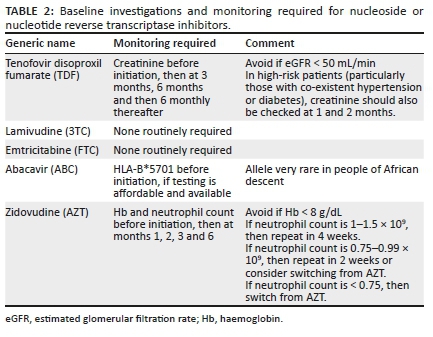
Lamivudine and emtricitabine
Lamivudine and FTC are well-tolerated drugs recommended as part of a first-line regimen. Although there are minor differences between them, 3TC and FTC are considered functionally interchangeable. Their use may be continued in the presence of 'high-level resistance' caused by the M184V mutation because this mutation impairs the replication ability of HIV, causing a ~0.5 log decrease in VL. Therefore, the drugs are often used in second- and third-line therapies (see the management of patients on second-line ART in section 13). Lamivudine and FTC are active against hepatitis B, but when used in the absence of a second drug active against hepatitis B, such as TDF, then resistance rates of approximately 50% at 1 year, and 90% at 5 years, are seen3. See section 20.
Tenofovir disoproxil fumarate
Tenofovir disoproxil fumarate is the preferred drug in this class for use with 3TC or FTC in first-line therapy because it aligns with public sector programmes, is widely available as an FDC and is generally well tolerated. Tenofovir disoproxil fumarate also offers durable therapy against hepatitis B virus (HBV). Hepatitis B virus resistance against TDF is extremely rare. In a minority of patients, TDF may cause a tubular wasting syndrome (including wasting of phosphate and potassium).4 If patients receiving TDF develop muscle weakness or other muscle symptoms, then potassium and phosphate levels must be assessed. Tenofovir disoproxil fumarate can also cause acute and chronic renal failure, but this is uncommon.5
Tenofovir disoproxil fumarate should be switched to ABC or an alternative NRTI immediately in patients with acute renal failure, as it may exacerbate injury even if it is not the primary cause. Consider recommencing TDF with careful monitoring when creatinine level is normal if an alternative cause of renal failure is established.
We recommend estimating the CrCl before commencing TDF; the drug should not be used if the estimated glomerular filtration rate (eGFR) or CrCl is < 50 mL/min. For monitoring whilst on TDF, see Table 2. Where TDF is avoided because CrCl is < 50 mL/min at baseline, it may be possible to switch to TDF at a later point if renal function improves. This is often the case where patients had diarrhoea or other opportunistic infections (OIs) at the time of ART initiation.
o Common pitfall: Permanently discontinuing TDF in patients with transiently decreased CrCl. Most cases of acute kidney injury (AKI) are not because of TDF, and if another cause of AKI is identified (e.g. severe diarrhoea or pneumonia), then TDF can be re-introduced with monitoring once renal function improves.
The long-term use of TDF together with other nephrotoxic agents (e.g. aminoglycosides or non-steroidal anti-inflammatory agents) should be avoided. Tenofovir disoproxil fumarate also causes a decrease in bone mineral density; however, this is generally mild and non-progressive, and most studies have not found an increase in fracture risk.
Abacavir
Abacavir can be used in patients with a CrCl < 50 mL/min at baseline, rather than TDF. Abacavir does not require dose adjustment in patients with renal failure and is especially useful in patients with chronic renal failure, where TDF is nephrotoxic and AZT could aggravate anaemia in patients with renal failure. A meta-analysis showed that virological suppression is equivalent with ABC- and TDF-containing first-line regimens regardless of baseline VL.6
oCommon pitfall: Avoiding ABC at high HIV VLs. This is unnecessary as viral suppression rates are equivalent in meta-analyses.
Abacavir has been associated with an increased risk of myocardial infarction in some but not all cohort studies; however, the association was not confirmed in a meta-analysis of randomised controlled trials (RCTs).6,7,8 Nevertheless, caution is recommended when considering ABC for patients who are at significant risk of or have established ischaemic heart disease. Abacavir hypersensitivity is a systemic reaction occurring within the first 8 weeks of therapy in approximately 3% of cases. Fatalities may occur on rechallenge. Abacavir must be discontinued and never re-introduced if hypersensitivity is suspected. The manifestations of hypersensitivity include fever, rash, fatigue and abdominal or respiratory symptoms. If there is any doubt concerning the diagnosis (e.g. if the patient has a cough with fever), then the patient should be admitted for observation of the next dose; symptoms progress if hypersensitivity is present. The hypersensitivity reaction has been shown to occur on a genetic basis, with a very strong association with the HLA-B*5701 allele. This allele is very uncommon in people of African descent; thus, ABC hypersensitivity is less frequent. If testing is affordable and available, then the presence of HLA-B*5701 should be excluded prior to prescribing ABC, especially in patients who are not of African descent.
Zidovudine
We now recommend reserving AZT for use only in special circumstances in first-line therapy. If both TDF and ABC are unavailable or contraindicated, then AZT should be used, provided that the Hb is > 8 g/dL.
Additional syndromes related to nucleoside reverse transcriptase inhibitors
Haematological toxicity
Cytopenias occur commonly in patients with HIV infection without exposure to ART. Patients receiving AZT or cotrimoxazole (CTX) may experience full blood count (FBC) abnormalities. Zidovudine can cause anaemia and neutropenia; platelet counts generally rise with the use of the drug. Monitoring is necessary with AZT (see Table 2). It is unusual, however, to see haematological toxicity develop after 6 months. Macrocytosis is usual with AZT therapy and is of little consequence. Routine measurement of vitamin B12 and folate concentrations is not needed.
oCommon pitfall: Discontinuing AZT because of macrocytosis. This is of little consequence and does not necessarily portend subsequent anaemia.
Pure red cell aplasia, which presents with severe anaemia and a low reticulocyte production index, has rarely been associated with 3TC and FTC.9,10 A bone marrow examination should be performed to confirm the condition. A polymerase chain reaction (PCR) test should be conducted to exclude the presence of parvovirus B19 infection. If 3TC and FTC are contraindicated because of pure red cell aplasia, then we suggest contacting an expert for advice about alternative regimens.
Hyperlactataemia and lactic acidosis
Lactic acidosis is a rare but serious and potentially fatal side effect of NRTIs, most commonly associated with stavudine (d4T), particularly when combined with didanosine (ddI). These drugs are no longer used. It can also occur occasionally with AZT. Symptomatic hyperlactatemia without acidosis is more common and is associated with the same drugs. Neither lactic acidosis nor hyperlactatemia without acidosis is seen with the newer, safer NRTIs such as TDF, ABC, 3TC or FTC. Symptoms are non-specific and include nausea and vomiting, abdominal pain, dyspnoea, fatigue and weight loss. A raised lactate (> 5 mmol/L) together with metabolic acidosis confirms the diagnosis of lactic acidosis. Low serum bicarbonate (< 20 mmol/L) is the most sensitive marker of acidosis. Patients receiving AZT who develop hyperlactatemia should be switched to alternative drugs, and lactate should be monitored serially until resolution. In severe patients, admission may be required.
Lipoatrophy
The thymidine analogue NRTIs (AZT and especially d4T) are associated with subcutaneous fat loss (most noticeable in the face, limbs and buttocks). Lipoatrophy improves when d4T/AZT are substituted with TDF or ABC, but resolution is very slow and often incomplete; therefore, it is important to recognise lipoatrophy early or, better still, to use NRTIs that are not associated with the condition. Although d4T is no longer used, patients who received it historically may still have lipoatrophy.
3. Integrase strand transfer inhibitor class of antiretroviral drugs
Key points
➢Two integrase strand transfer inhibitors (InSTIs) are available in southern Africa, namely, DTG and raltegravir (RAL).
➢ Dolutegravir is preferred to RAL because it has a higher barrier to resistance, is available in FDC formulation and can be taken once daily.
➢ Dolutegravir has been shown to have greater efficacy than efavirenz (EFV), driven largely by superior tolerability.
➢ Dolutegravir causes a small increase in serum creatinine (usually 10 mmol/L - 20 mmol/L) because of interference with tubular creatinine secretion; however, this does not represent a decline in renal function.
➢ Although definitive data are still lacking, DTG may be teratogenic in a small proportion of patients; thus, treatment decisions in women of reproductive age should be discussed and evaluated carefully (see section 19).
➢ Weight gain is a newly recognised side effect of InSTIs, more so with DTG than with RAL, and more so in black women and in patients with lower baseline CD4+ counts and higher VLs.
Overview of integrase strand transfer inhibitors
Integrase strand transfer inhibitors - often simply termed 'integrase inhibitors' - work by preventing the transfer of proviral DNA strands into the host chromosomal DNA. Currently, two InSTIs are available in southern Africa: DTG and RAL. Dolutegravir is preferred to RAL because of its higher barrier to resistance, its availability in FDC formulation and the ability to take the drug once daily. The SPRING-2 trial compared DTG- and RAL-containing first-line regimens and found no significant differences in virological suppression, and adverse effects were similar between treatment groups11; however, although no patients in the DTG arm were found to have developed resistance, one patient in the RAL arm developed InSTI resistance and four developed NRTI resistance. The high barrier to resistance of DTG-containing ART regimens has been replicated in other first-line studies and in a study of ART-experienced patients in which DTG was compared with RAL.12,13,14 In a meta-analysis that included clinical trials and observational studies, the emergence of InSTI resistance was more common with RAL than with DTG (3.9% vs. 0.1%).15 However, the emergence of InSTI resistance in patients receiving RAL can compromise second-generation InSTIs, such as DTG.
Dolutegravir use has been shown to be superior to EFV-based ART in the SINGLE trial.12 This difference was largely driven by the superior tolerability of the DTG arm: 2% in the DTG arm compared with 10% in the EFV arm had an adverse event leading to discontinuation of the study drug. Dolutegravir showed superior rates of viral suppression compared with EFV (71% vs. 63% at 144 weeks).
Dolutegravir-based regimens have also been shown to be superior to protease inhibitor (PI)-based regimens. As a first-line therapy, DTG was superior to darunavir/ritonavir (DRV/r) in terms of both viral suppression rates and side effect profile.13 The ARIA trial of ART-naive women demonstrated DTG's non-inferiority to atazanavir (ATV)/ritonavir (ATV/r), although with a statistically significantly higher rate of viral suppression and fewer side effects overall.16 In the DAWNING trial considering second-line regimens, DTG was found to be superior to lopinavir/ritonavir (LPV/r).17 Importantly, at least one fully active NRTI was genotypically confirmed at baseline in this trial.
Data from the Tsepamo surveillance study in Botswana demonstrated a statistically higher rate of neural-tube defects (NTDs) amongst women who were taking DTG at the time of conception (0.3% vs. 0.1% in women receiving other ARTs in the periconception period).18 Unlike in South Africa, folate fortification of staple foods does not occur in Botswana. In contrast to the Botswana data, no NTDs were reported in a Brazilian cohort of 1468 women, 382 of whom were DTG-exposed.19 Although additional data will undoubtedly be forthcoming, it should be noted that the absolute risk is < 0.5%, which may be outweighed by the additional benefits of DTG over alternative therapies. We recommend that women of childbearing potential (WOCP), particularly those who wish to become pregnant or who have no reliable access to effective contraception, should be counselled adequately about the potential risks and benefits of DTG- versus EFV-based ART and should be offered a choice of first-line regimens.
Common side effects
Dolutegravir and RAL are generally well tolerated, with most side effects being mild and very rarely leading to discontinuation. Dolutegravir may cause a mild increase in serum creatinine because of interference with tubular secretion. This does not represent renal damage and is not an indication of switching to another drug. The rise in creatinine occurs within the first few weeks and persists for as long as the patient remains on DTG.
oCommon pitfall: Assuming that the rise in creatinine seen in patients on DTG necessarily represents renal failure. In reality, the effect of DTG on creatinine secretion is of no consequence and does not represent a decline in renal function.
Raltegravir and DTG can cause headaches when started, but this usually resolves after sometime. These drugs may also cause insomnia and neuropsychiatric side effects. Raltegravir and DTG can occasionally cause hypersensitivity rashes, including life-threatening rashes. Weight gain is more pronounced in patients taking an InSTI as part of their ART regimen (with the exception of cabotegravir, which is not currently available in South Africa). Black women, patients with low baseline CD4+ counts and patients with high baseline VLs appear to be at greatest risk.20 The risk also appears to be moderated by the companion drugs in the patient's ART regimen. In the ADVANCE trial, women on a tenofovir alafenamide (TAF) + FTC + DTG regimen were found to gain a median of 10 kg over 96 weeks, with little evidence of a plateau in the increase.21 In women, median weight gain in the same period in the TDF + FTC + DTG arm was 5 kg, and 3 kg in the TDF + FTC + EFV arm. In men, weight gain was approximately half as much in each arm. The long-term health implications of these findings are currently unclear; however, clinicians should be aware of the possibility of weight gain and encourage appropriate exercise and dietary measures to limit this.
Dosage and common adverse drug reactions (ADRs) of InSTIs are described in Table 3.
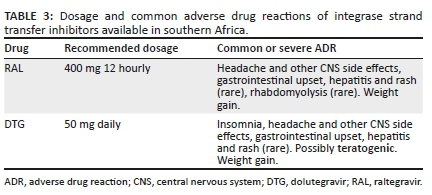
Key drug-drug interactions with dolutegravir
Key drug-drug interactions involving DTG are summarised in Table 4.
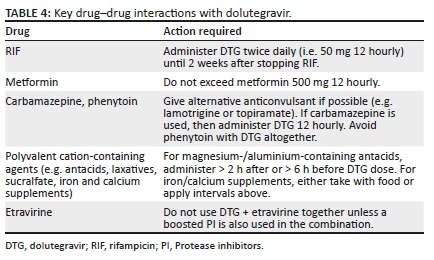
o Common pitfall: Forgetting to dose DTG twice daily when RIF-based tuberculosis treatment is commenced.
4. Non-nucleoside reverse transcriptase inhibitor class of antiretroviral drugs
Key points
➢ Efavirenz remains a good first-line ART option for patients who tolerate DTG poorly, or where DTG is contraindicated or declined.
➢ Efavirenz 400 mg is not inferior to EFV 600 mg and offers a somewhat improved side-effect profile. However, it is currently not available in FDC and has not been well studied in patients receiving rifampicin (RIF)-based TB treatment or in pregnant women.
➢ Rilpivirine (RPV) is another good first-line option, but it is not available in FDC, cannot be co-administered with RIF-based TB treatment and should not be started in patients with a VL > 100 000 copies/mL.
➢ Nevirapine (NVP) is no longer recommended for new patients because of its adverse side effect profile.
➢ Etravirine (ETR) may be used as part of third-line therapy where appropriate, but is not recommended as a first-line agent.
Overview of non-nucleoside reverse transcriptase inhibitors
Non-nucleoside reverse transcriptase inhibitors work by binding irreversibly to HIV's reverse transcriptase enzyme, which causes a conformational change in the enzyme's active site and impairs its functioning. The four NNRTIs currently available in southern Africa are EFV, NVP, RPV and ETR.
Individual non-nucleoside reverse transcriptase inhibitors
Efavirenz
Efavirenz is available in 600 mg and 400 mg formulations: Efavirenz 600 mg is available in public sector programmes in most countries in southern Africa. There is extensive clinical experience with the formulation, and it is available in FDC. Efavirenz 400 mg showed non-inferior efficacy with moderately improved tolerability in the ENCORE1 study.22 However, there are only limited pharmacokinetics data in pregnant patients, and in patients receiving RIF-based TB treatment. Efavirenz 400 mg is currently also not available in FDC. For these reasons, we do not recommend the routine use of EFV 400 mg in first-line ART. It remains an appropriate choice, however, in selected patients.
Efavirenz frequently causes neuropsychiatric effects in the first few weeks of therapy, typically presenting with insomnia, vivid dreams and dizziness. Both dysphoria and euphoria may occur. Patients starting on EFV should be warned about these symptoms and should be reassured that the symptoms usually resolve within the first few weeks, and if not, then an alternative can be substituted. Psychosis may occasionally occur. If the neuropsychiatric effects of EFV are not tolerated, then the patient should be switched to RPV, DTG or lower-dose EFV. Recently, a late-onset encephalopathy syndrome has been linked to EFV.23 This is characterised by a subacute encephalopathy and cerebellar dysfunction, frequently presenting months to years after commencing EFV, and is associated with supratherapeutic EFV levels. Patients who are genetically slow metabolisers of EFV may be predisposed to this syndrome. Two common CYP2B6 polymorphisms linked to slow EFV metabolism have been shown to occur with increased frequency in patients of African descent.24 This predisposition to toxic EFV levels may be further exacerbated in patients of low body weight and in those taking concomitant isoniazid, which inhibits an accessory EFV metabolism pathway via CYP2A6. Patients with a compatible clinical syndrome, in the absence of an alternative cause, should have plasma EFV levels measured and should be switched to a non-EFV-based regimen. Clinical improvement is typically seen within 10-21 days after stopping EFV.
Efavirenz may also cause a drug-induced hepatitis. A subset of these cases appears to occur relatively late, several months or even years after the drug has been initiated.25 It is important that this diagnosis is considered in the differential diagnosis of a subacute hepatitis syndrome. Gynaecomastia can occur with the use of EFV.26 This is not related to lipodystrophy. The onset occurs several months after initiation of ART and it may be bilateral or unilateral. The mechanism appears to be related to oestrogen receptor activation in breast tissue by EFV.27 It is important to exclude other common causes of gynaecomastia, such as other medications (including spironolactone, calcium channel blockers and metoclopramide). A serum testosterone test is useful in excluding hypogonadism as a possible cause. If serum testosterone is low, then other appropriate investigations should be carried out to identify the cause and manage accordingly; if serum testosterone is normal, then EFV should be substituted, bearing in mind the general principles of single-drug substitutions (patients who are virologically suppressed should be switched to DTG or RPV). Resolution of gynaecomastia is generally slow, taking months, and may be incomplete in a small percentage of patients.28 It is therefore important to manage the expectations of the patient in this regard.
Rilpivirine
Another option in first-line ART is RPV, a second-generation NNRTI: Rilpivirine is inexpensive, but not currently available in FDC in the region. An important drawback is that it should not be started in a patient with a VL > 100 000 copies/mL, as it is inferior to EFV in such patients.29 Rilpivirine has a lower incidence of neuropsychiatric side effects and rashes than EFV.30 There are several important drug-drug interactions with RPV. Amongst other considerations, RPV cannot be co-administered with RIF or proton pump inhibitors (PPIs). Histamine-2-receptor antagonists need to be administered 12 h before or 4 h after taking RPV. Rilpivirine should be taken with food to increase absorption.
oCommon pitfall: Prescribing RPV without first checking baseline VL. Rilpivirine is less efficacious than comparator drugs when VL is > 100 000 copies/mL.
Nevirapine
We no longer recommend NVP use for new patients starting ART because of the severe toxicity that may be associated with its use: In patients currently tolerating NVP, there is no reason to switch treatment because of toxicity concerns, as toxicity characteristically occurs in the first 3 months of NVP treatment and not later. However, switching for the purpose of simplification to a once-daily regimen should be considered, provided that there is virological suppression.
Etravirine
Etravirine is a second-generation NNRTI that has been studied in treatment-experienced patients rather than in ART-naive patients: As seen with RPV, the activity of ETR is not affected by the first-generation NNRTI's signature K103N resistance mutation.
Hypersensitivity with non-nucleoside reverse transcriptase inhibitors
Rash is common with NNRTIs in the first 6 weeks of therapy, notably more severely and frequently with NVP. If the rash is accompanied by systemic features (e.g. fever, elevated alanine transaminase [ALT] or hepatitis), mucosal involvement or blistering, then the NNRTI should be discontinued immediately and re-challenge must not be performed as these are features of life-threatening reactions. If the rash is mild and occurs without these features, then the NNRTI can be continued and the rash can be treated symptomatically with antihistamines and possibly topical steroids. Systemic steroids should not be used. If there is a severe reaction to EFV or NVP, then we do not recommend switching to RPV or ETR - rather use DTG or a PI.
Dosage and common ADRs of NNRTIs available in southern Africa are described in Table 5.
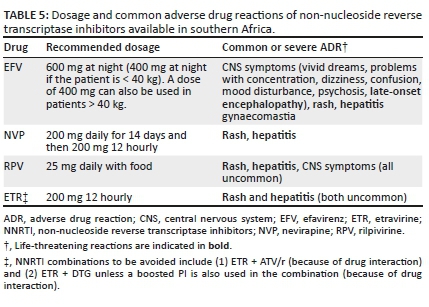
o Common pitfall: Immediately discontinuing NNRTIs in the case of a mild rash without systemic features. Such rashes often resolve if treatment is continued, although close monitoring is required.
5. Protease inhibitor class of antiretroviral drugs
Key points
➢ Three PI combinations are recommended in southern Africa: lopinavir (LPV), ATV or darunavir (DRV), each given with low-dose ritonavir (RTV, indicated as /r) for pharmacokinetic boosting.
➢ Ritonavir-boosted lopinavir is the only PI combination that can be used with RIF-based TB treatment, but the dose of LPV/r must be doubled.
➢ Atazanavir and DRV offer a better side effect profile than LPV.
➢ Darunavir has the highest barrier to resistance of any drug in this class.
Overview of protease inhibitors
Protease inhibitors are a class of agents that inhibit HIV's protease enzyme, which is required to cleave HIV's polyproteins into the final protein products that permit the production of infectious viral particles. Inhibition of this process results in immature, non-infectious virions.
Three PI combinations are recommended for use in southern Africa: LPV, ATV and DRV, each given with low-dose ritonavir.
Ritonavir is a PI in its own right, but is used principally as a pharmacokinetic 'booster'. As a potent inhibitor of CYP3A4, its use results in higher drug levels and prolonged half-lives of its companion PI. This allows for lower or less frequent PI dosing and decreases the chances of developing viral resistance. In rare situations, ATV is used without boosting in first-line therapy. However, this inhibition of CYP3A4, together with several other cytochrome P450 (CYP) enzymes and p-glycoprotein, results in numerous drug-drug interactions with other medications (see section 17).
o Common pitfall: Not using a drug interaction checker when prescribing PI-based ART with other medications. Clinically relevant drug-drug interactions are common with this class.
All PIs may be associated with cardiac conduction abnormalities (especially PR interval prolongation). This seldom results in clinically significant effects, but caution should be taken when co-prescribing other drugs that cause delayed cardiac conduction, such as macrolides or bedaquiline. All PIs are, to some extent, associated with metabolic side effects. Elevated triglycerides (TGs) and elevated low-density lipoprotein cholesterol (LDL-C) are class effects, although these side effects are more pronounced with LPV/r than with other PI combinations.31,32
Dosing and common ADRs of PIs are presented in Table 6.
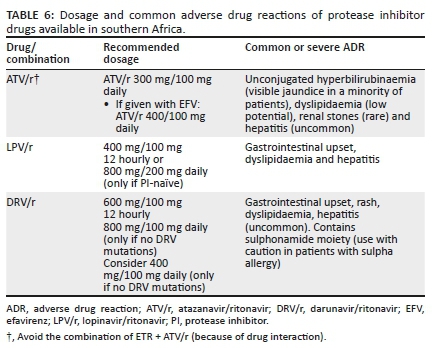
Individual protease inhibitors
Lopinavir
Lopinavir is co-formulated with ritonavir (e.g. Aluvia): In general, this twice-daily regimen has greater gastrointestinal (GI) side effects than other PI combinations, and is associated with a worse metabolic profile. Lopinavir is the only PI that can be used concurrently with RIF-based TB treatment; the LPV/r dose has to be doubled in this instance to 800 mg/200 mg twice daily until 2 weeks after RIF has been stopped (see section 18).
o Common pitfall: Forgetting to double the dose of LPV/r when starting RIF-based TB treatment.
Atazanavir
Atazanavir is generally better tolerated than LPV and can be taken once daily: It has important drug interactions with drugs that reduce stomach acidity, such as PPIs. Atazanavir may cause an unconjugated hyperbilirubinaemia as a result of inhibition of the hepatic enzyme Uridine 5›-diphospho-glucuronosyltransferase. Although the hyperbilirubinaemia is harmless and does not reflect a drug-induced liver injury (DILI), a minority of patients will become visibly jaundiced, and this may require changing ART regimens for cosmetic reasons.
oCommon pitfall: Mistaking the unconjugated hyperbilirubinaemia sometimes seen with ATV use with a DILI. Conversely, it is equally important to note that ARVs can also cause a true DILI, and therefore a complete liver function test (LFT) panel should be performed to distinguish between the two possibilities.
Darunavir
Darunavir has the highest barrier to resistance of any PI: Mutations selected by ATV or LPV can compromise DRV efficiency. For patients with mutations that confer any degree of resistance to DRV (e.g. I50V, L76V and I84V), the dose should be DRV/r 600 mg/100 mg twice daily. For patients without any DRV mutations, the drug can be taken at a dose of DRV/r 800 mg/100 mg once daily. There is evidence, however, that DRV/r 400 mg/100 mg once daily may be sufficient in this scenario, especially for patients with suppressed VLs at the time of the switch.33,34 Compared with a twice-daily dosing, a once-daily dosing offers the benefits of reduced pill burden and better side effect profile. As with ATV, DRV cannot be co-prescribed with RIF-based TB treatment.
o Common pitfall: Prescribing ATV or DRV in patients receiving RIF-based TB treatment. Lopinavir/ritonavir is the only PI combination that can be co-prescribed safely with RIF, but the dose of LPV/r must be adjusted as above.
6. Initiation and timing of antiretroviral therapy
Key points
➢ All individuals diagnosed with HIV should be initiated on ART.
➢ Delays to start ART should be minimised. Several studies have demonstrated that it is safe to initiate.
➢ ART on the same day as diagnosis or on receipt of CD4+ count result, with the main benefit being improved retention in care.
➢ Screening for TB, cryptococcal meningitis (CM) and other OIs prior to ART initiation is important, as these conditions may necessitate delaying ART initiation.
Overview
All patients who are diagnosed with HIV should be initiated on ART as soon as possible. Exceptions include patients presenting with CM or tuberculosis meningitis (TBM) - see below.
Benefits of antiretroviral therapy in reducing morbidity and mortality
With ART-induced viral suppression, the CD4+ lymphocyte count usually increases, which is accompanied by a restoration of pathogen-specific immune function. For most patients, this results in a dramatic reduction in the risk of HIV-associated morbidity and mortality. For patients who start ART with preserved CD4+ counts, ART is able to prevent the decline in CD4+ count observed in untreated patients and thereby prevent clinical complications of HIV infection. The benefits in morbidity and mortality extend to patients with relatively preserved CD4+ counts. The START and TEMPRANO ANRS 12136 trials showed significant individual clinical benefits when starting ART immediately in patients with CD4+ counts > 500 cells/µL rather than deferring until a certain lower CD4+ threshold or clinical indication was met.35,36
Benefits of antiretroviral therapy in reducing transmission
The HPTN 052 trial showed that treating the HIV-positive partner in a serodiscordant relationship with ART was associated with a 93% reduction in transmission risk to the uninfected partner, with the only linked transmissions occurring from partners without a suppressed VL.37 Further evidences in serodiscordant couples from the PARTNER, PARTNER2 and Opposites Attract trials have confirmed that HIV is essentially not transmittable when the VL is suppressed.38,39,40 Community-level evidence has also demonstrated a reduction in HIV incidence as ART rollout is scaled up. Therefore, early ART initiation has significant public health benefits.
Antiretroviral therapy in primary human immunodeficiency virus infection
In patients who are diagnosed with HIV during acute seroconversion, we advise counselling and initiating ART as soon as possible. Expedited ART initiation is preferable as there is evidence that this may limit the size of the HIV reservoir.41 Once the patient is established on ART, additional counselling may be required for patients who start ART in this acute stage because there is limited time for extensive pre-ART counselling, and there is often considerable psychological distress around this time.
Antiretroviral therapy initiation in 'elite controllers'
A minority of patients (< 1%) have very effective immune control of HIV infection and can control HIV viraemia at undetectable levels even in the absence of ART; these patients are termed 'elite controllers'.
Although definitive data are lacking for this patient subgroup, we advise initiating ART in elite controllers too, as indirect evidence suggests a potential benefit. Elite controllers still have evidence of chronic immune activation and inflammation that may drive non-infectious morbidities.42 Elite controllers have also been shown to have a higher rate of hospitalisation than patients who are virologically controlled by ART.43 Furthermore, a prospective study of HIV-positive 'controllers', who were able to control viral replication to < 500 copies/mL, showed that HIV therapy led to improvements in markers of immune activation and immune exhaustion, and a slightly improved self-reported quality of life.44 This trial included elite controllers.
One important consideration in such patients is that careful attention should be given to confirm the diagnosis of HIV before starting ART. These patients typically have a positive HIV enzyme-linked immunosorbent assay (ELISA) test, undetectable HIV VL, CD4+ count in the normal range and are clinically well. The possibility of a false-positive HIV ELISA test should be excluded either by qualitative HIV DNA PCR or Western Blot assay. If the patient previously had a detectable HIV VL, then this would also serve as confirmation. Such patients may need to be discussed with a laboratory virologist to assist with confirmation of HIV status.
o Common pitfall: Not confirming the HIV status of an 'elite controller'. If such patients have been diagnosed with HIV based on an HIV ELISA or rapid detection test, then confirmation of their HIV status should be sought by additional testing methods to exclude the possibility of a false-positive result.
Commencing antiretroviral therapy at the first clinic visit
Several studies have demonstrated that it is possible to initiate ART safely on the same day as HIV diagnosis or reporting of the CD4+ count result.45,46,47 These studies have demonstrated less overall loss to follow-up when ART is initiated immediately in selected patients. Now that treatment is recommended irrespective of CD4+ count, this same-day strategy should be considered as a means to improve retention in care.
When deciding to initiate ART on the same day as diagnosis, considerations should include the following:
•The patient should be motivated to start immediately.
•Same-day initiation is not an adherence support 'short cut'; ongoing support can occur in the days and weeks immediately after initiation.
•Patients starting TDF (who are the majority) should be contactable in the event of a CrCl < 50 mL/min and advised to return to the clinic immediately.
•A serum/plasma cryptococcal antigen (CrAg) test should be performed in patients with a CD4+ count < 200 cells/µL; again, the patient should be contactable in the event of a positive result and must be advised to return to the clinic immediately.
•Symptom screen for TB and CM before initiation of treatment remains important, and a positive screening requires further investigation prior to ART initiation.
Medical reasons to delay antiretroviral therapy initiation
Medical reasons to delay ART initiation are outlined in Table 7.
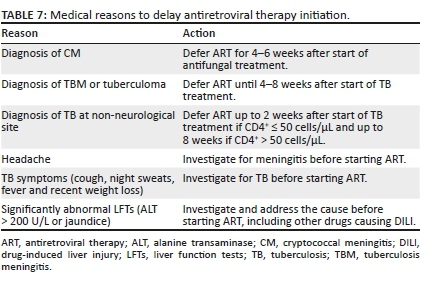
Tuberculosis: Decisions regarding the timing of ART in patients with TB should generally be based on the CD4+ count.
• CD4+count ≤ 50 cells/µL: Antiretroviral therapy should be regarded as urgent, with the aim to start therapy within 2 weeks following the commencement of TB treatment. A meta-analysis of RCTs has demonstrated that this approach reduces mortality.48 It is advised to commence ART after it is clear that the patient's TB symptoms are improving and that TB therapy is tolerated. The exception to this is the case of CM or TBM (see below).
• CD4+count > 50 cells/µL: Antiretroviral therapy can be delayed until 8 weeks after starting TB treatment, but no later. However, if the patient has other World Health Organization (WHO) stage 4 conditions, then ART should be initiated 2 weeks after TB treatment is started. The exception to this is CM or TBM. The longer delay before commencing ART in this group is anticipated to reduce the risk of IRIS (see section 26). The aforementioned meta-analysis of RCTs did not show a higher risk of acquired immune deficiency syndrome (AIDS) progression/mortality in this group when ART initiation was delayed until approximately 8 weeks after starting TB treatment, but a reduced risk of TB-IRIS.48
Tuberculosis meningitis: Patients with TBM are an exception to the above: starting ART immediately or at 2 months following the diagnosis was shown to have similar high mortality, with more complications in the immediate group.49 We recommend starting ART 4-8 weeks after TBM diagnosis.
There are important drug interactions and shared side effects when ART is co-administered with TB therapy (see section 18). When ART is commenced, patients should be warned that TB symptoms or signs may temporarily worsen and new features may occur in the first 3 months as a result of TB-IRIS (see section 26).
Cryptococcal disease: For patients with CM, the optimal time to start ART is 4-6 weeks from the time of starting CM treatment. The Cryptococcal Optimal ART Timing (COAT) trial demonstrated significantly higher mortality in patients who started ART in hospital 1-2 weeks after CM diagnosis than in those starting 5-6 weeks after diagnosis.50
For patients diagnosed with cryptococcal antigenaemia who have CM excluded by lumbar puncture (LP), ART can be commenced immediately.
Patients commenced on ART prior to a positive reflex CrAg result should be referred immediately for LP to exclude CM. In patients with a negative cerebrospinal fluid (CSF) CrAg result (i.e. CM is excluded), ART can be continued and fluconazole pre-emptive therapy should be initiated. It is unclear, however, whether to interrupt ART in patients with a positive CSF CrAg result.
For further details, refer to the 2019 Southern African HIV Clinicians Society guidelines for the prevention, diagnosis and management of cryptococcal disease amongst HIV-infected persons.
Starting antiretroviral therapy in patients with other opportunistic infections and acute illnesses: In the case of most OIs and acute illnesses (e.g. pneumocystis or bacterial pneumonia), the aim should be to initiate ART within 2 weeks of commencing treatment for that infection.51 In patients with severe Kaposi's sarcoma and lymphoma, ART counselling should be expedited and ART should be initiated as soon as possible.
In HIV-infected patients admitted to hospital and unable to take oral medications, for example, patients in intensive care unit (ICU):
•If the patient is receiving ART, then this should be continued - through nasogastric tube (NGT) if necessary - and only interrupted if the GI tract is not functional (e.g. ileus).
•If the patient is not yet received ART, then it should not be commenced if the reason for admission is an acute critical illness or injury. There are several potential problems associated with commencing ART in this setting: lack of adequate counselling, GI dysfunction, malabsorption and possible development of resistance.
•There are no intravenous options for ART. In patients admitted to the ICU for prolonged periods, ART initiation in the unit should be considered after multi-organ failure has resolved. Certain ART preparations should not be administered via NGT. In general, paediatric syrups can be administered via NGT. A pharmacist should always be consulted regarding which ART drugs can be administered via NGT and how to do this.
7. Baseline investigations
Confirming the diagnosis of human immunodeficiency virus
Prior to the initiation of lifelong ART, it is recommended that HIV infection is confirmed with two different testing methods, at least one of which should be a laboratory-based test. Acceptable combinations include the following:
•rapid detection test + ELISA
•rapid detection test + VL
•ELISA + VL.
Note that a VL may be undetectable in < 1% of patients not receiving ART, that is, 'elite controllers'.
Baseline investigations
Baseline investigations for ART are summarised in Table 8.
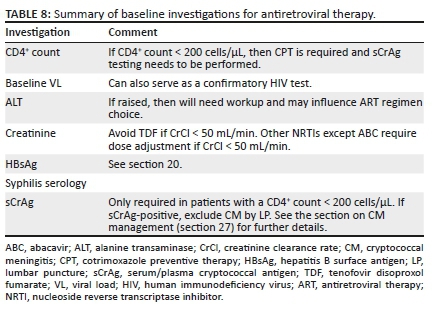
Symptom screen
We also advise a symptom screen for:
•Tuberculosis: patients should be asked about cough, weight loss, fever, night sweats and a possible TB contact. If any of these symptoms are present, then sputum should be sent for Xpert analysis, and if hospitalised or the CD4+ count is < 200 cells/µL, a urine lipoarabinomannan (LAM) assay should be performed.
•Cryptococcal meningitis: patients should be asked about new onset of headache; serum cryptococcal antigen (sCrAg) testing and possibly an LP should be performed if this symptom is present.
If the patient's symptom screen is positive, then ART should be deferred until the results of the Xpert, LAM, sCrAg test or LP (as indicated) are known. Delays in this process should, however, be kept to a minimum.
8. Viral load
Viral load monitoring is key to the success of ART. Decisions to change ART made on the basis of virological failure, rather than on clinical or immunological failure alone, have been shown to result in better patient outcomes.52 If the VL is undetectable, then the virus cannot mutate and develop resistance. A sustained VL < 50 copies/mL is associated with the most durable benefit. A suppressed VL also prevents the transmission of HIV to contacts.
Timing of viral load monitoring in the patient starting antiretroviral therapy (Figure 1)

We recommend a baseline VL for the following reasons:
1.The 3-month VL can then be compared with the baseline VL to detect > 2 log10 drop, and if this has not occurred, then it allows for early adherence intervention.
2.It may guide NNRTI selection (RPV should not be used if VL > 100 000 copies/mL).
3.It confirms the diagnosis of HIV (antibody tests may very rarely give a false-positive result).
A 3-month VL is desirable to detect adherence problems early before resistance develops. A subset of patients who start ART with a very high VL may not be fully suppressed at 3 months despite 100% adherence, but such patients would have had a > 2 log10 drop in VL from baseline if adherence is optimal and there is no resistance. Therefore, the 3-month result should be interpreted in relation to the baseline VL. All patients who have a detectable VL at 3 months should receive additional adherence interventions. In general, a patient's VL declines very fast on InSTI-based regimens.
