Serviços Personalizados
Artigo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares em Google
Similares em Google
Compartilhar
South African Journal of Child Health
versão On-line ISSN 1999-7671
versão impressa ISSN 1994-3032
S. Afr. j. child health vol.17 no.1 Pretoria 2023
http://dx.doi.org/10.7196/SAJCH.2023.V17I1.1899
RESEARCH
The cost-effectiveness and value of C-reactive protein in the diagnosis and management of neonatal late-onset sepsis in resource-limited settings
J ChandramatiI; S PonthenkandathII
IMD; Division of Neonatology, Amrita Institute of Medical Sciences, Amrita University, Amrita Viswavidyapeetam, Kochi, Kerala, India
IIMD, FAAP; ivision of Neonatology, Amrita Institute of Medical Sciences, Amrita University, Amrita Viswavidyapeetam, Kochi, Kerala, India
ABSTRACT
BACKGROUND: Although C-reactive protein (CRP) is used as a biomarker, its value in resource-limited settings for diagnosis and management of late-onset sepsis in neonates has not been reported previously
OBJECTIVE: To evaluate the value of CRP as a biomarker in identifying late-onset sepsis in symptomatic infants
METHOD: We performed a retrospective study to evaluate the value of CRP as a biomarker in identifying late-onset sepsis in symptomatic infants. Infants were classified into three groups (blood culture-proven sepsis (n=72), clinical sepsis (n=38) and no sepsis (n=114)). Infants underwent sepsis work-up consisting of complete blood count (CBC), blood culture, urine and cerebrospinal fluid (CSF) culture including CRP measurements
RESULTS: The overall sensitivity and specificity of CRP levels >10 |ig/mL was 94.5% and 91.2%, respectively, for the diagnosis of late-onset sepsis. Positive predictive value was 91.2% and negative predictive value was 92.8%. Total white blood cell (WBC) counts had poor sensitivity and specificity compared with CRP. The cost for CRP testing was only 2.5% of the total cost for sepsis work-up in neonates
CONCLUSION: Our study indicates that CRP has excellent sensitivity and specificity in the diagnosis of late-onset sepsis. In low- and middle-income countries, CRP testing perhaps offers more value compared with W14BC counts
Keywords: CRP; late-onset sepsis; neonates; cost-effectiveness; value.
Neonatal sepsis, classified as early onset (onset within 72 hours of age) and late onset (onset after 72 hours of age), is responsible for significant morbidity and mortality among high-risk newborns. The outcome of neonatal sepsis is dependent on early recognition and therapy[1] Because the presenting symptoms and signs are vague in neonatal sepsis, early recognition and prompt treatment are aided by laboratory tests.
C-reactive protein (CRP), first discovered in 1930, has been extensively studied as a biomarker for inflammation and sepsis in adults and children.[2-5] Previous studies have reported the usefulness of CRP in identifying cases of early-onset sepsis in neonates.[6-13] Serial CRP levels checked at 12 and 24 hours after the onset of illness have been reported to have high sensitivity in early-onset sepsis.[6,10-12]
Neonatal sepsis is widely prevalent in India, and is a major contributing factor with significant morbidity and mortality, particularly among high-risk neonates. Early-onset sepsis is due to vertical transmission from the mother, and late-onset sepsis is due to horizontal transmission from the environment and caregivers. Group B beta streptococcus (GBS) as a pathogen in early-onset sepsis is rare in India, owing to the low maternal carrier state, and consequently screening for GBS in pregnant women and intrapartum antibiotic prophylaxis for prevention of early-onset GBS are rarely carried out. The predominant organisms identified in late-onset sepsis in India are Gram-negative pathogens. In the present single-centre study we evaluated the accuracy and cost of CRP in diagnosing late-onset sepsis in preterm infants in a tertiary-care neonatal intensive care unit (NICU).
