Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of HIV Medicine
versión On-line ISSN 2078-6751
versión impresa ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.20 no.1 Johannesburg 2019
http://dx.doi.org/10.4102/sajhivmed.v20i1.1008
ORIGINAL RESEARCH
Neurodevelopment at 11 months after starting antiretroviral therapy within 3 weeks of life
Barbara LaughtonI; Shalena NaidooII; Els F.M.T. DobbelsI; Michael J. BoivinIII, IV; Anita Janse van RensburgI; Richard H. GlashoffII, V; Gert U. van ZylV, VI; Mariana KrugerI; Mark F. CottonI
IDepartment of Paediatrics and Child Health, Stellenbosch University, Cape Town, South Africa
IIDepartment of Pathology, Stellenbosch University, Cape Town, South Africa
IIIDepartment of Psychiatry, Michigan State University, East Lansing, United States
IVDepartment of Neurology and Ophthalmology, Michigan State University, East Lansing, United States
VNational Laboratory Services, Cape Town, South Africa
VIDivision of Virology, Faculty of Medicine and Health Sciences, Stellenbosch University, South Africa
ABSTRACT
BACKGROUND: Antiretroviral therapy (ART) started between 7 and 12 weeks of age improves neurodevelopmental outcomes in HIV-infected (HIV+) infants, but the impact of even earlier initiation is not yet described
OBJECTIVES: We assessed the early neurodevelopment of HIV+ infants who started ART within 21 days of life
METHOD: Participants were enrolled from the public sector birth HIV-diagnosis programme. Inclusion criteria included the following: birth weight > 2000 g, infant commencing ART < 6 weeks and no infant cytomegalovirus disease. Antiretroviral therapy included Zidovudine/Lamivudine/Nevirapine for the first 2 weeks, the latter then replaced by Lopinavir/Ritonavir. Once body weight > 3 kg and gestational age > 44 weeks, Abacavir replaced Zidovudine. The Griffiths mental development scales (GMDS) were administered at 10-12 months
RESULTS: Of 29 infants assessed, 23 (79%) were girls. Mean birth weight was 3002 ± 501 g. Twenty-four mothers (83%) received ART during pregnancy. Seven (24%) infants were diagnosed HIV+ within 48 h of birth. Median [interquartile range] viral load (VL) at diagnosis was 3904 [259-16 922] copies/mL, age starting ART was 6.0 [3-10] days and age at VL suppression was 19.1 [15-36] weeks. At the GMDS assessment, nine (31%) participants had detectable VL and 26 (90%) had World Health Organization (WHO) clinical stage I disease. The GMDS was performed at a mean age of 11.5 ± 0.8 months. Mean quotients were within the average range: Global Griffiths score was 103.6 ± 10.9 and mean quotients on the subscales ranged from lowest 95.9 ± 13.4 for locomotor to highest 112.8 ± 11.3 for hearing-and-language
CONCLUSION: Preliminary findings in this small group suggest that early neurodevelopmental scores are within the normal range in infants with perinatal HIV infection who started ART at a median of 6 days
Keywords: HIV; infants; antiretroviral therapy; neurodevelopment; paediatrics.
Introduction
HIV-infected (HIV+) children are at risk for neurodevelopmental delay. Neurologic insults develop after HIV enters the brain, creating an inflammatory state affecting neuronal and astrocyte growth and development1; the most severe manifestation being HIV encephalopathy (HIVE). HIV encephalopathy rates range from 6% to 30%, with higher rates in low- and middle-income countries especially with delayed initiation of combination antiretroviral therapy (ART).2,3,4,5 These consequences place a great burden on the social and healthcare systems.
