Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of HIV Medicine
versión On-line ISSN 2078-6751
versión impresa ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.19 no.1 Johannesburg 2018
http://dx.doi.org/10.4102/sajhivmed.v19i1.918
GUIDELINES
Appropriate clinical use of darunavir 800 mg
Michelle A. MoorhouseI; Sergio CarmonaII; Natasha DaviesI; Sipho DlaminiIII; Cloete van VuurenIV; Thandekile ManziniIV; Moeketsi MatheV; Yunus MoosaVI; Jennifer NashIV; Jeremy NelIV; Yoliswa PakadeIV; Joana WoodsI; Gert van ZylIV; Francesca ConradieI; Francois VenterI; Graeme MeintjesVII
IWits Reproductive Health and HIV Institute, Faculty of Health Sciences, University of the Witwatersrand, South Africa
IINational Health Laboratory Services, South Africa
IIIDepartment of Medicine, University of Cape Town, South Africa
IVSouthern African HIV Clinicians Society, South Africa
VPrivate Practice, Vereeniging, South Africa
VIDepartment of Infectious Diseases, University of KwaZulu-Natal, South Africa
VIIDepartment of Medicine and Institute of Infectious Disease and Molecular Medicine, University of ape Town, South Africa
Indication
Darunavir 400 mg tablets were recently approved by the South African Health Products Regulatory Authority (SAHPRA) f or the following indication:
PREZISTA, in combination with low dose ritonavir (DRV/r) and with other antiretroviral medicines, is indicated for the treatment of human immunodeficiency virus (HIV) infection in antiretroviral treatment experienced adult patients who are protease-inhibitor-naïve or after exclusion of darunavir resistance associated mutations (DRV-RAMs: V11I, V32I, L33F, I47V, I50V, I54M, I54L, T74P, L76V, I84V and L89V). Genotypic or phenotypic testing should guide the use of DRV/r. (Prezista package insert)
There is no information on the use of darunavir in combination with ritonavir in the paediatric population for the once-daily dose.
Southern African HIV Clinicians Society guidelines
Southern African HIV Clinicians Society adult antiretroviral therapy (ART) guidelines currently recommend ritonavir-boosted atazanavir (ATV/r) 300/100 mg as preferred boosted protease inhibitor (PI/r) for second-line ART. It was noted in the guidelines that once a suitable tablet for DRV/r 800/100 mg dosing became available, DRV/r 800/100 mg would be a feasible option in second-line ART, with fewer side effects than the DRV/r 600/100 mg twice-daily dosing.
Using darunavir/ritonavir 800/100 mg once-daily in clinical practice
In second-line antiretroviral therapy
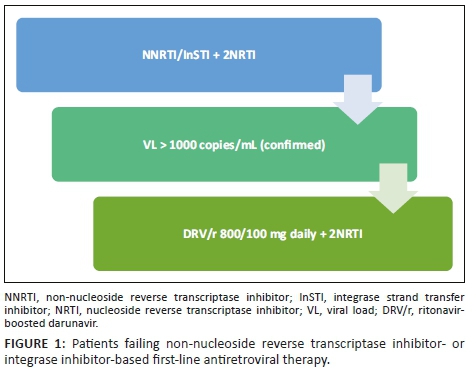
In patients failing first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)- or integrase strand transfer inhibitor (InSTI)-based regimens, switch to DRV/r 800/100 mg daily with two nucleoside reverse transcriptase inhibitors (NRTIs). Sequence the NRTIs as per guidelines (see Figure 1).
For those patients who are already on a second-line PI/r-based regimen, check the viral load (VL). If the VL is undetectable, then PI/r can be switched to DRV/r 800/100 mg daily, retaining the same NRTI backbone (see Figure 2).

If the VL is detectable, intensify adherence interventions and repeat the VL in 2-3 months. If the VL is undetectable, the PI/r can then be switched to DRV/r 800/100 mg daily. If VL > 1000 copies/mL, resistance genotype is needed to determine if the patient is eligible for third-line ART (see Figure 2).
Using darunavir/ritonavir 800/100 mg in third-line antiretroviral therapy
Currently, patients on DRV/r on third-line ART receive DRV/r 600/100 mg bid. However, a small proportion of third-line patients have no DRV resistance-associated mutations (RAMs), and in such patients it may be possible to use DRV/r 800/100 mg daily instead of DRV/r 600/100 mg bid to reduce pill burden, dosing frequency and side effects.
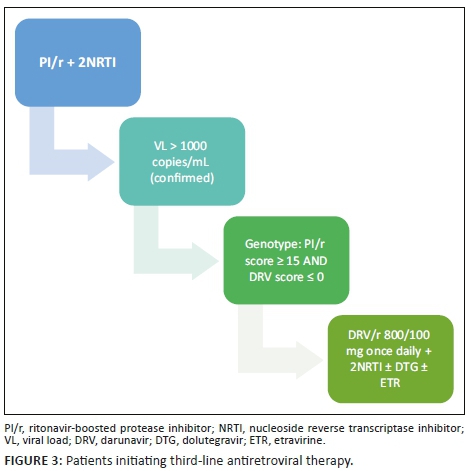
For patients initiating third-line ART, if the composite DRV score (Stanford) is zero on all genotypes, DRV/r 800/100 mg daily may be initiated (see Figure 3).
For those patients who are already on a third-line regimen, their VL must be checked. If the VL is undetectable, and the composite DRV score (Stanford) on all genotypes is zero, the patient may switch from DRV/r 600/100 mg twice daily to DRV/r 800/100 mg once daily. The rest of the regimen should not be changed (see Figure 4). If the VL is detectable, manage further as appropriate according to current guidelines.

 Correspondence:
Correspondence:
Michelle Moorhouse
mmoorhouse@wrhi.ac.za
Received: 20 Sept. 2018
Accepted: 21 Sept. 2018
Published: 18 Oct. 2018