If the 3-month VL is undetectable, then VL monitoring is recommended at 6 months and every 6 months thereafter. In patients who have an undetectable VL for more than 12 months, and who demonstrate reliable adherence and follow-up, it may be acceptable to reduce the frequency of VL monitoring to 12 monthly.
If the VL is > 50 copies/mL at any stage, then this should be an indication for urgent action: The patient should receive counselling and interventions should be implemented to improve adherence. A repeat measurement of VL should then be done in 2-3 months.
Interpreting viral load results
Virological criteria for treatment success
Treatment success is defined as a decline in VL to < 50 copies/mL within 6 months of commencing ART, and sustained thereafter.
Virological criteria for treatment failure
Treatment failure is defined as a confirmed VL > 50 copies/mL on two consecutive measurements taken 2-3 months apart: The decision to alter ART should therefore be based on the results of repeat testing after 2-3 months, following intensive adherence counselling. Although previous guidelines used a threshold of 1000 copies/mL to define virological failure, there is now good evidence that a VL > 50 copies/mL is robustly associated with subsequent virological failure, although this has not been established.53,54 Sustained viral replication, even at these low levels, can lead to the accumulation of resistance mutations (although this has not yet been definitively established in the case of DTG).
Viral blips
Isolated detectable HIV VLs < 1000 copies/mL, followed by an undetectable VL, are termed 'viral blips' and alone are not a reason to change the ART regimen.
•Viral blips can be caused by immune activation (such as from an acute infection), variability in the laboratory testing thresholds or intermittent poor adherence. Provided that they are infrequent, and the VL returns to being undetectable at the next measurement, they are not regarded as consequential.
Reasons for a high viral load
A high VL can be attributed to one or more of these three factors:
•inadequate patient adherence (most commonly)
•resistance to the prescribed ART - including both acquired and transmitted drug resistance
•inadequate ART drug levels as a result of altered pharmacokinetics, such as absorption difficulties, or drug-drug interactions.
These explanations are not mutually exclusive. For instance, inadequate patient adherence frequently leads to the development of resistance in patients on a non-DTG-containing regimen.
Transmitted drug resistance is currently increasing in the region.55 Such drug resistance is most frequently associated with the NNRTI class, as the signature K103N mutation has little effect on viral fitness and can therefore persist in the population even in the absence of drug pressure. Transmitted drug resistance to other drug classes is unusual; therefore, first-line therapy with a DTG-based regimen is unlikely to be affected by this phenomenon.
Interpreting a high viral load result of a patient receiving dolutegravir
Dolutegravir has been proved to be a remarkably robust drug in InSTI-naive patients when paired with at least one active NRTI. To date, less than five cases of DTG resistance have been described in this scenario. Thus, although a high VL has traditionally been a marker of possible resistance, this paradigm no longer applies for the most part in patients receiving a DTG-based regimen, provided that:
1.The patient has not had previous exposure to InSTIs as part of a failing regimen.
2.The patient is known to have at least one fully active NRTI as part of their regimen. (Note that patients who contract HIV whilst on pre-exposure prophylaxis [PrEP] are at risk of not having a fully active NRTI backbone).
3.The patient was not recently exposed to a scenario where a drug-drug interaction would have substantially decreased DTG concentrations (e.g. RIF-based TB therapy without increasing DTG dosing frequency to 12 hourly).
Provided that none of the above conditions are met, a detectable VL should not be assumed to reflect possible resistance. Rather, it can be assumed that the detectable VL, if not fulfilling criteria for a viral blip, merely represents poor adherence, and efforts to address this should be undertaken. We do not recommend performing resistance testing for patients on a DTG-based regimen within 2 years of commencing the drug, provided that the above conditions are met.
9. Cluster of differentiation 4 cell (CD4+) count
Key points
➢ All HIV-positive patients should be started on ART irrespective of their CD4+ counts.
➢ Cluster of differentiation 4 counts should be used only to establish whether CTX prophylaxis and sCrAg testing are required (CD4+ < 200 cells/µL).
➢ Monitoring ART efficacy is best established using VL, not CD4+ count.
➢ Most patients newly initiating ART with an abnormally low CD4 count will see a rapid initial CD4+ count increase (75 cells/µL - 100 cells/µL), followed by a more gradual rise thereafter (50 cells/µL - 100 cells/µL per year) until a normal CD4+ count > 500 cells/µL is achieved.
➢ If CD4+ count does not rise despite viral suppression, the ART regimen does not need to be altered. This phenomenon may reflect an 'immunological discordant response to ART'; however, if the patient is unwell, then other secondary causes should be sought.
Role of cluster of differentiation 4 count monitoring
A CD4+ count < 200 cells/mL indicates the need for CTX prophylaxis, principally to prevent Pneumocystis jirovecii pneumonia, although CTX is also active against other opportunistic pathogens, including Toxoplasma gondii, Cystoisospora belli and Nocardia spp. A baseline CD4+ count < 200 cells/mL is also an indication to reflexly perform sCrAg testing. If the CD4+ count is > 200 cells/µL at baseline or it increases above this threshold on ART, then CD4+ testing can be stopped, as therapeutic monitoring on ART is best accomplished with VL, not CD4+ count or clinical criteria. However, if virologic or clinical failure occurs, then the CD4+ count should be repeated, as CTX prophylaxis should be commenced if the count drops to < 200 cells/µL on ART.
oCommon pitfall: Routinely checking CD4+counts if the previous result was > 200 cells/µL. This is unnecessary unless virological or clinical failure subsequently occurs.
Timing of cluster of differentiation 4 count measurements
Cluster of differentiation 4 counts should be performed:
•at baseline (to guide decisions about CTX prophylaxis)
•every 6 months thereafter if the previous CD4+ count was < 200 cells/µL.
Cluster of differentiation 4 count response
In patients who start ART with an abnormally low CD4 count, the CD4+ count typically increases rapidly in the first month of ART, by ~75 cells/µL - 100 cells/µL, with a more gradual rise thereafter (50 cells/µL per year - 100 cells/µL per year).56 Most patients achieve a CD4+ count > 500 cells/µL after several years of ART, provided that the VL remains suppressed. However, CD4+ count responses are highly variable and may fail to increase despite virological suppression in about 10% - 20% of patients.57,58 Such patients have a delayed or absent CD4+ count response to ART despite viral suppression, which is termed an 'immunological discordant response to ART', previously 'immune non-responders'. Some studies have suggested that older patients are at a higher risk of this response. There is no evidence that such patients benefit from a change in ART regimen; therefore, the same regimen should be continued. Cotrimoxazole prophylaxis should be continued if the CD4+ count remains < 200 cells/µL. There is evidence that the prognosis of such patients is worse than in those who have a CD4+ response, but better than that of patients experiencing both virological and immunological failure.58 If patients with an immunological discordant response to ART are clinically unwell, then TB or lymphoma should be considered as the cause of persistent CD4+ lymphopenia. Cluster of differentiation 4 counts may remain stable in the presence of incomplete viral suppression in patients receiving ART until the VL is high (approximately ≥ 10 000 copies/mL).59
o Common pitfall: Confusing an 'immunological discordant response to ART' with treatment failure. There is no role for changing ART if the VL is suppressed.
Figure 2 shows the outline of the suggested approach to patients with low CD4+ counts despite a suppressed VL on ART.
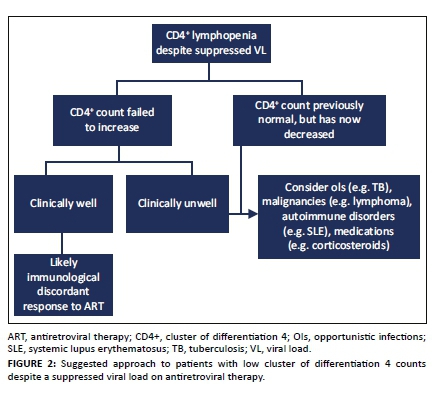
10. Resistance and genotyping
Key points
➢ Adherence is the key to prevent drug resistance.
➢ Resistance testing in patients failing a DTG-based therapy is unnecessary in the majority of cases and should only be undertaken if specific criteria are met.
➢ Resistance testing may not detect archived mutations to particular drugs if the patient is not receiving these drugs at the time of resistance testing.
Overview
As a result of transcription errors and recombination, HIV that is replicating can accumulate mutations, leading to drug resistance. Durable viral suppression by ART is required to limit the chances of developing drug resistance. Intermittent drug adherence, as opposed to a total lack of ART, provides a greater opportunity for resistance to develop, by exposing replicating virus to sub-therapeutic ART drug concentrations.
Antiretroviral drug resistance mutations are summarised in Table 9.
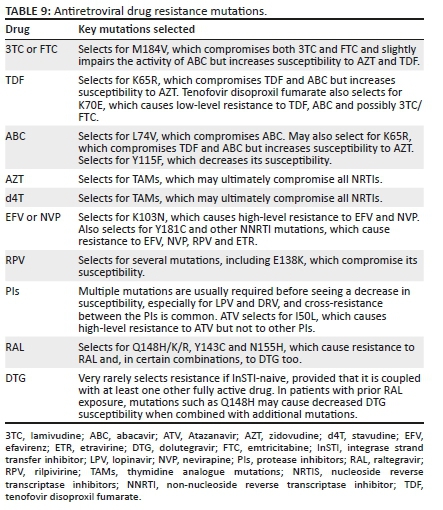
When to perform a resistance test
Baseline resistance test
A baseline resistance test is not generally indicated. We recommend a baseline resistance test to guide first-line regimen choice only in the following situations:
1.Pre-exposure prophylaxis received in the previous 6 months
2.History of sexual exposure to a person with known drug-resistant HIV or known to have failed an ART regimen.
Resistance testing at treatment failure
Resistance testing is generally only possible if the VL is > 500 copies/mL. Patients with two or more consecutive VL results of 50 copies/mL - 500 copies/mL are, however, still considered to have a virological failure (see section 8).
Recommendations for resistance testing are summarised in Table 10.
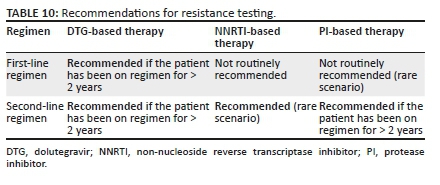
First-line therapy
Non-nucleoside reverse transcriptase inhibitor-based therapy: A resistance test at failure of first-line therapy is not routinely recommended. The EARNEST and SELECT trials showed that without the use of a resistance test to decide which NRTIs to use in second-line therapy, virological outcomes were good and equivalent to a boosted PI + RAL regimen.60,61 However, where funds permit, resistance testing will offer some advantages:
•A resistance test that shows no drug resistance may prevent having to switch unnecessarily to a second-line therapy.
•A resistance test may permit recycling of some first-line NRTIs (e.g. TDF), if they are shown to be susceptible. This is particularly useful if one wants to carry through TDF (or ABC) to a second-line DTG-based regimen.
•A resistance test will identify drug resistance that may be important to identify should the patient require a third-line ART in future.
Dolutegravir-based therapy: Because resistance to DTG in first-line therapy is extremely uncommon, we do not recommend resistance testing unless the patient has been on a first-line DTG-based regimen for more than 2 years, provided that they were not exposed to a scenario where a drug-drug interaction would have substantially decreased DTG concentrations (e.g. RIF-based TB therapy without increasing DTG dosing frequency to 12 hourly).62 (Other rare indications for performing a resistance test before 2 years include patients who were infected with HIV whilst receiving PrEP - see section 11). Because of the extreme rarity of first-line DTG-based resistance mutations, we suggest switching from a DTG-based first-line regimen to a second-line regimen only if a resistance testing shows DTG resistance.
Second-line therapy
Non-dolutegravir-containing regimens
•Resistance testing is recommended upon failure of a second-line therapy. This enables clinicians to individualise a treatment regimen for a third-line ART.
•For PI-based regimens, sufficient resistance mutations to cause virological failure typically take at least 2 years to develop; therefore, in most cases, we recommend only performing a resistance test after the patient has been on a PI-based regimen for at least this duration. Exceptions include exposure to sub-therapeutic PI drug levels as a result of drug-drug interactions (e.g. not doubling the dose of LPV/r when using RIF-based TB treatment). Patients on PI-based therapy with a VL of 50 copies/mL - 500 copies/mL pose a challenge as resistance testing is generally not possible. Such patients should remain on the same regimen with 2-3 monthly VL testing. If the VL rises to > 500 copies/mL, then resistance testing should be performed, whereas if the VL re-suppresses to < 50 copies/mL, then the patient may revert to 6-12 monthly VL testing.
Dolutegravir-based therapy: We do not recommend performing resistance testing for DTG-based second-line therapies within 2 years where at least one active NRTI is present. For instance, if the patient's first-line NRTIs were FTC and TDF, and the patient was changed to 3TC and AZT, then the strain of HIV can be assumed to be fully susceptible to AZT.
Scenarios in which to consider resistance testing when failing on a DTG-containing regimen include the following:
•The patient previously developed resistance to other InSTIs (e.g. RAL).
•The ART regimen may not contain any fully active NRTIs.
•Accidental exposure to sub-therapeutic levels of DTG (e.g. RIF-based therapy was commenced without the DTG being given twice daily).
Guide for interpreting a resistance test
Current commercial tests have been licensed for specimens with a VL of at least 1000 ribonucleic acid (RNA) copies/mL. Nevertheless, many in-house assays can detect VLs of 500 RNA copies/mL - 1000 RNA copies/mL. In general, most commercial HIV resistance tests detect mutations if they are present in > 10% - 20% of the HIV subpopulations in the sample.
oCommon pitfall: Performing a resistance test in patients with a low or undetectable VL. Commercial assays may not be successful in samples where the VL is < 500 copies/mL - 1000 copies/mL.
A key concept in interpreting resistance tests is archived resistance. After reverse transcription from its RNA template, HIV inserts a DNA copy of itself into the host genome. Some of the cells that HIV infects are extremely long-lived, and essentially provide an 'archive' of HIV variants over time. Thus, mutations that are known to have been present at one point in time can be assumed to be present for the lifetime of the patient, even if they are not visible on the patient's latest resistance test.
A second key concept is that of the wild-type virus, which is the naturally occurring HIV strain free of drug resistance mutations. In most cases, this form of the virus replicates more efficiently than viral strains that have acquired resistance. Therefore, when drug pressure is removed, the wild-type forms of the virus will predominate, even though the resistant strains have been archived and can become predominant again later if the drug pressure subsequently changes in ways favourable to these strains.
•A prominent exception to this is the signature mutation of EFV and NVP, namely K103N, which imposes no significant fitness cost on the virus. Even after these drugs are stopped, the K103N strains may persist at detectable levels for several years.
•Resistance testing should therefore only be performed when the patient is still taking his or her ART regimen, or up to a maximum of 4 weeks after discontinuation (see worked examples in Box 1).
•The absence of any identified resistance mutations implies that non-adherence is the cause of a raised VL.
•This does not exclude the possibility of archived resistance, however, which may only become detectable once the patient is back on ART that suppresses the wild-type strain.
•Any significant drug resistance mutations identified by resistance testing can be assumed to be present for the lifetime of the patient, even if subsequent resistance testings fail to show these mutations (as a result of worsened adherence or an ART switch, for instance).
•Conversely, it is only possible to identify mutations reliably for drugs that the patient was currently taking when the resistance testing was performed, and for drugs affected by cross-resistance. 'Susceptible' results to drugs for which there is no drug pressure may be unreliable because of archived resistance.
o Common pitfall: Performing a resistance test in the absence of drug pressure. If the patient has defaulted therapy for more than a few weeks, there is little purpose for a resistance test. In this scenario, it is highly likely that the replication of wild-type virus will overtake and obscure any resistant strain, rendering them undetectable by commercial resistance testing.
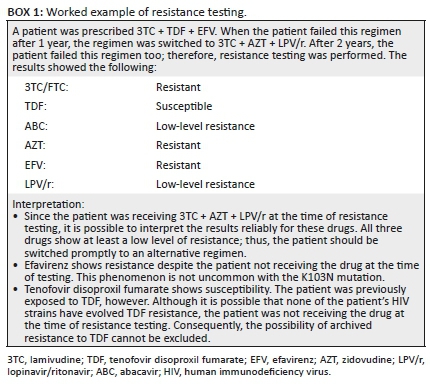
11. Initial antiretroviral therapy regimens for the previously untreated patient
Key points
➢ In ART-naive patients, the preferred initial regimen is TDF (300 mg) + 3TC (300 mg) (or FTC 200 mg) + DTG (50 mg) daily - available as a once-daily, one-tablet FDC.
➢ In patients receiving RIF, DTG dosing needs to be increased to 50 mg twice-daily until 2 weeks after stopping RIF.
➢ Dolutegravir has been associated with a small excess risk of NTDs in women taking the drug during conception - WOCP should be counselled accordingly.
Preferred initial antiretroviral therapy regimen
The preferred initial regimen for previously untreated patients is summarised in Table 11.
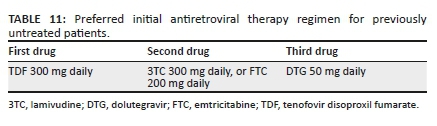
In patients receiving RIF, the DTG dose needs to be increased to 50 mg twice daily until 2 weeks after stopping RIF. Other drug-drug interactions with DTG are discussed in section 3 and section 17.
Reasons for this preferred regimen are:
•This combination is available as a once-daily, one-tablet FDC from several suppliers.
•Tenofovir disoproxil fumarate is preferred over ABC because of the risk of hypersensitivity reactions with ABC (HLA-B*5701 testing is not widely available in South Africa), certain studies showing lower VL suppression with ABC when baseline VL is > 100 000 copies/mL (although not confirmed in a meta-analysis)6 and cost.
•Lamivudine and FTC are regarded as interchangeable in terms of efficacy and safety.
•Dolutegravir is preferred over RAL and RPV because of its higher resistance barrier.63 Raltegravir also requires a twice-daily dosing and is not co-formulated in FDC. Dolutegravir is preferred over EFV and PIs because superior efficacy and tolerability were demonstrated in clinical trials.12,13
•There is an increasing prevalence of pre-treatment resistance to NNRTIs in South Africa (> 10% in some studies), which may compromise the efficacy of EFV-based regimens.64
Dolutegravir has been associated with a small but significant risk of NTDs in women taking the drug during conception (0.3% vs. 0.1% in women on EFV at conception in a large Botswana birth outcomes surveillance study).18 This risk is lower than what was originally reported (see section 19). Women of childbearing potential should be counselled about the risks and benefits of DTG and allowed to make an informed decision regarding the use of DTG and contraception, in line with WHO 2019 recommendations. Dolutegravir has also been associated with a greater weight gain than EFV.21 These issues have been discussed in section 3.
Alternative initial antiretroviral therapy regimens
Alternative regimens for previously untreated patients are summarised in Table 12.
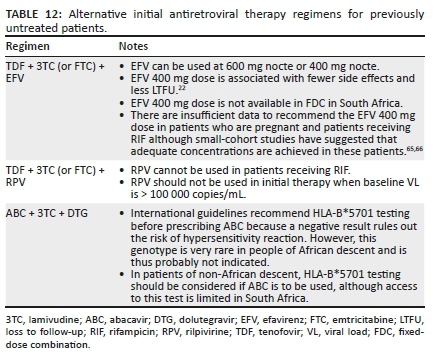
Alternative initial antiretroviral therapy regimens in specific clinical situations
There are specific clinical situations in which the preferred combination of TDF + 3TC (or FTC) + DTG cannot be used and the alternatives listed in Table 13 are advised instead.
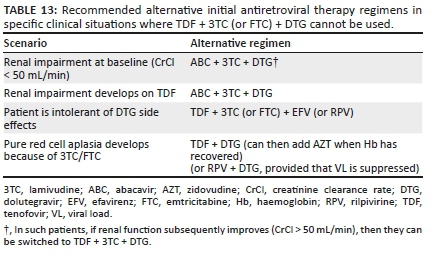
If both TDF and ABC are contraindicated and Hb is > 8 g/dL, then AZT can be considered as an alternative NRTI.
Considerations for two-drug first-line regimen of dolutegravir + lamivudine
This regimen was shown to have an efficacy not inferior to a three-drug regimen in RCTs.67 However, these trials did not include patients with a VL > 500 000 copies/mL, and there are no follow-up data beyond 3 years for this regimen. Furthermore, virological suppression was lower in patients with a CD4+ count ≤ 200 cells/µL. Therefore, we do not routinely recommend this regimen unless neither TDF nor ABC can be used. Importantly, hepatitis B must be excluded before considering this regimen as patients with hepatitis B must receive TDF + 3TC (or FTC) to prevent rapid emergence of 3TC resistance. The regimen should also not be used in patients receiving RIF.
12. Management of patients currently receiving first-line therapy
Key points
➢ Clinicians can consider switching patients who are virologically suppressed on NNRTI-based first-line therapy from an NNRTI to DTG, whilst maintaining the same two-drug NRTI backbone.
➢ In patients who are not virologically suppressed on an NNRTI-based regimen (VL > 50 copies/mL), we do not recommend an immediate switch to DTG, but rather an enhanced adherence counselling with repeat VL measurement in 2-3 months. If the VL remains > 50 copies/mL, then these patients should be switched to a second-line DTG regimen, which includes switching the NRTI back-bone.
➢ The typical criteria of two VL measurements greater than a certain threshold are not appropriate for DTG-based regimens, despite an adherence intervention to define virological failure. Rather, in patients started on a first-line DTG regimen, we recommend switching to a second-line therapy only if there is demonstrated InSTI resistance.
Patients currently on an efavirenz-, rilpivirine- or nevirapine-based first-line regimen
Patients on these treatment regimens should have VL measurements performed 6-12 monthly (see the sections 'Viral load' and 'Laboratory monitoring of the efficacy and safety of antiretroviral therapy'). Given that DTG is now readily available, clinicians can consider switching patients who are known to have virological suppression (VL < 50 copies/mL within last 6 months) from EFV (or NVP or RPV) to DTG whilst maintaining the same two-drug NRTI backbone. If the patient is tolerating the EFV (or RPV or NVP) regimen with no side effects, then such a switch is optional, as the patient may develop DTG-related side effects which he or she was not experiencing on the NNRTI (e.g. insomnia and weight gain). The benefit of such a switch is that a DTG regimen has a more robust resistance profile (DTG boasts a higher barrier to resistance than NNRTIs).12 An additional benefit of switching from NVP to DTG is that it is switching from a twice-daily to a once-daily regimen. In patients experiencing EFV-related side effects (even mild side effects), we encourage a change to DTG whilst maintaining the same NRTIs, provided that the VL is < 50 copies/mL within the last 6 months.
Another option in patients who are virologically suppressed (VL < 50 copies/mL) whilst receiving a regimen of NNRTI + two NRTIs, and who have never experienced virological failure, is a switch to the two-drug combination of DTG + RPV. Data from two clinical trials (SWORD I and II)68 showed that this regimen maintains virological suppression as a switch strategy in patients who have not previously experienced virological failure. This should not be done in patients who have chronic hepatitis B as TDF and 3TC (or FTC) should always form a part of their treatment.
The recommended protocol for switching from a first-line NNRTI-based regimen to a DTG-based regimen is shown in Figure 3.
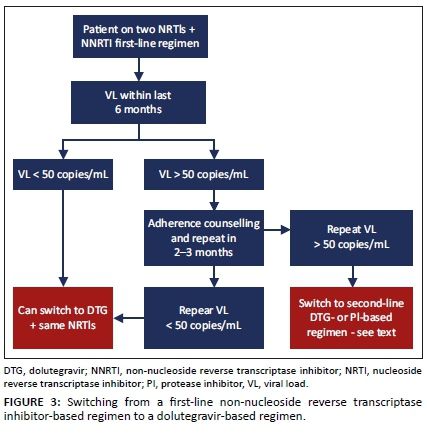
Antiretroviral therapy options in patients failing first-line non-nucleoside reverse transcriptase inhibitor-based therapy
In patients who are not virologically suppressed on an NNRTI-based regimen (VL > 50 copies/mL), we do not suggest an immediate switch to DTG with the same two NRTIs. The reason for this is that it is possible that they have developed resistance to the two NRTIs and may then be placed on DTG without a fully effective drug to accompany it. In this scenario, we recommend for enhanced adherence counselling and repeating the VL measurement in 2-3 months. If the VL is < 50 copies/mL, then the patient can be switched to DTG + the same two NRTIs. If the VL is > 50 copies/mL, then the patient should be switched to a second-line DTG regimen, which includes DTG + two NRTIs as follows:
•If the patient was receiving TDF (or ABC) + 3TC (or FTC) first-line therapy, then switch to AZT + 3TC in a second-line therapy with DTG.
•If the patient was receiving AZT (or D4T) + 3TC first-line therapy, then the decision regarding a second-line therapy should be based on a resistance test result. If the virus is susceptible to TDF on resistance testing, then the clinician can prescribe TDF + 3TC (or FTC) + DTG (provided that the patient has not potentially previously experienced virological failure on TDF and has not experienced TDF nephrotoxicity previously). If no fully active NRTI is available to accompany DTG, then it is best to switch to a PI-based second-line therapy with TDF + 3TC (or FTC). Advice is provided in section 13 regarding patients who cannot access a resistance test in this scenario.
Women of childbearing potential should be counselled about the potential risks and benefits of DTG and allowed to make an informed decision regarding the use of DTG and contraception (see section 3).
Patients started on a dolutegravir-based first-line regimen
These patients should have their VL measured 6-12 monthly (see the sections 'Viral load' and 'Laboratory monitoring of the efficacy and safety of antiretroviral therapy'). We have previously used the criteria of two VL measurements > 1000 copies/mL despite an adherence intervention to define virological failure and the need to switch from first- to second-line ART. This was appropriate for patients on NNRTI-based first-line regimens because of the low barrier to resistance of the NNRTI class. However, considerations are very different with DTG-based first-line regimens. In several clinical trials of DTG in first-line therapy, no DTG resistance has been described despite some patients having virological failure, and in clinical practice very few cases of DTG resistance (less than five cases worldwide at the time of writing this article) have been described when the drug has been used as part of a three-drug first-line regimen.63 Therefore, it would be inappropriate to use the same criteria for switching to second-line ART for DTG as it is likely that most patients with two unsuppressed VLs will not have resistance and rather require improved adherence on the same first-line regimen to achieve suppression. For that reason, we only recommend switching from a first-line DTG-based ART to a second-line regimen if resistance testing demonstrates InSTI resistance.
Until further data are available, in patients with an unsuppressed VL on DTG-based first-line ART, we recommend for enhanced adherence counselling. The tolerance of the regimen should also be addressed - the regimen may need to be switched because of side effects. Integrase strand transfer inhibitor resistance testing should be considered in these situations:
•Dolutegravir monotherapy for a period (DTG resistance has been more frequently described in this situation).63
•Co-administration of a drug that interacts with DTG without necessary dose adjustment (e.g. DTG given at 50 mg daily with RIF or given simultaneously with polyvalent cation-containing agents - see section 3).
•Viral load measurements > 50 copies/mL for > 2 years (despite adherence interventions, 100% pharmacy refills and self-reported adherence) and the current VL is > 500 copies/mL, thereby permitting a resistance test.
•The patient was infected whilst receiving PrEP (because of potential NRTI resistance at the time of infection).
•Sentinel surveillance projects or research studies, with the purpose of detecting emergence of DTG resistance.
If a resistance test is performed, then this should include sequencing of the integrase enzyme. The clinician should only switch from a DTG-based first-line regimen to a second-line therapy if resistance is detected, and the drugs used in the second-line regimen should depend on the resistance test result (see section 13).
These recommendations are based on accumulated information on the resistance barrier to DTG to date, suggesting that DTG resistance is extremely rare when the drug is used in a three-drug first-line regimen. These recommendations may be updated when more data become available regarding the incidence and risk factors of DTG resistance with more widespread use in routine clinical practice.
Figure 4 shows the outline of the virological monitoring of patients on DTG-based first-line ART and the recommended response to results.
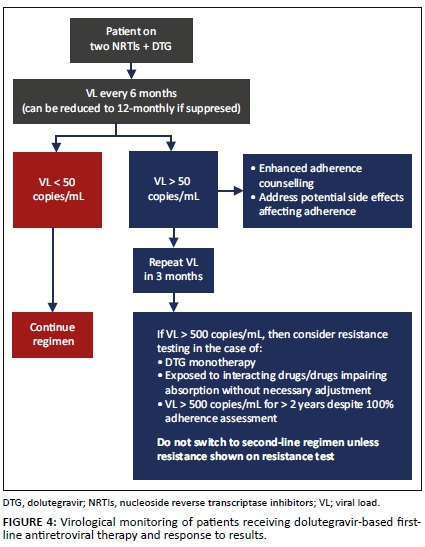
13. Management of patients starting or currently receiving second-line therapy
Key points
➢ When DTG is used in second-line therapy, there should be at least one fully active accompanying drug until further evidence is available.
➢ If the patient fails an NNRTI regimen with 3TC/FTC + either TDF or ABC, then AZT + 3TC + DTG is the recommended second-line regimen.
➢ If the patient fails other first-line regimens, then resistance testing is advised to decide on the choice of NRTIs in a DTG-based second-line regimen.
➢ Boosted PI + two NRTI second-line regimens are effective even if there is resistance to both NRTIs in the regimen.
➢ We advise DRV/r 800 mg/100 mg once daily as the first choice PI for use in second-line therapy.
Recommendations for patients failing a first-line regimen
Failed first-line regimen of two nucleoside reverse transcriptase inhibitors + non-nucleoside reverse transcriptase inhibitor
Table 14 shows the summary of the recommended second-line regimen to start in patients who have failed a first-line regimen consisting of two NRTIs + NNRTI.
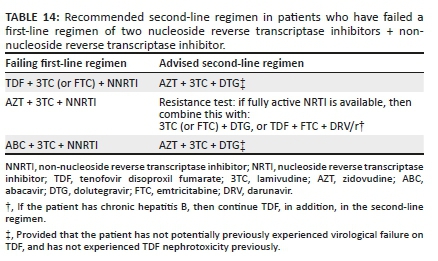
Based on the results of the DAWNING trial, it is preferable to use a DTG-based regimen rather than a PI/r regimen in second-line therapy.17 In this trial, a second-line regimen of DTG + two NRTIs was superior in terms of virological suppression and better tolerated than LPV/r + two NRTIs in patients who had failed a first-line regimen of NNRTI + two NRTIs. An important caveat is that all patients enrolled in this trial had a resistance test performed at entry and had to have at least one fully active NRTI to be eligible for inclusion. Thus, the current evidence supports a DTG-based regimen in second line only when used with at least one fully active NRTI. Whether a DTG-based second-line regimen would be equally effective with two NRTIs when there is resistance to both those NRTIs is currently a knowledge gap that is being addressed by several clinical trials - we do not advise such a strategy until the results of those trials are available.
In patients failing a first-line regimen in which the NRTIs are TDF + 3TC (or FTC), the mutations selected are typically M184V by 3TC (or FTC) and K65R (or K70E) by TDF. None of these mutations compromise AZT; in fact, they render the virus hyper-susceptible to it. Therefore, in this scenario, AZT remains fully active, and we can infer from the DAWNING trial results that a second-line regimen of AZT + 3TC + DTG will be optimally effective. The same applies for patients failing an ABC + 3TC + NNRTI regimen: AZT retains susceptibility and can be used with 3TC and DTG in a second-line regimen.
Where patients have failed an AZT (or d4T) + 3TC + NNRTI regimen, this could have resulted in the accumulation of thymidine analogue mutations (TAMs) and M184V. Certain TAMs compromise TDF, meaning that it is unpredictable whether there is a fully active NRTI if a regimen of TDF + 3TC (or FTC) + DTG is used in second-line therapy. We therefore advise resistance testing in such patients. If the resistance test demonstrates a fully active NRTI, then that NRTI can be used with 3TC (or FTC) + DTG in the second-line regimen. If there is no fully active NRTI or a resistance test is not possible in this situation (e.g. public sector), then we recommend a regimen of TDF + 3TC (or FTC) + DRV/r. The reason for this is that boosted PI + two compromised NRTIs retain activity as a second-line regimen (see below).
Women of childbearing potential should be counselled about the risks and benefits of DTG and allowed to make an informed decision regarding the use of DTG and contraception. If they choose not to use DTG in a second-line therapy, then DRV/r should be used in its place.
Failed first-line regimen of two nucleoside reverse transcriptase inhibitors + dolutegravir
The second-line regimen to commence in patients who have failed a first-line approach of two NRTIs + DTG is provided in Table 15.

If patients experience virological failure on a first-line DTG-based regimen, then we do not recommend switching to a second-line therapy unless a resistance test is performed that demonstrates DTG resistance. This is because DTG is a very robust drug and resistance is very rare when used in triple-drug combination first-line therapy. Therefore, it is far more likely that a VL > 50 copies/mL is attributed to adherence problems rather than resistance. If DTG resistance is demonstrated, then we advise a regimen of two NRTIs + DRV/r, with the two NRTIs selected based on the resistance test results.
Patients currently established on protease inhibitor-based second-line therapy
Viral load < 50 copies/mL
Clinicians can consider switching of patients currently on a second-line PI/r regimen to a DTG regimen, particularly if patients experience GI side effects of PIs. This switch may also simplify the regimen and reduce pill burden. However, before such a switch is made, we advise a careful review of the treatment and resistance (genotype test) history to ensure that there is at least one fully active NRTI to accompany DTG in the new regimen. If this cannot be assured, then we advise maintaining the current PI/r regimen - although a switch to an alternative PI/r can be considered to improve tolerance. If continuing a PI is not possible, then a switch to a DTG-based second-line regimen without an assured active NRTI could be considered with close VL monitoring. Refer to Table 16 for our advice when considering switching a patient on a PI/r-based regimen to DTG in a second-line therapy where the VL is < 50 copies/mL.
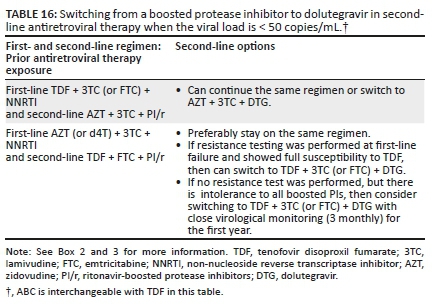
Viral load > 50 copies/mL
In patients on a second-line regimen containing a boosted PI, if the VL is not suppressed, then we do not advise switching to DTG. We advise for enhanced adherence counselling and switching to an alternative boosted PI if there is intolerance. If the VL subsequently re-suppresses to an undetectable VL < 50 copies/mL, then the advice in the section above should be followed. If the VL remains elevated, then the patient may be eligible for a resistance test, with consideration for a third-line therapy if they fulfil the criteria outlined in section 14.
14. Third-line antiretroviral therapy
Key points
➢ For patients with a detectable VL on second-line therapy for < 2 years, intensified adherence counselling and support are required rather than a switch to a third-line therapy.
➢ For a patient on a second-line regimen for > 2 years with two or three VL measurements > 50 copies/mL within a 6-month period despite adherence interventions that have been assessed to be satisfactory (see text), a resistance test should be performed. (N.B.A resistance test can only be performed if VL > 500 copies/mL.).
➢ The choice of the third-line regimen should be made in consultation with an HIV expert.
Management of patients with a detectable viral load on second-line therapy and initiation of third-line therapy
As outlined in the previous section, there should be documented PI or DTG resistance before switching to a third-line regimen. Resistance tests should be interpreted by an expert in conjunction with a full ART history. In many patients failing second-line regimens, there are no PI (or DTG) mutations. In these patients, improved adherence is required rather than switching to a third-line regimen. If side effects interfere with adherence, then consideration should be given to switching to a more tolerable regimen, provided that this regimen is predicted to be effective based on the treatment history (see section 13).
Figure 5 shows the outline of indications for performing resistance testing in second-line ART regimens.
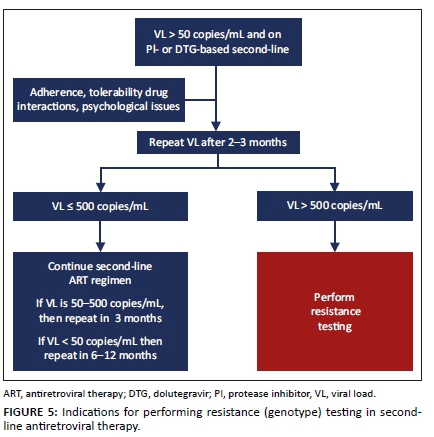
In a patient who has been on a second-line regimen for > 2 years, if there are two or three VL measurements > 50 copies/mL in a 6-month period, despite adherence interventions, and adherence is assessed to be satisfactory (e.g. 100% pharmacy claims over 6 months), then a resistance test should be performed (resistance test can only be performed if VL > 500 copies/mL). If a patient who has been on second-line therapy for < 2 years is found to have a detectable VL, then a resistance test should not be performed; rather, the same regimen should be continued and adherence counselling and support should be intensified. The regimen may be switched if there are significant side effects. It is unlikely that significant resistance to the PI or DTG will have developed within 2 years. The exceptions include a patient who in error was not prescribed LPV/r double dosing with concurrent RIF use, and subsequently demonstrates a detectable VL, and a patient who has been taking an incorrectly low dose of medication. Such patients should be eligible for resistance testing even if they have been on second-line therapy for < 2 years.
Adherence counselling before third-line therapy
Specific adherence counselling should be provided for patients preparing to start third-line ART, with a clear discussion that this regimen is likely to be their last option for the foreseeable future.
