Services on Demand
Article
Indicators
Related links
-
 Cited by Google
Cited by Google -
 Similars in Google
Similars in Google
Share
South African Journal of Child Health
On-line version ISSN 1999-7671
Print version ISSN 1994-3032
S. Afr. j. child health vol.17 n.3 Pretoria Sep. 2023
http://dx.doi.org/10.7196/sajch.2023.v17i3.1964
RESEARCH
In-hospital neonatal mortality in a level-2 hospital in Cape Town, South Africa
C GabrielsI; D M le RouxII
IMMed (Paed); Department of Paediatrics and Child and Health, University of Cape Town, South Africa; Department of Paediatrics, Mitchells Plain District Hospital, Cape Town, South Africa
IIMBChB, PhD; Department of Paediatrics and Child and Health, University of Cape Town, South Africa; Department of Paediatrics, Mitchells Plain District Hospital, Cape Town, South Africa
ABSTRACT
BACKGROUND: Neonatal mortality (death in the first 28 days of life) is a major contributor to under-5 mortality in South Africa. Many advances in neonatal care have been introduced, but the impact of these interventions has not been studied outside of tertiary academic centres
OBJECTIVE: To describe neonatal mortality in the neonatal high care unit at New Somerset Hospital in Cape Town, South Africa, over an 8-year period
METHODS: Records of neonatal deaths were captured and entered into a database; deaths were coded according to Perinatal Problem Identification Program categories
RESULTS: Neonatal deaths from 2011 to 2018 were analysed, excluding 2014. There were 296 neonatal deaths; median (interquartile range (IQR)) birthweight of neonatal deaths was 1 140 (790 - 2 420) g; median (IQR) gestation was 29 (25 - 38) weeks. Immaturity (132/296, 45%) was the most common cause of death, followed by hypoxia (67/296, 23%) and infections (61/296, 21%). There were 250 (84%) neonatal deaths in the first week of life. There was a trend towards a decreasing number of neonatal deaths (from 48 in 2011 to 34 in 2018), and rate of deaths (from 45.2 per 1 000 admissions to 28.2 per 1 000 admissions). This was driven by decreased deaths due to immaturity; number of deaths due to other causes remained approximately constant
CONCLUSION: We observed a decreasing number of neonatal deaths and rate of deaths per 1 000 admissions, with the largest decrease in deaths due to prematurity. Advances in respiratory care for preterm neonates may have contributed to decreased mortality due to immaturity. Upstream obstetric interventions will be required to address hypoxia-related causes of neonatal mortality
Neonatal mortality (death in the first 28 days of life) has been a major contributor to under-5 mortality in South Africa (SA), accounting for a third of under-5 deaths in 2015.[1] As a result of improvements in management of HIV, pneumonia and gastroenteritis,[2] under-5 child mortality in SA decreased dramatically over the last 20 years, from a peak of 80 deaths per 1 000 live births at the height of the HIV epidemic in 2004[3] to 34 deaths per 1 000 live births in 2018.[2] However, neonatal mortality in SA has remained static at 11 - 12 deaths per 1 000 live births for nearly 20 years,[2,3] so the relative contribution of neonatal mortality to total under-5 mortality has increased from 14% in 2002 to 32% in 2018.[2] Neonatal mortality must be addressed if SA is to meet target 3.2.1 of the Sustainable Development Goals (SDGs) by 2030, namely under-5 child mortality of <25 deaths per 1 000 live births.[1]
The leading causes of under-5 mortality in SA are prematurity, asphyxia, HIV/AIDS, pneumonia and diarrhoeal diseases;[1,3] neonatal sepsis is also a major contributor to both early (day 1 - 7) and late (day 8 - 28) neonatal deaths (NNDs).[4] Much
research into the cause of NNDs in SA and potential modifiable factors has been undertaken. Most pooled NND surveys focus on causes of early NNDs, and late NNDs are under-reported.[5,6] In a retrospective database review of 142 hospitals between October 1999 and September 2003, Velaphi and Pattinson[7] described 4 502 NNDs. Prematurity accounted for 35% of deaths and 32% of deaths were due to asphyxia-hypoxia. Early continuous positive airway pressure (CPAP)'81 and exogenous surfactant therapy[9] has improved survival of extremely low-birthweight (ELBW) neonates in tertiary neonatal units. High-flow nasal cannula (HFNC) oxygen has been used for preterm neonates instead of CPAP with good results.[10]
Furthermore, there is evidence that outborn neonates transferred to tertiary neonatal centres have worse outcomes than inborn neonates.
