Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.17 no.4 Pretoria Dez. 2023
http://dx.doi.org/10.7196/SAJCH.2023.v17i4.1986
ARTICLE
Prevalence of malnutrition and its impact on outcomes in children with cancer in a South African setting
N GeddaraI; L MubaiwaII; R ThejpalIII; C HendricksIV
IFCPaed (SA), MMed; Department of Paediatrics, Inkosi Albert Luthuli Central Hospital, University of KwaZulu-Natal, Durban, South Africa
IIFCPaed (SA), MA Child Development; Department of Paediatric Neurology, Inkosi Albert Luthuli Central Hospital, University of KwaZulu-Natal, Durban, South Africa
IIIFCPaed (SA), Cert Paed Clinical Haematology (SA); Department of Paediatric Haematology/Oncology, Inkosi Albert Luthuli Central Hospital, University of KwaZulu-Natal, Durban, South Africa
IVMMed, Cert Clinical Haem (SA) Paed; Department of Paediatric Haematology/Oncology, Inkosi Albert Luthuli Central Hospital, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. Malnutrition in children with cancer is a common problem, especially in developing countries. Determination of nutritional status on admission and during treatment is crucial to reduce morbidity and mortality.
OBJECTIVES. This study reports the prevalence of malnutrition in children with cancer and its impact on outcomes.
METHODS. A retrospective study identified newly diagnosed children with cancer between January 2017 and June 2018 at Inkosi Albert Luthuli Central Hospital (IALCH), an academic hospital in South Africa (SA). The cohort comprised 139 patients. Demographic, anthropometric and outcome data were collected from the hospital electronic database. World Health Organization (WHO) criteria were applied to classify nutritional status. The impact of nutritional status on mortality, length of hospital stay and infection status in the first year was assessed.
RESULTS. The prevalence of malnutrition in our cohort of 139 patients was 31.7% (17.3% wasted, 7.2% stunted and 7.2% wasted and stunted). There was a higher incidence of wasting in children with solid tumours than in those with haematological malignancies, but this was not statistically significant (21.2% v. 7.7%, respectively, p-value 0.242). No significant difference in early mortality, length of hospital stay or rate of infection was noted in malnourished patients compared with well-nourished patients.
CONCLUSION. The prevalence of malnutrition in our cohort with cancer was high but not associated with an increased risk of mortality, hospital stay or infection. A larger sample size using a combination of arm- and weight/height-based anthropometry is recommended to confirm these findings.
Malnutrition is defined as 'a state in which a deficiency (or excess) of energy, protein, and other nutrients causes a measurable adverse effect on the body form and function[1] and is a major problem to consider in children with cancer. Its clinical relevance has been accepted widely by professionals. In these patients, the cause of malnutrition is multifactorial and includes disease-related or more commonly treatment-related factors. Chemotherapy may affect nutritional status as a consequence of its side-effects (nausea, vomiting, mucositis and diarrhoea) and there is a strong correlation between malnutrition and intensity of treatment regimens.[2]
The reported prevalence of malnutrition in children with cancer varies widely and ranges from 6% to 50%.[3] This figure depends on the type of malignancy (less than 10% in acute lymphoblastic leukaemia (ALL) and up to 50% in advanced neuroblastoma),[4] the stage of the disease and methods used for nutritional assessment. [4] The higher prevalence of malnutrition in children with solid intra-abdominal tumours may be due to mass effect on the bowel (partial or complete obstruction) and systemic effects of the tumour. [5] The true prevalence of malnutrition for the different cancer types, however, remains elusive after more than three decades of research. The paucity of studies, small sample sizes and use of different methods to assess nutritional status, make it difficult to compare data and establish a true reflection of the problem. Additionally, most of the studies have focused on leukaemia and little is known about solid tumours, including brain tumours.[6]
The highest childhood cancer burden is borne by low- and middle-income countries (LMICs), where approximately 80% of children and adolescents with cancer live.[7] The prevalence of malnutrition in these children averages 50%,[4] and is compounded by lack of access to healthcare and a decreased chance of cure; this is due to a multitude of reasons such as late presentation, inadequate supportive care, abandonment of treatment, and sub-optimal healthcare delivery systems.[4] It is important to identify and monitor malnutrition in paediatric cancer patients, as there is a reported association with decreased survival rates, diminished response and tolerance to treatment (chemotherapy and radiotherapy), prolongation of hospital stay, increased rates of readmission and decreased quality of life.[4, 7, 8]
The present study reports the prevalence of malnutrition in children newly diagnosed with cancer, admitted to an academic hospital in South Africa (SA), and describes the impact of nutritional status on early outcomes in our setting.
