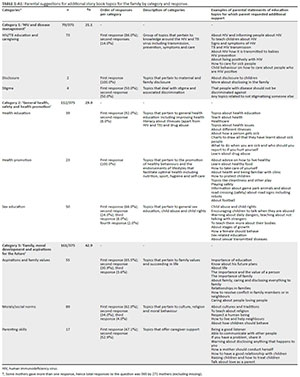
Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of HIV Medicine
versión On-line ISSN 2078-6751
versión impresa ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.21 no.1 Johannesburg 2020
http://dx.doi.org/10.4102/sajhivmed.v21i1.1039
11.Brooks RA, Kaplan RL, Lieber E, Landovitz RJ, Lee SJ, Leibowitz AA. Motivators, concerns, and barriers to adoption of preexposure prophylaxis for HIV prevention among gay and bisexual men in HIV-serodiscordant male relationships. AIDS Care. 2011;23(9):1136-1145. https://doi.org/10.1080/09540121.2011.554528 [ Links ]
12.Peng B, Yang X, Zhang Y, et al. Willingness to use pre-exposure prophylaxis for HIV prevention among female sex workers: A cross-sectional study in China. HIV AIDS. 2012;4:149-158. https://doi.org/10.2147/HIV.S33445 [ Links ]
13.Reza-Paul S, Lazarus L, Doshi M, et al. Prioritizing risk in preparation for a demonstration project: A mixed methods feasibility study of oral pre-exposure prophylaxis (PREP) among female sex workers in South India. PLoS One. 2016;11(11):e0166889. https://doi.org/10.1371/journal.pone.0166889 [ Links ]
14.Cohen SE, Vittinghoff E, Bacon O, et al. High interest in pre-exposure prophylaxis among men who have sex with men at risk for HIV-infection: Baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68(4):439-448. https://doi.org/10.1097/QAI.0000000000000479 [ Links ]
15.Whetham J, Taylor S, Charlwood L, et al. Pre-exposure prophylaxis for conception (PrEP-C) as a risk reduction strategy in HIV-positive men and HIV-negative women in the UK. AIDS Care. 2014;26(3):332-336. https://doi.org/10.1080/09540121.2013.819406 [ Links ]
16.Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS. 2011;25(16):2005-2008. https://doi.org/10.1097/QAD.0b013e32834a36d0 [ Links ]
17.Dolling DI, Desai M, McOwan A, et al. An analysis of baseline data from the PROUD study: An open-label randomised trial of pre-exposure prophylaxis. Trials. 2016;17:163. https://doi.org/10.1186/s13063-016-1286-4 [ Links ]
18.Elmes J, Nhongo K, Ward H, et al. The price of sex: Condom use and the determinants of the price of sex among female sex workers in eastern Zimbabwe. J Infect Dis. 2014;210 (Suppl 2):S569-S578. https://doi.org/10.1093/infdis/jiu493 [ Links ]
19.Wilton J, Senn H, Sharma M, Tan DH. Pre-exposure prophylaxis for sexually-acquired HIV risk management: A review. HIV AIDS. 2015;7:125-136. https://doi.org/10.2147/HIV.S50025 [ Links ]
20.Mack N, Odhiambo J, Wong CM, Agot K. Barriers and facilitators to pre-exposure prophylaxis (PrEP) eligibility screening and ongoing HIV testing among target populations in Bondo and Rarieda, Kenya: Results of a consultation with community stakeholders. BMC Health Serv Res. 2014;14:231. https://doi.org/10.1186/1472-6963-14-231 [ Links ]
21.Heffron R, Celum C, Mugo N, et al., editors. High initiation of PrEP and ART in a demonstration project among African HIV-discordant couples. 21st Conference on Retroviruses and Opportunistic Infections; 2014 March 03-06; Boston, MA: International Aids Society; 2014. [ Links ]
22.Restar AJ, Tocco JU, Mantell JE, et al. Perspectives on HIV pre- and post-exposure prophylaxes (PrEP and PEP) among female and male sex workers in Mombasa, Kenya: Implications for integrating biomedical prevention into sexual health services. AIDS Educ Prev. 2017;29(2):141-153. https://doi.org/10.1521/aeap.2017.29.2.141 [ Links ]
23.Auerbach JD, Kinsky S, Brown G, Charles V. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS. 2015;29(2):102-110. https://doi.org/10.1089/apc.2014.0142 [ Links ]
 Correspondence:
Correspondence:
Tinashe Mudzviti
tinashem@newlandsclinic.org.zw
Received: 22 Oct. 2019
Accepted: 11 Jan. 2020
Published: 19 Feb. 2020
ORIGINAL RESEARCH
Implementation of a PMTCT programme in a high HIV prevalence setting in Johannesburg, South Africa: 2002-2015
Coceka N. MnyaniI, II; Carol L. TaitIII; Remco P.H. PetersIII, IV; Helen StruthersIII, V; Avy ViolariVI; Glenda GrayVI; Eckhart J. BuchmannI; Matthew F. ChersichVII; James A. McIntyreIII, VIII
IDepartment of Obstetrics and Gynaecology, School of Clinical Medicine, University of the Witwatersrand, Johannesburg, South Africa
IISouth African Centre of Epidemiological Modelling and Analysis (SACEMA), DST-NRF Centre for Excellence, Epidemiological Modelling and Analysis, Stellenbosch University, Stellenbosch, South Africa
IIIAnova Health Institute, Johannesburg, South Africa
IVDepartment of Medical Microbiology, Maastricht University Medical Centre, Maastricht, The Netherlands
VDivision of Infectious Diseases and HIV Medicine, Department of Medicine, University of Cape Town, Cape Town, South Africa
VIPerinatal HIV Research Unit, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIWits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VIIISchool of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa
ABSTRACT
BACKGROUND: Great strides have been made in decreasing paediatric human immunodeficiency virus (HIV) infections, especially in sub-Saharan Africa. In South Africa, new paediatric HIV infections decreased by 84% between 2009 and 2015. This achievement is a result of a strong political will and the rapid evolution of the country's prevention of mother-to-child transmission (PMTCT) guidelines.
OBJECTIVES: In this paper we report on the implementation of a large PMTCT programme in Soweto, South Africa.
METHODS: We reviewed routinely collected PMTCT data from 13 healthcare facilities, for the period 2002-2015. Antiretroviral therapy (ART) coverage among pregnant women living with HIV (PWLHIV) and the mother-to-child transmission (MTCT) rate at early infant diagnosis were evaluated.
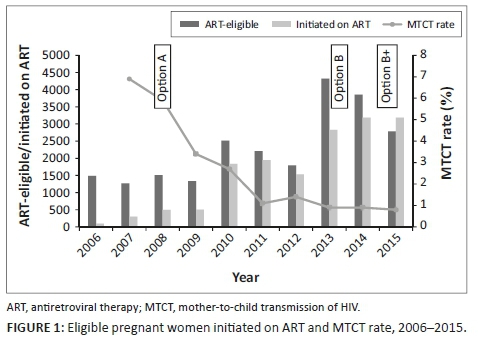
RESULTS: In total, 360 751 pregnant women attended the facilities during the review period, and the HIV prevalence remained high throughout at around 30%. The proportion of PWLHIV presenting with a known HIV status increased from 14.3% in 2009 when the indicator was first collected to 45% in 2015, p < 0.001. In 2006, less than 10% of the PWLHIV were initiated on ART, increasing to 88% by 2011. The MTCT rate decreased from 6.9% in 2007 to under 1% from 2013 to 2015, p < 0.001.
CONCLUSION: The achievements in decreasing paediatric HIV infections have been hailed as one of the greatest public health achievements of our times. While there are inherent limitations with using routinely collected aggregate data, the Soweto data reflect progress made in the implementation of PMTCT programmes in South Africa. Progress with PMTCT has, however, not been accompanied by a decline in HIV prevalence among pregnant women.
Keywords: PMTCT; South Africa; pregnant women living with HIV (PWLHIV); paediatric HIV infection; health systems.
Background
Great strides have been made in the global fight to decrease new paediatric human immunodeficiency virus (HIV) infections. In 2011, the Joint United Nations Programme on HIV/AIDS (UNAIDS) launched the Global Plan to reduce new paediatric HIV infections by 90%, by 2015, with the baseline year being 2009.1 The target was to decrease the rate of mother-to-child transmission (MTCT) of HIV to 2% or less among non-breastfeeding women, and to 5% or less among breastfeeding women.1 As part of the Global Plan, 22 priority countries, which together accounted for 90% of the global number of pregnant women living with HIV (PWLHIV) in 2009, were identified for intensified efforts for the elimination of MTCT.1 India and 21 countries in sub-Saharan Africa made up the 22 priority countries.1
According to the 2016 UNAIDS report, there was a 60% decrease in the overall MTCT rate in 21 priority countries, from 22.4% in 2009 to 8.9% in 2015 (data on India were not available).2 The decrease in the MTCT rate is largely because of the increased coverage of more efficacious antiretroviral regimens for the prevention of mother-to-child transmission (PMTCT) of HIV.2,3,4,5 South Africa was identified as one of the 22 priority countries, and through strong political will and rapid evolution of the country's PMTCT guidelines, new paediatric HIV infections decreased by 84% between 2009 and 2015, with an estimated 330 000 infections averted.2 The UNAIDS estimate for the MTCT rate for South Africa in 2015 was 2%. This was consistent with findings from a national survey conducted in 2012-2013, involving over 9000 infant-caregiver pairs, with an MTCT rate of 2.6% at 4-8 weeks.2,6
Donor funding has been critical in the establishment of the South African PMTCT and antiretroviral therapy (ART) programmes, with the country being the largest recipient of grants from the United States President's Emergency Plan for AIDS Relief (PEPFAR).7,8,9,10,11 President's Emergency Plan for AIDS Relief funding of HIV programmes in South Africa started in 2004, with direct service provision through the placement of staff and infrastructure in public healthcare facilities.8,10 From 2012, there was a transition in PEPFAR funding from direct service provision to technical support, with the South African government increasingly taking up ownership of the country's HIV programme.8,11 By 2016, more than 75% of South Africa's HIV response was funded by the government.12
This article reports on the outcomes of a large PMTCT programme in Soweto, South Africa, over time, including the coverage of ART among PWLHIV and the MTCT rate at approximately 6 weeks of age.
Methods
Study setting and design
We conducted a retrospective study of routinely collected PMTCT data from 13 public healthcare facilities that have been part of the Soweto PMTCT programme since its inception in 2002. Of the 13 facilities, one is a tertiary-level referral hospital (Chris Hani Baragwanath Academic Hospital), and 12 are primary healthcare facilities, of which six have delivery units. Soweto is an area of mixed urban and informal settlements, with an estimated population of approximately 1.7 million people.13
History of the Soweto prevention of mother-to-child transmission programme
The Soweto PMTCT programme was established in 2000 as the Demonstration of Antiretroviral Treatment (DART) programme initiated by the Perinatal HIV Research Unit (PHRU).14 The programme was initially funded by the Elizabeth Glaser Paediatric AIDS Foundation (EGPAF) with funding from the United States Agency for International Development (USAID), the Fonds De Solidarité Thérapeutique International (FSTI) and the Gauteng Department of Health, and from 2004 it was funded by PEPFAR, through the USAID.14 As part of the DART programme, pregnant women were offered voluntary counselling and testing for HIV and, if found to be HIV-positive, were issued a single-dose nevirapine (NVP) to be taken intrapartum, and also a single-dose NVP to be given to the infant immediately after birth. A free 6-month supply of infant formula was also available for women living with HIV who elected not to breastfeed.
The programme evolved in line with changes in the South African PMTCT and ART guidelines, and since 2009, the programme has been supported by the Anova Health Institute (Anova), a USAID/PEPFAR-funded non-profit organisation. The donor-funded support was initially through direct service provision with placement of staff - doctors, professional nurses, data collectors and lay counsellors - in public health facilities working alongside government employees. There was also infrastructure, pharmacy, and monitoring and evaluation support for the facilities providing HIV services. With the PEPFAR funding transitioning to technical support, the focus in support shifted to mentoring and quality improvement of the programmes through monitoring and evaluation.
Evolution of the South African prevention of mother-to-child transmission guidelines
Prior to 2002, no antiretroviral (ARV) prophylaxis or treatment was available in the South African public health sector, and ARVs were only available as part of research projects.15,16,17 From 2002 until 2007, only mother-infant single-dose NVP was available for PMTCT (Table 1). Additional zidovudine (AZT) monotherapy for PMTCT prophylaxis was introduced in 2008, initially started at 28 weeks' gestation, and from 2010 at 14 weeks' gestation.18,19,20 Antiretroviral therapy became available in South Africa in 2004 and the eligibility criterion was a CD4 count of < 200 cells/µL, or World Health Organization (WHO) stage 4 disease.21 CD4 count testing to assess ART eligibility became routinely available from 2005. The CD4 count threshold for ART initiation in pregnant women increased to ≤ 350 cells/µL in 2010.20
Up to September 2010, the antenatal clinics in the 12 primary healthcare facilities only provided antiretroviral prophylaxis for PMTCT, and PWLHIV who were eligible for lifelong ART were referred to a separate ART initiation site. In that time period, the only antenatal clinic that initiated ART was at Chris Hani Baragwanath Academic Hospital. At the primary health clinics, pregnant women diagnosed with HIV infection were referred to an ART initiation site, which could be in a different section of the same health facility, or in a different facility. Over a period of 18 months, beginning in October 2010, nurse-initiated and managed ART (NIMART) was introduced in the antenatal clinics, with PWLHIV receiving their antenatal and HIV care in the same facility. Nurse-initiated and managed ART, a task-shifting initiative to increase the number of patients initiated on ART, meant that professional nurses, including midwives, could initiate and manage patients on ART.22 Postpartum, the women were transitioned to adult HIV care for follow-up. From 2002 until 2011, a 6-month supply of free infant formula was available for all WLHIV who elected not to breastfeed.
In 2013, WHO Option B, where all pregnant and postpartum WLHIV were initiated on an efavirenz-based fixed-dose combination, was introduced.23 Treatment was stopped postpartum if not breastfeeding, or after cessation of breastfeeding, if the woman was not eligible for lifelong ART for her own health.23 The most recent PMTCT guideline change was in 2015 when all pregnant and postpartum women living with HIV became eligible for lifelong treatment regardless of CD4 count level, Option B+.24
The 2013 PMTCT guideline reinforced the recommendation that was first made in the 2010 guideline that pregnant women who initially tested HIV-negative were to be routinely retested during pregnancy at around 32 weeks' gestation. For women who presented intrapartum with an unknown HIV status, or with a negative HIV test done prior to 32 weeks' gestation, or done more than 12 weeks prior to delivery, the recommendation was for retesting intrapartum. Postpartum, the recommendation was to repeat the HIV test at 6 weeks postpartum and every 3 months during breastfeeding. In the 2015 guidelines, the recommendation is for routine repeat HIV testing every 3 months during pregnancy, intrapartum and postpartum as per the 2013 guidelines. Routine testing of HIV-exposed infants, using HIV polymerase chain reaction (PCR), became standard of care from 2004, and was offered at around 6 weeks of age. Also, as part of routine care, an HIV antibody test was done at 18 months in infants who initially tested negative at early infant diagnosis. Since 2015, routine testing of HIV-exposed infants was done at birth, 10 weeks of infant age, 6 weeks post-cessation of breastfeeding and at 18 months.
It is in this background of evolving guidelines and the changing focus of donor funding that we evaluated the Soweto PMTCT programme.
Data management and analysis
As part of routine reporting to the District Health and Information System (DHIS), PMTCT data were collected on PWLHIV and HIV-exposed infants, and the indicators collected changed over time with evolving PMTCT guidelines. We extracted aggregate data from paper-based and electronic registers on core PMTCT indicators for the period 2002-2015. The indicators that we collected data for include the following: pregnant women presenting for their first antenatal visit and gestational age at first visit; pregnant women tested for HIV during pregnancy and those presenting already known to be living with HIV and whether already on ART; PWLHIV who were issued with single-dose NVP prophylaxis antenatally; PWLHIV who had a CD4 count done during pregnancy and those who had a CD4 count of < 200 cells/µL and ≤ 350 cells/µL; the number of ART-eligible pregnant women who were initiated on ART; and the number of HIV-exposed infants who were tested for HIV and those found to be HIV-positive.
The prevalence of human immunodeficiency virus among pregnant women was calculated using the number of pregnant women presenting at the first antenatal visit already known to be living with HIV and those newly diagnosed as HIV-positive during pregnancy as the numerator and the total number of first visits as the denominator. The proportion of ART-eligible pregnant women was calculated using the number of PWLHIV who had a CD4 count of < 200 cells/µL or ≤ 350 cells/µL, depending on the CD4 count threshold at the time, divided by the total number who had a CD4 count done. For the 2013-2015 data, all PWLHIV who were not on ART at the first antenatal visit were regarded as eligible for treatment, in line with the guidelines. The number of HIV-exposed infants who were found to be HIV-positive at around 6 weeks of age was used to calculate the MTCT rate, using as the denominator the number of HIV-exposed infants tested in the 13 facilities. Data were analysed using Stata® version 14.0 (Stata Corporation, College Station, TX, USA).
Ethical consideration
The study was approved by the University of the Witwatersrand Human Research Ethics Committee (Reference No. M140461), and access to the facilities was granted by the Johannesburg Health District Office.
Results
From January 2002 to December 2008, around 30 000 pregnant women presenting for their first antenatal visit were seen in the programme annually (Table 2). As services became decentralised, and more facilities started providing antenatal services, and PMTCT and ART care, the number of pregnant women seen in the 13 facilities decreased from 2009 onwards. Most pregnant women presented for antenatal care after 20 weeks' gestation; the proportion doing so increased from 40.8% in 2010 when the indicator was first collected, to 51.3% in 2015, p < 0.001. There was also a progressive increase in pregnant women known to be living with HIV, presenting from 14.3% in 2009 to 45.0% 2015, p < 0.001. Human immunodeficiency virus testing rates during pregnancy were high throughout the study period, increasing from 95.8% in 2002 to 100% by 2010. The HIV prevalence was high from the beginning of the review period in 2002, with 28.9% of pregnant women found to be living with HIV, reaching a peak of 33.1% in 2009 (Table 2). From 2002 to 2007, when only single-dose NVP was available for PMTCT prophylaxis and issued at the first antenatal visit, the proportion of women who were issued prophylaxis peaked at 96.1% within 1 year of the implementation of the programme. Over time, the proportion of women who were issued NVP prophylaxis at the first antenatal visit decreased, as there was a shift to issuing the prophylaxis intrapartum.
With the CD4 count threshold for ART eligibility at < 200 cells/µL from 2005 to 2009, approximately 16% of PWLHIV were eligible for ART. When the CD4 count threshold was increased to ≤ 350 cells/µL in 2010, the proportion of ART-eligible pregnant women increased to around 40%. There was a progressive increase in the proportion of eligible women initiated on ART, from less than 10% in 2006 to over 80% by 2011 (Table 3 and Figure 1). From 2013, all PWLHIV were eligible to be started on ART regardless of the CD4 count level. Data for routine PCR testing of HIV-exposed infants, at around 6 weeks of age, are available for the period 2007-2015. A total of 41 948 PCR tests, with results, were reported, and of these, 1195 were found to be positive (Table 4). The MTCT rate at around 6 weeks of age decreased from 7.0% in 2007 to less than 1% in 2013-2015, p < 0.001 (Figure 1).
Discussion
In this 14-year review of the Soweto PMTCT programme, there was a progressive decline in the MTCT rate, at approximately 6 weeks of age, to under 1% by 2013. The decrease coincides with an increase in the proportion of PWLHIV initiated on ART, as the PMTCT guidelines evolved. Coverage of HIV testing of pregnant women was high throughout the study. The HIV prevalence remained high, with no discernible change over time. Donor funding and local non-governmental organisation (NGO) support were critical in the establishment of the Soweto PMTCT programme, and the collaboration with the South African Department of Health ensured sustainability of the programme.
While NVP and AZT monotherapy prophylaxis were important in decreasing the risk of MTCT in low-resource settings with limited access to ART, the greatest risk of transmission is in women with high viral loads who receive no or limited duration of ART during pregnancy.25 The decrease in the perinatal transmission rate in the Soweto programme became evident from 2008 to 2013, a period of rapid evolution of the South African PMTCT guidelines. The period saw the introduction of dual prophylaxis for PMTCT, an increase in the CD4 threshold for ART eligibility in pregnant women and also the introduction of NIMART within antenatal clinics.19,20,23 The decline in MTCT rate to below 2% coincided with the rapid increase in the number of ART-eligible pregnant women initiated on treatment in the programme. Integration of antenatal and HIV services, which was introduced in the programme in 2010, has been shown to increase the proportion of ART-eligible pregnant women initiated on ART.26,27 The infant HIV PCR testing reported does not reflect coverage, as testing was done in several other facilities in the Soweto area, not reported in this article. In spite of this, the trend in the decline in the MTCT rate is similar to that shown in published data in South Africa. It is also similar to the National Health Laboratory Services (NHLS) data for the Greater Soweto area which show a decline in the MTCT rate from 8.2% in 2007, to 1.6% in 2015 (personal communication, G. Sherman). While we did not have figures on breastfeeding transmission, postpartum MTCT remains a challenge with the 2016 UNAIDS report estimating that more than 50% of new HIV infections among children occur during the breastfeeding period.2
In spite of the gains made towards the elimination of MTCT in South Africa, several challenges remain.28 Only limited progress has been made in the prevention of new HIV infections among women of reproductive age, an important aspect of PMTCT.2 South Africa is reported to have had the highest number of new HIV infections among women of reproductive age globally in the period 2009-2015, and this is reflected in the high HIV prevalence among pregnant women, a consistent finding throughout the review period in our study.2,29 The finding of a consistently high HIV prevalence is similar to figures reported in the national antenatal sentinel HIV prevalence surveys that have been conducted in South Africa since 1990.29 Pregnant women in South Africa as a whole still present at an advanced gestational age for their first antenatal visit, with just over 50% reported to have presented before 20 weeks in 2014, albeit this being an increase from 36.7% in 2010.5 We found similar figures in our study.
The delayed presentation for antenatal care results in late ART initiation in those diagnosed as living with HIV during pregnancy. In our study, the majority of women were first diagnosed as living with HIV during their pregnancies, a finding reported in several studies conducted in South Africa and other sub-Saharan African countries, although this figure decreased over time.30,31,32,33 In spite of the late presentation for antenatal care, there was a steady increase in the proportion initiated on ART during pregnancy, a trend reported in published DHIS data.34 Among pregnant women who presented already known to be living with HIV, the proportion already on ART was high, a finding similar to that reported in the 2017 South African national antenatal sentinel HIV prevalence survey.29
One of the limitations of using aggregate data is that the denominators used to calculate rates for some of the indicators are proxy indicators. The data presented are the best available representation of the ideal, which would have been to have longitudinal data on each patient, from HIV diagnosis to the initiation of ART, and also have delivery details and linked infant HIV testing. The indicators collected also changed over time as guidelines changed. Details on the denominators used are presented in the 'Results' section. For HIV testing rates, no data were collected prior to 2009 on PWLHIV who already knew their HIV status and those who were already on ART. Hence, in this period, among those newly identified as HIV-positive, there will have been a proportion of PWLHIV who already knew their HIV status and may have been on treatment. Prior to 2013, for the indicators on PWLHIV assessed for ART eligibility and initiated on treatment, the numerator and denominator do not reflect the same group of women seen in 1 month, but the numbers even out over several months. Criteria for ART eligibility are reported in the 'Results' section. There were no data available on ART eligibility based on the WHO clinical staging.
There are also additional limitations with using routine, aggregate data, and these are related to the completeness and accuracy of the data.35,36 In their assessment of routinely collected PMTCT data from 57 public health facilities in South Africa, Nicol et al. raised concerns about the quality and consistency of reported data.36 The main discrepancies identified were between data in paper-based registers and the monthly facility reports.36 The discrepancies highlighted problems with data capturing related to lack of sufficient staff, and competence in recording and validation of data.36 In the Soweto PMTCT programme, there have always been dedicated data collectors and data managers involved in monitoring and evaluation of the programme. While recording of data in the facility registers remains primarily the responsibility of staff working at the healthcare facilities, data managers are involved in the validation of data and overseeing their work.
In spite of the limitations of the study, the Soweto PMTCT programme is a success story of the collaboration between donor-funded organisations and the South African Department of Health. The strength of this study is that it reports on a large PMTCT programme, over a long review period. While there are inherent inaccuracies with routinely collected aggregate data, the trends reported in this article are similar to those reported in published DHIS data and national surveys in South Africa.29,34,37 To our knowledge, no PMTCT data of this magnitude have been published from a low-resource, high HIV prevalence setting. Data from the programme illustrate that it is possible to significantly decrease the MTCT rate even in a high HIV prevalence setting. It is important to ensure that the gains made towards elimination of MTCT are sustained beyond the immediate postpartum period, and also ensure that HIV-exposed uninfected infants survive and thrive.38 There also needs to be a concerted effort to decrease the rate of new HIV infections, especially among women of reproductive age.
Acknowledgements
The authors would like to thank all the staff who were involved in the implementation of the Soweto PMTCT programme, and also all the mothers and infants who were part of the programme. They would also like to thank Prof. Gayle Sherman for sharing the National Health Laboratory Service (NHLS) data on infant HIV PCR testing in the Great Soweto area.
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
C.N.M. initiated the study and was the main investigator responsible for data collection and analysis, interpretation of the results and drafting of the manuscript. R.P.H.P. and C.L.T. were responsible for data collection and contributed to the drafting of the manuscript. H.S., A.V. and G.G. contributed to the initiation of the study, data collection and drafting of the manuscript. E.J.B. and M.F.C. contributed to data analysis and drafting of the manuscript. J.A.M. contributed to the initiation of the study, data collection and drafting of the manuscript. All authors reviewed, contributed to and approved the final manuscript.
Funding information
The Soweto PMTCT programme was initially funded by the Elizabeth Glaser Paediatric AIDS Foundation (EGPAF) with funding from the United States Agency for International Development (USAID), the Fonds De Solidarité Thérapeutique International (FSTI) and the Gauteng Department of Health, and from 2004 onwards it was funded by President's Emergency Plan for AIDS Relief (PEPFAR), via the USAID.
This study was funded by the US PEPFAR through the USAID under Cooperative Agreement number 674-A-12-00015 to the Anova Health Institute, Carnegie Corporation of New York PhD Fellowship (Grant number: B 8749.RO1) and SACEMA (DST/NRF Centre of Excellence in Epidemiological Modelling and Analysis), Stellenbosch University.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Joint United Nations Programme on HIV/AIDS (UNAIDS). Countdown to zero: Global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive, 2011-2015. Geneva: UNAIDS; 2011. [ Links ]
2.Joint United Nations Programme on HIV/AIDS (UNAIDS). On the fast-track to an AIDS-free generation. The incredible journey of the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive. Geneva: UNAIDS; 2016. [ Links ]
3.Burton R, Giddy J, Stinson K. Prevention of mother-to-child transmission in South Africa: An ever-changing landscape. Obstet Med. 2015;8(1):5-12. https://doi.org/10.1177/1753495X15570994 [ Links ]
4.Bhardwaj S, Barron P, Pillay Y, et al. Elimination of mother-to-child transmission of HIV in South Africa: Rapid scale-up using quality improvement. S Afr Med J. 2014;104(3):239-243. https://doi.org/10.7196/SAMJ.7605 [ Links ]
5.Millennium Development Goals 5: Improve maternal health [homepage on the Internet]. Pretoria: Statistics South Africa; 2015 [cited 2017 Jun]. Available from: http://www.statssa.gov.za/MDG/MDG_Goal5_report_2015_.pdf [ Links ]
6.Goga AE, Dinh TH, Jackson DJ, et al. South Africa PMTCT Evaluation (SAPMCTE) Team. Population-level effectiveness of PMTCT option A on early mother-to-child (MTCT) transmission of HIV in South Africa: Implications for eliminating MTCT. J Glob Health. 2016;6(2):020405. https://doi.org/10.7189/jogh.06.020405 [ Links ]
7.Johnson, K. The politics of AIDS policy development and implementation in post-apartheid South Africa. AfricaToday. 2004;51(2):107-128. https://doi.org/10.1353/at.2005.0007 [ Links ]
8.Katz IT, Bassett IV, Wright AA. PEPFAR in transition - Implications for HIV Care in South Africa. N Engl J Med. 2013;369(15):1385-1387. https://doi.org/10.1056/NEJMp1310982 [ Links ]
9.Simelela NP, Venter WD. A brief history of South Africa's response to AIDS. S Afr Med J. 2014;104(3):249-251. https://doi.org/10.7196/SAMJ.7700 [ Links ]
10.Kavanagh MM. The politics and epidemiology of transition: PEPFAR and AIDS in South Africa. J Acquir Immune Defic Syndr. 2014;65(3):247-250. https://doi.org/10.1097/QAI.0000000000000093 [ Links ]
11.Strauss M, Surgey G, Cohen S, for the South African National AIDS Council. A review of the South African Comprehensive HIV and AIDS Grant [homepage on the Internet]. 2015 [cited 2017 Jun]. Available from: http://sanac.org.za/wp-content/uploads/2015/11/A-review-of-the-South-African-Conditional-Grant-for-HIV-30Mar-vMS.pdf [ Links ]
12.PEPFAR investing more than $410 Million towards an AIDS-Free generation in South Africa [homepage on the Internet]. Media Advisory. 2016 [cited 2017 Jun]. Available from: https://za.usembassy.gov/pepfar-investing-410-million-towards-aids-free-generation-south-africa/ [ Links ]
13.Population of cities in South Africa [homepage on the Internet]. 2017 [cited 2018 Oct]. Available from: http://worldpopulationreview.com/countries/south-africa-population/cities/ [ Links ]
14.At the cutting edge of HIV/AIDS research: A review of the Perinatal HIV Research Unit, University of the Witwatersrand, 1996-2005 [homepage on the Internet]. [cited 2018 Oct]. Available from: https://www.phru.co.za/images/documents/phru_overview.pdf [ Links ]
15.Gray GE, Urban M, Chersich MF, et al. A randomized trial of two postexposure prophylaxis regimens to reduce mother-to-child HIV-1 transmission in infants of untreated mothers. AIDS. 2005;19(12):1289-1297. https://doi.org/10.1097/01.aids.0000180100.42770.a7 [ Links ]
16.Moodley D, Moodley J, Coovadia H, et al. A multicentre randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 2003;187(5):725-735. https://doi.org/10.1086/367898 [ Links ]
17.Petra Study Team. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): A randomised, double-blind, placebo-controlled trial. Lancet. 2002;359(9313):1178-1186. https://doi.org/10.1016/S0140-6736(02)08214-4 [ Links ]
18.Doherty T, Besser M, Donohue S, et al. An evaluation of the prevention of mother-to-child transmission (PMTCT) of HIV initiative in South Africa - Lessons and key recommendations [homepage on the Internet]. Durban: Health Systems Trust: 2003 [cited 2018 Oct]. Available from: http://www.hst.org.za/sites/default/files/pmtct_national.pdf [ Links ]
19.National Department of Health. Policy and guidelines for the implementation of the PMTCT Programme. Pretoria: South African National Department of Health; 2008. [ Links ]
20.National Department of Health, South Africa; South African National AIDS Council. Clinical guidelines: PMTCT (prevention of mother-to-child transmission), 2010 [homepage on the Internet]. Pretoria: South African National Department of Health; 2010 [cited 2018 Oct]. Available from: http://www.sahivsoc.org/upload/documents/NDOH_PMTCT.pdf [ Links ]
21.National antiretroviral treatment guidelines [homepage on the Internet]. Pretoria: Department of Health; 2004 [cited 2018 Oct]. Available from: http://www.doh.gov.za/docs/facts/2004/intro.pdf [ Links ]
22.Colvin CJ, Fairall L, Lewin S, et al. Expanding access to ART in South Africa: The role of nurse-initiated treatment. S Afr Med J. 2010;100(4):210-212. https://doi.org/10.7196/SAMJ.4124 [ Links ]
23.Revised antiretroviral treatment guideline update for frontline clinical health professionals [homepage on the Internet]. Pretoria: South African National Department of Health; 2013 [cited 2018 Oct]. Available from: http://www.sahivsoc.org/upload/documents/FDC%20Training%20Manual%2014%20March%202013 (1).pdf [ Links ]
24.National Consolidated Guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults [homepage on the Internet]. Pretoria: South African National Department of Health; 2015 [cited 2018 Oct]. Available from: http://www.sahivsoc.org/upload/documents/HIV%20guidelines%20_Jan%202015.pdf [ Links ]
25.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28:1049-1057. https://doi.org/10.1097/QAD.0000000000000212 [ Links ]
26.Killam WP, Bushimbwa TC, Namwinga C, et al. Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: A stepped-wedge evaluation. AIDS. 2010;24(1):85-91. https://doi.org/10.1097/QAD.0b013e32833298be [ Links ]
27.Stinson K, Jennings K, Myer L. Integration of antiretroviral therapy services into antenatal care increases treatment initiation during pregnancy: A cohort study. PLoS One. 2013;8(5):e63328. https://doi.org/10.1371/journal.pone.0063328 [ Links ]
28.Luzuriaga K, Mofenson LM. Challenges in the elimination of paediatric HIV-1 infection. N Engl J Med. 2016;374:761-770. https://doi.org/10.1056/NEJMra1505256 [ Links ]
29.Woldesenbet SA, Kufa T, Lombard C, et al. The 2017 National Antenatal Sentinel HIV Survey, South Africa. National Department of Health [homepage on the Internet]. 2019 [cited 2019 Jul]. Available from: http://www.nicd.ac.za/wp-content/uploads/2019/07/Antenatal_survey-report_24July19.pdf [ Links ]
30.Wettstein C, Mugglin C, Egger M, et al. Missed opportunities to prevent mother-to-child-transmission: Systematic review and meta-analysis. AIDS. 2012;26(18):2361-2373. https://doi.org/10.1097/QAD.0b013e328359ab0c [ Links ]
31.Technau KG, Kalk E, Coovadia A, et al. Timing of maternal HIV testing and uptake of prevention of mother-to-child transmission interventions among women and their infected infants in Johannesburg, South Africa. J Acquir Immune Defic Syndr. 2014;65(5):e170-e178. https://doi.org/10.1097/QAI.0000000000000068 [ Links ]
32.Kim MH, Ahmed S, Hosseinipour MC, et al. The impact of Option B+ on the antenatal PMTCT cascade in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2015;68:e77-e83. https://doi.org/10.1097/QAI.0000000000000517 [ Links ]
33.Herce ME, Mtande T, Chimbwandira F, et al. Supporting option B+ scale up and strengthening the prevention of mother-to-child transmission cascade in central Malawi: Results from a serial cross-sectional study. BMC Infect Dis. 2015;15:328. https://doi.org/10.1186/s12879-015-1065-y [ Links ]
34.Massyn N, Peer N, English R, Padarath A, Barron P, Day C, editors. District health barometer 2015/16. Durban: Health Systems Trust; 2016. [ Links ]
35.Barron P, Pillay Y, Doherty T, et al. Eliminating mother-to-child HIV transmission in South Africa. Bull World Health Organ. 2013;91(1):70-74. https://doi.org/10.2471/BLT.12.106807 [ Links ]
36.Nicol E, Dudley L, Bradshaw D. Assessing the quality of routine data for the prevention of mother-to-child transmission of HIV: An analytical observational study in two health districts with high HIV prevalence in South Africa. Int J Med Inform. 2016;95:60-70. https://doi.org/10.1016/j.ijmedinf.2016.09.006 [ Links ]
37.Sherman GG, Mazanderani AH, Barron P, et al. Toward elimination of mother-to-child transmission of HIV in South Africa: How best to monitor early infant infections within the Prevention of Mother-to-Child Transmission Program. J Glob Health. 2017;7(1):010701. https://doi.org/10.7189/jogh.07.010701 [ Links ]
38.Evans C, Jones CE, Prendergast AJ. HIV-exposed, uninfected infants: New global challenges in the era of paediatric HIV elimination. Lancet Infect Dis. 2016;16(6):e92-e107. https://doi.org/10.1016/S1473-3099(16)00055-4 [ Links ]
 Correspondence:
Correspondence:
Coceka Mnyani
nandipha.mnyani@gmail.com
Received: 22 Aug. 2019
Accepted: 20 Oct. 2019
Published: 23 Mar. 2020
SCIENTIFIC LETTER
Drug resistance after cessation of efavirenz-based antiretroviral treatment started in pregnancy
Globahan AjibolaI; Christopher RowleyI, II, III; Dorcas MaruapulaI; Jean LeidnerIV; Kara BennettV; Kathleen PowisI, II, VI, VII; Roger L. ShapiroI, II, III; Shahin LockmanI, II, VIII
IBotswana Harvard T.H. Chan School of Public Health AIDS Initiative Partnership, Gaborone, Botswana
IIDepartment of Immunology and Infectious Diseases, Harvard T.H. Chan School of Public Health, Boston, United States
IIIBeth Israel Deaconess Medical Center, Boston, United States
IVGoodtables Data Consulting, LLC., Norman, United States
VBennett Statistical Consulting, Inc., Ballston Lake, United States
VIDepartment of Medicine, Division of General Internal Medicine, Massachusetts General Hospital, Boston, United States
VIIDepartment of Pediatrics and Pediatric Surgery, Massachusetts General Hospital, Boston, United States
VIIIDivision of Infectious Disease, Brigham and Women's Hospital, Boston, United States
ABSTRACT
BACKGROUND: To reduce risk of antiretroviral resistance when stopping efavirenz (EFV)-based antiretroviral treatment (ART), staggered discontinuation of antiretrovirals (an NRTI tail) is recommended. However, no data directly support this recommendation
OBJECTIVES: We evaluated the prevalence of HIV drug resistance mutations in pregnant women living with HIV who stopped efavirenz (EFV)/emtricitabine (FTC)/tenofovir disoproxil fumarate (TDF) postpartum
METHOD: In accordance with the prevailing Botswana HIV guidelines at the time, women with pre-treatment CD4 > 350 cells/mm3, initiated EFV/FTC/TDF in pregnancy and stopped ART at 6 weeks postpartum if formula feeding, or 6 weeks after weaning. A 7-day tail of FTC/TDF was recommended per Botswana guidelines. HIV-1 RNA and genotypic resistance testing (bulk sequencing) were performed on samples obtained 4-6 weeks after stopping EFV. Stanford HIV Drug Resistance Database was used to identify major mutations
RESULTS: From April 2014 to May 2015, 74 women who had stopped EFV/FTC/TDF enrolled, with median nadir CD4 of 571 cells/mm3. The median time from cessation of EFV to sample draw for genotyping was 5 weeks (range: 3-13 weeks). Thirty-two (43%) women received a 1-week tail of FTC/TDF after stopping EFV. HIV-1 RNA was available from delivery in 70 (95%) women, 58 (83%) of whom had undetectable delivery HIV-1 RNA (< 40 copies/mL). HIV-1 RNA was available for 71 women at the time of genotyping, 45 (63%) of whom had HIV-1 RNA < 40 copies/mL. Thirty-five (47%) of 74 samples yielded a genotype result, and four (11%) had a major drug resistance mutation: two with K103N and two with V106M. All four resistance mutations occurred among women who did not receive an FTC/TDF tail (4/42, 10%), whereas no mutations occurred among 18 genotyped women who had received a 1-week FTC/TDF tail (p = 0.053
CONCLUSIONS: Viral rebound was slow following cessation of EFV/FTC/TDF in the postpartum period. Use of an FTC/TDF tail after stopping EFV was associated with the lower prevalence of subsequent NNRTI drug resistance mutation
Keywords: drug resistance; resistance mutations; HIV; antiretroviral treatment; Botswana.
Introduction
Cessation of antiretrovirals raises concerns about the potential for the development of drug-resistant viral strains, particularly for non-nucleoside reverse transcriptase inhibitors (NNRTIs). Non-nucleoside reverse transcriptase inhibitors, such as efavirenz (EFV), have long half-lives and a low barrier to the development of drug resistance. When used in combination with other classes of drugs such as nucleoside reverse transcriptase inhibitors (NRTIs), staggered discontinuation using a 4-7-day 'tail' of the NRTI backbone when stopping NNRTI-based antiretroviral treatment (ART) is advised.1 This is believed to mitigate the unintended period of monotherapy with NNRTIs that ensues because of the long half-life of most drugs in this class, thus reducing the risk of developing drug resistance.2,3 There are, however, very limited data to directly support this recommendation for a tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) 'tail': following cessation of EFV. Of note, pharmacokinetic variability (linked genetic factor - CYP2B6) in EFV metabolism between individuals has been observed, and EFV levels may be higher in persons of African descent.4 These higher drug levels may also impact risk of drug resistance when stopping EFV-based ART.
Prior to 2015, both the World Health Organization (WHO) and the Botswana programme for the prevention of mother-to-child transmission (PMTCT) recommended that pregnant women living with HIV with a CD4 cell count > 350 cells/mm3 without a WHO stage 3 or 4 illness take three-drug ART in pregnancy, but discontinue the ART postpartum or upon breastfeeding cessation (option B). Although both WHO and Botswana subsequently modified guidelines to recommend lifelong ART regardless of CD4 cell count or disease stage (option B+), the prior guidance for pregnant women, as well as the inconsistent application of an NRTI tail at the time of EFV cessation, offers an opportunity to evaluate the development of resistance and the performance of a TDF/FTC tail among women stopping an EFV/TDF/FTC regimen. Because this ART regimen remains widely used throughout the world, the natural experiment afforded by prior PMTCT guidelines is applicable to our understanding of the risk of cessation of NNRTI-based regimens generally.
Methods
Study population
Between April 2014 and May 2015, we enrolled a subset of pregnant women living with HIV participating in a trial of infant cotrimoxazole prophylaxis in Botswana (the 'Mpepu' study) into this resistance sub-study. Mpepu was a double-blinded randomised controlled trial designed to assess the efficacy and safety of infant cotrimoxazole prophylaxis versus placebo used daily from as early as 14 days of life through 15 months among HIV-exposed uninfected infants in Botswana.5
Women were eligible for this sub-study of drug resistance following cessation of EFV/FTC/TDF if they were living with HIV, had pre-treatment CD4 > 350 cells/mm3 without evidence of WHO stage 3 or 4 disease, initiated EFV/FTC/TDF in pregnancy and stopped ART postpartum according to Botswana guidelines at the time (at 4-6 weeks postpartum if formula feeding, or 6 weeks after weaning if breastfeeding). Per Botswana national HIV treatment guidelines, a 7-day tail of TDF/FTC after cessation of EFV was recommended. However, decisions regarding receipt and cessation of maternal ART occurred at government clinics (rather than study clinics), and the 7-day tail was inconsistently applied at the time of ART cessation.
Data collection and laboratory testing
Women eligible to participate in this sub-study were identified in the Mpepu study and scheduled for a clinic visit 4-6 weeks after ART cessation. Interested and eligible women consented to participate in the study and provided data (from self-report and medical records) on PMTCT regimen received; dates on which ART and individual antiretrovirals were started and stopped as well as provided blood specimens for drug resistance and HIV-1 RNA testing. HIV RNA results at the point of enrolment into the Mpepu study, and documented nadir CD4 counts, were retrieved and served as baseline values.
Prior to drug resistance testing, we first determined HIV-1 RNA viral load levels with the Abbott m2000sp/rt machine (limit of detection 40 copies/mL). Where HIV-1 RNA was detected, it was extracted using an automated validated technique.6 We then performed reverse transcription using in-house one-step RT-PCR technique.7 Polymerase chain reaction (PCR) products were then purified8 and sequenced.9 Consensus sequences were generated, and the Stanford HIV Drug Resistance Database was used to identify all drug resistance mutations in the protease and reverse transcriptase coding regions.
Statistical methods
Statistical Package for Social Sciences (SPSS) version 25 was used to perform statistical analyses, which were generally descriptive in nature; a two-sided Fisher's exact test was used to evaluate for differences in the proportion of drug resistance by the approach to ART cessation, either with a 7-day TDF/FTC tail or with abrupt cessation of all three drugs. The small number of women with drug resistance mutations precluded more detailed analysis of predictors of drug resistance.
Ethical onsiderations
The Botswana Health Research Development Committee and the Office of Human Research Administration at Harvard T. H. Chan School of Public Health approved this drug resistance sub-study (HRDC 00732 and IRB13-2772), and women provided written informed consent for participation.
Results
Baseline characteristics
Ninety women enrolled, 74 of whom discontinued EFV/FTC/TDF postpartum and had samples collected for genetic resistance testing and are included in this analysis. Sixteen of 90 women not included in this analysis had CD4 < 350 cell/mm3 post-delivery and had to continue ART for their own health per Botswana national protocol at the time. The median time from cessation of EFV (whether or not a tail period occurred) to time sample was collected for resistance testing was 5 weeks (range: 3-13 weeks). Median age at enrolment was 29 years (range: 20-45) with median nadir CD4 count of 571 cells/mm3 (range: 361-1236). Forty-seven (64%) women had no previous exposure to antiretrovirals, 25 (34%) reported previous exposure to antiretrovirals for PMTCT purposes in a prior pregnancy and 2 (2%) were previously exposed to EFV/FTC/TDF but stopped prior to conception and then re-started EFV/FTC/TDF as three-drug prophylaxis for the index pregnancy. Thirty-two (43%) stopped ART with the recommended 1-week tail of TDF/FTC after cessation of EFV and 42 (57%) stopped all treatment at once. Of 70 women with viral load results at enrolment into the Mpepu study, 58 (83%) had HIV-1 RNA < 40 copies/mL. At 4-6 weeks post-EFV/FTC/TDF cessation, 44 (62%) of 71 women with viral load result had HIV-1 RNA < 40 copies/mL. Thirty-three (47%) women had HIV-1 RNA < 40 copies/mL at delivery and at the time of sample draw for genotyping (post-ARV cessation).
Mutations
Thirty-five (47%) of 74 samples were successfully sequenced, with 4 (11%) of 35 having a major drug resistance mutation: 2 with K103N and 2 with V106M (Table 1). All four resistance mutations occurred among women who did not receive a TDF/FTC tail (4/42, 10%), whereas no mutations occurred among 18 genotyped women who had received a TDF/FTC tail (p = 0.053). Eighteen (58%) of the 31 women who had a genotype result and no drug resistance mutation took a TDF/FTC tail. Of 39 samples that could not be sequenced, 37 (95%) had HIV-1 RNA < 40 copies/mL in the sample being tested.
Discussion
A small proportion of women stopping EFV/FTC/TDF had a major NNRTI resistance mutation detected in plasma taken a little more than a month after stopping treatment. Among women receiving a 7-day tail of TDF/FTC after stopping EFV where genetic resistance testing was able to be performed, none had detectable mutations. Our findings thus support the recommended staggered discontinuation of antiretrovirals when an EFV/FTC/TDF regimen is being stopped. Our data support prior studies that evaluated the use of an NRTI tail when discontinuing EFV-based ART.
Our observation of prolonged viral suppression < 40 copies/mL for a median of 5 weeks in 62% of women post-ART cessation was similar to findings from other studies which have demonstrated that it is not unusual for viral suppression to persist for weeks after stopping ART.10 We believe this could be because of low pre-ART viral loads in our population (because of women with less advanced disease starting ART in pregnancy).
Our study had several limitations. Firstly, our results are based on a small number of observations making generalisability challenging. Secondly, we did not report on minor drug resistance variants and, finally, more than half (63%) of women tested at a median time of 5 weeks from EFV cessation were still virally suppressed, which may suggest that we might have tested for resistance strain development early. Thirdly, we could not determine why the 7-day tail was inconsistently applied at the time of ART cessation and could therefore have missed evaluating possible confounders related to this factor.
Conclusion
Although guidelines no longer recommend discontinuation of ART after pregnancy, our study has general applicability for those requiring permanent or temporary treatment discontinuation when receiving an EFV-based regimen, informing the risk of mutation emergence in the setting of abrupt three-drug discontinuation versus discontinuing with a TDF/FTC tail. Furthermore, despite the shift to the use of newer drugs such as dolutegravir for which there is less concern about the emergence of resistance than with NNRTIs, our data are relevant to millions of individuals still on EFV-based ART (and are of particular clinical importance in regions that have access to only a limited number of antiretroviral drugs and that are also generally unable to routinely assess for drug resistance mutations before initiating a new ART regimen).
Based upon our results, we conclude that an TDF/FTC tail may help prevent selection of major NNRTI drug resistance mutations that can occur with the abrupt discontinuation of an EFV/FTC/TDF ART regimen.
Acknowledgements
Many thanks to the management and staff of the Botswana Harvard AIDS institute partnership for their support and to all the participants enrolled in the study.
Competing interests
The authors declare that no competing interests exist.
Authors' contributions
S.L. was the project leader, and G.A. and S.L. were responsible for experimental and project design. G.A. and C.R. performed most of the experiments. R.L.S. and K.P. made conceptual contributions, while D.M. prepared the samples. Calculations were performed by G.A., J.L. and K.B. G.A, C.R. and S.L. co-wrote the article.
Funding information
This project was funded by the National Institute of Child Health and Human Development, and the National Institute of Allergy and Infectious Diseases, NIH grant: R01HD061265.
Data availability statement
Data and associated documentation from this study will be made available for external use only under a data-sharing agreement that provides for (1) a commitment to using the data only for research purposes and not to identify any individual participant; (2) a commitment to securing the data using appropriate computer technology; and (3) a commitment to destroying or returning the data after analyses are completed.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Lamorde M, Schapiro JM, Burger D, Back DJ. Antiretroviral drugs for prevention of mother-to-child transmission: Pharmacologic considerations for a public health approach. AIDS. 2014;28(17):2551-2563 https://doi.org/10.1097/QAD.0000000000000439 [ Links ]
2.McIntyre JA, Hopley M, Moodley D, et al. Efficacy of short-course AZT plus 3TC to reduce nevirapine resistance in the prevention of mother-to-child HIV transmission: A randomized clinical trial. PLoS Med. 2009;6(10):e1000172. https://doi.org/10.1371/journal.pmed.1000172 [ Links ]
3.Chi BH, Sinkala M, Mbewe F, et al. Single-dose tenofovir and emtricitabine for reduction of viral resistance to non-nucleoside reverse transcriptase inhibitor drugs in women given intrapartum nevirapine for perinatal HIV prevention: An open-label randomised trial. Lancet. 2007;370(9600):1698-1705. https://doi.org/10.1016/S0140-6736(07)61605-5 [ Links ]
4.Sinxadi PZ, Leger PD, McIlleron HM, et al. Pharmacogenetics of plasma efavirenz exposure in HIV infected adults and children in South Africa. Br J Clin Pharmacol. 2015;80(1):146-156. https://doi.org/10.1111/bcp.12590 [ Links ]
5.Lockman S, Hughes M, Powis K, Ajibola G, Bennett K, Moyo S. Effect of co-trimoxazole on mortality in HIV-exposed but uninfected children in Botswana (the Mpepu Study): A double-blind, randomized, placebo-controlled trial. Lancet Glob Health. 2017;5(5):e491-e500. https://doi.org/10.1016/S2214-109X(17)30143-2 [ Links ]
6.EZ1 Virus Mini kit v2.0, Virus Handbook. QIAGEN sample and assay technologies, USA [homepage on the Internet]. 2010 [cited 2019 Jul 04]. Available from: https://www.qiagen.com/cn/resources/download.aspx?id=ca13c92a-4b1b-4ced-9796-b0414a166803&lang=en [ Links ]
7.Rowley CF, MacLeod IJ, Maruapula D, et al. Sharp increase in rates of HIV transmitted drug resistance at antenatal clinics in Botswana demonstrates the need for routine surveillance. J Antimicrob Chemother. 2016;71(5):1361-1366. https://doi.org/10.1093/jac/dkv500 [ Links ]
8.BigDye Terminator v3.1 Cycle Sequencing Kit User Guide. Thermo-Fisher scientific [homepage on the Internet]. 2016 [cited 2019 Jul 04]. Available from: http://tools.thermofisher.com/content/sfs/manuals/cms_081527.pdf [ Links ]
9.Applied Biosystems 3130/3130xl Genetic Analyzers User Guide. Life technologies [homepage on the Internet]. 2012 [cited 2019 Jul 04]. Available from: http://tools.thermofisher.com/content/sfs/manuals/4477796.pdf [ Links ]
10.Graham TC, Aga E, Bosch RJ, et al. Brief report: Relationship among viral load outcomes in HIV treatment interruption trials. J Acquir Immune Defic Syndr. 1999;72(3):310-313. https://doi.org/10.1097/QAI.0000000000000964 [ Links ]
 Correspondence:
Correspondence:
Globahan Ajibola
gajibola@bhp.org.bw
Received: 19 Aug. 2019
Accepted: 16 Sept. 2019
Published: 27 Jan. 2020
Note: For editorial commentary from Prof. Gary Maartens (University of Cape Town) on this article, please see: https://doi.org/10.4102/sajhivmed.v21i1.1036.
^rND^sLamorde^nM^rND^sSchapiro^nJM^rND^sBurger^nD^rND^sBack^nDJ^rND^sMcIntyre^nJA^rND^sHopley^nM^rND^sMoodley^nD^rND^sChi^nBH^rND^sSinkala^nM^rND^sMbewe^nF^rND^sSinxadi^nPZ^rND^sLeger^nPD^rND^sMcIlleron^nHM^rND^sLockman^nS^rND^sHughes^nM^rND^sPowis^nK^rND^sAjibola^nG^rND^sBennett^nK^rND^sMoyo^nS^rND^sRowley^nCF^rND^sMacLeod^nIJ^rND^sMaruapula^nD^rND^sGraham^nTC^rND^sAga^nE^rND^sBosch^nRJ^rND^1A01^nNatasha^sKhamisa^rND^1A02^nMaboe^sMokgobi^rND^1A03^nTariro^sBasera^rND^1A01^nNatasha^sKhamisa^rND^1A02^nMaboe^sMokgobi^rND^1A03^nTariro^sBasera^rND^1A01^nNatasha^sKhamisa^rND^1A02^nMaboe^sMokgobi^rND^1A03^nTariro^sBaseraORIGINAL RESEARCH
Knowledge, attitudes and behaviours towards people with HIV and AIDS among private higher education students in Johannesburg, South Africa
Natasha KhamisaI; Maboe MokgobiII; Tariro BaseraIII
IDepartment of Public Health, School of Engineering, IT, Science and Health, IIE MSA, Johannesburg, South Africa
IIDepartment of Psychology, School of Social Science, IIE MSA, Johannesburg, South Africa
IIIMédecins Sans Frontières, Rustenburg, South Africa
ABSTRACT
BACKGROUND: Human immunodeficiency virus and acquired immunodeficiency syndrome (HIV and AIDS) is a global health and social problem, with South Africa having an estimated overall prevalence rate of 13.5%. Compared to young male participants, young female participants have been reported to have less knowledge about HIV and AIDS, including prevention strategies, and this is associated with risky sexual behaviours and negative attitudes towards condom use
OBJECTIVES: The study investigated gender differences in knowledge, attitudes and behaviours towards HIV and AIDS among 542 private higher education students in Johannesburg, South Africa
METHOD: Participants completed an online structured questionnaire measuring knowledge, attitudes and behaviours as well as demographics (including age, gender and relationship status
RESULTS: The results indicate that overall there were no significant differences between male and female students in terms of HIV and AIDS knowledge. However, female students had significantly less knowledge with regard to unprotected anal sex as a risk factor for HIV and AIDS. In addition, young female students reported condom use at last sex less frequently than male students. Nonetheless, both genders reported a positive attitude towards condom use and towards people living with HIV and AIDS
CONCLUSION: It is recommended that the relevant authorities at the state and the higher education level seriously consider implementing specific strategies for preventing HIV and AIDS through improved knowledge, attitudes and behaviours among young females
Keywords: attitudes about contraception; levels of knowledge; risky sexual behaviours; gender differences; young female students.
Introduction
Since the beginning of the epidemic, human immunodeficiency virus and acquired immunodeficiency syndrome (HIV and AIDS) has affected more than 70 million people globally, accounting for 35 million deaths.1 As of 2018, over 30% of the global HIV and AIDS prevalence has been among the youth aged 15-25 years, with 5 million young people currently living with HIV and AIDS.1 The youth between the ages of 15 and 24 years account for 45% of new infections.2 The burden of HIV and AIDS is concentrated in sub-Saharan Africa, where 71% of people living with HIV reside and where 65% of new infections reported in 2017 occurred.3 The female population is disproportionately affected by HIV, with three in four new infections reported among girls aged 15-19 years, while the young female population aged 15-24 years is twice as likely to be living with HIV and AIDS than their male counterparts.4
South Africa is known to have one of the highest rates of HIV and AIDS globally and on the African continent. It is estimated that 7.97 million South Africans (13.5%) are living with HIV and AIDS, and over a fifth of these are females of reproductive age (15-49 years). Gauteng Province, the most populous province in the country (25.8% of the population), is home to over 2 million young people, the majority of whom are females.5 The high infection rate among the young female population is attributed to a lack of knowledge as well as poor attitudes towards the use of condoms and risky sexual behaviour.6 Knowledge regarding HIV and AIDS infection is necessary to correct negative attitudes towards condom use and to encourage healthy sexual behaviour among the youth by improving their ability to practice safe sex. This is likely to improve the uptake of HIV prevention strategies to address the increase in the prevalence of HIV and AIDS in vulnerable populations.6,7 Young female participants are twice as likely to be infected with HIV and AIDS, compared to young male participants, with the most common means of transmission being unprotected sex, and the key barriers to prevention being lack of knowledge, negative attitudes and risky sexual behaviour.3,6,8
Knowledge facilitates familiarity with and awareness of HIV and AIDS, which influences attitudes (resulting in support and motivation for prevention) as well as behaviour (safer sex practices), thereby reducing the risk of infection.9 A high prevalence of HIV and AIDS is associated with lower levels of knowledge about the modes of transmission and condom use, negative attitudes towards condom use and risky sexual behaviours such as unsafe sex and multiple sex partners.10,11 The young female population has been shown to possess significantly lower levels of knowledge as well as misconceptions and erroneous beliefs about HIV and AIDS, compared to the male population.6,12 This is often associated with negative attitudes to prevention strategies, which have been shown to affect behaviours such as condom use.13 Studies have also confirmed that HIV and AIDS testing attitudes and the intention to use condoms are influenced by knowledge.14,15
Charles et al.16 reveal that although the young female population is more concerned about HIV and AIDS infection, they agree less than the young male population on condom use as a safe sex strategy. The attitudinal gender differences of the young female population with regard to transactional sex are thought to result in risky behaviours such as unprotected sex with older men.17
Although it is known that the young female population is more susceptible to HIV and AIDS infection, there is a paucity of research on gender differences in knowledge, attitudes and behaviours among young people in higher education settings, thereby inhibiting an in-depth understanding of factors contributing to HIV and AIDS infection among this population in South Africa. Such a gap in the literature negatively influences policy and practice aimed at reducing HIV and AIDS rates among vulnerable youth within this context. This study was aimed at identifying gender differences in knowledge, attitudes and behaviours among the youth at a private higher education institution in Johannesburg, South Africa. It is envisaged that this will allow for the development of specific strategies for preventing HIV and AIDS infection among the youth at private higher education institutions in South Africa. The research question seeks to determine differences between the male and female populations on knowledge, attitudes and behaviours towards HIV and AIDS. It is hypothesised that knowledge, attitudes and behaviours will differ between the male and the female populations.
Methods
Study design
This cross-sectional survey was conducted at a private higher education institution in Johannesburg, South Africa. Participants were invited via an online learning platform where they completed and submitted a structured questionnaire assessing sexual risk and sexual prevention behaviours.
Setting
Johannesburg is the capital of Gauteng province and is considered the largest and the wealthiest city in South Africa. With a population estimated at 5.6 million, it is the most populous city in the country, with 66% growth rate in population expected over the next 30 years. Racial profiles indicate that 76.4% of the population is black African, 5.6% is mixed race, 12.3% is white or of European descent and 4.9% is of Indian or Asian descent. Approximately, 7% of the population is illiterate, 3.4% have only a primary education, 41% have completed secondary education and 6% have a tertiary qualification.18
Study population and sampling
Random sampling was used to recruit 845 students enrolled at a private higher education institution in Johannesburg, South Africa. Random numbers were generated using a computer program to select participants from the sampling frame - enrolment records. A global email was sent to all potential participants inviting them to participate in the study. Of those invited to participate in the study, 542 responded - a response rate of 64%. Participants completed an online questionnaire via their online learning platform, which contained study information and instructions for accessing and completing the questionnaire.
Data collection and analysis
An online structured questionnaire measuring knowledge, attitudes and behaviours as well as demographics (including age, gender and relationship status) was completed by the participants. The questionnaire was developed as part of a larger study using existing literature and consisted of 93 questions of which 51 questions were on knowledge about HIV and AIDS transmission and prevention, attitudes towards HIV and AIDS, including treatment and prevention methods (six questions), and sexual behaviours (36 questions).
Data were cleaned and checked for errors before coding and analysing using STATA 14.0 (StataCorp, College Station, TX, USA). To evaluate knowledge and behaviours, respondents were required to provide mostly 'yes' or 'no' responses. For the knowledge score, a score of 1 was assigned for a correct answer and 0 for a wrong answer. For the attitude questions, a rank was assigned using the Relative Importance Index to obtain an overall rank for each attitude item. For knowledge, attitudes and practice questions (knowledge and awareness of HIV & AIDS, prevention and control of HIV, students' attitudes towards condom use and people living with HIV, risky sexual behaviours), the frequency of responses in each category was determined. A chi-square test was used to evaluate the variation in knowledge, attitude and behaviour between male and female students. For all tests, p < 0.05 was considered statistically significant.
Ethical considerations
Ethical approval to conduct the study was obtained from Monash University Human Research Ethics Committee (MUHREC) (approval number CF15/1095 - 2015000518). No identifying information was obtained from students when they completed the questionnaire. A unique identifier was generated when the questionnaire was submitted. All the data were de-identified for analysis. Data were stored in password-protected files and will be retained for up to 5 years after the study.
Results
Socio-demographic characteristics of the students
Data were collected from 542 students, 374 (69.0%) of whom were female students. The participants had a median age of 19 years (interquartile range [IQR] = 16-30 years), and their average knowledge score of HIV and AIDS and sexually transmitted infections (STIs) was 0.78 (standard deviation [SD] = 0.17); 397 (73.2%) students were black Africans, 67 (12.4%) students were whites, 44 (8.1%) students were of Indian/Asian descent and 29 (5.4%) students were of mixed race; 427 (80.6%) students were single and 88 (16.6%) students were in a stable relationship. Most of the students were in the Higher Certificate, Higher Education Studies' stream (n = 357, 71.1%), and 145 (28.9%) students were undergraduates (Table 1).

There were high levels of awareness and knowledge about biomedical methods of HIV prevention amongst the sample group. More female (77.1%) than male students (64.1%, p = 0.003) said that they had heard about medication that HIV-positive pregnant women could take to reduce the risk of infecting the baby with HIV, and more female (59.9%) than male students (47.6%, p = 0.015) had heard about medication that could help to reduce the risk of HIV infection if a woman had been raped. A higher proportion of male (95.8%) versus female students (85.7%, p = 0.001) said that they were able to obtain a condom. There was no significant difference between the proportion of male (97.0%) and female students (94.1%) about where to get condoms (Table 2).
Knowledge about HIV transmission was high, with 98.1% of female students and 96.4% of male students knowing that the virus could be passed on through unprotected sex. A high proportion of male students (92.9%) and female students (90.9%) knew that a person can have HIV and pass it on to others without showing symptoms. Also, most female students (77.4%) and male students (76.8%) knew that STIs put people at greater risk of HIV infection. However, only 33.6% of female students and 39.3% of male students admitted that they knew that anal sex increased the risk of HIV infection. A low proportion of female students (16.9%) and male students (21.4%) knew that people can reduce their chance of getting HIV by using a condom every time they have sex. More female (96.2%) than male students (91.7%) were aware that AIDS could not be cured. Most of the female students (n = 307, 82.5%) and male students (n = 119, 70.8%) indicated that partners could not have sexual intercourse if both partners were HIV-positive (p = 0.002) (Table 3).
As illustrated in Table 4, a substantial number of students expressed positive attitudes towards condom use. Across age and gender groups, a significant majority of students disagreed with the statement that a woman loses a man's respect if she asks him to use a condom (96.8% of female participants and 91.7% of male participants); that they only use condoms if their sexual partner wants to use them (95.7% female participants and 86.3% of male participants); and that condoms should only be used if having sex with a person who is not the main sexual partner (94.6% of female participants and 83.9% of male participants). About a third of the students and 41.7% of the male students said that condoms felt unnatural, and nearly a quarter of the students aged 20-32 years and 36.3% of the male students said that condoms alter climax or orgasm.
Most participants had positive attitudes towards HIV-positive people, but 38 (7%) participants were unwilling to be associated with or share living space with people living with HIV. Based on the relative importance index score, the most important attitudes towards people living with HIV, ranked in order of relative importance, were: (1) About 83.4% of students indicated that even if a family member had HIV, their relationship with them would remain good; (2) 20% of students said that sharing a house with HIV-positive people would be very difficult for them; and (3) 7.6% of students felt that people who get infected with HIV are promiscuous. Forty-one (7.6%) students also said they do not want to be associated with HIV-positive people, and 6.4% of students felt that HIV-negative people should not be allowed to socialise with HIV-positive people. Students had positive attitudes towards treatment for HIV and AIDS. Around two-thirds (63.1%) of the students agreed that HIV treatment would keep an HIV-positive person alive (ranked the most important attitude). Two hundred and seventy-four (50.8%) students agreed that HIV medication really works (with a relative importance score of 2). Most of the respondents (69% students) rejected the notion that antiretroviral (ARV) medication is poisonous (Table 5).
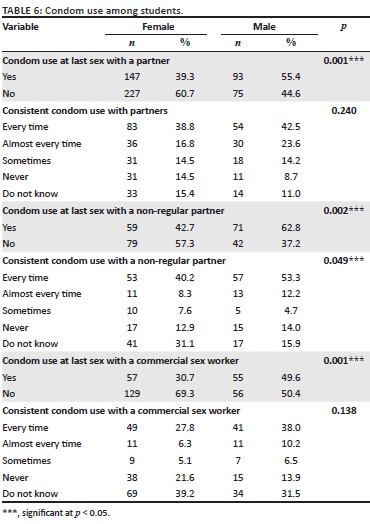
Condom use at last sex was higher when with a regular partner: female students (n = 147) and male students (n = 93). The difference is statistically significant (p < 0.001). Fewer female students (n = 53) and male students (n = 57) reported using condoms consistently with a non-regular partner (p = 0.049). More female students (n = 83) reported consistent condom usage (every time) with regular sex partners than male students (n = 54); however, the difference was not significant (p = 0.240) (Table 6).
Discussion
South Africa is battling an HIV and AIDS pandemic, which remains one of the primary social and health concerns in the country. Notwithstanding the outstanding effort by the Department of Health in implementing HIV and AIDS prevention strategies, South Africa is still the country worst affected by the HIV and AIDS pandemic, with the youth between the ages of 15 and 24 years being the hardest hit.5 Young female students are reported to have higher infection rates owing to a lack of knowledge and poor attitudes towards condom use and risky sexual behaviours,6 making them twice as likely as male students to be infected with HIV.3,6,8 This study was aimed at determining gender differences in knowledge, attitudes and behaviour in relation to HIV and AIDS among students at a private higher education institution in Johannesburg, South Africa.
Findings in this study indicate that there is no significant difference between male and female students in terms of their general knowledge of HIV and AIDS. However, it is noteworthy that female students had significantly less knowledge of unprotected anal sex as a risk factor for HIV and AIDS. In addition, a smaller proportion of female students reported condom use at last sex, compared to their male counterparts. This could be attributed to the female population having limited control over male condom usage.19 The complex power imbalance in this scenario, with most females not having the power to negotiate condom use with their partners, is the likely underlying cause.20,21
Moreover, 8.3% of male students and 3.8% of female students believed that AIDS can be cured. Although these percentages are comparatively low, they are equally as disconcerting as the results in Haroun et al.,22 who found that just over 20% of a sample of university students in the United Arab Emirates did not know whether HIV and AIDS could be cured or not. This poor knowledge of basic messages relating to HIV and AIDS is a likely reason as to why the youth engage in risky sexual behaviour and why the prevalence of HIV infection among them is high.23
Regarding risky sexual behaviour, this study revealed notable differences between male and female students, with the latter (57.3%) and the former (37.2%) reporting not having used a condom at last sex with a non-regular partner. As the chi-square test revealed no significant variation in attitudes between male and female students, we opted to investigate the entire sample's attitudes rather than to compare male and female students. Results revealed that the majority of participants (83.4%) had a positive attitude towards people living with HIV and AIDS. This differs from previous findings where the majority indicated negative attitudes towards people living with HIV and AIDS.22 Positive attitudes such as these have been attributed to parental and social communication aimed at promoting HIV and AIDS awareness among the youth.24
Limitations
There were a disproportionate number of female students, compared to male students in this study. It is possible that this might have affected the robustness of the chi-square test and therefore skewed the findings. In addition, the sample came from one private higher education institution in Johannesburg, South Africa. It would have been ideal for more institutions to be included in the study to get a better picture of the knowledge levels, attitudes and behaviours towards HIV and AIDS at private higher education institutions in the Johannesburg metropolitan area. Notwithstanding these limitations, these findings could serve as a springboard for national research that would be more representative of the wider student population in both private and public higher education institutions.
Recommendations
It is recommended that future research should sample students from several private and public higher education institutions with a representative number of both male and female students. This could then inform interventions that reduce HIV infection among the youth in South Africa through improved attitudes facilitated by communication at both social and parental levels.24 It is further recommended that sex education in secondary schools should be introduced to close the gender gap in knowledge and prevent risky sexual behaviours.
Conclusion
This study found that the level of HIV and AIDS knowledge in female students was not significantly different than in male students although risky sexual behaviour in female students was more frequent. In addition to the existing interventions aimed at reducing the prevalence of HIV and AIDS among the youth in South Africa, efforts towards implementing interventions at educational as well as social and family-based levels are imperative.
Acknowledgements
We would like to acknowledge the participants for their participation in this study.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
N.K. and M.M. conceptualised the study, collected data and did the write-up of the introduction, method and discussion sections. T.B. did data analysis and the write-up of the results section.
Funding Information
This study was funded by Monash South Africa research office.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views expressed in this article are the authors' own and not an official position of the institution or the funder.
References
1. WHO. HIV and AIDS. WHO [homepage on the Internet]. 2018 [cited 2019 May 17]. Available from: https://www.who.int/gho/hiv/en/ [ Links ]
2. Miller CL, Nkala B, Closson K, et al. The Botsha Bophelo Adolescent Health Study: A profile of adolescents in Soweto, South Africa. South Afr J HIV Med. 2017;18(1):1-10. https://doi.org/10.4102/sajhivmed.v18i1.731 [ Links ]
3. Dwyer-Lindgren L, Cork MA, Sligar A, et al. Mapping HIV prevalence in sub-Saharan Africa between 2000 and 2017. Nature [serial online]. [cited 2019 May 17]. Available from: http://www.nature.com/articles/s41586-019-1200-9 [ Links ]
4. UNAIDS. Global HIV & AIDS statistics - 2018 fact sheet [homepage on the Internet]. UNAIDS; 2019 [cited 2019 May 18]. Available from: https://www.unaids.org/en/resources/fact-sheet [ Links ]
5. Stats SA. Mid-year population estimates 2019 [homepage on the Internet]. [cited 2019 Nov 2]. Available from: http://www.statssa.gov.za [ Links ]
6. Hogg R, Nkala B, Dietrich J, et al. Conspiracy beliefs and knowledge about HIV origins among adolescents in Soweto, South Africa. PLoS One. 2017;12(2):1-9. https://doi.org/10.1371/journal.pone.0165087 [ Links ]
7. Yaya S, Ghose B, Udenigwe O, Shah V, Hudani A, Ekholuenetale M. Knowledge and attitude of HIV and AIDS among women in Nigeria: A cross-sectional study. Eur J Public Health. 2019;29(1):111-117. https://doi.org/10.1093/eurpub/cky131 [ Links ]
8. Kharsany ABM, Karim QA. HIV infection and AIDS in sub-Saharan Africa: Current status, challenges and opportunities. Open AIDS J. 2016;10(1):34-48. https://doi.org/10.2174/1874613601610010034 [ Links ]
9. Nubed CK, Akoachere J-FTK. Knowledge, attitudes and practices regarding HIV and AIDS among senior secondary school students in Fako Division, South West Region, Cameroon. BMC Public Health [serial online]. 2016;16(1):847. https://doi.org/10.1186/s12889-016-3516-9 [ Links ]
10. Wang SC, Lui JHL, Vega G, Waldrop M, Garris J. The moderating effect of alcohol use on protective and risky sex behaviors among college students in the Southeast United States. J Am Coll Heal. 2018;66(7):546-552. https://doi.org/10.1080/07448481.2018.1431916 [ Links ]
11. Younge SN, Wade BH, Geter A, Holliday RC, Trawick C. Condom attitudes and condom use among first year college men attending a historically black institution. Am J Health Stud [serial online]. 2018;33(2):80-88. [ Links ]
12. Vijay C, Johnson A, Rajitha K, Archana M, Rakesh J. Knowledge and beliefs regarding contraception, HIV and AIDS and sexually transmitted infections among young adults. Int J Recent Sci Res. 9(4):26211-26216. https://doi.org/10.24327/ijrsr.2018.0904.2014 [ Links ]
13. Egenti BN, Odiba EP, Dangana A, Yalma RM, Nasir IA. Knowledge, attitude and factors affecting voluntary HIV counseling and testing services among women in an Abuja suburb community. Heal Sci Res. 2018;5(2):50-58. [ Links ]
14. Dove M, Silver E, Swenson R. Beliefs associated with attitudes about HIV testing among adolescents at risk. J Adolesc Heal [serial online]. 2018 [cited 2019 May 17];62(2):S116. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1054139X17307656 [ Links ]
15. Protogerou C, Johnson B, Psychology MH-H. An integrated model of condom use in Sub-Saharan African youth: A meta-analysis. psycnet.apa.org [homepage on the Internet]. 2018 [cited 2019 May 17]. Available from: https://psycnet.apa.org/record/2018-19113-001 [ Links ]
16. Charles S, Drobatz L, Dorey R. Knowledge, attitudes, and beliefs about sexual health and behavior in Huye, Rwanda. 2018 [cited 2019 May 17]. Available from: https://jdc.jefferson.edu/cgi/viewcontent.cgi?filename=0&article=1027&context=si_phr_2021_phase1&type=additional [ Links ]
17. Sun CJ, Seloilwe ES, Magowe M, Dithole KS, Miller KS, St. Lawrence JS. Gender differences in sexual and reproductive health protective and risk factors of Batswana adolescents: Implications for parent and adolescent interventions. AIDS Educ Prev. 2018;30(1):35-46. https://doi.org/10.1521/aeap.2018.30.1.35 [ Links ]
18. World Population Review. Johannesburg population 2019 (demographics, maps, graphs) [homepage on the Internet]. 2019 [cited 2019 May 17]. Available from: http://worldpopulationreview.com/world-cities/johannesburg-population/ [ Links ]
19. Zuma K, Shisana O, Rehle TM, et al. New insights into HIV epidemic in South Africa: Key findings from the National HIV prevalence, incidence and behaviour survey, 2012. Afr J AIDS Res. 2016;15(1):67-75. https://doi.org/10.2989/16085906.2016.1153491 [ Links ]
20. Khuzwayo N, Taylor M. Exploring the socio-ecological levels for prevention of sexual risk behaviours of the youth in uMgungundlovu District Municipality, KwaZulu-Natal. Afr J Prim Health Care Fam Med. 2018;10(1):1-8. https://doi.org/10.4102/phcfm.v10i1.1590 [ Links ]
21. Mabaso M, Sokhela Z, Mohlabane N, Chibi B, Zuma K, Simbayi L. Determinants of HIV infection among adolescent girls and young women aged 15-24 years in South Africa: A 2012 population-based national household survey. BMC Public Health. 2018;18(1):183. https://doi.org/10.1186/s12889-018-5051-3 [ Links ]
22. Haroun D, El Saleh O, Wood L, Mechli R, Al Marzouqi N, Anouti S. Assessing knowledge of, and attitudes to, HIV and AIDS among university students in the United Arab Emirates. PLoS One. 2016;11(2):1-11. https://doi.org/10.1371/journal.pone.0149920 [ Links ]
23. Talwar P, Rahman MFBA. Assessment of HIV knowledge among university students using the HIV-KQ-18 scale: A cross-sectional study. South East Asia J Public Heal. 2015;5(1):33-38. https://doi.org/10.3329/seajph.v5i1.24849 [ Links ]
24. Adeleke IT, Azeez BA, Aliyu D, Ogundiran LM, Salami A, Adeoye WA. HIV and AIDS awareness among secondary schools' adolescents in south-western Nigeria: A correlate to strengthen advocacy and strategic sexuality education programs. Am J Health Res. 2015;3(1-1):61-67. https://doi.org/10.11648/j.ajhr.s.2015030101.19 [ Links ]
 Correspondence:
Correspondence:
Natasha Khamisa
natasha.khamisa@monash.edu
Received: 11 June 2019
Accepted: 11 Dec. 2019
Published: 24 Mar. 2020
Project Research Number: CF15/1095 - 2015000518c
^rND^sMiller^nCL^rND^sNkala^nB^rND^sClosson^nK^rND^sHogg^nR^rND^sNkala^nB^rND^sDietrich^nJ^rND^sYaya^nS^rND^sGhose^nB^rND^sUdenigwe^nO^rND^sShah^nV^rND^sHudani^nA^rND^sEkholuenetale^nM^rND^sKharsany^nABM^rND^sKarim^nQA^rND^sNubed^nCK^rND^sAkoachere^nJ-FTK^rND^sWang^nSC^rND^sLui^nJHL^rND^sVega^nG^rND^sWaldrop^nM^rND^sGarris^nJ^rND^sYounge^nSN^rND^sWade^nBH^rND^sGeter^nA^rND^sHolliday^nRC^rND^sTrawick^nC^rND^sVijay^nC^rND^sJohnson^nA^rND^sRajitha^nK^rND^sArchana^nM^rND^sRakesh^nJ^rND^sEgenti^nBN^rND^sOdiba^nEP^rND^sDangana^nA^rND^sYalma^nRM^rND^sNasir^nIA^rND^sDove^nM^rND^sSilver^nE^rND^sSwenson^nR^rND^sSun^nCJ^rND^sSeloilwe^nES^rND^sMagowe^nM^rND^sDithole^nKS^rND^sMiller^nKS^rND^sSt. Lawrence^nJS^rND^sZuma^nK^rND^sShisana^nO^rND^sRehle^nTM^rND^sKhuzwayo^nN^rND^sTaylor^nM^rND^sMabaso^nM^rND^sSokhela^nZ^rND^sMohlabane^nN^rND^sChibi^nB^rND^sZuma^nK^rND^sSimbayi^nL^rND^sHaroun^nD^rND^sEl Saleh^nO^rND^sWood^nL^rND^sMechli^nR^rND^sAl Marzouqi^nN^rND^sAnouti^nS^rND^sTalwar^nP^rND^sRahman^nMFBA^rND^sAdeleke^nIT^rND^sAzeez^nBA^rND^sAliyu^nD^rND^sOgundiran^nLM^rND^sSalami^nA^rND^sAdeoye^nWA^rND^1A01^nJillian C.^sPintye^rND^1A02 A03^nKathleen E.^sWirth^rND^1A04^nConrad^sNtsuape^rND^1A01 A02 A05 A06^nNora J.^sKleinman^rND^1A07 A08^nLisa^sSpees^rND^1A01 A02 A09^nBazghina-werq^sSemo^rND^1A01 A02^nShreshth^sMawandia^rND^1A01 A02^nJenny^sLedikwe^rND^1A01^nJillian C.^sPintye^rND^1A02 A03^nKathleen E.^sWirth^rND^1A04^nConrad^sNtsuape^rND^1A01 A02 A05 A06^nNora J.^sKleinman^rND^1A07 A08^nLisa^sSpees^rND^1A01 A02 A09^nBazghina-werq^sSemo^rND^1A01 A02^nShreshth^sMawandia^rND^1A01 A02^nJenny^sLedikwe^rND^1A01^nJillian C^sPintye^rND^1A02 A03^nKathleen E^sWirth^rND^1A04^nConrad^sNtsuape^rND^1A01 A02 A05 A06^nNora J^sKleinman^rND^1A07 A08^nLisa^sSpees^rND^1A01 A02 A09^nBazghina-werq^sSemo^rND^1A01 A02^nShreshth^sMawandia^rND^1A01 A02^nJenny^sLedikweORIGINAL RESEARCH
Sexual function after voluntary medical male circumcision for human immunodeficiency virus prevention: Results from a programmatic delivery setting in Botswana
Jillian C. PintyeI; Kathleen E. WirthII, III; Conrad NtsuapeIV; Nora J. KleinmanI, II, V, VI; Lisa SpeesVII, VIII; Bazghina-werq SemoI, II, IX; Shreshth MawandiaI, II; Jenny LedikweI, II
IDepartment of Global Health, University of Washington, Seattle, United States
IIBotswana International Training and Education Center for Health (I-TECH), Gaborone, Botswana
IIIDepartment of Biostatistics, Harvard T.H. Chan School of Public Health, Boston, Massachusetts, United States
IVDepartment of HIV/AIDS Prevention and Care, Botswana Ministry of Health and Wellness, Gaborone, Botswana
VNJK Consulting, Seattle, Washington, United States
VIAmgen Asia Holdings Ltd, Hong Kong, Japan
VIIDepartment of Health Policy and Management, University of North Carolina, Chapel Hill, United States,
VIIILineberger Comprehensive Cancer Center, University of North Carolina, Chapel Hill, United States
IXFHI 360, Washington, Washington, United States
ABSTRACT
BACKGROUND: Uptake of voluntary medical male circumcision (VMMC) remains modest in Botswana in spite of the government's commitment and service provision availability. Data on sexual function post-VMMC in programmatic settings could help guide messaging tailored to Botswana
OBJECTIVES: At 3-month post-VMMC, we evaluated changes in sexual function and satisfaction with the VMMC procedure amongst a cohort of HIV-negative, sexually active men aged 18-49 years who underwent VMMC in a public-sector clinic in Botswana
METHODS: We assessed whether each of the following domains of sexual function had improved, stayed the same or worsened since VMMC: sexual desire, ability to use condoms, ease of vaginal penetration, ease of ejaculation, ability to achieve and maintain an erection and hygiene or cleanliness
RESULTS: Data on sexual function were available for 378 men at 3-month post-VMMC. Median age was 27 years - 54% had a higher than secondary education, 72% were employed and 27% were married. Nearly all (96%) the men reported improvement in at least one domain of sexual function, while 19% reported improvement in all six domains. One-fourth (91/378, 24%) of the men reported that at least one domain of sexual function worsened post-VMMC. The most frequently reported domain that worsened was sexual desire (11%); in all other domains, < 10% of the men reported worsening. Men who reported any worsening sexual function were 2.3-fold as likely to be less than 'very satisfied' with the VMMC procedure (risk ratio 2.36, 95% confidence interval [CI] 1.66-3.34, p < 0.001
CONCLUSION: Emphasising improved sexual function experienced after VMMC in demand-creation efforts could potentially increase VMMC uptake in Botswana
Keywords: Botswana; voluntary medical male circumcision; human immunodeficiency virus (HIV) prevention; men; implementation science; program delivery.
Introduction
Continued promotion of voluntary medical male circumcision (VMMC) programmes in countries with high human immunodeficiency virus (HIV) burden and low male circumcision rates is needed to decrease population-level HIV incidences.1,2,3 More than 18.6 million men have been circumcised through VMMC programmes in 14 priority African countries to date, averting an estimated 230,000 new HIV infections by 2017.4,5,6,7,8 Achieving the global target of 27 million more VMMC procedures by 2021, translating to 90% of males aged 10-29 years being circumcised in priority countries, will depend in part on the continued acceptability of VMMC amongst target populations.3 Uptake of VMMC began slowly in Botswana9 and has remained modest in spite of government commitment, donor support and availability of service provision of VMMC since 2009. In 2018, Botswana achieved less than 50% of the country's target of 25 000 VMMC procedures.10
Studies exploring reasons for men's unwillingness to be circumcised have identified concerns related to potential effects of VMMC on sexual function (e.g. erection and orgasm) and sexual pleasure, the risk of surgical pain, reluctance to abstain from sex for at least 6 weeks during recovery and partners' responses.11,12,13 A systematic review that included > 40 000 men from cross-sectional, case-control and pre-post circumcision studies concluded that VMMC likely has little or no effect on male sexual function and satisfaction.14 However, ascertainment of sexual function data and findings varied across African settings15,16,17 and almost no data were included from the context of national VMMC programmes under 'real-world' conditions. One population-based cohort study in Kenya has found that the majority of the men who undergo VMMC are satisfied with the procedure and experience improvement in sexual function that increased over time.18 To date, no evaluations have examined sexual function amongst the men who undergo VMMC in Botswana, nor their perceptions of post-VMMC satisfaction beyond 7 days.19 Gathering evidence on these elements within the local context could inform VMMC messaging tailored to Botswana.
We previously assessed the frequency of adverse events at 7 days and early resumption of sexual intercourse amongst a cohort of adult men who underwent VMMC within a programmatic delivery setting in Botswana.19,20 This current analysis examines the post-VMMC experience at 3 months, including sexual function and satisfaction with the procedure. Given the suboptimal uptake of VMMC in Botswana and the concerns of its potentially negative impact on sexual function identified in qualitative studies,21,22 these new data could provide valuable evidence for VMMC demand-creation messaging in Botswana.
Methods
Study design and participants
We analysed data from a cohort study comprising HIV-negative, sexually active men aged 18-49 years who underwent VMMC through Botswana's National Safe Male Circumcision programme at two government-run clinics in Gaborone, Botswana. Between November 2013 and April 2015, the parent study enrolled men to prospectively assess sexual behaviours and adverse events following VMMC.19 Parent study procedures have been described previously in detail.19 Briefly, the study was collaboratively conducted by the Botswana Ministry of Health (MOH) and the International Training and Education Center for Health (I-TECH), a collaboration between the University of Washington and the University of California, San Francisco. After individuals completed group education about the risks and benefits of VMMC and received individual counselling from clinic staff (including HIV testing), they were screened for eligibility and offered enrolment into the study. Study staff collected information on demographic, clinical, relationship and sexual behaviour characteristics at enrolment. All clinical VMMC activities were conducted per MOH guidelines at no cost to participants and were not part of the study procedures.23
Data collection procedures
After circumcision, follow-up visits were scheduled in alignment with the Botswana MOH guidelines for adult VMMC (2 days, 7 days, 6 weeks, 3 months and 1 year).23 At study visits, participants were asked to self-administer a questionnaire about wound care, patient satisfaction of the procedure and resumption of sexual activities. At 3 months, questionnaire items included assessment of sexual functions. Each study participant was provided with a wallet-size reminder card noting the date of each follow-up visit. Prior to each visit, study staff telephoned participants to remind them of the upcoming scheduled appointment. At each follow-up visit, study staff performed a physical examination, including inspecting the circumcision site, and assessing for signs of sexually transmitted infections (STIs). Participants received BWP100 (approximately USD$8 at study initiation) at each visit as reimbursement for time and travel costs. In the event of a missed visit, study staff made telephone calls to reschedule the appointment.
Statistical analysis
The current analysis on sexual function and satisfaction with the VMMC procedure at 3 months was restricted to men who had data available from 3-month follow-up visits, as data on earlier outcomes from this cohort had previously been reported.23 We identified differences between men with and without data on sexual function available at 3-month post-VMMC using Chi-square tests for proportions and Kruskal-Wallis test for continuous measures. We assessed satisfaction with the VMMC procedure and the follow-up care using a four-point Likert scale (very satisfied, somewhat satisfied, somewhat dissatisfied and very dissatisfied). We compared the frequency distributions of satisfaction at 7-day and 3-month post-VMMC amongst men who had data available from both time points to describe the changes in satisfaction with the VMMC procedure over time. We assessed whether categories of sexual function improved, had no change or worsened, compared to before undergoing VMMC using a three-point Likert scale (better, no change and worse). Domains of sexual function included sexual desire, ability to put on a condom, ease of vaginal penetration, ease of ejaculation, ability to achieve and maintain an erection, and hygiene/cleanliness. Descriptive statistics were used to summarise the frequency distributions of each sexual function category.
We evaluated the following enrolment characteristics as potential predictors of reporting any worsened sexual function: demographic information (age, education, relationship status, employment, electricity in household) and behaviour (alcohol consumption, age of sexual debut, number sexual partners [lifetime, last 12 months], type of most recent relationship [regular or casual], history of buying sex and condom use) and primary motivation for VMMC (HIV prevention vs. other reasons). Variables were identified as predictors using univariate Poisson regression models with robust error variance, an approach used when the outcome prevalence was not rare (e.g. > 10%).24,25 The primary outcome of our Poisson regression models was reporting any worsening of sexual function in any domain since the VMMC procedure at 3-month post-VMMC (yes or no). We included all men with 3-month follow-up data in our primary models regardless of engaging in sexual activity or not, because sexual dysfunction could influence sexual activity. In a sensitivity analysis, we restricted our models to only men who reported sexual activity after undergoing VMMC. We also reran our primary models with the outcome excluding hygiene or cleanliness. We used Stata 15/SE (Stata Corporation, College Station, TX) to perform statistical analyses.
Ethical consideration
Ethical approvals were obtained from the Health Research and Development Committee at the Botswana Ministry of Health and Wellness (reference number: 00699) as well as the University of Washington Institutional Review Board (reference number: 42047). All participants provided written informed consent for participation in the study in addition to the consent obtained by the clinic staff for the circumcision procedure.
Results
Baseline characteristics
In total, 519 who were enrolled in the parent study underwent VMMC (Figure 1). Median age was 27 years (interquartile range [IQR] 23-31), 57% had completed secondary education or higher and 86% had electricity in the household, a proxy for socio-economic status in this setting. The most common relationship status was dating and not living together (53%), followed by being married and/or living together (28%). Over one-third (37%) of the men reported having ≥ 6 drinks at one time at least four times a month. The median age of sexual debut was 19 years (IQR 17-20), 20% of the men reported having ≥ 2 sexual partners in the previous month and 21% reported ≥ 10 lifetime sexual partners. Almost half (47%) reported that HIV prevention was their primary reason for becoming circumcised.
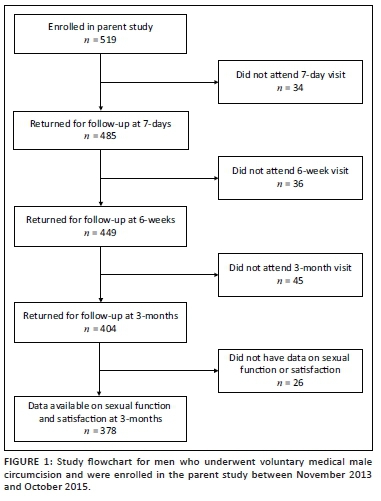
Overall, 378/519 (73%) men had data available on sexual function at 3-month post-VMMC and were included in the final analysis (Figure 1). Of the 141 men without 3-month sexual function data, 82% attended a prior follow-up visit at 7 days or 6 weeks post-VMMC. Compared with men without data available, men with 3-month post-VMMC sexual function data less frequently had higher than secondary education (54% vs. 66%, p = 0.005). There were no differences in any other baseline characteristics between those who did have and who did not have 3-month data available (Table 1).
Sexual function after voluntary medical male circumcision
Amongst men with sexual function data available at 3-month post-VMMC (n = 378), the majority (88%) reported having sex in the last 90 days. Amongst men who were sexually active in the last 90 days (n = 326), the median time since last sex was 7 days (IQR 7-14). Nearly all (96%) men reported better sexual function in at least one domain; 19% reported improvement in all six domains. The most frequently reported domain with improvement was hygiene/cleanliness (93%), followed by ease of vaginal penetration (57%); 44% - 50% of the men reported improvement in all other categories (Figure 2). One-fourth (91/378, 24%) of the men reported worsening sexual function post-VMMC in at least one domain. The most frequently reported domain to worsen was sexual desire (11%); for all other domains, less than 10% of the men reported any worsening (Figure 2). No change was reported by 36% - 44% of the men across all domains except for hygiene/cleanliness (5%). No baseline demographic, clinical or behavioural characteristics were predictive of worsening sexual function (Table 2); results were very similar in models restricted to sexually active men and when excluding hygiene/cleanliness from the domains included in the outcome (data not shown).
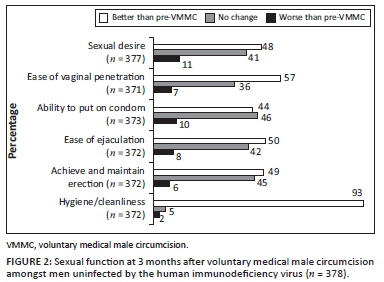
Satisfaction with voluntary medical male circumcision procedure
Overall, 84% of men were very satisfied with the VMMC procedure at 3 months, 14% were somewhat satisfied, 1% were somewhat dissatisfied and 1% were very dissatisfied. Frequency of being very satisfied with the VMMC procedure was slightly lower at 3 months, compared with 7-day post-VMMC procedure (Figure 3, 84% vs. 90%, p = 0.004). Almost all (93%) of the men reported being very satisfied with follow-up care at 3 months (Figure 4). Amongst men who were very satisfied with VMMC at 7 days and became less satisfied/dissatisfied at 3 months (n = 23), 17% reported worsening sexual function. At 3 months, men who reported any worsening of sexual function were 2.3-fold as likely to be less than 'very satisfied' with the VMMC procedure at 3 months (risk ratio 2.36, 95% confidence interval [CI] 1.66-3.34, p < 0.001). Amongst men who were overall somewhat or very dissatisfied with VMMC at 3 months (n = 7), non-mutually exclusive reasons for being dissatisfied included appearance (n = 4), wound care requirements (n = 2), aspects of the procedure (n = 3) and issues with pain (n = 2).
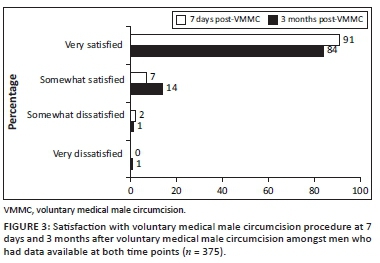
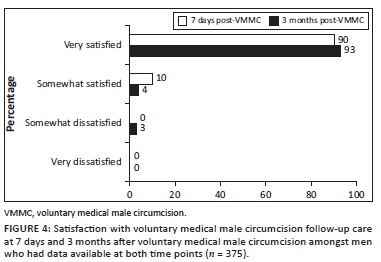
Discussion
In this longitudinal evaluation of the men who became circumcised within a programmatic VMMC setting in Botswana, we found very high overall satisfaction with the procedure at 3 months after VMMC accompanied by frequently reported improvement in sexual function. Consistent with prior data from research settings, our implementation evaluation found that nearly all (98%) men were at least somewhat satisfied overall with the VMMC procedure; almost one-fifth (19%) reported improved sexual function in every category assessed; and over one-third reported no change.26 Although few men reported being dissatisfied with VMMC, frequency of worsening sexual function post-VMMC was higher in this group. Our results highlight considerations for demand-creation messaging as VMMC programmes continue to roll out in countries such as Botswana that have high HIV burden and modest VMMC uptake.
