Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.17 n.3 Pretoria Sep. 2023
http://dx.doi.org/10.7196/sajch.2023.v17i3.1997
RESEARCH
Profiles of patients with myelomeningocele admitted to the neonatal unit at Universitas Academic Hospital in Bloemfontein, South Africa
D N PillayI; P MoodleyII
IFCPaed (SA); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
IIFCPaed (SA), Cert Neonatol (SA); Department of Paediatrics and Child Health, Faculty of Health Sciences, University of the Free State, Bloemfontein, South Africa
ABSTRACT
BACKGROUND: Myelomeningocele (MMC) is a common neural tube defect with significant sequelae. There are limited recent data on the mortality and morbidity of MMC in South Africa (SA
OBJECTIVE: To describe the outcomes and characteristics of patients with MMC admitted to the neonatal unit at Universitas Academic Hospital (UAH) in Bloemfontein, SA
METHODS: A retrospective, descriptive study which included 53 patients with MMC admitted to the neonatal unit between 1 January 2017 and 31 December 2019 was conducted. Electronic patient records were reviewed. Data included outcomes, length of stay, complications and maternal and infant characteristics
RESULTS: The inpatient mortality rate was 11.3% (n=6/53). The median length of stay was 18 days. Notable MMC complications included hydrocephalus (88.7%; n=47/53), Chiari malformation type II (44.7%; n=21/47), lower-limb paralysis (84.9%; n=45/53), lower-limb deformities (60.4%; n=32/53), meningitis (52.8%; n=28/53), neuropathic bladder (37.7%; n=20/53) and loss of anal tone (41.5%; n=22/53). MMC repair was performed in 62.2% (n=33/53) and 27.3% (n=9/33) developed complications. Wound sepsis and breakdown were the most common complications (18.2%, n=6/33), with a median 8 days to complications. Antenatal sonar was not performed in 62% (n=31/50) of cases. MMC was detected antenatally in 20% of cases
CONCLUSION: The inpatient mortality rate in this study was lower than the mortality rates reported in other low- and middle-income countries although significant morbidity was identified. A lack of quality antenatal care and access to antenatal sonars were barriers to early detection of MMC. Other healthcare system infrastructural failures may be contributory, which highlights the need for ongoing inter-sectoral collaboration for prevention, early detection and management of MMC to improve patient outcomes
Myelomeningocele (MMC) is the most commonly occurring congenital central nervous system malformation compatible with life but is associated with significant burden of disease, specifically with regards to morbidity and economic impact.[1-6] The multifactorial aetiology is well documented across the literature.[1,4,5] Management strategies prioritise prevention with optimal antenatal care, teratogen avoidance and periconception folic acid supplementation.[1,2,4-7] Antenatal sonograpic screening may be performed from as early as 11 - 14 weeks' gestation with detection rates of 80 - 90%, dependent on operator experience.[4] Fetal magnetic resonance imaging (MRI) is used as an adjuvant investigation to assist with postnatal surgical planning or in cases of diagnostic uncertainty.[8] Maternal serum alpha fetoprotein (AFP) levels may be useful in centers where access to reliable imaging is not readily available. Amniotic fluid AFP and acetylcholinesterase may also be useful.[5,8] Failing which, the evidence advocates for early closure of the spinal defect.[4,7,9] More recent literature indicates improved outcomes with fetal surgery, a prospect that presently remains distant to the South African (SA) public sector. [4,5,7]
Although food fortification has decreased the SA prevalence of MMC by 41.6%, the recent incidence of myelomeningocele is estimated at 9.9 per 10 000 live births and may be higher in rural areas of the country.[3-5,7,10,11] Two recent SA studies reported neonatal mortality rates of 7% and 9%.[11,12] Studies in other low- and middle-income countries (LMIC) quote MMC neonatal mortality rates between 25% and 29%.[11,13] The Chiari malformation type II is ubiquitous in MMC patients and leads to progressive herniation and resultant respiratory failure during a Chiari crisis[1,4,8] - this is a non-preventable cause of mortality.