Methods
A retrospective study was performed at the Paediatric Haematology Oncology unit at an academic hospital in SA. All children younger than 13 years of age in whom a new malignancy was diagnosed between January 2017 and June 2018 and managed in our hospital were included. Only the first hospitalisation after diagnosis was included in the analysis. No patients had received chemotherapy prior to admission. Data were collated by author NG from patient clinical records extracted from the hospital electronic database (Meditech, Westwood, Massachusetts, USA). For each patient, demographic information, diagnosis, nutritional status, human immunodeficiency virus (HIV) status, presence of infection during the hospital stay, length of hospital stay, and outcome were recorded.
Nutritional status was assessed based on recorded medical and dietary history including physical examination, available anthropometric measurements (height, weight, body mass index (BMI), Z-scores), mid-upper arm circumference (MUAC)) and serum albumin. BMI was calculated as weight in kg divided by square meters of the subject's height (kg/m2). Weight was measured in kg using a professional balance electronic scale, and height was measured in centimeters (cm) using a stature meter or infantometer for infants. If the child was less than two years old, weight for height (WFH) was used whereas, in older children, BMI was used for assessment of wasting.
All the values were plotted on standardised World Health Organization (WHO) growth charts and categorised as per WHO guidelines.[9] Patients were categorised as normal (weight for height Z-score between -2 and +2 or BMI Z-score between -2 and +2 and height for age Z-score between -2 and +2), wasted (weight for height Z-score <-2 or BMI Z-score <-2), stunted (height for age Z-score <-2) or wasted and stunted. MUAC was measured in centimeters (cm) with a non-stretch, flexible measuring tape and interpreted thus: for children under 60 months normal nutrition MUAC >13.5 cm, mild malnutrition MUAC 12.5 - 13.5 cm, moderate malnutrition MUAC 11.5 - 12.4 cm and severe malnutrition MUAC <11.5 cm. A normal serum albumin was defined as a level >35g/L and lower levels were interpreted in the context of other anthropometric measures.
The unit has a dedicated dietician who does ward rounds daily to identify in particular high-risk patients in whom early nutritional interventions such as nasogastric feeding can be initiated. Regular weights were done for all the patients at least weekly.
Infection was defined as at least one of the following criteria: a positive blood culture, a high procalcitonin (PCT), antibiotics received or a clinical assessment of infection. All data regarding infections reflect the first hospital admission only. The Department of Microbiology advised on likely contaminants on blood cultures, and these were excluded in the analysis. Outcome measures included the impact of nutritional status on length of hospital stay, presence and severity of infections and death. Early death (within the first hospital admission period) as well as later death (from diagnosis time to data collection time) were included.
All statistical analysis was conducted using R-3.6.3 software. Descriptive statistics are in the form of frequency tables. The associations between categorical variables were determined with the aid of Fisher's exact test. The study was approved by the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (ref. no. BE008/19).
Results
A total of 139 children with a newly diagnosed malignancy were included in this study. The patients' characteristics are shown in Table 1. Male patients outnumbered females (n=80, 57.6%). The median age at diagnosis was 4.3 years (range 6 months - 11.3 years). The patients between 1 and 5 years of age made up 49.6% of cases. Patients with solid tumours outnumbered those with haematological malignancies, making up 71.9% of the patients (data for sub-categories of solid and haematological subcategories are available as supplementary data). The most common diagnosis was B-cell ALL accounting for 13.8% of all cases, followed by retinoblastoma and Wilms' tumour (nephroblastoma) at 12.3% each. Ten patients (7.2%) were HIV seropositive.