However, there is little evidence regarding impacts of these recent improvements in neonatal care on neonatal mortality outside of tertiary academic centres. Also, outcomes of transfer of outborn neonates to non-tertiary neonatal units, where most neonatal care in SA is delivered, have not been reported.
For these reasons, we aimed to investigate neonatal mortality in a level-2 neonatal unit in Cape Town, SA, to better understand causes of early and late neonatal mortality, and to analyse inborn v. outborn neonatal mortality.
Methods
New Somerset Hospital (NSH) is a level-2 hospital in Cape Town, SA. NSH performs about 6 000 deliveries per year, and receives referrals from a wide geographical area, including local urban primary-level delivery facilities, and remote rural hospitals up to 200 km away. CPAP, surfactant and short-term ventilation (<3 days) are offered; HFNC oxygen was introduced in 2015, but longterm ventilation (>3 days), high-frequency oscillatory ventilation (HFOV), inotropic support, and total parenteral nutrition are not available. Neonates who require escalation of neonatal care are referred to the tertiary hospital, Groote Schuur Hospital (GSH); neonates requiring surgical intervention are referred to Red Cross War Memorial Children's Hospital (RCWMCH). Neonates born at other facilities and transferred to NSH were considered 'outborn.
A database of all NNDs was maintained by the lead consultant in the neonatal unit. Approval to maintain the database was obtained from the University of Cape Town (UCT) Human Research Ethics Committee (HREC ref. no. 391/2011). The neonatal mortality database included all neonates who died either on-site at NSH, or after transfer to a level-3 facility (GSH or RCWMCH); information about NNDs was captured in real time by doctors (registrars and medical officers) into a file kept in the neonatal high care unit (HCU), and entered into a password-protected database (initially an Excel spreadsheet, then an Access database) by the neonatal consultant on a weekly basis. Variables included gestational age, weight, sex, mode of delivery, and HIV exposure status. Data on early NNDs were submitted to the local co-ordinators of the Perinatal Problem Identification Program (PPIP), a structured national project for district-level longitudinal tracking of stillbirths and early NNDs.[4] Deaths were coded according to categories used by the PPIP for main causes of death (immaturity, hypoxia, sepsis, congenital anomalies and other). Birthweight was considered in the following categories: >2 500 g, 1 500 - 2 499 g, 1 000 - 1 499 g and <1 000 g. The survival status of neonates after transfer to level 3 was determined and entered monthly. An anonymous de-identified subset of data without any personal identifiers was analysed by the investigators. This analysis was restricted to deaths occurring in the first 28 days of life. NNDs that occurred in the labour ward were excluded, as the neonates had never been admitted to the neonatal unit. The study was approved by the UCT Human Research Ethics Committee (HREC ref. no. 71/2019). Permission to conduct the study was granted by the chief executive officer of NSH.
Statistical analysis
Categorical variables were compared as percentages and proportions, and by x2 test; means were compared by t-test. Continuous variables were presented as median and interquartile range (IQR) as they were not normally distributed, and compared by Mann-Whitney U test. Frequency tables, histograms and basic analyses were generated in Microsoft Excel (Microsoft Corp., SA); medians and IQRs were calculated in Stata version 16 (StataCorp, USA).
Results
NNDs from 2011 to 2018 were compiled, with the exclusion of 2014, owing to incomplete data capturing for several months of that year (supplementary Table 1). In the 7 years under review, there were 46 441 births at NSH, and 8 166 admissions to the neonatal unit: 6 205 (76%) of the neonatal admissions were inborn and 1 961 (24%) were transferred in from other birth units. There were 296 neonatal deaths associated with NSH HCU; 219 (74%) were inborn and 77 (26%) were outborn, either born before arrival or transferred in from a level-1 facility or maternal obstetric unit (Table 1). Most of the deaths (221; 75%) occurred at NSH, but 75 (25%) occurred after transfer to another unit. There were 171 (58%) males. Median (IQR) birthweight was 1 140 (790 - 2 420) g; birthweights for nearly half the deaths (130; 44%) were <1 000 g. Median (IQR) gestation of neonates who died was 29 (25 - 38) weeks, with no significant difference between inborn and outborn (p=0.86). Overall, the majority of neonates (181; 61%) who died were delivered by normal vertex delivery (NVD) and were not HIV exposed (220; 74%). Median (IQR) age at death was day 1 (1 - 4) for outborn v. day 2 (1 - 4) for inborn (p=0.20).