Antiretroviral therapy has decreased the incidence of HIVE,6 and when initiated between 3 and 9 months of age, it improves clinical and neurodevelopmental outcomes.7,8,9,10 Nevertheless, studies describing permanent deficits or lack of catch-up suggest that prevention is better than reversal of HIVE.11,12,13 From 2016, the World Health Organization (WHO) introduced birth testing and recommended starting ART as soon as possible once HIV infection is confirmed.14 Since the report of temporary remission in the Mississippi baby after very early ART,15 there is increasing evidence that early ART in perinatally infected children improves infant outcomes.16 Early ART can limit HIV reservoir size, and when started before 2 months of age, it is associated with fewer infected and transcriptionally active cells and less infectious virus recovery.17,18,19,20,21,22,23,24 However, administering ART in a neonate and young infant is not easy with potential drug resistance because of under-dosing, or neurotoxicity because of overdosing.25 The long-term outcomes of very early exposure to ART are still unknown. It is therefore imperative that neurodevelopmental testing be undertaken after early ART initiation. Our aim was to determine the neurodevelopmental outcomes of perinatally HIV-infected children after initiating ART within the first 3 weeks of life.
Research methods and design
We report early data from a prospective descriptive study conducted in the Family Centre for Research with Ubuntu (FAM-CRU) in Tygerberg Hospital, Cape Town, South Africa, with recruitment from the Médecins Sans Frontières service in Khayelitsha and elsewhere in the public sector. Antiretroviral therapy was started as soon as HIV infection was confirmed. HIV diagnosis was made by quantitative HIV-1 viral load (VL) testing and confirmed by a qualitative HIV-1 RNA PCR. Indeterminate samples were repeated until HIV diagnosis confirmation.26,27 Inclusion criteria were the following: birth weight > 2000 g, commencing ART < 6 weeks of age and no infant cytomegalovirus (CMV) infection. Mothers or legal guardians were consented in person in their language of choice according to Good Clinical Practice standards.
Participants were seen as frequently as needed until stable, monthly for 3 months and then 3 monthly. Visits included a medical examination, growth monitoring, adverse event assessment and social work support where needed. At each visit, a pharmacist calculated the percentage adherence for each drug from returned ART containers and an adherence counsellor established reasons for over or under-dosing with the parent or caregiver, offered advice on problems identified and reviewed measuring techniques. HIV viral load was performed at baseline, 3, 6 and 12 months. Undetectable VLs were reported as < 100 or < 40 copies/mL depending on the blood volume available for testing. CD4 cell counts were done at 3, 6 and 12 months. Antiretroviral therapy comprised Zidovudine, Lamivudine and Nevirapine, with Lopinavir/Ritonavir replacing Nevirapine after 2 weeks of age or gestational age of 42 weeks. Once weight exceeded 3 kg and gestational age was above 44 weeks, Abacavir replaced Zidovudine. Participants also received co-trimoxazole from 6 weeks of age.
The Griffiths mental development scales (GMDS) (0-2 years) were conducted by the same developmental paediatrician (B.L.) at 10-12 months of age.28 The GMDS assesses five subscales: locomotor, personal-social, hearing-and-language, eye-hand coordination and performance (visual-motor abilities). A global score, the General Griffiths, is also calculated. Raw scores are converted into quotients, derived from norms of healthy British children, with a mean of 100 and standard deviation (s.d.) of 16. While the GMDS is neither standardised nor validated in South Africa, it is the most widely used developmental assessment tool, is considered culturally fair and is used to assess young children including those exposed to HIV.29,30,31,32,33,34,35,36 Vision was assessed clinically during testing and through the ability to track small cake decorations ('hundreds and thousands' test), which implies visual acuity of 6/24 or better.37
Statistical analysis was performed using Stata release 11 (StataCorp, College Station, TX) and Statistica 13 (software.dell.com. Dell Inc. 2015). For descriptive statistics, mean and s.d. were reported for normally distributed data and median and interquartile range (IQR) for skewed data. Guided by distribution of the data, Spearman and Pearson correlations were used to explore correlation between various parameters and neurodevelopmental outcomes. For calculating age at VL suppression, those who had not yet achieved VL suppression were assigned a date 2 days after the GMDS. Regression analysis explored the contribution of five predictors of GMDS scores: birth weight, ART start age, baseline VL, baseline CD4% and age at first VL suppression.
Descriptive data and GMDS scores were also compared to those from the early treatment arms on Children with HIV Early antiRetroviral treatment (CHER) trial participating in a neurodevelopmental sub-study who received early ART from a median of age of 7.7 weeks and were assessed by the same investigators at 11 months of age.10
Ethical considerations
Mothers or legal guardians were consented in person in their language of choice according to Good Clinical Practice standards. The Stellenbosch University Health Research Ethics Committee approved the study (No.: M14/07/029).