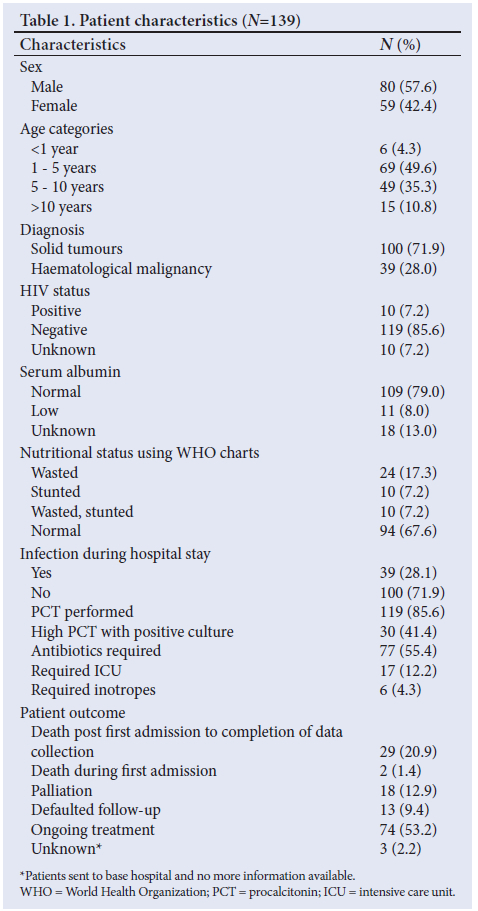
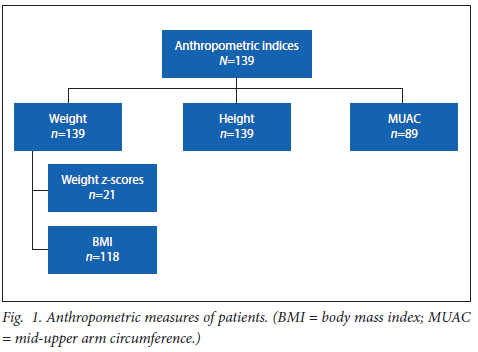
Forty-four patients (31.7%) were malnourished, of whom 24 were wasted (17.3%) and 10 each (7.2%) stunted and wasted-and-stunted. MUAC was not recorded in 89 (64%) patients and therefore further analysis using this measure was not performed. Weight was measured at two time points (first hospital admission and discharge); however, a negligible difference was found between these two time points. Serum albumin was normal in 109 (79%) of the patients and low in 11 (8%) patients. Thirty-nine patients had confirmed infection. All but two patients, who died, were discharged post their first hospital admission. The outcomes post first hospital admission to the end of the study period include a mortality rate of 20.86% (n=29/139) with a further 18 patients (12.94%) requiring palliation. Fig. 1 reflects anthropometric measures for the patients. Weight Z-score was used for children less than 5 years and BMI was used for those older than 5 years. MUAC was measured in only 89 patients.
In Table 2, nutritional status is correlated with diagnosis and survival. Survival data reflect those from throughout the 18 months of data collection. There was higher wasting among children with solid tumours compared with those with haematological malignancies, at 21.2% (n=21/99) v. 7.7% (n=3/39), respectively, but this was not statistically significant (p-value 0.24).
A mortality rate of 22.5% was found in our cohort. Nutritional status did not have an impact on survival (p-value 0.705) although a slightly higher rate of malnutrition was found in those who died (n=10/31=32.2%) compared with those who survived (n=18/70=25.7%)
Table 3 illustrates the correlation of nutritional status and infection. Malnutrition was not associated with an increased risk of infection. Thirty-eight patients (27.5%) had a positive blood culture, of whom 15 (39%) had malnutrition. Abnormal PCT levels were also not clearly associated with malnutrition, with 32% and 38% of malnourished patients contributing to high and normal values, respectively. Using the presence of a positive blood culture as sepsis confirmation, we found only a marginally higher rate of positive cultures in malnourished patients. With all malnutrition categories combined, the rates were 34% (n=15/44) compared with well-nourished patients at 24.5% (n=23/94), which was not statistically significant.
Durations of hospital stay varied widely between patients, ranging from 1 to 134 days, with the median duration of hospital stay being 23 days. (Data figures available in supplementary data.) There was no statistically significant difference between the malnourished and well-nourished groups.
