Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
Health SA Gesondheid (Online)
On-line version ISSN 2071-9736
Print version ISSN 1025-9848
Health SA Gesondheid (Online) vol.21 n.1 Cape Town 2016
http://dx.doi.org/10.1016/j.hsag.2016.06.003
FULL LENGTH ARTICLE
Knowledge of appropriate blood product use in perioperative patients among clinicians at a tertiary hospital
Bradley Yudelowitz*; Juan Scribante; Helen Perrie; Eddie Oosthuizen
Department of Anaesthesiology, Faculty of Health Sciences, University of the Witwatersrand, Johannesburg, South Africa
ABSTRACT
BACKGROUND: Blood products are an expensive and scarce resource with inherent risks to patients. The current knowledge of rational blood product use among clinicians in South Africa is unknown.
PURPOSE OF RESEARCH: To describe the level of clinicians' knowledge related to all aspects of the ordering and administration of blood products from the South African Blood Services for peri-operative patients at a tertiary hospital.
METHOD: A self-administered survey was distributed to 210 clinicians of different experience levels from the departments of Anaesthesiology, General Surgery and Trauma, Orthopaedic Surgery and Obstetrics and Gynaecology at the study hospital. The questions related to risks, cost, ordering procedures and transfusion triggers for red cell concentrate (RCC), fresh frozen plasma (FFP) and platelets.
RESULTS: A total of 172 (81.90%) surveys were returned. The overall mean for correctly answered questions was 16.76 (±4.58). The breakdown by specialty was: Anaesthesiology 19.98 (±3.84), General Surgery and Trauma 16.28 (±4.05), Orthopaedic Surgery 13.83 (±4.17) and Obstetrics and Gynaecology 15.63 (±3.51). Anaesthesiology performed better than other disciplines (p < 0.001) and consultants out-performed their junior colleagues (p < 0.001). Seventy percent correctly identified triggers for RCC transfusion and 50% for platelets. Administration protocols were correctly defined by 80% for RCC and FFP just over 50% for platelets. Thirty eight percent of respondents deemed infectious and non-infectious risk sufficient to obtain informed consent. Knowledge of costs and ordering was below 30%.
CONCLUSION: Clinician's knowledge of risks, resources, costs and ordering of blood products for perioperative patients is poor. Transfusion triggers and administration protocols had an acceptable correct response rate.
Keywords: Blood component transfusion; Blood products; Perioperative care; Knowledge; Complications
1. Introduction
Modern medicine has a continued reliance on allogeneic blood products. This is an expensive and scarce resource, with inherent risks to patients. Escalating costs and declining supplies have deepened the need to rationalise transfusion practice. Several transfusion guidelines have been developed, however, awareness and adherence to these guidelines seems to be lacking as demonstrated in a number of surveys (Hebert et al., 1998 and Matot et al., 2004; Nutall et al., 2003 and Stehling et al., 1987).
Between 5000 and 6000 blood products are ordered monthly from the South African National Blood Service (SANBS) at the study hospital and up to 30% of these orders are cancelled or wasted (SANBS 2012).
In South Africa it is of paramount importance that medical professionals have the competencies, skills and knowledge to administer the limited and expensive blood products safely to the most appropriate patients. There is no current literature evaluating the level of knowledge of rational blood product use in this country. The aim of this research was to describe the level of clinicians' knowledge related to the ordering and administration of blood products from the SANBS for perioperative patients at a tertiary hospital.
The primary objectives of the study were to determine the knowledge of clinicians with regard to:
• risk associated with the transfusion of blood products,
• resources and costs associated with the transfusion of blood products,
• donations, ordering and return of blood products,
• safe administration of blood products to a patient, and
• transfusion thresholds and triggers for blood product administration.
The secondary objectives were to compare knowledge levels among different specialty departments and clinician ranks.
2. Method
A prospective, descriptive, contextual study design was used. Ethics approval was obtained from the Human Research Ethics Committee (Medical) (M120748) of the University of the Witwatersrand and the other relevant authorities. The research was conducted according to the principles of the Declaration of Helsinki (2008).
The study hospital is a 2688 bed hospital where 65,000 surgeries are performed annually. The study population consisted of clinicians working with perioperative patients in the Anaesthesiology, General Surgery, Trauma, Orthopaedic Surgery and Obstetrics and Gynaecology Departments belonging to the professional ranks of intern, medical officer, registrar and consultant. A purposive sampling method was used and the sample size was realised by the number of respondents who completed the questionnaire. The exclusion criteria of the study were:
• clinicians who indicated that they have never been involved in the administration of blood products at the study hospital,
• who declined to take part in the study,
• who were on annual, special or sick leave and
• surveys that were less 50% complete.