Similar to prior studies that evaluated sexual function pre- and post-circumcision amongst African men,15,18 we found that the majority of the men reported improvement in some domains of sexual function after undergoing VMMC in Botswana. When asked to retrospectively compare with the condition before undergoing VMMC, approximately half of the men in our evaluation reported improved sexual desire, ease of vaginal penetration and ejaculation, and/or ability to achieve and maintain an erection post-VMMC. Studies amongst men who became circumcised in adulthood are especially useful in evaluating the impact of VMMC on sexual function because these men served as their own control.14 Data from randomised trials in Uganda and Kenya15,16 provide the highest quality evidence on the effects of VMMC on sexual function and found some improvement in sexual function after VMMC. There are important contextual differences between men who were willing to enrol in the early VMMC trials, when efficacy was unknown and VMMC was randomly assigned, and those who self-select VMMC delivered as part of national programmes. Our findings contribute to the evidence base supporting the benefits of VMMC in programmatic settings extended beyond HIV prevention and could broadly improve sexual health amongst African men.17 The future programmatic evaluations could improve design rigour by assessing sexual function prior to undergoing VMMC and comparing it with post-VMMC assessment.
In spite of the high levels of improvement in sexual function following VMMC, an appreciable proportion (19%) of the men in our evaluation reported at least one category of sexual function worsening after VMMC. A systematic review published in 2013 by Morris and Krieger14 that included 20 931 circumcised men and 19 542 uncircumcised men found no evidence for differences in any component of sexual function by circumcision status. However, almost no data were included from programmatic delivery settings in African countries that could provide different quality of services or reach a population unlike men enrolled in the initial VMMC randomised trials.15,16 In addition, prior randomised trials15,16 assessed prevalence of sexual dysfunctional components (erectile dysfunction, ease of ejaculation, etc.) up to 24 months post-VMMC and found a decrease in dysfunction over time. In our programmatic evaluation, we asked participants to retrospectively report changes in sexual functioning, if any, at the 3-month follow-up visit (as opposed to conducting a separate assessment of sexual functioning at baseline). Men who were less satisfied with the VMMC procedure were also more likely to report worsening of sexual function. Men currently experiencing sexual dysfunction may be more likely to report that their status had worsened after VMMC because of recall bias. It is also possible that men enrolled in our evaluation could experience better sexual function at later time points. We also found modestly lower, although still very high, satisfaction with the VMMC procedure at 3 months (84%), compared to 7-day (90%) post-VMMC. To our knowledge, no other programmatic evaluations amongst African men have evaluated satisfaction with VMMC over time. The future programmatic evaluations could identify areas for improving the VMMC procedure, counselling and follow-up care to prevent potential negative effects on sexual function and satisfaction with the procedure.
Over a decade after initiation, uptake of VMMC in Botswana's national programme remains modest.10 A recent systematic review from July 2019 by Ensor et al. has found that the most effective VMMC demand-creation interventions are financial incentives and education or counselling programmes delivered by community opinion leaders or individuals with personal experience of VMMC.27 To date, no VMMC demand-creation intervention studies have been conducted in Botswana and ongoing mass media campaigns focus on protection against HIV associated with VMMC. The future demand-creation messages in Botswana could be tailored to the values and preferences of the men at risk of HIV infection whilst extending beyond HIV prevention.28,29 Integrating evidence for improved sexual function and satisfaction following VMMC into mainstream messaging could potentially motivate Batswana men, who may not perceive their own HIV risk or for whom HIV prevention is not a motivator for VMMC, to seek VMMC. Our evaluation found high frequency of improved sexual function and overall satisfaction with VMMC within programmatic settings, which could be helpful for framing the holistic benefits of VMMC in future messaging.
Our study has limitations. We only ascertained sexual function information at 3-month post-VMMC and retrospectively asked men to compare aspects of sexual function with their pre-VMMC experiences. This approach was intended to capture how VMMC impacts sexual function, although a baseline assessment of sexual function would have improved the rigour of our findings. Recall bias is possible with over-reporting of worsening sexual function amongst dissatisfied men or potentially choice-supportive bias of improved function to rationalise the decision to undergo VMMC. Not all men returned for follow-up and/or had data on sexual function available at 3-month post-VMMC. This may bias our overall estimates of satisfaction and sexual function. We detected minimal baseline differences between men with and without 3-month data available, and no characteristics were predictive of worsening sexual function. Men who did not return for follow-up visits could potentially be more or less satisfied with their VMMC experience, which could bias our results. We intentionally conducted an implementation evaluation without intensive follow-up tracing to align with programmatic settings. The future evaluations could include more research procedures such as at-home follow-up tracing to collect data that are more complete.
Conclusion
To date, demand-creation messaging for VMMC in Botswana has primarily focussed on HIV prevention. However, as VMMC uptake remains modest amongst Batswana men, there is a need to effectively promote VMMC to men at-risk of HIV infection for whom protection against HIV may not be a motivator to undergo VMMC. Similar to prior studies from research settings in other African countries, our programmatic data show that the majority of the men report improved sexual function across multiple categories and high overall satisfaction after VMMC. As VMMC continues to roll out in Botswana, incorporating evidence of other non-HIV-related benefits of VMMC into demand-creation messaging may support maximising VMMC uptake.
Acknowledgements
The authors are grateful to all the participants for their participation in the study.
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
All authors contributed equally to this work.
Funding information
This work was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) through grant No. U91HA06801 from the US Department of Health and Human Services, Health Resources and Services Administration (HRSA), HIV/AIDS Bureau's Global Health Systems Branch.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1. Tobian AA, Kacker S, Quinn TC. Male circumcision: A globally relevant but under-utilized method for the prevention of HIV and other sexually transmitted infections. Annu Rev Med. 2014;65:293-306. https://doi.org/10.1146/annurev-med-092412-090539 [ Links ]
2. Grabowski MK, Serwadda DM, Gray RH, et al. HIV prevention efforts and incidence of HIV in Uganda. N Engl J Med. 2017;377(22):2154-2166. https://doi.org/10.1056/NEJMoa1702150 [ Links ]
3. Joint United Nations Programme on HIV/AIDS (UNAIDS). Policy brief. A framework for voluntary medical male circumcision: Effective HIV prevention and a gateway to improved adolescent boys' and men's health in eastern and southern Africa by 2021. Geneva: World Health Organisation; 2016 [ Links ]
4. World Health Organisation (WHO). WHO progress brief. Voluntary medical male circumcision for HIV prevention. Geneva: World Health Organization; 2018. [ Links ]
5. Hines JZ, Ntsuape OC, Malaba K, et al. Scale-up of voluntary medical male circumcision services for HIV prevention - 12 countries in southern and eastern Africa, 2013-2016. MMWR Morb Mortal Wkly Rep. 2017;66(47):1285-1290. https://doi.org/10.15585/mmwr.mm6647a2 [ Links ]
6. Davis SM, Hines JZ, Habel M, et al. Progress in voluntary medical male circumcision for HIV prevention supported by the US President's Emergency Plan for AIDS Relief through 2017: Longitudinal and recent cross-sectional programme data. BMJ Open. 2018;8(8):e021835. https://doi.org/10.1136/bmjopen-2018-021835 [ Links ]
7. Bochner AF, Baeten JM, Rustagi AS, et al. A cross-sectional analysis of Trichomonas vaginalis infection among heterosexual HIV-1 serodiscordant African couples. Sex Transm Infect. 2017;93(7):520-529. https://doi.org/10.1136/sextrans-2016-053034 [ Links ]
8. Pintye J, Drake AL, Unger JA, et al. Male partner circumcision associated with lower Trichomonas vaginalis incidence among pregnant and postpartum Kenyan women: A prospective cohort study. Sex Transm Infect. 2017;93(2):137-143. https://doi.org/10.1136/sextrans-2016-052629 [ Links ]
9. Sabone M, Magowe M, Busang L, Moalosi J, Binagwa B, Mwambona J. Impediments for the uptake of the Botswana government's male circumcision initiative for HIV prevention. Sci World J. 2013;2013:387508. https://doi.org/10.1155/2013/387508 [ Links ]
10. United States Department of State. FY 2019 PEPFAR planned allocation and strategic direction [homepage on the Internet]. Washington, DC;2019 [cited 2019 January 16]. Available from: https://www.pepfar.gov/documents/organization/289946.pdf [ Links ]
11. Westercamp M, Bailey RC, Bukusi EA, Montandon M, Kwena Z, Cohen CR. Male circumcision in the general population of Kisumu, Kenya: Beliefs about protection, risk behaviors, HIV, and STIs. PLoS One. 2010;5(12):e15552. https://doi.org/10.1371/journal.pone.0015552 [ Links ]
12. Toefy Y, Skinner D, Thomsen SC. 'What do you mean i've got to wait for six weeks?!' Understanding the sexual behaviour of men and their female partners after voluntary medical male circumcision in the Western Cape. PLoS One. 2015;10(7):e0133156. https://doi.org/10.1371/journal.pone.0133156 [ Links ]
13. Rogers JH, Odoyo-June E, Jaoko W, Bailey RC. Time to complete wound healing in HIV-positive and HIV-negative men following medical male circumcision in Kisumu, Kenya: A prospective cohort study. PLoS One. 2013;8(4):e61725. https://doi.org/10.1371/journal.pone.0061725 [ Links ]
14. Morris BJ, Krieger JN. Does male circumcision affect sexual function, sensitivity, or satisfaction? - A systematic review. J Sex Med. 2013;10(11):2644-2657. https://doi.org/10.1111/jsm.12293 [ Links ]
15. Krieger JN, Mehta SD, Bailey RC, et al. Adult male circumcision: Effects on sexual function and sexual satisfaction in Kisumu, Kenya. J Sex Med. 2008;5(11):2610-2622. https://doi.org/10.1111/j.1743-6109.2008.00979.x [ Links ]
16. Kigozi G, Watya S, Polis CB, et al. The effect of male circumcision on sexual satisfaction and function, results from a randomized trial of male circumcision for human immunodeficiency virus prevention, Rakai, Uganda. BJU Int. 2008;101(1):65-70. https://doi.org/10.1111/j.1464-410X.2007.07369.x [ Links ]
17. Zulu R, Jones D, Chitalu N, Cook R, Weiss S. Sexual satisfaction, performance, and partner response following voluntary medical male circumcision in Zambia: The spear and shield project. Glob Health Sci Pract. 2015;3(4):606-618. https://doi.org/10.9745/GHSP-D-15-00163 [ Links ]
18. Nordstrom MP, Westercamp N, Jaoko W, Okeyo T, Bailey RC. Medical male circumcision is associated with improvements in pain during intercourse and sexual satisfaction in Kenya. J Sex Med. 2017;14(4):601-612. https://doi.org/10.1016/j.jsxm.2017.02.014 [ Links ]
19. Wirth KE, Semo BW, Spees LP, Ntsuape C, Barnhart S, Ledikwe JH. A prospective cohort study of safety and patient satisfaction of voluntary medical male circumcision in Botswana. PLoS One. 2017;12(11):e0185904. https://doi.org/10.1371/journal.pone.0185904 [ Links ]
20. Pintye J, Wirth KE, Ntsuape C, et al. Early resumption of sex after voluntary medical male circumcision for HIV prevention within a programmatic delivery setting in Botswana. Int J STD AIDS. 2019;30(3):1275-1283. https://doi.org/10.1177/0956462419866051 [ Links ]
21. Green LW, Travis JW, McAllister RG, Peterson KW, Vardanyan AN, Craig A. Male circumcision and HIV prevention insufficient evidence and neglected external validity. Am J Prev Med. 2010;39(5):479-482. https://doi.org/10.1016/j.amepre.2010.07.010 [ Links ]
22. Wamai RG, Morris BJ, Bailey RC, Klausner JD, Boedicker MN. Male circumcision for protection against HIV infection in sub-Saharan Africa: The evidence in favour justifies the implementation now in progress. Glob Public Health. 2015;10(5-6):639-666. https://doi.org/10.1080/17441692.2014.989532 [ Links ]
23. Ministry of Health. Safe male circumcision: Additional strategy for HIV prevention: A national strategy. Gaborone: Ministry of Health; 2010. [ Links ]
24. Breslow NE. Generalized linear models: Checking assumptions and strengthening conclusions. Statistica Applicata. 1996;8(1):23-41 [ Links ]
25. Lee J, Chia KS. Estimation of prevalence rate ratios for cross-sectional data: An example in occupational epidemiology. Br J Ind Med. 1993;50(9):861-862. https://doi.org/10.1136/oem.50.9.861 [ Links ]
26. Herman-Roloff A, Bailey RC, Agot K. Factors associated with the early resumption of sexual activity following medical male circumcision in Nyanza province, Kenya. AIDS Behav. 2012;16(5):1173-1181. https://doi.org/10.1007/s10461-011-0073-1 [ Links ]
27. Ensor S, Davies B, Rai T, Ward H. The effectiveness of demand creation interventions for voluntary male medical circumcision for HIV prevention in sub-Saharan Africa: A mixed methods systematic review. J Int AIDS Soc. 2019;22 (Suppl 4):e25299. https://doi.org/10.1002/jia2.25299 [ Links ]
28. Ledikwe JH, Nyanga RO, Hagon J, Grignon JS, Mpofu M, Semo BW. Scaling-up voluntary medical male circumcision - What have we learned? HIV AIDS (Auckl). 2014;6:139-146. https://doi.org/10.2147/HIV.S65354 [ Links ]
29. Gurman TA, Dhillon P, Greene JL, Makadzange P, Khumlao P, Shekhar N. Informing the scaling up of voluntary medical male circumcision efforts through the use of theory of reasoned action: Survey findings among uncircumcised young men in Swaziland. AIDS Educ Prev. 2015;27(2):153-166. https://doi.org/10.1521/aeap.2015.27.2.153 [ Links ]
Received: 01 Nov. 2019
Accepted: 28 Nov. 2019
Published: 20 Apr. 2020
ORIGINAL RESEARCH
Tobacco smoking and associated factors in human immunodeficiency virus-infected adults attending human immunodeficiency virus clinics in the Western Cape province, South Africa
Muyunda MutemwaI; Nasheeta PeerI, II; Anniza de VilliersIII; Mieke FaberI; Andre-Pascal KengneI, II
INon-Communicable Diseases Research Unit, South African Medical Research Council, Cape Town, South Africa
IIDepartment of Medicine, University of Cape Town, Cape Town, South Africa
IIIResearch Capacity Development Division, South African Medical Research Council, Cape Town, South Africa
ABSTRACT
BACKGROUND: In human immunodeficiency virus (HIV)-infected individuals, smoking increases both HIV-related and non-related negative health outcomes
OBJECTIVES: To determine the prevalence and associations of smoking in HIV-infected adults receiving antiretroviral therapy at public healthcare facilities in the Western Cape province, South Africa
METHODS: Participants comprised 827 HIV-infected patients, who were > 18 years old and randomly selected from 17 HIV healthcare facilities. Self-reported smoking was defined as smoking tobacco daily or occasionally. Serum cotinine levels confirmed smoking status
RESULTS: Participants included 653 women and 174 men. The overall mean (standard deviation [SD]) age was 38.9 (9.0) years, 41.1 (8.9) years in men and 37.7 (8.9) years in women (p ˂ 0.001). The median diagnosed duration of HIV infection was 5 years. Smoking prevalence was 22% overall, and 26% in men and 21% in women (p = 0.022). The prevalence of former smoking was 14%. About a quarter of participants (185/751; 24.6%) had serum cotinine levels > 100 mg/mL with similar prevalence of high levels across smoking status (current smokers: 27.2%, former smokers: 29.6% and never smokers: 22.7%, p = 0.564) and did not vary by age, gender, cluster of differentiation 4 count or known duration of HIV. There was no agreement between self-reports and cotinine levels at ranking smoking exposure
CONCLUSIONS: Prevalence of current tobacco smoking in HIV-infected patients on care is within the range of that in the general population. This highlights the potential missed opportunity or challenges of co-addressing smoking cessation in individuals already in regular contact with the health system
Keywords: HIV and AIDS; smoking; cotinine; prevalence; South Africa.
Introduction
Globally, over 1.1 billion men and women ≥ 15 years of age currently smoke tobacco. Consequently, tobacco use is a growing public health burden worldwide that was responsible for 7.1 million deaths in 2016.1 Even in South Africa (SA), despite the introduction of comprehensive legislative action to discourage tobacco use since the early 1990s, tobacco smoking remains a major public health problem.2 In 2016, 37% of men and 8.0% of women ≥ 15 years of age smoked tobacco.3 Tobacco smoking contributes to a large burden of the preventable disease accounting for 8.0% - 9.0% of mortality in SA.2 While lung cancer had the largest attributable fraction because of tobacco smoking, cardiovascular diseases contributed to the largest proportion of deaths caused by smoking.
The impact of smoking extends beyond the well-known consequences of tobacco use to other conditions including human immunodeficiency virus (HIV) infection. In people living with HIV or acquired immunodeficiency syndrome (AIDS) (PLWHA), the harmful effects of smoking are greater and threaten efforts in controlling HIV and AIDS.1 Smoking increases both HIV-related and non-related outcomes and has been shown to impact HIV disease progression.4,5 Nevertheless, smoking prevalence in PLWHA is approximately twofold to threefold higher than in the general population in developed countries and ranges from 40% to 74%.6,7
The greater adverse effects of tobacco smoking in PLWHA are highly relevant to SA. The country has the greatest burden of HIV worldwide with approximately 7.97 million PLWHA in 2019.8 With almost 20% of 15-49-year-old South African adults being HIV positive, determining the burden of tobacco smoking in the HIV infected can inform strategies for tobacco cessation in this high-risk population. This is particularly pertinent in the era of widespread dissemination of antiretroviral therapy (ART) in SA9 and increased longevity of the HIV-infected population. People living with HIV and AIDS are now at an increased risk of dying from cardiovascular and other non-communicable diseases (NCDs), including tobacco-related conditions, rather than from AIDS.10,11,12
This study, therefore, aims to determine the prevalence of smoking and associated factors including HIV-specific factors in PLWHA receiving ART at public healthcare facilities in the Western Cape province of SA.
Methods
Population and sampling
This cross-sectional study was conducted in a sample of ≥ 18-year-old HIV-infected adults who were randomly selected from a list of patients attending the clinic on the study day. Participants were recruited between March 2014 and February 2015 from healthcare facilities in the Western Cape that provided ART to at least 325 HIV-infected patients per month. This was to ensure the recruitment of an adequate number of participants within a reasonable period. Of the 17 healthcare facilities selected, 10 were in Cape Town and seven were in the surrounding rural municipalities. Excluded participants were those who were pregnant, breastfeeding, bedridden, undergoing cancer treatment, on corticosteroid treatment, or unwilling or unable to provide consent. The detailed methods have been described previously.13
Data collection
Trained clinicians, nurses and fieldworkers collected data via standardised international questionnaires, clinical measurements and biochemical analyses. Data were captured on personal digital assistants (PDAs), using electronic case report forms with built-in checks for quality control. The interviews and physical examinations were conducted on the recruitment day, and following an overnight fast, participants returned the next day to have their blood samples taken.
Participants provided their socio-demographic history, including tobacco use, which was adapted from the World Health Organization's (WHO) STEPwise approach to Surveillance (STEPS) tool.14 Information on the duration of diagnosed HIV infection, cluster of differentiation 4 (CD4) counts and ART regimens was extracted from clinical records. Height to the nearest millimetre was measured using a Leicester Height Scale (Seca, UK) with the participant barefoot and in the upright position. Weight to the nearest gram was measured using Analog and Digital (A&D) Medical PersonalScale (Model UC-321, Japan) with the participant in light clothes and without shoes. Biochemical analyses included serum cotinine levels and lipid profiles, which were acquired at an ISO 15189-accredited pathology laboratory (Path Care, Reference Laboratory, Cape Town, SA), as previously described in detail.15
Definitions
Participants were classified as either 'current smoker', 'former smoker' or 'never a smoker', considering all forms of smoked tobacco, including cigarettes, cigars or pipes. Current smokers included participants who smoked daily or occasionally. Former smokers refer to participants who indicated that they had quit smoking at the time of the interview, regardless of the duration since quitting. 'Smokeless tobacco users' referred to the use of chewing tobacco, snuff or betel leaf and the areca nut at the time of the survey. Exposure to second-hand smoke was determined from 'household smoke'. Cotinine, a major metabolite of nicotine, is commonly used as a biomarker to identify exposure to tobacco.16,17 Serum cotinine levels were used to define the different smoking categories as follows: 'no tobacco exposure': cotinine <10 mg/mL, 'environmental smoke exposure or light smoking': cotinine levels of 10 ng/mL - 100 ng/mL and 'moderate to heavy smoking': cotinine >100 mg/mL, in line with the 2012 South African National Health and Nutrition Examination Survey (SANHANES).18
Alcohol use was defined as drinking at least one standard alcoholic drink per day. A standard alcoholic drink consists of a can (340 mL) of beer, one glass (125 mL) wine or 'one-shot' (25 mL) of spirits. Body mass index (BMI) was calculated as weight in kilograms divided by height in metres squared (kg/m2), and overweight and obesity was defined as BMI ≥ 25 kg/m2.19 Cut-points for HIV-related variables were set at median values, that is, ≥ 396 cell/mm3 for CD4+ counts and of ≥ 5 years duration of HIV diagnosis.
Statistical analysis
The Statistical Package for Social Sciences (IBM SPSS Inc, Chicago, IL, USA) V.25.0 software was used for the data analyses. Continuous variables are presented as means (± standard deviation [SD]) or medians (25th - 75th percentiles) and categorical variables are presented as counts and percentages. Analysis of variance (ANOVA), χ2 tests and non-parametric equivalents were used as appropriate for group comparisons. Logistic regression models adjusted for age and gender were used to determine associations with current smoking. A p-value < 0.05 defined statistically significant results.
Approval to conduct the study
Permission to conduct the survey was obtained from the Health Research Office of the Western Cape Department of Health and the relevant healthcare facilities. The study was approved by the South African Medical Research Council Ethics Committee and conducted in accordance with the principles of the Declaration of Helsinki. All participants signed informed consent. Data were anonymised to prevent identification of individual participants.
Ethical consideration
Permission to conduct the survey was obtained from the Health Research Office of the Western Cape Department of Health and the relevant healthcare facilities. The study was approved by the South African Medical Research Council Ethics Committee (reference number: EC021-11/2013) and conducted in accordance with the principles of the Declaration of Helsinki. All participants signed informed consent.
Results
The sample for this analysis comprised 827 participants, 653 (79%) women and 174 (21%) men after the exclusion of four with missing information on smoking status. The mean age was 38.4 years overall, with men significantly older than women (41.1 vs. 37.7 years, p < 0.001) (Table 1). Compared to men, women had fewer years of education, but a higher proportion was employed. Men were more likely to consume alcohol than women (54.0% vs. 34.3%, p < 0.001). Compared with men, women were more likely to have higher BMI and prevalence of overweight and obesity (both p < 0.001). Median duration of diagnosed HIV infection (p = 0.048) and median CD4 counts (p = 0.001) were also higher in women compared with men. The lipid profile did not vary by gender.
Current smoking prevalence was 22% overall and 26% in men and 21% in women (p = 0.022) (Table 1 and Figure 1). Overall, 14% of the participants were former smokers (Table 1). There were no significant trends in smoking status by age category (p = 0.649). Smoking status was also marginally related to the known duration of HIV infection (p = 0.040) but not the CD4 count (p = 0.151) (Figure 1).
Exposure to second-hand smoke was high with significantly higher rates in women (58.8%) compared with men (44.8%) (p = 0.001) (Table 1). The use of smokeless tobacco products was low, at < 3% for any sub-category, with no significant differences by gender or smoking status.
The median duration of diagnosed HIV infection was 6 years in current smokers, 5 years in non-smokers and 4 years in former smokers (p = 0.020). There were no significant differences in the median CD4 counts by smoking status. In age- and gender-adjusted logistic regression models, none of the general and HIV-predictive characteristics was associated with current smoking (Table 2).
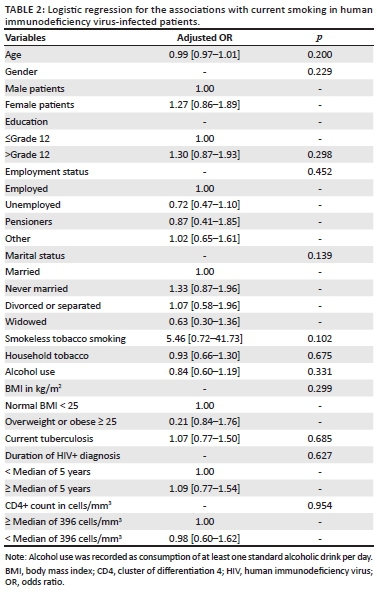
Data on serum cotinine were available for 751 participants. About a quarter of these participants had serum cotinine levels > 100 mg/mL, indicating exposure to tobacco smoke. Prevalence of high serum cotinine levels was similar across smoking status (current smokers: 28.5%, former smokers: 25.9% and never smokers: 23.1%) (p = 0.564) and did not differ between men and women (p = 0.940), between those above and below median duration of diagnosed HIV infection (p = 0.681) and between those above and below median CD4 count (p = 0.505) (Figure 2).
Figure 3 shows the cross-classification of smoking exposure by self-reports and serum cotinine levels, revealing the lack of agreement between the two classification methods (kappa = -0.014, p = 0.488). Among the 751 participants with data available on serum cotinine levels, 158 (21.0%) were current smokers based on self-reports, 108 (14.4%) were former smokers, and 485 (64.6%) had never smoked. Among current smokers based on self-reports, 95 (60.1%) had cotinine levels lower than 10 ng/mL, indicative of low tobacco exposure, while 45 (28.5%) had cotinine levels above 100 ng/mL, indicative of moderate-to-heavy smoking. Among those who had never smoked, 300 (61.9%) had no exposure to tobacco based on cotinine levels, while 112 (23.1%) had cotinine levels compatible with moderate-to-heavy smoking (Figure 3).
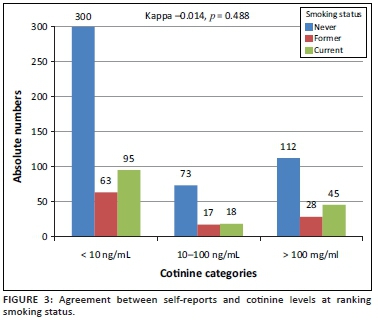
Discussion
Our data show that over one in five PLWHA currently smoke tobacco, with men being more likely to do so than women and with no indication that smoking habits were influenced either by the duration or by the time since HIV diagnosis and awareness of the nadir CD4 count. Furthermore, over half of the study samples (including those who had never smoked) were exposed to second-hand smoke, with such exposure being higher in women. For participants with data available on serum cotinine concentrations, about a quarter (including among self-declared never-smokers) had cotinine concentrations indicative of moderate-to-heavy tobacco smoke exposure. Altogether, our findings suggest that despite the frequent contact of PLWHA with the health system, multiple opportunities had been missed to address the harmful effects of smoking or implement smoking cessation programmes.
Current estimates of smoking habits in the South African population are, in general, based on the 2012 SANHANES18 and the 2016 South African Demographic Health Survey (SADHS).3 According to the SANHANES, 20.8% (32.8% in men and 10.1% in women) of the general population ever smoked (which include current and former smokers) with 38.5% in the Western Cape province. Furthermore, about two-thirds of participants had detectable cotinine in the blood, suggesting recent exposure to cigarette smoking. In the 2016 SADHS, 37% of men and 8% of women aged 15 years and above reported currently smoking tobacco products regularly or occasionally. Equivalent figures for the Western Cape province were 43% and 26%. The prevalence of smoking in our sample, therefore, seems to be generally in line with recent estimates in the general population at the national level.
Few other studies reported smoking habits among PLWHA in SA. In a sample of 1210 PLWHA in Klerksdorp, Elf and co-workers20 found a 34% prevalence of ever-smokers, with rates being higher in men than in women, in line with our findings. In an earlier study in a much smaller sample, Waweru and co-workers reported prevalence rates of 15% (men vs. women: 23.2% vs. 7.4%) for current smoking in Johannesburg.21 Studies from other African countries suggest rates of smoking in PLWHA lower than those reported in SA; likely reflecting the relatively lower overall prevalence of smoking in the general population in these countries.22,23 In an analysis of Demographic Health Survey data from 27 low- and middle-income countries including 24 African countries (excluding SA), the overall prevalence of tobacco smoking in PLWHA across African countries was 24.2% in men (ranging from 9.7% in Ethiopia to 54.8% in The Gambia) and 1.0% in women (ranging from 0% in 11 countries to 4.4% in Gabon).24 Across these surveys, the risk ratio (RR) comparing the prevalence of smoking in people with versus without HIV was in favour of a 47% (male) and 87% (female) relatively higher prevalence in PLWHA. In about half of the studies, however, the confidence interval around RR generally crossed the unity, indicating no significant difference.24 The male preponderance in smoking uptake in the general population has largely been described. This gender difference extends to PLWHA in some studies but narrows down (as in ours) or even disappears completely in some, suggesting an increased uptake of the habit in women.25
One observation from our study was the lack of agreement between self-reports and measured cotinine at ranking status for smoking exposure. Assuming that bias in self-reported status would tend to favour concealing current smoking as opposed to wrongly claiming such a status, applying cotinine levels selectively only in former or never smokers, would have identified nearly an additional 20% of the total samples who were likely current smokers. This would nearly double the proportion of current smokers, suggesting that the dependence on self-report alone is likely to underestimate the true magnitude of current smoking among PLWHA in care. This assumption, however, must be considered in the context of the validity of the cotinine cut-offs applied in our study.
The harmful effects of smoking in PLWHA have been largely described.26 Smoking-related health hazards seen in the general population are exacerbated in PLWHA, where smoking is also responsible for some harmful health effects that are specific to this vulnerable population. People living with HIV and AIDS who smoke are at high risk of cancers (including non-AIDS defining cancers), chronic obstructive pulmonary diseases (COPDs) and chest infections. There are suggestions that smoking can also limit the benefits of ART and decrease life expectancy even in the context of adequate viral suppression27; nevertheless, achieving smoking cessation in PLWHA is likely more challenging than in the general population. Successful and sustainable smoking strategies are therefore needed to mitigate the risk of adverse health outcomes in PLWHA.
Given the burden of cigarette smoking and its adverse health outcomes among HIV-positive patients, screening for smoking and support to quit should be integrated into HIV and AIDS treatment programmes. Currently, evidence exists for both pharmacological and non-pharmacological interventions for smoking cessation, but evidence is needed on how they can best be implemented for smoking cessation in PLWHA in African countries.26,28 One recent qualitative review of smoking cessation interventions in PLWHA identified 32 publications reporting on 28 interventions.29 These studies essentially originated from western countries and the USA in particular. Thirteen of the interventions tested resulted in improved smoking cessation outcomes, with information and communication technologies and clinic-based interventions having the greatest potential to achieve smoking cessation among PLWHA. This is a significant observation considering that with regard to HIV care, PLWHA constitute a highly medicalised population, and are familiar with mHealth interventions in HIV care and monitoring.30 Another recent comparative meta-analysis concluded that compared with face-to-face, interventions mHealth interventions could better achieve smoking cessation in the short term in PLWHA.31 Besides the inadequate knowledge on the efficacy of interventions to achieve smoking cessation in PLWHA, other identified barriers hampering smoking cessation interventions in PLWHA include the scepticism of healthcare providers regarding certain interventions such as nicotine replacement, their unpreparedness to co-address smoking cessation during routine HIV care and other competing priorities.26 In the specific case of SA, economic, social or interpersonal and individual-level factors including stress have been suggested as barriers hindering smoking cessation in PLWHA.32
Strengths and limitations
This study has some limitations. Participants were recruited from only one province of SA and included predominantly women. Smoking assessment inconsistently collected data on the age at initiation (or cessation) of smoking, limiting our ability to assess the potential effect of HIV diagnosis on the adoption or cessation of smoking habits. Data were missing on HIV characteristics (CD4 count) in an important number of participants, limiting our statistical power for some sub-group analyses. The study included only PLWHA and therefore did not offer the opportunity of comparing estimates with those in the non-HIV-infected population. Our study also has some strengths including the relatively large and randomly selected sample, which increased the generalisability of our findings. We also had data on blood cotinine in about 90% of our sample, which allowed us to substantiate that self-reports alone likely misclassify smoking status, with nearly a quarter of those reporting never smoking, having blood cotinine levels compatible with current moderate-to-heavy smoking.
Conclusion
People living with HIV and AIDS in care have current tobacco smoking rates within the range of those found in the general population. These rates appear similar regardless of the known duration of HIV infection and status of disease control. This highlights the potential of missed opportunities or the challenges of co-addressing smoking cessation in PLWHA who are already in regular contact with the health system for the management of HIV and related co-morbidities. With the improved survival of PLWHA on ART and the emergence of NCDs as a new threat to the health of this population, proactively addressing smoking and other major NCD risk factors must become an integral part of the routine care of PLWHA.
Acknowledgements
The authors are grateful to Deborah Jonathan and Erica April from the SAMRC's NCD Research Unit, and their team, for the huge effort to collect data for this study.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
A.P.K. conceived the study and acquired the funding. A.D.V. operationalised and supervised data collection in collaboration. M.M. and A.P.K. analysed the data and drafted the manuscript. N.P. and M.F. critically reviewed the data and revised the manuscript. All co-authors approved the submission. A.P.K. is the guarantor.
Funding information
This study was supported by Grand Challenges Canada, through the Global Alliance on Chronic Diseases initiative (Hypertension Grant #0169-04).
Data availability statement
Data are available upon request from the corresponding author.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1. Drope J, Schluger NW, Cahn Z, et al. The tobacco atlas: American Cancer Society and vital strategies. 6th ed. Atlanta, GA: American Cancer Society; 2018. [ Links ]
2. Groenewald P, Vos T, Norman R, et al. Estimating the burden of disease attributable to smoking in South Africa in 2000. S Afr Med J. 2007;97(8 Pt 2):674-681. [ Links ]
3. National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), ICF. South Africa demographic health survey 2016. Pretoria: NDoH, Stats SA, SAMRC, and ICF; 2019. [ Links ]
4. Crothers K, Goulet JL, Rodriguez-Barradas MC, et al. Impact of cigarette smoking on mortality in HIV-positive and HIV-negative veterans. AIDS Educ Prev. 2009;21(3 Suppl):40-53. https://doi.org/10.1521/aeap.2009.21.3_supp.40 [ Links ]
5. Davies T-L, Gompels M, Johnston S, Bovill B, May MT. Mind the gap: Difference between Framingham heart age and real age increases with age in HIV-positive individuals - A clinical cohort study. BMJ Open. 2013;3(10):e003245. https://doi.org/10.1136/bmjopen-2013-003245 [ Links ]
6. Broom J, Sowden D, Williams M, Taing K, Morwood K, McGill K. Moving from viral suppression to comprehensive patient-centered care: The high prevalence of comorbid conditions and health risk factors in HIV-1-infected patients in Australia. J Int Assoc Physicians AIDS Care (Chic). 2012;11(2):109-114. https://doi.org/10.1177/1545109711418832 [ Links ]
7. Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: Prevalence, frequency, and opportunities for cessation. AIDS Behav. 2010;14:824-835. https://doi.org/10.1007/s10461-008-9449-2 [ Links ]
8. Statistics South Africa. Mid-year population estimates, 2019. In: Statistics South Africa, editor. Pretoria: Statistics South Africa; 2019 [ Links ]
9. Mayosi BM, Lawn JE, Van Niekerk A, et al. Health in South Africa: Changes and challenges since 2009. Lancet. 2012;380(9858):2029-2043. https://doi.org/10.1016/S0140-6736(12)61814-5 [ Links ]
10. May MT, Gompels M, Delpech V, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS. 2014;28(8):1193-1202. https://doi.org/10.1097/QAD.0000000000000243 [ Links ]
11. Phillips AN, Neaton J, Lundgren JD. The role of HIV in serious diseases other than AIDS. AIDS. 2008;22(18):2409-2418. https://doi.org/10.1097/QAD.0b013e3283174636 [ Links ]
12. Lifson AR, Neuhaus J, Arribas JR, et al. Smoking-related health risks among persons with HIV in the strategies for management of antiretroviral therapy clinical trial. Am J Public Health. 2010;100(10):1896-1903. https://doi.org/10.2105/AJPH.2009.188664 [ Links ]
13. Mutemwa M, Peer N, De Villiers A, et al. Prevalence, detection, treatment, and control of hypertension in human immunodeficiency virus (HIV)-infected patients attending HIV clinics in the Western Cape province, South Africa. Medicine. 2018;97(35):e12121. https://doi.org/10.1097/MD.0000000000012121 [ Links ]
14. STEPS W. A framework for surveillance: The WHO STEPwise approach to surveillance of noncommunicable diseases (STEPS). Geneva: World Health Organization; 2003. [ Links ]
15. Nguyen KA, Peer N, De Villiers A, et al. The distribution of obesity phenotypes in HIV-infected African population. Nutrients. 2016;8(6):299. https://doi.org/10.3390/nu8060299 [ Links ]
16. Balhara YP, Jain R. A receiver operated curve-based evaluation of change in sensitivity and specificity of cotinine urinalysis for detecting active tobacco use. J Cancer Res Ther. 2013;9(1):84-89. https://doi.org/10.4103/0973-1482.110384 [ Links ]
17. Duque A, Martínez PJ, Giraldo A, et al. Accuracy of cotinine serum test to detect the smoking habit and its association with periodontal disease in a multicenter study. Med Oral Patol Oral Cir Bucal. 2017;22(4):e425-e431. https://doi.org/10.4317/medoral.21292 [ Links ]
18. Shisana O, Labadarios D, Rehle T, et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: The health and nutritional status of the nation. Paarl: HSRC Press; 2013. [ Links ]
19. World Health Organization. Obesity: Preventing and managing the global epidemic: Report of a WHO consultation. WHO technical report series. Geneva: World Health Organization; 2000. [ Links ]
20. Elf JL, Variava E, Chon S, et al. Prevalence and correlates of smoking among people living with HIV in South Africa. Nicotine Tobacco Res. 2017;20(9):1124-1131. https://doi.org/10.1093/ntr/ntx145 [ Links ]
21. Waweru P, Anderson R, Steel H, Venter WD, Murdoch D, Feldman C. The prevalence of smoking and the knowledge of smoking hazards and smoking cessation strategies among HIV-positive patients in Johannesburg, South Africa. S Afr Med J. 2013;103(11):858-860. https://doi.org/10.7196/SAMJ.7388 [ Links ]
22. Jaquet A, Ekouevi DK, Aboubakrine M, et al. Tobacco use and its determinants in HIV-infected patients on antiretroviral therapy in West African countries. Int J Tuberc Lung Dis. 2009;13(11):1433-1439. [ Links ]
23. Iliyasu Z, Gajida AU, Abubakar IS, Shittu O, Babashani M, Aliyu MH. Patterns and predictors of cigarette smoking among HIV-infected patients in northern Nigeria. Int J STD AIDS. 2012;23(12):849-852. https://doi.org/10.1258/ijsa.2012.012001 [ Links ]
24. Mdege ND, Shah S, Ayo-Yusuf OA, Hakim J, Siddiqi K. Tobacco use among people living with HIV: Analysis of data from Demographic and Health Surveys from 28 low-income and middle-income countries. Lancet Glob Health. 2017;5(6):e578-e592. https://doi.org/10.1016/S2214-109X(17)30170-5 [ Links ]
25. Smith PH, Zhang J, Weinberger AH, Mazure CM, McKee SA. Gender differences in the real-world effectiveness of smoking cessation medications: Findings from the 2010-2011 Tobacco use supplement to the current population survey. Drug Alcohol Depend. 2017;178:485-491. https://doi.org/10.1016/j.drugalcdep.2017.05.046 [ Links ]
26. Giles ML, Gartner C, Boyd MA. Smoking and HIV: What are the risks and what harm reduction strategies do we have at our disposal? AIDS Res Ther. 2018;15(1):26. https://doi.org/10.1186/s12981-018-0213-z [ Links ]
27. Mdodo R, Frazier EL, Dube SR, et al. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: Cross-sectional surveys. Ann Intern Med. 2015;162(5):335-344. https://doi.org/10.7326/M14-0954 [ Links ]
28. Ledgerwood DM, Yskes R. Smoking cessation for people living with HIV/AIDS: A literature review and synthesis. Nicotine Tob Res. 2016;18(12):2177-2184. https://doi.org/10.1093/ntr/ntw126 [ Links ]
29. Mann-Jackson L, Choi D, Sutfin EL, et al. A qualitative systematic review of cigarette smoking cessation interventions for persons living with HIV. J Cancer Educ. 2019;34(6):1045-1058. https://doi.org/10.1007/s13187-019-01525-2 [ Links ]
30. Cooper V, Clatworthy J, Whetham J, Consortium E. mHealth interventions to support self-management in HIV: A systematic review. Open AIDS J. 2017;11:119-132. https://doi.org/10.2174/1874613601711010119 [ Links ]
31. Uthman OA, Nduka CU, Abba M, et al. Comparison of mHealth and face-to-face interventions for smoking cessation among people living with HIV: Meta-analysis. JMIR Mhealth Uhealth. 2019;7(1):e203. https://doi.org/10.2196/mhealth.9329 [ Links ]
32. Krishnan N, Gittelsohn J, Ross A, et al. Qualitative exploration of a smoking cessation trial for people living with HIV in South Africa. Nicotine Tob Res. 2018;20(9):1117-1123. https://doi.org/10.1093/ntr/ntx139 [ Links ]
 Correspondence:
Correspondence:
Andre Kengne
andre.kengne@mrc.ac.za
Received: 15 Jan. 2020
Accepted: 14 Feb. 2020
Published: 21 Apr. 2020
ORIGINAL RESEARCH
Adolescent human immunodeficiency virus self-management: Associations with treatment adherence, viral suppression, sexual risk behaviours and health-related quality of life
Talitha CrowleyI; Anita van der MerweI; Martin KiddII; Donald SkinnerIII
IDepartment of Nursing and Midwifery, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
IICentre for Statistical Consultation, Stellenbosch University, Cape Town, South Africa
IIIDepartment of Public Health, Faculty of Medicine and Health Sciences, Stellenbosch University, Cape Town, South Africa
ABSTRACT
BACKGROUND: With the advent of access to antiretroviral treatment (ART), human immunodeficiency virus (HIV) has become a chronic disease and self-management is an important component of its care. Research to date has not explored associations between adolescent HIV self-management and treatment adherence, viral suppression, sexual risk behaviour and health-related quality of life (HRQoL
OBJECTIVES: To explore the associations between adolescent HIV self-management and treatment adherence, viral suppression, sexual risk behaviour and HRQoL
METHODS: A quantitative cross-sectional study of 385 adolescents living with HIV (ALHIV) aged 13-18 years, who were recruited from 11 healthcare facilities between March and August 2017 in the Cape Metropole of the Western Cape, South Africa, provided the data that were examined in this self-completed questionnaire. Validated scales were used to measure key variables. The most recent viral load (VL) was obtained from the participants' clinic folder, taking into account that VL is done annually
RESULTS: Adolescents who reported higher HIV self-management were more likely to be adherent to treatment (t = 4.435 [336], p < 0.01), virally suppressed (t = 2.376 [305], p = 0.02) and to practise consistent condom use (t = 1.947 [95], p = 0.54). Structural equation modelling (SEM) indicated a significant relationship between self-management and HRQoL (r = 0.43, p < 0.01), whilst non-adherent treatment taking behaviour, correlated with elevated VL log values. No significant correlation was found between self-management and sexual risk behaviour
CONCLUSION: Targeting adolescents' skills related to HIV self-management in the clinical setting may improve adolescents' treatment taking behaviour, viral suppression rates and their HRQoL
Keywords: HIV; adolescents; self-management; quality of life; antiretroviral treatment.
Background
Adolescents living with HIV (ALHIV) represent a growing proportion of the global population of people living with HIV. In 2018, 1 600 000 adolescents [1 100 000; 2 300 000] between the ages of 10 and 19 years were living with HIV. That year, 190 000 were newly infected.1 Sub-Saharan Africa (SSA) has the highest burden of HIV: 89% of the world's ALHIV reside in this region. Of South Africa's (SA) estimated 460 000 ALHIV, 52 000 new infections and 5 600 AIDS-related deaths were reported in 2018.1
Adolescents living with HIV can be divided into two groups: perinatally infected adolescents who are diagnosed as infants or children; and behaviourally/horizontally infected adolescents who likely acquired HIV through sexual transmission.2 One South African study reported 25.4% (n = 269) out of a sample of 1059 adolescents aged 10-19 years acquired HIV horizontally.3 Perinatally infected adolescents are usually treatment-experienced and more likely to suffer from the chronic effects of HIV infection such as delayed growth and development.4 Although the healthcare needs of perinatally and behaviourally infected adolescents may differ, shared healthcare concerns include medication non-adherence, risky sexual behaviour, psychosocial stressors and comorbid psychiatric illness.2,5
Adolescence is a complex developmental phase characterised by physical changes, cognitive and emotional advancement, sexual awakening and an increased sensitivity to relationships with peers.6 Adolescents have a need for autonomy and independence and ALHIV can be expected to begin to take responsibility for their care in preparation for transitioning from paediatric to adult care.2 Although cognitive ability and decision-making capacity have improved, adolescents remain vulnerable, that is, are preoccupied with social acceptance, may engage in risk-taking behaviour and the need to fit in with peers.6 Engagement in HIV care is further threatened by the perceived incongruence between HIV treatment and social goals.7 Deficits in cognitive function, memory and mental processing because of incompletely controlled HIV infection of the nervous system4 may further impair the self-management of ALHIV. Compared to adults, ALHIV have worse treatment outcomes.8 Adolescents living with HIV are more likely to be non-adherent or default their treatment. Evidence in support of specific approaches to the improved adherence of ALHIV is limited.5,9,10,11 Adolescents living with HIV require a differentiated care approach in clinical settings.5 In this regard, self-management is person-centred, an approach that may assist the adolescent to manage normal developmental tasks and to cope better with their HIV status,12 that is, with stigma, sexual health and behaviour, and emotional well-being.5,13
Self-management has been defined as a process by which individuals and families use knowledge and beliefs, self-regulate skills, abilities and social facilitation, to achieve health-related outcomes (Sawin, 2017:171).7 The Individual and Family Self-Management Theory (IFSMT) describes self-management as occurring in the context of various condition-specific, individual and environmental factors. The proximal outcome of self-management is behaviours such as engagement in treatment regimens (adherence). Distal outcomes include, for example, health status and health-related quality of life (HRQoL).7 Health-related quality of life includes perceived physical, emotional, mental, social and behavioural components of well-being and functioning.14
Adolescents living with HIV need skill to self-manage an array of challenges. These include being adherent to treatment (the medical management of their illness) as well as coping with HIV and stigma, namely, role and emotion management.15,16,17 Key self-management skills also include problem-solving, goal-setting and self-evaluation.7 Evidence in this regard, particularly in ALHIV in Africa and the SSA region, is limited.17 A Zambian study (2015) reported that ALHIV had few self-management skills to help them take antiretroviral treatment (ART) regularly.18
Self-management has been associated with better physical, psychological, knowledge and behavioural outcomes in people living with HIV.19 These outcomes have not yet been confirmed among ALHIV. A systematic review of the effectiveness of self-management interventions in youth with chronic conditions such as asthma, diabetes, HIV, cancer and cystic fibrosis found that self-management interventions that were focused on medical self-management and improved adherence to treatment.20 There is, however, little evidence of self-management interventions improving general coping with the chronic condition. Indeed, evidence from systematic reviews suggests that many self-management interventions do not have a sound theoretical basis12,20 and that HIV self-management has not been a research priority in SSA.21
The purpose of this article is to describe associations between adolescent HIV self-management and treatment adherence, viral suppression, sexual risk behaviour and HRQoL. The study was explorative in nature and is a secondary analysis of a larger study aimed to develop an instrument to measure adolescent HIV self-management.22 The theoretical hypotheses of associations between the construct of self-management and the proximal and distal outcomes as presented in the IFSMT are explored. We hypothesised that higher reported levels of self-management will be associated with treatment adherence, less risk-taking sexual behaviour, better HRQoL and better viral suppression rates.
Methods
Study population and design
This is a quantitative cross-sectional study of 385 ALHIV aged 13-18 years, from 11 healthcare facilities in the Western Cape, South Africa. Participants were required to complete a 'self-report' questionnaire. All healthcare facilities in the Cape Metropole with more than 50 adolescents on ART in care were canvassed. Adolescents who attended clinics for HIV care were recruited serially over a period of 5 months, from 13 March 2017 until 4 August 2017. Based on a previous study by Webel et al. that indicated a correlation between self-management and ART adherence as measured on a visual analogue scale, namely, r = 0.18, p < 0.01, a minimum sample size of 240 was required to provide 95% confidence interval.23 Participants were eligible if they knew their HIV status and had the capacity to complete the questionnaire. Of the participants approached, 27 either did not know their HIV status or parents informed the research team that their child was 'slow' and would not be able to comprehend the questions (Figure 1). No formal cognitive assessments were performed.

Data were collected by the researcher with the assistance of trained fieldworkers through paper-based self-report questionnaires. Participants either provided information on their own (70.4%, n = 271) or were assisted by fieldworkers (29.6%, n = 114). The questionnaires were available in the local languages (English, Afrikaans and isiXhosa) and were pretested with 33 participants prior to administration in the main study.
Measures
Demographic information
This section contained questions related to the individual, family and health background of the adolescent. Questions included gender, age, home language, highest grade completed and with whom the adolescent was residing. HIV-related information included how they became infected with HIV, when they were diagnosed with HIV, the age of disclosure, other health-related conditions (co-morbidities) and knowledge of their current CD4 count and viral load (VL) as a measure of their health literacy.2
Self-management
The measure of self-management presented in this article was developed based on the processes of self-management as identified in the IFSMT. The developed 35-item measure of Adolescent HIV Self-Management (AdHIVSM-35) included five components of adolescent HIV self-management (Table 1),24 which was found to be a valid and reliable measure in this population.22 Items were measured with a four-point Likert scale. Two scale options were used: strongly agree/always; agree/most of the time; disagree/sometimes and strongly disagree/never. The minimum score for each item was 1 = poor self-management and the maximum score 4 = good self-management. The Cronbach's alpha of the scale was 0.84 (subscales 0.55-0.76) and test-retest reliability 0.76.22

Sexual risk behaviours
Sexual risk behaviour questions included whether participants ever had penetrative vaginal/anal sex, the frequency of sex (in the past 3 months), number of partners, the use of condoms, diagnosis of sexually transmitted infections and pregnancy. It is made up of 16 questions derived from the Youth Questionnaire for persons aged 15-24 years used in the Third South African National HIV, Behaviour and Health survey.25 The sexual risk behaviour questions did not include questions about sexual abuse, although this was asked in another part of the questionnaire. As the questionnaires were anonymous, the researchers could not take action on these responses if not explicitly reported by the participants.
For the structural equation model (SEM), sexual risk behaviour was calculated as follows: (1) if the response to the two questions, whether they ever had vaginal or anal sex, was 'no' in both cases, then the score = 0. (2) The response to the question on the number of sexual events in the last 3 months provided a score of 1-5. In cases where there was a 'don't know' response, a score of 2 was assigned. (3) The responses on how often condoms were used were assigned the following scores: every time = 0, almost every time = 1, sometimes = 3 and never = 3; (4) Number of partners were scored 1-4. In conflicting cases where respondents indicated that they did have sex, but then responded with 'not applicable' to this question, the number of partners was assumed to be 1. (5) The final score was calculated by assigning a zero if case 1 above was applicable, or the sum of the numbers in cases 2, 3 and 4.
Viral suppression
The most recent documented VL was obtained from the participant clinic folder. A VL of < 50 copies/mL was considered to be viral suppression.26 For the SEM model, the VL log value was used in the analysis.
Adherence
Two Likert scale items were used. It included a rating of how often medication was missed over the past month and a rating of when was the last time the participant missed taking medication.27,28 The two items were dichotomised into adherent (indicating perfect adherence - never skipping or missing a dose) and non-adherent (reporting any missed dose).
Non-adherent behaviour: A list of reasons for non-adherence and the frequency thereof was taken from the Adult AIDS Clinical Trials Group (AACTG) Adherence questionnaire29 that was adapted for adolescents in 2004 by the Paediatric AIDS Clinical Trials Group.30 Response options included the following: never = 0; not often (1-2 times per month) = 1; sometimes (1-2 times per week) = 2 and often (more than 3 times per week) = 3. The total non-adherence score was calculated by adding the item codes for 0 = 'never' through 3 = 'often'. The Cronbach's alpha of this 17-item scale was 0.84.
In addition to the adherence questions, participants were asked how long they had taken ART, how many tablets they took each day and the frequency of daily doses. The current ART regimen was documented from the patient clinic folder.
Health-related quality of life
Health-related quality of life was measured with the KIDSCREEN-27 which consists of 27 items and measures health and well-being on a five-point Likert scale.14 The KIDSCREEN-27 has five latent concepts: physical activities and health; general mood and feelings about yourself; family and free time; friends and school and learning. The Cronbach's alpha coefficients of the KIDSCREEN-27 subscales range from 0.80 to 0.84 and test-retest reliability ranges from 0.61 to 0.74.14 In the present study, Cronbach's alpha was 0.89 (subscales 0.74-0.82).
Statistical analyses
Data were analysed with the Statistical Package for the Social Sciences (SPSS, version 25). Descriptive statistics included frequencies and percentages and means/medians and standard deviations (SDs)/interquartile ranges (IQRs). Bivariate Pearson's correlation was used to test for an association between the total self-management score and the HRQoL and non-adherence behaviour scores. The independent t-test was used to establish mean differences in self-management scores across binary categories of adherence, viral suppression and sexual risk behaviour as self-management scores were normally distributed. For reliability of the instruments used in this study, Cronbach's alphas were calculated and confirmatory factor analyses (CFAs) conducted using the R package Lavaan. Partial least squares structural equation modelling (PLS-SEM) using Smart PLS 3.2.6 was used to determine the relationships between self-management processes, proximal (non-adherence and sexual risk behaviour's) and distal outcomes (HRQoL, VL). The model was created based on the IFSMT (Figure 2).
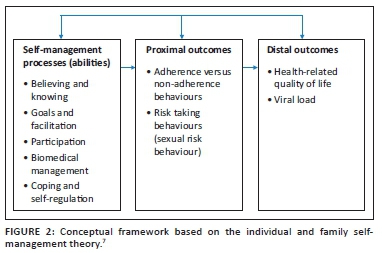
We tested for the direct influence of condition-specific, individual and environmental contextual factors on the self-management processes and outcomes in a separate SEM model. These factors included age, gender, adolescents' knowledge of the route of infection, years on treatment, frequency of treatment and whether they were staying with a biological parent. Because the path coefficients and p-values did not significantly differ between the model where the covariates were included and the model without the covariates (see Appendix 1), only the model without the covariates is reported. A level of significance of <0.05 was used.
Ethical consideration
Stellenbosch University Health Research Ethics Committee approval (Ref: S15/03/054) and Department of Health permission (Ref: WC_2015RP53_21) were obtained to conduct the study. Informed consent was obtained for all adult participants before data collection. Adolescent assent and parental consent (either in person or telephonically) were obtained for adolescents younger than 18.
Results
Reliability analysis
Reliability analyses were conducted on the measurement instruments that were used in this study, namely, self-management, non-adherence behaviour's and quality of life. Cronbach's alphas were calculated, and CFAs were conducted to determine whether this data set supported the latent structures of each of the instruments. In general, the reliability of the instruments was interpreted to be satisfactory (results not shown).
Demographics
The sample included 58.2% (n = 224) females and 77.1% (n = 296) participants were isiXhosa-speaking. The median age was 15 and the IQR range was 14-16. More than a third (n = 138, 36.2%) had not completed the appropriate grade for their age. Participants most frequently reported residing with their biological mother (n = 151, 39.4%). The researcher determined the most likely route of infection based on information provided in the questionnaire, including age of diagnosis and sexual history. The majority of adolescents (n = 344, 89.4%) appeared to have been infected either perinatally or early in life (Table 2).24

Adherence
Only 44.8% (n = 168) and 38% (n = 143) of participants, respectively, reported that they never miss a dose of ART in the past month or never skipped their treatment. Most were on a first-line regimen (Table 3).24 The most frequently reported reasons for missing a dose of ART (not often, sometimes or often) was forgetting (n = 196, 52.7%) because they fell asleep or were still sleeping (n = 135, 36.2%) and that taking antiretroviral drugs (ARVs) reminded them of HIV (n = 124, 33.4%).

Antiretroviral drugs
The frequency of taking tablets was significantly associated with self-management scores (F[3.335] = 3.381, p = 0.02). Those who take tablets once daily had higher self-management scores compared to those who did not know or those who took more than once daily doses.
Sexual risk behaviour
Almost a third (n = 121, 32%) of the participants in this study sample reported having penetrative vaginal sex, 26 (6.9%) penetrative anal sex and 45 (11.9%) oral sex. The mean age of sexual debut reported by 91 participants was 14.03 years (SD 2.14 and range 7-18); 38 participants indicated that they did not remember. Less than half of the participants used condoms every time they had sex (Table 4). Seventeen (12.9%) of the sexually active participants reported having a sexually transmitted infection in the past 3 months. Nine female adolescents (12.5% of sexually active females) reported being pregnant at the time of or before the completion of the questionnaire and nine male participants (15.5% of sexually active males) reported having made a female pregnant.
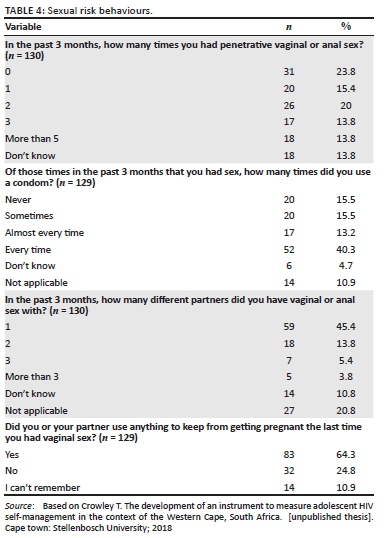
Of the 385 participants, 25 (n = 6.5%) reported sexual abuse within the past 3 months. Of these 25, 17 were female and 8 were male; 16 were in the age category of 16-18 years and 9 were in the age category of 13-15 years.
Health-related quality of life
Health-related quality of life is a subjective measure of one's own health and well-being. The majority (n = 354, 92.4%) of participants reported excellent, very good or good overall health (Table 5). Most participants reported very good or excellent levels of Physical activities and health. On the Mood and feelings scale, most participants reported that, for example, they very often or always enjoyed their life. The scores for the above-mentioned sub-scales were in the same range as international norms, indicating that the participants' HRQoL was similar to other population groups. Means for the Family and Free Time and Friends sub-scales were slightly lower compared to international norms and the mean for School and Learning higher. Lower scores on the Family and Free Time sub-scale seemed to be related to lower ratings with regard to the availability of money. Most participants reported that they were happy at school.
Self-management
Participants generally had high self-management ratings. Self-management items that participants seemed to struggle with (items with mean scores below 3) were coping with HIV stigma, participating in healthcare, communicating with healthcare providers about missing treatment or private issues, participating or finding help in the community, knowing the names of one's ARVs or one's VL, showing interest in understanding one's VL and remembering to take treatment (not relying on other people to remind them). The sub-scales with the lowest mean percentage scores were Biomedical management and Coping and self-regulation (Table 6). Participants who indicated that they did not know how they were infected had significantly lower self-management scores compared to those who knew (t[115.15] = −2.299, p = 0.02).
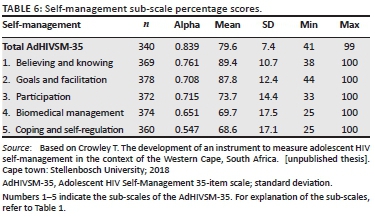
Adolescent HIV self-management had a correlation coefficient of medium strength with HRQoL (r = 0.450, p < 0.01) and a negative correlation with non-adherent behaviour (r = −0.249, p < 0.01). The sub-scale of self-management with the strongest correlation with HRQoL was Goals and facilitation, which includes setting goals, but importantly, obtaining support from family, friends and healthcare workers. Participation or being actively involved in one's care and in social pursuits was the sub-scale that had the strongest negative correlation with non-adherent behaviour.
Adolescents who reported higher HIV self-management were more likely to be adherent to treatment (t = 4.435 [336], p < 0.01), virally suppressed (t = 2.376 [305], p = 0.02) and practise consistent condom use (t = 1.947 [95], p = 0.05) (Table 7).
Structural equation modelling model
Figure 3 shows the PLS structural model indicating a significant relationship between self-management and HRQoL (r = 0.45, p < 0.01). Non-adherent behaviour appears to mediate the relationship between self-management and viral suppression. As shown in Figure 3, non-adherent behaviour was negatively correlated with self-management (r = −0.34, p < 0.01) and positively correlated with VL log (r = 0.17, p < 0.05). This means that lower self-management is associated with more non-adherent behaviour which, in turn, influences VL levels. Non-adherent behaviours had a moderate negative association with HRQoL. There also appears to be a positive correlation between sexual risk behaviour and the VL level (r = 0.15, p < 0.05).

The relationship between self-management and sexual behaviour was not significant.
Discussion
The current study explored relationships between variables based on a framework developed from the IFSMT and therefore cause and effect relationships cannot be inferred. However, the findings of this study support the theory and previous systematic reviews that higher self-management may influence treatment adherence, certain health behaviours, HRQoL and treatment outcome.12,19,20,21 The limitation identified in systematic reviews has been that most self-management interventions had no theoretical basis.12,20 The findings of this study may indicate that interventions that have a comprehensive focus, that include components to address the various self-management processes, may affect both the medical management, for example, adherence and the psychosocial outcomes, such as HRQoL.
The study also yielded some descriptive data with regard to self-management processes and the proximal (adherence and sexual risk behaviour) and distal outcomes (HRQoL and viral suppression). Self-management aspects that participants found challenging concerned knowledge of their treatment, for example, names of their ARVs and an understanding of whether they are doing well on treatment or not. It was challenging to manage HIV stigma, make decisions about disclosure and integrate taking treatment into their daily routine.
Less than half of the participants reported complete adherence in the two Likert scale items in this study. Low adherence rates amongst ALHIV have also been reported in other studies.3,28,31 This study supports the theory that low adherence rates are a concern and explains why adherence is a consistent component of self-management interventions for people living with HIV.13 Although self-management interventions that focus on adherence have been shown to improve treatment taking behaviour,20 self-management interventions must meet a broad range of needs.13,17 New interventions to address psychosocial support and mental health needs of ALHIV are needed. Currently, no single adherence strategy has been identified that improves adherence amongst ALHIV.11
Viral suppression rates (65.1%) in the present study were similar to other adolescent studies, namely, 32.5% - 76%.3,32,33 Other studies reported non-adherence between 30% and 45%31,32,33 whereas in our study it was between 55% and 62%. This may be because of differences in the measurement of non-adherence and the limitation of the current study that the VL was obtained from routine clinic records and not collected at the same time as questionnaires. We found that non-adherent behaviour mediates the relationship between self-management and the lack of viral suppression which is consistent with the IFSMT. Although biological markers have been the outcomes for some self-management interventions, VLs may be specifically related to medication self-management, which is only one component of chronic illness self-management. Self-management interventions may lead to improvement in the management of symptoms, coping, communication, participation and social roles without an effect on biological measures. Researchers should consider including outcomes such as quality of life or other psychological measures to measure the effect of self-management interventions whilst not excluding biological measures.13
In this study, almost a third of the participants reported having sex. The percentage is higher than in other studies amongst perinatally infected adolescents in Thailand, the United States/Porto Rico and South Africa.32,33,34 The present study included perinatally infected and behaviourally infected adolescents, which may be the reason for higher reported sexual activity. Sadly, a number of participants also reported sexual abuse, emphasising that clinicians should explicitly ask about sexual abuse during history taking. Further research is needed to explore sexuality and sexual risk behaviours amongst ALHIV. A study conducted in Botswana found that parents' inaccurate perception of their adolescent's sexual relationships was significantly associated with more risk-taking behaviours, emphasising the importance of parent-adolescent communication.35 Our study only found a borderline significant association between self-management and consistent condom use; according to the IFSMT, higher levels of self-management is associated with better health behaviour.7 Modelling in our study did not indicate a significant association between self-management and sexual behaviour. This may also be because the self-management scale used (AdHIVSM-35) did not specifically focus on sexual behaviour. Future studies should focus on developing instruments specifically for self-management of sexual behaviour.
Bernardin et al. (2013) recommended a culturally appropriate quality of life measurement as a key outcome for self-management interventions.13 Currently, there are no reference norms for HRQoL as measured by KIDSCREEN-27 amongst adolescents in South Africa. All the sub-scale mean scores were in the international range of 45-55, with SDs close to the international range of 10.36 This may indicate the subjective nature of HRQoL as well as the resilience of ALHIV. Nöstlinger et al. (2015) used the Family and Free Time (parents and home life) and Friends (social support by peers) sub-scales in their study in Kampala, Uganda and Western Kenya and reported mean values of 24 (SD 5.7) and 15.6 (SD 6.2), respectively, for the sub-scales, which is comparable to the mean values found in the present study.37 We found that there was a moderately significant relationship between self-management and HRQoL that is consistent with the IFSMT. However, evidence from systematic reviews suggests no clear effects of self-management interventions with regard to the HRQoL of young people living with chronic conditions20 or people living with HIV.19 More research is needed to explore this relationship.
Limitations
The limitations of this study include the cross-sectional nature thereof, the reliance on self-report, specifically with regard to adherence and sexual risk behaviours, and the use of documented VLs. We did not assess cognitive function in this study. Cognitive delay may be an important domain to assess and further research with regard to the relationship between cognitive functioning and self-management is needed. Although more than a third of the participants were not in the correct grade for their age, other factors such as missing school because of ill health or attending appointments may also influence educational delay.38 The timeframe between the last VL measure and completion of the self-report questionnaire was not recorded. Although correlation coefficients were not strong, it is similar to what is reported in other studies.23
Conclusion
Targeting adolescents' skills related to HIV self-management in the clinical setting may improve adolescents' adherence to treatment, viral suppression rates and their HRQoL. The relationship between self-management and sexual risk behaviour needs to be explored further. Sustainable self-management programmes for adolescents in primary healthcare settings should be developed and tested. Caregivers and healthcare workers can be involved and trained to support adolescents with self-management.
Acknowledgements
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
T.C. conducted the research and drafted the article. A.v.d.M. and D.S. were the supervisors for the research project and provided substantial feedback on the article. M.K. provided statistical support and assisted with the interpretation of the SEM model.
Funding information
The authors would like to acknowledge the following organisations that provided funding for this study: National Research Foundation (NRF) of South Africa (Grant number 97022); South-2-South through the President's Emergency Plan for AIDS Relief (PEPFAR) and Harry Crossley Foundation funding through Stellenbosch University. Opinions expressed and conclusions arrived at are those of the authors and are not attributed to the funders.
Data availability statement
Data sharing is not applicable to this article.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1. UNICEF. UNICEF data: Monitoring the situation of children and women [homepage on the Internet]. United Nations Children's Fund; 2019 [cited 2019 Oct 16]. Available from: https://data.unicef.org/topic/hivaids/adolescents-young-people/ [ Links ]
2. Sharer M, Fullem A. Transitioning of care and other services for adolescents living with HIV in sub-Saharan Africa. Arlington, VA: USAID's AIDS Support and Technical Assistance Resources, AIDSTAR-One, Task Order 1; 2012. [ Links ]
3. Cluver L, Pantelic M, Toska E, Orkin M, Casale M, Bungane N. Stacking the odds for adolescent survival: Health service factors associated with full retention in care and adherence amongst adolescents living with HIV in South Africa. J Int AIDS Soc. 2018;21(9):1-8. https://doi.org/10.1002/jia2.25176 [ Links ]
4. Lowenthal ED, Bakeera-Kitaka S, Marukutira T, Chapman J, Goldrath K, Ferrand RA. Saharan Africa: A review of emerging challenges. Lancet Infec Dis. 2014;14(7):627-639. https://doi.org/10.1016/S1473-3099(13)70363-3 [ Links ]
5. Armstrong A, Nagata JM, Vicari M, et al. A global research agenda for adolescents living with HIV. J Acquir Immune Defic Syndr. 2018;78 Suppl 1:S16-S21. [ Links ]
6. Newman B, Newman P. Development through life. 11th ed. New York, NY: Thomson Wadsworth; 2012. [ Links ]
7. Sawin KJ. Definitions, frameworks, and theoretical issues in self-management. J Pediatr Rehabil Med. 2017;10(3-4):169-176. [ Links ]
8. UNICEF. Children and AIDS: Statistical update [homepage on the Internet]. United Nations Children's Fund; 2017 [cited 2019 Oct 16]. Available from: https://data.unicef.org/resources/children-aids-statistical-update/ [ Links ]
9. Collaboration C, Slogrove AL, Schomaker M, et al. The epidemiology of adolescents living with perinatally acquired HIV: A cross-region global cohort analysis. The Collaborative Initiative for Paediatric HIV Education and Research (CIPHER) Global. PLoS Med. 2018;15(3):1-21. https://doi.org/10.1371/journal.pmed.1002514 [ Links ]
10. Hudelson C, Cluver L. Factors associated with adherence to antiretroviral therapy among adolescents living with HIV / AIDS in low- and middle-income countries: A systematic review. AIDS Care. 2015;27(7):805-816. https://doi.org/10.1080/09540121.2015.1011073 [ Links ]
11. Ridgeway K, Dulli LS, Murray KR, et al. Interventions to improve antiretroviral therapy adherence among adolescents in low- and middle-income countries: A systematic review of the literature. PLoS One. 2018;13(1):1-33. https://doi.org/10.1371/journal.pone.0189770 [ Links ]
12. Sattoe JNT, Bal MI, Roelofs PDDM, Bal R, Miedema HS, Van Staa A. Self-management interventions for young people with chronic conditions: A systematic overview. Patient Educ Couns. 2015;98(1):704-715. https://doi.org/10.1016/j.pec.2015.03.004 [ Links ]
13. Bernardin KN, Toews DN, Restall GJ, Vuongphan L. Self-management interventions for people living with human immunodeficiency virus: A scoping review. Can J Occup Ther. 2013;80(5):314-327. https://doi.org/10.1177/0008417413512792 [ Links ]
14. Ravens-Sieberer U, Herdman M, Devine J, et al. The European KIDSCREEN approach to measure quality of life and well-being in children: Development, current application, and future advances. Qual Life Res. 2014;23(3):791-803. https://doi.org/10.1007/s11136-013-0428-3 [ Links ]
15. Swendeman D, Ingram BL, Rotheram-Borus MJ. Common elements in self-management of HIV and other chronic illnesses: An integrative framework. AIDS Care. 2009;21(10):1321-1334. https://doi.org/10.1080/09540120902803158 [ Links ]
16. Holman H, Lorig K. Patient self-management: A key to effectiveness and efficiency in care of chronic disease. Public Health Rep. 2004;119(3):239-243. https://doi.org/10.1016/j.phr.2004.04.002 [ Links ]
17. Mutumba M, Mugerwa H, Musiime V, et al. Perceptions of strategies and intervention approaches for HIV self-management among Ugandan adolescents: A qualitative study. J Int AIDS Soc. 2019;18(1):1-8. https://doi.org/10.1177/2325958218823246 [ Links ]
18. Denison JA, Banda H, Dennis AC, et al. 'The sky is the limit': Adhering to antiretroviral therapy and HIV self-management from the perspectives of adolescents living with HIV and their adult caregivers. J Int AIDS Soc. 2015;18(1):1-6. https://doi.org/10.7448/IAS.18.1.19358 [ Links ]
19. Millard T, Hons OT, Elliott J, Girdler S. Self-management education programs for people living with HIV/AIDS. AIDS Patient Care STDS. 2013;27(2):103-113. https://doi.org/10.1089/apc.2012.0294 [ Links ]
20. Bal MI, Sattoe JNT, Roelofs PDDM, Bal R, Van Staa A, Miedema HS. Exploring effectiveness and effective components of self-management interventions for young people with chronic physical conditions: A systematic review. Patient Educ Couns. 2016;99(8):1293-1309. https://doi.org/10.1016/j.pec.2016.02.012 [ Links ]
21. Aantjes CJ, Ramerman L, Bunders JFG. A systematic review of the literature on self-management interventions and discussion of their potential relevance for people living with HIV in sub-Saharan Africa [homepage on the Internet]. [cited n.d.]. Patient Educ Couns. 2014;95(2):185-200. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0738399114000421?showall=true [ Links ]
22. Crowley T, Van Der Merwe A, Kidd M, Skinner D. Measuring adolescent HIV self-management: An instrument development study. AIDS Behav. 2019;24(20049):1-15. https://doi.org/10.1007/s10461-019-02490-z [ Links ]
23. Webel AR, Asher A, Cuca Y, et al. Measuring HIV self-management in women living with HIV/AIDS: A psychometric evaluation study of the HIV self- management scale. J Acquir Immune Defic Syndr. 2012;60(3):1-19. https://doi.org/10.1097/QAI.0b013e318256623d [ Links ]
24. Crowley T. The development of an instrument to measure adolescent HIV self-management in the context of the Western Cape, South Africa. [unpublished thesis]. Cape town: Stellenbosch University; 2018 [ Links ]
25. Health Sciences Research Council. Third South African National HIV, behavior and health survey [homepage on the Internet]. Human Sciences Research Council; 2008 [cited 2018 Oct 4]. Available from: https://www.hsrc.ac/za [ Links ]
26. Republic of South Africa. National Department of Health. 2019 ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates. 2019 [homepage on the Internet]. [cited 2019 Oct 16]. Available from: https://sahivsoc.org/Files/2019%20Abridged%20ART%20Guidelines%2010%20October%202019.pdf [ Links ]
27. Naar-King S, Frey M, Harris M, Arfken C. Psychological and socio-medical aspects of AIDS/HIV measuring adherence to treatment of paediatric HIV/AIDS. AIDS Care. 2005;17(3):37-41. https://doi.org/10.1080/09540120412331299753 [ Links ]
28. Usitalo A, Leister E, Tassiopoulos K, et al. AIDS care: Psychological and socio-medical aspects of AIDS/HIV relationship between viral load and self-report measures of medication adherence among youth with perinatal HIV infection. AIDS Care. 2014;26(1):37-41. https://doi.org/10.1080/09540121.2013.802280 [ Links ]
29. Chesney MA, Ickovics JR, Chambers DB. UK AIDS care: Psychological and socio-medical aspects of AIDS/HIV self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2010;12(3):255-266. [ Links ]
30. Paediatric AIDS Clinical Trials Group: Adherence questionnaires [homepage on the Internet]. 2016 [cited 2016 Jul 5]. Available from: https://www.frontierscience.org/apps/cfmx/apps/common/QOLAdherenceForms/index.cfm?project=IMPAACT [ Links ]
31. Kim MH, Mazenga AC, Yu X, et al. High self-reported non-adherence to antiretroviral therapy amongst adolescents living with HIV in Malawi: Barriers and associated factors. J Int AIDS Soc. 2017;20(Vl):1-12. https://doi.org/10.7448/IAS.20.1.21437 [ Links ]
32. Brittain K, Myer L, Phillips N, et al. Behavioural health risks during early adolescence among perinatally HIV-infected South African adolescents and same-age, HIV-uninfected peers. AIDS Care. 2019;31(1):131-140. https://doi.org/10.1080/09540121.2018.1533233 [ Links ]
33. Mellins CA, Tassiopoulos K, Malee K, et al. Behavioral health risks in perinatally HIV-exposed youth: Co-occurrence of sexual and drug use behavior, mental health problems, and nonadherence to antiretroviral treatment. AIDS Patient Care STDS. 2011;25(7):413-421. https://doi.org/10.1089/apc.2011.0025 [ Links ]
34. Lee B, Oberdorfer P. Risk-taking behaviours among vertically HIV-infected adolescents in Northern Thailand. AIDS Care. 2009;8(4):221-228. https://doi.org/10.1177/1545109709341082 [ Links ]
35. Sun CJ, Seloilwe ES, Magowe M, Dithole K, St Lawrence JS. Association of adolescent- and parent-reported relationship functioning with HIV sexual risk among adolescents in Botswana. AIDS Behav. 2020;24(3):975-983. https://doi.org/10.1007/s10461-019-02429-4 [ Links ]
36. KIDSCREEN Group Europe. The KIDSCREEN questionnaires [homepage on the Internet]. 2006 [cited 2019 Oct 16]. Available from: https://www.kidscreen.org/english/questionnaires/kidscreen-27-short-version/ [ Links ]
37. Nöstlinger C, Bakeera-Kitaka S, Buyze J, Loos J, Buvé A. Factors influencing social self-disclosure among adolescents living with HIV in Eastern Africa. AIDS Care. 2015;27(S1):36-46. https://doi.org/10.1080/09540121.2015.1051501 [ Links ]
38. Toska E, Cluver L, Orkin M, et al. Screening and supporting through schools: Educational experiences and needs of adolescents living with HIV in a South African cohort. BMC Public Health. 2019;19(1):1-10. https://doi.org/10.1186/s12889-019-6580-0 [ Links ]
 Correspondence:
Correspondence:
Talitha Crowley
tcrowley@sun.ac.za
Received: 10 Dec. 2019
Accepted: 27 Feb. 2020
Published: 29 Apr. 2020
Project Research Number: S15/03/054
APPENDIX 1: Structural equation modelling models.

Figure 1A-1 - Click to enlarge

^rND^sCluver^nL^rND^sPantelic^nM^rND^sToska^nE^rND^sOrkin^nM^rND^sCasale^nM^rND^sBungane^nN^rND^sLowenthal^nED^rND^sBakeera-Kitaka^nS^rND^sMarukutira^nT^rND^sChapman^nJ^rND^sGoldrath^nK^rND^sFerrand^nRA^rND^sArmstrong^nA^rND^sNagata^nJM^rND^sVicari^nM^rND^sSawin^nKJ^rND^sCollaboration^nC^rND^sSlogrove^nAL^rND^sSchomaker^nM^rND^sHudelson^nC^rND^sCluver^nL^rND^sRidgeway^nK^rND^sDulli^nLS^rND^sMurray^nKR^rND^sSattoe^nJNT^rND^sBal^nMI^rND^sRoelofs^nPDDM^rND^sBal^nR^rND^sMiedema^nHS^rND^sVan Staa^nA^rND^sBernardin^nKN^rND^sToews^nDN^rND^sRestall^nGJ^rND^sVuongphan^nL^rND^sRavens-Sieberer^nU^rND^sHerdman^nM^rND^sDevine^nJ^rND^sSwendeman^nD^rND^sIngram^nBL^rND^sRotheram-Borus^nMJ^rND^sHolman^nH^rND^sLorig^nK^rND^sMutumba^nM^rND^sMugerwa^nH^rND^sMusiime^nV^rND^sDenison^nJA^rND^sBanda^nH^rND^sDennis^nAC^rND^sMillard^nT^rND^sHons^nOT^rND^sElliott^nJ^rND^sGirdler^nS^rND^sBal^nMI^rND^sSattoe^nJNT^rND^sRoelofs^nPDDM^rND^sBal^nR^rND^sVan Staa^nA^rND^sMiedema^nHS^rND^sAantjes^nCJ^rND^sRamerman^nL^rND^sBunders^nJFG^rND^sCrowley^nT^rND^sVan Der Merwe^nA^rND^sKidd^nM^rND^sSkinner^nD^rND^sWebel^nAR^rND^sAsher^nA^rND^sCuca^nY^rND^sNaar-King^nS^rND^sFrey^nM^rND^sHarris^nM^rND^sArfken^nC^rND^sUsitalo^nA^rND^sLeister^nE^rND^sTassiopoulos^nK^rND^sChesney^nMA^rND^sIckovics^nJR^rND^sChambers^nDB^rND^sKim^nMH^rND^sMazenga^nAC^rND^sYu^nX^rND^sBrittain^nK^rND^sMyer^nL^rND^sPhillips^nN^rND^sMellins^nCA^rND^sTassiopoulos^nK^rND^sMalee^nK^rND^sLee^nB^rND^sOberdorfer^nP^rND^sSun^nCJ^rND^sSeloilwe^nES^rND^sMagowe^nM^rND^sDithole^nK^rND^sSt Lawrence^nJS^rND^sNöstlinger^nC^rND^sBakeera-Kitaka^nS^rND^sBuyze^nJ^rND^sLoos^nJ^rND^sBuvé^nA^rND^sToska^nE^rND^sCluver^nL^rND^sOrkin^nM^rND^1A01^nNondumiso^sMthembu^rND^1A01 A02^nJienchi^sDorward^rND^1A01^nNivashnee^sNaicker^rND^1A01^nFarzana^sOsman^rND^1A01^nSiphesihle^sGumede^rND^1A03^nYukteshwar^sSookrajh^rND^1A04 A05 A06^nPaul^sDrain^rND^1A01 A07^nNigel^sGarrett^rND^1A01^nNondumiso^sMthembu^rND^1A01 A02^nJienchi^sDorward^rND^1A01^nNivashnee^sNaicker^rND^1A01^nFarzana^sOsman^rND^1A01^nSiphesihle^sGumede^rND^1A03^nYukteshwar^sSookrajh^rND^1A04 A05 A06^nPaul^sDrain^rND^1A01 A07^nNigel^sGarrett^rND^1A01^nNondumiso^sMthembu^rND^1A01 A02^nJienchi^sDorward^rND^1A01^nNivashnee^sNaicker^rND^1A01^nFarzana^sOsman^rND^1A01^nSiphesihle^sGumede^rND^1A03^nYukteshwar^sSookrajh^rND^1A04 A05 A06^nPaul^sDrain^rND^1A01 A07^nNigel^sGarrett
SCIENTIFIC LETTER
Low CD4 count and educational status predict abnormal cervical smears amongst HIV-positive women initiating antiretroviral therapy in South Africa
Nondumiso MthembuI; Jienchi DorwardI, II; Nivashnee NaickerI; Farzana OsmanI; Siphesihle GumedeI; Yukteshwar SookrajhIII; Paul DrainIV, V, VI; Nigel GarrettI, VII
ICentre for the AIDS Programme of Research in South Africa (CAPRISA), University of KwaZulu-Natal, Durban, South Africa
IINuffield Department of Primary Care Health Sciences, University of Oxford, Oxford, United Kingdom
IIIPrince Cyril Zulu Communicable Disease Centre, eThekwini Municipality, Durban, South Africa
IVDepartment of Global Health, Schools of Medicine and Public Health, University of Washington, Seattle, United States
VDepartment of Medicine, School of Medicine, University of Washington, Seattle, United States
VIDepartment of Epidemiology, School of Public Health, University of Washington, Seattle, United States
VIIDepartment of Public Health Medicine, School of Nursing and Public Health, University of KwaZulu-Natal, Durban, South Africa
Introduction
Cervical cancer is common amongst human immunodeficiency virus (HIV)-positive women in low- and middle-income countries. In South Africa, more than 7500 cases are diagnosed annually and over 50% result in death, making cervical cancer the leading cause of cancer mortality.1 South Africa's high HIV prevalence contributes to this high burden, because HIV-positive women are more likely to have persistent human papilloma virus (HPV) infection and precancerous cervical changes.2 Cervical cancer is preventable either through HPV vaccination of girls before sexual debut, which was rolled out in South Africa from 2014, or screening and treatment of precancerous cervical lesions. South African guidelines recommend cervical screening for all HIV-positive women at HIV diagnosis and then every 3 years.1
Antiretroviral therapy (ART) causes immune reconstitution and may reduce the risk of cervical cancer amongst HIV-positive women by lowering HPV acquisition, increasing HPV clearance and slowing the progression to precancerous lesions.2 However, these effects may be diminished for women who initiate ART at low CD4 counts.3 Since 2016, when universal test and treat (UTT) was introduced in South Africa, women began initiating ART at CD4 counts > 500 cells/mm3 (early initiators) and may therefore be protected against precancerous cervical abnormalities and cancer.3
In this study, we aimed to assess whether early initiators of ART had a lower risk of abnormal cervical smears when compared to late initiators (women with a CD4 ≤ 500 cells/mm3), after introduction of UTT in South Africa.
Methods
Study design
We performed a cross-sectional analysis at enrolment into the Simplifying HIV TREAtment and Monitoring (STREAM) study, a randomised trial assessing point-of-care viral load testing amongst people living with HIV receiving ART (NCT03066128).4,5
Participants and setting
The STREAM study was conducted at the Prince Cyril Zulu Communicable Disease Centre (PCZ CDC), a large public clinic in central Durban, South Africa. At PCZ CDC, all women who test HIV-positive are referred for ART initiation and have CD4 cell count and Papanicolaou cervical smear testing performed using routine National Health Laboratory Services (NHLS). We enrolled non-pregnant, HIV-positive adults aged 18 years or older who were clinically stable on first-line ART for 6 months, and randomised them to receive point-of-care viral load monitoring and task-shifting to an enrolled nurse or standard laboratory monitoring and professional nurse care.4 All participants had been initiated on ART after the introduction of UTT. Cervical smear results were checked using NHLS records for all women at screening. Women with abnormal cervical smear results of high-grade squamous intraepithelial lesion (HSIL) who needed referral for colposcopy did not meet the STREAM study eligibility criteria of being clinically stable (as they required active care by a physician) and were excluded from STREAM, and therefore from this analysis.4 Those without a baseline cervical smear result had a smear test after entry into the study and continued follow-up, even if a high-grade lesion was detected.
Data source and data management
At enrolment, we recorded socio-demographic, laboratory and clinical information using a nurse-administered questionnaire and by retrospective review of clinical notes and NHLS records. We used the following validated questionnaires: Audit-C to assess alcohol use, Patient Health Questionnaire 2 (PHQ2) for depression and World Health Organization Violence Against Women for intimate partner violence. We captured data in the secure, password-protected iDataFax system (DF/Net Research, Inc., Seattle, WA, USA) and performed three rounds of data quality checks consisting of source document review, internal quality audits and weekly quality reports.
Statistical analysis
We assessed socio-demographic and clinical exposure variables including age, educational status, having a stable partner, parity, contraceptive use, time from diagnosis to ART initiation, previous tuberculosis, intimate partner violence, alcohol use or depression and CD4 count. The primary outcome for this analysis was the absence or the presence of an abnormal cervical smear, which included atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL) and HSIL. We used descriptive statistics to summarise socio-demographic and clinical variables and missing data. Associations between socio-demographic and clinical exposures, and the outcome of an abnormal cervical smear result were assessed using bivariable and multivariable Poisson regression models with robust variance. We included covariates with a p-value of < 0.15 in the bivariable analysis into the multivariable model. We used SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) for the statistical analysis.
Results
A total of 390 participants were enrolled into the STREAM study, of whom 235 (60.3%) were women, with a median age of 30 years (interquartile range [IQR] 26-37 years) and a median CD4 count of 401/cells/mm3 (IQR 215-608 cells/mm3). Eight women who had HSIL at ART initiation were not eligible for enrolment and thus were excluded from the STREAM study. Of the 235 women, 176 (74.9%) women with a conclusive cervical smear result were included in this analysis (Table 1), and of the 176 women included, 66 (37.5%) had abnormal cervical smears. Amongst the women with abnormal cervical smears, 62 women (35.2%) had LSIL, three (1.7%) had HSIL and one (0.6%) had ASCUS. Women with a CD4 count < 200 cells/mm3 had the highest proportion of abnormal cervical smears (20/40, 50.0%). Of the 59 women with no cervical smear test result available, eight (13.6%) tests were performed but no test result was available, 13 (22.0%) were inadequate for evaluation and 38 (64.4%) had no record of testing. Median CD4 count amongst women with no smear result was 405 (IQR 244-608), compared to 401 (IQR 207-611) amongst those with a smear result (p = 0.498).
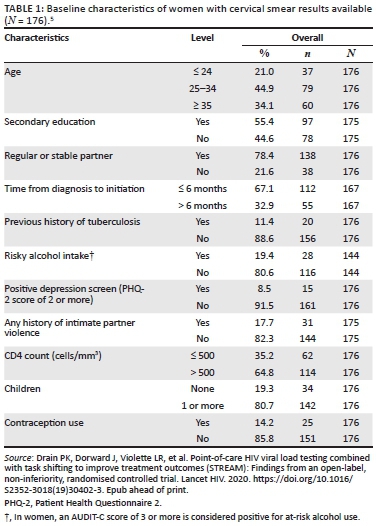
In bivariable analysis, women with an initiation CD4 count of ≤ 500 cells/mm3 had a higher risk of abnormal cervical smears, compared to women with a CD4 count > 500 cells/mm3 (risk ratio 1.84, 95% confidence interval [CI] 1.04-3.28, Table 2). Women who had not completed secondary education also had a higher risk of abnormal cervical smears. Time from diagnosis to ART initiation, having a stable partner, previous tuberculosis, contraceptive use, intimate partner violence, alcohol use or depression were not associated with abnormal cervical smear results.
In multivariable analyses, late initiators of ART had a higher risk of abnormal cervical smears when compared to early initiators (adjusted risk ratio [aRR] 1.71, 95% CI 1.04-2.80). Furthermore, independent of CD4 count, women with a lower educational status also had a higher risk of abnormal cervical smears (aRR 1.48, 95% Cl 1.02-2.14).
Discussion
In this cohort of women initiating ART after implementation of UTT, there was a high prevalence of abnormal cervical lesions, affecting over a third of all women successfully screened. Women who initiated ART at lower CD4 thresholds and those who did not reach secondary education had a higher risk for abnormal cervical lesions.
Our findings are similar to a systematic review, including studies mainly from Europe and North America, which found that women with lower CD4 counts had consistently higher incidence of abnormal cervical lesions.6 This review also showed an increased risk of progression of cervical lesions with declining CD4 count.6 A cross-sectional analysis of 1140 Nigerian women showed that both low- and high-grade cervical lesions were detected almost four times more frequently in women with CD4 < 200 cells/mm3.7 Therefore, earlier initiation of ART at higher CD4 counts may have a protective effect on the development and progression of abnormal cervical lesions in this setting.2,3,8 Of note, in spite of the implementation of UTT, the majority of women in this study presented with CD4 counts ≤ 500 cells/mm3, meaning that further efforts are needed to diagnose and initiate ART earlier amongst women living with HIV.
Similar to our finding, a cross-sectional study from Zambia, analysing data in over 14 000 women from a National Cervical Screening Programme, showed that women having at least secondary education were less likely to develop abnormal cervical lesions, compared to women with no formal education.9 The protective effect of attaining a higher level of education may be explained by increased awareness of the disease, delayed sexual debut and greater access to healthcare, including cervical screening services. While the recent roll-out of HPV vaccination to South African school girls through a public health initiative has somewhat created renewed awareness of cervical cancer, gaps remain in knowledge and prevention of the disease and access to screening services for secondary prevention.1,10
Limitations of our study included the cross-sectional design and a relatively small sample size, which means that the analysis may not be sufficiently powered to rule out weak associations between other exposure variables and abnormal smears. Furthermore, we excluded women with HSIL without a conclusive negative colposcopy result from the study. Even in this large HIV clinic, a quarter of women did not have the recommended cervical smear result available at ART initiation. A strength of our study is that we were able to assess the prevalence of abnormal cervical lesions in women initiating ART at higher CD4 thresholds in the era of UTT in a public health setting.
While HPV vaccination for young women has now been introduced in South Africa, the long-term impact on cervical cancer is still uncertain. In the meantime, cervical cancer screening remains a priority. Here, we highlight that early ART initiation and ensuring frequent cervical screening for those with lower CD4 counts and lower educational background should be prioritised. Universal test and treat will help reduce cervical abnormalities, but other measures, such as scaling up of existing screening services, better colposcopy and treatment provision, as well as the implementation of high-risk HPV screening strategies, are necessary to reduce the burden of this preventable disease.
Acknowledgements
The authors are grateful to the study participants and the staff at the Prince Cyril Zulu Communicable Disease Centre and Centre for the AIDS Programme of Research in South Africa (CAPRISA) eThekwini Clinical Research Site.
Competing interests
The authors report no real or perceived vested interests related to this article that could be construed as a conflict of interest.
Ethical consideration
Ethical approval to conduct the study was obtained by the University of KwaZulu-Natal Biomedical Research Ethics Committee (BFC296/16) and the University of Washington Institutional Review Board (STUDY00001466).
Authors' contributions
N.M., J.D., N.N., P.D. and N.G. conceived the study. N.M., J.D., N.N., S.G. and Y.S. collected the data. F.O. performed the statistical analysis. N.M. and J.D. drafted the manuscript. All authors critically reviewed and edited the manuscript and gave their consent for publication.
Funding information
The Simplifying HIV TREAtment and Monitoring (STREAM) study was funded by the US National Institutes for Health (NIH) (AI124719). The NIH had no role in the study design and manuscript submission, or collection, management, analysis or interpretation of study data.
