Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.17 no.3 Pretoria sep. 2023
http://dx.doi.org/10.7196/sajch.2023.v17i3.1962
RESEARCH
Outcome of two cohorts with nephroblastoma treated with consecutive International Society of Paediatric Oncology protocols in a South African paediatric oncology unit
K ReddyI; A van ZylI; R UysII; M KrugerIII
IMMed (Paed), FC Paed, Cert Med Oncol (Paed);Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
IIMB ChB; Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
IIIMMed (Paed), PhD; Department of Paediatrics and Child Health, Faculty of Medicine and Health Sciences, Stellenbosch University and Tygerberg Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND: Nephroblastoma is a common childhood solid tumour in South Africa
OBJECTIVE: The aim was to determine outcomes of patients diagnosed with nephroblastoma between 1990 and 2018 and compare outcomes of two cohorts treated with consecutive International Society of Paediatric Oncology (SIOP) nephroblastoma protocols
METHODS: This was a retrospective, descriptive study of two cohorts in Tygerberg Hospital. Cohort 1 (1990 - 2007) was treated with the SIOP 9 and SIOP 93-01 protocols, and Cohort 2 (2008 - 2018) with the SIOP 2001 protocol. Data included demographic data (age at diagnosis, sex), HIV status, pre- and postoperative staging, surgical complications, histological types, lymph node sampling, overall survival (OS) and event-free survival (EFS) with the end point two years after diagnosis
RESULTS: There were 60 children (M:F ratio 1:1.14) in Cohort 1 with an older mean age of 42 months (interquartile range (IQR) 16.25 - 56.5 months) v. 45 children (M:F ratio 1:0.8) in Cohort 2 with a mean age of 37 months (IQR 22 - 45.5 months). Cohort 2 had more patients with localised disease (76%) than Cohort 1 (55%) (trend towards significance p=0.076). Both cohorts had a good OS (respectively 88% and 93%) and EFS (respectively 82% and 80%). Half of Cohort 1 (50%; n=30/60) did not have lymph nodes sampled with four subsequent relapses, significantly associated with OS (p<0.001) and EFS (p=0.006). There was a significant association between OS and EFS and underlying histology (respectively p=0.006 and p=0.015) for Cohort 1, but only for EFS and histology (p=0.02) for Cohort 2
CONCLUSION: There was good OS for children with nephroblastoma treated with consecutive SIOP protocols in a single institution in South Africa
Nephroblastoma, a renal tumour, represents 6% of paediatric cancers.[1-3] It is the most common solid tumour, with its incidence exceeding 10% in Africa and South Africa.[4,5] Median age at diagnosis is 42 months[1,6,7] with a slight female predominance.[1,8] Axt et al.[9] reported an increased incidence among North American children of African-American descent.
Children usually present with an asymptomatic abdominal mass, discovered incidentally.[10-12] Associated signs and symptoms include abdominal pain, haematuria and hypertension, probably owing to an increase in renin activity.[10] Ekenze et al.[13] reported late presentation in Nigeria (average duration of symptoms 4.7 months). Soyemi et al.[14] reported that most nephroblastoma tumours exceeded 500 g, also indicating late presentation.
Nephroblastoma usually occurs sporadically; 1% of cases are familial.[15] Congenital abnormalities occur in 12 - 15% of cases.[16] Five percent of tumours occur in association with one of the 50 predisposing genetic syndromes described,[11,17] such as WAGR (Wilms' tumour, Aniridia, Genitourinary anomaly and mental Retardation), Denys-Drash and overgrowth syndromes, including Beckwith-Wiedemann, Simpson-Golabi-Behmel, and Sotos syndrome.[17,18] Some syndromes result from a disruption of the WT1 gene (crucial for renal and gonadal embryogenesis) which results in genitourinary abnormalities and a predisposition to nephroblastoma development.[17,18]
Nephroblastoma has an excellent five-year overall survival (OS) exceeding 90%[19] in localised disease and 70%[20] in metastatic disease. Treatment consists of chemotherapy, surgery and radiotherapy.[20] The International Society of Paediatric Oncology (SIOP) nephroblastoma studies offer pre-operative chemotherapy to reduce tumour size to prevent tumour rupture.[19] The SIOP-9 protocol demonstrated the non-inferiority of four v. eight weeks of pre-operative chemotherapy for localised disease. SIOP 93-01 showed that a reduced postoperative chemotherapy duration in stage 1 tumours with intermediate risk histology or anaplasia was not inferior to standard chemotherapy.[19] SIOP 2001 found that doxorubicin could be omitted for stages 2 and 3 intermediate-risk nephroblastoma when the histological response to preoperative chemotherapy was incorporated into risk stratification.[21]
SIOP nephroblastoma protocols were implemented since 1990 in the Tygerberg Hospital Paediatric Oncology Unit (POU). The primary objective of the present study was to compare OS and EFS for two cohorts treated with SIOP protocols respectively between 1990 and 2007 and 2008 and 2018. Secondary objectives investigated whether surgical complications, lymph node sampling or histological type had an association with OS and EFS.