A 20 question, self-administered, multiple-choice anonymous survey was drawn up based on a review of the literature (Hebert et al., 1998, Irving, 1992 and Matot et al., 2004; Nutall et al., 2003, Stehling et al., 1987, Turgeon et al., 2006 and Vlaar et al., 2009) and the SANBS Clinical Guidelines for the use of blood products ensuring content validity. Three senior anaesthesiologists and a senior haematologist, all with blood product expertise, reviewed the questionnaire ensuring face validity. Minor changes were made based on recommendations given. The adapted survey was given to 10 clinicians to assess for clarity. No further suggestions were made.
The survey assessed the following:
• formal blood product education attendance,
• professional rank and department of clinicians,
• knowledge of risks of blood product administration,
• knowledge of resources and costs associated with the transfusion of blood products,
• blood product donation, ordering and return administration of blood products according to the SANBS guideline, and
• transfusion thresholds and triggers for blood product administration.
The author (BY) addressed clinicians at departmental academic meetings (January to March 2013), explaining the study and inviting the clinicians to take part. The survey and an information letter were distributed to willing respondents. The completed surveys were collected at the meetings' conclusion in a sealed box. Return of surveys implied consent to take part in the study. The author (BY) was present during the meetings to prevent data contamination and answer any respondents' questions.
Data were analysed using descriptive and inferential statistics using Microsoft Excel for Mac 2011 and GraphPad InStat. For descriptive analysis of data that were normally distributed mean and standard deviation (SD) were used. ANOVA testing was used to compare means between groups. Bonferroni testing and correction procedure was used for post-testing to identify where the significant differences lie. A p-value < 0.05 was taken as statistically significant. Unanswered questions were assumed to be the 'don't know' option at data capture. No returned surveys were discarded as all had been more than half completed.
3. Results
There were 210 surveys distributed with 172 (81.90%) returned. Demographics of respondents are demonstrated in Table 1. Question, answers and number of correct responses are summarised in Table 2.
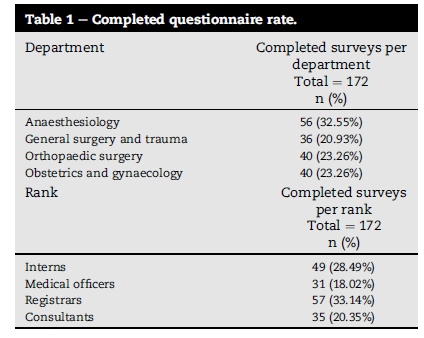
Of note, only 40-60% of respondents could quantify risk and complications of transfusion and therefore obtain informed consent. Knowledge of ordering processes and costs was also poor with only 8% of respondents able to define a crossmatch compared with type and screen.
RCC knowledge was more robust with 70-90% of respondents able to identify triggers, physiological response and appropriate temperatures for transfusion. A total of 123 (71.51%) respondents correctly responded to the haemoglobin trigger question with 7 or 8 g/dl. These data are represented in Fig. 1.
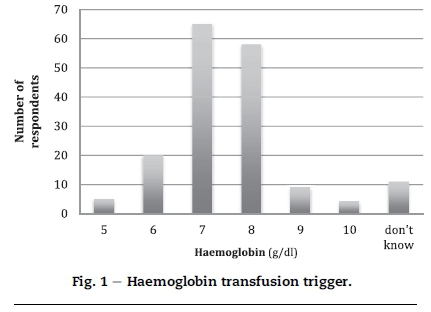
Similar questions regarding platelet administration were answered correctly by less than 50% of respondents. FFP knowledge seems haphazard.
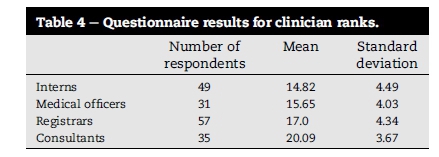
The mean and standard deviation (SD) for correctly answered questions was 16.76 (±4.58) from 32 questions for all respondents as shown in Table 3. Specialty department means were: Anaesthesiology 19.98 (±3.84), General Surgery and Trauma 16.28 (±4.05), Orthopaedic Surgery 13.83 (±4.17) and Obstetrics and Gynaecology 15.63 (±3.51). Clinician rank means were: interns' 14.82 (±4.49), medical officers' 15.65 (±4.03), registrars' 17.0 (±4.34) and consultants' 20.09 (±3.67) as shown in Table 4.