[4] A Nigerian study[4] noted that ~15% of children with MMC would die by 5 years of age due to neuropathic bladder-associated complications. In the presence of hindbrain dysfunction this figure could rise to 35%. MMC morbidity is significant and attributed to cognitive impairment, increased seizure propensity, lower-limb paralysis, spinal and limb deformities requiring orthopaedic intervention, bladder and bowel dysfunction, a lifelong increased risk for infections and treatment-related complications.[1,2,4,7] Hydrocephalus becomes evident within the first week of life in up to 85% of cases. Approximately 80% of neonates born with MMC require ventriculoperitoneal shunting to prevent further neurological and cognitive impairment.[4,7] Current guidelines advise closure ideally within 24 hours and not later than 72 hours.[2,4,7] Despite timely postnatal closure, complications may occur.[14,15] An Indian study[9] identified important postoperative complications: cerebrospinal fluid leak; meningitis; shunt infection; and worsening lower-limb paralysis. Delayed MMC repair is associated with a higher meningitis risk.[7]
There are limited recent data on the mortality, morbidity, rate of postoperative complications, maternal characteristics and infant characteristics of MMC infants in SA. Data from other LMIC demonstrate significant morbidity and mortality which constitute part of the burden of disease.[4,9,13] Universitas Academic Hospital (UAH) offers the only neurosurgical service for the Free State and Northern Cape provinces, as well as Lesotho, for management of complicated MMCs. Thus the UAH neonatal unit provides an adequate sample population to describe and provide data to bridge the identified gaps.
Methods
A retrospective descriptive study was conducted at the UAH neonatal unit in Bloemfontein, SA. Admissions to the unit are entered into a Microsoft Excel spreadsheet (Microsoft Corp., USA), as well as the unit's admission bo ok. Patients who were admitted to the neonatal unit with MMC were included. MMC patients admitted to the paediatric ward, paediatric intensive care unit and neurosurgical ward were not included. The study period was from 1 January 2017 to 31 December 2019. Data were captured using an anonymised collection sheet prior to entry into a Microsoft Excel spreadsheet. Statistical analysis was performed by the Biostatistics Department at the University of the Free State. All data were analysed descriptively, including medians and interquartile ranges (IQRs) for non-normally distributed continuous data, and frequencies and percentages for categorical data.
Ethics approvals
Ethics approval was obtained from the University of the Free State's Health Sciences Research Ethics Committee (ref. no. UFS-HSD2020/0316/3006) and from the Free State Department of Health.
Results
A total of 53 patients were included in the present study and the inpatient mortality rate was 11% (n=6/53). Early neonatal death cases were palliated owing to major congenital malformations, i.e. trisomy 18 and congenital diaphragmatic hernia. Chiari crisis occurred in 4 of the deaths and 2 of those cases had developed sepsis. Surgical correction was performed in 50% (n=3/6) of the patients who died. Two cases were not repaired owing to the decision to palliate based on concomitant major malformations. The third case was not repaired owing to early contamination of the MMC that progressed to meningitis. Most deaths (67%; n=4/6) were out-born referrals from regional hospitals (Table 1).
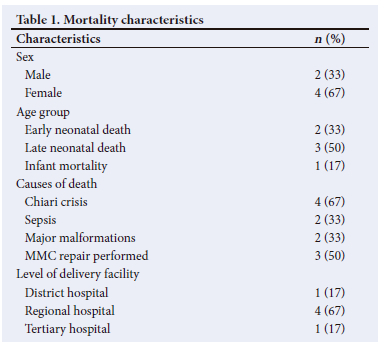
The median (IQR) length of stay was 18 (11 - 34) days. Hydrocephalus was diagnosed by cranial sonar in 89% (n=47/53) of the patients at a median (IQR) age of 2 (2 - 5) days. Further imaging was performed in 26% (n=12/47) to identify associated intracranial pathology. Lower-limb paralysis occurred in 85% (n=45/53) of patients, while 60% (n=32/53) had various limb deformities. Loss of anal tone was described in 42% (n=22/53). A neuropathic bladder was clinically noted in 38% (n=20/53). Renal sonography was performed on 74% (n=39/53) of patients to identify associated renal anomalies and 15% (n=6/39) received a micturating cystourethrogram. The associated malformations are detailed in a supplementary table (https://www.samedical.org/file/2070).