Patients and methods
This was a retrospective descriptive cohort study at the Tygerberg Hospital POU in the Western Cape, South Africa, including children under 16 years of age with newly diagnosed nephroblastoma. Parental consent for entry of data in the South African Children's Cancer Study Group (SACCSG) Tumour Registry was obtained. Data collected included demographics (age, sex, ethnicity, socioeconomic status), nutritional status at diagnosis, tumour laterality and disease stage. Surgical details included tumour spillage or rupture, intra-and postoperative complications, lymph node sampling and post-chemotherapy tumour histology. The electronic radiology system and the National Health Laboratory Services database were reviewed for imaging studies and histology. There were two cohorts: Cohort 1 (treated 1990 to 2007 with SIOP protocols 9 and 93-01)[22,23] and Cohort 2 (treated 2008 to 2018 with SIOP 2001).[24] The SIOP protocols use the terminology of localised, metastatic and bilateral disease for preoperative categorisation.[25] The OS and EFS end points were two years post diagnosis.
Categorical data were summarised using frequency tables determined with IBM SPSS version 26. Mean survival time and 95% confidence intervals were calculated using Kaplan-Meier survival analysis and demonstrated OS and EFS. Log rank tests were used to compare time to event between groups. A Cox proportional hazards model was used to model the effects of covariates for the outcome of time to EFS event while controlling for the cohort. Associations among variables were tested using Fisher's exact two-sided tests owing to small sample sizes and low frequency of events. A p-value less than 0.05 was statistically significant.
Results
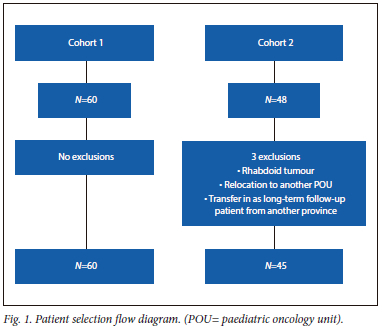
Cohort 1 included 60 patients. Cohort 2 included 45 patients with three exclusions: one rhabdoid tumour, one patient transferred in, and one transferred out (Fig. 1).
The overall male to female (M:F) ratio was 1:1.1, with a mean age of 40 months (median 34, interquartile range (IQR) 18 - 54.5 months) with a M:F ratio of 1:1.14 for Cohort 1 v. 1:08 for Cohort 2. The mean age for Cohort 1 was 42 months (median 35, IQR 16.25 - 56.5 months); older than for Cohort 2 (mean age of 37 months (median 33, IQR 22 - 45.5 months)) (p=0.815). The HIV status for most (95%; 57/60) Cohort 1 patients was unknown, with one patient infected, while HIV status was known in 100% for Cohort 2 (2% infected (n=1/45)).
Seven percent (n=4/60) of Cohort 2 patients required treatment for hypertension compared with 36% (n=16/45) of Cohort 1 patients. In Cohort 2, there was an association between stage at presentation and hypertension (p=0.013), with nine patients with limited disease (eight patients with stage 1 and one with stage 2) presenting with hypertension v. seven with advanced disease (four with stage 4; three with stage 5). There was no association between blood pressure at diagnosis and OS (p=0.757) or EFS (p=0.869).
Most patients presented with localised disease: 69% in Cohort 1 (n=41/60) and 75% in Cohort 2 (n=34/45), although not significant (p=0.440). Postoperative stages for Cohort 1 were respectively 38% (n=23/60) stage 1, 18% (n=11/60) stage 2, 20% (n=12/60) stage 3, 17% («=10/60) stage 4 and 5% (n=3/60) with stage 5 disease. One patient in Cohort 1 did not have surgery owing to sudden cardiovascular collapse and death. Four patients had high-risk disease: one each with stage 1 and 3 and two with stage 4 disease with blastemal predominant histology. Three were alive and one had died. Four patients with anaplasia were difficult to classify into a risk group as the extent of anaplasia (diffuse or focal) was not documented.
