Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
Southern African Journal of HIV Medicine
versión On-line ISSN 2078-6751
versión impresa ISSN 1608-9693
South. Afr. j. HIV med. (Online) vol.15 no.2 Johannesburg feb. 2014
ORIGINAL ARTICLE
Which clinical parameters predict a CSF diagnosis of meningitis in a population with high HIV prevalence?
W LoughboroughI; M AbouyannisII; L JonesIII; S GarachIV
IMB ChB (Hons), BSc, Bristol Royal Infirmary, UK
IIMB ChB, BMedSci, DTMH, Dip HIV Man (SA), Royal Liverpool University Hospital, UK
IIIMB ChB, BSc, Great Western Hospital, Swindon, UK
IVMB ChB, DA (SA), FCEM (SA) Stanger Provincial Hospital, KwaZulu-Natal, South Africa
ABSTRACT
BACKGROUND: The HIV epidemic has changed the aetiology of meningitis in sub-Saharan Africa, and frontline clinicians are faced with a variety of meningitic presentations. Doctors working in resource-limited settings have the challenge of appropriately selecting patients for lumbar puncture (LP), a potentially risky procedure that requires laboratory analysis.
METHODS: In a rural South African hospital, the practice of performing LPs was audited against local guidelines. Data were collected retrospectively between February and June 2013. Symptoms and signs of meningitis, HIV status, investigations performed prior to LP and cerebrospinal fluid (CSF) results were recorded. With the aim of determining statistically significant clinical predictors of meningitis, parameters were explored using univariate and multivariate logistic regression analyses
RESULTS: A total of 107 patients were included, of whom 43% had an abnormal CSF result. The majority (76%) of patients were HIV-positive (CD4+ cell count <200 cells/µl in 46%). Cryptococcal meningitis (CCM) was the most prevalent microbiological diagnosis, confirmed in 10 out of 12 patients. Of the non-microbiological diagnoses, lymphocytic predominance was the most common abnormality, present in 17 out of 33 patients. Confusion (p=0.011) was the most statistically significant predictor of an abnormal CSF result. Headache (p=0.355), fever (p=0.660) and photophobia (p=0.634) were not statistically predictive
CONCLUSION: The high incidence of CCM correlates with previous data from sub-Saharan Africa. In populations with high HIV prevalence, the classic meningitic symptoms of headache, fever and photophobia, while common presenting symptoms, are significantly less predictive of a meningitis diagnosis than confusion.
Meningitis contributes significantly to mortality in countries with high HIV prevalence.[1] Cryptococcal meningitis (CCM) has become a significant opportunistic infection, accounting for 12 - 44% of early mortality in HIV-infected cohorts in resource-limited countries.[2,3] It carries an estimated annual global mortality of 500 000, with a case fatality rate of 35 - 65% in sub-Saharan Africa.[1,4] Lumbar puncture (LP) and cerebrospinal fluid (CSF) analysis remain the cornerstones of diagnosis. Clinicians must be efficient in targeting patients to undergo this time-consuming and potentially risky procedure. Furthermore, laboratory CSF analysis is a cost consideration in resource-limited settings.
Studies exploring the presentation of meningitis in populations with high HIV prevalence generally lack statistical analysis of clinical predictors of an abnormal CSF result. Studies that analyse presenting features of meningitis are generally only from cohorts of patients with confirmed meningitis, without comparison with similar cohorts with normal CSF results.[5,6] The accuracy of variables to predict abnormal CSF results is not possible without this comparison. In addition, in studies determining clinical presentation of meningitis, there is a lack of logistic regression analysis; confounding features are not removed.[5,6] There is therefore little evidence that demonstrates statistically significant predictors of a positive meningitis diagnosis in populations with high HIV prevalence. Consequently, clinicians working in this setting have a paucity of evidence to use when trying to select patients for LP appropriately. Given the dangers associated with LP and the potential cost implications of CSF analysis, such literature is essential for guiding frontline clinicians in resource-limited settings as to when to suspect meningitis and when an LP is justified. Alongside this, clinicians must be aware of the contraindications to LP and must make appropriate use of tests such as platelet count, clotting and imaging, where available, to ensure patient safety.
Objective
Our first objective was to improve departmental practice through auditing the practice of performing diagnostic LPs against local guidelines in a regional referral hospital serving a rural South African (SA) population. We established local meningitis aetiology by reviewing the results of CSF analysis. The key objective was to determine which clinical parameters were statistically significant in predicting a positive CSF diagnosis of meningitis.