Third-line regimen choice after failing a protease inhibitor-based second-line regimen
A third-line regimen including a combination of DRV/r and other drugs decided on the basis of resistance testing results in virological suppression in the majority of patients, provided that adherence is optimal.74,75,76,77
For most patients who require third-line therapy (i.e. patients who experience virological failure on a second-line LPV/r or ATV/r regimen with a low-, intermediate- or high-level resistance to the PI, i.e. Stanford Score > 14), we recommend the third-line regimen outlined in Table 17. However, the final decision will be based on treatment history and resistance test results for the individual patient.
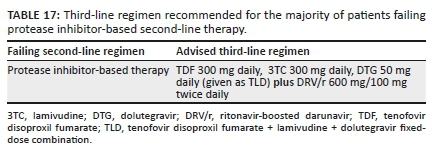
Exceptions
There are exceptions:
•In patients with renal impairment, replace TDF + FTC with ABC + 3TC, or AZT + 3TC. The choice between ABC and AZT will depend on Hb (anaemia: do not use AZT if Hb < 8 g/dL) and resistance testing.
•If the AZT Stanford score is lower than the TDF score, then use AZT + 3TC rather than TDF + FTC.
•Patients with a DRV score of 0 on Stanford score (and no DRV mutations, see section 5) can take DRV/r 800 mg/100 mg once daily.
•Patients with prior virological failure on RAL and/or with a DTG score > 0 on integrase resistance testing should receive DTG 50 mg twice daily.
•In patients with extensive resistance (e.g. DRV score > 29 and NRTI score > 29), consider adding RPV or ETR (provided that the score for these drugs is < 30) or maraviroc (MVC) (provided that the virus is CCR5-tropic on the tropism test).
Additional points and explanatory notes
•We generally advise the continuation of NRTIs in the third-line regimen, even if there is documented NRTI resistance.
•Lamivudine (or FTC) resistance with the M184V mutation impairs viral replication. Another NRTI (generally TDF, but based on resistance testing) should be added. This is not essential if there are more than two other active drugs in the regimen.78
•Because most patients are not receiving an NNRTI at the time of failing second-line therapy when a genotype resistance test is typically performed, prior NNRTI mutations related to first-line NNRTI failure may be archived at this time. Therefore, it is difficult to be certain from a (this) genotype performed at second-line ART failure whether ETR/RPV is still active; however, data from South Africa suggest that the majority of patients who have failed NVP or EFV are still susceptible to ETR/RPV.79
•In the SAILING trial, in treatment-experienced patients, a DTG regimen proved superior to RAL and fewer patients in the DTG arm developed treatment-emergent InSTI resistance. Consequently, we no longer recommend the use of RAL in third-line therapy unless DTG is not tolerated or otherwise contraindicated or unavailable. We also recommend switching patients currently using RAL in third-line therapy to DTG because of its higher barrier to resistance.14 If such patients have a suppressed VL, then they can be switched to standard dose DTG (50 mg daily); however, if they are not virologically suppressed, then we suggest a resistance test with a request for integrase sequencing before switching. If there are InSTI mutations present that are associated with reduced susceptibility to DTG, then the DTG dose should be 50 mg twice daily.
•Women of childbearing potential should be counselled about the risk and benefits of DTG and allowed to make an informed decision regarding the use of DTG and contraception.
•Maraviroc (a CCR5 blocker) is a consideration in third-line therapy; however, it is currently extremely costly and can only be used after a tropism test demonstrates that the patient's circulating virus has sole tropism for the CCR5 co-receptor. We advise only considering this when there is intermediate- or high-level resistance to all PIs, all NNRTIs and all NRTIs, and DTG is not fully susceptible.
•If viral suppression is not achieved on third-line therapy, then there is still benefit in continuing failing ART because of the residual partial activity and 'crippling' effect of such ART. 'Crippling' describes the fact that mutant viruses often have less replicative capacity. Provided that the VL can be maintained at < 10 000 copies/mL, the CD4+ count will usually be maintained or even increase.59
Third-line regimen choice after failing a dolutegravir-based second-line regimen
The choice of a regimen will be guided by resistance test results and should include DRV/r with two NRTIs (generally 3TC or FTC with the NRTI with the lowest Stanford score). In patients failing a second-line DTG regimen who have not failed a PI/r previously, it can be assumed that DRV/r is fully active and can be used at 800 mg/100 mg daily. Based on the results of the EARNEST, SELECT and SECOND LINE trials, it can be concluded that a DRV/r regimen with two NRTIs will be an active regimen even if there is documented resistance to the two NRTIs.60,61,72 However, decisions regarding the third-line therapy need to be individualised in consultation with an expert, taking into account the treatment history (which drugs and classes the patient previously failed) and previous and current resistance test results.
Raltegravir may still be active in patients with DTG resistance - it depends on the specific resistance mutations in the integrase gene - but it is usually not necessary to include it in the regimen. Etravirine and RPV are other drugs that can be considered depending on resistance test results and treatment history.
15. Laboratory monitoring of the efficacy and safety of antiretroviral therapy
Key points
➢ Antiretroviral therapy efficacy is monitored with VL and CD4+ count - discussed in the sections 'Viral load' and 'CD4+ cell count'.
➢ The key efficacy endpoint in ART is sustained virological suppression with a VL < 50 copies/mL.
➢ CD4+ count monitoring can be stopped when the CD4+ count is > 200 cells/µL and the VL is suppressed.
➢ Creatinine monitoring is advised in patients on TDF, and an FBC is advised in patients on AZT.
➢ In most patients taking PIs, only one lipid measurement is advised (at 3 months).
➢ In patients on TDF who are admitted to hospital, it is important to check creatinine even if it does not fall within these monitoring guidelines. This is because intercurrent illnesses with dehydration or sepsis may be associated with a deterioration in renal function, in which TDF may act as a co-factor.
Table 18 shows the list of the laboratory investigations and their frequency advised for monitoring of ART safety.

• Common pitfall: Monitoring of VL is not done at least annually. This results in a delayed detection of ART failure and intervention, with resultant clinical deterioration and increased risk of transmission.
16. Patients who return after stopping antiretroviral therapy
Key points
➢ Many patients return to care after treatment interruption when they experience clinical deterioration - screening for OIs should be performed.
➢ Viral load measurement should be performed before re-initiation and repeated 3-6 monthly.
➢ Choosing the appropriate regimen in patients who return to therapy depends on their previous regimen, their level of treatment adherence prior to disengaging with care, and their current CD4 and hospitalisation status.
It is common for patients receiving ART to interrupt their treatment for a number of reasons (e.g. treatment fatigue, denial, life event, depression, new job, relocation, etc.). Many patients return to care after an interruption, often precipitated by clinical deterioration. Patients who have clinical symptoms of an OI when returning to care should be investigated and, if appropriate, should be started on treatment for the infection before restarting ART. In particular, patients should be screened for headache and for TB symptoms when returning to care. Reasons for delaying ART re-initiation are the same as for delaying initiation in ART-naive patients (see section 6). Patients who are asymptomatic when returning to care could be re-initiated on ART the same day with appropriate counselling. A counselling plan should be implemented to ensure retention in care going forward and address reasons for disengagement.
We recommend performing a VL measurement before re-initiating ART, then 3-6 monthly thereafter. The choice of ART regimen to restart will depend on prior treatment history.
Return after stopping a TDF + FTC (or 3TC) + NNRTI regimen
In patients returning to treatment after disengaging from a TDF + FTC (or 3TC) + NNRTI regimen:
•If the patient had adhered to treatment prior to disengaging, with a suppressed VL, and has only disengaged once or twice, then he or she could either be restarted on the same regimen or restarted on TDF + 3TC (or FTC) + DTG. If a patient restarts an NNRTI-based regimen, then switching to a second-line regimen should be considered if the VL is not < 1000 copies/mL at 3 months after restarting.
•If the patient has a history of poor adherence with multiple episodes of disengaging, then we suggest re-initiating therapy with a second-line regimen of AZT + 3TC + DTG, AZT + 3TC + PI/r or TDF + FTC + PI/r. We do not recommend TDF + 3TC (or FTC) + DTG in this scenario, as there may be resistance to TDF and 3TC - multiple episodes of treatment interruption, particularly beyond the first year of ART, and poor adherence can result in resistance to all drugs in the first-line regimen.
•Hospitalisation with an AIDS-defining condition and a CD4+count < 50 cells/µL represents another scenario in which a patient may be restarted immediately on second-line ART when returning to care after disengaging. Such patients are considered to be at high risk of mortality if restarted on first-line therapy to which their virus may be resistant, and they require an ART regimen that is guaranteed to be effective immediately. This decision should typically be taken by the hospital-level clinician.
Return after stopping a dolutegravir-based regimen
In patients returning to treatment after disengaging from a DTG-based regimen:
•Restart the same regimen. Assess the VL at 6 months and follow standard guidance in response to the result.
Return after stopping a TDF + FTC (or 3TC) + PI/r regimen
In patients returning to treatment after disengaging from a second-line PI-based regimen:
•Either restart the patient on the same regimen or switch to AZT + 3TC + DTG (if the patient had failed a first-line TDF + 3TC (or FTC) + EFV regimen). Assess the VL at 6 months and follow standard guidance in response to the result.
Return after stopping a third-line regimen
In patients returning to treatment after disengaging from a third-line regimen:
•Restart the same regimen and assess the VL at 6 months. Follow standard guidance in response to the result.
oCommon pitfall: Performing a resistance test after an ART treatment interruption of > 4 weeks. Such a testing is of limited value. Many resistance mutations are overtaken by the wild-type virus when ART is stopped and thus the resistance test may not accurately reflect the true resistance pattern.
17. Drug-drug interactions
Key points
➢ Whenever patients start or switch antiretroviral drugs or start new concomitant medications, it is important to evaluate potential drug interactions.
➢ Many drugs and drug classes have clinically significant drug-drug interactions with ARVs.
➢ There are also important drug interactions between several ARVs.
➢ It is important to consult a regularly updated database to assess whether drugs can be co-administered and whether dose adjustment is required.
➢ Herbal medications may also have interactions with ART drugs (e.g. St John's wort and garlic), but data on herb-drug interactions are very limited.
Mechanisms of drug interactions
There are two main mechanisms of drug-drug interactions:
•Pharmacodynamic interactions: these interactions occur when one drug influences the action of another drug without altering its concentrations. Such interactions may be either beneficial, if drug effects are additive or synergistic; or harmful, if drug effects are antagonistic. Additive toxicity is also a pharmacodynamic interaction (e.g. AZT and linezolid both cause myelosuppression and should not be co-administered).
•Pharmacokinetic interactions: these interactions occur when a perpetrator drug alters the concentrations of a victim drug by affecting its absorption, distribution, metabolism or excretion. Inhibition is a direct chemical effect when a drug binds to the active site of drug-metabolising enzyme or drug transporter - typically only one or a few enzymes or transporters are inhibited. Inhibition is maximal when the inhibiting drug reaches steady state and wanes rapidly when the inhibiting drug is stopped. Strong inhibitors (e.g. ritonavir, clarithromycin and itraconazole) can cause significant increases in the concentrations of victim drugs, resulting in toxicity. Induction results in transcriptional activation of many genes involved in drug metabolism and transport, which takes about 2 weeks to be maximal and wanes in a similar time. Strong inducers (e.g. RIF, carbamazepine and phenytoin) can cause significant decreases in concentrations of victim drugs, resulting in reduced efficacy. Pharmacokinetic interactions are occasionally beneficial (e.g. RTV markedly increases the concentrations of other PIs). Data on herb-drug interactions are very limited - both St John's wort and garlic are known inducers. Clinically significant pharmacokinetic interactions require dose adjustment of the victim drug or, if the interaction is severe, avoiding co-administration with the perpetrator drug.
Overview of drug-drug interactions by antiretroviral class
•NRTIs. these are generally neither victims nor perpetrators of clinically significant pharmacokinetic interactions.
•PIs. ritonavir is a potent inhibitor of the key CYP enzyme 3A4 and the drug efflux transporter P-glycoprotein; it also induces several other drug-metabolising enzymes and drug transporters. Therefore, RTV-boosted PIs are frequent perpetrators of pharmacokinetic interactions, but can also be victims of such interactions when co-administered with strong inducers - co-administration with strong inhibitors does not add significantly to the inhibition by RTV. Atazanavir/ritonavir requires an acid pH in the stomach for absorption - it should be taken 2 h before or 1 h after antacids, and administration with PPIs is not advised.
•NNRTIs. these differ by individual drugs. Efavirenz is a moderate inducer. Rilpivirine can be the victim when co-administered with strong inducers. Although inhibitors increase the exposure to RPV, it is seldom necessary to adjust the dose. Etravirine induces CYP3A4 and also inhibits two CYP enzymes; it can also be the victim when co-administered with strong inducers.
•InSTIs. Polyvalent cations (calcium, magnesium, iron and aluminium) bind to InSTIs, reducing their absorption. Integrase strand transfer inhibitors can be taken 2 h before or 6 h after polyvalent cations. However, calcium and iron can be co-administered with InSTIs if taken with a meal, but not in the fasted state. Integrase strand transfer inhibitors are victim drugs when co-administered with strong inducers. InSTIs are not perpetrator drugs, except DTG that inhibits an efflux transporter important in the elimination of metformin (metformin dose should not exceed 500 mg 12 hourly).
There are many important pharmacokinetic drug interactions between ARVs and other drugs, as well as between different ARVs. Some of these drug-drug interactions are discussed in other sections of this article (e.g. interactions with RIF in section 18). The full list of all potential drug interactions is very long and beyond the scope of this article.
Knowledge of drug interactions is constantly evolving. Clinicians are advised to seek reliable information on drug-drug interactions when using non-standard ART regimens and when drugs are co-administered, using one or more of the resources listed in Box 4.
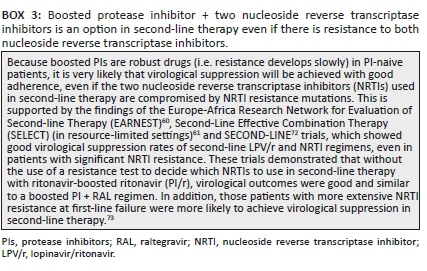

oCommon pitfalls:
oNot checking for interactions between concomitant drugs and current or newly initiated ARVs. Concomitant drugs may need dose adjustment or discontinuation when ART is switched, for example, switching from a moderate inducer (EFV) to a strong inhibitor (PI/r), or from either of these to an InSTI.
oNot considering marked increases in statin considerations when used concomitantly with PIs. There are major interactions between PIs and many statins, which result in marked increases in statin concentrations. Low-dose atorvastatin (not exceeding 10 mg, which will give an equivalent exposure to about 60 mg) can be used with PIs, but simvastatin cannot be used.
18. Tuberculosis
Key points
➢ Rifampicin is a potent inducer of certain drug-metabolising enzymes and drug transporters and reduces exposure to drugs in the InSTI, NNRTI and PI classes, necessitating dose adjustments of some of these drugs.
➢ LPV/r is the only PI that can be used with RIF, but the LPV/r dose needs to be doubled.
➢ Rifabutin (RFB) can be used with all PIs, but an RFB dose adjustment is required.
➢ Several side effects are shared between ARVs and TB drugs, including GI intolerance, hepatotoxicity, drug rashes, myelosuppression and neuropsychiatric side effects.
Considerations for antiretroviral therapy in the context of tuberculosis
Tuberculosis is the most frequent co-infection affecting HIV-positive people in southern Africa. Patients may be diagnosed with TB at entry or re-entry into HIV care, or diagnosed with active TB whilst on ART. Studies in South Africa have suggested that TB incidence remains higher in patients who are virally suppressed on long-term ART compared with HIV-negative people living in the same community, possibly because of persisting defects in anti-mycobacterial immunity. The co-treatment of HIV and TB is complex because of (1) drug-drug interactions (discussed below), (2) TB-IRIS (section 26) and (3) shared side effects (discussed below). These issues, which have recently been reviewed,80 affect decisions regarding the timing of ART in ART-naive patients with TB (section 6).
Certain ART regimens need to be modified for compatibility with RIF. Rifampicin is a critical component of the drug-sensitive TB regimen that substantially reduces the risk of relapse after completing TB treatment.
There are no significant interactions between NRTIs and RIF; however, InSTIs, NNRTIs, PIs and MVC all exhibit drug interactions with RIF. Dolutegravir can be used in patients receiving RIF, but a dose adjustment is required (Table 19).81 Efavirenz is the preferred NNRTI for use with RIF. Nevirapine was previously recommended as an alternative in patients with contraindications to EFV (e.g. psychosis), but it carries a higher risk of virological failure when used with RIF, and given the availability of the InSTI class, NVP is no longer recommended. Rilpivirine and ETR cannot be used with RIF. The plasma concentrations of all PI/r are reduced to subtherapeutic ranges with RIF. Dose adjustment of LPV/r can overcome this induction (Table 19), but there is a risk of hepatotoxicity; patients require counselling and ALT should be monitored frequently.82,83
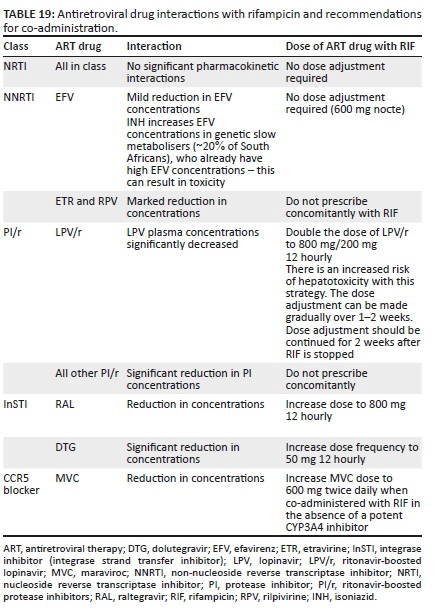
An alternative approach is to replace RIF with RFB in patients taking a PI/r. However, RFB is not co-formulated with other TB drugs, and the evidence base for RFB in the treatment of TB is much less substantial than that for RIF.84 There is also uncertainty regarding the optimal dose of RFB with PI/r; these guidelines recommend 150 mg daily (Table 20) for efficacy reasons, but careful monitoring for toxicity is required (ALT, neutrophil count and visual symptoms at least monthly).85 RFB may be considered in patients who are not able to tolerate co-treatment with double-dose LPV/r and RIF-based TB treatment (i.e. patients unable to tolerate the increased LPV/r dose because of hepatotoxicity or GI side effects) or in ART-experienced patients on an ART regimen that is not compatible with RIF (e.g. third-line ART with DRV/r). If RFB is unavailable and adjusted doses of LPV/r are poorly tolerated in patients receiving second-line ART, then DTG (50 mg 12 hourly) may be substituted for the PI. However, it should be noted that good evidence is lacking regarding the robustness of DTG in second-line therapy with both NRTIs compromised, as exists for PI/r (section 13). Nevertheless, the short-term use of DTG with two compromised NRTIs over 6 months is preferable to treating TB without RIF, which has a high risk of failure or relapse.
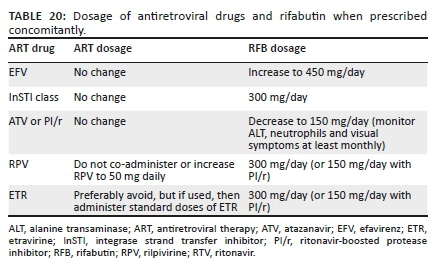
Antiretroviral therapy and TB medication share many side effects (Table 21).
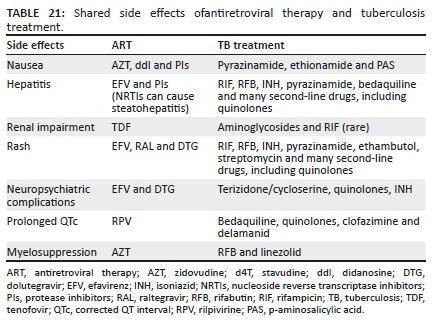
oCommon pitfalls:
o Rifampicin is co-administered with LPV/r, but the dose of LPV/r is not adjusted. This results in sub-therapeutic LPV concentrations and development of PI resistance. Rifampicin should not be co-administered with ATV/r or DRV/r at all.
o Combining linezolid and AZT. These drugs should not be combined because both can cause bone marrow suppression (especially anaemia and neutropenia).
19. Pregnancy and breastfeeding
Note: It is beyond the scope of these guidelines to provide comprehensive guidance for the management of pregnant women. Key recommendations relating to the mother are included, but providers are encouraged to refer to national guidelines. All women should be linked to routine antenatal care when pregnancy is confirmed.
•The prevention of mother-to-child transmission of HIV (PMTCT) programme includes periconception, pregnancy, delivery and breastfeeding and encompasses the prevention of unplanned pregnancies.
•In low-resource settings, breastfeeding commonly continues for up to 24 months. Breastfeeding transmission is now the most common mode of mother-to-child transmission of HIV in many parts of sub-Saharan Africa, rendering post-natal retention-in-care vital to successful PMTCT intervention.
•Virological suppression on ART is essential for maternal health, and to prevent HIV transmission to the infant. An elevated VL > 50 copies/mL in a pregnant or breastfeeding woman requires urgent action.
•In well-functioning PMTCT programmes, a significant proportion of infections in infants result from undetected seroconversion during pregnancy and breastfeeding. Repeated HIV testing throughout these periods is essential for women initially testing HIV-negative.
•Interventions that support HIV risk reduction in women include male partner HIV testing and linkage to ART for 'treatment as prevention', encouraging consistent condom use throughout pregnancy and breastfeeding, and providing PrEP to women who are at substantial risk of HIV infection.
•Maternal health is central to healthy infants, and is an essential focus of PMTCT services: advanced HIV results in life-threatening OIs, leading to miscarriage, stillbirth, premature delivery and maternal death.
Mother-to-child transmission of human immunodeficiency virus
Overall, the risk of mother-to-child transmission of HIV is ~ 40% in the absence of any intervention (see Box 5 for more information). Timing of such transmission is as follows: in utero: 5%; during delivery: 15% - 20%; up to 24 months of breastfeeding: 20%.

The time of the highest risk coincides with delivery, which spans a matter of hours; the risk during 24 months of breastfeeding is slightly higher, but over a significantly greater timespan. Breastfeeding should not be stopped because of a new diagnosis of HIV, or an elevated VL in women already on ART. Instead, initiation of ART and management of raised VL (together with infant prophylaxis) are interventions to 'make breastfeeding safer'.
Antiretroviral therapy for women of childbearing potential and during pregnancy and breastfeeding
All HIV-positive pregnant and breastfeeding women should be initiated on lifelong ART, ideally the same day that pregnancy is confirmed. Standard first-, second- and third-line regimens should be used in pregnancy (see the sections 'Initial antiretroviral therapy regimens for the previously untreated patient', 'Management of patients currently receiving first-line therapy', 'Management of patients starting or currently receiving second-line therapy' and 'Third-line antiretroviral therapy'). Regarding DTG use in pregnancy, it is important to note that the absolute risk of NTD is low (< 0.5%), and this risk may be outweighed by the additional benefits of DTG over alternative therapies. We currently recommend that WOCP who wish to become pregnant or who have no reliable access to effective contraception should be counselled adequately about the potential risks and benefits of DTG- versus EFV-based ART, and should be offered the choice of first-line regimens.
Other points regarding ART in pregnancy include the following:
•Efavirenz 600 mg is a safe and effective regimen for use by WOCP including during the time from conception to the end of the first trimester. There are insufficient data to recommend routine use of EFV 400 mg in pregnant women.
•The current guidelines no longer recommend initiating NVP in any patients. Maternal deaths in pregnant women have been associated with NVP because of liver and skin hypersensitivity reactions.
•NRTIs: Note that commonly used CrCl calculations are not validated for pregnant women; therefore, avoid TDF if serum Cr ≥ 85 µmol/L.
•Dose adjustment of ART during pregnancy is only indicated for women taking both TDF and ATV/r during the second/third trimester; the dose should be increased from ATV/r 300 mg/100 mg to 400 mg/100 mg.
•Women taking LPV/r 800 mg/200 mg daily should be advised to adjust this to 400 mg/100 mg 12 hourly (twice daily) during pregnancy because of altered pharmacokinetics. These women should also be informed about the association between LPV/r and premature labour and delivery.
•Particular importance should be placed on drug-drug interactions between DTG and divalent cation-containing medication in pregnancy, as pregnant women frequently receive iron supplements and/or magnesium-/aluminium-containing antacids.
Patients returning to care in pregnancy after defaulting a first-line regimen or those exposed to previous PMTCT regimens should generally be put directly on a DTG-based regimen, rather than retrying an NNRTI-regimen (section 16). As per current PMTCT guidelines, women not already on ART at the time of labour or delivery should commence TLD immediately and also receive an additional stat dose of NVP 200 mg. Women who are newly diagnosed with HIV during the breastfeeding period may continue breastfeeding as per maternal preference, provided that maternal ART and infant prophylaxis are initiated and adherence support is given.
Other key recommendations:
•All pregnant women should be screened at every visit for sexually transmitted infections and treated as needed.
•All pregnant and breastfeeding women should be screened for TB at every visit. If the TB screening is negative, then consider TB-preventive therapy during pregnancy only in women with a CD4+ count < 350 cells/µL (section 27).
o Common pitfalls:
o Not performing VL monitoring at appropriate time. See Table 22 for the appropriate monitoring intervals.
o An elevated VL is not acted upon urgently. Viral load results should be fast-tracked, and women failing their current regimen must be identified early and, if necessary, a regimen switch should be made without delay.
20. Liver disease
Antiretroviral dose adjustment in liver impairment
Key points
➢ There is no single blood test for accurate quantification of liver impairment.
➢ Child-Pugh class C may require dose adjustment for some ART drugs.
➢ The combination of TDF + 3TC (or FTC) + DTG (or RAL) is regarded as least hepatotoxic.
Antiretroviral dose adjustments
Table 23 shows the outline of dose adjustments for the relevant ART drugs in patients with Child-Pugh class C liver impairment.

Hepatitis B and human immunodeficiency virus co-infection
Key points
➢ All HIV-infected individuals should be screened for active HBV - hepatitis B surface antigen (HBsAg) screening is an appropriate test.
➢ The HBV VL correlates with disease progression and is used to monitor anti-HBV therapy.
➢ All children and adults eligible for HBV vaccination should be vaccinated.
➢ Antiretroviral therapy drugs with anti-HBV activity are TDF + 3TC (or FTC).
➢ For all HIV-infected HBsAg-positive patients, the ART regimen should include TDF + 3TC (or FTC).
➢ Using 3TC without TDF to treat HBV/HIV co-infection leads to HBV resistance in 80% - 90% of patients after 5 years of treatment.
➢ Interruption of TDF and/or 3TC (or FTC) has been associated with flares of life-threatening hepatitis in patients with hepatitis B in case reports.
➢ Adjust dosing frequency of TDF in patients with HBV infection and renal dysfunction; if renal function is severe or deteriorates with TDF, then 3TC monotherapy or other drugs with anti-HBV activity should be considered.
Hepatitis B virus is a common co-infection with HIV in southern Africa, with significant implications for progression to cirrhosis, as well as for treatment options. Access to vaccination, laboratory resources and treatment options is limited to some extent in southern African countries, and the recommendations below should be considered in the light of the local context.
Hepatitis B virus and HIV co-infection is associated with:
•an increased risk of chronic liver disease
•a higher HBV VL
•diminished responses to HBV vaccine
•an increased risk of drug-induced hepatotoxicity
•a flare of hepatitis within 3 months of commencing ART (because of HBV-related IRIS, which is difficult to differentiate from drug hepatotoxicity).
Drugs directed against HBV that have no or minimal anti-HIV activity (e.g. entecavir and telbivudine) are largely unavailable or extremely expensive in southern African region. Instead, it is usually necessary to use ART drugs that also have anti-HBV activity: TDF + 3TC (or FTC). As with HIV, these drugs suppress HBV but do not eradicate it. Effective treatment prevents or slows the progression to cirrhosis. For all HIV-infected HBsAg-positive patients, the ART regimen should include TDF + 3TC (or FTC). Using 3TC without including TDF leads to the development of HBV resistance in 80% - 90% of patients after 5 years of treatment. If a patient meets the criteria for switching to a second-line ART regimen (to treat HIV), then this combination - TDF + 3TC (or FTC) - should be continued to suppress HBV infection, as interruption of TDF and/or 3TC (or FTC) has been associated with flares of life-threatening hepatitis in case reports. The second-line ART regimen should be shaped around these two drugs.
In patients with HBV and renal dysfunction, the use of TDF may be considered with dosing frequency adjustment based on CrCl (Table 24) and more frequent creatinine monitoring. If renal dysfunction is severe or renal function deteriorates with TDF, then 3TC monotherapy or other drugs with anti-HBV activity should be considered.
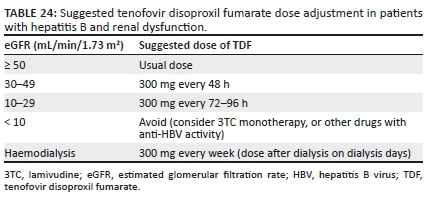
o Common pitfalls:
o Not continuing with TDF + 3TC (or FTC) combination when switching to second-line ART. The second-line ART regimen should be shaped around these two drugs.
oUsing 3TC without including TDF in the treatment of HIV/HBV co-infected patients.
21. Renal disease
Antiretroviral drug dose adjustment in renal disease
Key points
➢ Renal function is estimated by the modified Cockgraft-Gault formula or modification of diet in renal disease (MDRD) formula.
➢ For haemodialysis, the ART prescribed should be taken after dialysis.
In HIV-positive patients on chronic haemodialysis, there are a number of important ART considerations. The NRTI class is eliminated through the kidneys; thus, most NRTIs require dose adjustment as shown in Table 25.86,87,88 For suggested TDF dosing in patients with chronic hepatitis B, (section 20).
Antiretroviral drug choice and dosing in patients on chronic haemodialysis
Key points
➢ Patients with HIV may develop end-stage renal failure owing to HIV-associated nephropathy or an HIV-unrelated cause, necessitating chronic haemodialysis.
➢ Tenofovir disoproxil fumarate can be used in patients on chronic haemodialysis, but with once-weekly dosing which can be difficult for patients to remember.
➢ Zidovudine is generally avoided because of anaemia associated with renal failure.
➢ Integrase strand transfer inhibitors; and NNRTI drugs do not require dose adjustment.
➢ Atazanavir concentrations are reduced in patients on haemodialysis to a greater extent than LPV concentrations.
➢ Lopinavir/ritonavir requires a twice-daily dosing in patients on haemodialysis.
➢ Antiretroviral therapy drugs taken once daily, or the evening doses of drugs taken twice daily, should be given after haemodialysis session on dialysis days to prevent the drug from being dialysed out.
➢ Patients on chronic haemodialysis should be reviewed by a clinician experienced in ART management at least 6 monthly to monitor treatment efficacy and side effects and to adjust the regimen when needed.
Recommendations for antiretroviral therapy for patients on chronic haemodialysis
We recommend the following first-line option for patients on chronic haemodialysis: ABC (600 mg daily) + 3TC (50 mg first dose and thereafter 25 mg daily) + DTG (50 mg daily). On the days when haemodialysis is performed, the drugs should be given after the haemodialysis session.
•Common pitfall: Not giving daily doses or the evening doses of a twice-daily regimen after the haemodialysis session on dialysis days to prevent the drug from being dialysed out.
Antiretroviral therapy in patients with acute kidney injury
Key points
➢ In patients with AKI, NRTI dose adjustments should be implemented based on estimated CrCl calculation.
➢ Tenofovir disoproxil fumarate should be interrupted even if it is not thought to be the cause of the AKI.
➢ Re-challenge with TDF may be considered in patients 1-month post-resolution of AKI if TDF was not the cause and renal function returns to normal.
➢ In patients with AKI who have not yet received ART, initiation is preferably deferred until AKI has resolved. But avoid significant delays.
➢ Once renal function improves (i.e. creatinine is on a downward trend), standard NRTI doses should be re-introduced to avoid under-dosing.
Antiretroviral therapy in acute kidney injury
In patients with AKI, NRTI dose adjustments should be implemented based on estimated CrCl calculation (see Table 25). Tenofovir disoproxil fumarate should be interrupted even if it is not thought to be the cause of AKI. Care should be taken to identify other drugs that may affect renal function, such as aminoglycoside antibiotics, non-steroidal anti-inflammatory drugs, cotrimoxazole and iodinated radiocontrast; these drugs should be avoided where possible, including temporary discontinuation.Once there is clear evidence that renal function is improving (i.e. creatinine is on a downward trend), standard NRTI doses should be reintroduced to avoid under-dosing. In patients with AKI who have not yet received ART, initiation is preferably deferred until AKI has resolved. However, ART should not be delayed more than 2 weeks for this reason. If renal function does not show any improvement after treating an acute event (e.g. sepsis) and this persists beyond 3 months, then the patient should be assessed for chronic kidney disease and referred to a physician who can evaluate and investigate further.
oCommon pitfall: Not performing NRTI dose adjustment in patients with AKI.
22. Psychiatric disease
Key points
➢ Dolutegravir may cause insomnia, headache and neuropsychiatric side effects.
➢ Zidovudine and RAL frequently cause headaches when started, but this usually resolves.
➢ The majority of patients who experience neuropsychiatric features of EFV do so within the first 2-6 weeks, and thereafter the drug is better tolerated. Late neurological syndromes are described however (see section 4).
➢ Most neuropsychiatric effects relating to ART occur in the first few weeks of therapy.
➢ Depression and other mental illnesses are often undiagnosed or undertreated in HIV-infected individuals and may undermine adherence.
➢ Consider avoiding EFV- and RPV-based regimens in patients with psychiatric illness - these drugs can exacerbate psychiatric symptoms and may be associated with suicidality.
Mental health, especially mood and behaviour disorder, is associated with non-adherence to ART, leading to disability and poorer HIV treatment outcomes. There is a higher prevalence of depression in HIV-positive individuals, with a reported range of 20% - 40% versus 10% in the general population.89
Zidovudine and RAL frequently cause headaches when started, but this usually resolves after sometime. Efavirenz frequently causes neuropsychiatric effects in the first few weeks of therapy, typically presenting with insomnia, vivid dreams and dizziness. Both dysphoria and euphoria may occur. Fortunately, these features subside in the majority of patients within the first 4-6 weeks. Psychosis may occasionally occur. Dolutegravir may cause insomnia, headache and neuropsychiatric side effects. Raltegravir has been associated with similar central nervous system side effects.
o Common pitfalls:
o Not warning patients starting ART about potential neuropsychiatric symptoms. Patients must be informed about potential side effects.
o Unnecessary delays in initiating ART in patients with psychiatric illness.
23. Malaria
Key points
➢ There are several drug interactions between antimalarial agents and ART drugs.
➢ Efavirenz has a significant drug interaction with artemether-lumefantrine (Coartem) such that artemether (and its active metabolite) and lumefantrine concentrations are lowered, which can lead to failure of antimalarial therapy. Consider extending the course of artemether-lumefantrine to 6 days if administered concurrently with EFV.
➢ No artemether-lumefantrine dose adjustment is recommended for patients taking PIs or InSTIs.
➢ Protease inhibitors and NNRTIs exhibit several interactions with atovaquone-proguanil (Malanil) such that atovaquone concentrations are reduced - atovaquone-proguanil (Malanil) is best avoided in patients receiving these drugs.
➢ No significant drug interactions are predicted between InSTIs (DTG) and antimalarial drugs.
➢ Quinine is best avoided in patients on PIs or NNRTIs.
There are several drug interactions between antimalarials and ART drugs (see Table 26). Whilst artemether-lumefantrine (Coartem) can be administered safely with NVP, EFV significantly lowers the concentrations of artemether (and its active metabolite) and lumefantrine, which is likely to increase the risk of failure of antimalarial therapy. There is no clear guideline on how to overcome this interaction, but some experts recommend repeating the 3-day course of artemether-lumefantrine (i.e. treat for 6 days). Boosted PIs dramatically increase the plasma concentrations of lumefantrine, but a dose reduction is not recommended, as the toxicity threshold of lumefantrine seems to be high. Close monitoring for toxicity is recommended when co-administering artemether-lumefantrine with ART.
Quinine concentrations are significantly decreased by LPV/r, probably owing to induction of metabolism by RTV. It is likely that quinine concentrations will also be reduced by EFV and NVP; therefore, quinine should be avoided in patients receiving PIs or NNRTIs. Patients with severe malaria should receive artesunate, if available, and those with milder malaria should be treated with artemether-lumefantrine.
Amongst drugs used for chemoprophylaxis, there are no clinically significant pharmacokinetic interactions between ARVs and mefloquine or doxycycline. However, mefloquine and EFV both cause frequent neuropsychiatric side effects; therefore, doxycycline is the preferred chemoprophylactic agent for patients receiving EFV.
There are several interactions with atovaquone-proguanil (Malanil). Atovaquone concentrations are reduced by PIs and EFV, and also likely by NVP. Proguanil concentrations are also reduced by PIs and EFV. The use of atovaquone-proguanil is therefore best avoided in patients receiving PIs or NNRTIs.
No significant drug interactions are predicted between InSTIs and antimalarial drugs.
o Common pitfalls:
o Not advising patients receiving ART on chemoprophylaxis for malaria when travelling to malaria-endemic areas.
oNot providing ART recipients with intravenous artesunate or Coartem for malaria treatment despite the potential drug interactions.
24. Antiretroviral drug-induced liver injury
Key points
➢ All ART classes have been associated with hepatotoxicity and cause injury through an idiosyncratic reaction as the mechanism of injury.
➢ Alanine transaminase elevations greater than five times the upper limit of normal (ULN) are significant in the absence of symptoms.
➢ In the presence of symptoms of hepatitis, ALT elevations greater than 2.5 times the ULN are significant.
➢ Nevirapine is most frequently associated with DILI, with most cases occurring in the first few months after initiation. We no longer recommend NVP initiation.