The postoperative stages for Cohort 2 were 49% (n=22/45) stage 1, 13% (n=6/45) stage 2, 22% (10/45) stage 3, 9% (n=4/45) stage 4 and 5% (n=2/45) with stage 5 disease, respectively. One patient with stage 5 disease and Denys-Drash syndrome did not have surgery as tumours were unresectable (kidney transplant was impossible for this child in South Africa). The patient was still alive at last follow-up. Three patients had high-risk stage 1 disease (two alive; one unknown), three patients with stage 2 high-risk disease (alive) and one with stage 3 high-risk disease (died). Postoperative stage between the two cohorts did not differ significantly (p=0.799).
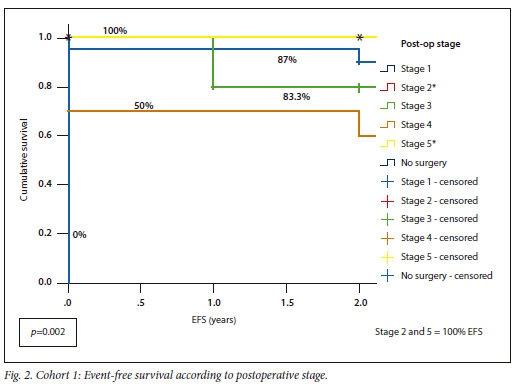
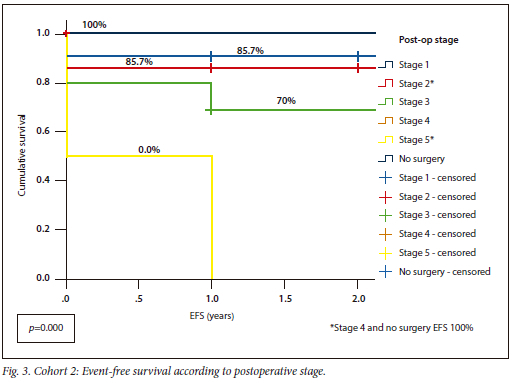
Cohort 1 had an OS of 88% v. 93% for Cohort 2, with similar EFS for both (Cohort 1 82%; Cohort 2 80%) (p=0.738). Cohort 1 had OS and EFS of 100% for stages 2 and 5, whereas Cohort 2 had 100% OS for stages 1 and 4, as well as the patient without surgery (Fig. 2, Fig. 3).
Intra-operative complications were present in 10% (6/60) of Cohort 1 v. 11% (n=5/45) ofCohort 2), but slightly more postoperative complications occurred in Cohort 2 (13%; n=6/45) than in Cohort 1 (7%; n=4/60). Cohort 1 complications included tumour spillage (n=1), tumour rupture (n=2), colectomy (n=1) and ileus (n=2), while Cohort 2 complications included tumour rupture (n=1), tumour spillage (n=1), intra-operative cardiac arrest (n=1), hemicolectomy (n=1) and diaphragmatic injury (n=1). The association between OS and EFS with intra- and postoperative complications was significant (respectively p<0.001 and p=0.004) for Cohort 1, but had no significance for Cohort 2 (OS and intra- and postoperative complications respectively p=0.069 and p=0.598), EFS and intra-and postoperative complications p=0.564). Tumour spillage was present in both cohorts (5%; 3/60 v. 4.4%; 2/45). For Cohort 1 there was an association with tumour spillage and OS (p<0.001) and EFS (p<0.001), but no association for Cohort 2 (tumour spillage and OS p=0.601; EFS p=0.486). In Cohort 1, 45% (n=27/60) of tumours were right sided, 42% (25/60) left sided, 12% (n=7/60) bilateral and 1% (n=1/60) were undocumented. In Cohort 2, 42% (19/45) were right sided, 51% (23/45) left sided and 7% (n=3/45) bilateral. Intra-operative complications and tumour laterality had no significant association (Cohort 1 p=0.681; Cohort 2 p=0.938).
Half of Cohort 1 (50%; «=29/60) did not have lymph node sampling, with four subsequent relapses significantly associated with OS (p<0.001) and EFS (p=0.006), probably owing to incorrect postoperative stage. Three with stage 1 disease had relapses: one peritoneal relapse, one lung relapse and one defaulted treatment.