Methods
Setting
Stanger Provincial Hospital is a regional centre located in Ilembe District, in rural KwaZulu-Natal, SA. It serves a population of approximately 700 000 and has 500 inpatient beds. Referrals are received from 15 smaller hospitals and satellite clinics. HIV prevalence in Ilembe district is 35.4%, one of the highest district rates in SA.[7] The emergency department (ED) treats ~2 500 patients a month, of whom 1 500 have a medical rather than a surgical complaint. Patients presenting with suspected meningitis are seen in the ED, where an LP is performed and CSF results are analysed, with referral to the Department of Internal Medicine where appropriate.
Data collection
Audit data were collected retrospectively between February and June 2013. All patients undergoing diagnostic LPs in the ED, regardless of indication, were eligible for inclusion. Doctors working in the department were asked to make a note of patients undergoing LP by documenting their hospital number. The audit team then used a data collection tool to gather information from the patient's notes, namely indication for LP, HIV status, presenting symptoms and signs, CD4+ cell count, platelet count, international normalised ratio (INR), whether a computed tomography (CT) head scan was performed before LP, and CSF result. As the data collection was primarily for audit purposes, identifying information such as name, age and sex was not recorded; unique study numbers were used to identify patients. If information from the medical notes did not provide all the key information for the data collection tool, the patient was excluded from the audit. When the audit period had finished, the results were summarised.
Clinicians did not record every patient undergoing LP. While every patient coming through the department was recorded in the ED admissions book, admitting diagnoses were not always clear in terms of whether meningitis was likely or an LP was performed. Therefore determining the audit sample size as a total number of patients undergoing LP could not be done accurately.
Patient management was audited against the Stanger Provincial Hospital Department of Internal Medicine 2010 guidelines for diagnostic LPs. According to these, relative contraindications were coagulopathy (platelets <50 x 109/l and INR >1.2) or suspicion of raised intracranial pressure (Glasgow Coma Scale (GCS) <12, focal neurological signs or papilloedema on fundoscopy). In these circumstances, platelet and/or freeze-dried plasma infusion or head CT, respectively, are advised prior to LP.
CSF diagnostic criteria were obtained from a study based in Cape Town in which 4 971 LPs were evaluated, the largest study of diagnostic LPs in SA.[8] A positive diagnosis for meningitis was made either microbiologically with staining, antigen testing and culture, or, if these were negative, through abnormal protein, glucose or chloride levels. Microbiological CSF diagnoses were defined as CCM (positive cryptococcal latex antigen test (CLAT) and/ or India ink stain) or bacterial meningitis (BM) (positive Gram stain and/ or culture). In this rural setting, samples are not consistently sent for tuberculosis (TB) culture, and therefore this outcome was omitted from the study. Abnormal biochemical and microscopic findings were classified as follows: lymphocytic (lymphocytes >6 x 106/l), pyogenic (neutrophils >2 x 106/l), mixed or normocellular with abnormal protein and/or glucose (protein >0.5 g/dl and/or glucose <1.5 mmol/l). Abnormal CSF was a binary variable, with a positive result defined as any of the above abnormalities in staining, culture and antigen testing, as well as raised protein (>0.5 g/dL), low glucose (<1.5 mmol/L) or low chloride (<110 mEq/L).
Data analysis
Statistical analysis included descriptive statistics with proportions, medians and interquartile ranges (IQRs) calculated for non-parametric data, and means and standard deviations presented for parametric data. Potential predictors of abnormal CSF were evaluated using univariate and multivariate logistic regression analyses, namely headache, confusion, terminal neck stiffness, photophobia, focal neurological signs, seizures, HIV status and CD4+ cell count. Odds ratios (ORs) were presented with 95% confidence intervals (CIs) and a statistical significance cut-off of p<0.05.
Stata 10.0 software (StataCorp, Stata Statistical Software, release 10, 2007) was used for the statistical analysis.
Ethical approval
As this project was an audit of departmental practice, we did not apply for formal ethics committee approval. Approval to undertake the audit was granted by the ED consultants and Head of Department. In terms of consent for the LP procedure, clinicians obtained and recorded informed consent from all patients, or a relative in cases where the patient lacked capacity (e.g. low GCS). In children aged between 16 and 18 years old, consent was obtained from a parent/guardian of the child.