➢ Patients on EFV may present with a delayed DILI many months after commencing therapy.
➢ Re-challenge is best avoided and may be considered in select cases in consultation with a specialist.
➢ If severe hepatitis occurs, or any hepatitis together with a rash, fever or systemic reaction occurs, then re-challenge with
➢ NNRTIs, ABC or CTX should not be attempted.
An ALT test should be performed in all patients at ART initiation. Repeat ALT testing is indicated in those who develop symptoms or signs suggestive of hepatitis. All ARV classes have been associated with hepatotoxicity - most commonly NNRTIs. Mild ALT elevations occur commonly and in general are transient. Alanine transaminase elevations greater than five times the ULN are significant in the absence of symptoms. In the presence of symptoms of hepatitis, ALT elevations greater than 2.5 times the ULN are also significant. In such patients, potentially hepatotoxic ARVs should be switched to alternative agents. Management guidelines are provided in Table 27.

Re-challenge may be considered, and in selected cases a specialist should be consulted. If severe hepatitis occurs, or any hepatitis with rash, fever or other systemic manifestation occurs, the opinion of a specialist should be sought. In this situation, re-challenge with NNRTIs, ABC or CTX should not be attempted.
Prolonged use of NRTIs, especially d4T and ddI (both of which are no longer used), may cause fatty liver. Typically, ALT concentration is more significantly elevated than aspartate transaminase (AST), and the concentrations of canalicular enzymes gamma-glutamyl transferase (GGT) and alkaline phosphatase (ALP) are more elevated than those of the transaminases. Non-tender hepatomegaly may be present. Ultrasound or computed tomography (CT) imaging may show decreased hepatic density. The condition is not benign, and fibrosis has been reported with long-term ddI use. Patients should be advised to avoid alcohol and should be switched to alternative drugs with lower potential for causing fatty liver.
In patients with severe hepatitis or jaundice, features of hepatic encephalopathy (i.e. features of hepatic failure) must be clinically assessed, and the international normalised ratio (INR) and serum glucose should be checked.
If the concentration of canalicular enzymes is more significantly elevated than that of ALT, or if conjugated bilirubin is elevated, then an ultrasound of the liver should be conducted to exclude biliary obstruction.
Isolated unconjugated hyperbilirubinaemia (drug-induced Gilbert's syndrome) is associated with ATV. In this case, all other LFTs are normal and the patient has no other symptoms of hepatitis. Although this is a benign condition (it does not reflect liver injury, but isolated competitive inhibition of the enzyme in the liver which conjugates bilirubin), it is often cosmetically unacceptable to patients, necessitating a switch from ATV to an alternative drug.
Although EFV has been recognised as an infrequent cause of DILI since it first became available, a novel pattern of DILI associated with the drug has recently been recognised in South Africa.25 Amongst such patients, many had a particularly severe pattern of liver injury found on liver biopsy (termed 'submassive necrosis', and associated with severe jaundice and a raised INR). The overall mortality was 11%. Whilst severe EFV-related DILI is likely to be uncommon, clinicians should be aware of the features observed:
1.The diagnosis of DILI was generally made after a longer duration on EFV (~ 3-6 months) than what is seen with DILI related to NVP or TB medication.
2.The DILI was not associated with features of hypersensitivity (e.g. drug rash) and often the first symptom was jaundice rather than abdominal symptoms.
3.Once EFV was stopped, it typically took several months for LFTs to normalise (median resolution > 6 months).
We do not advise routine LFT monitoring in patients on ART, as there is no evidence that this would lead to early detection of this DILI or improve outcomes. Those managing patients on EFV should monitor for symptoms and signs of hepatitis (nausea, vomiting, right-sided abdominal pain or jaundice). If these occur, then ALT should be assessed, and the patient should be examined for jaundice. The patient should be managed appropriately for DILI if there are hepatitis symptoms with ALT > 120 U/L, or if there is jaundice. Efavirenz should be switched to an alternative drug (e.g. DTG).
Many other drugs can cause hepatotoxicity, notably anti-tuberculous agents (including prophylactic isoniazid) and azoles. Cotrimoxazole is an uncommon cause of hepatitis, often as part of a systemic hypersensitivity reaction.
Recommendations for the management of DILI in patients receiving TB treatment have been published by the Southern African HIV Clinicians Society in 2013 (see link here).90
o Common pitfalls:
o Failing to recognise other drugs apart from ART as a cause of hepatotoxicity.
o Performing routine LFT monitoring in patients on ART, in an attempt to detect DILI earlier. There is no evidence to support this approach.
25. Dyslipidaemia
Key points
➢ Protease inhibitors can cause hypertriglyceridaemia and elevated LDL-C.
➢ Atazanavir/ritonavir and DRV/r are associated with less significant lipid abnormalities than LPV/r.
➢ Efavirenz can cause elevated total cholesterol and mild hypertriglyceridaemia.
➢ Dolutegravir does not significantly affect cholesterol.
➢ Lipids should be assessed routinely after 3 months on a PI regimen.
Protease inhibitors can cause hypertriglyceridaemia and elevated LDL-C. Atazanavir/ritonavir and once-daily DRV/r (800 mg DRV/100 mg once daily) are associated with less severe dyslipidaemia than other boosted PIs; AZT can cause mild hypertriglyceridaemia, and EFV can cause elevated total cholesterol and mild hypertriglyceridaemia.
We suggest that lipids should be assessed routinely after 3 months on a PI regimen. If normal at this stage, then re-assessment should be performed only in those with cardiovascular risk factors. Diet and lifestyle modification should always be advised. Diet is more effective for controlling hypertriglyceridaemia than hypercholesterolaemia. Other cardiovascular risk factors should be addressed. Clinicians should consider and investigate secondary causes of hypertriglyceridaemia and hypercholesterolaemia (e.g. diabetes, nephrotic syndrome, alcohol abuse and hypothyroidism).
If patients receiving LPV/r develop significant dyslipidaemia, they should be switched to DRV/r or ATV/r, rather than adding lipid-lowering therapy. However, lipid-lowering therapy is indicated in patients with persistent elevations despite switching to DRV/r or ATV/r. Switching the PI to DTG is another option because DTG has a more favourable lipid profile than PIs. However, DTG should only be used in a regimen in which at least one other ART drug is known to be fully active. In patients with hyperlipidaemia on EFV, the drug should be switched to DTG or RPV.
Marked hypertriglyceridaemia (> 10 mmol/L) can cause pancreatitis and requires urgent treatment with diet modification (restrict total TG intake to < 30 g/day), fibrates and switching LPV/r to DRV/r, ATV/r or DTG (fibrates can be stopped after 1 month, followed by reassessment within 4-6 weeks).
Indications for statin therapy in HIV-positive patients should be the same as in HIV-negative patients, using the Framingham heart disease risk score. As a general rule, in young patients with isolated elevated cholesterol but no other cardiovascular risk factors, a threshold of total cholesterol > 7.5 mmol/L (or LDL cholesterol > 5.0 mmol/L) should be used for initiating statin therapy, and the patient should be referred to a lipid clinic for investigation if feasible. In patients with cardiovascular risk factors (e.g. smoking, diabetes and hypertension), decisions should be made using the Framingham heart disease risk score. All patients with established atherosclerotic disease (coronary, cerebral or peripheral) or familial hypercholesterolaemia should be started on statin treatment. In addition, type 2 diabetics should also be started on a statin treatment if they have chronic kidney disease, or if they are older than 40 years of age (or have had diabetes for more than 10 years) and have one or more additional cardiovascular risk factors.91
Many statins have interactions with PIs that can lead to potentially toxic statin concentrations, with the exception of pravastatin and fluvastatin. Atorvastatin concentrations are significantly raised by PIs, but low doses (maximum 10 mg daily) can be used with monitoring for symptoms of myalgia. Lovastatin and simvastatin should not be co-administered with PIs, as their concentrations are dramatically increased, and severe rhabdomyolysis has been reported. We also advise against the use of rosuvastatin with PIs because of a complex drug-drug interaction; PIs increase the plasma concentrations of rosuvastatin whilst reducing their efficacy in the liver.
o Common pitfalls:
o Not routinely assessing lipids whilst the patient is receiving PI-based ART.
oFailure to recognise that many statins have interactions with PIs that lead to toxic statin concentrations.
oMonitoring of LDL-C in patients on a high-dose statin for secondary prevention. Such monitoring is not necessary.
26. Immune reconstitution inflammatory syndrome
Key points
➢ Approximately 10% - 20% of patients who start ART with advanced immunosuppression experience IRIS in the first few months of treatment.
➢ Two forms of IRIS have been recognised, namely unmasking and paradoxical IRIS.
➢ Immune reconstitution inflammatory syndrome is most frequently described in association with TB and CM.
➢ Integrase strand transfer inhibitors are not associated with an increased risk of TB-IRIS in clinical trials.
➢ Early ART initiation (defined as 1-4 weeks after anti-tuberculous therapy) doubles the risk of TB-IRIS compared with late ART initiation (defined as 8-12 weeks after anti-tuberculous therapy), but ART should not be delayed for this reason in patients with CD4 < 50 cells/mL.
➢ There is no confirmatory diagnostic test for IRIS.
➢ In most instances, ART is continued in cases of IRIS, unless IRIS is life-threatening (e.g. neurological involvement in TB-IRIS with depressed level of consciousness).
➢ Corticosteroids have been shown to reduce morbidity and improve symptoms in paradoxical TB-IRIS.
Approximately 10% - 20% of patients who start ART with advanced immunosuppression experience clinical deterioration during the first few months because of IRIS. Most presentations of IRIS occur within the first 3 months of ART. Two forms are recognised:
•Unmasking IRIS occurs in patients who have an unrecognised OI when ART is initiated, and who then present with exaggerated inflammatory features of that infection during early ART because of it being 'unmasked' by recovering immunity.
•Paradoxical IRIS occurs in patients who are being treated for an OI when they start ART, but who develop an immune-mediated worsening or recurrence of features of that infection after starting ART.
Immune reconstitution inflammatory syndrome is most frequently described in association with TB and CM. Skin conditions such as molluscum contagiosum and Kaposi's sarcoma may also worsen because of IRIS. The diagnosis of IRIS can be difficult, mainly because there is no confirmatory diagnostic test. Diagnosis relies on recognition of the characteristic clinical presentation, ensuring that OIs are correctly diagnosed, and excluding alternative causes of deterioration, such as drug resistance (e.g. multidrug-resistant TB). Case definitions for TB and cryptococcal IRIS have been published.92,93 It is important to ensure that the underlying OI is treated appropriately. Antiretroviral therapy should be continued, unless the IRIS is life-threatening (e.g. neurological involvement in TB-IRIS with depressed level of consciousness). Corticosteroids have been shown to reduce morbidity and improve symptoms in paradoxical TB-IRIS,94 and can be used in mycobacterial and fungal forms of IRIS when other causes of deterioration have been excluded, and particularly when IRIS features are severe.
For paradoxical TB-IRIS, prednisone can be commenced at a dose of 1.5 mg/kg/day and weaned over 4 weeks, but a longer course may be required if symptoms recur on weaning.95 Steroids should not be used in patients with Kaposi's sarcoma.
oCommon pitfall: Using steroids in patients with Kaposi's sarcoma.
Prophylactic prednisone
Key points
➢ Patients with active TB and who are improving on TB therapy with a CD4+ count 100 cells/mL upon starting ART can be initiated on prednisone 40 mg daily for 14 days, followed by 20 mg daily for 14 days to prevent paradoxical TB-IRIS.
➢ The use of prednisone in this context is not associated with high risk of severe infections, cancers or adverse events.
The use of prophylactic prednisone for the prevention of paradoxical TB-associated IRIS in adults with a CD4+ count 100 cells/mL has been shown in a randomised trial to be associated with a 30% lower relative incidence of TB-IRIS.96 Importantly, this did not come at the expense of any excess risk of severe infections or cancers. The recommended prednisone regimen is 40 mg daily for 14 days, followed by 20 mg daily for 14 days, and prednisone should be started concurrently with ART. Certain patient groups should be excluded from receiving prednisone; however, including patients with Kaposi's sarcoma and patients with RIF-resistant TB, or whose TB has not improved prior to starting ART.
o Common pitfall: Using prophylactic prednisone in patients who are not improving on TB therapy.
27. Opportunistic infection prophylaxis
Key points
➢ The use of appropriate prophylaxis (primary or secondary) is essential in patients initiating ART.
➢ In general, prophylaxis can be discontinued once the CD4+ count has increased to 200 cells/µL, but certain minimal durations of prophylaxis apply for secondary prophylaxis.
➢ Local and international guidelines should be consulted.
Cotrimoxazole primary prophylaxis
Prophylactic CTX is indicated for HIV-positive patients with a CD4+ count < 200 cells/µL, or with WHO stage 3 or 4 conditions (including TB). Cotrimoxazole offers protection against Pneumocystis jirovecii, toxoplasmosis, isosporiasis and certain bacterial infections. The recommended dose is 160/800 mg daily. Patients who develop a hypersensitivity reaction to CTX can be given dapsone instead, although this is best avoided if the reaction to CTX is life-threatening. Cotrimoxazole can be discontinued once the patient's CD4+ count is > 200 cells/µL.
Cotrimoxazole is a common cause of cutaneous and systemic hypersensitivity reactions, indistinguishable from hypersensitivity reactions to ART drugs. Cotrimoxazole should be interrupted when treating mild suspected NNRTI cutaneous hypersensitivity rashes, and permanently discontinued if severe hypersensitivity reactions occur. If CTX is prescribed for secondary prophylaxis or used for primary prophylaxis in those with severe immunosuppression, then an alternative should be substituted.
o Common pitfall: Prescribing CTX for newly diagnosed HIV-positive patients with a high CD4+count (> 200 cells/mL).
Cryptococcal antigen screening and pre-emptive treatment
Key points
➢ Cryptococcal antigenaemia screening should be performed for all adults or adolescents with a CD4+ count < 200 cells/mL who are initiating or re-initiating ART.
➢ Reflex laboratory screening is the preferred approach in South Africa.
➢ Lumbar puncture is recommended for all patients with a new positive CrAg screening test.
Screening for subclinical cryptococcal disease has been shown to have a benefit in reducing mortality in HIV-infected patients with a CD4+ count < 200 cells/mL. It is recommended that HIV-seropositive adults or adolescents (≥ 10 years) with a CD4+ count < 200 cells/mL should be screened for CrAg on serum or plasma by reflex laboratory testing (preferred) or clinician-initiated testing. If the clinician-initiated testing is performed, then it is recommended that screening should be restricted to adults or adolescents without prior cryptococcal disease who are initiating or re-initiating ART. For patients with a new positive CrAg result and an LP that rules out CM, oral fluconazole alone as induction therapy should be given (adults 1200 mg daily for 2 weeks). In these patients with a negative CSF CrAg result, ART can be started immediately with fluconazole. Patients diagnosed with CM should be managed as per the latest Southern African HIV Clinicians' Society guideline for the prevention, diagnosis and management of cryptococcal disease among HIV-infected persons: 2019 update.
oCommon pitfall: Not performing an LP in all patients who are newly diagnosed as CrAg positive. The absence of any symptoms of meningitis does not exclude CM; approximately one in three patients with asymptomatic antigenaemia has concurrent CM.
Isoniazid preventive therapy
Key points
➢ Isoniazid preventive therapy should be started at ART initiation or added to the treatment regimen of patients already on ART who have not yet received IPT, once active TB has been excluded.
➢ There is no need to test for latent TB prior to commencing IPT.
➢ Isoniazid preventive therapy should not be started during pregnancy, except in pregnant women where the CD4+ count is < 350 cells/mL and who are at high risk of death from TB.
➢ Before commencing IPT, active TB infection should always be excluded.
Clinical trials conducted in South Africa and Cote d'Ivoire have shown that IPT has an additive effect with ART in preventing incident TB in HIV-infected patients.97,98 In the South African trial, there was a 37% reduction in incident TB when patients receiving ART were prescribed IPT (vs. placebo) for 12 months. This benefit applied irrespective of tuberculin skin test (TST) status, and the trial included patients established on ART. All patients receiving ART should be considered for IPT and screened for active TB using a symptom screen99 - IPT should be deferred and investigations should be conducted for active TB if any of the four symptoms (current cough, fever, night sweats or weight loss) is present. In patients receiving IPT, monitoring for neuropathy and hepatitis symptoms should be performed. Routine ALT monitoring is not indicated, but ALT should be tested if hepatitis symptoms occur.
A recent trial of IPT in pregnant women receiving ART, the TB APPRISE study, showed that IPT resulted in worse pregnancy outcomes.100 However, this was not confirmed in a larger observational study from the Western Cape, which showed that IPT use was associated with better pregnancy outcomes, and that incident TB was reduced in women on IPT who had CD4+ counts < 350 cells/mL.101 The duration of IPT is now 12 months irrespective of TST status, as outlined in Table 28.
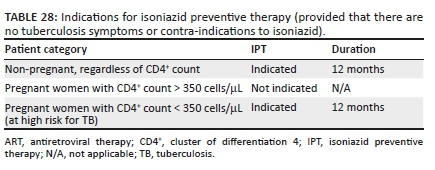
28. Adherence
Patient readiness for antiretroviral therapy
Key points
➢ Each patient commencing ART needs to be prepared for treatment before or during early ART period.
•➢ Barriers to adherence (e.g. depression, alcohol use, non-disclosure and food security) and any mis-conceptions about ART must be identified in the preparation for ART or during the early period of ART.
Preparing patients for lifelong ART with good adherence is a critical component of achieving long-term efficacy and preventing treatment resistance. To accommodate counselling, traditionally two or three visits are required, staggered closely together, before ART. However, it is now considered acceptable to do some of the counselling during early ART rather than delaying initiation (same-day initiation is described in section 6). Prolonged delays in commencing ART should be avoided. Antiretroviral therapy should be delayed only if concerns about adherence are severe enough to outweigh the risk of HIV disease progression.
The patient should be provided with details regarding:
•the benefits of ART
•that ART is a life-long therapy
•the importance of good adherence
•a list of ART side effects relevant to the drugs they will use, including what to do and who to contact if serious side effects occur
•viral load monitoring on ART.
The counselling approach should also ensure that the patient has a good understanding of HIV (the virus, the potential clinical complications and transmission) and should cover safer sex practices and address issues related to reproductive health (i.e. family planning, contraception, condom use and pregnancy). Clinicians should check family-planning choices at follow-up visits and ensure adequate access to safe and effective contraception. It is important to discuss the concept of 'Undetectable = Untransmittable' with patients and ensure that they have a correct understanding of this concept and that ART will only prevent onward transmission if there is optimal adherence with VL suppression.
Active depression, other mental health issues or substance abuse should be detected actively and treated. A personal treatment plan should be formulated for each patient, specifying drug storage, strategies for missed doses and how to integrate taking medication into their daily routine. The patient must be made aware of scheduling in terms of clinical follow-up.
Disclosure of HIV status (to a partner and/or other household members) should strongly be encouraged; it is an important determinant of treatment adherence and assists in the provision of patient-directed support. Disclosure also identifies exposed contacts for screening and support. This issue needs to be handled carefully in situations where disclosure may have harmful consequences, particularly for women. The patient should be encouraged to join a support group and/or identify a treatment 'buddy'. However, neither disclosure nor support group participation is a prerequisite for good adherence and should not be a reason for deferring ART. Clinicians should ensure that they have the contact details of each patient and their treatment buddy.
oCommon pitfalls:
oDelaying ART because the patient has not completed three clinic visits or not disclosed his or her HIV status.
oNot outlining the goals of ART with the patient. These are to:
■provide maximal and durable suppression of VL
■restore and preserve immune function
■reduce HIV-related infectious and non-infectious morbidity
■prolong life expectancy and improve quality of life
■prevent onward transmission of HIV
■minimise adverse effects of the treatment.
Support and counselling
Key points
➢ Success of ART hinges on how well the tablets are taken; at least 90%, preferably more, of treatment doses need to be taken.
➢ Support should be provided to ensure high levels of treatment adherence.
➢ None of the commonly used first- and second-line options have meaningful food restrictions.
➢ Delayed dosing is rarely a problem; even if out by many hours, most of the drugs have long half-lives, and patients should be encouraged and supported to take their dose once they remember to do so in these instances.
➢ Disclosure is not a prerequisite for ART.
➢ Heavy alcohol use may affect adherence and may potentiate ART hepatotoxicity and other hepatic pathology; however, responsible alcohol use is not prohibited in patients established on or starting ART.
Patients should be informed about the benefits of ART and that side effects are usually minor and transient, or manageable. They should be given a treatment plan, specifying the drugs to be used (with names and details including the appearance of each drug, when and how they are to be taken, and a brief indication of anticipated side effects and toxicity).
The causes of poor adherence are often complex and linked to social issues. Common causes are outlined in Table 29.
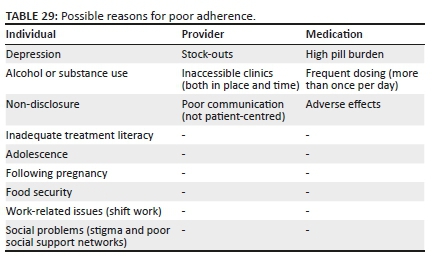
o Common pitfalls:
oNot informing patients about the benefits of ART. This includes not only reduced mortality and morbidity, but also prevention of HIV transmission.
oNot informing patients that side effects are usually minor and transient, or manageable.
oNot advising patients on how to deal with delayed dosing.
oNot providing patients with a treatment plan specifying the drugs to be used.
29. Acknowledgements
The authors would like to acknowledge the Southern African HIV Clinicians Society for their support.
Competing interests
The authors have declared that no competing interests exist.
Authors' contribution
J.N., S.D. and G.M. constituted the writing committee and were the primary authors of the manuscript. The other authors reviewed the manuscript and offered constructive criticisms.
Ethical consideration
This article followed all ethical standards for carrying out research without direct contact with human or animal subjects.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article, as no new data were created or analysed in this study.
Disclaimer
Specific recommendations provided here are intended only as a guide to clinical management, based on expert consensus and best current evidence. Treatment decisions for patients should be made by their responsible clinicians, with due consideration of individual circumstances. The most current version of this document should always be consulted.
30. Abbreviations
/r ritonavir-boosted
3TC lamivudine
ABC abacavir
ADR adverse drug reaction
AKI acute kidney injury
ALP alkaline phosphatase
ALT alanine transaminase
ART antiretroviral therapy
ARV antiretroviral
AST aspartate transaminase
ATV atazanavir
ATV/r atazanavir/ritonavir
AZT zidovudine
bd twice daily
CD4+ cluster of differentiation 4
CM cryptococcal meningitis
CrAg cryptococcal antigen
CrCl creatinine clearance rate
CSF cerebrospinal fluid
CT computed tomography
CTX Cotrimoxazole
CVS cardiovascular
CYP cytochrome P450
d4T stavudine
ddI didanosine
DILI drug-induced liver injury
DNA deoxyribonucleic acid
DRV darunavir
DRV/r darunavir/ritonavir
DTG dolutegravir
eGFR estimated glomerular filtration rate
ELISA enzyme-linked immunosorbent assay
ETR etravirine
FBC full blood count
FTC emtricitabine
GGT gamma-glutamyl transferase
GI gastrointestinal
Hb haemoglobin
HBsAg hepatitis B surface antigen
HBV hepatitis B virus
HIV human immunodeficiency virus
ICU intensive care unit
INR international normalised ratio
InSTI integrase strand transfer inhibitor
IPT isoniazid preventive therapy
LAM lipoarabinomannan
LDL-C low-density lipoprotein cholesterol
LP lumbar puncture
LPV lopinavir
LPV/r lopinavir/ritonavir
MDRD modification of diet in renal disease
MVC maraviroc
NGT nasogastric tube
NNRTI non-nucleoside reverse transcriptase inhibitor
NRTI nucleoside reverse transcriptase inhibitor
NTDs neural-tube defects
NtRTI nucleotide reverse transcriptase inhibitor
NVP nevirapine
OI opportunistic infection
PCR polymerase chain reaction
PI protease inhibitor
PI/r ritonavir-boosted ritonavir
PMTCT prevention of mother-to-child transmission of HIV
PPIs proton pump inhibitors
PrEP pre-exposure prophylaxis
RAL raltegravir
RCTs randomised controlled trials
RIF rifampicin
RFB rifabutin
RNA ribonucleic acid
RPV rilpivirine
RTV or /r ritonavir
sCr serum creatinine
sCrAg serum cryptococcal antigen
TAF tenofovir alafenamide
TAM thymidine analogue mutation
TB tuberculosis
TB-IRIS tuberculosis immune reconstitution inflammatory syndrome
TBM tuberculosis meningitis
TC total cholesterol
TDF tenofovir disoproxil fumarate
TG triglycerides
TST tuberculin skin test
ULN upper limit of normal
VL viral load
WHO World Health Organization
WOCP women of childbearing potential
31. References
1.Johnson LF, Mossong J, Dorrington RE, et al. Life expectancies of South African adults starting antiretroviral treatment: Collaborative analysis of cohort studies. PLoS Med. 2013;10(4):e1001418. https://doi.org/10.1371/journal.pmed.1001418 [ Links ]
2.Maartens G. 'Covering the tail' after stopping efavirenz-based antiretroviral therapy. S Afr J HIV Med. 2020;21(1):a1036. https://doi.org/10.4102/sajhivmed.v21i1.1036 [ Links ]
3.Benhamou Y, Bochet M, Thibault V, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30(5):1302-1306. https://doi.org/10.1002/hep.510300525 [ Links ]
4.Fernandez-Fernandez B, Montoya-Ferrer A, Sanz AB, et al. Tenofovir nephrotoxicity: 2011 update. AIDS Res Treat. 2011;2011:354908. https://doi.org/10.1155/2011/354908 [ Links ]
5.Venter WDF, Fabian J, Feldman C. An overview of tenofovir and renal disease for the HIV-treating clinician. South Afr J HIV Med. 2018;19(1):817. https://doi.org/10.4102/sajhivmed.v19i1.817 [ Links ]
6.Cruciani M, Mengoli C, Malena M, et al. Virological efficacy of abacavir: Systematic review and meta-analysis. J Antimicrob Chemother. 2014;69(12):3169-3180. https://doi.org/10.1093/jac/dku279 [ Links ]
7.D. A. D. Study Group, Sabin CA, Worm SW, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: A multi-cohort collaboration. Lancet. 2008;371(9622):1417-1426. https://doi.org/10.1016/S0140-6736(08)60423-7 [ Links ]
8.Ribaudo HJ, Benson CA, Zheng Y, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: Short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis. 2011;52(7):929-940. https://doi.org/10.1093/cid/ciq244 [ Links ]
9.Majluf-Cruz A, Luna-Castanos G, Trevino-Perez S, Santoscoy M, Nieto-Cisneros L. Lamivudine-induced pure red cell aplasia. Am J Hematol. 2000;65(3):189-191. https://doi.org/10.1002/1096-8652(200011)65:3<189::AID-AJH2>3.0.CO;2-6 [ Links ]
10.Cohen K, Viljoen C, Njuguna C, Maartens G. Emtricitabine-associated red cell aplasia. AIDS. 2019;33(6):1095-1096. https://doi.org/10.1097/QAD.0000000000002136 [ Links ]
11.Raffi F, Jaeger H, Quiros-Roldan E, et al. Once-daily dolutegravir versus twice-daily raltegravir in antiretroviral-naive adults with HIV-1 infection (SPRING-2 study): 96 week results from a randomised, double-blind, non-inferiority trial. Lancet Infect Dis. 2013;13(11):927-935. https://doi.org/10.1016/S1473-3099(13)70257-3 [ Links ]
12.Walmsley SL, Antela A, Clumeck N, et al. Dolutegravir plus abacavir-lamivudine for the treatment of HIV-1 infection. N Engl J Med. 2013;369(19):1807-1818. https://doi.org/10.1056/NEJMoa1215541 [ Links ]
13.Molina JM, Clotet B, Van Lunzen J, et al. Once-daily dolutegravir versus darunavir plus ritonavir for treatment-naive adults with HIV-1 infection (FLAMINGO): 96 week results from a randomised, open-label, phase 3b study. Lancet HIV. 2015;2(4):e127-e136. https://doi.org/10.1016/S2352-3018(15)00027-2 [ Links ]
14.Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700-708. https://doi.org/10.1016/S0140-6736(13)61221-0 [ Links ]
15.You J, Wang H, Huang X, et al. Therapy-emergent drug resistance to integrase strand transfer inhibitors in HIV-1 patients: A subgroup meta-analysis of clinical trials. PLoS One. 2016;11(8):e0160087. https://doi.org/10.1371/journal.pone.0160087 [ Links ]
16.Orrell C, Hagins DP, Belonosova E, et al. Fixed-dose combination dolutegravir, abacavir, and lamivudine versus ritonavir-boosted atazanavir plus tenofovir disoproxil fumarate and emtricitabine in previously untreated women with HIV-1 infection (ARIA): Week 48 results from a randomised, open-label, non-inferiority, phase 3b study. Lancet HIV. 2017;4(12):e536-e546. https://doi.org/10.1093/ofid/ofw194.89 [ Links ]
17.Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): An open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253-264. https://doi.org/10.1016/S1473-3099(19)30036-2 [ Links ]
18.Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. N Engl J Med. 2019;381(9):827-840. https://doi.org/10.1056/NEJMoa1905230 [ Links ]
19.Raesima MM, Ogbuabo CM, Thomas V, et al. Dolutegravir use at conception - Additional surveillance data from Botswana. N Engl J Med. 2019;381(9):885-887. https://doi.org/10.1056/NEJMc1908155 [ Links ]
20.Hill A, Waters L, Pozniak A. Are new antiretroviral treatments increasing the risks of clinical obesity? J Virus Erad. 2019;5(1):41-43. [ Links ]
21.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803-815. https://doi.org/10.1056/NEJMoa1902824 [ Links ]
22.Encore Study Group, Carey D, Puls R, et al. Efficacy and safety of efavirenz 400 mg daily versus 600 mg daily: 96-week data from the randomised, double-blind, placebo-controlled, non-inferiority ENCORE1 study. Lancet Infect Dis. 2015;15(7):793-802. [ Links ]
23.Variava E, Sigauke FR, Norman J, et al. Brief report: Late Efavirenz-induced ataxia and encephalopathy: A case series. J Acquir Immune Defic Syndr. 2017;75(5):577-579. https://doi.org/10.1097/QAI.0000000000001451 [ Links ]
24.Sinxadi PZ, Leger PD, McIlleron HM, et al. Pharmacogenetics of plasma efavirenz exposure in HIV-infected adults and children in South Africa. Br J Clin Pharmacol. 2015;80(1):146-156. https://doi.org/10.1111/bcp.12590 [ Links ]
25.Sonderup MW, Maughan D, Gogela N, et al. Identification of a novel and severe pattern of Efavirenz drug-induced liver injury in South Africa. AIDS. 2016;30(9):1483-1485. https://doi.org/10.1097/QAD.0000000000001084 [ Links ]
26.Jover F, Cuadrado JM, Roig P, Rodriguez M, Andreu L, Merino J. Efavirenz-associated gynecomastia: Report of five cases and review of the literature. Breast J. 2004;10(3):244-246. [ Links ]
27.Sikora MJ, Rae JM, Johnson MD, Desta Z. Efavirenz directly modulates the oestrogen receptor and induces breast cancer cell growth. HIV Med. 2010;11(9):603-607. https://doi.org/10.1111/j.1468-1293.2010.00831.x [ Links ]
28.Njuguna C, Swart A, Blockman M, et al. Cases of antiretroviral-associated gynaecomastia reported to the National HIV & Tuberculosis Health Care Worker Hotline in South Africa. AIDS Res Ther. 2016;13:40. [ Links ]
29.Cohen CJ, Molina JM, Cassetti I, et al. Week 96 efficacy and safety of rilpivirine in treatment-naive, HIV-1 patients in two Phase III randomized trials. AIDS. 2013;27(6):939-950. [ Links ]
30.Behrens G, Rijnders B, Nelson M, et al. Rilpivirine versus efavirenz with emtricitabine/tenofovir disoproxil fumarate in treatment-naive HIV-1-infected patients with HIV-1 RNA </=100,000 copies/mL: Week 96 pooled ECHO/THRIVE subanalysis. AIDS Patient Care STDS. 2014;28(4):168-175. https://doi.org/10.1089/apc.2013.0310 [ Links ]
31.Cohen C, Nieto-Cisneros L, Zala C, et al. Comparison of atazanavir with lopinavir/ritonavir in patients with prior protease inhibitor failure: A randomized multinational trial. Curr Med Res Opin. 2005;21(10):1683-1692. https://doi.org/10.1185/030079905X65439 [ Links ]
32.Mills AM, Nelson M, Jayaweera D, et al. Once-daily darunavir/ritonavir vs. lopinavir/ritonavir in treatment-naive, HIV-1-infected patients: 96-week analysis. AIDS. 2009;23(13):1679-1688. [ Links ]
33.Venter WDF, Moorhouse M, Sokhela S, et al. Low-dose ritonavir-boosted darunavir once daily versus ritonavir-boosted lopinavir for participants with less than 50 HIV RNA copies per mL (WRHI 052): A randomised, open-label, phase 3, non-inferiority trial. Lancet HIV. 2019;6(7):e428-e437. https://doi.org/10.1016/S2352-3018(19)30081-5 [ Links ]
34.Molina JM, Gallien S, Chaix ML, et al. Low-dose ritonavir-boosted darunavir in virologically suppressed HIV-1-infected adults: An open-label trial (ANRS 165 Darulight). J Antimicrob Chemother. 2018;73(8):2129-2136. https://doi.org/10.1093/jac/dky181 [ Links ]
35.Insight Start Study Group, Lundgren JD, Babiker AG, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795-807. [ Links ]
36.Temprano Anrs Study Group, Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808-822. [ Links ]
37.Cohen MS, Chen YQ, McCauley M, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. N Engl J Med. 2016;375(9):830-839. https://doi.org/10.1056/NEJMoa1600693 [ Links ]
38.Rodger AJ, Cambiano V, Bruun T, et al. Sexual activity without condoms and risk of HIV transmission in serodifferent couples when the HIV-positive partner is using suppressive antiretroviral therapy. JAMA. 2016;316(2):171-181. [ Links ]
39.Rodger AJ, Cambiano V, Bruun T, et al. Risk of HIV transmission through condomless sex in serodifferent gay couples with the HIV-positive partner taking suppressive antiretroviral therapy (PARTNER): Final results of a multicentre, prospective, observational study. Lancet. 2019;393(10189):2428-2438. [ Links ]
40.Bavinton BR, Pinto AN, Phanuphak N, et al. Viral suppression and HIV transmission in serodiscordant male couples: An international, prospective, observational, cohort study. Lancet HIV. 2018;5(8):e438-e447. https://doi.org/10.1016/S2352-3018(18)30132-2 [ Links ]
41.O'Brien M, Markowitz M. Should we treat acute HIV infection? Curr HIV/AIDS Rep. 2012;9(2):101-110. https://doi.org/10.1007/s11904-012-0113-0 [ Links ]
42.Krishnan S, Wilson EM, Sheikh V, et al. Evidence for innate immune system activation in HIV type 1-infected elite controllers. J Infect Dis. 2014;209(6):931-939. [ Links ]
43.Crowell TA, Gebo KA, Blankson JN, et al. Hospitalization rates and reasons among HIV elite controllers and persons with medically controlled HIV infection. J Infect Dis. 2015;211(11):1692-1702. https://doi.org/10.1093/infdis/jiu809 [ Links ]
44.Li JZ, Segal FP, Bosch RJ, et al. ART reduces T cell activation and immune exhaustion markers in HIV controllers. Clin Infect Dis. 2019;70:1636-1642. [ Links ]
45.Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: A stepped-wedge cluster-randomised trial. Lancet HIV. 2016;3(11):e539-e548. [ Links ]
46.Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a US public health setting. J Acquir Immune Defic Syndr. 2017;74(1):44-51. https://doi.org/10.1097/QAI.0000000000001134 [ Links ]
47.Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: The RapIT randomized controlled trial. PLoS Med. 2016;13(5):e1002015. [ Links ]
48.Uthman OA, Okwundu C, Gbenga K, et al. Optimal timing of antiretroviral therapy initiation for HIV-infected adults with newly diagnosed pulmonary tuberculosis: A systematic review and meta-analysis. Ann Intern Med. 2015;163(1):32-39. [ Links ]
49.Torok ME, Yen NT, Chau TT, et al. Timing of initiation of antiretroviral therapy in human immunodeficiency virus (HIV)-associated tuberculous meningitis. Clin Infect Dis. 2011;52(11):1374-1383. https://doi.org/10.1093/cid/cir230 [ Links ]
50.Boulware DR, Meya DB, Muzoora C, et al. Timing of antiretroviral therapy after diagnosis of cryptococcal meningitis. N Engl J Med. 2014;370(26):2487-2498. [ Links ]
51.Zolopa A, Andersen J, Powderly W, et al. Early antiretroviral therapy reduces AIDS progression/death in individuals with acute opportunistic infections: A multicenter randomized strategy trial. PLoS One. 2009;4(5):e5575. https://doi.org/10.1371/journal.pone.0005575 [ Links ]
52.Tucker JD, Bien CH, Easterbrook PJ, et al. Optimal strategies for monitoring response to antiretroviral therapy in HIV-infected adults, adolescents, children and pregnant women: A systematic review. AIDS. 2014;28 Suppl 2:S151-S160. [ Links ]
53.Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: A multicentre cohort study. Lancet Infect Dis. 2018;18(2):188-197. https://doi.org/10.1016/S1473-3099(17)30681-3 [ Links ]
54.Laprise C, De Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: Results from 12 years of observation. Clin Infect Dis. 2013;57(10):1489-1496. https://doi.org/10.1093/cid/cit529 [ Links ]
55.Chimukangara B, Lessells RJ, Rhee SY, et al. Trends in pretreatment HIV-1 drug resistance in antiretroviral therapy-naive Adults in South Africa, 2000-2016: A pooled sequence analysis. EClinicalMedicine. 2019;9:26-34. [ Links ]
56.Smith CJ, Sabin CA, Lampe FC, et al. The potential for CD4 cell increases in HIV-positive individuals who control viraemia with highly active antiretroviral therapy. AIDS. 2003;17(7):963-969. https://doi.org/10.1097/00002030-200305020-00004 [ Links ]
