Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.17 no.2 Pretoria Jun. 2023
http://dx.doi.org/10.7196/SAJCH.2023.v17i2.1951
ARTICLE
The role of β-2-microglobulin and cystatin C as urinary biomarkers of focal segmental glomerulosclerosis in the setting of paediatric HIV infection
K PersadI; L NandlalII; R BhimmaIII; T NaickerII
IMMedSci; Discipline of Optics and Imaging, College of Health Sciences, Doris Duke Medical Research Institute, University of KwaZulu-Natal, Durban, South Africa
IIPhD; Discipline of Optics and Imaging, College of Health Sciences, Doris Duke Medical Research Institute, University of KwaZulu-Natal, Durban, South Africa
IIIPhD, MD; Department of Paediatrics and Child Health, Nelson R. Mandela School of Medicine, College of Health Sciences, University of KwaZulu-Natal, Durban, South Africa
ABSTRACT
BACKGROUND. Africa has the highest rate of HIV infection, and HIV-associated nephropathy (HIVAN) is one of the most frequent kidney diseases observed in children. HIVAN in children usually presents as a form of nephrotic syndrome, predominantly focal segmental glomerulosclerosis (FSGS) on histopathology, that often leads to chronic kidney failure.
OBJECTIVE. This study determined the urinary concentrations of β-2-microglobulin (β2M) and cystatin C proteins in children with HIVAN and primary FSGS.
METHODS. The study group comprised 34 children; 14 with HIVAN and 20 with primary FSGS. The control groups were 20 HIV-positive and 20 HIV-negative children with no kidney disease. Urine samples collected from these 74 children were stored at -80°C. Bio-Plex technology was used to analyse the urinary protein concentration of cystatin C and β2M.
RESULTS. A significant increase in urinary β2M levels was observed in the HIVAN group compared with the HIV-negative group (p=0.0240). No other statistically significant differences in urinary β2M concentrations were noted across the study groups. Urinary cystatin C levels were significantly increased in primary FSGS children compared with both HIV-negative (p=0.0041) and HIV-positive controls (p=0.0256). Urinary cystatin C displayed a significant increase in the primary FSGS compared with the HIVAN group (p=0.0150). No significant differences in urinary cystatin C levels were noted in the HIVAN group compared with the HIV-negative and HIV-positive control groups.
CONCLUSION. Urinary cystatin C has promising prognostic value to predict primary FSGS from HIVAN.
Keywords: Biomarkers, paediatric, FSGS, HIV, urinary proteins.
Africa has the highest incidence of HIV infection, affecting ~37.6 million people.[1] Moreover, 1.7 million children (<14 years old) are HIV infected, with a further 160 000 being newly infected annually.[1]-Currently, 1.7 million children (<15 years old) are infected with HIV, most of these infections occurring in Africa.[2] Notably, a 50% decrease in new paediatric HIV infections has been reported owing to access to antiretroviral therapy (ART).[3] Globally, kidney disease is rapidly becoming a major public health concern as a cause of morbidity and mortality in children.[3] Kidney disease in the setting of HIV that is untreated often leads to rapid progression to chronic kidney failure (CKF).[4]
In one of the largest studies of HIV-related kidney diseases in children in Africa, Ramsuran et al.[4] reported that nephrotic syndrome owing to HIV-associated nephropathy (HIVAN) is the most common form of kidney disease in the setting of HIV. The prevalence of HIVAN has increased in both adults and children.[5] Previous studies have indicated that the proportion of children with HIVAN was 10 - 15%.[26]
Focal segmental glomerulosclerosis (FSGS) is a common histopathological form of primary steroid-resistant nephrotic syndrome with a high propensity for progression to CKF in children and adolescents.[7] Approximately 30% to 60% of patients who are diagnosed with primary FSGS are likely to progress to CKF over a period of 5 - 10 years.[8] While a kidney biopsy is the gold standard for providing a histopathological assessment of the type of pattern of kidney disease, it is an invasive procedure with attendant complications such as bleeding, infection, visceral perforation, or arterio-venous fistula formation. The current strategy for detection and monitoring the effect of treatment in kidney diseases in children and adults includes the utilisation of serum creatinine levels.[9,10] However, several factors limit its use, as serum creatinine values are influenced by protein intake, nutritional status, muscle mass and body weight, all of which are affected in HIV-infected children and primary nephrotic syndrome. Hence, the use of a non-invasive biomarker to detect kidney disease is urgently warranted.[11]
β-2-microglobulin (β2M) is an 11kDa polypeptide protein that is freely filtered in the glomeruli and reabsorbed and metabolised in the proximal tubule. Although urinary excretion of β2M is an indicator of underlying kidney disease, it is nonspecific as increased urinary excretion may occur in other diseases such as autoimmune diseases, malignancies and especially in AIDS. [12] There is, however, a lack of data on the reliability of β2M as a biomarker to differentiate between various forms of kidney disease.