Results
Of 29 children studied, 23 (79%) were female. Mean birth weight was 3002 ± 501 g and gestation was 37.9 ± 2.3 weeks. HIV+ diagnosis was made by 48 h of birth in 7 (24%) and within 7 days of birth in 17 (59%) infants. Median [IQR] age for starting ART was 6.0 [3-10] days (range 0-21) from birth. Twenty-three achieved VL suppression at median [IQR] 19.1 [14.7-35.9] weeks of age (range 2-53) (Table 1).

The GMDS was performed at a mean of 11.5 ± 0.8 months (range 10.2-13.1) and scores are described in Table 2. Mean GMDS quotients were in the average range and within 1 s.d. of the standardised scores. The locomotor subscale had the lowest mean quotient. No children were suspected of having hearing or vision problems.

Clinical status at the time of GMDS is described in Table 3. One child had progressed to WHO stage II HIV disease (persistent oral candida), and two to stage III (chronic suppurative otitis media and pulmonary tuberculosis). Nine children (31%) had detectable VL at the time of GMDS testing, six (21%) had not yet achieved viral suppression and three had previously suppressed (one at 27 weeks and two at 19 weeks of age), but rebounded to log 5.44 (273 328 copies/mL), log 3.18 (1519 copies/mL) and log 4.46 (28 649 copies/mL), respectively. Another participant suppressed at 3 months, and had a viral blip to 118 copies/mL at 6 months, with the VL undetectable 6 months later at GMDS.

A number of demographic and exposure issues with potential to influence neurodevelopmental outcomes were identified. These included two without antenatal care, one with an unsupervised home birth and three with maternal substance abuse: two methamphetamine and one methamphetamine and alcohol (over time these children were fostered by caring relatives). Medical problems included one each of the following: congenital pneumonia of unknown aetiology, intrauterine growth retardation, neonatal jaundice above exchange transfusion levels (resolved without exchange), congenital syphilis with mild hypoxia and suspected seizure, mild birth asphyxia (low birth Apgar scores and cord blood pH = 7.17) and suspected hypoglycaemia (but glucose level not recorded) (these data not shown in any table).
The following adverse events, which could negatively impact neurodevelopment, were documented before the GMDS assessment: six with otitis media (one had two episodes), six with anaemia and three with neutropenia (Zidovudine was discontinued). One infant recovered fully after treatment for suspected bacterial meningitis and another was hospitalised for 6 months with pulmonary tuberculosis. Lastly, failure to thrive because of poor feeding and insufficient caloric intake occurred in one infant.
Adherence was calculated at a median [IQR] of 10 [9-11] visits. Only one participant had acceptable adherence percentages for all drugs at all visits. Three participants had poor adherence for more than half of the visits, with the rest over or under-dosing at various times. For the former, the infant would spit syrups out and caregivers were unsure how much to replace. For the latter, the caregivers either measured syrups incorrectly or were non-compliant. This prompted clinicians to encourage treatment supporters for the caregivers.
Correlations between GMDS scores and possible predictors of developmental outcomes (birth weight, gestation, maternal age, baseline VL, age starting ART, time to suppression and CD4 parameters at baseline) were not significant. The five predictors of GMDS scores entered into the regression model also did not show significant relationships, that is, birth weight, ART start age, baseline VL, baseline CD4% and age at first suppression. CD8 count at the time of GMDS showed a negative correlation with personal-social (Pearson r = −0.41; p = 0.03) and a negative trend with General Griffiths (Pearson r = −0.6; p = 0.06).
For growth parameters closest to the GMDS assessment, head circumference z-scores correlated significantly with the performance (visual-spatial) scores (Pearson's r = 0.4; p = 0.02) and weight z-score correlated with eye-hand coordination scores (Pearson's r = 0.36; p = 0.05). There was a positive trend between weight for age z-score and General Griffiths score (Pearson's r = 0.34; p = 0.07).