57.Loutfy MR, Genebat M, Moore D, et al. A CD4+ cell count <200 cells per cubic millimeter at 2 years after initiation of combination antiretroviral therapy is associated with increased mortality in HIV-infected individuals with viral suppression. J Acquir Immune Defic Syndr. 2010;55(4):451-459. [ Links ]
58.Tuboi SH, Pacheco AG, Harrison LH, et al. Mortality associated with discordant responses to antiretroviral therapy in resource-constrained settings. J Acquir Immune Defic Syndr. 2010;53(1):70-77. https://doi.org/10.1097/QAI.0b013e3181c22d19 [ Links ]
59.Ledergerber B, Lundgren JD, Walker AS, et al. Predictors of trend in CD4-positive T-cell count and mortality among HIV-1-infected individuals with virological failure to all three antiretroviral-drug classes. Lancet. 2004;364(9428):51-62. [ Links ]
60.Paton NI, Kityo C, Hoppe A, et al. Assessment of second-line antiretroviral regimens for HIV therapy in Africa. N Engl J Med. 2014;371(3):234-247. [ Links ]
61.La Rosa AM, Harrison LJ, Taiwo B, et al. Raltegravir in second-line antiretroviral therapy in resource-limited settings (SELECT): A randomised, phase 3, non-inferiority study. Lancet HIV. 2016;3(6):e247-e258. https://doi.org/10.1016/S2352-3018(16)30011-X [ Links ]
62.Cohen K, Stewart A, Kengne AP, et al. A clinical prediction rule for protease inhibitor resistance in patients failing second-line antiretroviral therapy. J Acquir Immune Defic Syndr. 2019;80(3):325-329. [ Links ]
63.Rhee SY, Grant PM, Tzou PL, et al. A systematic review of the genetic mechanisms of dolutegravir resistance. J Antimicrob Chemother. 2019;74(11):3135-3149. https://doi.org/10.1093/jac/dkz256 [ Links ]
64.Gupta RK, Gregson J, Parkin N, et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: A systematic review and meta-regression analysis. Lancet Infect Dis. 2018;18(3):346-355. [ Links ]
65.Lamorde M, Wang X, Neary M, et al. Pharmacokinetics, pharmacodynamics, and pharmacogenetics of Efavirenz 400 mg once daily during pregnancy and post-partum. Clin Infect Dis. 2018;67(5):785-790. https://doi.org/10.1093/cid/ciy161 [ Links ]
66.Kaboggoza JP, Wang X, Neary M, et al. A lower dose of Efavirenz can be coadministered with Rifampicin and Isoniazid in tuberculosis patients. Open Forum Infect Dis. 2019;6(2):ofz035. https://doi.org/10.1093/ofid/ofz035 [ Links ]
67.Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): Week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. 2019;393(10167):143-155. [ Links ]
68.Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: Phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet. 2018;391(10123):839-849. [ Links ]
69.Cahn P, Fourie J, Grinsztejn B, et al. Week 48 analysis of once-daily vs. twice-daily darunavir/ritonavir in treatment-experienced HIV-1-infected patients. AIDS. 2011;25(7):929-939. [ Links ]
70.Molina JM, Andrade-Villanueva J, Echevarria J, et al. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr. 2010;53(3):323-332. https://doi.org/10.1097/QAI.0b013e3181c990bf [ Links ]
71.Johnson M, Grinsztejn B, Rodriguez C, et al. 96-week comparison of once-daily atazanavir/ritonavir and twice-daily lopinavir/ritonavir in patients with multiple virologic failures. AIDS. 2006;20(5):711-718. [ Links ]
72.Boyd MA, Moore CL, Molina JM, et al. Baseline HIV-1 resistance, virological outcomes, and emergent resistance in the SECOND-LINE trial: An exploratory analysis. Lancet HIV. 2015;2(2):e42-e51. https://doi.org/10.1016/S2352-3018(14)00061-7 [ Links ]
73.Hill AM, Venter F. The unexpected success of NRTIs in second-line treatment. Lancet Infect Dis. 2018;18(1):3-5. https://doi.org/10.1016/S1473-3099(17)30631-X [ Links ]
74.Yazdanpanah Y, Fagard C, Descamps D, et al. High rate of virologic suppression with raltegravir plus etravirine and darunavir/ritonavir among treatment-experienced patients infected with multidrug-resistant HIV: Results of the ANRS 139 TRIO trial. Clin Infect Dis. 2009;49(9):1441-1449. [ Links ]
75.Moorhouse M, Maartens G, Venter WDF, et al. Third-line antiretroviral therapy program in the South African public sector: Cohort description and virological outcomes. J Acquir Immune Defic Syndr. 2019;80(1):73-78. https://doi.org/10.1097/QAI.0000000000001883 [ Links ]
76.Grinsztejn B, Hughes MD, Ritz J, et al. Third-line antiretroviral therapy in low-income and middle-income countries (ACTG A5288): A prospective strategy study. Lancet HIV. 2019;6(9):e588-e600. [ Links ]
77.Meintjes G, Dunn L, Coetsee M, et al. Third-line antiretroviral therapy in Africa: Effectiveness in a Southern African retrospective cohort study. AIDS Res Ther. 2015;12:39. https://doi.org/10.1186/s12981-015-0081-8 [ Links ]
78.Gandhi RT, Tashima KT, Smeaton LM, et al. Long-term outcomes in a large randomized trial of HIV-1 salvage therapy: 96-week results of AIDS Clinical Trials Group A5241 (OPTIONS). J Infect Dis. 2019;221:1407-1415. https://doi.org/10.1093/infdis/jiz281 [ Links ]
79.Stevens WS, Wallis CL, Sanne I, Venter F. Will etravirine work in patients failing nonnucleoside reverse transcriptase inhibitor-based treatment in southern Africa? J Acquir Immune Defic Syndr. 2009;52(5):655-656. [ Links ]
80.Meintjes G, Brust JCM, Nuttall J, Maartens G. Management of active tuberculosis in adults with HIV. Lancet HIV. 2019;6(7):e463-e474. https://doi.org/10.1016/S2352-3018(19)30154-7 [ Links ]
81.Dooley KE, Kaplan R, Mwelase N, et al. Dolutegravir-based antiretroviral therapy for patients co-infected with tuberculosis and HIV: A multicenter, noncomparative, open-label, randomized trial. Clin Infect Dis. 2019;70:549-556. https://doi.org/10.2139/ssrn.3244943 [ Links ]
82.la Porte CJ, Colbers EP, Bertz R, et al. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Antimicrob Agents Chemother. 2004;48(5):1553-1560. [ Links ]
83.Decloedt EH, Maartens G, Smith P, Merry C, Bango F, McIlleron H. The safety, effectiveness and concentrations of adjusted lopinavir/ritonavir in HIV-infected adults on rifampicin-based antitubercular therapy. PLoS One. 2012;7(3):e32173. [ Links ]
84.Davies G, Cerri S, Richeldi L. Rifabutin for treating pulmonary tuberculosis. Cochrane Database Syst Rev. 2007(4):CD005159. https://doi.org/10.1002/14651858.CD005159.pub2 [ Links ]
85.Hennig S, Svensson EM, Niebecker R, et al. Population pharmacokinetic drug-drug interaction pooled analysis of existing data for rifabutin and HIV PIs. J Antimicrob Chemother. 2016;71(5):1330-1340. https://doi.org/10.1093/jac/dkv470 [ Links ]
86.Bartlett JG, Gallant JE, Pham PA (eds). Medical management of HIV infection. 15th ed. Baltimore, MD: John Hopkins University Press, 2009-2010; 556 pp. [ Links ]
87.Gilbert DN, Moellering RC, Eliopoulos GM, et al. (eds). The Sanford Guide to antimicrobial therapy. 42nd ed. Sperryville, Virginia: Antimicrobial Therapy, Inc., 2012; 232 pp. [ Links ]
88.HIV Medicine Association of the Infectious Diseases Society of America. Clinical practice guideline for the management of chronic kidney disease in patients infected with HIV: 2014 update. Clin Infect Dis. 2014;59(9):e96-e138. [ Links ]
89.Van Coppenhagen B, Duvenage HS. Prevalence of depression in people living with HIV and AIDS at the Kalafong Provincial Tertiary Hospital Antiretroviral Clinic. S Afr J Psychiatr. 2019;25:1175. [ Links ]
90.Jong E, Conradie F, Berhanu R, et al. Consensus statement: Management of drug-induced liver injury in HIV-positive patients treated for TB. S Afr J HIV Med. 2013;14(3):7. https://doi.org/10.4102/sajhivmed.v14i3.63 [ Links ]
91.SEMDSA Type 2 Diabetes Guidelines Expert Committee. SEMDSA 2017 guidelines for the management of type 2 diabetes mellitus2017 [homepage on the Internet] [cited 2020 June 1]. Available from: https://www.semdsa.org.za/images/647-4385-1-PB.pdf [ Links ]
92.Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: Proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791-802. [ Links ]
93.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: Case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516-523. https://doi.org/10.1016/S1473-3099(08)70184-1 [ Links ]
94.Meintjes G, Wilkinson RJ, Morroni C, et al. Randomized placebo-controlled trial of prednisone for paradoxical tuberculosis-associated immune reconstitution inflammatory syndrome. AIDS. 2010;24(15):2381-2390. https://doi.org/10.1097/QAD.0b013e32833dfc68 [ Links ]
95.Meintjes G, Sonderup MW. A practical approach to the diagnosis and management of paradoxical tuberculosis immune reconstitution inflammatory syndrome. Contin Med Educ. 2011;29(10):410-417. [ Links ]
96.Meintjes G, Stek C, Blumenthal L, et al. Prednisone for the prevention of paradoxical tuberculosis-associated IRIS. N Engl J Med. 2018;379(20):1915-1925. https://doi.org/10.1056/NEJMoa1800762 [ Links ]
97.Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: A randomised double-blind, placebo-controlled trial. Lancet. 2014;384(9944):682-690. https://doi.org/10.1016/S0140-6736(14)60162-8 [ Links ]
98.Group TAS, Danel C, Moh R, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808-822. https://doi.org/10.1056/NEJMoa1507198 [ Links ]
99.Getahun H, Kittikraisak W, Heilig CM, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: Individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. https://doi.org/10.1371/journal.pmed.1000391 [ Links ]
100.Gupta A, Montepiedra G, Aaron L, et al. Isoniazid preventive therapy in HIV-infected pregnant and postpartum women. N Engl J Med. 2019;381(14):1333-1346. https://doi.org/10.1056/NEJMoa1813060 [ Links ]
101.Kalk E, Heekes A, Mehta U, et al. Safety and effectiveness of isoniazid preventive therapy in HIV-positive pregnant women on art: An observational study using linked population data. Clin Infect Dis. 2020. https://doi.org/10.1093/cid/ciz1224 [ Links ]
 Correspondence:
Correspondence:
Jeremy Nel
jeremynel@hotmail.com
Received: 18 June 2020
Accepted: 21 June 2020
Published: 16 Sept. 2020
ORIGINAL RESEARCH
Healthcare worker compliance with cervical cancer screening guidelines. An audit at district and regional level of care in the Pietermaritzburg Metropolitan area of KwaZulu-Natal
Mbali T. MakhuboI; Thinagrin D. NaidooII, III
IDepartment of Obstetrics and Gynaecology, Nelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
IIDepartment of Obstetrics and Gynaecology, Grey's Hospital, Pietermaritzburg, South Africa
IIINelson R. Mandela School of Medicine, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: In South Africa (SA) there are screening guidelines for cervical cancer in women living with HIV (WLWH). To our knowledge there is lack of data concerning the knowledge of health care workers (HCWs) about cervical cancer screening guidelines before the initiation of antiretroviral therapy (ART) in WLWH.
OBJECTIVES: To investigate the knowledge and familiarity of HCWs regarding cervical cancer screening guidelines in WLWH.
METHODS: A cross-sectional questionnaire-based study exploring compliance with cervical cancer screening guidelines before initiating ART was conducted with 85 HCWs in the antiretroviral (ARV) clinics of a district and regional hospitals in KwaZulu-Natal, SA. Data were analysed using Stata V13 and a p-value of ≤ 0.05 was considered statistically significant.
RESULTS: Eighty-five HCWs were included in the study. Health care workers' responses to knowledge about cervical cancer screening in WLWH were suboptimal and revealed significant gaps. Most HCWs did not know the screening intervals of WLWH. Statistically significant associations were found between an HCW's occupation and responses to the Likert scale questions.
CONCLUSION: Although the majority of HCWs were familiar with cervical cancer screening guidelines in WLWH, the study highlights that there are deficiencies in both knowledge and practice. Creating awareness among HCWs regarding the current methods of cervical cancer screening is a necessary to reduce morbidity and mortality from cervical cancer in WLWH.
Keywords: questionnaire; Likert scale; healthcare worker; cervical cancer; HIV; guidelines.
Introduction
The 'dual epidemics' of the human immunodeficiency virus (HIV) and cervical cancer have been responsible for the premature deaths of thousands of women in resource-constrained countries (RCCs).1 Screening is a vital key to the early detection and prevention of cervical cancer. Given the enormous burden of HIV and human papillomavirus (HPV) in South Africa (SA), healthcare workers' (HCWs) knowledge of cervical screening and the management of both cervical cancer and HIV is vital to ensure the survival and well-being of women living with HIV (WLWH). The risk of HPV infection is increased, as are precancerous cervical lesions (risk increased by 2-12 times), in WLWH versus HIV-uninfected women.2 Linking cervical screening to antiretroviral therapy(ART) treatment initiation in WLWH provides HCWs with a unique opportunity to diagnose and manage both viral infections. If followed properly, the screening guidelines will reduce the risk of cervical cancer in RCCs.3 Premalignant lesions are reportedly four to five times more likely in WLWH than in uninfected women.4 Furthermore, cervical cancer in South African WLWH presents earlier (up to 18 years earlier) than HIV-uninfected peers.5 Well-implemented national cervical screening guidelines and programmes significantly reduce the attributable morbidity and mortality of cervical cancer in WLWH and HIV-uninfected women.6
Cervical screening is most effective when undertaken within an organised programme. In RCCs, including SA, screening rates and subsequent follow-up care remain suboptimal. Screening rates as low as 1% have been reported.7 Screening coverage is crucial for effective prevention. The problem is compounded in RCCs by poor call-back systems, weak infrastructure and inadequately skilled staff. Indeed, it is unethical to offer screening without ensuring that follow-up and treatment services are available for all women. It is essential therefore that the HCWs with appropriate training and skills who provide these services are familiar with the screening guidelines. To our understanding, there is a lack of data on the knowledge of HCWs in this regard. This study aimed to investigate the knowledge and familiarity of HCWs with cervical cancer guidelines in WLWH before initiating ART.
Methodology
This study was a cross-sectional questionnaire-based descriptive survey of 85 HCWs in ART clinics in the Pietermaritzburg Metropolitan area of KwaZulu-Natal province, SA. The study population was stratified by profession into three groups: interns, medical officers and professional nurses. The cohort was recruited from staff members attached to the antiretroviral (ARV) clinics of the Northdale (District) and Edendale (Regional) hospitals. The study participants were randomly selected from each group. A survey cover sheet explaining the purpose of the study was attached to the questionnaire and was signed by those who completed the questionnaire. To ensure anonymity and confidentiality, personal-subject identifiers were not used in the questionnaire itself. The principal investigator enrolled only participants who willingly and voluntarily filled in and returned the questionnaire.
Data collection
A structured and pretested, self-administered questionnaire was used for data collection. The collected data were checked for completeness and consistency by the principal investigator and the supervisor. Concerning the guidelines, experts in research methodology, obstetrics and gynaecology, and oncology confirmed the validity of the questionnaire before the pilot study commenced. The instrument was pretested on 10 study participants working in other health facilities who were not part of the actual study. Findings from the pre-test were used to modify the instrument and clarify the questions. The questionnaire was divided into two parts: the first part dealt with the socio-demographic profile and professional status of the respondents and the second part dealt with knowledge about the practice of cervical cancer screening in WLWH before initiating ART. The questionnaire was written in English.
Healthcare workers' responses were assessed by asking them to rate each of the following statements on a five-point Likert scale:
•All WLWH must have a yearly pap smear.
•A pap smear must be done at the age of 21 years irrespective of HIV status.
•A pap smear in WLWH is only done after the age of 30 years.
•Cervical cancer screening in WLWH can be done anytime.
•Cervical cancer screening in WLWH can only be done after 3 years of initiating ART.
•If the results come back with high-grade squamous intraepithelial lesion (HSIL), the patient must repeat the pap smear.
•If the results come back with persistent low-grade squamous intraepithelial lesion (LSIL), keep the patient at the clinic without any further action.
•The incidence of cervical cancer in our population is very high.
Healthcare workers were asked to choose one of the following options for each of the statements listed above: 'strongly agree', 'agree', 'neither agree nor disagree', 'disagree' or 'strongly disagree'. For ease of interpreting and presenting results, responses for 'strongly agree' and 'agree' and for 'disagree' and 'strongly disagree' were combined. Participants' practices were assessed by asking specific questions about practices regarding cervical cancer screening.
Statistical analysis
A descriptive analysis of the HCW's demographic characteristics is presented. Frequencies and percentages were used to summarise categorical variables, such as the type of HCWs. Frequency distributions of numeric variables, such as age and years of experience, were examined for normality and means or medians used as appropriate. The outcome of the study was a knowledge score composed of the sum of the responses of each HCW to the questions posed and measured on the Likert scale. Subgroups were assessed by age, type of HCW and experience (years) and were compared with responses to individual questions, and an overall score was determined using the Chi-square test. Data were analysed using Stata V13 and a p-value of < 0.05 was considered statistically significant.
Ethical consideration
Ethical clearance was obtained from the Biomedical Research Ethics Committee, the University of Natal (BE: 572/18), Department of Health and the various institutions before commencing the study. All participants provided written informed consent before enrolment.
Results
Eighty-five HCWs were interviewed and enrolled in the study.
Demographic characteristics and professional standing of participants
The majority of the HCWs were 35 years old or younger (60%), women (71.8%) and black people (52.9%). Professional nurses and medical officers accounted for 41.2% and 22.1% of the HCWs, respectively. A total of 66% of the HCWs had less than 3 years' experience in the initiation of ARV drugs and only 19 (22.4%) had formal training in HIV management. A total of 51.8% of the HCWs practised at a district hospital (Northdale), whereas the other half practised at the regional hospital (Edendale) (Table 1). Of the 19 HCWs who had formal training in HIV management, 7 (36.8%) had a diploma and 12 (62.2%) had a certificate in HIV management.
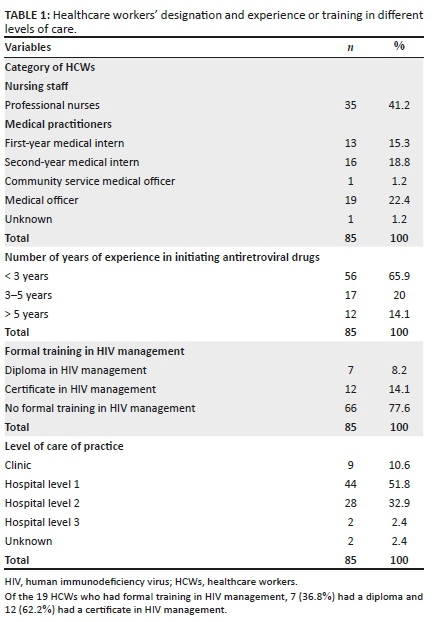
Awareness of cervical cancer screening guidelines and local protocols
Only two (2.4%) of the HCWs were aware of the KwaZulu-Natal cervical cancer screening guidelines, and 20 (23.5%) were aware of the World Health Organization (WHO) cervical cancer screening guidelines. Fifty-one (60%) of the HCWs considered that the guidelines on screening WLWH provided by their health facilities were adequate (Table 2).
Twenty-three (27.1%) HCWs were aware of the uMgungundlovu district protocol on cervical cancer screening, of which 19 were professional nurses. Fifty-one (60.0%) HCWs felt that patients who had abnormal pap smear results should be referred for colposcopy (33 professional nurses and 17 medical officers), and 76 (89.4%) participants believed that the health facilities where they were currently working were compliant with the cervical cancer screening programme for HIV-positive patients. Only two (2.4%) participants (one first-year medical intern and one medical officer) were aware of more than one set of cervical cancer screening guidelines. The KwaZulu-Natal guidelines were the least known of all screening protocols.
The knowledge and the implementation of cervical cancer screening guidelines
The knowledge of the respondents about cervical cancer screening in WLWH was assessed on a Likert scale. Healthcare workers' responses to the various statements that were designed to test their knowledge and implementation of cervical cancer screening varied (see Table 3).
Most of the HCWs were familiar with cervical cancer screening in WLWH infection in general; however, specific gaps in the knowledge were identified. Some of the identified gaps included lack of knowledge about screening intervals in WLWH, when to refer a patient with an abnormal pap smear or when to repeat a smear.
There was a statistically significant association betweenHCWs and the awareness of the uMgungundlovu District policy (only 2.1% were aware) on cervical cancer screening (p = 0.00), and the perception of oneself being compliant with the cervical cancer screening in WLWH (p = 0.00). Statistically significant associations were also found between healthcare category and certain Likert scale questions (Table 4).
Discussion
To our understanding, this is the first study to evaluate the knowledge and implementation of cervical cancer screening guidelines by HCWs in WLWH in KwaZulu-Natal province. The most crucial finding is that 70% of HCWs knew that screening in WLWH could be done at any age, including at the time of the diagnosis of HIV infection. This is important because this differs from the previous policy that all women should be screened from the age of 30 years onwards. This finding highlights an awareness that WLWH are at a higher risk and should be managed differently. The National Department of Health (NDOH) guidelines were used as the yardstick.
More than 80% of the HCWs agreed that all WLWH must have a yearly pap smear. Current South African recommendations encourage all WLWH who are at increased risk of developing cervical cancer to have yearly pap smears in high-resource settings, and 3 yearly in low-resource settings.8 Similarly, Canadian guidelines recommend annual pap tests for WLWH.9 One-third of the HCWs agreed that a pap smear should be done at 21 years of age irrespective of HIV status. In this regard, the South African guidelines report that the pap smear must be done at the age of 25 years in HIV-uninfected women whilst in WLWH smears should be done at the time of testing HIV-seropositive.8 At present, the NDOH guidelines for the cervical cancer programme offer three cervical cytology smears per lifetime at public health facilities, starting from 30 years, and then at 10-year intervals, in HIV-uninfected women. Guidelines for WLWH include more frequent cytology tests. The National Department of Health recommends screening for WLWH at HIV diagnosis and every 3 years then if screening is normal and yearly if abnormal (LSIL). The WHO advocates at least one smear test (at 30-35 years of age) to be performed in a woman's lifetime. Women Living with HIV who are < 21 years of age and are sexually active may have a high rate of progression of abnormal cytology.9 Brogly and colleagues reported that 30% of adolescents had atypical squamous cells of undetermined significance (ASC-US) or greater on their first cervical pap test.10
A small percentage of the HCWs agreed that a pap smear in WLWH is only performed after the age of 30 years, whereas the NDOH guidelines state that in WLWH pap smears should be done at the time of diagnosis of the HIV infection.8
More than 40% of the HCWs stated that if the results returned as HSIL, the patient must repeat the pap smear. And more than 17% of HCWs agreed that if the results returned as a persistent LSIL, the patient should be kept at the clinic without any further action. This is not what the guidelines state. It is recommended that for a persistent LSIL or worse (including ASC and atypical glandular cells [AGCs]) and HSIL, women with abnormal pap smears should be referred for a colposcopy assessment.11,12
Our study found that HCWs' responses to cervical screening in WLWH were suboptimal. Most of the HCWs had less than 3 years of experience in initiating ARV drugs and more than 20% had formal training in HIV management, which might have negatively impacted the results.
All the HCWs in our study were aware of the existence of different cervical cancer screening guidelines. In SA, these guidelines are published by the NDOH. Several South African provinces and hospitals have guidelines which they have adapted from the NDOH. It is concerning, however, to note that only 2.1% of KwaZulu-Natal HCWs in this study were familiar with their provincial guidelines. This article recommends that greater attention should be given to the continuing medical education of cervical cancer screening and cervical cancer prevention.
Most of our HCWs were in the early years of their careers. They were aware of both the NDOH and WHO guidelines. The former is used in all South African public sector hospitals and clinics. Although two HCWs answered that there were 'separate' KZN guidelines, this was an error.
The study did not find any statistically significant association between the HCWs' category and their knowledge of the guidelines. This demonstrates the need, regardless of category, for courses in the workplace that target these concerns. On the other hand, significant differences were revealed between HCWs and their awareness of district guidelines or policy, the screening intervals of WLWH and when to refer for colposcopy. Although the majority of participants indicated that their facilities were compliant with the guidelines, the gaps that have been uncovered indicate the need for ongoing education in this regard.
Limitations
The main limitations of the study are as follows:
•The small number of HCWs: results should be interpreted with caution.
•The use of a questionnaire may not reflect the actual workplace competency of the participants.
•The answers reflect a local or specific context and therefore generalisation of the study results may be limited.
•An operational bias exists in the mixing of different groups of HCWs with different levels of professional experience. Nonetheless and surprisingly, the gaps were widespread and did not link with individual categories of HCWs.
Conclusion
Healthcare workers were knowledgeable about cervical cancer screening in WLWH but displayed gaps in practice and knowledge. Most of them were well informed about the recommended screening intervals. However, a certain number of HCWs did not know when to refer a patient for further management. A delay in the management of precancerous lesions could lead to morbidity and mortality, particularly in WLWH. Healthcare workers require guidelines customised to local contexts, in particular for the care of WLWH. Recommendations emerging from this study include regular workplace refresher training that highlights the guidelines and related institutional protocols and renewed attention to the monitoring and evaluation of institutional compliance with cervical cancer screening and prevention.
Acknowledgements
We would like to thank both facilities and staff members who participated in our study.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
Both authors contributed equally o this work.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data presented in this article can be available upon reasonable request to the corresponding author, provided that the source of data is acknowledged.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.FIGO. International Federation of Gynaecology and Obstetrics Cervical cancer and HIV [homepage on the Internet]. c2018 [cited 2020 May]. Available from: https://www.figo.org/news/cervical-cancer-and-hiv [ Links ]
2.Clifford GM, Polesel J, Rickenbach M. Cancer risk in the Swiss HIV cohort study: Associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97(6):425-432. https://doi.org/10.1093/jnci/dji072 [ Links ]
3.Atashili J, Smith JS, Adimora AA, Eron J, Miller WC, Myers E. Potential impact of antiretroviral therapy and screening on cervical cancer mortality in HIV-positive women in sub-Saharan Africa: A simulation. PLoS One. 2011;6(4):e18527. [ Links ]
4.Kafuruki L, Rambau PF, Massinde A, Masalu N. Prevalence and predictors of cervical intraepithelial neoplasia among HIV infected women at Bugando Medical Centre, Mwanza-Tanzania. Infect Agent Cancer. 2013;8(1):45. https://doi.org/10.1186/1750-9378-8-45 [ Links ]
5.Van Bogaert LJ. Age at diagnosis of preinvasive and invasive cervical neoplasia in South Africa HIV-positive versus HIV-negative women. Int J Gynecol Cancer. 2011;21(2):363-366. https://doi.org/10.1097/IGC.0b013e3182094d78 [ Links ]
6.Kamal EM, El Sayed GA, El Behery MM, El Shennawy GA. HPV detection in a self-collected vaginal swab with VIA for cervical cancer screening with correlation to histologically confirmed CIN. Arch Gynecol Obstet. 2014;290(6):1207-1213. https://doi.org/10.1007/s00404-014-3321-6 [ Links ]
7.Van Schalkwyk SL, Maree JE, Wright SCD. Cervical cancer: The route from signs and symptoms to treatment in South Africa. Reprod Health Matters. 2008;16(32):9-17. https://doi.org/10.1016/S0968-8080(08)32399-4 [ Links ]
8.Botha MH, Dreyer G. Guidelines for cervical cancer screening in South Africa. S Afr J Gynaecol Oncol. 2017;9(1):8-12. [ Links ]
9.De Pokomandy A, Burchell AN, Salters K, et al. Cervical cancer screening among women living with HIV: A cross-sectional study using the baseline questionnaire data from the Canadian HIV women's sexual and reproductive health cohort study (CHIWOS). CMAJ Open. 2019;7(2):E217-E226. https://doi.org/10.9778/cmajo.20180151 [ Links ]
10.AIDS Info. Guidelines for the prevention and treatment of opportunistic in adults and adolescents with HIV [homepage on the Internet]. No date [cited 2020 February 01]. Available from: https://aidsinfo.nih.gov/guidelines [ Links ]
11.Brogly SB, Watts DH, Ylitalo N, et al. Reproductive health of adolescent girls perinatally infected with HIV. Am J Public Health. 2007;97(6):1047-1052. [ Links ]
12.Moodley J, Constant D, Botha MH, Van der Merwe FH, Edwards A, Momberg M. Exploring the feasibility of using mobile phones to improve the management of clients with cervical cancer precursor lesions. BMC Womens' Health 2019;19(1):2. https://doi.org/10.1186/s12905-018-0702-1 [ Links ]
 Correspondence:
Correspondence:
Mbali Makhubo
makhubo26@gmail.com
Received: 11 May 2020
Accepted: 26 June 2020
Published: 02 Sept. 2020
ORIGINAL RESEARCH
Effects of HIV and non-communicable disease comorbidity on healthcare costs and health experiences in people living with HIV in Zimbabwe
Laston Gonah; Indres Moodley; Khumbulani Hlongwana
Health Outcomes Research Unit, Discipline of Public Health Medicine, School of Nursing and Public Health, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: The effects of HIV and non-communicable disease (NCD) comorbidities on healthcare costs and health experiences have been documented in most high-income countries. However, little similar data are available for Zimbabwe and most countries in sub-Saharan Africa. Untreated or under-treated NCDs can potentially negate the gains achieved from the control of HIV.
OBJECTIVES: The study sought to determine the effects of HIV-NCD comorbidity on healthcare costs, health experiences and treatment options for people living with HIV (PLWH) in Zimbabwe.
METHODS: A repeated-measures, quantitative study was conducted at six antiretroviral therapy (ART) sites in the Gweru District of Zimbabwe. Simple random sampling was used to enrol 100 PLWH concurrently diagnosed with hypertension and/or diabetes mellitus (cases). Cases were matched by age, sex and viral load to an equal number of PLWH without hypertension and/or diabetes mellitus (controls). Quantitative data were collected using an interviewer-administered questionnaire at monthly intervals for 6 months. The questionnaire survey sought to compare healthcare costs, health-related experiences and treatment options between cases and controls. Data were analysed using Stata Version 13.1®. A logistic model was used to examine other factors such as demographic, clinical and behavioural data that were assumed to be unchanged over the study period. A random-effects model, including costs and other covariates, was used to compare groups in the final analysis.
RESULTS: Non-communicable disease status was associated with the length of time on ART. Cases spent significantly more on transport (p = 0.0001) and medication (adjusted odds ratio [AOR] = 4.4, 95% confidence interval [CI]: 3.2-7.3); spent more days without doing usual daily activities because of sickness (AOR = 4.2, 95% CI: 3.3-7.6) and were more likely to use alternative medication (AOR = 3.4, 95% CI: 2.3-4.6) when compared with controls. Unemployment, female gender, age of 60 years and above, and living in rural areas were associated with failure to purchase prescribed medication.
CONCLUSIONS: HIV-NCD comorbidity causes an additional burden to PLWH because of increased transport costs, NCD prescribed medication expenses and more productive days lost due to illness. The success of HIV programmes does not only rely on improving access to the diagnosis and treatment of HIV. Addressing the complications of HIV-related NCDs, and the long-term costs of ART and its occasional potential for harm will be essential if health outcomes in Zimbabweans living with HIV are to be optimised.
Keywords: human immunodeficiency virus; non-communicable disease; Zimbabwe; antiretroviral therapy; unemployment; diagnosis and treatment of HIV; ART.
Introduction
Unprecedented donor and government funding to address the HIV and AIDS pandemic resulted in more than 20 million people receiving antiretroviral therapy (ART) by mid-2017, against a total of 36.7 million people living with HIV (PLWH) worldwide.1 Whilst ART has markedly increased survival, PLWH have been found to be at greater risk of developing non-communicable diseases (NCDs).2
People living with HIV have a threefold increased risk of developing NCDs because of three main reasons: (1) inflammatory and infectious sequelae of HIV infection, (2) the effects of ART treatment itself and (3) finally, the increased risk associated with ageing.3 Successful ART roll-out has resulted in most PLWH living longer, possibly driving the onset of NCDs, because of long-term viral infection, accumulation of drug toxins in the body and acceleration of age-related degenerative changes by HIV.4,5,6 According to the World Health Organization,7 NCDs may be present before HIV infection but could be worsened by HIV or the side effects of some of the antiretroviral drugs.
HIV-NCD comorbidity could have implications for healthcare costs, health experiences and survival compared with HIV alone. Although the effects of HIV-NCD comorbidity on healthcare costs, health experiences and treatment options have previously been documented, especially in high-income countries, this is not true for most sub-Saharan African countries, including Zimbabwe.2 Conducting such studies provides valuable information so that targeted intervention strategies can be developed. Studies on HIV-NCD comorbidity are limited to a few cross-sectional surveys that are mainly based on self-reported data without a comparison group.2,8
HIV-NCD comorbidity causes an additional healthcare burden. Whilst significant efforts have been made towards HIV control in Zimbabwe, untreated or under-treated NCDs can potentially negate the gains achieved through the national ART roll-out. Currently, no integrated care for HIV-NCD comorbidity is available in Zimbabwe. Despite accessing free services for HIV and AIDS, PLWH have to pay for the treatment of NCDs, mostly through out-of-pocket expenditure, which because of the financial burden, may prevent many patients from seeking care for NCDs.
The study sought to determine the effects of HIV-NCD comorbidity, using hypertension (HTN) and diabetes mellitus (DM) as NCD-tracer conditions, on healthcare costs, health experiences and treatment options in PLWH in the Gweru district of Zimbabwe.
Materials and methods
Study design
A repeated-measures, longitudinal quantitative study design was employed.
Study setting
The study was conducted at six high-volume government ART sites that had the highest number of PLWH and collectively representing over 80% of all PLWH on ART in Gweru district. The six study sites consisted of four urban and two rural sites. The four urban sites are directly administered by Gweru City Council Health Department, whilst the two rural sites are administered by the Ministry of Health and Child Care.
Study population and participant selection procedures
In this study, HIV patients of either gender, aged ≥18 years, registered for ART programme in the electronic Patient Monitoring System (ePMS) and able to respond to the study questionnaire in Shona, Ndebele or the English language, were considered.
In addition to the age and gender inclusion criteria above, the cases had to be PLWH and concurrently diagnosed with DM and/or HTN. All subjects provided written informed consent to participate in the study. Those with a confirmed diagnosis other than the NCD-tracer conditions of HTN and DM, as required for the study, were excluded. Mentally impaired PLWH and those not on ART were also excluded from the study.
As the ePMS and the Chronic Disease Register are maintained separately, the identification and linkage of PLWH with the tracer NCDs were time-consuming and laborious. A total of 2969 PLWH and comorbid NCDs were identified during a 3-month screening or verification exercise among patients registered for the ART programme. The 3-month period (December 2018 to February 2019) was ideal because all patients were expected to have visited the ART sites for their monthly ART supply. After applying the eligibility criteria, 842 eligible participants from the six sites were identified. Proportional representation, as guided by the total number of eligible participants per site, was followed when selecting participants from rural and urban regions, and from among men and women, within all six sites. Simple random sampling was then performed to obtain a representative sample per site, coming up with a total of the required sample size.
Sample size and sampling criteria
A sample size of 186 (93 per group) was required to detect a characteristic difference of ± 20% between the two groups with a probability of 95% and power of 80%, assuming 50% in the control group. A 10% assumption was made for missing data and lost to follow-up. As such, a final sample size of 208 (104 per group) was needed to ensure sufficient numbers for analysis.
Two hundred of those providing informed consent, namely 100 pairs, instead of the initially calculated 208 participants or 104 pairs, were enrolled in the study and followed up for 6 months. (It is anticipated that the use of 100 pairs instead of the initially calculated 104 would not significantly affect the study power because 100 per group is still larger than the originally calculated sample size of 93 pairs needed to achieve the given power at the desired standard error.)
The 100 cases were purposively matched by viral load (± 5 copies per milliliter of blood [copies/ml]), age (± 1 years), gender (male, female), distance to ART site (± 2 km) and geographical location (rural, urban) in the ratio of 1 case : 1 control to get a total of 200 study participants.