Cystatin C is a 13kDa plasma protein that functions as a cysteine protease inhibitor,[13] and its dysregulation has been implicated in the detection of impaired kidney function.[14] In patients with a kidney transplant, diabetes mellitus and chronic kidney disease (CKD), cystatin C is found much earlier in the urine than creatinine. The measurement of blood cystatin C is believed to be of better prognostic value than creatinine for estimating the glomerular filtration rate (GFR), especially in patients with more advanced kidney disease who have a GFR <60 mL/min/1.73 m2. This has successfully been demonstrated in children with kidney disorders caused by various conditions.[15]
To evaluate the accuracy of ß2M and cystatin C as predictors of kidney disease in HIV-infected children, notably, HIVAN, we compared the urinary levels of ß2M and cystatin C in children with HIVAN and primary FSGS with a control group of children (HIVpositive and HIV-negative) with no kidney disease.
Methods
Study design
Ethical permission to conduct this study was obtained from the Biomedical Research Ethics Committee of the College of Health Sciences, University of KwaZulu-Natal, (BREC reference: BE202/17). Urine samples were collected from children attending the Inkosi Albert Luthuli Central Hospital and King Edward VIII Hospital in Durban, KwaZulu-Natal, South Africa (SA). Informed consent was obtained from the parent or guardian and assent (where applicable) from the patient prior to collection of urine samples. Samples were collected 2 - 4 years after the kidney biopsy was done, aliquoted and stored in cryovials at -80°C for a period of ~3 months until analysed.
Study population
Seventy-four black SA children aged 1 - 16 years were recruited. The study group (N=34) consisted of children with biopsy-proven HIVAN (n=14) and primary FSGS (n=20). At the time of sample collection, none of the children had a fever or any other evidence of secondary infections. In children with HIVAN, comorbidities included cardiomyopathy (n=6), chronic lung disease (n=4) and stunting (n=4). The control group (n=40) consisted of children who were HIV-positive with no kidney disease (n=20) and HIV-negative with no kidney disease (n=20). The HIV-negative children with no kidney disease were recruited from follow-up clinics, e.g. neurology, endocrine and respiratory clinics.
Prior to recruitment, all 14 children with HIVAN were on combined ART and angiotensin-converting enzyme antagonists for a minimum of 2 years. At the time of sample collection, the 20 children with primary FSGS were on low-dose steroids, angiotensin-converting enzyme inhibitors as well as additional immunosuppressants such as calcineurin inhibitors (cyclosporin or tacrolimus) and/or mycophenolate mofetil.
