Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of HIV Medicine
versión On-line ISSN 2078-6751
versión impresa ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.16 no.1 Johannesburg 2015
http://dx.doi.org/10.4102/sajhivmed.v16i1.386
FORUM
http://dx.doi.org/10.4102/sajhivmed.v16i1.386
How ready are our health systems to implement prevention of mother to child transmission Option B+?
Palesa NkomoI; Natasha DaviesII; Gayle ShermanIII, IV; Sanjana BhardwajV; Vundli RamokoloI; Nobubelo K. NganduI; Nobuntu NoveveI; Trisha RamrajI; Vuyolwethu MagasanaI; Yages SinghI; Duduzile NsibandeI; Ameena E. GogaI, VI
IHealth Systems Research Unit, South African Medical Research Council, South Africa
IIWits Reproductive Health and HIV Institute, University of the Witwatersrand, South Africa
IIINational Institute for Communicable Diseases, Johannesburg, South Africa
IVDepartment of Paediatrics and Child Health, University of the Witwatersrand, South Africa
VThe United Nations Children's Fund, Pretoria, South Africa
VIDepartment of Paediatrics, University of Pretoria, South Africa
ABSTRACT
In January 2015, the South African National Department of Health released new consolidated guidelines for the prevention of mother to child transmission (PMTCT) of HIV, in line with the World Health Organization's (WHO) PMTCT Option B+. Implementing these guidelines should make it possible to eliminate mother to child transmission (MTCT) of HIV and improve long-term maternal and infant outcomes. The present article summarises the key recommendations of the 2015 guidelines and highlights current gaps that hinder optimal implementation; these include late antenatal booking (as a result of poor staff attitudes towards 'early bookers' and foreigners, unsuitable clinic hours, lack of transport to facilities, quota systems being applied to antenatal clients and clinic staff shortages); poor compliance with rapid HIV testing protocols; weak referral systems with inadequate follow-up; inadequate numbers of laboratory staff to handle HIV-related monitoring procedures and return of results to the correct facility; and inadequate supply chain management, leading to interrupted supplies of antiretroviral drugs. Additionally, recommendations are proposed on how to address these gaps. There is a need to evaluate the implementation of the 2015 guidelines and proactively communicate with ground-level implementers to identify operational bottlenecks, test solutions to these bottlenecks, and develop realistic implementation plans.
Introduction
Context and summary of 2015 prevention of mother to child transmission guidelines
South Africa has the highest HIV incidence rates globally, and is the largest provider of antiretroviral therapy in the world.1,2 In January 2015, the South African National Department of Health (NDoH) released new national consolidated guidelines, including an approach akin to World Health Organization (WHO) Option B+ for the prevention of mother to child transmission (PMTCT) of HIV.3 These guidelines harmonise triple antiretroviral treatment (ART) regimens for infants and young children, adolescents, pregnant and breastfeeding women, and adults to facilitate continuity of care. The guidelines stipulate lifelong ART for all pregnant and breastfeeding women and HIV-positive infants regardless of their CD4 cell count.3 Box 1 summarises the main differences between the 20153 and 20134 PMTCT guidelines. Specific algorithms have been developed for women with comorbidities (e.g. active psychiatric illness, renal dysfunction and/or anaemia) and these remain unchanged compared with the 2013 South African guidelines.3,4 The 2015 guidelines highlight the need to improve access to testing and treatment in general, to achieve the 90/90/90 target (90% coverage for HIV testing, 90% coverage for ART uptake amongst HIV-positive patients, and viral suppression of 90% of patients on ART) and to prioritise HIV prevention and treatment amongst adolescents.3 Despite the complexity of the new policy, its 'treatment as prevention' approach amongst pregnant and breastfeeding women could move South Africa closer to achieving the fourth and fifth millennium development goals and the post-2015 sustainable development goals.