MMC repair was performed during the neonatal admission in 62% (n=33/53) of patients and complications occurred in 27% (n=9/33). The median (IQR) time for postoperative complications was 8 (7 - 14) days. A VP shunt was inserted in 42% (n=22/53), of which 36% (n=8/22) developed shunt-related complications with overlap of complications in some patients. Meningitis was observed in 53% (n=28/53) of patients, of which 50% (n=14/28) had a positive culture. Pre-operative pus swabs of the MMC were performed in 61% (n=31/53) of cases and 39% (n=12/31) of those swabs were culture-positive, with two polymicrobial specimens. Nine patients had a postoperative swab performed with a variety of organisms cultured but there were no polymicrobial specimens (Table 2).
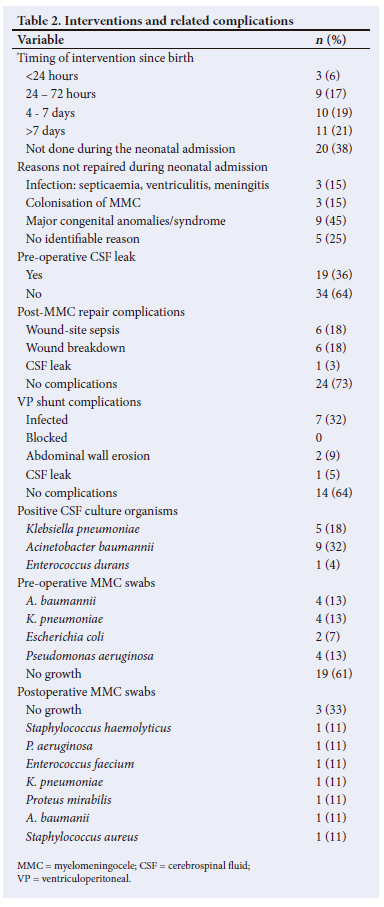
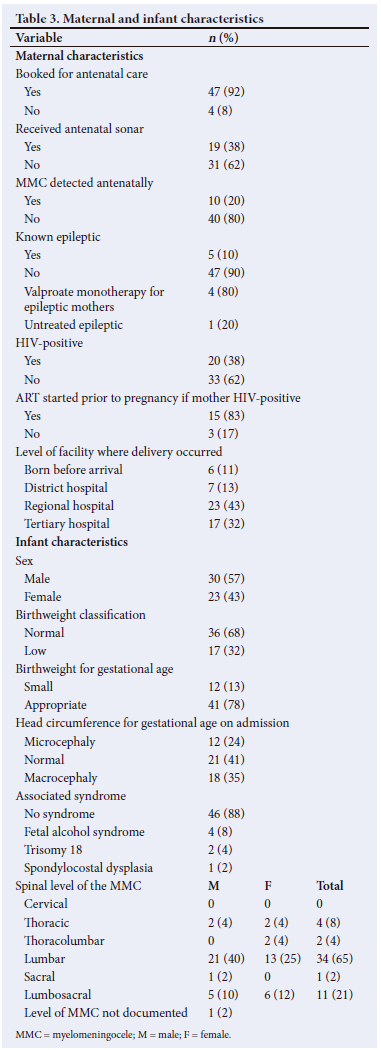
The median (IQR) maternal age was 29.5 (23 - 34) years. Mothers were referred from various districts in the Free State, with a preponderance of participants from the Mangaung District (24%; n=13/53). Furthermore, 15% (n=8/53) of the cases were referred from outside the Free State (7 cases from Lesotho and 1 case from the Northern Cape). The median (IQR) gestational age at booking was 21.5 (15.5 - 27.5) weeks. Maternal MRI identified MMC in 3 cases, all of whom had prior fetal anomaly sonography. None of the mothers had received a serum AFP testing or an amniocentesis. HIV infection was observed in 37.7% (n=20/53) of mothers. Only an efavirenz-based regimen was used. None of the mothers had used co-trimoxazole and none of them were diabetic or obese. The median gestational age at delivery was 38 (37 - 38) weeks, with a median (IQR) birthweight of 2 770 (2 260 - 3 100) g and a male-to-female ratio of 1.3:1. Maternal and infant characteristics are summarised in Table 3.