Data availability statement
The data that support the findings of this study are available from the corresponding author (J.D.) upon reasonable request and subject to CAPRISA data availability policies.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1. The South African National Department of Health. Cervical cancer prevention and control [homepage on the Internet]. Pretoria; 2017 [cited 21 Jan 2020]. Available from: http://www.health.gov.za/index.php/2014-08-15-12-53-24?download=1393:cervical-cancer-policy-pdf [ Links ]
2. Liu G, Sharma M, Tan N, et al. HIV-positive women have higher risk of human papilloma virus infection, precancerous lesions, and cervical cancer. AIDS. 2018;32(6):795-808. https://doi.org/10.1097/QAD.0000000000001765 [ Links ]
3. Kelly H, Weiss HA, Benavente Y, et al. Association of antiretroviral therapy with high-risk human papillomavirus, cervical intraepithelial neoplasia, and invasive cervical cancer in women living with HIV: A systematic review and meta-analysis. Lancet HIV. 2018;5(1):e45-e58. https://doi.org/10.1016/S2352-3018(17)30149-2 [ Links ]
4. Dorward J, Garrett N, Quame-Amaglo J, et al. Protocol for a randomised controlled implementation trial of point-of-care viral load testing and task shifting: The Simplifying HIV TREAtment and Monitoring (STREAM) study. BMJ Open. 2017;7(9):e017507. https://doi.org/10.1136/bmjopen-2017-017507 [ Links ]
5. Drain PK, Dorward J, Violette LR, et al. Point-of-care HIV viral load testing combined with task shifting to improve treatment outcomes (STREAM): Findings from an open-label, non-inferiority, randomised controlled trial. Lancet HIV. 2020. https://doi.org/10.1016/S2352-3018(19)30402-3. Epub ahead of print. [ Links ]
6. Denslow SA, Rositch AF, Firnhaber C, et al. Incidence and progression of cervical lesions in women with HIV: A systematic global review. Int J STD AIDS. 2014;25(3):163-177. https://doi.org/10.1177/0956462413491735 [ Links ]
7. Ezechi OC, Pettersson KO, Okolo CA, et al. The association between HIV infection, antiretroviral therapy and cervical squamous intraepithelial lesions in South Western Nigerian women. PLoS One. 2014;9(5):e97150. https://doi.org/10.1371/journal.pone.0097150 [ Links ]
8. Kelly HA, Sawadogo B, Chikandiwa A, et al. Epidemiology of high-risk human papillomavirus and cervical lesions in African women living with HIV/AIDS. AIDS. 2017;31(2):273-285. https://doi.org/10.1097/QAD.0000000000001301 [ Links ]
9. Hamoonga TE, Likwa RN, Musonda P, et al. Higher educational attainment associated with reduced likelihood of abnormal cervical lesions among Zambian women - A cross sectional study. BMC Cancer. 2017;17(1):681. https://doi.org/10.1186/s12885-017-3680-z [ Links ]
10. Ramathuba DU, Ngambi D. Knowledge and attitudes of women towards human papilloma virus and HPV vaccine in thulamela municipality of vhembe district in Limpopo province, South Africa. Afr J Reprod Health. 2018;22(3):111-119. https://doi.org/10.29063/ajrh2018/v22i3.12 [ Links ]
 Correspondence:
Correspondence:
Jienchi Dorward
jienchi.dorward@caprisa.org
Received: 18 Nov. 2019
Accepted: 22 Jan. 2020
Published: 30 Mar. 2020
Project Research Number: NCT03066128
^rND^sLiu^nG^rND^sSharma^nM^rND^sTan^nN^rND^sKelly^nH^rND^sWeiss^nHA^rND^sBenavente^nY^rND^sDorward^nJ^rND^sGarrett^nN^rND^sQuame-Amaglo^nJ^rND^sDrain^nPK^rND^sDorward^nJ^rND^sViolette^nLR^rND^sDenslow^nSA^rND^sRositch^nAF^rND^sFirnhaber^nC^rND^sEzechi^nOC^rND^sPettersson^nKO^rND^sOkolo^nCA^rND^sKelly^nHA^rND^sSawadogo^nB^rND^sChikandiwa^nA^rND^sHamoonga^nTE^rND^sLikwa^nRN^rND^sMusonda^nP^rND^sRamathuba^nDU^rND^sNgambi^nD^rND^1A01 A02^nVidya^sKalawan^rND^1A03^nKevindra^sNaidoo^rND^1A01 A04^nMoherndran^sArchary^rND^1A01 A02^nVidya^sKalawan^rND^1A03^nKevindra^sNaidoo^rND^1A01 A04^nMoherndran^sArchary^rND^1A01 A02^nVidya^sKalawan^rND^1A03^nKevindra^sNaidoo^rND^1A01 A04^nMoherndran^sArcharyORIGINAL RESEARCH
Impact of routine birth early infant diagnosis on neonatal HIV treatment cascade in eThekwini district, South Africa
Vidya KalawanI, II; Kevindra NaidooIII; Moherndran ArcharyI, IV
IDepartment of Paediatrics and Children Health, University of KwaZulu-Natal, Durban, South Africa
IIKing Dinizulu Hospital, Durban, South Africa
IIIMaternal Adolescent and Child Health (MatCH), University of the Witwatersrand, Johannesburg, South Africa
IVKing Edward VIII Hospital, Durban, South Africa
ABSTRACT
BACKGROUND: Early infant diagnosis (EID) of human immunodeficiency virus (HIV) and early initiation of antiretroviral therapy (ART) in HIV-infected infants can reduce the risk of mortality and improve clinical outcomes. Infant testing guidelines in KwaZulu-Natal, South Africa, changed from targeted birth EID (T-EID) only in high-risk infants to a routine birth EID (R-EID) testing strategy in 2015.
OBJECTIVES: To describe the impact of the implementation of R-EID on the infant treatment cascade.
METHOD: A retrospective analysis of a facility-based clinical database for the eThekwini district and the National Health Laboratory Services (NHLS) was conducted. All data on neonates (< 4 weeks of age) diagnosed with HIV between January 2013 and December 2017 (T-EID [2013-2015] and R-EID [2016-2017]) were extracted including follow-up until 1 year post-diagnosis.
RESULTS: A total of 503 neonates were diagnosed HIV-infected, with 468 (93.0%) initiated on ART within a median of 6 days. There was a significant increase in the estimated percentage of HIV-infected neonates diagnosed (21% vs. 86%, p < 0.001) and initiated on ART (90% vs. 94.3%, p < 0.001) between the T-EID and R-EID periods. Despite achieving over 90% of HIV-infected neonates diagnosed and initiated on ART in 2017, retention in care and viral suppression remained low.
CONCLUSION: Implementation of R-EID in eThekwini district improved diagnosis and initiation of ART in HIV-infected neonates and should be recommended as part of diagnostic guidelines. These gains are, however, lost because of poor retention in care and viral suppression rates and therefore required urgent attention.
Keywords: early infant diagnosis; birth HIV testing; HIV PCR; treatment cascade; paediatrics; LMIC.
Introduction
Early infant diagnosis (EID) of human immunodeficiency virus (HIV) and initiation of antiretroviral therapy (ART) are essential in reducing morbidity and mortality in HIV-infected infants.1,2,3,4,5,6,7,8,9,10 In 2018, the Joint United Nations Programme on HIV/AIDS (UNAIDS) reported that 89% of HIV-exposed infants were tested before 8 weeks of age. However, only 63% of HIV-infected children under 14 years were on ART in South Africa.11 Strategies to increase the uptake of EID and linkage to ART care are essential in achieving the UNAIDS 95-95-95 targets for testing, treatment and viral suppression.
In 2013, the World Health Organization (WHO) recommended an HIV nucleic acid polymerase chain reaction (PCR) assay at 4-6 weeks of age, or at the earliest opportunity for EID in resource-limited settings, although birth HIV PCR testing was a conditional recommendation.12 Despite the improved capacity for EID, only half of all HIV-exposed infants were tested within 2 months of life in the 21 African Global Plan countries.13 Multiple factors contribute to the low rates of early testing of HIV-exposed infants, including the lack of testing and/or sample collection sites, stock-outs of HIV testing commodities at facility and central laboratory levels, poorly functioning sample transport networks, and delays and gaps in returning results to carers.14,15,16,17,18 Access to timely HIV diagnosis for HIV-exposed infants is a critical step to close the treatment coverage gap and reduce HIV-associated mortality for children.19
South Africa's national HIV management guideline was revised in June 2015, with the change in the EID recommendation from targeted birth EID (T-EID) testing in high-risk infants to a routine birth EID (R-EID) testing for all HIV-exposed neonates, in addition to virological testing at 10 or 18 weeks.20 High-risk infants include all premature (born before 37 weeks' gestational age), low-birth-weight (LBW < 2500 g) or symptomatic HIV-exposed neonates, or those born to women who were un-booked or received a late diagnosis of HIV or received < 4 weeks of ART, or had viral loads (VLs) of > 1000 copies/mL.21 Universal birth testing of all HIV-exposed infants is simpler to implement than targeted birth testing.21 Within 1 year of implementation of birth testing, the national birth testing coverage exceeded 90%.22
eThekwini district, KwaZulu-Natal (KZN), has used an electronic data management system (Tier.Net) to record all patient data at a facility level to monitor HIV and ART services and provides a system of monitoring and evaluating the ART programme. A retrospective analysis of routinely collected data from the Tier.Net database over the period of January 2013-December 2017 was carried out. The aim was to determine the proportion of infants with a positive HIV PCR who are successfully initiated on ART and to evaluate the time taken to ART initiation before and after the initiation of birth EID in eThekwini district, South Africa. This study also evaluated the immunological and virological response and outcomes at 12 months after ART initiation.
Methods
The databases of the facility-based patient management system (Tier.Net) and the National Health Laboratory Services (NHLS) were retrospectively analysed to identify all infants diagnosed HIV positive at birth or less than 4 weeks of age between January 2013 and December 2017 at any facility in eThekwini district. Date of ART initiation and clinical and laboratory investigations for the first year after ART initiation were further extracted.
The Tier.Net system is a facility-based patient management system implemented in 2011 by the South African National Department of Health to monitor patients accessing ART from public health facilities. Data on all infants (< 1 year of age) diagnosed and/or initiating ART during the study period were extracted onto a password-protected Excel spreadsheet from Tier.Net.
The Tier.Net data were first filtered to include neonates and then compared with the NHLS EID district report by the investigator to ensure accuracy and to remove duplications. The database included demographic information (e.g. age, sex and facility), age at ART initiation, CD4 T-lymphocyte count and HIV VL results from 1 year after ART initiation. In addition, all biochemical laboratory test results were collected. The database was de-identified prior to analysis. The NHLS performed all laboratory tests as part of the standard of care by using standard commercial laboratory kits.
eThekwini district is the largest district in KZN with a population of 3.7 million and includes urban, peri-urban and semi-rural populations. An estimated 1.9 million people are living with HIV in KZN, with 34% living in eThekwini district.23 Categorical variables were described by percentages, and continuous variables were described by medians and interquartile ranges (IQRs). Univariate analysis of characteristics associated with HIV diagnosis, ART initiation and viral suppression was performed by using Fisher's exact test or the Mantel-Haenszel test for categorical variables. The analysis was stratified into T-EID (from 2013 to 2015) and R-EID (from 2016 to 2017) periods. p values < 0.05 were considered significant. All two-way interactions were evaluated by using STATA IC version 15.
The estimate of the number of HIV-infected neonates in eThekwini was calculated by using the total number of live births in the respective years from Statistics South Africa with 41% of these neonates being HIV-exposed based on the HIV antenatal seroprevalence survey, and an in-utero HIV transmission rate of 0.9%.24,25
Ethical consideration
A patient consent waiver was obtained for this study because of the retrospective nature of the database audit. Ethical approval for the study was obtained from the University of KwaZulu-Natal Biomedical Research Ethics Committee (BREC Ref No: BE023/18), and it was approved by the KwaZulu-Natal Department of Health (HRKM Ref: 183/18, NHRD Ref: KZ_201805_010) and eThekwini district (Ref: 22/02/18).
Results
During the study period, 4049 HIV-infected infants were captured from the Tier.Net database in eThekwini district, and 503 were less than 4 weeks of age at diagnosis. There was a female predominance within the R-EID infants (318/503, 63.2%). A total of 468 (93%) neonates were initiated on ART in the first month of life. Of the 224 (44.5%) infants who initiated ART within the first month of life and those remained in care at 1 year of age, 91 (40.6%) infants had a documented HIV VL. Fifty-six (61.5%) infants had a VL of <1000 copies/mL and 25 (27.5%) infants had a VL of < 50 copies/mL.
Differences between the T-EID and R-EID periods are shown in Table 1. The number of neonates initiating ART increased from 135 (90%) in the T-EID period to 333 (94.3%) in the R-EID (p = 0.081). Median age at ART initiation was at 6 days of life (IQR 0-16) overall. In the R-EID period, the HIV VL was significantly lower and fewer patients were lost to follow-up (Table 1).
Viral suppression in patients with a VL available at 1 year remained low in both periods, with 6 (0.8%) versus 19 (4.6%) infants who were retained in care having a VL of < 1000 copies/mL in the T-EID and R-EID periods, respectively (p < 0.005). Similarly, 7 (1.0%) versus 34 (8.0%) infants who were retained in care had a VL of < 50 copies/mL in the T-EID and R-EID periods, respectively (p < 0.005).
By using the Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Paediatric Adverse Events, we found 12 infants with a low haemoglobin level, eight with grade 3 and two with grade 4. Four infants had grade 1 alanine aminotransferase levels during the study period.
The DAIDS toxicity table is internationally recognised. It contains parameters or adverse events with severity grading guidance.26
On the basis of the estimated number of HIV-infected neonates born in eThekwini district during the study period, there was a significant increase in the percentage of neonates tested (20.7% vs. 85.7%, p < 0.001) and initiated on ART (90.0% vs. 94.3%, p < 0.001) between the T-EID and R-EID periods (Figure 1). In 2017, the percentage of neonates tested and initiated on ART had reached above 90%; however, the percentage of infants with viral suppression (HIV VL < 1000 copies/mL) at 1 year remained low at 10%.
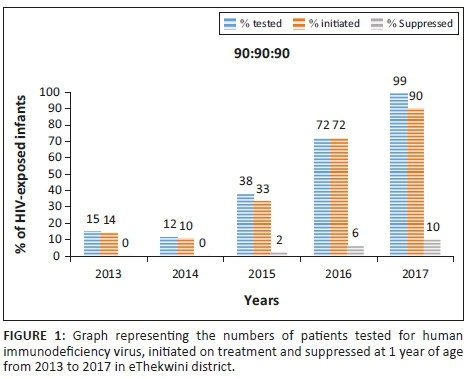
Discussion
In this retrospective analysis of all HIV-infected neonates diagnosed within the first 4 weeks of age and initiated on ART in eThekwini district over 5 years, we found a significant increase in the percentage of neonates diagnosed and initiating ART with the introduction of routine birth EID; however, viral suppression at 1 year after ART initiation remained low.
Achieving the WHO 90:90:90 targets for the infant treatment cascade is vital for improving the outcomes of HIV-infected infants. Because of a lack of evidence, the WHO conditionally recommends the addition of birth testing to existing EID strategies to identify HIV infection in HIV-exposed infants. In a study modelling the impact of the implementation of birth EID, a positive impact on both uptake and timing of ART initiation in HIV-infected infants was predicted.27 Several studies have reported both increases in the number of HIV-exposed infants tested and earlier age of HIV diagnosis in programmes that have implemented birth HIV testing.28,29
In this study, we found a significant increase in the percentage of infants infected during the peripartum and intrauterine period following the implementation of R-EID compared with during the T-EID in KZN. The prevention of mother-to-child transmission (PMTCT) guidelines in South Africa changed in 2013 with the implementation of Option B+ resulting in more HIV-infected women maintained on ART and retained in the PMTCT cascade.30 However, Option B+ was consistently implemented throughout the study period and would not account for the differences noted. Targeted birth EID for high-risk HIV-exposed infants is challenging to implement programmatically, because of the complexity in stratifying according to risk profile in often busy and under-resourced facilities.
Earlier diagnosis of HIV, through the R-EID approach, facilitates improved linkage to care and higher rates of ART initiation. In KZN, the majority of deliveries occur in healthcare facilities with labour wards where birth PCR is performed. In contrast, infant follow-up occurs in well-baby clinics, often at different facilities. Linkage to care is vitally important, and the process starts with obtaining patient contact details in an HIV PCR register when performing the birth PCR following delivery, providing a linkage form to facilitate the transfer of information between the labour ward and well-baby clinics, and active identification facilitated by weekly electronic lists of HIV PCR results supplied by the NHLS directly to key healthcare workers at the facility, district and province. Although a central laboratory for HIV PCR testing is the current testing model in KZN, this may be difficult in areas with logistic problems in tracking, transporting samples and returning the results to the facility and patients. The availability of point-of-care (POC) HIV PCR testing is feasible in several healthcare settings in sub-Saharan Africa and would further improve linkage to care.31,32
Early ART initiation in HIV-infected infants decreases the HIV reservoir and improves morbidity and mortality in these children. In this study, the lower baseline HIV VL and higher CD4 count at ART start during the R-EID period is a finding similar to other studies and likely reflects the very early identification of asymptomatic HIV-infected infants. A study conducted in Zambia between 2006 and 2016 also noted better HIV care and a decrease in the average time from diagnosis to treatment initiation from 220 days in 2006 to 9 days in 2015.33 Increased exposure to antiretroviral drugs through maternal ART and infant prophylaxis is an additional factor that may have contributed to the lower baseline HIV VL. The performance of POC HIV PCR testing in patients with low-level HIV viraemia is an area of concern resulting in diagnostic dilemmas and delays in ART initiation.34
Despite a significant reduction in the number of patients lost to follow-up during the R-EID period, the percentage of patients virally suppressed remained low at 1 year. This finding is similar to other cohorts describing ART initiation during the first 4 weeks of life despite the lower baseline HIV VLs.35,36 Bad tasting liquid lopinavir-ritonavir may contribute to the poor VL suppression.
The challenges associated with improving viral suppression and retention in care require urgent attention to fully realise the benefits of earlier HIV diagnosis and ART initiation seen with R-EID. These strategies include improving patient tracking and linkage to care by utilising community health workers and mobile Health applications, for example a messaging system to remind carers of follow-up appointments.31 HIV stigmatisation and discrimination can interrupt adherence to treatment and retention in care. Therefore, support is required from local organisations that are involved with HIV education, advocacy groups, faith-based organisations and members of support groups for people living with HIV.37,38 Strengthening of referral networks, possibly through the use of an improved patient-held record that is used at all ART facilities, may assist mothers and infants to continue care elsewhere and assist providers at different sites to coordinate care.39
An interesting finding was the disproportionate number of HIV-infected women than men identified. Previous studies show that female sex is more susceptible to intrauterine HIV infection.40 Women are infected by viruses of lower replication capacity in utero than men, but have a higher interferon (IFN) expression and accelerated antiviral immune response to the virus, which is IFN-resistant.40 The possible mechanism underlying this increased female susceptibility is that the sex difference in in-utero infection is most marked in the setting of recent maternal infection. Recently infected mothers are more likely to harbour IFN-resistant virus and women in utero, because of their higher level of immune activation, are more susceptible to the virus.40
Limitations
There are several limitations of the study and the findings because of the retrospective analysis of the data. The facility-based Tier.Net data were dependent on the completeness of the data entered by the facility and may have potentially missed infants not registered. Many patients were captured in the Tier.Net database system that could not be located on the NHLS system despite by using at least two identifiers. Further, as data were limited to eThekwini district, patients who self-transferred or those transferred to other provinces or districts were lost to follow-up. This likely contributed to the observed high loss to follow-up rates. Many patients who were still in care did not have an HIV VL performed at 1 year, further limiting the conclusions related to viral suppression rates in this patient population.
Conclusion
HIV-infected infants can be identified at birth and ART can be initiated within the first 7 days of life. Despite most infants in our cohort starting ART early, the retention of patients in care was suboptimal and viral suppression was very low. Major challenges moving forward are improving retention in care and viral suppression. Adequate maternal counselling during antenatal care is essential and needs to include a clear explanation about EID and infant ART. This discussion needs to continue through each step in the care cascade.
Acknowledgements
The authors would like to acknowledge and thank Mr P. Tinarwo, for his contribution as the statistician.
Competing interests
The authors declare that they have no competing interest.
Authors' contributions
V.K. was involved in concept development, data collection, data analysis and manuscript writing. K.N. conducted data collection and data analysis. M.A. was involved in the concept development and manuscript writing. All authors have read and approved the final manuscript.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Marston M, Becquet R, Zaba B, et al. Net survival of perinatally and postnatally HIV-infected children: A pooled analysis of individual data from sub-Saharan Africa. Int J Epidemiol. 2011;40(2):385-396. https://doi.org/10.1093/ije/dyq255 [ Links ]
2.Luzuriaga K, McManus M, Catalina M, et al. Early therapy of vertical human immunodeficiency virus type 1 (HIV-1) infection: Control of viral replication and absence of persistent HIV-1-specific immune responses. J Virol. 2000;74(1):6984-6991. https://doi.org10.1128/JVI.74.15.6984-6991.2000 [ Links ]
3.Goetghebuer T, Le Chenadec J, Haelterman E, et al. Short and long term immunological and virological outcome in HIV-infected infants according to the age at antiretroviral treatment initiation. Clin Infect Dis. 2012;54(6):878-881. https://doi.org/10.1093/cid/cir950 [ Links ]
4.Luzuriaga K, Tabak B, Garber M, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained control in HIV-1 infected children who received early treatment. J Infect Dis. 2014;210(10):1529-1538. https://doi.org/10.1093/infdis/jiu297 [ Links ]
5.Luzuriaga K, McManus M, Mofenson L, et al. A trial of the three antiretroviral regimens in HIV-1 infected children. N Engl J Med. 2004;350(1):2471-2480. https://doi.org/10.1056/NEJMoa032706 [ Links ]
6.Newell ML, Patel D, Goetghebuer T, et al. CD4 cell response to antiretroviral therapy in children with vertically acquired HIV infection: Is it associated with age initiation? J Infect Dis. 2006;193(1):954-962. [ Links ]
7.Picat MQ, Lewis J, Musiime V, et al. Predicting patterns of long term CD4 reconstitution in HIV-infected children starting antiretroviral therapy in sub-Saharan Africa: A cohort-based modelling study. PLos Med. 2013;10(10):e1001542. https://doi.org/10.1371/journal.pmed.1001542 [ Links ]
8.Chiappin E, Galli L, Tovo PA, et al. Virologic, immunologic and clinical benefits from early combined antiretroviral therapy in infants with perinatal HIV-1 infection. AIDS. 2006;20(13):207-215. https://doi.org/10.1097/01.aids.0000242833.12743.78 [ Links ]
9.Shiau S, Arpadi S, Strehlau R, et al. Initiation of antiretroviral therapy before 6 months of age is associated with faster growth recovery in South African children perinatally infected with human immunodeficiency virus. J Pediatr. 2013;162(6):1138-1145. https://doi.org/10.1016/j.jpeds.2012.11.025 [ Links ]
10.Crowell CS, Huo Y, Tassiopoulos K, et al. Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS. 2015;29:295-304. [ Links ]
11.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS factsheet for South Africa [homepage on the Internet]. 2018 [cited 2020 Feb 12]. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica [ Links ]
12.World Health Organization (WHO). Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach [homepage on the Internet]. Geneva: World Health Organization; 2013 [cited 2020 Feb 12]. Available from: https://www.who.int/hiv/pub/arv/arv-2016/en/ [ Links ]
13.Joint United Nations Programme on HIV/AIDS (UNAIDS). On theFast-track to an AIDS Free Generation: The incredible journey of the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive [homepage on the Internet]. Geneva: Joint United Nations Programme on HIV/AIDS; 2016 [cited 2020 Feb 12]. Available from: https://www.unaids.org/sites/default/files/media_asset/GlobalPlan2016_en.pdf [ Links ]
14.Ciaranello AL, Park JE, Ramirez-Avila L, Freedberg KA, Walensky RP, Leroy V. Early infant HIV-1 diagnosis programs in resource-limited settings: Opportunities for improved outcomes and more cost-effective interventions. BMC Med. 2011;9(1):59. https://doi.org/10.1186/1741-7015-9-59 [ Links ]
15.Penazzato M, Revill P, Prendergast AJ, et al. Early infant diagnosis of HIV infection in low-income and middle-income countries: Does one size fit all? Lancet Infect Dis. 2014;14(1):650-655. [ Links ]
16.Sherman GG, Stevens G, Jones SA, Horsfield P, Stevens WS. Dried blood spots improve access to HIV diagnosis and care for infants in low-resource settings. J Acquir Immune Defic Syndr. 2005;38(5):615-617. https://doi.org/10.1097/01.qai.0000143604.71857.5d [ Links ]
17.Wolpaw BJ, Mathews C, Chopra M, et al. The failure of routine rapid HIV testing: A case study of improving low sensitivity in the field. BMC Health Serv Res. 2010;10(1):73. https://doi.org/10.1186/1472-6963-10-73 [ Links ]
18.Goga A, Chirinda W, Ngandu NI, et al. Closing the gaps to eliminate mother-to-child-transmission of HIV (MTCT) in South Africa: Understanding MTCT case rates, factors that hinder the monitoring and attainment of targets, and potential games changers. S Afr Med J. 2018;108(3 Suppl 1):S17-S24. [ Links ]
19.Diallo K, Modi S, Hurlston M, Beard RS, Nkengasong JN. A proposed framework for the implementation of early infant diagnosis point-of-care. AIDS Rese Hum Retroviruses. 2017;33(3):203-210. https://doi.org/10.1089/aid.2016.0021 [ Links ]
20.National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission (PMTCT) of HIV and the management of HIV in children. Adolescents and adults [homepage on the Internet]. Pretoria: National Department of Health; 2015 [cited 2020 Feb 12]. Available from: https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf [ Links ]
21.Sherman GG. HIV testing during the neonatal period. S Afr J HIV Med. 2015;16(1):362. https://doi.org/10.4102/sajhivmed.v16i1.362 [ Links ]
22.Moyo F, Haeri Mazanderani A, Barron P, et al. Introduction of routine HIV birth testing in the South African National Consolidated Guidelines. Pediatr Infect Dis J. 2018;37(6):559-563. https://doi.org/10.1097/INF.0000000000001840 [ Links ]
23.eThekwini District AIDS Council Quarter1,2017/2018 Report [homepage on the Internet]. [cited 2020 Feb 12]. Available from: http://www.kznonline.gov.za/hivaids/councils/Provincial-Councils-on-AIDS/2017/September/eThekwini%20presentation.pdf [ Links ]
24.Statistics South Africa. Media Release. Recorded live births [homepage on the Internet]. 2016 [cited 2017 Sep 28]. Available from: http://www.statssa.gov.za/?p=10524 [ Links ]
25.Goga A, Chirinda W, Ngandu NI, et al. Closing the gaps to eliminate mother-to-child-transmission of HIV (MTCT) in South Africa: Understanding MTCT case rates, factors that hinder the monitoring and attainment of targets, and potential games changers. S Afr Med J. 2018;108(3 Suppl 1):S17-S24. [ Links ]
26.Division of AIDS (DAIDS). Table forgrading the severity of adult and pediatric adverse events [homepage on the Internet]. Corrected version 2.1, July 2017 [cited 2020 Feb 12]. Available from: https://rsc.niaid.nih.gov/sites/default/files/daidsgradingcorrectedv21.pdf [ Links ]
27.Chiu A, Modi S, Rivadeneira ED, Koumans EH. Optimising infant hiv diagnosis in resource-limited settings: Modeling the impact of HIV DNA PCR testing at birth. J Acquir Immune Defic Syndr. 2016;73(4):454-462. https://doi.org/10.1097/QAI.0000000000001126 [ Links ]
28.Kalk E, Kroon M, Boulle A, et al. Neonatal and infant diagnostic HIV-PCR uptake and associations during three sequential policy periods in Cape Town, South Africa: A longitudinal analysis. J Int AIDS Soc. 2018;21(11):e25212. https://doi.org/10.1002/jia2.25212 [ Links ]
29.Chatterjee A, Tripathi S, Gass R, et al. Implementing services for Early Infant Diagnosis (EID) of HIV: A comparative descriptive analysis of national programs in four countries. BMC Public Health. 2011;11(1):553. https://doi.org/10.1186/1471-2458-11-553 [ Links ]
30.Abrams EJ, Langwenya N, Gachuhi A, et al. Impact of universal antiretroviral therapy for pregnant and postpartum woman on antiretroviral therapy uptake and retention. AIDS. 2019;33(1):45-54. https://doi.org/10.1097/QAD.0000000000002027 [ Links ]
31.Jean-Philippe P, Spiegel H, Gnanashanmugam D, et al. HIV birth testing and linkage to care for HIV-infected infants. AIDS. 2017;31(13):1797-1807. https://doi.org/10.1097/QAD.0000000000001561 [ Links ]
32.Spooner E, Govender K, Reddy R, et al. Point-of-care HIV testing best practice for early infant diagnosis: An implementation study. BMC Public Health. 2019;19:731. https://doi.org/10.1186/s12889-019-6990-z [ Links ]
33.Singh J, Filteau S, Todd J, Gumede-Moyo S. Progress in the performance of HIV early infant diagnosis services in Zambia using routinely collected data from 2006 to 2016. BMC Public Health. 2018;18(1):1297. https://doi.org/10.1186/s12889-018-6222-y [ Links ]
34.Agutu CA, Ngetsa CJ, Price MA, et al. Systematic review of the performance and clinical utility of point of care HIV-1 RNA testing for diagnosis and care. PLoS One. 2019;14(6):e0218369. https://doi.org/10.1371/journal.pone.0218369 [ Links ]
35.Millar JR, Bengu N, Fillis R, et al. High-frequency failure of combination antiretroviral therapy in paediatric HIV infection is associated with unmet maternal needs causing maternal non-adherence. EClinicalMedicine. 2020. https://doi.org/10.1016/j.eclinm.2020.100344 [ Links ]
36.Technau K-G, Strehlau R, Kuhn L. 12 month outcomes of HIV infected infants identified a birth at one maternity site in Johannesburg, South Africa: An observational cohort study. Lancet HIV. 2018;5(12):e706-e714. https://doi.org/10.1016/S2352-3018(18)30251-0 [ Links ]
37.Frigati L, Wynberg E, Maritz J, Holgate S, Cotton MF, Rabie H. Antiretroviral treatment initiated in the first month of life. Pediatr Infect Dis J. 2017;36(6):584-587. https://doi.org/10.1097/INF.0000000000001504 [ Links ]
38.Kinuthia J, Kiarie JN, Farquhar C, et al. Uptake of prevention of mother to child transmission interventions in Kenya: Health systems are influential than stigma. J Int AIDS Soc. 2011;14(1):61. https://doi.org/10.1186/1758-2652-14-61 [ Links ]
39.Pharr JR, Obiefune MC. Linkage to care, early infant diagnosis and perinatal transmission among infants born to HIV-infected Nigerian mothers: Evidence from the healthy beginning initiative. J Acquir Immune Defic Syndr. 2016;72 (Suppl 2):S154-S160. https://doi.org/10.1097/QAI.0000000000001051 [ Links ]
40.Adland E, Millar J, Bengu N, et al. Sex-specific innate immune selection of HIV-1 in utero is associated with increased female susceptibility to infection. Nat Commun. 2020;11:1767. https://doi.org/10.1038/s41467-020-15632-y [ Links ]
 Correspondence:
Correspondence:
Moherndran Archary
Archary@ukzn.ac.za
Received: 05 Mar. 2020
Accepted: 19 Mar. 2020
Published: 02 June 2020
Project Number: BE023/18
^rND^sMarston^nM^rND^sBecquet^nR^rND^sZaba^nB^rND^sLuzuriaga^nK^rND^sMcManus^nM^rND^sCatalina^nM^rND^sGoetghebuer^nT^rND^sLe Chenadec^nJ^rND^sHaelterman^nE^rND^sLuzuriaga^nK^rND^sTabak^nB^rND^sGarber^nM^rND^sLuzuriaga^nK^rND^sMcManus^nM^rND^sMofenson^nL^rND^sNewell^nML^rND^sPatel^nD^rND^sGoetghebuer^nT^rND^sPicat^nMQ^rND^sLewis^nJ^rND^sMusiime^nV^rND^set^nal^rND^sChiappin^nE^rND^sGalli^nL^rND^sTovo^nPA^rND^sShiau^nS^rND^sArpadi^nS^rND^sStrehlau^nR^rND^sCrowell^nCS^rND^sHuo^nY^rND^sTassiopoulos^nK^rND^sCiaranello^nAL^rND^sPark^nJE^rND^sRamirez-Avila^nL^rND^sFreedberg^nKA^rND^sWalensky^nRP^rND^sLeroy^nV^rND^sPenazzato^nM^rND^sRevill^nP^rND^sPrendergast^nAJ^rND^sSherman^nGG^rND^sStevens^nG^rND^sJones^nSA^rND^sHorsfield^nP^rND^sStevens^nWS^rND^sWolpaw^nBJ^rND^sMathews^nC^rND^sChopra^nM^rND^sGoga^nA^rND^sChirinda^nW^rND^sNgandu^nNI^rND^sDiallo^nK^rND^sModi^nS^rND^sHurlston^nM^rND^sBeard^nRS^rND^sNkengasong^nJN^rND^sSherman^nGG^rND^sMoyo^nF^rND^sHaeri Mazanderani^nA^rND^sBarron^nP^rND^sGoga^nA^rND^sChirinda^nW^rND^sNgandu^nNI^rND^sChiu^nA^rND^sModi^nS^rND^sRivadeneira^nED^rND^sKoumans^nEH^rND^sKalk^nE^rND^sKroon^nM^rND^sBoulle^nA^rND^sChatterjee^nA^rND^sTripathi^nS^rND^sGass^nR^rND^sAbrams^nEJ^rND^sLangwenya^nN^rND^sGachuhi^nA^rND^sJean-Philippe^nP^rND^sSpiegel^nH^rND^sGnanashanmugam^nD^rND^sSpooner^nE^rND^sGovender^nK^rND^sReddy^nR^rND^sSingh^nJ^rND^sFilteau^nS^rND^sTodd^nJ^rND^sGumede-Moyo^nS^rND^sAgutu^nCA^rND^sNgetsa^nCJ^rND^sPrice^nMA^rND^sMillar^nJR^rND^sBengu^nN^rND^sFillis^nR^rND^sTechnau^nK-G^rND^sStrehlau^nR^rND^sKuhn^nL^rND^sFrigati^nL^rND^sWynberg^nE^rND^sMaritz^nJ^rND^sHolgate^nS^rND^sCotton^nMF^rND^sRabie^nH^rND^sKinuthia^nJ^rND^sKiarie^nJN^rND^sFarquhar^nC^rND^sPharr^nJR^rND^sObiefune^nMC^rND^sAdland^nE^rND^sMillar^nJ^rND^sBengu^nN^rND^1A01^nJeannette^sWessels^rND^1A02 A03^nGayle^sSherman^rND^1A04 A05^nLesley^sBamford^rND^1A06^nManala^sMakua^rND^1A06^nMathilda^sNtloana^rND^1A07^nJames^sNuttall^rND^1A06^nYogan^sPillay^rND^1A08 A09 A10^nAmeena^sGoga^rND^1A01 A09 A10 A11^nUte^sFeucht^rND^1A01^nJeannette^sWessels^rND^1A02 A03^nGayle^sSherman^rND^1A04 A05^nLesley^sBamford^rND^1A06^nManala^sMakua^rND^1A06^nMathilda^sNtloana^rND^1A07^nJames^sNuttall^rND^1A06^nYogan^sPillay^rND^1A08 A09 A10^nAmeena^sGoga^rND^1A01 A09 A10 A11^nUte^sFeucht^rND^1A01^nJeannette^sWessels^rND^1A02 A03^nGayle^sSherman^rND^1A04 A05^nLesley^sBamford^rND^1A06^nManala^sMakua^rND^1A06^nMathilda^sNtloana^rND^1A07^nJames^sNuttall^rND^1A06^nYogan^sPillay^rND^1A08 A09 A10^nAmeena^sGoga^rND^1A01 A09 A10 A11^nUte^sFeuchtGUIDELINE
The updated South African National Guideline for the Prevention of Mother to Child Transmission of Communicable Infections (2019)
Jeannette WesselsI; Gayle ShermanII, III; Lesley BamfordIV, V; Manala MakuaVI; Mathilda NtloanaVI; James NuttallVII; Yogan PillayVI; Ameena GogaVIII, IX, X; Ute FeuchtI, IX, XI, XII
IResearch Centre for Maternal, Fetal, Newborn and Child Health Care Strategies, University of Pretoria, Pretoria, South Africa
IIDepartment of Paediatrics & Child Health, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IIICentre for HIV & STI, National Institute for Communicable Diseases, Division of the National Health Laboratory Services, Johannesburg, South Africa
IVChild, Youth, and School Health Chief Directorate, National Department of Health, Pretoria, South Africa
VSchool of Health Systems and Public Health, University of Pretoria, Pretoria, South Africa
VICommunicable and Non-Communicable Diseases Branch, National Department of Health, Pretoria
VIIDepartment of Paediatrics and Child Health, Red Cross War Memorial Children's Hospital, University of Cape Town, Cape Town, South Africa
VIIIHealth Systems Research Unit, South African Research Council, Cape Town, South Africa
IXDepartment of Paediatrics and Child Health, University of Pretoria, Pretoria, South Africa
XHIV Prevention Research Unit, South African Medical Research Council, Cape Town, South Africa
XITshwane District Health Services, Gauteng Department of Health, Tshwane, South Africa
XIIMaternal and Infant Health Care Strategies Research Unit, South African Medical Research Council, Pretoria, South Africa
Introduction
South Africa has made great strides in reducing the vertical transmission of human immunodeficiency virus (HIV) in the first two months of life from 23% (2003) to 0.7% (2019), despite a persistently high antenatal HIV prevalence of around 30%. Improving access to antiretroviral therapy during antenatal care has significantly contributed to this success, but has led to an increase in the relative proportion of vertical transmissions due to breastfeeding in the first six months post-delivery. Yet, due to the short- and long-term benefits of breastfeeding and risks associated with not breastfeeding, mothers need to be supported to breastfeed their infants for the longest duration possible, while maintaining virological suppression to reduce the vertical transmission risk.
The new South African National Guideline for the Prevention of Mother to Child Transmission of Communicable Infections (2019) outlines three major strategies for programme improvement. These are 1) prevention of primary HIV infection and unintended pregnancies in women of childbearing potential, 2) improvement of maternal viral suppression rates at delivery and in the post-delivery period through potent, well-tolerated antiretroviral regimens, strategic use of maternal viral load monitoring, linking of mothers to post-delivery HIV care and integration of mother-infant health care, and 3) provision of enhanced prophylaxis to infants of mothers with elevated HIV viral loads in the breastfeeding period, while every effort is made to regain maternal viral suppression.
Rigorous implementation of this guideline can potentially move South Africa closer to the goal of eliminating mother-to-child transmission and making an HIV-free generation a reality.
South Africa's (SA) programme of prevention of mother-to-child transmission (PMTCT) of HIV has achieved remarkable successes in recent years in ensuring good outcomes for pregnant women living with HIV and reducing the risk of vertical HIV transmission to their children.1 The most critical intervention to prevent vertical transmission is the maintenance of the undetectable maternal HIV viral load (VL) levels through effective antiretroviral therapy (ART).2 This has necessitated a considerable scale-up of HIV testing services (HTS) within antenatal care to identify women living with HIV (WLWH). The PMTCT 'Option B Plus', namely the provision of lifelong ART irrespective of CD4 count or clinical disease severity, was implemented in South Africa in January 2015, and significantly improved access to ART for pregnant women in the public sector.3,4 As a result, more than 95% of women with unknown HIV status are currently tested for HIV during antenatal care, and more than 90% of WLWH are on ART.5 Vertical transmission rates within the first two months of life dropped dramatically from 23% in 20036 to 0.7% in 2019.7
Whilst the achievements in reducing HIV transmission at birth are noteworthy, the Global Plan target1 of elimination of vertical transmission will remain elusive due to SA's high HIV prevalence rates. This brings into stark focus the need for primary prevention of HIV in all women of reproductive potential, before, during, and after pregnancy, as well as the urgent need to intensify measures to prevent unintended pregnancies.
According to data published in 2016, the cumulative vertical transmission rate by 18 months of age is 4.3%.8 The largest proportion (> 80%) of these transmissions occur during the first six months of the breastfeeding period,8 when women may experience pronounced challenges to adherence and retention in care, impacting negatively on viral suppression.9,10,11,12 At the same time, breastfeeding remains a key strategy to ensure that South African children survive and thrive. The evidence indicates that the benefits of breastfeeding outweigh the risks of not breastfeeding, regardless of the maternal HIV status.13,14,15 As the HIV epidemic matures, it is clear that the breastfeeding period must be one of the main priorities in the prevention of vertical transmission of HIV. New innovative strategies are required to achieve and maintain maternal viral suppression in the period after birth, whilst simultaneously promoting breastfeeding as a major child survival strategy. In addition, sustained maternal viral suppression will allow the realisation of the longer-term advantages of 'Option B Plus,' including improved maternal health, viral suppression in subsequent pregnancies, and reduced HIV transmission to sexual partners.
To this end, the South African Department of Health has revised the Guideline for the Prevention of Mother to Child Transmission of Communicable Infections (2019). This standalone guideline also forms part of the revised National Consolidated Guidelines for the Management of HIV in Adults, Adolescents, Children and Infants and for the Prevention of Mother-to-Child Transmission (2019). The guideline incorporates new evidence, both scientific and operational, to ensure that South Africa's HIV PMTCT programme remains relevant, practical, and evidence-based. A concerted effort has been made to ensure alignment between these guidelines and other national guidelines, including the Standard Treatment Guidelines and Essential Medicines List for South Africa. It includes a strong focus on the prevention of HIV and unintended pregnancies in women of childbearing potential, maternal viral suppression, preventing MTCT during the breastfeeding period, and care integration for the mother-infant pair. A summary of the major changes in the guideline is illustrated in Table 1, together with the rationale for major changes for WLWH, being provided in the text.
Specific guidelines changes
Antiretroviral therapy during pregnancy and the breastfeeding period
The risk of vertical HIV-transmission correlates strongly with maternal HIV VL levels.16,17 For every additional week on suppressive ART during antenatal care, the risk of MTCT is reduced by 10%.2 Therefore, the prescription of antiretroviral drugs that rapidly and safely achieve and sustain maternal viral suppression during pregnancy and the breastfeeding period is of greatest importance to the prevention of vertical transmission. In this regard, the newly introduced integrase inhibitor dolutegravir (DTG) offers improved tolerability, few drug interactions, and the reduced risk of viral drug resistance.18 The time to viral suppression is approximately halved by DTG when compared to the currently administered drug efavirenz (EFV).18
Recent data from Botswana indicates that DTG may increase the risk of neural tube defects (NTDs).19 The absolute risk is low, currently documented at 0.3% for mothers conceiving on a DTG-containing ART compared to a risk of 0.1% for mothers conceiving on alternative regimens.20 Whilst the World Health Organization (WHO) has recommended DTG as the preferred first-line option for all populations (weight ≥ 20 kg) without exceptions, South Africa has opted for a more conservative approach, and recommends that DTG should be used with caution in women wanting to conceive, and be avoided in the first six weeks of pregnancy, that is, following her last menstrual period and before closure of the foetal neural tube approximately four weeks after conception. However, recent evidence indicates that DTG is likely to have health and cost benefits over EFV even in women who intend pregnancy,21 providing further confirmation for the WHO's more inclusive approach. Whilst South Africa's position on DTG is likely to change as evidence evolves, confusion around the use of DTG in women of childbearing potential has resulted in the suboptimal uptake of effective ART in the women who need it most.
As a positive consequence, the current DTG recommendations will require that family planning services be better integrated into ART care. Health care workers should regularly discuss issues of childbearing and contraception with their clients in order to understand current fertility intentions and contraceptive needs. All women require appropriate counselling on the risks and benefits of DTG and should make an informed choice (Box 1). Women may choose to use DTG; for those women who choose not to use DTG, EFV remains a safe, efficacious and cost-effective option. Concurrent use of effective contraception is recommended for all non-pregnant women not currently desiring a pregnancy.
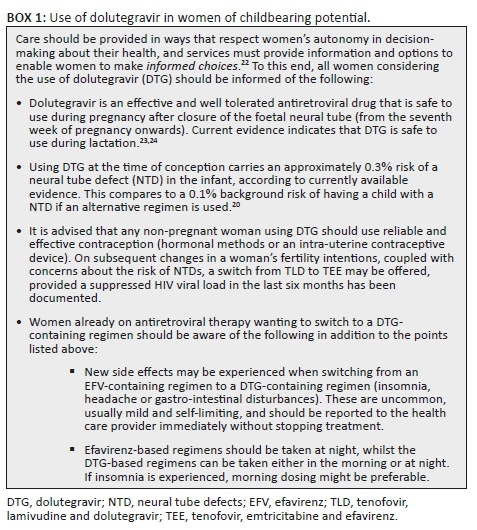
Regimen switches during pregnancy
A single drug switch from an EFV-containing regimen to a DTG-containing regimen should only be considered if the client has a suppressed VL (in the last six months), irrespective of pregnancy status. Therefore, pregnant women already on ART should continue their current ART regimen, pending the result of their HIV VL at entry into antenatal care. If the VL is below 50 copies/mL, and the woman has progressed past the initial six weeks of pregnancy, that is, six weeks since her last normal menstruation, switching to a DTG-containing regimen may be offered. Appropriate counselling as outlined in Box 1 should precede the use of DTG in any women of childbearing potential.
Antiretroviral therapy for women with previous antiretroviral exposure
Women presenting during pregnancy and breastfeeding who are not on ART but with previous exposure to ART present a unique dilemma, given the urgency for maternal VL suppression. They may already have developed viral resistance, with the potential of delayed viral suppression on the re-starting of EFV-containing regimens. For this reason, women known to be living with HIV who are currently not on ART, but who are ART-exposed, for example previous PMTCT, or previous loss-to-follow-up on ART, should initiate a DTG-containing regimen. The fixed-dose combination of tenofovir (TDF), lamivudine (3TC), and DTG, known as TLD, is recommended with previously documented VL suppression on ART. Without proof of prior viral suppression (with previous VL results either not suppressed or not available), zidovudine (AZT), 3TC and DTG should be initiated, due to the higher likelihood of concomitant resistance to both the TDF and EFV components of their first-line fixed-dose combination regimen.
Antiretroviral therapy for women presenting in labour
For women initiating ART around the time of labour and delivery, earlier guidelines recommended one dose of nevirapine (NVP) and TDF/emtricitabine (FTC), together with three-hourly AZT during labour.25 This has been replaced with a single dose of NVP, together with a dose of TLD (See Table 1, row 7: ART for the mother presenting in labour). The dose of NVP is retained due to existing evidence.26 Previously, in the era where not all women were eligible for ART post-delivery, the TDF/FTC combination was used to 'cover the tail' and reduce the risk of viral resistance. However, all women now qualify for ART. The TLD given in labour provides the first dose of lifelong ART and removes the need for both TDF/FTC and three-hourly AZT during labour. Antiretroviral therapy is to be continued the following day after understanding the woman's fertility intentions and appropriate counselling on the risk of DTG-associated NTDs for her subsequent pregnancies. The recommended regimen in the period after birth is TLD. Concurrent use of effective contraception is recommended for all women at discharge from labour wards.
Maternal human immunodeficiency virus viral load monitoring
Potent ART regimens are most effective when coupled with HIV VL monitoring to confirm good adherence and viral suppression or to allow the timely detection of factors that may negatively affect viral suppression (e.g. suboptimal treatment adherence, drug interactions, intercurrent infections, etc.). Importantly, VL monitoring can only be effective if coupled with a response to the test results, and women with detectable VL results (VL > 50 c/mL) must be followed up for urgent intervention, directed to ensure immediate viral suppression. Accurate national surveillance of maternal VL suppression will require a means to distinguish between VLs done during antenatal care, at delivery, and after birth. Within the public sector this will be achieved through the use of electronic gatekeeping codes submitted on National Health Laboratory Service (NHLS) requisition forms (code C#DELIVERY applied to VLs at delivery, and C#PMTCT applied to VLs during the antenatal and postpartum periods). Of great importance, colleagues in the private sector may wish to discuss with their laboratory service the value or feasibility of implementing such a coding system in their practice.
Viral load monitoring during the antenatal period
Women initiating or re-initiating ART in pregnancy should have their first VL measured three months after starting ART. If suppressed, the VL should be repeated at delivery. For WLWH and who are already on ART, the VL should be measured at the first antenatal care visit, as per previous guidelines. If suppressed, the VL is repeated at delivery, unless subsequent VL non-suppression is suspected. Accurate recording, of antenatal maternal VL testing data in the maternity case record, is crucial to ensure communication and continuity of care between antenatal and delivery services.
Women initiating ART at any time after 28 weeks gestation will have a VL measurement done at delivery and repeated three months after delivery. If the mother-infant pair is receiving integrated care, this VL should be aligned to the 10-week well-child immunisation visit.
Viral load monitoring at delivery and six months after delivery
Previous implementation of VL monitoring in the PMTCT programme has been suboptimal. Thus, the VL monitoring schedule, that had been linked to the ART history and timing of ANC booking, has been changed to fixed time points linked to the pregnancy itself. A VL at delivery has, therefore, been introduced for all WLWH. Much debate has ensued with regard to the best timing of a VL around the time of delivery. Whilst results of a VL done at 36 weeks' gestation would be available by the time of delivery, a significant proportion of women, including those with premature labour, those with uncertain gestational age, and un-booked deliveries, would not receive a VL at 36 weeks' gestation. Additionally, antenatal care usually occurs at a different facility from delivery, and antenatal VL results may not be available at the delivery site.
A VL done at the actual time of delivery has the following advantages:
•High in-facility birth rates, and previous rapid uptake and high coverage of infant birth PCR testing within labour wards, infer the possibility of near-universal coverage of the delivery VL.
•Uniformly performed VLs at time-point of delivery will enable the health system to monitor and better enforce the coverage of maternal VL testing.
•Viral load testing at delivery allows for placing of the reference to the maternal laboratory testing into the infant's Road-to-Health Booklet to provide a potential link for PMTCT interventions, promoting integrated care for the mother-infant pair and ensuring consideration of maternal laboratory results during infant health care provision.
•It provides a means of epidemiological surveillance of vertical transmission risk at delivery.
Given that more than half of vertical transmissions now occur after delivery,8,27 linked to postpartum challenges to adherence and retention in care,9,10,11,12 a maternal VL should be done at six months after delivery for all WLWH, aligned to the six-month well-child immunisation visit, regardless of breastfeeding status. As the maternal VL impacts directly on the care required for the infant, breastfeeding mothers who are not receiving integrated care as a mother-infant pair and who are receiving care at a separate ART clinic face a significant challenge. General ART services have, to date, not effectively identified breastfeeding mothers, nor monitored their VLs at six-monthly intervals. If breastfeeding is to be protected by providing enhanced infant antiretroviral prophylaxis in mothers who have detectable VLs, much will need to be done to improve the identification of breastfeeding mothers and their infants, VL monitoring, and communication with providers caring for their infants.
Clinical interventions alone will not improve postpartum viral suppression. Health workers, supported by community engagement and media campaigns, should empower mothers with knowledge regarding the importance of continued viral suppression for both maternal and child health benefits. Women need to be equipped to anticipate adherence challenges in the postpartum period, including disrupted routines, sleep deprivation, and postnatal depression. Linkages to available resources should occur, for example community health workers, mentor mothers, MomConnect, or other support groups and clubs. A concerted effort should be made by all in the care team to ensure that the mother is retained in care, adherent to ART, maintains VL suppression and has access to effective contraceptive services.
Management of an elevated maternal human immunodeficiency virus viral load
Viral load suppression across the South African ART programme is now defined as a VL of < 50 copies/mL. Any VL ≥ 50 copies/mL implies viral replication and risk to the mother and infant. If the mother is on ART and her VL is detectable, drug resistance must be considered. But such a situation needs to trigger a thorough assessment, for example evaluation of adherence, a check of medication dosages, a review of potential drug-drug interactions and the exclusion of intercurrent infection. A cause needs to be identified and corrected. An elevated maternal VL of ≥ 1000 copies/mL at delivery or during the breastfeeding period warrants initiating, extending, or re-starting high-risk infant ART prophylaxis (see below).
The threshold for virological failure and switch to second-line ART is influenced by the treatment regimen, namely an EFV or a DTG-containing regimen, and the prior duration of ART. The definition of virological failure whilst on an EFV-based regimen remains a VL of ≥ 1000 c/mL on two consecutive occasions ideally not longer than three months apart, despite attention to adherence issues. The definition of failure on a DTG-based regimen requires at least three VLs ≥ 1000 copies/mL over a two-year period together with a focused effort to improve the patient's adherence. Women failing to suppress on second- or third-line ART warrant expert discussion or referral. These clients are likely to be experiencing complex clinical and psychosocial challenges and will likely benefit from an individualised approach to maternal management, infant prophylaxis, and recommendations for possible breastfeeding cessation and the prescription of infant formula.
Infant post-exposure prophylaxis to be provided at birth
The delivery VL will determine the infant's HIV transmission risk category. However, many mother-infant pairs will be discharged after birth but before the delivery VL results are available. Then the most recently available VL result, that is, within the last 12 weeks, will determine the infant's risk category prior to discharge at birth. The laboratory barcode sticker or similar indicator of the delivery VL, clearly labelled 'maternal' to distinguish it from the infant's HIV PCR barcode sticker, should be placed in the infant's patient records, together with the Road-to-Health Booklet, to ensure access to both maternal and infant birth-PCR results at the 3-6-day postnatal visit. Adjustments can then also be made to the infant prophylaxis regimen, as appropriate.
•Low-risk infants, namely infants born to mothers with a suppressed VL within the last 12 weeks of delivery, or at delivery, should receive NVP daily for six weeks.
•High-risk infants identified at birth, namely no recent VL available in the last 12 weeks of pregnancy or maternal VL ≥ 1000 copies/mL, should receive high-risk prophylaxis: NVP daily for a minimum of 12 weeks and AZT twice daily for six weeks.
•A persistently elevated maternal VL in the breastfeeding period is a further risk for HIV acquisition in the infant.16,17 For this reason, NVP should only be stopped after 12 weeks post-delivery if the maternal VL is < 1000 copies/mL. Otherwise NVP should be continued until a maternal VL of < 1000 copies/mL is achieved or until four weeks after breastfeeding cessation.
Table 2 provides a summary of infant post-exposure prophylaxis to be given at birth, based on the infant's risk profile for HIV transmission and chosen feeding method.
Infant human immunodeficiency virus testing
Infant HIV PCR testing at birth is recommended in all HIV-exposed infants, to identify intra-uterine transmission. Wherever possible, birth HIV PCR tests should not be submitted with the mother's name and details such as 'Baby of'. Mothers should be counselled during antenatal care to provide the permanent name and surname of the child at delivery. Correct identification information for the child will allow the birth PCR to be linked to later test records, and will facilitate registration of the birth with home affairs before discharge.
HIV PCR testing at age 10 weeks is recommended to identify perinatal HIV transmission. This remains part of the standard schedule of testing for HIV-exposed infants (Table 3). However, the HIV PCR test at 18 weeks, previously performed in infants who received high-risk prophylaxis, has been discontinued due to inadequate uptake and non-alignment with any child immunisation visit. Instead, all HIV-exposed infants, both low and high risk, are to receive an HIV PCR test at the six-month integrated well-child visit. Over 80% of infants who acquire HIV do so by six months of age, highlighting the importance of this six-month HIV PCR test.8 However, the risk of infant HIV acquisition may shift to more than 20% after six months of age if longer periods of breastfeeding are achieved. Breastfed infants who test HIV negative, particularly those on antiretroviral prophylaxis, should not be assumed to be uninfected until after the age-appropriate post-weaning HIV test has been performed.
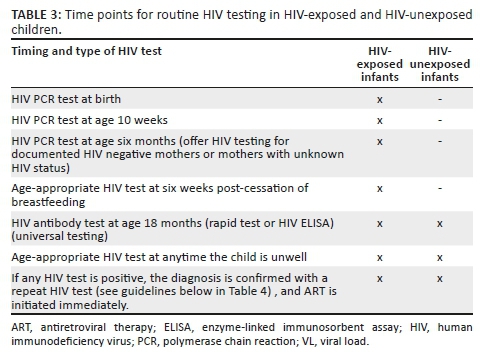
At six months of age, the HIV status of all infants not already known to be HIV-exposed should be established by offering an HIV test to the mother. This maternal HIV test should fall into the routine three-monthly HIV testing schedule for all breastfeeding mothers who are not yet known to be living with HIV. If a maternal HIV test is not feasible, consent should be obtained to perform a rapid test on the child. Care that is provided in an integrated manner to the mother-infant pair greatly facilitates this process of (1) identifying a women as breastfeeding, (2) identifying those women who require HIV testing, and (3) determining the care required by the infant as informed by the mother's results. Breastfeeding mothers who are not receiving integrated care face a significant challenge with regard to repeat HIV testing. Much will need to be done to ensure that breastfeeding mothers are able to access HIV testing services within family planning clinics and other general health services, and that her infant receives the appropriate care according to her test results.
An HIV antibody test (HIV rapid or ELISA [enzyme-linked immunosorbent assay] test) is used as a screening test above 18 months of age. However, in a small percentage of children the maternal antibodies persist beyond 18 months of age, potentially resulting in false-positive HIV diagnoses and inappropriate initiation of lifelong ART.22 Therefore, HIV PCR testing is now recommended as confirmatory testing in all HIV-positive HIV rapid or ELISA tests in children under two years of age. A summary of initial and confirmatory HIV testing is outlined in Table 4.
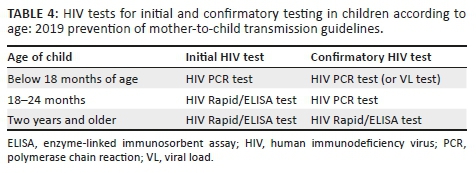
At the clinician's discretion, the HIV PCR may be replaced by a VL test, which has the advantage of both confirming the HIV diagnosis and providing a baseline VL for monitoring the child's response to ART. Diagnostic difficulties, for example indeterminate HIV PCR test results, require urgent expert advice.
Breastfeeding
Due to the expanded access to ART in South Africa, the country-level recommendations for breastfeeding are now the same whether or not the mother is living with HIV.15,28 These recommendations include exclusive breastfeeding for the first six months, the introduction of appropriate complementary foods thereafter, and continued breastfeeding for two years or longer. Given the numerous benefits of breastfeeding for the health and well-being of all children, it is imperative that mothers are supported to breastfeed their infants for the longest possible duration whilst maintaining viral suppression and reducing the risk of HIV-transmission through breastmilk exposure.
To reduce HIV transmission during breastfeeding, the guideline outlines two major strategies. The first is to improve viral suppression rates in the period after birth by (1) providing potent and well-tolerated ART regimens - including DTG, (2) outlining mechanisms for linking mothers back into appropriate HIV care post-delivery, (3) integrating services for mother-infant pairs to promote adherence and retention in care, and (4) using VL monitoring strategically for the timely detection of, and response to, elevated HIV VLs. Whilst maternal viral suppression remains the gold standard, elevated HIV VLs will occur in selected women in the breastfeeding period. The second strategy is to provide enhanced infant prophylaxis whilst every effort is made to regain maternal viral suppression.
Prophylaxis for infants of breastfeeding mothers with an elevated human immunodeficiency virus viral load
An elevated maternal HIV VL during breastfeeding can occur either at the time of a new HIV diagnosis or because of an unsuppressed VL on ART. Regardless of the cause, an elevated HIV VL in a breastfeeding mother requires urgent clinical intervention and more frequent VL monitoring. With pre-treatment resistance to non-nucleoside reverse transcriptase inhibitors documented to be on the increase, and the high likelihood (up to 40%) of viral resistance in the mother with an elevated VL on ART,29,30 these infants should receive high-risk prophylaxis consisting of AZT twice daily for six weeks, and NVP daily for a minimum of 12 weeks, with infant NVP only stopped after confirmed maternal HIV VL < 1000 c/mL, or breastfeeding cessation.
Consolidated HIV PCR and VL Results for Action reports, generated daily or weekly from the NHLS data warehouse, can be used to fast track high-risk clients, including pregnant women with high VLs and HIV-infected infants. Reports for the public sector can be accessed by registering at http://www.nicd.ac.za.
Conclusions
Whilst much progress has been made in preventing vertical transmission of HIV, much remains to be done. Overcoming the 'next frontier' and preventing vertical HIV transmission during the breastfeeding period may well be the most challenging phase yet. In response, the new 2019 PMTCT guideline outlines strategies that focus strongly on maintaining maternal HIV viral suppression, strengthening and integrating care for mother-infant pairs, and supporting and protecting breastfeeding as a major child survival strategy. Creating an environment that enables the rigorous implementation of this guideline will move South Africa closer to the goal of eliminating vertical HIV transmission and making an HIV-free generation a reality.
The Guideline for the Prevention of Mother to Child Transmission of Communicable Infections is available at http://bit/ly/2019-PMTCT-Guidelines.
Acknowledgements
The authors would like to acknowledge the National Department of Health Prevention of Mother-to-Child Transmission (NDOH PMTCT) Technical Working Group, and all experts and health care workers who provided inputs into the guideline.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
U.D.F. conceptualised the paper and provided strategic guidance to J.W. As lead author, J.W. synthesised all drafts, managed input and prepared the paper in final form. U.D.F. reviewed all drafts and provided editorial support. A.G., G.S., L.B. and J.N. provided strategic inputs and contributed to all drafts. All authors read and commented on drafts and approved the final version.
Ethical consideration
This article followed all ethical standards for carrying out research without direct contact with human or animal subjects.
Funding information
This paper received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in preparation of this manuscript.
Disclaimer
The information presented in these guidelines conforms to the current medical, nursing and pharmaceutical practice.
Contributors and editors cannot be held responsible for errors, individual responses to medicines, and other consequences.
References
1.UNAIDS. 2015 progress report on the global plan [homepage on the Internet]. Geneva: UNAIDS; 2015 [cited 2019 Oct 17]. Available from: https://www.unaids.org/sites/default/files/media_asset/JC2774_2015ProgressReport_GlobalPlan_en.pdf [ Links ]
2.Townsend CL, Byrne L, Cortina-Borja M, et al. Earlier initiation of ART and further decline in mother-to-child HIV transmission rates, 2000-2011. AIDS. 2014;28(7):1049-1057. https://doi.org/10.1097/qad.0000000000000212 [ Links ]
3.Kim MH, Ahmed S, Abrams EJ. Pediatric HIV: Progress on prevention, treatment, and cure. Curr Pediatr Rep. 2015;3(3):219-229. https://doi.org/10.1007/s40124-015-0087-7 [ Links ]
4.Myer L, Phillips TK. Beyond 'Option B+'. JAIDS. 2017;75(Suppl 2):S115-S122. https://doi.org/10.1097/qai.0000000000001343 [ Links ]
5.Massyn N, Peer N, English R, Padarath A, Barron P, Day C. District health barometer 2015/16 [homepage on the Internet]. Durban: Health Systems Trust; 2016 [cited 2019 Oct 17]. Available from: https://www.hst.org.za/publications/District%20Health%20Barometers/District%20Health%20 Barometer%202015_16.pdf [ Links ]
6.Sherman GG, Lilian RR, Bhardwaj S, Candy S, Barron P. Laboratory information system data demonstrate successful implementation of the prevention of mother-to-child transmission programme in South Africa. S Afr Med J. 2014;104(3):235. https://doi.org/10.7196/samj.7598 [ Links ]
7.Moyo F, Haeri Mazanderani A, Barron P, et al. Introduction of routine HIV birth testing in the South African National consolidated guidelines. Pediatr Infect Dis J. 2018;37(6):559-563. https://doi.org/10.1097/inf.0000000000001840 [ Links ]
8.Goga A, Jackson C. Highest risk of mother-to-child transmission of HIV or death in the first 6 months postpartum: Results from 18-month follow-up of an HIV-exposed national cohort, South Africa [homepage on the Internet]. Presented at: AIDS 2016 Conference, 2016 [cited 2019 Dec 13]. Available from: https://programme.aids2016.org/Abstract/Abstract/6477 [ Links ]
9.Gertsch A, Michel O, Locatelli I, et al. Adherence to antiretroviral treatment decreases during postpartum compared to pregnancy: A longitudinal electronic monitoring study. AIDS Patient Care STDs. 2013;27(4):208-210. https://doi.org/10.1089/apc.2013.0005 [ Links ]
10.Nachega JB, Uthman OA, Anderson J, et al. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high-income countries. AIDS. 2012;26(16):2039-2052. https://doi.org/10.1097/qad.0b013e328359590f [ Links ]
11.Haas A, Msukwa M, Egger M, et al. Adherence to antiretroviral therapy during and after pregnancy: Cohort study on women receiving care in Malawi's Option B+ Program. Clin Infect Dis. 2016;63(9):1227-1235. https://doi.org/10.1093/cid/ciw500 [ Links ]
12.Henegar CE, Westreich DJ, Maskew M, Miller WC, Brookhart MA, Van Rie A. Effect of pregnancy and the postpartum period on adherence to antiretroviral therapy amongst HIV-infected women established on treatment. JAIDS. 2015;68(4):477-480. https://doi.org/10.1097/qai.0000000000000501 [ Links ]
13.Nieuwoudt SJ, Ngandu CB, Manderson L, Norris SA. Exclusive breastfeeding policy, practice and influences in South Africa, 1980 to 2018: A mixed-methods systematic review. Doherty T, editor. PLoS One. 2019;14(10):e0224029. https://doi.org/10.1371/journal.pone.0224029 [ Links ]
14.Rollins NC, Bhandari N, Hajeebhoy N, et al. Why invest, and what it will take to improve breastfeeding practices? Lancet. Elsevier BV; 2016;387(10017):491-504. https://doi.org/10.1016/s0140-6736(15)01044-2 [ Links ]
15.WHO, UNICEF. Guideline: Updates on HIV and infant feeding [homepage on the Internet]. World Health Organisation; 2016 [cited 2019 Oct 17]. Available from: https://apps.who.int/iris/bitstream/10665/246260/1/9789241549707-eng.pdf [ Links ]
16.Myer L, Phillips T, McIntyre JA, et al. HIV viraemia and mother-to-child transmission risk after antiretroviral therapy initiation in pregnancy in Cape Town, South Africa. HIV Med; 2016;18(2):80-88. https://doi.org/10.1111/hiv.12397 [ Links ]
17.Ewing AC, Ellington SR, Wiener JB, et al. Predictors of perinatal HIV transmission among women without prior antiretroviral therapy in a resource-limited setting. Pediatr Infect Dis J. 2019;38(5):508-512. https://doi.org/10.1097/inf.0000000000002220 [ Links ]
18.Khoo S. dolPHIN-1: Randomised controlled trial of dolutegravir (DTG)-versus efavirenz (EFV)-based therapy in mothers initiating antiretroviral treatment in late pregnancy [homepage on the Internet]. Presented at: AIDS 2018 [cited 2019 Oct 18]. Available from: https://programme.aids2018.org/Abstract/Abstract/13144 [ Links ]
19.Zash R, Holmes L, Diseko M, et al. Neural-tube defects and antiretroviral treatment regimens in Botswana. New Engl J Med. 2019;381(9):827-840. https://doi.org/10.1056/NEJMoa1905230 [ Links ]
20.WHO. Update of recommendations on first- and second-line antiretroviral regimens [homepage on the Internet]. 2019 [cited 2019 Sept 29]. Available from: https://www.who.int/hiv/pub/arv/arv-update-2019-policy/en/ [ Links ]
21.Phillips AN, Bansi-Matharu L, Venter F, et al. Updated assessment of risks and benefits of dolutegravir versus efavirenz in new antiretroviral treatment initiators in sub-Saharan Africa: Modelling to inform treatment guidelines. Lancet HIV. Elsevier BV; 2020;7(3):e193-e200. https://doi.org/10.1016/s2352-3018(19)30400-x [ Links ]
22.WHO. Policy brief: Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV [homepage on the Internet]. Geneva: World Health Organisation; 2018 [cited 2019 Sept 28]. Available from: https://www.who.int/hiv/pub/guidelines/en/ [ Links ]
23.Mofenson L. Web Annex C. Safety of dolutegravir in pregnancy and breastfeeding [homepage on the Internet]. In: Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: Interim guidelines. Supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. World Health Organisation; 2018 [cited 2019 Sept 28]. Available from: https://apps.who.int/iris/handle/10665/276491 [ Links ]
24.Waitt C, Orrell C, Walimbwa S, et al. Safety and pharmacokinetics of dolutegravir in pregnant mothers with HIV infection and their neonates: A randomised trial (DolPHIN-1 study). Mofenson LM, editor. PLoS Med. 2019;16(9):e1002895. https://doi.org/10.1371/journal.pmed.1002895 [ Links ]
25.SANDoH. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults [homepage on the Internet]. South Africa National Department of Health; 2015 [cited 2018 May]. Available from: https://www.health.gov.za/index.php/2014-03-17-09-09-38/policies-and-guidelines/category/230-2015p?download=937:national-art-guidelines-2015final [ Links ]
26.Abrams EJ, Myer L. Can we achieve an AIDS-free generation? Perspectives on the global campaign to eliminate new pediatric HIV infections. JAIDS. 2013;63(Suppl 2):S208-S212. https://doi.org/10.1097/qai.0b013e3182986f55 [ Links ]
27.Myer L, Phillips TK. Beyond 'Option B+'. JAIDS; 2017;75(1):S115-S122. https://doi.org/10.1097/qai.0000000000001343 [ Links ]
28.Bispo S, Chikhungu L, Rollins N, Siegfried N, Newell M-L. Postnatal HIV transmission in breastfed infants of HIV-infected women on ART: A systematic review and meta-analysis. JIAS. 2017;20(1):21251. https://doi.org/10.7448/ias.20.1.21251 [ Links ]
29.Mancinelli S, Galluzzo CM, Andreotti M, et al. Virological response and drug resistance 1 and 2 years post-partum in HIV-infected women initiated on life-long antiretroviral therapy in Malawi. AIDS Res Hum Retroviruses. 2016;32(8):737-742. https://doi.org/10.1089/aid.2015.0366 [ Links ]
30.Fogel J, Li Q, Taha TE, et al. Initiation of antiretroviral treatment in women after delivery can induce multiclass drug resistance in breastfeeding HIV-infected infants. Clin Infect Dis. 2011;52(8):1069-1076. https://doi.org/10.1093/cid/cir008 [ Links ]
 Correspondence:
Correspondence:
Jeannette Wessels
Jeannettewessels75@gmail.com
Received: 24 Feb. 2020
Accepted: 03 May 2020
Published: 08 July 2020
CASE REPORT
Spectrum of HIV-associated infectious diseases: A case series through the eyes of the histopathologist
Reena MohanlalI, II; Denasha L. ReddyIII
IDepartment of Anatomical Pathology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
IINational Health Laboratory Services, Chris Hani Baragwanath Academic Hospital, Johannesburg, South Africa
IIIDivision of Infectious Diseases, Department of Internal Medicine, Chris Hani Baragwanath Academic Hospital, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Human immunodeficiency virus (HIV) infection increases the risk of infection by a host of other opportunistic pathogens. The clinical presentations of these co-infections in immunocompromised patients are often atypical; therefore diagnosis is delayed in the absence of investigations such as tissue biopsy. Infection may involve sites that are difficult to access for biopsy and, as a consequence, there is limited diagnostic tissue available for analysis. The histopathologist, aided by ancillary tests, is relied upon to make a timeous and accurate diagnosis.
OBJECTIVES: To illustrate key histological features of HIV-associated infectious diseases encountered in a histopathology laboratory and to highlight, with the aid of literature, the relevance of histopathology in diagnosis.
METHOD: A retrospective descriptive case series of biopsies histologically diagnosed with HIV-associated infectious diseases over four years (2015-2019) was performed at the Chris Hani Baragwanath Academic Hospital National Health Laboratory Services Histopathology department. These cases have been photographed to illustrate microscopic aspects and will be accompanied by a literature review of opportunistic infections in the context of HIV infection.
RESULTS: This article highlights aspects of fungal, parasitic, viral and selected bacterial infections of people living with HIV for whom the histopathological examination of tissue was an essential component of the clinical diagnosis. Histological features are noted on routine slides and accompanied by diagnostic features revealed with histochemical and immunohistochemical stains.
CONCLUSION: Medical practitioners working in areas of high HIV endemicity should be familiar with the variety of infectious diseases that are encountered and with the diagnostic importance of the histopathologist in clinical management.
Keywords: infectious diseases; histology; HIV; opportunistic infections; diagnosis.
Introduction
People living with human immunodeficiency virus (HIV) are at risk from multiple infective pathogens. The clinical presentation of these infections is often atypical. This can result in costly diagnostic and therapeutic delays. Adequate tissue is sometimes inaccessible for re-biopsy, is of limited quantity, or may have been fixed in formalin and is therefore useless for routine microbiological culture. What the histopathologist has may be all the material there is. In the absence of a confirmatory microbiological 'answer', the histopathologist must maintain a high index of suspicion for infectious diseases, perform special stains and exclude multiple pathogens even after one has been identified.1 There is limited exposure to histopathology in undergraduate medical training in South Africa (SA) and clinicopathological meetings for postgraduate teaching are mostly confined to large academic centres. This article highlights aspects of fungal, parasitic, viral and selected bacterial infections encountered in the context of HIV infection and immunosuppression. A series of cases diagnosed with the aid of the histopathology laboratory of the National Health Laboratory Service (NHLS) of the Chris Hani Baragwanath Academic Hospital (CHBAH) in Soweto, SA, has been collected to demonstrate the importance of the histopathologist to the HIV clinician and infectious diseases specialist.
Fungal infections
Case 1
A bone marrow trephine biopsy from an HIV-positive man with bi-cytopaenia was received. Intracellular fungal yeasts were noted on special stains (Figure 1). A diagnosis of fungal infection was made and correlation with fungal culture was advised.

Case 2
A skin punch biopsy was submitted from a 32-year-old woman with umbilicated skin lesions. Cryptococcal latex antigen on serum and cerebrospinal fluid (CSF) were positive and CSF fungal culture revealed Cryptococcus neoformans. Her CD4 count was 1 cell/µL. Numerous extracellular yeasts were seen on haematoxylin and eosin stained section (Figure 2). The capsules and cell walls were highlighted on Alcian Blue Periodic Acid Schiff (ABPAS) special stain (Figure 2, inset) and the diagnosis of cryptococcosis was made.
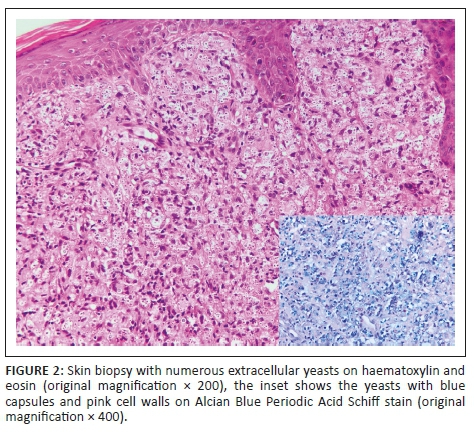
Comment: The histopathologist is often relied upon to make a timeous diagnosis of a fungal infection as tissue may not have been submitted for fungal culture or an 'extended' culture may be required, leading to a longer diagnostic turnaround time. Although fungal culture remains the gold standard, the diagnosis of a fungal infection can be reliably made on histological examination by identifying fungal yeasts (see Figure 1) or hyphae using histochemical stains such as Grocott and Periodic Acid Schiff (PAS). Histological features of fungal infection that should prompt special staining for fungal elements include granulomatous inflammation, neutrophilic micro-abscesses, foamy histiocytes, ulceration, pseudoepitheliomatous hyperplasia and suppurative inflammation.2 Some morphological clues may point towards a specific fungus; for example Cryptococcus yeasts, which are extracellular and variably sized. The typical staining on ABPAS special stain, as was noted in case 2 (see Figure 2), is supportive of the diagnosis. Pneumocystis jirovecii is typically present within a foamy exudate, the organisms are Grocott positive and appear as collapsed 'helmets' with a central dot. In a South African cohort of patients from the Western Cape, emergomycosis (previously emmonsiosis) was the most common systemic mycoses, followed by sporotrichosis and histoplasmosis.3 It is not possible to distinguish emergomycosis from other fungi on histological examination.4 Serum β-D-glucan and urine Histoplasma capsulatum antigen testing can be used as adjuncts when a fungal infection is clinically suspected. It is worthwhile remembering that urine H. capsulatum antigen can be positive in patients with emergomycosis due to cross-reactivity.3,4 Once the histological diagnosis of a fungal infection is made, further material should be submitted for fungal culture or polymerase chain reaction (PCR). Although further confirmatory investigations were not performed in case 1, definitive identification of fungal species by these methods is critical as they will impact the choice and duration of antifungal therapy.
Bacterial infections
Mycobacterial infection
Case 3
A 40-year-old HIV-positive woman had bi-cytopaenia on full blood count. Histological examination of the bone marrow trephine biopsy showed an infiltrate of foamy histiocytes. Numerous, clumped intracellular acid-fast bacilli were noted on Ziehl Neelsen (ZN) stain (Figure 3). Culture yielded growth of a non-tuberculous mycobacterium and PCR confirmed Mycobacterium avium complex.
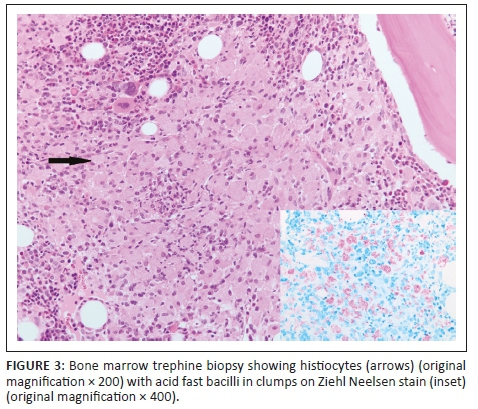
Comment: The synergy between the Mycobacterium species and HIV is well documented. Patients with HIV have progressive and disseminated mycobacterial diseases, and, in turn, mycobacterial infection increases HIV replication.5 The histological presentations of mycobacterial infection are varied. The prototypic feature noted on microscopic examination is granulomatous inflammation. However, with advanced immunosuppression, granulomas are usually absent and neutrophilic infiltration and necrosis are prominent.6 Mycobacterial spindle cell pseudotumour is another manifestation of mycobacterial infection seen more commonly in lymph node biopsies. This entity is characterised by a proliferation of spindled histiocytes and fibroblasts and positive ZN stain. It may mimic a host of mesenchymal tumours due to the spindled appearance of the cells, thus leading to misdiagnosis.6,7 Bacille Calmette-Guérin (BCG) infection may manifest as regional (BCGitis) or systemic disease (BCGosis) following BCG vaccination.8 In addition, BCGitis may occur after commencement of antiretroviral therapy (ART) as part of immune reconstitution. This should be borne in mind, especially when children present with lymphadenitis involving axillary or supraclavicular nodes and granulomatous inflammation is noted on histological examination.8,9 Testing for Mycobacterium bovis is indicated in this setting.8 Tuberculids such as erythema induratum are hypersensitivity reactions to mycobacterial antigens and no acid-fast bacilli are demonstrated in tissue biopsies from these lesions.10 Although definitive mycobacterial species identification is not possible on histological examination, the finding of sheets of foamy histiocytes containing ZN and PAS-positive bacilli are suggestive of Mycobacterium avium complex as noted in case 3 (see Figure 3). Acid-fast bacilli with a long beaded appearance may indicate infection with Mycobacterium kansasii.5 In cases where granulomatous inflammation morphologically in keeping with mycobacterial infection or acid-fast bacilli are noted on histological examination, samples should be submitted for mycobacterial culture and sensitivity, and when appropriate - molecular testing. It should be noted that there may be false positives on ZN stain due to laboratory contamination that occurs during preparation. Histopathologists are always sensitised to this possibility and contaminants are recognised as such as they lie on a different plane, are clumped or are free-lying, away from the tissue section.5
Bartonella infection
Case 4
A 34-year-old man on ART presented with a fungating mass on the right foot. He had a CD4 count of 21 cells/µL. Features of bacillary angiomatosis were noted on routinely stained skin biopsy and Warthin-Starry stain confirmed the presence of bacilli; PCR was not requested.
Comment: Histologically, bacillary angiomatosis comprises a lobular proliferation of vascular spaces with plump endothelial cells and neutrophilic debris.11 The bacilli in bacillary angiomatosis are noted as basophilic clumps in the tissue stroma where they proliferate (Figure 4). The bacteria induce endothelial anti-apoptosis and a pro-inflammatory state which accounts for the histological features that are noted.12 Warthin-Starry special stain or PCR for Bartonella genus can be used to confirm the diagnosis. The microscopic differential diagnosis includes a pyogenic granuloma or Kaposi sarcoma. While Bartonella infection manifests most commonly as bacillary angiomatosis in immunocompromised patients, it may also cause peliosis in the liver, endocarditis, osteomyelitis and cat-scratch disease.12

Syphilis
Case 5
A 32-year-old woman who was recently commenced on ART presented to the dermatology clinic with a generalised maculopapular rash involving her palms and soles. Her CD4 count was 219 cells/µL. A lichenoid lymphoplasmacytic infiltrate was noted on haematoxylin and eosin stained section (Figure 5, inset). Numerous spirochaetes were identified on Treponema pallidum immunohistochemistry, confirming the diagnosis of secondary syphilis (Figure 5). Serum T. pallidum antibody was positive and rapid plasma reagin test was reactive with a titer of 1024.

Comment: The prevalence of syphilis is increasing. In Canada, the United States and the United Kingdom, an increasing incidence is noted in men who have sex with men. Skin lesions are the most amenable to biopsy in suspected syphilis. Papulosquamous lesions with palmo-plantar involvement are the typical clinical findings but atypical presentations such as alopecia, pustular lesions, annular rash and nodules have also been described. Patients with HIV infection are more likely to have atypical presentations and secondary syphilis at the time of diagnosis.13 Histopathological findings in secondary syphilis include epidermal hyperplasia and a moderate to dense lymphoplasmacytic infiltrate as were noted in case 5 (Figure 5, inset). The T. pallidum immunohistochemical stain (Figure 5) is more sensitive than a silver stain. The sensitivity of immunohistochemistry was 64% compared to 9% for the silver stain, in detecting spirochaetes in one study. The same study also showed that patients with CD4 counts less than 250 cells/mL had more organisms (> 100 treponemes in 10 high power fields) demonstrated on skin biopsy than those with CD4 counts above 250 cells/mL.14
Viral infections
Case 6
A 32-year-old woman presented with a one-month history of a perianal lesion. She had a CD4 count of 18 cells/µL. Herpes simplex virus (HSV) and Cytomegalovirus (CMV) inclusions were both present in the perianal biopsy (Figure 6). No serological testing for HSV or CMV were performed.
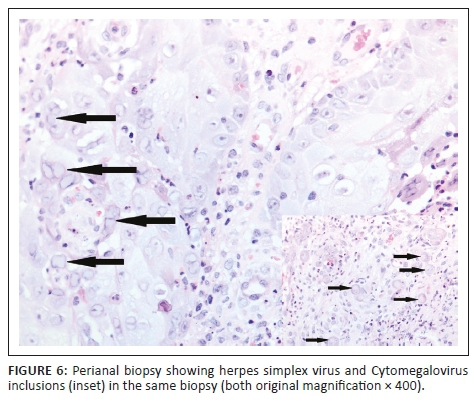
Comment
Infection with HSV2 typically presents as genital ulcers. There is a strong association between HIV and HSV2 infection.15 Individuals infected with HSV2 have sixfold higher odds of HIV infection compared with those uninfected with HSV2, and 68% of patients with genital ulcers caused by HSV2 were found to be co-infected with HIV. Locally, HSV2 remains the leading cause of pathogen detectable genital ulcer disease.16 Histologically, cells infected with HSV2 show intranuclear inclusions with a glassy appearance, margination of chromatin and multinucleation. Infected keratinocytes are best demonstrated at the ulcer edge (Figure 6). However, in hypertrophic or tumourous HSV2 lesions, the dense inflammatory cell infiltrate may obscure the viral inclusions. Careful search of multiple levels through the tissue block and immunohistochemistry may be required to identify the virally infected cells. Sparse infected cells within the deep dermis derived from ruptured hair follicles may be seen. It has been postulated that the florid inflammation noted in these hypertrophic lesions is due to immune reconstitution.17 Tissue may be submitted for drug resistance testing in those cases not showing clinical response to standard therapy. Cells infected with HSV and varicella-zoster virus show identical features on histological examination. Immunohistochemical stains are available to help distinguish among them.
Characteristic intracytoplasmic and intranuclear CMV inclusions were also seen in case 6 (Figure 6, inset) but atypical histological features are well documented.18 Immunohistochemistry can also be used in those cases that are densely inflamed. The role of CMV that is detected in mucocutaneous lesions is controversial. It is thought by some that the virus does not cause the lesion but is merely a bystander and signifies that there is generalised CMV infection. Its presence in genito-anal lesions may be as a result of autoinoculation of virus shed in faeces. Possible re-activation of latent virus in endothelial cells or haematogenous spread of the virus to granulation tissue is also postulated.19 Cytomegalovirus gastrointestinal tract disease can manifest as ulceration or polyps endoscopically and can show co-infection with other organisms such as Cryptosporidium.20 Viral load testing for CMV may be useful in confirming disease and in monitoring treatment response.
Case 7
A bone marrow trephine biopsy was submitted from a 29-year-old HIV-positive woman known to have pulmonary tuberculosis and a CD4 count of 80 cells/µL. Her red cell count was 2.31 × 1012/L, her reticulocyte count was 7.41% and her haemoglobin was 7.0 g/dL. Numerous parvovirus inclusions were noted on microscopic examination (Figure 7).
Comment
Parvovirus B19 infection of the bone marrow can manifest as a transient aplastic crisis or persistent infection with pure red cell aplasia. On microscopic examination of a bone marrow trephine biopsy with parvovirus infection, erythroid precursors are absent and giant pro-normoblasts21 are seen (Figure 7). Morphologically suspicious cases can be confirmed with immunohistochemistry. While PCR testing for parvovirus B19 is very sensitive, detection of parvovirus B19 DNA in the blood does not equate to acute infection.22 Parvovirus B19 DNA has also been detected in asymptomatic, parvovirus B19 IgM negative individuals in solid organs such as skin, myocardium, synovium and bone marrow.23 No PCR or viral load testing was performed in our case.
Helminthic infections
Case 8
A fallopian tube was excised for an ectopic pregnancy and submitted for histology. Schistosomal ova were noted incidentally within the fallopian tube on microscopic examination (Figure 8).
Comment
Schistosomiasis is diagnosed on histology in biopsy specimens from the urinary bladder, cervix, fallopian tube, appendix, liver and colon. The ova are elliptical in shape and may be calcified (Figure 8). A Schistosoma haematobium ovum has a terminal spine, while a Schistosoma mansoni ovum has a lateral spine. This distinguishing feature is difficult to apply in histology due to variability in the plane of sectioning. Schistosoma mansoni ova are positive with a ZN stain while S. haematobium ova are negative. The ova elicit a granulomatous or eosinophilic inflammatory response. Haemazoin pigment, a fine black non-refractile pigment, is another useful clue to the presence of schistosomiasis. It is formed by digestion of haemoglobin present in the worm gut following ingestion of red blood cells.24 Schistosomiasis is thought to increase susceptibility to HIV infection by disrupting the mucosal barrier and increasing vascularisation and recruitment of CD4 positive T cells.25 Although treatment kills the adult worms, the lesions may persist. Tissue diagnosis in female genital schistosomiasis is crucial as it is thought to impact fertility.26 Stool and urine microscopy are the gold standard to assess for infection but suffer from a lack of sensitivity as the eggs are not always detectable in the urine.27 Bladder biopsies are usually submitted to the laboratory with the clinical information of the characteristic 'sandy patches' appearance noted on cystoscopy. In other sites, however, the diagnosis is often rendered incidentally in biopsies submitted for other pathologies such as in case 8.
Other helminthic infections that can be readily diagnosed by the histopathologist are neurocysticercosis and hydatid disease. HIV-positive individuals with neurocysticercosis may present with multiple parenchymal lesions and co-infection with HIV has been reported in almost a third of cases of neurocysticercosis.28 On microscopic examination, the cysts have three layers, including outer cuticular, middle cellular and inner reticular layers.29 Hydatid disease is caused by species of the Echinococcus genus.30 Histologically proven cases of hydatid disease have increased and may be due to the increase in HIV prevalence. Lungs and liver are the more commonly affected organs.31 The cyst wall appears thin, smooth and white macroscopically, and on histological examination appears eosinophilic, acellular and lamellar. Cytological examination of the cyst fluid shows refractile hooklets and scolices.30 While HIV is a risk factor for infection with Strongyloides stercoralis, hyperinfection with autoinfection and disseminated disease that is associated with immunosuppression are rarely reported in HIV-positive patients.32 The histopathologist plays a limited role in the diagnosis of strongyloidiasis as stool samples are usually submitted for confirmation. An eosinophilic infiltrate with microabscess formation and degranulation on histological examination of mesenteric lymph nodes may be a clue to the presence of strongyloides infection.33
Conclusion
Histopathology plays an important role in the diagnosis of infectious diseases as many have characteristic histological features. Pathologists practising in areas with a high HIV prevalence are attuned to the importance of investigating inflamed tissue biopsies for an underlying infectious agent with special and immunohistochemical stains and liaising with clinicians to initiate timeous therapy. Correlation with relevant investigations such as PCR, culture and sensitivity, is advised in all cases to inform treatment decisions. With the aid of this case series and accompanying histology images, some key features of infections encountered in practice are conveyed to demonstrate the relevance of histopathology.
Acknowledgements
Mr Eric Liebenberg for his assistance with photography, members of the Histopathology Department at NHLS Chris Hani Baragwanath Academic Hospital and the staff at the Chris Hani Baragwanath Academic Hospital who work tirelessly under challenging circumstances.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
R.M. and D.L.R. conceived the design, R.M. collected and photographed cases and drafted the manuscript, and D.L.R. revised the manuscript.
Ethical consideration
Ethics approval was obtained from the Human Research Ethics Committee (Medical) of the University of the Witwatersrand (Clearance Certificate Number M190484). Specific histology slides were retrieved from the archives of the histopathology department at CHBAH NHLS and photographed. A literature review was conducted using PubMed.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not for profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or any affiliated agency of the authors.
References
1.Grayson W. Recognition of dual or multiple pathology in skin biopsies from patients with HIV/AIDs. Patholog Res Int. 2011;2011:398546. https://doi.org/10.4061/2011/398546 [ Links ]
2.Fernandez-Flores A, Saeb-Lima M, Arenas-Guzman R. Morphological findings of deep cutaneous fungal infections. Am J Dermatopathol. 2014;36(7):531-556. https://doi.org/10.1097/DAD.0b013e31829cc6f3 [ Links ]
3.Schwartz I, Kenyon C, Lehloenya R, et al. AID-related endemic mycoses in Western Cape, South Africa and clinical mimics: A cross sectional study of adults with advanced HIV and recent onset widespread skin lesions. Open Forum Infect Dis. 2017;4(4):1-7. https://doi.org/10.1093/ofid/ofx186 [ Links ]
4.Schwartz I, Govender N, Corcoran C, et al. Clinical characteristic, diagnosis, management and outcomes of disseminated emmonsiosis: A retrospective case series. Clin Infect Dis. 2015;61(6):1004-1012. https://doi.org/10.1093/cid/civ439 [ Links ]
5.Procop G. HIV and mycobacteria. Semin Diagn Pathol. 2017;34(4):332-339. https://doi.org/10.1053/j.semdp.2017.04.006 [ Links ]
6.Michelow P, Omar T, Field A, Wright C. The cytopathology of Mycobacterial infection. Diagn Cytopathol. 2016;44(3):255-262. https://doi.org/10.1002/dc.23410 [ Links ]
7.Sfeir M, Shuetz A, Van Besien K, et al. Mycobacterial spindle cell pseudotumour: Epidemiology and clinical outcomes. J Clin Path. 2018;71(7):626-630. https://doi.org/10.1136/jclinpath-2017-204777 [ Links ]
8.Hassanzad M, Valinejadi A, Darougar S, Hashemitari S, Velayati A. Disseminated Bacilli-Calmette Guerin infection at a glance: A mini review of the literature. Adv Respir Med. 2019;87(4):239-242. https://doi.org/10.5603/ARM.2019.0040 [ Links ]
9.Fernandes R, Medina-Acosta E. BCG-itis in two antiretroviral-treated HIV-infected infants. Int J STD and AIDS. 2010;21(9):662-663. https://doi.org/10.1258/ijsa.2010.010267 [ Links ]
10.Chen Q, Chen W, Hao F. Cutaneous tuberculosis: A great imitator. Clin Dermatol. 2019;37(3):192-199. https://doi.org/10.1016/j.clindermatol.2019.01.008 [ Links ]
11.LeBoit P, Berger T, Egbert B, Beckstead J, Yen T, Stoler M. Bacillary angiomatosis. The histopathology and differential diagnosis of a pseudoneoplastic infection in patients with human immunodeficiency virus disease. Am J Surg Pathol. 1989;13(11):909-920. https://doi.org/10.1097/00000478-198911000-00001 [ Links ]
12.Mosepele M, Mazo D, Cohn J. Bartonella infection in immunocompromised hosts: Immunology of vascular infection and vasoproliferation. Clin Dev Immunol. 2012;2012:612809. https://doi.org/10.1155/2012/612809 [ Links ]
13.Balagula Y, Mattei P, Wisco O, Erdag G, Chien A. The great imitator revisited: The spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53(12):1434-1441. https://doi.org/10.1111/ijd.12518 [ Links ]
14.Rosa G, Procop G, Schold J, Piliang M. Secondary syphilis in HIV positive individuals: Correlation with histopathologic findings, CD4 counts, and quantity of treponemes in microscopic sections. J Cutan Pathol. 2016;43(10):847-851. https://doi.org/10.1111/cup.12756 [ Links ]
15.Kouyoumjian S, Heijnen M, Chaabna K, et al. Global population-level association between herpes simplex virus 2 prevalence and HIV prevalence. AIDS. 2018;32(10):1343-1352. https://doi.org/10.1097/QAD.0000000000001828 [ Links ]
16.Kularatne R, Muller E, Maseko D, Kufa-Chakezha T, Lewis D. Trends in the relative prevalence of genital ulcer disease pathogens and association with HIV infection in Johannesburg, South Africa 2007-2015. PLoS One. 2018;13(4):e0194125. https://doi.org/10.1371/journal.pone.0194125 [ Links ]
17.Sbidian E, Battistella M, eGoff J, et al. Recalcitrant pseudotumoral anogenital herpes simplex virus type 2 in HIV infected patient: Evidence for predominant B lymphoplasmacytic infiltration and immunomodulators as effective therapeutic strategy. Clin Infect Dis. 2013;57(11):1648-1655. https://doi.org/10.1093/cid/cit592 [ Links ]
18.Yan Z, Wang L, Dennis J, Doern C, Baker J, Park J. Clinical significance of isolated cytomegalovirus-infected gastrointestinal cells. Int J Surg Pathol. 2014;22(6):492-498. https://doi.org/10.1177/1066896914537681 [ Links ]
19.Dauden E, Fernandez-Buezo G, Fraga J, Cardenoso L, Garcia-Diez A. Mucocutaneous presence of cytomegalovirus associated with Human Immunodeficiency Virus infection. Arch Dermatol. 2001;137(4):443-448. [ Links ]
20.Mohanlal R, Karstaedt A. Cytomegalovirus infection of the gastrointestinal tract in Soweto, South Africa: A look back at the clinical and histological features over 8 years. J Infect Dis Epidemiol. 2017;3:038. https://doi.org/10.23937/2474-3658/1510038 [ Links ]
21.Young N, Brown K. Parvovirus B19. N Engl J Med. 2004;350(6):586-597. https://doi.org/10.1056/NEJMra030840 [ Links ]
22.Molenaar-de Backer M, Russcher A, Kroes A, Koppelman M, Lanfermeijer M, Zaaijer H. Detection of parvovirus B9 DNA in blood: Viruses or DNA remnants. J Clin Virol. 2016;84:19-23. https://doi.org/10.1016/j.jcv.2016.09.004 [ Links ]
23.Corcioli F, Zakrzewska K, Rinieri A, et al. Tissue persistence of Parvovirus B19 Genotypes in asymptomatic persons. J Med Virol. 2008;80(11):2005-2011. https://doi.org/10.1002/jmv.21289 [ Links ]
24.Shu-hua X, Sun J. Schistosoma hemozoin and its possible roles. Int J Parasitol. 2017;47(4):171-183. https://doi.org/10.1016/j.ijpara.2016.10.005 [ Links ]
25.Secor W. The effects of schistosomiasis on HIV/AIDS infection, progression and transmission. Curr Opin HIV AIDS. 2012;7(3):254-259. https://doi.org/10.1097/COH.0b013e328351b9e3 [ Links ]
26.Kjetland E, Leutscher P, Ndhlovu P. A review of female genital schistosomiasis. Trends Parasitol. 2012;28(2):58-65. https://doi.org/10.1016/j.pt.2011.10.008 [ Links ]
27.Le L, Hsieh M. Diagnosing urogenital schistosomiasis: Dealing with diminishing returns. Trends Parasitol. 2017;33(5):378-387. https://doi.org/10.1016/j.pt.2016.12.009 [ Links ]
28.Serpa J, Moran A, Goodman J, Giordano T, White A. Neurocysticerosis in the HIV era: A case report and review of the literature. Am J Trop Med Hyg. 2007;77(1):113-117. https://doi.org/10.4269/ajtmh.2007.77.113 [ Links ]
29.Gyure K. Infections. In: Prayson R, editor. Neuropathology. 2nd ed. Philadelphia, PA: Elsevier, 2012; p. 365-367. [ Links ]
30.Dissanayake P, Chennuri R, Tarjan G. Fine-needle aspiration diagnosis of primary hydatid disease of the thyroid; first reported case in the USA. Diagn Cytopathol. 2016;44(4):334-337. https://doi.org/10.1002/dc.23421 [ Links ]
31.Wahlers K, Menezes C, Wong M, et al. Human cystic echinococcosis in South Africa. Acta Trop. 2011;120(3):179-184. https://doi.org/10.1016/j.actatropica.2011.08.006 [ Links ]
32.Von Braun, Trawinski H, Wendt S, Lubbert C. Schistosoma and other relevant helminth infections in HIV-positive individuals - An overview. Trop Med Infect Dis. 2019;4(2):pii: E65. https://doi.org/10.3390/tropicalmed4020065 [ Links ]
33.Ramdial P, Hlatshwayo N, Singh B. Strongyloides stercoralis mesenteric lymphadenopathy: Clue to the etiopathogenesis of intestinal pseudo-obstruction in HIV-infected patient. Ann Diagn Pathol. 2006;10(4):209-214. https://doi.org/10.1016/j.anndiagpath.2005.11.008 [ Links ]
 Correspondence:
Correspondence:
Reena Mohanlal
reena.mohanlal@nhls.ac.za
Received: 20 Mar. 2020
Accepted: 23 Apr. 2020
Published: 29 June 2020
CASE REPORT
Case report: Emergence of dolutegravir resistance in a patient on second-line antiretroviral therapy
Kairoonisha MahomedI; Carole L. WallisII; Liezl DunnIII; Shavani MaharajIII; Gary MaartensIV; Graeme MeintjesIV
IPrivate practice, Johannesburg, South Africa
IIDepartment of Molecular Pathology, BARC-SA and Lancet Laboratories, Johannesburg, South Africa
IIIAid for AIDS Management (Pty) Ltd, Cape Town, South Africa
IVDepartment of Medicine, Faculty of Health Sciences, University of Cape Town, Cape Town, South Africa
ABSTRACT
INTRODUCTION: The integrase strand transfer inhibitor dolutegravir (DTG) has a high genetic barrier to resistance. Only rare cases of resistance to DTG have been reported when it is used as a component of antiretroviral therapy regimens in treatment-experienced patients unless there was prior use of a first-generation integrase inhibitor.
