Servicios Personalizados
Articulo
Indicadores
Links relacionados
-
 Citado por Google
Citado por Google -
 Similares en Google
Similares en Google
Compartir
South African Journal of Child Health
versión On-line ISSN 1999-7671
versión impresa ISSN 1994-3032
S. Afr. j. child health vol.17 no.1 Pretoria 2023
http://dx.doi.org/10.7196/SAJCH.2023.v17i1.1884
RESEARCH
An atypical presentation of non-IgE-mediated cow's milk allergy associated with Staphylococcus aureus: A case study
T MabelaneI; M C KhabaII; E KiraguIII
IFCFP (Cert. Allerg.); Sefako Makgato Health Science University, Ga-Rankuwa, South Africa
IIFC Path (Anat.); Department of Anatomical Pathology, Dr George Mukhari Academic Laboratory, National Health Laboratory Service, Ga-Rankuwa, South Africa
IIIMMed Paeds (Cert. Allerg.); Department of Paediatrics and Child Health, Aga Khan University Hospital, Nairobi, Kenya
ABSTRACT
Cow's milk allergy (CMA) is one of the most common food allergies in the first years of life. CMA is classified as immunoglobulin E (IgE) or non-IgE-mediated reactions. IgE-mediated reactions are typically of immediate onset, with symptoms manifesting within 2 hours after ingestion. However, non-IgE-mediated reactions are delayed and mostly occur between 2 and 48 hours post exposure. This report describes a 17-year-old who presented with a long-standing history of pustular lesions immediately after cow's milk ingestion. Allergy tests, which included skin-prick test (SPT); specific IgE to cow's milk; cow's milk IgE components; and Cellular antigen stimulation test (CAST) yielded insignificant results. However, an oral food challenge resulted in pustular lesions and abdominal pain within 30 and 120 minutes, respectively. A swab from a pustule cultured Staphylococcus aureus. CMA was confirmed and managed with dietary restriction. It is uncommon for non-IgE reactions to occur with immediate symptoms. S. aureus may be associated with atypical skin manifestation of CMA.
The World Allergy Organization (WAO) estimates that 1.9 to 4.9% of children suffer from cow's milk allergy (CMA).[1,2] Approximately 80 - 90% of children develop tolerance before 3 years of age.[3] However, in some cases, CMA persists and may be associated with later development of other allergic diseases such as atopic dermatitis, asthma and rhino-conjunctivitis.[4]
CMA is an immune-mediated reaction to one or more cow's milk proteins (CMPs) and has a variable presentation based on the underlying immune mechanism.[5] However, there is clinical overlap between some presentations of CMA as indicated by the United States food allergy guidelines.[6] Immunoglobulin E (IgE)-mediated reactions have well defined clinical criteria with validated diagnostic tests that confirm sensitisation such as the skin-prick and specific IgE tests. The basophil activation test is also useful in selected patients although the gold standard of diagnosis is the oral food challenge (OFC) test.[7-9] Non-IgE-mediated reactions are more difficult to recognise and lack validated diagnostic tests, resulting in diagnostic challenges.[10]
The present case describes an atypical presentation of non-IgE-mediated CMA with manifestation of pustular lesions within 30 minutes of ingestion. A skin-prick test (SPT), specific IgE test, as well as a basophil activation test (BAT) were all negative despite a recurrent history of immediate cutaneous reactions. In this case, the OFC was especially useful in confirming the diagnosis.
Case
A 17-year-old girl presented with a suspected CMA persisting since infancy. She reported development of itchy skin and pustular lesions soon after consumption of cow's milk and other dairy products. She did not report other associated skin, respiratory or gastrointestinal symptoms. She was exclusively breastfed for 6 months, after which cow's milk formula was introduced, resulting in immediate development of pustular lesions which resolved after 48 hours. Cow's milk and other dairy products were eliminated from her diet and reintroduced in later childhood with similar results. Her past medical history was significant for childhood asthma and atopic dermatitis - both conditions resolved before puberty. Her father also reported allergic ocular symptoms following consumption of dairy products.