Diagnosis of HIVAN
The classification of HIVAN was based on the confirmation of HIV-1 infection and presence of persistent proteinuria >1+ on urinary dipstick examination with histopathological findings of FSGS and one or more of the following: (i) abnormal urinary sediment; (ii) presence of enlarged echogenic kidney on ultrasound; and (iii) microcystic tubular dilation.[4,15]
BioPlex Multiplex
The urine samples were analysed for ß2M and cystatin C using the Bio-Plex Pro RBM kidney toxicity assay (panel 2) (Bio-Rad Laboratories, USA) according to the manufacturer's instructions.[17]
The detection of reaction was carried out using the Bio-Plex MAGPIX 200 reader system (Bio Rad Laboratories, 2017). Raw data were collated using Bio-Plex Manager software version 4.1. A standard curve was generated using the known concentration (ng/mL) of each analyte by plotting the median fluorescent intensity (MFI) signal against concentration. These standards were used to interpolate the concentration of the unknown samples. Intra-plate variability was determined with CV <20% and (X100) between 70% and 130% (r=0.8, p=0.05). All data were imported to a Microsoft Excel (version 2018; Microsoft Corp., USA) spreadsheet for statistical analysis.
Statistical analysis
Non-parametric tests (Mann-Whitney U) were performed for statistical analysis using GraphPad Prism version 5 (GraphPad software version 5, USA). One-way ANOVA and Dunn's post hoc multiple comparison test were used. Spearman's coefficients were used to evaluate correlations. The level of statistical significance was considered as JK0.05. Graphical data were represented as median and interquartile range.
Results
The study group comprised 34 children with biopsy-proven FSGS with a histopathological pattern not otherwise specified based on the Columbia classification.'181 Fourteen children (41%) were HIV-positive and were confirmed to have paediatric HIVAN, and 20 children (59%) had primary FSGS. The mean age (standard deviation (SD)) for HIVAN and primary FSGS was 10 (3.62) years (range 6.00 - 19.00) and 9 (3.11) years (range 4.00 - 13.00), respectively (Table 1). The control groups consisted of 40 children with no kidney disease; 20 (50%) children were HIV-negative with a mean (SD) age of 7 (3.87) years (range 2.00 - 14.00) and 20 (50%) HIV-positive children with a mean age of 11 (3.52) years (range 5.00 - 15.00).
The patients with known FSGS had stages 1 to 4 CKD. In the primary FSGS group, 11 patients had CKD stage 1, 4 stage 2, 2 stage 3, and 3 stage 4, according to the KDIGO classification of CKD.[10] In the HIVAN group, 8 patients were CKD stage 1, 1 stage 2, 3 stage 3 and 2 stage 4. These patients were diagnosed with FSGS for a mean of 2.8 years with a range of 2.1 - 4.3 years prior to study entry. Kidney biopsy showed FSGS (not otherwise specified) in all patients with >80% of glomeruli having more than 50% sclerosis.
To determine the associations with the variability, we compared the urinary protein concentration of ß2M and cystatin C with age, weight, creatinine, urine creatinine, eGFR, urea, albumin and cholesterol in the four groups of children. No statistically significant correlation was observed in ß2M and cystatin C when compared with the above clinical and biochemical findings; this indicates that these factors had no major impact on the concentration of these urinary proteins in children.
Urinary concentrations β2M and cystatin C
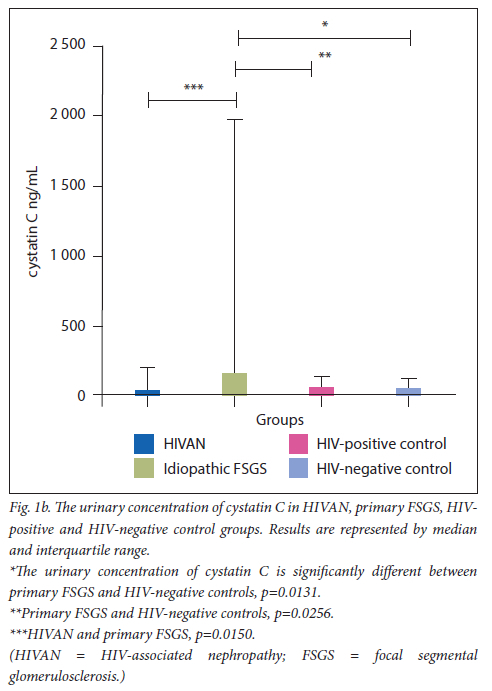
The urinary concentrations of ß2M and cystatin C are displayed in Figs 1a and 1b, respectively.