'Immaturity' was the most commonly coded cause of death (132; 45%); this category included neonates who had been recorded as having extreme prematurity (94; 32%), hyaline membrane disease (31; 11%) and preterm intraventricular haemorrhage (7; 2%). 'Perinatal hypoxia' included hypoxic ischaemic encephalopathy (32; 11%) and meconium aspiration syndrome (MAS) with or without persistent pulmonary hypertension of the newborn (PPHN) (25; 8%). Perinatal hypoxia was a more common cause of death among inborn neonates (54/219; 25%) compared with outborn neonates (13/77; 17%), but this difference was not statistically significant (p=0.16). Infection-related causes included 19 cases (6%) of necrotising enterocolitis (NEC), 2 of which had an organism identified (one each of Serratia marcescens and Escherischia coli) and 17 were culture-negative. There were 38 neonatal deaths due to non-abdominal sepsis; 31/38 (82%) were culture-negative. Those with positive cultures included 3 cases of Pseudomonas aeruginosa, and 1 case of each of Streptococcus agalactiae, Candida albicans, Enterobacter cloacae, and a mixed infection of Klebsiella pneumoniae and Acinetobacter baumanii. There were four (1%) neonatal deaths due to congenital syphilis. Infection-related causes of death were more common among outborn neonates (25/77; 32%) v. inborn neonates (36/219; 16%) (p=0.003). Of the 30 neonates who died with congenital abnormalities: there were 4 with trisomy 18; 12 had multiple anomalies but not a recognisable syndrome; 7 had congenital cardiac lesions, 2 of whom also had trisomy 21; 5 had pulmonary hypoplasia; and 1 each had a central nervous system lesion and congenital anaemia. The category of 'other' causes of death included 4 neonates who had haemorrhages (1 subaponeurotic, 1 intracerebral, 2 other exsanguinating haemorrhages) and 2 with metabolic disorders.
Among neonates of different of birthweight categories, there were differences in method of delivery and cause of death: most ELBW deaths (107/130; 82%) followed NVD, and immaturity was the main cause of death (104/130; 80%); whereas for deaths where birthweight was >2 500 g, hypoxia was the main cause (50/72; 69%) and caesarean section was the most common delivery method (39/72; 54%) (Table 1). There were no significant differences in age at death among neonates of different birthweight categories.
Throughout the study period, antenatal HIV prevalence among pregnant women remained constant at about 20%; there were similar numbers of deaths of HIV-exposed neonates in all weight categories (Table 1). A higher proportion of HIV-exposed neonates died owing to infectious causes (26/61; 43%) compared with other causes (50/235; 21%) (p=0.001) (supplementary Fig. 1).
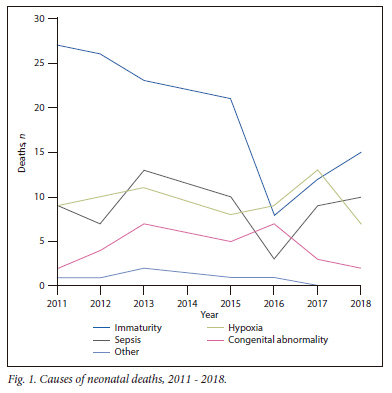
There was a gradual trend towards a decreasing number of NNDs (from 48 in 2011 to 34 in 2018) and rate of deaths (from 45.2/1 000 admissions to 28.2/1 000 admissions (Table 2). This was largely driven by decreased deaths due to immaturity, from 27 (56%) in 2011 to 15 (44%) in 2018 (Fig. 1); deaths among ELBW neonates decreased from 25 (52%) to 12 (35%) (Table 2). The number of deaths in other birthweight categories and due to other causes remained approximately constant (Table 2). The mean number of deaths due to immaturity before 2014 (25.3 per year) was significantly less than the mean number of deaths after 2014 (14.0 per year) (p=0.01). The decrease in number of deaths due to immaturity was more marked in neonates who were inborn (from 21 in 2011 to 11 in 2018) compared with those who were outborn (from 6 to 4) (supplementary Fig. 2). There were four deaths due to congenital syphilis prior to 2015 and none after 2015. Age at death after 2015 was lower (median (IQR) 1 (1 - 5) day), compared with neonates born before 2015 (median (IQR) 2 (1 - 4) days) (supplementary Fig. 3), but this was not statistically significant (p=0.86).