Results
From February to June 2013, 107 patient cases were audited. An HIV test was refused by 15 out of 107 patients (14%); among those who consented to the test, 81 out of 92 (88%) were seropositive. Many HIV-positive patients had significant immunosuppression, with 46% (n=37) having a CD4+ cell count below 200 cells/µl and 34% (n=28) below 100 cells/µl.
The most common symptom was headache, present in 76% (n=80) of patients. Approximately half (51%) described the headache as focal, and the median duration was 4 days (IQR 2 - 7). Neck stiffness was reported by 56% (n=60), and 38% (n=41) reported photophobia. Seizures were recalled by 5.6% (n=6), and confusion was present in 41% (n=44) (Table 1).
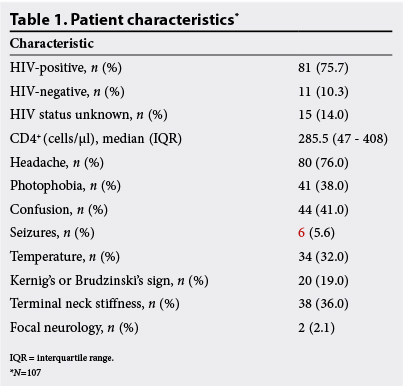
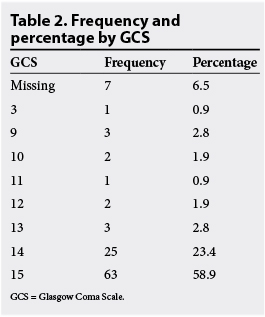
On examination, 32% (n=34) were pyrexial. Kernig's or Brudzinski's signs were present in 19% (n=20) and terminal neck stiffness was felt in 36% (n=38). There were two cases with focal neurology and one case with papilloedema, who underwent an LP. Papilloedema was not examined for in 86% (n=92) of patients. Fifty-nine per cent (n=63) had a GCS of 15, 26% (n=28) had a GCS of between 13 and 14, and 8.4% (n=16) had a GCS of <12 (Table 2). Of patients with a GCS <12, three had a CT prior to LP and three did not. The indication for the LP was meningitis in 83% (n=89), seizures in 6.2% (n=6) and psychosis in 11% (n=12).
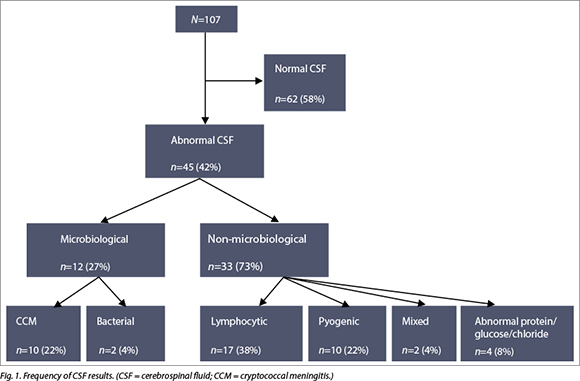
Prior to LP, 89% (n=94) had platelets checked, and of these one patient had a significantly reduced count at 14 x 109/l (1%). Only 7.5% (n=8) of patients had their INR checked prior to LP and of these, three patients had a result of 1.3 - 1.4 units. Prior to LP, 6.5% (n=7) underwent a CT scan. Out of the 107 patients, 42% (n=45) had an abnormal CSF result. Of these, 27% (n=12) had confirmed microbiological diagnoses - 10 CCM and 2 BM. The remaining abnormal results were 17 lymphocytic, 10 mixed, 2 pyogenic and 4 normocellular with abnormal protein and/or glucose (Fig. 1).