Health systems' readiness to implement the new guidelines
System gaps that may hinder successful implementation of the 2015 South African prevention of mother to child transmission guidelines
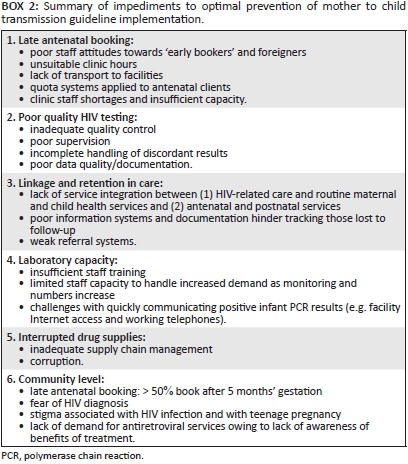
In our opinion, there are five main requirements for successful implementation of the 2015 PMTCT guidelines: (1) early presentation at the health facility to access care (i.e. early antenatal booking), (2) universal antenatal HIV testing based on high-quality standardised operating procedures, with repeat testing of HIV-negative women, (3) immediate referral into appropriate care and retention in care, (4) adequate coverage of appropriate laboratory systems and (5) uninterrupted drug supplies. These require appropriate actions within the health system and amongst sufficiently informed and empowered individual mother-infant pairs. Box 2 presents a summary of the main health system and community gaps in optimal implementation of the 2015 PMTCT guidelines; these are explained in more detail below.
Late antenatal booking
Since 2001, South Africa has improved access to antenatal care, HIV testing and ART provision for pregnant women. Currently, antenatal care uptake is over 95%; HIV testing is offered by over 95% of health facilities, and more than 87% of HIV-positive pregnant women receive some form of ART.3,5 However, the District Health Information System shows that in 2011/2012 only 40.2% (range 33.6% in Eastern Cape to 56.2% in Western Cape) of pregnant women had their first antenatal booking visit before 20 weeks' gestation, highlighting the first key bottleneck to successful guideline implementation.6 A study in North-West Province identified a variety of reasons for late booking, including late pregnancy disclosure amongst teenagers, fear of HIV testing, non-caring nurse attitudes, cultural beliefs that dissuade early revealing of pregnancy, lack of transport and unsuitable clinic opening hours.6 These findings were corroborated by research in Johannesburg which showed that 54% of pregnant women sought antenatal care later than 5 months' gestation.7 Solarin and Black found that almost half of new mothers interviewed reported that their first antenatal booking was not accepted by health facilities for various reasons including (1) they needed 'to make a booking appointment', (2) they did not have a South African identity document and (3) clinics had reached their quota for the day.7 These obstacles delay first antenatal booking and thus HIV screening, ART initiation and detection of treatment failure amongst pregnant women on ART as recommended by the 2015 South African PMTCT guidelines.3
Late HIV testing and poor quality HIV testing
There are grave concerns about quality control of HIV counselling and testing (HCT) at facilities, as shown by a study conducted in 455 sites (primary health care clinics, community healthcare centres and hospital gateway clinics) in Limpopo Province (Adrian Puren, personal communication, 11 March 2015). Poor quality control increases the risk of false-positive and -negative HIV results within the PMTCT programme. Concerns identified included inadequate training, frequent rotation of staff, lack of supervision and on-site quality control, incorrect storage of control samples, poor adherence to standard operating procedures (SOP) and improper stock control (Adrian Puren, personal communication, 11 March 2015). Anecdotal information gathered during healthcare provider (HCP) PMTCT guideline training found that HCPs are not waiting the required time before reading the HIV result, increasing the risk of false-negatives.