Discussion
In this retrospective observational study, we observed decreasing numbers of NNDs and a decreased neonatal mortality rate among both inborn and outborn neonates; the decrease in number deaths was more marked for deaths due to immaturity than for other cause-of-death codes. Over the same period of time, the NND rate in SA remained almost unchanged (between 11 and 12 deaths per 1 000 live births[2]); in Cape Town (Metro West), early NNDs decreased from 7.6 to 6.4 deaths per 1 000 live births (NSH 2018 PPIP report, Dr Lizel Jacobs, personal communication).
Possible reasons for the observed decrease in NNDs due to immaturity and in neonates in the lowest birthweight category through the course of the study period may be changes in policy regarding respiratory support for preterm neonates. Prior to 2015, neonates needing respiratory support received low-flow, non-humidified, blended oxygen. Limited CPAP machines were available for neonates weighing >1 000 g and if gestation was at least 28 weeks. From 2015, warmed humidified blended HFNC oxygen was available for all neonates, irrespective of weight or gestational age. From 2017, CPAP and surfactant were made available to neonates with birthweights >800 g if gestational age was at least 28 weeks. HFNC oxygen therapy in preterm infants has been associated with similar outcomes to nasal CPAP with fewer complications and less nasal trauma.[12] It is possible that the introduction of early HFNC oxygen in 2015 was associated with improved survival of preterm inborn neonates. The reason that deaths of outborn preterm neonates did not decrease to the same extent as inborn preterm neonates may be delays in transport from other facilities; neonates may have spent many hours receiving unheated non-humidified oxygen before admission to NSH, which may have caused hypothermia, atelectasis and lung inflammation.[11] Outborn neonates were also more likely to have died of infection-related causes: it is possible that potential improvements in respiratory care were mitigated by ongoing high risk of exposure to infection while in transit to NSH. It is difficult to see from these data whether decreasing the threshold to qualify for CPAP and surfactant in 2017 had any further impact on neonatal mortality. There had been some concern that introduction of HFNC oxygen in 2014 might prolong survival of ELBW neonates beyond day 7, but that most of these neonates would subsequently die of NEC or sepsis. However, since we did not observe an increase in late deaths from other causes, and the age at death did not change throughout the study period, we believe that the observed decrease in early deaths due to immaturity is real, and is not simply due to shifting the mortality burden into a different category. There was some turnover in doctors working in the unit, but there was low turnover of nursing staff; there were no other major changes in practice or policy regarding management of perinatal hypoxia, suspected sepsis or other neonatal conditions during the period under review.
The number of deaths due to hypoxia did not change much over the study period. Throughout the study period, NSH neonatal unit practised therapeutic hypothermia for moderate/severe hypoxic ischaemic encephalopathy, and used amplitude-integrated electroencephalograms to monitor cerebral function in neonates with brain injury. However, addressing deaths due to perinatal hypoxia will require obstetric interventions and labour ward management; improved neonatal care will not be sufficient to substantially reduce hypoxia-related deaths.[13] There were relatively more deaths due to hypoxia among inborn neonates compared with those referred in from other facilities. It is likely that the most severely brain-injured neonates who were born at other facilities demised within a few hours of life, before the ambulance transport could bring them to NSH.
The striking decrease in hospital-related NNDs has not been seen in the annual PPIP reports. There are a number of reasons for this. PPIP reports early NNDs according to the delivery unit, and includes all neonates who die in the labour ward or after admission to a neonatal unit. In this analysis, as we were only considering in-hospital mortality in the neonatal unit, we excluded all labour-ward deaths, but we included outborn neonates who were admitted to our unit if they subsequently died. For this reason, PPIP stats are a more sensitive longitudinal indicator of trends for delivery units and labour wards. The current analysis is better suited to detect trends in survival that are affected by changes in neonatal practice, not labour ward management.
There was a low rate of blood culture positivity among neonates who were attributed an 'infection-related' cause of death. Although the unit policy is to always draw blood for culture before starting or escalating antibiotics, it is possible that some critically ill neonates may have died before blood could be drawn, or that inadequate blood volumes were drawn.[14] This may have resulted in low culture positivity, or true pathogen growth may have been masked by skin contaminants.[15] It is also possible that some NNDs labelled as 'infection-related' may have been misclassified: neonates who had a clinical deterioration and demised may actually have had underlying cardiac or metabolic disease that was not diagnosed.