The physical examination was non-revealing. SPT showed the following results: 0 mm saline negative control; 4 mm histamine positive control; and no reaction to both fresh cow's milk and cow's milk extract.. Delayed SPT readings were not documented. Specific IgE to whole cow's milk, casein, lactoglobulin and lactoalbumin were also below detectable levels, with values <0.1 kUA/L (positive is >0.35 kUA/L). Cellular antigen stimulation test (CAST) was negative, with a value of 7.10% (>9.00% is positive).
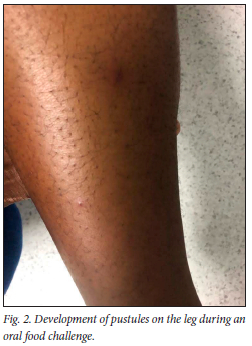
The patient was admitted for a graded OFC with increasing doses of cow's milk administered every 15 minutes. Thirty minutes into the challenge, the patient reported a sensation of itchy skin, followed by the development of multiple pustules on her face, neck (Fig. 1), arms (Fig. 2) legs and torso (Fig. 3). Her vital signs remained stable throughout the challenge which ended with a final dose of 100 mL and a total of 160 mL. After ingestion of the final dose, the patient reported abdominal pain without associated nausea, vomiting or diarrhoea. Oral antihistamine (10 mg cetirizine) was administered and she was observed for 2 hours after the challenge. The abdominal pain resolved within the observation period. A pus swab collected from skin pustules during the OFC cultured Staphyloccocus aureus. The pustules resolved within 8 days and the patient discontinued oral histamine use. No other medication was administered during the allergic reaction. CMA was confirmed and the patient was advised to continue with cow's milk protein elimination. Management included nutritional counselling and adequate calcium supplementation.


Discussion
IgE-mediated reactions are divided into immediate-onset, which arise within 2 hours of food ingestion, and immediate plus late-phase, in which immediate-onset symptoms are followed by prolonged or ongoing symptoms. Clinical manifestations include skin (urticaria, angioedema, eczema) abdominal (vomiting, diarrhoea, cramps) respiratory (rhinits, cough, wheeze, shortness of breath) and anaphylaxis.[11,12] Contrarily, non-IgE-mediated reactions are poorly defined clinically and scientifically and may be T-cell-mediated. Symptoms of non-IgE reactions are of delayed onset and occur 4 to 48 hours after ingestion of the allergen. Non-IgE-mediated food allergy may manifest as gastroesophageal reflux, vomiting, constipation, and diarrhoea and include clinical entities such as pulmonary haemosiderosis, gastroenteropathy, eosinophilic oesophagitis, enterocolitis and proctocolitis.[11-13]
Our patient presented with pustular lesions and abdominal pain within 2 hours of ingestion of cow's milk. Abdominal pain is a typical immediate-onset symptom and this is further supported by her response to H1 antihistamines. The pustular lesions, though of immediate onset, are not characteristic of immediate reactions and failed to respond to antihistamine therapy. Pustules are more likely to occur as a delayed reaction.
It is evident that early age of onset and severity of eczema are both associated with food allergy. [14] In the present case, a previous history of non-severe early-onset eczema was noted. S. aureus is a part of the normal microbiota of the skin; however, during eczema flares, this organism is isolated, although the mechanism remains unclear. A study by Tsilochristou et al.[15] showed that S. aureus, independent of eczema severity, is associated with food sensitisation and allergy and can impair tolerance to foods. This case study illustrates the influence of S. aureus on food allergy independent of eczema severity.
CMA is an immunologically mediated reaction to one or more milk proteins. These proteins are either casein or whey.[16] The diagnosis of an IgE-mediated CMA is based on the combination of clinical history and confirmatory sensitisation tests, including an SPT, specific IgE levels and, where indicated, basophil activation tests (BAT). The total IgE assay is nonspecific and provides only gross information,[17] therefore; it was not performed in our patient. Serum-specific IgE assays against allergen sources are the most commonly used in vitro diagnostic approaches.[17] OFCs are considered to be the gold standard.[18] In cases of nonspecific symptoms with a low risk of CMA or a high likelihood of non-IgE-mediated immune responses owing to CMP allergy (e.g. frequent regurgitation, constipation or bloody stools), allergy tests for CMA are not required in the primary diagnostic workup.[19-22]
Our patient's clinical presentation was not typical of non-IgE reactions, and hence an OFC was needed to confirm allergic reaction to CMP. It is imperative to rule out lactose intolerance in cases with isolated abdominal symptoms with negative SPTs or specific IgE tests for milk protein. Furthermore, hyper-IgE syndrome has to be considered in patients over 8 years of age, presenting with a clinical triad of recurrent staphylococcal abscesses, recurrent airway infections and increased serum IgE concentration.[23] Our patient did not meet the criteria for this syndrome as she presented with skin pustules during milk protein antigen exposure only and had no history of recurrent respiratory infections or skin abscesses.