Late referral into care and poor retention in care
Appropriate, timely referrals and linkages to care are needed antenatally and post delivery to facilitate uptake of and retention in care. Over 90% of facilities assessed during a national South African Medical Research Council (SAMRC) review conducted in 2010 had a referral system for infant and adult ART clients.5 Similarly, an Eastern Cape study found that 100% of facilities reported appropriate referral mechanisms for HIV-positive women.8 However, in the MRC review, 38% of facilities did not make appointments for their patients at referral centres, and only 50% of clinics followed up whether clients engaged with care at the referral site.5 From our research and clinical experience, postnatal loss to follow-up is particularly high in South Africa. Poor information systems and documentation contribute to difficulties with tracing such patients. In Malawi, loss to follow-up was five times higher for women who started ART during pregnancy and two times higher for women who started ART whilst breastfeeding than for women who started ART with WHO stage 3/4 disease or CD4 cell count ≤ 350 cells/µL.9 These data highlight the risk of poor retention in care postnatally and emphasise the need for robust referral systems, integrated services and effective tracking mechanisms to retain, or re-engage, women in ART care post delivery.
Limited laboratory capacity
Laboratory capacity is needed for (1) viral load monitoring to identify poor adherence and treatment failure - a vitally important step in PMTCT programme success, (2) CD4 cell count to identify women who need cryptococcal antigen screening or cotrimoxazole prophylaxis, (3) routine antenatal bloods for ART toxicity monitoring and (4) repeated polymerase chain reaction (PCR) testing of HIV-exposed infants from birth (for symptomatic infants) to 18 months;3 laboratory capacity is accordingly a critical component of the PMTCT programme, and is key to timely referral into appropriate care. Previous challenges to implementing laboratory-based HIV tests in South Africa included difficulties processing large numbers of HIV-related specimens, high staff turn-over, and insufficient training in PCR techniques and CD4 measurements.10 Consequently, experienced staff carry the burden of training new staff10 and processing high quantities of specimens.
Interrupted drug supplies
By mid-2014, an estimated 2.6 million people were on ART in South Africa.3 This number will further increase following the 2015 guideline implementation, creating additional demand for ART stock. Sustaining such ART programme expansion will necessitate more efficient, effective supply chain management and increased human resources. Yet the healthcare system remains plagued by frequent HIV medicines stock-outs and clinic staff shortages. Inadequate supply chain management and 'corruption' contribute to avoidable stock-out-related treatment interruptions,11 resulting in regimen modification at best or, at worst, drug discontinuation.12 Considering the complexity of the new guidelines in part reflects South Africa's mature HIV epidemic, including increasing rates of highly experienced ART patients with treatment failure and drug resistance. The effects of recurrent stock-outs on adherence, viral loads and drug resistance should not be underestimated.
Recommendations
In light of the gaps identified above, we make several recommendations for optimal 2015 guideline implementation (Box 3).
Conclusion
The January 2015 PMTCT guideline recommendations are of a very high standard and based on the best intentions to improve the management of both HIV-positive and HIV-negative women. By implementing these guidelines, it should be possible to eliminate MTCT, improve maternal and infant outcomes, and ensure that women remain virologically suppressed and engaged in lifelong ART care. However, the implementation challenges might have been underestimated. Evaluation of the implementation process is needed to identify key bottlenecks and develop realistic implementation plans. A proactive process of communicating with ground-level implementers is needed to understand their challenges and to address these through well recognised, quality improvement processes.
Acknowledgements
Our sincere thanks to Professor Adrian Puren from the National Institute of Communicable Diseases for help with data on the Internal Quality Control (IQC) Implementation Report in Limpopo (April 2014). This work was supported by funds from the South African Medical Research Council.
Competing interests
The authors declare that they have no financial or personal relationships which may have inappropriately influenced them in writing this article.
Authors' contributions
P.N. (South African Medical Research Council) conceptualised and designed, drafted, wrote and finalised the article. N.D. (Wits Reproductive Health and HIV Institute) designed, contributed to and assisted with finalisation of the article. G.S. (National Institute for Communicable Diseases); S.B. (The United Nations Children's Fund) and V.R. (South African Medical Research Council), N.K.N. (South African Medical Research Council), T.R. (South African Medical Research Council), N.N. (South African Medical Research Council), V.M. (South African Medical Research Council), Y.S. (South African Medical Research Council) and D.N. (South African Medical Research Council) reviewed, commented on and approved the final version of the article. A.E.G. (South African Medical Research Council) conceptualised and designed, contributed to and assisted with finalisation of the article.