There is good evidence that both HIV-infected and HIV-exposed uninfected (HEU) infants have higher rates of infectious morbidity than HIV-unexposed infants.[16,17] However, the higher proportion of infection-related deaths among HIV-exposed neonates is difficult to interpret, as most neonates did not have nucleic-acid testing at the time of death. Universal antiretroviral therapy for pregnant women for prevention of mother-to-child transmission of HIV (PMTCT) was introduced in 2013[18] but universal birth polymerase chain reaction (PCR) testing was only introduced in 2015;[19] it is possible that some considered to be 'HIV-exposed' were actually HIV-infected in utero, and already had profound immune compromise at the time of death.
There are a number of important limitations of this data analysis. This was a retrospective review of an existing database; therefore no patient folders were reviewed or interrogated regarding possible adverse outcomes. We accepted the clinical judgement of the attending clinician at the time the data were captured regarding the likely cause and category of death. However, misclassification of causes of death is possible, as very few autopsies were performed. As mentioned above, cardiac or metabolic diseases may have been misclassified as 'infection-related' deaths. Annual total numbers of births in labour ward and admissions to the neonatal unit were available; however, as this was not disaggregated by birthweight categories, it is not possible to calculate a neonatal mortality rate per birthweight category as the denominator for each birthweight category is not known. It would have been valuable to calculate early and late NND rates by weight category, to observe if any changes occurred during the period under review. Data collection in 2014 was obviously incomplete; for 11 months of that year the number of deaths was far below the mean number of deaths for the rest of the study period, and there were 3 months with no deaths recorded at all. It would have been inappropriate to include that year.
However, it is possible that some deaths were missed and not entered into the database. The same paediatrician supervised the unit from the end of 2014 till 2018, and the same data capture systems were in place, but with a manual system of data capture and data entry, it is possible that some deaths may have been missed. PPIP does not capture access to neonatal therapies (HFNC, CPAP, surfactant, therapeutic hypothermia). It was not possible to compare impact of neonatal therapies within the neonatal unit over time as these data were not available.
Conclusion
In this retrospective analysis of an existing database, we observed a decreasing number of NNDs over the period 2011 - 2018; the category with the largest decrease was inborn deaths due to prematurity, while all other numbers of deaths due to all other causes remained approximately the same. This period coincided with the introduction of HFNC oxygen into the neonatal unit, and subsequent expansion of CPAP eligibility criteria from 1 000 g to 800 g. These advances in respiratory support may have contributed to some of the observed decreased deaths due to prematurity. Combinations of interventions may be required to reduce the residual burden of neonatal mortality in SA. Expansion of access to HFNC oxygen and CPAP may reduce deaths due to prematurity, but other upstream interventions, including improved access to antenatal care (like steroids for preterm labour) and other obstetric interventions in the labour ward will be required to address the residual burden of immaturity- and hypoxia-related causes of neonatal mortality.
Declaration. None.
Acknowledgements. The authors would like to thank Dr Geoff Moller, who started the original electronic neonatal unit database, and the nursing staff in the New Somerset Hospital neonatal unit, for their tireless efforts, and for teaching us so much about caring for neonates.
Author contributions. CG wrote the study protocol, obtained ethical approval, did the initial data analysis and wrote the first draft of the manuscript. DLR cleaned the data from 2011, and maintained the database from 2014; he helped with secondary analysis and statistics, and helped shape the direction of the discussion.
Funding. None.
Conflicts of interest. None.