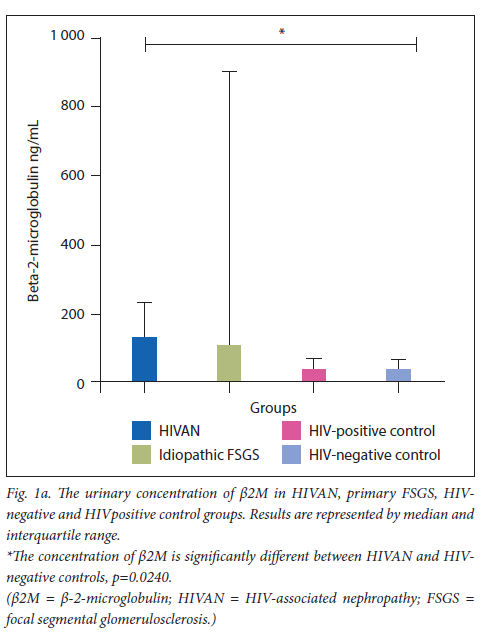
A statistically significant increase was observed in urinary ß2M excretion in the HIVAN (mean=169.7 ng/mL, 95% CI 272.3 - 67.15) group compared with the-HIV negative control (mean=52.15ng/mL, 95% CI 75.19 - 29.10) (Mann-Whitney U=75.00; p=0.0240). No other statistically significant differences of urinary ß2M concentrations were noted across the study groups (Table 2).
There was a significant increase of cystatin C in the primary FSGS group (mean=987.7 ng/mL, 95% CI 1 689 - 286.7) compared with the HIV-negative control group (mean=87.49 ng/mL; 95% CI 1 44.0 - 30.97) (Mann-Whitney U=93.50; p=0.0041). There was a statistically significant increase in the primary FSGS group (mean=987.7 ng/mL, 95% CI 1 689 - 286.7) compared with the HIVpositive control (mean=104.5 ng/mL, 95% CI 152.5 - 56.41) (Mann Whitney U=117.0; p=0.0256). There was also a significant increase in the primary FSGS group (mean=987.7 ng/mL, 95% CI 1 689 - 286.7) compared with the HIVAN group (mean=203.5 ng/mL, 95% CI 400.5 - 6.522) (Mann-Whitney U=70.00; p=0.0150).
Cystatin C levels were down-regulated in the HIVAN group (mean=203.5 ng/mL, 95% CI 400.5 - 6.522) compared with the HIV-negative control group (mean=87.49 ng/mL; 95% CI 144.0 - 30.97); however, this did not reach a statistical significance (Mann- Whitney U=139.0; p=0.9860). A non-significant decrease (Mann Whitney U=117.0; p=0.4311) was observed in the HIVAN group (mean=203.5 ng/mL, 95% CI 400.5 - 6.522) compared with the HIVpositive control group (mean=104.5 ng/mL, 95% CI 152.5 - 56.41).
Discussion
In this study, we report on two candidate urinary biomarkers (β2M and cystatin C) in HIVAN and primary FSGS (all presenting as nototherwise- specified variants of FSGS on histopathology) compared with HIV-positive and -negative controls.
The expression of urinary β2M was only significantly upregulated in HIVAN compared with the HIV-negative control group. Our results are corroborated by previous studies by Nishijima et al. who reported high levels of β2M and α1M as biomarkers in the detection of kidney tubulopathy in patients with HIV-1 infection.[19] The latter study, however, was not on patients with HIVAN. A study conducted by Garcia et al. reported an increase in β2M in urine of children with HIVAN.[20]
Of note, in kidney disease, urinary β2M is generally elevated, reflecting a dysfunction in proximal tubular reabsorption.[21] In healthy individuals, owing to the low molecular mass of these proteins, they are easily filtered through the glomerular filtration apparatus and are reabsorbed in the proximal convoluted tubules.[21] These results indicate that the upregulation of β2M noted in our study may be attributed to either abnormal glomerular filtration or proximal tubular reabsorptive dysfunction in children with HIVAN. In primary FSGS, there is also tubular involvement to varying degrees.[22] It is possible that the degree of proximal tubular dysfunction may not have been enough in our group of patients to show significant differences between this group and healthy controls. A study by Kim and Lim, however, reported higher urinary levels of β2M in children with FSGS.[23] This finding may be attributed to proximal convoluted tubular pathology where they are unable to absorb and transfer β2M back into the interstitial capillaries and into the general circulation.