Lymph node sampling for Cohort 2 was done in 75% (n=34/45) with no association with OS (p=0.340) and EFS (p=0.497), probably indicating correct postoperative stage. In some patients, no lymph nodes were found intra-operatively; for others there was no documented reason for non-sampling. Three patients in Cohort 2 who had no lymph node sampling done, relapsed.
Cohort 1 and histology: Most had intermediate-risk triphasic histology (68%; n=41/60), followed by high-risk blastemal predominant (7%; n=4/60)
extent (diffuse or focal involvement) was unspecified; these cases were added to the 'other' subtypes group (18%; n=11/60). Triphasic subtype had an OS of 89.5% and EFS of 82.9% (two deaths). The blastemal predominant group had a lower OS of 85.7% (one death) and an EFS of 75%. The EFS and OS for the 'other' group was 57%. The patients with unspecified anaplasia had an OS and EFS of 75% (p=0.006) and EFS (p=0.015) (Table 1). The four patients with a blastemal predominance were aged 8, 40, 47 and 74 months, respectively, whereas the patients with subtype containing an unknown extent of anaplasia were aged 44, 55, 66 and 115 months. The deaths present in each group occurred in the oldest patients.
Cohort 2 and histology: Intermediate-risk triphasic histology (42%; n=19/45) was predominant, while the rest were respectively epithelial (20%; 9/45), stromal (9%; n=4/45) and high-risk blastemal predominant (16%; n =7/45) types. Epithelial and stromal subtypes had 100% OS compared with 89.5% for triphasic histology with an EFS of 73.7% owing to five events, including two deaths. The blastemal predominant type had an OS and EFS of 86% (one death) and the stromal group had an EFS of 75% with one event occurring. There were no anaplasia subtypes in this cohort. There was a significant difference in EFS in association with histological subtype (p=0.02) but no association with OS (p=0.97) for Cohort 2 (Table 1). There were seven patients in the blastemal predominant group, with ages 7, 32, 39, 46, 56, 68 and 71 months. Similarly, the one death occurred in the oldest patient aged 71 months.
Cohort 1 had 11 events: seven deaths secondary to relapse, a cardiovascular collapse, two secondary malignancies. Cohort 2 had nine events: three deaths secondary to relapse and six alive after relapses (two pulmonary, three hepatic and one peritoneal carcinomatosis).
Healthcare was free for most children in both cohorts (Cohort 1 68%; Cohort 2 80%) as they were under six years of age.[26] The Cohort 1 families (18% unemployed parents, 34% some income, 20% medical insurance and 28% unknown socioeconomic status) were at a greater socioeconomic disadvantage than Cohort 2 families (9% unemployed parents, 67% some income, 18% medical insurance and 6% unknown socioeconomic status). There was a significant improvement in families' socioeconomic circumstances between the two time periods (p=0.014).
Nutritional status was superior for Cohort 2 (no acute malnutrition in 96% of Cohort 2 v. 58% in Cohort 1; 80% in Cohort 2 with normal height v. 55% in Cohort 1; 93% with normal weight in Cohort 2 v. 62% in Cohort 1). There were significant differences between the nutritional status of the two cohorts with regards to weight for age (p=0.002), height for age (p=0.004) and weight for height (p<0.001). There was no association between EFS and OS and weight for age and weight for height or nutritional status and relapses or deaths.
In summary, this study demonstrated slightly better OS in Cohort 2, but similar EFS. Intra- and postoperative complications were associated with a poorer OS for Cohort 1. Lymph node sampling was important for postoperative staging as three patients with no sampling relapsed. Histology subtype was only significantly associated with OS and EFS in Cohort 1. Nutritional status at diagnosis had no significant association with either OS or EFS.
Discussion
Visser et al.[27] reported a better OS (86.4%) with SIOP protocols than with a National Wilms' Tumor Study Group (NWTSG) protocol (61.5%) in a previous nephroblastoma review at the same POU. A similar study from Johannesburg demonstrated an OS of 89% for limited disease, utilising the SIOP 9 protocol.[28] Our study demonstrated similar improved OS for both cohorts.