Methods
This single-centre retrospective study of neonates who were admitted to a tertiary care NICU (Amrita Institute of Medical Sciences) in India was approved by the institutional review board (research and ethics committee). All infants admitted to the tertiary care NICU for 36 months (2013 - 2016) were included. The inclusion criteria were all infants who developed clinical signs of sepsis after 72 hours of age and underwent diagnostic laboratory evaluation and treatment. Infants who did not develop clinical signs of sepsis were excluded. Late-onset sepsis was suspected whenever there was an unexplained change in the clinical condition of the infant. The clinical findings included temperature instability, unexplained tachycardia, lethargy, feeding intolerance - increased gastric residuals (more than 30% of prior feeding volume three times consecutively, abdominal distension with or without emesis or bloody stools, apnoea with or without hypoxic spells requiring oxygen supplementation, poor ventilatory effort requiring assisted ventilation, metabolic acidosis, or hyperglycaemia. These infants underwent a septic work-up which included complete blood count (CBC), differential count, CRP blood, urine and cerebrospinal fluid (CSF) cultures, and were treated with antibiotics (usually ampicillin and amikacin). CRP levels were repeated at 24-hour intervals for two days and if abnormal on the third day also.
We collected the following information from the medical records: Apgar scores, presence of chorioamnionitis, gestational age, birthweight, age, results of the blood, urine and CSF cultures, duration of antibiotic therapy, total white blood cell (WBC) count, platelet count, IT ratio (immature to total neutrophil ratio), and CRP levels. We tried to correlate the CRP levels to clinical symptoms and laboratory findings. Infants were classified into three groups based upon the clinical outcome and laboratory findings.
Group 1. Blood culture-proven sepsis. In this group, blood cultures were repeated at 48 - 72-hour intervals until they were negative. Antibiotic therapy was discontinued 7 days after negative blood culture; most infants received treatment for 10 - 14 days.
Group 2. Clinical sepsis. This group comprised infants with negative blood culture but with an abnormal CBC with either elevated WBC count (>30 000/and/ or leukopenia (<5 000/|iL), and/or abnormal IT ratio (>0.2) and/or thrombocytopenia (<100 000/nL) and/or elevated CRP(>10|ig/ ml). CBC was repeated 24 hours after the first test. A decrease in the platelet count from the first CBC by 20% or higher in 24 hours was also considered significant. Infants in this group were treated for 7 - 8 days as dictated by the resolution of clinical symptoms, usually 4-5 days after initiation of antibiotic therapy and normalisation/improvement in laboratory test results.
Group 3. No-sepsis group. This group comprised infants with negative blood culture and normal laboratory data whose symptoms resolved in less than 24 hours. Antibiotic therapy was discontinued after 48 - 72 hours when blood culture results were available.
For data analysis of sepsis v. no-sepsis, we combined groups 1 and 2 as the sepsis group and group 3 as the no-sepsis group.
Results
During the study period, 1 492 infants were admitted to the NICU. Excluded were 110 infants <3 days of age and 1 158 infants who did not have clinical sepsis after 72 hours of age (Fig. 1).
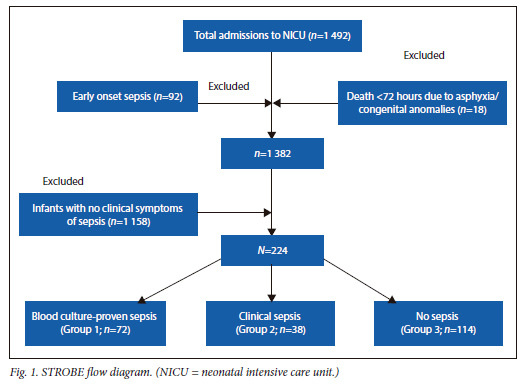
There were 224 infants who developed clinical signs and symptoms of sepsis included in this study. The overall incidence of late-onset sepsis was 16.2%. The mean birthweight (BW) of the whole cohort (n=224) was 2 170 ± 885 g and gestational age was 35.5 ± 4.5 weeks. The postnatal age of onset of symptoms was 13 ± 10 days. Overall mortality was 9.8% (n=22). The mean birthweight of infants in groups 1 and 2 combined (culture-proven and clinical sepsis) was 1 980 ± 910 g (range 570 -4 200 g) which was lower than that of infants in group 3 (non-septic group), of 2 330 ± 800 g (range 620 - 3 800 g) (p=0.007). Among the pathogens in the blood cultures, 62 (86%) were Gram-negative organisms, 8 (11 %) were Gram-positive organisms and 2 (3%) were Candida species. The types of organism isolated are given in the appendix. There were no cases of meningitis, necrotising enterocolitis (NEC), isolated urinary tract infection (UTI), bone and joint infections or pneumonia. There were 10 cases of central line-associated blood stream infections.