Discussion
The inpatient mortality rate was lower in the present study (11.3%) compared with studies from other LMICs, which reported mortality rates of ~25%.[11,13] Although the inpatient mortality rate of the present study was higher than other SA studies (7% and 9%), comparison was difficult as mortality characteristics were not provided in previous studies.[11,12] One explanation for the observed difference may be that those studies only included mortalities during the neonatal period while our study included patients between the neonatal and early infancy periods. The decision to describe the inpatient mortality rate in this study, thus including early infancy deaths, was informed by the existing body of evidence that MMC patients have a protracted length of stay.[2,16]
Chiari crisis was contributory to all mortalities, in keeping with the trend in the literature of a higher mortality risk in patients with hindbrain herniation.[4] Half of the patients who died (n=3/6) had received surgical repair of the MMC. However, all the repairs occurred after the first 24 hours. Higher rates of wound sepsis (18.2%), meningitis (52.8%) and shunt infection (31.8%) were observed in our study compared with studies in other LMICs (7%, 19% and 9.8%, respectively).[4,9] In the current study, 63.6% of patients underwent MMC repair after the optimal 72-hour window period, with resultant MMC colonisation as evidenced by pre-operative culture-positive swabs.[4,9] The literature demonstrates improved mortality and morbidity when surgical closure is performed within the first 24 hours of life.[2,4,7] Logistical difficulties with patient referral from elsewhere in the province (supplementary Fig. 1: https://www.samedical.org/file/2071) to a centralised tertiary centre for repair, as well as across the border from Lesotho, presented barriers to timeous intervention and highlights the need for intersectoral collaboration to improve MMC patient outcomes.
The number of patients who had surgical repairs in the present study was lower compared with other SA studies.[11] Reasons for lower repair rates in the present study included the presence of major congenital anomalies and syndromes that led to palliative decisions (45%), pre-operative MMC colonisation (15%) and early-onset sepsis (15%). Improved antenatal care practices and the previously mentioned logistical difficulties may have been contributory in this regard. However, in 25% of cases who did not undergo surgical repair, no reason could be identified. Infrastructural constraints may be contributory but further studies are needed to investigate the causes for the difference in repair rates. The postoperative wound sepsis rate in the present study was lower than rates reported in other SA studies (30.1%).[11] The difference could be explained by lower repair rates in the current study, the subjective nature of a clinical wound assessment and a small sample size.
During the course of the present study, 42% of the patients underwent surgery within 72 hours of life, which was comparable with 43% of patients in an upper-middle income setting in Turkey.[16] However, the median length of stay in the current study (18 days) was lower than that of the mean length of stay for the Turkish study.[16] There are limited SA data on neonatal length of stay for MMC patients and comparison is difficult, as the definition for prolonged length of stay is controversial.[11]
Comparative rates of hydrocephalus were observed between our study (88.7%) and the literature (85%).[4] VP shunt insertion rates were higher in the current study (41.5%) compared with previous SA studies (28%). However, both studies described lower rates than the existing evidence base of ~85%.[4,11] This could be explained by resource and infrastructural constraints in the SA setting.