PATIENT PRESENTATION: A 38-year-old woman diagnosed with tuberculosis was switched to a second-line antiretroviral regimen of zidovudine, lamivudine and dolutegravir 50 mg 12-hourly together with rifampicin-based TB treatment. Based on treatment history and a previous resistance test there was resistance to lamivudine but full susceptibility to zidovudine. The patient did not suppress her viral load on this regimen and later admitted to only taking dolutegravir 50 mg in the morning because of insomnia.
MANAGEMENT AND OUTCOME: A second resistance test was performed which showed intermediate level of resistance to dolutegravir. Her regimen was changed to tenofovir, emtricitabine and ritonavir-boosted atazanavir with rifabutin replacing rifampicin for the remainder of her TB treatment. She achieved viral suppression on this regimen.
CONCLUSION: To our knowledge this is the first case report from South Africa of emergent dolutegravir resistance in a treatment-experienced, integrase inhibitor-naïve patient. Factors that may have contributed to resistance emergence in this patient were that there was only one fully active nucleoside reverse transcriptase inhibitor in the regimen and lower exposure to dolutegravir because of the reduced dosing frequency while on rifampicin.
Keywords: HIV drug resistance; antiretroviral therapy; regimens; dolutegravir; rifampicin;
Case presentation
A 38-year-old woman started antiretroviral therapy (ART) in 2007 with zidovudine (AZT), lamivudine (3TC) and efavirenz. Her baseline human immunodeficiency virus (HIV) viral load and cluster of differentiation 4 (CD4) cell count results were not available. In September 2009, she experienced virological failure (this HIV viral load result is not available), and a genotypic antiretroviral resistance test showed a thymidine analogue mutation (TAM, K219KE [i.e. mixed population of mutant and wild type at that codon]), M184M/V and three non-nucleoside reverse transcriptase inhibitor (NNRTI) mutations (A98G, E138A and K238T) (see Table 1).
Based on this genotype, her regimen was changed to tenofovir (TDF), emtricitabine (FTC) and ritonavir-boosted atazanavir. She then transitioned from HIV care in the private sector to the public sector where she was changed to abacavir (ABC), 3TC and ritonavir-boosted lopinavir. When she returned to private sector care in May 2017, she reported severe diarrhoea, resulting in poor adherence to ART. Her medication was switched to ABC, 3TC and ritonavir-boosted atazanavir and the diarrhoea settled.
In June 2017, she was diagnosed with tuberculosis (TB) and was started on a rifampicin-based TB treatment. Her HIV viral load at this time was 345 406 copies/mL. As she already had significant diarrhoea on standard dose ritonavir-boosted lopinavir, it was felt that double-dose ritonavir-boosted lopinavir during TB treatment would not be tolerated. Given that AZT was still fully susceptible at the time of first-line failure, it was thought that AZT would still be susceptible and therefore providing one active nucleoside reverse transcriptase inhibitor (NRTI) to accompany dolutegravir (DTG). The patient was therefore switched to AZT, 3TC and DTG 50 mg 12-hourly. The patient informed her doctor in July 2017 that she was experiencing insomnia on this regimen. She was reassured and advised to continue until she had completed TB treatment.
There were regular pharmacy claims for treatment from June 2017 until November 2017 when her viral load was 2800 copies/mL. A resistance test was performed in February 2018, and the following mutations were observed: three integrase mutations (T66TI, G118R and E138EK) resulting in high-level resistance to raltegravir and elvitegravir and intermediate resistance to DTG (based on the Stanford score), M184V and two NNRTI mutations (A98G and E138A). A sample was also collected for phenotypic testing at monogram and the results showed that there was a 21-fold reduction in DTG susceptibility. Furthermore, all the other available integrase inhibitors had reduced susceptibility (3.92; 32- and 24-fold reduction to bictegravir, elvitegravir and raltegravir, respectively).
She then admitted to only taking DTG 50 mg in the morning because she had experienced insomnia with twice daily dosing, which resolved if she skipped the evening dose. Based on the resistance test result, her ART regimen was changed to TDF, FTC and ritonavir-boosted atazanavir in February 2018 with rifabutin replacing rifampicin for the remaining months of TB treatment. An HIV viral load performed 3 months after the change was 230 copies/mL, and subsequent viral loads have been below 20 copies/mL for about 12 months.
Discussion
The integrase strand transfer inhibitor DTG has a high genetic barrier to resistance. In clinical trials evaluating DTG as a component of triple drug therapy in ART-naïve patients, no emergence of resistance to DTG has been reported. Only rare cases of emergence of resistance to DTG have been reported when it has been used as a component of ART regimens in treatment-experienced patients, unless there has been prior use of the first-generation integrase inhibitors, raltegravir and elvitegravir.1
In the case reported here of a treatment-experienced patient, DTG resistance was detected after 8 months on a regimen of AZT, 3TC and DTG. Based on the patient's treatment history and resistance test results, this patient's virus (at the time of starting this regimen) had resistance to 3TC (through the M184V mutation, which also re-sensitises the virus to AZT in the presence of TAMs), but was fully susceptible to AZT. The Stanford score for AZT on the 2009 resistance test was 0 (the one TAM that was present, K219E, results in potential low-level resistance to AZT, but is counteracted by the re-sensitising effect of M184V) and between 2009 and 2017, the patient received only TDF/FTC and ABC/3TC as NRTIs. These NRTI combinations typically do not select for TAMs, the mutations that may compromise AZT. In addition, there were no TAMs found on the 2018 resistance test.
Based on the Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING) trial results, it would be predicted that her regimen of DTG with two NRTIs, one of which (AZT) was fully active, should be effective. The DAWNING trial evaluated DTG with NRTIs in second-line ART and demonstrated that when there was at least one fully active NRTI accompanying DTG, then this regimen was superior to ritonavir-boosted lopinavir combined with NRTIs: at week 48, 84% in the DTG arm achieved viral suppression compared with 70% in the lopinavir arm.2 Whilst no protease inhibitor mutations were detected in patients in the ritonavir-boosted lopinavir arm, 2/283 patients in the per-protocol analysis and in the DTG arm developed integrase resistance mutations (11 patients in the DTG arm who met virologic withdrawal criteria had a resistance test performed). One patient developed H51HY, G118R, E138EK and R263K mutations and the other patient developed the G118R mutation. Like these two cases in DAWNING, the patient reported here developed DTG resistance on second line despite there being a fully active NRTI in the regimen.
Another factor that may have contributed to the development of DTG resistance in this patient was the drug interaction with rifampicin. Rifampicin is a potent inducer of UGT1A1 and CYP3A4, the enzymes that metabolise DTG and thereby reduces concentrations of DTG (the trough DTG concentration is reduced by 85%).3 Studies in healthy volunteers and patients with HIV and TB have shown that this reduction can be compensated by increasing the frequency of DTG dosing from 50 mg daily to 50 mg 12-hourly in patients on rifampicin, which results in trough concentrations similar to patients taking DTG 50 mg daily without rifampicin, and resulted in adequate virological responses in patients with HIV and TB in the INSPIRING trial.4,5 Our patient decreased the DTG dose to 50 mg daily whilst on rifampicin because of insomnia, which would have resulted in considerably lower exposure to DTG and may have contributed to the emergence of resistance. A case has been reported in which DTG resistance emerged in a patient on DTG-containing first-line regimen who was taking rifampicin for treatment of a staphylococcal infection. The DTG was increased to 50 mg twice daily, but despite this, the patient had unexpectedly lower DTG concentrations.6
It is important to note that the need for this dose adjustment of DTG with rifampicin is currently being investigated. Because DTG was shown to have antiviral efficacy at doses as low as 10 mg daily in phase 2 trials,7,8 it is possible that a dose increase is not required in patients on first-line DTG-based ART and rifampicin. This is supported by a recent pharmacokinetic study in healthy volunteers that showed that in patients taking DTG 50 mg once daily with rifampicin, all had DTG trough concentrations above the protein-adjusted IC90.3 A recent observational study from Botswana reported similar virologic outcomes in patients on rifampicin taking DTG 50 mg 12-hourly versus 50 mg daily.9 The RADIANT-TB trial is being conducted in Cape Town and is enrolling patients on TB treatment and starting first-line ART who are being randomised to TDF/FTC/DTG 50 mg 12-hourly versus TDF/FTC/DTG 50 mg daily (https://clinicaltrials.gov/ct2/show/NCT03851588). Until data from this trial are available, we recommend DTG be dosed at 50 mg 12-hourly in all patients on rifampicin.
Whilst very few cases of DTG resistance have been reported in patients taking DTG as part of a triple-drug first-line ART regimen in routine clinical practice, one feature of the cases reported has been a high baseline viral load.6,10,11 This may also have been a contributing factor in the patient described in this case report.
In conclusion, although there is a very low risk of DTG resistance when used as part of the first-line combination therapy, DTG resistance is not uncommon after monotherapy and when used in patients previously exposed to raltegravir or elvitegravir therapy. However, apart from the DAWNING and Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV (SAILING) trials,2,12 there is limited data on the risk of DTG resistance in treatment-experienced, integrase inhibitor-naïve patients, especially in routine clinical practice, with concomitant usage of rifampicin and variable adherence and when used long term. To our knowledge, this is the first case report from South Africa of DTG resistance emerging in a patient who was integrase inhibitor-naïve when starting DTG. Factors that may have contributed to resistance emergence in this patient were that there was only one fully active NRTI in the regimen in a treatment-experienced patient (based on population-based genotyping) and the lowered exposure to DTG because the patient reduced the DTG dosing frequency from 50 mg 12-hourly to 50 mg daily against her doctor's advice whilst on rifampicin because of insomnia.
Acknowledgements
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
All authors contributed equally to this work.
Ethical consideration
This article followed all ethical standards for carrying out a research without direct contact with human or animal subjects.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Rhee SY, Grant PM, Tzou PL, et al. A systematic review of the genetic mechanisms of dolutegravir resistance. J Antimicrob Chemother. 2019;74(11):3135-3149. https://doi.org/10.1093/jac/dkz256 [ Links ]
2.Aboud M, Kaplan R, Lombaard J, et al. Dolutegravir versus ritonavir-boosted lopinavir both with dual nucleoside reverse transcriptase inhibitor therapy in adults with HIV-1 infection in whom first-line therapy has failed (DAWNING): An open-label, non-inferiority, phase 3b trial. Lancet Infect Dis. 2019;19(3):253-264. https://doi.org/10.1016/S1473-3099(19)30036-2 [ Links ]
3.Wang X, Cerrone M, Ferretti F, et al. Pharmacokinetics of dolutegravir 100 mg once daily with rifampicin. Int J Antimicrob Agents. 2019;54(2):202-206. https://doi.org/10.1016/j.ijantimicag.2019.04.009 [ Links ]
4.Dooley KE, Sayre P, Borland J, et al. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: Results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62(1):21-27. https://doi.org/10.1097/QAI.0b013e318276cda9 [ Links ]
5.Dooley KE, Kaplan R, Mwelase N, et al. Dolutegravir-based antiretroviral therapy for patients co-infected with tuberculosis and HIV: A multicenter, noncomparative, open-label, randomized trial. Clin Infect Dis. 2020;70(4):549-556. [ Links ]
6.Pena MJ, Chueca N, D'Avolio A, Zarzalejos JM, Garcia F. Virological failure in HIV to triple therapy with dolutegravir-based firstline treatment: Rare but possible. Open Forum Infect Dis. 2018;6(1):ofy332. https://doi.org/10.1093/ofid/ofy332 [ Links ]
7.Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1-infected adults. AIDS. 2011;25(14):1737-1745. https://doi.org/10.1097/QAD.0b013e32834a1dd9 [ Links ]
8.Van Lunzen J, Maggiolo F, Arribas JR, et al. Once daily dolutegravir (S/GSK1349572) in combination therapy in antiretroviral-naive adults with HIV: Planned interim 48 week results from SPRING-1, a dose-ranging, randomised, phase 2b trial. Lancet Infect Dis. 2012;12(2):111-118. https://doi.org/10.1016/S1473-3099(11)70290-0 [ Links ]
9.Modongo C, Wang Q, Dima M, et al. Clinical and virological outcomes of TB/HIV coinfected patients treated with dolutegravir-based HIV antiretroviral regimens: Programmatic experience from Botswana. J Acquir Immune Defic Syndr. 2019;82(2):111-115. https://doi.org/10.1097/QAI.0000000000002126 [ Links ]
10.Fulcher JA, Du Y, Zhang TH, Sun R, Landovitz RJ. Emergence of integrase resistance mutations during initial therapy containing dolutegravir. Clin Infect Dis. 2018;67(5):791-794. https://doi.org/10.1093/cid/ciy228 [ Links ]
11.Lübke N, Jensen B, Hüttig F, et al. Failure of dolutegravir first-line ART with selection of virus carrying R263K and G118R. N Engl J Med. 2019;381(9):887-889. https://doi.org/10.1056/NEJMc1806554 [ Links ]
12.Cahn P, Pozniak AL, Mingrone H, et al. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase-inhibitor-naive adults with HIV: Week 48 results from the randomised, double-blind, non-inferiority SAILING study. Lancet. 2013;382(9893):700-708. https://doi.org/10.1016/S0140-6736(13)61221-0 [ Links ]
 Correspondence:
Correspondence:
Liezl Dunn
liezld@afadm.co.za
Received: 19 Dec. 2019
Accepted: 02 Mar. 2020
Published: 02 July 2020
ORIGINAL RESEARCH
Changes in the incidence and prevalence of human immunodeficiency virus or acquired immunodeficiency syndrome in the South African medical schemes environment: 2005-2015
Floidy WafawanakaI; Martha S. LubbeI; Irma KotzéI; Marike CockeranII
IMedicine Usage in South Africa (MUSA), Faculty of Health Sciences, North-West University, Potchefstroom, South Africa
IIDepartment of Statistics, School of Computer, Statistical and Mathematical Sciences, North-West University, Potchefstroom, South Africa
ABSTRACT
BACKGROUND: The South African (SA) private medical schemes environment has over the past two decades respond to the evolving needs of people living with the human immunodeficiency virus (PLWH) or acquired immunodeficiency syndrome (AIDS).
OBJECTIVE: To determine changes in the incidence and prevalence rate of human immunodeficiency virus (HIV) or AIDS in the SA private medical schemes environment from 2005 to 2015.
METHOD: In this observational study, a single, pharmaceutical benefit management (PBM) company's medicine-claims database of members with HIV or AIDS has been retrospectively analysed from January 2005 to December 2015. The cohort includes all patients identified by the HIV or AIDS-related diagnostic ICD-10 codes, B20-B24, who also claimed antiretroviral medication during that period.
RESULTS: From 2005 to 2015, the proportion of HIV or AIDS patients enrolled in the PBM-company increased from 0.63% to 2.10%, and the incidence rate of new cases among the beneficiaries increased 2.3 times. The highest HIV or AIDS prevalence and incidence rates were found in the age group ≥ 40 and < 60 years, followed by the age group ≥ 60 and < 70 years. The highest prevalence rates in 2015 were recorded in Gauteng, namely, 422.4/1000 beneficiaries, followed by Western Cape (149.4/1000), and KwaZulu-Natal (118.4/1000).
CONCLUSION: There has been an increase in the number of SA-PLWH accessing treatment in the medical scheme environment. The high prevalence of HIV infection among older members should signal concern that HIV-related comorbid conditions are likely to become a growing component of care required by PLWH utilizing the SA private healthcare sector.
Keywords: incidence; prevalence; HIV or AIDS; medical schemes; South Africa.
Introduction
Human immunodeficiency virus or acquired immunodeficiency syndrome (HIV or AIDS) is a significant cause of death in Africa, and the fourth-largest cause of death worldwide.1,2 Access to antiretroviral treatment (ART) in the South Africa's public healthcare sector is carefully monitored,3 yet much remains unknown regarding the number of HIV-positive individuals on ART outside this sector.4 The South African Medical Schemes Act, No. 131 of 1998,4 ensures that the diagnostic and treatment costs of specified medical conditions are addressed in the so-called, 'prescribed minimum benefits (PMBs)' regardless of the benefit-option selected by the member.4,5 Prescribed minimum benefits cover conditions that meet the definition of a medical emergency, certain specifically defined medical conditions (n = 270) and additional chronic conditions (n = 26) as per the Chronic Disease' List (CDL).4,5 Furthermore, medical aid schemes in South Africa have a legal obligation to provide PMBs to their members with HIV or AIDS and to pay in full without recourse to co-payment or deductibles.4,5 Since 2005, ART has been included as part of PMBs.4,5 These benefits also cover HIV voluntary counselling and testing, pain management in palliative care, preventive therapies, hospitalisation and treatment for HIV or AIDS-related opportunistic conditions.4,5
Before 2005, South Africans (SAs) obtained ART privately through disease-management, workplace or community treatment programmes.5,6 In 2004, the SA public sector started providing ART to its citizens, and whilst ART has resulted in the global decrease of new HIV infections, it has been accompanied by the increased survival of people living with the HIV (PLWH), that is, an increase in the number of persons on long-term care.1 The increase in PLWH is also seen in the SA medical schemes environment.7,8 Between 2012 and 2017, the Council for Medical Schemes (CMS) reported that the number of its members on ART increased by 72.4%, that is an average annual growth rate of about 11.51%, to 25.12/1000 beneficiaries in 2017.7 In spite of managed HIV or AIDS being ranked fourth after hypertension, hyperlipidaemia and diabetes mellitus on SA-CDL,7,8 and being the 'best managed' chronic condition in the SA private sector,9 the influence of HIV or AIDS remains poorly studied in the private healthcare environment. Understanding the epidemic of HIV or AIDS in this context is important to follow and monitor. Hence, against this background, this observational study sought to determine possible changes in the incidence and prevalence rates of treated SA-PLWH who accessed private medical schemes care from 2005 to 2015.
Research method and design
Study design and setting
The study design incorporates a longitudinal and retrospective review of data of an open cohort of PLWH from 01 January 2005 up to 31 December 2015. The data were sourced from a large SA pharmaceutical benefit management (PBM) company with more than 1.8 million beneficiaries in 42 medical schemes and capitation plans. To ensure the quality of its data, the PBM company applies several automated confirmatory validation steps to the data. The cohort includes all its members who claimed ART and whose International Classification of Diseases-10 (ICD-10) diagnostic codes, namely, B20-B24, confirmed the presence of HIV and/or an HIV-related condition. The research database includes only those PBM members who claimed one or more prescriptions during the study period. Table 1 summarises the yearly demographic profile of the study population. The dataset includes the following fields: patient's demography, namely, date of birth, gender, a unique code for the medical scheme member and beneficiary, prescription number, date of dispensing, trade name of the medication, the National Pharmaceutical Product Index (NAPPI) code of each medicine, the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10) code10 and name of the province where each item was dispensed.
Statistics: Variables and measurements
The number of HIV or AIDS patients on the database was stratified by year, gender, age group and province. The patient's age was determined at the time of the first dispensing in the index year, namely, 2005. It was thereafter divided into seven age groups: > 0 and < 6 years; ≥ 6 and < 12 years, ≥ 12 and < 18 years; ≥ 18 and < 40 years; ≥ 40 and < 60 years; ≥ 60 and < 70 years; and ≥ 70 years. Patients were also grouped into two categories according to their gender (male and female) and province.
In this study, the prevalence rate of treated PLWH was calculated per 1000 medical scheme beneficiaries annually who claimed one or more prescriptions during the specific year11:

The population in Equation 1 includes the total population or the population of specific gender or age group on the database who claimed one or more prescriptions during the specific year.
The incidence rate was used to determine the proportion of study participants who had newly registered their HIV or AIDS status with their medical schemes during the study period without taking into account when participants contracted the disease. Each participant was followed from the time he or she was registered with the central database. Participants who cancelled their membership with a specific medical scheme did not contribute to the year's denominator, whereas new members did.
The HIV or AIDS incidence rate was calculated as the number of new cases per 1000 medical scheme beneficiaries who claimed one or more prescriptions during the specific year. The incidence rate was calculated as follows:11

The population in Equation 2 includes the total population or the population of specific gender or age group on the database who claimed one or more prescriptions during the particular year.
Data analysis
The Statistical Analysis System® (SAS 9.4®) software (SAS Institute Inc., 2002-2012) was used to analyse the data. Variables were expressed using descriptive statistics, which include numbers (n) and proportions presented as percentages (%).
Ethical consideration
This study was approved by the Health Research Ethics Committee of the North-West University (certificate number NWU-00179-14-A1), and the 'Goodwill Permission' to perform the study was obtained from the board of directors of the company. All data were anonymised prior to the incorporation in this study.
Results
The study population and those on the PBM database who claimed one or more prescriptions in a specific year were stratified by gender and age group as shown in Table 1.
In 2005 and 2015, 1 213 676 and 843 972 patients claimed medicines, respectively. In 2005, 7665 (0.63%) of patients on the PBM database were PLWH. This number increased to 17 302 (2.05%) in 2015. In 2005, of the total of 675 812 females, 4395 (0.65%) were PLWH, and of the total of 537 864 males, 3270 (0.61%) were PLWH. In 2015, female patients totalled 445 626, of whom 9092 (2.04%) were PLWH, and of the 398 166 male patients, 8250 (2.07%) were PLWH.
The results in Figure 1 show the prevalence rate of HIV or AIDS patients per 1000 medical scheme beneficiaries, which had increased from 6.3/1000 in 2005 to 20.5/1000 in 2015. The prevalence rate of female HIV or AIDS patients was 6.5/1000 in 2005, which increased to 20.4/1000 by the end of 2015. In males, the prevalence rate of HIV or AIDS increased from 6.0/1000 (in 2005) to 21.7/1000 in 2015.
Figure 2 demonstrates change in the incidence rate of new HIV or AIDS cases on the database per 1000 medical scheme beneficiaries from 2005 to 2015. The combined incidence rate increased from 3.9/1000 in 2006 to 9.1/1000 in 2015. The HIV or AIDS incidence in females increased from 4.0/1000 in 2006 to 8.5/1000 in 2015, whilst that of males rose from 3.9 in 2006 to 9.9/1000 in 2015. The intermittent increase ('spikes') in the incidence rate of new HIV or AIDS cases on the database of both genders is an artefact that reflects changes during the period in the overall number of members of contributing medical aid schemes.
Figures 3 and 4 illustrate the respective prevalence of HIV or AIDS patients and incidence rates of new HIV or AIDS cases by age on the database per 1000 medical scheme beneficiaries.
Age group ≥ 40 and < 60 years had the highest HIV or AIDS prevalence rates of 14.4 in 2005 and 38.3 in 2015, followed by the age group ≥ 60 and < 70 years. The age group ≥ 0 and < 6 years had the lowest HIV or AIDS prevalence rate followed by the ≥ 6 and < 12 years age group with prevalence rates of 2.1/1000 and 2.6/1000 in 2005 and 2015, respectively. In the age group ≥ 18 and < 40 years, the HIV or AIDS prevalence rate increased by 2.9/1000 beneficiaries between 2005 and 2015. The prevalence rate in elderly patients, ≥ 70 years, was 2.7/1000 in 2015, which is an increase of 2.1 from 2005.
The incidence rate of new HIV or AIDS cases in the < 6 years age group declined from 1.71/1000 in 2006 to 1.51/1000 in 2015. Incidence rates in the age group ≥ 6 and < 12 years remained similar: < 1/1000 beneficiaries in 2006 and 1.1/1000 in 2015, whilst rates in the ≥ 12 and < 18 years and the ≥ 18 and < 40 years age groups increased marginally. The highest increase occurred in the ≥ 40 - ≤ 60-year age group: 8-18/1000 new HIV or AIDS cases. A large increase was also recorded in the ≥ 60 and < 70 years age group: 4-11/1000. The age group ≥ 70 years recorded the lowest HIV or AIDS incidence rate, that is, less than 1/1000 medical scheme beneficiaries in 2006 and 2015. The intermittent 'spikes' in the incidence rate are an artefact created by changes in the overall numbers of contributing schemes.
Beneficiary-prevalence data per province are presented in Figure 5. The highest HIV or AIDS prevalence rate was noted in Gauteng at 372.9/1000 in 2005. This increased to 422.4/1000 in 2015. A decline in prevalence rate was noted in KwaZulu-Natal: from 140.4/1000 in 2005 to < 118.4 in 2015. The recorded prevalence amongst beneficiaries increased in both Northern Cape and the Free State, 15.9-23.5/1000 from 42.6-65.5/1000, respectively, in 2015. Prevalence rates have increased in Mpumalanga but decreased modestly in the North West, Limpopo and the Eastern Cape.
Discussion
This study aimed to identify changes in the SA HIV or AIDS incidence and prevalence rates in a population that was covered by private medical schemes between 2005 and 2015.
Studies determining both incidence and prevalence rates of HIV or AIDS in the medical schemes environment in South Africa are few. Human immunodeficiency virus and ART studies in developed regions, for instance, the United Kingdom and Australia,12,13 seldom provide an adequate comparator arm as patients, healthcare systems and individual circumstances are too dissimilar. The results of this study do nonetheless indicate that both incidence and prevalence rates of SA-PLWH who claimed ART through PBM company have increased over the last decade. Specifically, the prevalence rate of HIV or AIDS increased by > 3.3 times and the incidence rate by 2.3 times in this population. These results correlate well with the general data reported by the SA-CMS that confirm the following annual increase in HIV prevalence rates in the managed healthcare sector: 15.36/1000 beneficiaries in 2011, 17.31/1000 in 2015 and 22.08/1000 in 2016.8,9,14
Our data confirm the preponderance of females over males - certainly a consequence of the generalised character of HIV epidemic in sub-Saharan Africa. Our results mirror the results of public sector.15,16 Yet the results also suggest that the female predominance of earlier incidence studies - a fact that is visible in our data from 2005 to 2009 (Table 1) - has begun to narrow and at times show reverse trend. Is the SA HIV epidemic gradually changing and perhaps entering a new phase? Alternatively, is the nature of employment in the SA private sector currently favouring a greater representation of males? Answers to these questions are currently unclear.
With regard to age, the following points are made. Representation of HIV in the youngest groups, namely, 0-12 years is low. The fact that the prevalence has fallen steadily in the 6-12-year age group as well is testimony to the success of vertical transmission prevention efforts introduced almost 15 years ago. These findings are similar to those reported in the CMS annual report of 2015-2016.9 Furthermore, the adults in our cohort are ageing, and those with the highest prevalence rates of HIV are moving out of the child-bearing category, namely, most are aged 40+ years.
A review of the demographics of adolescents (age 10-19 years) and young adults in this study indicates that this group is not represented adequately. This is not surprising for a country (such as South Africa) where one-third of youth aged below 30 years is unemployed and up to 50% of school leavers cannot find work, that is, cannot access private healthcare. This group is nonetheless a high-risk group for HIV transmission, infection and failure of HIV-management.
The highest HIV prevalence occurred amongst middle-aged and older persons, namely, 40-70 years old. Figure 4 clearly indicates that this group has consistently accounted for 80% - 90% of HIV-infected population managed by private medical schemes. Figure 4 also provides graphically the annual new incident infections in this age group. If these data are duplicated in all SA medical schemes, then the private sector must begin to view their 40+-year-old HIV-infected patients as a key population for whom the message of HIV prevention must become a priority.
The 40-70-year age group is at risk of comorbid diseases. These include diabetes, hypertension, renal dysfunction, neurocognitive decline, life-threatening cardiovascular events, fragility fractures and non-AIDS defining cancers. These may occur a decade earlier than occurring in uninfected peers and result from the persistently inflammatory milieu that cannot be corrected currently by ART.17,18,19 Our data indicate that the adult HIV-infected group is ageing and is likely to develop one or more comorbid conditions. While this condition cannot be fully reversed, it could be mitigated with, for example, changes in medication, lifestyle and dietary changes, and regular medical assessments to evaluate risk. Long-term consequences of comorbid disease in PLWH means greater exposure to drug-drug interactions, drug toxicities and increased healthcare costs. The 40+-year age group needs to be monitored closely in this regard.
According to the 2017-2018 CMS annual report, maximum number of healthcare service providers, healthcare-related visits and beneficiaries were found in Gauteng, followed by the Western Cape. Mpumalanga, the Northern Cape, Western Cape and Limpopo consistently have lower proportions.8 Each of the other provinces made up less than 10% of covered beneficiaries. Disparity in medical scheme coverage according to province is likely to reflect the urban-rural divide, employment status and lack of opportunity of those in rural areas. It is to be noted that membership of a medical scheme is linked with the availability of employment.
Study's strengths and limitations
An important limitation of this study is the fact that data were obtained from only one PMB. Generalisations may, therefore, be inappropriate. Furthermore, the data we have been able to obtain are restricted to broad demographic categories and excludes other data with a specific clinical interest, for example, long-term survival, morbidity and so on. The data are also limited by its retrospective nature/capture. Nevertheless, the data provide an important overview of the incidences and prevalence of HIV infection in the SA private sector, namely, a large South African PBM company. The number of PLWH registered with companies that supply PMBs and provide disease management programmes has increased significantly during the last decade, that is, this sector is important for the successful delivery of HIV care to the nation. Indeed, the provision of PMBs - strength of programme - highlights positive benefits that members are able to access.
Conclusion
Our study indicates an increase in the number of SA-PLWH accessing treatment in private healthcare sector and utilising PMBs. The latter has proven to be successful in managing HIV or AIDS in the private medical schemes' environment. The growing prevalence of middle-aged and older adults with HIV or AIDS warrants further studies as this group is sexually active and presents an opportunity to re-emphasise HIV-prevention messages. In addition, this group is at risk of comorbid diseases that would affect their risk-profile assessments and their long-term survival.
Acknowledgements
The authors thank the Statistical Consultation Services, North-West University, Potchefstroom Campus, for statistical assistance, and Anne-Marie Bekker for administrative support concerning the database.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
All authors contributed equally to this work.
Funding information
The authors acknowledge the North-West University and the Water Foundation and National Research Foundation (NRF) for financial support.
Data availability statement
Data sharing is not applicable to this article because the data source, the PBM, does not allow it according to the current contract.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.GBD 2017 HIV Collaborators. Global, regional, and national incidence, prevalence, and mortality of HIV, 1980-2017, and forecasts to 2030, for 195 countries and territories: A systematic analysis for the Global Burden of Diseases, injuries, and risk factors 2017 study. Lancet HIV. 2019;16(12):e831-e859. https://doi.org/10.1016/S2352-3018(19)30196-1 [ Links ]
2.Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: Analysis for the global burden of disease study. Lancet. 2020;395(10219):200-211. https://doi.org/10.1016/S0140-6736(19)32989-7 [ Links ]
3.Department of Health. HIV & AIDS and STI national strategic plan for South Africa 2017-2022 [homepage on the Internet]. Pretoria: Department of Health; c2007 [cited 2020 Jan 29]. [ Links ] Available from: https://sanac.org.za//wp-content/uploads/2017/06/NSP_FullDocument_FINAL.pdf
4.Council of Medical Schemes, South Africa. Medical Schemes Act 131 of 1998: Regulations. (Government notice No. R.1397, 2003) [homepage on the Internet]. Government Gazette, 25537; 2003 [cited 2019 May 10]. [ Links ] Available from: http://www.medicalchemes.com/publicationsZipPublicalions/Acts%20and%20Regu lations/MSREGS19July2004.pdf
5.Da Silva R, Wayburne L. Effects of HIV/AIDS on medical schemes in South Africa: Population covered. SA Actuarial J. 2008;8(1):35-91. https://doi.org/10.4314/saaj.v8i1.24513 [ Links ]
6.Stevens M, Sinanovic E, Regensberg L, et al. HIV and AIDS: STI and TB in the private sector. In: Harrison S, Bhana R, Ntuli A, editors, South African health review 2007 [homepage on the Internet]. Durban: Health Systems Trust; c2007 [cited 2020 Jan 29]. [ Links ] Available from: http://www.hst.org.za/publications/711
7.Council of Medical Schemes, Research and Monitoring Unit of the Council for Medical Schemes, South Africa. Prevalence of chronic diseases in the population covered by medical aid schemes in South Africa [homepage on the Internet]. c2019 [cited 2020 Jan 28]. [ Links ] Available from: http://www.medicalschemes.com/Publications.aspx
8.Council of Medical Schemes, South Africa. Annual report 2017/18 [homepage on the Internet]. [ Links ] c2017 [cited 2020 Jan 28]. Available from: http://www.medicalschemes.com/Publications.aspx
9.Council for Medical Schemes, South Africa. Annual report 2015/2016 [homepage on the Internet]. c2016 [cited 2019 May 10]. [ Links ] Available from: http://www.medicalschemes.com/Publications.aspx
10.World Health Organization. International statistical classification of diseases and related health problems [homepage on the Internet]. 10th revised ed. c2011 [cited 2019 Jun 25]. [ Links ] Available from: https://www.who.int/classifications/icd/ICD10Volume2_en_2010.pdf
11.Centre for Disease Control and Prevention. Lesson 3: Measure risk. Section 1: Frequency measures. In: Principle of epidemiology in public health practice. 3rd ed. An introduction to applied epidemiology and biostatistics [homepage on the Internet]. [ Links ] c2018 [cited 2019 May 10]. Available from: https://www.cdc.gov/ophss/csels/dsepd/ss1978/lesson3/section1.html
12.McManus H, Hoy JF, Woolley I, et al. Recent trends in early-stage response to combination antiretroviral therapy in Australia. Antivir Ther. 2015;20(2):131-139. https://doi.org/10.3851/IMP2774 [ Links ]
13.Williams I, Churchill D, Anderson J, et al. British HIV Association (BHIVA) guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy. HIV Med. 2012;13(2):1-85. https://doi.org/10.1111/j.1468-1293.2012.01029.x [ Links ]
14.Council of Medical Schemes, South Africa. Annual report 2016/17 [homepage on the Internet]. [ Links ] c2017 [cited 2019 May 10]. Available from: http://www.medicalschemes.com/Publications.aspx
15.Lessells RJ, Mutevedzi PC, Iwuji C, Newell ML. Reduction in early mortality on antiretroviral therapy for adults in rural South Africa since the change in CD4+ cell count eligibility criteria. J Acquir Immune Defic Syndr. 2014;65(1):e17-e24. https://doi.org/10.1097/QAI.0b013e31829ceb14 [ Links ]
16.Tanser F, Barnighausen T, Graspar E, et al. High coverage of ART associated with a decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966-971. https://doi.org/10.1126/science.1228160 [ Links ]
17.Maggi P, Biagio A, Rusconi S, et al. Cardiovascular risk and dyslipidemia among persons living with HIV. A review. BMC Infect Dis. 2017;17(1):551. https://doi.org/10.1186/s12879-017-2626-z [ Links ]
18.Duncan AD, Groff LM, Peters BS. Type 2 diabetes prevalence and its risk factors in HIV: A cross-sectional study. PLoS One. 2018;13(3):e0194199. https://doi.org/10.1371/journal.pone.0194199 [ Links ]
19.Goodkin K, Miller EN, Cox C, et al. Effect of aging in neurocognitive function by stage of HIV infection: Evidence from the Multi-Center AIDS Cohort Study. Lancet HIV. 2017;4(9):e411-e422. https://doi.org/10.1016/52352-3018(17)30098-X [ Links ]
 Correspondence:
Correspondence:
Martha Lubbe
martie.lubbe@nwu.ac.za
Received: 06 July 2019
Accepted: 05 Feb. 2020
Published: 29 June 2020
ORIGINAL RESEARCH
Empowering parents for human immunodeficiency virus prevention: Health and sex education at home
Taygen EdwardsI, II; Ntombizodumo MkwanaziIII, IV; Joanie MitchellV; Ruth M. BlandVI, VII, VIII; Tamsen J. RochatIV, IX
IAfrica Health Research Institute, Somkhele, South Africa
IILiggins Institute, University of Auckland, Auckland, New Zealand
IIIHuman and Social Capabilities Division, Human Sciences Research Council, Durban, South Africa
IVDSI-NRF Centre of Excellence in Human Development, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
VLentegeur Psychiatric Hospital, Department of Health, Government of the Western Cape, Cape Town, South Africa
VIRoyal Hospital for Sick Children, Glasgow, Scotland
VIIInstitute of Health and Wellbeing, University of Glasgow, Glasgow, Scotland
VIIISchool of Public Health, University of the Witwatersrand, Johannesburg, South Africa
IXSAMRC Developmental Pathways to Health Research Unit (DPHRU), Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Improving health literacy amongst human immunodeficiency virus (HIV)-positive mothers could strengthen child and adolescent HIV prevention. The Amagugu intervention included health literacy materials to strengthen maternal communication and has demonstrated success in low-resource HIV-endemic settings.
OBJECTIVES: Our aims were to (1) evaluate whether Amagugu materials improved health literacy leading to changes in parental behaviour towards communicating on topics such as HIV, health behaviours and sex education, and (2) explore what additional information and materials mothers would find helpful.
METHOD: The Amagugu evaluation included 281 HIV-positive mothers and their HIV-uninfected children (6-10 years). Process evaluation data from exit interviews were analysed using content analysis and logistic regression techniques.
RESULTS: Of 281 mothers, 276 (98.0%) requested more educational storybooks: 99 (35.2%) on moral development/future aspirations, 92 (32.7%) on general health, safety and health promotion, and 67 (23.8%) on HIV and disease management. Compared to baseline, mothers reported that the materials increased discussion on the risks of bullying from friends (150; 53.4%), teacher problems (142; 50.5%), physical abuse (147; 52.3%) and sexual abuse (126; 44.8%). Most mothers used the 'HIV Body Map' for health (274; 97.5%) and sex education (267; 95.0%). The use of a low-cost doll was reported to enhance mother-child communication by increasing mother-child play (264; 94.3%) and maternal attentiveness to the child's feelings (262; 93.6%.
CONCLUSION: Parent-led health education in the home seems feasible, acceptable and effective and should be capitalised on in HIV prevention strategies. Further testing in controlled studies is recommended.
Keywords: health education; sex education; intervention materials; HIV prevention; HIV-uninfected children; parent-child communication.
Introduction
Prevention of paediatric and adolescent human immunodeficiency virus (HIV) is a global priority.1 In South Africa, substantial investment in reducing vertical transmission of HIV has led to more children being born HIV-negative.2 However, the rate of new infections, particularly amongst adolescents, remain high.3,4
Children who are HIV-negative but have an HIV-positive parent are especially vulnerable. Some evidence has shown that parental HIV may be associated with increased sexual risk behaviour and HIV infection amongst HIV-exposed or HIV-affected adolescents.5,6 Although the process by which these risks may be conferred (i.e. parenting, parental illness, parental death) is still unclear, it is plausible, given international evidence, that at least some of these effects may occur as a consequence of parenting capacity or the absence thereof.7,8,9,10 The proportion of South African children who live with a parent infected with HIV is large4 and the burden on the South African health system is high,11 thus limiting the feasibility of providing parental health literacy training in primary health or HIV treatment care settings.12 Therefore, it is important to consider task shifting to lay workers as a means to deliver training and education that enable HIV-positive parents to use developmentally appropriate health literacy strategies in the home setting.11 In turn, this could increase preadolescent children's capacity to remain free from HIV in later life.10 Utilising caregivers to strengthen HIV prevention in the home during the preadolescent years, before the onset of the high-risk adolescent period, has substantial potential but has been underexploited and under-researched in South Africa.
In South Africa, the predominant HIV health literacy strategies are implemented in school-based education models which tend to target older children aged 14-16 years.13 These strategies have been moderately successful in targeting HIV risk behaviours and increasing HIV-related knowledge in low- and middle-income countries.14 However, the quality and delivery of HIV, health and sex education in rural South African schools are considered to be poor, and inconsistent, as the teachers themselves often lack the capacity to fulfil this educational role.15 Evidence has shown that targeting the caregiver in the home setting is a cost-effective response to increasing health education, in particular, in resource-scarce settings.16 Globally, interventions which target parents in the home are on the increase, for example, countries such as France, the Netherlands, Australia and the United States are increasingly focused on parental capacity as a strategy for promoting healthy ideas around reproductive health in young people.17,18 Research to date has shown that caregivers have an influential role in HIV prevention and in ensuring the optimal development of children.8,18
Central to achieving increased health promotion by caregivers is providing them with the necessary skills and training. Health promotion is broadly defined as a process of enabling people to increase control over and improve their health.19 The World Health Organisation includes three key elements in its definition of health promotion: (1) governance to ensure the removal of structural barriers to adequate access to health; (2) healthy cities which limit geographical hindrances to health and (3) most relevant to this study is to ensuring health literacy. Health literacy refers to the knowledge, skills and information individuals need to make healthy choices and central to improving health literacy is ensuring that individuals have the capacity to obtain, process and understand health information.20 More recently, the literature has begun to emphasise the need to go beyond simply providing health information and to move towards ensuring capacity to change behaviour, which from a psycho-social perspective is critical.21
However, very little is available to support parent's health literacy, with many parents reporting that they feel ill-equipped to provide education to their children with HIV or sex education.22 In response, the South African National Strategic Plan for HIV, tuburculosis (TB) and sexually transmitted infections (STI) 2017-2022 has included a focus on early parenting interventions to support resilience in children.23 However, these focus predominantly on the early years, while little is known about the support needs of caregivers in contexts of parental HIV in South Africa, and almost no interventions have focused on primary school-aged children.11,24 One maternal HIV-disclosure intervention (Amagugu) focused on supporting maternal disclosure to HIV-uninfected primary school-aged children has been shown to be effective in improving mother-led health behaviours and health promotional activities (such as taking children to clinic to learn about health services); in improving maternal HIV-disclosure rates; and in strengthening the quality of the mother-child relationship in South Africa.11,24,25,26 This manuscript undertakes a detailed analysis of the Amagugu process evaluation with the aims of (1) evaluating whether Amagugu materials improved health literacy leading to changes in parental behaviour towards communicating on topics such as HIV, health behaviours and sex education, and (2) identifying what additional informational needs (over and above existing Amagugu content) might be helpful for parents.
Methods
Study design and intervention
The Amagugu intervention was based on a health literacy and promotion conceptual framework that was informed by an extensive body of literature.10 In summary, the conceptual framework hypothesised that the relationship between parental HIV and child outcomes was mediated through parenting and that parent-child communication is central to improving parent-led health promotion. Specifically, Amagugu hypothesised that non-disclosure and avoidant coping leads to low-quality parent-child communication which, in turn, decreases the likelihood of health and sex education. These children then enter adolescence with a diminished capacity for healthy behaviours resulting in increased risk-taking and adverse outcomes such as HIV infection. The conceptual framework has been described in detail elsewhere.10
The intervention model (see Figure 1) was designed to disrupt these risk pathways by targeting the avoidant behaviour, facilitating maternal HIV-disclosure thereby improving parent-child communication, and fostering parent-led health education, engagement with primary healthcare services and custody planning for their child. Mothers were supported to disclose their HIV status to their child at a level with which they felt comfortable. This included either 'partial disclosure' using the word 'virus', or 'full disclosure' using the word 'HIV'.25,27
Amagugu involved six home-based sessions delivered by experienced lay counsellors.11,25 Lay counsellors had completed high school, had previous counselling experience and were trained on Amagugu.11 The intervention package (Figure 1) included low-cost, age-appropriate materials that were given to the mother to use with her child. The health literacy materials supported HIV disclosure and education, health information on the importance of nutrition, hygiene, physical activity and health promotion activities which encouraged parental engagement with health services through a series of parent-led activities undertaken in a primary healthcare setting.10 A central part of the Amagugu approach involved training parents in health literacy and encouraging change in parental behaviour towards health promotion and communication. As such, counsellors did not interact directly with children, but rather the mothers were trained on the use of the materials so that they could lead the activities with their child without the involvement of the counsellor. This aimed to ensure the transfer of learning, encourage behaviour change and increase parental confidence.
Each mother-child pair received one intervention package consisting of 17 materials. The health literacy materials included a variety of activity cards, educational games and storybooks, such as the 'Family Treasures Story Book', an illustrated 14-page English-isiZulu storybook designed to foster closeness between the mother and child; the 'Disclosure Safety Hand', which served as a tool to create a confidante circle for the child that helped discourage the child's disclosure of maternal HIV status to others beyond her confidante circle, in a way easily understood by the child. The tool also encouraged the child to feel safe to disclose any risks at home or school to 'safety hand' adults in the household; an 'HIV Body Map', a tool for sex and health education including how to explain HIV to a child; and a culturally appropriate doll which facilitated play and parent-child communication. Non-index children in a household were also given a doll. The families were able to keep the intervention materials after the intervention had ended.11
Amagugu has been implemented successfully in a pilot study with 24 mothers11; in a large-scale evaluation with 281 mothers25,26 and in a randomised controlled trial with 464 mothers.24 This analysis used data from the large-scale evaluation; specifically, the process evaluation data collected during the exit interviews with the 281 mothers.
Study setting and population
This study was conducted between 2010 and 2012 at the Africa Health Research Institute, previously known as the Africa Centre for Population Health ('Africa Centre'), situated in a rural community in northern KwaZulu-Natal with a high HIV prevalence rate.28 A Prevention of Mother-to-Child Transmission (PMTCT) programme was implemented in 2001,29,30 followed by a decentralised HIV treatment and prevention programme in 2004.30,31,32
The sample for the Amagugu evaluation was purposively recruited from an existing cohort in the Vertical Transmission Study (VTS; 2001-2006), a non-randomised intervention study which supported exclusive breastfeeding for the first 6 months post-birth.33 Prior participation in the VTS study meant that the mothers' HIV status during the perinatal period, and hence the child's HIV exposure status, was known. At the time of VTS, these mothers had given consent to be re-contacted at a later date and for the purpose of this Amagugu study, they were physically traced and invited to participate.25 Inclusion criteria were that the mothers were HIV-positive, and their children were HIV-uninfected and between the ages of 6-10 years. In addition, the mother-child pair needed to be in reasonable physical and mental health and reside in the study area. In cases where the mother migrated for work, to be eligible for enrolment, she needed to be staying with her child for a minimum of two nights per week.10 The consort diagram is shown in Figure 2. Out of a total available pool of 525 mothers who consented to be contacted at the end of the VTS, 375 women were approached, of whom 291 were enrolled and 281 completed follow-up.
Data collection
Data in the Amagugu evaluation were collected by questionnaires at 4 time-points: a baseline and post-intervention assessment to collect outcome data. A further two process evaluation semi-structured interviews were completed: the first immediately after disclosure (a post-disclosure interview) and the second conducted 1 week after a health clinic promotion visit (post-clinic visit).11,25 This study reports on data from the baseline questionnaires and process evaluation interviews.
During the baseline assessment, data were collected using questionnaires (collected in an interview format) covering information on maternal and child characteristics, including socio-economic, demographic and health information. This included treatment status and CD4 count; partner HIV status and previous HIV disclosure to the index child and family. Process evaluation data included disclosure outcome and type ('partial'; 'full'); and post-disclosure questions and reactions of the children. Informational needs of the mothers were derived from the open question of 'Would you like more storybooks for you and your family? If so, what topics would you like to be covered?' which was asked in the context of the 'Family Treasures Story Book'.
At the end-line assessment, questionnaires (collected in an interview format) also collected data on intervention material usefulness including a pre-post evaluation question on whether the 'Disclosure Safety Hand' had helped the mothers to talk to their children about the risk of bullying from friends, teacher-child problems, or physical and sexual abuse; whether the participant thought that the 'HIV Body Map' could be used to teach about health or sex education. Lastly, information was gathered about whether there were any dolls in the household before the intervention; if the child played with the doll provided by Amagugu; and whether the doll helped the mother to spend more time with her child, listen to her child more and know when her child was worried, happy or excited.
Other data collected in this Amagugu evaluation are detailed and published elsewhere.25,26,34
Analysis
We used a process evaluation design to analyse data which has not previously been analysed.
Data were transformed, coded and analysed in two phases to address each research aim.
In phase 1, we analysed the qualitative data collected through end-line questionnaires on intervention resource use to evaluate which materials in their current form fostered mother-child communication about HIV, health behaviours and sex education. We used descriptive statistics including cross-tabulations and chi-square tests to investigate whether there were significant differences amongst mothers who chose to use the intervention materials, whether those materials were used to discuss health- and sex-related topics with their children, and whether it fostered mother-child communication and maternal attentiveness to the child's feelings. Where a cross-tabulation contained multiple cells, adjusted standardised residuals were calculated to determine which cells did not differ by chance. The analysis was undertaken using Stata version 13.35
In phase 2, we analysed and reported on the process evaluation data collected during the two semi-structured interviews on what other storybook topics the mothers would like to be trained on, to identify additional information that would be useful to mothers. The data were systematically categorised and quantified using content analysis36 with the following steps: the third author repeatedly read the data and identified recurrent codes of informational topics requested by the mothers, once an exhaustive codebook was finalised through review by the third and last author to check consistency and saturation of codes, all of the responses were re-analysed and coded. Thereafter the first author independently reviewed the coded data and queries and discrepancies were resolved by consensus between first, third and last authors. Secondly, the codes were then grouped into categories by the first and third author and were reviewed together with the last author. The analysis was conducted using Microsoft Excel. Because some mothers provided more than one response for future storybook topics, a Z-test was conducted to determine whether there was a significant difference between the first and all responses. Logistic regression models were computed to test for the effects of maternal characteristics, child characteristics, post-disclosure reactions of children and post-disclosure questions of children on the likelihood of mothers asking for more information on each of the categories derived from the content analysis. Regression models were run controlling for disclosure type (partial; full), with and without controls.
Ethical consideration
Ethical approval was obtained from the Biomedical Research Ethics Committee (BREC) of the University of KwaZulu-Natal (Ref: BF 144/010).
Results
Sample characteristics
Table 1 shows the sample characteristics by post-intervention disclosure level.26 All mothers had engaged in some degree of disclosure at that time, with 110 (39%) mothers reporting 'partial' and 171 (61%) reporting 'full' disclosure to their child.26 Almost all had completed at least some primary school education, were in a current relationship and did not have a regular source of income. A large proportion of the mothers were in relatively good health, defined both objectively (having a CD4 count above the eligibility criteria for antiretroviral therapy [ART] at that time of 350 cells/mL) and subjectively (perceiving their current health to be 'excellent'), although over half of the sample was not yet on ART. The median age of children was 7.0 (interquartile range [IQR] = 7-8) years, and most had a father who was still alive and contributed financially to their care. Disclosure to children prior to the intervention was low.
The descriptive results suggested that the intervention materials in their current form improved parental capacity for health and sex education. Table 2 shows descriptive statistics on whether the 'Disclosure Safety Hand' increased mother-child communication during the Amagugu evaluation on the topics of risks of bullying from friends, teacher-child problems and physical or sexual abuse. Overall, the findings indicated that the intervention material did increase communication across all categories. The intervention material facilitated maternal discussion amongst approximately half of the mothers post-intervention who had never engaged in such discussions pre-intervention with their boy children. Child sex was associated with differences for the categories of 'talked before and talked during' and 'did not talk before or during' on communication about the risks of sexual abuse (p < 0.01). The category of 'talked before and during' for communication about the risks of physical abuse (p < 0.05) also differed by child sex.
Most of the mothers reported having used the 'HIV Body Map' for sex (n = 267; 95.0%), and health education (n = 274; 97.5%) with no gender differentials being observed. When reporting on the use of the Amagugu doll, only 94 (33.6%) mothers reported that there had been a doll in the household pre-intervention. After the introduction of the intervention doll, almost all mothers reported that their child had played with the doll (n = 270; 96.4%). Most mothers reported that since receiving the doll they had played with their child more (n = 264; 94.3%). In relation to whether the doll helped foster mother-child communication, mothers reported that the doll had helped them listen to their child more (n = 262; 93.6%), and to know when their child was worried (n = 256; 91.4%), or happy or excited (n = 257; 91.8%). Encouragingly, the doll was equally popular with both boy and girl children as no gender differences were observed (p > 0.05).
In total, 276 (98.6%) mothers responded that they would like more educational storybooks. In response to the question of what topic they would like for future books, 281 mothers gave 363 suggestions, excluding 'missing' or 'not applicable' responses. These topics were coded and grouped into three main categories and further broken down into three sub-categories: 'HIV and disease management' (HIV and TB education and caregiving/disclosure/stigma); 'General health, safety and health promotion' (health education/health promotion/sex education); and 'Family, moral development and aspirations for the future' (aspirations and family values/morals and social norms/parenting skills).
Table 3 shows categories of story book topics suggested by the mothers. The most popular topics amongst mothers were those relating to 'Family, moral development and aspirations'. Mothers expressed interest in learning parenting skills with topics such as 'how to have a good relationship with children', 'raising children and how to treat children' and 'talk about love as a parent' being requested for future books. The second most requested category comprised topics related to 'General health, safety and health promotion'. In this category, requests for books covering health and sex education were prominent with examples, including 'what to do when you are sick…', 'learn about drug abuse', 'child abuse and rights', 'sexually transmitted diseases' and 'encouraging children to talk when they are abused'. The category with the least counts was 'HIV and disease management', which covered topics related to the aetiology, prevention and treatment of HIV/TB, maternal and family disclosure, and stigma surrounding illness, with parents' requests ranging from 'how HIV is transmitted to babies', 'more about disclosing in the family' and 'any topics related to not stigmatising someone else'.
Maternal and child characteristics, post-disclosure reactions and post-disclosure questions were regressed against the outcome of storybook category. No characteristics were found to be significant predictors of storybook topic selection, and therefore the results were not included in this article.
Discussion
This study showed that the mothers found the current Amagugu intervention materials to be useful in leading communication with their preadolescant children around HIV and health behaviours which may include sex education as part of reproductive health and HIV prevention.8 These results suggest that parents may be able to overcome their expressed discomfort, embarrassment and lack of knowledge on how to engage their children in discussions about sex-related matters with appropriate and user-friendly materials.8,15 These results are encouraging because discussing sex-related issues with children is often reported to be a taboo in many settings.8,15,22
Building parental capacity for sex education has been identified in the literature as a key method through which increased health education and prevention occurs in the family context.8,18 This involves providing parents and caregivers with the necessary practical tools for laying the early foundation in health needed for a positive trajectory over the life course.18 The literature suggests that because family, especially parents, plays an important role in the sexual socialisation of children, their role should be capitalised upon when designing programmes to improve the sexual and reproductive health of children and adolescents.37,38 Although research on parent-child communication regarding sexuality with younger children is limited, a multi-site study conducted with adolescents in Burkina Faso, Ghana, Malawi and Uganda demonstrated parental influence on adolescents' sexual and reproductive health.37 It is promising that studies in other parts of the world have demonstrated that parents also agree that the basis for sex education should be the home, supplemented by external facilities such as schools.39 In a study conducted in a rural area of the United States, 80% of parents believed that the family should provide sex education to children; 94% reported to have talked to their children about sex, and 87% regarded themselves as the primary source of sexual information for their adolescents.39
This research is one of the first to demonstrate that South African HIV-positive mothers are willing and interested in being involved in providing such health education (including education on HIV and sexuality) to their preadolescent and adolescent children. Most studies on parent-child communication on sexual issues have been conducted in the United States, Europe and Australia.8,15 According to the World Health Organization, studies on parent-child communication on sexual matters in sub-Saharan Africa are limited, but there is a growing literature on this issue.38 Our findings align with existing research which suggests that if parents are equipped with adequate support, they can communicate with their children about HIV/AIDS and sexuality matters.8 This would, in turn, assist in HIV prevention in young people who may also be exposed to multiple risks as they enter adolescence, including ill-health, depression and substance abuse.38
Parent-child communication is a recognised protective factor during the high-risk developmental stage of adolescence, especially concerning HIV infection, and other sexual and reproductive health outcomes.8,24,37 The Amagugu evaluation was shown to increase mother-child communication on topics, including the risks of bullying from friends, teacher-child problems, physical abuse and sexual abuse. This finding is important as these childhood events have been linked to adverse outcomes, including behavioural problems,40 mental health disorders,41,42,43 substance abuse disorders, sexual risk behaviour and increased risk of HIV infection and interpersonal violence,41 especially amongst HIV-affected children who are particularly vulnerable to bullying and abuse.44,45
It is encouraging to note that in comparison to baseline, mother-child communication increased for all topics. Importantly, we also found that there were gender-specific and significant increases amongst mothers with boys, towards increased education of the potential risk for sexual abuse amongst their boy children. This is an important finding given that a recent national representative cross-sectional study of sexual abuse in South Africa found that 10% of boys and 14% of girls aged between 15 and 17 years reported some sexual victimisation in their lifetime.46 This risk of early sexual abuse amongst boys has also been shown in longitudinal research in South Africa to start early.47 Thus, intervention such as Amagugu, which encourage communication with boy children about the risk of sexual abuse, has important potential beyond the context of HIV.
Conversely, we found no gender differentials in the use of the 'HIV Body Map' for sex education. This is encouraging as the finding suggests that the distribution of educational resources has the potential to make sex education more gender-inclusive, and overcome the accepted norm that the education of boys is often regarded as the responsibility of the father or male caregiver. This is particularly relevant in the context of rural South Africa, not only where patriarchal gender norms pervade but also where the role of education falls to the mother because the father may often be absent from the household.48 A plausible explanation for our finding is that the age-appropriate resources boost a mother's confidence and empowers her to undertake this task.49
An important finding was that a simple tool such as a doll could foster parent-child communication and strengthen the parent-child relationship. This is because a doll can build parental capacity by providing an opportunity for interactive play, and insight into a child's emotions and thoughts, fundamental to capacitating mothers as agents of early prevention.50 Based on existing evidence on the usefulness of dolls in counselling with children, it may also provide children with a valuable tool for expressing concerns about emerging risks to which the child is exposed, which they feel afraid to disclose, but may disclose inadvertently through play.51,52 For example, illustrating to the parent through projective play concerns about bullying at school or undisclosed sexual abuse.
In contexts of parental HIV in South Africa, there is very little information on what practical support mothers may need to support them in optimising health education with their children.11,24 This study provided novel insights into the type of informational needs the mothers themselves identified to be important for their children as they entered into adolescence. This included requests for more information on family, moral development and aspirations for the future, in addition to information on health and sex. This is an original finding as prior evidence has mainly been restricted to its exploration of parent-led educational topics to the domain of sexual and reproductive health.50,53 In comparison, our results, which allowed parents to use an open-ended question to define the topics, illustrate the importance of considering a more value-based approach to health education because this emerged as a parental priority and preference. Interventions which are responsive to this parental desire may benefit from increased engagement and motivation amongst parents to undertake what can be a challenging task.
It is important to consider these parental preferences within the context of South Africa and the culture of the study population. The black South African Zulu population have a sense of pride in traditional values which emphasise morality. Broadly, in African cultures, values emphasise collectivist consciousness where the value of the individual (self-achievement) is not as important as the value of one's relationship with others and how it contributes to a collective or societal achievement, known as 'Ubuntu' which represents a moral philosophy of life.54 This may explain why mothers considered the moral developmental of children as important requesting material on topics about the importance of family, culture and traditions, as well as education. With regard to the latter, it is also possible that given the South African history of exclusion and discrimination, parents would be particularly motivated to ensure that their children succeed in post-apartheid South Africa.55
Practical implications
The under-utilisation of caregivers in health education and disease prevention in current approaches is a missed opportunity. These findings provide insight into how parental capacities can be strengthened in a cost-effective way which may guide both researchers and policy makers. Targeting mothers in the home context has the potential to be a feasible and cost-effective point of entry for intervention in early prevention of disease. Specific tools may be useful in engaging and encouraging parental education in areas which are traditionally difficult for parents to handle.
Limitations of the study
Limitations include that sample size was small and context-specific, thereby limiting generalisability. The sample was also restricted to mothers, a practical decision because as mentioned previously, children in rural South Africa are mostly raised by their mothers.48 Hence, we are not able to determine whether fathers would find such approaches helpful or appropriate. However, Amagugu is easily adapted to target fathers and other family members24 if it were demonstrated to be acceptable to them.
An additional limitation was that because the data in this evaluation study were based on self-report, social desirability bias may have influenced the responses of the mothers regarding health communication and the usefulness of the materials in achieving behavioural change towards increased communication. The Amagugu evaluation is thus limited in its ability to determine the extent to which the intervention materials or activities played a direct role in changing parental behaviours. This is because the data on disclosure and communication outcomes were not measured objectively, and there was no control group. However, the descriptive findings of the analysis do suggest that the intervention materials in their current form helped to improve the mothers' capacity for health and sex education, contributing to their (the mothers) capacity for making behavioural changes towards increased health communication. It is thus plausible to infer that potentially the content and activities of the package could have led, at least in part, to the increased rates of disclosure and health education that were reported at the end line assessment. This is particularly so because additional research on the Amagugu intervention using a randomised controlled design has since, indeed, demonstrated efficacy in changing parent behaviour towards disclosure and health promotion.24
Recommendations
A long-term follow-up study, when the children in the sample will reach adolescence, would provide further information about whether Amagugu is an effective intervention for HIV prevention in later life. Moreover, studies should be conducted to ascertain whether these findings are replicated in other population settings. National surveys such as the National Attitudes Survey56 could help illuminate parental perceptions in this area and determine broader acceptability. A partial economic evaluation should be conducted to make a stronger case for investment in prevention at home.
Conclusion
This study provided valuable information on what HIV-positive mothers need to support their HIV-uninfected children's health education as well as the type of interventional resources they found useful in leading communication around health and sex. The capacity of HIV-positive mothers to lead health and sex education in the home should be capitalised on in HIV prevention strategies. The intervention materials were low cost and administered by lay counsellors, which suggests that early prevention does not have to be a costly and complicated endeavour.
Acknowledgements
The authors wish to thank the Amagugu team including Samu Dube, Hlengiwe Mtolo, Bonnie Gumede and Philani Sithole (field team); Colin Newell (database design and management); Dickman Gareta and Siyabonga Nxumalo (data extraction team); and the Africa Centre Community Liaison Office and the Community Advisory Board for their assistance in this research. They would also like to acknowledge all the mothers and families who agreed to participate in Amagugu without whom this study would not have been possible.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
T.E. contributed to the analysis and interpretation of data, drafted and revised the article. N.M. contributed to drafting the article and critically revised the final version. J.M. contributed to the coding of the data and reviewed the final version. R.B. contributed to drafting of the article and critically reviewed the final version. T.J.R. contributed to the article concept, interpretation of the data and critically reviewed the article.
Funding information
This study was funded by the Canadian International Development Agency (CIDA). T.E. receives salary support from the Africa Health Research Institute. N.M. receives a post-doctoral scholarship from the DST-NRF Centre of Excellence in Human Development, University of the Witwatersrand. T.J.R is a Wellcome Trust Intermediate Fellow in Public Health and Tropical Medicine and recieves salary support from the Wellcome Trust.
Data availability statement
Data is available on the Africa Health Research Institute data repository for researchers who meet the criteria for access to confidential data.
Disclaimer
The views expressed in this article are those of the authors and not an official position of the institution or funder.
References
1.Menzies NA, Berruti AA, Blandford JM. The determinants of HIV treatment costs in resource limited settings. PLoS One. 2012;7(11):e48726. https://doi.org/10.1371/journal.pone.0048726 [ Links ]
2.McNally LM, Hadingham J, Archary D, Moodley R, Coovadia HM. HIV-exposed but uninfected children: Why are they vulnerable? Vulnerable Child Youth Stud. 2006;1(2):139-148. https://doi.org/10.1080/17450120600872241 [ Links ]
3.Goga AE, Dinh T, Jackson DJ, et al. Population-level effectiveness of PMTCT Option A on early mother-to-child (MTCT) transmission of HIV in South Africa: Implications for eliminating MTCT. J Glob Health. 2016;6(2):020405. https://doi.org/10.7189/jogh.06.020405 [ Links ]
4.UNAIDS. Global AIDS update [homepage on the Internet]. [ Links ] 2018 [cited 2017 Nov 06]. Available from: https://www.unaids.org/sites/default/files/media_asset/miles-to-go_en.pdf
5.Sherr L, Cluver LD, Betancourt TS, Kellerman SE, Richter LM, Desmond C. Evidence of impact. AIDS. 2014;28:S251-S259. https://doi.org/10.1097/QAD.0000000000000327 [ Links ]
6.Chi P, Li X. Impact of parental HIV/AIDS on children's psychological well-being: A systematic review of global literature. AIDS Behav. 2013;17(7):2554-2574. https://doi.org/10.1007/s10461-012-0290-2 [ Links ]
7.Sutton MY, Lasswell SM, Lanier Y, Miller KS. Impact of parent-child communication interventions on sex behaviors and cognitive outcomes for black/African-American and Hispanic/Latino youth: A systematic review, 1988-2012. J Adolesc Health. 2014;54(4):369-384. https://doi.org/10.1016/j.jadohealth.2013.11.004 [ Links ]
8.Bastien S, Kajula LJ, Muhwezi WW. A review of studies of parent-child communication about sexuality and HIV/AIDS in sub-Saharan Africa. Reprod Health. 2011;8(1):25. https://doi.org/10.1186/1742-4755-8-25 [ Links ]
9.Pettifor A, Stoner M, Pike C, Bekker L-G. Adolescent lives matter: Preventing HIV in adolescents. Curr Opin HIV AIDS. 2018;13(3):265. https://doi.org/10.1097/COH.0000000000000453 [ Links ]
10.Rochat TJ, Mitchell J, Stein A, Mkwanazi NB, Bland RM. The Amagugu intervention: A conceptual framework for increasing HIV disclosure and parent-led communication about health among HIV-infected parents with HIV-uninfected primary school-aged children. Front Public Health. 2016;4:1-12. https://doi.org/10.3389/fpubh.2016.00183 [ Links ]
11.Rochat TJ, Mkwanazi N, Bland R. Maternal HIV disclosure to HIV-uninfected children in rural South Africa: A pilot study of a family-based intervention. BMC Public Health. 2013;13(1):147. https://doi.org/10.1186/1471-2458-13-147 [ Links ]
12.Coetzee B, Kagee A, Bland R. Shortcomings of adherence counselling provided to caregivers of children receiving antiretroviral therapy in rural South Africa. AIDS Care. 2016;28(Suppl. 2):60-65. https://doi.org/10.1080/09540121.2016.1176675 [ Links ]
13.Department of Basic Education. Integrated strategy on HIV, STIs and TB 2012-2016 [homepage on the Internet]. [ Links ] 2012 [cited 2017 Apr 23]; p. 1-84. Available from: https://www.gov.za/sites/default/files/gcis_document/201409/national-strategic-plan-hiv-stis-and-tb0.pdf
14.Fonner VA, Armstrong KS, Kennedy CE, O'Reilly KR, Sweat MD. School based sex education and HIV prevention in low-and middle-income countries: A systematic review and meta-analysis. PLoS One. 2014;9(3):e89692. https://doi.org/10.1371/journal.pone.0089692 [ Links ]
15.Phetla G, Busza J, Hargreaves JR, et al. 'They have opened our mouths': Increasing women's skills and motivation for sexual communication with young people in rural South Africa. AIDS Educ Prev. 2008;20(6):504-518. https://doi.org/10.1521/aeap.2008.20.6.504 [ Links ]
16.Desmond C, Viviers A, Edwards T, Rich K, Martin P, Richter L. Priority-setting in the roll out of South Africa's National Integrated ECD Policy. Early Years. 2019;39(3):276-294. https://doi.org/10.1080/09575146.2019.1572074 [ Links ]
17.Weaver H, Smith G, Kippax S. School-based sex education policies and indicators of sexual health among young people: A comparison of the Netherlands, France, Australia and the United States. Sex Educ. 2005;5(2):171-188. https://doi.org/10.1080/14681810500038889 [ Links ]
18.Mistry KB, Minkovitz CS, Riley AW, et al. A new framework for childhood health promotion: The role of policies and programs in building capacity and foundations of early childhood health. Am J Public Health. 2012;102(9):1688-1696. https://doi.org/10.2105/AJPH.2012.300687 [ Links ]
19.WHO. What is health promotion? [homepage on the Internet]. [ Links ] 2016 [cited 2019 Feb 18]. Available from: https://www.who.int/features/qa/health-promotion/en/
20.Kindig DA, Panzer AM, Nielsen-Bohlman L. Health literacy: A prescription to end confusion. Washington, DC: National Academies Press; 2004. [ Links ]
21.Rademakers J, Heijmans M. Beyond reading and understanding: Health literacy as the capacity to act. Int J Environ Res Public Health. 2018;15(8):1676. https://doi.org/10.3390/ijerph15081676 [ Links ]
22.Nambambi NM, Mufune P. What is talked about when parents discuss sex with children: Family based sex education in Windhoek, Namibia. Afr J Reprod Health. 2011;15(4):120-129. [ Links ]
23.SANAC. South Africa's national strategic plan for HIV, TB and STIs 2017-2022 [homepage on the Internet]. [ Links ] 2017 [cited 2019 Feb 18]. Available from: https://www.gov.za/sites/default/files/gcis_document/201705/nsp-hiv-tb-stia.pdf
24.Rochat TJ, Stein A, Cortina-Borja M, Tanser F, Bland RM. The Amagugu intervention for disclosure of maternal HIV to uninfected primary school-aged children in South Africa: A randomised controlled trial. Lancet HIV. 2017;4(12):e566-e576. https://doi.org/10.1016/S2352-3018(17)30133-9 [ Links ]
25.Rochat TJ, Arteche AX, Stein A, Mkwanazi N, Bland RM. Maternal HIV disclosure to young HIV-uninfected children: An evaluation of a family-centred intervention in South Africa. AIDS. 2014;28(Suppl. 3):S331-S341. https://doi.org/10.1097/QAD.0000000000000333 [ Links ]
26.Rochat TJ, Arteche AX, Stein A, Mitchell J, Bland RM. Maternal and child psychological outcomes of HIV disclosure to young children in rural South Africa: The Amagugu intervention. AIDS. 2015;29(Suppl. 1):S67-S79. https://doi.org/10.1097/QAD.0000000000000668 [ Links ]
27.Qiao S, Li X, Stanton B. Disclosure of parental HIV infection to children: A systematic review of global literature. AIDS Behav. 2013;17(1):369-389. https://doi.org/10.1007/s10461-011-0069-x [ Links ]
28.Zaidi J, Grapsa E, Tanser F, Newell M-L, Bärnighausen T. Dramatic increase in HIV prevalence after scale-up of antiretroviral treatment. AIDS. 2013;27(14):2301-2305. https://doi.org/10.1097/QAD.0b013e328362e832 [ Links ]
29.Mkwanazi NB, Patel D, Newell M-L, et al. Rapid testing may not improve uptake of HIV testing and same day results in a rural South African community: A cohort study of 12,000 women. PLoS One. 2008;3(10):e3501. https://doi.org/10.1371/journal.pone.0003501 [ Links ]
30.Janssen N, Ndirangu J, Newell M-L, Bland RM. Successful paediatric HIV treatment in rural primary care in Africa. Arch Dis Child. 2010;95(6):414-421. https://doi.org/10.1136/adc.2009.169367 [ Links ]
31.Houlihan CF, Bland RM, Mutevedzi PC, et al. Cohort profile: Hlabisa HIV treatment and care programme. Int J Epidemiol. 2010;40(2):318-326. https://doi.org/10.1093/ije/dyp402 [ Links ]
32.Tanser F, Bärnighausen T, Grapsa E, Zaidi J, Newell M-L. High coverage of ART associated with decline in risk of HIV acquisition in rural KwaZulu-Natal, South Africa. Science. 2013;339(6122):966-971. https://doi.org/10.1126/science.1228160 [ Links ]
33.Bland RM, Coovadia HM, Coutsoudis A, Rollins NC, Newell ML. Cohort profile: Mamanengane or the Africa centre vertical transmission study. Int J Epidemiol. 2010;39(2):351-360. https://doi.org/10.1093/ije/dyp165 [ Links ]
34.Rochat TJ, Mitchell J, Lubbe AM, Stein A, Tomlinson M, Bland RM. Communication about HIV and death: Maternal reports of primary school-aged children's questions after maternal HIV disclosure in rural South Africa. Soc Sci Med. 2017;172:124-134. https://doi.org/10.1016/j.socscimed.2016.10.031 [ Links ]
35.StataCorp. Stata statistical software: Release 12. College Station, TX: StataCorp LP; 2011. [ Links ]
36.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277-1288. https://doi.org/10.1177/1049732305276687 [ Links ]
37.Biddlecom A, Awusabo-Asare K, Akinrinola B. Role of parents in adolescent sexual activity and contraceptive use in four African countries. Int Perspect Sex Reprod Health. 2009;35(2):72-81. https://doi.org/10.1363/3507209 [ Links ]
38.WHO. Helping parents in developing countries improve adolescents' health [homepage on the Internet]. [ Links ] 2007 [cited 2019 Feb 18]. Available from: https://apps.who.int/iris/bitstream/handle/10665/43725/9789241595841_eng.pdf;jsession id=4343D19A7B9B649B3EC58AC02FE1931D?sequence=1
39.Jordan TR, Price JH, Fitzgerald S. Rural parents' communication with their teenagers about sexual issues. J Sch Health. 2000;70(8):338-344. https://doi.org/10.1111/j.1746-1561.2000.tb07269.x [ Links ]
40.O'Connor EE, Dearing E, Collins BA. Teacher-child relationship and behavior problem trajectories in elementary school. Am Educ Res J. 2011;48(1):120-162. https://doi.org/10.3102/0002831210365008 [ Links ]
41.Anda RF, Felitti VJ, Bremner JD, et al. The enduring effects of abuse and related adverse experiences in childhood. Eur Arch Psychiatry Clin Neurosci. 2006;256(3):174-186. https://doi.org/10.1007/s00406-005-0624-4 [ Links ]
42.Takizawa R, Maughan B, Arseneault L. Adult health outcomes of childhood bullying victimization: Evidence from a five-decade longitudinal British birth cohort. Am J Psychiatry. 2014;171(7):777-784. https://doi.org/10.1176/appi.ajp.2014.13101401 [ Links ]
43.Ttofi MM, Farrington DP, Lösel F, Loeber R. Do the victims of school bullies tend to become depressed later in life? A systematic review and meta-analysis of longitudinal studies. J Aggress Confl Peace Res. 2011;3(2):63-73. https://doi.org/10.1108/17596591111132873 [ Links ]
44.Cluver L, Bowes L, Gardner F. Risk and protective factors for bullying victimization among AIDS-affected and vulnerable children in South Africa. Child Abuse Negl. 2010;34(10):793-803. https://doi.org/10.1016/j.chiabu.2010.04.002 [ Links ]
45.Cluver L, Orkin M, Boyes M, Gardner F, Meinck F. Transactional sex amongst AIDS-orphaned and AIDS-affected adolescents predicted by abuse and extreme poverty. J Acquir Immune Defic Syndr. 2011;58(3):336-343. https://doi.org/10.1097/QAI.0b013e31822f0d82 [ Links ]
46.Ward CL, Artz L, Leoschut L, Kassanjee R, Burton P. Sexual violence against children in South Africa: A nationally representative cross-sectional study of prevalence and correlates. Lancet Glob Health. 2018;6(4):e460-e468. https://doi.org/10.1016/S2214-109X(18)30060-3 [ Links ]
47.Richter LM, Mathews S, Kagura J, Nonterah E. A longitudinal perspective on violence in the lives of South African children from the birth to twenty plus cohort study in Johannesburg-Soweto. S Afr Med J. 2018;108(3):181-186. https://doi.org/10.7196/SAMJ.2018.v108i3.12661 [ Links ]
48.Richter L, Chikovore J, Makusha T. The status of fatherhood and fathering in South Africa. Child Educ. 2010;86(6):360-365. https://doi.org/10.1080/00094056.2010.10523170 [ Links ]
49.Schuster MA, Corona R, Elliott MN, et al. Evaluation of talking parents, healthy teens, a new worksite based parenting programme to promote parent-adolescent communication about sexual health: Randomised controlled trial. BMJ. 2008;337:a308. https://doi.org/10.1136/bmj.39609.657581.25 [ Links ]
50.Wamoyi J, Fenwick A, Urassa M, Zaba B, Stones W. Parent-child communication about sexual and reproductive health in rural Tanzania: Implications for young people's sexual health interventions. Reprod Health. 2010;7(1):1-18. https://doi.org/10.1186/1742-4755-7-6 [ Links ]
51.Rozentals-Thresher R, Hemmens R, Stone J. Play with us: Bringing hope and healing to KwaZulu-Natal's children. In: Bunston W, Jones SJ, editors. Supporting vulnerable babies and young children: Interventions for working with trauma, mental health, illness and other complex challenges. London: Jessica Kingsley Publishers, 2019; p. 175-190. [ Links ]
52.Mkwanazi NB, Rochat TJ, Bland RM. The Amagugu intervention: A qualitative investigation into maternal experiences and perspectives of a maternal HIV disclosure support intervention in rural South Africa. Health Policy Plan. 2017;32(9):1231-1240. https://doi.org/10.1093/heapol/czx056 [ Links ]
53.Turnbull T, Van Wersch A, Van Schaik P. Parents as educators of sex and relationship education: The role for effective communication in British families. Health Educ J. 2011;70(3):240-248. https://doi.org/10.1177/0017896911398817 [ Links ]
54.Gade CBN. What is ubuntu? Different interpretations among South Africans of African descent. S Afr J Philos. 2012;31(3):484-503. https://doi.org/10.1080/02580136.2012.10751789 [ Links ]
55.Barbarin OA, Richter LM. Mandela's children: Growing up in post-apartheid South Africa. London: Routledge; 2001. [ Links ]
56.Pillay U, Roberts B, Rule SP. South African social attitudes: Changing times, diverse voices. Cape town: HSRC Press; 2006. [ Links ]
 Correspondence:
Correspondence:
Tamsen Rochat
tamsen.rochat@wits.ac.za
Received: 19 Mar. 2019
Accepted: 24 Nov. 2019
Published: 29 June 2020
Appendix 1: Supplementary material
^rND^sMenzies^nNA^rND^sBerruti^nAA^rND^sBlandford^nJM^rND^sMcNally^nLM^rND^sHadingham^nJ^rND^sArchary^nD^rND^sMoodley^nR^rND^sCoovadia^nHM^rND^sGoga^nAE^rND^sDinh^nT^rND^sJackson^nDJ^rND^sSherr^nL^rND^sCluver^nLD^rND^sBetancourt^nTS^rND^sKellerman^nSE^rND^sRichter^nLM^rND^sDesmond^nC^rND^sChi^nP^rND^sLi^nX^rND^sSutton^nMY^rND^sLasswell^nSM^rND^sLanier^nY^rND^sMiller^nKS^rND^sBastien^nS^rND^sKajula^nLJ^rND^sMuhwezi^nWW^rND^sPettifor^nA^rND^sStoner^nM^rND^sPike^nC^rND^sBekker^nL-G^rND^sRochat^nTJ^rND^sMitchell^nJ^rND^sStein^nA^rND^sMkwanazi^nNB^rND^sBland^nRM^rND^sRochat^nTJ^rND^sMkwanazi^nN^rND^sBland^nR^rND^sCoetzee^nB^rND^sKagee^nA^rND^sBland^nR^rND^sFonner^nVA^rND^sArmstrong^nKS^rND^sKennedy^nCE^rND^sO'Reilly^nKR^rND^sSweat^nMD^rND^sPhetla^nG^rND^sBusza^nJ^rND^sHargreaves^nJR^rND^sDesmond^nC^rND^sViviers^nA^rND^sEdwards^nT^rND^sRich^nK^rND^sMartin^nP^rND^sRichter^nL^rND^sWeaver^nH^rND^sSmith^nG^rND^sKippax^nS^rND^sMistry^nKB^rND^sMinkovitz^nCS^rND^sRiley^nAW^rND^sRademakers^nJ^rND^sHeijmans^nM^rND^sNambambi^nNM^rND^sMufune^nP^rND^sRochat^nTJ^rND^sStein^nA^rND^sCortina-Borja^nM^rND^sTanser^nF^rND^sBland^nRM^rND^sRochat^nTJ^rND^sArteche^nAX^rND^sStein^nA^rND^sMkwanazi^nN^rND^sBland^nRM^rND^sRochat^nTJ^rND^sArteche^nAX^rND^sStein^nA^rND^sMitchell^nJ^rND^sBland^nRM^rND^sQiao^nS^rND^sLi^nX^rND^sStanton^nB^rND^sZaidi^nJ^rND^sGrapsa^nE^rND^sTanser^nF^rND^sNewell^nM-L^rND^sBärnighausen^nT^rND^sMkwanazi^nNB^rND^sPatel^nD^rND^sNewell^nM-L^rND^sJanssen^nN^rND^sNdirangu^nJ^rND^sNewell^nM-L^rND^sBland^nRM^rND^sHoulihan^nCF^rND^sBland^nRM^rND^sMutevedzi^nPC^rND^sTanser^nF^rND^sBärnighausen^nT^rND^sGrapsa^nE^rND^sZaidi^nJ^rND^sNewell^nM-L^rND^sBland^nRM^rND^sCoovadia^nHM^rND^sCoutsoudis^nA^rND^sRollins^nNC^rND^sNewell^nML^rND^sRochat^nTJ^rND^sMitchell^nJ^rND^sLubbe^nAM^rND^sStein^nA^rND^sTomlinson^nM^rND^sBland^nRM^rND^sHsieh^nH-F^rND^sShannon^nSE^rND^sBiddlecom^nA^rND^sAwusabo-Asare^nK^rND^sAkinrinola^nB^rND^sJordan^nTR^rND^sPrice^nJH^rND^sFitzgerald^nS^rND^sO'Connor^nEE^rND^sDearing^nE^rND^sCollins^nBA^rND^sAnda^nRF^rND^sFelitti^nVJ^rND^sBremner^nJD^rND^sTakizawa^nR^rND^sMaughan^nB^rND^sArseneault^nL^rND^sTtofi^nMM^rND^sFarrington^nDP^rND^sLösel^nF^rND^sLoeber^nR^rND^sCluver^nL^rND^sBowes^nL^rND^sGardner^nF^rND^sCluver^nL^rND^sOrkin^nM^rND^sBoyes^nM^rND^sGardner^nF^rND^sMeinck^nF^rND^sWard^nCL^rND^sArtz^nL^rND^sLeoschut^nL^rND^sKassanjee^nR^rND^sBurton^nP^rND^sRichter^nLM^rND^sMathews^nS^rND^sKagura^nJ^rND^sNonterah^nE^rND^sRichter^nL^rND^sChikovore^nJ^rND^sMakusha^nT^rND^sSchuster^nMA^rND^sCorona^nR^rND^sElliott^nMN^rND^sWamoyi^nJ^rND^sFenwick^nA^rND^sUrassa^nM^rND^sZaba^nB^rND^sStones^nW^rND^sRozentals-Thresher^nR^rND^sHemmens^nR^rND^sStone^nJ^rND^sMkwanazi^nNB^rND^sRochat^nTJ^rND^sBland^nRM^rND^sTurnbull^nT^rND^sVan Wersch^nA^rND^sVan Schaik^nP^rND^sGade^nCBN^rND^1A01^nIsabel A.^sMichaelis^rND^1A01 A02 A04^nMaryke^sNielsen^rND^1A05^nCraig^sCarty^rND^1A06^nMarkus^sWolff^rND^1A07^nCaroline A.^sSabin^rND^1A08^nJohn S.^sLambert^rND^1A01^nIsabel A.^sMichaelis^rND^1A01 A02 A04^nMaryke^sNielsen^rND^1A05^nCraig^sCarty^rND^1A06^nMarkus^sWolff^rND^1A07^nCaroline A.^sSabin^rND^1A08^nJohn S.^sLambert^rND^1A01^nIsabel A^sMichaelis^rND^1A01 A02 A04^nMaryke^sNielsen^rND^1A05^nCraig^sCarty^rND^1A06^nMarkus^sWolff^rND^1A07^nCaroline A^sSabin^rND^1A08^nJohn S^sLambert
ORIGINAL RESEARCH
Late diagnosis of human immunodeficiency virus infection is linked to higher rates of epilepsy in children in the Eastern Cape of South Africa
Isabel A. MichaelisI; Maryke NielsenII, III, IV; Craig CartyV; Markus WolffVI; Caroline A. SabinVII; John S. LambertVIII
IDepartment of Health, Faculty of Paediatrics, Walter Sisulu University, Mthatha, South Africa
IIDepartment of Paediatrics and Child Health, Faculty of Infectious Disease, Malawi- Liverpool-Wellcome Clinical Research Facility, Blantyre, Malawi
IIIInstitute of Infection and Global Health, Faculty of Clinical Infection, Immunology and Microbiology, University of Liverpool, Liverpool, United Kingdom
IVDepartment of Paediatrics, Queen Elizabeth Central Hospital, Blantyre, Malawi
VDepartment of Evidence- Based Social Intervention, Faculty of Sociology, University of Oxford, Oxford, United Kingdom
VIDepartment of Neuropaediatrics and Social Paediatrics, Faculty of Paediatrics, Vivantes Klinikum Neukolln, Berlin, Germany
VIIDepartment of Medical Statistics and Epidemiology, Faculty of Population Health Sciences, University College London, London, United Kingdom
VIIIDepartment of Infectious Diseases, Faculty of Infectious Diseases and Genitourinary Medicine, UCD School of Medicine, Dublin, Ireland
ABSTRACT
BACKGROUND: Human immunodeficiency virus (HIV)-positive children may present with a wide range of neurological disorders. Among these, epilepsy is of key concern because of its lifelong impact and potential for damage to the central nervous system (CNS). Few studies in developing regions have investigated the prevalence and aetiology of epilepsy in HIV-infected children as a key population.
OBJECTIVES: We describe the prevalence of epilepsy, associated neurological disabilities, immunological status, clinical stage and history of CNS infection at epilepsy diagnosis in a cohort of HIV-infected children receiving antiretroviral therapy (ART) in the Eastern Cape of South Africa.
METHODS: We conducted a retrospective study (2004-2014) at two major referral sites for HIV-infected children diagnosed with epilepsy aged 0-16 years. Eligible subjects were extracted from the electronic medicine bridging access to care in excellence (EMBRACE) Paediatric Cohort using the Paediatric ART Data Management Tool (PADMT). Fixed data fields were interrogated for exposures to antiepileptic drugs. Unstructured 'comments' fields were searched for the terms: epilepsy, seizures, fits and szs, as well as abbreviated versions of common antiepileptic drug names. Eligible subject folders were then retrieved to validate the digital data.
RESULTS: From 2139 children enrolled in the two sites, 53 children were diagnosed with epilepsy (2.48%). In these, the median CD4 count was 591 cells/mm3, and the mean viral load was 4.9 log copies/mL, with undetectable viral loads in only seven children (14.0%). World Health Organization (WHO) clinical HIV stage was available for 46 patients of the sample, with 3, 6, 26 and 11 children graded at stages 1, 2, 3 and 4, respectively. Forty percent children had a history of CNS infection prior to the epilepsy diagnosis, and 55% children were reported to have school problems.
CONCLUSIONS: In this descriptive study, the prevalence of epilepsy among children with HIV was 2.48%, mostly diagnosed in advanced HIV-disease stages. Our findings support the usefulness of early detection and initiation of ART in HIV-infected children in order to reduce the risk of epilepsy. In addition, our study demonstrates that novel techniques are effective in accessing cohort-level data that allow interrogation of both structured and unstructured clinical data.
Keywords: epilepsy; HIV-infection; children; WHO staging; paediatric ART data management tool.
Introduction
The latest World Health Organization (WHO) report on human immunodeficiency virus (HIV) and acquired immunodeficiency syndrome (AIDS) estimates that 3.2 million children are infected with HIV worldwide, of whom 91% are living in sub-Saharan Africa.1 South Africa is regarded as having the largest HIV and AIDS burden in the world, with an estimated 17.6% of the population living with the disease; between 370 000 and 450 000 children younger than 14 years in South Africa are thought to be infected with the virus.1
Human immunodeficiency virus-related neurological manifestations are common in both adults and children.2,3,4,5,6 They include opportunistic central nervous system (CNS) infections, behavioural problems and psychiatric disorders, as well as neurodevelopmental delay and epilepsy.4 Epilepsyis a potentially disabling disease whose treatment is challenging in sub-Saharan Africa,7,8,9,10,11,12,13 particularly among children with HIV infection, who are often orphaned and have to face poverty.14
The prevalence of seizures in children with confirmed HIV infection has been reported to range from 2% to 14%,9,15,16,17,18 and the prevalence of epilepsy is estimated between 0.29% and 11.3%.19,20,21 This wide variation is believed to be a result of differences of risk factors in different regions.22
Epilepsy in HIV-infected persons may occur as a result of several different processes: direct viral damage to the brain by uncontrolled viral replication; following a CNS infection by opportunistic pathogens; as a result of malignancies; following the use of some medications; as a result of metabolic and electrolyte derangements; or as a secondary acquired pathology because of HIV encephalopathy.4
Of interest, Bearden et al.19 argued that earlier initiation of antiretroviral therapy (ART) might prevent the manifestation of epilepsy in children with HIV infection. This study was conducted in Botswana, and there is no other study on record so far to confirm or challenge these results in a different population.
We describe the prevalence of epilepsy, including associated neurological disabilities, immunological and clinical status and history of CNS infection in children with HIV infection on ART in a mixed rural/urban area in the Eastern Cape. The aim is to show that late diagnosis of HIV infection and delayed initiation of ART in children are linked to higher rates of epilepsy.
Material and methods
Setting
We conducted a retrospective study (2004-2014) at two major referral sites for HIV-infected children aged 0-16 years in the Eastern Cape, South Africa. One centre is a paediatric and adolescent HIV referral clinic in a tertiary hospital in East London, and the other is the HIV clinic for children and adolescents in the township of Mdantsane, which serves as a major referral centre as well as the local health clinic.
The Eastern Cape belongs to one of the most underprivileged parts of South Africa, and its health service is among the most under-resourced. Together, the two hospitals serve around 3.5 m people, including more than 890 000 children younger than 15 years (Census 2011 Municipal report).
In South Africa, ART became available in state health facilities from 2004. At this time, the criteria for ART required that children had advanced HIV disease, assessed by WHO staging.23,24 The eligibility criteria for initiation of ART and the type of first-line regimen for infants and children with HIV infection following HIV guidelines changed in 2004, 2010, 2013 and 2015.25 The clinicians in our setting used the appropriate guidelines for the specific year the children were firstly seen in the clinics. Since 2014, every child younger than 5 years with confirmed HIV infection was eligible for treatment, regardless of his/her clinical (WHO staging) and laboratory status (CD4 counts)26; since 2017, all people in South Africa with confirmed HIV infection have immediate access to ART.
In our setting, the routine approach to children presenting with seizures consists of a detailed clinical history and physical examination, including blood pressure, as well as assessment of blood glucose and, if indicated, antiepileptic drug plasma levels. Emergency computed tomography (CT) is performed if the child has new-onset focal seizures, encephalopathy or prolonged neurological fallout. In children with suspected meningitis, encephalitis or other inflammations, appropriate serum markers are investigated, and a lumbar puncture is performed unless contraindicated. Human immunodeficiency virus testing is offered to all children. In children known to be HIV infected, CD4 count and HIV viral loads (VLs) are assessed.
Cases were identified by screening all available subfiles for 'epilepsy' and by interrogating the fixed data fields of an electronic database, the Paediatric ART Data Management Tool (PADMT), for exposure to antiepileptic drugs. Unstructured 'comments' fields were hand-searched for relevant terms like epilepsy, seizures, fits and szs, paroxysmal events, faints or syncope or electroencephalogram (EEG). Eligible subject folders were then retrieved to validate the digital data.
Children with a single seizure or single seizure episode during acute illness because of metabolic disturbances, hypertension, neurocysticercosis or infection, or those with the diagnosis of febrile seizures were excluded from further analysis.
Those children with a diagnosis of epilepsy, as defined by Fisher et al.,27 were further assessed for aetiology by reviewing clinical information, laboratory results, brain imaging and EEG studies, if available. In epileptic children diagnosed with HIV encephalopathy with no other clinical, laboratory or imaging findings, the neurotoxic effect of HIV was considered as the cause of epilepsy.