Discussion
A paucity of data exists on malnutrition in children with cancer in SA and this impedes the development of guidelines to aid in the unique management requirements of these children. Our study reports 139 new cases of a malignancy over the course of 18 months. Patients with solid tumours outnumbered those with haematological malignancies, making up 71.7% of the patients, in keeping with a study done in Italy, where this number was 73%,[10] but higher than reported figures from the USA, where 51% of patients had solid tumours.[11] We have to consider whether the lower rate is not due to a large proportion of children in our province of KwaZulu-Natal (KZN) living below the poverty line, which may be a proxy for poor access to healthcare and therefore decreased numbers of patients with cancer presenting to a health facility.[12]
The prevalence of malnutrition in our cohort was 31.7% (17.3% wasted, 7.2% stunted and 7.2% wasted-and-stunted). Males were affected marginally higher than females (57.6 % and 42.4%, respectively) and similar results were found by Stones et al.[13] in SA, with males making up 53.5% of that cohort. The overall prevalence is higher than that of primary malnutrition in SA children without cancer (9.3% undernourished and 2.6% wasting[14]) but lower than stunting in children without cancer, which is reported as 22.9%.[14] In a study from Malawi using the same measurements, Israels et al. '[15] reported 17.2% wasted and 44.5% stunted patients.[15] The rate of stunting in normal Malawian children is high at 45%, which reflects the rate of chronic malnutrition in the general paediatric population, while 22% were underweight and 5% were wasted.'151 In Switzerland, the prevalence of malnutrition in paediatric cancer patients was much less at 5.8%, using weight/height-based anthropometry.[2] The difference in the prevalence of malnutrition between HICs and LMICs could be due to LMICs having an already high prevalence of primary malnutrition compounded by the diagnosis of cancer. Additionally, children are more likely to present at later stages of their malignancy than in HICs, allowing for cancer-associated wasting.[15]
Unfortunately, 64% of our cohort did not have MUACs recorded. This may have influenced the results, particularly in patients where the BMI could have been affected by the presence of a large mass. In Malawi, by using arm anthropometry (AMA=arm muscle area), the documented prevalence of malnutrition was 55.1% and 59% when using both triceps skinfold (TSF) and MUAC, respectively.[15] These findings are similar to those in Casablanca, Morocco, where 50% of patients were classified as malnourished using TSF, 39% using MUAC and only 33% when using weight/ height-based anthropometry.[16] These studies are evidence for the need for the incorporation of this anthropometric measure in the accurate determination of nutritional assessment in children with cancer. Indeed, arm anthropometry rather than weight/height-based measurements has been advised by many as it is independent of ethnicity and unaffected by tumour weight.[5,15]
Serum albumin is the most widely used biochemical marker for nutritional assessment. It may, however, be affected by hydration status and liver function.[17] In our cohort, we found serum albumin to be a poor marker for the detection of malnutrition, with low values found in only 8% of patients. These findings are corroborated by Tazi et al.[16]in Casablanca and Murphy et al.[17,20]in Australia, with the latter concluding that serum albumin was not a valid indicator of malnutrition in children with cancer.
There are numerous studies which highlight the association between risk of infection and malnutrition.[18,19] In the present study, there were no significantly higher rates of positive cultures and raised PCT in malnourished children. This finding contrasts with a study from Bangladesh, where malnourished children with ALL were 2 - 3 times more susceptible to infection.[20] In addition, a study including 20 patients with leukaemia showed a statistically significant and inverse correlation between nutritional status and infection rate but no similar correlation in patients with solid tumours.[21] In Nicaragua, researchers also showed a significant association between malnutrition and severe infection.[19] At our facility, most patients who are newly diagnosed and those who are at an increased risk of infection, have access to isolation facilities. Antibiotic protocols are in place and rapidly escalated if necessary. These factors might have played a role in these results.
In the present study, malnourished patients did not have prolonged hospital stays as compared with their well-nourished counterparts. Yazbeck et al.[22]in Lebanon reported similar results in children diagnosed with ALL. Length of hospital stay depends on many factors, viz. (i) the cancer diagnosis and chemotherapy protocol duration; (ii) chemosensitivity of each tumour type; (iii) chemotherapy tolerance; (iv) mass effect of the primary tumour on the gastrointestinal tract or respiratory airway; and (v) disease stage and metastasis.'2,4,51 All these factors may lead to prolongation of hospital stay in both well-nourished as well as malnourished groups. Further studies with larger cohorts of patients are required in this field to assess the effect of malnutrition on length of hospital stay in children with cancer.
Malnourished patients did not have worse outcomes in our study compared with well-nourished patients. In a study by Sala et al.,[23] survival was recorded to be worse in malnourished children, with 65% of adequately nourished patients being alive at two years from diagnosis compared with only 48% of severely malnourished patients.[23] This finding is supported by Loeffen et al.[18] who also showed worse survival in malnourished patients. Pedrosa et al.,[24] however, documented no significant difference in survival rate between malnourished and well-nourished groups. Our results are in keeping with the latter. We propose that the availability of a dedicated dietician to support malnourished children daily in the ward was a major advantage which mitigated the impact of malnutrition and was likely to have had a positive effect on patient outcomes.[25] Additionally, the early initiation of nasogastric feeding in patients with poor appetites is routine practice, as is regular weighing.[25]
Most of the patients were HIV negative in our cohort (only 7.2% were positive). HIV was therefore not a factor that influenced the outcomes of our patients.