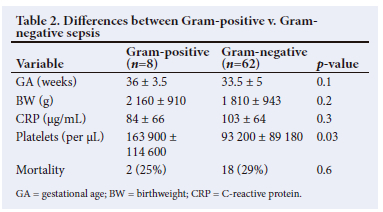
Mortality was confined to Group 1. Mortality among infants with Gram-negative sepsis was 29% (n=18). Two infants with Gram-positive sepsis died (25%) and 2 infants with Candida sepsis died. There was no difference in mortality rate between Gram-negative and Gram-positive infections (29% v. 25% (p=0.6)). The differences in variables between survivors and non-survivors are given in Table 1, and differences between Gram-negative and Gram-positive septic infants are given in Table 2.
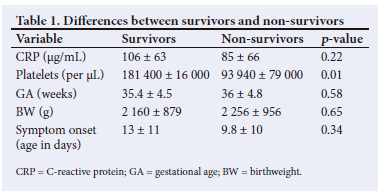
CRP levels rose from 75.7 ± 64 to 108 ± 64 µg/mL (p=0.008) in 48 hours in the sepsis group (Groups 1 and 2). There was no significant difference in CRP levels between 48 and 72 hours. Overall, there was a marginal decrease in CRP levels by 72 hours compared with 48-hour levels. In Group 3 infants, there was no difference in CRP levels between 24 and 48 hours. There was no significant difference in CRP levels between Gram-positive and Gram-negative sepsis (83 ± 66 v. 103 ± 64 µg/mL) (p=0 0.4). CRP levels were not different between survivors and non-survivors (106 ± 63 v. 85 ± 66) (p=0.22). Total WBC counts were not different between the groups. There was no relationship of CRP levels to WBC counts (Table 3).
Platelet counts had a negative correlation to CRP levels (r-0.4; p=0.002).
The mean CRP level of the infants in the septic group (Groups 1+2) was 87.4 ± 62.3 µg/mL compared with 5.2 ± 8 µg/mL (p=0.001) in Group 3. The platelet counts were significantly lower in infants in Group 1 compared with Group 2 and the platelet counts in Group 2 were significantly lower than in Group 3. There were no differences in the total WBC count among all three groups. The mean platelet count of the septic group (1+2) was lower than in Group 3: 132 700 ± 15 200/µL v. 212 000 ± 5 000/µL (p=0.0001). These data suggest that platelet counts were significantly lower and CRP levels higher in infants with sepsis (Groups 1 and 2) than in infants who had no sepsis (Group 3). We studied leukopenia (WBC <5 000/µL) and leukocytosis (WBC >30 000/µL) as markers of infection. The sensitivity and specificity of leukopenia (WBC <5 000/µL) was 1.82% and 93.86%, respectively while the sensitivity and specificity of leukocytosis (WBC >30 000/µL) were 7.27% and 94.74%, respectively.
We used two cut-off points for the CRP to determine the sensitivity and specificity at these two levels. Table 4 shows the sensitivity, specificity, positive and negative likelihood ratios, positive and negative predictive values, and accuracy for CRP values >10/µg/mL), and >15 µg/mL.
As expected, when the normal CRP cut-off value was changed from 10 to 15 µg/mL, the sensitivity of the test decreased from 94.5% to 90%, the specificity increased slightly from 91.2% to 93.8% and the accuracy changed from 92.8% to 90.9% (Table 4).
Accuracy is the overall probability that an elevated CRP will be indicative of sepsis in neonates.
In our institution, the cost for obtaining a CRP is US$3.00. The cost for CBC without manual differential count is US$5.00 and CBC with manual differential count is US$20.00. The cost of blood culture is US$25.00. Lumbar puncture with CSF analysis including culture is US$50.00 and urine analysis with culture is US$20.00. Thus, the total septic work-up evaluation costs approximately US$118.00, of which the charge for CRP constitutes only 2.5%. These charges may be considerably lower than in developed countries, but these are high when patients must pay out of their pocket, as the majority of the population in India do not have health insurance. With both sensitivity and specificity above 92% and accuracy of around 92%, we feel that CRP is a very valuable and cost-effective tool in the assessment of late-onset sepsis in preterm infants.