Donadio reported a significant elevation of urinary β2M concentrations in patients with CKD at stage 4 and 5.[24] It is also documented that high β2M is evident in kidney infection, chronic kidney failure and various connective tissue diseases.[21] Also, this outcome in our study may be due to the small sample size that was used, making it difficult to detect significant differences across the groups.
In our study, we also report a statistically significant increase of urinary cystatin C concentration in the primary FSGS group compared with the HIV-negative control group, as well as the HIVpositive control group. In a study on lupus nephritis, a condition that causes glomerular injury similar to FSGS, Tony et al. observed a significant increase of urinary cystatin C excretion in patients with lupus nephritis compared with controls.[25] Further, Donadio reported a significant elevation of urinary cystatin C concentrations in patients with CKD at stage 4 and 5, compared with individuals with normal GFR.[24] These findings on elevated cystatin C excretion were similar to our study. Urinary cystatin C proteins are known to protect tissues and cells from damage owing to intracellular enzymes released from apoptosis or malignancy. However, when glomerular sclerosis is present, the levels of cystatin C may increase,[15] as was observed in our study.
We demonstrated a significant down-regulation of urinary cystatin C levels in the HIVAN group compared with the primary FSGS group. In contrast to our study, elevated levels of cystatin C in the urine of HIV-infected children with proteinuria have been reported by Garcia et al., suggesting a compromised capacity of the proximal tubular epithelial cells to reabsorb and metabolise cystatin C in these patients.[26,27] This apparent contradiction with our study results may also be explained by a discordance between the immunological and clinical stages of HIV disease as all patients were on ART.[28]
Nevertheless, in one study, serum cystatin C, which reflects kidney proximal tubular dysfunction, directly correlated with HIV viral load.[28] On the other hand, patients with a very low viral load, including those receiving kidney transplants, may also develop HIVAN.[27] Further, even though cystatin C is a potent marker for inflammation and kidney disease, it has also been shown to have antiviral activity.[29,30] It is plausible that in HIV-infected patients, as in our cohort, there may have been a down-regulation of serum cystatin C owing to its interaction with the virus, and hence low urinary cystatin C excretion in the HIVAN group.
The limitations of our study were sample size and absence of viral load; hence we were not able to correlate our data with severity of HIV infection. The findings of this study may not apply to other population groups as this was a single-centre study in a homogeneous group of black African children. Also, all HIV-infected patients recruited into the study were on treatment, which could have affected the levels of urinary biomarkers studied. Future investigations should also consider an assessment of nutritional state.
Conclusion
This novel study demonstrates significant difference of urinary cystatin C expression in children with primary FSGS compared with HIVAN and with controls. Moreover, β2M was significantly different between HIVAN v. HIV-negative controls. Larger prospective studies to determine the role of cystatin C in early detection of HIVAN, thus obviating the need for kidney biopsy, and allowing early institution of appropriate therapy, thereby improving clinical outcome and survival, are needed.
Declaration. None.
Acknowledgements. We gratefully acknowledge the patients who consented to participate in this study; Inkosi Albert Luthuli and King Edward VIII Hospitals; and Drs E Naicker and K Naidoo for their assistance during the sample collection. We also thank the Optics and Imaging Centre at the Doris Duke Medical Research Institute, of the Nelson R Mandela School of Medicine in South Africa, for use of the Bioplex equipment.