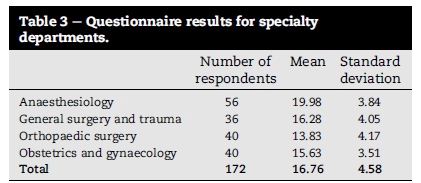
tatistical analysis did identify a significant difference between specialty departments with Anaesthesiology performing significantly better (p < 0.001) than the other departments. No significant differences in performance were demonstrated between General Surgery and Trauma, Orthopaedic Surgery and Obstetrics and Gynaecology (p > 0.05).
A significant difference between clinician ranks was shown. Consultants performed better than other ranks (p < 0.005). Interns, medical officers and registrars performed similarly with no significant difference between them demonstrated (p > 0.005).
In the last two years formal education on blood products had been attended by 34.30% of respondents. The annual seminar (SANBS 2011) is the most likely setting for this education.
4. Discussion
The WHO launched a patient safety programme in 2008 with the slogan of 'Better knowledge for safer care' (WHO Patient Safety 2008). This programme urges the prioritisation of patient safety and identified inadequate competencies and skills as well as the lack of appropriate knowledge and transfer of knowledge among the top six research priorities in developed and developing countries. These research priorities are specifically appropriate for the transfusion of blood products, which is a distinctive technology that blends science and altruism.
Various surveys have highlighted the variations in clinical practice of blood product transfusion despite the multitude of available guidelines (Hebert et al., 1998, Irving, 1992, Matot et al., 2004 and Nutall et al., 2003; Stehling et al. 1987). There is, however a paucity in the literature with regard to describing physicians' knowledge of risk, cost appropriate ordering, administration, guidelines and physiology of blood product transfusion. Knowledge of these aspects of blood product transfusion is critical to ensure that the use of these scarce resources are safe and cost-effective.
The safety of blood products, obtained from the SANBS, appears to show parity with international standards and are certainly the safest in sub-Saharan Africa (Jayaraman et al., 2010, South African National Blood Service, 2012, South African National Blood Service, 2009 and South African National Blood Service, 2008). However, risks are not completely eliminated and clinicians' knowledge of the risks associated with blood product administration appears to be poor with only 38.37% of respondents able to accurately quantify both the infectious and non-infectious risk. Just over half of the respondents identified the most common cause of an adverse reaction to blood product transfusion as clerical or laboratory error and a similar number appreciated that blood product administration is immunosuppressive. These findings are similar to a South African survey published by Irving in 1992 where 30-60% of respondents were able to appropriately quantify risks of blood product transfusion.
Poor awareness of costs was also demonstrated with approximately half of all respondents overestimating costs of FFP, underestimating costs of RCC and platelets and 73.84% underestimating the cost of a crossmatch. If clinicians were aware of the price of these products, for example, including the price on the ordering form, or the availability of the SANBS hamper system they might be more cognisant of only ordering blood when appropriate or use the hamper system if available.
A lack of understanding of the definitions and difference between a crossmatch and a type and screen was poor with only 48.26% defining a crossmatch correctly and 54.65% defining a type and screen correctly. A concerning 29.65% and 16.28% of respondents indicated they did not know these definitions at all. This may contribute to inappropriate ordering of blood products. In 2011, 30% of blood products that were ordered were not used at the study hospital (SANBS 2012).
It would seem that respondents' knowledge of RCC is better than that of platelets and FFP with 97.67% of respondents indicating an acceptable transfusion temperature, 71.51% indicating an appropriate haemoglobin level transfusion trigger of 7-8 g/dl and 90.69% stating that a single unit of RCC would raise the haemoglobin by 1-2 g/dl. The trend of accepting a lower haemoglobin, as in previous surveys, appears to be sustained and is reflected in this study while platelet and FFP knowledge was not as robust in keeping with other surveys (Hebert et al., 1998, Irving, 1992, Matot et al., 2004 and Nutall et al., 2003; Stehling et al. 1987). FFP knowledge appears to be the weakest. Guidelines of FFP transfusion triggers are very vague. Platelet trigger guidelines are more well defined but used less often. This may contribute to these results.