References
1. Human Sciences Research Council. Launch of the 2012 South African national HIV prevalence, incidence and behaviour survey report. April 2014 [cited 2015 Mar 02]. Available from http://www.hsrc.ac.za/en/media-briefs/hiv-aids-stis-and-tb/sabssm4-launch. Full report available from http://www.hsrc.ac.za/uploads/pageContent/4565/SABSSM%20IV%20LEO%20final.pdf [ Links ]
2. UNAIDS. Report of the global AIDS epidemic 2013 [cited 2015 Mar 02]. Available from http://www.unaids.org/sites/default/files/en/media/unaids/contentassets/documents/epidemiology/ 2013/gr2013/UNAIDS_Global_Report_2013_en.pdf [ Links ]
3. South African National Department of Health. 2014 [cited 2015 Mar 02]. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Available from http://www.sahivsoc.org/upload/documents/HIV%20guidelines%20_Jan%202015.pdf [ Links ]
4. South African antiretroviral treatment guidelines 2013. PMTCT guidelines: Revised March 2013 [cited 2015 Aug 27]. Available from http://www.sahivsoc.org/upload/documents/2013%20ART%20Guidelines-Short%20Combined%20FINAL%20draft%20guidelines%2014%20March%202013.pdf [ Links ]
5. Goga AE, Dinh TH, Jackson DJ for the SAPMTCTE study group. Early (4-8 weeks post-delivery) population-level effectiveness of WHO PMTCT Option A, South Africa, 2011. South African Medical Research Council, National Department of Health of South Africa and PEPFAR/US Centers for Disease Control and Prevention. 2013 [cited 2015 Mar 12]. Available from http://www.mrc.ac.za/healthsystems/SAPMTCTE2011.pdf [ Links ]
6. District Health Barometer 2011/2012. Durban: Health Systems Trust; 2013 [cited 2015 Apr 02]. Available from http://www.health-e.org.za/wp-content/uploads/2013/04/DHB2011_12lowres.pdf [ Links ]
7. Solarin I, Black V. 'They told me to come back': Women's antenatal care booking experience in inner-city Johannesburg. Matern Child Health J. 2013;17:359-367. PMID: 22527767, http://dx.doi.org/10.1007/s10995-012-1019-6 [ Links ]
8. Rispel LC, Peltzer K, Phaswana-Mafuya N, Metcalf CA, Treger L. Assessing missed opportunities for the prevention of mother-to-child HIV transmission in an Eastern Cape local service area. S Afr Med J. 2009;99:174-179. PMID: 19563095 [ Links ]
9. Tenthani L, Haas AD, Tweya H, et al. Retention in care under universal antiretroviral therapy for HIV-infected pregnant and breastfeeding women ('Option B+') in Malawi. AIDS. 2014;28:589-598. PMID: 24468999, http://dx.doi.org/10.1097/QAD.0000000000000143 [ Links ]
10. Stevens WS, Marshall TM. Challenges in implementing HIV load testing in South Africa. J Infect Dis. 2010;201(suppl. 1):S78-S84. PMID: 20225952, http://dx.doi.org/10.1086/650383 [ Links ]
11. Bateman C. Drug stock-outs: Inept supply-chain management and corruption. S Afr Med J. 2013;103:600-602. PMID: 24344422, http://dx.doi.org/10.7196/samj.7332 [ Links ]
12. Pasquet A, Messou E, Gabillard D, et al. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan, Côte d'Ivoire. PLoS ONE. 2010;5:e13414. PMID: 20976211, http://dx.doi.org/10.1371/journal.pone.0013414 [ Links ]
 Correspondence:
Correspondence:
Palesa Nkomo
Private Bag X385
Pretoria 0002
South Africa
Email: palesa.nkomo@mrc.ac.za
Received: 15 Apr. 2015
Accepted: 06 Aug. 2015
Published: 07 Oct. 2015