The lack of validated diagnostic tests for non-IgE reactions requires elimination and reintroduction of the suspected allergen to confirm if symptoms reoccur upon reexposure. Although there may be a role for atopy-patch testing in the future for patients with negative CMP-specific IgE, there is no agreement on standardisation of the preparation and application of antigens.[24-26]
Conclusion
Non-IgE-mediated CMA is often a challenge to diagnose and may be missed in primary care settings. Non-IgE reactions to cow's milk are typically delayed; however, an immediate reaction was observed in our patient. Clinical presentation of pustular lesions has not been described in non-IgE-mediated reactions to food allergens. The present case study demonstrates an atypical presentation, associated with an abundance of S. aureus in pustular lesions.
Declaration. Written assent was obtained from the patient and informed consent was obtained from the patient's parents for publication of this case report.
Acknowledgements. We would like to thank the nursing team that assisted with the oral food challenge test.
Author contributions. TM conceptualised the report. TM, MCK and KE wrote the manuscript. All authors read and approved the manuscript for submission.
Funding. None.
Conflicts of interest. None.
References
1. World Health Organization. Global surveillance, prevention, and control of chronic respiratory diseases: A comprehensive approach. Geneva: WHO, 2007. http://www.who.int/gard/publications/GARD%20Book%202007.pdf (accessed 26 May 2021). [ Links ]
2. Fiocchi A, Brozek J, Schunemann H, et al. World Allergy Organization (WAO) diagnosis and rationale for action against Cow's milk allergy (DRACMA) guidelines. World Allergy Organ J 2010;3(4):57161. https://doi.org/10.1097/WOX.0b013e3181defeb9. [ Links ]
3. Venter C, Arshad SH. Epidemiology of food allergy. Pediatr Clin North Am 2011;58(2):327-349. https://doi.org/10.1016/j.pcl.2011.02.011 [ Links ]
4. Sampaio G, Marinho S, Prates S, Morais-Almeida M, Rosado-Pinto J. Transient vs. persistent cow's milk allergy and development of other allergic diseases. Allergy 2005;60(3):411-412. https://doi.org/10.1111/j.1398-9995.2004.00690.x [ Links ]
5. Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow's-milk protein allergy in infants and children: ESPGHAN GI Committee practical guidelines. J Pediatr Gastroenterol Nutr 2012,55(2):221-229. https://doi.org/10.1097/MPG.0b013e31825c9482 [ Links ]
6. Boyce JA, Assa'ad A, Burks AW, et al. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol 2010;126(6 Suppl):S1-S58 [ Links ]
7. Rueff F, KC, Brockow K, et al. Skin tests for diagnostics of allergic immediate-type reactions. Guideline of the German Society for Allergology and Clinical Immunology. 2011 ;65(8) : 484-95. https://doi.org/10.1055/s-0030-1256476. [ Links ]
8. Renz H, Biedermann T, Bufe A, et al. In vitro Allergiediagnostik. Allergol J 2010;19:110.28. https://doi.org/10.1007/s40629-015-0067-z [ Links ]
9. Uyttebroek AP, Sabato V, Faber MA, et al. Basophil activation tests: Time for a reconsideration. Expert Rev Clin Immunol 2014;10(10):1325-1335. https://doi.org/10.1586/1744666X.2014.959498 [ Links ]
10. Benhamou AH, Scháppi Tempia MG, Belli DC, Eigenmann PA. An overview of cow's milk allergy in children. Swiss Med Week 2009;139(21-22):300-307. https://doi.org/10.4414/smw.2009.12258 [ Links ]
11. Vandenplas Y, Abuabat A, Al-Hammadi S, et al. Middle East Consensus Statement on the Prevention, Diagnosis, and Management of Cow's Milk Protein Allergy. Pediatr Gastroenterol Hepatol Nutr 2014;17(2):61-73. https://doi.org/10.5223/pghn.2014.17.2.61 [ Links ]