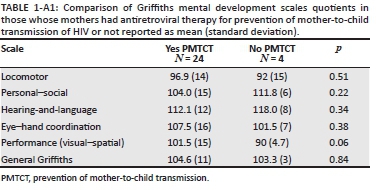
We compared the GMDS scores of those whose mothers had ART for prevention of mother-to-child transmission of HIV (PMTCT) and those who did not and found no difference between the groups (see Appendix 1, Table 1-A1). There were also no significant differences on the GMDS scores between those with detectable VL and undetectable VL at the time of the test, despite the mean scores in the hearing-and-language and eye-hand coordination subtests being 5 points lower for the nine with detectable VL compared to the 20 with undetectable VL at testing (Table 4). We also compared the following participant demographics between the detectable VL and undetectable VL groups: birth weight, baseline VL copies and CD4 parameters, ART start age, CD4, CD8 and growth parameters at the time of GMDS, and found no difference (see Appendix 1, Table 2-A1).

The GMDS scores achieved by this cohort were similar to those from the CHER cohort (children on ART commenced at 7 weeks of age) at a mean age of 11.3 months,10 apart from personal-social subscale, where the CHER cohort had mean quotients 7 points above that of the current study population (Table 5). Post hoc item comparison for personal-social showed that CHER participants were more likely to help with dressing, hold an open cup for drinking, try to use a spoon for feeding and obey simple requests. Participants on the current study were more likely to clap hands and enjoy an adult showing a book.

Significant differences between the two groups are shown in Table 5, with CHER having higher VLs and lower CD4 counts at baseline and a longer time to undetectable VL compared to participants in the current study. Abacavir also replaces Zidovudine use in the CHER participants.
Discussion
These findings from the first 29 infants who started ART at a median age of 6 days are encouraging and show potential for normal neurodevelopmental outcomes, despite other medical conditions in nine infants that may impair neurological development. These scores are well within 1 s.d. of the UK norms, and are comparable to other South African infants assessed at similar ages using the GMDS29,30,32,33,34 (see Appendix 1, Table 3-A1 for summary of scores). This finding is despite almost a third not being virologically suppressed at testing. However, VLs in this cohort indicated low exposure to HIV because of maternal ART.38
We previously described neurodevelopmental outcomes in the CHER trial at 11 months.10 We compared children on delayed ART to those who started early ART at a median [IQR] of 7.7 [7.1-9.5] weeks. The GMDS scores from the CHER early treatment arms are comparable to this very early treatment group, apart from the personal-social subscale (Table 5), which is the most subjective as caregiver report items are used, and may reflect a change in child-rearing practices over time with less emphasis on self-care skills. The CHER early treatment arms had a mean baseline VL of log10 copies/mL 5.64 which is far higher than the current study and baseline mean CD percentage of 35% which is lower than the current study, and longer time to undetectable VL. This may suggest that there is a safe window period for starting ART - between birth and a median of 7.7 weeks; however, these are early neurodevelopmental outcomes. Alternatively, were it not for adverse in utero exposures and non-suppressed VLs in six infants, the scores may have been higher than CHER early treatment participants. The early diagnosis of HIV+ infants within 48 h in 24% and by 7 days of age in 59% reflects high proportion of prenatal HIV infection, which also negatively impacts outcomes. In the CHER trial, in utero infection could not be assessed as infant screening began at 4-6 weeks of age for HIV.
An important finding is that we identified a number of challenges within the context of perinatal HIV infection, despite good PMTCT programmes. In those perinatally infected infants, a number of secondary effects, including systemic illnesses and environmental affects, may negatively impact a child's early neurodevelopment.39,40,41 In our sample, we identified three with no prenatal care, three substance abuse, two congenital infections (syphilis and pneumonia of unknown aetiology), one co-infected with tuberculosis and one nutritional failure. Growth in participants was appropriate for weight and head circumference, but mean length z-score was −1.1.
We noted variability of ART adherence and the delay in attaining competence with ART dosing and adherence, with six children not yet suppressed at the time of GMDS assessment. Management of these young children was challenging as caregivers were non-compliant, under-skilled and found difficulty administering liquid formulations. Solid or dispersible formulations would certainly improve adherence.42,43 Our findings do not suggest neurotoxicity from ART.