Human immunodeficiency virus encephalopathy is defined as damage or malfunction of the brain because of HIV infection. Human immunodeficiency virus encephalopathy in children must include at least one of the following findings present for at least 2 months in the absence of a concurrent illness other than HIV infection: (1) failure to attain or loss of developmental milestones or loss of intellectual ability; (2) impaired brain growth or acquired microcephaly; or (3) acquired symmetric motor deficit.28
The intent of this study was to see how many children in this cohort have epilepsy, even if it was not caused by the HIV infection.
Absolute CD4 count and CD4%, baseline VL at the start of ART, the VL between 6 and 12 months after initiating ART, the VL nearest to the diagnosis of epilepsy (for those diagnosed with epilepsy while on ART and where the value was available within a window of 5 months before or after diagnosis) and VL at last available follow-up assessment were retrieved. Neurological deficits and indicators of developmental delay were also recorded, as well as reports of previous CNS infection. Prescribed antiepileptic drugs were also recorded. Clinical staging of HIV infection as assessed by the attending doctor following the WHO guidelines were documented.
Children attending the clinics were followed up at regular 3-monthly intervals (monthly for children with social problems or adherence issues).
Ethical consideration
This article followed all ethical standards for a research without direct contact with human or animal subjects.
Results
Of the 2137 children with confirmed HIV infection enrolled in the two clinics, 53 (2.5%) were diagnosed with epilepsy. Comprehensive medical records were available in 49 (92%) of the 53 patients. Only those children were included in this study. The age of the children ranged from 1 month to 12 years (median 4 years) at the time of diagnosis of epilepsy, and 26 (53%) were boys.
All children and infants with HIV infection and epilepsy were initiated on ART during the study assessment period. Eighty-one per cent of the children were on first-line treatment, which comprised abacavir, lamivudine and lopinavir/ritonavir (n = 18) or efavirenz (n = 22), depending on the age of the child at initiation and the year of treatment initiation. In 16 of the children, stavudine was replaced by abacavir as part of the change in guidelines for first-line treatment. Four (8%) of the children were placed on lamivudine-holding regimes, mostly for treatment failure because of non-compliance after extensive counselling of the family. The others (11%) were started on second-line treatment following treatment guidelines.
Antiretroviral therapy was started either after (20 patients; 40%), at (15 patients; 31%) or before (14 patients; 29%) diagnosis of epilepsy.
The WHO clinical staging at diagnosis of HIV infection was available in the records for 46 children, with 4 (8%), 5 (10%), 26 (53%) and 11 (23%) children graded at stages 1, 2, 3 and 4, respectively. Staging data were missing in three (6%) children (Table 1).

For children who developed epilepsy at the beginning of or after initiating ART (n = 29; 59%), the median CD4 count was 591 cells/mm3 (range 15 cells/mm3 - 1980 cells/mm3), and the mean VL was 782 768 copies/mL (range from lower than detectable limit [LDL] to 10 000 000), at the time of diagnosis of epilepsy.
In nine of the 14 children (64%) who were diagnosed with epilepsy while already on ART, the VL was lower than 800 copies/mL or undetectable at the time of epilepsy diagnosis. In four (29%) children, VL was > 800 copies/mL at the time of diagnosis, with one child having no VL data available in the time period. In six (43%) children, the CD4 percentage was < 25%, even though they had been on ART for at least 7 months at the time of diagnosis of epilepsy. Five (36%) children had CD4% > 25%, while CD4% data were not available for three (21%) of the children (Table 2).
About 74% (26/35) of the children who were diagnosed with epilepsy before or at ART initiation had a VL that was below the limit of detection or below 800 copies/mL between 6 and 12 months after ART initiation, with only six (17% [6/35]) having VL values > 800 copies/mL. In the group which developed epilepsy at least 7 months after initiation of ART, viral suppression was seen only in 57% (8/14). For three children (9% [3/35]), no data were available.
Nineteen children (39%) were found to have a CNS infection, mainly tuberculosis (n = 13), but also other bacterial CNS infections. Three children were diagnosed with neurocysticercosis. Eighteen per cent of the children were diagnosed with HIV encephalopathy by the Centers for Disease Control and Prevention (CDC) criteria,25 where neurotoxic effect of HIV was considered as the cause of epilepsy. In one child with spastic quadriplegic cerebral palsy, it was assumed that intra-partum hypoxic ischaemic encephalopathy caused the epilepsy as the child developed seizures right after birth. Two children presenting with cerebrovascular accidents, both caused by persistent severe thrombocytopenia, developed epilepsy thereafter. The average duration between CNS infection and diagnosis of epilepsy was 11 months (range: 1-24 months). In only one case, the diagnosis of epilepsy was made prior to CNS infection. For more than one-third of children (37%), no cause could be found.
Features of neurodevelopmental delay, as assessed by the medical officer in the antiretroviral therapy (ARV) clinic, were present in 17 (34%) of the epileptic children. Several children were referred either for assessment in the neurodevelopmental clinic or to the occupational, physio- or speech therapist. One of the eight children seen in the neurodevelopmental clinic was diagnosed with hemiplegic cerebral palsy post-stroke, one with quadriplegic cerebral palsy because of hypoxic ischemic encephalopathy, two with speech and cognitive impairment of unknown cause and four with HIV encephalopathy.
School failure, school problems, reports from the educational psychologist of intellectual impairment or the notice of attendance of a special school was noted for 27 (55%) of the epileptic children (70% of whom were boys).
Most children were diagnosed with epilepsy before or at the time of diagnosis of HIV infection (35/49). Almost all (48 of 49) children with epilepsy were treated with sodium valproate; 18 received other antiepileptic drugs either before sodium valproate or as a dual- or multi-drug regime, including phenobarbitone (n = 16), carbamazepine (n = 2), clonazepam (n = 2), lamotrigine (n = 1) and/or ethosuximide (n = 1). The two children treated with carbamazepine were referred from other health facilities on carbamazepine and were changed to sodium valproate in our clinic. Half (24/49) of the children became seizure-free with the use of antiepileptic drugs and eight (16%) had a significant (50% - 75%) reduction in seizure frequency.
Discussion
In this retrospective survey, we were able to show a 2.5% prevalence of epilepsy in children with HIV infection, which is similar to other areas in South Africa and sub-Saharan Africa, as well as in India.19,20,21 This number is about two to three times greater compared to the overall prevalence of epilepsy in Africa and South Africa, which is estimated between 0.73% and 1.2%.13,28,29 However, this prevalence might still be underestimated as the data were retrospectively collected from case notes, which is a key limitation of this study.
Most children (76%) with epilepsy were classified as stage 3 or 4 according to the WHO staging system for HIV/AIDS at diagnosis of HIV. Thus, most of the children with epilepsy were diagnosed at an advanced stage of HIV infection. In contrast, 80% of the children in the group without epilepsy were assessed as stage 1 at diagnosis. (see Table 1 and Figure 1)
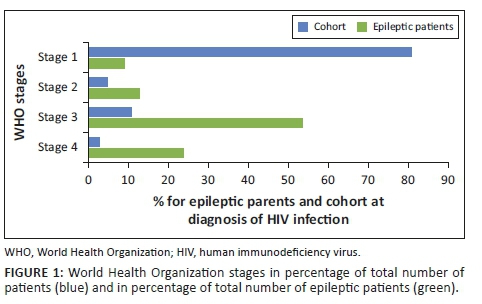
In about half of the patients with epilepsy (48%), treatment for HIV infection was initiated at the time of diagnosis. Twenty-eight per cent had treatment initiation months, and sometimes years after HIV diagnosis, probably because of the guidelines for that specific time period. For the rest, the exact date of diagnosis of HIV infection was not available, which is another limitation of this study.
None of the children with epilepsy and stage 1 HIV infection had prior CNS infection, while 81% of the children with epilepsy and stage 4 HIV infection had prior CNS infection. Thirty-seven (75%) of the children were diagnosed with epilepsy before or at initiation of ART. Retrospectively, it was not possible to differentiate in how many children the complaint of seizures was the cause of further investigations, followed by the discovery of HIV infection. These findings might nevertheless indicate not only that many children were only diagnosed as HIV-positive when presenting at the hospital with seizures or CNS infection, but also that some of them did not yet qualify for ART according to the specific HIV treatment guidelines in place at the time. This led to progression of HIV infection in these children and made them vulnerable to opportunistic infections and increased the risk of developing epilepsy. The above findings also support the findings of a prior study,19 where early initiation of ART was assumed to be protective for epilepsy and seizures.
The most common specific aetiology for epilepsy was a prior CNS infection, with meningitis caused by Mycobacterium tuberculosis being the most frequent. Again, CNS infection was probably because of late diagnosis of HIV infection, and/or late initiation of ART, making the children vulnerable to opportunistic infections. Human immunodeficiency virus neurotoxicity was the second most common suspected cause. Those data correlate well to those of Bearden et al. in Botswana.19 Two children had infection-independent strokes and developed seizures and epilepsy afterwards, and one patient developed seizures as a neonate after suffering from a hypoxic-ischaemic event during birth. As the type of epilepsy was mostly not described in the notes and as most children were unable to receive EEG readings, it is likely that epilepsy syndromes like absence epilepsy or childhood epilepsy with centro-temporal spikes may have been missed. The prevalence of these disorders, however, is low in high resource settings,30 but unknown in South Africa. Furthermore, besides CD4 counts and VL, limited laboratory investigations were available for most children, which is a further limitation of this study. In more than one-third of children, the aetiology could not be established, which is slightly higher than that in other studies in children and adults.19,31,32,33
In our cohort, almost half of the children with epilepsy became seizure-free and another 16% had a significant reduction (50% - 75%) of seizure frequency. The tight follow-up schedule to assure adherence to ART, and thus to antiepileptic treatment, might have contributed to this outcome.
One big concern is the high percentage of the epileptic children in our cohort with school problems (55%). It is known that many children with perinatal acquired HIV infection show neurodevelopmental delay.3,15,16,34 Human immunodeficiency virus-positive preschool and school children had global deficits in all measures of neurodevelopment, except gross motor skills, in a study from Uganda.35,36,37 Compared to their non-exposed and non-infected classmates, two to three times more HIV-infected children struggled with language, visual perception and fine motor skills. A study conducted in Congo showed that children infected with HIV had a significantly higher incidence, up to 91%, of neurodevelopmental deficits in all domains compared to uninfected children.37 Newer studies could show that early start of treatment with ART in perinatal infected children correlates with higher neuro-cognitive performance in children and adolescents,38,39,40,41 supporting the recommendation to preferably start ART in the first 3 months of life.42,43
Conclusion
In this retrospective survey, we were able to show a 2.5% prevalence of epilepsy in infants and children with confirmed HIV infection on ART in a semi-urban-rural part of South Africa. Many of the children who developed epilepsy during the course of their HIV infection had prior CNS infection, or HIV encephalopathy and advanced disease, as demonstrated by the high WHO staging at the start of ART. In addition, more than half (55%) of the children were found to have educational difficulties including referral to a special school.
As shown, HIV-infected infants and children in low-resource settings face an array of additional challenges besides HIV infection, which need to be addressed in a comprehensive manner. It is clear that in a setting with a high prevalence of HIV, early detection of HIV infection and swift initiation of ART in children are crucial, and delays will impact on school performance and thus on future socio-economic status, keeping the spiral of poverty going. The new South African HIV guidelines from 2017, which made it possible to start immediate highly active antiretroviral therapy (HAART) on every person diagnosed with HIV infection, address this issue. Unfortunately, in low-resource areas like the Eastern Cape, early diagnosis in children and infants is still a challenge and this needs to be addressed.
Acknowledgements
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
All authors contributed equally to this work.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.UNAIDS. The gap report [homepage on the Internet]. [ Links ] c2014 [cited 2019 Feb 01]. Available from https://www.unaids.org/sites/default/files/en/media/unaids/contentassets/docum ents/unaidspublication/2014/UNAIDS_Gap_report_en.pdf
2.Aarli JA, Diop AG, Lochmüller H. Neurology in sub-Saharan Africa: A challenge for World Federation of Neurology. Neurology. 2007 Oct 23;69(17):1715-1718. https://doi.org/10.1212/01.wnl.0000285102.47543.02 [ Links ]
3.Nassen R, Donald K, Walker K, et al. Management of mental health disorders and central nervous system sequelae in HIV-positive children and adolescents. By the Southern African HIV Clinicians Society. SAJHIVMED. 2014 Sep;15(3):82-96. https://doi.org/10.4102/sajhivmed.v15i3.7 [ Links ]
4.Donald KA, Hoare J, Eley B, et al. Neurologic complications of pediatric human immunodeficiency virus: Implications for clinical practice and management challenges in the African setting. Semin Pediatr Neurol. 2014;21(1):3-11. https://doi.org/10.1016/j.spen.2014.01.004 [ Links ]
5.Mitchell W. Neurological and developmental effects of HIV and AIDS in children and adolescents. Ment Retard Dev Disabil Res Rev. 2001;7(3):211-216. https://doi.org/10.1002/mrdd.1029 [ Links ]
6.Habibi P, Strobel S, Smith I, et al. Neurodevelopmental delay and focal seizures as presenting symptoms of human immunodeficiency virus 1 infection. Eur J Pediatr. 1989;148(4):315-317. https://doi.org/10.1007/BF00444122 [ Links ]
7.Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. Lancet Neurol. 2005;4(1):21-33. https://doi.org/10.1016/S1474-4422(04)00963-9 [ Links ]
8.Ba-Diop A, Marin B, Druet-Cabanac M, Ngoungou EB, Newton CR, Preux PM. Epidemiology, causes, and treatment of epilepsy in sub-Saharan Africa. Lancet Neurol. 2014 Oct;13(10):1029-1044. https://doi.org/10.1016/S1474-4422(14)70114-0 [ Links ]
9.Rotta NT, Silva C, Ohlweiler L, et al. Aids neurologic manifestations in childhood. Rev Neurol. 1999;29(4):319-322. https://doi.org/10.1007/BF03023703 [ Links ]
10.Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: A systematic review of the magnitude, causes, and intervention strategies. Epilepsia. 2008;49(9):1491-1503. https://doi.org/10.1111/j.1528-1167.2008.01693.x [ Links ]
11.Carpio A, Hauser WA. Epilepsy in the developing world. Curr Neurol Neurosci Rep. 2009;9(4):319-326. https://doi.org/10.1007/s11910-009-0048-z [ Links ]
12.Wilmshurst JM, Birbeck GL, Newton CR. Epilepsy is ubiquitous, but more devastating in the poorer regions of the world… or is it? Epilepsia. 2014 Sep;55(9):1322-1325. https://doi.org/10.1111/epi.12602 [ Links ]
13.Wagner RG, Bottomley C, Ngugi AK, et al. Incidence, remission and mortality of convulsive epilepsy in rural northeast South Africa. PLoS ONE. 2015;10(6):e0129097. https://doi.org/10.1371/journal.pone.0129097 [ Links ]
14.Grantham-McGregor S, Cheung YB, Cueto S, et al. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007 Jan 06;369(9555):60-70. https://doi.org/10.1016/S0140-6736(07)60032-4 [ Links ]
15.Civitello L, Brouwers P, Pizzo PA. Neurological and neuropsychological manifestations of 120 children with symptomatic HIV infection. Ann Neurol. 1993;34:481. [ Links ]
16.Tellechea-Rotta N, Legido A. Acquired immunodeficiency syndrome by vertical transmission: Neurological disorders. Rev Neurol. 2003;36:255-263. https://doi.org/10.33588/rn.3603.2002246 [ Links ]
17.Samia P, Petersen R, Walker K, Eley B, Wilmshurst JM. Prevalence of seizures in children infected with human immunodeficiency virus. J Child Neurol. 2013;28(3):297-302. https://doi.org/10.1177/0883073812446161 [ Links ]
18.Bhigjee AI. Seizures in HIV/AIDS: A southern African perspective. Acta Neurol Scand Suppl. 2005;122(181):4-7. https://doi.org/10.1111/j.1600-0404.2005.00500.x [ Links ]
19.Bearden D, Steenhoff AP, Dlugos DJ, et al. Early antiretroviral therapy is protective against epilepsy in children with human immunodeficiency virus infection in Botswana. J Acquir Immune Defic Syndr. 2015;69(2):193-199. https://doi.org/10.1097/QAI.0000000000000563 [ Links ]
20.Govender R, Eley B, Walker K, et al. Neurologic and neurobehavioral sequelae in children with human immunodeficiency virus (HIV-1) infection. J Child Neurol. 2011;26(11):1355-1364. https://doi.org/10.1177/0883073811405203 [ Links ]
21.Gupta S, Shah DM, Shah I. Neurological disorders in HIV-infected children in India. Ann Trop Paediatr. 2009;29(3):177-181. https://doi.org/10.1179/027249309X12467994693734 [ Links ]
22.Nquqi AK, Bottomley C, Kleinschmidt I, et al. Prevalence of active convulsive epilepsy in sub-Saharan Africa and associated risk factors: Cross-sectional and case-control studies. Lancet Neurol. 2013 Mar 13;12(3):253-263. https://doi.org/10.1016/S1474-4422(13)70003-6 [ Links ]
23.World Health Organization. Interim WHO clinical staging of HIV/AIDS and HIV/AIDS case definitions for surveillance: African region. Geneva, Switzerland: World Health Organization; 2005. [ Links ]
24.World Health Organization. WHO case definitions of HIV for surveillance and revised clinical staging and immunological classification of HIV-related disease in adults and children. Geneva: WHO Library Cataloguing-in-Publication Data; 2007. [ Links ]
25.Johnson L. Access to antiretroviral treatment in South Africa, 2004 - 2011. South Afr J HIV Med [serial online]. 2012 Mar 13 [cited 2017 Oct 21];13(1):a156. Available from: https://sajhivmed.org.za/index.php/hivmed/article/view/156/261
26.Dr Pillay Y. National Consolidated Guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children adolescents and adults. Pretoria: National Department of Health; April 2015. Available from: www.doh.gov.za [ Links ]
27.Fisher RS, Acevedo C, Arzimanoglou A, et al. A practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475-482. https://doi.org/10.1111/epi.12550 [ Links ]
28.Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: a global perspective. Eur J Paediatr Neurol. 2007;11(1):1-9. https://doi.org/10.1016/j.ejpn.2006.10.006 [ Links ]
29.Christianson AL, Zwane ME, Manga P, Rosen E, Venter A, Kromberg JG. Epilepsy in rural South African children: Prevalence, associated disability and management. S Afr Med J. 2000;90(3):262-266. [ Links ]
30.Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46(Suppl 9):10-14. https://doi.org/10.1111/j.1528-1167.2005.00309.x [ Links ]
31.Kellinghaus C, Engbring C, Kovac S, et al. Frequency of seizures and epilepsy in neurological HIV-infected patients. Seizure. 2008;17(1):27-33. https://doi.org/10.1016/j.seizure.2007.05.017 [ Links ]
32.Weisberg LA. Neurologic abnormalities in human immunodeficiency virus infection. South Med J. 2001;94(3):266-275. https://doi.org/10.1097/00007611-200194030-00001 [ Links ]
33.Modi M. New onset seizures in HIV. Epilepsia. 2009;50(5):1266-1269. https://doi.org/10.1111/j.1528-1167.2008.01942.x [ Links ]
34.Walker SY, Pierre RB, Christie CDC, Chang SM. Neurocognitive function in HIV-positive children in a developing country. Int J Infect Dis. 2013 Oct;17(10):e862-e867. https://doi.org/10.1016/j.ijid.2013.02.014 [ Links ]
35.Brahmbhatt H, Boivin M, Ssempijja V, et al. Neurodevelopmental benefits of antiretroviral therapy in Ugandan children aged 0-6 years with HIV. J Acquir Immune Defic Syndr. 2014;67(3):316-322. https://doi.org/10.1097/QAI.0000000000000295 [ Links ]
36.Brahmbhatt H, Boivin M, Ssempijja V, et al. Impact of HIV and antiretroviral therapy on neurocognitive outcomes among school aged children. J Acquir Immune Defic Syndr. 2017;75(1):1-8. https://doi.org/10.1097/QAI.0000000000001305 [ Links ]
37.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122(1):e123-e128. https://doi.org/10.1542/peds.2007-2558 [ Links ]
38.Crowell CS, Huo Y, Tassiopulos K, et al. Early viral suppression improves neurocognitive outcomes in HIV-infected children. AIDS. 2015 Jan;29(3):295-304. https://doi.org/10.1097/QAD.0000000000000528 [ Links ]
39.Lowick S, Sawry S, Meyers T. Neurodevelopmental delay among HIV-infected preschool children receiving antiretroviral therapy and healthy preschool children in Soweto, South Africa. Psychol Health Med. 2012;17(5):599-610. https://doi.org/10.1080/13548506.2011.648201 [ Links ]
40.Weber V, Radeloff D, Reimers B, et al. Neurocognitive development in HIV-positive children is correlated with plasma viral loads in early childhood. Medicine. 2017 Jun;96(23):e6867. https://doi.org/10.1097/MD.0000000000006867 [ Links ]
41.Brahmbhatt H, Boivin M, Ssempijja V, et al. Neurodevelopmental benefits of antiretroviral therapy in Ugandan children aged 0-6 years with HIV. J Acquir Immune Defic Syndr. 2014;67(3):316-322. https://doi.org/10.1097/QAI.0000000000000295 [ Links ]
42.Puthanakit T, Ananworanich J, Vonthanak S, et al. Cognitive function and neurodevelopmental outcomes in HIV-infected children older than 1 year of age randomized to early versus deferred antiretroviral therapy: The PREDICT neurodevelopmental study. Pediatr Infect Dis J. 2013 May;32(5):501-508. https://doi.org/10.1097/INF.0b013e31827fb19d [ Links ]
43.Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012 Aug 24;26(13):1685-1690. https://doi.org/10.1097/QAD.0b013e328355d0ce [ Links ]
 Correspondence:
Correspondence:
Isabel Michaelis
isabel.michaelis@echealth.gov.za
Received: 23 Nov. 2019
Accepted: 07 Jan. 2020
Published: 30 June 2020
ORIGINAL RESEARCH
Evaluation of a mobile application to support HIV self-testing in Johannesburg, South Africa
Natasha GousI; Alex E. FischerII; Naleni RhagnathII; Mothepane PhatsoaneII; Mohammed MajamII; Samanta T. Lalla-EdwardII
ISystemOne, LLC, Weltevreden Park, South Africa
IIEzintsha, Wits Reproductive Health and HIV Institute, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Human immunodeficiency virus self-testing (HIVST) reduces barriers associated with facility-based testing; however, no formal mechanism exists for users to self-report results or link to care. The AspectTM HIVST mobile application (app) was developed for use in South Africa.
OBJECTIVES: This study evaluated the acceptability and feasibility of the AspectTM HIVST app for individuals from the inner city of Johannesburg.
METHOD: This cross-sectional pilot, with a convenience sample of 300 adults, was conducted in July 2018. Participants were provided an OraQuick HIVST kit and a smartphone preloaded with the app, then asked to follow the in-app instructions for use (IFU) to complete the HIVST and upload results. Trained healthcare workers (HCWs) observed and recorded any deviations from the IFU, and conducted a post-test survey to assess acceptability. Feasibility was evaluated by the number of participants who agreed to participate, completed the self-test, and uploaded all information onto the app correctly.
RESULTS: Most participants (98.7%) found the app easy to use. To reduce difficulties related to the IFU (26; 8.7%), participants suggested multimedia supplements (4; 1.3%), additional languages (4; 1.3%) and simplified instructions (5; 1.7%). All individuals approached, agreed to participate, 267 (89.0%) correctly completed all steps and 210 (78.7%) successfully captured all information on the app. Most errors (26; 8.7%) were testing errors and 1 (0.3%) was from the app sequence. Twelve (4.5%) errors were with test strip imaging and 72 (27.0%) discordances were with demographic information.
CONCLUSION: Despite some challenges with IFU interpretation and data capture via the app, this pilot showed that the AspectTM HIVST app is an acceptable way to upload mobile HIVST results and demographic information to a central database.
Keywords: HIV self-test; digitisation; mobile app; monitoring and evaluation; digital health.
Introduction
In 2012, the OraQuick ADVANCE Rapid HIV-1/2 Antibody Test (OraSure Technologies Inc, Bethlehem, USA) was the first HIV self-test (HIVST) approved for sale in the United States as an over-the-counter HIVST rapid diagnostic test (RDT) for individuals with no prior HIV testing experience.1 Since then, over 2.5 million HIVST kits have been sold globally and more than 4 million have been distributed through donor funded programmes.2 The World Health Organization (WHO) strongly recommends that HIVST be utilised as a way to complement existing HIV services3 as self-testing may reduce barriers associated with traditional facility-based testing, like travel, wait times and privacy concerns.4,5
Based on this growing body of evidence, South Africa became one of over 40 countries to have incorporated HIV self-testing into their national HIV policies,6,7 with self-testing introduced as a way to help close the gap between the 84.9% of adults living with HIV who know their HIV status and the 90% target of the UNAIDS 90-90-90 initiative.5,8,9,10 The introduction of HIVST programmes will improve access to further HIV diagnostic services, prompting an increase in testing uptake and frequency, which could lead to earlier diagnosis.11
There are, however, several concerns related to HIVST, as there is no formal pipeline for users to self-report their results or be linked to care following the self-test. These HIVST kits are not diagnostic, but rather considered tests for triage, and all positive results should prompt the user to seek confirmatory testing by a trained healthcare professional.12 Furthermore, the independence of HIVST presents considerable challenges surrounding the monitoring and evaluation (M&E) of HIVST programmes, which are required by public health stakeholders to understand the uptake and effectiveness.13
Strong mobile phone penetration in low- and middle-income countries (LMIC)14,15 has led to the development of a variety of mobile health (mHealth) interventions to complement HIVST. These include telephone hotlines, short message service interventions, internet-based platforms and mobile applications (apps).16,17,18,19,20 A Brazilian study conducted in 2019 showed that an internet-based intervention targeting men who have sex with men led to 21.4% of online participants self-reporting, whilst an interactive voice response telephone line in South Africa was found to link 9.8% of participants to care.21 Whilst these platforms have shown varied success, the introduction of mHealth interventions for linkage to care and M&E are in line with the South African National Department of Health mHealth Strategy (2015), and should be explored further.22
Despite data concerns in LMICs,23 recent trends are towards the development of downloadable apps due to their agility and scalability.24 The app interface also provides developers with a malleable platform that can be tailored to individual users, allowing them to curate a collection of HIVST information, resources and guidance for testers, whilst also capturing the HIVST result data.19,20 Recently, HIVSmartTM, a Canadian app, was developed to guide users through the testing process, link them to care, and store the HIVST result data. Preliminary evaluations in key Canadian populations, as well as healthcare workers in South Africa have shown the app to be feasible and acceptable; however, neither HIVSmartTM, nor any other app, has been developed or tested for the general population in LMICs.9,20,25
South Africa has shown previous acceptance of HIV-related mHealth interventions with SmartLink, an app that improved linkage to care for clinic-based HIV testing in participants under 30 years of age.26 Another successful mHealth intervention, MomConnect, has been used by over 2 million pregnant South African women with information regarding their pregnancy, whilst also creating a national pregnancy registry.27,28
The AspectTM HIVST app was developed to help strengthen and complement HIVST programmes by supporting self-testers through testing, facilitating linkage to care and digitising the reporting of HIVST results through an operational dashboard for M&E. The specific objective of this pilot study was to evaluate the acceptability and feasibility of the AspectTM HIVST app for individuals from the inner city of Johannesburg, in order to advise further scale-up. We present the findings from this pilot.
Methods
Study design
This evaluation was a cross-sectional pilot study that ran for four weeks in July 2018. A convenience sample of 300 consenting adults was recruited from inner-city Johannesburg, South Africa. Recruitment was based around the Hillbrow Health Clinic by trained healthcare workers (HCW) who went into the surrounding communities and spoke to the public about the current study. Those interested were screened against inclusion/exclusion criteria, then brought to the Hillbrow Clinic to provide consent and complete the study. Participants were included if they owned a mobile phone (feature phones, or higher, for app compatibility) and could provide a valid mobile phone number, were 18 years or older, able to read English and able to provide written informed consent. Participants were excluded if they did not meet the inclusion criteria, were currently on a pre-exposure prophylaxis (PrEP) regime or any HIV treatment medication, could not provide valid identification or had any condition that may have interfered with the testing process (such as intoxication or poor vision).
Development of the AspectTM HIV-self-testing mobile app
The AspectTM HIVST app was designed for Android and deployed by SystemOne, LLC (Northampton, MA, USA), a diagnostic connectivity and disease intelligence company. The AspectTM HIVST app was designed to be integrated with the existing AspectTM software platform, a system designed to integrate directly with diagnostic instruments in order to collect digital results for real-time monitoring and reporting via an operational dashboard. The AspectTM API can also communicate with RedCap, an existing South African healthcare database, and this application is already being used for reporting HIV viral load results and early infant HIV diagnosis (EID).
The AspectTM HIVST app was developed using Dimagi Commcare (Washington, USA), a common data-gathering platform. The app was structured to allow the self-tester to collect their own demographic information, provide the tester with instructions on how to perform self-testing, input their interpretation of the test result, and capture a photo of the HIVST strip (Figure 1). Demographic data were collected with one question per page and included the self-tester's age, gender, mobile number, education level and whether they had self-tested before. The instructions, which were developed in English, provided the tester with step-by-step guidance, presented pictorially with simple wording taken directly from the HIVST kit manufacturer's instruction sheet, so that self-testing could be performed independently of a clinical setting.
All data gathered by the app was automatically uploaded via a secure server to the AspectTM data management platform for viewing and review by the research team. Data collected in AspectTM was presented in aggregate form on a data dashboard that could be configured to display any relevant statistics for the research team. The app security was implemented with privacy by design methodology as per Protection of Personal Information (POPI) guidelines30 with patient data encrypted in transit and at rest, and also followed best practice guidelines in accordance with General Data Protection Regulation recommendations.31
Data collection
Trained HCWs obtained voluntary informed consent from the participant in a private room, then uploaded the participant's unique study identification number on the app. Once uploaded, the participant was handed a Samsung J5 smartphone, preloaded with the AspectTM HIVST app, and an accompanying HIVST kit. The sealed test kit contained an English brochure with instructions for use (IFU) as part of the standard packaging; however, the participant was requested to perform the HIVST by following the IFU included in the HIVST kit and the digital version of the IFU provided on the app. Obtaining the sample takes 5-8 min when using the IFU (either paper or digital), followed by a 20 min incubation period. The OraQuick HIVST kit (Orasure Technologies Inc., Bethlehem, USA) was used for the study as it had already undergone full evaluation and was approved for use in South Africa.32 In a private room at a clinic, participants were asked to navigate the app and perform the HIVST with no assistance, whilst the HCW observed the process and recorded any deviations from the app instructions. Following the test, the HCW asked the participant a number of questions to obtain feedback on the app design and willingness to use an app for HIVST in future.
After the 28 min test was completed, the participant returned the phone to the HCW, who then uploaded their professional interpretation of the HIVST result on the app. Regardless of the HIVST result, the HCW performed confirmatory testing using a commercial HIV rapid test (Advanced Quality, InTec Products, Inc., Xiaman, China). If the participant's self-test and HCW confirmatory tests were discordant, a third test was performed (Abon 1/2/O Tri-line, Abon Biopharm Hangzhou Co., Hangshou China). The HCW uploaded all results, as applicable, on the app for reporting purposes. Participants with HIV-positive results (based on the confirmatory testing) were referred to a clinic as per standard of care.7
Evaluation of HIV-self-testing and mobile app usage
Acceptability outcomes
The evaluation of mobile apps may provide challenges to researchers due to the nature of their varied users, objectives, interfaces and mobility.33 In many cases, app developers and researchers develop data collection tools that are app-specific, in order to explore concepts exclusive to their app.34,35 For this pilot study, a survey was developed to advise on the preliminary scale-up of the app, which looked at general acceptability and asked a set of closed-ended (yes/no) and open-ended questions, similar to the methodologies found in other mHealth app evaluations.21,36 The survey collected participant demographic information and included questions on whether the app was easy to use; which steps, if any, were difficult to understand; would they use the app again; would they be willing to download this app in the future and if they had any suggestions to improve the app. The demographic information collected by the survey and recorded by the HCW was also used to reference the accuracy of data capture on the app.
Feasibility outcomes
Similar to acceptability, there is no universal measure for determining the feasibility of an app; however, the generally accepted formula for feasibility includes three criteria: the participant's acceptance of using the app, the ability of the participant to complete tasks on the app and the ability of the app to perform the required tasks.37 These variables inevitably change based on the functionality of the app and its intended users, and for this pilot the feasibility criteria were as follows:
•User acceptance of the app: The number of participants who agreed to use the app.
•Successful test completion using the app: The number of participants who completed the testing through the app without error (i.e. experiencing difficulties or asking the HCW for assistance).
•Success of data capture through the app: The number of participants who captured their demographic information (when compared to the original records collected by the HCW), uploaded their interpreted test result and captured their test-strip images correctly.
The final feasibility score is then presented as a percentage of the final criteria.37
Data analysis
All data extracted from the survey questionnaire (paper based) were entered into an access controlled Excel spreadsheet. The quantitative data captured on AspectTM were extracted into a separate access controlled Excel spreadsheet. Quality control checks involved a 10% randomised check comparing paper-based tools against data on the spreadsheet. This was performed by the quality control officer on a daily basis. All data were coded and then exported to Stata version 15.1 (StataCorp, USA) for descriptive analysis. Data were grouped into categories to define demographic characteristics, then presented as frequency counts and percentages.
Ethical consideration
Ethics approval was obtained from the University of the Witwatersrand Human Research Ethics Committee (reference number 180504). All participants provided informed consent and were compensated ZAR150 for their time.
Results
Demographics
Of the 300 participants, over two-thirds (211; 70.3%) were younger than 36 years old, there were 134 (44.7%) female participants and 231 (77.0%) participants who were educated up to at least high school level. Only 35 (11.7%) participants indicated that they had previously self-tested. This information is presented in Table 1.
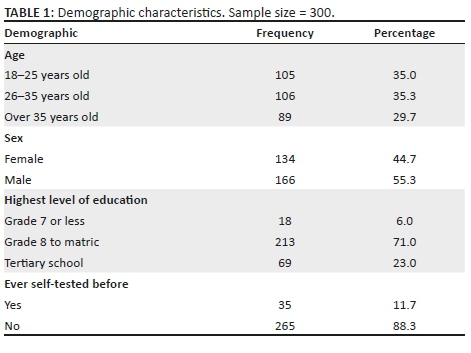
HIV test outcomes
Forty-two (14%) participants interpreted their self-test result as HIV positive; however, there were 5 (1.7%) discordant interpretations between participants and HCWs (Table 2). Three (1.0%) results were interpreted as positive by the HCW but were interpreted as either invalid (1; 0.3%) or negative (2; 0.7%) by the participant, and 2 (0.7%) results were interpreted as negative by the HCW but interpreted as either indeterminate (1; 0.3%) or positive (1; 0.3%) by the participant. Manual review of these discordant test result images, on the AspectTM dashboard by a senior researcher, confirmed the HCW interpretation in all discordances. The confirmatory testing of all participants conclusively diagnosed 43 (14.3%) as HIV positive, all of whom were referred to care by the HCW.
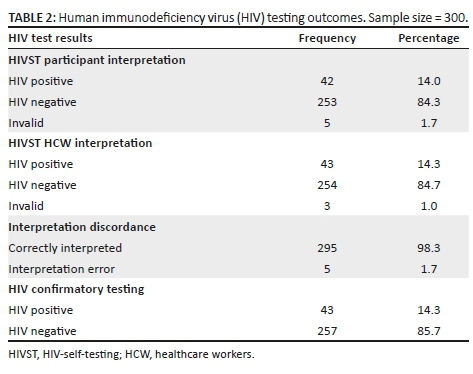
Acceptability
Nearly all participants (296/300; 98.7%) found the AspectTM HIVST app easy to use, when surveyed; however, 26 (8.7%) participants experienced some difficulty working through the testing steps as outlined in the app (Table 3). Almost all of the difficulties were related to the self-testing procedures, as 18 (6.0%) participants had difficulty sliding the tube into the stand, eight (2.7%) had difficulties swabbing their gums and three (1.0%) stated that the instructions were not clear. Another four (1.3%) participants had difficulty taking and uploading the picture of the test to the app. When asked for suggestions to make the app easier to use, five (1.7%) participants recommended that the instructions and steps be clarified, whilst four (1.3%) participants specifically suggested adding a multimedia component to the instructions. Another four (1.3%) participants suggested that the app be available in local languages and two (0.7%) participants stated that the phone memory requirements should be decreased. All but one (299/300; 99.7%) participants were willing to use the app again and only two (0.7%) participants stated that they would not be willing to download the app in the future.
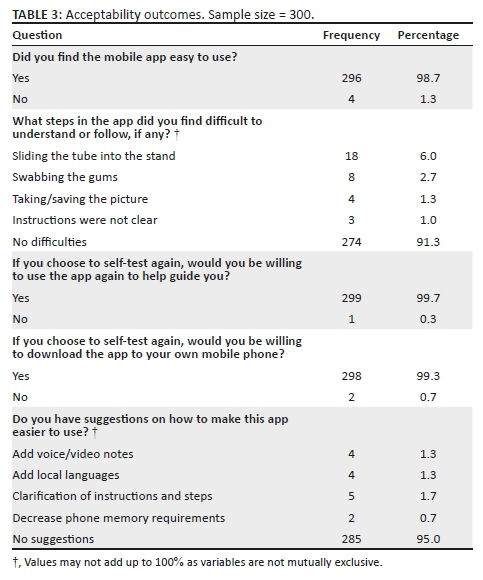
Feasibility
The final feasibility score was 70.0%. All 300 individuals approached for this study agreed to participate in the evaluation of the AspectTM HIVST app (Table 4). Of the 300 participants, 267 (89.0%) successfully completed the HIVST by following all of the steps on the app without error. The majority of errors (26; 8.7%) came from participants performing the testing procedures incorrectly, after reading the instructions on the app, which included sliding the tube into the stand (18; 6.0%) and swabbing the gums (8; 2.7%). Another four (1.3%) participants had difficulties with the language of the instructions, whilst eight (2.7%) participants made errors interpreting their HIVST results and one participant (0.3%) could not properly navigate the pages of the app.
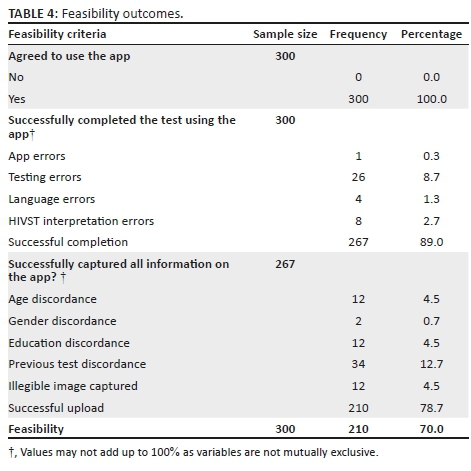
Of the 267 participants who completed the testing (Table 4), 210 (78.7%) participants successfully captured all information on the app. The most erroneous variable was previous testing history, where 34 (12.7%) participants submitted information that did not correlate with what they stated to the HCW during the survey. The variables of age and highest level of education each had 12 (4.5%) participants who exhibited discordance and there were also two (0.7%) discordances with gender compared with HCW-recorded data. Twelve (4.5%) participants also uploaded an illegible image of the HIVST strip to the app.
Discussion
This pilot study is the first investigation of an mHealth app to enhance monitoring and evaluation of HIVSTs for individuals from the inner city of Johannesburg, and the findings from this pilot have established that participants showed high acceptability of the intervention, whilst also identifying challenges that can be targeted for improvement as the platform scales up. The high acceptability was similar to that of the HIVSmartTM app and a Brazilian internet-based intervention; however, these studies only evaluated the feasibility of using the app to link patients to care or increase testing uptake, respectively.9,20,21,37
The AspectTM HIVST app, instead, aimed to guide participants through the testing process, then upload the results to a central server for M&E, and this additional layer of complexity has introduced more opportunities for user error. The majority of errors, however, were not as a result of the app functionality, but rather test usability and the IFU that guided the self-testing process. Errors stemming from the IFUs have been well documented in a number of HIVST studies, including ones from South Africa.38,39,40 Suggestions like clarifying the instructions, incorporating video or voice notes, and offering additional languages should all be taken into consideration, especially as more HIVSTs, each with specific IFUs, become available to the market. Some of these suggestions have already been implemented by other platforms, as the HIVSmartTM app is already available in both of Canada's national languages, and provides supplemental video content.20
There were a number of discrepancies between HCW-recorded and app-captured data on participant demographic information. There were also some difficulties in the uploading of the test strip photo via the app. A simple summary page, similar to that seen on a banking app, before completing a transaction, could provide the user with an opportunity to review their information before submitting it through the app. This additional checkpoint should help prevent any data entry errors. One variable, however, previous HIV testing history, had 34 (12.7%) discordant entries between what the HCW recorded and what the app captured; all 34 entries reported never having HIV tested to the HCW, but were captured in the app as having previously tested. It is possible that privacy of the app has revealed an interviewer bias, where some participants may not have felt comfortable sharing sensitive information with the HCW, but felt free to do so through the app. Previous mHealth studies have also found that self-administered tools may decrease interview bias;41 however, further evaluation of this app and its users would be required before stating that the app is responsible for removing or decreasing this interviewer bias.
Some participants also had difficulty understanding how to take a picture of the test strip. When test images were reviewed on the AspectTM dashboard, the images were quite variable in terms of quality. The purpose of this functionality was to allow a third party to manually review test images and flag potential discordant results for follow-up. However, similarly to other studies,20,42 we had high concordance between participant and HCW interpretation of the self-test and, thus, this step may not even be necessary if lay persons are able to interpret results as accurately as trained HCWs. In low bandwidth environments, the requirement to upload images may also incur additional data charges and may not be cost effective.
With the number of countries adopting HIVST policies being on the rise, the M&E of these programmes poses a unique set of challenges12 and measurement of uptake and effectiveness becomes difficult. The AspectTM HIVST app facilitated the capture of HIVST data directly to an operational dashboard, namely AspectTM. This dashboard was developed by SystemOne and is currently being used to report tuberculosis and HIV viral load results from over 3000 diagnostic instruments across 43 countries.29 For this study, the dashboard displayed very basic summary HIV statistics, a list of individual test results and also supported the downloading of automated reports. This could allow a programme manager to remotely monitor indicators such as uptake, demographics of the testing population, HIV positivity rates, invalid rates and improve reporting against key performance indicators. The functionality of the dashboard also allows for the pushing of automated SMS notifications directly to the tester based on their HIV result, which could be used to promote confirmatory testing and help link them to care.43 This is especially important for HIVST, as one of the problems with home testing is that people receiving a positive diagnosis are suddenly faced with a serious diagnosis and no immediate access to information, counselling or treatment resources.11 The feasibility of these dashboard features should be considered for future research.
Data concerns are also an important issue in South Africa, with previous mHealth studies highlighting data costs and phone memory as a barrier to entry.26,44 Future app development should focus on keeping storage requirements minimal to ensure that the app is available for as many individuals as possible. Furthermore, the necessity to upload images may also incur additional data charges and may not be affordable for all users.
Limitations
The study had several limitations. Convenience sampling from one sub-district from inner-city Johannesburg was used to recruit participants limiting the generalisability of the findings, and the compensation of participants may have accounted for the very high participation rate. Furthermore, the majority of participants were under 35 years old, which may have made it easier for them to navigate a mobile app as they may be more tech-savvy than older age groups. The AspectTM HIVST app was only available in English. It was also only tested on a Samsung phone, and it may not reflect the usability of the app on other phones owned by the general population, especially across different operating systems and memory capacities. The discordance between HCW-recorded and app-captured demographics may reflect an interviewer bias, whilst the process of testing in front of a HCW may have increased the number of forced errors due to the pressures of being observed. Performing the HIVST with the app in a clinic, with a HCW present, may also present bias, as the app is intended to be used independently of a clinic setting. Another limitation of the pilot process was that the HCWs did not record the participants' interpretation on paper and, thus, results discordance could not be verified, as was done for the other variables.
Although recent studies have introduced validated data collection tools for mHealth usability,45 at the time of this study, there were also no validated data collection tools to measure the acceptability and feasibility of mHealth apps for HIVST, hence the study-specific questions may not be used to reproduce these results in similar settings. Similarly, the use of only one HIVST kit and its accompanying IFU means that these results cannot be generalised across all HIVSTs, especially since many of the errors were related to the interpretation of the IFU.
Conclusions
With millions of HIVST kits distributed worldwide without adequate tracking, the need for M&E of these kits is ever increasing. On an individual level, this may lead to better linkage to care and follow-up with patients and, on a national level, tracking can identify areas of need to optimise kit distribution, marketing and supplementary information. Despite some challenges with IFU interpretation and data capture via the app, this pilot study has shown that the AspectTM HIVST app is an acceptable way to upload mobile HIVST results and demographic information to a central database.
Acknowledgements
The investigators would like to thank the study implementation team and the study participants.
Competing interests
N.G. works for SystemOne and was involved in the design of the Aspect HIVST app.
Authors' contributions
N.G., N.R., M.P. and M.M. designed the study. N.G., N.R. and M.P. collected data, N.G., N.R., M.P. and A.E.F. were involved in the data cleaning and analysis; A.E.F., S.T.L.-E. and N.G. wrote the initial draft of the manuscript. All authors critically reviewed and approved the final draft.
Funding information
Funding to carry out the research project has been obtained through a Gates Grand Challenges Award to SystemOne (grant number: OPP1182240) and Grand to Wits RHI received from the Bill and Melinda Gates Foundation (grant number OPP1132929).
Data availability statement
Data are available upon reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Richter M, Venter WD, Gray A. Enabling HIV self-testing in South Africa. S Afr J Med. 2016;13(4):186-187. https://doi.org/10.7196/sajhivmed.858 [ Links ]
2.Unitaid, World Health Organization. Market and technology landscape: HIV rapid diagnostic tests for self-testing. 4th ed. Geneva: Unitaid; 2018. [ Links ]
3.Wong V, Jenkins E, Ford N, Ingold H. To thine own test be true: HIV self-testing and the global reach for the undiagnosed. J Int AIDS Soc. 2019;22(S1):e25256. https://doi.org/10.1002/jia2.25256 [ Links ]
4.Figueroa C, Johnson C, Verster A, Baggaley R. Attitudes and acceptability on HIV self-testing among key populations: A literature review. AIDS Behav. 2015;19(11):1949. https://doi.org/10.1007/s10461-015-1097-8 [ Links ]
5.Makusha T, Knight L, Taegtmeyer M, et al. HIV self-testing could 'revolutionize testing in South Africa, but it has got to be done properly': Perceptions of key stakeholders. PLoS One. 2015;10(3):e0122783. https://doi.org/10.1371/journal.pone.0122783 [ Links ]
6.World Health Organization. Number of countries adopting HIV self-testing policies rises sharply [homepage on the Internet]. 2020 [updated 2017 Jul 25; [ Links ] cited 2020 Jan 15]. Available from: https://www.who.int/hiv/mediacentre/news/hiv-self-testing-increases/en/
7.National Department of Health South Africa. National HIV self screening guidelines 2018. Pretoria: Department of Health Republic of South Africa; 2018. [ Links ]
8.AIDS. Global AIDS update. UNAIDS [homepage on the Internet]. [ Links ] 2020 [cited 2020 Feb 1]. Available from: http://www.unaids.org/en/resources/documents/2017/%202017_data_book
9.Pai NP, Behlim T, Abrahams L, et al. Will an unsupervised self-testing strategy for HIV work in health care workers of South Africa? A cross sectional pilot feasibility study. PLoS One. 2013;8(11):e79772. https://doi.org/10.1371/journal.pone.0079772 [ Links ]
10.Human Sciences Research Council. The Fifth South African National HIV prevalence, incidence, behaviour and communication survey, 2017: HIV impact assessment summary report. Pretoria: Human Sciences Research Council; 2018. [ Links ]
11.Ritchwood D, Selin A, Pettifor A, et al. HIV self-testing: South African young adults' recommendations for ease of use, test kit contents, accessibility, and supportive resources. BMC Public Health. 2019;19(1):123. https://doi.org/10.1186/s12889-019-6402-4 [ Links ]
12.Venter F, Majam M, Jankelowitz L, et al. South African HIV self-testing policy and guidance considerations. Southern Afr J HIV Med. 2017;18(1):775. https://doi.org/10.4102/sajhivmed.v18i1.775 [ Links ]
13.Fionet: Mobile diagnostics integrated with cloud information services. USAID mHealth compendium, Volume 2; pp. 62-63 [homepage on the Internet]. [ Links ] 2017 [cited 2019 Nov 13]. Available from: http://www.africanstrategies4health.org/uploads/1/3/5/3/13538666/fionet_-_mobile_diagnostics.pdf
14.GSMA. The mobile economy Sub-Saharan Africa. London: GMSA; 2017. [ Links ]
15.Wedderburn CJ, Murtagh M, Toskin I, Peeling RW. Using electronic readers to monitor progress toward elimination of mother-to-child transmission of HIV and syphilis: An opinion piece. Int J Gynecol Obstet. 2015;130(S1):S81-S83. https://doi.org/10.1016/j.ijgo.2015.04.006 [ Links ]
16.Mokgatle MM, Madiba S. High acceptability of HIV self-testing among technical vocational education and training college students in Gauteng and North West Province: What are the implications for the scale up in South Africa? PLoS One. 2017;12(1):e0169765. https://doi.org/10.1371/journal.pone.0169765 [ Links ]
17.Majam M, Quaife M, Phatsoane M, et al. High self-reporting of HIV self-test results through an interactive voice response telephone line in inner city Johannesburg. Poster session presented at: IAS Conference on HIV Science, Mexico; 2019 Jul 21-24. [ Links ]
18.De Boni RB, Lentini N, Santelli AC, et al. Self-testing, communication and information technology to promote HIV diagnosis among young gay and other men who have sex with men (MSM) in Brazil. J Int AIDS Soc. 2018;21(5):e25116. https://doi.org/10.1002/jia2.25116 [ Links ]
19.Janssen R, Engel N, Esmail A, et al. Alone but supported: A qualitative study of an HIV self-testing app in an observational cohort study in South Africa. AIDS Behav. 2020;24(2):467-474. https://doi.org/10.1007/s10461-019-02516-6 [ Links ]
20.Pai PN, Smallwood M, Desjardins L, et al. An unsupervised smart app-optimized HIV self-testing program in Montreal, Canada: Cross-sectional study. J Med Internet Res. 2018;20(11):e10258. https://doi.org/10.2196/10258 [ Links ]
21.De Boni RB, Veloso VG, Fernandes NM, et al. An internet-based HIV self-testing program to increase HIV testing uptake among men who have sex with men in Brazil: Descriptive cross-sectional analysis. J Med Internet Res. 2019;21(8):e14145. https://doi.org/10.2196/14145 [ Links ]
22.National Department of Health, South Africa. mHealth Strategy 2015-2019. Pretoria: National Department of Health; 2015. [ Links ]
23.ResearchICTafrica.net. Cape Town: Research ICT Africa; 2017. State of prepaid market in South Africa: Submission to parliament of South Africa on the cost to communicate in South Africa [homepage on the Internet]. [ Links ] 2016 [cited 2019 Dec 8]. Available from: https://www.ellipsis.co.za/wpcontent/uploads/2016/09/cost_to_communicate_research_ict_africa.pdf
24.Gough A, Hunter RF, Ajao O, et al. Tweet for behavior change: Using social media for the dissemination of public health messages. JMIR Public Health Surveill. 2017;3(1):e14. https://doi.org/10.2196/publichealth.6313 [ Links ]
25.McGill University Health Centre Foundation. HIV Smart App [homepage on the Internet]. [ Links ] 2016 [cited 2020 Jan 12]. Available from: https://www.muhcfoundation.com/current-projects/hiv-smart-app/
26.Venter WDF, Fischer A, Lalla-Edward ST, et al. Improving linkage to and retention in care in newly diagnosed HIV-positive patients using smartphones in South Africa: Randomized controlled trial. JMIR Mhealth Uhealth. 2019;7(4):e12652. https://doi.org/10.2196/12652 [ Links ]
27.Seebregts C, Dane P, Parsons AN, et al. Designing for scale: Optimising the health information system architecture for mobile maternal health messaging in South Africa (MomConnect). BMJ Glob Health. 2018;3(S2):e000563. https://doi.org/10.1136/bmjgh-2017-000563 [ Links ]
28.Praekelt. MomConnect for nurses and midwives programme status report (Quarter 2) 1 April 2017-30 June. Johannesburg: Praekelt; 2017. [ Links ]
29.SystemOne. Aspect, see what matters [homepage on the Internet]. [ Links ] 2020 [cited 2020 Mar 24]. Available from: https://systemone.id/aspect
30.Republic of South Africa. Government Gazette, Act No 4 of 2013: Protection of Personal Information Act, 2013. Cape Town: The South African Government; 2013. [ Links ]
31.The EU General Data Protection Regulation (GDPR) (Regulation (EU) 2016/679). European Parliament and Council [homepage on the Internet]. [ Links ] 2016 [cited 2019 Nov 3]. Available from: http://www.eugdpr.org/
32.Ingold H, Mwerinde O, Ross AL, et al. The Self-Testing AfRica (STAR) Initiative: Accelerating global access and scale-up of HIV self-testing. J Int AIDS Soc. 2019; 22(S1):e25249. https://doi.org/10.1002/jia2.25249 [ Links ]
33.Baharuddin R, Singh D, Razali R. Usability dimensions for mobile applications - A review res. J. Appl Sci Eng Technol. 2013;5(6):2225-2231. https://doi.org/10.19026/rjaset.5.4776 [ Links ]
34.Reynoldson C, Stones C, Allsop M, et al. Assessing the quality and usability of smartphone apps for pain self-management. Pain Med. 2004;15(6):898-909. https://doi.org/10.1111/pme.12327 [ Links ]
35.Jake-Schoffman DE, Silfee VJ, Waring ME, et al. Methods for evaluating the content, usability, and efficacy of commercial mobile health apps. JMIR Mhealth Uhealth. 2017;5(12):e190. https://doi.org/10.2196/mhealth.8758 [ Links ]
36.Birkhoff S, Cantrell MA, Moriarty H, Lustig R. The usability and acceptability of a patient-centered mobile health tracking app among a sample of adult radiation oncology patients. ANS Adv Nurs Sci. 2018;(41)3:243-259. https://doi.org/10.1097/ANS.0000000000000202 [ Links ]
37.Pai NP, Bhrgava M, Joseph L, et al. Will an unsupervised self-testing strategy be feasible to operationalize in Canada? Results from a pilot study in students of a large Canadian University. AIDS Res Treat. 2014;2014(747619):1-8. https://doi.org/10.1155/2014/747619 [ Links ]
38.Simwinga M, Kumwenda MKRD, Kayira L, et al. Ability to understand and correctly follow HIV self-test kit instructions for use: Applying the cognitive interview technique in Malawi and Zambia. J Int AIDS Soc. 2019;22(S1):e25253. https://doi.org/10.1002/jia2.25253 [ Links ]
39.Smith P, Wallace M, Bekker LG. Adolescents' experience of a rapid HIV self-testing device in youth-friendly clinic settings in Cape Town South Africa: A cross-sectional community based usability study. JIAS. 2016;19(1):28406597. https://doi.org/10.7448/IAS.19.1.21111 [ Links ]
40.Majam M, Mazzola L, Rhagnath N, et al. Usability assessment of seven HIV self-test devices conducted with lay-users in Johannesburg, South Africa. PLoS One. 2020;15(1):e0227198. https://doi.org/10.1371/journal.pone.0227198 [ Links ]
41.Kim H, Xie B. Health literacy and internet- and mobile app-based health services: A systematic review of the literature. Proc Assoc Info Sci Tech. 2015;52(1):1-4. https://doi.org/10.1002/pra2.2015.145052010075 [ Links ]
42.Bwana P, Ochieng L, Mwau M. Performance and usability evaluation of the INSTI HIV self-test in Kenya for qualitative detection of antibodies to HIV. PLoS One. 2018;13(9):e0202491. https://doi.org/10.1371/journal.pone.0202491 [ Links ]
43.Siedner M, Santorino D, Lankowski A, et al. An SMS intervention to improve HIV linkage to care: A randomized, comparative effectiveness trial. 2014 Presented at: 21st Conference on Retroviruses and Opportunistic Infections; 2014 March; Boston, MA. [ Links ]
44.Fischer AE, Sebidi J, Barron P, Lalla-Edward ST. The MomConnect nurses and midwives support platform (NurseConnect): A qualitative process evaluation. JMIR Mhealth Uhealth. 2019;7(2):e11644. https://doi.org/10.2196/11644 [ Links ]
45.Zhoe L, Bao J, Setiawan MA, Saptono A, Parmanto B. The mHealth app usability questionnaire (MAUQ): Development and validation study. JMIR Mhealth Uhealth. 2019;7(4):e11500. https://doi.org/10.2196/11500 [ Links ]
 Correspondence:
Correspondence:
Alex Fischer
afischer@wrhi.ac.za
Received: 25 Mar. 2020
Accepted: 23 Apr. 2020
Published: 30 June 2020
ORIGINAL RESEARCH
Characteristics and outcomes of older people on antiretroviral therapy in Tlokwe Clinics, South Africa
Mareike Rabe; Huibrecht C. Lion-Cachet; Melaku A. Eyassu
Department of Family Medicine and Primary Care, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: South Africa (SA) has a large human immunodeficiency virus (HIV) epidemic but little is known of its effect on those ≥ 60 years of age viz. 'older-persons' living with HIV (OPLWH). Numbers in this age group are increasing and are expected to place a greater strain on existing resources.
OBJECTIVES: To describe the demographic features and the co-morbidities of OPLWH in Tlokwe. This included an assessment of viral load (VL) suppression and the identification of associations between patient characteristics and clinical outcomes
METHODS: A retrospective file review was undertaken to cover the period 01 May 2017 to 30 April 2018. Descriptive statistics were applied to demographic and clinical data and to treatment outcomes. Statistically significant associations were subjected to logistic regression analysis.
RESULTS: Of the 191 participants, 111/191 (58.1%) were female and 167/191 (87.4%) were 60 ̶70 years of age. Of the participants, 154/191 (81.9%) were virally suppressed (< 400 copies/mL). Hypertension (n = 106/191, 55.5%) was the most frequently identified co-morbidity. A CD4 cell count of ≥ 350 cells/mm3 at last assessment correlated positively with VL suppression (odds ratio 2.3, confidence interval 1.05-5.02, p = 0.037.
CONCLUSION: Although the level of VL suppression in this cohort was high, greater effort is required to bring this in line with the Joint United Nations Programme on HIV/AIDS (UNAIDS) recommendations viz. 90% viral suppression in PLWH by 2030. Further research is needed to define the evolving long-term needs of OPLWH and to facilitate entry into care of those currently not in care.
Keywords: HIV; older adults; characteristics; outcomes; antiretroviral therapy.
Introduction
The term 'older-persons' is defined by the United Nations (UN) as persons aged ≥ 60-65 years.1 This definition has been used in this study because South African citizens become eligible for an 'older-persons' government grant at 60 years of age.2 Globally, the number of people in this age group is increasing. Over the next 30 years, a growth rate of 218% of older persons, that is from 32 million in 2019 to 101 million in 2050, is predicted for sub-Saharan Africa (SSA).1 Over the same period, South Africa's (SA) older-person population is likely to increase from 8% currently, to 10.5% in 2030, and to 15.4% in 2050.3 The drivers of this change include lower fertility rates (improved access to contraception), reduced childhood mortality and better access to healthcare for all.4 The number of people living with human immunodeficiency virus (HIV) (PLWH) in SA - including older persons living with HIV (OPLWH) - will also increase. These survival gains have followed the widespread use of antiretroviral therapy (ART) and the implementation of government programmes such as Universal Test and Treat (UTT).5 For many, HIV infection has become a chronic condition.6
South African doctors are ill equipped to provide care to OPLWH, as they are not adequately trained in gerontology7 and trained geriatricians in SA tend to work in academic centres. Medical training centres in SA do not offer sub-speciality training that combines geriatrics and HIV. The lack of trained infectious disease specialists in the sphere of geriatric medicine may pose a problem in future, and primary care clinicians will likely treat these patients, as they do currently. The treatment and care OPLWH need is laborious and complex. The persistence of subclinical inflammation even when a patient is on effective ART leads to persistent immune dysfunction. This is believed to result in cardiovascular dysfunction, end-organ failure and non-AIDS defining malignancy viz. the increase of co-morbid conditions. Old age increases health risks. These include multiple pathologies: drug-drug interactions and the predisposition to drug-toxicity (renal and liver), greater likelihood of neurovascular impairment, frailty and fractures, limited immune recovery (especially CD4 cell count), less social security (finances and support) and greater dependency on the state and local health services.8 On the other hand, OPLWH are usually adherent to treatment and likely to follow up at their health care centres. They are likely to remain in their current residence and, thus, are generally contactable. In light of the above, it is necessary for researchers in SA to understand this cohort in all settings in order to develop feasible and sustainable care packages. There is a paucity of data for OPLWH in the SA context.
Identifying HIV in the elderly is important. Benefits include reducing the risk of transmission as many are still sexually active and practise poor prevention strategies. Early entry into care will reduce the risk of opportunistic conditions and aid in the timely diagnosis of co-morbidities. In the SA public sector, OPLWH have access to specialised healthcare and ART at minimal cost. If retained in-care, costs such as hospitalisation and end-of-life care are more readily anticipated and mitigated.8 Nonetheless, immune reconstitution is often incomplete in this group, despite ART and viral suppression, particularly where presenting nadir CD4 levels, were low.8
Two regional studies provide data on the prevalence and outcome of HIV infection in SA-OPLWH.9,10 A retrospective study of 7295 OPLWH (study-total n = 83 566 PLWH) who started ART between 2004 and 2013 found that those ≥ 50 years of age increased from 6% in 2004 to 10% in 2012/2013. These finally constituted 9% of the entire cohort.9 This study assessed only three of SA's nine provinces and, in particular, excluded the North West Province (NWP). A second study confirmed a significantly higher prevalence of diabetes mellitus (DM) and hypertension (HPT) in its OPLWH: DM, n = 16/262 (6.3%) versus 24/3741 (0.7%) and HPT, n = 55/262 (21.5%) versus 79/3741 (2.2%), p < 0.001, respectively.10
The prevalence of HIV infection in Tlokwe's older citizens is unknown. Those aged ≥ 65 years comprise 5.7% of the population of the town.11 Data from the 2019 mid-year population census described only the 15-49 year age group.12 In 2012, the Human Sciences Research Council (HSRC) reported the general prevalence of HIV in South Africans of ≥ 50 years to be 7.6% (95% CI 6.5-8.8).13
The objectives of this study were the following:
•To describe the demographic characteristics of the study population
•To describe their co-morbidities
•To assess viral load (VL) suppression rates
•To determine the relationship, if any, of patient characteristics to the following four outcomes viz.: VL suppression and immune (CD4 cell count) recovery on ART, loss to follow-up (LTFU) and death.
Research methods and design
Study design and study population
This was a retrospective study of OPLWH undertaken to cover the period 01 May 2017 to 30 April 2018 at three healthcare sites in Tlokwe (Potchefstroom), a town in the NWP of SA. The study subjects were aged ≥ 60 years and were on ART for a minimum of one year prior to the period under review, hence, they had to be initiated on ART prior to 30 April 2017. The three clinics were chosen to represent, as closely as possible, the diversity of people who utilise the public health service in the town.
Clinic A: a primary care clinic situated close to the municipal hospital that serves residents and patients referred from hospital clinics. Services provided by the clinic include counselling, HIV testing and the initiation of treatment of HIV. Afrikaans and Setswana are spoken by the majority of patients accessing clinic services.
Clinic B: a community health centre serving a predominantly Setswana-speaking population.
Clinic C: a community health centre serving both Afrikaans and Setswana-speaking residents.
Inclusion criteria
•Older persons living with HIV aged ≥ 60 years at their last birthday and with the time of their latest clinic visit being between 01 May 2017 and 30 April 2018.
•Older persons living with HIV who had been on ART for at least 12 months prior to the study census that is to include as a minimum a second VL test whilst on ART.14
•Confirmation as an adherence measure, that the patient or a nominated representative of the patient, had collected treatment on their behalf, at the regular three-monthly medicines collection visits in the year under study.
Exclusion criteria
•Patient clinic file 'missing' for the study period under review.
•Patients who did not meet the inclusion criteria.
Data collection
A collection sheet developed by the researcher was used to capture data as per the study's objectives. All information was captured manually and patient identifiers were removed. Data were then transferred to Excel 2016 (Microsoft, US). Study subjects comprised all available patients recruited from the study sites who fulfilled entry criteria. A prior calculation of the 'sample size' was not undertaken. All files were retrieved with the help of data capturers and clerks at the research sites. TIER.Net.,15 an electronic patient management system, was used to access additional (missing) patient information. Further laboratory data were located on the TrakCare service of the SA National Health Laboratory Services (NHLS). Some data that were more than 1 year old, were excluded as these did not represent the patient's status at the time of the study census. These data included laboratory values of creatinine, haemoglobin and total cholesterol blood results.
Data analysis
A list of study definitions can be found in Appendix 1. Stata/IC 16.0 software (STATA Corporation, LLC, TX, US 2019) was used to analyse data. For descriptive statistics numbers, percentages, medians, minimum and maximum values and interquartile ranges (IQR) were used. For evaluating associations between demographic, clinical and laboratory characteristics with four treatment outcomes viz. the most recent VL and CD4 cell count, LTFU and death, Pearson's chi-square, Fisher's exact, Mann-Whitney (Wilcoxon rank-sum), Kruskall-Wallis or Spearman's correlation tests were used where appropriate. Most values for the continuous variables were shown to be non-normally distributed as per the Shapiro-Wilk normality test. Even after logarithmic transformation, the data were non-normally distributed. All variables were initially assessed individually against the treatment outcomes. If p values were ≤ 0.05 or there were > 0.5 correlations for measures of association, these predictors were entered into a linear or logistic regression model where appropriate. A level of ≤ 0.05 was considered statistically significant. During the initial analysis, the VL and latest CD4 cell count outcomes were assessed as continuous variables as well as clinical categories. Whenever there was a statistically significant relationship between any continuous or categorical outcome in the initial analysis, it was used in the binomial logistic regression model provided that the number of observations was ≥ 10 per cell. The binomial logistic regression model categorised the VL as suppressed (< 400 copies/mL) or unsuppressed (≥ 400 copies/mL) and the latest CD4 cell as high (≥ 350 cells/mm3) or low (< 350 cells/mm3).
Ethical consideration
Ethical clearance was granted by both the Human Research Ethics Committee (Medical) of the University of the Witwatersrand, Johannesburg (M180304) and the North West Health Research Committee (NW_2018_006). The Tlokwe sub-district Office of the Primary Health Care Manager also granted permission for the research. Owing to the retrospective nature of the study, patient's anonymised data were evaluated. Consequently, informed consent was waived for this study.
Results
Data from a total of 191 clinic files of OPLWH were examined. Clinic A provided n = 38/191 (19.9%), Clinic B, n = 96/191 (50.2%) and Clinic C, n = 57/191 (29.8%). One patient file from Clinic A had to be excluded as this was a duplicate file. Eleven patient files were missing from Clinic B, as were 12 files from Clinic C. An overview of the demographic, treatment, clinical and laboratory characteristics is provided in Table 1. All participants were pensioners.
Antiretroviral therapy treatment, clinical and laboratory characteristics
Out of 157 participants for whom the ART regimens and the latest serum creatinine blood results were available, some 12/150 participants (8%) had an eGFR of ≤ 50 mL/min/1.73m3 whilst on first-line ART. Those on second-line ART showed a low eGFR in 1/7 participants (14.3%). Not a single participant with a serum creatinine of ≤ 100 µmol/L had renal dysfunction as per the chronic kidney disease (CKD)- Epidemiology Collaboration Equation (EPI) equation, whereas 13/36 (36.1%) of those with a creatinine of ≥ 100 µmol/L had renal dysfunction, according to this equation.
Twenty-nine out of 34 participants (85.3%) with an unsuppressed VL (≥ 400 copies/mL) remained on a non-nucleoside reverse transcriptase inhibitor (NNRTI)-based ART regimen, whereas 5/34 (14.7%) were on a second-line regimen with a protease inhibitor (PI) boosted with ritonavir. The PI was lopinavir in all cases. Of the participants with a VL of 400 copies/mL - 999 copies/mL, 14/16 (87.5%) were on a NNRTI first-line regimen, whereas for those with a VL of ≥ 1000 copies/mL, 15/18 (83.3%) were on a NNRTI first-line regimen.
The median CD4 cell count improved from a baseline of 279.5 cells/mm3 (IQR 167-433) to 536 cells/mm3 (IQR 337.5-703.5) at the most recent CD4 cell count monitoring visit, resulting in a median improvement of 256.5 cells/mm3. Moreover, some 33% of the cohort had a CD4 cell count of ≥ 350 cells/mm3 at baseline compared to 70.7% at the most recent CD4 cell count monitoring visit. Close to 10% of the cohort still had a CD4 cell count of < 200 cells/mm3 at the latest CD4 cell count monitoring visit. For this group, 10/19 (52.3%) participants had an unsuppressed VL of ≥ 400 cells/mL. This group also showed that 12/19 (63.2%) suffered from a defined co-morbidity. The majority of this group (n = 16/19, 84.2%) were on first-line ART.
Recent haemoglobin blood results were available for 24 participants. Of these, 4/24 (16.7%) had a haemoglobin of < 8g/dL, whilst 3/24 (12.5%) had a haemoglobin of 8 g/dL - 12 g/dL and 17/24 (70.8%) had a haemoglobin of ≥ 12g/dL. Total cholesterol blood results were available for 75 participants. Of these, 40/74 (53.3%) had readings of < 5 mmol/L and 35/74 (46.7%) had readings of ≥ 5 mmol/L. For participants who were classified with hypercholesterolaemia, 9/14 (64.3%) had recent total cholesterol readings of ≥ 5 mmol/L whereas 26/61 (42.6%) participants who were not classified to have hypercholesterolaemia had recent total cholesterol readings of ≥ 5 mmol/L.
Co-morbidities
One or more co-morbidity was found in 123/199 (64.4%) participants. Eighty-four participants (44%) had one chronic condition, a number of 34 participants (17.8%) had two chronic conditions and five participants (2.6%) had three chronic conditions. The majority of the participants (n = 106/191, 55.5%) had HPT. The following other co-morbidities were found: hypercholesterolaemia (n = 18/191, 9.4%), DM (n = 15/191, 7.9%), CKD (n = 7/191, 3.7%), a mental health problem (n = 6/191, 3.1%), asthma (n = 5/191, 2.6%), post-herpetic neuralgia (PHN) (n = 2/191, 1.0%), epilepsy and chronic obstructive airway disease (n = 1/191, 0.5%). Six participants (3.1%) had tuberculosis (TB) during the study period and five participants (2.6%) previously had TB. Renal dysfunction was found in 4/7 (57.1%) participants who were classified with CKD.
Virologic suppression
The VL results were available for all but three participants in the cohort (98.4%). Female participants showed 81.7% VL suppression rates compared to 82.3% for male participants. The overall VL suppression rate was 81.9%. Table 2 depicts the proportion of participants with VL suppression considering their most recent CD4 cell counts. Nineteen per cent of participants still had a CD4 cell count of < 350 cells/mm3, even when they were found to have VL suppression. One hundred and eleven participants (59%) had a VL of ≤ 50 copies/mL.
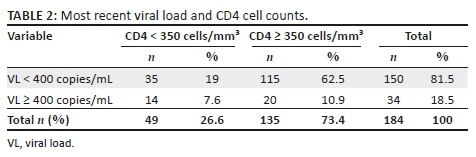
Relationships between patient characteristics and outcomes
There were no strong correlations or meaningful linear regression results amongst the most recent VL, latest CD4 cell count and other continuous variables (age, duration of ART in months, weight, baseline CD4 count, creatinine, haemoglobin and total cholesterol). During the study period, six participants were LTFU (3.1%) and four died (2.1%) with a mean age of 65.5 years at death. No statistically significant relationships were evident for the outcome LTFU. Table 3 shows the statistically significant relationships that were apparent between patient characteristics and outcomes. Hospitalisation in the past year was positively correlated with dying (50% of participants who died during the study period also had been hospitalised in that year). Moreover, participants with lower haemoglobin levels (< 8 g/dL) had a greater association with dying than those with higher haemoglobin levels where there were no mortalities. Statistically significant relationships for VL were: firstly, participants tended to have higher CD4 cell counts when their VL was suppressed and, secondly, PHN was associated with an unsuppressed VL, although only two participants in the cohort were noted to have PHN. Participants on first-line ART had 2.78 greater odds of having higher CD4 cell counts, whereas those on ART for > 5 years had 3.15 greater odds of having higher CD4 cell counts. Although not statistically significant in the logistic regression model, it is still worth noting that female participants had 2.24 times higher odds of having higher CD4 cell counts than male participants and that the odds of having a lower creatinine were 1.67 times more likely in those with higher CD4 cell counts. A high baseline CD4 cell count was associated with a high recent CD4 cell count, and current TB infection was associated with lower recent CD4 cell counts.
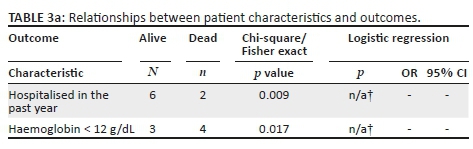
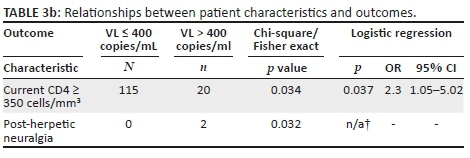
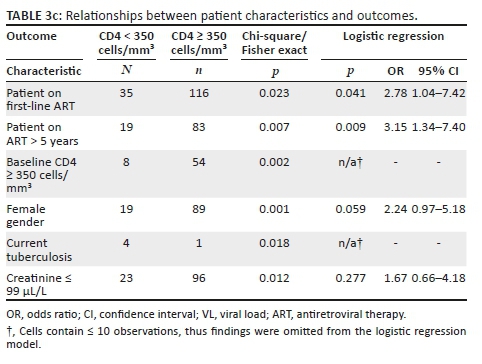
Discussion
According to our knowledge, this study was the first to describe patient characteristics and outcomes of PLWH ≥ 60 years old on ART in Tlokwe. Participants 60-69 years old and female participants comprised the largest proportion of our sample. The VL suppression rate in this cohort needs to be improved. Almost two-thirds of our sample had one or more co-morbidity. As a small proportion of participants were LTFU or had died during the study period, it is unsurprising that there were few significant relationships between these outcomes and patient characteristics. In this cohort, a suppressed VL was associated with a good CD4 cell response. A good CD4 cell response was also associated with first-line ART and longer treatment duration.
Participants 60-69 years old and female participants comprised the largest proportion of this study's sample. Female gender seemed to predominate in South African studies evaluating OPLWH from the age of 50 years old.10,16,17 Interestingly, the only other study in SSA that showed a female predominance was a recent Ugandan study.18 Studies from other countries in SSA painted a slightly different picture. Auld et al.19 pooled data from seven SSA countries. Male participants aged ≥ 50 years formed the majority of the cohort in six of the seven countries that were studied. Nigeria was the marginal exception, where 51% of participants ≥ 50 years old were female participants. A male predominance was also evident in Burkina Faso20 and Malawi.21 Both studies used the age of ≥ 50 years to define older adults.
In 2012, the SA National HIV prevalence incidence and behaviour survey reported a higher HIV prevalence in female participants aged 55-60 years, but male participants still predominated in the age categories of 50-55 years and ≥ 60 years, in contrast to our findings. In the aforementioned survey, the proportion of men who reported having more than one sexual partner was eight times that of women. Older men also reported low condom use during their last sexual encounter. These factors may explain why men are more likely to become HIV infected when they are older. On the other hand, women are known to test for HIV more readily than men and are more aware of their status, which could increase their HIV incidence. This was reiterated in the report.13 Efforts relating to HIV screening and testing, as well as condom distribution amongst older men, need to be scaled up to ensure they do not continue spreading the infection to their older and younger female sexual partners. Another intervention strategy could include a mass medical male circumcision campaign, aimed specifically at older men.
The majority of this study cohort had been on ART for ≤ 10 years. It was evident from the file reviews that changing treatment guidelines over the years has had an influence on ART initiation in this population, as they were more likely to be initiated in line with their CD4 cell count values at a later stage of infection. Dates of HIV diagnoses were unavailable for many participants, which made it impossible to report on the timeline from receiving a diagnosis of HIV and initiating treatment. In the era of UTT, ART should be initiated as soon as possible for all those who qualify for it, especially in OPLWH. This could potentially negate a poor immune reconstitution effort and improve the overall clinical status for OPLWH. Moreover, this could limit the spread of the disease to their sexual partners. This may also empower them to learn more about the disease and educate their families about it.
It may be challenging to comment on the current ART regimen of this cohort, as it was unclear if and when regimens were changed from a stavudine-based regimen for those taking treatment for longer, as per evolving guidelines. It was clear, however, that an NNRTI-based regimen was the most common treatment regimen prescribed for these participants in line with guidelines used at the time. The tenofovir component was not included in 16.2% of participants' ART and 6.8% of the cohort had renal dysfunction. Only 3.7% of the cohort was classified with CKD. One explanation for the low prevalence of CKD may be that renal function improved when tenofovir was substituted with an agent that is known to be renal protective, and these participants were subsequently not classified as having CKD, but this does not explain the higher percentage of renal dysfunction in this cohort. Another explanation may be that primary care clinicians simply did not diagnose or document CKD in this cohort. The prevalence of CKD was one in five PLWH ≥ 60 years old in a study conducted in 12 French hospitals and was independently associated with an increased 5-year mortality.22 These findings may warrant more rigorous screening and documentation of kidney function in our setting, especially in light of the fact that CKD is a known risk factor for adverse cardiovascular outcomes.23
Knowing the nutritional status of a patient might affect 21% - 82% of treatment decisions,24 with poor nutritional status strongly associated with mortality.25 The presence of anaemia and poor nutritional status are interconnected, and in this cohort the presence of anaemia was associated with the risk of dying. Unfortunately, height and thus body mass index (BMI) and/or mid-upper arm circumference (MUAC) measurements were not routinely measured in the care of this study's participants. Haemoglobin levels were also not readily available for this cohort. The weight (median and IQR) nevertheless correlated well with that of participants in another South African study.9 Nutritional status is one of the components that needs to be assessed in a comprehensive geriatric assessment (CGA). This tool includes 11 components that address biomedical, social and economic concerns for HIV care providers relating to OPLWH.24 Recent hospitalisation is a known risk factor for dying in OPLWH, and this was echoed in this study. A meta-analysis showed that those who underwent a CGA whilst hospitalised were more likely to be alive after 12 months than those who did not.25 An explanation for this may be that the teams who performed CGA were more experienced and specialised than the teams who typically worked on the wards. Long-term follow-up also appeared to be more comprehensive in the participants who underwent CGA in the hospital setting. This is a novel tool in HIV care; it has been used successfully in other disciplines and may prove to be a crucial tool in the HIV care sphere pertaining to the ageing population in years to come.26 Hence, there are ample reasons for incorporating anthropometric measures, including those related to nutritional status and anaemia, into the clinical guide for the care of OPLWH. However, clinicians in better resourced settings are struggling to adhere to CGA recommendations24 and its rollout to resource-constrained settings may come with challenges, such as lack of experience with the tool, time and resource limitations and insufficient evidence for its effectiveness in African settings.
Even in this reasonably small cohort, the prevalence of HPT and DM were high compared with other studies.9,17 In these studies, the prevalence of HPT ranged from 21.5% - 33.3%, the latter percentage being for participants aged ≥ 70 years. In our study population, there were 55.5% of OPLWH on treatment for HPT. The differences may be due to the other studies actively measuring participants' blood pressure at ART initiation, whereas the current study relied on clinical records and could not account for what might have come first: HIV or HPT. The same could be said about DM, where the prevalence ranged from 2.2% - 6.3% in the other cohorts, compared to 7.9% in the current cohort. Again, conclusive interpretation of these results is elusive because the current study inception was not at ART initiation. It is well established that ART and ageing both accelerate cardiovascular disease risk in older adults.8 Added to the cardiovascular disease burden of HPT, DM and CKD, it stands to reason that older adults require tailor-made interventions to address their cardiovascular health. The administration of a novel polypill (including a statin, aspirin and anti-HPT medications) is a potential option to reduce cardiovascular and cancer morbidity and mortality in OPLWH.8
The VL suppression in this cohort was lower than previously reported in SA.9,10,16 Moreover, VL suppression was attained in 86% - 89.5% of older adults in other settings after 12-60 months of treatment. It was perturbing that only 81.9% of this cohort had a suppressed VL, especially as a larger proportion of them were on second-line treatment than found in another South African cohort (5.2% versus 0.88)16; and female participants, who are historically more adherent to treatment, had lower VL suppression rates. Only 5.2% of the cohort were on second-line ART. The rest were still on a NNRTI-based regimen. Moreover, over 85% of those with an unsuppressed VL were still on an NNRTI-based regimen. HIV treatment and monitoring guidelines in SA have changed since this study was conducted. The new guidelines define a suppressed VL as < 50 copies/ml.27 If these criteria were used to assess VL suppression, only 59% of this cohort would be suppressed. Although high-range low-level viraemia (VL 400 copies/mL - 999 copies/mL) in this cohort was low (8.4%), a previous study performed in SA showed a five-times increased risk of virological failure in participants who had VL readings in this range.28 It is imperative to monitor these patients closely and act appropriately if and when true virological failure (VL ≥ 1000 copies/mL) and attenuated CD4 cells count responses develop. Appropriate action, that is changing to a second-line ART regimen, seemed to be lacking in this cohort. There was, likely, treatment failure and the development of resistance to ART in this cohort. This may hamper the 90-90-90 UNAIDS target of achieving a 90% VL suppression rate in all age groups globally by 2030. South Africa currently stands at 87% VL suppression in 54% of all PLWH.29 More attention should be given to OPLWH in order to attain the UNAIDS goals and ensure their overall well-being and prevent the spread of drug-resistant HIV.
Malaza et al.30 found that the median CD4 cell count in OPLWH was 367 cells/mm3 after a median duration of 2.3 years on ART. Similarly, Fatti et al.16 found that OPLWH had a median CD4 cell count of 377 cells/mm3 after 3 years on ART. The median CD4 cell count increased by 256.5 cells/mm3 from the baseline in this cohort. Fatti et al.16 also found that the median CD4 cell count increased from about 100 cells/mm3 after 6 months to over 300 cells/mm3 after 48 months, since ART initiation. It is well-established that CD4 cell recovery is attenuated in OPLWH compared to younger PLWH.16,30 In this study, it was evident that higher recent CD4 cell counts were more likely in those who had been taking ART for > 5 years (OR = 3.15, 95% CI 1.34-7.40, p = 0.009) and those on the usual first-line ART (OR = 2.78, 95% CI 1.04-7.42, p = 0.041). The former may have been an obvious finding because of improved immune reconstitution, the sooner a patient is initiated on ART,8 but further work may be needed to evaluate the latter.
Strengths and limitations
Most previous studies in the field of OPLWH have defined them to be aged ≥ 50 years. However, seeing as assumptions of life expectancy without HIV and total fertility rates were estimated to be 61.5 years for males and 67.7 years for females in 2019,13 it was imperative to assess older age groups and exclude those still eligible to work for future planning of care interventions for these individuals. This is one of the first local studies to use this novel approach. Also, a paucity of data was available on the subject in the NWP according to the researcher's knowledge, and this is the first study to begin to address this gap. Most previous studies published about OPLWH have compared their response since ART initiation to younger cohorts. The current study did not address this. The sample was chosen conveniently and thus could have introduced selection bias. Also, the retrospective design has disadvantages. Previous prospective studies on the subject could compare baseline characteristics and outcomes in terms of ART initiation and follow-up. This approach was not possible in the current study due to time constraints in terms of data collection, and there were many missing data (e.g. files and blood results) which could have influenced study findings. The sample size was small and this could perhaps be seen as a pilot study for future studies in OPLWH in the NWP. Generalisability was affected by limited patient numbers and the exclusion of a more rural cohort. Unmeasured covariates could have resulted in hidden bias.
Conclusion
To our knowledge, this was the first study to assess characteristics and outcomes in older PLWH in the NWP. Older men and women living with HIV need to be approached using different strategies, tailored to their specific needs. There is an urgent need for South African clinicians to consider the implementation of standardised CGA in all OPLWH, as this has the potential to improve outcomes of older patients after hospital admission. Chronic kidney disease needs to be actively excluded in OPLWH as it has major clinical implications, especially in the context of a high burden of other co-morbidities and high cardiovascular morbidity and mortality. Considering the study limitations, further prospective studies with larger samples are necessary in the NWP to confirm or refute the findings of low VL suppression rates in OPLWH.
Acknowledgements
The authors would like to thank Professor Olufemi Omole for his valuable contribution to the final draft of the article.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
This article was submitted by M.R as a requirement for partial fulfilment of the MMed (Family Medicine). M.R. was the main author. C.L.-C. was the primary supervisor and M.E. the secondary supervisor. All authors commented on drafts of the article and approved the final version to be published.
Funding information
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.UN. World Population Ageing 2019: Highlights (ST/ESA/SER.A/430) [homepage on the Internet]. Department of Economic and Social Affairs, Population Division, New York: The United Nations; 2019 [cited 2019 Dec 03]. [ Links ] Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf
2.SASSA. Grants for older persons [homepage on the Internet]. South African Department of Social Development; n.d. [ Links ] [cited 2019 Oct 23]. Available from: https://www.sassa.gov.za/Pages/Older-Persons-Grant.aspx
3.UN. World Population Ageing 2015 (ST/ESA/SER.A/390) [homepage on the Internet]. [ Links ] Department of Economic and Social Affairs, Population Division, New York: The United Nations; 2015 [cited 2019 Dec 03]. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf
4.WHO. World report on ageing and health [homepage on the Internet]. Geneva: World Health Organisation; 2015 [cited 2019 Dec 03]. [ Links ] Available from: https://apps.who.int/iris/bitstream/handle/10665/186463/9789240694811_eng.pdf;jsession id=564128D768AA8195EA13923FA66A80CB?sequence=1
5.NDoH. Implementation of the universal test and treat strategy for HIV positive patients and differentiated care for stable patients [homepage on the Internet]. Pretoria: South African National Department of Health; 2016 [cited 2019 Mar 03]. [ Links ] Available from: https://www.sahivsoc.org/Files/22%208%2016%20Circular%20UTT%20%20%20Dec ongestion%20CCMT%20Directorate%20(2).pdf
6.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet. 2013;382(9903):1525-1533. https://doi.org/10.1016/S0140-6736(13)61809-7 [ Links ]
7.Tanyi PL, Pelser A. The missing link: Finding space for gerontology content into university curricula in South Africa. Gerontol Geriatr Educ. 2018;40(4):491-507. https://doi.org/10.1080/02701960.2018.14285799 [ Links ]
8.Bendavid E, Ford N, Mills EJ. HIV and Africa's elderly: The problems and possibilities. AIDS. 2012;26(Suppl 1):S85-S91. https://doi.org/10.1097/QAD.0b013e3283558513 [ Links ]
9.Cornell M, Johnson LF, Schomaker M, et al. Age in antiretroviral therapy programmes in South Africa: A multicentre observational cohort study. Lancet HIV. 2015;2(9):pe368-e375. https://doi.org/10.1016/S2352-3018(15)00113-7 [ Links ]
10.Dawood H, Hassan-Moosa R, Zuma N-Y, et al. Mortality and treatment response amongst HIV-infected patients 50 years and older accessing antiretroviral services in South Africa. BMC Infect Dis. 2018;18(168):1-9. https://doi.org/10.1186/s12879-018-3083-z [ Links ]
11.STATSSA. Statistics South Africa. Tlokwe city council [homepage on the Internet]. [ Links ] Department Statistics South Africa; no date [cited 2019 Oct 23]. Available from: https://www.statssa.gov.za/?page_id=993&id=tlokwe-city-council-municipality
12.STATSSA. Mid-year population estimates [homepage on the Internet]. [ Links ] Department Statistics South Africa; 2019 [cited 2019 Oct 22]. Available from: https://www.statssa.gov.za/publications/P0302/P03022019.pdf
13.Shisana O, Rehle T, Simbayi L, et al. South African National HIV prevalence, incidence and behaviour survey, 2012. Cape Town: HSRC Press; 2014. [ Links ]
14.NDoH. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults [homepage on the Internet]. Republic of South Africa: South African Department of Health; 2015 [cited 2019 Dec 05]. [ Links ] Available from: https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf
15.Osler M, Hilderbrand K, Hennessey C, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc. 2014;17(1):18908. https://doi.org/10.7448/IAS.17.1.18908 [ Links ]
16.Fatti G, Mothibi E, Meintjies G, Grimwood A. Antiretroviral treatment outcomes amongst older adults in a large multicentre cohort in South Africa. PLoS One. 2014;9(6):e100273. https://doi.org/10.1371/journal.pone.0100273 [ Links ]
17.Butler I, MacLeod W, Majuba PP, Tipping B. Human immunodeficiency virus infection and older adults: A retrospective single-site cohort study from Johannesburg, South Africa. S Afr J HIV Med. 2018;19(1):e838. https://doi.org/10.4102/sajhivmed.v19i1.838 [ Links ]
18.Mugisha JO, Schatz EJ, Randell M, et al. Chronic disease, risk factors and disability in adults aged 50 and above living with and without HIV: Findings from the wellbeing of older people study in Uganda. Glob Health Action. 2016;9(1):31098. https://doi.org/10.3402/gha.v9.31098 [ Links ]
19.Auld AF, Agolory SG, Shirashi RW, et al. Antiretroviral therapy enrolment characteristics and outcomes among HIV-infected adolescents and young adults compared with older adults: Seven African countries, 2004 - 2013. MMWR Morb Mortal Wkly Rep [serial online]. 2014 [cited 2019 Dec 12];63(47):1097-1103. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5779521/
20.Diallo I, Meda N, Quédraogo S, et al. Profiles of elderly people infected with HIV and response to antiretroviral treatment in Burkina Faso: A retrospective cohort study. J Int Assoc Provid AIDS Care. 2017;16(4):405-411. https://doi.org/10.1177/2325957417709088 [ Links ]
21.Tweya H, Feldacker C, Heller T, et al. Characteristics and outcomes of older HIV-infected patients receiving antiretroviral therapy in Malawi: A retrospective observation cohort study. PLoS One. 2017;12(7):e0180232. https://doi.org/10.1371/journal.pone.0180232 [ Links ]
22.Hentzien M, Dramé M, Allavena C, et al. Impact of age-related comorbidities in five-year overall mortality among elderly HIV-infected patients in the late HAART era - role of chronic renal disease. J Nutr Health Aging. 2016;20(4):408-414. https://doi.org.10.1007/s12603-015-0608-7 [ Links ]
23.Davids MR, Chothia MY. Chronic kidney disease for the primary care clinician. S Afr Fam Pract [serial online]. 2019 [cited 2019 Dec 11];61(5):19-23. Available from: https://safpj.co.za/index.php/safpj/article/view/4941/5837
24.Bitas C, Jones S, Singh HK, et al. Adherence to recommendations from comprehensive geriatric assessment of older individuals with HIV. J Int Assoc Provid AIDS Care. 2019;18:1-8. https://doi.org/10.1177/2325958218821656 [ Links ]
25.Ellis G, Whitehead MA, O'Neil D, Ramirez M, Siegler E, Glesby M. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev 2011;(7):CD006211. https://doi.org/10.1002/14651858.CD006211.pub2 [ Links ]
26.Singh HK, Carmen TD, Freeman R, Glesby MJ, Siegler EL. From one syndrome to many: Incorporating geriatric consultation into HIV care. Clin Infect Dis. 2017;65(3):501-506. https://doi.org/10.1093/cid/cix311 [ Links ]
27.DOH e-Library. 2019 ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates [homepage on the Internet]. Department of Health; 2019 [cited 2019 Dec 11]. [ Links ] Available from: https://www.knowledgehub.org.za/elibrary/2019-art-clinical-guidelines-management-hiv-adults-pregnancy-adolescents-children-infants
28.Hermans LE, Moorhouse M, Carmona S, et al. Effect of HIV-1 low level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: A multicentre cohort study. Lancet Infect Dis. 2018;18(2):188-197. https://doi.org/10.1016/s1473-3099(17)30681-3 [ Links ]
29.Avert. South Africa 90-90-90 progress [homepage on the Internet]. [ Links ] 2019 [cited 2019 Dec 12]. Available: https://www.avert.org/infographics/south-africa-90-90-90-progress-2019
30.Malaza A, Mossong J, Bärnighausen T, Johannes V, Newell M-L. Population-based CD4 counts in a rural area in South Africa with high HIV prevalence and high antiretroviral treatment coverage. PLoS One. 2013;8(7):e70126. https://doi.org/10.1371/journal.pone.0070126 [ Links ]
 Correspondence:
Correspondence:
Mareike Rabe
mareikerabe@gmail.com
Received: 06 Jan. 2020
Accepted: 28 Apr. 2020
Published: 07 July 2020
^rND^sDeeks^nSG^rND^sLewin^nSR^rND^sHavlir^nDV^rND^sTanyi^nPL^rND^sPelser^nA^rND^sBendavid^nE^rND^sFord^nN^rND^sMills^nEJ^rND^sCornell^nM^rND^sJohnson^nLF^rND^sSchomaker^nM^rND^sDawood^nH^rND^sHassan-Moosa^nR^rND^sZuma^nN-Y^rND^sOsler^nM^rND^sHilderbrand^nK^rND^sHennessey^nC^rND^sFatti^nG^rND^sMothibi^nE^rND^sMeintjies^nG^rND^sGrimwood^nA^rND^sButler^nI^rND^sMacLeod^nW^rND^sMajuba^nPP^rND^sTipping^nB^rND^sMugisha^nJO^rND^sSchatz^nEJ^rND^sRandell^nM^rND^sAuld^nAF^rND^sAgolory^nSG^rND^sShirashi^nRW^rND^sDiallo^nI^rND^sMeda^nN^rND^sQuédraogo^nS^rND^sTweya^nH^rND^sFeldacker^nC^rND^sHeller^nT^rND^sHentzien^nM^rND^sDramé^nM^rND^sAllavena^nC^rND^sDavids^nMR^rND^sChothia^nMY^rND^sBitas^nC^rND^sJones^nS^rND^sSingh^nHK^rND^sEllis^nG^rND^sWhitehead^nMA^rND^sO'Neil^nD^rND^sRamirez^nM^rND^sSiegler^nE^rND^sGlesby^nM^rND^sSingh^nHK^rND^sCarmen^nTD^rND^sFreeman^nR^rND^sGlesby^nMJ^rND^sSiegler^nEL^rND^sHermans^nLE^rND^sMoorhouse^nM^rND^sCarmona^nS^rND^sMalaza^nA^rND^sMossong^nJ^rND^sBärnighausen^nT^rND^sJohannes^nV^rND^sNewell^nM-L^rND^1A01^nAhmed^sCordie^rND^1A02^nMenna-t-allah^sEl-Kotamy^rND^1A01^nGamal^sEsmat^rND^1A01^nAhmed^sCordie^rND^1A02^nMenna-t-allah^sEl-Kotamy^rND^1A01^nGamal^sEsmat^rND^1A01^nAhmed^sCordie^rND^1A02^nMenna-t-allah^sEl-Kotamy^rND^1A01^nGamal^sEsmat•Previous defaulter: Past single or serial failure by the patient to collect his or her treatment as documented in the clinical record or on TIER.Net.