Attempts have been made to improve transfusion practices of blood products. Verlicchi (2010) is of the opinion that passive distribution of recommendations and guidelines are ineffective. Recently Joubert, Joubert, Raubenheimer, and Louw (2014) conducted a follow up audit of red cell concentrate utilisation at a South African hospital. Their 2010 audit, with subsequent interventions and training, seems to demonstrate a sustained improvement in practice and guideline adherence among clinicians in transfusing patients with chronic anaemias. A series of publications (Louw, 2014a, Louw, 2014b, Louw et al., 2013a and Louw et al., 2013b) discuss the status of transfusion education in South Africa outlining international challenges and local shortcomings. Again, the emphasis of patient safety is made and these authors suggest a formalised education programme to educate leaders in the field. The requirements of a formal education programme are debated and identified encompassing transfusion science, blood banking, ethics, haematology and clinical transfusion medicine. The differing education needs of specialists in transfusion medicine compared with clinicians occasionally involved in transfusion needs to be considered and implemented appropriately as these authors suggest. However, would the top-down approach allow for a change in practice for the clinicians actually involved at the transfusion-patient interface?
In this study respondents from Anaesthesiology performed better than their colleagues although overall results remain disappointing. Perhaps the better performance demonstrated by Anaesthesiology respondents can be attributed to observing a clinical response to administration of blood products acutely with haemodynamic monitoring. Among the clinician ranks, consultants performed significantly better than their junior colleagues. This is almost certainly due to experience.
The study design was contextual at the study hospital in selected disciplines and therefore the results may not be generalizable to other hospitals and disciplines. As the study was reliant on a self-administered survey it depended on the integrity of respondents not giving socially acceptable answers. The study also aimed to assess awareness of guidelines, however, awareness cannot be interpreted as adherence to these guidelines.
Regular formal education on risk, resources, blood product ordering and administration with appropriate feedback may be of value and are recommended. Regular audits and feedback are also recommended. A review of the annual transfusion summary is planned after discussion with the Local Blood Committee and Hospital Management. Alternative education methods will also be discussed. Attaching information on costs, definitions and risks to the SANBS ordering form may be of benefit to clinicians and patients.
Blood products are a scarce resource with inherent risks. Patient safety must be a priority. It is recommended that a similar survey should be conducted nationally.
5. Conclusion
Currently much emphasis is placed on patient safety, furthermore escalating costs and a declining supply of blood products require that clinicians rationalise their transfusion practices. The results from this study has shown that clinicians' knowledge of risks, resources, costs, ordering and return of blood products is poor, especially regarding FFP administration. Ensuring that clinicians have knowledge of appropriate blood product use in perioperative patients can make a meaningful contribution to patient safety and cost-effective care.
The study has addressed a relevant and particular knowledge deficit within the CHBAH and therefore is of value to the SANBS, management of the institution and clinical departments.
Research into reasons for the apparent poor knowledge and whether guidelines are actually implemented should be undertaken. Implementation and impact of any educational intervention must be followed up. The blood product transfusion seminar (SANBS 2011) is a potential area for intervention.
This study was done in partial fulfilment of a Masters of Medicine.
Acknowledgements
Professor Moosa Patel, Wits Health Sciences Library, South African National Blood Service.