This work had some limitations. As multiple factors may influence outcomes, 29 children starting ART very early are too few to assess weak associations with neurodevelopmental outcomes, including our finding of lower locomotor scores compared to other subscales. More girls than boys were enrolled in the sample; although previously described,44 this may be because of small sample size. We were not able to determine reliable predictors for neurodevelopmental outcomes, or compare the outcomes of suppressed and unsuppressed participants. This was also hindered by time to suppression being inaccurate as VLs were only done at baseline 3, 6 and 12 months. We did not collect information on maternal health, immune status, VL or antiretroviral therapies. In the absence of South African normative data on the GMDS, a control or comparison group would have been helpful. However, we have experience in this community using the GMDS and are able to use these results for comparison30,32 (Appendix 1, Table 3-A1). The confounding problems of mothers with substance abuse did not seem to have a major impact, but the limitation is probably sample size.
Our findings are relevant to upscaling neonatal HIV identification and care.45,46 While the number of HIV+ infants is decreasing, this population remains at high risk because of structural and behavioural challenges in providing appropriate care. As liquid Lopinavir/Ritonavir formulation is poorly tolerated, newer formulations and other alternatives such as integrase inhibitors will be better accepted. Healthcare planners should not downscale programmes according to decreasing numbers, as those failing PMTCT require a higher level of care and intensive intervention to enable benefit from early ART. With the potential of early ART to limit HIV reservoir seeding, and potential to contribute to functional cures, treatment programmes need to support these vulnerable infants and their caregivers.47 Mentor mothers as treatment supporters may decrease the burden of HIV care and consequences of developmental delay, and could be very important when planning programmes. If these needs can be met, our findings are encouraging.48,49
Conclusion
Preliminary findings in this small group suggest that despite PMTCT failure, children infected perinatally with HIV may have typical neurodevelopment if starting ART at a median age of 6 days, and similar to those starting ART at a median of 7 weeks. Good supportive care, including for ART adherence, is essential. A larger cohort that includes controls is in study and the findings at 18 months of age will inform on the influence of time to VL suppression and reservoir size and also the influence of social factors and demographic factors on neurodevelopmental outcomes. This may also allow for more precise study of locomotor outcomes.
Acknowledgements
The authors thank the parents and babies who were willing to be part of this study, as well as the research support team at the Family Centre for Research with Ubuntu and Médecins Sans Frontières [Doctors Without Borders] (MSF) Khayelitsha for providing excellent care; Martin Kidd for statistics help; and Helen Payne, Di Gibb, Nigel Klein, Jean Maritz and Wolfgang Preiser who contributed to the original study design.
Competing interests
The authors have declared that no competing interests exist.
Authors' contributions
B.L. was the primary author of the article and was responsible for neurodevelopmental testing; S.N. was responsible for lymphocyte subtest testing and provided input into the article writing; E.F.M.T.D. was the clinician on study managing infants and assisted with the article writing; M.J.B. provided input into study assessments and assisted with the article writing; A.J.v.R. was the project manager on study and assisted with the article writing; R.H.G.M. was the immunologist on study and assisted with the article writing; G.U.v.Z. was the virologist on study and assisted with the article writing; M.K. assisted with the article writing; and M.F.C. was the principal investigator of the study and assisted with the article writing.
Funding
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health, USA, under Award Number R01MH105134.
Data availibility statement
Data sharing for these preliminary outcomes is not applicable until the study is completed.
Disclaimer
The submitted article is the own work of the authors and not an official position of their institutions and does not necessarily represent the official views of the funders from National Institutes of Health.