References
1. Bamford LJ, McKerrow NH, Barron P, Aung Y. Child mortality in South Africa: Fewer deaths, but better data are needed. S Afr Med J 2018;108(3a):s25-s32. https://doi.org/10.7196/SAMJ.2017.v108i3b.12779 [ Links ]
2. Dorrington R, Bradshaw D, Laubscher R, Nannan N. Rapid mortality surveillance report 2018; South African Medical Research Council. www.mrc.ac.za/bod/reports.htm (accessed 16 December 2021). [ Links ]
3. Rhoda NR, Verlapi S, Gebhardt GS, Kauchali S, Barron P. Reducing neonatal deaths in South Africa: Progress and challenges. S Afr Med J 2018;108(3 suppl 1):s9-s16. https://doi.org/10.7196/SAMJ.2018.v108i3.12804 [ Links ]
4. Saving Babies 2012-2013: Ninth report on perinatal care in South Africa. Secondary Saving Babies 2012-2013: Ninth report on perinatal care in South Africa 2014. https://www.ppip.co.za/wp-content/uploads/Saving-Babies-2012-2013.pdf. (accessed 25 January 2019) [ Links ]
5. Lehtonen L, Gimeno A, Parra-Llorca A, Vento M. Early neonatal death: A challenge worldwide. Semin Fetal Neonatal Med 2017;22(3):153-160. https://doi.org/10.1016/j.siny.2017.02.006 [ Links ]
6. Damian DJ, Njau B, Lisasi E, Msuya SE, Boulle A. Trends in maternal and neonatal mortality in South Africa: A systematic review protocol. Syst Rev 2017;6(1):165. https://doi.org/10.1186/s13643-017-0560-1 [ Links ]
7. Velaphi S, Pattinson R. Avoidable factors and causes of neonatal deaths from perinatal asphyxia-hypoxia in South Africa: National perinatal survey. Ann Trop Paediatr 2007;27(2):99-106. https://doi.org/10.1179/146532807x192462 [ Links ]
8. Pieper CH, Smith J, Maree D, Pohl FC. Is nCPAP of value in extreme preterms with no access to neonatal intensive care? J Trop Pediatr 2003;49(3):148-52. https://doi.org/10.1093/tropej/49.3.148 [ Links ]
9. Kirsten GF, Kirsten CL, Henning PA, et al. The outcome of ELBW infants treated with NCPAP and InSurE in a resource-limited institution. Pediatrics 2012;129(4):e952-9. https://doi.org/10.1542/peds.2011-1365 [ Links ]
10. Hodgson KA, Manley BJ, Davis PG. Is Nasal high flow inferior to continuous positive airway pressure for neonates? Clin Perinatol 2019;46(3):537-551. https://doi.org/10.1016/j.clp.2019.05.005 [ Links ]
11. Gibbs L, Tooke L, Harrison MC. Short-term outcomes of inborn v. outborn very-low-birth-weight neonates (<1 500 g) in the neonatal nursery at Groote Schuur Hospital, Cape Town, South Africa. S Afr Med J 2017;107(10):900-903. https://doi.org/10.7196/SAMJ.2017.v107i10.12463 [ Links ]
12. Lavizzari A, Colnaghi M, Ciuffini F, et al. Heated, humidified high-flow nasal cannula vs nasal continuous positive airway pressure for respiratory distress syndrome of prematurity: A randomised clinical noninferiority trial. JAMA Pediatr 2016. https://doi.org/10.1001/jamapediatrics.2016.1243 [ Links ]
13. Moshiro R, Mdoe P, Perlman JM. A global view of neonatal asphyxia and resuscitation. Front Pediatr 2019;7:489. https://doi.org/10.3389/fped.2019.00489 [ Links ]
14. Hazen KC, Polage CR. Using data to optimise blood bottle fill volumes and pathogen detection: Making blood cultures great again. Clin Infect Dis 2020;70(2):269-270. https://doi.org/10.1093/cid/ciz203 [ Links ]
15. Hamilton LF, Gillett HE, Smith-Collins A, Davis JW. A sterile collection bundle intervention reduces the recovery of bacteria from neonatal blood culture. Biomed Hub 2018;3(1):1-7. https://doi.org/10.1159/000486703 [ Links ]
16. Slogrove AL, Goetghebuer T, Cotton MF, Singer J, Bettinger JA. Pattern of infectious morbidity in HIV-exposed uninfected infants and children. Front Immun 2016;7:164. https://doi.org/10.3389/fimmu.2016.00164 [ Links ]
17. Le Roux SM, Abrams EJ, Donald KA, et al. Infectious morbidity of breastfed, HIV-exposed uninfected infants under conditions of universal antiretroviral therapy in South Africa: A prospective cohort study. Lancet Child Adolesc Health 2020;4(3):220-231. https://doi.org/10.1016/S2352-4642(19)30375-X [ Links ]
18. National Department of Health, South Africa. South African Antiretrovial Treatment Guidelines, 2013. https://sahivsoc.org/Files/2013%20ART%20Treatment%20Guidelines%20Final%2025%20March%202013%20corrected.pdf (accessed 20 September 2017). [ Links ]
19. National Department of Health, South Africa. National Consolidated Guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults, 2015. https://sahivsoc.org/Files/ART%20Guidelines%2015052015.pdf (accessed 28 July 2018). [ Links ]
 Correspondence:
Correspondence:
D M le Roux
dave.leroux@uct.ac.za
C Gabriels
cgabriels4@icloud.com
Accepted 8 May 2023