•Ward-based outreach team (WBOT): A clinic-based team that collected treatment on the patient's behalf at any time during the study period. A patient could thus be categorised in one, two or three categories in terms of treatment collection during the study period.
•Transfer/move out as per clinical record or TIER.Net: A distinction was made about whether or not the new treatment collection site was still the primary care facility or at a higher level of care.
•Co-morbidity as documented on ART stationery: If the co-morbidity was unstated but treatment was prescribed during the period under review at any time, the patient was considered as having the related co-morbidity.
•Recent hospitalisation: A discharge summary spanning the previous 12 months recorded in the patient file or specified in clinical notes.
•Suppressed viral load: A reading of < 400 copies/mL.
•Adequate immunological response: A CD4 cell count of ≥ 350 cells/mm3 within 1 year of the data census being documented in the clinical record.
•Loss to follow-up: The patient's treatment was not collected within 3 months (90 days) of the latest (last) study visit at the study census and the patient could not be traced either telephonically or at his or her place of residence by community health workers during data collection period.
•Died: The patient's date of death was documented in the clinical records or on TIER.Net.
EDITORIAL
Antiretroviral therapy optimisation in the time of COVID-19: Is it really different in North and South Africa?
Ahmed CordieI; Menna-t-allah El-KotamyII; Gamal EsmatI
IEndemic Medicine Department, Cairo University Hospitals, Cairo, Egypt
IIEgyptian Patent Office, Academy of Scientific Research and Technology, Cairo, Egypt
Dear editors, we are attentively pursuing calls for an urgent need to have global and national actions to adopt differentiated service delivery (DSD) to ensure continuity of human immunodeficiency virus (HIV) services, especially uninterrupted antiretroviral therapy (ART) supply, during the COVID-19 (Coronavirus Disease 2019) pandemic.1 Location modification, longer refill times and tailored packages of clinical services, including ART optimisation, are the main pillars of the transition toward DSD.2
However, these interventions alone are not sufficient in low- to middle-income countries (LMICs), where the lockdown and restrictions applied to international movement may affect ART supply, especially for imported medications. Medicine stock-outs are an unfortunate possibility for treatment discontinuation and the emergence of drug resistance.3
Following the World Health Organization (WHO) recommendations, the Egyptian guidelines set the combination of tenofovir disoproxil fumarate (TDF) and emtricitabine (FTC) plus dolutegravir (DTG) as the preferred first-line treatment regimen for adults and TDF/FTC/efavirenz (EFV) as an alternative first-line regimen.4,5
Egypt is reported to have very low HIV prevalence; however, it has the fastest increasing epidemic in the Middle East and North African regions.6,7 The Medicines Policy and Standards (PSM) system can guarantee uninterrupted ART supply when the framework cycle is properly functioning at all levels of the healthcare system. Unfortunately, LMICs usually have underdeveloped PSMs, and hence face the risk of stock-outs.3
During the COVID-19 crisis, the situation is expected to be more complicated; therefore, resilience is needed to enable the health system to follow the WHO treatment recommendation guidelines and keep treatment for all as a first priority,4 at the same time as the context is rapidly evolving. Therefore, ART included in national treatment regimens may need to be locally manufactured for the time being.
Our search revealed that FTC and EFV are not patented in Egypt, and the only patent on lamivudine (3TC) has expired. Tenofovir disoproxil fumarate, 3TC and EFV are all locally manufactured and available at an affordable price. Dolutegravir (a ViiV product), provided under voluntary license in South Africa, is part of a patent filed in Egypt that was technically rejected and is still under appeal; however, it is also provided under voluntary license. Gilead Sciences have patents on TDF/FTC in a number of countries, but not in Egypt or South Africa.8
Neither DTG nor the TDF/FTC 2-in-1 combination is locally manufactured in Egypt. In these critical times, local pharmaceutical companies should be encouraged to produce these medicines to avoid dependence on the originators, who do not even have patent rights in Egypt.
Currently, Egypt and many LMICs are in an extraordinary situation. These desperate times demand extraordinary measures. With respect to the WHO first-line ART recommendation for adults during the COVID-19 crisis, to ensure stable ART supply, we recommend the following:
•Antiretroviral therapy-naïve patients should start locally produced TDF + 3TC + EFV, as this stock may be less threatened than the TDF/FTC 2-in-1 combination and DTG-based regimens.
•In contradiction to what is recommended in South Africa and many sub-Saharan countries, we recommend slowing down the transition from EFV to DTG to save it for those already using it.
•In the case of stock-outs, virally suppressed patients on the TDF/FTC 2-in-1 combination-based regimen can be switched safely to locally produced TDF + 3TC, as the available evidence confirms the interchangeability between FTC and 3TC.9 Healthcare workers can follow ART-switching guidelines that can be applied by means of telemedicine.10
We should highlight that increased pill burden (four instead of two pills) is the main limitation of this recommended locally manufactured regimen. However, the priority under the current circumstances is to ensure uninterrupted ART supply.
Acknowledgements
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
All authors contributed equally to this work.
Ethical consideration
This article followed all ethical standards for a research without direct contact with human or animal subjects.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Wilkinson L, Grimard A. The time is now: Expedited HIV differentiated service delivery during the COVID-19 pandemic. J Int AIDS Soc. 2020;23(5):e25503. https://doi.org/10.1002/jia2.25503 [ Links ]
2.World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Recommendations for a public health approach [homepage on the Internet]. [ Links ] Geneva: WHO; 2016 [cited 15 June 2020]. Available from: https://www.who.int/hiv/pub/arv/arv-2016/en/
3.World Health Organization. Access to antiretroviral drugs in low- and middle-income countries: Technical report [homepage on the Internet]. [ Links ] Geneva: WHO; 2014 [cited 15 June 2020]. Available from: https://www.who.int/hiv/pub/amds/access-arv-2014/en/
4.World Health Organization. Update of recommendations on first- and second-line antiretroviral regimens. Policy brief [homepage on the Internet]. [ Links ] Geneva: WHO; 2019 [cited 15 June 2020]. Available from: https://apps.who.int/iris/bitstream/handle/10665/325892/WHO-CDS-HIV-19.15-eng.pdf
5.National AIDS Program in Egypt. Antiretroviral guidelines for adults with HIV: Update. Cairo: Egyptian Ministry of Health, 2017; p. 91-94. [ Links ]
6.A map showing the distribution of the number of PLHIV [homepage on the Internet] [cited 15 June 2020]. [ Links ] Available from: http://aidsinfo.unaids.org/
7.UNAIDS. Miles to go - closing gaps, breaking barriers, righting injustices: Global AIDS update 2018 [homepage on the Internet]. [ Links ] Geneva: UNAIDS, 2018; p. 233. Available from: https://www.unaids.org/en/resources/documents/2018/global-aids-update
8.Checking the validity of license status and information regarding the patent of DTG and TDF/FTC combination [homepage on the Internet] [cited 15 June 2020]. [ Links ] Available from: https://www.medspal.org/?country_name%5B%5D=Egypt&product_standardized_name%5B%5D=Dol utegravir+50+mg&product_standardized_ name%5B%5D=Tenofovir%2FEmtricitabine+300%2F200+mg&page=1
9.World Health Organization. Appropriate medicines: Options for pre-exposure prophylaxis [homepage on the Internet]. [ Links ] Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO [cited 15 June 2020]. Available from: https://www.who.int/hiv/pub/journal_articles/3tc-ftc-interchangeable/en/
10.US DHHS. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents [homepage on the Internet]. [ Links ] 2019 [cited 15 June 2020]. Available from: http://aidsinfo.nih.gov/guidelines
 Correspondence:
Correspondence:
Ahmed Cordie
ahmedcordie@gmail.com
CASE REPORTS
Paediatric antiretroviral overdose: A case report from a resource-poor area
Bryan A. OgotiI; Angela A. OtedoII; Thomas M. ChokweI
IDepartment of Anaesthesia, Faculty of Medicine, University of Nairobi, Nairobi, Kenya
IIDepartment of Medical Services, Avenue Healthcare, Nairobi, Kenya
ABSTRACT
INTRODUCTION: Toxic side effects from antiretroviral overdose in children have not been widely reported. Antiretroviral drugs are widely used as oral medications throughout sub-Saharan Africa.
PATIENT PRESENTATION: We describe the clinical presentation and management of a 3-year-old male in rural Kenya, who accidentally overdosed on abacavir/lamivudine combination pills. The number of pills taken was approximately 250 tablets, that is 15 g of abacavir and 7.5 g of lamivudine. He presented 24 hours later to Homabay County Referral Hospital, with unresponsiveness, inability to feed and absence of playfulness. Physical examination revealed a sick-looking, 'unconscious' child, responding only to voice, with tachycardia, hypertension and moderate dehydration.
MANAGEMENT AND OUTCOME: He was managed conservatively with rehydration, namely intravenous 1125 mL of 5% dextrose in 0.9% saline, and the monitoring of his neurologic status, urine output and all vital signs. He regained normal neurological function after 24 hours, and recovered uneventfully, but was lost to follow-up.
CONCLUSION: In an area endemic for HIV and where antiretroviral drug use is commonplace, there is a need for health education to ensure that parents keep drugs out of the reach of children. In the case of a suspected overdose, parents need to be reminded to seek medical attention immediately. Physician awareness of the clinical presentation, management and challenges with an antiretroviral drug overdose is also important
Keywords: antiretroviral; abacavir; lamivudine; poisoning; overdose; paediatrics; resource-poor.
Introduction
Antiretroviral therapy has changed infection with HIV from a fatal illness to one that is manageable.1 According to the UNAIDS report of 2019, 61% of new HIV infections are in sub-Saharan Africa where the incidence in Kenya is 1.02 per 1000 persons, and the overall prevalence is 4.7%. Antiretroviral drugs are used by 91% of pregnant Kenyan women living with HIV, 61% of infected children are on treatment and the number of AIDS-related deaths is now declining.2
Despite the widespread use of antiretroviral drugs (ARVs), paediatric overdose-related toxicity remains unreported in resource-poor settings. In 2018, Van Dam et al. described an adult parasuicide with dolutegravir/abacavir/lamivudine that resulted in lactic acidosis and hypokalemia. This was managed with intravenous fluids, potassium supplementation, the monitoring of drug levels and blood chemistries.3 A second adult parasuicide-overdose with dolutegravir/tenofovir/emtricitabine has also been reported.4 In this report the patient had a minor airway obstruction and required monitoring in an intensive care unit.
Abacavir and lamivudine are ARVs that are well absorbed orally.5 Abacavir is metabolised in the liver, but lamivudine is excreted unchanged in urine. Nausea, vomiting, diarrhoea, cough, fever and fatigue are side effects of both drugs.5,6
We report a case of suspected abacavir/lamivudine toxicity managed conservatively in a resource-poor setting. We highlight the scarcity of information and the challenges faced.
Case report
A 3-year-old male from rural Kenya, weighing 12.5 kg, was admitted to the paediatric ward of the Homabay County Referral Hospital. This was 24 hours after ingesting four and a half bottles of abacavir 600 mg/lamivudine 300 mg combination pills prescribed for his stepbrother. Each bottle contained 60 tablets, that is, a total of 250 tablets or 15 g of abacavir and 7.5 g of lamivudine. He had been playing unattended when he ingested the tablets and afterwards appeared well and as playful as usual. He did not have a fever, rash, diarrhoea, vomiting or difficulty with breathing that would have suggested an abacavir hypersensitivity reaction. There was no report of confusion, drowsiness or seizures. He ate and slept well but the next morning was drowsy, unable to walk and feed.
He arrived looking sick, lethargic and responding only to voice commands. His airway was intact and breathing and circulation were normal. There was no pallor, jaundice, cyanosis or oedema. The eyes were sunken and the skin turgor was reduced suggesting moderate dehydration. He had a tachycardia of 156/min, a respiratory rate of 27/min, SPaO2 of 99% - 100%, an elevated blood pressure of 131/78 mmHg. His temperature was 37.1°C. Physical examination was unremarkable and all systems were normal. Blood glucose and full hemogram were normal. His HIV test was negative. Serum drug levels, renal and other metabolic tests were unavailable.
The child was admitted to the acute room and re-hydrated as per the Holliday-Segar method, with 1125 mL of dextrose 5% in 0.9% sodium chloride over 24 h. Vital signs and urine output were monitored hourly. Activated charcoal and gastric lavage were omitted because of the late presentation. There was improvement within 24 h. He became ambulant, fed orally and his urine output normalised. He was discharged after 24 hours of observation.
The child's mother was counselled and advised on the safe handling of household poisons.The stepbrother's prescription was refilled. She was single, uneducated and unemployed. She avoided discussions around paternity, marital status and HIV status. She provided no reliable contacts and never returned for follow-up.
Ethical consideration
This article followed all ethical standards for research without direct contact with human or animal subjects.
Discussion
Abacavir and lamivudine are commonly used in treating children with HIV. They are well absorbed orally with short half-lives of about 2 hours. While abacavir is metabolised in the liver, lamivudine is excreted unchanged in the urine. Nausea, vomiting, diarrhoea, cough, fever and fatigue are side effects of both drugs.5,6 They cause fatigue, and this patient had lethargy with altered consciousness. Hypersensitivity to abacavir was absent.7
Therapeutic daily paediatric doses of abacavir and lamivudine are 16 mg/kg and 10 mg/kg, not exceeding 600 mg and 300 mg per day.7 Individual doses are weight-adjusted at clinics and dispensed incrementally to reduce toxicity. The doses taken in this case far exceeded the recommended upper limit.
There is no known antidote for overdose. Management is supportive, and ideally the patient should be admitted to a high dependency unit for continuous monitoring of urine output, neurologic status and vital signs. Liver and renal function tests, with abacavir/lamivudine levels would immediately be useful to assess excretion.8,9 Lamivudine is dialysable,10 but dialysis was unavailable. The two drugs are bases and urinary alkalinisation would fail. These resources were unavailable, but he nevertheless recovered well. The few reports of adult antiretroviral overdose recommend supportive management, including intensive care. Although applicable to children, resources are often scarce. The temporal relationship between drug ingestion and presentation with signs and symptoms suggested overdose toxicity. The scarcity of investigational resources makes it difficult to be certain this was the cause of the patient's clinical presentation.
Fatal overdoses have reduced since the introduction of child-resistant containers.9 These containers should have been used. Parental education on safe storage of medications away from children can prevent such cases. Inadequate maternal education likely resulted in this child's mother being poorly informed on the proper handling of household poisons. The mother and child were lost to follow-up making it difficult to know whether the education provided to the mother had impacted on safe practices in the home.
Conclusion
There is limited awareness of ARV overdose toxicity in children, its social factors, and management options in resource-poor settings. Antiretroviral drugs are supplied orally for prolonged periods, reducing mortality and HIV transmission. The potential for accidental overdosing remains and parental education must include the safe storage and handling of ARVs in the home.
Acknowledgements
The authors wish to thank Dr Antony Gatheru and Prof. Otieno C.F. of the University of Nairobi for taking their time to review and intellectually critique the article prior to submission.
Competing interests
The authors declare that they have no financial or personal relationships that might have inappropriately influenced them in writing this article.
Authors' contributions
All authors contributed equally to this work.
Funding information
The authors did not receive financial support for the research, authorship, or publication of this article.
Data availability statement
Data sharing is not applicable to this article, as no new data were created or analysed in this study.
Disclaimer
The authors declare that the views expressed in the submitted article are their own and not an official position of the their affiliate institutions.
References
1.Margolis AM, Heverling H, Pham PA, Stolbach A. A review of the toxicity of HIV medications. J Med Toxicol [serial online]. 2014 [cited 2019 Aug 17];10(1):26-39. https://doi.org/10.1007/s13181-013-0325-8
2.Joint United Nations Programme on HIV/AIDS (UNAIDS). UNAIDS data 2019. Geneva: Joint United Nations Programme on HIV/AIDS; 2019. [ Links ]
3.Van Dam PMEL, Van Geffen MWL, Havenith TRA, Posthouwer D. Intentional overdose of dolutegravir/abacavir/ lamivudine (Triumeq) in a 26-year-old man. Antivir Ther. 2018;23(6):549-552. https://doi.org/10.3851/IMP3229 [ Links ]
4.Blanch J, Corbella B, Garcıa F, Parellada E, Gatell JM. Manic syndrome associated with Efavirenz overdose. Clin Infect Dis. 2001;33(2):270-271. https://doi.org/10.1086/321828 [ Links ]
5.Volberding PA. Overview of antiretroviral therapy. In: Volberding PA, Sande MA, Lange J, Greene WC, Gallant J, editors. Global HIV/AIDS medicine. New York: Elsevier Saunders, 2008; p. 135-160. [ Links ]
6.National AIDS & STI Control Programme. Guidelines on use of antiretroviral drugs for treating and preventing HIV infection in Kenya [homepage on the Internet]. c2016 [updated 2016 Jul; [ Links ] cited 2020 Jan 08]. Available from: https://aidsfree.usaid.gov/sites/default/files/kenya_art_2016.pdf
7.Nolan D, Mallal S, Reiss P. Prevention, diagnosis and treatment of HIV Infection. In: Volberding PA, Greene WC, Lange JMA, Gallant JE, Sewankambo N, editors. Sande's HIV/AIDS medicine medical management of AIDS in 2012. Elsevier Saunders, 2012; p. 178-179. [ Links ]
8.Francis JD. Clinical approach to the poisoned patient. In: Shaw LM, Kwong TC, Magnani B, Rosano TG, editors. The clinical toxicology laboratory: Contemporary practice of poisoning evaluation. Washington, DC: AACC Press, 2001; p. 27-33. [ Links ]
9.Penny L, Moriarty T. Poisoning in children. BJA Educ [serial online]. 2009 [cited 2020 Mar 15];9(4):109-113. Available from: Science Direct
10.Electronic Medicines Compendium. Lamivudine [homepage on the Internet]. [ Links ] c1999 [updated 2018 Jun 24; cited 2020 Apr 12]. Available from: https://www.medicines.org.uk/emc/product/4383/smpc
 Correspondence:
Correspondence:
Bryan Ogoti
bryanatandi@yahoo.com
Received: 23 Apr. 2020
Accepted: 08 May 2020
Published: 21 July 2020
ORIGINAL RESEARCH
Evaluation of the impact of delayed centrifugation on the diagnostic performance of serum creatinine as a baseline measure of renal function before antiretroviral treatment
Chemedzai E. ChikombaI; Carolyn J. PadoaI; Donald TanyanyiwaII
IDepartment of Chemical Pathology, National Health Laboratory Services, Faculty of Health Science, University of the Witwatersrand, Johannesburg, South Africa
IIDepartment of Chemical Pathology, National Health Laboratory Service, Faculty of Health Science, Sefako Makgatho Health Sciences University, Pretoria, South Africa
ABSTRACT
BACKGROUND: The measurement of serum creatinine is a standard requirement of the medical management of people living with HIV. Renal dysfunction is common, both as a complication of HIV-infection and as a result of its treatment. The detection of abnormal renal function before the start of antiretroviral therapy will impact patient management and the outcome of treatment.
OBJECTIVES: This study aimed to determine if a time delay in the centrifugation of serum samples affected the creatinine level and the estimated glomerular filtration rate as recorded on the analytical platforms used in the laboratory.
METHODS: Twenty-two (n = 22) HIV-positive, newly diagnosed and treatment-naïve patients were randomly recruited from Alexandra Health Clinic, Johannesburg, South Africa. Serum samples were centrifuged at six time intervals following receipt of the sample viz. < 4 h (baseline), 6 h, 24 h, 48 h, 72 h and 96 h. Creatinine concentrations were measured on the Roche platform utilising the enzymatic and kinetic Jaffe methods. Whole blood samples were also analysed with the Abbott i-STAT point-of-care instrument. The estimated glomerular filtration rate was calculated using the Cockcroft Gault, CKD-Epidemiology Collaboration and Modified Diet and Renal Disease v3/4 equations.
RESULTS: At baseline (< 4 h) there was good agreement between the enzymatic and kinetic Jaffe methods: bias 1.7 µmol/l. The enzymatic and i-STAT creatinine concentrations were stable over 96 h viz. changes of 1.8% and 5.7%. However, from 24 h onwards agreement between the enzymatic and kinetic Jaffe methods was poor with the latter measuring 43.7 µmol/l higher than the enzymatic method at 96 h. Creatinine concentrations measured with the kinetic Jaffe method increased significantly in samples centrifuged after 6 h (p < 0.001, 61.7% change), and resulted in a 95% decline in eGFR at 96 h as determined with the CKD-Epidemiology Collaboration equation.
CONCLUSION: The analysis of serum creatinine using the isotope dilution mass spectrometry traceable kinetic Jaffe method is unreliable if performed on samples centrifuged ≥ 6 h after collection. The raised creatinine concentration can affect clinical decisions such as renal functional assessment, choice of antiretroviral drug or regimen, and the dose and frequency of medication.
Keywords: Kidney function; serum creatinine; antiretroviral; estimated GFR; kinetic Jaffe; i-STAT; ROCHE.
Introduction
South Africa (SA) has one of the highest HIV infection rates in the world. In 2016, the overall prevalence of HIV infection in SA was 12.7%.1,2 The use of antiretroviral drugs (ARVs) has slowed the progression of the infection and increased the life expectancy of people living with HIV.3,4,5 In SA's public health sector, patients are started on antiretroviral treatment (ART) with combinations of different ARV drug classes, prescribed as a single, once-a-day tablet.6 This has led to a reduction of the pill-burden of therapy and to improved adherence.7,8 In the southern African region the most frequently prescribed initial combination is the nucleotide reverse transcriptase inhibitor (NtRTI), tenofovir disoproxil fumarate (TDF), the nucleoside reverse transcriptase inhibitors (NRTIs) emtricitabine (FTC) or lamivudine (3TC) and the non-nucleoside reverse transcriptase inhibitor (NNRTI), efavirenz (EFV). This is first-line ART.6 Current SA national ART guidelines indicate that the integrase strand transfer inhibitor (INSTI), dolutegravir (DTG), has replaced efavirenz for all except women of child-bearing age who may fall pregnant or those initiating ART in the first trimester of pregnancy.9,10 Tenofovir however remains the standard NRTI-backbone of all first-line ART in southern Africa.
TDF is an acyclic nucleoside phosphate prodrug. Its long half-life permits once-daily dosing. Although the drug is extensively filtered by the kidneys, 20% - 30% is actively reabsorbed at the proximal tubule.11 TDF is an occasional cause of renal injury viz. a proximal renal tubulopathy and a salt-wasting syndrome (Fanconi Syndrome and renal tubular acidosis), drug-induced acute kidney injury and a slower, general decline in glomerular filtration, diabetes insipidus, and a dysregulation of the kidney's calcium and phosphorus metabolism.11,12 Impaired renal function may also result from the use of other drugs, such as aminoglycosides, sulphonamides, and amphotericin B, the presence of HIV-associated nephropathy (HIVAN), blood-borne infection, for example tuberculosis, bacteraemia and fungaemia, HIV-associated immune-complex kidney disease and common comorbidities such as hypertension and diabetes mellitus.13,14 Pre-existing mild renal dysfunction may increase susceptibility to the toxicity of TDF. Kidney function must be evaluated before starting ART: the use of TDF is contraindicated if the estimated glomerular filtration rate (eGFR) is < 50 mL/min. Patients on TDF have serum creatinine measured at 3 and 6 months following the initiation of ART and biannually thereafter. In high-risk patients, for example with coexistent hypertension or diabetes, the creatinine is measured more frequently.9
The measurement of the GFR confirms and stages the degree of renal impairment and provides a platform for the ongoing monitoring of kidney function. Although the urinary clearance of inulin is the gold standard of GFR measurement,15 the method is time-consuming, expensive and impractical in most clinical settings. Endogenous substances such as serum creatinine and cystatin C are cheaper, provide more rapid results and are widely available.16 Although the National Institute for Health and Care Excellence (NICE) and the Kidney Disease Improving Global Outcomes (KDIGO) groups both base their eGFR assessment (equations) on cystatin C, its cost and lack of standardisation excludes its general use.17,18 Creatinine is produced at a constant rate, is present in all body fluids, and is filtered by the glomerulus. Its serum level is influenced by several biological factors, such as active secretion by the renal tubules in the presence of declining renal function, diet, extremes of muscle mass, age, gender, drugs and the use of creatine supplements.19 Several equations control for some of these variables.20 The SA National Department of Health (SANDOH) 2019 guidelines recommend therefore that the Counahan-Barratt equation be used to measure the eGFR of youths aged 10-16 years and the Modifications of Diet in Renal Disease (MDRD) equation be used for adolescents and adults 16 years of age and older.9
The Cockcroft-Gault (CG) equation incorporates the serum creatinine level and the variables of weight, age and sex in the measurement of the eGFR.21 The equation has limitations: in pregnancy, at the extremes of weight, and in those on dialysis for acute renal failure. Its principal use is in drug dosing and pharmacokinetic studies.20 The MDRD equation adds a fourth variable: race.22 This ethnicity adjustment-factor is based on African Americans and tends to overestimate the GFR in sub-Saharan (African) populations.23 The CKD-Epidemiology Collaboration (EPI) equation is recommended by the KDIGO guidelines group and has been validated in participants with and without impaired renal function with eGFR < 90 mL/min/1.73 min2.17,24 The SA National Health Laboratory Service (SA-NHLS) currently reports both the MDRD and CKD-EPI eGFR without ethnic adjustment. The adoption of the CKD-EPI equation in SA has been delayed due to limited local data.
Routine laboratory serum creatinine measurements are analysed on Jaffe and enzymatic assays. These methods should be traceable to isotope dilution mass spectrometry (IDMS) method and the Standard Reference Material for creatinine in serum (SRM 967). NHLS laboratories in SA generally use the modified kinetic Jaffe method, as opposed to the enzymatic assay, as it is more affordable. The World Health Organization's (WHO) guidelines indicate that at room temperature, creatinine is stable for 2-3 days.25 However, a report from Shepherd et al.26 noted a significant increase in creatinine levels measured on the kinetic Jaffe method when processing occurred later than 24 h.26 This impacted the study's eGFR results and had caused the renal misclassification of patients.26 A turnaround time for serum creatinine measurements from local clinics in Gauteng of 12-72 h has been observed at NHLS laboratories, as reported in the internal TAT reports. This delay increases with the remoteness of clinics due to the poor transport networks, the restricted working hours at referral laboratories, and the greater workload of regional laboratories. The aim of this study was to determine, in HIV-positive black South Africans not on ART, the stability limit (SL), that is, the time at room temperature, when the serum creatinine results are still within the maximum permissible instability (MPI) range.27
Methods
Study population
Twenty-two (n = 22) newly diagnosed and treatment-naïve people living with HIV between the ages of 18 and 70 years were randomly recruited from the Alexandra Health Community Centre in Alexandra, Johannesburg, SA. The minimum number of participants required for testing the stability of biochemical analytes is 10.27 Furthermore, multiple samples were required from each participant for analysis on three analysers at six time points, providing 396 creatinine measurement data points. Patients with comorbidities such as diabetes mellitus, pregnancy, urinary tract infection, hypertension and proteinuria were excluded from the study. De-identified demographic and anthropometric data were transcribed from the patient files.
Sample collection and processing
Each patient provided 13 tubes of blood: 12 × approximately 2 mL in 5 mL serum separator-tubes (SST) and 1 × 5 mL in a heparin tube (Becton Dickenson, Plymouth, UK). Serum was isolated from SST tubes following centrifugation at 1370 × g for 5 min at defined time intervals viz. < 4 h, 6 h, 24 h, 48 h, 72 h and 96 h. Prior to centrifugation, samples were stored at room temperature. Whole blood heparin tubes were stored at room temperature until required.
Measurement of creatinine concentrations
Serum creatinine concentrations were measured immediately after each centrifugation (as above) using the IDMS-traceable enzymatic and IDMS-traceable kinetic Jaffe method on the Roche COBAS 8000 module 702 and Roche COBAS 6000 module 502 instruments at the NHLS laboratory of the Chris Hani Baragwanath Hospital, Soweto, SA. The enzymatic method test principle is based on a series of coupled reactions that result in the conversion of creatinine to hydrogen peroxide. In the final reaction, catalysed by peroxidase, a quinone imine chromogen is formed. The colour intensity of this chromogen is measured at 550 nm and is directly proportional to the concentration of creatinine in the sample. The kinetic Jaffe method test principle is based on the reaction of creatinine and picrate under alkaline conditions forming a yellow-orange complex. The rate of formation of this complex is proportional to the amount of creatinine in the sample. The kinetic Jaffe method is prone to interference from non-creatinine chromogens such as proteins and ketones. A correction factor of -26 µmol/L is, therefore, universally applied to correct for these inherent chromogens. Bilirubin is the major negative interferent in the kinetic Jaffe method, and this is overcome by rate blanking. The internal quality control for creatinine in our laboratory is performed twice daily. The laboratory adheres to the Royal College of Pathologists of Australasia (RCPA) quality assurance programmes. External quality assurance submissions during the study period were within allowable limits.
Creatinine concentrations were also measured using whole blood samples on the Abbott i-STAT point-of-care instrument immediately after collection and at 6 h, 24 h, 48 h, 72 h and 96 h. This method is based on the conversion of creatinine to hydrogen peroxide, via hydrolysis and oxidation. The hydrogen peroxide is oxidized (at the platinum electrode) to produce a current that is proportional to the concentration of creatinine in the sample.
Estimated glomerular filtration rate measurements
eGFR was calculated using the MDRDv3 without ethnic adjustment (age, sex, and serum creatinine), MDRDv4 (age, sex, ethnicity, and serum creatinine), CG and CKD-EPI equations with ethnic adjustment.
Statistical analysis
All analyses were performed using Medcalc software. The Shapiro-Wilks test was used to test for normality. Normally distributed continuous variables (creatinine) were presented as mean ± standard deviation and continuous variables that were not normally distributed (age, weight) were presented as median [interquartile range]. Categorical variables are presented as proportions and percentages. The Student paired t-test was used to compare baseline creatinine concentrations between the three different methods (Bonferroni corrected p-value). The serum creatinine results were evaluated for analytically significant changes using the uncertainty of measurement (UOM; calculated for the laboratory quality control data in the preceding 6 months), total allowable error (TAE) of 15% (Clinical Laboratory Improvement Amendments [CLIA] and coefficient of variation [CV]). Passing-Bablok linear regression and Bland-Altman plots were generated to evaluate correlation and method agreement over time, using the enzymatic method as the reference method. For all analyses, p < 0.05 was considered statistically significant.
Ethical consideration
Ethical approval was obtained from the University of the Witwatersrand Human Research Ethic Committee, reference number: M1711109. R14/49.
Results
Characteristics of the study population
A total of 22 randomly recruited HIV-positive, treatment-naïve black South African individuals consented to participate in this study. The median age of the participants was 37 years. The majority (54.5%; 12 of 22) of participants were younger than 40 years of age. The age distribution is consistent with the current demographics published by Statistics South Africa with respect to individuals mostly affected by HIV/AIDS. All participants were black Africans (ethnicity self-reported). The median weight for the participants was 70.0 kg (range 45-110 kg) and 59.1% (13 of 22) of participants were male.
Creatinine assay performance
The mean baseline (< 4 h) creatinine concentrations for the study cohort obtained using the enzymatic, kinetic Jaffe method and i-STAT methods were 78.73 µmol/L ±14.63 µmol/L, 77.05 µmol/L ± 13.12 µmol/L and 70.14 µmol/L ± 15.3 µmol/L: p > 0.05 for all comparisons (Table 1). The mean creatinine concentrations analysed using the kinetic Jaffe method were significantly higher than baseline when blood samples were processed after 6 h: p ≤ 0.001 for all comparisons. Creatinine concentrations did not change significantly over the 96 h when measured using the enzymatic method or i-STAT system: p > 0.05 for all comparisons (Table 1).
The kinetic Jaffe method exhibited the widest intra-individual CV (range: 12% - 40% vs 1.2% - 12% enzymatic and 1.9% - 12% i-STAT method) as well as the greatest inter-assay variation (22.7% vs 6.0% enzymatic and 6.6% i-STAT method), as seen in Figure 1. When creatinine results were assessed against the 10% UOM for each respective assay, 8.9% of the enzymatic creatinine results fell outside the UOM for the duration of the study compared to 65% of the results for the kinetic Jaffe method. The i-STAT results could not be evaluated as UOM has not been established for this assay.
Based on the positive trend of increasing creatinine concentrations over time observed for the Jaffe method, we explored the data further to determine if a correction factor could be established to adjust creatinine results obtained from samples with delayed processing time. The percentage change of the serum creatinine results for the participants over the 96-h time frame was inconsistent and a correction factor could, therefore, not be established based on the current sample size (Figure 2).
Method comparison
Passing-Bablok regression and Bland-Altman plots were used to determine the correlation and agreement of the kinetic Jaffe method and i-STAT creatinine results compared to the enzymatic creatinine results at different time intervals (see Table 2, Figure 3 and Figure 4). The enzymatic method was chosen as the reference method as it had the best analytical CV in our study and previous studies have shown that this method correlated well with the standard reference method (isotopic dilution mass spectrometry).27,28,29
The Jaffe and enzymatic method showed a strong correlation at < 4 h (r = 0.953); however, this correlation became weaker with time. The higher r values at 72 h and 96 h may be due to the missing creatinine data (enzymatic 72 h n = 3, enzymatic 96 h n = 6, Jaffe 72 h n = 1, Jaffe 96 h n = 4) for participants at these time intervals. The slope and the intercept increased over the time interval (Table 2), and this demonstrated the increase in the magnitude of the systematic error. At 4 h, there was a strong agreement between the kinetic Jaffe method and enzymatic methods with a small negative bias of 1.8% (1.7 µmol/L). With an increased delay in sample separation, the kinetic Jaffe method resulted in an overestimation of creatinine concentrations with a positive bias of 48.6% (49.9 µmol/l) at 96 h (Figure 3).
Creatinine results from the i-STAT correlated well with the enzymatic creatinine results over the six time intervals used in the study: r-value, 0.836-0.948. The i-STAT method had a negative bias ranging from 7.4% to 18.0% throughout the study. The i-STAT method had a negative bias of 12.2% (8.6 µmol/L) at 4 h and 18.0% (12.0 µmol/L) at 96 h.
Comparison of estimated glomerular filtration rate equation performance
The impact on the classification of renal dysfunction of the delay in centrifugation of blood and serum samples measured for creatinine levels by means of three different laboratory methsods was evaluated with four eGFR equations viz. CG, MDRD v4, MDRD v3, and CKD-EPI. The serum creatinine eGFR results of the enzymatic and i-STAT methods performed well (to within the 10% TAE for eGFR throughout the study). However, the eGFR from the Jaffe data decreased over time. At baseline, viz. < 4 h, there was consensus among all four equations. All 22 participants had an eGFR > 60 mL/min per 1.73 m2 using the CG and MDRD v4 equation. Similarly, 21 participants had an eGFR > 60 mL/min per 1.73 m2 with the MDRD v3 and the CKD-EPI equations (Table 3). One participant had an eGFR of < 60 mL/min per 1.73 m2. This person was classified as having stage 3A renal failure with an eGFR 45 mL/min - 59 mL/min per 1.73 m2, according to the KDIGO guidelines.
The change in eGFR varied over time among the different equations. The CG equation was the least sensitive to the increase in serum creatinine: only 27% (n = 6/22) of eGFR-based 96-h creatinine concentrations resulted in a change in the Chronic Kidney Disease (CKD) stage when compared with baseline (i.e. the < 4-h eGFR; see Figure 5). The MDRD v4 equation gave the greatest change in eGFR over 96 h: 50% (n = 11/22) of participants at 24 h and 100% (n = 22/22) at 96 h showed an eGFR decline compared to baseline. The most significant change of eGFR was noted with the MDRDv3 equation: 25% eGFR at 48 h and 10% at 96 h compared to the baseline eGFR.
Using the CKD-EPI equation as recommended by the KDIGO guidelines, enzymatic and i-STAT renal classification was noted at different time intervals with changes between stages 1 and 2, which are not clinically significant for ARV drug choice. Using the kinetic Jaffe method however, changes in the renal classification were observed for 21 participants over the 96-h study period. At baseline, < 4 h, 21 of 22 (95%) participants had an eGFR > 60 mL/min/1.73 m2 (renal stage 1 and 2) while at 96 h only 1 of 18 (6%) participant was classified as stage 2. The remaining 17 participants (94%) were classified as stage 3a or higher (eGFR < 60 mL/min/1.73 m2). Figure 6 demonstrates that renal staging deteriorated over the study period, with all participants (100%) eligible for the tenofovir based regimen with an eGFR > 50 mL/min/1.73 m2 at 4 h while only four participants (22%) would have been eligible for this regimen at 96 h.
Discussion
The accurate measurement of serum and whole-blood creatinine is essential to the determination of the eGFR. Laboratory methods have been standardised, but concern remains regarding the impact on results of nonspecific analytical biases.30,31 This study from a clinical laboratory based at a large public hospital in SA reports levels of serum creatinine as assessed by three standard methods of measurement, following a delay in sample separation and centrifugation. The clinical significance of the delay was evaluated by calculating the eGFR of each of the 22 patient's serum samples by means of the following four equations: the CG, MDRD v4, MDRD v3, and CKD-EPI. Serum and whole blood creatinine as assessed by the enzymatic and i-STAT methods were stable throughout the study. However, we found that a delay in centrifugation and sample separation of > 6 h resulted in significantly raised creatinine concentrations when using the kinetic Jaffe method. This led to the misclassification of patients when the eGFR was determined using all four formulas (equations), and was consistent with previous studies.29,32
The Roche enzymatic creatinine results were stable over the duration of the study, with a CV of 6%, which was consistent with a previous study which showed that the enzymatic method meets the analytical performance specifications as stipulated by the National Kidney Disease Education Program (NKDEP) (USA).33,34 The i-STAT method showed a mean negative bias of 13.6 µmol/L compared to the enzymatic method. Studies comparing the performance of the i-STAT device to other platforms have been inconsistent.35,36,37,38 One study showed that the i-STAT overestimated creatinine results by 3.88 µmol/L in comparison to the Roche enzymatic creatinine results. This overestimation occurred predominantly at higher creatinine concentrations. In contrast, a study conducted on oncology patients found that mean creatinine concentrations obtained on the i-STAT system were 42.4 µmol/lower than those obtained using the core laboratory method.35 The negative bias may be attributed to whole blood creatinine negative interferences.39 POCT creatinine whole blood samples are affected by the matrix effect (such as haematocrit) and are prone to negative bias.40
Despite the negative bias seen with the i-STAT device, the creatinine results were sufficiently sensitive to detect the participant with renal dysfunction using the MDRD v3 and CKD-EPI equations. There are currently no POCT analytical goals for creatinine and no UOM was available as this was a new platform. However, the negative bias and the CV were within the acceptable 'Laboratory Working Group of the NKDEP' total error goal of less than 10% for the eGFR (CV < 8% and an analytical bias relative to IDMS < 5%).41 Previous studies have cautioned utilisation of POCT creatinine due to failure to detect renal dysfunction; however, in our study the i-STAT did not erroneously classify any of the participants.42 It is worth noting that the equations for eGFR were derived using serum samples, therefore utilisation of these equations with whole blood samples should still be validated using measured GFR.
While standardisation of creatinine methods has addressed calibration biases, method-specific interferences remain problematic.30 In the current study, the Roche kinetic Jaffe method showed analytical, clinically significant imprecision and a positive bias for creatinine results when analysed 24 h after collection. This finding is consistent with previous studies that showed delayed separation of serum from cellular components following specimen collection resulted in a positive interference with different Jaffe methods and could be attributed to the accumulation of pyruvate and other non-creatinine chromogens.26 Ford et al.32 established that on the Roche kinetic Jaffe method the serum creatinine results were significantly increased from 16 h and recommended that creatinine results with a delay in separation should not be reported as they impacted CKD staging. Similarly, Shepherd et al.26showed an overestimation of Jaffe creatinine results when assessing the impact of delayed sample separation using five different analytical platforms.26 These results are in contrast to the WHO guidelines that state that creatinine in whole blood is stable at room temperature for 2-3 days.25 In the current study, the overestimation by the kinetic Jaffe method over time was clinically significant as this led to a higher CKD staging (i.e. lower eGFR). This is consistent with a study by Drion et al. where they found that creatinine results from Jaffe techniques were more biased, imprecise and overestimated serum creatinine concentrations, especially at low concentrations compared to enzymatic techniques.29 The authors recommended that clinical laboratories consider the health costs associated with inappropriate referral and further suggested using the enzymatic assay that had a minimal bias.
The inconsistencies observed in renal classification using the four GFR equations shown in this study highlight the fact that these equations cannot be used interchangeably for identifying kidney disease, monitoring of renal function, drug dosing and selection of ARV drug regimens. Measured GFR was not required for this study as ascertaining the GFR was not pertinent, but rather the study sought to quantify the clinical significance of the impact of the instability on the calculated GFR. The CG equation was the least sensitive to the effect of the increased creatinine concentrations on eGFR in our study. This is consistent with a previous finding that demonstrated that the CG equation overestimated the eGFR in a large cohort of Europeans, resulting in the misclassification of 29.2% of individuals.43 In contrast, the CKD-EPI equation was sensitive to the serum creatinine changes of the kinetic Jaffe method, and the impact on the eGFR was noted at 24 h. This result is consistent with a previous study by Seape and colleagues who found that the CKD-EPI equation performed better than other creatinine-based eGFR equations when assessing renal function in 100 treatment-naïve HIV-positive individuals.44
Strengths and limitations
This study was able to show the impact of delayed processing of samples on creatinine concentrations on three platforms with a larger sample size than previous studies. In addition, blood samples were taken from participants at a single time point and the pre-analytical conditions were standardised. A limitation of this study is that serum indices (such as bilirubin which can falsely elevate creatinine results) were not considered when evaluating the creatinine result. Furthermore, we did not exclude all confounders to creatinine measurements such as drugs and pre-existing renal dysfunction which may have adversely affected the creatinine results at all time intervals. In addition, we did not use the reference creatinine method (IDMS) for comparison purposes; we used an enzymatic method traceable to IDMS. A further limitation is that we did not have a wide range of creatinine concentrations to see how a delay in centrifugation would affect the higher concentrations. Furthermore, this study did not explore all the risks associated with POCT devices such as lot-to-lot variation (one lot number was used), operator variability, quality control, analytical traceability, impact of temperature, humidity, different sample types, and the different analytical performance of different POCT devices which have previously been shown to have a significant impact on the eGFR.45
Conclusion
This study demonstrated that the Roche kinetic Jaffe method creatinine assay gave falsely elevated serum creatinine levels if samples were not separated and analysed within 24 h of collection. The increase in the serum creatinine concentration over time was clinically significant resulting in misclassification of renal status which would, in turn, lead to incorrect clinical decisions regarding ARV regimen choice. Based on the outputs of this study, and previously published literature,26,44 laboratories currently analysing creatinine on any Jaffe method should reject (not analyse) samples reaching the laboratory after the stability index time from collection (< 24 h), or if these laboratories decide to analyse these serum creatinine samples an appropriate interpretative comment should be appended to the result as done for other biochemical analytes such as potassium, glucose, and phosphate. The enzymatic creatinine method is highly recommended in clinical laboratories that analyse samples more than 24 h after collection due to transport delays and excessive workload. A major deterrent concerning the utility of the enzymatic method is its high cost. However, the health costs associated with the misclassification of patients using the Jaffe method may outweigh the analytical costs of the enzymatic method. Increased demand for the enzymatic method will result in competition between suppliers and ultimately reduce the cost. This study demonstrated that although creatinine assays have been standardised the extra analytical considerations which are not standardised may result in clinically significant creatinine results, thus the use of POCT, which performed well in this study, in remote clinics may avert the need to perform the laboratory-based kinetic Jaffe.
Measurement of creatinine with a POCT provides an accurate, timely result, and has eliminated the pre-analytical problems noted with the kinetic Jaffe method; therefore, adopting of POCT creatinine should be envisaged. The i-STAT POCT creatinine, however, had a negative bias which resulted in the misdiagnosis of a renal dysfunction for one participant with stage 3a renal failure while the eGFR of the other 21 participants was not affected. Its utilisation in HIV/AIDS programmes could, therefore, be adopted once the eGFR equations have been validated for the sample type, confounders of POCT mentioned previously and infrastructural issues such as integration with the laboratory information systems have been addressed. POCT and enzymatic assays will reduce unnecessary clinic visits due to renal misdiagnoses (false positives) and importantly reduce the pill burden, thereby ultimately reducing health costs to both the government and the patient.
Acknowledgements
NHLS medical technologist team from Chris Hani Baragwanath Hospital, the Alexandra Health Clinic chronic care team, Mr Philani Buthelezi
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
C.E.C. recruited patients, collected data, obtained ethics approval and wrote the first draft. C.P. reviewed and edited the manuscript. D.T. conceptualised the study, reviewed the statistical analysis, reviewed and edited the manuscript and approved the final version of the manuscript.
Funding information
We received the i-STAT machine and the cartridges from Obsidian Health (Pty) Ltd the official distributor of Abbott Point of Care South Africa. The company did not influence the protocol formulation, recruitment of participants, data analysis or the final write-up.
Data availability statement
Data from this study is available.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Meintjes G, Moorhouse MA, Carmona S, et al. Adult antiretroviral therapy guidelines 2017. South Afr J HIV Med. 2017;18(1):1-24. https://doi.org/10.4102/sajhivmed.v18i1.776 [ Links ]
2.HIV Country Profiles. [ Links ] [cited 2019 Sep 17]. Available from: http://cfs.hivci.org/country-factsheet.html
3.Kitahata MM, Gange SJ, Abraham AG, et al. Effect of early versus deferred antiretroviral therapy for HIV on survival. N Engl J Med. 2009;360(18):1815-1826. https://doi.org/10.1056/NEJMoa0807252 [ Links ]
4.Jamieson DJ, King CC, Kourtis AP. Human immunodeficiency virus infection. Clin Gynecol. 2015;(2)360-370. https://doi.org/10.1017/CBO9781139628938.026 [ Links ]
5.Capeau J. Premature aging and premature age-related comorbidities in HIV-infected patients: Facts and hypotheses. Clin Infect Dis. 2011;53(11):1127-1129. https://doi.org/10.1093/cid/cir628 [ Links ]
6.Department of Health, the Republic of South Africa. The South African antiretroviral treatment guidelines, 2013 [homepage on the Internet]. [ Links ] [cited 2019 Aug 3] Available from: http://www.kznhealth.gov.za/medicine/2013_ART_Guidelines.pdf
7.Cotte L, Ferry T, Pugliese P, Valantin M-A. Effectiveness and tolerance of single tablet versus once daily multiple tablet regimens as first-line antiretroviral therapy - Results from a large french multicenter cohort study. PLoS One. 12(2):e0170661. https://doi.org/10.1371/journal.pone.0170661 [ Links ]
8.Ghandi M, Ghandi RT. Single-pill combination regimens for treatment of HIV-1 infection. N Engl J Med. 2014;371:248-259. https://doi.org/10.1056/NEJMct1215532 [ Links ]
9.Department of Health, Republic of South Africa. 2019 ART clinical guidelines for the management of HIV in adults, pregnancy, adolescents, children, infants and neonates [homepage on the Internet]. [ Links ] Dolutegravir Overview pg. 8 ff. 2019. Available from: https://sahivsoc.org/Files/2019%20Abridged%20ART%20Guidelines%2010%20October%202019.pdf
10.Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med. 2019;381(9):803-815. https://doi.org/10.1056/NEJMoa1902824 [ Links ]
11.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of hiv-infected patients: A double-edged sword? J Am Soc Nephrol. 2013;24(10):1519-1527. https://doi.org/10.1681/ASN.2012080857 [ Links ]
12.Kohler JJ, Hosseini SH, Hoying-Brandt A, et al. Tenofovir renal toxicity targets mitochondria of renal proximal tubules. Lab Investig. 2009;89:513-519. https://doi.org/10.1038/labinvest.2009.14 [ Links ]
13.Wyatt CM, Klotman PE. Kidney disease in HIV infection: Introduction. Semin Nephrol. 2008;28(6):511-512. https://doi.org/10.1016/j.semnephrol.2008.08.004 [ Links ]
14.Foy MC, Estrella MM, Lucas GM, et al. Article comparison of risk factors and outcomes in HIV immune complex kidney disease and HIV-associated nephropathy. Clin J Am Soc Nephrol. 2013;8(9):1524-1532. https://doi.org/10.2215/CJN.10991012 [ Links ]
15.Alving AS, Miller BF. A practical method for the measurement of glomerular filtration rate (inulin clearance): With an evaluation of the clinical significance of this determination. Arch Intern Med. 1940;66(2):306-318. https://doi.org/10.1001/archinte.1940.00190140014002 [ Links ]
16.Traynor J, Mactier R, Geddes CC, et al. Clinical review how to measure renal function in clinical practice. Br Med J. 2006;333(7571):733-737. https://doi.org/10.1136/bmj.38975.390370.7C [ Links ]
17.Official Journal of the international society of nephrology KDIGO 2012. Clinical practice guideline for the evaluation and management of chronic kidney disease [homepage on the Internet]. [ Links ] [cited 2020 Mar 06] Available from: https://www.publicationethics.org
18.National Institute for Health and Care Excellence. 2014 clinical guideline. Chronic kidney disease in adults: Assessment and management [homepage on the Internet]. [ Links ] Available from: https://www.nice.org.uk/guidance/cg182
19.Bargnoux A-S, Kuster N, Cavalier E, et al. Serum creatinine: advantages and pitfalls. J Lab Precis Med. 2018;3(8):71. [ Links ]
20.Naicker J. Glomerular filtration rate (GFR) and estimation of the GFR (eGFR) in adults. Continuing Med Educ [serial online]. 2012;30(7). Available from: https://www.cmej.org.za/index.php/cmej/article/view/2514/2430
21.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41. https://doi.org/10.1159/000180580 [ Links ]
22.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med. 1999;130(6):461-470. https://doi.org/10.7326/0003-4819-130-6-199903160-00002 [ Links ]
23.Van Deventer HE, George JA, Paiker JE, et al. Estimating glomerular filtration rate in black South Africans by use of the modification of diet in renal disease and Cockcroft-Gault equations. Clin Chem. 2008;54(7):1197-202. https://doi.org/10.1373/clinchem.2007.099085 [ Links ]
24.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604-612. https://doi.org/10.7326/0003-4819-150-9-200905050-00006 [ Links ]
25.World Health Organization. Diagnostic Imaging and Laboratory Technology. Use of anticoagulants in diagnostic laboratory investigations [homepage on the Internet]. [ Links ] World Health Organization; 2002. Available from: https://apps.who.int/iris/handle/10665/65957
26.Shepherd J, Warner MH, Kilpatrick ES. Stability of creatinine with delayed separation of whole blood and implications for eGFR. Ann Clin Biochem. 2007;44(4):384-387. https://doi.org/10.1258/000456307780945660 [ Links ]
27.Gómez-Rioja R, Segovia Amaro M, Diaz-Garzón J, et al. Guidelines and recommendations from scientific societies a protocol for testing the stability of biochemical analytes. Technical document. Clin Chem Lab Med. 2019;57(12):1829-1836. https://doi.org/10.1515/cclm-2019-0586 [ Links ]
28.Badiou S, Dupuy AM, Descomps B, Cristol JP. Comparison between the enzymatic vitros assay for creatinine determination and three other methods adapted on the olympus analyzer. J Clin Lab Anal. 2003;17(6):235-240. https://doi.org/10.1002/jcla.10103 [ Links ]
29.Drion I, Cobbaert C, Groenier KH, et al. Clinical evaluation of analytical variations in serum creatinine measurements: Why laboratories should abandon Jaffe techniques. BMC Nephrol. 2012;13(1):1-8. https://doi.org/10.1186/1471-2369-13-133 [ Links ]
30.Jassam N, Weykamp C, Thomas A, et al. Post-standardization of routine creatinine assays: Are they suitable for clinical applications. Ann Clin Biochem. 2017;54(3):386-394. https://doi.org/10.1177%2F0004563216664541 [ Links ]
31.Greenberg N, Roberts WL, Bachmann LM, et al. Specificity characteristics of 7 commercial creatinine measurement procedures by enzymatic and jaffe method principles. Clin Chem. 2012;58(2):391-401. https://doi.org/10.1373/clinchem.2011.172288 [ Links ]
32.Ford L, Berg J. Delay in separating blood samples affects creatinine measurement using the Roche kinetic Jaffe method. Ann Clin Biochem. 2008;45(1):83-87. https://doi.org/10.1258%2Facb.2007.007109 [ Links ]
33.Boutten A, Bargnoux AS, Carlier MC, et al. Enzymatic but not compensated Jaffe methods reach the desirable specifications of NKDEP at normal levels of creatinine. Results of the French multicentric evaluation. Clin Chim Acta. 2013;419:132-135. https://doi.org/10.1016/j.cca.2013.01.021 [ Links ]
34.Küme T, Sağlam B, Ergon C, Sisman AR. Evaluation and comparison of Abbott Jaffe and enzymatic creatinine methods: Could the old method meet the new requirements? J Clin Lab Anal. 2018;32(1):1-8. https://doi.org/10.1002/jcla.22168 [ Links ]
35.Bahar B, DeChristopher PJ, Holmes EW, Kahn SE. Investigation of falsely decreased creatinine results observed from the abbott i-Stat point-of-care device in use for testing specimens from ambulatory oncology patients. Point Care J Near-Patient Test Technol. 2016;15(2):72-77. https://doi.org/10.1097/POC.0000000000000093 [ Links ]
36.Korpi-Steiner NL, Williamson EE, Karon BS. Comparison of three whole blood creatinine methods for estimation of glomerular filtration rate before radiographic contrast administration. Am J Clin Pathol. 2009;132(6):920-966. https://doi.org/10.1309/AJCPTE5FEY0VCGOZ [ Links ]
37.Snaith B, Harris MA, Shinkins B, Jordaan M, Messenger M, Lewington A. Point-of-care creatinine testing for kidney function measurement prior to contrast-enhanced diagnostic imaging: Evaluation of the performance of three systems for clinical utility. Clin Chem Lab Med. 2018;56(8):1269-1276. https://doi.org/10.1515/cclm-2018-0128 [ Links ]
38.van der Heijden C, Roosens L, Cluckers H, Van Craenenbroeck AH, Peeters B. Analytical and clinical performance of three hand-held point-of-care creatinine analyzers for renal function measurements prior to contrast-enhanced imaging. Clin Chim Acta. 2019;497(March):13-19. https://doi.org/10.1016/j.cca.2019.06.025 [ Links ]
39.Straseski JA, Lyon ME, Clarke W, DuBois JA, Phelan LA, Lyon AW. Investigating interferences of a whole-blood point-of-care creatinine analyzer: Comparison to plasma enzymatic and definitive creatinine methods in an acute-care setting. Clin Chem. 2011;57(11):1566-1573. https://doi.org/10.1373/clinchem.2011.165480 [ Links ]
40.Shephard MDS. Point-of-care testing and creatinine measurement. Clin Biochem Rev. 2011;32(2):109-114. [ Links ]
41.Myers GL. Recommendations for improving serum creatinine measurement: A report from the laboratory working group of the national kidney disease education program. Clin Chem. 2006;52(1):5-18. https://doi.org/10.1373/clinchem.2005.0525144 [ Links ]
42.Moreno M, Schwartz A, Dvorkin R. The accuracy of point-of-care creatinine testing in the emergency department. Adv Emerg Med. 2015;965368:5. https://doi.org/10.1155/2015/965368 [ Links ]
43.Froissart M, Rossert J, Jacquot C, Paillard M, Houillier P. Predictive performance of the modification of diet in renal disease and Cockcroft-Gault equations for estimating renal function. J Am Soc Nephrol. 2005;16(3):763-773. https://doi.org/10.1681/ASN.2004070549 [ Links ]
44.Seape T, Gounden V, van Deventer HE, Candy GP, George JA. Cystatin C- and creatinine-based equations in the assessment of renal function in HIV-positive patients prior to commencing highly active antiretroviral therapy. Ann Clin Biochem. 2016;53(1):58-66. https://doi.org/10.1177/0004563215579695 [ Links ]
45.Nichols JH, Nichols JH. Risk management for point-of-care testing. 2014;25(2):154-161. [ Links ]
 Correspondence:
Correspondence:
Chemedzai Chikomba
drcheme@gmail.com
Received: 12 Dec. 2019
Accepted: 11 Apr. 2020
Published: 16 July 2020
Project Number: M1711109
^rND^sMeintjes^nG^rND^sMoorhouse^nMA^rND^sCarmona^nS^rND^sKitahata^nMM^rND^sGange^nSJ^rND^sAbraham^nAG^rND^sJamieson^nDJ^rND^sKing^nCC^rND^sKourtis^nAP^rND^sCapeau^nJ^rND^sCotte^nL^rND^sFerry^nT^rND^sPugliese^nP^rND^sValantin^nM-A^rND^sGhandi^nM^rND^sGhandi^nRT^rND^sVenter^nWDF^rND^sMoorhouse^nM^rND^sSokhela^nS^rND^sTourret^nJ^rND^sDeray^nG^rND^sIsnard-Bagnis^nC^rND^sKohler^nJJ^rND^sHosseini^nSH^rND^sHoying-Brandt^nA^rND^sWyatt^nCM^rND^sKlotman^nPE^rND^sFoy^nMC^rND^sEstrella^nMM^rND^sLucas^nGM^rND^sAlving^nAS^rND^sMiller^nBF^rND^sTraynor^nJ^rND^sMactier^nR^rND^sGeddes^nCC^rND^sBargnoux^nA-S^rND^sKuster^nN^rND^sCavalier^nE^rND^sNaicker^nJ^rND^sCockcroft^nDW^rND^sGault^nMH^rND^sLevey^nAS^rND^sBosch^nJP^rND^sLewis^nJB^rND^sVan Deventer^nHE^rND^sGeorge^nJA^rND^sPaiker^nJE^rND^sLevey^nAS^rND^sStevens^nLA^rND^sSchmid^nCH^rND^sShepherd^nJ^rND^sWarner^nMH^rND^sKilpatrick^nES^rND^sGómez-Rioja^nR^rND^sSegovia Amaro^nM^rND^sDiaz-Garzón^nJ^rND^set^nal^rND^sBadiou^nS^rND^sDupuy^nAM^rND^sDescomps^nB^rND^sCristol^nJP^rND^sDrion^nI^rND^sCobbaert^nC^rND^sGroenier^nKH^rND^sJassam^nN^rND^sWeykamp^nC^rND^sThomas^nA^rND^sGreenberg^nN^rND^sRoberts^nWL^rND^sBachmann^nLM^rND^sFord^nL^rND^sBerg^nJ^rND^sBoutten^nA^rND^sBargnoux^nAS^rND^sCarlier^nMC^rND^sKüme^nT^rND^sSağlam^nB^rND^sErgon^nC^rND^sSisman^nAR^rND^sBahar^nB^rND^sDeChristopher^nPJ^rND^sHolmes^nEW^rND^sKahn^nSE^rND^sKorpi-Steiner^nNL^rND^sWilliamson^nEE^rND^sKaron^nBS^rND^sSnaith^nB^rND^sHarris^nMA^rND^sShinkins^nB^rND^sJordaan^nM^rND^sMessenger^nM^rND^sLewington^nA^rND^svan der Heijden^nC^rND^sRoosens^nL^rND^sCluckers^nH^rND^sVan Craenenbroeck^nAH^rND^sPeeters^nB^rND^sStraseski^nJA^rND^sLyon^nME^rND^sClarke^nW^rND^sDuBois^nJA^rND^sPhelan^nLA^rND^sLyon^nAW^rND^sShephard^nMDS^rND^sMyers^nGL^rND^sMoreno^nM^rND^sSchwartz^nA^rND^sDvorkin^nR^rND^sFroissart^nM^rND^sRossert^nJ^rND^sJacquot^nC^rND^sPaillard^nM^rND^sHouillier^nP^rND^sSeape^nT^rND^sGounden^nV^rND^svan Deventer^nHE^rND^sCandy^nGP^rND^sGeorge^nJAORIGINAL RESEARCH
Patient acceptance of HIV testing services in rural emergency departments in South Africa
Aditi RaoI; Caitlin KennedyI; Pamela MdaII; Thomas C. QuinnIII, IV; David SteadV, VI; Bhakti HansotiI, III
IDepartment of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, United States of America
IINelson Mandela Academic Clinical Research Unit, Mthatha, South Africa
IIIDepartment of Medicine, Johns Hopkins University School of Medicine, Baltimore, United States of America
IVDivision of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, United States of America
VDepartment of Medicine, Frere and Cecilia Makiwane Hospitals, East London, South Africa
VIDepartment of Medicine, Faculty of Health Sciences, Walter Sisulu University, East London, South Africa
ABSTRACT
BACKGROUND: South Africa faces the highest burden of HIV infection globally. The National Strategic Plan on HIV recommends provider-initiated HIV counselling and testing (HCT) in all healthcare facilities. However, HIV continues to overwhelm the healthcare system. Emergency department (ED)-based HCT could address unmet testing needs.
OBJECTIVES: This study examines the reasons for accepting or declining HCT in South African EDs to inform the development of HCT implementation strategies.
METHOD: We conducted a prospective observational study in two rural EDs, from June to September 2017. Patients presenting to the ED were systematically approached and offered a point-of-care test in accordance with national guidelines. Patients demographics, presenting compaint, medical history and reasons for accepting/declining testing, were recorded. A pooled analysis is presented.
RESULTS: Across sites, 2074 adult, non-critical patients in the ED were approached; 1880 were enrolled in the study. Of those enrolled, 19.7% had a previously known positive diagnosis, and 80.3% were unaware of their HIV status. Of those unaware, 90% patients accepted and 10% declined testing. The primary reasons for declining testing were 'does not want to know status' (37.6%), 'in too much pain' (34%) and 'does not believe they are at risk' (19.9%).
CONCLUSIONS: Despite national guidelines, a high proportion of individuals remain undiagnosed, of which a majority are young men. Our study demonstrated high patient acceptance of ED-based HCT. There is a need for investment and innovation regarding effective pain management and confidential service delivery to address patient barriers. Findings support a routine, non-targeted HCT strategy in EDs.
Keywords: HIV counselling and testing; South Africa; emergency department; patient acceptance; implementation research; linkage to care.
Introduction
South Africa (SA) faces the highest burden of HIV infection globally, with 7.7 million people living with HIV (PLWH) and prevalence ranging from 12.6% to 27% across the country.1,2 In 2018, SA had 240 000 new HIV infections and 71 000 AIDS-related deaths.1 The Joint United Nations Programme on HIV/AIDS (UNAIDS) adopted the ambitious treatment target of 90-90-90, wherein by 2020 90% of all PLWH would know their status, 90% of whom would be receiving sustained antiretroviral therapy (ART), of whom 90% would have achieved viral suppression.3 Currently, in SA, an estimated 90% of PLWH know their status, of whom 68% are accessing ART, and 87% of these have achieved viral suppression.1 Over the past two decades, numerous steps have been taken by the government to deliver evidence-based interventions focusing on HIV prevention, treatment, and retention. These efforts include the expansion of condom distribution, a national voluntary medical male circumcision program, prevention of mother-to-child transmission, as well as initiatives to increase knowledge and awareness of HIV/AIDS in communities utilising healthcare, in educational infrastructures and social media.2,4 However, despite sustained efforts and innovative measures, critical coverage gaps remain, with an estimated 10% of HIV-positive South Africans unaware of their status5 and with 15% of new global infections occurring in the country.2
The first critical step to meeting the 90-90-90 target is HIV testing. Early detection of undiagnosed HIV infection followed by effective linkage to care and treatment extends life expectancy, improves the quality of life, and reduces HIV transmission.6 Since 2015, the South African National Strategic Plan on HIV, sexually transmitted infections and tuberculosis has recommended provider-initiated HIV counselling and testing (HCT) to all persons attending healthcare facilities as a standard component of medical care, including trauma, casualty, and specialty clinics.6,7 Nonetheless, the provision of HCT in healthcare facilities is often hindered by the lack of standardised training and by competing clinical care priorities that prohibit effective service delivery.5,7 In addition, resources for HCT have largely been directed to primary healthcare centres and antenatal clinics or are focused on high-risk populations such as sex workers, men who have sex with men, injection drug users, and prisoners.8,9 As a result, individuals who do not interact with the healthcare system through these channels, such as young men, often miss being tested.
In SA, 90% of the population accesses healthcare through the public sector. For 28%, the emergency department (ED), a setting that provides high-volume care, is their only point of contact.10 In the United States of America, the ED is recognised by the Centers for Disease Control and Prevention to be a crucial venue in implementing the national HIV testing strategy.11 Seminal studies have not only quantified the burden of HIV infection in EDs but also have been critical to shaping the US national strategy for HIV; they could similarly address unmet testing needs in SA.11,12,13,14 In low- and middle-income countries (LMICs), HIV prevalence in EDs may be high, for example, 19% in Papua New Guinea and 50% in Uganda.15,16 Provision of HIV testing in the ED could thus be a critical intervention in curbing the epidemic. Its acceptance in acute care settings, however, has not been widely evaluated in sub-Saharan Africa. Studies have primarily focused on rates of acceptance, without exploring the reasons behind patients' decisions. Ascertaining the perspectives of patients, especially of those who decline testing, enables the identification of barriers to service delivery and the development of effective strategies to increase HIV diagnosis and linkage to care.
In this exploratory observational study, to determine the feasibility of expanding an ED-based HIV testing strategy in SA, we investigated patient perspectives on accepting or declining HCT and quantified the burden of HIV infection in the ED while implementing the nationally recommended HCT programme. This study will assist policymakers and healthcare providers to inform the integration of HCT in the clinical care pathway and optimise HCT service delivery in this venue, resulting in early engagement in care and treatment initiation, ultimately reducing HIV-associated morbidity and mortality.
Methods
The Walter Sisulu Infectious Diseases Screening in Emergency Departments (WISE) Study was a prospective observational study. HIV counselling and testing was implemented in the EDs of the Nelson Mandela Academic Hospital (NMAH) and the Mthatha Regional Hospital (MRH) in the Eastern Cape Province, from 27 June to 03 September 2017.
Study site
The study was conducted in Mthatha, a rural town in the South African province of the Eastern Cape, a region that supports 12.6% of the country's population.10 The area faces a disproportionate burden of acute injuries and illnesses with high rates of HIV and tuberculosis.7 It is also one of SA's poorest provinces and is a key priority area for HIV research and capacity building.7 Both hospitals are affiliated with the Walter Sisulu University. Nelson Mandela Academic Hospital is a large tertiary-care referral centre with 24-h trauma services, seeing only patients requiring specialty or surgical interventions referred from other district-level facilities. Mthatha Regional Hospital is a district-level facility that provides care to walk-in patients and referrals from adjacent maternal and childcare facilities. The EDs provide 24-h coverage and see 100-150 patients daily from the surrounding 100-km catchment area. Both sites are relatively low-resourced and not equipped with an electronic medical record (EMR) system, patient tracking system or standardised triage processes. Furthermore, there are no providers specialising in emergency medicine at these sites.
Study population
Patients presenting to the ED who were aged 18 years and older and clinically stable (defined as the South African Triage Scale designation of 'non-emergent') were included in the study. Triage scores were assigned by trained study staff, based on the South African Triage Scale (SATS).17 Patients younger than 18 years, not able to provide informed consent (i.e. patients with a depressed level of consciousness or mentally altered) or undergoing active resuscitation were excluded.
Recruitment and sampling
All patients presenting to the ED during the study period who met the inclusion criteria were approached by trained HCT staff, informed of the ongoing study and offered a point-of-care HIV test. Written informed consent was sought for testing and participation in a survey that asked about reasons for accepting or declining the test. Patients with a known HIV-positive diagnosis were asked if they had access to an antiretroviral (ARV) clinic, if they were on regular treatment and whether they were aware of having developed AIDS or being virally suppressed. Data were also collected on patient demographics, presenting complaint, presenting symptoms and past medical history.
HIV counsellors approached all eligible patients in a large waiting room after they underwent initial triage and administrative processes. Patients consenting to the study were escorted to a private room for testing if possible, whereas patients assigned a bed were tested at the bedside with curtains drawn where possible. Given the lack of an EMR or patient tracking system, HCT staff placed a small dot on the folders of all patients who were approached and offered HCT. Every 4 h, the study supervisors audited the folders of patients located within the ED to ensure that all eligible patients had been approached.
Based on recent survey data from the 2017 South African national HIV prevalence study, HIV prevalence amongst South Africans of all ages was estimated at 14%.2 Our study aimed to recruit a sample size of 700 patients at each site. This would present a large enough sample to capture the variation in testing preferences in the study setting, allowing us to detect a difference of greater than 5% from the baseline estimate of 14%, assuming a two-sided α of 0.05 and 80% power, for a period of 7 weeks at each site.
Intervention
Patients were offered point-of-care HIV testing following the South African national HIV testing guidelines.7 Patients who consented to the test provided a blood sample obtained through a lancet finger prick. Following the recommended testing algorithm, patients were first tested using the Advanced Quality Anti-HIV 1&2 rapid test (InTec Products, Inc., Fujian, China). Non-reactive samples were reported as an HIV-negative result. Reactive samples were confirmed with an HIV 1/2/O Tri-line HIV rapid test (ABON Biopharm, Hangzhou, China). Confirmed reactive samples were reported as an HIV-positive result, and patients were provided with a referral letter to a local ARV clinic. Confirmed non-reactive samples were reported as an indeterminate result, and patients were counselled to repeat the test in 4-6 weeks. Counselling preceded and followed all tests and included education on HIV transmission, prevention, and management. Results were available within 10-15 min of testing, whereas counselling required an additional 10-15 min, depending on the HIV test result.
Data collection
Ten local research assistants were hired and trained in rapid point-of-care HCT, good clinical practice and data collection, and were familiarised with the study protocol before the start of the study. Research assistants and study staff worked in shifts to ensure 24-h coverage of the ED.
In tandem with offering HIV testing, HCT staff administered a brief survey. Patient responses to questions about their gender, past medical history, mode of arrival, reason for visit, presenting complaint, and symptoms were recorded as pre-determined binary or categorical options, age was recorded as free text, and reasons for accepting or declining testing were captured via pre-determined categorical options derived from the literature or as free text. Data were recorded on case report forms. These forms were scanned and uploaded onto iDatafax (DF/Net Research, Inc., Seattle, WA, USA) by trained study staff. Following validation and cleaning, data were exported into Excel v.16.9 (Microsoft, Inc., Redmond, WA, USA), and then imported into Stata v.14 (StataCorp, TX, USA) for analysis.
The outcome of interest, declining HCT, was measured as a binary variable ('no' = 0 and 'yes' = 1). The independent variables measured were age (18-30, 31-50, 51-70, 70+), sex (male, female), presenting complaint (trauma, medical), South African triage score (death, routine visit, urgent, very urgent, emergent), access to primary care (yes, no), past medical history (hypertension, coronary artery disease, tuberculosis, diabetes, asthma, chronic obstructive pulmonary disorder, cancer), visit time (within regular operating hours, 9 am to 5 pm, or out of regular operating hours), visit reason (new complaint, return visit, referral), mode of transport (self-transport, ambulance, police), presenting symptoms (pain, fever) and disposition (death, intensive care unit admission, general admission, emergent surgery, transfer, discharge, absconded).
Data analysis and statistics
Analysis was conducted on patients unaware of their status, to examine the relationship between the outcome of interest and all other independent variables. Chi-square tests were used to explore individual variable associations with declining HCT. Logistic regression analysis was conducted to assess the contribution of each variable to declining HCT. Bivariate analysis was conducted to estimate the association between the outcome and each predictor variable, as well as multivariate analysis to estimate the independent effect of each predictor variable, adjusting for all others. All variables were included in the final model, following checks for collinearity and goodness of fit and performing a best-subsets variable selection. Sub-group analysis was completed on the top reasons for accepting and declining HCT by gender.
A reference level was selected for categorical variables with multiple responses, and other levels were accordingly compared. Associations were assessed using odds ratios (ORs), 95% confidence intervals (CIs) and p-values. A p-value of ≤ 0.05 was regarded as statistically significant. A pooled analysis of data collected from both sites is presented; no significant differences were observed between the two sites (Table 1).
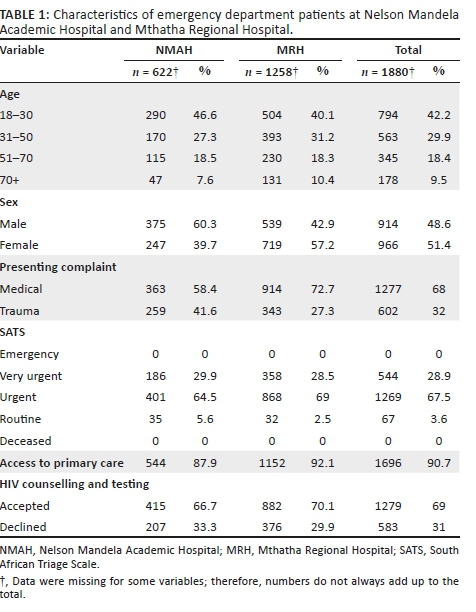
Ethical consideration
The study was approved by the Johns Hopkins University School of Medicine Institutional Review Board (reference number IRB00105801), the Human Research Ethics Committee from the University of Cape Town (MREC reference number 856/2015), the Human Research Committee of Walter Sisulu University (reference number 069/2015) and the Eastern Cape Department of Health. Written consent was obtained from all participants who enrolled in the study and was required for the collection of demographic data, HCT and a follow-up call for newly diagnosed patients, separately.
Results
A total of 1010 patients presented to the NMAH ED between 27 June and 13 August 2017. Of these, 727 (72%) patients were approached by HCT staff, and 622 (61.6%) were enrolled in the study. A total of 3245 patients presented to the MRH ED between 24 July and 03 September 2017; of these, 1347 (41.5%) patients were approached by HCT staff, and 1258 (38.8%) were enrolled in the study (Table 1).
Across both sites, 2074 patients were approached by the HCT staff, and 1880 (90.6%) were enrolled in the study. Patients enrolled were slightly female predominant (966, 51.4%), with a median age of 33 years (interquartile range [IQR]:24-59). Most patients presented with medical complaints (1278, 67.9%), received a triage designation of 'urgent' (1269, 67.5%) and reported having access to primary care services (1696, 90.2%; Table 1).
Of the 1880 patients enrolled, 465 (24.7%) patients were aware of their HIV status (defined as a known HIV-positive diagnosis [371, 19.7%] or tested HIV negative within the last 12 months [94, 5%]), and 1415 (75.3%) patients were unaware of their HIV status. Of patients with a known HIV-positive diagnosis, 351 (94.9%) said they were regularly accessing an ARV clinic. Of patients who were regularly accessing an ARV clinic, 23 (6.5%) reported being virally suppressed, 46 (13.1%) reported not being virally suppressed and 282 (80.3%) were unsure (Figure 1). In addition, 20 (5.4%) patients who had a known HIV-positive diagnosis wanted to get retested to confirm if they were truly/still HIV positive.
Of the 1415 patients unaware of their status, 141 (10%) declined HCT, and 1274 (90%) accepted. Of the patients who accepted HCT, 159 (12.5%) were diagnosed as HIV positive, 1102 (86.5%) were diagnosed as HIV negative and 13 (1%) had an indeterminate result. The overall prevalence of HIV in the study population was 28.1%. Patients declining and those accepting HCT both largely presented with medical complaints (912, 64.5%), received a triage designation of 'urgent' (954, 67.4%), had stated access to primary care services (1255, 89.2%), had no past medical history (929, 65.7%), visited the ED outside of regular hours (799, 56.5%), had a new complaint (878, 62.4%), used self-transport (881, 62.7%), had symptoms of pain (790, 55.8%), had no symptoms of fever (1388, 98.1%), were ultimately discharged from the ED (718, 53.9%) and were aged 18-30 years (629, 44.5%; Table 2).
The top reasons for accepting HCT were 'has not tested in the past year' (451, 35.4%), 'has never been tested' (242, 18.9%) and 'test is rapid and free' (237, 18.6%) (Figure 1). Patients accepting testing were largely male (672, 52.7%). The top reasons for declining HCT were 'does not want to know status' (53, 37.6%), 'in too much pain' (48, 34%) and 'does not believe they are at risk' (28, 19.9%; Figure 1). Patients declining testing were largely female (85, 60.3%).
Sub-group analysis of the reasons for accepting and declining HCT by gender showed slight differences between men and women in the reported reasons (Table 3). The primary reason for accepting HCT for both men and women was 'has not tested in the past year', 35.8% and 34.9%, respectively, followed by 'has never been tested' (21.4%) for men and 'test is rapid and free' (20.3%) for women. The primary reason for declining HCT given by men was 'does not want to know status' (66.1%) and 'in too much pain' for women (22.4%), followed by 'in too much pain' for men (42.9%) and 'does not believe they are at risk' (17.6%) for women.
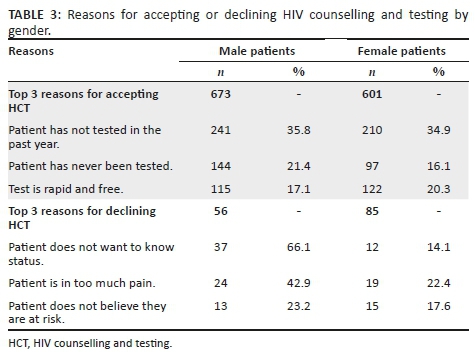
Univariate analysis showed that compared with male patients, female patients were more likely to decline HCT Associations were assessed using odds ratio (OR: 1.7; 95%) Confidence Intervals (CI:1.2-2.4). Patients who complained of pain compared with patients who did not (OR: 1.7; 95% CI: 1.2-2.5) and those arriving at the ED by ambulance compared to self-transport or with the police (OR: 1.4; 95% CI: 1.1-2.1) were also more likely to decline HCT. In addition, patients presenting with traumatic injuries compared with medical complaints (OR: 1.6; 95% CI: 1.1-2.2) were more likely to decline HCT. Other factors including age, triage score, access to primary care, past medical history, visit time, visit reason and final disposition did not show a statistically significant correlation with declining HCT (Table 2).
Multivariate analysis showed that patients who complained of pain compared with patients who did not (OR: 1.6; 95% CI: 1.1-2.6) were slightly more likely to decline HCT. Other variables did not show a statistically significant correlation with declining HCT (Table 2).
Discussion
We found acceptance of HCT services in the ED to be reassuringly high. Our study revealed that 90% of patients who were unaware of their HIV status accepted HIV testing. This was observed despite patients being in a clinical environment where HIV testing is not routinely offered and where patients present with acute injury or illness and are often in pain or moderate distress.11,18 These findings are consistent with results from other LMICs. In Kenya and Guyana, it was found that 97.7% and 75.5% of non-critical patients accepted HCT in the ED, respectively.19,20 Our results also highlight a substantial burden of undiagnosed HIV: 8.5% of enrolled patients were newly diagnosed as HIV positive. These patients presented to the ED for various clinical complaints, and a positive diagnosis of HIV was an incidental finding.
The two most common reasons for accepting HCT in the ED were 'has not tested in the past year', followed by 'has never been tested'. This positively implies the need for HCT in acute care settings, to cover the existing testing gap, as we are able to capture patients who are currently missed by other testing venues within the healthcare system. Recently, novel approaches for HCT - such as home-based, community-based and couples testing - have been added to traditional facility-based HCT delivery systems, with good acceptance rates.21,22 A systematic review of HCT strategies in sub-Saharan Africa reported an acceptance rate of 70% for home-based testing and 76% for community-based testing.21 However, despite the variety of testing strategies and venues, a testing gap remains, particularly amongst young adults and men.23,24 Considering that 44.1% of all patients accepting testing were young adults (18-30 years) and 52.7% were male, our study demonstrates that the ED is an opportune venue to capture this missed population.
Another factor leading to testing acceptance, reported by a fifth of patients accepting HCT, was 'the test is rapid and free'. This measure combines both cost and ease/limited time lost to testing. While it is hard to separate the two and determine which is a more significant factor, ensuring that both are addressed is a likely key to maintaining high acceptance of HCT in a fast-moving environment such as the ED. This is supported by a study in Uganda, where 25% of ED patients reported not knowing their HIV status because of the lack of access to free testing services.16 At present, HCT services are offered free of cost in all government healthcare facilities in SA; however, maintaining free services can be burdensome for the government, especially if testing services are to be further expanded. Furthermore, ensuring that testing and counselling are not time-consuming and are part of routine clinical care in the ED might address the barrier of having to seek out testing as a discrete task in itself.
Acceptance, however, was not universal. In our study population, women were significantly more likely to decline to test, whereas men were more likely to accept. Similar findings were observed in other studies examining the acceptability of testing in EDs, in both high- and low-resource settings.20,25 This might be because women are aware of having access to testing services during antenatal visits, through preventing mother-to-child transmission (PMTCT) programmes or through family planning services, and hence do not need to test in the ED.1 This is supported by the finding that more women were already aware of their HIV status; 71.6% of the patients with a known HIV-positive diagnosis were women. In addition, a significant proportion of women presenting to the ED in our context were diagnosed with pregnancy complications or injury wounds, likely justifying 'in too much pain' as the primary reason reported for women to decline HCT. On the contrary, though women were more likely to decline HCT, a majority still accepted testing when offered. While it is difficult to generalise individual motivations, factors including lack of social support and fear of stigma or rejection if tested HIV positive, especially in the presence of their partner or family accompanying them to the ED, may otherwise underlie the greater tendency of women to decline HCT.25
The top reasons reported for declining HCT in our ED, 'does not want to know status' and 'does not believe they are at risk', are interestingly established findings from high-income countries and LMICs, across healthcare settings.16,20,26,27,28,29 It could be that patients prefer uncertainty rather than facing the psychosocial consequences of an HIV-positive diagnosis, especially considering the imaginable stigma attached to such a diagnosis.30 This could be tackled through targeted pre- and post-counselling efforts. On the contrary, it is also possible that the small proportion of patients who declined to be tested are not at risk of contracting HIV and were accurately perceiving their risk. We did not include any risk measures in our survey and are thus unable to precisely indicate individuals who should have been tested.
A significant barrier to HCT in the ED and other healthcare facilities frequently described in the literature are stigma and the lack of confidentiality.20,26,31 This finding is supported by contextual knowledge, wherein anthropological studies exploring cultural perceptions and practices around HIV in Mthatha have reported pervasive stigma attached to HIV/AIDS, resulting in multiple forms of exclusion based on sexism, racism and homophobia,30 The National HIV Prevalence Survey indicated that 26% of people would not be willing to share a meal, 18% were unwilling to sleep in the same room and 6% would not speak to PLWH.2 Yet, none of the patients declining testing in our study reported reasons implying real or perceived stigma or the lack of confidentiality. The studies supporting this notion conducted in-depth interviews or had one-on-one conversations with patients, which likely allowed for deeper exploration of patient perspectives on HCT services, whereas given the patient volumes, high turnover and the lack of any coordinated processes in our study setting, it is possible that patients were less likely to report stigma as a reason for declining testing.
Pain was a notable justification for declining testing in this context and showed significant correlation through bivariate and multivariate analysis. The second most common reported reason, 'in too much pain', was not surprising, as a high proportion of cases presented with acute traumatic injuries. In addition, traumatic injuries and arriving at the ED in an ambulance, which are critical proxies to pain and the seriousness of a patient's condition, were positively correlated with declining testing. This finding is specific to declining HCT in the ED and has not been previously reported as a barrier in other testing venues. To address this barrier, the integration of pain management before HCT is recommended. If a patient's presenting complaint has been addressed by providers, and appropriate action taken, patients might be more likely to accept testing.
Linkage to care is the next critical step following testing. As part of our study, all newly diagnosed patients were counselled extensively on the importance of seeking follow-up care and were given a referral letter. Given that both NMAH and MRH see patients from a 100-km radius, it was challenging to ensure linkage to care, as it would depend on the area individuals came from and the presence and ease of access to an ART clinic. For patients who were local to Mthatha, we were able to direct them to the Gateway Clinic - an ART centre - located within the same campus as the hospital. With the consent of all patients who were newly diagnosed as HIV positive, we collected their names and contact details to conduct follow-up calls after 1 month, 6 months and a year. The follow-up calls will allow us to assess whether individuals have been able to link to care and/or what challenges they are facing in doing so. Results from the follow-up calls will be collated and analysed post-completion. Despite these challenges, 94.9% of patients with a known HIV-positive diagnosis presenting to the EDs reported having access to an ART clinic, and 85.4% of those individuals reported regularly accessing the clinic. These rates are commendable and imply a willingness of patients in this setting to seek follow-up care. However, these are self-reported statistics and could be inflated as a result of social-desirability bias.
Another interesting finding was that a small proportion of patients with a known HIV-positive diagnosis (20, 5.4%) requested a repeat test to confirm their diagnosis. Patients stated that they wanted to confirm whether they were truly HIV positive and/or if they were still HIV positive. Upon retesting, all 20 patients were HIV positive. The desire to retest when an opportunity presented could likely be a result of mistrust in the healthcare system or a result of the low health literacy in the region, which are both potential barriers to achieving high rates of testing and sustained linkage to care.30,31
There are several study limitations to consider. The protocol was to approach every patient presenting in the ED who met the inclusion criteria. However, the lack of infrastructure, systematic medical record-keeping or a patient tracking process made it challenging to retain all patients who presented to the ED. Many patients were missing from the records, whereas others were entered multiple times, making it difficult to keep count of the total number of patients. HIV counselling and testing services were provided 24 h a day, yet we were only able to approach 48% of patients who presented for care. We believe this is, in part, a result of the high volumes of patients and the quick turnaround time, as well as the time-consuming nature of counselling. Patients enrolled in the study may be a biased subset of the ED population, namely, easier to approach, spoke the same language as the HCT counsellors, had milder injuries or conditions and presented at times when the patient volume was lower. Maintaining confidentiality was challenging given the limited space - the EDs in both hospitals were in essence one big room, with beds lined up against each other. Lastly, the study was human-resource intensive. We had a team of four dedicated HCT staff at all times. Nevertheless, greater staff numbers would have allowed the capture of more study subjects. Such a situation would be difficult to sustain in a low-resource setting such as Mthatha.
To optimise our strategy and accurately capture data, given the lack of organisation and clear processes, our data were collected prospectively, whereby we relied less on recorded data and were able to capture most of it in real time. As the ED is busy and sees high patient volumes, we attempted to collect as much data as efficiently as possible, using a survey format with mostly 'yes' and 'no' questions. However, to have had a better understanding of patient perspectives, the study might have been enhanced by in-depth telephone interviews with a smaller number of patients after they had left the ED.
Conclusion
Our study demonstrated high patient acceptance of the nationally recommended HCT strategy in an ED setting. The overall adult prevalence of HIV in the ED was high at 28.1%. Patients who were male, young and not in pain or critically injured were more likely to accept HCT, critically supporting the provision of HCT in acute care settings, as it successfully captured an important demographic that has generally been missed through other testing venues. In addition, the lack of significant correlation in demographic or clinical characteristics and HCT uptake argues for a routine, non-targeted strategy in the ED. Our study further reveals the need for continued investment to ensure that HCT is widely available, with provision to effectively identify and manage pain and trauma. Finally, critical to embedding HCT in the routine clinical care offered in the ED will be the confidential conduct of HCT that permits stigma around HIV infection and testing to be appropriately addressed - something that will require further innovation and implementation research.
Acknowledgements
The authors acknowledge the staff in the Nelson Mandela Academic Hospital and Mthatha Regional Hospital emergency departments for making this research possible and the HIV Counselling and Testing team for their dedication and hard work during the study. The authors also acknowledge the contributions of Nomzamo Mvandaba for her assistance as a study coordinator for the WISE study and those of Victoria Chen and Kathryn Clark in data collection and data validation.
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors' contributions
B.H. conceived the original idea for the parent study and designed the protocol. A.R., P.M. and B.H. coordinated the study and data collection. A.R. carried out data analysis and prepared the manuscript. C.K., T.C.Q., D.S. and B.H. provided substantial edits and revisions.
Funding information
This research was supported by the South African Medical Research Council, the Division of Intramural Research, the National Institute of Allergy and Infectious Diseases, National Institutes of Health, and the Johns Hopkins Center for Global Health.
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, A.R. The data are not publicly available because they contain sensitive information that could compromise the privacy of research participants.
Disclaimer
All views expressed in the submitted article are the authors' own and not an official position of the institutions represented or the funders.
References
1.UNAIDS. UNAIDS data 2019. UNAIDS, Geneva, Switzerland; 2019. [ Links ]
2.Simbayi LC, Zuma K, Zungu N, et al. South African National HIV prevalence, incidence and behaviour, and communication survey, 2017. Cape Town: Human Sciences Research Council Press; 2019. [ Links ]
3.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. Geneva: UNAIDS; 2014. [ Links ]
4.UNAIDS. Ending AIDS: Progress towards the 90-90-90 targets. Geneva: UNAIDS; 2017. [ Links ]
5.Johnson LF, Chiu C, Myer L, et al. Prospects for HIV control in South Africa: A model-based analysis. Glob Health Action. 2016;9(1):30314. https://doi.org/10.3402/gha.v9.30314 [ Links ]
6.DoHRoS A. National HIV counselling and testing (HCT) policy guidelines 2015. Pretoria: National Department of Health; 2015. [ Links ]
7.SANAC. Let our actions count: South Africa's National strategic plan for HIV, TB and STIs 2017-2022. Pretoria: SANAC; 2017. [ Links ]
8.Telisinghe L, Charalambous S, Topp SM, et al. HIV and tuberculosis in prisons in sub-Saharan Africa. Lancet. 2016;388(10050):1215-1227. https://doi.org/10.1016/S0140-6736(16)30578-5 [ Links ]
9.Wolfe D, Carrieri MP, Shepard D. Treatment and care for injecting drug users with HIV infection: A review of barriers and ways forward. Lancet. 2010;376(9738):355-366. https://doi.org/10.1016/S0140-6736(10)60832-X [ Links ]
10.DoHRoS A. Mid-year population estimates. Pretoria: National Department of Health; 2015. [ Links ]
11.Rothman RE, Ketlogetswe KS, Dolan T, Wyer PC, Kelen GD. Preventive care in the emergency department: Should emergency departments conduct routine HIV screening? A systematic review. Acad Emerg Med. 2003;10(3):278-285. https://doi.org/10.1111/j.1553-2712.2003.tb02004.x [ Links ]
12.Hsieh YH, Kelen GD, Beck KJ, et al. Evaluation of hidden HIV infections in an urban ED with a rapid HIV screening program. Am J Emerg Med. 2016;34(2):180-184. https://doi.org/10.1016/j.ajem.2015.10.002 [ Links ]
13.Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: Experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33(2):147-155. https://doi.org/10.1016/s0196-0644(99)70387-2 [ Links ]
14.Haukoos JS, Rowan SE. Screening for HIV infection. BMJ. 2016;532(1):i1. https://doi.org/10.1136/bmj.i1 [ Links ]
15.Curry C, Bunungam P, Annerud C, Babona D. HIV antibody seroprevalence in the emergency department at Port Moresby General Hospital, Papua New Guinea. Emerg Med Australas. 2005;17(4):359-362. https://doi.org/10.1111/j.1742-6723.2005.00757.x [ Links ]
16.Nakanjako D, Kamya M, Daniel K, et al. Acceptance of routine testing for HIV among adult patients at the medical emergency unit at a national referral hospital in Kampala, Uganda. AIDS Behav. 2007;11(5):753-758. https://doi.org/10.1007/s10461-006-9180-9 [ Links ]
17.Twomey M, Wallis LA, Thompson ML, Myers JE. The South African Triage Scale (adult version) provides reliable acuity ratings. Int Emerg Nurs. 2012;20(3):142-150. https://doi.org/10.1016/j.ienj.2011.08.002 [ Links ]
18.Hansoti B, Kelen GD, Quinn TC, et al. A systematic review of emergency department based HIV testing and linkage to care initiatives in low resource settings. PLoS One. 2017;12(11). e0187443. https://doi.org/10.1371/journal.pone.0187443 [ Links ]
19.Waxman MJ, Muganda P, Carter EJ, Ongaro N. The role of emergency department HIV care in resource-poor settings: Lessons learned in western Kenya. Int J Emerg Med. 2008;1(4):317-320. https://doi.org/10.1007/s12245-008-0065-8 [ Links ]
20.Christensen A, Russ S, Rambaran N, Wright SW. Patient perspectives on opt-out HIV screening in a Guyanese emergency department. Int Health. 2012;4(3):185-191. https://doi.org/10.1016/j.inhe.2012.03.001 [ Links ]
21.Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77-S85. https://doi.org/10.1038/nature16044 [ Links ]
22.Jurgensen M, Sandoy IF, Michelo C, Fylkesnes K, Group ZS. Effects of home-based voluntary counselling and testing on HIV-related stigma: Findings from a cluster-randomized trial in Zambia. Soc Sci Med. 2013;81(1):18-25. https://doi.org/10.1016/j.socscimed.2013.01.011 [ Links ]
23.Nglazi MD, Van Schaik N, Kranzer K, Lawn SD, Wood R, Bekker LG. An incentivized HIV counseling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. J Acquir Immune Defic Syndr. 2012;59(3):e28-e34. https://doi.org/10.1097/QAI.0b013e31824445f0 [ Links ]
24.Johnson LF, Rehle TM, Jooste S, Bekker LG. Rates of HIV testing and diagnosis in South Africa: Successes and challenges. AIDS. 2015;29(11):1401-1409. https://doi.org/10.1097/QAD.0000000000000721 [ Links ]
25.Pisculli ML, Reichmann WM, Losina E, et al. Factors associated with refusal of rapid HIV testing in an emergency department. AIDS Behav. 2011;15(4):734-742. https://doi.org/10.1007/s10461-010-9837-2 [ Links ]
26.Chimoyi L, Tshuma N, Muloongo K, Setswe G, Sarfo B, Nyasulu PS. HIV-related knowledge, perceptions, attitudes, and utilisation of HIV counselling and testing: A venue-based intercept commuter population survey in the inner city of Johannesburg, South Africa. Glob Health Action. 2015;8(1):26950. https://doi.org/10.3402/gha.v8.26950 [ Links ]
27.Schechter-Perkins EM, Koppelman E, Mitchell PM, Morgan JR, Kutzen R, Drainoni ML. Characteristics of patients who accept and decline ED rapid HIV testing. Am J Emerg Med. 2014;32(9):1109-1112. https://doi.org/10.1016/j.ajem.2014.05.034 [ Links ]
28.Christopoulos KA, Weiser SD, Koester KA, et al. Understanding patient acceptance and refusal of HIV testing in the emergency department. BMC Public Health. 2012;12(1):3. https://doi.org/10.1186/1471-2458-12-3 [ Links ]
29.Gazimbi MM. A multilevel analysis of the determinants of HIV testing in Zimbabwe: Evidence from the demographic and health surveys. HIV/AIDS Res Treat Open J. 2017;4(1):17. http://doi.org/10.17140/HARTOJ-4-124 [ Links ]
30.Leclerc-Madlala S, Simbayi LC, Cloete A. The sociocultural aspects of HIV/AIDS in South Africa. In: HIV/AIDS in South Africa 25 years on: Psychosocial perspectives. 2009; p. 13-25. [ Links ]
31.Nombembe C. Music-making of the Xhosa diasporic community: A focus on the Umguyo tradition in Zimbabwe. Cape Town: University of Witwatersrand, Faculty of Humanities, Wits School of Music in Cape Town; 2013. [ Links ]
 Correspondence:
Correspondence:
Aditi Rao
aditi.rao@jhmi.edu
Received: 15 May 2020
Accepted: 02 June 2020
Published: 22 July 2020
ORIGINAL RESEARCH
Retention in care for adolescents who were newly initiated on antiretroviral therapy in the Cape Metropole in South Africa
Brian van Wyk; Ebrahim Kriel; Ferdinand Mukumbang
School of Public Health, Faculty of Community and Health Sciences, University of the Western Cape, Cape Town, South Africa
ABSTRACT
BACKGROUND: Long-term retention of adolescents aged 10 -19 years on antiretroviral therapy (ART) is crucial to achieve viral load suppression. However, it is reported globally that adolescents have lower retention in care (RiC) on ART, compared with children and adults.