Cunningham et al.[29] reported that OS ranged from 70% to 97% in high-income countries (HICs) v. 61% to 94% in upper-middle-income countries, 0% to 85% in lower-middle-income countries (LMICs) and 25% to 53% in low-income countries. The Groupe Franco-Africain d'Oncologie Pediatrique (GFAOP)(GFAOP-NEPHRO-02 study) used an adapted SIOP 2001 protocol and reported a three-year OS of 72% and EFS of 69%.[30] Advanced tumour presentation was reportedly more common in Africa.[13,31] Diagnostic delay, lack of treatment and inadequate follow-up contributed to these differences.[29] OS in the present study corresponds to that of HICs (OS respectively 88% and 93% for Cohort 1 and Cohort 2; EFS respectively 82% and 80% for Cohort 1 and Cohort 2). The outcome for stage 4 in this study was superior to that reported in SIOP 2001[32] but the sample was very small.
Financial constraints[13,33] have contributed to poor outcomes for patients in Africa, emphasising the importance of socioeconomic status. The present study demonstrated an improvement in both socioeconomic and nutritional status for the two time periods. Nutritional improvement was probably due to food fortification and national school nutrition programmes, improved primary healthcare, supplement provision and dietetic involvement in communities.[34] In Cape Town, the Integrated Nutrition Programme aims to improve the nutritional status of residents.[34] Though the study showed an improved nutritional status, it is important to note that in children with cancer, specifically those with abdominal tumours, weight is an inadequate marker of nutritional status.[35]
The complication rate for nephroblastoma surgery varies from 13% to 28%[36,37] and includes intestinal obstruction, haemorrhage, infection and vascular injury. Complications were more likely if the tumour extended into the inferior vena cava or right atrium, if a different surgical approach was used, if a tumour was larger than 10 cm or if the surgery was performed by a general surgeon instead of a paediatric surgeon. SIOP publications have shown that complications are reduced to below 8% if preoperative chemotherapy is given.[37] The current study found an intra- and postoperative surgical complication rate for Cohort 1 of 17% and 24% for Cohort 2. This increase needs further investigation in prospective studies.
Intra-operative spillage (IOS) from the primary tumour or renal vein, or tumour rupture is an important surgical complication. Tumour size larger than 10 cm or tumour volume greater than 1 000 cc and right-sided location are associated with increased risk.[38,39] In the present study, post-nephrectomy tumour volume was not calculated owing to incomplete data. There was no association between tumour laterality and intra-operative complications, but there was an association between OS and EFS and tumour spillage for Cohort 1. Surgeries were performed by paediatric surgeons; therefore, this was not the reason for more complications.
Lymph node sampling provides important prognostic and staging information.[40] In the present study, lymph node sampling was increased in Cohort 2 (75% v. 50%) while reduced sampling negatively affected OS and EFS in Cohort 1. Reasons for non-sampling include the surgeons' impression that it was unnecessary in metastatic or early disease or those with positive tumour margins.[41] The reasons for incomplete lymph node sampling in the present study are unknown and need to be elucidated further.
In the present study, triphasic-type histology was predominant in both cohorts, a similar finding reported by Weirich et al.[42] with triphasic variant predominant (45.1%), followed by blastemal type (39.4) with a poorer outcome. In studies from Nigeria, Egypt and Kenya, blastemal predominant type (44.4%) was most frequent,[43] also associated with a poor outcome.[44] In the present study, the blastemal predominant type occurrence increased from Cohorts 1 to 2 (7% to 16%) and was higher than the reported incidence of about 9% in the SIOP93-01 and SIOPWT2001 trials.[45] This finding may indicate a larger poor prognostic group in the South African population.
Anaplasia is a high-risk nephroblastoma factor which confers a worse prognosis. Diffuse anaplasia is classified as high risk and focal anaplasia as intermediate risk.[46] In the present study, distinction between focal and diffuse anaplasia was missing from the records; therefore the subtype could not be accurately classified. As the OS and EFS was 75% for this unspecified anaplasia group in Cohort 1, it could be speculated that the cases most likely had focal anaplasia. The deaths in the blastemal predominant and unspecified anaplasia cases occurred in children older than five years of age, who have a poorer outcome, as expected.[47]
A new treatment protocol for nephroblastoma was recently developed by the Renal Tumour Study Group of SIOP (SIOP-RTSG) (UMBRELLA SIOP-RTSG 2016).[25] Despite the excellent outcomes of patients with nephroblastoma, there is a group of patients with increased relapse rates and poor prognosis. Identification of these patients is important to improve treatment stratification and outcomes and to decrease late effects of chemotherapy for those with a better outcome. The UMBRELLA protocol aims to validate new prognostic factors; for example, blastemal tumour volume and molecular markers.