References
1. Bednar MM, Sturdevant CB, Tompkins LA, et al. Compartmentalization, viral evolution, and viral latency of HIV in the CNS. Curr HIV/AIDS Rep. 2015;12(2):262-271. https://doi.org/10.1007/s11904-015-0265-9 [ Links ]
2. Brahmbhatt H, Boivin M, Ssempijja V, et al. Neurodevelopmental benefits of antiretroviral therapy in Ugandan children aged 0-6 years with HIV. J Acquir Immune Defic Syndr. 2014;67(3):316-322. https://doi.org/10.1097/QAI.0000000000000295 [ Links ]
3. Boivin MJ, Barlow-Mosha L, Chernoff MC, et al. Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS. 2018;32(2):189-204. https://doi.org/10.1097/01.aids.0000530201.64167.b7 [ Links ]
4. Donald KA, Hoare J, Eley B, Wilmshurst JM. Neurologic complications of pediatric human immunodeficiency virus: Implications for clinical practice and management challenges in the African setting. Semin Pediatr Neurol. 2014;21(1):3-11. https://doi.org/10.1016/j.spen.2014.01.004 [ Links ]
5. Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: A global perspective. Eur J Paediatr Neurol. 2007;11(1):1-9. https://doi.org/10.1016/j.ejpn.2006.10.006 [ Links ]
6. Patel K, Ming X, Williams PL, et al. Impact of HAART and CNS-penetrating antiretroviral regimens on HIV encephalopathy among perinatally infected children and adolescents. AIDS. 2009;23(14):1893-1901. https://doi.org/10.1097/QAD.0b013e32832dc041 [ Links ]
7. Brahmbhatt H, Boivin M, Ssempijja V, et al. Impact of HIV and atiretroviral therapy on neurocognitive outcomes among school-aged children. J Acquir Immune Defic Syndr. 2017;75(1):1-8. https://doi.org/10.1097/QAI.0000000000001305 [ Links ]
8. Benki-Nugent S, Wamalwa D, Langat A, et al. Comparison of developmental milestone attainment in early treated HIV-infected infants versus HIV-unexposed infants: A prospective cohort study. BMC Pediatr. 2017;17(1):24. https://doi.org/10.1186/s12887-017-0776-1 [ Links ]
9. Collins IJ, Judd A, Gibb DM. Immediate antiretroviral therapy in young HIV-infected children: Benefits and risks. Curr Opin HIV AIDS. 2014;9(1):87-94. https://doi.org/10.1097/COH.0000000000000027 [ Links ]
10. Laughton B, Cornell M, Grove D, et al. Early antiretroviral therapy improves neurodevelopmental outcomes in infants. AIDS. 2012;26(13):1685-1690. https://doi.org/10.1097/QAD.0b013e328355d0ce [ Links ]
11. Strehlau R, Kuhn L, Abrams EJ, Coovadia A. HIV-associated neurodevelopmental delay: Prevalence, predictors and persistence in relation to antiretroviral therapy initiation and viral suppression. Child Care Health Dev. 2016;42(6):881-889. https://doi.org/10.1111/cch.12399 [ Links ]
12. Van Arnhem LA, Bunders MJ, Scherpbier HJ, et al. Neurologic abnormalities in HIV-1 infected children in the era of combination antiretroviral therapy. PLoS One. 2013;8(5):e64398. https://doi.org/10.1371/journal.pone.0064398 [ Links ]
13. Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. 2014;26(4):497-504. https://doi.org/10.1080/09540121.2013.841828 [ Links ]
14. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Geneva: World Health Organization; June 2016. [ Links ]
15. Persaud D, Gay H, Ziemniak C, et al. Absence of detectable HIV-1 viremia after treatment cessation in an infant. N Engl J Med. 2013;369(19):1828-1835. https://doi.org/10.1056/NEJMoa1302976 [ Links ]
16. Cotton MF, Rabie H. Impact of earlier combination antiretroviral therapy on outcomes in children. Curr Opin HIV AIDS. 2015;10(1):12-17. https://doi.org/10.1097/COH.0000000000000117 [ Links ]
17. Bitnun A, Samson L, Chun TW, et al. Early initiation of combination antiretroviral therapy in HIV-1-infected newborns can achieve sustained virologic suppression with low frequency of CD4+ T cells carrying HIV in peripheral blood. Clin Infect Dis. 2014;59(7):1012-1019. https://doi.org/10.1093/cid/ciu432 [ Links ]