12. Burks AW, Tang M, Sicherer S, et al. ICON: Food allergy. J Allergy Clin Immunol 2012;129(4):906-920. https://doi.org/10.1016/j.jaci.2012.02.001 [ Links ]
13. Shek LP, Bardina L, Castro R, et al. Humoral and cellular responses to cow milk proteins in patients with milk-induced IgE-mediated and nonIgE-mediated disorders. Allergy 2005;60:912-919. https://doi.org/10.1111/j.1398-9995.2005.00705.x [ Links ]
14. Hill DJ, Hosking CS, de Benedictis FM; EPAAC Study Group. Confirmation of the association between high levels of immunoglobulin E food sensitisation and eczema in infancy: An international study. Clin Exp Allergy 2008;38(1):161-168. https://doi.org/10.1111/j.1365-2222.2007.02861.x [ Links ]
15. Tsilochristou O, du Toit G, Sayre PH, et al. Association of Staphylococcus aureus colonisation with food allergy occurs independently of eczema severity. J Allergy Clin Immunol 2019;144(2):494-503. https://doi.org/10.1016/j.jaci.2019.04.025. [ Links ]
16. Hill DJ, Firer MA, Shelton MJ, et al. Manifestations of milk allergy in infancy: Clinical and immunologic findings. J Pediatr 1986;109(2):270-276. https://doi.org/10.1016/s0022-3476(86)80384-5 [ Links ]
17. Ansotegui IJ, Melioli G, Canonica GW, et al. IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper. World Allergy Organization J 2020;13(2):100080. https://doi.org/10.1016/j.waojou.2019.100080 [ Links ]
18. Kattan JD, Cocco RR, Jarvinen KM. Milk and soy allergy. Pediatr Clin North Am 2011;58:407-426. https://doi.org/10.1016/j.pcl.2011.02.005 [ Links ]
19. Arvola T, Ruuska T, Keranen J, et al. Rectal bleeding in infancy: Clinical, allergological, and microbiological examination. Pediatrics 2006;117:e760- 768. https://doi.org/10.1542/peds.2005-1069 [ Links ]
20. Iacono G, Cavataio F, Montalto G, et al. Intolerance of cow's milk and chronic constipation in children. N Engl J Med 1998;339(16):1100-1104. https://doi.org/10.1056/NEJM199810153391602 [ Links ]
21. Heine RG. Gastroesophageal reflux disease, colic and constipation in infants with food allergy. Curr Opin Allergy Clin Immunol 2006;6(3):220-225. https://doi.org/10.1097/01.all.0000225164.06016.5d [ Links ]
22. Heine RG. Allergic gastrointestinal motility disorders in infancy and early childhood. Pediatr Allergy Immunol 2008;19(5):383-391. https://doi.org/10.1111/j.1399-3038.2008.00785.x [ Links ]
23. Grimbacher B, Holland SM, Puck JM. Hyper-IgE syndromes. Immunol Rev 2005;203(X):244-250. https://doi.org/10.1111/j.0105-2896.2005.00228.x [ Links ]
24. Kalach N, Soulaines P, de Boissieu D, et al. A pilot study of the usefulness and safety of a ready-to-use atopy patch test (Diallertest) versus a comparator (Finn Chamber) during cow's milk allergy in children. J Allergy Clin Immunol 2005;116(6):1321-1326. https://doi.org/10.1016/j.jaci.2005.08.033 [ Links ]
25. Mehl A, Rolinck-Werninghaus C, Staden U, et al. The atopy patch test in the diagnostic workup of suspected food-related symptoms in children. J Allergy Clin Immunol 2006;118:923-929. https://doi.org/10.1016/j.jaci.2006.07.003 [ Links ]
26. Dupont C, Soulaines P, Lapillonne A, et al. Atopy patch test for early diagnosis of cow's milk allergy in preterm infants. J Pediatr Gastroenterol Nutr 2010;50(4):463-464. https://doi.org/10.1097/MPG.0b013e3181b97bed [ Links ]
 Correspondence:
Correspondence:
T Mabelane
tmabelane003@gmail.com
Accepted 10 February 2022