The rate of Chiari type II malformation in the current study (44.7%) was lower than what has been reported elsewhere (>75%).[4,8] The literature reports that MRI is superior to cranial ultrasound for evaluation of the posterior fossa.[8,17] Owing to limited availability and long waiting times for MRI, patients admitted to the UAH neonatal unit with MMC receive a cranial sonar to expedite intracranial visualisation. MRI is performed on an outpatient basis. As these reports were not included during the neonatal admission , this may account for the spuriously lower rates.
Our study demonstrated a higher rate of lower-limb paralysis (84.9%) compared with a previous SA study (78.6%).[11] A possible reason for the observed difference may be the difference in timing to repair, which has been reported in the literature to affect motor outcomes adversely.[4,7]
Although most mothers (92.2%) booked for their antenatal assessments, 62% of them did not receive an antenatal sonar. The World Health Organization recommends antenatal sonars in every pregnancy.[18] Limited resources and other infrastructural constraints pose persistent challenges to accessing antenatal sonars and achieving this benchmark for antenatal care. In the absence of access to antenatal sonars, evidence indicates that the sensitivity of serum AFP retains viability for antenatal MMC detection.[4,5,8] The current study demonstrates no usage of serum AFP or amniocentesis during antenatal assessments. This represents a modifiable factor that could improve antenatal MMC detection, which in turn would allow delivery of affected neonates in a tertiary institution to further decrease the delay to repair. Despite the barriers to early detection, the antenatal MMC detection rate in the current study (20%) was double that of other local studies (10.1%).[11]
The present study had a M:F ratio (1.3:1), which was similar to more recent SA studies (1.5:1)[11] and in contrast to trends noted by Teckie et al.[10] (0.82:1). The study also showed a higher rate of lower-spinal MMC in males (1.4:1) which adds to the existing body of research on the topic.[1,4,10]
Study limitations
This retrospective study was subject to data variation such as incomplete records as well as subjective clinical assessments.
In addition, the study comprised a small sample. Owing to the descriptive nature of the study, it highlights key areas that require further investigation by means of an analytical study design. The present study refers to a neonatal unit at a specific tertiary institution and the findings may not be generalisable to other populations. Patients admitted to the paediatric intensive care unit and other wards were not included in the study population. This may affect the measured outcomes of the neonatal population in Bloemfontein.
Conclusion
The present study determined an inpatient mortality rate of 11.3%, significantly lower than reports from other LMICs. The observed morbidity features and maternal and infant characteristics add to the existing body of research in the SA context. However, further efforts are required to improve access to quality antenatal care to detect these cases. Intersectoral collaboration is essential to circumvent the critical challenges identified in the present study. Further areas of research from this study could investigate the long-term follow-up and outcomes of the identified population as well as the direct and indirect cost implications to delineate the economic impact which would inform a more comprehensive MMC burden of disease assessment.
Declaration. Submitted in partial fulfilment of the requirements for DNPs MMed (Paed) degree.
Acknowledgements. We gratefully acknowledge Mrs. A. Bouwer for her assistance with formatting and Mr. C. van Rooyen for assistance with statistical analyses.
Author contributions. DNP: data collection, review and analysis; wrote the initial draft and revised the manuscript. PM: research supervision, critically reviewed and assisted with revisions of the manuscript. Both authors approved the final version of the manuscript.
Funding. None.
Conflicts of interest. None.