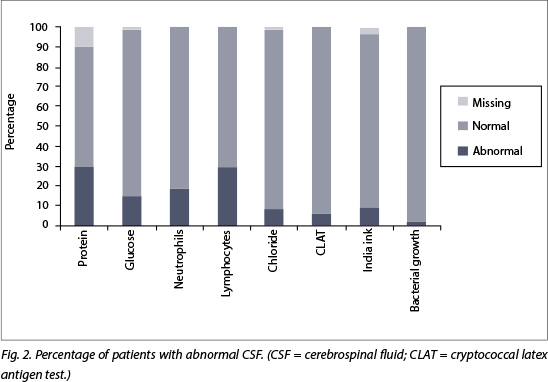
Overall, polymorphs were >2 x 106/l in 18% (n=19) and the lymphocytes were >6 x 106/l in 29% (n=31). Chloride was <110 in 7.6% (n=8) of cases. CSF glucose was <1.5 mmol/l in 14% (n=15) and protein was >0.5 g/dl in 33% (n=35). Gram staining revealed Gram-positive diplococci (streptococcus pneumoniae on culture) in one case and Gram-positive cocci (failed to culture) in another case. India ink staining identified Cryptococcal neoformans in five patients, and all of these plus an additional four cases had a positive CLAT. (Fig. 2)
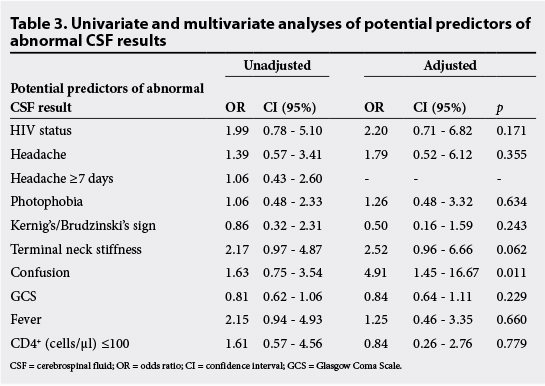
Potential clinical predictors of abnormal CSF results were evaluated using univariate and multivariate logistic regression analyses (Table 3). Seizures and papilloedema were excluded from these analyses, as numbers were too small for these variables. In the unadjusted analysis, none of the factors were significantly associated with abnormal CSF results. Terminal neck stiffness was almost statistically significant, with an OR of 2.17 (95% CI 0.97 - 4.87), as was raised temperature with an OR of 2.15 (95% CI 0.94 - 4.93). In the multivariate analysis, terminal neck stiffness remained almost statistically significant with an OR of 2.52 (95% CI 0.96 - 6.66). Confused patients were found to be significantly more likely to have abnormal CSF, with an OR of 4.91 (95% CI 1.45 - 16.67) and p=0.011.
Discussion
The primary objective of this study was to determine statistically significant clinical predictors of meningitis through logistic regression analysis. Previous research has generally lacked this analysis and therefore has failed to account for confounding factors. Unlike previous studies, which only analysed presenting features from a cohort of patients with abnormal CSF results, in our study patients were selected before LP, allowing for proper estimation of the accuracy of each variable predicting abnormal CSF results. The logistic regression analyses demonstrated confusion as a strong predictor, with an OR of 4.91 and p=0.011. Previous papers that focused on sub-populations with HIV and meningitis have found headache and fever as the most common presenting features, with a lower incidence of confusion.[5,6] For example, one study from Zambia found headache or abnormal temperature present in 91% of their HIV-positive patients with CCM, but only 13% were confused.[6] Our study has highlighted that although headache and fever were common symptoms in patients presenting for LP, they lacked specificity in predicting meningitis, as they were often present in patients with a normal CSF result (headache OR 1.39, p=0.355; fever OR 1.24, p=0.660). An explanation for this lack of specificity is that patients with HIV are at increased risk of multiple pathologies, of which headache and fever are symptoms. For example, migraines are more common in HIV-positive patients.[9]
Therefore, these symptoms lack specificity in predicting meningitis. Photophobia, another symptom classically associated with meningitis and common in patients presenting for LP, was not statistically significant in predicting meningitis (OR 1.06, p=0.634). These findings highlighted that meningitis is difficult to predict in HIV-positive patients. Furthermore, the varied aetiology showed that the classic presentation of meningitis is unlikely, proven by the lack of statistical predictability of classic meningitis symptoms. The fact that the incidence of meningitis is high (42% in our cohort) reveals that clinicians should have a low threshold for undertaking LP when not contraindicated. In resource-limited settings, selecting patients with more specific features, such as confusion rather than headache, may allow for a higher identification rate of abnormal CSF results. More research is needed in similar populations of high HIV prevalence to validate these findings and support clinicians in their decision to undertake LP.