Discussion
Late-onset sepsis is reported to cause significant morbidity and mortality in high-risk infants.[17] The outcome of infants with sepsis is dependent on prompt recognition and treatment. The diagnosis of sepsis is difficult, based solely on clinical signs and symptoms because clinical presentation is vague and non specific in infants. This is the reason why biomarkers are important adjuncts in the early diagnosis of sepsis. Unfortunately, none of these tests is 100% sensitive and specific.[18 22] An ideal biomarker should be cost-effective and can be performed with a small volume of blood with fast turnaround time and has high sensitivity and specificity. Currently there is no biomarker that will meet all these requirements.
We included all infants who developed symptoms suspected to be due to sepsis. In neonates, several factors can give rise to such symptoms besides sepsis. These include environmental factors, anaemia, pain, metabolic factors, drugs, and infections. Although the gold standard for diagnosing sepsis is a positive blood culture, it takes 12 - 72 hours or longer for the resultsto become available. Delaying treatment until culture results are available leads to the high morbidity and mortality and hence is not the standard of care. Therefore, at the earliest suspicion of sepsis, neonates are treated with broad-spectrum antibiotics after work-up for sepsis which includes blood, urine and CSF cultures. To improve diagnostic accuracy, total and differential WBC count, platelet count and biomarkers such as CRP are routinely performed.[23]
The present study indicates that CRP has good sensitivity and specificity and can be used as an adjunct in diagnosing and managing late-onset sepsis in neonates. The number of infants with Gram-negative infections was significantly higher compared with Gram-positive sepsis. Although statistically not significant, the CRP levels tended to be higher in Gram-negative sepsis compared with Gram-positive sepsis. Whether Gram-negative sepsis evokes a higher inflammatory response is not certain. There was no difference in CRP levels between survivors and non-survivors, which suggests that CRP levels cannot be used to predict outcome in neonatal sepsis.
Limitations
The manual differential WBC counts and I:T ratios were performed in less than 40% of the cases owing to logistical reasons. Manual differential count is performed by the pathologist, who is not available to perform this during nights and weekends. The cost of a manual differential count is US$15.00; hence it was not routinely done. Therefore, we did not analyse the I:T ratio as a marker for sepsis in most of the babies. Consequently, the correlation of CRP to WBC differential count and I:T ratio (immature to total neutrophil) was not done. We included only infants who had symptoms and were screened for sepsis. There were no asymptomatic healthy infants included in this study, and hence the sensitivity and specificity results may be different. Because this is a retrospective study, the results need to be tested for reproducibility in a larger study prospectively.
In summary: our study indicates that, in infants with unexplained vague symptoms suggestive of sepsis, CRP is a valuable, low-cost biomarker. In low- and middle-income countries, CRP testing perhaps offers more value compared with WBC counts in the diagnosis of late-onset sepsis in neonates. We suggest that a prospective multi-centre study in low- and middle-income countries should be carried out to evaluate the clinical significance and cost-effectiveness of low-cost biomarkers such as CRP in the diagnosis of late-onset sepsis in neonates.
Appendix
Microbiology: blood culture pathogens: Gram-negative: Klebsiella (n=32), Ralstonia picketii (n=10), Acinetobacter (n=7), Serratia marcessans (n=2), Enterobacter (n=6), Burkholderia cepacian (n=1), Stenotrophomonas maltophilia (n=2), Pseudomonas (n=2), CoNS (n=6), Staphylococcus aureus (n=2), Candida (n=2).
Author contributions. SP contributed to the study conception and design. Material preparation, data collection and analysis were performed by IC. The first draft of the manuscript was written by IC and both authors commented on previous versions of the manuscript. Both authors read and approved the final manuscript.
Funding. No funding to report.
Conflicts of interest. No conflicts of interest or financial disclosures to report.