References
1. Greene NDE, Copp AJ. Neural tube defects NTDs: Neural tube defects. Ann Rev Neurosci 2014;37:221-242. https://doi:10.1146/annurev-neuro-062012-170354 [ Links ]
2. Yi Y, Lindemann M, Colligs A, Snowball C. Economic burden of neural tube defects and impact of prevention with folic acid: A literature review. Eur J Pediatr 2011;170(11):1391-1400. https://doi:10.1007/s00431-011-1492-8 [ Links ]
3. Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res Part A - Clin Mol Teratol 2008;82(4):211-216. https://doi.org/10.1002/bdra.20442 [ Links ]
4. Ntimbani J, Kelly A, Lekgwara P. Myelomeningocele - a literature review. Interdiscip Neurosurg 2020;19:100502. https://doi.org/10.1016/j.inat.2019.100502 [ Links ]
5. Fieggen K, Stewart C. Aetiology and antenatal diagnosis of spina bifida. S Afr Med J 2014;104(3):218. https://doi.org/10.7196/SAMJ.8039 [ Links ]
6. Blom HJ, Shaw GM, Den Heijer M, Finnell RH. Neural tube defects and folate: Case far from closed. Nat Rev Neurosci 2006;7(9):724-731. https://doi.org/10.1038/nrn1986 [ Links ]
7. Fieggen G, Fieggen K, Stewart C, et al. Spina bifida: A multidisciplinary perspective on a many-faceted condition. S Afr Med J 2014;104(3):213-217. https://doi.org/10.7196%2FSAMJ.8079 [ Links ]
8. Zugazaga Cortazar A, Martín Martinez C, Duran Feliubadalo C, Bella Cueto MR, Serra L. Magnetic resonance imaging in the prenatal diagnosis of neural tube defects. Insights Imaging 2013;4(2):225-237. https://doi:10.1007/s13244-013-0223-2 [ Links ]
9. Kumar R, Singhal N. Outcome of meningomyelocele/lipomeningomyelocele in children of Northern India. Pediatr Neurosurg 2007;43(1):7-14. https://doi.org/10.1159/000097518 [ Links ]
10. Teckie G, Krause A, Kromberg JGR. Neural tube defects in Gauteng, South Africa: Recurrence risks and associated factors. S Afr Med J 2013;103(12 Suppl 1):973-977. https://doi.org/10.7196%2FSAMJ.7119 [ Links ]
11. Mashiloane PC, Masekela R. A review of the epidemiology, post-neurosurgical closure complications and outcomes of neonates with open spina bifida. S Afr J Child Health 2020;14(2):75-81. https://doi.org/10.7196%2FSAJCH.2020.v14i2.1638 [ Links ]
12. Mnguni MN, Enicker BC, Madiba TE. A perspective in the management of myelomeningocoele in the KwaZulu-Natal Province of South Africa. Childs Nerv Syst 2020;36(7):1521-1527. https://doi:10.1007/s00381-020-04506-9 [ Links ]
13. Kancherla V, Oakley GP. Total prevention of folic acid-preventable spina bifida and anencephaly would reduce child mortality in India: Implications in achieving Target 3.2 of the Sustainable Development Goals. Birth Defects Res 2018;110(5):421-428. https://doi.org/10.1002/bdr2.1175 [ Links ]
14. Schroeder HK, Nunes JC, Madeira L, Moritz JLW, Walz R, Linhares MN. Postsurgical infection after myelomeningocele repair: A multivariate analysis of 60 consecutive cases. Clin Neurol Neurosurg 2012;114(7):981-985. https://doi.org/10.1016/j.clineuro.2012.02.034 [ Links ]
15. Shehu BB, Ameh EA, Ismail NJ. Spina bifida cystica: Selective management of Zaria, Nigeria. Ann Trop Paediatr 2000;20(3):239-242. https://doi.org/10.1080/02724936.2000.11748142 [ Links ]
16. Oncel MY, Ozdemir R, Kahilogullari G, Yurttutan S, Erdeve O, Dilmen U. The effect of surgery time on prognosis in newborns with meningomyelocele. J Korean Neurosurg Soc 2012;51(6):359-362. https://doi:10.3340/jkns.2012.51.6.359 [ Links ]
17. Fumagalli M, Parodi A, Ramenghi L, et al. Ultrasound of acquired posterior fossa abnormalities in the newborn. Pediatr Res 2020;87:25-36. https://doi.org/10.1038/s41390-020-0778-9 [ Links ]
18. World Health Organization. Recommendations on Antenatal Care for a Positive Pregnancy Experience. Geneva: WHO, 2016;58-60. [ Links ]
 Correspondence:
Correspondence:
D N Pillay
dhesan.n.pillay@gmail.com
Accepted 8 May 2023