Author contributions. Conceptualisation and conceiving of idea: RB, TN. Writing the article: KN, LN. Research: KN, LN, RB, TN. Editing of article: TN, RB. All authors contributed to the article and approved the submitted version.
Funding. Funding was received from the College of Health Sciences, University of KwaZulu-Natal.
Conflicts of interest. The authors have no conflicts of interest to declare.
References
1. UNAIDS Data 2020. 2021. https://www.unaids.org/en/resources/fact-sheet (accessed 25 October 2021). [ Links ]
2. Global Statistics. The Global HIV/AIDS Epidemic. Last updated 25 June 2021. https://www.hiv.gov/hiv-basics/overview/data-and-trends/global-statistics (accessed 31 October 2021). [ Links ]
3. Jindal AK, Tiewsoh K, Pilania RK. A review of renal disease in children with HIV infection. Infect Dis 2017;1-12. https://doi.org/10.1080/23744235.2017.1371852 [ Links ]
4. HIVAN, Pediatric. In: Trachtman H, Herlitz L, Lerma E, Hogan J, editors. Glomerulonephritis. Cham: Springer, 2019. https://doi.org/10.1007/978-3-319-49379 [ Links ]
5. Ramsuran D, Bhimma R, Ramdial PK, et al. The spectrum of HIV-related nephropathy in children. Pediatric Nephrol 2012;27(5):21-27. https://doi.org/10.1007/s00467-011-2074-8 [ Links ]
6. Soler-Palacín P, Melendo S, Noguera-Julian A, et al. Prospective study of renal function in HIV-infected pediatric patients receiving tenofovir-containing HAART regimens. AIDS 2011;25(2):171-176. https://doi.org/10.1097/QAD.0b013e328340fdca [ Links ]
7. Sharma M, Sharma R, McCarthy ET, Savin VJ. The focal segmental glomerulosclerosis permeability factor: Biochemical characteristics and biological effects. Exper Biol Med 2004;229(1):85-98. https://doi.org/10.1177/153537020422900111 [ Links ]
8. Kiffel J, Rahimzada Y, Trachtman H. Focal segmental glomerulosclerosis and chronic kidney disease in pediatric patients. Adv Chronic Kidney Dis 2011;18(5):332-382. https://doi.org/10.1053/j.ackd.2011.03.005 [ Links ]
9. Coca SG, Parikh CR. Urinary biomarkers for acute kidney injury: Perspectives on translation. Clin J Am Soc Nephrol 2008;3(2):481-490. https://doi.org/10.2215/CJN.03520807 [ Links ]
10. Levin A, Stevens PE. Summary of KDIGO 2012 CKD Guideline: Behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014;85(1):49-61. https://doi.org/10.1038/ki.2013.444 [ Links ]
11. Giorgi A. Renal Biopsy. 2016. http://www.healthline.com/health/renal-biopsy#ReadThisNext8 (accessed 25 July 2017). [ Links ]
12. Bethea M, Forman D. Beta 2-microglobulin: Its significance and clinical usefulness. Ann Clin Lab Sci 1990;20(3):163-168. [ Links ]
13. Dajak M, Ignjatovic S, Stojimirovic B, Gajic S, Majkic-Singh N. Beta-trace protein as a marker of renal dysfunction in patients with chronic kidney disease: Comparison with other renal markers. J Med Biochem 2010;29(2):66-72. [ Links ]
14. Wu I, Parikh CR. Screening for kidney diseases: Older measures versus novel biomarkers. Clin J Am Soc Nephrol 2008;3(6):1895-1901. https://doi.org/10.2215/CJN.02030408 [ Links ]
15. Deyà-Martínez À, Fortuny C, Soler-Palacín P, et al. Cystatin C: A marker for inflammation and renal function among HIV-infected children and adolescents. Pediatr Infect Dis J 2016;35(2):196-200. https://doi.org/10.1097/INF.0000000000000960 [ Links ]