Study limitations
This was a retrospective study from a single institution in South Africa with missing data, limiting investigation into associations between lymph node sampling, radiological tumour volume, post-nephrectomy volume and OS.
Conclusion
The use of SIOP protocols yielded excellent patient outcomes in this study, confirming this to be a feasible and appropriate treatment approach in an upper-middle-income country. It is imperative to strictly adhere to the treatment protocol guidelines to identify high-risk patients and to ensure that lymph node sampling is undertaken during surgical resection. The implementation of a national nephroblastoma treatment protocol would assist in ensuring uniformity in patient care across all paediatric oncology units in South Africa and should result in improved outcomes.
Declaration. None.
Acknowledgements. Thanks to Mrs Anita Fourie for administrative support.
Author contributions. MK and KR conceptualised the study. KR, a MPhil student, designed the study, collected data, analysed the data, and wrote the manuscript. RU assisted with data collection and analysis. MK and AvZ assisted with the design of the study and critically reviewed the manuscript.
Funding. None.
Conflict of interest. None.
References
1. Breslow NE, Olshan A, Beckwith JB, Green DM. Epidemiology of Wilms' tumor. Med Pediatr Oncol 1993;21(3):172-181. https://doi.org/10.1002/mpo.2950210305 [ Links ]
2. Pastore G, Znaor A, Spreafico F, et al. Malignant renal tumours incidence and survival in European children (1978-1997): Report from the Automated Childhood Cancer Information System project. Eur J Cancer 2006;42(13):2103-2114. https://doi.org/10.1016/j.ejca.2006.05.010 [ Links ]
3. Davidoff AM. Wilms Tumor. Adv Pediatr 2012;59(1):247-267. https://doi.org/10.1016/j.yapd.2012.04.001 [ Links ]
4. Stefan DC. Patterns of distribution of childhood cancer in Africa. J Trop Pediatr 2015;61:165-173. https://doi.org/10.1093/tropej/fmv005 [ Links ]
5. Stefan DC. Epidemiology of childhood cancer and the SACCSG tumour registry. Contin Med Educ 2010;28(7):317-319. [ Links ]
6. Stefan DC, Stones DK, van Zyl A, Uys R. The cost of nephroblastoma treatment in South Africa : A very cost-effective investment with guidelines for the rest of Africa. S Afr J Child Heal 2014;8(4):2-6. https://doi.org/10.7196/SAJCH.749 [ Links ]
7. Stones DK, Hadley GP, Wainwright RD, Stefan DC. The impact of ethnicity on Wilms' tumor : Characteristics and outcome of a South African cohort. Int J Pediatr 2015;706058. https://doi.org/10.1155/2015/706058 [ Links ]
8. Kaste SC, Dome JS, Babyn PS, et al. Wilms tumour : Prognostic factors, staging, therapy and late effects. Pediatr Radiol 2008;38:2-17. https://doi.org/10.1007/s00247-007-0687-7 [ Links ]
9. Axt J, Murphy AJ, Seeley EH, et al. Race disparities in Wilms tumor incidence and biology. J Surg Res 2011;170(1):112-119. https://doi.org/10.1016/j.jss.2011.03.011 [ Links ]
10. Davidoff A. Wilms' tumor. Curr Opin Pediatr 2009;21(3):357-364. [ Links ]
11. Szychot E, Apps J, Pritchard-Jones K. Wilms' tumor : Biology, diagnosis and treatment. Transl Pediatr 2014;3(1):12-24. https://doi.org/10.3978%2Fj.issn.2224-4336.2014.01.09 [ Links ]
12. Kalapurakal JA, Dome JS, Perlman EJ, et al. Management of Wilms ' tumour : Current practice and future goals. Lancet Oncol 2004;5:37-46. [ Links ]
13. Ekenze S, Obianyo NEN. The challenge of nephroblastoma in a developing country. Ann Oncol 2006;17(10):1598-1600. https://doi.org/10.1016/s1470-2045(03)01322-6 [ Links ]