Limitations of the study were that MUAC was recorded in only a small proportion of patients and might have influenced the data, had all the values been known. Furthermore, the sample size was small, making it difficult to draw firm conclusions from the data, and data were only collected for the first admission.
Conclusion
The data reported in the present study confirm that the prevalence of malnutrition in children with cancer is high. Patients with solid tumours were more likely to be wasted compared with those with haematological malignancies. Malnutrition in this study was not associated with an increased risk of infection, mortality or hospital stay. We propose that the early and aggressive interventions to support malnourished patients might have played a role in this study. The advantage of this study was the heterogeneous cohort with different cancer types and stages as well as different courses and treatment. We recommend further study in this field, with a larger sample size using both arm and weight/height-based anthropometry for a better understanding of malnutrition in children with cancer and its impact on the outcome.
Declaration. None.
Acknowledgements. We thank the University of KwaZulu-Natal and Mr Partson Tinarwo (university biostatistician) for his help in the analysis; haematology/oncology consultant Dr Beverley Neethling; and hospital management at IALCH for allowing electronic access to the patient medical files.
Author contributions. Concepualisation: NG, CH, LM, RT. Data collection: NG. Data analysis: NG, PT. Writing the original draft: NG, CH, LM, RT. Writing the review and editing: NG, CH, LM, RT. Supervision: LM, RT, CH.
Funding. None.
Conflicts of interest. There are no conflicts of interest.
References
1. Lochs H, Allison SP, Meier R, et al. Introductory to the ESPEN Guidelines on Enteral Nutrition: Terminology, Definitions and General Topics. Clin Nutr 2006;25(2):180-186. https://linkinghub.elsevier.com/retrieve/pii/S0261561406000513 [ Links ]
2. Zimmermann K, Ammann RA, Kuehni CE, De Geest S, Cignacco E. Malnutrition in pediatric patients with cancer at diagnosis and throughout therapy: A multicenter cohort study. Pediatr Blood Cancer 2013;60(4):642-649. http://doi.wiley.com/10.1002/pbc.24409 [ Links ]
3. Ladas EJ, Sacks N, Meacham L, et al. A multidisciplinary review of nutrition considerations in the pediatric oncology population: A perspective from children's oncology group. Nutr Clin Pract 2005;20(4):377-393. http://doi.wiley.com/10.1177/0115426505020004377 [ Links ]
4. Sala A, Pencharz P, Barr RD. Children, cancer, and nutrition - a dynamic triangle in review. Cancer 2004;100(4):677-687. http://doi.wiley.com/10.1002/cncr.11833 [ Links ]
5. Oguz A, Karadeniz C, Pelit M, Hasanoglu A. Arm anthropometry in evaluation of malnutrition in children with cancer. Pediatr Hematol Oncol 1999;16(1):35-41. [ Links ]
6. Brinksma A, Huizinga G, Sulkers E, et al. Malnutrition in childhood cancer patients: A review on its prevalence and possible causes. Crit Rev Oncol Hematol 2012;83(2):249-275. https://linkinghub.elsevier.com/retrieve/pii/S1040842811002770 [ Links ]
7. Peccatori N, Ortiz R, Rossi E, et al. Oral nutritional supplementation in children treated for cancer in low- and middle-income countries is feasible and effective: The experience of the Children's Hospital Manuel De Jesus Rivera 'La Mascota' in Nicaragua. Mediterr J Hematol Infect Dis 2018;10(1):e2018038. https://www.mjhid.org/index.php/mjhid/article/view/2018.038 [ Links ]
8. Bauer J, Jürgens H, Frühwald MC. Important aspects of nutrition in children with cancer. Adv Nutr 2011;2(2):67-77. https://academic.oup.com/advances/article/2/2/67/4591585 [ Links ]
9. World Health Organization. Nutrition Landscape Information System (NLIS) Interpretation Guide [Internet]. Nutrition Landscape Information System (NLIS). 2019. p. 134. https://apps.who.int/iris/bitstream/handle/10665/332223/9789241516952-eng.pdf [ Links ]