The audit compared the practice of performing LPs with current hospital guidelines. The checking of platelets was thorough, with 89% of patients checked prior to LP. However, checking of the INR was poor, with only 7.5% checked; among those with an INR result, 37.5% of the results were >1.2 (hospital guidelines stated that these patients required freeze-dried plasma prior to LP). The aetiology of these clotting disorders is unknown but may be explained by comorbidities such as viral hepatitis. The lack of INR checking can be explained by the lack of suspicion of coagulopathy on admission. Warfarin prescription and sepsis at presentation were rare, so iatrogenic or sepsis-induced coagulopathies were not suspected clinically. While there has not been any published research examining the risk of bleeding from LP in patients with coagulopathy, it is considered best practice to correct an INR of >1.4 prior to LP.[10] In one literature review, 47% of 21 published cases of spinal haematoma following LP occurred in patients with a coagulopathy.[11] Following these audit results, new departmental protocol was to perform an INR on all patients and correct abnormalities accordingly.
In this study, local guidelines stated that patients with a GCS of <12 required a head CT prior to LP, but a CT was performed on only 50% of such patients, which shows that practice clearly falls short of local standards. However, the reasoning behind performing a CT prior to LP remains controversial. A CT may identify an alternative pathology such as a mass lesion or indications of raised intracranial pressure, which could contraindicate LP. However, a normal CT scan does not exclude raised intracranial pressure and the scanning of patients potentially delays LP and antibiotic administration.[12] Guidelines need to be set locally. Following presentation and discussion of these audit findings at a departmental meeting, it was decided to perform a CT on any patient with a fluctuating GCS or GCS <12 prior to LP.
In this rural SA setting with high HIV prevalence, 42% of patients had abnormal CSF results. The Cape Town study had a similarly high incidence of abnormal results, at 35%.[8] Of the abnormal results, 27% had definitive microbiological diagnoses, compared with 47% in Cape Town. Of the positive microbiological diagnoses, 83% cultures grew Cryptococcus neoformans and 17% pyogenic bacteria (BM), in contrast to 63% and 8%, respectively, in Cape Town.'81 We did not have culture results for tuberculous meningitis (TBM), for which the Cape Town figure was 28%.'81 Recent studies from other high HIV prevalence locations in Africa have found similar high rates of CCM; it is now the most prevalent cause of meningitis in sub-Saharan Africa (25 - 47%).[1]
As TB CSF culture was excluded from this study, direct comparisons could not be made with regard to TBM and total microbiological diagnoses - this was a limitation as discussed below. However, the high levels of lymphocytic predominance in the non-microbiological diagnoses almost certainly correlated with high levels of TBM, in the context of high HIV prevalence.[11]
Limitations
A key limitation of this study was the lack of TB culture results due to the lack of TB culturing facilities at Stanger Provincial Hospital. TB meningitis is a significant cause of mortality in cohorts with high HIV prevalence and has a variety of initial CSF findings.[13] The high levels of lymphocytic predominance and raised protein would fit with high levels of TBM.[13] Therefore, our results almost certainly underestimated the prevalence of TBM in our population. While this clearly affected our aetiological results, it had less of an impact on our statistical analysis as this was based on a positive CSF diagnosis in general (microbiological and non-microbiological results), rather than specific microbiological diagnoses. The lack of TB culturing at a facility is a common issue in the developing world - clinicians often have only their clinical impression plus initial CSF results with which to make diagnostic decisions. Even if culturing facilities are available, TB culture often takes some time to grow. This is why the outcome from this study is relevant for clinicians working in this setting - it demonstrates which presenting features are predictive of the CSF results on the basis of which diagnoses are made.
Another limitation was the quality control in the clinicians' documentation. Because this was a retrospective departmental audit, we could not ensure that all information recorded in the notes was consistent. The GCS was the most common area of subjectivity, for example several patients had GCS 15 recorded in the notes while they were identified as confused in the history (therefore GCS <14). However, in general, the major presenting features audited were objective. One area inconsistently recorded was antiretroviral therapy use, which therefore had to be excluded from the results. This would have been a valuable set of results, comparing the presentation and aetiology of meningitis in HIV-positive patients currently on or not on ART.