14. Soyemi SS, Osuoji RI, Faduyile FA, et al. Morphological features of Wilms' tumour in a tertiary health care institution: Our findings. Clin Exp Pathol 2013;3(3):1-3. [ Links ]
15. Tay J. Molecular genetics of Wilms' tumor. J Pediatr Child Heal 1995;31:379-383. https://doi.org/10.1111/j.1440-1754.1995.tb00841.x [ Links ]
16. Poole JE. Wilms' tumour (nephroblastoma). Contin Med Educ 2010;28(7):3-5. [ Links ]
17. Szychot E, Pritchard-Jones K. Review of current approaches to the management of Wilms' tumor. CML-Urology 2012;18(3):65-75. https://doi.org/10.21037%2Ftau.2020.03.03 [ Links ]
18. Scott RH, Stiller CA, Walker L, Rahman N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J Med Genet 2006;43:705-715. https://doi.org/10.1136/jmg.2006.041723 [ Links ]
19. Bhatnagar S. Management of Wilms' tumor : NWTS vs SIOP. J Indian Assoc Pediatr Surg 2009;14(1):6-14. https://doi.org/10.4103%2F0971-9261.54811 [ Links ]
20. Pritchard-Jones K. Controversies and advances in the management of Wilms' tumour. Arch Dis Child 2002;87:241-244. https://doi.org/10.1136/adc.87.3.241 [ Links ]
21. Pritchard-Jones K, Bergeron C, De Camargo B, et al. Omission of doxorubicin from the treatment of stage II-III, intermediate-risk Wilms' tumour (SIOP WT 2001): An open-label, non-inferiority, randomised controlled trial. Lancet 2015;386(9999):1156-1164. https://doi.org/10.1016/s0140-6736(14)62395-3 [ Links ]
22. Tournade MF, Com-Nougué C, de Kraker J, et al. Optimal duration of preoperative therapy in unilateral and nonmetastatic Wilms' tumor in children older than 6 months: Results of the Ninth International Society of Pediatric Oncology Wilms' Tumor Trial and Study. J Clin Oncol 2001;19(2):488-500. https://doi.org/10.1200/jco.2001.19.2.488 [ Links ]
23. De Kraker J, Graf N van TH, Pein F, Sandstedt B, Godzinski JTM. Reduction of postoperative chemotherapy in children with stage I intermediate-risk and anaplastic Wilms' tumour (SIOP 93-01 trial): A randomised controlled trial. Lancet 2004;364(9441):1229-1235. https://doi.org/10.1016/s0140-6736(04)17139-0 [ Links ]
24. De Kraker J, Graf N, Pritchard-Jones K. Nephroblastoma clinical trial and study SIOP 2001, Protocol 2001;1-11. [ Links ]
25. Van Den Heuvel-Eibrink MM, Hol JA, Pritchard-Jones K, et al. Position Paper: Rationale for the treatment of Wilms tumour in the UMBRELLA SIOP-RTSG 2016 protocol. Nat Rev Urol 2017;14(12):743-752. https://doi.org/10.1038/nrurol.2017.163 [ Links ]
26. National Health Act 61 of 2003. Government Gazette, Republic of South Africa 2004. [ Links ]
27. Visser YT, Uys R, Van Zyl A SD. Nephroblastoma - a 25-year review of a South African unit. J Med Life 2014;7(3):445-449. [ Links ]
28. Rogers T, Bowley DM, Poole J, et al. Experience and outcomes of nephroblastoma in Johannesburg, 1998-2003. Eur J Pediatr Surg 2007;17:41-44. https://doi.org/10.1055/s-2007-964917 [ Links ]
29. Cunningham ME, Klug TD, Nuchtern JG, et al. Global disparities in Wilms tumor. J Surg Res 2020;247(832):34-51. https://doi.org/10.1016/j.jss.2019.10.044 [ Links ]
30. Yao AJJ, Moreira C, Traoré F, et al. Treatment of Wilms tumor in sub-Saharan Africa: Results of the second French African pediatric oncology group study. J Glob Oncol 2019;2019(5):1-8. https://doi.org/10.1200/jgo.18.00204 [ Links ]
31. Abdallah FK Macharia WM. Clinical presentation and treatment outcome in children with nephroblastoma in Kenya. East Afr Med J 2001;78(7 Suppl):S43-S47. [ Links ]