OBJECTIVES: To determine the prevalence and predictors of RiC of adolescents over 2 years following initiation onto ART in public health facilities in the Metropole District Health Services of the Western Cape province in 2013.
METHODS: Data of 220 adolescent patients who were newly initiated on ART in 2013 were extracted from the provincial electronic database, and subjected to univariate and bivariate analyses using SPSS.
RESULTS: The rate of RiC post-initiation was low throughout the study period, that is, 68.6%, 50.5% and 36.4% at 4, 12 and 24 months, respectively. The corresponding post-initiation viral load suppression levels on ART of those remaining in care and who had viral loads monitored were 84.1%, 77.4% and 68.8% at 4, 12 and 24 months, respectively. Retention in care after initiation on ART was higher amongst younger adolescents (10-14 years), compared with older adolescents (15-19 years). Male adolescents were significantly more likely to be retained, compared with females. Pregnant adolescents were significantly less likely to be retained compared with those who were not pregnant.
CONCLUSION: Key interventions are needed to motivate adolescents to remain in care, and to adhere to their treatment regimen to achieve the target of 90% viral load suppression, with specific emphasis on older and pregnant adolescents.
Keywords: HIV; AIDS; adolescents; youth; retention in care.
Introduction
Globally, it has been estimated that 190 000 (59 000-380 000) adolescents, between the ages of 10 and 19 years, were newly infected with human immunodeficiency virus (HIV) in 2018, and the total number of adolescents living with HIV (ALWH) was 1.6 million (1.1-2.3 million), which accounts for 4% of all people living with HIV (PLWH).1 Sub-Saharan Africa has the highest number of HIV-infected adolescents with about 1.5 million of them. In South Africa (SA), it is estimated that 280 000 children aged between 0 and 14 years were living with HIV in 2017, and that just under 7 million persons aged ≥ 15 years were PLWH.2 The national HIV household survey estimated HIV prevalence in 0-14-year-olds to be 3.0% and 2.4% for females and males, respectively.3 In 15-19-year-olds, it was 5.8% in females and 4.7% in males. The number of adolescents aged 15-19 years receiving antiretroviral therapy (ART) in SA increased tenfold between 2005-2008 and 2013-2016.4 This increase is attributed to perinatally infected infants surviving into adolescence and to a rising incidence of HIV in behaviourally infected 15-19-year-olds.
Despite success in ART roll-out in most countries over the last decade, acquired immune deficiency syndrome (AIDS)-related deaths amongst adolescents have increased whilst declining in other age groups.5 To prevent AIDS-related deaths, the infected must be diagnosed, receive ART and remain in care to maintain viral load (VL) suppression. This would help achieve the 90-90-90 targets of the Joint United Nations Programme on HIV and AIDS (UNAIDS).6 Retention on ART is particularly challenging for key populations, such as adolescents, amongst others, and has been noted as a global priority for action.7,8 Previous studies confirm that adherence, retention in care (RiC) and treatment outcomes for adolescents in southern Africa are worse, compared with adults.9,10,11
However, routine monitoring of HIV treatment programmes does not report RiC and treatment outcomes for adolescents (10-19 years); they report only for children (0-14 years) and adults (15 years and older).11 In this way, adolescent problems with RiC and treatment outcomes remain undetected in the monitoring of HIV programmes. It is argued that because of the general adherence problems faced by adolescents globally, specific analysis is needed to report outcomes for this age group at a health-systems level.8
Objectives
This article reports on the RiC of ALWH aged 10-19 years who were newly initiated on ART in public health facilities in the Western Cape Metropole, SA, in 2013, and the risk factors associated with remaining in care at 4, 12 and 24 months post-initiation on ART.
Methods
Design
We conducted a retrospective cohort analysis of adolescents aged 10-19 years, who were initiated on ART in 2013 in the Western Cape Metropole.
Study context
The Western Cape's ART programme has been in existence for over 10 years. The 2013 version of the ART guidelines was updated in 2016, and had consolidated adult, adolescent, paediatric and prevention of mother-to-child transmission of HIV guidelines.12 In the province of the Western Cape, most adults and children access ART services at primary healthcare facilities, such as clinics, community day centres and community health centres. At the end of June 2017, this province had 237 285 patients on ART, of whom 229 171 were aged ≥ 15 years and 8114 were aged < 15 years. The Cape Town Metropole accounts for 74.3% (167 833) of the total number of ART patients in the Western Cape, that is 162 092 aged ≥ 15 years and 5741 aged < 15 years.13 Antiretroviral therapy services are rendered at the various Western Cape facilities, as outlined in Table 1.13
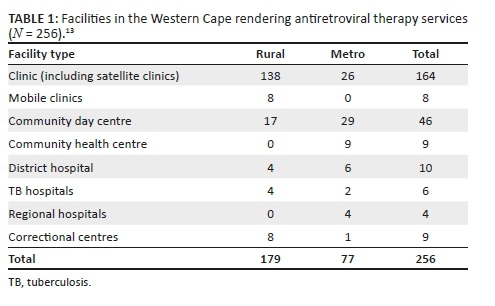
However, services rendered at these facilities vary. Some provide only adult ART services. Others offer both adult and paediatric support. Some facilities offer ART daily, whilst others provide ART only on certain days of the week. Human resource capacity also varies. Not all facilities have resident medical officers or clinical nurse practitioners. Hence, the need for outreach support arises. The capacity to care for adults in the Western Cape has improved with the implementation of the nurse-initiated management of antiretroviral treatment (NIMART) programme, whereby nurses are trained and receive structured mentorship and accreditation to initiate first-line ART. A challenge in the rural areas is the irregular access to competent and skilled clinicians to manage paediatric patients and complicated adult and adolescent patients. As previously stated, most ART services are designated as paediatric or adult. Adolescent-specific ART services are not yet part of standard care being offered at all ART facilities. Those that do are limited to services initiated and/or supported by tertiary hospitals or non-profit organisations.
Data source
Two data sources were used for this analysis: the Three Interlinked Electronic Registers.Net (TIER.Net),14 an electronic ART database developed by the University of Cape Town's Centre for Infectious Disease Epidemiology and Research, and patients' folders accessed at the treating facilities. Tier.Net is used operationally in the public health facilities of SA to monitor baseline clinical care and client outcomes over time. It is also the platform on which HIV tests are electronically captured in the public sector.15
We first visited the Tier.net platform to obtain data on all those who met the inclusion criteria. With the use of our data-capturing form, we searched for the relevant information from the Tier.net platform. Where information was missing, we accessed the patient's folder to confirm the availability of the required information.
Study participants
Data were found for 332 ALWH newly registered on ART from 29 facilities across the Western Cape Metropole and extracted from the provincial Tier.net register. Only 220 participants were included in the final analysis. Of the 112 excluded, for 68, folders could not be found in spite of making numerous attempts to trace these documents at the various facilities. Furthermore, 28 patients were incorrectly captured as new patients when they had been transferred in from other facilities; 4 patients were not adolescents, and their birth dates had been incorrectly captured; and 12 patients had been incorrectly captured as having initiated ART in 2013.
Main outcome measures and analysis
We extracted data on sociodemographic characteristics (age, sex, source of income, type of dwelling, disclosure to significant other and reported alcohol or other drug use) and clinical characteristics (CD4 count, WHO stage, pregnancy and ART regimen).
Bivariate analysis was conducted to determine the significance and strength of association between RiC at 4, 12 and 24 months and various sociodemographic and clinical characteristics. Statistical significance was tested by using the chi-square test, with significance set at p < 0.05, and where significant, the strength of association was calculated as risk ratios (RRs) with 95% confidence interval (CI), using SPSS v23. Our use of 4, 12 and 24 months rather than 6, 12 and 24 months is informed by the operational guidelines of the Western Cape's ART programme, which requires the first VL test to be conducted at 4 months and the patient to return for results in month 5.16 Patients would receive 1 month's medication if they have unsuppressed VL, and 2 months' supply if their VL is suppressed. The reason for the 4-month RiC measurement is to identify how many ALWH return for their VL tests. The subsequent RiC behaviour of the patients is measured annually.
Survival analysis was assessed with lost to follow-up (LTFU) as the outcome of interest. We did a comparative survival analysis for the age and sex of the study participants. We reported the hazard ratios and p-values. An ALWH was considered LTFU if they had not made contact with a treating healthcare facility within 90 days since their last registered contact for HIV-related treatment and care. The LTFU date was determined from the day when the patient was last seen at the clinic where they were provided with their last medication. Therefore, by using the intention-to-treat population in this study, the RiC definition was the proportion of HIV-infected adolescents alive and on ART at months 4, 12 and 24 in the entire study sample.
Ethical consideration
The protocol was approved by the University of the Western Cape Biomedical Research Ethics Committee (Reference number: BM/17/1/15) and the Government Health Impact Assessment (Reference number: WC_2017RP58_418) Committee.
Results
Of the 220 adolescents who were newly initiated on ART in 2013, the majority were 'older' adolescents, 15-19 years (n = 179, 81.4%) and female (n = 182, 82.7%) (Table 2). Most were financially supported by their families and friends (n = 129, 58.6%) and lived in a formal house (n = 116, 52.7%). As per HIV clinical treatment guidelines, the overwhelming majority (n = 182, 87%) had disclosed their HIV status to a significant other.
The median CD4 count at ART initiation was 292.5 cells/mm3 (interquartile range [IQR]: 228.8-391.3). Only two participants had no baseline CD4 count recorded. Half of the participants (n = 109) were initiated with a CD4 count between 200 cells/mm3 and 349 cells/mm3, and 19% (n = 42) had a baseline CD4 count of < 200 cells/mm3 as per the HIV treatment guidelines of 2013.
Of the 213 participants who had WHO staging done at ART initiation, 46.5% and 22.5% were WHO stages I and II, respectively, and 23.2% and 6.8% were WHO stages III and IV, respectively. As with the CD4 counts, clinical staging is taken into consideration in the universal test and treat era.
Observed RiC was low throughout the study period with 68.6%, 50.5% and 36.4% adolescents being retained in care at 4, 12 and 24 months post-initiation on ART, respectively. Figure 1 illustrates the comparison of RiC at 4, 12 and 24 months between younger (10-14 years of age) and older (15-19 years of age) adolescents (90.2% vs. 63.7%, 82.9% vs. 43.0% and 68.3% vs. 29.1%, respectively). However, RiC of the younger adolescents at month 4 was just over 90%, but the younger adolescents at months 12 and 24 fell short of 90%. The older adolescents showed poorer rates of RiC at months 4, 12 and 24, compared with the younger adolescents at the same time periods.
Table 3 shows that a significantly higher number of younger adolescents (10-14 years) were retained in care at 4, 12 and 24 months post-initiation on ART, compared with older adolescents (15-19 years). At 4 months post-initiation on ART, younger adolescents had 37% higher risk (likelihood) of RiC (RR = 1.37, 95% CI: 1.17-1.60) compared with older adolescents. At 12 months post-initiation on ART, younger adolescents had 85% higher risk of RiC (RR = 1.85, 95% CI: 1.48-2.31), compared with older adolescents, and more than two times higher risk (RR = 2.35, 95% CI: 1.73-3.20) at 24 months.
Male adolescents had higher rates of RiC post-initiation of ART at 4 months (RR = 1.29, 95% CI: 1.08-1.53), 12 months (RR = 1.39, 95% CI: 1.06-1.83) and 24 months (RR = 1.60, 95% CI: 1.11-2.30), compared with female adolescents.
Adolescents who were pregnant had significantly lower rates of RiC post-initiation of ART, compared with all other adolescents at 4 months (RR = 0.73, 95% CI: 0.59-0.90), 12 months (RR = 0.60, 95% CI: 0.44-0.83) and 24 months (RR = 0.47, 95% CI: 0.30-0.74).
Adolescents who disclosed their HIV status to a significant other were two times more likely to be retained in care at month 12 (RR = 2.06, 95% CI: 1.07-3.95) than adolescents who did not disclose to a significant other.
Adolescents classified as WHO stage I at ART initiation had significantly lower rates of RiC at 4 months post-initiation, compared with adolescents who were classified as WHO stage III (RR = 1.29, 95% CI: 1.14-1.42). Those classified as WHO stages II and IV also had better rates of RiC at month 4, compared with adolescents classified as WHO stage I at baseline, but these did not reach statistical significance. At 12 months post-initiation of ART, those who were at WHO stage II (RR = 1.35, 95% CI: 1.09-1.53) as well as WHO stage III (RR = 1.35, 95% CI: 1.10-1.53) at baseline had a 35% greater risk (likelihood) of RiC, compared with those who were WHO stage I at ART initiation. Adolescents who were at WHO stage I at baseline also showed significantly lower RiC rates at 24 months post-initiation of ART, compared with those who were at WHO stage II (RR = 1.52, 95% CI: 1.24-1.69) and WHO stage III (RR = 1.46, 95% CI: 1.15-1.66) at baseline. At 24 months post-initiation of ART, adolescents who were classified as WHO stage II (RR = 1.35, 95% CI: 1.09-1.53) and those who were WHO stage III (RR = 1.35, 95% CI: 1.10-1.53) at ART initiation had a 35% greater risk of RiC, compared with those who were WHO stage I at ART initiation. Those adolescents who were classified as WHO stage IV at ART initiation showed better RiC rates at months 4, 12 and 24 post-initiation, but none of these reached statistical significance.
Figures 2, 3 and 4 show the survival curves of the study participants. The overall person-time at risk of being LTFU was 3303 months, with an incidence of 119/3807 (4/100) person-months. The hazard ratio for males compared with females was 0.71 (95% CI: 0.41-1.26). The hazard ratio for those aged 15-19 years compared with those aged 10-14 years was 2.53 (95% CI: 1.30-4.91).
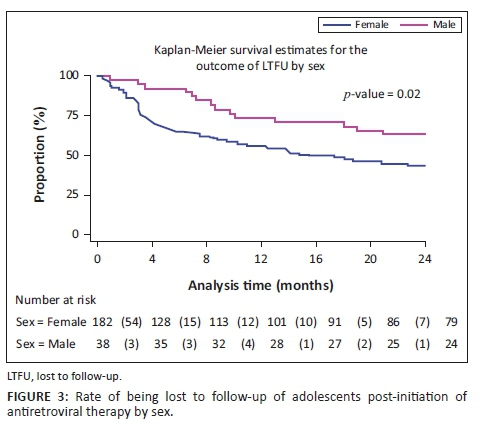
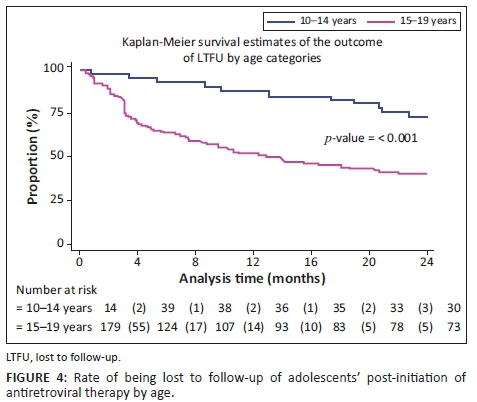
Discussion
In 2014, the UNAIDS set a global ART target of 90-90-90. This includes the goal that 90% of persons who test HIV-positive should be initiated on ART.6 Having successfully tested and initiated ALWH onto ART, their RiC and the maintenance of VL suppression of at least 90% are the ongoing challenges. Our study reports that the overall RiC of ALWH was low throughout the 24-month observation period. Contrary to our findings, Nabukeera-Barungi et al. found that 90.4% of Ugandan adolescents demonstrated good RiC with an LTFU of only 5%.17 A meta-analysis of six South African studies also reported a relatively high ALWH retention rate of 83% (95% CI: 68% - 94%) in the first 2 years on ART.18 The results of our study are reported with the intention-to-treat population as the denominator at every time point, that is, months 4, 12 and 24, and without the exclusion of transfer-outs, LTFU patients and the numerator being those alive and on ART. The above-mentioned studies did not measure RiC in the same manner. Nabukeera-Barungi et al.17 determined RiC by dividing those still active in care by the total number that started after subtracting those who died and transferred out from both the numerator and denominator.
Younger adolescents (10-14 years) demonstrated better RiC rates, compared with the older group. This observation could be attributed to the disproportionate attention offered to younger adolescents. In spite of the unique challenges posed by adolescents and ART, there is a dearth of comprehensive health services for adolescents, including interventions to improve RiC in sub-Saharan Africa.19 Nevertheless, younger adolescents show better RiC rates because they depend, to a greater extent, on their caregivers to handle their treatment journey. In this way, the RiC of the young adolescent is an extension of the dedication and understanding of the caregiver. Another study designed to investigate the RiC rates between younger and older adolescents in Zimbabwe demonstrated no differences in attrition amongst younger versus older adolescents.20
We found that older adolescents (15-19 years) were significantly less likely to be retained in care over the first 24 months, compared with younger adolescents. This finding is congruent with the trends reported in other studies21,22 and corresponds to the transition of adolescents from paediatric to adult HIV programmes - a known high-risk period for disengagement with care.23,24,25 Several authors have argued that patient-level challenges, such as developmental delays, mental health issues, stigma and social support at home and school, must be adequately addressed for a successful transition to take place.26 A supported transition requires a skilful adult treatment team and the provision of facilitated care aimed at overcoming the disruptions of the patient-paediatric provider relationship. The loss of ancillary support is required to foster independence, the exercise of autonomy and the growth of personal responsibility.27,28
Although male adolescents constituted a smaller proportion of the study sample, on average, they had greater RiC throughout the observation period, compared with females. Just under half of the female adolescents (n = 84/182, 46%) were initiated on ART whilst pregnant. They exited care at an alarming rate, that is, 44%, 64% and 79% at 4, 12 and 24 months, respectively. These findings correspond to those of Nuwagaba-Biribonwoha et al. who found a greater rate of LTFU amongst pregnant and non-pregnant female adolescents, compared with male adolescents.29 The current study reports lower RiC rates, compared with the 76.4% RiC at 12 months noted in a recent systematic review of pregnant and post-partum women in Africa.30 This report found younger age and same-day ART initiation to be risk factors for poor retention, as was initiating during pregnancy, particularly late pregnancy.
Our findings indicate that adolescents who were classified as WHO stage IV at ART initiation showed better RiC rates at months 4, 12 and 24 post-initiation, although no statistical significance was achieved. Individuals at WHO stages III and IV are likely to remain in care because they are motivated by their health status and by the association between treatment and health outcomes. Clinicians tend to monitor individuals who are at WHO stages III and IV more closely because of other comorbidities such as tuberculosis and other opportunistic infections requiring clinical assessments. However, Matyanga et al. found that a low CD4 count and advanced HIV infection at initiation were associated with LTFU.20 We also found that adolescents classified as WHO stage I at ART initiation had significantly lower rates of RiC at 4 months post-initiation versus those with a WHO stage III. Contrary to the results in our study, another Ugandan study found that the risk of LTFU of adolescents at 12 months was significantly greater amongst those on WHO clinical stages III and IV, compared with those on WHO stages I and II.31 People living with HIV at WHO stage I hardly display signs and symptoms associated with AIDS. The literature has attributed this low RiC behaviour amongst adolescents at stage I to not feeling 'sick' or feeling 'well' as a proxy of nothing being wrong.
Although the primary focus of our study was not on pregnant, HIV-infected adolescents, many in this sub-group were captured in our sample. This could be explained by the fact that pregnant, HIV-infected adolescents are often horizontally infected and receive their positive HIV test result for the first time when booking for antenatal care. Although vertical transmission of HIV is common amongst younger ALWH, horizontal transmission is a frequent mode of transmission in older adolescents. Adolescent boys tend to not access HIV treatment because they mostly remain asymptomatic at this stage.
Interventions such as task shifting, community-based adherence support, mHealth platforms and group adherence counselling emerged as strategies in adult populations that could be adapted for adolescents.32,33 These interventions may benefit older adolescents, especially those transitioning to adult programmes that utilise them. However, the effectiveness of, for example, 'teen clubs', has had mixed results. MacKenzie et al.34 reported that Malawian ALWH who were not in a teen club were less likely to be retained than those in teen clubs. On the other hand, Munyayi and van Wyk35 found that group-based adherence interventions such as teen clubs did not improve retention rates for younger adolescents in specialised paediatric ART clinics in Namibia but did hold potential for improving rates in older adolescents. Adolescent-only clinics and monthly meetings have been shown to improve the RiC of adolescents.36 To this end, we support the calls of other authors for interventions, especially targeting older adolescents whose needs are increased during the transition period.23
Conclusion
Our study highlights low RiC for adolescents over the first 2 years after initiation on ART. Critical intervention is needed to motivate adolescents to remain in care, adhere to treatment and ultimately to achieve and maintain VL suppression (even when they are not feeling sick). Targeted interventions to address transition coordination - pre- and post-transition from paediatric to adult HIV programmes - are needed to counter older adolescents dropping out of care. Female adolescents who initiate ART whilst pregnant should receive special attention. This aligns with the increased need to provide and integrate appropriate sexual reproductive health services to ALWH. Behavioural interventions to improve adherence and RiC should ideally be embedded in community health services so that this forms part of an 'extended' HIV treatment package.
Acknowledgements
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
E.K. conducted the research under the supervision of B.v.W., and F.M. and B.v.W. drafted the first manuscript. E.K. and F.M. commented on all drafts. All authors approved the final version.
Funding information
This project was supported under a Medical Research Council of South Africa Self-Initiated Research grant.
Data availability statement
Data are available upon request to the author.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the South African Medical Research Council or University of the Western Cape.
References
1.UNICEF. Turning the tide against AIDS will require more concentrated focus on adolescents and young people [homepage on the Internet]. 2019 [cited 2019 Oct 21]. [ Links ] Available from: https://data.unicef.org/topic/hivaids/adolescents-young-people/
2.Joint United Nations Programme on HIV/AIDS (UNAIDS). Data [homepage on the Internet]. [ Links ] 2018 [cited 2019 Oct 21]. Available from: https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf
3.South African National HIV prevalence, incidence, behaviour and communication survey [homepage on the Internet]. [ Links ] 2017 [cited 2019 Dec 03]. Available from: http://www.hsrc.ac.za/uploads/pageContent/9234/SABSSMV_Impact_Assessment_ Summary_ZA_ADS_cleared_PDFA4.pdf
4.Maskew M, Bor J, MacLeod W, Carmona S, Sherman GG, Fox MP. Adolescent HHIV treatment in South Africa's national HIV programme: A retrospective cohort study. Lancet HIV. 2019;6(11):e760-e768. https://doi.org/10.1016/S2352-3018(19)30234-6 [ Links ]
5.UNICEF. Children and AIDS [homepage on the Internet]. 2017 [cited 2018 Aug 18]. [ Links ] Available from: https://data.unicef.org/wp-content/uploads/2017/11/HIVAIDS-Statistical-Update-2017.pdf
6.UNAIDS. 90-90-90: An ambitious treatment target to help end the AIDS epidemic [homepage on the Internet]. [ Links ] UNAIDS; 2014 [cited 2019 Oct 15]. Available from: http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf
7.Mugglin C, Haas AD, Van Oosterhout JJ, et al. Long-term retention on antiretroviral therapy among infants, children, adolescents and adults in Malawi-term retention on antiretroviral therapy among infants, children, adolescents and adults in Malawi: A cohort study. PLoS One. 2019;14(11):e0224837. https://doi.org/10.1371/journal.pone.0224837 [ Links ]
8.Wong VJ, Murray KR, Phelps BR, Vermund SH, McCarraher DR. Adolescents, young people, and the 90-90-90 goals: A call to improve HIV testing and linkage to treatment. AIDS. 2019;31(Suppl. 3):S191-S194. https://doi.org/10.1097/QAD.0000000000001539 [ Links ]
9.Nglazi MD, Kranzer K, Holele P, et al. Treatment outcomes in HIV-infected adolescents attending a community-based antiretroviral therapy clinic in South Africa. BMC Infect Dis. 2012;12:21. https://doi.org/10.1186/1471-2334-12-21 [ Links ]
10.Nachega JB, Hislop M, Nguyen H, Dowdy DW, Chaisson RE, Regensberg L. Antiretroviral therapy adherence, virologic and immunological outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65-71. https://doi.org/10.1097/QAI.0b013e318199072e [ Links ]
11.Kariminia A, Law M, Davies M, et al. Mortality and losses to follow-up among adolescents living with HIV in the IeDEA global cohort collaboration. JIAS. 2018;21:e25215. https://doi.org/10.1002/jia2.25215 [ Links ]
12.Western Cape Government Health. 2016. The Western Cape consolidated guidelines for HIV treatment: Prevention of mother-to-child transmission of HIV (PMTCT), children, adolescents and adults. Western Cape: Western Cape Government Health. [ Links ]
13.Western Cape Government Health: HAST. June 2017 ART data sign-off document. Western Cape: Western Cape Government Health; 2017. [ Links ]
14.Osler M, Hilderbrand K, Hennessey C, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. JIAS. 2014;17:18908. https://doi.org/10.7448/IAS.17.1.18908 [ Links ]
15.Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RPH. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: Analysis of routine data. PLoS One. 2020;15(1):e0227572. https://doi.org/10.1371/journal.pone.0227572 [ Links ]
16.Western Cape Government Health. Update to antiretroviral guidelines: Circular H116 of 2012 [homepage on the Internet]. [ Links ] 2012 [cited 2017 Oct 06]. Available from: http://www.doh.gov.za.
17.Nabukeera-Barungi N, Elyanu P, Asire B, et al. Adherence to antiretroviral therapy and retention in care for adolescents living with HIV from 10 districts in Uganda. BMC Infect Dis. 2015;15:520. https://doi.org/10.1186/s12879-015-1265-5 [ Links ]
18.Zanoni BC, Archary M, Buchan S, et al. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: The Cresting Wave. BMJ Global Health. 2016;1:e000004. https://doi.org/10.1136/bmjgh-2015-000004 [ Links ]
19.Adejumo OA, Malee KM, Ryscavage P, Hunter SJ, Taiwo BO. Contemporary issues on the epidemiology and antiretroviral adherence of HIV-infected adolescents in sub-Saharan Africa: A narrative review. J Int AIDS Soc. 2015;18(1):20049. https://doi.org/10.7448/IAS.18.1.20049 [ Links ]
20.Matyanga CMJ, Takarinda KC, Owiti P, et al. Outcomes of antiretroviral therapy among younger versus older adolescents and adults in an urban clinic, Zimbabwe. Public Health Action. 2016;6(2):97-104. https://doi.org/10.5588/pha.15.0077 [ Links ]
21.Kranzer K, Bradley J, Musaazi J, et al. Loss to follow-up among children and adolescents growing up with HIV infection: Age really matters. J Int AIDS Soc. 2017;20(1):21737. https://doi.org/10.7448/IAS.20.1.21737 [ Links ]
22.Okoboi S, Ssali L, Yansaneh A, et al. Factors associated with long-term antiretroviral therapy attrition among adolescents in rural Uganda: A retrospective study. J Int AIDS Soc. 2016;19(554):20841. https://doi.org/10.7448/IAS.19.5.20841 [ Links ]
23.Dahourou DL, Gautier-Lafaye C, Teasdale CA, et al. Transition from paediatric to adult care of adolescents living with HIV in sub-Saharan Africa: Challenges, youth-friendly models, and outcomes. J Int AIDS Soc. 2017;20(Suppl. 3):21528. https://doi.org/10.7448/IAS.20.4.21528 [ Links ]
24.Cervia JS. Easing the transition of HIV infected adolescents to adult care. AIDS Patient Care STDS. 2013;27(12):692-696. https://doi.org/10.1089/apc.2013.0253 [ Links ]
25.Pinzón-Iregui MC, Ibanez G, Beck-Sagué C, Halpern M, Mendoza RM. '… like because you are grownup, you do not need help': Experiences of transition from pediatric to adult care among youth with perinatal HIV infection, their caregivers, and health care providers in the Dominican Republic. J Int Assoc Provid AIDS Care. 2017;16(6):579-587. https://doi.org/10.1177/2325957417729749 [ Links ]
26.Vijayan T, Benin AL, Wagner K, et al. We never thought this would happen: Transitioning care of adolescents with perinatally acquired HIV infection from paediatrics to internal medicine. AIDS Care. 2009;21(10):1222-1229. https://doi.org/10.1080/09540120902730054 [ Links ]
27.Jones C, Ritchwood TD, Taggart T. Barriers and facilitators to the successful transition of adolescents living with HIV from pediatric to adult care in low and middle-income countries: A systematic review and policy analysis. AIDS Behav. 2019;23(9):2498-2513. https://doi.org/10.1007/s10461-019-02621-6 [ Links ]
28.Kung TH, Wallace ML, Snyder KL, et al. South African healthcare provider perspectives on transitioning adolescents into adult HIV care. SAMJ. 2016;106(8):804-808. https://doi.org/10.7196/SAMJ.2016.v106i8.10496 [ Links ]
29.Nuwagaba-Biribonwoha H, Kiragga AN, Yiannoutsos CT, et al. Adolescent pregnancy at antiretroviral therapy (ART) initiation: A critical barrier to retention on ART. J Int AIDS Soc. 2018;21:e25178. https://doi.org/10.1002/jia2.25178 [ Links ]
30.Knettel BA, Cichowitz C, Ngocho JS, et al. Retention in HIV care during pregnancy and the postpartum period in the option B+ era: A systematic review and meta-analysis of studies in Africa. J Acquir Immune Defic Syndr. 2018;77(5):427-438. https://doi.org/10.1097/QAI.000000000001616 [ Links ]
31.Ssali L, Kalibala S, Birungi J, et al. Retention of adolescents living with HIV in care, treatment, and support programs in Uganda. 2014; Washington, DC: United States Agency for International Development. Available from: https://www.popcouncil.org/uploads/pdfs/2014HIVCore_UgandaAdolHAART.pdf. [ Links ]
32.Ridgeway K, Dulli LS, Murray KR, et al. Interventions to improve antiretroviral therapy adherence among adolescents in low- and middle-income countries: A systematic review of the literature. PLoS One. 2018;13(1):e0189770. https://doi.org/10.1371/journal.pone.0189770 [ Links ]
33.Murray KR, Dulli LS, Ridgeway K, et al. Improving retention in HIV care among adolescents and adults in low-and middle-income countries: A systematic review of the literature. PLoS One. 2017;12(9):e0184879. https://doi.org/10.1371/journal.pone.0184879 [ Links ]
34.MacKenzie RK, Van Lettow M, Gondwe C, et al. Greater retention in care amongst adolescents on antiretroviral treatment accessing 'Teen Club' an adolescent-centred differentiated care model compared with standard of care: A nested case-control study at a tertiary referral hospital in Malawi. J Int AIDS Soc. 2017;20(3):e25028. https://doi.org/10.1002/jia2.25028 [ Links ]
35.Munyayi F, Van Wyk B. The effects of teen clubs on retention in HIV care amongst adolescents in Windhoek, Namibia. S Afr J HIV Med. 2020;21(1):a1031. https://doi.org/10.4102/sajhivmed.v21i1.1031 [ Links ]
36.Izudi J, Mugenyi J, Mugabekazi M, Spector VT, Katawera A, Kekitiinwa A. Retention of HIV-positive adolescents in care: A quality improvement intervention in mid-western Uganda. BioMed Res Int. 2018;2018:1524016. https://doi.org/10.1155/2018/1524016 [ Links ]
 Correspondence:
Correspondence:
Brian van Wyk
bvanwyk@uwc.ac.za
Received: 12 Feb. 2020
Accepted: 23 May 2020
Published: 22 July 2020
ORIGINAL RESEARCH
The spectrum of electrolyte abnormalities in black African people living with human immunodeficiency virus and diabetes mellitus at Edendale Hospital, Pietermaritzburg, South Africa
Preyanka PillayI, II; Somasundram PillayIII, IV; Nobuhle MchunuV, VI
IDepartment of Internal Medicine, Greys Hospital, Pietermaritzburg, South Africa
IISchool of Clinical Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
IIIDepartment of Internal Medicine, Edendale Hospital, Pietermaritzburg, South Africa
IVDepartment of Internal Medicine, King Edward Hospital, Durban, South Africa
VDepartment of Biostatistics, Faculty of Statistics, South African Medical Research Council, Durban, South Africa
VIDepartment of Statistics, School of Mathematics, Statistics and Computer Science, University of KwaZulu-Natal, Pietermaritzburg, South Africa
ABSTRACT
BACKGROUND: Serum electrolyte abnormalities in black African people living with human immunodeficiency virus (HIV) and diabetes mellitus (PLWH/DM) is unknown.
OBJECTIVES: The aim of this study was to analyse serum electrolytes (sodium, potassium, calcium and phosphate) and factors associated with electrolyte abnormalities in black African PLWH/DM versus HIV-uninfected patients with DM.
METHODS: We conducted a retrospective case-control study in 96 black African PLWH/DM (cases) and 192 HIV-uninfected patients with DM (controls), who were visiting the Edendale Hospital DM clinic, from 01 January 2016 to 31 December 2016. Pearson's correlation, multivariate linear and logistic regression analyses were utilised.
RESULTS: Hypocalcaemia was the most frequent electrolyte abnormality in PLWH/DM and HIV-uninfected patients with DM (31.25% vs. 22.91%), followed by hyponatraemia (18.75% vs. 13.54%). Median (IQR) corrected serum calcium levels were significantly lower in PLWH/DM compared with HIV-uninfected patients with DM (2.24 [2.18-2.30] mmol/L vs. 2.29 [2.20-2.36] mmol/L; p = 0.001). For every per cent increase in glycated haemoglobin, the odds of hyponatraemia significantly increased in both PLWH/DM (odds ratio [OR]: 1.55; 95% confidence interval [CI]: 1.19 -2.02; p = 0.003) and HIV-uninfected patients with DM (OR: 1.26; 95% CI: 1.04 -1.54; p = 0.009.
CONCLUSION: Hypocalcaemia and hyponatraemia were the most frequent electrolyte abnormalities and occurred more frequently in PLWH/DM compared with HIV-uninfected patients with DM. People living with HIV and DM have significantly lower corrected serum calcium levels compared with HIV-uninfected patients with DM. Furthermore, hyponatraemia is a marker of impaired glycaemic control.
Keywords: HIV; diabetes mellitus; electrolytes; sodium; potassium; calcium; phosphate; black African.
Introduction
Low- and middle-income countries account for 80% of the global diabetes mellitus (DM) burden.1 The International Diabetes Federation (IDF) estimates that Africa will experience the greatest global upsurge of DM by 2045.1 In 2015, DM was the second leading cause of mortality in South Africa after tuberculosis.2 Moreover, South Africa has the highest prevalence of human immunodeficiency virus (HIV) globally and recorded 7.06 million people living with HIV (PLWH) in 2017, with KwaZulu-Natal province having the majority of these cases.3,4 Following the introduction of antiretroviral therapy (ART), the prevalence of comorbid HIV and DM is on the rise because of an increase in life expectancy and the adverse metabolic effects of ART.5 A study in the United States of America (USA) determined that the prevalence of DM in PLWH was 10.3%, and the prevalence of DM was 3.8% higher in PLWH compared with the general population.6
Electrolytes play a vital role in maintaining homeostasis and are paramount in mediating enzymatic reactions, cellular function and electrical gradients.7 Patients with HIV or DM are predisposed to electrolyte abnormalities because of multifactorial pathophysiological factors.8,9 The risk of nephropathy, with subsequent electrolyte abnormalities, increases in the setting of comorbid HIV and DM. Furthermore, the black African population has distinct electrolyte physiology and a predisposition to chronic kidney disease and HIV-associated nephropathy (HIVAN).10 In addition, the use of tenofovir (TDF) increases the risk of proximal tubular dysfunction and subsequent hypokalaemia and hypophosphataemia.11
The Atherosclerosis Risk in Communities (ARIC) study concluded that African American patients had an approximately twofold greater incidence of type 2 DM compared with white patients.12 Although this racial disparity is multifactorial, lower vitamin D13 and serum potassium levels14 in the black population are being explored as possible contributory factors. Importantly, the prevalence of vitamin D deficiency in type 2 DM is disproportionately elevated in African American people compared with other ethnic groups in the USA.15
Electrolyte abnormalities are associated with increased morbidity and mortality, even if they are chronic or of mild severity and may remain clinically silent until an advanced stage.8,9 However, there is a paucity of data from Africa regarding electrolyte abnormalities in HIV or DM. Furthermore, there are no studies assessing electrolyte abnormalities in black African people living with HIV and diabetes mellitus (PLWH/DM). Determining and understanding the spectrum of electrolyte abnormalities in black African PLWH/DM are of crucial significance, particularly in South Africa, which has a large burden of HIV and DM and is undergoing an epidemiological transition in a resource-limited setting.
The objective of this retrospective case-control study was to determine, compare and identify associated factors regarding serum electrolyte abnormalities (sodium, potassium, calcium and phosphate) in black African PLWH/DM versus black African HIV-uninfected patients with DM who attended the Edendale Hospital DM clinic from 01 January to 31 December 2016.
Methods
This quantitative retrospective case-control study was conducted in 96 black African PLWH/DM (cases) and 192 black African HIV-uninfected patients with DM (controls) attending the Edendale Hospital DM clinic, Pietermaritzburg, KwaZulu-Natal, South Africa, over 1 year from 01 January to 31 December 2016. Records of patients attending the DM clinic were analysed retrospectively from datasheets. Electrolytes were measured during routine outpatient visits at the Edendale Hospital DM clinic. Black African PLWH/DM included in the study could have either type 1 or type 2 DM, be on ART or antiretroviral-naïve, have any degree of renal function that was determined by the estimated glomerular filtration rate (eGFR) and be on medication for comorbidities. Patients with incomplete records were excluded from the study. Sample sizes were determined by applying a power analysis in G*Power, which used an alpha of 0.05, a power of 0.80 and a medium effect size of 0.4. The ratio of cases and controls was 1:2, and participants were selected by random sampling, matched by eGFR. Estimated glomerular filtration rate was stratified by the Kidney Disease Outcomes Quality Initiative (KDOQI) classification. Data were anonymised with reference numbers. Variables analysed included the following:
• age (years)
• sex
• HIV status
• type of DM
• duration of DM (years)
• duration of HIV (years)
• duration of ART (years)
• type of ART
• eGFR (mL/min/1.73m2)
• levels of serum sodium, potassium, corrected calcium and phosphate (mmol/L)
• levels of glycated haemoglobin (HbA1c) (%).
The following serum electrolyte reference ranges, as per the National Health Laboratory Services (NHLS), were utilised:
• sodium: 136 mmol/L - 145 mmol/L
• potassium: 3.5 mmol/L - 5.1 mmol/L
• calcium: 2.20 mmol/L - 2.55 mmol/L
• phosphate: 0.78 mmol/L - 1.42 mmol/L
Any electrolyte values below the lower limit of normal were considered hypo-electrolyte abnormalities, whilst those above the upper limit of normal were considered hyper-electrolyte abnormalities. Corrected serum calcium levels were utilised and calculated as follows: measured total calcium (mmol/L) + 0.02 (40 [g/L] - serum albumin [g/L]). The 2017 Society for Endocrinology, Metabolism and Diabetes of South Africa (SEMDSA) guidelines advocate for an HbA1c ≤ 7% to prevent micro- and macro-vascular complications. Therefore, in this study, adequate glycaemic control was defined as HbA1c ≤ 7%. Estimated glomerular filtration rate was calculated by using the Modification of Diet in Renal Disease (MDRD) formula. Serum electrolytes, eGFR and HbA1c were measured by using the Siemens Dimension® analyser.
Statistical analysis
Data were captured by using Microsoft Excel, version 2016 (Microsoft, USA). Statistical analyses were conducted by using Statistical Analysis Software (SAS), version 9.4 (SAS Institute Inc., Cary, NC, USA). Continuous variables were expressed as medians with interquartile ranges (IQRs). Categorical variables were expressed as frequencies and percentages. Continuous variables were compared by using the Wilcoxon rank-sum test as data were asymmetrically distributed. Categorical variables were compared by using either the Chi-square test or Fisher's exact test if there were less than five observations in any cell. Pearson's correlation coefficient assessed the correlation between HbA1c and electrolytes. Multinomial logistic regression assessed factors associated with electrolyte abnormalities. Linear regression analyses assessed the effect of measured covariates on electrolytes. Multivariable models were stratified by HIV status and were adjusted for gender, age, use of TDF, type of DM, duration of DM, duration of HIV, HbA1c and eGFR. A two-tailed value of p < 0.05 was considered to indicate statistical significance.
Ethical consideration
Ethical approval to conduct the study was obtained from the Biomedical Research and Ethics Committee (BREC) of the University of KwaZulu-Natal (reference number BE576/18). Permission was obtained from Edendale hospital to utilise the DM clinic datasheet for data collection.
Results
Descriptive data
Ninety-six black African PLWH/DM (cases) and 192 black African HIV-uninfected patients with DM (controls) were reviewed. People living with HIV and DM were significantly younger than HIV-uninfected patients with DM (median [IQR]: 46.5 [39-53.5] years vs. 56 [47-64] years; p < 0.001) (Table 1). HIV-uninfected patients had a significantly longer duration of DM compared with PLWH/DM (median [IQR]: 7 [3-13] years vs. 5 [2-9] years; p = 0.006). People living with HIV and DM had a median (IQR) duration of HIV of 7 (3-10) years and 86 (89.58%) patients were on ART, which included 65 (67.7%) patients on TDF (Table 1). Eighty-four (87.5%) PLWH/DM and 172 (89.58%) HIV-uninfected patients had type 2 DM. Seventy-three (76.0%) PLWH/DM and 153 (79.7%) HIV-uninfected patients with DM had an HbA1c > 7%, which was considered uncontrolled. The median (IQR) HbA1c amongst PLWH/DM and HIV-uninfected patients with DM was 9.45% (7.1% - 11.45%) and 9.70% (7.35% - 11.5%), respectively (p = 0.836). An eGFR less than 60 mL/min/1.73m2 was present in 12 (12.5%) PLWH/DM and 24 (12.5%) HIV-uninfected patients with DM, respectively (Table 1).
Analysis of electrolytes
Sodium
Hyponatraemia was the second most frequent electrolyte abnormality, which occurred in 18 (18.75%) PLWH/DM and 26 (13.54%) HIV-uninfected patients with DM (Table 1). Serum sodium was the only electrolyte significantly negatively correlated with HbA1c in both PLWH/DM (r = −0.34; p = 0.001) and HIV-uninfected patients with DM (r = −0.28; p < 0.001) (Table 2). Adjusted multinomial logistic regression analysis amongst PLWH/DM suggests that for every per cent increase in HbA1c, the odds of hyponatraemia significantly increased by 55% (odds ratio [OR]: 1.55; 95% confidence interval [CI]: 1.19-2.02; p = 0.003), whilst in HIV-uninfected patients with DM the odds of hyponatraemia significantly increased by 26% (OR: 1.26; 95% CI: 1.04-1.54; p = 0.009) (Table 3). Multivariate linear regression showed significant associations between serum sodium and HbA1c. Amongst PLWH/DM, for every per cent increase in HbA1c, serum sodium decreased by 0.51 mmol/L (β = −0.51; p = 0.004), and for every per cent increase in HbA1c amongst HIV-uninfected patients with DM, serum sodium decreased by 0.45 mmol/L (β = −0.45; p < 0.001) (Table 4).
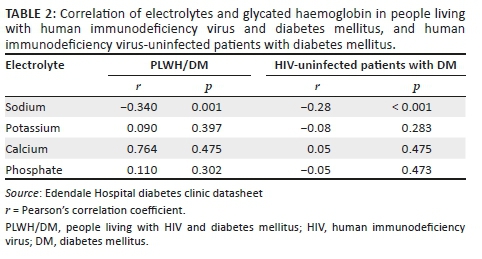
Potassium
The duration of HIV was not significantly associated with hypokalaemia on adjusted multinomial logistic regression analysis (OR: 0.85; 95% CI: 0.59-1.23; p = 0.645). Furthermore, the odds of hypokalaemia in PLWH using TDF compared with non-TDF-based ART were not significant (OR: 0.87; 95% CI: 0.06-13.12; p = 0.766). Adjusted multinomial logistic regression determined that the duration of DM was significantly associated with potassium abnormalities. In PLWH/DM, for every year increase in the duration of DM, the odds of hypokalaemia increased by 97% (OR: 1.97; 95% CI: 1.13-3.43; p = 0.025). However, in HIV-uninfected patients with DM, the odds of hyperkalaemia increased by 10% (OR: 1.10; 95% CI: 1.02-1.19; p = 0.048). Multivariate linear regression also showed significant associations between serum potassium levels and the duration of DM. For every year increase in the duration of DM, serum potassium decreased by 0.04 mmol/L amongst PLWH/DM (β = −0.04; p = 0.018) and increased by 0.01 mmol/L amongst HIV-uninfected patients with DM (β = 0.01; p = 0.042) (Table 4).
Calcium
Serum-corrected calcium was the only electrolyte with median (IQR) levels significantly lower in PLWH/DM compared with HIV-uninfected patients with DM (2.24 [2.18-2.30] mmol/L vs. 2.29 [2.20-2.36] mmol/L; p = 0.001). Furthermore, the most frequent electrolyte abnormality in PLWH/DM and HIV-uninfected patients with DM was hypocalcaemia (31.25% vs. 22.91%) (Table 1). Adjusted multinomial logistic regression in PLWH/DM and HIV-uninfected patients with DM found no factors significantly associated with hypocalcaemia or hypercalcaemia. However, multivariate linear regression analysis in HIV-uninfected patients with DM showed that for every year increase in age, serum calcium decreased by 0.01 mmol/L (β = −0.01; p = 0.031) (Table 4).
Phosphate
Adjusted multinomial logistic regression in PLWH/DM and HIV-uninfected patients with DM found no factors significantly associated with hypophosphataemia or hyperphosphataemia. Notably, the use of TDF was not significantly associated with hypophosphataemia in PLWH/DM (OR: 1.69; 95% CI: 0.20-14.12; p = 0.800). Multivariate linear regression in PLWH/DM determined that for every year increase in age, serum phosphate decreased by 0.01 mmol/L (β = −0.01; p = 0.033). Moreover, on average, serum phosphate was 0.09 mmol/L higher in women than in men amongst HIV-uninfected patients with DM, and all other variables were constant (β = 0.09; p = 0.006) (Table 4).
Discussion
Sodium
Serum sodium abnormalities in DM vary depending on the degree of water and sodium change.9 Serum glucose is an osmotically active substance; therefore, hyponatraemia in DM is mostly attributed to hyperglycaemia-induced hyper-osmolality, resulting in a dilutional effect or osmotic diuresis with hypovolemic hyponatraemia.9 Hypernatraemia may occur if water loss exceeds sodium loss.9 Our study identified serum sodium to be the only electrolyte significantly associated with HbA1c levels in both PLWH/DM and HIV-uninfected patients with DM. Furthermore, elevated HbA1c levels significantly increased the odds of hyponatraemia, with the odds being greater in PLWH/DM compared with their HIV-uninfected counterparts. Although pseudo-hyponatraemia in DM is common, hyponatraemia could be utilised as a marker of impaired DM control. Our finding regarding the association between HbA1c and serum sodium levels is comparable with that of a study conducted in India, which determined that mean (standard deviation [s.d.]) serum sodium levels were significantly lower in patients with DM compared with non-DM controls (127.92 [0.45] mmol/L vs. 135.82 [0.34] mmol/L; p = 0.0001) and that HbA1c was significantly inversely correlated with serum sodium levels (r = 0.640; p = 0.0001).7 However, no regression analysis was performed in the study conducted in India.7 Other causes of hyponatraemia in DM include side effects of drugs such as diuretics, diabetic nephropathy and the syndrome of inappropriate antidiuretic hormone secretion (SIADH).9
Our study demonstrated that PLWH/DM had a higher frequency of hyponatraemia compared with HIV-uninfected patients with DM (18.75% vs. 13.4%). The increased frequency of hyponatraemia in PLWH/DM could be attributed to the additive effect of HIV on sodium homeostasis. Hyponatraemia is a common electrolyte disorder in PLWH and a possible marker of HIV severity, as patients with hyponatraemia have significantly lower CD4 counts, higher viral loads and an increased prevalence of acquired immunodeficiency syndrome (AIDS).16 In PLWH, the main causes of hyponatraemia include opportunistic infections which predispose to SIADH, adrenal insufficiency, diarrhoea and vomiting.17 Furthermore, dysfunction of the thick ascending limb of the loop of Henle secondary to HIV and inflammation results in impaired free water clearance and dilutional hyponatraemia.8,18
People living with HIV and DM may be at a higher risk of hyponatraemia because of contributory factors from both HIV and DM. Furthermore, hyponatraemia in the black African population may indicate a greater degree of sodium imbalance compared with other ethnic groups as the black population physiologically have increased sodium retention with lower plasma renin and aldosterone levels.19,20
Potassium
Hyperkalaemia is common in DM.7,21,22 Conversely, studies conducted in Nigeria and Saudi Arabia found a predominant hypokalaemia and no significant association between serum potassium levels and glycaemic control, respectively.23,24 Similarly, our study found no significant association between HbA1c and serum potassium levels. Common causes of hyperkalaemia in DM and HIV include hyporeninaemic hypo-aldosteronism, acidosis, renal impairment and drugs such as angiotensin-converting enzyme (ACE) inhibitors, potassium-sparing diuretics and beta-blockers8,9 Hypokalaemia in DM is frequently caused by insulin administration, malabsorption, osmotic diuresis and hypomagnesaemia.9 In PLWH, hypokalaemia is commonly caused by vomiting, diarrhoea and proximal tubular dysfunction secondary to TDF.25,26,27 However, in our study, TDF was not significantly associated with hypokalaemia. This could be attributed to our study having relatively young PLWH that were on ART for a median duration of 6 years and only 12.5% of PLWH having an eGFR < 60ml/min/1.73m2, which may reduce the risk of TDF induced nephrotoxicity.
Notably, our study determined that for every unit increase in DM duration, the odds of hypokalaemia significantly increased by 97% in PLWH/DM. This could be attributed to patients with comorbid HIV and DM developing a greater degree of insulin resistance, as both conditions progress,28 and therefore require higher doses of insulin to achieve glycaemic control with a propensity for hypokalaemia. This possible contributory factor needs to be explored further as our study did not evaluate the use of insulin. In HIV-uninfected patients with DM, the likelihood of hyperkalaemia significantly increased by 10% for every unit increase in DM duration. This could be attributed to dysautonomia in long-standing DM which impairs the conversion of prorenin to renin and predisposes to hyporeninaemic hypo-aldosteronism and associated hyperkalaemia.29
Calcium
Calcium homeostasis is strongly regulated by parathyroid hormone and vitamin D. Factors contributing to hypocalcaemia in HIV and DM include vitamin D deficiency, hypoparathyroidism and hypomagnesaemia.9,30 Our study identified hypocalcaemia as the most common electrolyte abnormality in both PLWH/DM and HIV-uninfected patients with DM. Furthermore, serum calcium was the only electrolyte with median levels significantly lower in PLWH/DM compared with HIV-uninfected patients with DM. Similarly, Keuhn et al. identified mean serum calcium levels to be significantly lower in HIV-infected patients compared with controls (p < 0.0001), irrespective of serum albumin levels.30 Hypocalcaemia is also common in patients with DM, with a study in Sudan showing significantly lower mean serum calcium levels in patients with DM compared with controls (p < 0.05).31 Furthermore, vitamin D deficiency in the elderly is common despite consistent vitamin D intake and may predispose to hypocalcaemia.32 Notably, our study demonstrated a significant inverse association between serum calcium and age in HIV-uninfected patients with DM. A significant association between serum calcium and age may have not been detected in PLWH/DM as they were significantly younger. The degree of sunlight exposure was not documented in this study. Although PLWH/DM were significantly younger than HIV-uninfected patients with DM, clinically this difference in age should not usually result in a greater proportion of HIV-uninfected patients being housebound. Therefore, the significant inverse association between serum calcium levels and age in HIV-uninfected patients may be influenced by factors besides sun exposure.
The mechanism of vitamin D deficiency in HIV is multifactorial and involves the inhibitory effect of pro-inflammatory cytokines that reduces renal 1-α hydroxylation of vitamin D and the consumption of vitamin D by macrophages and lymphocytes.33 Furthermore, vitamin D has a significant immunomodulatory role, and deficiencies in PLWH are associated with lower CD4 cell counts, higher viral loads, HIV progression and an increased risk of opportunistic infections.34 Moreover, studies have suggested that vitamin D deficiency and low calcium levels result in impaired insulin synthesis and secretion with subsequent glucose intolerance and insulin resistance.15 The European AIDS Clinical Society (EACS) has vitamin D supplementation recommendations and reports that the prevalence of low vitamin D levels was up to 80% in HIV cohorts and was associated with an increased risk of osteoporosis, type 2 DM, mortality and AIDS events.35 This is in contrast to South African HIV and DM guidelines, which do not have vitamin D deficiency recommendations despite our susceptible population.36,37
Consequently, the presence of comorbid HIV and DM in the black African population potentially increases the risk of vitamin D deficiency and hypocalcaemia, which might negatively impact HIV and DM control. The role of vitamin D in the pathogenesis and control of HIV and DM and the effect of vitamin D supplementation need to be further explored, particularly in the black African population.
Phosphate
The risk of TDF-induced nephrotoxicity with isolated hypophosphataemia, proximal tubular dysfunction or Fanconi syndrome increases in the presence of renal impairment.38 This is of particular concern in patients with comorbid HIV and DM, advancing age, lower CD4 cell counts and elevated baseline creatinine levels.38 Our study did not find TDF to be significantly associated with hypophosphataemia or serum phosphate levels in PLWH/DM. This could be attributed to the fact that our study had only 12.5% of PLWH/DM with an eGFR <60 mL/min/1.73m2, patients were using ART for a median duration of only 6 years and PLWH/DM were relatively young. A study by Day et al. observed the frequency of hypophosphataemia in TDF recipients to be higher than non-TDF ART recipients (31% vs. 22%). However, no independent association was found between TDF use and the frequency or severity of hypophosphataemia.39 The recognition that hypophosphataemia in PLWH on TDF is multifactorial must be considered to avoid unnecessary TDF cessation in a resource-limited setting.
Current guidelines
This study determined that electrolyte abnormalities in black African PLWH/DM are common, with hypocalcaemia and hyponatraemia being the most frequent electrolyte abnormalities. However, the current SEMDSA guidelines only recommend that serum potassium needs to be measured at diagnosis and monitored annually.37 Furthermore, South African HIV guidelines only recommend the monitoring of serum potassium and phosphate in high-risk patients and patients with features of tubular wasting.36
Limitations
The limitations of this study included CD4 count and viral load not being documented for a majority of patients as management and monitoring of HIV occurs at designated HIV clinics. Therefore, the association between HIV control and electrolyte abnormalities could not be determined. Possible TDF-induced proximal tubular dysfunction and electrolyte loss were not assessed with urine electrolytes as they were not routinely performed in the DM clinic. Patients in this study could have been on medication or could have comorbidities which may affect electrolytes. However, by including these patients the study was more representable and reproducible as the majority of patients suffering from DM were usually part of a metabolic syndrome which requires chronic treatment to improve outcomes. In addition, the use of oral antidiabetic medication or insulin and the respective doses were not included. This could have been used to compare the DM treatment requirements in PLWH/DM and HIV-uninfected patients as both groups had similar HbA1c levels. Lastly, because of the retrospective nature of the study, causality could not be determined. However, this is a newly explored topic, and this study is useful in providing preliminary data for future prospective studies.
Conclusion
Serum electrolyte abnormalities in black African PLWH/DM are common. Hypocalcaemia and hyponatraemia were the most frequent electrolyte abnormalities and occurred more frequently in PLWH/DM compared with HIV-uninfected patients with DM. Serum calcium levels were significantly lower in black African PLWH/DM compared with HIV-uninfected patients with DM. Importantly, hyponatraemia is a potential marker of impaired glycaemic control as elevated HbA1c levels significantly increased the odds of hyponatraemia in both groups; however, the odds were greater in PLWH/DM. Ultimately, black African PLWH/DM are highly vulnerable to electrolyte abnormalities because of multifactorial pathophysiological factors. Further large prospective studies regarding electrolyte abnormalities in black African PLWH/DM will assist in identifying contributory factors and implementing tailored guidelines that could facilitate prevention, earlier detection, closer monitoring and appropriate intervention to reduce associated adverse effects in this high-risk population, particularly in the South African context.
Acknowledgements
This study contributes towards the Master of Medical Science degree of Dr Preyanka Pillay, which is supervised by Dr Somasundram Pillay.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
P.P. contributed to the conception and design of the study as well as the collection, analysis and interpretation of the data. P.P. wrote and edited the manuscript. S.P. contributed to the conception and design of the study and critically reviewed and edited the manuscript. N.M. contributed to the statistical analysis and interpretation of data; she also critically reviewed and edited the manuscript. All authors gave final approval of the version to be published.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data are available upon request from the corresponding author.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.International Diabetes Federation. IDF Diabetes Atlas, 8th ed. [ Links ] [homepage on the Internet]. Brussels: International Diabetes Federation; 2017 [cited 2019 Mar 03]. Available from: http://www.diabetesatlas.org
2.Statistics South Africa. Media release: Mortality and causes of death, 2015 [hompage on the Internet]. [ Links ] Statistics South Africa [cited 2019 Mar 03]. Available from: http://www.statssa.gov.za/?p=9604
3.UNAIDS. South Africa overview [homepage on the Internet]. [ Links ] [cited 2019 Mar 03]. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica
4.Statistics South Africa. Mid-year population estimates 2017 [homepage on the Internet]. [ Links ] [cited 2019 Mar 03]. Available from: http://www.statssa.gov.za/publications/P0302/P03022017.pdf
5.Samad F, Harris M, Puskas C, et al. Incidence of diabetes mellitus and factors associated with its development in HIV-positive patients over the age of 50. BMJ Open Diabetes Res Care. 2017;5(1):e000457. https://doi.org/10.1136/bmjdrc-2017-000457 [ Links ]
6.Hernandez-Romieu A, Garg S, Rosenberg E, Thompson-Paul A, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009-2010. BMJ Open Diabetes Res Care. 2017;5(1):e000304. https://doi.org/10.1136/bmjdrc-2016-000304 [ Links ]
7.Rajagopal L, Ganesan V, Abdullah S, et al. Exploring the interrelationship between electrolytes, anemia, and glycosylated hemoglobin (HbA1c) levels in type 2 diabetics. Asian J Pharm Clin Res. 2018;11(1):251. https://doi.org/10.22159/ajpcr.2017.v11i1.22533 [ Links ]
8.Musso C, Belloso W, Glassock R. Water, electrolytes, and acid-base alterations in human immunodeficiency virus infected patients. World J Nephrol. 2016;5(1):33-42. https://doi.org/10.5527/wjn.v5.i1.33 [ Links ]
9.Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases: WJCC. 2014;2(10):488-496. https://doi.org/10.12998/wjcc.v2.i10.488 [ Links ]
10.Naicker S, Rahmania S, Kopp J. HIV and chronic kidney disease. Clin Nephrol. 2015;83(Suppl 1):32-38. https://doi.org/10.5414/cnp83s032 [ Links ]
11.Labarga P, Barreiro P, Martin-Carbonero L, et al. Kidney tubular abnormalities in the absence of impaired glomerular function in HIV patients treated with tenofovir. AIDS. 2009;23(6):689-696. https://doi.org/10.1097/qad.0b013e3283262a6 [ Links ]
12.Brancati F, Kao W, Folsom A, et el. Incident type 2 diabetes mellitus in African American and white adults: The atherosclerosis risk in communities study. JAMA. 2000;283(17):2253-2259. https://doi.org/10.1001/jama.283.17.2253 [ Links ]
13.Alvarez J, Bush N, Choquette S, et al. Vitamin D intake is associated with insulin sensitivity in African American, but not European American, women. Nutr Metab. 2010;7(1):28. https://doi.org/10.1186/1743-7075-7-28 [ Links ]
14.Chatterjee R, Yeh H-C, Shafi T, et al. Serum potassium and the racial disparity in diabetes risk: The Atherosclerosis Risk in Communities (ARIC) study. Am J Clin Nutr. 2011;93(5):1087-1091. https://doi.org/10.3945/ajcn.110.007286 [ Links ]
15.Davis S. Vitamin D deficiency and type 2 diabetes in African Americans: The common denominators. Diabetes Spectrum. 2011;24(3):148. https://doi.org/10.2337/diaspect.24.3.148 [ Links ]
16.Braconnier P, Delforge M, Garjau M, Wissing K, De Wit S. Hyponatremia is a marker of disease severity in HIV-infected patients: A retrospective cohort study. BMC Infect Dis. 2017;17(1):98. https://doi.org/10.1186/s12879-017-2191-5 [ Links ]
17.Shu Z, Tian Z, Chen J, et al. HIV/AIDS-related hyponatremia: An old but still serious problem. Renal Failure. 2018;40(1):68-74. https://doi.org/10.1080/0886022x.2017.1419975 [ Links ]
18.Belloso W, de Paz Sierra M, Navarro M, Sanchez M, Perelsztein A, Musso C. Impaired urine dilution capability in HIV stable patients. Int J Nephrol. 2014;2014:1-8. https://doi.org/10.1155/2014/381985 [ Links ]
19.Sagnella G. Why is plasma renin activity lower in populations of African origin? J Hum Hypertens. 2001;15(1):17-25. https://doi.org/10.1038/sj.jhh.1001127 [ Links ]
20.Rayner B, Myers J, Opie L, Trinder Y, Davidson J. Screening for primary aldosteronism - Normal ranges for aldosterone and renin in three South African population groups. S Afr Med J. 2001;91(7):594-599. https://doi.org/10.1177/1470320312463833 [ Links ]
21.Anago E, Medehouenou T, Akpovi CD, Tchehouenou H. Electrolyte disturbances in diabetic patients in Cotonou, Benin. Int J Res Med Sci. 2016;4:5430-5435. https://doi.org/10.18203/2320-6012.ijrms20164223 [ Links ]
22.McNair P, Madsbad S, Christiansen C, Christensen M, Transbol I. Hyponatremia and hyperkalemia in relation to hyperglycemia in insulin-treated diabetic out-patients. Clin Chim Acta. 1982;120(2):243-250. https://doi.org/10.1016/0009-8981(82)90161-9 [ Links ]
23.E. Ugwuja N. A comparative study of serum electrolytes, total protein, calcium and phosphate among diabetic and HIV/AIDS patients in Abakaliki, Southeastern, Nigeria. Internet J Lab Med. 2007;2(1):1-5. https://doi.org/10.5580/15e5 [ Links ]
24.Al-Rubeaan K, Siddiqui K, Abu Risheh K, et al. Correlation between serum electrolytes and fasting glucose and Hba1c in Saudi diabetic patients. Biol Trace Element Res. 2011;144(1-3):463-468. https://doi.org/10.1007/s12011-011-9144-4 [ Links ]
25.Eshiet E, Okogun G. Plasma urea and electrolytes profile in different stages of hiv infection in Ekpoma, Nigeria. Afr J Cell Pathol. 2015;4(1):1-5. https://doi.org/10.5897/ajcpath15.001 [ Links ]
26.Narayanan Ramesh V. To study the renal and electrolyte disturbances in HIV infected patients. IOSR Jof Dent Med Sci. 2017;16(8):91-94. [ Links ]
27.Emejulu A, Onwuliri V, Ojiako O. Electrolyte abnormalities and renal impairment in asymptomatic HIV-infected patients in Owerri, South Eastern Nigeria. Aust J Basic Appl Sci. 2011;5(3):257-260. [ Links ]
28.Kalra S, Kalra B, Agrawal N, Unnikrishnan A. Understanding diabetes in patients with HIV/AIDS. Diabetol Metab Syndr. 2011;3(1):2. https://doi.org/10.1186/-1758-5996-3-2 [ Links ]
29.McFarlane S, Sowers J. Aldosterone function in diabetes mellitus: Effects on cardiovascular and renal disease. J Clin Endocrinol Metab. 2003;88(2):516-523. https://doi.org/10.1210/jc.2002-021443 [ Links ]
30.Kuehn E, Anders H, Bogner J, Obermaier J, Goebel F, Schlondorff D. Hypocalcaemia in HIV infection and AIDS. J Intern Med. 1999;245(1):69-73. https://doi.org/10.1046/j.1365-2796.1999.00407.x [ Links ]
31.Hassan S, Elsheikh W, Rahman N, ElBagir N. Serum calcium levels in correlation with glycated hemoglobin in type 2 diabetic sudanese patients. Adv Diabetes Metab. 2016;4(4):59-64. [ Links ]
32.Linnebur S, Vondracek S, Vande Griend J, Ruscin J, McDermott M. Prevalence of vitamin D insufficiency in elderly ambulatory outpatients in Denver, Colorado. Am J Geriatr Pharmacother. 2007;5(1):1-8. https://doi.org/10.1016/j.amjopharm.2007.03.005 [ Links ]
33.Villamor E. A potential role for vitamin D on HIV infection? Nutr Rev. 2006;64(5):226-233. https://doi.org/10.1111/j.1753-4887.2006.tb00205.x [ Links ]
34.Alvarez N, Aguilar-Jimenez W, Rugeles M. The potential protective role of vitamin D supplementation on HIV-1 infection. Front Immunol. 2019;10. https://doi.org/10.3389/fimmu.2019.02291 [ Links ]
35.European AIDS Clinical Society guidelines 2018 [homepage on the Internet]; [ Links ] version 9.1:49-51. [cited 2019 Mar 03]. Available from: www.eacsociety.org/files/2018_guidelines-9.1-english.pdf
36.Meintjes G, Moorhouse M, Carmona S, et al. Adult antiretroviral therapy guidelines 2017. S Afr J HIV Med. 2017;18(1):a776. https://doi.org/10.4102/sajhivmed.v18i1.776 [ Links ]
37.SEMDSA 2017 guidelines for the management of type 2 diabetes mellitus. SEMDSA type 2 Diabetes Guidelines Expert Committee. JEMSDA [serial online] 2017 [cited 2019 Mar 03];22(1):1-196. Available from: https://www.semdsa.org.za/images/647-4385-1-pb.pdf
38.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: A double-edged sword? J Am Soc Nephrol. 2013;24(10):1519-1527. https://doi.org/10.1681/asn.2012080857 [ Links ]
39.Day S, Leake Date H, Bannister A, Hankins M, Fisher M. Serum hypophosphatemia in tenofovir disoproxil fumarate recipients is multifactorial in origin, questioning the utility of its monitoring in clinical practice. JAIDS J Acquir Immune Defic Syndr. 2005;38(3):301-304. [ Links ]
 Correspondence:
Correspondence:
Preyanka Pillay
preyankapillay@yahoo.com
Received: 26 Apr. 2020
Accepted: 07 June 2020
Published: 23 July 2020
ORIGINAL RESEARCH
Understanding adherence in virally suppressed and unsuppressed human immunodeficiency virus-positive urban patients on second-line antiretroviral treatment
Siphamandla B. GumedeI, II; Willem D.F. VenterI; Samanta T. Lalla-EdwardI
IEzintsha, a sub-division of Wits Reproductive Health and HIV Institute, University of the Witwatersrand, Johannesburg, South Africa
IIDepartment of Interdisciplinary Social Science, Public Health, Utrecht University, Utrecht, The Netherlands
ABSTRACT
BACKGROUND: Understanding antiretroviral therapy (ART) adherence may assist in designing effective support interventions
OBJECTIVES: This study elicited perspectives on how to promote treatment adherence from virologically suppressed and unsuppressed patients receiving second-line ART
METHODS: This was a cross-sectional study conducted with randomly selected patients active on second-line ART, from five public health facilities in the Johannesburg inner city. Data were collected on demographics, clinical information, participant's experiences and ART knowledge. Virological failure was defined as exceeding 1000 copies/mL
RESULTS: The study sample comprised 149 participants; of which 47.7% (n = 71) were virally unsuppressed and 69.1% (n = 103) were women; the median age of the participants was 42 years (interquartile range [IQR] 36-47 years). Experiencing medication-related difficulties in taking second-line ART (p = 0.003), finding second-line regimen more difficult to take than a first-line regimen (p = 0.001) and experiencing side effects (p < 0.001) were all subjective predictors of virological failure. Participants' recommendations for improving adherence included the introduction of a single tablet regimen (31.6%, n = 55), reducing the dosage to once daily (26.4%, n = 46) and reducing the pill size for second-line regimen (4.0%, n = 7
CONCLUSION: The results of this study highlight the importance of improving patients' knowledge about adherence and motivation to continue ART use despite the persistence of side effects and difficulties with taking medication
Keywords: adherence; viral load suppression; virological failure; antiretroviral therapy; South Africa.
Introduction
The World Health Organization (WHO) defines adherence as the degree to which a patient is able to follow a treatment plan and take medication at prescribed times.1
Factors that affect treatment adherence include changes in daily routines, forgetting to take medication, side effects, depression, being away from home, comorbidity, lack of knowledge and desire to take treatment.2,3,4 In addition, patients experiencing financial constraints, social issues such as the fear of disclosure and lack of understanding of treatment benefits are more prone to non-adherence to treatment and illnesses.5,6 Some studies have reported disclosure and relationship with the person being disclosed to as predictors of adherence.7,8
The South African government's adherence promotion strategies include routine viral load monitoring, adherence counselling, pill counting, adherence clubs and routine completion of clinical stationery.9,10,11 Despite all these antiretroviral therapy (ART) adherence strategies being implemented, treatment failure amongst patients on first- and second-line ART remains an issue.12 Lapses in ART medication adherence can lead to viral rebound with ongoing immunosuppression and viral resistance.1,13,14 However, not much is understood about the perspectives of patients regarding adherence challenges.15,16
Therefore, this study sought to describe and obtain perspectives on treatment adherence from two separate groups of patients on second-line therapy, those who were suppressed and those who were not, to understand treatment-taking behaviour.
Materials and methods
Study design and setting
This was a cross-sectional study conducted between July and August 2018 in a sub-population of patients receiving second-line ART at the end of June 2018. Five public health facilities in inner-city Johannesburg (two hospitals, one community health centre and two primary healthcare clinics) were included in the study.
Study population
The study population comprised patients aged 18 years and older who were on second-line ART for at least 1 month or longer.
Data collection
Sample selection and recruitment
We randomly sampled 10% of the population of 1500 eligible patients. The total number of active patients on second-line treatment per facility was divided by the total sample size (n = 150) to determine the interval that needed to be used to select the eligible patients. Using this formula, every nth (different for each facility) record from the register or list of active patients on second-line treatment in each facility was selected and recruited to the study until the facility sample size was reached. Once the eligible patients were identified, they were invited to participate in the study in one of the two ways: telephonically or in facility recruitment where researchers met them at the facility during their scheduled clinic visit. For the patients who refused to participate in the study, the next nth patient was recruited.
Data collection, tool and variables
A pretested semi-structured questionnaire was used, which consisted of five sections: (1) demographic data, (2) comorbidity information, (3) human immunodeficiency virus (HIV) diagnosis and care information, (4) experiences on the first-line regimen and adherence and (5) experiences on second-line regimen and adherence. Information collected included demographic information (facility name, sex, age, relationship status, employment status and education level), comorbidity information, experiences on both first- and second-line treatment, disclosure information, duration on ART, reasons for starting ART, side effects, self-reported treatment interruptions, challenges with taking second-line treatment, treatment supporter information and insights into how adherence could be improved.
Questionnaire administration
Data were collected by the principal investigator and a trained research assistant. The interviews were conducted in English as it was the most commonly spoken language in the study setting and all participants could speak it.
Data entry, cleaning and analysis
Data were captured into REDCap immediately after interviews were conducted. The research team conducted data clean-up by running data quality checks in REDCap and STATA (quantitative data). For the closed-ended questions, we assessed the association between outcome variables and selected socio-demographic and health-related characteristics. Pearson's chi-squared test was used to assess trend associations between categorical variables. Continuous data were summarised and analysed using the median and interquartile ranges (IQRs). Logistic and multiple logistic regression models (bivariate and multivariate logistic regression) were built for key outcome variables, such as viral load, difficulties in taking second-line regimen and side effects, to identify independent predictors. We reported unadjusted and adjusted odds ratios (ORs), 95% confidence interval (CI) and p-values - p-values that were less than 0.05 were considered significant. Open-ended questions were analysed using qualitative data analysis methods. Data were coded and thematic analysis was performed. Where appropriate, quotations have been included to support the reported results.
Ethical consideration
Ethical approval to conduct the study was obtained from the University of the Witwatersrand Human Research Ethics Committee (ethical clearance number: M170691). Approval was also granted by the Johannesburg Health District (DRC Ref No. 2017-08-003 and NHRD Ref No. GP_201708_030). Participants were informed that participation in the study was voluntary and that refusal would not affect their relationship with their healthcare provider or facility. All patients who agreed to participate in the study signed an informed consent form. To ensure confidentiality, there were no linkages between the data collected in the questionnaire and the patients' clinic information. Participants were reimbursed for their travel.
Results
Sample characteristics
A total of 150 out of 1500 active patients on second-line ART across the five public health facilities were interviewed (69.1%, n = 103 women). During the quality checking processes, we found that one of the participants was younger than 18 years and was subsequently omitted from the analysis. The median age of the participants was 42 years (IQR 36-47 years). Most of the participants were single (38.1%, n = 57); 30.2% (n = 45) participants were married. Nearly two-thirds of the participants were born in South Africa (61.1%, n = 91), whilst almost one-third of the participants were born in Zimbabwe (32.9%, n = 49). The majority (87.2%, n = 130) of participants had completed at least their secondary or high school-level education. A minority (8.1%, n = 12) of participants had completed tertiary qualifications; 4.7% (n = 7) participants had never attended a school. Of the total participants, 45.6% (n = 68) were unemployed. The majority of participants were identified as Christian (87.9%, n = 131). Hypertension (65.1%, n = 28/43), diabetes (9.3%, n = 4/43) and hypercholesterolaemia (9.3%, n = 4/43) were the most common concomitant conditions reported by the participants. The average distance travelled to reach a health facility was 5 km (IQR: 2 km - 15 km), with 57.7% (n = 86) participants travelling 5 km or less to reach the health facility.
Socio-demographic and virological status
Table 1 shows the socio-demographic characteristics disaggregated by virological status. Nearly half (47.7%, n = 71) of the participants interviewed had virological failure (VLF), with women accounting for 73.2% (n = 52). With regard to age, of the total unsuppressed participants, 39.4% (n = 28) were between 30 and 39 years. Of the participants with virological suppression (VLS), 29.5% (n = 23) had comorbidity compared to VLF participants (28.2%, n = 20).
Disclosure, treatment support and virological status
Almost all the participants' first disclosure of their HIV status was to a partner or relative (98.7%, n = 147 combined). More VLS participants (55.1%, n = 43) disclosed about their HIV status to a family member first, whilst most VLF participants (52.1%, n = 37) chose to disclose to their partners first. Almost no disclosure to friends was reported. Disclosure did not show any statistical significance. Participants typically (63.1%, n = 94) disclosed within 1 week after HIV diagnoses, with more VLF participants reporting early disclosure than VLS participants (64.8%, n = 46 vs. 61.5%, n = 48). Most participants (79.2%, n = 118) had treatment supporters (VLF = 80.3%, n = 57; VLS = 78.2%, n = 61). Whilst 10.1% (n = 15) of participants felt like stopping treatment completely at some point, only 3.4% (n = 5) stopped treatment for longer than 1 month, more in VLS participants, although this was not statistically significant.
Factors of virological failure and adherence
Overall, there were more participants (52.3%, n = 78) who felt that taking a second-line regimen was difficult compared to the first-line regimen, with the VLF group (66.2%, n = 47, p = 0.001) predominantly reporting this challenge. Generally, 38.3% (n = 57/149) experienced difficulties in taking the second-line regimen (p = 0.003). Of these, about two-thirds (63.2%, n = 36) were VLF participants (p = 0.003). Just under half (47.7%, n = 71/149) of the participants experienced side effects whilst taking their second-line regimen, and of these, 63.4% (n = 45) were VLF participants (p < 0.001).
Table 2 presents results from both bivariate and multivariate logistic regression analysis. No association was detected between VLF and relationship status in bivariate analysis. However, in multivariate analysis, participants who cohabit were three times more likely to have a VLF than those who are married (adjusted odds ratios [AORs] 3.1, 95% CI = 1.1-8.9; p = 0.035). Unemployed participants were two and a half times more likely to have treatment-related side effects compared to employed participants (AOR 2.5, 95% CI = 1.1-5.7; p = 0.023). Results for age did not show any statistical significance but older people were less likely to be unsuppressed.
Treatment-taking behaviour
Table 3 presents the reported treatment behaviour of the participants for the duration of receiving ART. There were more reports of treatment interruption whilst participants were on first-line treatment (n = 52). With respect to the reported treatment interruption whilst on second-line regimen, there was no distinct difference between the failing (n = 8) and suppressed (n = 5) groups. Both groups equally relied on themselves to remember to take their treatment ('naturally [I] remember taking my pills [VLF, woman, 31 years]; [I am] experienced on remembering my time' [VLS, man, 41 years]). Some of the reasons for interrupting treatment included stopping to take tuberculosis medication ('yes, interruption due to TB recurrence' [VLF, woman, 22 years]) and no medication availability whilst relocating ('I once interrupted my treatment due to the shortage of drugs as I relocated in South Africa and I did not have a proper transfer letter' [VLF, man, 38 years]). In the group with no reported second-line treatment interruption, more virologically suppressed participants (n = 21) listed using an alarm as a reminder. Several participants used the timeslots of popular local television programmes or the news (n = 14) as reminders to take their medication.
Table 4 presents the bivariate and multivariate logistic regression analyses for ART-taking behaviour. There was no association between participants' treatment-taking behaviour (i.e. medication alert, daily frequencies and pill storage) and VLF. However, participants who relied on their memory as a reminder to take medication (whilst on first-line regimen) were almost three times more likely to interrupt their first-line treatment than those who relied on an alarm (AOR: 2.6, 95% CI = 1.0-6.4, p = 0.042). There was no association found between medication alert or frequency of taking medication and second-line treatment interruption. Participants who used their handbag to store their medication were the least likely to experience second-line treatment interruption (OR: 0.2, 95% CI = 0.05-1.0, p = 0.054).
Recommendations from participants
The study participants made 175 recommendations for improving adherence (see Table 5). Coformulation in single tablets, only needing to take one dose of medication daily (preferably at night) and education about being adherent were listed as the most effective mechanisms to improve adherence on second-line treatment. Some examples of recommendations from participants include the following:
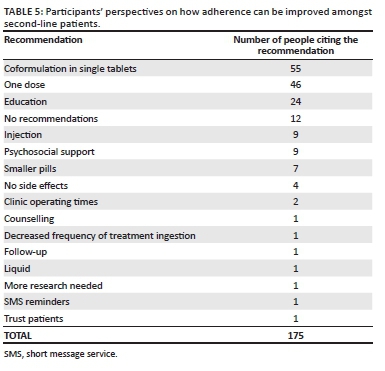
'Education should be emphasised through adherence classes. Reinforce on the benefits of ART'. (VLS, female, 38 years old)
'Availing a single-dose treatment for the second-line patients would enable them to adhere to treatment. Further ongoing education would also help'. (VLF, female, 39 years old)
Other recommendations included the development of injectable ART (n = 9, seven women and two men) and the provision of psychosocial support (particularly related to poverty and ensuring food supply):
'Should consider addressing the psychosocial needs of patients on second line as they have to adhere to treatment but sometimes they do not have enough food to eat'. (VLF, female, 45 years old)
Single recommendations to improve adherence included treatment reminders (n = 1), additional counselling (n = 1) and minimising the number of times second-line regimen is taken per day (n = 1). One patient felt that healthcare workers trusting that their patients took their medication as prescribed would promote treatment adherence. Lastly, 12 participants did not have any recommendations (mainly because of not experiencing any pill-taking challenges), as shown in the following statement:
'The current regimen is fine with me, therefore, I will suggest no change'. (VLS, male, 41 years old)
Discussion
This study sought to describe and understand treatment adherence and possible treatment support interventions from patients receiving second-line ART.
It has been reported that relationship dynamics influence ART adherence and VLS in that being married or having a committed and supportive partner tended to foster an environment for better clinical outcomes in HIV-positive people.17,18 Studies from South Africa and the United Kingdom found that HIV-positive married individuals had better clinical outcomes compared to any other relationship status.19,20 Similarly, our study found that single and unmarried people living with their partners were more likely to be virally unsuppressed.
Not statistically significant but important for consideration in adherence strengthening, our study showed that being younger was a predictor of VLF, which was congruent with previous studies.21,22,23 We noted VLS in those participants who resided further away from the health facilities. This is not in agreement with findings of studies conducted in Uganda, Ghana and Burkina Faso,24,25,26 which reported that individuals who resided closer to a health facility were more likely to seek healthcare.
Late disclosure may hinder adherence or treatment support and subsequently yield poor clinical outcomes.27 Whilst the majority of participants (63%) disclosed their HIV status 1 week after diagnoses, about 28% took longer than 4 weeks to disclose. Early disclosure, particularly to a family member or partner, has been strongly associated with improved adherence.8,28,29 Disclosure to a family member or partner has been linked with adequate psychosocial support which in turn facilitates adherence to treatment.8,29,30,31,32 However, the findings of our study suggest that disclosure and dependence on a treatment supporter are likely not to produce desired adherence levels (and did not feature in the list of participant recommendations), indicating that disclosure and treatment support should be assessed in combination with other adherence strategies instead of as a single consideration or mechanism.33
Unsurprisingly, the more toxic the second-line multi-pill, and regimens requiring medication to be taken multiple times a day, were seen as significantly harder to take than a single tablet daily well-tolerated first-line regimen. These views were consistent with reports from other studies that attributed similar challenges to taking second-line regimen.34,35,36
Participants who did not interrupt ART mainly reported using an alarm as a reminder for taking their medication. This finding suggests the need to explore external reminder mechanisms for improving adherence in this setting, considering that about 15% of VLF participants reported not using any external reminders. Various studies have also found a trend towards better adherence amongst patients who used external reminders.37,38,39 In addition, our study showed that participants who used their handbags to store their medication were more likely to adhere to treatment. This finding is in line with other studies that have reported having a handbag to have pills all the time as the preferred ART storage by patients.40,41,42
Side effects are an important predictor of poor adherence, and cumulative toxicity associated with ART, especially in second-line regimens.43,44,45 We found that participants with VLF were more likely to have treatment-related side effects. Furthermore, those participants with side effects were more likely to be unemployed. Although this was not explored further in our study, various studies have reported that employed patients can manage their health and side effects better than their unemployed counterparts.46,47,48
Participants had ideas regarding drug formulation that may improve adherence. These included a fixed-dose combination, a dosage taken once a day and reducing the pill size. Furthermore, the participants suggested that education on the benefits of taking ART could improve adherence, whilst a few participants also suggested the implementation of injectable ART. Various studies have recommended similar strategies,49,50,51 with the effectiveness of some of these strategies being previously reported for first-line regimens.51,52
Study limitations and strengths
This study had several limitations. Firstly, the study relied on participants' self-reports, prompting a likelihood that socially desirable answers may have been provided. However, to control for this, information such as viral load, side effects and comorbidity was verified by checking participants' medical records as part of data quality checks for the study. Secondly, the clinical measure for adherence considered viral load only. Finally, the sample might be small for the results to be generalised to all patients receiving second-line ART. However, the direction and size of effect were generally consistent, suggesting that the study findings may be robust despite these limitations.
Conclusion
Participants on a second-line antiretroviral regimen had firm recommendations regarding improving adherence, largely focused on administration, reduced dosing and pill burden. The study results suggest the importance of improving patients' knowledge about treatment and adherence and motivation to continue ART use despite the persistence of side effects. Drug manufacturers and health programmers must consider such recommendations as they modify and implement new ART regimens and programmes. Lastly, treatment support interventions recommended in this study need to be tested in practice to determine their efficacy for large-scale implementation.
Acknowledgements
The authors would like to thank all the facilities and relevant health and research authorities from the City of Johannesburg for allowing the research team to engage in a partnership to strengthen health service delivery through technical assistance and research. They also thank the participants for sharing their experiences, views and recommendations, and Jean Claude Nkembi for his assistance with data collection and capture.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
S.B.G. and S.T.L.-E. designed the study and supervised the data extraction at the facilities. S.B.G. analysed the quantitative data and S.T.L.-E. analysed the qualitative data. S.B.G. interpreted the data and prepared the first draft of the manuscript, which was revised by S.T.L.-E. and W.D.F.V. All authors read and approved the final manuscript.
Funding information
This study was funded through the OPTIMIZE project. OPTIMIZE (AID-OAA-A-15-00069) is funded by the United States Agency for International Development (USAID) under the US President's Emergency Plan for AIDS Relief (PEPFAR). S.B.G. was supported by the Consortium for Advanced Research Training in Africa (CARTA). It is jointly led by the African Population and Health Research Center and the University of the Witwatersrand and funded by the Carnegie Corporation of New York (Grant No. B 8606.R02), Swedish International Development Cooperation Agency (SIDA) (Grant No. 54100113), the Developing Excellence in Leadership, Training and Science (DELTAS) Africa Initiative (Grant No. 107768/Z/15/Z) and Deutscher Akademischer Austauschdienst (DAAD). The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences' (AAS) Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (the United Kingdom [UK]) and the UK government.
Data availability statement
The data sets used or analysed in this study are available from the corresponding author upon reasonable request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach [homepage on the Internet]. [ Links ] WHO Guidelines. 2013 [cited 2019 Aug 14]. Available from: https://apps.who.int/iris/bitstream/handle/10665/85321/9789241505727_eng.pdf?sequence=1
2. Fox MP, Berhanu R, Steegen K, et al. Intensive adherence counselling for HIV-infected individuals failing second-line antiretroviral therapy in Johannesburg, South Africa. Trop Med Int Heal. 2016;21(9):1131-1137. https://doi.org/10.1111/tmi.12741 [ Links ]
3. Nieuwlaat R, Wilczynski N, Navarro T, et al. Interventions for enhancing medication adherence. Cochrane Database Syst Rev. 2014;11:1-538. https://doi.org/10.1002/14651858.CD000011.pub4 [ Links ]
4. World Health Organization. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV [homepage on the Internet]. [ Links ] World Health Organization; 2015 [cited 2019 Aug 14]. Available from: https://apps.who.int/iris/bitstream/handle/10665/186275/9789241509565_eng.pdf?sequence=1
5. Goudge J, Ngoma B. Exploring antiretroviral treatment adherence in an urban setting in South Africa. J Public Health Policy. 2011;32:S52-S64. https://doi.org/10.1057/jphp.2011.22 [ Links ]
6. Elliott JH, Lynen L, Calmy A, et al. Rational use of antiretroviral therapy in low-income and middle-income countries: Optimizing regimen sequencing and switching. AIDS. 2008;22(16):2053-2067. https://doi.org/10.1097/QAD.0b013e328309520d [ Links ]
7. Gross R, Zheng L, Rosa A La, et al. Partner-based adherence intervention for second-line antiretroviral therapy (ACTG A5234): A multinational randomised trial. Lancet HIV. 2(1):e12-19. https://doi.org/10.1016/S2352-3018(14)00007-1 [ Links ]
8. Onoya D, Nattey C, Budgell E, et al. Predicting the need for third-line antiretroviral therapy by identifying patients at high risk for failing second-line antiretroviral therapy in South Africa. AIDS Patient Care STDS. 31(5):205-212. https://doi.org/10.1089/apc.2016.0291 [ Links ]
9. Gill CJ, Hamer DH, Simon JL, Thea DM, Sabin LL. No room for complacency about adherence to antiretroviral therapy in sub-Saharan Africa. AIDS. 2005;19(12):1243-1249. https://doi.org/10.1097/01.aids.0000180094.04652.3b [ Links ]
10. South African National Department of Health. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults [homepage on the Internet]. [ Links ] Health Policy. 2015 [cited 2019 Aug 20]. Available from: https://sahivsoc.org/Files/ARTGuidelines15052015.pdf
11. Nnambalirwa M, Govathson C, Evans D, McNamara L, Maskew M, Nyasulu P. Markers of poor adherence among adults with HIV attending Themba Lethu HIV Clinic, Helen Joseph Hospital, Johannesburg, South Africa. Trans R Soc Trop Med Hyg. 2016;110(12):696-704. https://doi.org/10.1093/trstmh/trx003 [ Links ]
12. World Health Organization. HIV drug resistance report 2017 [homepage on the Internet]. [ Links ] World Health Organization; 2017 [cited 2019 Aug 20]. Available from: https://apps.who.int/iris/bitstream/handle/10665/255896/9789241512831-eng.pdf?sequence=1
13. Hertogs K, Bloor S, De Vroey V, et al. A novel human immunodeficiency virus type 1 reverse transcriptase mutational pattern confers phenotypic lamivudine resistance in the absence of mutation 184V. Antimicrob Agents Chemother. 2000; 44(3):568-573. https://doi.org/10.1128/AAC.44.3.568-573.2000 [ Links ]
14. Achappa B, Madi D, Bhaskaran U, Ramapuram JT, Rao S, Mahalingam S. Adherence to antiretroviral therapy among people living with HIV. N Am J Med Sci. 2013;5(3):220-223. https://doi.org/10.4103/1947-2714.109196 [ Links ]
15. Pagès-Puigdemont N, Mangues MA, Masip M, et al. Patients' perspective of medication adherence in chronic conditions: A qualitative study. Adv Ther. 2016; 33:1740-1754. https://doi.org/10.1007/s12325-016-0394-6 [ Links ]
16. Laba TL, Lehnbom E, Brien J-A, Jan S. Understanding if, how and why non-adherent decisions are made in an Australian community sample: A key to sustaining medication adherence in chronic disease? Res Social Adm Pharm. 2015;11(2):154-162. https://doi.org/10.1016/j.sapharm.2014.06.006 [ Links ]
17. Conroy A, Leddy A, Johnson M, Ngubane T, Van Rooyen H, Darbes L. 'I told her this is your life': Relationship dynamics, partner support and adherence to antiretroviral therapy among South African couples. Cult Heal Sex. 2017;19(11):1239-1253. https://doi.org/10.1080/13691058.2017.1309460 [ Links ]
18. Johnson MO, Dilworth SE, Taylor JM, Darbes LA, Comfort ML, Neilands TB. Primary relationships, HIV treatment adherence, and virologic control. AIDS Behav. 2012;16:1511-1521. https://doi.org/10.1007/s10461-011-0021-0 [ Links ]
19. Robards J, Evandrou M, Falkingham J, Vlachantoni A. Marital status, health and mortality. Maturitas. 2012. https://doi.org/10.1016/j.maturitas.2012.08.007 [ Links ]
20. Shisana O. South African national HIV prevalence, incidence and behaviour survey, 2012 [homepage on the Internet]. [ Links ] HSRC Press; 2014 [cited 2019 Aug 21]. Available from: http://repository.hsrc.ac.za/bitstream/handle/20.500.11910/2490/8162.pdf?sequence=1&isAllowed=y
21. Hadland SE, Milloy M-J, Kerr T, et al. Young age predicts poor antiretroviral adherence and viral load suppression among injection drug users. AIDS Patient Care STDS. 2012;26(5):274-280. https://doi.org/10.1089/apc.2011.0196 [ Links ]
22. Bulage L, Ssewanyana I, Nankabirwa V, et al. Factors associated with virological non-suppression among HIV-positive patients on antiretroviral therapy in Uganda, August 2014-July 2015. BMC Infect Dis. 2017;17(1):326. https://doi.org/10.1186/s12879-017-2428-3 [ Links ]
23. Mujugira A, Celum C, Tappero JW, Ronald A, Mugo N, Baeten JM. Younger age predicts failure to achieve viral suppression and virologic rebound among HIV-1-infected persons in serodiscordant partnerships. AIDS Res Hum Retroviruses. 2016; 32(2):148-154. https://doi.org/10.1089/aid.2015.0296 [ Links ]
24. Akullian AN, Mukose A, Levine GA, Babigumira JB. People living with HIV travel farther to access healthcare: A population-based geographic analysis from rural Uganda. J Int AIDS Soc. 2016;19(1):20171. https://doi.org/10.7448/IAS.19.1.20171 [ Links ]
25. Buor D. Analysing the primacy of distance in the utilization of health services in the Ahafo-Ano South district, Ghana. Int J Health Plann Manage. 2003;18(4):293-311. https://doi.org/10.1002/hpm.729 [ Links ]
26. Schoeps A, Gabrysch S, Niamba L, Sié A, Becher H. The effect of distance to health-care facilities on childhood mortality in rural Burkina Faso. Am J Epidemiol. 2011;173(5):492-498. https://doi.org/10.1093/aje/kwq386 [ Links ]
27. Cluver LD, Hodes RJ, Toska E, et al. 'HIV is like a tsotsi. ARVs are your guns': Associations between HIV-disclosure and adherence to antiretroviral treatment among adolescents in South Africa. AIDS. 2015;29 Suppl 1:S57-65. https://doi.org/10.1097/QAD.0000000000000695 [ Links ]
28. Rochat TJ, Mkwanazi N, Bland R. Maternal HIV disclosure to HIV-uninfected children in rural South Africa: A pilot study of a family-based intervention. BMC Public Health. 2015;29 Suppl 1:S57-65. https://doi.org/10.1186/1471-2458-13-147 [ Links ]
29. Wouters E, Van Loon F, Van Rensburg D, Meulemans H. Community support and disclosure of HIV serostatus to family members by public-sector antiretroviral treatment patients in the Free State Province of South Africa. AIDS Patient Care STDS. 2009;23(5):357-364. https://doi.org/10.1089/apc.2008.0201 [ Links ]
30. Kunutsor S, Walley J, Katabira E, et al. Improving clinic attendance and adherence to antiretroviral therapy through a treatment supporter intervention in Uganda: A randomized controlled trial. AIDS Behav. 2011;15(8):1795-1802. https://doi.org/10.1007/s10461-011-9927-9 [ Links ]
31. Nachega JB, Chaisson RE, Goliath R, et al. Randomized controlled trial of trained patient-nominated treatment supporters providing partial directly observed antiretroviral therapy. AIDS. 24(9):1273-1280. https://doi.org/10.1097/QAD.0b013e328339e20e [ Links ]
32. Duwell MM, Knowlton AR, Nachega JB, et al. Patient-nominated, community-based HIV treatment supporters: Patient perspectives, feasibility, challenges, and factors for success in HIV-infected South African adults. AIDS Patient Care STDS. 2013;27(2):96-102. https://doi.org/10.1089/apc.2012.0348 [ Links ]
33. Kanters S, Park JJH, Chan K, et al. Interventions to improve adherence to antiretroviral therapy: A systematic review and network meta-analysis. Lancet HIV. 2017; 4(1):e31-e40. https://doi.org/10.1016/S2352-3018(16)30206-5 [ Links ]
34. Mills EJ, Nachega JB, Bangsberg DR, et al. Adherence to HAART: A systematic review of developed and developing nation patient-reported barriers and facilitators. PLoS Med. 2006;3(11):e438. https://doi.org/10.1371/journal.pmed.0030438 [ Links ]
35. Ajose O, Mookerjee S, Mills EJ, Boulle A, Ford N. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: A systematic review and meta-analysis. AIDS. 2012. https://doi.org/10.1097/QAD.0b013e328351f5b2 [ Links ]
36. Cheng Y, Nickman NA, Jamjian C, et al. Predicting poor adherence to antiretroviral therapy among treatment-naïve veterans infected with human immunodeficiency virus. Medicine. 2018;97(2):e9495. https://doi.org/10.1097/MD.0000000000009495 [ Links ]
37. Petersen ML, Wang Y, Van der Laan MJ, Guzman D, Riley E, Bangsberg DR. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: A marginal structural model analysis. Clin Infect Dis. 2007;45(7):908-915. https://doi.org/10.1086/521250 [ Links ]
38. Azia IN, Mukumbang FC, Van Wyk B. Barriers to adherence to antiretroviral treatment in a regional hospital in Vredenburg, Western Cape, South Africa. South Afr J HIV Med. 2016;17(1):476. https://doi.org/10.4102/sajhivmed.v17i1.476 [ Links ]
39. Nozaki I, Dube C, Kakimoto K, Yamada N, Simpungwe JB. Social factors affecting ART adherence in rural settings in Zambia. AIDS Care. 2011;23(7):831-838. https://doi.org/10.1080/09540121.2010.542121 [ Links ]
40. Nassir Azmach N. Adherence to antiretroviral therapy and associated factors among adult ARV users in Arba Minch Hospital, Southern Ethiopia. Cent African J Public Heal. 2017;3(2):19-26. https://doi.org/10.11648/j.cajph.20170302.12 [ Links ]
41. Stawarz K, Rodríguez MD, Cox AL, Blandford A. Understanding the use of contextual cues: Design implications for medication adherence technologies that support remembering. Digit Heal. 2016; 1;2:2055207616678707. https://doi.org/10.1177/2055207616678707 [ Links ]
42. Nkomo G, Mosalo A, Thupayagale-Tshweneagae GB. Adherence to treatment and retention to care of adult patients on antiretroviral therapy. Afr J Nurs Midwifery. 2018;20(1):1-13. https://doi.org/10.25159/2520-5293/1590 [ Links ]
43. Wasti SP, Simkhada P, Randall J, Freeman JV, Van Teijlingen E. Factors influencing adherence to antiretroviral treatment in Nepal: A mixed-methods study. PLoS One. 2012;7(5):e35547. https://doi.org/10.1371/journal.pone.0035547 [ Links ]
44. Fonsah JY, Njamnshi AK, Kouanfack C, et al. Adherence to antiretroviral therapy (ART) in Yaoundé-Cameroon: Association with opportunistic infections, depression, ART regimen and side effects. PLoS One. 2017;12(1):e0170893. https://doi.org/10.1371/journal.pone.0170893 [ Links ]
45. Johnson MO, Dilworth SE, Taylor JM, Neilands TB. Improving coping skills for self-management of treatment side effects can reduce antiretroviral medication nonadherence among people living with HIV. Ann Behav Med. 2011;41(1):83-91. https://doi.org/10.1007/s12160-010-9230-4 [ Links ]
46. Moyo F, Chasela C, Brennan AT, et al. Treatment outcomes of HIV-positive patients on first-line antiretroviral therapy in private versus public HIV clinics in Johannesburg, South Africa. Clin Epidemiol. 2016;8:37-47. https://doi.org/10.2147/CLEP.S93014 [ Links ]
47. Goodman N. The impact of employment on the health status and health care costs of working-age people with disabilities. LEAD Cent Policy Br. 2015. [ Links ]
48. Kilian R, Lauber C, Kalkan R, et al. The relationships between employment, clinical status, and psychiatric hospitalisation in patients with schizophrenia receiving either IPS or a conventional vocational rehabilitation programme. Soc Psychiatry Psychiatr Epidemiol. 2012;47(9):1381-1389. https://doi.org/10.1007/s00127-011-0451-z [ Links ]
49. Barnett W, Patten G, Kerschberger B, et al. Perceived adherence barriers among patients failing second-line antiretroviral therapy in Khayelitsha, South Africa. South Afr J HIV Med. 2013;14(4):170-176. https://doi.org/10.4102/sajhivmed.v14i4.51 [ Links ]
50. Scanlon ML, Vreeman RC. Current strategies for improving access and adherence to antiretroviral therapies in resource-limited settings. HIV/AIDS. 2013;5:1-17. https://doi.org/10.2147/HIV.S28912 [ Links ]
51. Chaiyachati KH, Ogbuoji O, Price M, Suthar AB, Negussie EK, Bärnighausen T. Interventions to improve adherence to antiretroviral therapy: A rapid systematic review. AIDS. 2014; 28:S187-S204. https://doi.org/10.1097/QAD.0000000000000252 [ Links ]
52. Ridgeway K, Dulli LS, Murray KR, et al. Interventions to improve antiretroviral therapy adherence among adolescents in low- and middle-income countries: A systematic review of the literature. PLoS One. 2018;13(1):e0189770. https://doi.org/10.1371/journal.pone.0189770 [ Links ]
 Correspondence:
Correspondence:
Siphamandla Gumede
sgumede@cartafrica.org
Received: 24 May 2020
Accepted: 13 June 2020
Published: 11 Aug. 2020
ORIGINAL RESEARCH
Feasibility of implementing same-day antiretroviral therapy initiation during routine care in Ekurhuleni District, South Africa: Retention and viral load suppression
Nolundi Mshweshwe-PakelaI; Bhakti HansotiII, III; Tonderai MabutoI, IV; Deanna KerriganV; Griffiths KubekaI; Elizabeth HahnIII; Salome CharalambousI, IV; Christopher J. HoffmannI, VI, VII
IImplementation Research Division, The Aurum Institute, Johannesburg, South Africa
IIDepartment of Emergency Medicine, Johns Hopkins School of Medicine, Baltimore, United States of America
IIIDepartment of International Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, United States of America
IVSchool of Public Health, University of the Witwatersrand, Johannesburg, South Africa
VDepartment of Sociology, American University, Washington, United States of America
VIDepartment of Medicine, Johns Hopkins University School of Medicine, Baltimore, United States of America
VIIDepartment of Health, Behavior, and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, United States of America
ABSTRACT
BACKGROUND: Same-day initiation (SDI) of antiretroviral therapy (ART) has been advocated as an approach to increase linkage to care and overall ART initiation. Clinical trials have demonstrated impressive benefits. However, questions regarding patient preparedness and retention in care remain for routine implementation of this approach
OBJECTIVES: In this study, we sought to describe SDI of ART during routine care delivery and compare time to ART initiation on longitudinal care outcomes
METHOD: We performed a retrospective chart review of 100 consecutive individuals, newly diagnosed with HIV, from 10 health facilities across Ekurhuleni, from January to July 2017. Records were reviewed for a period of 1 year post-diagnosis. Abstracted data included demographics, time to ART initiation, clinic visits and laboratory test results (including viral load testing
RESULTS: A total of 993 patient records were reviewed, of which 826 were included in the analysis. The majority of patients (752, 91%) had ART initiation recorded, of which 654 (79%) had ART initiated within 30 days, and 224 (27%) had SDI. Uptake of SDI of ART was higher among women (36% vs. 10.4%; p < 0.001) and in younger patients (33.7% in those < 29 years; p < 0.01). Retention in care at 6 months was achieved in 477 (58%) patients. Of those with 6-month viral loads, 350/430 (73%) had a viral load < 400 c/m. Retention in care and viral suppression were similar among those with SDI of ART and later ART initiation
CONCLUSION: Same-day initiation of ART was successfully delivered with similar retention and viral load outcomes as subsequent initiation, providing re-assurance for scale-up of this strategy in routine care
Keywords: HIV testing; primary care; SDI; ARV initiation; implementation.