Conclusion
This audit demonstrated that LP practice in the ED did not adhere to local guidelines in certain areas. Following presentation of the results and discussion at a departmental meeting, it was agreed that prior to LP, an INR should be measured routinely, and a head CT should be performed on any patient with fluctuating GCS or GCS <12. The high prevalence of CCM from our data was consistent with other studies, which demonstrated that cryptococcosis has become the leading cause of adult meningitis in sub-Saharan Africa.[1] While there were limitations to the project, the data analysis has demonstrated some valuable findings. Current literature analysing the presentation of meningitis in cohorts with high HIV prevalence generally fails to compare normal with abnormal CSF groups and lacks logistic regression analysis. Our statistical analysis demonstrated confusion to be a significant predictor of an abnormal CSF result. While headache, fever and photophobia were common presenting symptoms, they were not statistically significant predictors of meningitis. Clinicians working in populations with high HIV prevalence need to have a broad differential diagnosis for patients presenting with headache and have a low threshold for performing LP or empirically treating meningitis in confused patients.
References
1. World Health Organization. Rapid Advice: Diagnosis, Prevention and Management of Cryptococcal Disease in HIV-infected Adults, Adolescents and Children. Geneva: World Health Organization, 2011. http://www.who.int/hiv/pub/cryptococcal_disease2011/en/ (accessed 10 November 2013). [ Links ]
2. French N, Gray K, Watera C, et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 2002;16(7):1031-1038. 'http://dx.doi.org/10.1097/00002030-200205030-000091 [ Links ]
3. Okongo M, Morgan D, Mayanja B, Ross A, Whitworth J. Causes of death in a rural, population-based human immunodeficiency virus type 1 (HIV-1) natural history cohort in Uganda. Int J Epidemiol 1998;27(4):698-702. [ Links ]
4. Park BJ, Wannemuehler KA, Marston BJ, Govender N, Pappas PG, Chiller TM. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 2009;23(4):525-530. 'http://dx.doi.org/10.1097/QAD.0b013e328322ffac1 [ Links ]
5. Baradkar V, Mathur M, De A, Kumar S, Rathi M. Prevalence and clinical presentation of cryptococcal meningitis among HIV seropositive patients. Indian J Sex Transm Dis AIDS 2009;30(1):19. 'http://dx.doi.org/10.4103/0253-7184.554741 [ Links ]
6. Mwaba P, Mwansa J, Chintu C, et al. Clinical presentation, natural history, and cumulative death rates of 230 adults with primary cryptococcal meningitis in Zambian AIDS patients treated under local conditions. Postgrad Med J 2001;77(914):769-773. 'http://dx.doi.org/10.1136/pmj.77.914.7691 [ Links ]
7. South African National Department of Health. The 2011 National Antenatal HIV and Syphilis Prevalence Survey, 2011. http://www.health-e.org.za/2012/12/11/2011-national-antenatal-hiv-syphilis-prevalence-survey-released/ (accessed 1 April 2014). [ Links ]
8. Jarvis JN, Meintjes G, Williams A, Brown Y, Crede T, Harrison TS. Adult meningitis in a setting of high HIV and TB prevalence: Findings from 4 961 suspected cases. BMC Infect Dis 2010;10:67. 'http://dx.doi.org/10.1186/1471-2334-10-671 [ Links ]
9. Kirkland KE, Kirkland K, Many WJ, Smitherman TA. Headache among patients with HIV disease: Prevalence, characteristics, and associations. Headache 2012;52(3):455-466. 'http://dx.doi.org/10.1111/j.1526-4610.2011.02025.x1 [ Links ]
10. Longmoore M, Wilkinson IB, Davidson EH. Oxford Handbook of Clinical Medicine. 8th ed. Oxford: Oxford University Press, 2010. [ Links ]
11. Sinclair AJ, Carroll C, Davies B. Cauda equina syndrome following a lumbar puncture. J Clin Neurosci 2009;16(5):714-716. 'http://dx.doi.org/10.1016/j.jocn.2008.07.0791 [ Links ]
12. Joffe AR. Lumbar puncture and brain herniation in acute bacterial meningitis: A review. J Intensive Care Med 2007;22(4):194-207. 'http://dx.doi.org/10.1177/08850666072995161 [ Links ]
13. Vinnard C, Macgregor RR. Tuberculous meningitis in HIV-infected individuals. Curr HIV/AIDS Rep 2009;6(3):139-145. 'http://dx.doi.org/10.1007/s11904-009-0019-71 [ Links ]
 Correspondence:
Correspondence:
WLoughborough
wloughborough84@gmail.com