16. Senguttuvan P. Human immunodeficiency virus-associated nephropathy (HIVAN) in Indian children. Open Urology Nephrol J 2014;7(1). [ Links ]
17. Inc BRL. Bio-Plex Pro RBM Kidney Toxicity Assays, Instruction Manual. www.bio-rad.com/webroot/web/pdf/lsr/literature/10028258.pdf (accessed 27 February 2017). [ Links ]
18. DAgati V, Fogo AB, Bruijn JA, Jennette JC. Pathologic classification of focal segmental glomerulosclerosis: A working proposal. Am J Kidney Dis 2004;43(2):368-382. https://doi.org/10.1053/j.ajkd.2003.10.024 [ Links ]
19. Nishijima T, Shimbo T, Komatsu H, et al. Urinary beta-2 microglobulin and alpha-1 microglobulin are useful screening markers for tenofovir-induced kidney tubulopathy in patients with HIV-1 infection: A diagnostic accuracy study. J Infect Chemother 2013;19(5):850-857. https://doi.org/10.1007/s10156-013-0576-y [ Links ]
20. Soler-García ÁA, Johnson D, Hathout Y, Ray PE. Iron-related proteins: Candidate urine biomarkers in childhood HIV-associated renal diseases. Clin J Am Soc Nephrol 2009;4(4):763-771. https://doi.org/10.2215/CJN.0200608 [ Links ]
21. Sonkar G, Singh R. A preliminary study on the significant value of beta-2-microglobulin over serum creatinine in renal transplant rejection and renal failure. Singapore Med J 2008;49(10):786. [ Links ]
22. Erkan E, Garcia CD, Patterson LT, et al. Induction of renal tubular cell apoptosis in focal segmental glomerulosclerosis: Roles of proteinuria and Fas-dependent pathways. J Am Soc Nephrol 2005;16(2):398-407. https://doi.org/10.1681/ASN.2003100861. [ Links ]
23. Kim DW, Lim IS. Analysis of urine ß2-microglobulin in pediatric renal disease. Korean J Pediatr 2007;50(4):369-375. [ Links ]
24. Donadio C. Serum and urinary markers of early impairment of GFR in chronic kidney disease patients: Diagnostic accuracy of urinary beta-trace protein. Am J Physiology-Renal Physiol 2010. https://doi.org/10.1152/ajprenal.00507.2009 [ Links ]
25. Tony E, Mohammed H, Fathi N, Tony A, Afifi O. Serum and urinary biomarkers endothelin-1, beta-2 microglobulin, cystatin C, galectin-3 and alpha-1-acid glycoprotein; can they surrogate clinical and histological staging in lupus nephritis patients? J Arthritis 2016;5(223):2. [ Links ]
26. Soler-García ÁA, Rakhmanina NY, Mattison PC, Ray PE. A urinary biomarker profile for children with HIV-associated renal diseases. Kidney Int 2009;76(2):207-214. https://doi.org/10.1038/ki.2009.115 [ Links ]
27. Perazzo S, Soler-García ÁA, Hathout Y, Das JR, Ray PE. Urinary biomarkers of kidney diseases in HIV-infected children. PROTEOMICS-clinical applications. 2015;9(5-6):490-500. https://doi.org/10.1002/prca.201400193 [ Links ]
28. Jaroszewicz J, Wiercinska-Drapalo A, Lapinski TW, Prokopowicz D, Rogalska M, Parfieniuk A. Short communication. Does HAART improve renal function? An association between serum cystatin C concentration, HIV viral load and HAART duration. Antiviral Therapy 2006;11:641-645. [ Links ]
29. Luthra K. Antiviral activity of cystatin C against HIV. Indian J Med Res 2015;141(4):383. https://doi.org/10.4103/0971-5916.159242 [ Links ]
30. Vernekar V, Velhal S, Bandivdekar A. Evaluation of cystatin C activities against HIV. Indian J Med Res 2015;141(4):423. https://doi.org/10.4103/0971-5916.159282 [ Links ]
 Correspondence:
Correspondence:
T Naicker
naickera@ukzn.ac.za
Accepted 7 February 2023