Introduction
Timely linkage to antiretroviral therapy (ART) is pivotal to decreasing human immunodeficiency virus (HIV) transmission and reducing HIV-associated morbidity and mortality.1 Increasing evidence indicates that same-day initiation (SDI) of ART can increase overall linkage to care (LTC) and the proportion of patients with viral load suppression.2,3,4,5,6,7 Randomised studies from South Africa, Haiti and Lesotho have demonstrated the feasibility, acceptability and improved retention over 6 months with SDI of ART when compared with standard practices in research settings.5,8,9 In response to clinical trial evidence of the benefits of SDI of ART, the South African Department of Health announced a policy of universal testing and treatment (UTT) in public health facilities in September 2016 and endorsed SDI of ART.10 The UTT directorate specified: 'ART should be started as soon as the patient is ready and within 2 weeks', with immediate (same-day) initiation prioritised for pregnant or breastfeeding women.10 Same-day initiation of ART was further highlighted as a priority in a 2017 South African Department of Health circular.10,11 and in the 2017 World Health Organization rapid ART initiation guidelines.12
As a counterpoint to SDI of ART enthusiasm, there are concerns regarding the operational feasibility of SDI in public health facilities providing routine care. These concerns include failure to rule-out important opportunistic infections, challenges with assuring adequate renal function for some primary ART regimens and patient readiness to cope with a new HIV diagnosis and initiate life-long ART.1,13,14,15,16 Furthermore, SDI of ART may not allow time (and focussed time) to provide important HIV education and adherence counselling that may be valuable for long-term ART success.16,17,18 Moreover, a recent South African study demonstrated a reduced rate of retention among same-day initiators.19 These reports of early findings warrant further investigation to understand the impact of routine delivery of same-day ART. Lastly, SDI of ART adds a further service burden to an already overwhelmed system. The shortage of 'nurse-initiation and management of ART' (NIMART) trained staff, limited resources and long waiting times in facilities pose system-level barriers that may impede the effective implementation of SDI of ART and contribute to the negative outcomes already reported.
In spite of the challenges associated with SDI of ART, it still offers important benefits to both the patient and healthcare system. In addition to early access to a definitive management strategy, SDI obviates the need for repeat visits and is likely a more attractive option for young working adults.19 Furthermore, routinisation of SDI may in some way normalise ART initiation, by providing a similar approach to HIV management as other chronic health conditions (i.e. high blood pressure management where antihypertensive medications are often started during the clinic visit without the need for extensive repeat visits or referral to a specialised clinical setting).
This study seeks to describe our experience with SDI across 10 healthcare facilities in the Ekurhuleni District of South Africa and characterise both patients- and health facility-associated factors that impact SDI success, namely retention in care and viral load suppression.
Materials and methods
Study design
This is a retrospective clinical review of patients who presented for routine care and tested HIV positive at 10 public sector health facilities in the Ekurhuleni District of South Africa between 01 January 2017 and 31 July 2017. We abstracted clinical data from patient charts (including patient files and HIV testing registers) and electronic databases (Tier.Net20 and the South African National Health Laboratory Service [NHLS] database). All data were collected after the pathway of care was completed, there was no interaction with patients and written informed consent was not obtained. All data were de-identified prior to data capture and data analysis.
Study setting and population
The study took place in the Ekurhuleni District (Gauteng Province) of South Africa. This district has the second-highest district-level HIV prevalence (14.3%) in South Africa and is comprised of urban and peri-urban settings.21,22 The study sites consisted of six primary healthcare clinics, three community healthcare centres and one outpatient department at a district-level hospital. Facilities were selected to achieve geographic diversity within the district. All facilities had HIV counselling and testing personnel (lay counsellors) and routinely provide free HIV testing services (HTS) according to the 2015 National HIV Counselling and Testing (HCT) and the UTT policy guidelines.23,24
Sampling and data collection
We aimed to include 100 consecutive adult patients with an HIV-positive diagnosis from each of the 10 health facilities for a total sample of approximately 1000 patients. This sample size was selected to provide an estimate of LTC within 20% of the point estimate, assuming 50% LTC, an alpha of 0.05 and 80% power. Consecutive patients were identified from the HIV testing registers and were included if they were aged 18 or older and had a positive HIV test recorded in the clinic HIV testing register between January 1st and July 31st 2017. Approximately 1 year after the date of diagnosis, paper and electronic clinical records were abstracted for each selected patient, from the clinic at which HIV testing occurred. The data abstracted included sex, age, CD4 count, viral load (HIV RNA), creatinine, ART initiation, HIV care visits, tuberculosis (TB) testing, TB test results and TB preventive therapy prescribing.
Outcome measures
Linkage to care was defined as documentation of ART prescription. Same-day initiation of ART was defined as having the same date of HIV diagnosis and ART initiation.25 Rapid ART initiation that did not occur on the day of HIV testing was defined as initiation between 1 and 7 days after HIV diagnosis.12 Retention in care was defined as evidence of a care visit (in the same facility where patients had initiated ART) or HIV-related laboratory testing between 91 and 365 days after ART initiation. Six-month viral suppression was defined as a viral load of < 400 c/mL (for consistency with prior reports) between 4 and 8 months after ART initiation.
Statistical analyses
Descriptive statistics were used to explore the relationship between patient characteristics and the timing of ART initiation (same-day, 1-7, 8-30, 31-90; > 90 days). Comparisons of factors associated with the timing of ART initiation were conducted using chi-square tests with particular interest in clinic attended, sex, age group, CD4 count and TB testing. Log binomial regression modelling (used to approximate relative risk) was used to assess for associations between the timing of ART initiation and viral suppression and retention in care while adjusting for age, sex, CD4 count and facility. Data were analysed using STATA© v.14 (StataCorp, LLC, Texas).
Ethical consideration
The study was approved by the University of the Witwatersrand Human Research Ethics Committee, the Johns Hopkins University School of Medicine Institutional Review Board and the Ekurhuleni District Research Committee.
Results
A total of 993 patient records were identified and abstracted during the study period. Of these, 826 (83.2%) patients were included in the analysis and 167 (16.8%) were excluded. Reasons for exclusion included: 111 (66.5%) had ART recorded prior to testing date, 49 (29.3%) were documented to have transferred out of the healthcare facility and 7 (4.2%) were documented to have died. Of those included, 528 (63.9%) were women, and the median age was 32 years (interquartile range [IQR]; 27, 39). Of the 10 study clinics, the number of patients included in the analysis ranged from 65 to 99 per clinic.
Patient characteristics at human immunodeficiency virus testing
The majority of patients had CD4 count documentation (610, 73.8%; Table 1) with a median CD4 count of 312 cells/mm3 (IQR; 156, 490). Of those with CD4 count documentation, nearly a third had a CD4 count of ≤ 200 cells/mm3 (192, 31.5%) (Table 1). Serum creatinine was documented for less than half of the patients (414, 50.1%), of which 14 (3.4%) had an estimated glomerular filtration rate of < 50 ml/min/m2. Tuberculosis testing (GeneXpert MTB/Rif) was documented for 45 (5.4%) patients, of which 10 tested positive for TB.
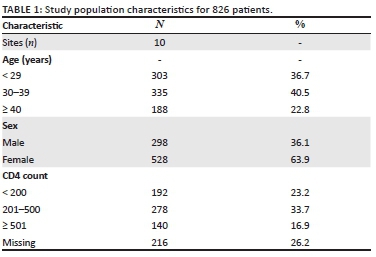
Timing of antiretroviral therapy initiation
Of the 826 patients included in the analysis, ART initiation within a year of HIV testing was documented for 710 (85.9%; Table 1). Of these, the majority, 654 (92.1%), of patients initiated ART within 30 days after HIV testing (median of 8 days; IQR: 0, 15). A similar proportion of men and women initiated ART (83% and 88%, respectively; p = 0.06). Of the 10 patients testing positive for TB, seven were initiated on ART within 30 days with the remaining three initiated on ART within 90 days.
Antiretroviral therapy was initiated on the same day as HIV testing for 221 patients (31.1%). The proportion of patients initiated on ART who had SDI varied considerably by facility, ranging from 8.1% to 61% (10% - 69% for women and 0% - 45% for men). A significantly higher proportion of women, compared with men, initiated ART on the same day (36% vs. 10.4%, p < 0.001) (Table 2). Same-day initiation of ART was also more common among those aged < 29 years (102, 33.7%), compared with those aged 30-40 years (89, 26.6%), and those older than 40 years (30, 16%; p = 0.009). Lastly, a greater proportion of patients with a higher CD4 count initiated on the same-day: 42 (30%) of those with a CD4 count > 500 cells/mm3 compared with 60 (21.6%) among those with a CD4 count between 200 and 500 cells/mm3 and 23 (12%) of those with CD4 count < 200 cells/mm3 (p < 0.001; Table 3). Notably, the CD4 count results were not available to clinicians on the day of HIV testing, so decisions around SDI were not influenced by the CD4 count.
Retention in care by timing of antiretroviral therapy initiation
A total of 477 patients (67.2% of patients who initiated ART) met our definition of retention in care at 6 months. There was no association between retention in care and time of ART initiation; 69.2% (153) of those who initiated same-day ART were retained at 6 months compared with 66.1% (286) who initiated ART between 1 and 30 days post-diagnosis (p = 0.47; Table 3). In a model-adjusted for sex, age and CD4 count, time of ART initiation did not significantly impact the relative likelihood retention in care at 6 months (Table 4).
Viral load suppression by timing of antiretroviral therapy initiation
A total of 455 patients (64.1% of patients who initiated ART) had a 6-month viral load result documented, of which 359 (79%) were virally suppressed (viral load < 400 c/mL; Table 3). There was no significant association between viral suppression and time to ART initiation; 78.1% (118) of patients who initiated same-day ART were virally suppressed compared with 80.3% (216) patients who initiated ART between 1 and 30 days post-diagnosis (p = 0.5; Table 4). In a model adjusted for sex, age group and CD4 count, time of ART initiation did not significantly impact the relative likelihood of viral suppression (Table 4).
Discussion
We found that just over a quarter of patients at 10 routine care facilities in South Africa initiated same-day ART. Of those initiating ART, 58% were retained in care at the same clinic at 6 months, and 58% of those retained in care at 6 months had a viral load of < 400 c/mL (80% of those with viral load test results). There was no significant association between the timing of ART initiation and viral suppression or retention in care. Furthermore, we identified that same-day ART achieves similar retention in care and viral suppression when compared with later initiation. These findings suggest that provision of SDI does not adversely impact long-term care outcomes. It is plausible that the overall retention and viral load suppression is a superior strategy to delayed ART initiation, because of less time lost from care, by reducing the time between the testing visit and subsequent clinic visit. These are important and encouraging early findings from the routine implementation of SDI that help to expand the understanding of the use of SDI beyond clinical trial settings.
We found several patient-level characteristics that impacted the likelihood of SDI, namely sex, age and CD4 count. Healthier, young women were more likely to be initiated on ART and initiated on the same day. It is unclear if this was because of provider biases in terms of who was offered SDI of ART, or a potential pitfall when engaging older male patients into SDI of ART. It is possible that higher rates of ART initiation in women are accounted for by same-day ART among pregnant women as part of prevention of mother-to-child transmission (PMTCT); the overall lower engagement in care among men may also have contributed to our findings.26,27,28 In contrast to general ART initiation studies and an SDI study from Zimbabwe, we observed that younger patients (men and women) were more likely to have SDI compared with older patients.29 This is an intriguing finding and contrasts with findings that older individuals are more likely to initiate ART.30,31 It is plausible that SDI may have an especially important role in engaging young adult patients in care.30 Overall, the most substantial variation in SDI was by facility (10 - 69% for women and 0 - 45% for men). This variability was not associated with the type of health facility or structure, nor daily headcount, suggesting that health facility practice and provider discretion were the primary determinants of whether or not a patient received SDI. Further qualitative research within healthcare facilities to understand facility-based barriers to SDI is needed to support this initiative.
Studies of retention in care with SDI are limited to studies of PMTCT and research studies.32 Research studies on SDI have reported increased retention in care, whereas some PMTCT studies have reported a higher loss from care with same-day ART.5,6,32,33,34,35,36,37 Same-day ART may improve outcomes by reducing the cost of care and enhancing the perceived value of ART because of the immediacy of receiving the therapy.38,39 The findings of our study present a stark contrast to a recently published study led by Lilian et al., which presents data on the national impact of SDI on retention in care.19 Routinely collected data from TIER.Net (n = 32 290) were analysed for HIV-infected adults who were newly initiating ART in Johannesburg or Mopani districts between October 2017 and June 2018. Their study identified a significant increase in lost to follow-up (LTFU) at 30.1% in the SDI group compared with 22.4%, 19.8% and 21.9% among clients initiating ART 1-7 days, 8-21 days and ≥ 22 days after HIV diagnosis, respectively.19 Although LTFU was similar to our study (overall LTFU was 32.8%, 233/710), the association between the timing of ART initiation and LTFU was not found. Although our study was smaller, it benefitted from the triangulation of multiple data sources that may capture individuals missed in the Tier.Net system. This likely reveals the bias of using a single data source, which may underestimate LTC. Additionally, it is also possible that there is a heterogeneity in the success of SDI by district and even by clinic. Our study within a single district (Ekurhuleni) had more promising LTC results than those in the neighbouring districts of Johannesburg and Mopani. A deeper dive into the implementation practices across districts and their relative LTC outcomes is required to understand this phenomenon further.
This study has the strength of assessing multiple clinics that provided routine care in a real-world setting without augmentation from research or academic staff. We also used multiple sources for data collection, paper and electronic and both local and national, to the covert possibility of missing or incorrect data in one source. Some notable limitations arise from the use of chart review to assess successful LTC. Linkage to care and ART initiation required documentation in a file at the same clinic as HIV testing occurred. Patients who initiated ART at other facilities were considered as not having initiated ART. Furthermore, we also did not abstract a reason for visit at the time of HIV testing, making it impossible to compare ART for PMTCT with other initiation reasons. Finally, time to ART initiation was not randomised. As a result, more motivated individuals may have started on the same day. It is also plausible that some same-day initiators were just doing what they were told and were not motivated. Comparing early ART (1-7 days after diagnosis) with same-day ART provides a group with motivation demonstrated by a prompt return to the clinic to the potentially more heterogeneous same-day group. However, the similar outcomes between these two groups are reassuring.
Conclusion
This study demonstrates the feasibility of SDI of ART and similar outcomes with subsequent initiation. These findings are reassuring and should support continued scale-up of SDI of ART. The finding of higher SDI of ART among younger individuals may suggest that this approach is especially promising to improve care engagement among this population. Operational research should be continued to identify challenges and barriers to inform programmatic delivery of same-day ART.
Acknowledgements
Competing interests
The authors have declared that no competing interest exists.
Authors' contributions
N.M-P., T.M., C.H., D.K. and S.C. designed the research study; T.M., N.M-P, G.K. and S.C. performed the research; B.H., C.H. and E.H. analysed the data; B.H., N.M-P., T.M. and C.H. wrote the article. All authors have read and approved the final manuscript.
Funding information
The study was funded by Project SOAR (Supporting Operational AIDS Research). Project SOAR (Cooperative Agreement AID-OAA-A-14-00060) was made possible by the generous support of the American people through the United States President's Emergency Plan for AIDS Relief (PEPFAR) and United States Agency for International Development (USAID).
Data availability statement
De-identified data will be available upon written request to the corresponding author, Dr Christopher J. Hoffmann (choffmann@jhmi.edu).
Disclaimer
The contents of this article are the sole responsibility of the authors and do not necessarily reflect the views of PEPFAR, USAID or the United States Government.
References
1. Ford N, Migone C, Calmy A, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. 2018;32(1):17-23. https://doi.org/10.1097/QAD.0000000000001671 [ Links ]
2. Govindasamy D, Meghij J, Negussi EK, Baggaley RC, Ford N, Kranzer K. Interventions to improve or facilitate linkage to or retention in pre-ART (HIV) care and initiation of ART in low-and middle-income settings - A systematic review. J Int AIDS Soc. 2014;17(1):19032. https://doi.org/10.7448/IAS.17.1.19032 [ Links ]
3. Kranzer K, Govindasamy D, Ford N, Johnston V, Lawn SD. Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: A systematic review. J Int AIDS Soc. 2012;15(2):17383. https://doi.org/10.7448/IAS.15.2.17383 [ Links ]
4. Fox MP, Rosen S. A new cascade of HIV care for the era of 'treat all'. PLoS Med. 2017;14(4):e1002268. https://doi.org/10.1371/journal.pmed.1002268 [ Links ]
5. Rosen S, Maskew M, Fox MP, et al. Initiating antiretroviral therapy for HIV at a patient's first clinic visit: The RapIT randomised controlled trial. PLoS Med. 2016;13(5):e1002015. https://doi.org/10.1371/journal.pmed.1002015 [ Links ]
6. Koenig SP, Dorvil N, Devieux JG, et al. Same-day HIV testing with initiation of antiretroviral therapy versus standard care for persons living with HIV: A randomised unblinded trial. PLoS Med. 2017;14(7):e1002357. https://doi.org/10.1371/journal.pmed.1002357 [ Links ]
7. Blanc F-X, Sok T, Laureillard D, et al. Earlier versus later start of antiretroviral therapy in HIV-infected adults with tuberculosis. N Engl J Med. 2011;365(16):1471-1481. [ Links ]
8. Amanyire G, Semitala FC, Namusobya J, et al. Effects of a multicomponent intervention to streamline initiation of antiretroviral therapy in Africa: A stepped-wedge cluster-randomised trial. Lancet HIV. 2016;3(11):e539-e48. https://doi.org/10.1016/S2352-3018(16)30090-X [ Links ]
9. Pilcher CD, Ospina-Norvell C, Dasgupta A, et al. The effect of same-day observed initiation of antiretroviral therapy on HIV viral load and treatment outcomes in a U.S. public health setting. J Acquir Immune Defic Syndr. 2017;74(1):44-51. https://doi.org/10.1097/QAI.0000000000001134 [ Links ]
10. Pillay Y, Pillay A. Implementation of the universal test and treat strategy for HIV positive patients and differentiated care for stable patients. Pretoria: BMJ Publishing Group; 2016. [ Links ]
11. SANAC. South Africa's National Strategic Plan for HIV, T.B. and STIs 2017-2022. Pretoria: South African National AIDS Council; 2017. [ Links ]
12. World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva: WHO; 2017. [ Links ]
13. Schulz S, Draper H, Naidoo P. A comparative study of tuberculosis patients initiated on ART and receiving different models of TB-HIV care. Int J Tuberc Lung Dis. 2013;17(12):1558-1563. https://doi.org/10.5588/ijtld.13.0247 [ Links ]
14. Cooper RD, Wiebe N, Smith N, Keiser P, Naicker S, Tonelli M. Systematic review and meta-analysis: Renal safety of tenofovir disoproxil fumarate in HIV-infected patients. Clin Infect Dis. 2010;51(5):496-505. https://doi.org/10.1086/655681 [ Links ]
15. Black S, Zulliger R, Marcus R, Mark D, Myer L, Bekker L-G. Acceptability and challenges of rapid ART initiation among pregnant women in a pilot programme, Cape Town, South Africa. AIDS Care. 2014;26(6):736-741. https://doi.org/10.1080/09540121.2013.855300 [ Links ]
16. Helova A, Akama E, Bukusi EA, et al. Health facility challenges to the provision of Option B+ in western Kenya: A qualitative study. Health Policy Plan. 2016;32(2):283-291. https://doi.org/10.1093/heapol/czw122 [ Links ]
17. Chan AK, Kanike E, Bedell R, et al. Same day HIV diagnosis and antiretroviral therapy initiation affects retention in Option B+ prevention of mother-to-child transmission services at antenatal care in Zomba District, Malawi. J Int AIDS Soc. 2016;19(1):20672. https://doi.org/10.7448/IAS.19.1.20672 [ Links ]
18. Langwenya N, Phillips TK, Brittain K, Zerbe A, Abrams EJ, Myer L. Same-day antiretroviral therapy (ART) initiation in pregnancy is not associated with viral suppression or engagement in care: A cohort study. J Int AIDS Soc. 2018;21(6):e25133. https://doi.org/10.1002/jia2.25133 [ Links ]
19. Lilian RR, Rees K, McIntyre JA, Struthers HE, Peters RP. Same-day antiretroviral therapy initiation for HIV-infected adults in South Africa: Analysis of routine data. PloS One. 2020;15(1):e0227572. https://doi.org/10.1371/journal.pone.0227572 [ Links ]
20. Osler M, Hilderbrand K, Hennessey C, et al. A three-tier framework for monitoring antiretroviral therapy in high HIV burden settings. J Int AIDS Soc. 2014;17(1):18908. https://doi.org/10.7448/IAS.17.1.18908 [ Links ]
21. Shisana O, Rehle T, Simbayi LC, et al. South African national HIV prevalence, incidence and behaviour survey. Pretoria: BMJ Publishing Group; 2012. [ Links ]
22. Zuma K, Shisana O, Rehle TM, et al. New insights into HIV epidemic in South Africa: Key findings from the National HIV prevalence, incidence and behaviour survey, 2012. Afr J AIDS Res. 2016;15(1):67-75. https://doi.org/10.2989/16085906.2016.1153491 [ Links ]
23. National Department of Health. National HIV Counselling and Testing (HCT) Policy Guidelines. Pretoria: Department of Health; 2015. [ Links ]
24. HSRC. HSRC: Research methodology and data centre, S.A. [ Links ] [homepage on the Internet]. 2018. [cited 2020 Jan 01]. Available from: http://www.hsrc.ac.za/en/departments/rmdc
25. PEPFAR. South Africa Country Operational Plan 2017(COP17): PEPFAR [homepage on the Internet]. [ Links ] 2019 [cited 2019 Jun 04]. Available from: https://www.pepfar.gov/documents/organization/272022.pdf
26. Johannessen A. Are men the losers of the antiretroviral treatment scale-up? AIDS. 2011;25(9):1225-1226. https://doi.org/10.1097/QAD.0b013e32834403b8 [ Links ]
27. Mills EJ, Beyrer C, Birungi J, Dybul MR. Engaging men in prevention and care for HIV/AIDS in Africa. PLoS Med. 2012;9(2):e1001167. https://doi.org/10.1371/journal.pmed.1001167 [ Links ]
28. Taylor-Smith K, Tweya H, Harries A, Schoutene E, Jahn A. Gender differences in retention and survival on antiretroviral therapy of HIV-1 infected adults in Malawi. Malawi Med J. 2010; 22(2);49-56. https://doi.org/10.4314/mmj.v22i2.58794 [ Links ]
29. Rufu A, Chitimbire V, Nzou C, et al. Implementation of the 'Test and Treat' policy for newly diagnosed people living with HIV in Zimbabwe in 2017. Public Health Action. 2018;8(3):145-150. https://doi.org/10.5588/pha.18.0030 [ Links ]
30. Nachega JB, Hislop M, Nguyen H, et al. Antiretroviral therapy adherence, virologic and immunologic outcomes in adolescents compared with adults in southern Africa. J Acquir Immune Defic Syndr. 2009;51(1):65. https://doi.org/10.1097/QAI.0b013e318199072e [ Links ]
31. Eduardo E, Lamb MR, Kandula S, et al. Characteristics and outcomes among older HIV-positive adults enrolled in HIV programs in four sub-Saharan African countries. PloS One. 2014;9(7):e103864. https://doi.org/10.1371/journal.pone.0103864 [ Links ]
32. McNaghten A, Mneimneh AS, Farirai T, et al. Strengthening HIV test access and treatment uptake study (Project STATUS): A randomised trial of HIV testing and counseling interventions. J Acquir Immune Defic Syndr. 2015;70(4):e140. https://doi.org/10.1097/QAI.0000000000000785 [ Links ]
33. Sharma M, Ying R, Tarr G, Barnabas R. Systematic review and meta-analysis of community and facility-based HIV testing to address linkage to care gaps in sub-Saharan Africa. Nature. 2015;528(7580):S77. https://doi.org/10.1038/nature16044 [ Links ]
34. Genberg BL, Naanyu V, Wachira J, et al. Linkage to and engagement in HIV care in western Kenya: An observational study using population-based estimates from home-based counselling and testing. Lancet HIV. 2015;2(1):e20-e6. https://doi.org/10.1016/S2352-3018(14)00034-4 [ Links ]
35. Rosen S, Fox MP, Larson BA, et al. Simplified clinical algorithm for identifying patients eligible for immediate initiation of antiretroviral therapy for HIV (SLATE): Protocol for a randomised evaluation. BMJ Open. 2017;7(5):e016340. https://doi.org/10.1136/bmjopen-2017-016340 [ Links ]
36. Labhardt ND, Ringera I, Lejone TI, Masethothi P, Kamele M, Gupta RS, et al. Same day ART initiation versus clinic-based pre-ART assessment and counselling for individuals newly tested HIV-positive during community-based HIV testing in rural Lesotho-a randomised controlled trial (CASCADE trial). BMC Public Health. 2016;16(1):329. https://doi.org/10.1186/s12889-016-2972-6 [ Links ]
37. Labhardt ND, Ringera I, Lejone TI, et al. Effect of offering same-day ART vs usual health facility referral during home-based HIV testing on linkage to care and viral suppression among adults with HIV in Lesotho: The CASCADE randomised clinical trial. JAMA. 2018;319(11):1103-1112. https://doi.org/10.1001/jama.2018.1818 [ Links ]
38. Mabuto T, Latka MH, Kuwane B, Churchyard GJ, Charalambous S, Hoffmann CJ. Four models of HIV counseling and testing: Utilisation and test results in South Africa. PLoS One. 2014;9(7):e102267. https://doi.org/10.1371/journal.pone.0102267 [ Links ]
39. Biadgilign S, Deribew A, Amberbir A, Deribe K. Adherence to highly active antiretroviral therapy and its correlates among HIV infected pediatric patients in Ethiopia. BMC Pediatr. 2008;8(1):53. https://doi.org/10.1186/1471-2431-8-53 [ Links ]
 Correspondence:
Correspondence:
Christopher Hoffmann
choffmann@jhmi.edu
Received: 10 Mar. 2020
Accepted: 07 May 2020
Published: 20 Aug. 2020
REVIEW ARTICLE
Future approaches to clearing the latent human immunodeficiency virus reservoir: Beyond latency reversal
Alexander M.L. Hayes
Medical Sciences Division, Faculty of Clinical Medicine, University of Oxford, Oxford, United Kingdom
ABSTRACT
BACKGROUND: While combined antiretroviral therapy (cART) allows near-normal life expectancy for people living with human immunodeficiency virus (HIV), it is unable to cure the infection and so life long treatment is required
OBJECTIVES: The main barrier to curing HIV is the latent reservoir of cells, which is stable and resistant to cART
METHOD: Current approaches under investigation for clearing this reservoir propose a 'Shock and Kill' mechanism, in which active replication is induced in latent cells by latency reversal agents, theoretically allowing killing of the newly active cells
RESULTS: However, previous studies have failed to achieve depletion of the T central memory cell reservoir, are unable to target other latent reservoirs and may be causing neurological damage to participants
CONCLUSION: Future approaches to clearing the latent reservoir may bypass latency reversal through the use of drugs that selectively induce apoptosis in infected cells. Several classes of these pro-apoptotic drugs have shown promise in in vitro and ex vivo studies, and may represent the basis of a future functional cure for HIV
Keywords: HIV; latency reversal; viral reservoir; pro-apoptotic drugs; cART.
Introduction
The global HIV/AIDS pandemic has caused over 25 million deaths since its origins in the 20th century and remains without a cure. Combined antiretroviral therapy (cART) is the current treatment of choice for human immunodeficiency virus (HIV) infection1 and suppresses viral load below detectable levels with correct administration.1,2 However, lifelong treatment is required, at considerable expense to healthcare systems,1 as the cessation of therapy causes a rapid rebound of viraemia within days3 and eventual progression of the disease to AIDS. Furthermore, escape mutations of the virus are common and reduce efficacy,4,5 necessitating expensive combination therapy with multiple drugs. Viral rebound occurs due to the presence of latent viral reservoirs, rather than continuing low-level replication during cART, as shown by clonal evolutionary studies.6 On therapy cessation these latently infected cells clonally expand, seeding a population of infected cells containing intact provirus.7 Whilst cART has been successful in extending life expectancy of people living with HIV in high-income countries, this remains impaired compared to healthy adults.8 As cART is limited by mutations, is an expensive lifelong treatment and may not be available to those in countries with poorly developed healthcare systems, development of a cure for HIV is desirable. Cure research would be of considerable impact in South Africa, which has the highest number of people living with HIV in the world, at 7.7 m as of 2018, a prevalence of 20.4% in adults (UNAIDS 2019)9. In that year 77 000 deaths were recorded due to AIDS-related illnesses (UNAIDS 2019), although this number is coming down and life expectancy is rising as the cART programme continues to grow.
Reservoirs of latently infected cells are the main barrier to cART curing HIV. The first latently infected cells to be identified were CD4+ central memory T cells (TCM) containing replication-competent, integrated provirus.10 These were found to be present in patients on cART, with no change in reservoir size with continuous therapy11 as the quiescent cells do not contain actively replicating virus, and so are unaffected by cART. The TCM- reservoir is present even if cART is initiated within the first week post exposure,12 so is established early in infection. It is also extremely stable, as the latent cells have a half-life of around 44 months,13 so is unlikely to be cleared in a patient's lifetime. The TCM- reservoir contains the majority of latent cells, but other reservoirs are also present and form barriers to cure research. Several myeloid cell lines have been shown to harbour latent HIV in chronic infection and contribute to viral rebound on cART cessation.14,15 There is evidence for the presence of latent reservoirs in tissue macrophages16 and microglial cells and astrocytes in the central nervous system (CNS).17,18 There is no in vivo evidence of latently infected tissue dendritic cells but infection has been shown in vitro,19 and it has been proposed that this may be transferred to CD4+ cells via a virological synapse.20 Follicular dendritic cells have been shown to maintain a stable pool of virus on their surface without being infected, providing a latent reservoir within secondary lymphoid tissue.21 Further reservoirs have been suggested, such as within fibrocytes,20 but it is now apparent that the TCM reservoir is not the only source of latently infected cells. Potential cures must, therefore, target all identified reservoirs to be successful.
Several approaches to clearing the latent reservoirs have been investigated, with the 'Shock and Kill' combination receiving particular interest. However, recent findings seem to suggest this is unlikely to ever provide a cure for HIV22,23,24,25,26,27,28,29,30 and may have unresearched deleterious side effects.31,32,33 An emerging class of drugs targeting the apoptosis pathways in latently infected cells may allow reservoir depletion without latency reversal, and therefore merit further research as a potential cure for HIV.
Shock and Kill
A potential approach to clearing HIV latent reservoirs is 'Shock and Kill', in which the reservoir would be 'shocked' with latency-reversing agents (LRAs) to induce replication of the provirus. 'Killing' of the activated cells would then be achieved through viral cytopathic effects, CD8+ T cells, cART or other agent, resulting in clearance of the latent cells and curing the infection. This mechanism is outlined in Figure 1. Several LRAs have been developed, many using Yang's in vitro model of latently infected CD4+ TCM34 to identify compounds that selectively induce viral replication in latent cells. These include disulfiram22 and histone deacetylase inhibitors (HDACIs) such as vorinostat and panobionstat. Early in vivo studies were promising as latency reversal was demonstrated with an increase in viral gene expression in the resting cells observed with disulfiram,23,24 vorinostat25 and panbionstat26 administration. However, the key caveat is that none of these studies decreased reservoir size in vivo as the number of latent cells did not decrease following latency reversal. Whilst replication was induced in a proportion of the latent cells, this did not lead to immune or viral-mediated killing of reactivated cells.
Several reasons have been proposed for the failure of LRAs alone to reduce reservoir size. The initial principle of 'Shock and Kill' was that viral cytopathic effects and lysis, due to HIV replication, aided by cytotoxic T lymphocyte (CTL)-mediated killing of actively infected cells, would be sufficient to kill reactivated, formerly latent, cells. Shan et al.27 showed that the viral cytopathic effect alone is insufficient to kill cells, and the vigorous CTL response is lost in chronic HIV infection. However, CTL stimulation increased latent cell clearance, leading to the suggestion that some form of immune stimulation may be required alongside latency reversal to achieve a reduction in reservoir size. The CTL response to reactivated cells may be insufficient to reduce reservoir size on latency reversal, due to 98% of latent T cells carrying escape mutations to CTL killing28 and T cell exhaustion in chronic infection29 reducing the efficacy of the CTL response. Furthermore, LRAs may not affect non-TCM- reservoirs30 as macrophages are resistant to the cytopathic effects of HIV, and dendritic cells do not integrate viral DNA, meaning that LRAs would be unable to cause viral replication and subsequent targeting by the immune system. It has also been proposed that continued cART may not completely inhibit infection of further cells by virions produced from reactivated, formerly latent cells.30 Thus, LRA administration may lead to infection of previously uninfected cells. It is therefore clear that LRA administration alone does not reduce reservoir size, due to a lack of viral cytopathic effects and lysis, insufficient CTL response and the potential failure of cART to suppress replication. As such, stages 3 and 4 from Figure 1 are unlikely to be successful and an additional killing agent is required to achieve the second goal of 'Shock and Kill'. This may involve immune stimulation, with natural killer (NK) cell stimulation showing some promise ex vivo35 or other agents, such as broadly neutralising antibodies (bNAbs), which appear to mediate antibody-dependent cellular cytotoxicity of activated latently infected cells in mice36 but human studies are required to assess their efficacy. Killing agents that selectively induce apoptosis of HIV-infected cells have also shown promise in vitro (see Pro-apoptotic drugs [PADs]).
Limitations of latency-reversing agents
Whilst it has been repeatedly shown that LRAs alone are unable to reduce the size of the latent HIV reservoir, administration of a killing agent alongside an LRA has been proposed as a method of clearing the reactivated cells.37 However, it seems likely that even with killing agents LRAs cannot clear enough latent cells to achieve a functional cure and may cause complications due to non-T cell HIV reservoirs. Future studies in humans to test the efficacy of killing agents administrated alongside LRAs could be carried out by administering a previously tested LRA, such as disulfiram, alongside a killing agent such as a bNAb to participants living with HIV and measuring the change in reservoir size using a technique such as TILDA.38 However, the combination of an LRA with, for example, a bNAb, is unlikely to clear the myeloid reservoir as dendritic cells are likely to be unaffected by LRAs.30 Therefore, even if functional elimination of the TCM reservoir is achieved, viral rebound on cART cessation may still occur due to the presence of the myeloid reservoir, which would likely not be cleared by current 'Shock and Kill' approaches under investigation.
Human immunodeficiency virus-associated neurological disorders (HANDs) are well-observed in patients on cART and may lead to widespread neurological impairment,39 suggesting significant damage to the CNS even when viraemia elsewhere is suppressed below detectable levels. The cause of this appears to be relatively poor blood-brain barrier (BBB) penetrance by antiretrovirals,31 allowing continued HIV replication alongside the presence of latently infected microglial cells, perivascular macrophages and astrocytes.20 Recent research suggests that impaired neurogenesis may underpin HAND persistence even with cART.32 A study on the CNS penetrance of LRAs33 showed that disulfiram and vorinostat are able to cross the BBB relatively easily. Whilst this could enable targeting of the CNS latent reservoir, it may mean that current in vivo studies of LRAs are damaging to the patients involved, as there is no reduction in latent reservoir size, whilst LRAs are able to penetrate the CNS and increase viral replication with no inhibition from antiretroviral drugs. This may potentially lead to an increase in viral protein, inflammatory cytokine (CK) and neurotoxin release from infected macrophages and microglia, which could increase the probability of HAND development (personal communication, Grant Campbell) as shown in Figure 2. Therefore, in addition to their inability to affect all HIV reservoirs, even with the accompanying administration of killing agents, LRAs may cause increased neurological impairment of patients. Furthermore, if the killing agent used in a hypothetical cure is unable to penetrate the BBB, there would be no reduction in the size of the CNS reservoir. This makes the development of a cure strategy distinct from 'Shock and Kill', involving the direct killing of latent cells without the need for latency reversal, desirable. The PAD class mentioned earlier has shown promise in achieving this.
Interactions of human immunodeficiency virus with apoptosis
The human immunodeficiency virus has complex interactions with apoptosis pathways in infected cells.40 In latently infected cells the increase in longevity observed is partially the result of avoidance of apoptosis.37 A number of specific effects on apoptosis pathways have been suggested as the cause of this. The intrinsic apoptosis pathway is a response to cellular damage and involves pro-apoptotic factors causing mitochondrial outer membrane permeabilisation (MOMP), triggering the release of soluble proteins from the mitochondrial intermembrane space. These include the cytochrome c and second mitochondria-derived activator of caspase (SMAC, also known as DIABLO41). Cytochrome c binds cytosolic proteins to form the apoptosome, which activates the 'initiator' enzyme caspase-9. This, in turn, cleaves the 'executioner' enzymes caspase-3 and caspase-7 which trigger the cleavage cascade of other caspase enzymes resulting in cell death. The second mitochondria-derived activator of caspase activates this process through disinhibition of caspases 3, 7 and 9 via binding and inactivating the X-linked inhibitor of apoptosis (XIAP). The X-linked inhibitor of apoptosis binds these caspases in the absence of SMAC, inhibiting their activity and directing their degradation by the proteasome. The second mitochondria-derived activator of caspase antagonises this activity, allowing the progression of the apoptotic process.41 Several other IAPs are also inhibited by SMAC, including the baculoviral IAP repeat-containing protein 2 (BIRC, also known as cellular IAP1).41
Viral proteins, in particular Tat and Nef, cause inhibition of this intrinsic apoptosis pathway in latently infected cells. Tat upregulates host production of anti-apoptotic proteins including XIAP and Bcl2.42 The X-linked inhibitor of apoptosis inhibits caspase activity, as detailed earlier, whilst Bcl2 inhibits MOMP,43 thus preventing the release of cytochrome c and SMAC. Tat also inhibits the pro-apoptotic proteins p53 and the Bcl2-associated antagonist of cell death (BAD).40 Nef causes p21-activated kinase activation, leading to phosphorylation of BAD, thus inhibiting its pro-apoptotic activity (binding to and inhibition of Bcl2),44 and also inhibits caspase-8 and caspase-10.40 Conversely, in the CNS, it appears that Tat and Nef may induce autophagy and apoptosis in HAND pathogenesis.45,46 Expression and activation of anti-apoptotic proteins are therefore increased in latently infected cells, making this an attractive therapeutic target.
Pro-apoptotic drugs
Several classes of pro-apoptotic compounds have been developed recently for cancer treatment,47,48 as tumour cells often over-express or activate IAPs allowing the evasion of apoptosis, a key hallmark of cancer. Some of these have been shown to induce apoptosis of latent HIV infected cells in vitro, leading to speculation that PADs may contribute to a future cure.
Second mitochondria-derived activator of caspase mimetics (SMs) are a class of drugs which bind IAPs in a similar fashion to the endogenous molecule.48 They cause caspase activation via IAP binding49 and appear to reduce IAP activity through both degradation and inhibition of the proteins,50 and therefore induce apoptosis. Second mitochondrias have recently been developed for cancer treatment, inducing apoptosis48 of tumour cells which over-express or activate IAPs. The SMs birinapant, embelin and GDC-0152 were investigated for their efficacy to induce apoptosis in latently infected TCM by Campbell et al.51 All were found to cause rapid degradation of XIAP and BIRC2, which were upregulated in the infected cells, and importantly showed selective killing of HIV-infected TCM compared to uninfected TCM, with the doses required for 90% clearance of HIV-infected cells causing a small increase of TCM cell death, of 3.5% - 4.6% above basal levels. This occurred in the absence of increased virus production, despite findings in models of latency by Pache et al.52 suggesting that SMs may act as LRAs due to the reduction in BIRC2 leading to increased NFκβ signalling, resulting in an increased viral transcription. However, in latent HIV-TCM from patients undergoing cART, the Pache study showed that SMs only caused latency reversal when used in combination with an LRA such as vorinostat. Therefore, it appears that the major effect of SMs on latently infected TCM- -is to induce apoptosis rather than reactivate transcription.
Furthermore, Campbell et al. demonstrated the mechanism of apoptosis on SM administration is dependent on the induction (but not completion) of autophagy. Wortmannin (an inhibitor of early stages of autophagy) led to a reduction in apoptosis despite the degradation of IAPs by SMs. Further findings suggest that, following IAP degradation, autophagy proteins form a scaffold for the assembly of a ripoptosome-like death-inducing signaling complex (DISC), which can then initiate apoptosis via caspase 8 induction. This is an additional mechanism to the simple removal of IAP inhibition due to their degradation and may explain the selectivity of SMs for inducing death of HIV-TCM. Inhibitors of later stages of autophagy did not reduce apoptosis, suggesting that the process of autophagy itself is not required for this mechanism of cell death. Induction of autophagy is sufficient for apoptosis, as autophagy proteins allow DISC formation. IAPs are degraded by SMs in uninfected TCM but this does not lead to the same degree of cell death due to reduced autophagy induction and therefore decreased probability of DISC formation. HIV-TCM are more prone to autophagy induction than uninfected cells as a result of the effects of viral proteins. Gag and Nef interact with autophagy factors to increase autophagy induction, which in turn augments HIV yields in cells containing the actively replicating virus.53 Nef and Vif inhibit later stages of autophagy to prevent HIV degradation.53,54 Therefore, HIV-TCM are more prone to autophagy induction than uninfected cells, providing a potential explanation for the selective apoptosis observed in latently infected cells on SM administration. Over-expression or activation of IAPs in infected cells may also contribute to the selectivity of SMs.
These findings present ex vivo evidence of the selective killing of latently infected TCM by SMs, along with a convincing mechanism of how uninfected cells are spared. Clinical trials in humans may now be justified, but should be performed with caution as the side effects of these drugs in people living with HIV are unknown. A number of SMs have been used in phase I trials for various cancers, with a generally tolerable safety profile, although the occasional occurrence of cytokine release syndrome in patients is of some concern.55In vitro and ex vivo findings suggest SMs may be able to deplete the TCM reservoir in vivo, and, whilst if 90% of latently infected cells are killed (as in the Campbell study) the patient will not be cured of HIV, this eclipses reservoir clearance demonstrated by current 'Shock and Kill' approaches. However, it is unclear if these drugs would induce apoptosis in non-T cell reservoirs to the same degree, which may prevent the development of a functional cure. Future in vitro and ex vivo studies of PADs using latently infected myeloid cells from people living with HIV would be advisable to further test their suitability.
In vitro and ex vivo studies of other PADs have also shown promise. Bcl-2 antagonists such as venetoclax caused disinhibition of MOMP and led to the depletion of latent HIV-TCM from patients on cART in an ex vivo study.56 P13K/Akt inhibitors increase the expression of pro-apoptotic genes downregulated by HIV and have shown promise in predisposing latently infected macrophages and microglia to cell death.57,58 RIG-1 inducers activate an innate immune response to viral RNA, triggering apoptosis. An ex vivo study demonstrated selective depletion of latent HIV-TCM using the RIG-1 inducer acitretin.59 These classes of PADs also merit further investigation and could be used in combination with SMs in a future approach to clearing the latent reservoir to induce apoptosis in a greater portion of latently infected cells.
Conclusions
Recent 'Shock and Kill' research has failed to demonstrate significant depletion of the viral reservoir, and it remains unclear if the use of LRAs may cause HANDs. PADs may represent a promising future approach to curing HIV given their in vitro and ex vivo success in selectively causing apoptosis in latently infected cells, with SMs appearing effective against the TCM reservoir and P13K/Akt inhibitors against myeloid reservoirs. Several of these drugs have been used in clinical trials for other conditions, and further investigation into their potential for people living with HIV is merited.
Acknowledgements
Grant Campbell provided theoretical assistance via email correspondence for the limitations of the LRAs section, specifically about the potential neurological side effects LRAs may have.
Professor William James, Dunn School of Pathology, University of Oxford, was my supervisor for the review, and so offered me advice and guidance on selecting a subject area and how to go about writing the review. He has not read or edited the review since I began writing it due to university examination regulations.
Competing interests
I declare that no competing interests exists.
Author's contributions
I am the sole author for this work.
Ethical consideration
This article followed all ethical standards for carrying out research without direct contact with human or animal subjects.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data sharing is not applicable to this article as no new data were created or analysed in this study.
Disclaimer
The views and opinions expressed in this article are those of the author and do not necessarily reflect the official policy or position of any affiliated agency of the author.
References
1. Volberding PA, Deeks SG. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376(9734):49-62. https://doi.org/10.1016/s0140-6736(10)60676-9 [ Links ]
2. Palmer S, Maldarelli F, Wiegand A, et al. Low-level viremia persists for at least 7 years in patients onsuppressive antiretroviral therapy. Proc Natl Acad Sci U S A. 2008;105(10):3879-3884. https://doi.org/10.1073/pnas.0800050105 [ Links ]
3. Fischer M, Hafner R, Schneider C, et al. HIV RNA in plasma rebounds within days during structuredtreatment interruptions. AIDS. 2003;17(2):195-199. https://doi.org/10.1097/01.aids.0000042945.95433.4b [ Links ]
4. Poggensee G, Kücherer C, Werning J, et al. Impact of transmission of drug-resistant HIV on the course of infection and the treatment success. Data from the German HIV-1 seroconverter study. HIV Med. 2007;8(8):511-519. https://doi.org/10.1111/j.1468-1293.2007.00504.x [ Links ]
5. Wittkop L, Günthard H, de Wolf F, et al. Effect of transmitted drug resistance on virological and immunological response to initial combination antiretroviral therapy for HIV (EuroCoord-CHAIN joint project): A European multicohort study. Lancet Infect Dis. 2011;11(5):363-371. https://doi.org/10.1016/S1473-3099(11)70032-9 [ Links ]
6. Joos B, Fischer M, Kuster H, et al. HIV rebounds from latently infected cells, rather than from continuing low-level replication. Proc Natl Acad Sci U S A. 2008;105(43):16725-16730. https://doi.org/10.1073/pnas.0804192105 [ Links ]
7. Simonetti FR, Sobolewski M, Fyne E, et al. Clonally expanded CD4+ T cells can produce infectious HIV-1 invivo. Proc Natl Acad Sci U S A. 2016;113(7):1883-1888. https://doi.org/10.1073/pnas.1522675113 [ Links ]
8. Collaboration A TC. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: A collaborative analysis of 14 cohort studies. Lancet. 2008;372(9635):293-299. https://doi.org/10.1016/S0140-6736(08)61113-7 [ Links ]
9. UNAIDS website: https://www.unaids.org/en/regionscountries/countries/southafrica [ Links ]
10. Chun TW, Stuyver L, Mizell S, et al. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997;94(24):13193-13197. https://doi.org/10.1073/pnas.94.24.13193 [ Links ]
11. Finzi D, Hermankova M, Pierson T, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278(5341):1295-1300. https://doi.org/10.1126/science.278.5341.1295 [ Links ]
12. Chun TW, Engel D, Berrey M, et al. Early establishment of a pool of latently infected, resting CD4(+) T cells during primary HIV-1 infection. Proc Natl Acad Sci U S A. 1998;95(15):8869-8873. https://doi.org/10.1073/pnas.95.15.8869 [ Links ]
13. Siliciano JD, Kajdas J, Finzi D, et al. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(1):727-728. https://doi.org/10.1038/nm880 [ Links ]
14. Abbas W, Tariq M, Iqbal M, Kumar A, Herbein G. Eradication of HIV-1 from the macrophage reservoir: An uncertain goal? Viruses. 2015;7(4):1578-1598. https://doi.org/10.3390/v7041578 [ Links ]
15. Gama L, Abreu C, Shirk E, et al. SIV Latency in macrophages in the CNS. Curr Top Microbiol Immunol. 2018;417:111-130. https://doi.org/10.1007/82_2018_89 [ Links ]
16. Honeycutt JB, Thayer W, Baker C, et al. HIV persistence in tissue macrophages of humanized myeloidonly mice during antiretroviral therapy. Nat Med. 2017;23:638-643. https://doi.org/10.1038/nm.4319 [ Links ]
17. Churchill MJ, Gorry P, Cowley D, et al. Use of laser capture microdissection to detect integrated HIV-1 DNA in macrophages and astrocytes from autopsy brain tissues. J Neurovirol. 2006;12:146-152. https://doi.org/10.1080/13550280600748946 [ Links ]
18. Kramer-Hämmerle S, Rothenaigner I, Wolff H, Bell JE, Brack-Werner R. Cells of the central nervous system as targets and reservoirs of the human immunodeficiency virus. Virus Res. 2005;111(2):194-213. https://doi.org/10.1016/j.virusres.2005.04.009 [ Links ]
19. Kawamura T, Gulden F, Sugaya M, et al. R5 HIV productively infects Langerhans cells, and infection levels are regulated by compound CCR5 polymorphisms. Proc Natl Acad Sci U S A. 2003;100(14):8401-8406. https://doi.org/10.1073/pnas.1432450100 [ Links ]
20. Kandathil AJ, Sugawara S, Balagopal A. Are T cells the only HIV-1 reservoir? Retrovirology. 2016;13:86. https://doi.org/10.1186/s12977-016-0323-4 [ Links ]
21. Spiegel H, Herbst H, Niedobitek G, Foss HD, Stein H. Follicular dendritic cells are a major reservoir for human immunodeficiency virus type 1 in lymphoid tissues facilitating infection of CD4+ T-helper cells. Am J Pathol. 1992;140:15-22. [ Links ]
22. Xing S, Bullen C, Shroff N, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85(12):6060-6064. https://doi.org/10.1128/JVI.02033-10 [ Links ]
23. Spivak AM, Andrade A, Eisele E, et al. A pilot study assessing the safety and latency-reversing activity of disulfiram in HIV-1-infected adults on antiretroviral therapy. Clin Infect Dis. 2014;58(6):883-890. https://doi.org/10.1093/cid/cit813 [ Links ]
24. Elliott JH, McMahon J, Chang C, et al. Short-term administration of disulfiram for reversal of latent HIV infection: A phase 2 dose-escalation study. Lancet HIV. 2015;2(12):e520-529. https://doi.org/10.1016/S2352-3018(15)00226-X [ Links ]
25. Archin NM, Liberty A, Kashuba A et al. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487:482-485. https://doi.org/10.1038/nature11286 [ Links ]
26. Rasmussen TA, Tolstrup M, Brinkmann C, et al. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: A phase 1/2, single group, clinical trial. Lancet HIV. 2014;1(1):e13-e21. https://doi.org/10.1016/S2352-3018(14)70014-1 [ Links ]
27. Shan L, Deng K, Shroff N, et al. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity. 2012;36(3):491-501. https://doi.org/10.1016/j.immuni.2012.01.014 [ Links ]
28. Deng K, Pertea M, Rongvaux A, et al. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517:381-385. https://doi.org/10.1038/nature14053 [ Links ]
29. Chew GM, Fujita T, Webb G, et al. TIGIT marks exhausted T cells, correlates with disease progression, and serves as a target for immune restoration in HIV and SIV infection. PLoS Pathog. 2016;12:e1005349. https://doi.org/10.1371/journal.ppat.1005349 [ Links ]
30. Thorlund K, Horwitz MS, Fife BT, Lester R, Cameron DW. Landscape review of current HIV 'kick and kill' cure research - Some kicking, not enough killing. BMC Infect Dis. 2017;17:595. https://doi.org/10.1186/s12879-017-2683-3 [ Links ]
31. Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65(1):65-70. https://doi.org/10.1001/archneurol.2007.31 [ Links ]
32. Putatunda L, Ho WZ, Hu W. HIV-1 and compromised adult neurogenesis: Emerging evidence for a new paradigm of HAND persistence. AIDS Rev. 2019;21:11-22. https://doi.org/10.24875/AIDSRev.19000003 [ Links ]
33. Churchill MJ, Cowley DJ, Wesselingh SL, Gorry PR, Gray LR. HIV-1 transcriptional regulation in the central nervous system and implications for HIV cure research. J Neurovirol. 2015;21:290-300. https://doi.org/10.1007/s13365-014-0271-5 [ Links ]
34. Yang HC, Xing S, Shan L, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473-3486. https://doi.org/10.1172/JCI39199 [ Links ]
35. Garrido C, Abad-Fernandez M, Tuyishime M, et al. Interleukin-15-stimulated natural killer cells clear HIV-1-infected cells following latency reversal ex vivo. J Virol. 2018;92(12):e00235-18. https://doi.org/10.1128/JVI.00235-18 [ Links ]
36. Halper-Stromberg A, Lu C, Klein F, et al. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell. 2014;158(5):989-999. https://doi.org/10.1016/j.cell.2014.07.043 [ Links ]
37. Kim Y, Anderson JL, Lewin SR. Getting the 'Kill' into 'Shock and Kill': Strategies to eliminate latent HIV. Cell Host Microbe. 2018;23(1):14-26. https://doi.org/10.1016/j.chom.2017.12.004 [ Links ]
38. Procopio FA, Fromentin R, Kulpa D, et al. A novel assay to measure the magnitude of the inducible viral reservoir in HIV-infected individuals. EBioMedicine. 2015;2(8):874-883. https://doi.org/10.1016/j.ebiom.2015.06.019 [ Links ]
39. Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087-2096. https://doi.org/10.1212/WNL.0b013e318200d727 [ Links ]
40. Timilsina U, Gaur R. Modulation of apoptosis and viral latency - An axis to be well understood for successful cure of human immunodeficiency virus. J Gen Virol. 2016;97(4):813-824. https://doi.org/10.1099/jgv.0.000402 [ Links ]
41. Green DR, Llambi F. Cell death signaling. Cold Spring Harb Perspect Biol. 2015;7. https://doi.org/10.1101/cshperspect.a006080 [ Links ]
42. López-Huertas MR, Mateos E, Sánchez del Cojo M et al. The presence of HIV-1 Tat protein second exon delays fas protein-mediated apoptosis in CD4+ T lymphocytes: A potential mechanism for persistent viral production. J Biol Chem. 2013;288:7626-7644. https://doi.org/10.1074/jbc.M112.408294 [ Links ]
43. Llambi F, Moldoveanu T, Tait S, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44(4):517-531. https://doi.org/10.1016/j.molcel.2011.10.001 [ Links ]
44. Wolf D, Witte V, Laffert B, et al. HIV-1 Nef associated PAK and PI3-kinases stimulate Akt-independent Bad-phosphorylation to induce anti-apoptotic signals. Nat Med. 2001;7:1217-1224. https://doi.org/10.1038/nm1101-1217 [ Links ]
45. Wu X, Dong H, Ye X, et al. HIV-1 Tat increases BAG3 via NF-κB signaling to induce autophagy during HIV-associated neurocognitive disorder. Cell Cycle. 2018;13:1614-1623. https://doi.org/10.1080/15384101.2018.1480219 [ Links ]
46. Dong H, Ye X, Zhong L, et al. Role of FOXO3 activated by HIV-1 tat in HIV-associated neurocognitive disorder neuronal apoptosis. Front Neurosci. 2019;4:44. https://doi.org/10.3389/fnins.2019.00044 [ Links ]
47. Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;150845:23. https://doi.org/10.1155/2014/150845 [ Links ]
48. Fulda S. Smac mimetics to therapeutically target IAP proteins in cancer. Int Rev Cell Mol Biol. 2017;330:157-169. https://doi.org/10.1016/bs.ircmb.2016.09.004 [ Links ]
49. Gao Z, Tian Y, Wang J, et al. A dimeric Smac/diablo peptide directly relieves caspase-3 inhibition by XIAP. Dynamic and cooperative regulation of XIAP by Smac/Diablo. J Biol Chem. 2007;282:30718-30727. https://doi.org/10.1074/jbc.M705258200 [ Links ]
50. Bertrand MJ, Milutinovic S, Dickson K, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30(6):689-700. https://doi.org/10.1016/j.molcel.2008.05.014 [ Links ]
51. Campbell GR, Bruckman RS, Chu YL, Trout RN, Spector SA. SMAC mimetics induce autophagy-dependent apoptosis of HIV-1-infected resting memory CD4+ T cells. Cell Host Microbe. 2018;24(5):689-702. https://doi.org/10.1016/j.chom.2018.09.007 [ Links ]
52. Pache L, Dutra MS, Spivak AM, et al. BIRC2/cIAP1 is a negative regulator of HIV-1 transcription and can be targeted by smac mimetics to promote reversal of viral latency. Cell Host Microbe. 2015;18(3):345-353. https://doi.org/10.1016/j.chom.2015.08.009 [ Links ]
53. Kyei GB, Dinkins C, Davis AS, et al. Autophagy pathway intersects with HIV-1 biosynthesis and regulates viral yields in macrophages. J Cell Biol. 2009;186(2):255-268. https://doi.org/10.1083/jcb.200903070 [ Links ]
54. Borel S, Robert-Hebmann V, Alfaisal J, et al. HIV-1 viral infectivity factor interacts with microtubule-associated protein light chain 3 and inhibits autophagy. AIDS. 2015;29(3):275-286. https://doi.org/10.1097/QAD.0000000000000554 [ Links ]
55. Boddu P, Carter BZ, Verstovek S, Pemmaraju N. SMAC mimetics as potential cancer therapeutics in myeloid malignancies. Br J Haematol. 2019;185(2):219-231. https://doi.org/10.1111/bjh.15829 [ Links ]
56. Cummins NW, Sainski AM, Dai H, et al. Prime, shock, and kill: Priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol. 2016;90:4032-4048. https://doi.org/10.1128/JVI.03179-15 [ Links ]
57. Lucas A, Kim Y, Rivera-Pabon O, et al. Targeting the PI3K/Akt cell survival pathway to induce cell death of HIV-1 infected macrophages with alkylphospholipid compounds. PLoS One. 2010;5(9):e13121. https://doi.org/10.1371/journal.pone.0013121 [ Links ]
58. Kim Y, Hollenbaugh JA, Kim DH, Kim B. Novel PI3K/Akt inhibitors screened by the cytoprotective function of human immunodeficiency virus type 1 Tat. PLoS One. 2011;6(7):e21781. https://doi.org/10.1371/journal.pone.0021781 [ Links ]
59. Li P, Kaiser P, Lampiris HW, et al. Stimulating the RIG-I pathway to kill cells in the latent HIV reservoir following viral reactivation. Nat Med. 2016;22:807-811. https://doi.org/10.1038/nm.4124 [ Links ]
 Correspondence:
Correspondence:
Alexander Hayes
Alexander-hayes@keble.ox.ac.uk
Received: 25 Mar. 2020
Accepted: 12 May 2020
Published: 12 Aug. 2020
ORIGINAL RESEARCH
Perspectives on oral pre-exposure prophylaxis use amongst female sex workers in Harare, Zimbabwe
Tinashe MudzvitiI, II; Anesu DhliwayoI; Byrone ChingombeIII; Bernard NgaraIV; Tsitsi G. Monera-PendukaI; Charles C. MapongaI, III, V; Gene D. MorseV, VI
ISchool of Pharmacy, University of Zimbabwe, Harare, Zimbabwe
IINewlands Clinic, Harare, Zimbabwe
IIIPopulation Services International, Harare, Zimbabwe
IVDepartment of Community Medicine, College of Health Sciences, University of Zimbabwe, Harare, Zimbabwe
VCenter for Integrated Global Biomedical Sciences, University at Buffalo, New York, United States
VITranslational Pharmacology Research Core, University at Buffalo, New York, United States
ABSTRACT
BACKGROUND: Pre-exposure prophylaxis (PrEP) could provide protection from human immunodeficiency virus (HIV) infection in sexually active persons at risk. Limited data are available in Zimbabwe with regard to the perceptions about PrEP amongst female sex workers (FSWs
OBJECTIVES: The aim of this study was to evaluate the knowledge levels of oral PrEP and the likelihood of its use amongst FSWs.
METHOD: This was a cross-sectional study in the peri-urban areas of Harare, Zimbabwe. Human immunodeficiency virus-negative FSWs were interviewed to assess their awareness of and likelihood to use PrEP. The relative importance index was used to evaluate the levels of knowledge and the likelihood of, and barriers to, PrEP use. A set of 10 questions was designed and validated that evaluated participants' understanding of PrEP. A bivariate logistic regression model was utilised to identify predictors of PrEP use.
RESULTS: A total of 131 FSWs with a median age of 25 years (interquartile range: 21-31) participated in this study. Of the 71 (54%) FSWs who had heard about PrEP, 46 (35%) participants had adequate knowledge of its use. A total of 102 (78%) participants revealed that they would be willing to continuously use PrEP if it was provided free of cost. Increasing age of the participants was associated with an increase in the likelihood of using PrEP (r = 0.0033, p = 0.038). More knowledge about PrEP increased the likelihood of its use (r = 0.21, p = 0.0153). This likelihood increased amongst participants with an unprotected sexual intercourse encounter in the preceding 3 months (r = 0.0448, p = 0.026).
CONCLUSION: Knowledge of PrEP amongst FSWs was low. To increase the uptake of PrEP, there is a need to further sensitise FSWs about this intervention. Programmes should also promote awareness training in FSW subgroups that are less likely to use PrEP.
Keywords: female sex workers; HIV; pre-exposure prophylaxis; barriers; Truvada.
Background
Whilst the adult prevalence of human immunodeficiency virus (HIV) in the general population of Zimbabwe is 15%, the prevalence in key populations is higher.1 In populations of female sex workers (FSWs), the HIV prevalence in 2013 was 50% - 70% in different parts of Zimbabwe.2 In many settings, key populations are hidden and stigmatised, and their representation in national surveillance data is limited. It has been cited that key populations and their sex partners not only make up the largest proportion of people living with HIV (PLWH) but also represent a significant proportion of new infections in sub-Saharan Africa.3
Pre-exposure prophylaxis (PrEP) can become a female-controlled HIV prevention method for FSWs and others who are unable to negotiate condom use. The Ministry of Health and Child Care (MoHCC) has developed a PrEP framework policy that prioritises access for 'at-risk' populations. Groups that need to be offered PrEP include female and male sex workers; serodiscordant couples, that is, the HIV seronegative partner; adolescent girls and young women; pregnant women in relationships with men of unknown status and high-risk men, for example, men who have sex with men (MSM); prisoners; long-distance truck drivers; and transgender people.4 A fixed-dose regimen of either Tenofovir 300 mg and Emtricitabine 200 mg (TDF/FTC) or Tenofovir 300 mg and Lamivudine 300 mg (TDF/3TC) has been recommended for once-daily oral administration during the period an individual is at risk of contracting HIV infection.4 Zimbabwe has adopted The Joint United Nations Programme on HIV and AIDS 90-90-90 global goals to help reduce new infections and end the HIV pandemic.5 This target will be difficult to reach without reducing transmission amongst high-incidence populations.
Although it is well established that effective PrEP could provide an additional safety net to sexually active persons at risk,6 limited data are available in Zimbabwe regarding the knowledge and the likelihood of PrEP use amongst FSWs. There is a need to understand both the acceptability of PrEP amongst FSWs and the factors likely to determine uptake. Most research efforts to date have focussed on clinical aspects of PrEP. Little attention has been focussed on the factors that influence FSWs' willingness to take it. It is estimated that there are 40 000 (plausibility bounds [PBs] 28 000-59 000) active FSWs in Zimbabwe, that is, 1.23% (PB: 0.86% - 1.79%) of the adult female population. A total of 20 000 (50%) are in Harare and Bulawayo.7 This study was conducted to assess the levels of knowledge, barriers to and likelihood of oral PrEP use amongst FSWs as a preventative method in reducing the risk of acquiring HIV in Harare, Zimbabwe.
Methods
Study design and setting
This was a cross-sectional study of FSWs in seven peri-urban areas of Harare province, Zimbabwe. These sites were specifically chosen because they are high-density areas, and apart from the central business district, they are areas from which FSWs frequently operate.8
Study population and recruitment
This cross-sectional study was conducted between December 2016 and February 2017 in partnership with a local private voluntary organisation (PVO) that offers PrEP services. The PVO had been offering PrEP using TDF/FTC (TruvadaTM) tablets as an HIV prevention method in six Zimbabwean districts since August 2016. This was a demonstration project introduced to inform people of the new HIV prevention strategy of the MoHCC. The primary target populations included adolescent girls and young women aged 15-24 years, FSWs, MSM and serodiscordant couples.9
Human immunodeficiency virus-negative FSWs were defined as those who had been tested for HIV in the previous 3 months and had tested negative or those who perceived themselves to be HIV-negative. The PrEP intervention that is defined for the purpose of this study refers to oral PrEP with Truvada, which is indicated for 'at-risk' individuals with a laboratory-confirmed HIV-negative result. None of the participants was already receiving PrEP from the PVO.
Snowball sampling was used to locate and enrol FSWs from the peri-urban Harare sites. A peer referral system whereby 'seed' subjects previously identified by the PVO and 'queens' (the leaders of a significant group of sex workers based on their location in a certain area) provided referrals to enable further recruitment and mobilisation of other FSWs. This technique was utilised because of laws and policies that criminalise sex work in Zimbabwe. Female sex workers were also identified during community outreach HIV testing and counselling activities by the PVO. Once identified, FSWs were asked to provide written consent and to complete a 48-item questionnaire. The questionnaire was administered by the interviewer. The questionnaire had sections enquiring about socio-demographic factors, sexual behavioural characteristics, HIV testing, PrEP knowledge, perceptions, barriers and the likelihood of its use. Sexual behavioural characteristics were investigated to determine the HIV acquisition risk profile of the FSWs. The interviews were conducted in the language that the participant was most comfortable with. 'Sufficient knowledge' was judged by means of an initial self-report and an additional nine technical questions designed to test the level of knowledge. These technical questions included the participant's knowledge that PrEP is used by HIV-negative individuals, the dosing frequency, potential drug interactions and whether there are other reproductive health benefits. Once the level of knowledge was ascertained, the participants were then educated about PrEP use. The perceived barriers that were evaluated included stigma, cost of PrEP and the side-effects of Truvada.
Data management and statistical analysis
Research Electronic Data Capture (REDCap) was used to manage data. This was hosted by the College of Health Sciences of the University of Zimbabwe. The contribution of each factor, namely, age, cost of PrEP and drug side-effects, with regard to improving PrEP uptake amongst FSWs was examined. The importance of each factor as perceived by the respondents was assessed by computing the relative importance index (RII). The RII is a statistical measure recorded on a scale of 0 < RII ≤ 1, where '0' or any value close to '0' is defined as a poor knowledge of PrEP use, the participant is less likely to use PrEP or barriers associated with PrEP are likely to affect uptake. If the RII score was '1' or close to '1', it means that participants were knowledgeable about PrEP, were more likely to use PrEP and that barriers associated with PrEP use were unlikely to affect its uptake.
The RII was computed using the equation  , where Scorei was the score for each question given by the participants and ranged from 0 to 4 (where '0' was 'very much disagree, strongly disagree or never' and '4' was 'strongly agree, very much agree or almost always'). K was the maximum possible score and n was the total number of questions. According to Johnson and LeBreton,10 RII is the proportionate contribution each predictor makes to R2 (where R2 is the extent to which the dependent variable can be predicted by the predictor variables), considering both its direct effect (correlation with the dependent variable) and its effect when combined with other variables.
, where Scorei was the score for each question given by the participants and ranged from 0 to 4 (where '0' was 'very much disagree, strongly disagree or never' and '4' was 'strongly agree, very much agree or almost always'). K was the maximum possible score and n was the total number of questions. According to Johnson and LeBreton,10 RII is the proportionate contribution each predictor makes to R2 (where R2 is the extent to which the dependent variable can be predicted by the predictor variables), considering both its direct effect (correlation with the dependent variable) and its effect when combined with other variables.
All data analysis was performed using Stata version 13 (StataCorp LP, TX, USA) software package. Bivariate linear regression was used to determine if there was a relationship between the RII scores of knowledge, likelihood and barriers (KLBs) and exploratory factor variables that were collected in the study. To identify the relationship between KLB RII scores, a matrix of Spearman correlation coefficients was used.
Ethical consideration
Prior to conducting the survey, the study protocol including the data collecting tool was reviewed and approved by the Joint Research Ethics Committee of the University of Zimbabwe and the Parirenyatwa Group of Hospitals (JREC/328/16). All participants provided written informed consent before participating in the study.
Results
A total of 131 presumed HIV-negative, adult FSWs were recruited to participate in this study, and their demographic characteristics are shown in Table 1. All study participants were self-identified as residents of the Harare province.
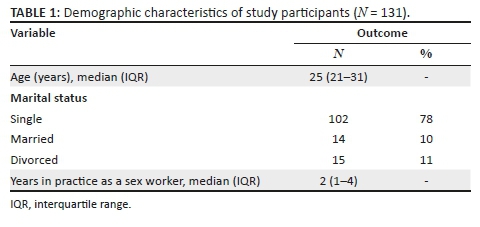
Participant's characteristics - Sexual risk factors and human immunodeficiency virus testing
The median number of sexual encounters by the participants was five [interquartile range (IQR): 3-6] partners per day. Half of the participants (50%) did not know their partner's HIV serostatus and only 42% of the FSWs would talk about HIV with their clients or partners. All participants perceived the use of condoms as a necessary tool when engaging in sexual activity with their partners or clients, 86% used condoms with the last three partners they encountered and 44% reported having ever had a condom burst at least once during sexual intercourse. The variables affecting HIV acquisition risk are depicted in Table 2.
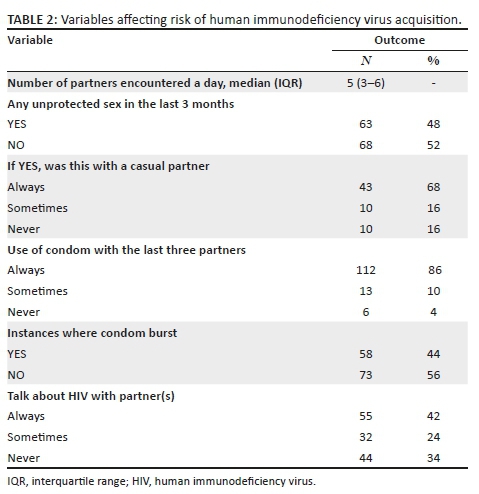
Pre-exposure prophylaxis knowledge
Of the 131 participants, 71 (54%) had heard about PrEP and of those only 46 (35%) had sufficient knowledge about PrEP (RII > 0.5). Participants mostly heard about PrEP from non-governmental organisations (59%), from friends (35%) and only 6% of the participants had heard about PrEP through clinics.
Likelihood of pre-exposure prophylaxis use
The participants' responses when asked about the likelihood of PrEP use are shown in Table 3. Regardless of a likelihood to use PrEP, potential barriers were cited, such as stigma, costs, side-effects associated with the PrEP tablet and poor knowledge, as shown in Table 3.
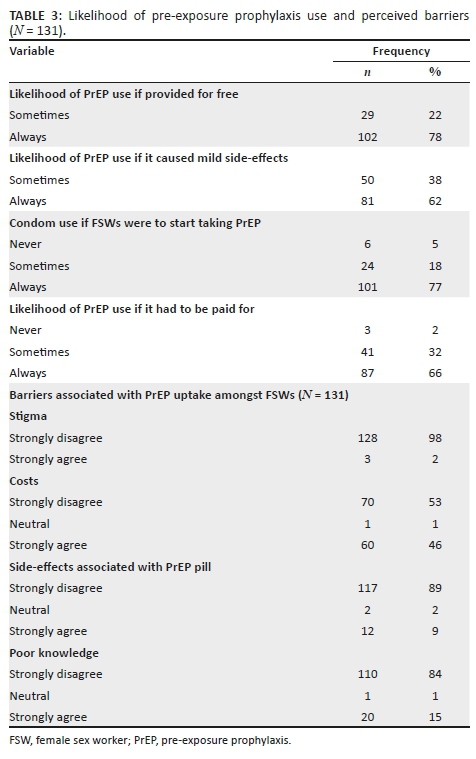
Relative importance index
On a scale of RII, the median score for PrEP knowledge was '0', indicating that participants had limited knowledge about PrEP. Participants perceived the use of PrEP as an important component in the ideal HIV prevention strategy once educated about it. In relation to the likelihood of PrEP use, the median score for the likelihood of PrEP use was 0.89 ranging from 0.48 to 1. Therefore, the likelihood of PrEP use amongst the participants was high. The RII median score for barriers associated with PrEP use was 0.29 (IQR: 0-0.63). This indicated that the barriers associated with PrEP uptake were less likely than knowledge to affect participants' use of PrEP. Results that were statistically significant in the bivariate analysis are shown in Table 4.
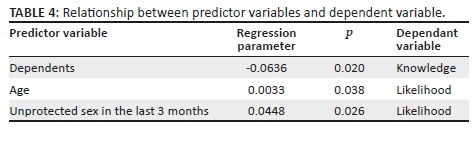
An increase in the number of dependents was associated with a reduced knowledge about PrEP, as shown in Table 4. There was a statistically significant association between age and likelihood of PrEP use. As participants became older, there was an increase in the likelihood of PrEP use. This increased amongst participants who had unprotected sex in the last 3 months.
There was, however, no statistically significant association between knowledge RII with age, marital status, education, change in place of residence, income and years of practice as a sex worker (p > 0.05).
Considering the likelihood of PrEP use amongst the participants, there was no association noted between the likelihood RII score and the number of dependents, marital status, education, change in place of residence, income and years of practice as a sex worker (p > 0.05). Spearman's rank correlation coefficient was computed to identify and test the strength of the relationship between knowledge and barriers with the likelihood of PrEP use. Table 5 shows the Spearman correlation coefficients for the relationship between RII scores of KLBs associated with PrEP use.
Considering likelihood and knowledge RII scores, there was a positive correlation whereby more knowledge about PrEP moderately increased the likelihood of PrEP use (r = 0.21, p = 0.0153). There was a negative correlation between barriers and the likelihood RII score, that is, as barriers associated with PrEP use decreased, the likelihood of PrEP use moderately increased (r = 0.23, p = 0.0074).
Discussion
In a real-world setting, the effectiveness of PrEP will depend on its acceptability, adoption and sustained use by high-risk populations. Pre-exposure prophylaxis medication will have little impact in reducing HIV infections if these components are not addressed.11 Findings from this study indicate that FSWs continue to engage in risky sexual behaviours. In the last 3 months, 53 (40%) of the participants reported having unprotected sexual intercourse with a casual partner.
In our study, 54% of FSWs had heard of PrEP before participating. In spite of having little knowledge of PrEP, the majority of FSWs were willing to use PrEP (median RII = 0.89; range 0.48-1) to reduce their risk of contracting HIV infection. In a similar study conducted in China, only 16.5% had heard of PrEP before participation, and only 1.4% had used PrEP before.12 Nevertheless, 69% and 95% of the respective FSW populations of China and India reported a willingness to use PrEP.12,13 These estimates were consistent with our findings that 89% of the participants were willing to use PrEP. Educating HIV-uninfected FSWs about PrEP is likely to support the uptake of PrEP and assist in decreasing the incidence of HIV in Zimbabwe. Pre-exposure prophylaxis programmes have been incorporated into sexually transmitted disease clinics, reproductive health programmes and genitourinary medicine clinics in high-income countries.14,15,16,17
Findings from this study indicated that there was a significant association between the likelihood of PrEP use, age and unprotected sexual intercourse in the preceding 3 months. Older participants were more likely to adopt PrEP as an HIV prevention strategy. This suggests that those who perceive themselves to be at a higher risk of HIV infection are more likely to adopt PrEP as an HIV intervention. Elmes et al. evaluated condom use by FSWs in Eastern Zimbabwe and reported that older participants were less likely to request condom use from partners.18 Older FSWs may therefore be at a higher risk of acquiring HIV and should be prioritised for PrEP access.
In spite of the high levels of interest in PrEP, potential barriers were cited including cost, side-effects and poor knowledge of PrEP use. Whilst PrEP had to be bought, 46% of the participants strongly agreed that lack of money would pose a challenge to PrEP uptake. Many participants felt that PrEP ought to be provided free in view of the severity of the HIV epidemic in Zimbabwe. The high cost of PrEP is undoubtedly a major barrier to its uptake worldwide, particularly in high-income countries where only branded TDF/FTC is available.19 In low-income countries that have established PrEP programmes, cost is not identified as a barrier to access because the medicines are free. In the Kenyan setting, FSWs were more concerned about the stigma of being tested for HIV and the barrier posed by the test with regard to the success of the intervention.20 In our study, stigma was not identified as a possible barrier to taking PrEP. This contrasted with the Kenyan setting and is a distinct finding for the Zimbabwean FSWs. With a reduced fear of stigmatisation, there is scope for improved acceptability of PrEP in FSWs.
Our results indicate that participants were generally willing to accept PrEP and adopt it as soon as it was made available. Information emerging from trial projects and open-label extensions where PrEP has been offered free of charge by knowledgeable providers suggests that uptake may be high in settings where cost and provider-related barriers had been removed.15,16,21
Our results showed that participants were willing to take PrEP even when reminded of potential side-effects. Participants cited that the side-effects of PrEP could have an impact on one's quality of life but that would not hinder taking PrEP. Only 9% of the FSWs strongly expressed that side-effects would affect their lifestyle and possibly prevent PrEP use. Sex workers in Mombasa, Kenya, voiced concerns about the potential negative side-effects of PrEP. In this qualitative study, a minority of participants were deterred from using PrEP because of the side-effects.22 Women from six US cities where female HIV infection is highly prevalent viewed PrEP as an important prevention option, provided that side-effects and cost to the consumer were minimal.23
The feasibility of oral PrEP implementation in Zimbabwe has been proven in ongoing and completed demonstration projects and clinical trials. By the end of 2017, a total of 3073 clients were initiated on PrEP in Zimbabwe. Ninety per cent of the clients initiated on PrEP were women, with the majority of them in the 25-49 years age group. The majority (52%) of the clients initiated on PrEP were FSWs. The accessibility of PrEP outside of demonstration projects has been limited.9
Whilst the sample size recruited for participation in this study was small, the information generated provides a foundation in the development of further programming for PrEP implementation in FSWs. The information was also generated from peri-urban, high-density areas in Harare (the capital city of Zimbabwe). Perceptions and knowledge levels might differ across other diverse geographical locations in Zimbabwe, and more studies need to be conducted. Because of the legal status of sex work in Zimbabwe, we used a snowball sampling technique in order to reach FSWs who might otherwise have been unwilling to participate in the study for fear of litigation. This sampling technique has the potential to introduce selection bias.
The Zimbabwean MoHCC has considered addressing the knowledge gap about PrEP in the general population through different channels whilst at the same time raising awareness to increase risk perception, especially amongst adolescent girls and young women. These considerations have been made as part of an implementation plan for PrEP in Zimbabwe between 2018 and 2020 (inclusive). Our study provides guidance on the progress made on addressing the knowledge gap about PrEP.
Conclusion
We set out to evaluate the knowledge levels of PrEP and the likelihood of its use amongst FSWs. Whilst the knowledge level was low, the majority of FSWs would be willing to use PrEP for the purpose of HIV/AIDS prevention. Non-governmental organisations are playing a major role in sensitising FSWs about PrEP. The local clinics need to increase their visibility as information dissemination institutions for PrEP. The clinics are strategically positioned to enlighten key populations about PrEP and to prescribe medication to those who might need it. Successful dissemination of information can be achieved if PrEP programmes are incorporated into other programmes within the clinics.
Acknowledgements
The authors thank all the team members, field officers and FSWs who participated in the study.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
T.M., A.D., C.C.M. and B.C. provided leadership for the project. T.M., A.D. and B.C. were responsible for conceptualisation of the study. A.D., B.N. and B.C. were responsible for data collection. T.M., C.C.M., B.N., T.G.M.-P. and A.D. were responsible for data analysis. G.D.M., T.G.M.-P. and C.C.M. provided technical expertise. T.M. and B.C. provided clinical expertise for the project. All authors contributed to the writing of the manuscript and approved the final version for publication.
Funding information
This research was made possible through core services and support from the University of Rochester Center for AIDS Research, a programme (P30 AI078498) funded by the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Data availability statement
Data sets are available from the corresponding author upon request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
References
1.Takarinda KC, Madyira LK, Mhangara M, et al. Factors associated with ever being HIV-tested in Zimbabwe: An extended analysis of the Zimbabwe Demographic and Health Survey (2010-2011). PLoS One. 2016;11(1):e0147828. https://doi.org/10.1371/journal.pone.0147828 [ Links ]
2.Cowan FM, Mtetwa S, Davey C, et al. Engagement with HIV prevention treatment and care among female sex workers in Zimbabwe: A respondent driven sampling survey. PLoS One. 2013;8(10):e77080. https://doi.org/10.1371/journal.pone.0077080 [ Links ]
3.Beyrer C, Baral SD, Collins C, et al. The global response to HIV in men who have sex with men. Lancet. 2016;388(10040):198-206. https://doi.org/10.1016/S0140-6736(16)30781-4 [ Links ]
4.National Medicines and Therapeutics Policy Advisory Committee (NMTPAC). Guidelines for antiretroviral therapy for the prevention and treatment of HIV in Zimbabwe, Harare: Ministry of Health and Child Care; 2016. [ Links ]
5.Joint United Nations Programme on HIV/AIDS (UNAIDS). 90-90-90 an ambitious treatment target to help end the AIDS epidemic, Geneva: UNAIDS; 2014. [ Links ]
6.Desai M, Field N, Grant R, McCormack S. State of the art review: Recent advances in PrEP for HIV. BMJ. 2017;359:j5011. https://doi.org/10.1136/bmj.j5011 [ Links ]
7.Cowan FM, Chabata ST, Musemburi S, et al. Strengthening the scale- p and uptake of effective interventions for sex workers for population impact in Zimbabwe. J Int AIDS Soc. 2019;22 (Suppl 4):e25320. https://doi.org/10.1002/jia2.25320 [ Links ]
8.Chiyaka T, Mushati P, Hensen B, et al. Reaching young women who sell sex: Methods and results of social mapping to describe and identify young women for DREAMS impact evaluation in Zimbabwe. PLoS One. 2018;13(3):e0194301. https://doi.org/10.1371/journal.pone.0194301 [ Links ]
9.Gwavava E. Understanding the uptake and retention patterns of PrEP users in Zimbabwe by subpopulation. AIDS 2018. Amsterdam: International AIDS Society; 2018. [ Links ]
10.Johnson JW, LeBreton JM. History and use of relative importance indices in organizational research. Organ Res Methods. 2004;7(3):238-257. https://doi.org/10.1177/1094428104266510 [ Links ]
11.Brooks RA, Kaplan RL, Lieber E, Landovitz RJ, Lee SJ, Leibowitz AA. Motivators, concerns, and barriers to adoption of preexposure prophylaxis for HIV prevention among gay and bisexual men in HIV-serodiscordant male relationships. AIDS Care. 2011;23(9):1136-1145. https://doi.org/10.1080/09540121.2011.554528 [ Links ]
12.Peng B, Yang X, Zhang Y, et al. Willingness to use pre-exposure prophylaxis for HIV prevention among female sex workers: A cross-sectional study in China. HIV AIDS. 2012;4:149-158. https://doi.org/10.2147/HIV.S33445 [ Links ]
13.Reza-Paul S, Lazarus L, Doshi M, et al. Prioritizing risk in preparation for a demonstration project: A mixed methods feasibility study of oral pre-exposure prophylaxis (PREP) among female sex workers in South India. PLoS One. 2016;11(11):e0166889. https://doi.org/10.1371/journal.pone.0166889 [ Links ]
14.Cohen SE, Vittinghoff E, Bacon O, et al. High interest in pre-exposure prophylaxis among men who have sex with men at risk for HIV-infection: Baseline data from the US PrEP demonstration project. J Acquir Immune Defic Syndr. 2015;68(4):439-448. https://doi.org/10.1097/QAI.0000000000000479 [ Links ]
15.Whetham J, Taylor S, Charlwood L, et al. Pre-exposure prophylaxis for conception (PrEP-C) as a risk reduction strategy in HIV-positive men and HIV-negative women in the UK. AIDS Care. 2014;26(3):332-336. https://doi.org/10.1080/09540121.2013.819406 [ Links ]
16.Vernazza PL, Graf I, Sonnenberg-Schwan U, Geit M, Meurer A. Preexposure prophylaxis and timed intercourse for HIV-discordant couples willing to conceive a child. AIDS. 2011;25(16):2005-2008. https://doi.org/10.1097/QAD.0b013e32834a36d0 [ Links ]
17.Dolling DI, Desai M, McOwan A, et al. An analysis of baseline data from the PROUD study: An open-label randomised trial of pre-exposure prophylaxis. Trials. 2016;17:163. https://doi.org/10.1186/s13063-016-1286-4 [ Links ]
18.Elmes J, Nhongo K, Ward H, et al. The price of sex: Condom use and the determinants of the price of sex among female sex workers in eastern Zimbabwe. J Infect Dis. 2014;210 (Suppl 2):S569-S578. https://doi.org/10.1093/infdis/jiu493 [ Links ]
19.Wilton J, Senn H, Sharma M, Tan DH. Pre-exposure prophylaxis for sexually-acquired HIV risk management: A review. HIV AIDS. 2015;7:125-136. https://doi.org/10.2147/HIV.S50025 [ Links ]
20.Mack N, Odhiambo J, Wong CM, Agot K. Barriers and facilitators to pre-exposure prophylaxis (PrEP) eligibility screening and ongoing HIV testing among target populations in Bondo and Rarieda, Kenya: Results of a consultation with community stakeholders. BMC Health Serv Res. 2014;14:231. https://doi.org/10.1186/1472-6963-14-231 [ Links ]
21.Heffron R, Celum C, Mugo N, et al., editors. High initiation of PrEP and ART in a demonstration project among African HIV-discordant couples. 21st Conference on Retroviruses and Opportunistic Infections; 2014 March 03-06; Boston, MA: International Aids Society; 2014. [ Links ]
22.Restar AJ, Tocco JU, Mantell JE, et al. Perspectives on HIV pre- and post-exposure prophylaxes (PrEP and PEP) among female and male sex workers in Mombasa, Kenya: Implications for integrating biomedical prevention into sexual health services. AIDS Educ Prev. 2017;29(2):141-153. https://doi.org/10.1521/aeap.2017.29.2.141 [ Links ]
23.Auerbach JD, Kinsky S, Brown G, Charles V. Knowledge, attitudes, and likelihood of pre-exposure prophylaxis (PrEP) use among US women at risk of acquiring HIV. AIDS Patient Care STDS. 2015;29(2):102-110. https://doi.org/10.1089/apc.2014.0142 [ Links ]
 Correspondence:
Correspondence:
Tinashe Mudzviti
tinashem@newlandsclinic.org.zw
Received: 22 Oct. 2019
Accepted: 11 Jan. 2020
Published: 19 Feb. 2020