32. Niedzielski J, Taran K, Mtynarski W, Sitkiewicz A. Is the SIOP-2001 classification of renal tumors of childhood accurate with regard to prognosis? A problem revisited. Arch Med Sci 2012;8(4):684-689. https://doi.org/10.5114/aoms.2012.30292 [ Links ]
33. Libes J, Oruko O, Abdallah F, et al. Risk factors for abandonment of Wilms tumor therapy in Kenya. Pediatr Blood Cancer 2015;62(2):252-256. https://doi.org/10.1002/pbc.25312 [ Links ]
34. Western Cape Government. Integrated Nutrition Programme. 2020 (accessed 13 August 2020). https://www.westerncape.gov.za/service/integrated-nutrition-programme [ Links ]
35. Bauer J, Jürgens H, Frühwald MC. Important aspects of nutrition in children with cancer. Adv Nutr 2011;2(2):67-77. https://doi.org/10.3945/an.110.000141 [ Links ]
36. Günther P, Tröger J, Holland-Cunz S, et al. Surgical complications in abdominal tumor surgery in children. Experiences at a single oncological center. Eur J Pediatr Surg 2009;19(5):297-303. https://doi.org/10.1055/s-0029-1220681 [ Links ]
37. Huddart SN. Wilms tumour - the surgical issues. Paediatr Child Health (Oxford) 2020;24(4):137-142.https://doi.org/10.1016/j.paed.2013.09.001 [ Links ]
38. Gow KW, Barnhart DC, Hamilton TE, et al. Primary nephrectomy and intraoperative tumor spill: Report from the Children's Oncology Group (COG) renal tumors committee. J Pediatr Surg 2015;48(1):34-38. https://doi.org/10.1016/j.jpedsurg.2012.10.015 [ Links ]
39. Barber TD, Derinkuyu BE, Wickiser J, Joglar J, Koral K, Baker LA. Wilms tumor : Preoperative risk factors identified for intraoperative tumor spill. J Urol 2011;185(4):1414-1418. https://doi.org/10.1016/j.juro.2010.11.047 [ Links ]
40. Kieran K, Anderson JR, Domec JS, et al. Lymph node involvement in Wilms tumor: Results from National Wilms Tumor Studies 4 and 5. J Pediatr Surg 2012;47(4):700-706. https://doi.org/10.1016%2Fj.jpedsurg.2011.08.017 [ Links ]
41. Stewart AK, Winchester DP, Ko CY. Nodal evaluation in Wilms' tumors. Ann Surg 2010;251(3):559-565. https://doi.org/10.1097/sla.0b013e3181cc95d7 [ Links ]
42. Weirich A, Leuschner I, Harms D, et al. Clinical impact of histologic subtypes in localised nonanaplastic nephroblastoma treated according to the trial and study SIOP-9/GPOH. Ann Oncol 2001;12:311-319. https://doi.org/10.1023/a:1011167924230 [ Links ]
43. Atanda AT, Anyanwu LJC, Atanda OJ, Mohammad AM, Abdullahi LB, Farinyaro AU. Wilms' tumour: Determinants of prognosis in an African setting. African J Paediatr Surg 2015;12:171-176. https://doi.org/10.4103%2F0189-6725.170185 [ Links ]
44. Seeley EH, Caprioli RM, Newton MW, de Caestecker MP. Molecular characterisation of Wilms tumor from a resource constrained region of sub-Saharan Africa. 2012;131(6):983-994. https://doi.org/10.1002/ijc.27544 [ Links ]
45. Van den Heuvel-Eibrink MM, Van Tinteren H, Bergeron C, et al. Outcome of localised blastemal-type Wilms tumour patients treated according to intensified treatment in the SIOP WT 2001 protocol, a report of the SIOP Renal Tumour Study Group (SIOP-RTSG). Eur J Cancer 2015;51(4):498-506. https://doi.org/10.1016/j.ejca.2015.03.005 [ Links ]
46. Vujanic GM, Gessler M, Ooms AHAG, et al. The UMBRELLA SIOP-RTSG 2016 Wilms tumour pathology and molecular biology protocol. Nat Rev Urol 2018;15(11):693-701. https://doi.org/10.1038/s41585-018-0100-3 [ Links ]
47. Roshin DA, Matveev V, Volkova M, Glekov IV. Outcome anlaysis of treatment of nephroblastoma in children older than 5 years and adults. J Urol 2008;179(4):381. https://doi.org/10.1002/ijc.31399 [ Links ]
 Correspondence:
Correspondence:
M Kruger
marianakruger@sun.ac.za
Accepted 17 May 2023