References
1. Stoll B, Gordon T, Shankaran S, et al. Incidence, presenting features, risks factors, and significance of late onset septicemia in very low birth weight infants. The NICHD neonatal research network. Pediatr Infect Dis J 1998;17(7):593-598. [ Links ]
2. Tillett WS, Francis T. Serological reactions in pneumonia with a non-protein somatic fraction of Pneumococcus. J ExperimMed 1930;52(4):561-571. [ Links ]
3. Volanakis JE. Human C-reactive protein: expression, structure, and function. Molec Immun 2001;38(2-3):189-197. [ Links ]
4. Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Invest 2003;111(12):1805-1812. [ Links ]
5. Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pedia Infect Dis J 1997;16(8):735-746. [ Links ]
6. Chirico G, Loda C. Laboratory aid to the diagnosis and therapy of infection in the neonate. Pediatric Rep 2011;3(1): el. [ Links ]
7. Mishra UK, Jacobs SE, Doyle LW, Garland SM. Newer approaches to the diagnosis of early onset neonatal sepsis. Arch Dis Childhood Fetal Neon Edition 2006;91(3):F208-212. [ Links ]
8. Kaapa P, Koistinen E. Maternal and neonatal C-reactive protein after interventions during delivery. Acta Obstetrícia Et Gynecologica Scand 1993;72(7):543-546. [ Links ]
9. Hengst JM. The role of C-reactive protein in the evaluation and management of infants with suspected sepsis. Adv Neon Care 2003;3(1):3-13. [ Links ]
10. Hofer N, Zacharias E, Muller W, Resch B. An update on the use of C-reactive protein in early-onset neonatal sepsis: Current insights and new tasks. Neonatology 2012; 102:25-36. [ Links ]
11. Beltempo M, Viel-Theriault I, Thibeault R, Julien A, Piedboeuf B. C-reactive protein for late-onset sepsis diagnosis in very low birth weight infants. BMC Pedia (2018):18:16. [ Links ]
12. Brown JVE, Meader N, Cleminson J, McGuire W. C-reactive protein for diagnosing late onset infection in newborn infants. Cochrane Database of Systematic Reviews 2019, Issue 1. Art. No.: CD012126. [ Links ]
13. Perrone S, Lotti F, Longini M, et al. C reactive protein in healthy term newborns during the first 48 hours of life. Arch Dis Child Fetal Neonatal Ed 2018;103(2):F163-F166. [ Links ]
14. Benitz WE, Han MY, Madan A, Ramachandra P. Serial serum C-reactive protein levels in the diagnosis of neonatal infection. Pediatrics 1998; 102(4) :E41. [ Links ]
15. Pourcyrous M, Bada HS, Korones SB, Baselski V, Wong SP. Significance of serial C-reactive protein responses in neonatal infection and other disorders. Pediatric 1993;92(3):431-435. [ Links ]
16. Kawamur aM, Nishida H. The usefulness of serial C-reactive protein measurement in managing neonatal infection. Acta Paediatrica 1995;84(1):10-13. [ Links ]
17. Stoll BJ, Hansen NI, Adams-Chapman I, et al. Neuro developmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA (2004);292:2357-2365. [ Links ]
18. Ng PC, Li K, Leung TF, et al. Early prediction of sepsis induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem 2006;52(6):1181-1189. [ Links ]
19. Laborada G, Rego M, Jain A, et al. Diagnostic value of cytokines and C-reactive protein in the first 24 hours of neonatal sepsis. Am J Perina 200 3;20(8) :491-501. [ Links ]
20. Sharma D, Farahbakhshb N, Shastric S, Sharmad P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J Matern-Fetal Neon Med 2018;131(12):1646-1659. [ Links ]
21. Ng PC, Cheng SH, Chui KM, et al. Diagnosis of late onset neonatal sepsis with cytokines, adhesion molecule, and C-reactive protein in preterm very low birthweight infants. Arch Dis Childhood 1997;77:F221-F227. [ Links ]
22. Hisamuddin E, Hisam A, Wahid S, Raza G. Validity of C-reactive protein (CRP) for diagnosis of neonatal sepsis. Pak J Med Sci 2015;31(3):527-531. [ Links ]
23. Amon S, Litmanovitz I, Regev R, Lis M, Shainkin-Kestenbaum R, Dolin T. The prognostic value of inflammatory markers during late-onset sepsis in preterm infants. J Perinat Med 2004;32(2):176-180. [ Links ]
 Correspondence:
Correspondence:
S Ponthenkandath
psasidha@gmail.com
Accepted 6 December 2021