10. Triarico S, Rinninella E, Cintoni M, et al. Impact of malnutrition on survival and infections among pediatric patients with cancer: A retrospective study. Eur Rev Med Pharmacol Sci 2019;23(3):1165-1175. [ Links ]
11. Pietsch JB, Ford C. Children with cancer: Measurements of nutritional status at diagnosis. Nutr Clin Pract 2000;15(4):185-188. [ Links ]
12. Meintjes H, John-Langba L. Demography of South Africa's children. Population 2007. http://www.ci.uct.ac.za/sites/default/files/image_tool/images/367/Child_Gauge/Sout h_African_Child_Gauge_20072008/demography.pdf [ Links ]
13. Stones DK, De Bruin GP, Esterhuizen TM, Stefan DC. Childhood cancer survival rates in two South African units. S Afr Med J 2014 ;104(7):501. http://www.samj.org.za/index.php/samj/article/view/7882 [ Links ]
14. Bourne LT, Hendricks MK, Marais D, Eley B. Addressing malnutrition in young children in South Africa. Setting the national context for paediatric food-based dietary guidelines. Matern Child Nutr 2007;3(4):230-238. http://doi.wiley.com/10.1111/j.1740-8709.2007.00108.x [ Links ]
15. Israels T, Chirambo C, Caron HN, Molyneux EM. Nutritional status at admission of children with cancer in Malawi. Pediatr Blood Cancer 2008;51(5):626-628. https://onlinelibrary.wiley.com/doi/10.1002/pbc.21697 [ Links ]
16. Tazi I, Hidane Z, Zafad S, et al. Nutritional status at diagnosis of children with malignancies in Casablanca. Pediatr Blood Cancer 2008;51(4):495-498. http://doi.wiley.com/10.1002/pbc.21689 [ Links ]
17. Murphy AJ, White M, Davies PSW. The validity of simple methods to detect poor nutritional status in paediatric oncology patients. Br J Nutr 2009;101(09):1388. http://www.journals.cambridge.org/abstract_S0007114508076241 [ Links ]
18. Loeffen EAH, Brinksma A, Miedema KGE, de Bock GH, Tissing WJE. Clinical implications of malnutrition in childhood cancer patients-infections and mortality. Support Care Cancer 2015;23(1):143-150. http://link.springer.com/10.1007/s00520-014-2350-9 [ Links ]
19. Pribnow AK, Ortiz R, Báez LF, Mendieta L, Luna-Fineman S. Effects of malnutrition on treatment-related morbidity and survival of children with cancer in Nicaragua. Pediatr Blood Cancer 2017;64(11):e26590. http://doi.wiley.com/10.1002/pbc.26590 [ Links ]
20. Hafiz MG, Mannan MA. Nutritional status at initial presentation in childhood acute lymphoblastic leukemia and its effect on induction of remission. Mymensingh Med J 2008;17(2 Suppl):46-51. [ Links ]
21. Taj MM, Pearson ADJ, Mumford DB, Price L. Effect of nutritional status on the incidence of infection in childhood cancer. Pediatr Hematol Oncol 1993;10(3):283-287. http://www.tandfonline.com/doi/full/10.3109/08880019309029498 [ Links ]
22. Yazbeck N, Samia L, Saab R, et al. Effect of malnutrition at diagnosis on clinical outcomes of children with acute lymphoblastic leukemia. J Pediatr Hematol Oncol 2016;38(2):107-110. [ Links ]
23. Sala A, Rossi E, Antillon F, et al. Nutritional status at diagnosis is related to clinical outcomes in children and adolescents with cancer: A perspective from Central America. Eur J Cancer 2012;48(2):243-252. https://doi.org/10.1016/j.ejca.2011.06.006 [ Links ]
24. Pedrosa F, Bonilla M, Liu A, et al. Effect of malnutrition at the time of diagnosis on the survival of children treated for cancer in El Salvador and Northern Brazil. J Pediatr Hematol Oncol 2000;22(6):502-505. http://journals.lww.com/00043426-200011000-00005 [ Links ]
25. Holzinger TT, Shaik AS, Hadley GP. The role of nutritional intervention in children with nephroblastoma. S Afr J Clin Nutr 2007;20(3):96-99. http://www.tandfonline.com/doi/full/10.1080/16070658.2007.11734133 [ Links ]
 Correspondence:
Correspondence:
N Geddara
nagibgeddara@gmail.com
Accepted 3 August 2022