Data collection methods
Quantitative data on participants' demographic profile, disease-related factors, healthcare costs and health experiences were collected using an interviewer-administered questionnaire. Health experiences were measured as the number of days spent by participants without carrying out usual daily activities because of ill health. The questionnaire intended for use was pretested among 10 consenting individuals and adjusted according to pretest findings before final use. Time to complete a questionnaire survey ranged from 15 minutes to 20 minutes per participant. Cases and controls were followed up for 6 months, and data were collected at 1-month intervals. The 6-month follow-up period allowed for repeated measurements, thereby providing sufficient data 'points' to determine disease burden, healthcare costs and health experiences. To ensure that participants kept an accurate record of their healthcare costs during the study, participants were requested to keep all receipts of their medical expenses and note down expenditure events within the month. Medications for conditions under study required once-off monthly refills, thereby reducing chances of recall bias.
Data analysis methods
Data were analysed using Stata Version 13.1®. Descriptive statistics were used to summarise the data for each analysis group. Frequencies and percentages were used for categorical data. Frequencies of numeric data were examined for normality, and means, standard deviation or medians were used as appropriate. A logistic model was used to examine other factors such as demographics, clinical data and behavioural data of the two groups that had been assumed to be unchanged over the study period. A random-effects mixed model including costs and other covariates was used to compare the groups in the final analysis.
Ethical consideration
Ethics approval for the study was obtained from the Biomedical Research Ethics Committee (University of KwaZulu-Natal) - Ethical Clearance Number: BE086/19, and Ministry of Health and Child Care (from both the District Office in Gweru and the Head Office in Harare). Written informed consent was sought from participants and confidentiality was maintained throughout the study by removal of personal identifiers after entry into the electronic database, and the use of non-identifiable coded numbers. Furthermore, all data were password protected in the electronic subject-storage database.
Results
Demographics of study participants
Equal numbers of cases and controls (100 participants each) were enrolled into each arm. The age of the participants ranged from 33 to 80 years, with a mean age of 57 (SD ± 10.79) years. The proportion of female participants was greater than that of male participants (Table 1). The majority were unemployed at the time of the study, with no significant difference in financial status between cases and controls. Mean monthly income per participant was $17.56 (SD ± 28.37), and grants received from relatives had a mean of $6.66 (SD ± 24.26). Thirty-eight per cent of participants had viral loads of 0 copies/mL (undetectable viral load), whilst 34% had viral loads of below 20 copies/mL (Table 1). Average duration on ART at baseline for cases was 10.22 years (SD ± 3.165) and was 4.32 years (SD ± 2.12) among controls.

Over 98% of study participants were followed for the entire 6 months of the study with n = 3 deaths among the cases, and no loss to follow-up. In the analysis, the mixed-effects model was employed to deal with deaths, and all observations that contributed to the results were measured up to the point of the participant's death. Table 1 shows demographic and other key characteristics of study participants.
The non-communicable disease study group, data and outcomes
Table 2 shows that the proportion of cases with HTN alone was greater than that with DM alone, both at baseline and at the end of the study. None of the controls had a diagnosis of any NCD at baseline. The presence of 'new' NCDs in cases and controls at the 6-month follow-up census is shown in Table 2. Three (n = 3) deaths (HIV or comorbid disease related) were recorded among cases during the study period.
During the follow-up period, three new diagnoses of DM were recorded in the case group, whereas four new diagnoses of DM were recorded in the control group. Again, four new cases of HTN were diagnosed in the case group, and four new cases in the control arm. Other new diagnostic conditions recorded during the study were peptic (gastrointestinal tract) ulcers (four among cases and two among controls), asthma (two among cases and none among controls), arthritis (three among cases and one among controls) and cataract (one among cases and none among controls).
The average monthly number of productive days lost because of sickness was compared between cases and controls. On average, cases spent 11 more days unable to do usual daily activities because of self-reported illness when compared with controls (Figure 1).
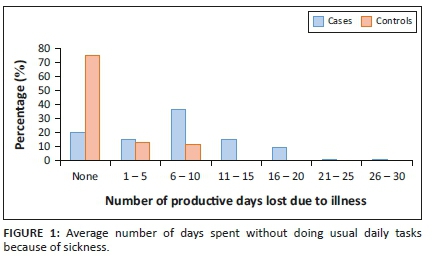
Out-of-pocket expenses
Average monthly amount of money spent on transport by participants for travelling to access healthcare or medication was compared between cases and controls. In general, 52% more cases spent money on transport compared with controls (Figure 2).
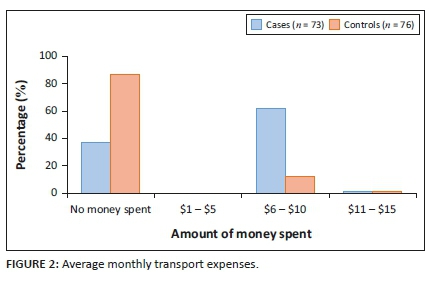
The participants' ability to purchase DM and antihypertensive medications was assessed. More than 68% reported an inability to purchase prescribed medication for their conditions during the study period (Table 3).

The average monthly amount of money spent on prescription medication for HTN and DM was assessed for the study period in both the cases and the controls (Table 4). A significant number of cases did not have money to purchase their prescribed medication. Only 14.8% reported consistently getting antihypertensive medication for free at their local health centre during the study period. None of the cases and controls reported receiving free diabetic medication from a health facility during the study period.
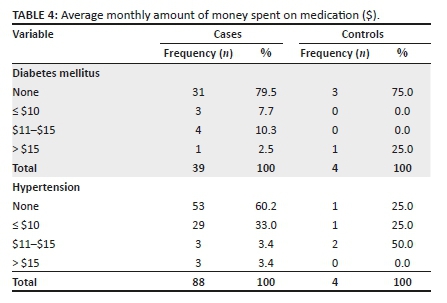
Concerning the use of traditional medicines, more cases (83.4%) reported using traditional medicines for the treatment and management of ill health compared to the control group (5.3%). Similarly, the majority (> 73.0%) reported using traditional medication for the management of DM and/or HTN in the case group, than in the control group who developed HTN or DM (see Table 5).
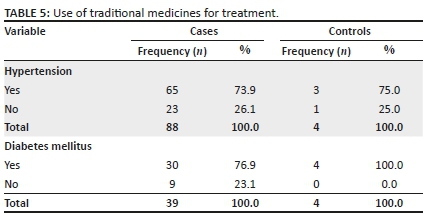
Usual diet using the 24-hour food recall method
Participants were asked to name the type of food they had consumed in the previous 24 hours prior to the questionnaire survey, and subsequently for all the six (6-Xs) monthly visits. The usual diet for more than 80% of both cases and controls was carbohydrate staples, such as rice and maize meal (sadza), which often included green leafy vegetables. Less commonly consumed foods, consumed by less than 30% of the participants, were meat (all types), fruits, cereals, milk and milk products and legumes (Figure 3).
Analytical statistics of demographic and personal characteristics according to non-communicable diseases status and type, days spent without usual daily tasks and the ability to purchase non-communicable disease medication
Our analysis compared healthcare costs and the health-related experiences of cases versus controls. The parameters that were assessed included the NCD diagnosis, the number of days spent without doing usual daily tasks or activities and the ability to purchase prescribed medication. Covariates included demographic variables such as age, sex, employment status, marital status, health insurance status, use of alternative medication, transport cost, viral load, duration on ART, distance from the ART centre, smoking and alcohol consumption.
Non-communicable disease status in relation to the use of alternative medication, transport costs and hospitalisation
Compared with the control group, the presence of target NCDs in the case group was associated with a longer duration on ART, that is, > 5 years, after controlling for other factors (p = 0.0023). As cases were matched to controls by age, sex, employment status, viral load and area of residence, these covariates were not associated with NCD status between the two groups. However, subgroup analysis of the case group indicated that two or more NCDs were more common in female cases compared with male cases (p = 0.0021).
Use of alternative or traditional medicines was compared between cases and controls. Cases were significantly more associated with use of alternative or traditional medication compared with controls (adjusted odds ratio [AOR] = 3.4, 95% confidence interval [CI]: 2.3-4.6, p = 0.0001).
Having an NCD was significantly associated with higher monthly transport costs (p = 0.0001). Rural cases in particular were associated with higher transport costs compared with urban cases, urban controls and rural controls (p = 0.0041). The average distance to the ART centre was 15 km for rural cases as compared with 7 km for urban cases. The usual source of NCD medication (HTN and DM) for the majority of cases (over 85%) was private pharmacies, except for only 14.8% of hypertensive patients whose usual source was other public health facilities.
Cases, whether diagnosed with DM, HTN or both, were more likely to be hospitalised compared with controls (AOR = 2.4, 95% CI: 1.2-3.3). The average number of hospitalisation days in cases was 5 days compared with 1 day in controls.
Days spent without usual daily tasks or activities because of illness
To assess the effect of NCDs on productivity, average monthly number of days spent without doing usual daily activities because of illness was compared between cases and controls. Cases were more likely to spend more days without doing usual daily activities compared with controls (AOR = 4.2, 95% CI: 3.3-7.6, p = 0.0000).
Days spent without doing usual daily tasks or activities were associated with female gender, inability to purchase medication and unemployment status. Women spent more days without usual daily activities when compared with men in both cases and controls (p = 0.0031). Again, the inability to purchase prescribed medication was associated with more days spent without doing usual daily activities because of illness in both cases and controls (p = 0.0023). Unemployed participants spent more days without doing 'usual' daily activities as compared with formally employed and informally employed participants (p = 0.001). Subgroup analysis of cases showed that cases with more than two NCDs spent more days without doing usual daily activities (AOR = 2.1, 95% CI: 1.3-4.4).
Ability to purchase non-communicable disease medication
Not all patients could afford their prescribed medication. Therefore, simply estimating costs on the basis of monthly prescriptions would not have represented the actual situation on the ground. Participants - cases and controls - were asked to indicate monthly medication expenses and to record for the duration of the study, which medicines had not been purchased each month. This inability to purchase prescribed medication was used in this study as an adjunctive measure of the effect of NCDs on healthcare costs in PLWH.
Cases were less likely than controls to be able to purchase medication for ill health. Inability to purchase medication was associated with having an NCD (AOR = 4.4, 95% CI: 3.2-7.3). Among the cases, the inability to purchase NCD medication was associated with the number of NCDs per patient, sex, age and employment status. Cases with two or more NCDs were more unlikely to purchase medication compared with those with one NCD (p = 0.000). Female participants were less likely than male cases to afford monthly medication requirements (p = 0.0011). Participants living in rural areas and those in the age categories 60-69 years and ≥70 years were similarly less likely to afford medications, than urban participants and those aged <60 years (p = 0.0031, 0.0001 and 0.0000, respectively). Formal employment was significantly associated with the ability to purchase medication, after adjusting for potential confounders (p = 0.000). Estimated average monthly expense on medication among cases was $12 compared with $1 among controls, considering only those who were able to purchase all the prescribed medication.
Assessment of usual diet
There was no significant difference in usual diet between cases and controls, using the 24-hour food recall method. The usual diet for both cases and controls was carbohydrate staples and green leafy vegetables, with less protein sources of food. Employment status was significantly associated with consuming a balanced diet, rich in all the required nutrients, in both cases and controls (p = 0.0003). Consumption of a balanced diet was not associated with other demographic characteristics and NCD status.
Discussion
The study compared healthcare costs, health experiences and care-related outcomes in PLWH diagnosed with HTN and/or DM with a matched control group of PLWH without NCDs. This study observed numerous important findings of relevance to PLWH in Africa (Zimbabwe) who are on ART and virally suppressed:
•The concurrent presence of comorbid disease is a function of time on ART.
•HIV-NCD comorbidity undermines the goals of HIV treatment, which is to control the virus (HIV) and promote wellness: (1) expenses increase, for example, medication and travel costs; (2) impaired management of comorbid conditions, where over 68% of the case group were unable to afford DM or HTN medication, which were not supplied free-of-charge; (3) increasing vulnerability to non-evidence-based health options and (4) HIV-NCD comorbidity results in greater risk of morbidity and mortality.
•Women bear the greater burden of comorbid disease, and experience greater 'disability' - unable to do usual daily tasks.
•Rural citizens and the elderly appear to experience a greater negative impact of economic hardship from comorbid conditions.
In general, controls, namely, virally suppressed PLWH without HTN, DM or both, were associated with lower average monthly expenses on prescription medication and spent fewer number of days without carrying out usual daily activities or tasks because of illness, compared with virally suppressed PLWH with HTN and/or DM. In Zimbabwe, most PLWH access ART free-of-charge in public health facilities.9 However, medication for the NCDs is usually not available free-of-charge. These patients have to access medication largely through out-of-pocket expenses. Ability to pay for NCD medication, therefore, becomes a major determinant for access to medication, which results in poor management of the NCDs and the generally observed higher number of productive days lost because of illness. This has potential to impact negatively on the gains achieved so far in controlling HIV through ART.
Unemployment, gender, age and distance to a healthcare facility are well-known key determinants of health.10 In this study, significant associations were found between these key determinants of health and the ability to purchase prescribed medication, as well as days spent without carrying out usual daily activities because of illness. This finding can be explained in part by the fact that the majority of employed participants in the study were men, below the age of 60 years. Given that antihypertensives and diabetic medications were largely accessible through out-of-pocket payment, employment enables those with NCDs to purchase the prescribed medications and take control of their health. In patriarchal societies, such as Zimbabwe, opportunities for income and control of resources are biased towards the male gender compared with females.11 The female gender and unemployment are inextricably linked together in such societies, predisposing unemployed female participants to negative health outcome, as observed in this study.
On the other hand, the lower proportion of male participants and the fewer number of days these male participants spent without undertaking usual daily activities because of illness, compared with female participants, needs further inquiry. Observed findings could have been influenced in part by poor health seeking behaviours, which are commonly associated with the male gender.12 Because of their masculinity, characteristic of men, participants might deliberately under-report the number of days they spent without carrying out usual daily activities. Perhaps, the perception of serious ill health in men might be different from that in women.12,13,14
In general, cases were found to spend more days without undertaking usual daily activities because of illness compared with controls. A study conducted in Namibia found similar results, where sickness because of HTN and DM was the top cause of absenteeism among workers in the formal sector.15 Cases aged 60-69 years and ≥ 70 years were more likely to spend more days without carrying out usual daily activities or tasks because of illness. They were more at risk of not being able to purchase NCD medication, compared with other age categories, and compared with similar age categories in controls, after controlling for potential confounders.
Productivity days lost because of illness are linked with lower income or loss of employment or limited opportunities for work, which translates to lack of income to purchase the needed medication. A study conducted in USA16 found out-of-pocket medication costs to be a significant challenge faced by older adults with DM, often forcing them to cut back on medication use, forgoing food or other basic necessities or borrowing money. The age group ≥60 years is usually associated with retirement and diminished ability and opportunities for employment.17 When medication for NCDs has to be purchased through out-of-pocket payments, this age group is at risk because of inability to afford medication and consequently face a greater risk for the observed poorer health outcomes.
Having two or more NCDs was markedly associated with more days spent without undertaking usual daily activities and more money spent on medication, compared with cases with one NCD. Having more than one NCD naturally translates to more money needed to purchase medication, thus increasing the possibility of inability to purchase the required medication, especially given the fact that the majority of the participants were either unemployed or had an unstable source of income. Without access to prescribed medication to manage their health, patients would be predisposed to poorer health outcomes as indicated by productive days lost because of illness observed in this study. Patients were more likely to opt for alternative medication from informal sources to manage their health in the face of limited or no income to purchase prescribed medication.
Use of alternative medication was commonly reported among cases as compared with controls. However, use of alternative medication was not associated with positive improvements on days spent without undertaking daily activities because of sickness. Use of alternative medication may have been influenced to some extent by the cases' inability to purchase NCD medication, forcing patients to resort to cheaper alternatives to manage ill health. This could be assumed, given that the controls did not report use of alternative medication for management of HIV other than ART. Common alternative medication included home remedies and traditional herbs that are readily available at lower costs. Toxicity, maximum safe dosage and clinical efficacy for these treatment options are usually not known, thus potentially predisposing the users to other unknown negative health consequences.18 Future studies should assess the various types and sources of alternative medications commonly used by this patient population to gain a better understanding about this issue.
Higher out-of-pocket medication and transport expenses faced by cases can push patients to employ various coping mechanisms, including cutting back on medication use to adopt traditional medication or forgoing other basic necessities including food.16 Finally, we need further enquiry to better understand challenges experienced by people with HIV-NCD comorbidity (especially the difficulties faced in accessing medication and care) and the coping mechanisms that they employ in dealing with these challenges.
Study limitations
•Measurement of the outcome variable, 'number of days spent by participants without doing usual daily activities or tasks', was based on self-reported responses by participants and not objectively ascertained. Although effort was put in corroborating individual responses with further follow-up questions, there is a possibility that variations in personal characteristics could have affected how participants responded to ill health (to do or not to do usual daily tasks). Again, recall bias cannot be ruled out in the measurement of this variable. However, we believe that self-reported episodes of serious sickness within a month are less likely to vary significantly from the actual episodes.
•Adherence to ART use in this study was not assessed, but rather assumed, based on satisfactory HIV viral load measurements of participants. There is a possibility that the observed health experiences measured in this study might not have been sorely because of inability to purchase NCD medication alone, but because of interaction with non-compliance to NCD prescribed medication or use of traditional medication for NCDs that was common among cases in this study.
•Survival was not assessed in this study to compare survival rates between cases and controls. Whilst the 6-month follow-up was adequate to assess trends in health expenses and health experiences, it was not long enough for survival analysis to be performed. Survival analysis would have required longer a follow-up period of 5-15 years, which would not have been feasible for this study.
•Representativeness of our research data to other African countries with stable economies might be limited, in part, because of the economic crisis faced by Zimbabwe, which makes it unique in sub-Saharan Africa.
Conclusion
HIV-NCD comorbidity causes an additional burden to PLWH because of increased transport costs, NCD prescribed medication expenses and more productive days lost because of illness. Access to antihypertensives and diabetic medications is influenced by interplay of the key determinants of health, which include income, gender, age and geographical location. The success of HIV programmes does not only rely on improving access to the diagnosis and treatment of HIV. Addressing the complications of HIV-related NCDs and the long-term costs of ART and its occasional potential for harm will be essential if health outcomes in Zimbabweans living with HIV are to be optimised.
Acknowledgements
The authors acknowledge the support received from the University of KwaZulu-Natal in conducting the study.
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
All authors contributed equally to this work.
Funding information
The study was funded by University of KwaZulu-Natal, under PhD research grants.
Data availability statement
Due to the private nature of the data, data for the study will be available only upon request and approval of Authorising Ministry of Health.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global report: UNAIDS report on the global AIDS epidemic 2010. Geneva: UNAIDS; 2010. [ Links ]
2.Harris TG, Rabkin M, El-Sadr WM. Achieving the fourth 90: Healthy aging for people living with HIV. AIDS. 2018;32(12):1563. https://doi.org/10.1097/QAD.0000000000001870 [ Links ]
3.Narayan KV, Miotti PG, Anand NP, et al. HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: A vital agenda for research in low-and middle-income country settings. AIDS. 2014;67:S2-S7. https://doi.org/10.1097/QAI.0000000000000267 [ Links ]
4.Negin J, Mills EJ, Bärnighausen T. Aging with HIV in Africa: The challenges of living longer. AIDS. 2012;26(01):S1. https://doi.org/10.1097/QAD.0b013e3283560f54 [ Links ]
5.Nugent R, Barnabas RV, Golovaty I, et al. Costs and cost-effectiveness of HIV/NCD integration in Africa: From theory to practice. AIDS. 2018;32(Suppl 1):S83. https://doi.org/10.1097/QAD.0000000000001884 [ Links ]
6.Shade SB, Osmand T, Luo A, et al. Cost of integrating non-communicable disease (NCD) care into Ugandan HIV/medical clinics in the SEARCH study. 9th International AIDS Society (IAS) Conference 23-26 July 2017; Paris, France: IAS. 2017 [ Links ]
7.WHO. Working group on the inclusion of NCDs in other programmatic areas: World Health Organization global coordination mechanism on the prevention and control of noncommunicable diseases. Working group 3.1 (2016-2017). Geneva: World Health Organization; 2018. [ Links ]
8.Haregu TN, Oldenburg B, Sestwe G, Elliott J, Nanayakkara V. Epidemiology of comorbidity of HIV/AIDS and non-communicable diseases in developing countries: A systematic review. J Glob Health Care Syst. 2012;2(1):1-2. [ Links ]
9.Zimbabwe. The National Health Strategy for Zimbabwe, 2009-2013: Equity and quality in health: A people's right. Harare: Ministry of Health & Child Welfare; 2009. [ Links ]
10.WHO. Closing the gap in a generation: Health equity through action on the social determinants of health: WHO commission on social determinants of health final report. Geneva: World Health Organization; 2008. [ Links ]
11.Türmen T. Gender and HIV/AIDS. Int J Gynaecol Obstet. 2003;82(3):411-418. https://doi.org/10.1016/S0020-7292(03)00202-9 [ Links ]
12.Peacock D, Stemple L, Sawires S, Coates TJ. Men, HIV/AIDS, and human rights. J Acquir Immune Defic Syndr. 2009;51(Suppl 3):S119-S125. https://doi.org/10.1097/QAI.0b013e3181aafd8a [ Links ]
13.Camlin CS, Ssemmondo E, Chamie G, et al. Men 'missing' from population-based HIV testing: Insights from qualitative research. AIDS Care. 2016;28(Suppl 3):67-73. https://doi.org/10.1080/09540121.2016.1164806 [ Links ]
14.Dworkin SL. Who is epidemiologically fathomable in the HIV/AIDS epidemic?. Gender, sexuality, and intersectionality in public health. Cult Health Sex. 2005;7(6):615-623. https://doi.org/10.1080/13691050500100385 [ Links ]
15.Guariguata L, de Beer I, Hough R, et al. Diabetes, HIV and other health determinants associated with absenteeism among formal sector workers in Namibia. BMC Public Health. 2012;12(1):44. https://doi.org/10.1186/1471-2458-12-44 [ Links ]
16.Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care. 2004;27(2):384-391. https://doi.org/10.2337/diacare.27.2.384 [ Links ]
17.Maciel RA, Klück HM, Durand M, Sprinz E. Comorbidity is more common and occurs earlier in persons living with HIV than in HIV-uninfected matched controls, aged 50 years and older: A cross-sectional study. Int J Infect Dis. 2018;70:30-35. https://doi.org/10.1016/j.ijid.2018.02.009 [ Links ]
18.Peltzer K, Pengpid S. Utilization and practice of traditional/complementary/alternative medicine (T/CAM) in Southeast Asian nations (ASEAN) member states. Stud Ethno-Med. 2015;9(2):209-218. https://doi.org/10.1080/09735070.2015.11905437 [ Links ]
 Correspondence:
Correspondence:
Laston Gonah
lggonah@gmail.com
Received: 04 May 2020
Accepted: 23 June 2020
Published: 04 Sept. 2020
ORIGINAL RESEARCH
Capacity of antiretroviral therapy sites for managing NCDs in people living with HIV in Zimbabwe
Laston Gonah; Indres Moodley; Khumbulani Hlongwana
Health Outcomes Research Unit, Discipline of Public Health Medicine, School of Nursing and Public Health, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND: There are marked inconsistencies in prevalence data for human immunodeficiency virus and non-communicable disease (HIV-NCD) comorbidity in Zimbabwe.
OBJECTIVES: To explain these discrepancies, we investigated the capacity of antiretroviral therapy (ART) sites in managing hypertension (HTN) and diabetes mellitus (DM) in people living with HIV (PLWH) in Gweru district, Zimbabwe.
METHOD: This was a qualitative research design in which key informant interviews were conducted with eight health managers, and 12 focus group discussions (FGDs) were conducted with 72 PLWH concurrently diagnosed with HTN and/or DM. Thematic data analysis was performed in NVivo version 12®.
RESULTS: Routine screening for HTN and targeted screening for DM were often interrupted by dysfunctional machines and intermittent supply of necessary consumables, impacting negatively on the capacity of the sites to monitor and screen for the NCDs. Erratic hypertensive and diabetic medication availability at study sites were also reported, forcing patients to turn to other treatment options (medication rationing or overdose or sharing, use of home remedies and traditional medicines, and reliance on faith and traditional healers.
CONCLUSION: Findings demonstrate that the quality of observed incidence and prevalence data for HTN and DM in LMICs is a function of the capacity of health centres to screen for NCDs. Given the ageing population of PLWH in sub-Saharan Africa, coupled with increasing trends in the prevalence of NCDs in HIV-infected people, HIV programmes have not evolved with the changing needs of PLWH. Attention to the holistic management of PLWH is long overdue.
Keywords: hypertension; diabetes mellitus; HIV-NCD comorbidity; NCD management protocols; NCD screening and treatment.
Introduction
Unprecedented global improvement in enabling access to antiretroviral therapy (ART) by people living with human immunodeficiency virus (PLWH), contributing to increased viral suppression and increased survival in PLWH, is well documented.1 By the end of 2018, more than 61% of the 37.9 million PLWH, globally, were accessing ART, which is a substantial increase from 45% in 2010.1 Over the same period, acquired immunodeficiency syndrome (AIDS)-related deaths decreased by about 45%,1 thereby demonstrating the significant contribution of ART to increased survival in PLWH.
While there is empirical evidence showing the positive contribution of ART to improved survival in PLWH, new threats associated with non-communicable diseases (NCDs) co-existing with human immunodeficiency virus (HIV) in PLWH have emerged.2 This co-existence is likely to impact negatively on the gains already achieved in controlling HIV, especially in many low- to middle-income countries (LMICs) where the majority of PLWH on ART live and where health care systems are known to be fragile.2 The real effects of NCDs co-existence with HIV in PLWH in many settings may be undermined by the lack of accurate data to understand this phenomenon fully. For example, there are marked inconsistencies in the Zimbabwean prevalence data for HIV-NCD comorbidity.3,4,5 These epidemiological discrepancies can be best explained by studying the capacity of ART sites to manage NCDs in PLWH, that is, the screening, diagnosis, treatment and data management. Limited capacity to screen and diagnose diseases or conditions may compromise the quality of the prevalence data generated.
In Zimbabwe, more robust and consistent evidence exists for the management of tuberculosis (TB) - a well-known leading cause of death in PLWH.6,7 Contrary to the worldwide situation with TB, which is well integrated into HIV care allowing active TB case finding and better case management, there is limited evidence on how HIV-NCD comorbidity is being managed in Zimbabwe.6,7
Against this background of discrepancies as reported in the current literature, a qualitative study was conducted in Gweru district of Zimbabwe among healthcare workers and PLWH. The study sought to explore the treatment of hypertension (HTN) and diabetes mellitus (DM) in PLWH attending ART sites and to assess the current capacity of these sites to manage HTN and DM. We also investigated the challenges faced by PLWH diagnosed with HTN and/or DM in accessing care at the study sites and their ways of coping up with the challenges.
Gweru district is one of the unique districts in Zimbabwe in the sense that it comprises both urban and rural populations, a phenomenon different from the other major metropolitan provinces such as Harare and Bulawayo, which consist of urban populations in their entirety. Gweru district is in the Midlands Province, where the prevalence of HIV is relatively higher than some other provinces.8
Methods
Design and setting
This was an exploratory, qualitative study utilising data collected from key informant interviews and focus-group discussions (FGDs). Six high-volume public ART sites situated at primary healthcare clinics were selected for the study. These were those with the highest number of PLWH (≥1000 PLWH) registered for ART in the Gweru district. Four sites were situated in the urban part. The remaining two sites were in the rural sector.
Sampling and data collection
Key-informant interviews were conducted with the District Medical Officer (DMO), the City Health Director and a Nurse-in-Charge of each of the six ART sites. Each of the key informants had special expertise and experience in managing NCDs and HIV in the district. A total of eight key-informant interviews were conducted in the English language, using a key-informant interview guide. The guide was designed to assess how HTN and DM were being managed at the ART sites and to assess the capacity of the sites to manage PLWH with these comorbidities. Each interview lasted an average of 50 minutes. Where necessary, observations were conducted with key informants to corroborate data. The English language was used given its status as the official language of the workplace.
Focus group discussions were conducted among PLWH with HTN and/or DM. A standard FGD guide was used to elicit care perspectives on comorbid disease care and experiences and/or challenges encountered while accessing care at the ART centres. We also enquired about the coping strategies employed in dealing with the identified challenges. Purposive sampling criteria were used to identify the FGD participants.9 These were ART-registered PLWH who had HTN and/or DM and had reported for care at least once a month for the previous 3 months prior to the study - confirmed by patient site records. The average time taken to complete each FGD was 65 minutes. The data collection tools, namely the interview guide and the FGD guide, were validated by (1) having them separately checked for appropriateness by three doctorate holders, who had particular experience in health sciences research, (2) pilot testing the interview guide among health managers from another district and (3) pilot testing the FGD guide among other potential participants who were later excluded from the final study. The data collection tools were modified according to pilot test responses before being used in the final data collection.
Written informed consent was sought from potential participants. Age (30-49; and >50 years), gender (male and female) and geographical background (rural and urban) were considered as key characteristics that could influence free and natural discussion. Therefore, homogeneity of characteristics (similar age group, gender and geographical background) within FGDs was ensured to promote unrestricted group member participation. Heterogeneity of participants in terms of age, gender and geographic background was ensured across all FGDs to capture diverse perspectives. Each FGD was composed of six participants. All FGDs were conducted in local languages (Shona and/or Ndebele) using an FGD guide. Translation of FGD data to the English language was performed in consultation with three experts of English, Shona and Ndebele languages during data transcription.
Both interviews and FGDs were conducted by the principal investigator who has some training in qualitative research methods. Furthermore, the co-author who guided the research process had experience as a qualitative researcher. Notes were taken during interviews, and FGDs, whereas session summaries and debriefing notes were produced from each interview and FGD conducted. All interview and FGD sessions were recorded using two audio recorders. Analysis and data collection were iterative, with interviews and FGDs continuing until code saturation, that is, until no new codes were emerging.10 When the last Nurse-in-Charge was interviewed at the sixth clinic, it was found that no new themes or information had emerged and that code saturation had been reached with the eight interviews. No further interviews were conducted with additional employees. As for FGDs, 12 FGDs (six participants per one FGD) were conducted before reaching the saturation point.
Data management and analyses
Data from the interviews and FGDs were stored as audio-recordings, interview notes, FGD notes, session summaries and debriefing notes. Audiotapes were transcribed verbatim onto a word-processing programme. Analyst triangulation was achieved through the two researchers independently analysing all emerging codes and issues across interviews and FGDs, and later discussing them to ensure that the results were grounded on data rather than the researchers' perceptions. Thematic analysis of qualitative data was performed in NVivo version 12®, involving familiarisation with the data, generation of initial codes, searching of themes within the codes, reviewing, defining and naming themes. Findings were presented according to emerging themes, using verbatim quotes to illustrate and support the analysis.
Findings
Participant characteristics
A total of eight interviews were conducted with key informants, whereas 12 FGDs were conducted with PLWH concurrently diagnosed with HTN and/or DM (Table 1). The basic requirement for a Nurse-in-Charge is a diploma, whereas that for a DMO and/or City Health Director is a degree.
Findings from key informant interviews
Key themes that emerged from key-informant interviews pointed on issues to do with how HTN and DM were being managed, and the capacity of ART sites to screen, diagnose and treat HTN and DM. The themes and subthemes emerging from each FGD are summarised in Table 2.
Management of hypertension and diabetes mellitus
Findings from key informants consistently concurred on how screening services for HTN and DM were being offered:
'Ministry [of Health] regulations require vital observations (including blood pressure, pulse rate, weight, and temperature measurements) to be done at every consultation as part of routine screening, to all patients regardless of HIV status …. However, the regulations require targeted screening for diabetes mellitus where random blood sugar (RBS) measurements are done only when a patient presents with signs and symptoms suggestive of diabetes mellitus.' (Male, 45 years old, Health Executive)
It was found that both BP and RBS measurements were being offered as part of the consultation process at all ART sites, where HIV-negative people pay a consultation fee equivalent to USD$1, and PLWH do not pay any consultation fee in urban sites. In rural sites, both HIV negative and PLWH do not pay any consultation fee.
Nurses-in-Charge for all the study sites consistently mentioned existence of Community ART Review Groups (CARGs):
'There are CARGs, of 7-10 PLWH per group. Through CARGS, only one group member collects medication on behalf of other group members, thereby reducing transport cost for collection of ART medication. This is useful especially when the patient does not require other services like viral load measurements, HTN/DM screening or other consultation services.' (Female, Nurse-in-Charge, with over 10 years' experience)
The capacity of antiretroviral therapy sites to screen, diagnose and treat non-communicable diseases
With regard to the capacity of ART sites to manage HTN and DM, the availability of human, financial and material (equipment and drugs) resources for screening, diagnosis and treatment was assessed at each ART centre.
Human resources
All key informants confirmed that the general nurses present at all ART sites were capable of screening for HTN and DM, even though they lacked at least one speciality, namely training in endocrinology. However, the lack of equipment remained a leading challenge:
'All the ART sites are manned by nurses who can screen for hypertension and diabetes mellitus if they have the required equipment. However, we do not have specialised endocrinology nurses for NCD care at any of ART sites in the district.' (Male, District Health Executive, with over 6 years' experience)
Material resources (equipment and drugs)
Screening for HTN requires a blood pressure (BP) machine, whereas screening for DM requires a random blood sugar (RBS) machine and glucostrips. Some BP machines used in the study sites were battery-powered, while others were electric-powered. Random blood sugar machines at all study sites were battery-powered. All sites had at least one BP and RBS machine.
Interview findings from all study sites consistently found that availability of screening services for HTN and DM was generally limited and periodically interrupted by various challenges. These included running out of machine batteries without timely replacement for HTN screening and frequent electrical power cuts that affected the use of electric-powered BP machines:
'…. Yes, we have a BP machine here. But we have not been using it for the past 2 months, because it doesn't have batteries.' (Female, Nurse-in-Charge, at a rural ART site)
Another reported challenge was the dysfunctional BP machines caused by the machine breakdowns. In three (two urban and one rural site) of the six study sites, the availability of only one BP machine was insufficient to service all clinic departments, including the outpatients and the maternity departments. For targeted screening for DM, all the sites reported that the service was generally not available, largely because of non-availability of glucostrips:
'The major challenge affecting screening for diabetes mellitus is the unavailability of glucostrips. There is a general shortage of glucostrips in our health centres, so patients might not have their blood glucose levels checked when they require the service …. For hypertension screening, some health centres do not have functional BP machines due to issues like machine breakdowns, electricity power cuts, and unavailability of batteries, among other reasons.' (Male, District Health Executive, with 5 years' experience)
Concerning the availability of drugs for HTN and DM, the interview findings revealed the partial availability of HTN drugs and the general non-availability of DM drugs. There was a convergence of views from all the study sites in that some hypertensive patients usually on hydrochlorothiazide (HCT) drug sometimes had access to the drug. However, hypertensive patients may need more than one type of drug, where some combinations might not include HCT. None of the key informants reported having any diabetic medication in stock:
'Stock-outs for hypertensive and diabetic medication are generally common due to limited funding for NCDs. Most patients usually get HCT from most of our health centres.' (Male, Health Executive, with over 6 years' experience)
While patients are unable to access screening services or medication for either HTN or DM or both, they are usually referred to private pharmacies where they must pay transportation costs, and the cost of the screening service and/or the medication costs:
'If they can't get medication here, we write them a prescription to purchase in town [in private pharmacies] if they have the funds.' (Male, Nurse-in-Charge, with over 15 years' experience at an urban ART site)
Financial resources
Responses pointed to limited financial resources for NCD management. Interviewees consistently reported that funding was inadequate to meet the needs of people at risk of or living with NCDs:
'The main source of funds for NCD management is the government of Zimbabwe (GoZ). Unlike the integrated HIV and TB care programmes, which receive additional funding from Non-governmental organizations (NGOs) like the Global Fund and USAID, NCD programmes are funded largely by the government, where the funding is usually limited. Funding is required for maintenance of equipment, procurement of equipment, drugs and medical sundries, … even training and re-training of our healthcare workers on NCD management.' (Male, Health Executive, with 5 years' experience)
Focus group discussion findings from patients
A total of 12 FGDs (consisting of six participants each) were conducted. More FGDs (n = 8) were conducted among female participants than among male participants (n = 4). Other characteristics of FGD participants are shown in Table 3.

Challenges faced by patients in accessing non-communicable diseases care and related coping mechanisms
The analysis of data generated from the FGDs revealed the non-availability of BP and RBS measurement services, the non-availability of HTN and DM medication at ART sites, the unaffordable cost of medication at private pharmacies and the challenges of transportation costs when seeking medical care or medication, as common challenges faced by patients in accessing care for HTN and DM. The saturation grid for themes and subthemes emerging from the FGDs is presented in Table 4. The FGD findings at both urban and rural sites were consistent with those from the key informant interviews.
Challenges in accessing healthcare
There was consensus across all FGDs that BP and RBS measurement services were not always available at all study sites. Participants pointed to repeated power cuts affecting the electric-powered BP machines, the battery-powered machines running out of batteries or service non-availability because of use of the machine in other clinic departments:
'…. As for me, my BP was only checked once this year. Most of the time I come here there will be no electricity so the BP machine will not be working. Sometimes you come here, and you are told the machine is being used in the maternity ward. As for sugar [diabetes mellitus], I don't even know whether the machine is here or not because I have never received the service here.' (Female, 55 years old, participant with HTN & DM)
The other commonly emerging subtheme from all FGDs was of medication availability for HTN and DM. Participants from all the ART sites confirmed that HCT is the only medication for HTN, which some might access. However, some hypertensive patients are not prescribed HCT, while others take more than one variety of medicine, none of which can be accessed at the clinic. None of the patients from all the 12 FGDs reported to have accessed DM medication:
'Sometimes they give HCT, but it is not everyone who gets it. Again, some of us do not take HCT, I take 4 other drugs for hypertension. For sugar [diabetes mellitus], I have never received medication from this clinic.' (Male, 62 years old, participant with HTN and DM)
When they cannot get medication from the clinic, participants reported getting prescriptions to purchase the required medication from private pharmacies, where the main challenge is the high cost of medication and transportation:
'They give a prescription for you to buy in the [private] pharmacies in town. There drugs are very expensive and some of us cannot even afford them. To go there you need transport fare again. It's really not easy.' (Female, 68 years old, participant with diabetes mellitus)
Coping mechanisms
Common coping subthemes included taking multiple doses to compensate for missed doses, sharing and rationing the medication. Furthermore, the use of home remedies, traditional herbs and consultation of traditional and faith healers emerged as coping strategies to deal with the identified challenges in managing HTN and DM.