References
Hebert, P. C., Wells, G., Martin, C., Tweeddale, M., Marshall, J., Blajchman, M., et al. (1998). A Canadian survey of transfusion practices in critically ill patients. Transfusion requirements in critical care investigators and the Canadian critical care trials group. Critical Care Medicine, 26(3), 482e487. Mar http://dx.doi.org/10.1097/00003246-199803000-00019. [ Links ]
Irving, G. (1992). A survey of the use of blood and blood components among South African anaesthetists working in teaching hospitals. South African Medical Journal, 82(5), 324e328. Nov. [ Links ]
Jayaraman, S., Chalabi, Z., Perel, P., Guerriero, C., & Roberts, I. (2010). The risk of transfusion-transmitted infections in sub-Saharan Africa. Transfusion, 50(2), 433e442. Feb http://dx.doi.org/10.1111/j.1537-2995.2009.002402.x. [ Links ]
Joubert, J., Joubert, S., Raubenheimer, J., & Louw, V. (2014). The long-term effects of training interventions on transfusion practice: A follow-up audit of red cell concentrate utilisation at Kimberley hospital, South Africa. Transfusion and Apheresis Science, 51(3), 25e32. http://dx.doi.org/10.1016/j.transci.2014.10.013]. [ Links ]
Louw, V. J. (2014a). The difference in scope of practice between a specialist in transfusion medicine and the clinician who deals with transfusion on an ad hoc basis. Transfusion and Apheresis Science, 51(3), 33e37. http://dx.doi.org/10.1016/j.transci.2014.10.008]. [ Links ]
Louw, V. J. (2014b). Determining the outcomes for clinicians completing a postgraduate diploma in transfusion medicine. Transfusion and Apheresis Science, 51(3), 38e43. http://dx.doi.org/10.1016/j.transci.2014.10.009]. [ Links ]
Louw, V. J., Nel, M. M., & Hay, J. F. (2013a). Factors affecting the current status of transfusion medicine education in South Africa. Transfusion and Apheresis Science, 49(3), 665e672, 2013 http://dx.doi.org/10.1016/j.transci.2013.05.003]. [ Links ]
Louw, V. J., Nel, M. M., & Hay, J. F. (2013b). Postgraduate education in transfusion medicine in the absence of formal residency training: Assessment of factors needed to develop and sustain a postgraduate diploma program. Transfusion and Apheresis Science, 49(3), 681e686. http://dx.doi.org/10.1016/j.transci.2012.07.003]. [ Links ]
Matot, I., Einav, S., Goodman, S., Zeldin, A., Weissman, C., & Elchalal, U. (2004). A survey of physicians' attitudes toward blood transfusion in patients undergoing cesarean section. American Journal of Obstetrics and Gynecology, 190(2), 462e467. Feb http://dx.doi.org/10.1016/j.ajog.2003.07.028. [ Links ]
Nutall, G. A., Stehling, L. C., Beighley, C. M., & Faust, R. J. (2003). Current transfusion practices of members of the American society of anesthesiologists: A survey. Anesthesiology, 99(6), 1433e1443. Dec http://dx.doi.org/10.1097/00000542-200312000-00028. [ Links ]
South African National Blood Service. (2008). In A. Bird, & L. Mpuntsha (Eds.), Clinical guidelines for the use of blood products in South Africa (4th ed.). Johannesburg: SANBS. [ Links ]
South African National Blood Service. (2009). Haemovigilance report. SANBS. [ Links ]
South African National Blood Service. (2011). Chris Hani Baragwanath Academic Hospital blood transfusion seminar evaluation 2011. Johannesburg: SANBS. [ Links ]
South African National Blood Service. (2012). Chris Hani Baragwanath Academic Hospital blood bank data 2011. Johannesburg: SANBS. [ Links ]
Stehling, L. C., Ellison, N., Faust, R. J., Grotta, A. W., & Moyers, J. R. (1987). A survey of transfusion practices among anesthesiologists. Vox Sanguinis, 52(1e2), 60e62, 1987 http://dx.doi.org/10.1111/j.1423-0410.1987.tb02990.x. [ Links ]
Turgeon, A. F., Fergusson, D. A., Doucette, S., Khanna, M. P., Tinmouth, A., Aziz, A., et al. (2006 Apr). Red blood cell transfusion practices amongst Canadian anesthesiologists: A survey. Canadian Journal of Anesthesia, 53(4), 344e352. [ Links ]
Verlicchi, F. (2010). Evaluation of clinical appropriateness of blood transfusion. Blood Transfusion, 8(2), 89e93. Apr http://dx.doi.org/10.2450/2009.0123-09]. [ Links ]
Vlaar, A. P., in der Maur, A. L., Binnekade, J. M., Schultz, M. J., & Juffermans, N. P. (2009). A survey of physicians' reasons to transfuse plasma and platelets in the critically ill: A prospective single-centre cohort study. Transfusion Medicine, 19(4), 207e212. Aug http://dx.doi.org/10.1111/j.1365-3148.2009.00928.x. [ Links ]
World Health Organisation research priority setting working group. (2008). Global priorities for research in patient safety (1st ed.). Geneva: WHO Patient Safety. [ Links ]
World Medical Assembly. (2008). Declaration of Helsinki: Ethical principles for medical research involving human subjects. [ Links ]
Received 26 January 2016
Accepted 24 June 2016
Available online 21 August 2016
Abbreviations: RCC, Red Cell Concentrate; FFP, Fresh Frozen Plasma; SANBS, South African National Blood Service; ANOVA, Analysis of Variance
Peer review under responsibility of Johannesburg University.
* Corresponding author. PO Box 821, Wendywood, 2144, South Africa.