18. Goulder PJ, Lewin SR, Leitman EM. Paediatric HIV infection: The potential for cure. Nat Rev Immunol. 2016;16(4):259-271. https://doi.org/10.1038/nri.2016.19 [ Links ]
19. Kuhn L, Paximadis M, Da Costa Dias B, et al. Age at antiretroviral therapy initiation and cell-associated HIV-1 DNA levels in HIV-1-infected children. PLoS One. 2018;13(4):e0195514. https://doi.org/10.1371/journal.pone.0195514 [ Links ]
20. Luzuriaga K, Tabak B, Garber M, et al. HIV type 1 (HIV-1) proviral reservoirs decay continuously under sustained virologic control in HIV-1-infected children who received early treatment. J Infect Dis. 2014;210(10):1529-1538. https://doi.org/10.1093/infdis/jiu297 [ Links ]
21. McManus M, Mick E, Hudson R, et al. Early combination antiretroviral therapy limits exposure to HIV-1 replication and cell-associated HIV-1 DNA levels in infants. PLoS One. 2016;11(4):e0154391. https://doi.org/10.1371/journal.pone.0154391 [ Links ]
22. Persaud D, Palumbo PE, Ziemniak C, et al. Dynamics of the resting CD4(+) T-cell latent HIV reservoir in infants initiating HAART less than 6 months of age. AIDS. 2012;26(12):1483-1490. https://doi.org/10.1097/QAD.0b013e3283553638 [ Links ]
23. Van Zyl GU, Bedison MA, Van Rensburg AJ, Laughton B, Cotton MF, Mellors JW. Early antiretroviral therapy in South African children reduces HIV-1-infected cells and cell-associated HIV-1 RNA in blood mononuclear cells. J Infect Dis. 2015;212(1):39-43. https://doi.org/10.1093/infdis/jiu827 [ Links ]
24. Veldsman KA, Maritz J, Isaacs S, et al. Rapid decline of HIV-1 DNA and RNA in infants starting very early antiretroviral therapy may pose a diagnostic challenge. AIDS. 2018;32(5):629-634. https://doi.org/10.1097/QAD.0000000000001739 [ Links ]
25. Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388-399. https://doi.org/10.1007/s13365-012-0120-3 [ Links ]
26. Martiz J, Van Zyl G, Mellors JW, et al. Feasibility of a targeted, very early infant HIV diagnosis algorithm in a resource-limited setting. Conference on retroviruses and opportunistic infections. Boston: International Journal of Infectious Diseases; 2014. p. 129. [ Links ]
27. Nelson A, Maritz J, Giddy J, et al. HIV testing and antiretroviral therapy initiation at birth: Views from a primary care setting in Khayelitsha. South Afr J HIV Med. 2015;16(1):376. https://doi.org/10.4102/sajhivmed.v16i1.376 [ Links ]
28. Griffiths R. The Griffiths mental development scales: From birth to 2 years. Revision by Huntley M. London: The Test Agency Ltd; 1996. [ Links ]
29. Amod ZCK, Soelaart B. Use of the 1996 Griffiths mental developmental scales for infants: A pilot study with a Black, South African sample. J Child Adolescent Ment Health. 2007;19(21):123-130. https://doi.org/10.2989/17280580709486647 [ Links ]
30. Laughton B, Cornell M, Kidd M, et al. Five year neurodevelopment outcomes of perinatally HIV-infected children on early limited or deferred continuous antiretroviral therapy. J Int AIDS Soc. 2018;21(5):e25106. https://doi.org/10.1002/jia2.25106 [ Links ]
31. Potterton J, Hilburn N, Strehlau R. Developmental status of preschool children receiving cART: A descriptive cohort study. Child Care Health Dev. 2016;42(3):410-414. https://doi.org/10.1111/cch.12321 [ Links ]
32. Springer PE, Laughton B, Harvey J, Esser M. Neurodevelopmental status of HIV-exposed but uninfected children: A pilot study. S Afr J Child Health. 2012;6(2):51-55. [ Links ]
33. Davies L, Dunn M, Chersich M, et al. Developmental delay of infants and young children with and without fetal alcohol spectrum disorder in the Northern Cape Province, South Africa. Afr J Psychiatry (Johannesbg). 2011;14(4):298-305. https://doi.org/10.4314/ajpsy.v14i4.7 [ Links ]