It emerged that when patients do not have enough medication to last the whole month, there was a general consensus that they ration the medication by only taking medication when they experience serious illness. Also reported was that patients often took multiple doses of HCT to complement the missing medication, which would not have been purchased, or when they perceive symptoms to be associated with their hypertension:
'You go to the clinic and they give you only HCT. Apart from HCT, I need 3 more types of BP drugs. So when it gets serious, I take 4 HCT pills at once to relieve the pain, and it works.' (Male, 57 years old, participant diagnosed with HTN 10 years ago)
Others reported borrowing medications when they run out of medication:
'Last time I fell seriously ill and I could feel I was dying. I did not have the medication and transport fare to go to the clinic either. I ended up borrowing the medication from my neighbour, that is how I got saved. We always help each other that way.' (Female, 68 years old, participant diagnosed with HTN and DM)
Concerning home remedies, the use of ginger, garlic, cassia abbreviata (murumanyama in local Shona language) and smashed avocado seeds commonly reported remedies used to manage HTN. Diabetic patients often used ginger, boiled black-jack and diet monitoring as remedies or alternatives for managing DM when they could not access medication:
'I rely a lot on those [remedies]… especially garlic, murumanyama [cassia abbreviata] and [smashed] avocado seeds to manage my [high] BP … ginger and boiled black-jack also works for sugar [DM]… What can we do?… I can't afford these [allopathic] medicines.' (Female, 57 years old, participant diagnosed with HTN and DM)
Patients also reported that they relied on faith and traditional healers for the management of HTN and DM. Most participants across all FGDs mentioned that faith and traditional healers play an important role in managing their HTN and DM. Although all generally believed that allopathic medicines were more effective, they reported resorting to faith and traditional healers because they were cheaper alternatives. Participants also reported that these faith and traditional healers provided 'spiritual-uplifting' in the absence of medical drugs:
'I know that [prescribed] drugs are effective… but one needs money to buy them. Prophets, traditional healers … and home remedies are cheaper or even free, … so I can't watch myself die when these options are there.' (Male, 61 years old, participant with DM)
In some worst-case scenario, some reported avoiding formal healthcare for fear of what they called 'unnecessary costs':
'I do not even go to the [provincial] hospital, because when I go there, sometimes they tell me my [blood] sugar [level] is very high and give me bed-rest, but not the medication. The next day, they give me a huge bill to pay for the bed-rest when I did not have medication. Better I rest at home.' (Female, 48 years old, participant with DM)
Discussion
This study assessed the capacity of ART sites to manage HTN and DM in PLWH, from the perspectives and experiences of key healthcare workers and affected patients in the Gweru district of Zimbabwe. Also assessed were patients' experiences on the challenges they face in accessing care and the coping mechanisms they employ in dealing with the challenges. Several important findings emerged: (1) ART sites had limited capacity to screen for HTN and DM in PLWH, together with non-availability of HTN and DM medication, because of limited funding available for NCDs care; (2) PLWH who have HTN and/or DM are deterred from accessing healthcare for HTN and DM at public ART sites, mainly because of frequent unavailability of screening and treatment services and the high costs of medication in private pharmacies; (3) coping strategies employed in addressing encountered challenges include taking multiple doses to compensate for missed doses, sharing medication with others, medication rationing, the use of home remedies and traditional herbs, and the consultation of traditional or faith healers.
In this study, HTN screening was found to be integrated into routine screening as part of vital measurements which should be performed at every clinic consultation regardless of HIV status. In principle, this is a strategic arrangement to increase chances of hypertensive case finding as well as reducing stigma associated with screening only one group of patients.7 For DM, the Zimbabwean Ministry of Health's regulations require targeted screening where RBS measurements are taken from patients presenting with signs and symptoms suggestive of high blood sugar levels.7 While the protocols and cost of screening for HTN and DM could promote active case finding, in this study, effective implementation of the process was threatened by non-functional equipment secondary to limited funds. Interrupted provision of screening services can negatively affect diagnosis of new cases and ultimately result in lower than actual incidence and prevalence rates being observed for HTN and DM, as has been found in other studies.11
Erratic availability of HTN and DM medication at ART sites was reported as another common challenge faced by patients, as observed in other similar studies.4,12,13 For the majority of patients who could not afford medication from private pharmacies, alternative approaches were taken. The reported options included medication rationing or sharing or overdose, for example, multiple doses to compensate when the medication became available and the use of home remedies and herbs. These options do not offer effective treatment for HTN and DM because they are not regulated, nor is their effectiveness subjected to empirical research.7 If HTN and DM are not managed well in PLWH, the survival and wellness gains of ART will be sacrificed.
The unprecedented scale-up of ART in sub-Saharan Africa (SSA) has resulted in more PLWH achieving virologic suppression and increased survival.14 Therefore, the incidence of diseases associated with ageing such as HIV-HTN, DM, chronic kidney and heart disease will also increase.2,14,15,16,17,18 A study conducted in Malawi revealed that the lack of screening equipment and services, and medication stock-outs were frequent challenges to the care of PLWH and HTN/DM.19 Other studies conducted in SSA focused more on the feasibility of integrating HIV/AIDS programme with NCD care, and key findings point to the existence of challenges in screening for HTN and DM compared to those for HIV/AIDS care.17 There is limited research into the capacity of SSA to manage the future challenges faced by the long-term survivors of HIV.
Strengths and limitations
Use of purposive sampling based on set criteria to identify participants; involvement of more than one experienced qualitative researcher to reduce the chances of subjective bias; and attempts to attain code saturation in data collection represent methodological strengths of the study.
The weakness of the study was the use of cross-sectional study design that prevents long-term follow-up of participants and the absence of 'hard' clinical end points - actual BP, glucose monitoring and viral load suppression of the study cohort over time - to support the contention that the care of this group of Zimbabwean patients is being compromised.
Conclusion
Findings from this study demonstrate that the observed incidence and prevalence of HTN and DM among PLWH in Gweru district is a function of the capacity of its healthcare system to screen for and adequately manage NCDs. Given the ageing population of PLWH in SSA, coupled with the growing prevalence of NCDs in this group, HIV programmes require strengthening. While the social and economic problems of Zimbabwe are dire, this study gives voice to some of its PLWH. We feel that this is a voice that others need to hear too.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this research article.
Authors' contributions
All authors contributed equally to this work.
Ethical consideration
Ethical approval for this study was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (Ethical Clearance Number: BE086/19) and the Ministry of Health and Child Care Head office in Harare. Participation in the study was voluntary. Written informed consent was sought from potential participants, and confidentiality was maintained throughout the study by removal of personal identifiers after entry into the electronic database and use of non-identifiable coded numbers. All data were password-protected and stored in the electronic participant database.
Funding information
The research received funding from University of KwaZulu- Natal, under PhD research grant.
Data availability statement
Because of the private nature of the data, data for the study will be available only upon request and approval of Authorising Ministry of Health.
Disclaimer
The views and opinions expressed in this article are those of the authors and not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Joint United Nations Programme on HIV/AIDS (UNAIDS). Global HIV & AIDS statistics - 2018 fact sheet [homepage on the Internet]. UNAIDS; 2018. Available from: https://www.unaids.org/en/resources/fact-sheet [ Links ]
2.World Health Organization. Working Group on the inclusion of NCDs in other programmatic areas: WHO global coordination mechanism on the prevention and control of non-communicable diseases (Working group 3.1, 2016-2017). Geneva: World Health Organization; 2018. [ Links ]
3.Magodoro IM, Esterhuizen TM, Chivese T. A cross-sectional, facility based study of comorbid non-communicable diseases among adults living with HIV infection in Zimbabwe. BMC Res Notes. 2016;9(1):379. https://doi.org/10.1186/s13104-016-2187-z [ Links ]
4.Mutede BR, Magure T, Gombe NT, Bangure D, Tshimanga M. Prevalence and factors associated with hypertension among anti-retroviral therapy patients aged 15 years and above in Makonde District, Zimbabwe, 2012: An analytic cross sectional study. World J Cardiovasc Dis. 2015;5(9):266. https://doi.org/10.4236/wjcd.2015.59030 [ Links ]
5.Smit M, Olney J, Ford NP, et al. The growing burden of non-communicable disease among persons living with HIV in Zimbabwe. AIDS. 2018;32(6):773. https://doi.org/10.1097/QAD.0000000000001754 [ Links ]
6.Lazarus JV, Olsen M, Ditiu L, Matic S. Tuberculosis - HIV co-infection: Policy and epidemiology in 25 countries in the WHO European region. HIV Med. 2008;9(6):406-414. https://doi.org/10.1111/j.1468-1293.2008.00567.x [ Links ]
7.Ministry of Health and Child Care (MoHCC). The National Health Strategy for Zimbabwe (2016-2020): Equity and quality in health: leaving no one behind. Harare: Ministry of Health and Child Care; 2016 [cited no date]. Available from: https://malariaelimination8.org/wp-content/uploads/2017/02/National%20Health%20Strategy%20for%20Zimbabwe%202016-2020.pdf [ Links ]
8.Zimbabwe population-based HIV impact assessment: ZIMPHIA 2015-2016, Final Report [homepage on the Internet]. ICAP at Columbia University. 2019 [cited 2020 Feb 5]. Available from: https://phia.icap.columbia.edu/wp-content/uploads/2019/08/ZIMPHIA-Final-Report_integrated_Web-1.pdf [ Links ]
9.Marshall MN. Sampling for qualitative research. Family Prac. 1996;13(6):522-526. https://doi.org/10.1093/fampra/13.6.522 [ Links ]
10.Hennink MM, Kaiser BN, Marconi VC. Code saturation versus meaning saturation: How many interviews are enough? Qual Health Res. 2017;27(4):591-608. https://doi.org/10.1177/1049732316665344 [ Links ]
11.Aschengrau A, Seage GR. Essentials of epidemiology in public health. Sudbury, Massachusetts: Jones & Bartlett Publishers; 2013. [ Links ]
12.Mungati M, Manangazira P, Takundwa L, et al. Factors affecting diagnosis and management of hypertension in Mazowe District of Mashonaland Central Province in Zimbabwe: 2012. BMC Cardiovasc Disord. 2014;14(1):102. https://doi.org/10.1186/1471-2261-14-102 [ Links ]
13.Peltzer K. Utilization and practice of traditional/complementary/alternative medicine (TM/CAM) in South Africa. Afr J Tradit Complemen Altern Med. 2009;6(2):175. [ Links ]
14.Chamie G, Kwarisiima D, Clark TD, et al. Leveraging rapid community-based HIV testing campaigns for non-communicable diseases in rural Uganda. PLoS One. 2012;7(8):e43400. https://doi.org/10.1371/journal.pone.0043400 [ Links ]
15.Chhoun P, Tuot S, Harries AD, et al. High prevalence of non-communicable diseases and associated risk factors amongst adults living with HIV in Cambodia. PLoS One. 2017;12(11):e0187591. https://doi.org/10.1371/journal.pone.0187591 [ Links ]
16.Coetzee L, Bogler L, De Neve JW, Bärnighausen T, Geldsetzer P, Vollmer S. HIV, antiretroviral therapy and non-communicable diseases in sub-Saharan Africa: Empirical evidence from 44 countries over the period 2000 to 2016. J Int AIDS Soc. 2019;22(7):e25364. https://doi.org/10.1002/jia2.25364 [ Links ]
17.Mc Conalogue D, Nxumalo B, Busawa-la F, Sharp A, Walley J. Implementation of a non-communicable disease (NCD) screening programme in a rural African HIV clinic. HIV/AIDS Res Treat Open J. 2017;4(1):32-39. [ Links ]
18.Patel P, Speight C, Maida A, et al. Integrating HIV and hypertension management in low-resource settings: Lessons from Malawi. PLoS Med. 2018;15(3):e1002523. https://doi.org/0.1371/journal.pmed.1002523 [ Links ]
19.Pfaff C, Scott V, Hoffman R, Mwagomba B. You can treat my HIV-but can you treat my blood pressure? Availability of integrated HIV and non-communicable disease care in northern Malawi. Afr J Prim Health Care Fam Med. 2017;9(1):1-8. [ Links ]
 Correspondence:
Correspondence:
Laston Gonah
lggonah@gmail.com
Received: 11 June 2020
Accepted: 06 July 2020
Published: 04 Sept. 2020
ORIGINAL RESEARCH
Neural tube defects in the Free State province from 2012 to 2016. Is there an increase?
Nické TheronI; Gina JoubertII; Bertram D. HendersonIII
IDepartment of Paediatrics and Child Health, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIDepartment of Biostatistics, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIIDivision of Clinical Genetics, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
ABSTRACT
BACKGROUND: Neural tube defects (NTDs) are anomalies of the central nervous system caused by the defective closure of the neural tube during early embryogenesis. A significant decline in the incidence of NTDs after folic acid fortification of food in South Africa was previously shown. Recently, clinical geneticists have voiced concerns that there is a possible resurgence in the number of NTDs.
OBJECTIVES: The aim of this study was to determine the incidence of NTDs at a South African Hospital from 2012 to 2016.
METHODS: This is a retrospective cross-sectional study where all babies with NTDs born in, or referred to Universitas Hospital were included as study participants. Information was collected for both the mother and the baby from hospital records and data forms
RESULTS: Seventy-seven cases of NTDs were captured from 2012 to 2016. The incidence of NTDs was 0.34/1000 births in the Free State province, and 1.21/1000 births if only the data for babies born in Universitas Hospital and Pelonomi Hospital were used. Further analysis showed a male: female ratio of 1:1. Open spina bifida was the most common defect at 71.4%.
CONCLUSION: The incidence of NTDs in the Free State province was low compared to other South African and international studies. The incidence for the metropolitan hospitals is comparable to that of previous studies. This discrepancy is a marker of poor data recording and will impact healthcare planning. A statistically significant increase in NTDs could not be proven.
Keywords: neural tube defects; birth defects; data collection; Free State province; South Africa; antiretroviral treatment.
Introduction
Neural tube defects (NTDs) are severe anomalies of the central nervous system caused by the defective closure of the neural tube during early embryogenesis.1 An estimated minimum of 300 000 neonates are affected worldwide each year.2 According to UNICEF and the South African Burden of Disease study in 2000,3 numerous different congenital defects are ranked in the first 20 specific-causes of under-five childhood mortality in South Africa (SA). The specific disorders were congenital heart disease (8th), NTDs (12th), chromosomal defects (18th) and congenital disorders of the gastrointestinal tract (19th).
Despite many studies and growth in the knowledge of neural tube embryology, this multifactorial disorder and the complex genetic and environmental factors interactions that cause or prevent it remain poorly understood.4
Neural tube defects are classified as open or closed (membrane-covered) lesions. The type of lesion determines the clinical impact. Open lesions affecting the brain such as anencephaly (absence of major part of the brain, skull and scalp) and craniorachischisis (anencephaly and NTDs that extend to the neck) are lethal antenatally or shortly after birth. The morbidity of encephalocoeles (protrusion of either brain tissue, its covering membranes or both through a defect in the skull) depends on the extent, position and contents of the protrusion. Spina bifida is an NTD that is restricted to the caudal portion of the neural tube. Its morbidity depends on the extent and level of the lesion and the degree of neurological impairment below that level.5 Spina bifida can be divided into three common types. Spina bifida occulta results from the failure of vertebral fusion and has no opening on the back (also called a closed NTD). There might be a clue to the defect in the form of a tuft of hair or a dimple in the overlying skin. This is the mildest form and usually does not cause any disability. A protruding sac filled with fluid that does not involve the spinal cord is called a meningocoele. Myelomeningocoele is the most common and severest form. This involves incomplete vertebral and neural tube closure and thus exposure of the spinal cord and meninges which may be open or closed.6 This necessitates early surgical treatment of the lesion and the associated complications (such as hydrocephalus) and requires long-term rehabilitation and follow-up.7
Numerous risk factors have been identified for NTDs, including both genetic and environmental factors. Maternal vitamin B12 deficiency has been shown to increase the risk of NTDs.2 Maternal exposure to teratogens, such as methotrexate, valproic acid, other anticonvulsants and aminopterin, as well as hyperthermia early in pregnancy, low socioeconomic status, maternal obesity, pre-gestational diabetes and genetic predisposition are factors that increase the risk.4 Neural tube defects can also occur as part of genetic syndromes as one of the multiple congenital malformations.1,2
Since the seminal work of Czeizel and Dudás,8 folic acid (vitamin B9) has become the accepted norm for primary prevention of NTDs.4,7 Sustainable progress has been made in the primary prevention of NTDs resulting from folic acid supplementation/food fortification. One of the first studies to support this was done in China from 1993 to 1995.9 The daily intake of folic acid during the periconceptional period was found to reduce a woman's risk of having a foetus or infant with an NTD. The effect of this fortification was greatest in the high-prevalence rural regions. In 2003, SA legislated a programme of folic acid fortification of staple foods. An ecological study in the country, from 2002 to 2005, confirmed a significant decline in the incidence of NTDs post fortification, together with a reduction in the related financial and health burden.10
There are no current studies on the incidence or prevalence of NTDs in SA. Ncayiyana conducted a study from 1980 to 1984 in a rural Transkei district that showed an incidence of 6.1 NTDs/1000 births.11 A lower prevalence was found in urban regions such as Cape Town, namely 1.3/1000 births.12 According to Sayed et al.,10 the prevalence between January 2003 and June 2004 (pre-fortification) was 1.41/1000 births and between October 2004 and June 2005 (post-fortification) was 0.98/1000 births. This is comparable to the 1.67/1000 prevalence in low- and middle-income countries reported between 2000 and 2013.2
Since the study of Sayed et al.,10 there have been many changes in SA's healthcare. Clinical geneticists 'have the impression' that there has been a resurgence of NTDs in recent years: Christianson AL, personal communication, Johannesburg, SA, May 2016. The Sayed study10 took place in an environment of high HIV infection rates. The slow rollout of antiretroviral treatment (ART) in the region is unlikely to have influenced the study findings. Antiretroviral treatment protocols have changed numerous times in the past 11 years. If an increase in NTDs is demonstrated, a causal relationship will need to be investigated.
The SA-National ART programme was launched in April 2004. Adult regimens comprised of two nucleoside/tide reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTI): initially stavudine and lamivudine (NRTIs) and efavirenz/nevirapine (NNRTIs).13 In 2010, first-line ART was changed to tenofovir and lamivudine/emtricitabine with nevirapine still preferred in women of child-bearing age.14 By 2013, the guideline was changed to tenofovir, emtricitabine/lamivudine and efavirenz for all adults, irrespective of gender or pregnancy.15 This is generally given as a fixed-dose combination tablet as recommended in the 2015 National Guidelines.16
An analysis in 2013 found no evidence of an increased risk of central nervous system congenital anomalies associated with first-trimester exposure to efavirenz in low- and middle-income countries.17 The incidence of NTDs was low and similar to that of the general population in this systematic review and meta-analysis.
Since 7 October 2003, SA legislation namely, the Foodstuffs, Cosmetics and Disinfectants Act Number 54, has required any person who manufactures, imports or sells bread, wheat-flour and maize-meal, to fortify it with vitamins and minerals.18 The included vitamins are vitamin A, thiamine (vitamin B1), riboflavin (vitamin B2), niacin (vitamin B3), folic acid (vitamin B9) and pyridoxine (vitamin B6). The included minerals are iron and zinc. The regulations also stipulate that a miller should keep monthly records and store the fortification mixture under hygienic conditions. Contravention of these conditions may result in a fine.19 Environmental Health Practitioners were trained and mobilised to carry out routine inspections of mills to ensure compliance with food fortification regulations. The success of this fortification programme depends on a multitude of factors as outlined in 'A reflection of the South African Maize Meal and Wheat Flour Fortification Program (2004-2007)'.19
Aim and objectives
This study aimed to determine if there was an increase in the incidence of NTDs in live and stillbirths in the Free State province (FS) from 2012 to 2016 as reflected by Universitas Hospital's (UH) records.
Specific objectives:
•The incidence of NTDs found in this study was compared with the incidence of NTDs found in the FS in 2005 by Sayed et al.10
•Data were collected to identify recurrent factors that could be used in further research studies as possible risk factors.
•To determine the incidence of cranial versus spinal NTDs.
Methods
This was a retrospective cross-sectional study set at UH, Bloemfontein, which is the referral hospital in the FS for the treatment of all NTDs as well as antenatal diagnoses of congenital defects. All babies with NTDs (including stillbirths, late terminations and live births) from pregnancies reaching viability born in UH or referred to UH from 2012 to 2016 were included in this study. At UH, viability is defined as later than 28 weeks' pregnancy duration or more than 800 g birth weight.
The Department of Health Information Systems (DHIS) defines a delivery later than 28 weeks as a birth, and not a miscarriage. Early miscarriages or termination of pregnancy before 28 weeks' gestation were excluded to enable comparison with the Sayed et al. study.10 Patients referred from outside the FS were also excluded.
Measurement
Neural tube defect cases were identified and data were collected retrospectively from the following sources:
•Surveillance of Birth Defects reporting forms - UH.
•MEDITECH records: the Electronic system for patient data capturing at UH.
•Hospital records from the Neonatal unit and Obstetric unit, including admission registers as well as the records-archive of UH.
•Number of births in the FS for 2012 to 2016 - DHIS.
•The birth data for UH and Pelonomi Hospital (PH) were collected from the specific maternity wards and calculated separately to that from the rest of the FS.
Once the NTD cases were identified, data from their summaries on MEDITECH as well as hospital files were collected and entered into a data collection sheet. Each case was given a unique research number for the information of the mother and the baby to maintain the confidentiality of the patients. The data collection sheets were then entered into an Excel® spreadsheet.
The first three cases were used for the pilot study to test the data collection sheet. The data-sheet was adapted to include the gender and the weight of the baby. These three cases were included in the study.
Analysis of data
The University analysed the data. The results were summarised as frequencies, percentages (categorical variables) and medians (numerical variables due to skew distributions). Ninety five per cent confidence intervals (95% CI) and appropriate hypothesis testing were used for comparison with other studies.
Ethical consideration
Approval to undertake this study was obtained from the Health Sciences Research Ethics Committee of the University of the Free State (HSREC 53/2016) and permission to conduct this study was also obtained from the Free State province Department of Health. The information of each patient was managed with strict confidentiality. The hospital numbers of each patient and mother and the date of birth were transcribed onto a separate list and a specific research number was allocated for each mother and baby (e.g. M1, B1). On the data-sheet and the Excel spread-sheet, only these research numbers were used to ensure confidentiality. These data-sheets were kept in a locked cabinet at the Department of Paediatrics.
Results
In total, 77 cases of NTDs were captured from 2012 to 2016 at UH. The number of cases per year indicated that most were born in 2013 and 2014, with 10 in 2012, 19 in 2013, 19 in 2014, 15 in 2015 and 14 in 2016. See Figure 1.
Twenty-six (26; 33.8%) of the cases were born in UH, and 51 in other hospitals. The highest number of referrals was from the Mangaung area (32.7%). This is displayed in Figure 2.
The incidence of NTDs was calculated using the number of births in the FS per year as well as the number of NTDs per year, as shown in Table 1. Thus, over the 5 years, the incidence of NTDs was 0.34/1000 births.
Data for UH and PH were calculated separately from data of the rest of the FS as both had been sentinel sites for the Sayed et al. study.10 There were 27 222 births from 2012 to 2016 and 33 cases born at these hospitals. The incidence for NTDs was 1.21/1000 births for babies born in PH and UH, which represents the metropolitan areas of the FS. The relative risk of an NTD from 2012 to 2016 in comparison to the Sayed et al. study is 1.18 (95% CI 0.52-2.68, p = 0.69). There is no significant increase.
The demographics of the NTD cases revealed a male: female ratio of 1:1 with the gender known in 68 (88.3%).
Fifteen of the cases were firstborn (15; 22.4%), 20 were second-born (29.9%) and 15 third-born (22.4%). The birth order was known in 67 (87.0%).
Sixty-two (62; 80.5%) of the NTD cases were live births and 15 (19.5%) were late terminations or stillbirths.
All the different types of NTDs were represented in these cases. Open spina bifida (meningocoele or myelomeningocoele) was the most common at 71.4%, 2.6% had spina bifida occulta, 13% had anencephaly and 9.1% had an encephalocoele. Some babies were reported to have more than one NTD as identified by the examining doctor: 1.3% had both anencephaly and an encephalocoele, and 2.6% had both an encephalocoele and open spina bifida. See Figure 3.
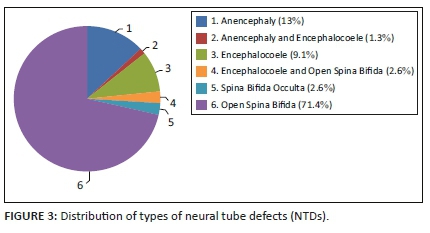
Other congenital defects were identified in addition to the NTD in many cases. These were classified as part of the NTD sequence (found in 55.8% of cases) or part of other syndromes and associations (found in 16.9% of cases). Open spina bifida is often associated with other central nervous system anomalies, such as Chiari Type 2 malformation, hydrocephalus and club feet. These are considered as a sequence. Isolated NTDs occurred in 27.3% of cases. See Figure 4.
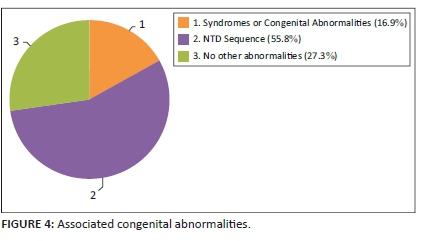
The other specific syndromes or patterns of congenital abnormalities most frequently associated with NTDs were VACTERL association (30.8%) and Trisomy 18 (15.4%). See Figure 5.
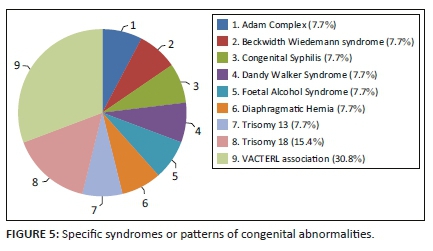
The data collected from the maternal files were analysed to identify risk factors that might play a role in the development of NTDs. The maternal age showed a skewed distribution with a median of 28.5 years. The maximum age was 43 years and the minimum was 16 years. The maternal age was known in 76 cases (98.7%).
Teratogen exposure was known for 63 mothers (81.8%) and 15 (23.8%) were exposed to teratogens. Three mothers were on anti-epileptic treatment, all used valproate. The epilepsy history of 67 (87.0%) mothers was known. Twelve mothers (15.6%) were exposed to other medications or toxins during their pregnancy including alcohol (n = 1), smoking (n = 1), antihypertensive treatment (n = 6), penicillin (n = 3) and traditional medicine (n = 1). One mother was diagnosed with diabetes mellitus during pregnancy.
The HIV data were captured for most mothers (94.8%). The prevalence of HIV-positive mothers in this study was 34.3% (25/73). The 95% CI for the prevalence of HIV in this study was 24.4% - 45.7%, which encapsulates both the FS and national prevalence. There is, therefore, no statistically significant difference in the HIV status of mothers who delivered babies with NTDs and the general population. Of the mothers who were living with HIV, most were taking antiretroviral drugs (ARVs) (n = 23), one mother was not on ARVs during pregnancy and the treatment status of one mother was unknown. See Figure 6.
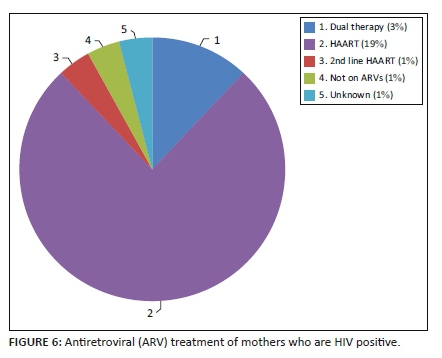
Family history, including previous pregnancy outcomes, was available for 34 cases (44.2%). One mother had a previous pregnancy-related NTD. Neural tube defects in the extended family were absent in the 17 cases where the data were recorded.
One mother of seven with known data had used folic acid during her pregnancy. It was unknown whether any of the mothers used folic acid during the peri-conception period.
Discussion
This study determined the incidence of NTDs in the FS from 2012 to 2016 and looked at the maternal and foetal profiles of the babies with these defects. According to the authors' knowledge, this is the first study to determine the incidence of NTDs in the FS. Other available data in SA are for rural Transkei (6.1 NTDs/1000 births in 1980-1984)11 and Cape Town (1.3/1000 in 1994)12. Sayed et al.10 used only the number of births at UH and PH, and the number of babies with an NTD born in these sentinel sites to obtain an incidence of 1.03/1000 for the FS and an incidence of 0.98 for SA using sentinel sites in three other provinces.
Compared to the above incidences, the FS incidence of 0.34/1000 found in this study is low. As only the cases referred to UH were captured, it can be postulated that this incidence is a false representation of the actual situation. Babies may die before referral or not been referred to as in the case of anencephaly, or the cases with spina bifida occulta may have been missed. An attempt was made to supplement the data with data from the DHIS where the causes of death are captured. This was unsuccessful. The entries were either not made or wrongly entered as searches indicated one death per year due to NTDs.
Comparing the incidence for UH and PH to the Sayed et al.10 data indicated an increase in the incidence of NTDs from 1.03/1000 in 2004/2005 (shortly after food fortification with folic acid was introduced) to 1.21/1000 in 2012 to 2016. This tends to support the impression that there is a resurgence in the number of NTD cases in the FS, but this was not statistically significant.
The referrals from outside UH were classified according to the municipal healthcare districts where the baby was born. Most referrals were from the Mangaung area as expected from the population distribution of the FS.20 See Figure 2.
The demographics of the NTD cases in this study contradicted most of the other studies done in SA. Both the study performed in Cape Town over 20 years ago12 and a study performed in Gauteng21 that looked at the profile of NTD cases showed that it was more prevalent in females than males, whereas this study had an equal female to male ratio. The birth order also differed from the study performed in Gauteng21 where the firstborn and lastborn infants were more at risk of NTDs, whereas it was the second-born infants who were mostly affected in this study. A possible explanation for the contradictions would be our small sample size.
The distribution of the type of NTD is in keeping with other studies.2,10,11,22 A study performed in Tunisia22 also showed that spina bifida was the most common defect, followed by anencephaly. The difference between the two was much smaller, however, (38.9% for spina bifida, 22.8% for anencephaly) and this can be accounted for by the fact that most anencephaly patients are probably not referred from the peripheral hospitals due to the poor prognosis.
When interpreting the maternal data, it is important to note that the data available for the mothers were poor. They were collected from the neonatal and obstetric summaries found in the electronic data-keeping system as well as the UH admission books, which were often incomplete. Most of the summaries regularly did not include the family history of NTDs or whether the mother used supplements before or during pregnancy. The data for the mother's medication history and other chronic diseases (e.g. diabetes mellitus) that could be risk factors were also poorly represented. It is, thus, not possible to draw definitive conclusions. If the data were not recorded in the summaries, these were probably not considered whilst the patient was admitted to the hospital and the mother was, thus, also not properly counselled regarding recurrence and prevention in future pregnancies. This should be addressed by the different departments to optimise patient care.
The HIV data were captured for most mothers. HIV status was known for 94.8%. The prevalence of HIV-positive mothers in this study was 34.3%. This is higher than the prevalence in pregnant females in the FS from 2009 to 2013 which ranged between 29.8% (2013) and 32.5% (2011) according to 'The 2013 National Antenatal Sentinel HIV Prevalence Survey South Africa'.23 The overall HIV prevalence in pregnant females in SA in 2013 was 29.7%, which is also lower than the prevalence found in this study. These differences were, however, not statistically significant.
The type of ARVs and the duration of ARV use were also poorly captured in the data sources. It is, therefore, not possible to say whether a specific type of ARV or a certain treatment regimen contributed to a higher rate of NTDs.
On 21 May 2018, after the completion of this study, the FDA issued a warning that women treated with dolutegravir in the first trimester of pregnancy are at higher risk for NTDs.24 This report also indicated that the National Institutes of Health has launched an international study to compare the safety and efficacy of three ARV treatments for pregnant women with HIV.
A recent study highlights the need for proper data surveillance and recording of congenital disorders to accurately demonstrate the contribution thereof to the burden of disease on our health care system.25 The Congenital Disorder Surveillance was already implemented in SA in 1980 with several changes to the system in 2001 namely, the Birth Defect Notification Tool of the National Department of Health, and a coding classification added in 2006. It was found in the study performed by Lebese et al.25 that the implementation of these systems was poor. When compared to expected congenital disorder notifications, there was an underreporting of more than 99%. Kwa-Zulu Natal recorded the highest number of congenital disorders per year (total of 7219 over 9 years) whilst the FS only recorded 744 cases during the same period. This highlights the need for training of healthcare providers as well as coordinators to report congenital defects so that relevant health policies can be developed. The poor data availability in this study highlighted the same limitations and issues.
Conclusion
There is a clinical impression that the incidence of NTDs is increasing. The data obtained in this study appear to support this impression, but no significant statistical difference could be proven.
A major finding of this study was, however, the poor data capturing and recording in the FS, which will impact the planning and funding for the healthcare of congenital defects in the FS and SA as a whole.
The incidence for NTDs in the FS was found to be 0.34/1000 births, which is low compared to other South African and international data.1,2,10,11,12,22 The discrepancy between the incidence of NTDs in the FS and the metropolitan hospitals serves as a marker of the poor data recording.
The incidence for UH and PH for 2012 to 2016 (1.21/1000 births) is comparable to the incidence found in the Sayed et al. study.10 No specific correlation could be drawn between known risk factors and the NTD cases.
A prospective study in this field, with a larger study population, will be required to confirm or refute the clinical impression that there is an increase in the incidence of NTDs in the FS and to identify possible explanations, if so.
Acknowledgements
The authors would like to thank Ms. A Steinhobel, Operational Manager, Universitas Hospital, and her staff at the Neonatal High Care for filing all the congenital abnormality report forms and permitting access to them, and Ms. T Mulder, Faculty of Health Sciences, UFS, for reviewing the document and editing it for publication.
This research was conducted for the fulfilment of the MMed in Paediatrics (NT) and supervised by BDH.
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
All authors contributed equally to this work.