34. Perez EM, Hendricks MK, Beard JL, et al. Mother-infant interactions and infant development are altered by maternal iron deficiency anaemia. J Nutr. 2005;135:850-855. https://doi.org/10.1093/jn/135.4.850 [ Links ]
35. Luiz D, editor. Griffiths scales of mental development: South African studies. Research Papers. Port Elizabeth: University of Port Elizabeth; 1997, C 25. [ Links ]
36. Laher S, Cockroft K, editors. Psychological assessment in South Africa, research and applications. Johannesburg, South Africa: Wits University Press; 2013. [ Links ]
37. Hall SM, Pugh AG, Hall DM. Vision screening in the under-5s. Br Med J (Clin Res Ed). 1982;285(6348):1096-1098. https://doi.org/10.1136/bmj.285.6348.1096 [ Links ]
38. Mazanderani AH, Moyo F, Kufa T, Sherman GG. Brief report: Declining baseline viremia and escalating discordant HIV-1 confirmatory results within South Africa's early infant diagnosis program, 2010-2016. J Acquir Immune Defic Syndr. 2018;77(2):212-216. https://doi.org/10.1097/QAI.0000000000001581 [ Links ]
39. Boyede GO, Lesi FE, Ezeaka VC, Umeh CS. Impact of sociodemographic factors on cognitive function in school-aged HIV-infected Nigerian children. HIV AIDS (Auckl). 2013;5:145-152. https://doi.org/10.2147/HIV.S43260 [ Links ]
40. Richter LM, Mofenson LM. Children born into families affected by HIV. AIDS. 2014;28(Suppl 3):S241-S244. https://doi.org/10.1097/QAD.0000000000000361 [ Links ]
41. Suchdev PS, Boivin MJ, Forsyth BW, Georgieff MK, Guerrant RL, Nelson CA, 3rd. Assessment of neurodevelopment, nutrition, and inflammation from fetal life to adolescence in low-resource settings. Pediatrics. 2017;139(Suppl 1):S23-S37. https://doi.org/10.1542/peds.2016-2828E [ Links ]
42. Abrams EJ, Ananworanich J, Archary M, Ngongondo M, Brouwers P. Propelling the pediatric HIV therapeutic agenda with science, innovation, and collaboration. J Acquir Immune Defic Syndr. 2018;78(Suppl 1):S32-S39. https://doi.org/10.1097/QAI.0000000000001747 [ Links ]
43. Shiau S, Kuhn L. Antiretroviral treatment in HIV-infected infants and young children: Novel issues raised by the Mississippi baby. Expert Rev Anti Infect Ther. 2014;12(3):307-318. https://doi.org/10.1586/14787210.2014.888311 [ Links ]
44. Taha TE, Nour S, Kumwenda NI, et al. Gender differences in perinatal HIV acquisition among African infants. Pediatrics. 2005;115(2):e167-e172. https://doi.org/10.1542/peds.2004-1590 [ Links ]
45. Davies MA. Research gaps in neonatal HIV-related care. South Afr J HIV Med. 2015;16(1):375. https://doi.org/10.4102/sajhivmed.v16i1.375 [ Links ]
46. Kuhn L, Shiau S. The pharmacological treatment of acute HIV infections in neonates. Expert Rev Clin Pharmacol. 2017;10(12):1353-1361. https://doi.org/10.1080/17512433.2017.1398645 [ Links ]
47. Goga AE, Singh Y, Singh M, et al. Enhancing HIV treatment access and outcomes amongst HIV infected children and adolescents in resource limited settings. Matern Child Health J. 2017;21(1):1-8. https://doi.org/10.1007/s10995-016-2074-1 [ Links ]
48. Ford ND, Stein AD. Risk factors affecting child cognitive development: A summary of nutrition, environment, and maternal-child interaction indicators for sub-Saharan Africa. J Dev Orig Health Dis. 2016;7(2):197-217. https://doi.org/10.1017/S2040174415001427 [ Links ]
49. Walker SP, Wachs TD, Grantham-McGregor S, et al. Inequality in early childhood: Risk and protective factors for early child development. Lancet. 2011;378(9799):1325-1338. https://doi.org/10.1016/S0140-6736(11)60555-2 [ Links ]
 Correspondence:
Correspondence:
Barbara Laughton
bl2@sun.ac.za
Received: 08 July 2019
Accepted: 18 Aug. 2019
Published: 30 Oct. 2019
Appendix 1