Funding information
The research was funded from the research funds of the Department of Paediatrics and Child Health, UFS, and the Division Clinical Genetics, UFS.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Public Health Agency of Canada. Congenital anomalies in Canada 2013: A perinatal health surveillance report [homepage on the Internet]. c2013 [cited 2015 Mar 17]. Available from: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP35-40-2013-eng.pdf [ Links ]
2.Lo A, Polšek D, Sidhu S. Estimating the burden of neural tube defects in low- and middle-income countries. J Glob Health. 2014;4(1):010402. https://doi.org/10.7189/jogh.04.010402 [ Links ]
3.Bradshaw D, Bourne D, Nannan N. What are the leading causes of death among South African children? MRC policy brief no 3 [homepage on the Internet]. c2003 [cited 2016 Feb 07]. Available from: www.unicef.org/southafrica/SAF_publications_mrc.pdf [ Links ]
4.Wallingford JB, Niswander LA, Shaw GM, Finnell RH. The continuing challenge of understanding, preventing and treating neural tube defects. Science. 2013;339(6123):1047-1055. https://doi.org/10.1126/science.1222002 [ Links ]
5.Copp AJ, Stanier P, Greene ND. Neural tube defects' recent advances, unsolved questions, and controversies. Lancet Neurol. 2014;12(8):799-810. https://doi.org/10.1016/s1474-4422(13)70110-8 [ Links ]
6.NIH Eunice Kennedy Shriver National Institute of Child Health and Human Development. Neural tube defects (NTDs): Condition information [homepage on the Internet]. c2012 [cited 2015 Mar 17]. Available from: https://www.nichd.nih.gov/health/topics/ntds/conditioninfo [ Links ]
7.MRC Vitamin Study Research Group. Prevention of neural tube defects: Results of the Medical Research Council Vitamin study. Lancet. 1991;338(8760):131-137. https://doi.org/10.1016/0140-6736(91)90133-A [ Links ]
8.Czeizel AE, Dudás I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327(26):1832-1835. https://doi.org/10.1097/00006254-199306000-00005 [ Links ]
9.Berry RJ, Li Z, Erickson JD, et al. Prevention of neural tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341(20):1485-1490. https://doi.org/10.1056/nejm199911113412001 [ Links ]
10.Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res Part A Clin Mol Teratol. 2008;82(4):211-216. https://doi.org/10.1002/bdra.20442 [ Links ]
11.Ncayiyana DJ. Neural tube defects among rural blacks in a Transkei District. A preliminary report and analysis. S Afr Med J. 1986;69(10):618-620. [ Links ]
12.Buccimazza SS, Molteno CD, Dunne TT, Viljoen DL. Prevalence of neural tube defects in Cape Town, South Africa. Teratology. 1994;50(3):194-199. https://doi.org/10.1002/tera.1420500304 [ Links ]
13.Boulle A, Bock P, Osler M, et al. Antiretroviral therapy and early mortality in South Africa. Bull World Health Organ. 2008;86(9):678-687. https://doi.org/10.2471/blt.07.045294 [ Links ]
14.National Department of Health. Republic of South Africa. The South African antiretroviral treatment guidelines. Specialist [homepage on the Internet]. c2010 [cited 2015 Mar 17]. Available from: https://apps.who.int/medicinedocs/documents/s19153en/s19153en.pdf [ Links ]
15.National Department of Health. Republic of South Africa. The South African antiretroviral treatment guidelines [homepage on the Internet]. c2013 [cited 2015 Mar 17]. pp. 1-21. Available from: https://sahivsoc.org/Files/2013%20ART%20Treatment%20Guidelines%20Final%2025%20 March%202013%20corrected.pdf [ Links ]
16.National Department of Health. Republic of South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults [homepage on the Internet]. c2014 [cited 2015 Mar 17]. pp. 1-119. Available from: https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf [ Links ]
17.Ford N, Calmy A, Mofenson L. Safety of efavirenz in the first trimester of pregnancy: An updated systematic review and meta-analysis. Aids. 2011;25(18):2301-2304. https://doi.org/10.1097/QAD.0b013e32834cdb71 [ Links ]
18.National Department of Health. Republic of South Africa. Foodstuffs, cosmetics and disinfectants act 54/1972, 2003. Regulations relating to the fortification of certain foodstuffs. Government Notice R504. Government Gazette. Vol. 454. No 24715. Pretoria: Government Printer, 07 April 2003, p. 1-23. [ Links ]
19.National Department of Health. Republic of South Africa, UNICEF South Africa. A reflection of the South African Maize Meal and Wheat Flour Fortification Programme (2004 to 2007) [homepage on the Internet]. c2007 [cited 2016 Feb 18]. Available from: https://www.unicef.org/southafrica/SAF_resources_wheatfortificationn.pdf [ Links ]
20.Free State Development Corporation. Regional overview of the free state [homepage on the Internet]. n.d. [cited 2017 Jun 07]. Available from: https://municipalities.co.za/provinces/view/2/free-state [ Links ]
21.Teckie G, Krause A, Kromberg JGR. Neural tube defects in Gauteng, South Africa: Recurrence risks and associated factors. S Afr Med J. 2013;103(Suppl. 1):973-977. https://doi.org/10.7196/samj.7119 [ Links ]
22.Nasri K, Ben Fradj MK, Hamdi T, et al. Epidemiology of neural tube defect subtypes in Tunisia, 1991-2011. Pathol Res Pract. 2014;210(12):944-952. https://doi.org/10.1016/j.prp.2014.06.027 [ Links ]
23.National Department of Health. Republic of South Africa Epidemiology and Surveillance Directorate. The 2013 National Antenatal Sentinel HIV Prevalence Survey South Africa [homepage on the Internet]. c2013 [cited 2017 Jun 08]. Available from: http://www.kznhealth.gov.za/data/The-2013-National-Antental-Sentinel-HIV-Prevalence-Survey-South-Africa.pdf [ Links ]
24.Rosenberg J. FDA warns of neural tube birth defects from HIV drug dolutegravir [homepage on the Internet]. AJMC Managed Markets Network. 21 May 2018. c2018 [cited 2018 May 25]. Available from: https://ajmc.com/newsroom/fda-warns-of-neural-tube-birth-defects-from-hiv-drug-dolutegravir [ Links ]
25.Lebese V, Aldous C, Malherbe HL. South African congenital disorders data, 2006-2014. S Afr Med J. 2016;106(10):992-995. https://doi.org/10.7196/samj.2016.v106i10.11314 [ Links ]
 Correspondence:
Correspondence:
Bertram Henderson
rozanne@discoverymail.co.za
Received: 14 July 2020
Accepted: 03 Aug. 2020
Published: 25 Sept. 2020
ORIGINAL RESEARCH
Patient acceptance of HIV testing services in rural emergency departments in South Africa
Aditi RaoI; Caitlin KennedyI; Pamela MdaII; Thomas C. QuinnIII, IV; David SteadV, VI; Bhakti HansotiI, III
IDepartment of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, United States of America
IINelson Mandela Academic Clinical Research Unit, Mthatha, South Africa
IIIDepartment of Medicine, Johns Hopkins University School of Medicine, Baltimore, United States of America
IVDivision of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, United States of America
VDepartment of Medicine, Frere and Cecilia Makiwane Hospitals, East London, South Africa
VIDepartment of Medicine, Faculty of Health Sciences, Walter Sisulu University, East London, South Africa
ABSTRACT
BACKGROUND: South Africa faces the highest burden of HIV infection globally. The National Strategic Plan on HIV recommends provider-initiated HIV counselling and testing (HCT) in all healthcare facilities. However, HIV continues to overwhelm the healthcare system. Emergency department (ED)-based HCT could address unmet testing needs.
OBJECTIVES: This study examines the reasons for accepting or declining HCT in South African EDs to inform the development of HCT implementation strategies.
METHOD: We conducted a prospective observational study in two rural EDs, from June to September 2017. Patients presenting to the ED were systematically approached and offered a point-of-care test in accordance with national guidelines. Patients demographics, presenting compaint, medical history and reasons for accepting/declining testing, were recorded. A pooled analysis is presented.
RESULTS: Across sites, 2074 adult, non-critical patients in the ED were approached; 1880 were enrolled in the study. Of those enrolled, 19.7% had a previously known positive diagnosis, and 80.3% were unaware of their HIV status. Of those unaware, 90% patients accepted and 10% declined testing. The primary reasons for declining testing were 'does not want to know status' (37.6%), 'in too much pain' (34%) and 'does not believe they are at risk' (19.9%).
CONCLUSIONS: Despite national guidelines, a high proportion of individuals remain undiagnosed, of which a majority are young men. Our study demonstrated high patient acceptance of ED-based HCT. There is a need for investment and innovation regarding effective pain management and confidential service delivery to address patient barriers. Findings support a routine, non-targeted HCT strategy in EDs.
Keywords: HIV counselling and testing; South Africa; emergency department; patient acceptance; implementation research; linkage to care.
Introduction
South Africa (SA) faces the highest burden of HIV infection globally, with 7.7 million people living with HIV (PLWH) and prevalence ranging from 12.6% to 27% across the country.1,2 In 2018, SA had 240 000 new HIV infections and 71 000 AIDS-related deaths.1 The Joint United Nations Programme on HIV/AIDS (UNAIDS) adopted the ambitious treatment target of 90-90-90, wherein by 2020 90% of all PLWH would know their status, 90% of whom would be receiving sustained antiretroviral therapy (ART), of whom 90% would have achieved viral suppression.3 Currently, in SA, an estimated 90% of PLWH know their status, of whom 68% are accessing ART, and 87% of these have achieved viral suppression.1 Over the past two decades, numerous steps have been taken by the government to deliver evidence-based interventions focusing on HIV prevention, treatment, and retention. These efforts include the expansion of condom distribution, a national voluntary medical male circumcision program, prevention of mother-to-child transmission, as well as initiatives to increase knowledge and awareness of HIV/AIDS in communities utilising healthcare, in educational infrastructures and social media.2,4 However, despite sustained efforts and innovative measures, critical coverage gaps remain, with an estimated 10% of HIV-positive South Africans unaware of their status5 and with 15% of new global infections occurring in the country.2
The first critical step to meeting the 90-90-90 target is HIV testing. Early detection of undiagnosed HIV infection followed by effective linkage to care and treatment extends life expectancy, improves the quality of life, and reduces HIV transmission.6 Since 2015, the South African National Strategic Plan on HIV, sexually transmitted infections and tuberculosis has recommended provider-initiated HIV counselling and testing (HCT) to all persons attending healthcare facilities as a standard component of medical care, including trauma, casualty, and specialty clinics.6,7 Nonetheless, the provision of HCT in healthcare facilities is often hindered by the lack of standardised training and by competing clinical care priorities that prohibit effective service delivery.5,7 In addition, resources for HCT have largely been directed to primary healthcare centres and antenatal clinics or are focused on high-risk populations such as sex workers, men who have sex with men, injection drug users, and prisoners.8,9 As a result, individuals who do not interact with the healthcare system through these channels, such as young men, often miss being tested.
In SA, 90% of the population accesses healthcare through the public sector. For 28%, the emergency department (ED), a setting that provides high-volume care, is their only point of contact.10 In the United States of America, the ED is recognised by the Centers for Disease Control and Prevention to be a crucial venue in implementing the national HIV testing strategy.11 Seminal studies have not only quantified the burden of HIV infection in EDs but also have been critical to shaping the US national strategy for HIV; they could similarly address unmet testing needs in SA.11,12,13,14 In low- and middle-income countries (LMICs), HIV prevalence in EDs may be high, for example, 19% in Papua New Guinea and 50% in Uganda.15,16 Provision of HIV testing in the ED could thus be a critical intervention in curbing the epidemic. Its acceptance in acute care settings, however, has not been widely evaluated in sub-Saharan Africa. Studies have primarily focused on rates of acceptance, without exploring the reasons behind patients' decisions. Ascertaining the perspectives of patients, especially of those who decline testing, enables the identification of barriers to service delivery and the development of effective strategies to increase HIV diagnosis and linkage to care.
In this exploratory observational study, to determine the feasibility of expanding an ED-based HIV testing strategy in SA, we investigated patient perspectives on accepting or declining HCT and quantified the burden of HIV infection in the ED while implementing the nationally recommended HCT programme. This study will assist policymakers and healthcare providers to inform the integration of HCT in the clinical care pathway and optimise HCT service delivery in this venue, resulting in early engagement in care and treatment initiation, ultimately reducing HIV-associated morbidity and mortality.
Methods
The Walter Sisulu Infectious Diseases Screening in Emergency Departments (WISE) Study was a prospective observational study. HIV counselling and testing was implemented in the EDs of the Nelson Mandela Academic Hospital (NMAH) and the Mthatha Regional Hospital (MRH) in the Eastern Cape Province, from 27 June to 03 September 2017.
Study site
The study was conducted in Mthatha, a rural town in the South African province of the Eastern Cape, a region that supports 12.6% of the country's population.10 The area faces a disproportionate burden of acute injuries and illnesses with high rates of HIV and tuberculosis.7 It is also one of SA's poorest provinces and is a key priority area for HIV research and capacity building.7 Both hospitals are affiliated with the Walter Sisulu University. Nelson Mandela Academic Hospital is a large tertiary-care referral centre with 24-h trauma services, seeing only patients requiring specialty or surgical interventions referred from other district-level facilities. Mthatha Regional Hospital is a district-level facility that provides care to walk-in patients and referrals from adjacent maternal and childcare facilities. The EDs provide 24-h coverage and see 100-150 patients daily from the surrounding 100-km catchment area. Both sites are relatively low-resourced and not equipped with an electronic medical record (EMR) system, patient tracking system or standardised triage processes. Furthermore, there are no providers specialising in emergency medicine at these sites.
Study population
Patients presenting to the ED who were aged 18 years and older and clinically stable (defined as the South African Triage Scale designation of 'non-emergent') were included in the study. Triage scores were assigned by trained study staff, based on the South African Triage Scale (SATS).17 Patients younger than 18 years, not able to provide informed consent (i.e. patients with a depressed level of consciousness or mentally altered) or undergoing active resuscitation were excluded.
Recruitment and sampling
All patients presenting to the ED during the study period who met the inclusion criteria were approached by trained HCT staff, informed of the ongoing study and offered a point-of-care HIV test. Written informed consent was sought for testing and participation in a survey that asked about reasons for accepting or declining the test. Patients with a known HIV-positive diagnosis were asked if they had access to an antiretroviral (ARV) clinic, if they were on regular treatment and whether they were aware of having developed AIDS or being virally suppressed. Data were also collected on patient demographics, presenting complaint, presenting symptoms and past medical history.
HIV counsellors approached all eligible patients in a large waiting room after they underwent initial triage and administrative processes. Patients consenting to the study were escorted to a private room for testing if possible, whereas patients assigned a bed were tested at the bedside with curtains drawn where possible. Given the lack of an EMR or patient tracking system, HCT staff placed a small dot on the folders of all patients who were approached and offered HCT. Every 4 h, the study supervisors audited the folders of patients located within the ED to ensure that all eligible patients had been approached.
Based on recent survey data from the 2017 South African national HIV prevalence study, HIV prevalence amongst South Africans of all ages was estimated at 14%.2 Our study aimed to recruit a sample size of 700 patients at each site. This would present a large enough sample to capture the variation in testing preferences in the study setting, allowing us to detect a difference of greater than 5% from the baseline estimate of 14%, assuming a two-sided α of 0.05 and 80% power, for a period of 7 weeks at each site.
Intervention
Patients were offered point-of-care HIV testing following the South African national HIV testing guidelines.7 Patients who consented to the test provided a blood sample obtained through a lancet finger prick. Following the recommended testing algorithm, patients were first tested using the Advanced Quality Anti-HIV 1&2 rapid test (InTec Products, Inc., Fujian, China). Non-reactive samples were reported as an HIV-negative result. Reactive samples were confirmed with an HIV 1/2/O Tri-line HIV rapid test (ABON Biopharm, Hangzhou, China). Confirmed reactive samples were reported as an HIV-positive result, and patients were provided with a referral letter to a local ARV clinic. Confirmed non-reactive samples were reported as an indeterminate result, and patients were counselled to repeat the test in 4-6 weeks. Counselling preceded and followed all tests and included education on HIV transmission, prevention, and management. Results were available within 10-15 min of testing, whereas counselling required an additional 10-15 min, depending on the HIV test result.
Data collection
Ten local research assistants were hired and trained in rapid point-of-care HCT, good clinical practice and data collection, and were familiarised with the study protocol before the start of the study. Research assistants and study staff worked in shifts to ensure 24-h coverage of the ED.
In tandem with offering HIV testing, HCT staff administered a brief survey. Patient responses to questions about their gender, past medical history, mode of arrival, reason for visit, presenting complaint, and symptoms were recorded as pre-determined binary or categorical options, age was recorded as free text, and reasons for accepting or declining testing were captured via pre-determined categorical options derived from the literature or as free text. Data were recorded on case report forms. These forms were scanned and uploaded onto iDatafax (DF/Net Research, Inc., Seattle, WA, USA) by trained study staff. Following validation and cleaning, data were exported into Excel v.16.9 (Microsoft, Inc., Redmond, WA, USA), and then imported into Stata v.14 (StataCorp, TX, USA) for analysis.
The outcome of interest, declining HCT, was measured as a binary variable ('no' = 0 and 'yes' = 1). The independent variables measured were age (18-30, 31-50, 51-70, 70+), sex (male, female), presenting complaint (trauma, medical), South African triage score (death, routine visit, urgent, very urgent, emergent), access to primary care (yes, no), past medical history (hypertension, coronary artery disease, tuberculosis, diabetes, asthma, chronic obstructive pulmonary disorder, cancer), visit time (within regular operating hours, 9 am to 5 pm, or out of regular operating hours), visit reason (new complaint, return visit, referral), mode of transport (self-transport, ambulance, police), presenting symptoms (pain, fever) and disposition (death, intensive care unit admission, general admission, emergent surgery, transfer, discharge, absconded).
Data analysis and statistics
Analysis was conducted on patients unaware of their status, to examine the relationship between the outcome of interest and all other independent variables. Chi-square tests were used to explore individual variable associations with declining HCT. Logistic regression analysis was conducted to assess the contribution of each variable to declining HCT. Bivariate analysis was conducted to estimate the association between the outcome and each predictor variable, as well as multivariate analysis to estimate the independent effect of each predictor variable, adjusting for all others. All variables were included in the final model, following checks for collinearity and goodness of fit and performing a best-subsets variable selection. Sub-group analysis was completed on the top reasons for accepting and declining HCT by gender.
A reference level was selected for categorical variables with multiple responses, and other levels were accordingly compared. Associations were assessed using odds ratios (ORs), 95% confidence intervals (CIs) and p-values. A p-value of ≤ 0.05 was regarded as statistically significant. A pooled analysis of data collected from both sites is presented; no significant differences were observed between the two sites (Table 1).

Ethical consideration
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board (reference number IRB00105801), the Human Research Ethics Committee from the University of Cape Town (MREC reference number 856/2015), the Human Research Committee of Walter Sisulu University (reference number 069/2015) and the Eastern Cape Department of Health. Written consent was obtained from all participants who enrolled in the study and was required for the collection of demographic data, HCT and a follow-up call for newly diagnosed patients, separately.
Results
A total of 1010 patients presented to the NMAH ED between 27 June and 13 August 2017. Of these, 727 (72%) patients were approached by HCT staff, and 622 (61.6%) were enrolled in the study. A total of 3245 patients presented to the MRH ED between 24 July and 03 September 2017; of these, 1347 (41.5%) patients were approached by HCT staff, and 1258 (38.8%) were enrolled in the study (Table 1).
Across both sites, 2074 patients were approached by the HCT staff, and 1880 (90.6%) were enrolled in the study. Patients enrolled were slightly female predominant (966, 51.4%), with a median age of 33 years (interquartile range [IQR]:24-59). Most patients presented with medical complaints (1278, 67.9%), received a triage designation of 'urgent' (1269, 67.5%) and reported having access to primary care services (1696, 90.2%; Table 1).
Of the 1880 patients enrolled, 465 (24.7%) patients were aware of their HIV status (defined as a known HIV-positive diagnosis [371, 19.7%] or tested HIV negative within the last 12 months [94, 5%]), and 1415 (75.3%) patients were unaware of their HIV status. Of patients with a known HIV-positive diagnosis, 351 (94.9%) said they were regularly accessing an ARV clinic. Of patients who were regularly accessing an ARV clinic, 23 (6.5%) reported being virally suppressed, 46 (13.1%) reported not being virally suppressed and 282 (80.3%) were unsure (Figure 1). In addition, 20 (5.4%) patients who had a known HIV-positive diagnosis wanted to get retested to confirm if they were truly/still HIV positive.
Of the 1415 patients unaware of their status, 141 (10%) declined HCT, and 1274 (90%) accepted. Of the patients who accepted HCT, 159 (12.5%) were diagnosed as HIV positive, 1102 (86.5%) were diagnosed as HIV negative and 13 (1%) had an indeterminate result. The overall prevalence of HIV in the study population was 28.1%. Patients declining and those accepting HCT both largely presented with medical complaints (912, 64.5%), received a triage designation of 'urgent' (954, 67.4%), had stated access to primary care services (1255, 89.2%), had no past medical history (929, 65.7%), visited the ED outside of regular hours (799, 56.5%), had a new complaint (878, 62.4%), used self-transport (881, 62.7%), had symptoms of pain (790, 55.8%), had no symptoms of fever (1388, 98.1%), were ultimately discharged from the ED (718, 53.9%) and were aged 18-30 years (629, 44.5%; Table 2).
The top reasons for accepting HCT were 'has not tested in the past year' (451, 35.4%), 'has never been tested' (242, 18.9%) and 'test is rapid and free' (237, 18.6%) (Figure 1). Patients accepting testing were largely male (672, 52.7%). The top reasons for declining HCT were 'does not want to know status' (53, 37.6%), 'in too much pain' (48, 34%) and 'does not believe they are at risk' (28, 19.9%; Figure 1). Patients declining testing were largely female (85, 60.3%).
Sub-group analysis of the reasons for accepting and declining HCT by gender showed slight differences between men and women in the reported reasons (Table 3). The primary reason for accepting HCT for both men and women was 'has not tested in the past year', 35.8% and 34.9%, respectively, followed by 'has never been tested' (21.4%) for men and 'test is rapid and free' (20.3%) for women. The primary reason for declining HCT given by men was 'does not want to know status' (66.1%) and 'in too much pain' for women (22.4%), followed by 'in too much pain' for men (42.9%) and 'does not believe they are at risk' (17.6%) for women.

Univariate analysis showed that compared with male patients, female patients were more likely to decline HCT Associations were assessed using odds ratio (OR: 1.7; 95%) Confidence Intervals (CI:1.2-2.4). Patients who complained of pain compared with patients who did not (OR: 1.7; 95% CI: 1.2-2.5) and those arriving at the ED by ambulance compared to self-transport or with the police (OR: 1.4; 95% CI: 1.1-2.1) were also more likely to decline HCT. In addition, patients presenting with traumatic injuries compared with medical complaints (OR: 1.6; 95% CI: 1.1-2.2) were more likely to decline HCT. Other factors including age, triage score, access to primary care, past medical history, visit time, visit reason and final disposition did not show a statistically significant correlation with declining HCT (Table 2).
Multivariate analysis showed that patients who complained of pain compared with patients who did not (OR: 1.6; 95% CI: 1.1-2.6) were slightly more likely to decline HCT. Other variables did not show a statistically significant correlation with declining HCT (Table 2).
Discussion
We found acceptance of HCT services in the ED to be reassuringly high. Our study revealed that 90% of patients who were unaware of their HIV status accepted HIV testing. This was observed despite patients being in a clinical environment where HIV testing is not routinely offered and where patients present with acute injury or illness and are often in pain or moderate distress.11,18 These findings are consistent with results from other LMICs. In Kenya and Guyana, it was found that 97.7% and 75.5% of non-critical patients accepted HCT in the ED, respectively.19,20 Our results also highlight a substantial burden of undiagnosed HIV: 8.5% of enrolled patients were newly diagnosed as HIV positive. These patients presented to the ED for various clinical complaints, and a positive diagnosis of HIV was an incidental finding.
The two most common reasons for accepting HCT in the ED were 'has not tested in the past year', followed by 'has never been tested'. This positively implies the need for HCT in acute care settings, to cover the existing testing gap, as we are able to capture patients who are currently missed by other testing venues within the healthcare system. Recently, novel approaches for HCT - such as home-based, community-based and couples testing - have been added to traditional facility-based HCT delivery systems, with good acceptance rates.21,22 A systematic review of HCT strategies in sub-Saharan Africa reported an acceptance rate of 70% for home-based testing and 76% for community-based testing.21 However, despite the variety of testing strategies and venues, a testing gap remains, particularly amongst young adults and men.23,24 Considering that 44.1% of all patients accepting testing were young adults (18-30 years) and 52.7% were male, our study demonstrates that the ED is an opportune venue to capture this missed population.
Another factor leading to testing acceptance, reported by a fifth of patients accepting HCT, was 'the test is rapid and free'. This measure combines both cost and ease/limited time lost to testing. While it is hard to separate the two and determine which is a more significant factor, ensuring that both are addressed is a likely key to maintaining high acceptance of HCT in a fast-moving environment such as the ED. This is supported by a study in Uganda, where 25% of ED patients reported not knowing their HIV status because of the lack of access to free testing services.16 At present, HCT services are offered free of cost in all government healthcare facilities in SA; however, maintaining free services can be burdensome for the government, especially if testing services are to be further expanded. Furthermore, ensuring that testing and counselling are not time-consuming and are part of routine clinical care in the ED might address the barrier of having to seek out testing as a discrete task in itself.
Acceptance, however, was not universal. In our study population, women were significantly more likely to decline to test, whereas men were more likely to accept. Similar findings were observed in other studies examining the acceptability of testing in EDs, in both high- and low-resource settings.20,25 This might be because women are aware of having access to testing services during antenatal visits, through preventing mother-to-child transmission (PMTCT) programmes or through family planning services, and hence do not need to test in the ED.1 This is supported by the finding that more women were already aware of their HIV status; 71.6% of the patients with a known HIV-positive diagnosis were women. In addition, a significant proportion of women presenting to the ED in our context were diagnosed with pregnancy complications or injury wounds, likely justifying 'in too much pain' as the primary reason reported for women to decline HCT. On the contrary, though women were more likely to decline HCT, a majority still accepted testing when offered. While it is difficult to generalise individual motivations, factors including lack of social support and fear of stigma or rejection if tested HIV positive, especially in the presence of their partner or family accompanying them to the ED, may otherwise underlie the greater tendency of women to decline HCT.25
The top reasons reported for declining HCT in our ED, 'does not want to know status' and 'does not believe they are at risk', are interestingly established findings from high-income countries and LMICs, across healthcare settings.16,20,26,27,28,29 It could be that patients prefer uncertainty rather than facing the psychosocial consequences of an HIV-positive diagnosis, especially considering the imaginable stigma attached to such a diagnosis.30 This could be tackled through targeted pre- and post-counselling efforts. On the contrary, it is also possible that the small proportion of patients who declined to be tested are not at risk of contracting HIV and were accurately perceiving their risk. We did not include any risk measures in our survey and are thus unable to precisely indicate individuals who should have been tested.
A significant barrier to HCT in the ED and other healthcare facilities frequently described in the literature are stigma and the lack of confidentiality.20,26,31 This finding is supported by contextual knowledge, wherein anthropological studies exploring cultural perceptions and practices around HIV in Mthatha have reported pervasive stigma attached to HIV/AIDS, resulting in multiple forms of exclusion based on sexism, racism and homophobia,30 The National HIV Prevalence Survey indicated that 26% of people would not be willing to share a meal, 18% were unwilling to sleep in the same room and 6% would not speak to PLWH.2 Yet, none of the patients declining testing in our study reported reasons implying real or perceived stigma or the lack of confidentiality. The studies supporting this notion conducted in-depth interviews or had one-on-one conversations with patients, which likely allowed for deeper exploration of patient perspectives on HCT services, whereas given the patient volumes, high turnover and the lack of any coordinated processes in our study setting, it is possible that patients were less likely to report stigma as a reason for declining testing.
Pain was a notable justification for declining testing in this context and showed significant correlation through bivariate and multivariate analysis. The second most common reported reason, 'in too much pain', was not surprising, as a high proportion of cases presented with acute traumatic injuries. In addition, traumatic injuries and arriving at the ED in an ambulance, which are critical proxies to pain and the seriousness of a patient's condition, were positively correlated with declining testing. This finding is specific to declining HCT in the ED and has not been previously reported as a barrier in other testing venues. To address this barrier, the integration of pain management before HCT is recommended. If a patient's presenting complaint has been addressed by providers, and appropriate action taken, patients might be more likely to accept testing.
Linkage to care is the next critical step following testing. As part of our study, all newly diagnosed patients were counselled extensively on the importance of seeking follow-up care and were given a referral letter. Given that both NMAH and MRH see patients from a 100-km radius, it was challenging to ensure linkage to care, as it would depend on the area individuals came from and the presence and ease of access to an ART clinic. For patients who were local to Mthatha, we were able to direct them to the Gateway Clinic - an ART centre - located within the same campus as the hospital. With the consent of all patients who were newly diagnosed as HIV positive, we collected their names and contact details to conduct follow-up calls after 1 month, 6 months and a year. The follow-up calls will allow us to assess whether individuals have been able to link to care and/or what challenges they are facing in doing so. Results from the follow-up calls will be collated and analysed post-completion. Despite these challenges, 94.9% of patients with a known HIV-positive diagnosis presenting to the EDs reported having access to an ART clinic, and 85.4% of those individuals reported regularly accessing the clinic. These rates are commendable and imply a willingness of patients in this setting to seek follow-up care. However, these are self-reported statistics and could be inflated as a result of social-desirability bias.
Another interesting finding was that a small proportion of patients with a known HIV-positive diagnosis (20, 5.4%) requested a repeat test to confirm their diagnosis. Patients stated that they wanted to confirm whether they were truly HIV positive and/or if they were still HIV positive. Upon retesting, all 20 patients were HIV positive. The desire to retest when an opportunity presented could likely be a result of mistrust in the healthcare system or a result of the low health literacy in the region, which are both potential barriers to achieving high rates of testing and sustained linkage to care.30,31
There are several study limitations to consider. The protocol was to approach every patient presenting in the ED who met the inclusion criteria. However, the lack of infrastructure, systematic medical record-keeping or a patient tracking process made it challenging to retain all patients who presented to the ED. Many patients were missing from the records, whereas others were entered multiple times, making it difficult to keep count of the total number of patients. HIV counselling and testing services were provided 24 h a day, yet we were only able to approach 48% of patients who presented for care. We believe this is, in part, a result of the high volumes of patients and the quick turnaround time, as well as the time-consuming nature of counselling. Patients enrolled in the study may be a biased subset of the ED population, namely, easier to approach, spoke the same language as the HCT counsellors, had milder injuries or conditions and presented at times when the patient volume was lower. Maintaining confidentiality was challenging given the limited space - the EDs in both hospitals were in essence one big room, with beds lined up against each other. Lastly, the study was human-resource intensive. We had a team of four dedicated HCT staff at all times. Nevertheless, greater staff numbers would have allowed the capture of more study subjects. Such a situation would be difficult to sustain in a low-resource setting such as Mthatha.
To optimise our strategy and accurately capture data, given the lack of organisation and clear processes, our data were collected prospectively, whereby we relied less on recorded data and were able to capture most of it in real time. As the ED is busy and sees high patient volumes, we attempted to collect as much data as efficiently as possible, using a survey format with mostly 'yes' and 'no' questions. However, to have had a better understanding of patient perspectives, the study might have been enhanced by in-depth telephone interviews with a smaller number of patients after they had left the ED.
Conclusion
Our study demonstrated high patient acceptance of the nationally recommended HCT strategy in an ED setting. The overall adult prevalence of HIV in the ED was high at 28.1%. Patients who were male, young and not in pain or critically injured were more likely to accept HCT, critically supporting the provision of HCT in acute care settings, as it successfully captured an important demographic that has generally been missed through other testing venues. In addition, the lack of significant correlation in demographic or clinical characteristics and HCT uptake argues for a routine, non-targeted strategy in the ED. Our study further reveals the need for continued investment to ensure that HCT is widely available, with provision to effectively identify and manage pain and trauma. Finally, critical to embedding HCT in the routine clinical care offered in the ED will be the confidential conduct of HCT that permits stigma around HIV infection and testing to be appropriately addressed - something that will require further innovation and implementation research.
Acknowledgements
The authors acknowledge the staff in the Nelson Mandela Academic Hospital and Mthatha Regional Hospital emergency departments for making this research possible and the HIV Counselling and Testing team for their dedication and hard work during the study. The authors also acknowledge the contributions of Nomzamo Mvandaba for her assistance as a study coordinator for the WISE study and those of Victoria Chen and Kathryn Clark in data collection and data validation.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
B.H. conceived the original idea for the parent study and designed the protocol. A.R., P.M. and B.H. coordinated the study and data collection. A.R. carried out data analysis and prepared the manuscript. C.K., T.C.Q., D.S. and B.H. provided substantial edits and revisions.
Funding information
This research was supported by the South African Medical Research Council, the Division of Intramural Research, the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and the Johns Hopkins Center for Global Health.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, A.R. The data are not publicly available because they contain sensitive information that could compromise the privacy of research participants.
Disclaimer
All views expressed in the submitted article are the authors' own and not an official position of the institutions represented or the funders.
References
1.UNAIDS. UNAIDS data 2019. UNAIDS, Geneva, Switzerland; 2019. [ Links ]
2.Simbayi LC, Zuma K, Zungu N, et al. South African National HIV prevalence, incidence and behaviour, and communication survey, 2017. Cape Town: Human Sciences Research Council Press; 2019. [ Links ]
3.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [ Links ]
4.UNAIDS. Ending AIDS: Progress towards the 90-90-90 targets. Geneva: UNAIDS; 2017. [ Links ]
5.Johnson LF, Chiu C, Myer L, et al. Prospects for HIV control in South Africa: A model-based analysis. Glob Health Action. 2016;9(1):30314. https://doi.org/10.3402/gha.v9.30314 [ Links ]
6.DoHRoS A. National HIV counselling and testing (HCT) policy guidelines 2015. Pretoria: National Department of Health; 2015. [ Links ]
7.SANAC. Let our actions count: South Africa's National strategic plan for HIV, TB and STIs 2017-2022. Pretoria: SANAC; 2017. [ Links ]
8.Telisinghe L, Charalambous S, Topp SM, et al. HIV and tuberculosis in prisons in sub-Saharan Africa. Lancet. 2016;388(10050):1215-1227. https://doi.org/10.1016/S0140-6736(16)30578-5 [ Links ]
9.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. Lancet. 2010;376(9738):355-366. https://doi.org/10.1016/S0140-6736(10)60832-X [ Links ]
10.DoHRoS A. Mid-year population estimates. Pretoria: National Department of Health; 2015. [ Links ]
11.Rothman RE, Ketlogetswe KS, Dolan T, Wyer PC, Kelen GD. Preventive care in the emergency department: Should emergency departments conduct routine HIV screening? A systematic review. Acad Emerg Med. 2003;10(3):278-285. https://doi.org/10.1111/j.1553-2712.2003.tb02004.x [ Links ]
12.Hsieh YH, Kelen GD, Beck KJ, et al. Evaluation of hidden HIV infections in an urban ED with a rapid HIV screening program. Am J Emerg Med. 2016;34(2):180-184. https://doi.org/10.1016/j.ajem.2015.10.002 [ Links ]
13.Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: Experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33(2):147-155. https://doi.org/10.1016/s0196-0644(99)70387-2 [ Links ]
14.Haukoos JS, Rowan SE. Screening for HIV infection. BMJ. 2016;532(1):i1. https://doi.org/10.1136/bmj.i1 [ Links ]
15.Curry C, Bunungam P, Annerud C, Babona D. HIV antibody seroprevalence in the emergency department at Port Moresby General Hospital, Papua New Guinea. Emerg Med Australas. 2005;17(4):359-362. https://doi.org/10.1111/j.1742-6723.2005.00757.x [ Links ]
16.Nakanjako D, Kamya M, Daniel K, et al. Acceptance of routine testing for HIV among adult patients at the medical emergency unit at a national referral hospital in Kampala, Uganda. AIDS Behav. 2007;11(5):753-758. https://doi.org/10.1007/s10461-006-9180-9 [ Links ]
17.Twomey M, Wallis LA, Thompson ML, Myers JE. The South African Triage Scale (adult version) provides reliable acuity ratings. Int Emerg Nurs. 2012;20(3):142-150. https://doi.org/10.1016/j.ienj.2011.08.002 [ Links ]
18.Hansoti B, Kelen GD, Quinn TC, et al. A systematic review of emergency department based HIV testing and linkage to care initiatives in low resource settings. PLoS One. 2017;12(11). e0187443. https://doi.org/10.1371/journal.pone.0187443 [ Links ]
19.Waxman MJ, Muganda P, Carter EJ, Ongaro N. The role of emergency department HIV care in resource-poor settings: Lessons learned in western Kenya. Int J Emerg Med. 2008;1(4):317-320. https://doi.org/10.1007/s12245-008-0065-8 [ Links ]
20.Christensen A, Russ S, Rambaran N, Wright SW. Patient perspectives on opt-out HIV screening in a Guyanese emergency department. Int Health. 2012;4(3):185-191. https://doi.org/10.1016/j.inhe.2012.03.001 [ Links ]
21.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77-S85. https://doi.org/10.1038/nature16044 [ Links ]
22.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Group ZS. Effects of home-based voluntary counselling and testing on HIV-related stigma: Findings from a cluster-randomized trial in Zambia. Soc Sci Med. 2013;81(1):18-25. https://doi.org/10.1016/j.socscimed.2013.01.011 [ Links ]
23.Nglazi MD, Van Schaik N, Kranzer K, Lawn SD, Wood R, Bekker LG. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2012;59(3):e28-e34. https://doi.org/10.1097/QAI.0b013e31824445f0 [ Links ]
24.Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: Successes and challenges. AIDS. 2015;29(11):1401-1409. https://doi.org/10.1097/QAD.0000000000000721 [ Links ]
25.Pisculli ML, Reichmann WM, Losina E, et al. Factors associated with refusal of rapid HIV testing in an emergency department. AIDS Behav. 2011;15(4):734-742. https://doi.org/10.1007/s10461-010-9837-2 [ Links ]
26.Chimoyi L, Tshuma N, Muloongo K, Setswe G, Sarfo B, Nyasulu PS. HIV-related knowledge, perceptions, attitudes, and utilisation of HIV counselling and testing: A venue-based intercept commuter population survey in the inner city of Johannesburg, South Africa. Glob Health Action. 2015;8(1):26950. https://doi.org/10.3402/gha.v8.26950 [ Links ]
27.Schechter-Perkins EM, Koppelman E, Mitchell PM, Morgan JR, Kutzen R, Drainoni ML. Characteristics of patients who accept and decline ED rapid HIV testing. Am J Emerg Med. 2014;32(9):1109-1112. https://doi.org/10.1016/j.ajem.2014.05.034 [ Links ]
28.Christopoulos KA, Weiser SD, Koester KA, et al. Understanding patient acceptance and refusal of HIV testing in the emergency department. BMC Public Health. 2012;12(1):3. https://doi.org/10.1186/1471-2458-12-3 [ Links ]
29.Gazimbi MM. A multilevel analysis of the determinants of HIV testing in Zimbabwe: Evidence from the demographic and health surveys. HIV/AIDS Res Treat Open J. 2017;4(1):17. http://doi.org/10.17140/HARTOJ-4-124 [ Links ]
30.Leclerc-Madlala S, Simbayi LC, Cloete A. The sociocultural aspects of HIV/AIDS in South Africa. In: HIV/AIDS in South Africa 25 years on: Psychosocial perspectives. 2009; p. 13-25. [ Links ]
31.Nombembe C. Music-making of the Xhosa diasporic community: A focus on the Umguyo tradition in Zimbabwe. Cape Town: University of Witwatersrand, Faculty of Humanities, Wits School of Music in Cape Town; 2013. [ Links ]
 Correspondence:
Correspondence:
Aditi Rao
aditi.rao@jhmi.edu
Received: 15 May 2020
Accepted: 02 June 2020
Published: 22 July 2020














